5 Blood supply of the brain
Arterial Supply of the Forebrain
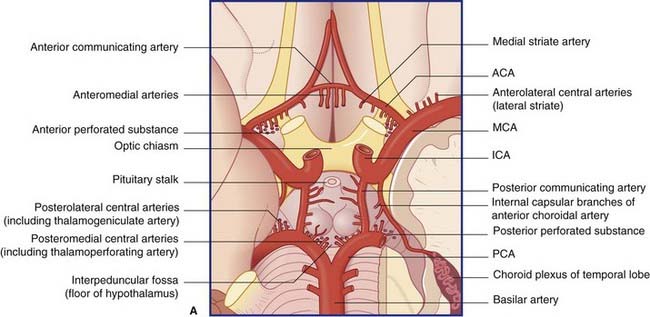
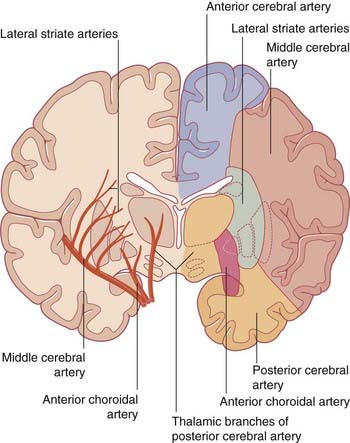
The blood supply to the forebrain is derived from the two internal carotid arteries and from the basilar artery (Figure 5.1).
Anterior cerebral artery (Figure 5.2)
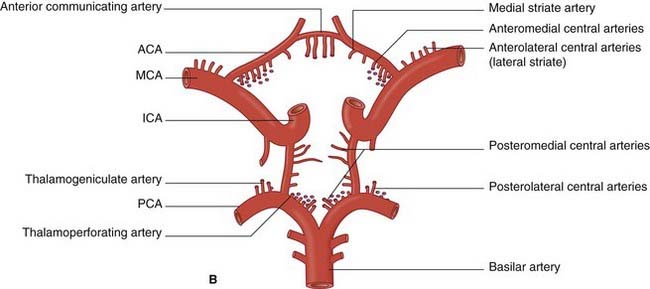
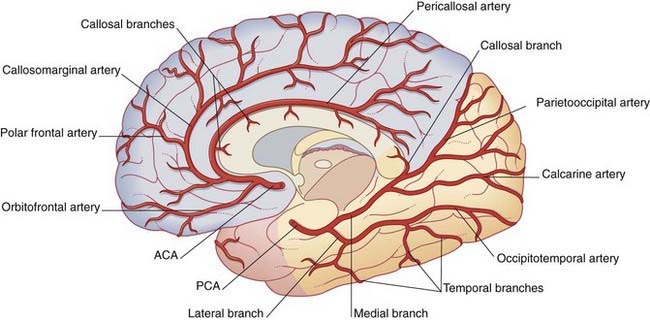
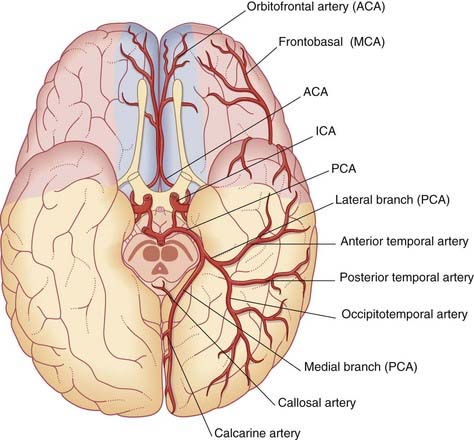
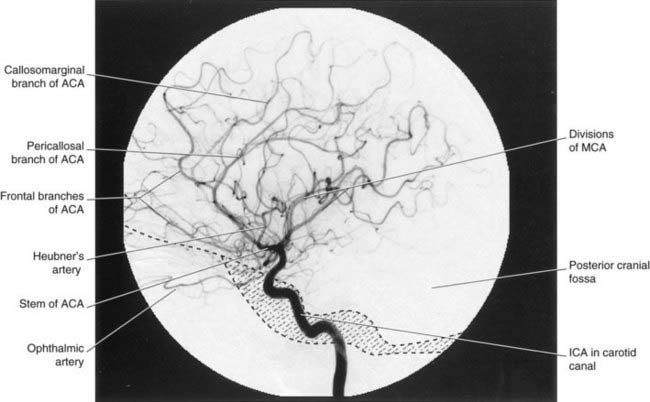
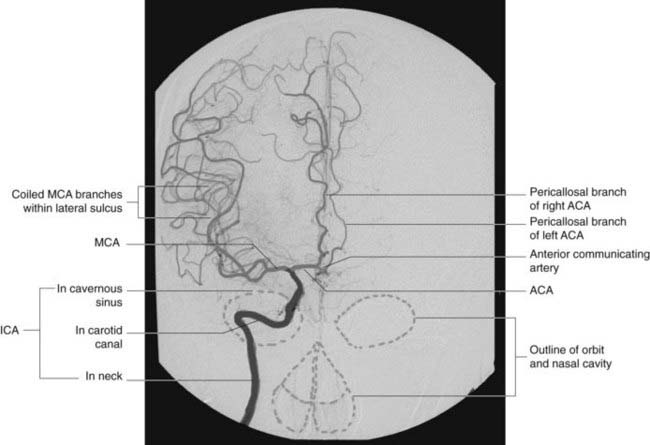
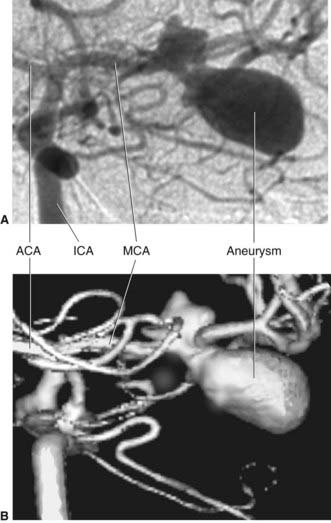
The anterior cerebral artery passes above the optic chiasm to gain the medial surface of the cerebral hemisphere. It forms an arch around the genu of the corpus callosum, making it easy to identify in a carotid angiogram (see later). Close to the anterior communicating artery, it gives off the medial striate artery, also known as the recurrent artery of Heubner (pron.‘Hoibner’), which contributes to the blood supply of the internal capsule. Cortical branches of the anterior cerebral artery supply the medial surface of the hemisphere as far back as the parietooccipital sulcus (Table 5.1). The branches overlap on to the orbital and lateral surfaces of the hemisphere.
Table 5.1 Named corticala branches of the anterior cerebral artery
| Branch | Territory |
|---|---|
| Orbitofrontal | Orbital surface of frontal lobe |
| Polar frontal | Frontal pole |
| Callosomarginal | Cingulate and superior frontal gyri; paracentral lobule |
| Pericallosal | Corpus callosum |
a The term cortical is conventional. Terminal is better, because these arteries also supply the underlying white matter.
Middle cerebral artery (Figure 5.3)
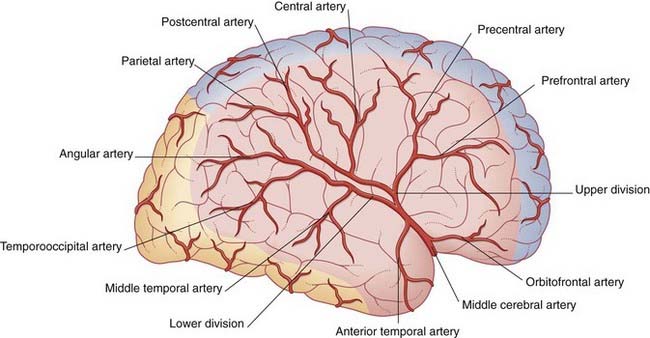
The middle cerebral artery is the main continuation of the internal carotid, receiving 60–80% of the carotid blood flow. It immediately gives off important central branches, then passes along the depth of the lateral fissure to reach the surface of the insula. There it usually breaks into upper and lower divisions. The upper division supplies the frontal lobe, the lower division supplies the parietal and temporal lobes and the midregion of the optic radiation. Named branches and their territories are listed in Table 5.2. Overall, the middle cerebral supplies two-thirds of the lateral surface of the brain.
| Origin | Branch(es) | Territory |
|---|---|---|
| Stem | Frontobasal | Orbital surface of frontal lobe |
| Anterior temporal | Anterior temporal cortex | |
| Upper division | Prefrontal | Prefrontal cortex |
| Precentral | Premotor areas | |
| Central | Pre- and postcentral gyri | |
| Postcentral | Postcentral and anterior parietal cortex | |
| Parietal | Posterior parietal cortex | |
| Lower division | Middle temporal | Midtemporal cortex |
| Temporooccipital | Temporal and occipital cortex | |
| Angular | Angular and neighboring gyri |
The central branches of the middle cerebral include the lateral striate arteries (Figure 5.4). These arteries supply the corpus striatum, internal capsule, and thalamus. Occlusion of one of the lateral striate arteries is the chief cause of classic stroke, where damage to the pyramidal tract in the posterior limb of the internal capsule causes hemiplegia, a term denoting paralysis of the contralateral arm, leg, and lower part of face.
Note: Additional information on the blood supply of the internal capsule is provided in Chapter 35.
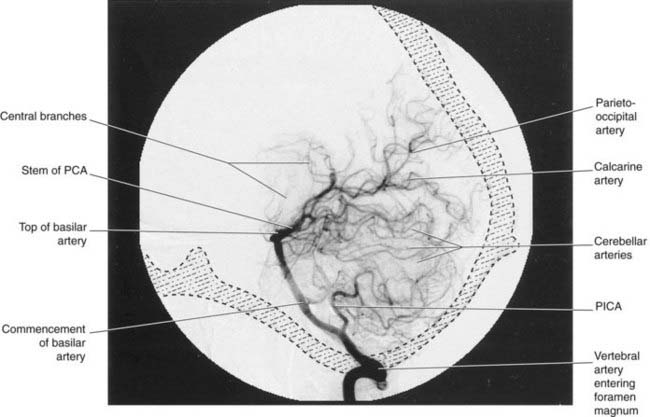
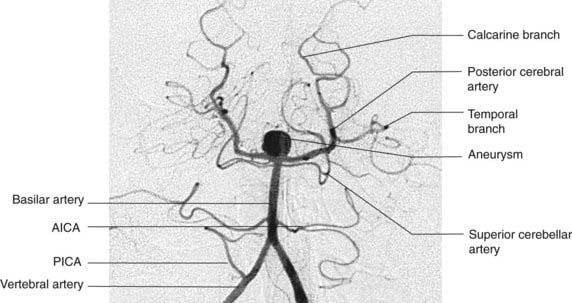
Posterior cerebral artery (Figures 5.2 and 5.5)
Close to its origin, each posterior cerebral artery gives branches to the midbrain and a posterior choroidal artery to the choroid plexus of the lateral ventricle. Additional, central branches are sent into the posterior perforated substance (Figure 5.1). The main artery winds around the midbrain in company with the optic tract. It supplies the splenium of the corpus callosum and the cortex of the occipital and temporal lobes. Named cortical branches and their territories are given in Table 5.3.
Table 5.3 Named cortical branches of the posterior cerebral artery
| Branch | Artery | Territory |
|---|---|---|
| Lateral | Anterior temporal | Anterior temporal cortex |
| Posterior temporal | Posterior temporal cortex | |
| Occipitotemporal | Posterior temporal and occipital cortex | |
| Medial | Calcarine | Calcarine cortex |
| Parietooccipital | Cuneus and precuneus | |
| Callosal | Splenium of corpus callosum |
Note: Additional information on the central branches is provided in Chapter 35.
Neuroangiography
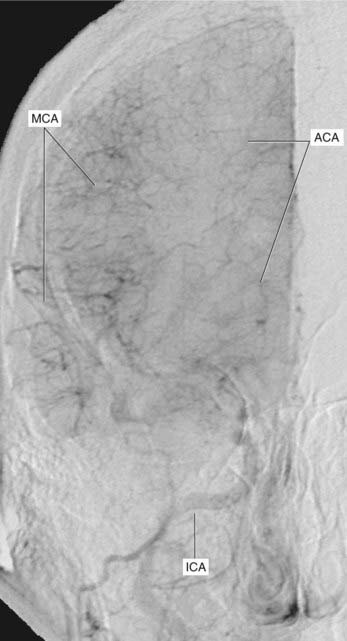
Arterial phases of carotid angiograms are shown in Figures 5.6–5.8.
Figure 5.9 was taken at the parenchymal phase, when the dye is filling a web of minute terminal branches of the anterior and middle cerebral arteries, some of these anastomosing on the brain surface but most occupying the parenchyma, i.e cortex and subjacent white matter.
Arterial Supply to Hindbrain
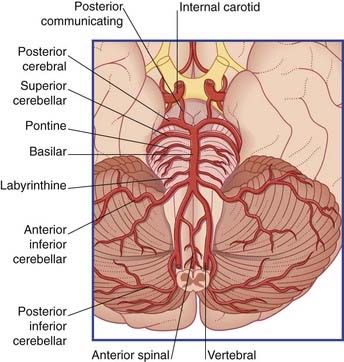
The brainstem and cerebellum are supplied by the vertebral and basilar arteries and their branches (Figure 5.10).
The two vertebral arteries arise from the subclavian arteries and ascend the neck in the foramina transversaria of the upper six cervical vertebrae. They enter the skull through the foramen magnum and unite at the lower border of the pons to form the basilar artery. The basilar artery ascends to the upper border of the pons and divides into two posterior cerebral arteries (Figures 5.11 and 5.12).
All primary branches of the vertebral and basilar arteries give branches to the brainstem.
Venous Drainage of the Brain
The venous drainage of the brain is of great importance in relation to neurosurgical procedures. It is also important to the professional neurologist, because a variety of clinical syndromes can be produced by venous obstruction, venous thrombosis, and congenital arteriovenous communications. In general medical practice, however, problems (other than subdural hematomas, Ch. 4) caused by cerebral veins are rare in comparison with arterial disease.
Superficial veins
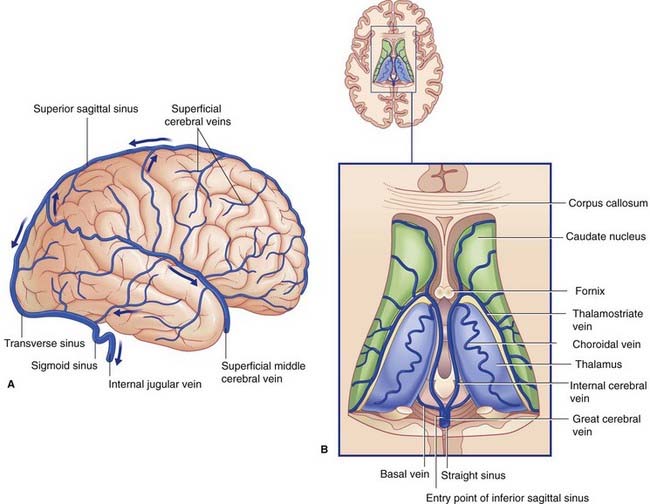
The superficial cerebral veins lie in the subarachnoid space overlying the hemispheres. They drain the cerebral cortex and underlying white matter, and empty into intracranial venous sinuses (Figures 5.13A, 5.14, and 5.15).
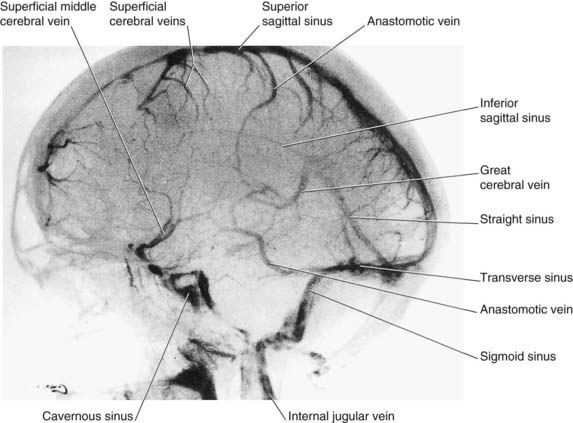
Figure 5.14 Internal carotid angiogram, venous phase, lateral view. The dye is draining into the dural venous sinuses.
(Photograph kindly provided by Dr. James Toland, Department of Radiology, Beaumont Hospital, Dublin, Ireland.)
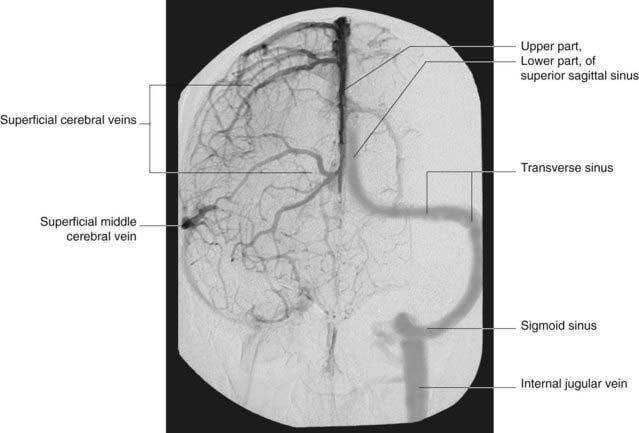
Figure 5.15 Internal carotid angiogram, venous phase, anteroposterior view. Same patient as in Figure 5.6; this picture taken circa 8 s later. The vascular pattern is unusual, in that the left rather than the right transverse sinus is dominant.
(Angiogram kindly provided by Dr. Pearse Morris, Director, Interventional Neuroradiology, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.)
The Blood–Brain Barrier
The extracellular compartments of the CNS are shown diagrammatically in Figure 5.16. As previously described (Ch. 4), CSF secreted by the choroid plexuses circulates through the ventricular system and the subarachnoid space before passing through the arachnoid villi into the dural venous sinuses. In addition, CSF diffuses passively through the ependyma–glial membrane lining the ventricles and enters the brain extracellular spaces. It adds to the ECF produced by the capillary bed and by cell metabolism, and it diffuses through the pia–glial membrane into the subarachnoid space. This ‘sink’ movement of fluid compensates for the absence of lymphatics in the CNS.
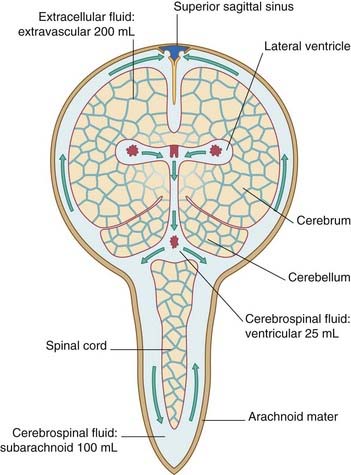
Figure 5.16 Extracellular compartments of the brain. Arrows indicate circulation of cerebrospinal fluid.
Relative contributions to the CSF obtained from a spinal tap are approximately as follow:
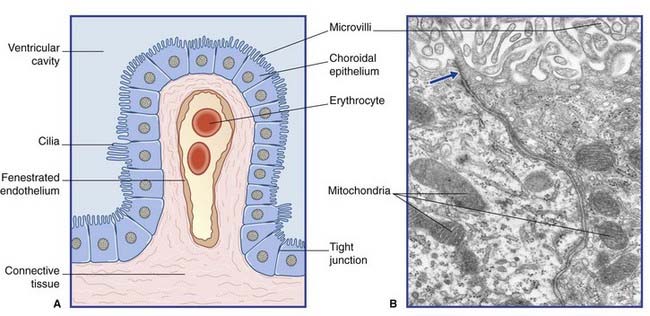
Blood–CSF barrier (Figure 5.17)
Blood–ECF barrier (Figure 5.18)
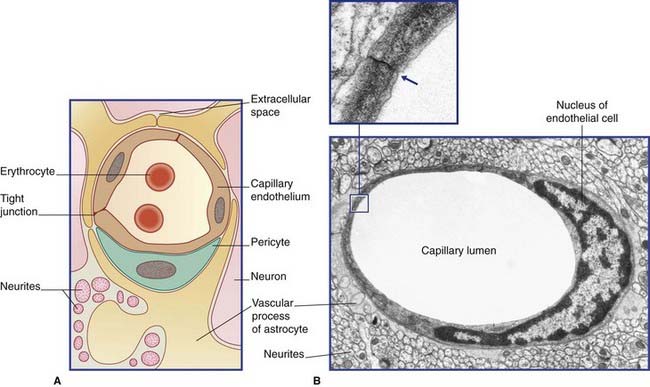
Figure 5.18 (A) Diagram of blood–extracellular fluid barrier. (Astrocytes are described in Ch. 6.) (B) Central nervous system capillary. In this transverse section, a single endothelial cell completely surrounds the lumen, its edges being sealed by a tight junction (inset). Outside its basement membrane, the capillary is invested with an astrocytic sheath.
(From Pannese 1994, with permission of Thieme.)
Roles of microvascular pericytes
Pericytes are also phagocytic, and possess immunoregulatory cytokines.
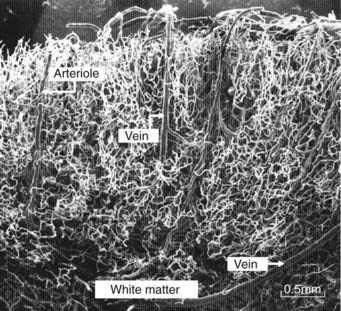
The surface area of the brain capillary bed is about the size of a tennis court! This huge area accounts for the brain’s consumption of 20% of basal oxygen intake by the lungs. The density of the cortical capillary bed is demonstrated in the latex cast shown in Figure 5.19.
Functions of the blood–brain barrier
For some clinical notes concerning the blood–brain barrier, see Clinical Panel 5.1. Clinical Panel 5.2 describes the knock-on effects of raised intracranial pressure.
Clinical Panel 5.1 Blood–brain barrier pathology
The following five conditions are associated with breakdown of the blood–brain barrier:
Clinical Panel 5.2 Intracranial pressure curve
Figure CP 5.2.1 represents progressive ventricular expansion in an adult case of hydrocephalus. As noted in Clinical Panel 4.3, hydrocephalus can result from obstruction of the fourth ventricular outlets by leptomeningeal webs caused by meningitis. The same effect can be induced by accumulated blood around the base of the brain following spontaneous arterial hemorrhage into the subarachnoid space.
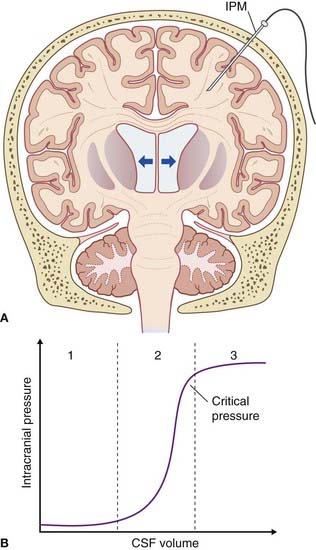
Figure CP 5.2.1 (A) Adult hydrocephalus. Arrows indicate compression of cerebral parenchyma by expanding lateral ventricles. IPM, intraparencymal pressure monitor. (B) Intracranial pressure–CSF volume curve. (Based on Steiner and Andrews 2006.)
Balabanonov B, Dore-Duffy P. Role of the microvascular pericyte in the blood–brain barrier. J Neurosci Res. 1998;53:637-644.
Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519-530.
Pannese E. Neurocytology. Fine structure of neurons, nerve processes and neuroglial cells. New York: Thieme; 1994.
Popescu BO, Toescu EC, Popescu LM, et al. Blood–brain barrier alterations in ageing and dementia. J Neurol Sci. 2009;283:99-106.
Scremin IU. Cerebral vascular system. In Paxinos G, Mai JK, editors: The human nervous system, ed 2, Amsterdam: Elsevier, 2004.
Steiner LA, Andrews PJD. Monitoring the injured brain: ICP and CBF. Br J Anaesth. 2006;1:26-38.