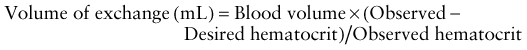Chapter 97 Blood Disorders
97.1 Anemia in the Newborn Infant
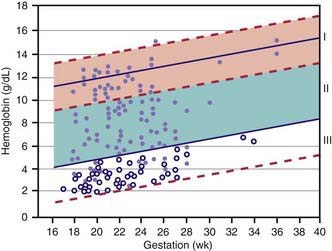
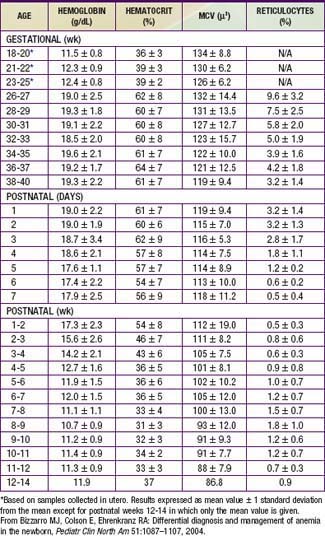
Hemoglobin increases with advancing gestational age: at term, cord blood hemoglobin is 16.8 g/dL (14-20 g/dL); hemoglobin levels in very low birthweight (VLBW) infants are 1-2 g/dL below those in term infants (Fig. 97-1). A hemoglobin value less than the normal range of hemoglobin for birthweight and postnatal age is defined as anemia (Table 97-1). A “physiologic” decrease in hemoglobin content is noticed at 8-12 wk in term infants (hemoglobin, 11 g/dL) and at about 6 wk in premature infants (7-10 g/dL).
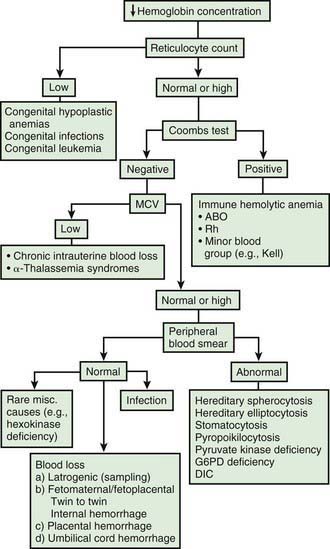
Infants born by cesarean section may have a lower hematocrit (Hct) than those born vaginally. Anemia at birth is manifested as pallor, heart failure, or shock (Fig. 97-2). It may be due to acute or chronic fetal blood loss, hemolysis, or underproduction of erythrocytes. Specific causes include hemolytic disease of the newborn, tearing or cutting of the umbilical cord during delivery, abnormal cord insertion, communicating placental vessels, placenta previa or abruptio, nuchal cord, incision into the placenta, internal hemorrhage (liver, spleen, intracranial), α-thalassemia, congenital parvovirus infection or other hypoplastic anemias, and twin-twin transfusion in monozygotic twins with arteriovenous placental connections (Chapter 92).
Treatment of neonatal anemia by blood transfusion depends on the severity of symptoms, the hemoglobin level, and the presence of co-morbid diseases (bronchopulmonary dysplasia, cyanotic congenital heart disease, respiratory distress syndrome) that interfere with oxygen delivery. The need for treatment with blood should be balanced against the risks of transfusion, including hemolytic transfusion reactions, exposure to blood product preservatives and other potential toxins, volume overload, possible increased risk of retinopathy of prematurity and necrotizing enterocolitis, graft-versus-host (GVH) reaction, and transfusion-acquired infection (cytomegalovirus [CMV], HIV, parvovirus, hepatitis B and C) (Chapter 468). The risk of CMV infection can be almost eliminated by the use of leukoreduced blood. In the infant <1,500 g, CMV antibody-negative leukoreduced blood should be used. The risk of acquiring HIV and hepatitis B and C viruses is reduced but not eliminated by antibody screening of donated blood. Blood banking techniques that limit multiple donor exposure should be encouraged.
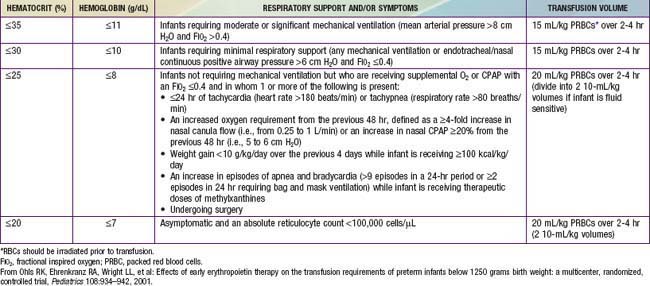
Although transfusion guidelines for preterm infants have been proposed (Table 97-2), they have not been subjected to rigorous clinical study. Nonetheless, these guidelines have led to a decline in the number of unnecessary transfusions. The use of restrictive vs more liberal transfusion guidelines has been examined in two randomized trials, one conducted at University of Iowa and a second multicentric trial known as the PINT (Premature Infants in Need of Transfusion) study. The restrictive guidelines in the two groups were generally similar. In the Iowa trial, the transfusion thresholds in the liberal- and restrictive-transfusion groups were <46% and <34%, respectively, in tracheally intubated infants receiving assisted ventilation; <38% and <28%, respectively, in infants receiving nasal continuous positive airway pressure or supplemental oxygen; and <30% and <22%, respectively, in infants breathing room air. The transfusion thresholds for the liberal groups were higher in the Iowa trial than in the PINT study. In both trials, the use of restrictive thresholds resulted in fewer transfusions and also increased the number of infants who received no transfusions at all. However, in the Iowa trial (but not in the PINT study), restrictive transfusion thresholds were associated with increases in major cranial ultrasonographic abnormalities and in the frequency of apneic spells. Although these findings need further evaluation in clinical studies, the issue of finding an appropriate transfusion threshold in premature infants remains unresolved.
Aher S, Ohlsson A: Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants, Cochrane Database Syst Rev (3):CD004868, 2006.
Anderson C. Critical haemoglobin thresholds in premature infants. Arch Dis Child Fetal Neonatal Ed. 2001;84:F146-F148.
Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685-1691.
Bizzarro MJ, Colson E, Ehrenkranz RA. Differential diagnosis and management of anemia in the newborn. Pediatr Clin North Am. 2004;51:1087-1107.
Christensen RD, Henry E. Hereditary spherocytosis on neonates with hyperbilirubinemia. Pediatrics. 2010;125:120-125.
Ferguson D, Hébert PC, Lee SK, et al. Clinical outcomes following institution of universal leukoreduction of blood transfusions for premature infants. JAMA. 2003;289:1950-1956.
Hébert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941-1949.
Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates. JAMA. 2007;297:1241-1252.
Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301-307.
Nicaise C, Gire C, Casha P, et al. Erythropoietin as treatment for late hyporegenerative anemia in neonates with Rh hemolytic disease after in utero exchange transfusion. Fetal Diagn Ther. 2002;17:22-24.
Ohlsson A, Aher SM: Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants, Cochrane Database Syst Rev (3):CD004863, 2006.
Rabe H, Reynolds G, Diaz-Rossello J: Early versus delayed umbilical cord clamping in preterm infants, Cochrane Database Syst Rev (4):CD003248, 2004.
97.2 Hemolytic Disease of the Newborn (Erythroblastosis Fetalis)
Hemolytic Disease of the Newborn Caused by Rh Incompatibility
Clinical Manifestations
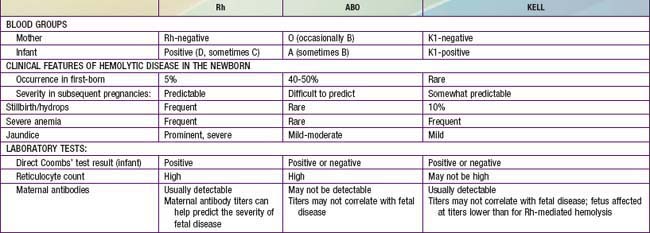
A wide spectrum of hemolytic disease occurs in affected infants born to sensitized mothers, depending on the nature of the individual immune response. The severity of the disease may range from only laboratory evidence of mild hemolysis (15% of cases) to severe anemia with compensatory hyperplasia of erythropoietic tissue leading to massive enlargement of the liver and spleen. When the compensatory capacity of the hematopoietic system is exceeded, profound anemia occurs and results in pallor, signs of cardiac decompensation (cardiomegaly, respiratory distress), massive anasarca, and circulatory collapse. This clinical picture of excessive abnormal fluid in two or more fetal compartments (skin, pleura, pericardium, placenta, peritoneum, amniotic fluid), termed hydrops fetalis, frequently results in death in utero or shortly after birth. With the use of anti-D gamma globulin to prevent Rh sensitization, nonimmune (nonhemolytic) conditions have become frequent causes of hydrops (Table 97-3). The severity of hydrops is related to the level of anemia and the degree of reduction in serum albumin (oncotic pressure), which is due in part to hepatic dysfunction. Alternatively, heart failure may increase right heart pressure, with the subsequent development of edema and ascites. Failure to initiate spontaneous effective ventilation because of pulmonary edema or bilateral pleural effusions results in birth asphyxia; after successful resuscitation, severe respiratory distress may develop. Petechiae, purpura, and thrombocytopenia may also be present in severe cases as a result of decreased platelet production or the presence of concurrent disseminated intravascular coagulation.
| CATEGORY | DISORDER(S) |
|---|---|
| Anemia | Immune (Rh, Kell) hemolysis |
| α-Thalassemia | |
| Red blood cell enzyme deficiencies (glucose-6-phosphate dehydrogenase) | |
| Fetomaternal hemorrhage | |
| Donor in twin-to-twin transfusion | |
| Diamond-Blackfan syndrome | |
| Cardiac dysrhythmias | Supraventricular tachycardia |
| Atrial flutter | |
| Congenital heart block | |
| Structural heart lesions | Premature closure of foramen ovale |
| Tricuspid insufficiency | |
| Hypoplastic left heart | |
| Endocardial cushion defect | |
| Cardiomyopathy | |
| Endocardial fibroelastosis | |
| Tuberous sclerosis with cardiac rhabdomyoma | |
| Pericardial teratoma | |
| Vascular | Chorioangioma of placenta, chorionic vessels, or umbilical vessels |
| Umbilical artery aneurysm | |
| Angiomyxoma of umbilical cord | |
| True knot of umbilical cord | |
| Hepatic hemangioma | |
| Cerebral arteriovenous malformation (aneurysm of vein of Galen) | |
| Angiosteohypertrophy (Klippel-Trénaunay syndrome) | |
| Thrombosis of renal or umbilical vein or inferior vena cava | |
| Recipient in twin-to-twin transfusion | |
| Lymphatic | Lymphangiectasia |
| Cystic hygroma | |
| Chylothorax, chylous ascites | |
| Noonan syndrome | |
| Multiple pterygium syndrome | |
| Central nervous system | Absent corpus callosum |
| Encephalocele | |
| Intracranial hemorrhage | |
| Holoprosencephaly | |
| Thoracic lesions | Cystic adenomatoid malformation of lung |
| Mediastinal teratoma | |
| Diaphragmatic hernia | |
| Sequestered lung | |
| Teratomas | Choriocarcinoma |
| Sacrococcygeal teratoma | |
| Tumors and storage diseases | Neuroblastoma |
| Hepatoblastoma | |
| Gaucher disease | |
| Niemann-Pick disease | |
| Mucolipidosis | |
| GM1 gangliosidosis | |
| Mucopolysaccharidosis | |
| Chromosome abnormalities | Trisomy 13, 15, 16, 18, 21 |
| XX/XY, 45XO | |
| Partial duplication of chromosome 11, 15, 17, 18 | |
| Partial deletion of chromosome 13, 18 | |
| Triploidy | |
| Tetraploidy | |
| Bone diseases | Osteogenesis imperfecta |
| Asphyxiating thoracic dystrophy | |
| Skeletal dysplasias | |
| Congenital infections | Cytomegalovirus |
| Parvovirus | |
| Rubella | |
| Toxoplasmosis | |
| Syphilis | |
| Leptospirosis | |
| Chagas disease | |
| Others | Bowel obstruction with perforation and meconium peritonitis, volvulus |
| Hepatic fibrosis | |
| Beckwith-Wiedemann syndrome | |
| Prune-belly syndrome | |
| Congenital nephrosis | |
| Infant of a diabetic mother | |
| Myotonic dystrophy | |
| Neu-Laxova syndrome | |
| Maternal therapy with indomethacin | |
| Fetal akinesia | |
| Idiopathic | Multiple congenital anomaly syndromes |
* The incidence of nonimmune (nonhemolytic) hydrops fetalis is 1/2,000-1/3,500 births.
Modified from Phibbs R. In Polin N, Fox W, editors: Fetal and neonatal physiology, ed 2, Philadelphia, 1998, WB Saunders.
Diagnosis
Antenatal Diagnosis
In Rh-negative women, a history of previous transfusions, abortion, or pregnancy should suggest the possibility of sensitization. Expectant parents’ blood types should be tested for potential incompatibility, and the maternal titer of IgG antibodies to D antigen should be assayed at 12-16, 28-32, and 36 wk of gestation. Fetal Rh status may be determined by isolating fetal cells or fetal DNA (plasma) from the maternal circulation. The presence of elevated antibody titers at the beginning of pregnancy, a rapid rise in titer, or a titer of 1:64 or greater suggests significant hemolytic disease, although the exact titer correlates poorly with the severity of disease. If a mother is found to have antibody against D antigen at a titer of 1:16 (15 IU/mL in Europe) or greater at any time during a subsequent pregnancy, the severity of fetal disease should be monitored by Doppler ultrasonography of the middle cerebral artery and then percutaneous umbilical blood sampling (PUBS) if indicated (Chapter 90). If the mother has a history of a previously affected infant or a stillbirth, an Rh-positive infant is usually equally or more severely affected than the previous infant, and the severity of disease in the fetus should be monitored.
Assessment of the fetus may require information obtained from ultrasonography and PUBS. Real-time ultrasonography is used to detect the progression of disease, with hydrops defined as skin or scalp edema, pleural or pericardial effusions, and ascites. Early ultrasonographic signs of hydrops include organomegaly (liver, spleen, heart), the double–bowel wall sign (bowel edema), and placental thickening. Progression to polyhydramnios, ascites, pleural or pericardial effusions, and skin or scalp edema may then follow. If pleural effusions precede ascites and hydrops by a significant time, causes other than fetal anemia should be suspected (see Table 97-4). Extramedullary hematopoiesis and, less so, hepatic congestion compress the intrahepatic vessels and produce venous stasis with portal hypertension, hepatocellular dysfunction, and decreased albumin synthesis.
Hydrops is present with a fetal hemoglobin level <5 g/dL, frequent with a level <7 g/dL, and variable with levels between 7 and 9 g/dL. Real-time ultrasonography predicts fetal well-being by means of the biophysical profile (see Table 90-2), whereas Doppler ultrasonography assesses fetal distress by demonstrating increased vascular resistance in fetal arteries (middle cerebral). In pregnancies with ultrasonographic evidence of hemolysis (hepatosplenomegaly), early or late hydrops, or fetal distress, further and more direct assessment of fetal hemolysis should be performed.
Treatment
Treatment of An Unborn Infant
Survival of severely affected fetuses has been improved by the use of fetal ultrasonography to identify the need for in utero transfusion. Intravascular (umbilical vein) transfusion of packed RBCs is the treatment of choice for fetal anemia, replacing intrauterine transfusion into the fetal peritoneal cavity. Hydrops or fetal anemia (Hct < 30%) is an indication for umbilical vein transfusion in infants with pulmonary immaturity (see Fig. 97-1). Intravascular fetal transfusion is facilitated by maternal and hence fetal sedation with diazepam and by fetal paralysis with pancuronium. Packed RBCs are given by slow-push infusion after being cross-matched against the mother’s serum. The cells should be obtained from a CMV-negative donor and irradiated to kill lymphocytes to avoid GVH disease. Of note, leukoreduction alone (without irradiation) does not prevent GVH disease. Transfusions should achieve a post-transfusion Hct of 45-55% and can be repeated every 3-5 wk. Indications for delivery include pulmonary maturity, fetal distress, complications of PUBS, and 35-37 wk of gestation. The survival rate for intrauterine transfusions is 89%; the complication rate is 3%. Complications include rupture of the membranes and preterm delivery, infection, fetal distress requiring emergency cesarean section, and perinatal death.
Exchange Transfusion
When an infant’s clinical condition at birth does not require an immediate full or partial exchange transfusion, the decision to perform one should be based on a judgment that the infant has a high risk of rapid development of a dangerous degree of anemia or hyperbilirubinemia. Cord hemoglobin value of 10 g/dL or less and bilirubin concentration of 5 mg/dL or more suggest severe hemolysis but inconsistently predict the need for exchange transfusion. Some physicians consider previous kernicterus or severe erythroblastosis in a sibling, reticulocyte counts >15%, and prematurity to be additional factors supporting a decision for early exchange transfusion (Chapters 96.3 and 96.4). Intrauterine, intravascular transfusions have decreased the need for exchange transfusion.
The hemoglobin concentration, Hct, and serum bilirubin level should be measured at 4-6 hr intervals initially, with extension to longer intervals if and as the rate of change diminishes. The decision to perform an exchange transfusion is based on the likelihood that the trend of bilirubin levels plotted against hours of age indicates that serum bilirubin will reach the levels indicated in Figure 96-12 and Table 96-7. Term infants with bilirubin levels ≥20 mg/dL have an increased risk of kernicterus. Ordinary transfusions of compatible Rh-negative, leukoreduced, and irradiated RBCs may be necessary to correct anemia at any stage of the disease up to 6-8 wk of age, when the infant’s own blood-forming mechanism may be expected to take over. Weekly determinations of hemoglobin or Hct values should be performed until a spontaneous rise has been demonstrated.
Careful monitoring of the serum bilirubin level is essential until a falling trend has been demonstrated in the absence of phototherapy (Chapter 96.3). Even then, an occasional infant, particularly if premature, may experience an unpredicted significant rise in serum bilirubin as late as the 7th day of life. Attempts to predict the attainment of dangerously high levels of serum bilirubin on the basis of observed levels exceeding 6 mg/dL in the 1st 6 hr or 10 mg/dL in the 2nd 6 hr of life or on rates of rise exceeding 0.5-1.0 mg/dL/hr can be unreliable. Measurement of unbound bilirubin may be a more sensitive predictor of the risk associated with hyperbilirubinemia.
After exchange transfusion, the bilirubin level must be determined at frequent intervals (every 4-8 hr) because bilirubin may rebound 40-50% within hours. Repeated exchange transfusions should be carried out to keep the indirect fraction from exceeding the levels indicated in Table 96-7 for preterm infants and 20 mg/dL for term infants. Symptoms suggestive of kernicterus are mandatory indications for exchange transfusion at any time.
Hemolytic Disease of the Newborn Caused by Blood Group A and B Incompatibility
Treatment
Phototherapy may be effective in lowering serum bilirubin levels (Chapter 96.4). In severe cases, IVIG administration can reduce the rate of hemolysis and the need for exchange transfusion. Exchange transfusions with type O blood of the same Rh type as the infant may be needed in some cases to correct dangerous degrees of anemia or hyperbilirubinemia. Indications for this procedure are similar to those previously described for hemolytic disease due to Rh incompatibility. Some infants with ABO hemolytic disease may require transfusion of packed RBCs at several weeks of age because of slowly progressive anemia. Post-discharge monitoring of hemoglobin or Hct is essential in newborns with ABO hemolytic disease.
Other Forms of Hemolytic Disease
Blood group incompatibilities other than Rh or ABO account for < 5% of hemolytic disease of the newborn. The direct Coombs test result is invariably positive, and exchange transfusion may be indicated for hyperbilirubinemia and anemia. Hemolytic disease, anemia, and hydrops fetalis as a result of anti-Kell antibodies are not predictable from the previous obstetric history, amniotic fluid bilirubin determinants, or the maternal antibody titer. Erythroid suppression may contribute to the anemia; PUBS is beneficial in actually measuring the fetal Hct. Kell-alloimmunized infants often have inappropriately low numbers of circulating reticulocytes in comparison with other forms of hemolytic disease, which can cause difficulties in the laboratory confirmation of the hemolytic etiology of hyperbilirubinemia. The clinical characteristics of hemolytic disease due to Rh, ABO, and Kell antigen systems are summarized in Table 97-4.
Kumar S. Universal RHD genotyping in fetuses. BMJ. 2008;336:783-784.
Kumar S, Regan F. Management of pregnancies with RhD alloimmunization. Br Med J. 2005;330:1255-1258.
Moise KJJr. Diagnosing hemolytic disease of the fetus—time to put the needles away. N Engl J Med. 2006;355:192-194.
Patra K, Storfer-Isser A, Siner B, et al. Adverse events associated with neonatal exchange transfusion in the 1990s. J Pediatr. 2004;144:626-631.
van Kamp IL, Klumper FJ, Oepkes D, et al. Complications of intrauterine intravascular transfusion for fetal anemia due to maternal red-cell alloimmunization. Am J Obstet Gynecol. 2005;192:171-177.
97.3 Plethora in the Newborn Infant (Polycythemia) (See Also Chapter 461)
Dempsey EM, Barrington K. Short and long term outcomes following partial exchange transfusion in the polycythaemic newborn: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2006;91:F2-F6.
Pappas A, Delaney-Black V. Differential diagnosis and management of polycythemia. Pediatr Clin North Am. 2004;51:1063-1086.
Schimmel MS, Bromiker R, Soll RF. Neonatal polycythemia: is partial exchange transfusion justified? Clin Perinatol. 2004;31:545-553.
Wong W, Fok TF, Lee CH, et al. Randomized controlled trial: comparison of colloid or crystalloid for partial exchange transfusion for treatment of neonatal polycythaemia. Arch Dis Child. 1997;77:F115-F118.
97.4 Hemorrhage in the Newborn Infant
Hemorrhagic Disease of the Newborn
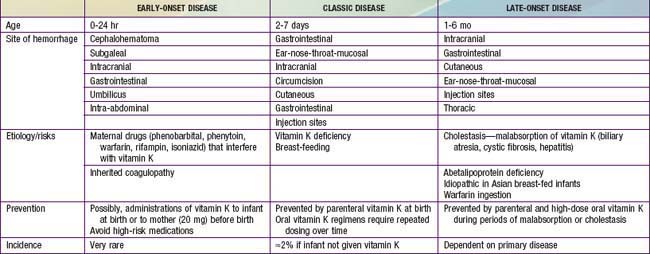
A moderate decrease in factors II, VII, IX, and X normally occurs in all newborn infants by 48-72 hr after birth, with a gradual return to birth levels by 7-10 days of age. This transient deficiency of vitamin K–dependent factors is probably due to lack of free vitamin K from the mother and absence of the bacterial intestinal flora normally responsible for the synthesis of vitamin K. Rarely in term infants and more frequently in premature infants, accentuation and prolongation of this deficiency between the 2nd and 7th days of life result in spontaneous and prolonged bleeding. Breast milk is a poor source of vitamin K, but hemorrhagic complications are more frequent in breast-fed than in formula-fed infants. This classic form of hemorrhagic disease of the newborn, which is responsive to and prevented by vitamin K therapy, must be distinguished from disseminated intravascular coagulopathy and from the more infrequent congenital deficiencies of one or more of the other factors that are unresponsive to vitamin K (Chapter 470). Early-onset life-threatening vitamin K deficiency–induced bleeding (onset from birth to 24 hr) also occurs if the mother has been treated with drugs (phenobarbital, phenytoin) that interfere with vitamin K function. Late onset (>2 wk) is often associated with vitamin K malabsorption, as noted in neonatal hepatitis or biliary atresia (Table 97-5).
Other forms of bleeding may be clinically indistinguishable from hemorrhagic disease of the newborn responsive to vitamin K, but they are neither prevented nor successfully treated with vitamin K. A clinical pattern identical to that of hemorrhagic disease of the newborn may also result from any of the congenital defects in blood coagulation (Chapters 470 and 471). Hematomas, melena, and post-circumcision and umbilical cord bleeding may be present; only 5-35% of cases of factor VIII and IX deficiency become clinically apparent in the newborn period. Treatment of the rare congenital deficiencies of coagulation factors requires fresh frozen plasma or specific factor replacement.
Disseminated intravascular coagulopathy in newborn infants results in consumption of coagulation factors and bleeding. Affected infants are often premature; the clinical course is frequently characterized by asphyxia, hypoxia, acidosis, shock, hemangiomas, or infection. Treatment is directed at correcting the primary clinical problem, such as infection, interrupting consumption of clotting factors, and replacing them (Chapter 477).
American Academy of Pediatrics Committee on Fetus and Newborn. Controversies concerning vitamin K and the newborn. Pediatrics. 2003;112:191-192.
Puckett RM, Offringa M: Prophylactic vitamin K for vitamin K deficiency bleeding in neonates, Cochrane Database Syst Rev (4):CD002776, 2000.
van Hasselt PM, de Koning TJ, Kvist N, et al. Prevention of vitamin K deficiency bleeding in breastfed infants: lessons from the Dutch and Danish biliary atresia registries. Pediatrics. 2008;121:e857-e863.