Blood and Tissue Protozoa
1. Explain the general life cycle of Plasmodium spp. including both asexual and sexual stages, exoerythrocytic and erythrocytic cycle trophozoites, schizonts, hypnozoites, merozoites, gametocytes, and sporozoites.
2. Describe the distinguishing morphologic characteristics, clinical disease, vectors, stages of infectivity, and laboratory diagnosis for Plasmodium spp., Babesia spp., and Trypanosoma and Leishmania spp.
3. Define paroxysm in malarial periodicity.
4. Compare and contrast recrudescence and relapse including the physiologic basis for each during infection with malaria.
5. Compare and contrast the pathogenesis of infections with P. falciparum, P. malariae, P. ovale, P. vivax, and P. knowlesi including variation in signs and symptoms.
6. Differentiate intracellular forms of Babesia spp. from Plasmodium spp.
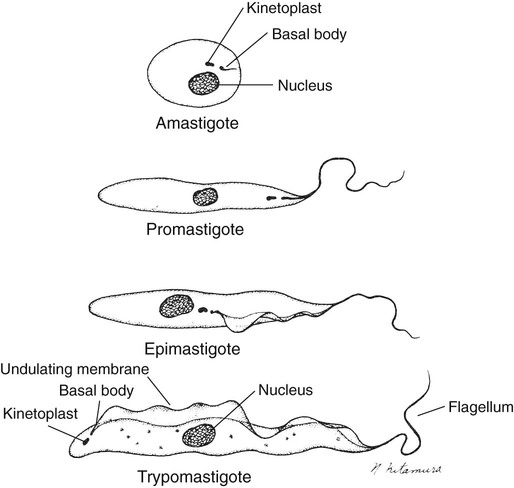
7. Define and describe the life cycle stages of Trypanosoma and Leishmania spp. including amastigotes, promastigotes, trypomastigotes, epimastigotes, and metacyclic trypanosome forms when appropriate.
Plasmodium spp.
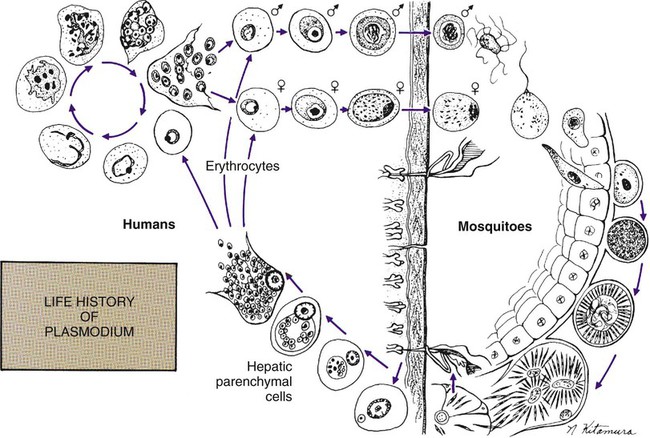
The vector for malaria is the female anopheline mosquito. When the vector takes a blood meal, sporozoites contained in the salivary glands of the mosquito are discharged into the puncture wound (Figure 49-1). Within an hour, these infective sporozoites are carried via the blood to the liver, where they penetrate hepatocytes and begin to grow, initiating the preerythrocytic or primary exoerythrocytic cycle. The sporozoites become round or oval and begin dividing repeatedly. Schizogony results in large numbers of exoerythrocytic merozoites. Once these merozoites leave the liver, they invade the red blood cells (RBCs), initiating the erythrocytic cycle. A dormant schizogony may occur in P. vivax and P. ovale organisms, which remain quiescent in the liver. These resting stages have been termed hypnozoites and lead to a true relapse, often within 1 year or up to more than 5 years later. Delayed schizogony does not occur in P. falciparum, P. malariae, or P. knowlesi.
Plasmodium Vivax (Benign Tertian Malaria)
General Characteristics
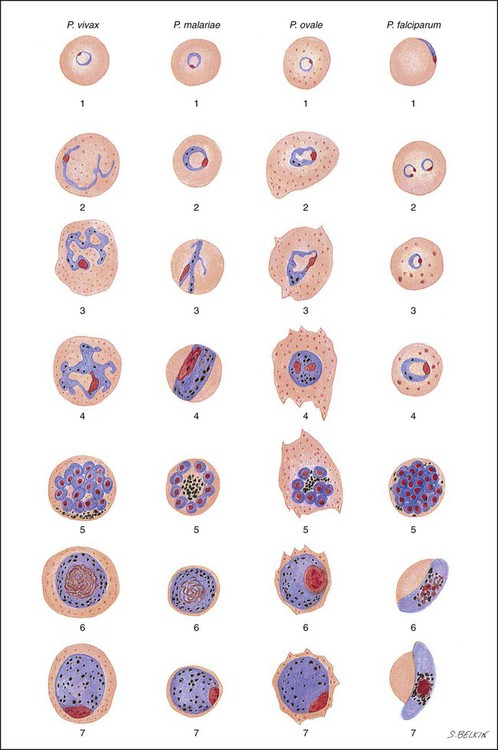
P. vivax infects only the reticulocytes; thus, the parasitemia is limited to approximately 2% to 5% of the available RBCs (Tables 49-1 to 49-3, Figures 49-2 and 49-3). Splenomegaly occurs during the first few weeks of infection, and the spleen will progress from being soft and palpable to hard, with continued enlargement during a chronic infection. If the infection is treated during the early phases, the spleen will return to its normal size. A secondary or dormant schizogony occurs in P. vivax and P. ovale, which remain quiescent in the liver. These resting stages have been termed hypnozoites.
TABLE 49-1
Plasmodium spp.: Clinical Characteristics of the Five Human Infections
| Infection | P. vivax | P. ovale | P. malariae | P. falciparum | P. knowlesi | Comments |
| Incubation period | 8-17 days | 10-17 days | 18-40 days | 8-11 days | 9-12 days | All may be extended for months to years |
| Prodromal symptoms Severity Initial fever pattern |
Mild to moderate Irregular (48 hr) |
Mild Irregular (48 hr) |
Mild to moderate Regular (72 hr) |
Mild Continuous remittent |
Mild to moderate Regular (24 hr) |
All may mimic influenza symptoms Early symptoms may reflect lack of regular periodicity |
| Symptom periodicity | 48 hr | 48 hr | 72 hr | 36-48 hr | 24-27 hr | |
| Initial paroxysm Severity Mean duration |
Moderate to severe 10 h |
Mild 10 h |
Moderate to severe 11 h |
Severe 16-36 h |
Moderate to severe Not available |
P. knowlesi might increase/lose virulence on passage in humans |
| Duration of untreated primary attack | 3-8+ wk | 2-3 wk | 3-24 wk | 2-3 wk | Not available | |
| Duration of untreated infection | 5-7 yr | 12 mo | 20+ yr | 6-17 mo | Not available | |
| Parasitemia limitations | Young RBCs | Young RBCs | Old RBCs | All RBCs | All RBCs | |
| Anemia | Mild to moderate | Mild | Mild to moderate | Severe | Moderate to severe | P. knowlesi can be as dangerous as P. falciparum |
| CNS involvement | Rare | Possible | Rare | Very common | Possible | |
| Nephrotic syndrome | Possible | Rare | Very common | Rare | Probably common |
TABLE 49-2
Plasmodia in Giemsa-Stained Thin Blood Smears
| Plasmodium vivax | Plasmodium malariae | Plasmodium falciparum | Plasmodium ovale | Plasmodium knowlesi | |
| Persistence of exoerythrocytic cycle | Yes | No | No | Yes | No |
| Relapses | Yes | No, but long-term recrudescence is recognized | No long-term relapses | Possible, but usually spontaneous recovery | No |
| Time of cycle | 44-48 hr | 72 hr | 36-48 hr | 48 hr | 24 hr |
| Appearance of parasitized RBCs; size and shape | 1.5-2 times larger than normal; oval to normal; may be normal size until ring fills half of cell | Normal shape; size may be normal or slightly smaller | Both normal | 60% of cells larger than normal and oval; 20% have irregular, frayed edges | Normal shape, size |
| Schüffner’s dots (eosinophilic stippling) | Usually present in all cells except early ring forms | None | None; occasionally comma-like red dots are present (Maurer’s dots) | Present in all stages including early ring forms; dots may be larger and darker than in P. vivax | No true stippling; occasional faint dots |
| Color of cytoplasm | Decolorized, pale | Normal | Normal, bluish tinge at times | Decolorized, pale | Normal |
| Multiple rings/cell | Occasional | Rare | Common | Occasional | Common |
| All developmental stages present in peripheral blood | All stages present | Ring forms few, since ring stage brief; mostly growing and mature trophozoites and schizonts | Young ring forms and no older stages; few gametocytes | All stages present | All stages present |
| Appearance of parasite; young trophozoite (early ring form) | Ring is 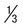 diameter of cell, cytoplasmic circle around vacuole; heavy chromatin dot diameter of cell, cytoplasmic circle around vacuole; heavy chromatin dot |
Ring often smaller than in P. vivax, occupying 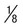 of cell; heavy chromatin dot; vacuole at times “filled in”; pigment forms early of cell; heavy chromatin dot; vacuole at times “filled in”; pigment forms early |
Delicate, small ring with small chromatin dot (frequently 2); scanty cytoplasm around small vacuoles; sometimes at edge of red cell (appliqué form) or filamentous slender form; may have multiple rings per cell | Ring is larger and more ameboid than in P. vivax; otherwise similar to P. vivax | Rings 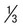 to to 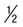 diameter of RBC; double chromatin dots; appliqué forms rare; multiple rings per RBC diameter of RBC; double chromatin dots; appliqué forms rare; multiple rings per RBC |
| Growing trophozoite | Multishaped irregular ameboid parasite; streamers of cytoplasm close to large chromatin dot; vacuole retained until close to maturity; increasing amounts of brown pigment | Non-ameboid rounded or band-shaped solid forms; chromatin may be hidden by coarse dark brown pigment | Heavy ring forms; fine pigment grains | Ring shape maintained until late in development; non-ameboid compared to P. vivax | Slightly ameboid and irregular; band forms seen; very little pigment |
| Mature trophozoite | Irregular ameboid mass; 1 or more small vacuoles retained until schizont stage; fills almost entire cell; fine brown pigment | Vacuoles disappear early; cytoplasm compact, oval, band shaped, or nearly round and almost filling cell; chromatin may be hidden by peripheral coarse dark brown pigment | Not seen in peripheral blood (except in severe infections); development of all phases following ring form occurs in capillaries of viscera | Compact; vacuoles disappear; pigment dark brown, less than in P. malariae | Denser cytoplasm (slightly ameboid) band forms seen; little to no malaria pigment (scattered, fine brown grains) |
| Schizont (pre-segmenter) | Progressive chromatin division; cytoplasmic bands containing clumps of brown pigment | Similar to P. vivax except smaller; darker, larger pigment granules peripheral or central | Not seen in peripheral blood (see above) | Smaller and more compact than P. vivax | Between 2 and 5 divided nuclear chromatin masses; abundant pigment granules occupy 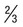 of RBC of RBC |
| Mature schizont | 16 (12-24) merozoites, each with chromatin and cytoplasm, filling entire red cell, which can hardly be seen | 8 (6-12) merozoites in rosettes or irregular clusters filling normal-sized cells, which can hardly be seen; central arrangement of brown-green pigment | Not seen in peripheral blood | 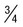 of cells occupied by 8 (8-12) merozoites in rosettes or irregular clusters of cells occupied by 8 (8-12) merozoites in rosettes or irregular clusters |
RBCs normal size; distorted/fimbriated RBCs very rare; occupy whole RBC; maximum of 16 merozoites; no rosettes; grapelike clusters |
| Macrogametocyte | Rounded or oval homogeneous cytoplasm; diffuse delicate light brown pigment throughout parasite; eccentric compact chromatin | Similar to P. vivax, but fewer in number; pigment darker and more coarse | Gender differentiation difficult; “crescent” or “sausage” shapes characteristic; may appear in “showers” with black pigment near chromatin dot, which is often central | Smaller than P. vivax | Occupy most of RBC; bluish cytoplasm; dense pink chromatin at periphery of parasite |
| Microgametocyte | Large pink to purple chromatin mass surrounded by pale or colorless halo; evenly distributed pigment | Similar to P. vivax, but fewer in number; pigment darker and more coarse | Same as macrogametocyte (described above) | Smaller than P. vivax | Occupy most of RBC; cytoplasm pinkish purple; early forms similar to mature trophozoite |
| Main criteria | Large pale red cell; trophozoite irregular; pigment usually present; Schüffner’s dots not always present; several phases of growth seen in one smear; gametocytes appear as early as third day | Red cell normal in size and color; trophozoites compact, stain usually intense, band forms not always seen; coarse pigment; no stippling of red cells; gametocytes appear after a few weeks | Development following ring stage takes place in blood vessels of internal organs; delicate ring forms and crescent-shaped gametocytes are only forms normally seen in peripheral blood; gametocytes appear after 7-10 days | Red cell enlarged, oval, with fimbriated edges; Schüffner’s dots seen in all stages; gametocytes appear after 4 days or as late as 18 days | Ring forms compact; single/double chromatin dots, appliqué forms, multiple rings/RBC (mimic P. falciparum); overall RBCs not enlarged; developing stages mimic P. malariae (band forms, 16 merozoites in mature schizont, but no rosettes) |
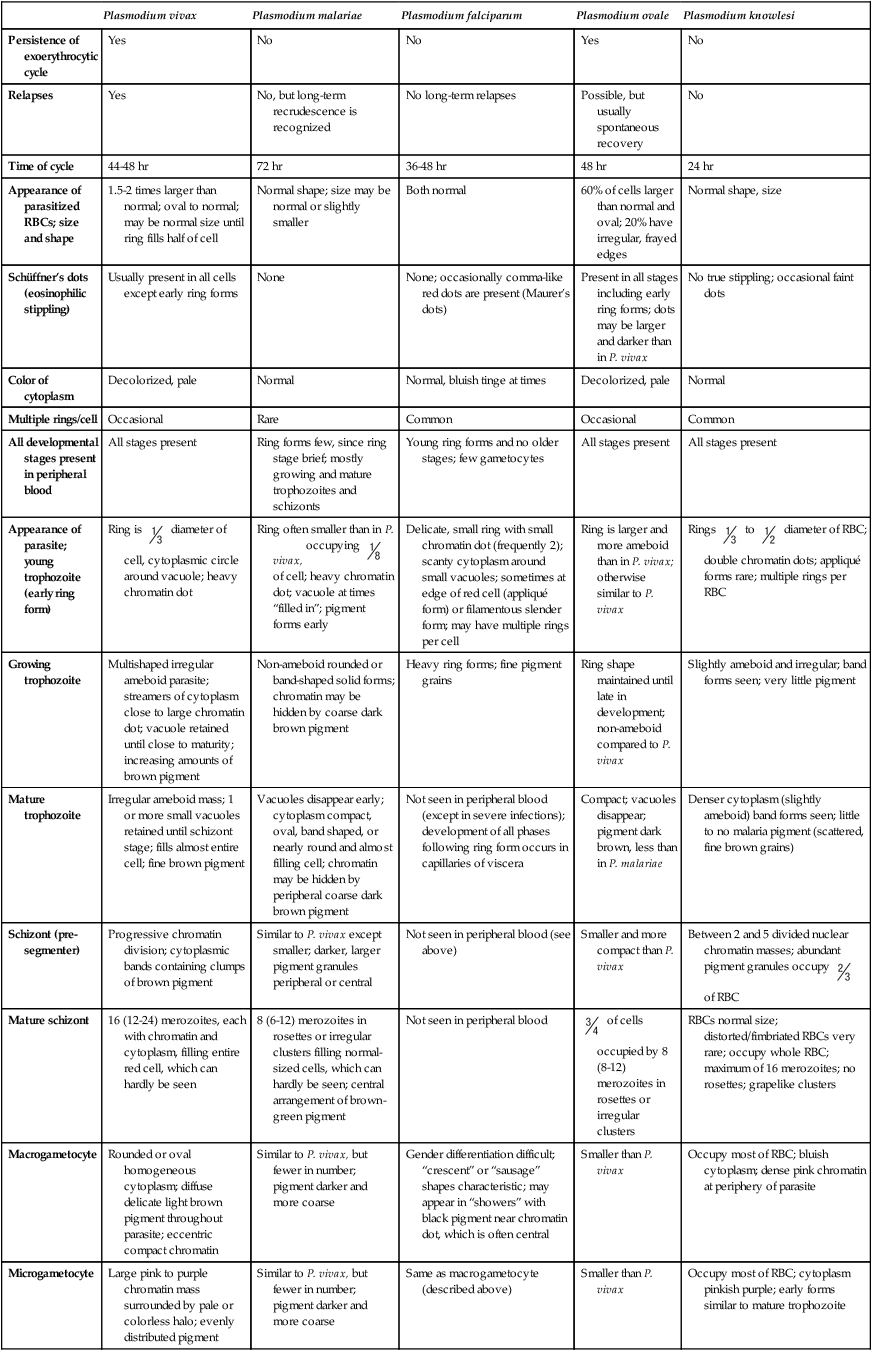
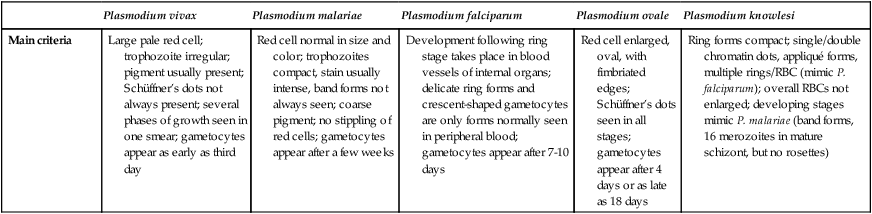
TABLE 49-3
Malaria Characteristics with Fresh Blood or Blood Collected Using EDTA with No Extended Lag Time*
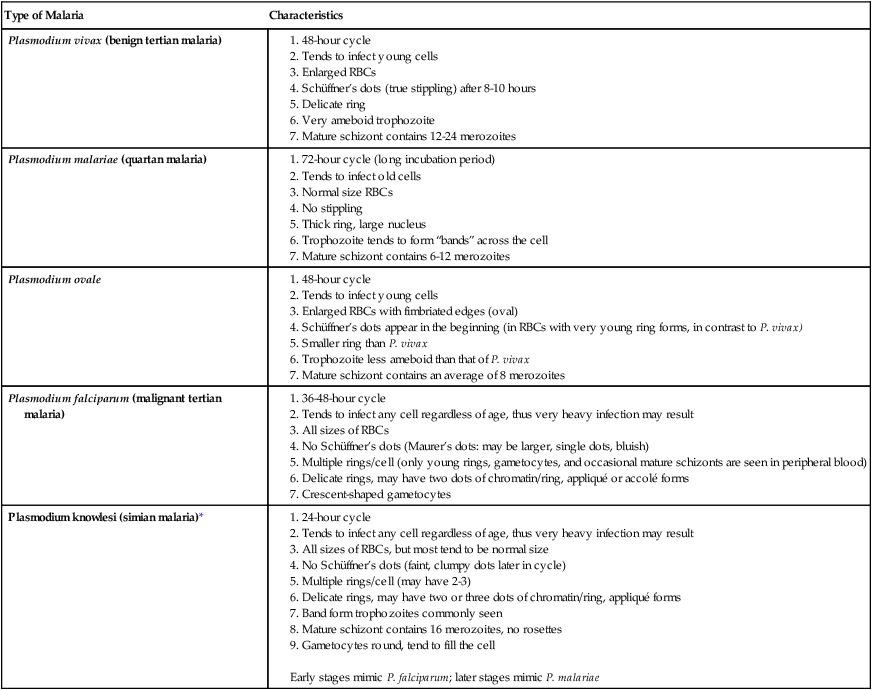
| Type of Malaria | Characteristics |
| Plasmodium vivax (benign tertian malaria) |
2. Tends to infect any cell regardless of age, thus very heavy infection may result
4. No Schüffner’s dots (Maurer’s dots: may be larger, single dots, bluish)
5. Multiple rings/cell (only young rings, gametocytes, and occasional mature schizonts are seen in peripheral blood)
6. Delicate rings, may have two dots of chromatin/ring, appliqué or accolé forms
2. Tends to infect any cell regardless of age, thus very heavy infection may result
3. All sizes of RBCs, but most tend to be normal size
4. No Schüffner’s dots (faint, clumpy dots later in cycle)
5. Multiple rings/cell (may have 2-3)
6. Delicate rings, may have two or three dots of chromatin/ring, appliqué forms
7. Band form trophozoites commonly seen
Early stages mimic P. falciparum; later stages mimic P. malariae
*Preparation of thick and thin blood films within <60 min of collection.
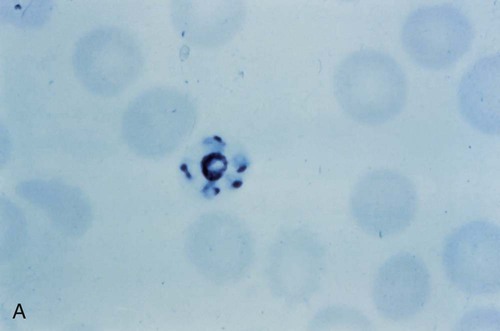
After a few days of irregular periodicity, a regular 48-hour cycle is established. An untreated primary attack may last from 3 weeks to 2 months or longer. Over time, the paroxysms (symptomatic period) become less severe and more irregular in frequency and then cease altogether. In approximately 50% of patients infected with P. vivax, relapses occur after weeks, months, or even after 5 years or more. The RBCs tend to be enlarged (young RBCs), there may be Schüffner’s dots (exclusively found in P. vivax and P. ovale) after 8 to 10 hours, the developing rings are ameboid, and the mature schizont contains 12 to 24 merozoites (Figure 49-3 [3]).
Plasmodium Ovale
General Characteristics
Although P. ovale and P. vivax infections are clinically similar, P. ovale malaria is usually less severe, tends to relapse less frequently, and usually ends with spontaneous recovery, often after no more than 6 to 10 paroxysms (see Tables 49-1 to 49-3, Figures 49-2 and 49-3). Like P. vivax, P. ovale infects only the reticulocytes, so that the parasitemia is limited to approximately 2% to 5% of the available RBCs. For many years the literature has stated that as with P. vivax, a secondary or dormant schizogony occurs in P. ovale, which remain quiescent in the liver. However, newer findings indicate that hypnozoites have never been demonstrated by biologic experiments.
Plasmodium Malariae (Quartan Malaria)
General Characteristics
P. malariae invades primarily the older RBCs, limiting the number of infected cells (see Tables 49-1 to 49-3, Figures 49-2 and 49-3). The incubation period between infection and symptoms may be much longer than that for P. vivax or P. ovale malaria, ranging from about 27 to 40 days. A regular periodicity is seen from the beginning, with a more severe paroxysm, including a longer cold stage and more severe symptoms during the hot stage. Collapse during the sweating phase is not uncommon.
Plasmodium Falciparum (Malignant Tertian Malaria)
General Characteristics
Plasmodium falciparum invades all ages of RBCs, and the number of infected cells may exceed 50% (see Tables 49-1 to 49-3, Figures 49-2 to 49-4). Schizogony occurs in the spleen, liver, and bone marrow rather than in the circulating blood. Ischemia caused by the obstruction of vessels within these organs by parasitized RBCs will produce various symptoms, depending on the organ involved. A decrease in the ability of the RBCs to change shape when passing through capillaries or the splenic filter may lead to plugging of the vessels Also, only P. falciparum causes cytoadherence, a feature that is associated with severe malaria.
Plasmodium Knowlesi (Simian Malaria, the Fifth Human Malaria)
General Characteristics
The early blood stages of P. knowlesi resemble those of P. falciparum, whereas the mature blood stages and gametocytes resemble those of P. malariae (see Tables 49-1 to 49-3, Figures 49-2 to 49-4). Unfortunately, these infections are often misdiagnosed as the relatively benign P. malariae; however, infections with P. knowlesi can be fatal. The RBCs are all sizes, there is no true stippling (fine, granular, blue stippling in RBCs stained with Wright’s stain or red when using eosin hematoxylin as seen in Figure 49-3 (P. vivax photo, third from the top), often there are multiple rings per RBC (there may be 2 to 3 rings), the rings are delicate and often have 2 to 3 dots of chromatin, band forms are typically seen with the developing trophozoites, and the mature schizont contains 16 merozoites. The early stages mimic P. falciparum, whereas the later stages mimic P. malariae.
Laboratory Diagnosis (All Species)
Routine Methods
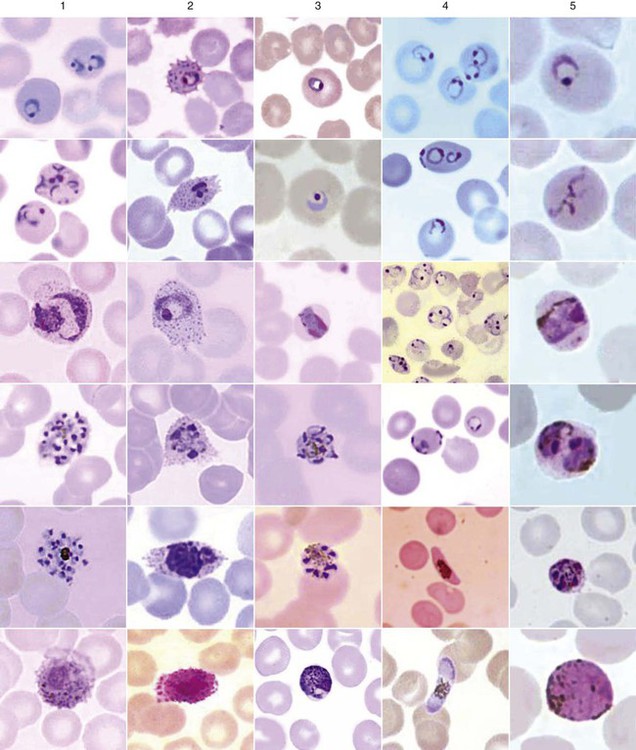
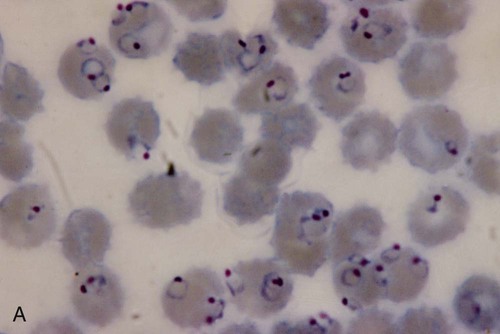
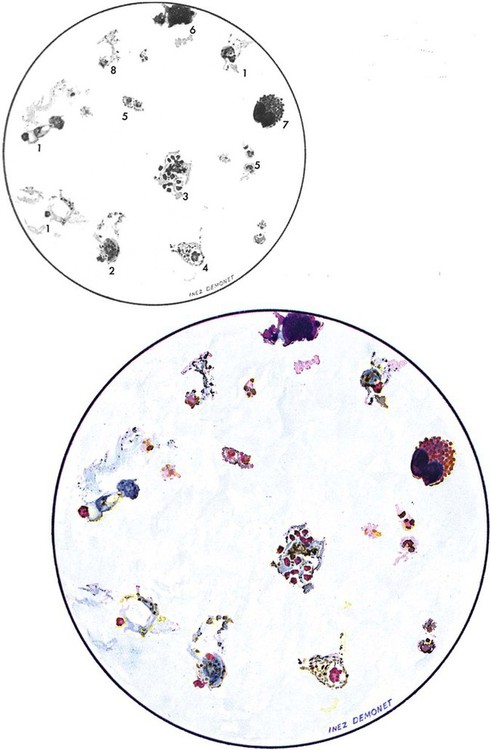
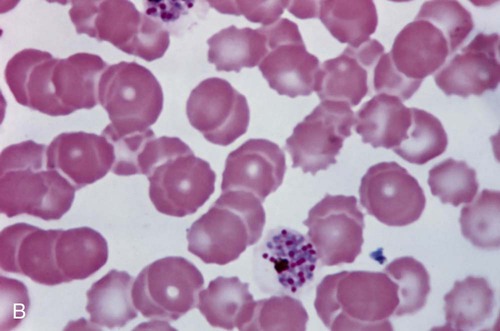
Examination of a single blood specimen is not sufficient to exclude the diagnosis of malaria, especially when the patient has received partial prophylaxis or therapy and has a low number of organisms in the blood. Patients with a relapse case or an early primary case may also have few organisms in the blood smear. Regardless of the presence or absence of any fever periodicity, both thick (Figure 49-5) and thin blood films should be prepared immediately, and at least 200 to 300 oil immersion fields should be examined on both films before a negative report is issued. If the initial specimen is negative, additional blood specimens should be examined over a 36-hour time frame. Although Giemsa stain is recommended for all parasitic blood work, the organisms can also be seen with other blood stains, such as Wright’s stain. Using any of the blood stains, the white blood cells (WBCs) serve as the built-in quality control; if the WBCs look good, any parasites present will also look good. Figure 49-6 compares the multinucleated stages (schizont) of Plasmodium malariae and Plasmodium vivax. Fluorescent nucleic acid stains, such as acridine orange, may also be used to identify organisms in infected RBCs. However, this may be more difficult to interpret because of the presence of white blood cell nuclei or RBC Howell-Jolly bodies.
Therapy
Chloroquine-resistant P. falciparum is present in almost all endemic areas other than Central America and the Caribbean. Increasing resistance to sulfadoxine-pyrimethamine and mefloquine has also been identified in P. falciparum. Therefore, treatment with ACT—including artesunate-mefloquine, artemether-lumefantrine (Coartem), and artesunate-amodiaquine—has been instituted against P. falciparum. However, resistance to artesunate-mefloquine has already appeared in Southeast Asia. Current information on the distribution of drug-resistant P. falciparum is available from the Centers for Disease Control and Prevention (CDC) malaria hotline in Atlanta, Georgia [phone (770) 488-7788]. Therapy for chloroquine-resistant P. falciparum remains very complex with continual changes; thus consultation with an infectious disease specialist is highly recommended. Current treatment guidelines from the CDC are available at www.cdc.gov/malaria/pdf/clinicalguidance.pdf. Chloroquine resistance continues to evolve and spread. Primaquine tolerance has also been documented.
Babesia Spp.
General Characteristics
Organism
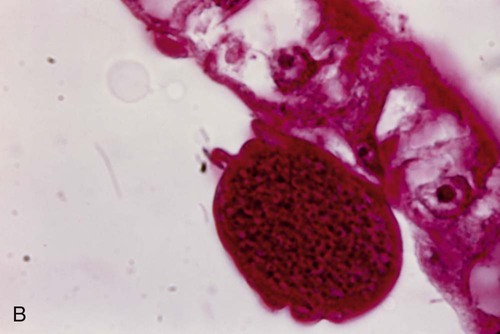
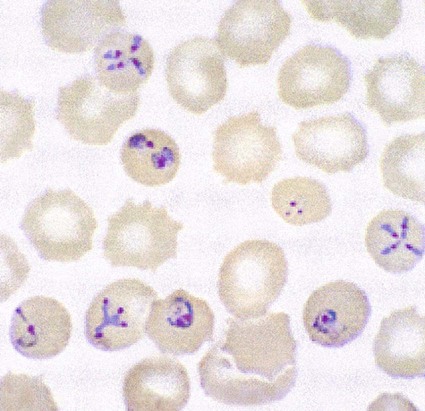
The trophozoites of Babesia can mimic P. falciparum rings; however, there are differences that can help differentiate the two organisms (Figure 49-7). Babesia trophozoites vary in size from 1 to 5 µm; the smallest are smaller than P. falciparum rings. Also, ring forms outside of the RBCs and two to three rings per RBC are much more common in Babesia. The ring forms of Babesia tend to be very pleomorphic and range in size, even within a single RBC. The diagnostic tetrads, the Maltese Cross, though not seen in every specimen or species, may be present (see Figure 49-7).
Trypanosoma Spp.
Trypanosoma spp. are hemoflagellate protozoa that live in the blood and tissue of the human host (Tables 49-4 and 49-5, Figures 49-8 to 49-10). African trypanosomiasis (sleeping sickness) is caused by Trypanosoma brucei gambiense and T. brucei rhodesiense species belonging to the family Trypanozoon and is confined to the central belt of Africa. American trypanosomiasis (Chagas’ disease) is produced by Trypanosoma cruzi, which belongs to the family Schizotrypanum and is confined to the American continent. Trypanosoma rangeli belongs to the family Tejaria, produces an asymptomatic infection, and is also present only on the American continent. African trypanosomes and T. rangeli are transmitted directly into the bite wound by salivary secretions from the insect vector, whereas T. cruzi is transmitted through contamination of the bite wound with the feces from the reduviid bug.
TABLE 49-4
Characteristics of American Trypanosomiasis
| Characteristic | CAUSATIVE ORGANISM | |
| Trypanosoma cruzi | Trypanosoma rangeli | |
| Vector | Reduviid bug | Reduviid bug |
| Primary reservoirs | Opossums, dogs, cats, wild rodents | Wild rodents |
| Illness | Symptomatic (acute, chronic) | Asymptomatic |
| Diagnostic stage | ||
| Blood | Trypomastigote | Trypomastigote |
| Tissue | Amastigote | None |
| Recommended specimens | Blood, lymph node aspirate, chagoma | Blood |
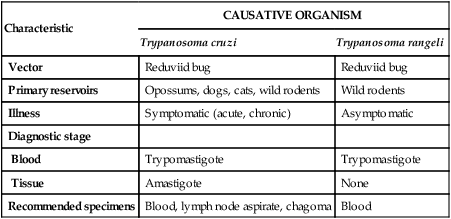
TABLE 49-5
Characteristics of East and West African Trypanosomiasis
| Characteristic | East African | West African |
| Organism | Trypanosoma brucei rhodesiense | Trypanosoma brucei gambiense |
| Vector | Tsetse fly, Glossina morsitans group | Tsetse fly, Glossina palpalis group |
| Primary reservoirs | Animals | Humans |
| Illness | Acute (early CNS invasion), <9 months | Chronic (late CNS invasion), months to years |
| Lymphadenopathy | Minimal | Prominent |
| Parasitemia | High | Low |
| Epidemiology | Anthropozoonosis, game parks | Anthroponosis, rural populations |
| Diagnostic stage | Trypomastigote | Trypomastigote |
| Recommended specimens | Chancre aspirate, lymph node aspirate, blood, CSF | Chancre aspirate, lymph node aspirate, blood, CSF |
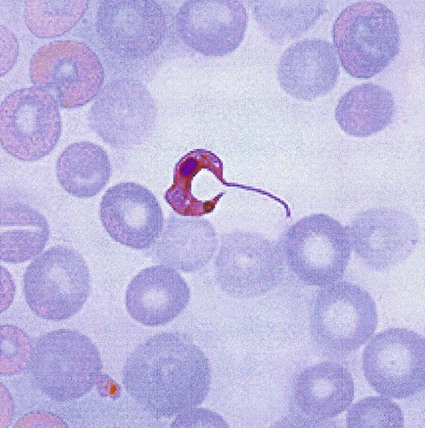
African Trypanosomiasis
General Characteristics
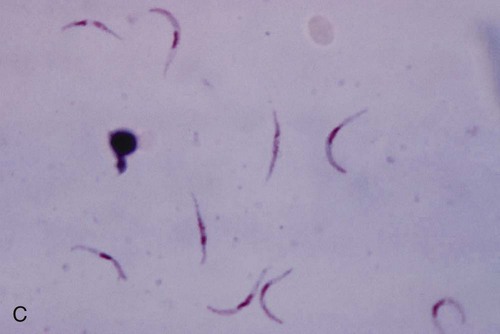
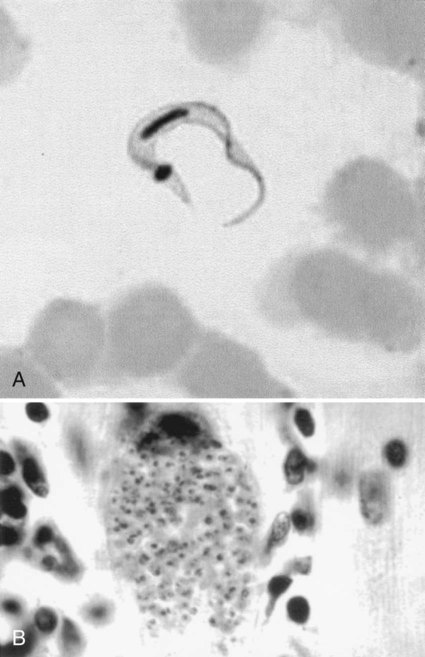
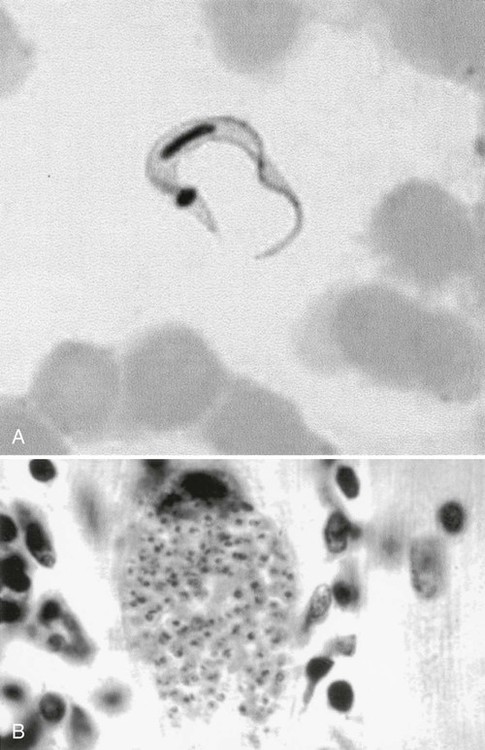
In fresh blood, the trypanosomes move rapidly among the red blood cells. An undulating membrane and flagellum may be seen with slower moving organisms. The trypomastigote forms are 14 to 33 µm long and 1.5 to 3.5 µm wide (see Table 49-4, Figure 49-8). With a blood stain, the granular cytoplasm stains pale blue. The centrally located nucleus stains reddish. At the posterior end of the organism is the kinetoplast, which also stains reddish, and the remaining intracytoplasmic flagellum (axoneme), which may not be noticeable. The flagellum arises from the kinetoplast, as does the undulating membrane. The flagellum runs along the edge of the undulating membrane until the undulating membrane merges with the trypanosome body at the anterior end of the organism. At this point, the flagellum becomes free to extend beyond the body.
American Trypanosomiasis
Trypanosoma cruzi
General Characteristics.
Trypomastigotes (see Table 49-5 and Figures 49-9 and 49-10) are ingested by the reduviid bug (triatomids, kissing bugs, or conenose bugs) as it obtains a blood meal. The trypomastigotes transform into epimastigotes (see Figure 49-8) that multiply in the posterior portion of the bug’s midgut. After 8 to 10 days, trypomastigotes develop from the epimastigotes. Humans contract Chagas’ disease when the reduviid bug defecates while taking a blood meal and the parasites in the feces are rubbed or scratched into the bite wound or onto mucosal surfaces.
In humans, T. cruzi is found in two forms: amastigotes and trypomastigotes (see Figure 49-8). The trypomastigote form is present in the blood and infects the host cells. The amastigote form multiplies within the cell, eventually destroying the cell, and both amastigotes and trypomastigotes are released into the blood.
The trypomastigote (see Figures 49-9 and 49-10) is approximately 20 µm long, and it usually assumes a C or U shape in stained blood films. Trypomastigotes occur in the blood in two forms: a long slender form and a short stubby one. The nucleus is situated in the center of the body, with a large oval kinetoplast located at the posterior extremity. A flagellum arises from the kinetoplast and extends along the outer edge of an undulating membrane until it reaches the anterior end of the body, where it projects as a free flagellum. When the trypomastigotes are stained with any of the blood stains, the cytoplasm stains blue and the nucleus, kinetoplast, and flagellum stain red or violet.
When the trypomastigote penetrates a cell, it loses its flagellum and undulating membrane and divides by binary fission to form an amastigote (see Figure 49-10). The amastigote continues to divide and eventually fills and destroys the infected cell. Both amastigote and trypomastigote forms are released from the cell. The amastigote is indistinguishable from those found in leishmanial infections. It is 2 to 6 µm in diameter and contains a large nucleus and rod-shaped kinetoplast that stains red or violet with blood stains. The cytoplasm stains blue. Only the trypomastigotes are found free in the peripheral blood.
Pathogenesis and Spectrum of Disease.
Acute symptoms occur 2 to 3 weeks after infection and include high fevers, enlarged spleen and liver, myalgia, erythematous rash, acute myocarditis, lymphadenopathy, keratitis, and subcutaneous edema of the face, legs, and feet. There may be symptoms of CNS involvement, which carry a very poor prognosis. Myocarditis is confirmed by electrocardiographic changes, tachycardia, chest pain, and weakness. Amastigotes proliferate within the cardiac muscle cells and destroy the cells, leading to conduction defects and a loss of heart contractility (see Figure 49-10). Death may occur due to myocardial insufficiency or cardiac arrest. In infants and very young children, swelling of the brain can develop, causing death.
Leishmania Spp.
General Characteristics
The parasite has two distinct phases in its life cycle: amastigote and promastigote (Table 49-6, Figure 49-11). The amastigote form is an intracellular parasite in the cells of the reticuloendothelial system and is oval, measuring 1.5 to 5 µm, and contains a nucleus and kinetoplast. Leishmania spp. exist as the amastigote in humans and as the promastigote in the insect host. As the vector takes a blood meal, promastigotes are introduced into the human host. Depending on the species, the parasites then move from the bite site to the organs within the reticuloendothelial system (bone marrow, spleen, liver) or to the macrophages of the skin or mucous membranes.
TABLE 49-6
Features of Human Leishmanial Infectionsa
| Species | Disease Type | Humoral Antibodies | Delayed Hypersensitivity | Parasite Quantity | Self-Cure | Recommended Specimen |
| Leishmania donovani | VL | Abundant | Absent | Absent | Rare | Bone marrow, spleen |
| CL | Variable | Present | Present | Yes | Skin macrophages | |
| DL | Variable | Variable | Variable | Variable | Skin macrophages | |
| L. tropica | CL | Variable | Present | Present | Yes | Skin macrophages |
| L. major | CL | Present | Present | Present | Rapid | Skin macrophages |
| L. aethiopica | CL | Variable | Weak | Present | Slow | Skin macrophages |
| DCL | Variable | Absent | Abundant | No | Skin macrophages | |
| L. mexicana | CL | Variable | Present | Present | Yes | Skin macrophages |
| DCL | Variable | Absent | Abundant | No | Skin macrophages | |
| L. braziliensis | CL | Present | Present | Present | Yes | Skin macrophages |
| MCL | Present | Present | Scant | No | Skin macrophages |
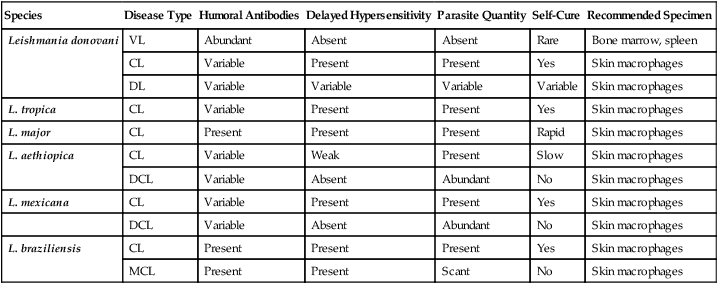
CL, Cutaneous leishmaniasis; DCL, diffuse cutaneous leishmaniasis; DL, dermal leishmanoid; MCL, mucocutaneous leishmaniasis; VL, visceral leishmaniasis.
aFor culture, specimens must be collected aseptically; in older lesions, the number of parasites may be scant and difficult to recover. Isolation: Hamsters; culture media (Novy-MacNeal-Nicolle medium and Schneider’s Drosophila medium with 30% fetal bovine serum). Serology: Most suitable for visceral leishmaniasis; little value for cutaneous leishmaniasis; limited value for mucocutaneous leishmaniasis. Montenegro test: Delayed hypersensitivity reaction to intradermal injection of cultured parasites.