Chapter 54 Blood Products and Coagulation
3 What are the main red blood cell surface antigen systems?
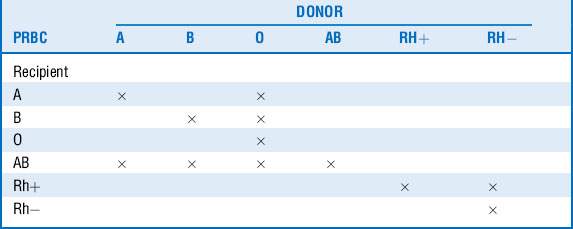
An individual’s red cells may express A, B, both, or no surface antigens, which determine that individual’s blood type. Those who do not express an antigen will eventually develop antibodies against it. People carrying anti-A or anti-B antibodies cannot receive red blood cells with the corresponding surface antigens, or immunologic destruction of the transfused red cells may occur. Consequently, type O individuals are considered universal donors, whereas AB individuals may donate only to other AB recipients. Similar to the ABO system, a separate Rh surface antigen exists that may be either present (Rh+) or absent (Rh−) from the red cell plasma membrane. Individuals who are Rh negative will develop antibodies to the Rh factor when exposed to Rh+ blood. This is not a problem with the initial exposure, but hemolysis may occur with subsequent transfusions (Table 54-1).
5 What are potential transfusion hazards?
10 What are the infectious risks of transfusion?
 The incidence of transmission of hepatitis C, human T-lymphotropic virus, and human immunodeficiency virus in the United States through a blood component transfusion is approximately 1:2,000,000 units transfused.
The incidence of transmission of hepatitis C, human T-lymphotropic virus, and human immunodeficiency virus in the United States through a blood component transfusion is approximately 1:2,000,000 units transfused.
 Hepatitis B is transmitted in 1:270,000 transfusions.
Hepatitis B is transmitted in 1:270,000 transfusions.
 The risk of transfusion-related bacterial infections is much higher at 1:2000 units of platelets transfused (platelets carry a higher risk of contamination because they are stored at room temperature).
The risk of transfusion-related bacterial infections is much higher at 1:2000 units of platelets transfused (platelets carry a higher risk of contamination because they are stored at room temperature).
 Transmission of other infectious agents (e.g., Babesia spp, variant Creutzfeldt-Jakob disease agent, West Nile virus) for which blood products are not routinely tested is possible, yet even rarer.
Transmission of other infectious agents (e.g., Babesia spp, variant Creutzfeldt-Jakob disease agent, West Nile virus) for which blood products are not routinely tested is possible, yet even rarer.
13 What are the most commonly used techniques of autologous transfusion?
 Preoperative autologous blood donation (PABD). Blood is donated at frequent intervals (as often as every 3 days) starting 4 to 6 weeks before surgery and transfused after surgery as needed. Benefits include freedom from hemolytic, allergic, and febrile reactions, as well as alloimmunization and transfusion-related infections.
Preoperative autologous blood donation (PABD). Blood is donated at frequent intervals (as often as every 3 days) starting 4 to 6 weeks before surgery and transfused after surgery as needed. Benefits include freedom from hemolytic, allergic, and febrile reactions, as well as alloimmunization and transfusion-related infections.
 Acute normovolemic intraoperative hemodilution (ANH). This blood conservation technique entails the removal of blood (typically 500-1500 mL) from a patient immediately before surgery, with maintenance of normovolemia with crystalloids and/or colloids. Intraoperative blood loss leads to smaller hemoglobin losses due to hemodilution. The removed blood, which is anticoagulated and stored for up to 8 hours, is reinfused during or after surgery as needed.
Acute normovolemic intraoperative hemodilution (ANH). This blood conservation technique entails the removal of blood (typically 500-1500 mL) from a patient immediately before surgery, with maintenance of normovolemia with crystalloids and/or colloids. Intraoperative blood loss leads to smaller hemoglobin losses due to hemodilution. The removed blood, which is anticoagulated and stored for up to 8 hours, is reinfused during or after surgery as needed.
 Intraoperative blood salvage (Cell Saver) (IBS). Blood is aspirated from the surgical field, anticoagulated, and collected for centrifuging. Salvaged red cells are washed and reinfused to the patient as needed. Contraindications include bacteremia, gross operative field contamination, and cancer.
Intraoperative blood salvage (Cell Saver) (IBS). Blood is aspirated from the surgical field, anticoagulated, and collected for centrifuging. Salvaged red cells are washed and reinfused to the patient as needed. Contraindications include bacteremia, gross operative field contamination, and cancer.
14 What else can be done to minimize blood loss and transfusion requirements?
 Antifibrinolytic agents (ε-aminocaproic acid and tranexamic acid [TXA]) are synthetic lysine analogs that have been used extensively, mainly in cardiac surgery, to minimize blood loss and decrease transfusion requirements. They also appear to minimize blood loss and improve survival in trauma patients, and their use has been increasing in the field. (Aprotinin, an older-generation antifibrinolytic, was withdrawn from the market in 2008 when it was found to be associated with a higher risk for cardiovascular complications and death). A recent multiinstitutional large prospective randomized clinical trial has shown survival advantage in trauma patients treated early with TXA.
Antifibrinolytic agents (ε-aminocaproic acid and tranexamic acid [TXA]) are synthetic lysine analogs that have been used extensively, mainly in cardiac surgery, to minimize blood loss and decrease transfusion requirements. They also appear to minimize blood loss and improve survival in trauma patients, and their use has been increasing in the field. (Aprotinin, an older-generation antifibrinolytic, was withdrawn from the market in 2008 when it was found to be associated with a higher risk for cardiovascular complications and death). A recent multiinstitutional large prospective randomized clinical trial has shown survival advantage in trauma patients treated early with TXA.
 Recombinant erythropoietin, a normally endogenously produced hormone that stimulates erythropoiesis, was previously thought to decrease tranfusion requirements and possibly improve survival in the critically ill. However, recent evidence suggests that the benefit may be too small to outweigh risks (thrombotic events). This finding, in addition to the fact that it does not work quickly enough to have a role in the management of acute blood loss, has led to the abandonment of its routine use in modern intensive care units.
Recombinant erythropoietin, a normally endogenously produced hormone that stimulates erythropoiesis, was previously thought to decrease tranfusion requirements and possibly improve survival in the critically ill. However, recent evidence suggests that the benefit may be too small to outweigh risks (thrombotic events). This finding, in addition to the fact that it does not work quickly enough to have a role in the management of acute blood loss, has led to the abandonment of its routine use in modern intensive care units.
 Recombinant human factor VIIa is licensed for use in patients with hemophilia but has gained momentum in recent years as a potent agent in controlling life-threatening hemorrhage, usually after trauma. However, significant complications (thromboembolic episodes), along with two prospective randomized trials in trauma patients that failed to show any clear benefits, have dampened enthusiasm for its use.
Recombinant human factor VIIa is licensed for use in patients with hemophilia but has gained momentum in recent years as a potent agent in controlling life-threatening hemorrhage, usually after trauma. However, significant complications (thromboembolic episodes), along with two prospective randomized trials in trauma patients that failed to show any clear benefits, have dampened enthusiasm for its use.
 Desmopressin increases plasma levels of von Willebrand factor (vWF) and factor VIII and is licensed for use in von Willebrand disease and hemophilia A. It can also be used to control bleeding in patients with uremia.
Desmopressin increases plasma levels of von Willebrand factor (vWF) and factor VIII and is licensed for use in von Willebrand disease and hemophilia A. It can also be used to control bleeding in patients with uremia.
 Factor concentrates (fibrinogen concentrate [FC], prothrombin complex concentrate [PCC]) have emerged recently as potential adjuvant therapies in the management of the acute, massive bleeding with associated hypofibrinogenemia (the former) and emergent reversal of warfarin anticoagulation (the latter). FC contains fibrinogen at very high concentrations (even higher than cryoprecipitate), and PCCs are preparations containing near-physiologic concentrations of factors II, IX, and X and proteins C and S and variable levels of factor VII. FC has been approved for management of acute bleeding episodes in patients with congenital fibrinogen deficiency, but PCC, although its use in Europe and Canada is on the rise, is still undergoing phase III testing in the United States.
Factor concentrates (fibrinogen concentrate [FC], prothrombin complex concentrate [PCC]) have emerged recently as potential adjuvant therapies in the management of the acute, massive bleeding with associated hypofibrinogenemia (the former) and emergent reversal of warfarin anticoagulation (the latter). FC contains fibrinogen at very high concentrations (even higher than cryoprecipitate), and PCCs are preparations containing near-physiologic concentrations of factors II, IX, and X and proteins C and S and variable levels of factor VII. FC has been approved for management of acute bleeding episodes in patients with congenital fibrinogen deficiency, but PCC, although its use in Europe and Canada is on the rise, is still undergoing phase III testing in the United States.
17 What is FFP?
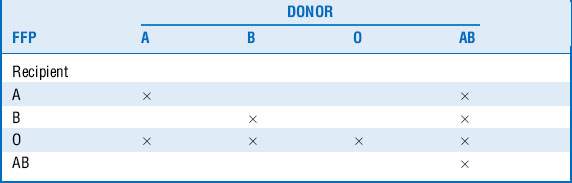
FFP is plasma obtained from single units of whole blood collected by apheresis. It is frozen and maintained at −18° to −30° C to preserve the labile coagulation factors. FFP contains all of the coagulation factors present in blood, along with antithrombin III and proteins C and S, at near-physiologic concentrations. It has a half life of approximately 1 year and has to be thawed before administration. It has to be matched for ABO system compatibility (Table 54-2) but not Rh compatibility.
21 What is measured by prothrombin time (PT)? What is international normalized ratio (INR)?
 PT is used to assess the extrinsic pathway of clotting, namely the activity of factor VII and the common pathway factors (fibrinogen and factors II, V, and X). It is prolonged in patients with liver disease, vitamin K deficiency, or circulating lupus anticoagulants or receiving warfarin therapy.
PT is used to assess the extrinsic pathway of clotting, namely the activity of factor VII and the common pathway factors (fibrinogen and factors II, V, and X). It is prolonged in patients with liver disease, vitamin K deficiency, or circulating lupus anticoagulants or receiving warfarin therapy.
 INR is a standardized method of reporting the PT, so that values from various laboratories can be directly comparable. It is most commonly used to monitor patients receiving oral warfarin therapy.
INR is a standardized method of reporting the PT, so that values from various laboratories can be directly comparable. It is most commonly used to monitor patients receiving oral warfarin therapy.
28 What is damage control resuscitation?
The basic tenets of damage control resuscitation are as follows:
 Avoid crystalloid resuscitation.
Avoid crystalloid resuscitation.
 Aim for permissive hypotension whenever possible.
Aim for permissive hypotension whenever possible.
 Prevent coagulopathy through early use of blood products.
Prevent coagulopathy through early use of blood products.
 Aggressively break the vicious cycle of acidosis, coagulopathy, and hypothermia.
Aggressively break the vicious cycle of acidosis, coagulopathy, and hypothermia.
Key Points Blood Products and Coagulation
1. Controlling the bleeding is more important than replacing the losses.
2. Blood products carry significant risks. Transfuse only when necessary.
3. Know the mechanism of warfarin and heparin anticoagulants and how they can be reversed.
4. Early use of blood component therapy can prevent development of coagulopathy in massively bleeding patients.
5. Excessive crystalloid resuscitation can worsen coagulopathy.
1 Alter H.J., Stramer S.L., Dodd R.Y. Emerging infectious diseases that threaten the blood supply. Semin Hematol. 2007;44:32–41.
2 Brown C.V., Foulkrod K.H., Sadler H.T., et al. Autologous blood transfusion during emergency trauma operations. Arch Surg. 2010;145:690–694.
3 Carless P.A., Henry D.A., Carson J.L., et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 10, 2010. CD002042, 2010
4 Carson J.L., Noveck H., Berlin J.A., et al. Mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion. Transfusion. 2002;42:812–818.
5 CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32.
6 Duchesne J.C., Hunt J.P., Wahl G., et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–276.
7 Hauser C.J., Boffard K., Dutton R., et al. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500.
8 Henry D.A., Carless P.A., Moxey A.J., et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 3, 2011. CD001886, 2011
9 Holcomb J.B., Wade C.E., Michalek J.E., et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458.
10 Inaba K., Lustenberger T., Rhee P., et al. The impact of platelet transfusion in massively transfused trauma patients. J Am Coll Surg. 2010;211:573–579.
11 Kennedy L.D., Case L.D., Hurd D.D., et al. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48:2285–2291.
12 Levi M., Levy J.H., Andersen H.F., et al. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800.
13 Martí-Carvajal A.J., Solà I., González L.E., et al. Pharmacological interventions for the prevention of allergic and febrile non-haemolytic transfusion reactions. Cochrane Database Syst Rev. 6, 2010. CD007539, 2010
14 Roberts I., Shakur H., Ker K., et al. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 1, 2011. CD004896, 2010
15 Schulman S., Kearon C., Kakkar A.K., et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352.
16 Vanderlinde E.S., Heal J.M., Blumberg N. Autologous transfusion. BMJ. 2002;324:772–775.