Chapter 52 Bladder Cancer
Etiology and Epidemiology
Bladder cancer is the most common malignant disease involving the urinary system and the second most common genitourinary cancer. It is the fourth most common cancer among men and the ninth most common cancer among women. It is estimated that there will be 70,530 new cases and 14,680 bladder cancer deaths in 2010.1 There is little or no association between socioeconomic status and bladder cancer.2
Internationally, incidence rates of bladder cancer vary almost tenfold. The highest rates occur in western Europe and North America; relatively low rates are found in eastern Europe and several areas of Asia (i.e., China, India, the Philippines).3,4
Cancer of the bladder occurs primarily in older men, with a median age at diagnosis of 69 years. Two thirds of cases occur among persons aged 65 years and older. The incidence rate among African-American and Hispanic men is about 50% of that among whites. The male to female ratio is at least 3 : 1, approaching 4 : 1 in whites.5
From the period 1985 to 2005, the incidence rates in the United States increased by over 50%; from 1975 to 1996, the 5-year survival rate increased from 75% to 81%.1 The incidence of locally advanced and metastatic bladder cancer remained virtually constant.6
Risk Factors
Cigarette smoking increases the risk of bladder cancer twofold to threefold. Risk estimates for moderate to heavy smokers range from 2 to 10 compared with nonsmokers.7–11 Some studies indicate a reduction in risk by over 30% within the first 1 to 4 years after cessation of smoking, and over 60% 25 years after cessation, but even after 25 years the risk had not reached the background level of never smokers.9,11 Smoking cessation may decrease recurrence rates for patients with non–muscle-invasive disease.12 Exposure to secondhand smoke in women may be a risk factor for the development of bladder cancer.13
In the 1950s, occupational exposures to aromatic amines in the British dyestuff and rubber industries were found to be associated with bladder cancer.14,15 The mean time from start of exposure to death was 25 years, with the greatest risk for workers who started before age 25 years. Workers in the leather industry are at increased risk for bladder cancer, but the carcinogen has not yet been identified.16,17 The increased risk for painters seems to be due to many known carcinogens in paint such as benzidine, polychlorinated biphenyls, formaldehyde, asbestos, and solvents such as benzene, dioxane, and methylene chloride.18 Drivers of trucks, buses, or taxicabs are suspected to have an increased exposure to exhaust emissions containing polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs.19
It was first recognized in the 1970s that chlorination of drinking water generates a wide array of halogenated organic compounds. A number of epidemiologic studies investigated the possible association between exposure to these compounds and cancer. A 1992 meta-analysis of these studies found a significant association between exposure to chlorination by-products and bladder cancer.20 Several studies published subsequently supported this conclusion.21,22 High levels of arsenic in drinking water have also been linked to the subsequent development of bladder cancer.23
Aromatic amines are detoxified by N-acetylation. The N-acetyltransferase enzyme in the liver is polymorphic, displaying a slow and a rapid acetylator phenotype. The allele that confers a slow acetylator phenotype is assumed to be associated with an increased risk of bladder cancer (relative risk, 1.1 to 1.9).24
There are several other well-documented risk factors. Exposure to S. haematobium infection is associated with a predominantly increased risk of squamous cell carcinoma of the bladder.25 Heavy consumption of phenacetin-containing analgesics was linked to cancer of the renal pelvis, ureter, and bladder.26 Cyclophosphamide, an alkylating agent, and pelvic radiation have also been linked to the risk of bladder cancer.27–29 Other risk factors include low total daily fluid intake, chronic cystitis, and consumption of Chinese herbs containing aristolochic acid.30,31
Prevention and Early Detection
Primary prevention involves identification and avoidance of cancer-causing factors.32 In public health terms, avoidance and cessation of cigarette smoking is the most effective means available for the prevention of bladder cancer. Decreasing exposure to workplace carcinogens and increasing fluid intake are other useful approaches.
Secondary prevention involves screening individuals at high risk for a particular cancer with the goal of early detection and treatment. Urinary cytologic testing as a screening method has a low cost and low risk and has excellent specificity (>98%) but relatively poor sensitivity, particularly for low-grade tumors (overall sensitivity, 34%, with sensitivities for grade 1, 2, and 3 tumors of 12%, 26%, and 64%, respectively).33 Furthermore, the prevalence of preclinical bladder cancer is probably too low in the general population for large-scale screening programs to be rewarding. Testing for asymptomatic hematuria does not appear to be a useful method for screening. A single test has a low sensitivity. Repeat testing at short intervals, such as weeks, has been advocated but yields unacceptably high rates of false-positive results.34,35
Chemoprevention involves the administration of a natural or synthetic agent to healthy people (primary) or patients with precancerous conditions who are at high risk for bladder cancer (secondary) to prevent or retard the development or progression of cancer. Retinoids (vitamin A derivatives) as well as high-dose multivitamins, pyridoxine (vitamin B6), alpha-tocopherol (vitamin E), ascorbic acid, polyamine synthesis inhibitors, polyphenols (green tea extract), COX-2 inhibitors (celecoxib), EGFR tyrosine kinase inhibitors (erlotinib), and oltipraz have been or are under investigation as potential chemopreventive agents,36–46 but nothing definitive has been established at present. Additional studies are under way.
Pathology and Pathways Of Spread
In T2 lesions, muscle invasion is present and the probability of nodal and distant spread is increased. T2 disease is divided into superficial (T2a) or deep (T2b) invasion.47
TCC is often multifocal both in place and in time (polychronotropism). This is a striking characteristic of this disease, but the etiology is uncertain. Two of the most commonly held explanatory theories are a genetic field defect with multiple new tumors spontaneously arising or, alternatively, the local reimplantation of tumor cells (local metastasis). Evidence strongly suggests that tumor reimplantation or submucosal migration is the predominant mechanism. Multifocal tumors as well as upper tract and lower tract lesions arising in one individual demonstrate clonality.48
External and internal iliac chains as well as perivesical lymph nodes are first-echelon lymph nodes. Common iliac, para-aortic, and paracaval lymph nodes are considered second-echelon lymph nodes. Lymph node sampling in patients undergoing cystectomy should include excision of 12 or more lymph nodes.47
Molecular Biology
Newly discovered cellular mechanisms and their associated proteins have become subjects of intense interest. This is partly for what they tell us about the biology of a tumor but also because of their potential role as prognostic markers and/or therapeutic targets. It has long been recognized that invasive bladder tumors are extremely heterogeneous in nature, despite almost always having high-grade, transitional cell morphologic characteristics because of complex genetic and epigenetic interactions that play a direct role in the initiation and progression of TCC. When the range of genetic alterations is considered, deletions (e.g., loss of tumor suppressor genes) generally arise in early-stage disease, whereas gains and amplifications (e.g., activation of oncogenes) may be more frequent in advanced stages of disease. Genetic factors may also modify the risk associated with exogenous agents through activation or detoxification of potential carcinogens. The search to understand the heterogeneity and use it to therapeutic advantage is only just beginning. At the moment, the best prognostic indicators we have in bladder cancer are clinical ones of stage, grade, tumor size, unifocality or multifocality, and the presence or absence of either hydronephrosis or lymph node involvement (discussed further later in this chapter). No molecular biomarker yet comes close to improving upon these clinical markers, but they likely will in the next decade.49 This will probably involve a combination of multiple independent markers, and an individual tumor’s gene expression pattern is an intriguing area of research.
Apoptosis and Cell Cycle Receptors
These receptors provide malignant cells with an evolutionary selective advantage. These include cellular immortalization, escape from G1 checkpoint control, loss of DNA damage checkpoints (G2/M), loss of normal DNA repair mechanisms, loss of apoptotic response, and the development of invasive capabilities.50–52 The pRB and TP53 pathways, which involve several common molecules, provide G1 checkpoint control and regulate the response to DNA damage. Though the RB gene itself has mutated in only a few specific cancers, it is thought that abnormalities of its function are far more common and that transitional cell bladder cancer represents one of the abnormalities.51,53–55 Disruption of the gene’s function is required to overcome the restraint it imposes on cell cycle progression. The proapoptotic tumor suppressor gene eliminates cells with abnormalities in their RB pathways. Tumors therefore commonly have abnormalities in p16 and p14ARF, and abnormalities at these loci may also be required to overcome the checkpoints that protect the cell from aberrant stimuli. TP53 gene mutations, which may be detected by nuclear immunohistochemical testing, are present in over half of bladder tumors and appear to be related to the progression of bladder cancer and to the outcome following treatment.53,56,57–59 A meta-analysis of 168 studies assessing TP53 overexpression showed a weak, although significant, association with the overall risk of recurrence and mortality.60 Cooperative group trials are under way to test the role of three cycles of combination chemotherapy in patients with negative nodes but mutated TP53 genes. Work from the Radiation Therapy Oncology Group (RTOG) on patients who were treated with chemotherapy and irradiation has not, however, shown any clear association between pRB, p16, or TP53 status and outcome.61 Clearly, the implication of the expression of these markers will vary according to the treatment planned.
Other genes regulating apoptotic function or involved in the initiation of bladder carcinogenesis and its progression have been studied, such as the BAX, BCL2, and p21 genes, survivin, and matrix metalloproteinases.62,63,64,65–69 In examining a large database containing bladder cancer of different stages all treated by a variety of methods, it did seem that those who had BAX or CD40L tumors lived longer. BCL2 positivity appeared to predict worse survival times. The caveat, however, is that most of these were not prospective studies balanced for other prognostic variables.
Researchers from Germany have suggested that one does not have to analyze the molecular pathways relating to apoptosis to predict a response; a measure of the baseline spontaneous apoptotic rate of the tumor may be easier and quite sufficient. The inability to undergo spontaneous apoptosis whether due to overexpression of BCL2 or survivin predicts local failure after irradiation and chemotherapy.70
The RAS family of transforming genes is often overexpressed in bladder cancer. Their presence may be related to the original development of the tumor. In a mouse model, farnesyltransferase inhibitors that inhibit the post-translational modifications of HRAS worked synergistically with irradiation in reducing tumor recurrence.71
Growth Factor Receptors
Both EGFR and HER2 are commonly overexpressed in urothelial tumors. Numerous immunohistochemical studies have investigated the expression of HER2 in bladder cancer and have found overexpression in 40% to 80% of tumors.60,72–75 There are, however, conflicting data on the relationship between the expression of these receptors with response to treatment and clinical outcome. A recent RTOG study evaluated the clinical relevance of EGFR and HER2 expression as measured by immunohistochemical staining on slides from 73 patients treated on four of the RTOG bladder preservation trials. Results pointed to a more favorable prognosis in patients with overexpression of EGFR but a lower complete response rate in patients with TCC with overexpression of HER2.61 EGFR positivity correlated with an improved overall survival (OS) rate (p = .044), disease-specific survival rate (p = .042), and survival rate with an intact bladder (p = .021). A trend toward a decreased incidence of distant metastases was also associated with EGFR expression. On multivariate analysis, adding tumor stage, tumor grade, whether or not a visibly complete TURBT was done, and patient age to the model, EGFR positivity remained significantly associated with an improved disease-specific survival rate. HER2 overexpression correlated with reduced complete response rates to chemoradiation (50% vs. 81%; p = .026), which remained significant on multivariate analysis. Unlike findings in other studies, TP53 and p16 had no prognostic significance. These patients had all been treated with chemotherapy and irradiation, and it may be that EGFR has a more direct involvement in improving the response of TCC to these agents than it does other tumors. It will be crucial to elucidate this relationship clearly because a growth factor receptor may be either a favorable or an unfavorable marker, depending on the proposed therapy. The RTOG data would suggest that targeting the HER2 pathway may be more profitable than EGFR in the initial therapy of muscle-invasive bladder cancer. There is certainly experimental evidence that HER2 has an antiapoptotic effect and that tumor radiosensitization can result from targeting this mechanism.76 An ongoing RTOG trial (05-24) is evaluating the addition of trastuzumab to paclitaxel and daily radiation therapy following TURBT in the treatment of noncystectomy candidates with muscle-invasive bladder cancer.
Recent data suggest that the presence of mutations in fibroblast growth factor receptor 3 (FGFR3) identifies a subgroup of patients with a favorable prognosis.77 Further investigation into the potential diagnostic and therapeutic implications of such findings is warranted and may lead to the rational development of targeted therapies.
Hypoxia
Hypoxia is well recognized to be strongly associated with both irradiation and chemotherapy resistance. It results in the up-regulation of genes that facilitate anaerobic metabolism and promote tumor vascularization. One such hypoxia-inducible factor is carbonic anhydrase IX (CA IX). It appears to be useful as a surrogate marker of hypoxia within bladder tumors. Several studies have shown that the majority of bladder tumors express this marker, although it appears, strangely, to be more strongly expressed in superficial tumors than invasive tumors. Evidence on its role as a prognostic marker has been conflicting.78,79
Clinical Presentation, Patient Evaluation, and Staging
Clinical Presentation
Painless, gross, and episodic hematuria is the most frequent presenting manifestation of bladder cancer (80%), typically present through the entire course of micturition. It also may be associated with irritative symptoms (e.g., dysuria, frequency, or urgency), which can occur in up to one third of patients and are suggestive of bladder CIS. Ten percent to 20% of patients presenting with gross hematuria are diagnosed with bladder cancer.80,81 Some asymptomatic patients proven to have bladder cancer are found to have microscopic hematuria or, less frequently, pyuria. Bladder tumors can cause strangury (pain on straining to urinate), urinary retention, ureteral obstruction, sepsis, and, rarely, life-threatening hematuria. Regional pelvic disease may cause flank pain because of nerve invasion, edema, and ureteral obstruction. The signs and symptoms of bone, liver, pulmonary, and central nervous system metastases may be present if disease has disseminated.
Workup of Patients with Hematuria or Suspected Bladder Tumors
Endoscopy
The endoscopic evaluation forms the mainstay of diagnosis and staging. Initially, an outpatient cystoscopic examination with biopsy is performed, and urine is obtained for cytologic studies. Cystoscopy provides direct visualization of the bladder and facilitates biopsy of the bladder. The number, size, shape, and location of tumors as well as the appearance of the surrounding mucosa, urethra, and urethral orifice are documented. The tumor size (<2 cm, >5 cm) and shape (papillary or flat) and the presence of associated Tcis lesions are of predictive importance. Using an illustrative bladder map, the location, number, size, and characteristics (papillary vs. sessile) of the bladder tumor as well as the presence or absence of CIS should be noted. Fluorescence cystoscopy after intravesical instillation of a photoactive substance (e.g., a porphyrin such as 5-aminolevulinic acid [5-ALA]) that preferentially accumulates in neoplastic tissue may be more effective than white light cystoscopy. Randomized trials have shown that fluorescence endoscopy detects more tumors (particularly CIS), and this improved tumor identification may result in better patient management.82 This procedure has been approved in Europe but remains investigational in the United States.
Urinary Cytology
Urinary cytologic findings should be used to complement the findings of cystoscopy. It is extremely valuable for the diagnosis of high-grade TCCs, especially CIS that might be difficult for the endoscopist to visualize, and upper tract malignant disease. The presence of high-grade TCC in the cytologic specimen in patients with low-grade papillary TCC suggests either unrecognized CIS or, less commonly, high-grade disease in the upper urinary tracts.83,84 For the diagnosis of low-grade papillary tumors, cytologic testing is not sensitive, because the appearance of grade 1 TCC is identical to that of normal urethral cells.33
Cytology has been regarded as the gold standard for noninvasive screening of urine for bladder cancer. It has a sensitivity of around 40% to 60%, with a specificity in excess of 98%.33 Cytology is illustrative of the problems of noninvasive screening. Poorly differentiated tumors have a false-negative detection rate of 20%, whereas well-differentiated tumors have a false-negative detection rate of up to 80%.
Multiple urine-based biomarkers have been developed because of the low sensitivity of cytologic testing, and they perhaps benefit from the lack of subjectivity by the person reading the test.85–90 These tests identify proteins in urine, detect cellular antigens by immunohistochemical or cytochemical testing, or use fluorescence in situ hybridization (FISH) to identify genetic alterations associated with bladder cancer. Markers include nuclear matrix proteins (e.g., NMP22), fibrin/fibrinogen degradation products (FDPs), cytokeratins,8,18–20 hyaluronic acid, urinary bladder tumor antigen (BTA), basic fetoprotein, survivin, telomerase, microsatellite DNA, and chromosomal abnormalities (3, 7, 17, 9p21). These approaches have demonstrated a higher sensitivity than urinary cytologic testing but a lower specificity. None of these markers, however, have sufficient sensitivity (especially for low-grade small tumors) and diagnostic reliability to replace cystoscopy, and their clinical use has not been recommended by consensus panels. Additional trials are needed to evaluate these newer techniques and their clinical effectiveness and cost-effectiveness as they are integrated into patient management.
Imaging
Although fewer than 60% of bladder cancers are seen on intravenous urography, this imaging modality provides valuable information about the status of the upper urinary tract.91 The presence of ureteral obstruction or a nonfunctioning kidney predicts muscle-invasive disease in 90% of cases.92 Concomitant or subsequent upper urinary tract tumors are found in 2% to 5% of patients. CT urography has replaced IVP as the procedure of choice, though IVP remains an appropriate alternative when CT is not available.
Evaluation of a patient with potential muscle-invading bladder cancer should include CT scanning of the abdomen and pelvis (with and without contrast and with delayed images). Although it is not sensitive in reliably describing the depth of invasion or lymph node involvement, it can be helpful in determining extravesical extension and the planning for subsequent treatment. Notably, the detection of lymph node metastases with CT imaging is poor (high false-negative rate). About 20% to 30% of patients with node-negative disease by CT scanning have positive nodes at the time of cystectomy with lymphadenectomy.93,94
Gadolinium-enhanced MRI studies may prove useful in distinguishing superficial from invasive disease and intravesical from extravesical tumors.95–99 Lymphangiography using iron nanoparticles and MRI might be particularly useful in detecting early nodal metastases.100 Positron emission tomography (PET) scanning has limited value. All bladder-specific imaging should be performed before TURBT is attempted to avoid postsurgical edema.
Staging
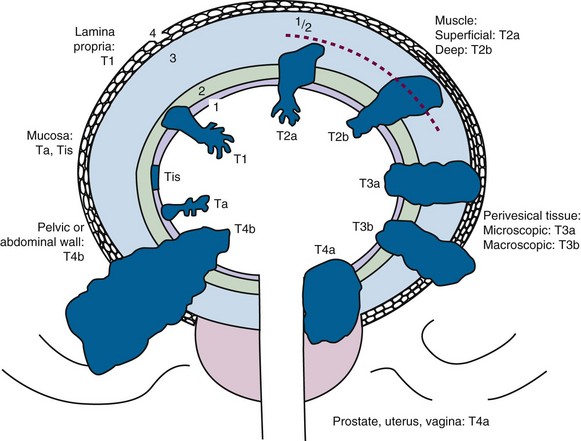
Stage is the most important independent prognostic variable for progression and OS for invasive bladder cancer. The basis for the major staging systems is the classic clinicopathologic study by Jewett and Strong,101 which correlates the probability of regional lymph node and distant metastases with the depth of invasion of the primary bladder tumor. The TNM (tumor, node, metastasis) system of 2010102 is shown in Table 52-1 and Figure 52-1. Of note, CT and MRI are not used for staging of the primary tumor in the TNM system. If lymphadenectomy is performed, sampling should include excision of an average of more than 12 lymph nodes.
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Ta | Noninvasive papillary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades the lamina propria (subepithelial connective tissue) but not beyond |
| T2 | Tumor invades the muscularis propria |
| pT2a | Tumor invades superficial muscle (inner half) |
| pT2b | Tumor invades deep muscle (outer half) |
| T3 | Tumor invades perivesical tissue |
| pT3a | Microscopically |
| pT3b | Macroscopically (extravesical mass) |
| T4 | Tumor invades any of the following: prostate, seminal vesicles, uterus, vagina, pelvic or abdominal wall |
| T4a | Tumor invades prostatic stroma, uterus, vagina |
| T4b | Tumor invades pelvic or abdominal wall |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Single regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral) |
| N2 | Multiple regional lymph node metastases in the true pelvis (hypogastric, obturator, external iliac, or presacral) |
| N3 | Lymph node metastasis to the common iliac region |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
In clinical practice, the correlation between depth of invasion, based on cystoscopic evaluation, and the final cystectomy specimen is only 50% to 60%. That is to say that 40% to 50% of patients undergo pathologic upstaging.103 The accuracy of the determination of degree of muscle infiltration is, therefore, modest at best. The clinical staging system as proposed by the Union Internationale Contre le Cancer was often more practical. Cancer without residual induration after TURBT was staged as T2 and cancer with palpable residual induration as T3. The most important determination of treatment outcome seems to be whether the tumor is confined to the organ (≤T2) or has spread beyond it (≥T3). When comparing outcomes for different treatment modalities, it is very important to consider whether the disease was staged clinically or whether a cystectomy specimen was available for thorough pathologic staging.
Primary Therapy
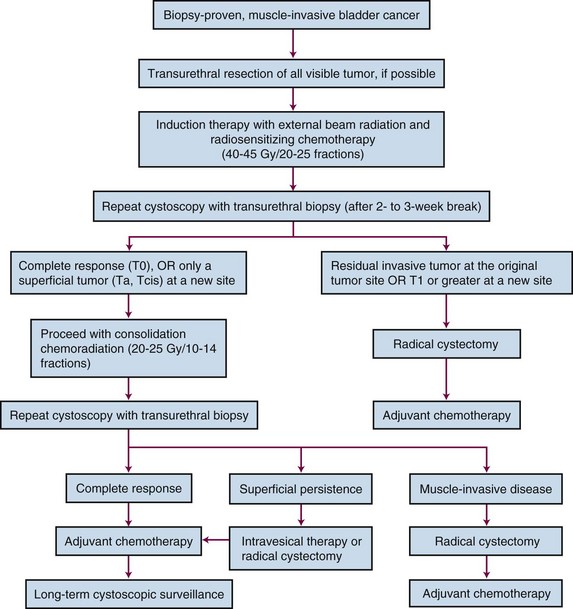
The most appropriate treatment algorithm for muscle-invading disease remains controversial. Although radical cystectomy has long been the standard treatment in the United States, organ-preserving regimens using irradiation with concurrent chemotherapy are emerging as viable alternatives in a subset of patients (Fig. 52-2). These two approaches appear to have similar survival rates in uncontrolled comparisons, but strong physician and patient preferences have so far prevented a randomized, controlled trial comparing these treatment options.
Superficial Bladder Cancer (Ta, T1, Tcis Disease)
Transurethral Resection (TURBT)
The majority (40% to 80%) of patients with superficial TCC will eventually experience recurrent disease after TURBT.104 Progression to invasive disease after TURBT alone occurs in 10% to 25% of patients and is closely associated with tumor type, stage, and grade.105,106 Other factors include multicentric disease, frequency of recurrence, tumor size, and the presence or absence of concomitant CIS.
The risk of progression is substantially higher for tumors of a higher grade or stage. Ta, G2 to G3 disease will progress to invasive TCC in 20% of cases at 5 years after treatment with TURBT alone. Nearly all patients (>70%) with T1 disease have high-grade tumors, and approximately 50% experience invasive disease at 5 years. In patients with T1 disease with CIS in the pathologic specimen, the rate of progression rises to 80% at 5 years.107,108
Intravesical Therapy
Bacille Calmette-Guérin
Several randomized, controlled trials compared TURBT alone with TURBT plus BCG.109–113 Those treated with BCG had significantly fewer recurrences at 12 months. For patients with Ta, G2 to G3, and T1 disease, adjuvant therapy with BCG reduces the rate of progression to 5% to 15% at 5 years. Nonetheless, 50% of patients with T1/CIS will experience muscle-invading cancer at 10 years. Progression rates to muscle invasion among patients who respond to BCG are 1% at 1 year, 5% at 3 years, and 15% at 5 years.
The positive impact on progression translates into a survival advantage.114,115 With long-term follow-up, adjuvant intravesical BCG improved survival rates to 88%, compared with 63% with TURBT alone, and reduced the cystectomy rates from 42% to 20%.114
Intravesical therapy is the first-line treatment for diffuse CIS. The average complete response rate of CIS to BCG is greater than 70% for more than 1 year.116 This treatment prevents subsequent disease in 60% of cases for up to 5 years and in 40% of cases for up to 10 years. Particularly in CIS of the prostatic urethra, BCG has spared many patients from having to undergo cystectomy. TURBT is recommended to stage the disease and to open the bladder neck to allow BCG to bathe the prostatic urethra. Low-grade superficial disease with frequent recurrence is also considered an indication for intravesical therapy.
One consequence of controlling the bladder component of disease in a patient with a TCC of the bladder is an increase in the frequency of extravesical relapses117; hence, careful follow-up is required. Relapses can develop at any point at which transitional epithelium is found: the renal pelvis, ureters, and urethra. The risk for an upper tract tumor is 20% at 5 years, 25% at 10 years, and 33% at 15 years. The risk of prostatic urethra and duct involvement at 5 years is 10% to 15% and at 10 years, 20%. If complete resection of tumors of the urethra cannot be obtained, patients are managed with cystectomy. Patients with positive cytologic testing and no obvious bladder tumor need careful monitoring of the upper tracts. Ureteroscopic resection and instillation of BCG through the renal pelvis is possible.
Other Agents
Although BCG is the treatment of choice for intravesical therapy, a variety of other agents have been investigated, such as mitomycin, doxorubicin, epirubicin, valrubicin, thiotepa, gemcitabine, interferon, and docetaxel. None has proved consistently superior to BCG,116,118 especially in cases of high-risk disease features (high grade, T1, Tis), though they may have a role in BCG failures and as an immediate single postoperative dose for patients at low risk of recurrence following TURBT. Novel approaches to decrease recurrence following initial treatment for non–-muscle-invasive bladder cancer include photodynamic therapy.
Role of Irradiation in High-Risk Superficial Bladder Cancer
It is well recognized that TURBT may clinically understage tumors as superficial when they have already invaded the muscle wall (≈20% to 30%). Radiation therapy, therefore, has an advantage in that it can reach tumor deposits too deep for instillation therapy. There have been no randomized trials comparing radiotherapy with current intravesical treatment options. The Dutch South Eastern Bladder Cancer Group has presented data in 121 patients with T1G3 cancers.119 EBRT with 50 Gy/25 fractions was one treatment option, and 17 patients received this. Though the numbers are limited, the treatment appeared to be as effective as intravesical BCG or mitomycin. Good results have also been achieved at the University of Rotterdam with interstitial implants.120 In this selective but prospective series, patients with single T1 tumors of less than 5 cm in diameter underwent TURBT and subsequent local irradiation of the tumor area in the bladder wall by an interstitial radium implant. The definitive local control rate was 82% and the 10-year OS was 76%. Another group in the Netherlands has published their results using a combination of TUR, EBRT at a dose of 3.5 Gy in 3 to 4 fractions, and interstitial radiotherapy at a mean implant dose of 55 Gy in patients with solitary T1G3 and T2 tumors.121 The local control rate was 70% in the whole patient population. The 5-year disease-specific survival (DSS) was 80% for stage T1 and 60% for T2a tumors. One randomized trial of radiation therapy alone (60 Gy in 30 fractions) in the management of high-grade T1 TCC of the bladder did not seem to indicate a benefit over observation for patients with unifocal disease or over BCG for those with multifocal disease or CIS.122
A large series of 141 patients with high-risk T1 cancers treated with either TURBT plus EBRT with or without platinum-based chemotherapy was conducted at the University of Erlangen.123,124 In this series, 88% of patients achieved a complete response; about 15% progressed to muscle-invasive disease after 5 years and 30% at 10 years, which compares favorably to most studies of TURBT and instillation therapy. DSS were 82% and 73% at 5 and 10 years. In more than 80% of survivors, the bladder was preserved, and 70% were “delighted” or “pleased” with their urinary function. These results suggest that irradiation may have a role to play in patients with high-grade or recurrent T1 lesions and may be attempted ahead of cystectomy on protocol or in place of cystectomy in those who are medically inoperable.
Muscle-Invading Bladder Cancer (T2-4 Disease)
Radical Cystectomy
Surgical Methods
Radical cystectomy entails the surgical removal of the bladder, adjacent organs, and regional lymph nodes. Although traditionally performed as an open procedure, laparoscopic or robotic radical cystectomy has been investigated as a less invasive alternative. In men, the bladder, prostate, seminal vesicles, proximal vas deferens, and proximal urethra, with a margin of adipose tissue and peritoneum, are resected en bloc. In women, the procedure involves an anterior pelvic exenteration to remove the bladder, urethra, uterus, fallopian tubes, ovaries, anterior vaginal wall, and surrounding fascia en bloc. The extent of pelvic lymph node dissection is an important predictor of outcome, and many centers have adopted an extended template dissection to include presacral and common iliac lymph nodes to the aortic bifurcation and often more proximal to the origin of the inferior mesenteric artery, in addition to pelvic lymph nodes distal to the common iliac bifurcation.125 Before cystectomy, grossly abnormal lymph nodes are sampled. In cases of metastases, cystectomy is aborted unless urinary diversion is required to relieve symptoms. Urinary diversion is usually accomplished through an ileal conduit opening onto the anterior abdominal wall through a urostoma.
Continent Urinary Tract Reconstruction
Cutaneous Urinary Reservoir
The two main options are reservoirs made from the colon and small bowel (Indiana pouch) or entirely from the small bowel (Kock pouch).126,127 The Indiana pouch consists of approximately 25 cm of right colon, often including some distal ileum, which is brought to the abdominal wall. It relies on the ileocecal valve for continence. Patients catheterize the pouch on an average of four to six times per day.
Orthotopic Diversion
A neobladder is constructed from ileum, ileocolon, or sigmoid colon and anastomosed to the native urethra as opposed to the abdominal wall. This permits the patient to void in a relatively normal fashion per the urethra. The Mainz pouch (neobladder) consists of an ileocecal segment, which is detubularized and anastomosed to the urethra.128 The Hautmann pouch consists of a W-shaped ileoneobladder, in which 60 cm of ileum is configured into a sphere.129
The choice of urinary diversion is based upon patient and surgeon preferences and may be influenced by the extent of cancer. Orthotopic bladders are contraindicated when the tumor involves the urethra. Orthotopic neobladders are becoming increasingly common in patients undergoing cystectomy but have been predominantly used in men. They are less common in women because of the technical difficulties in maintaining continence and the risk of urethral recurrence given the shorter length of the female urethra.130
Morbidity of Cystectomy
Operative mortality from cystectomy in modern series ranges from 1% to 3%, with postoperative complication rates ranging from 15% to 64% and a readmission rate of 26%.131,132,133,134 Complications include hemorrhage, rectal injury, deep venous thrombosis, pulmonary embolus, pelvic abscess, sepsis from urine or bowel leak, wound infection/dehiscence, and small bowel obstruction. The frequency of complications appears to be related to surgeon experience, hospital volume, age, and presence of comorbidities.
The need for urinary diversion and loss of sexual function in patients undergoing radical cystectomy are the most serious side effects and may impair the quality of life dramatically. In women, loss of sexual function occurs secondary to subtotal vaginectomy and in men, secondary to prostatectomy with loss of vascular supply or nerves serving penile erection. Newer nerve-sparing techniques for men allow for approximately 50% of selected patients to regain their erectile potency.135
Cancer Outcome after Cystectomy
Contemporary large series provide the best data on outcome after radical cystectomy.136,137,138,139 The University of Southern California reported on 633 patients undergoing radical cystectomy with pathologic categories pT2 to T4a with an actuarial OS at 5 years of 48% and at 10 years of 32%.136 The series from the Memorial Sloan-Kettering Cancer Center showed that in 184 patients with tumors of pT2 to T4, 5-year OS was 36%. Five-year OS for all 269 patients with pathologic categories ranging from pT0 to pT4 was 45%.137
The presence or absence of lymph node disease and its extent and the lymph node density (ratio of number of positive lymph nodes to the number of lymph nodes removed) are also significant determinants of survival rates.140 The estimated probability of 5-year OS of patients with nodal metastases is 29%; with one to five nodes positive, 35%, and with six or more nodes positive, 17%.141 The incidence of positive lymph nodes varies according to pathologic tumor stage. In cystectomy series, it ranged from 0% to 6% for superficial disease, 18% to 22% for muscle-invasive disease (pT2, 6% to 20%; pT3a, 30% to 31%), 30% to 64% for disease invading the perivesical fat (pT3b), and 45% to 59% for disease invading adjacent organs (pT4).142,143,144
Other factors that may influence the outcome include the quality of surgery, margin status, presence of lymphovascular invasion, and marker status (TP53, p21, pRB, p16). Nomograms that consider multiple prognostic factors such as stage, nodal status, tumor grade and histologic type, lymphovascular invasion, and patient age have been developed.145
Irradiation as an Adjunct to Cystectomy
Preoperative Radiation Therapy
The recognition that moderate-dose irradiation (20 to 50 Gy) may reduce the volume of gross disease and eradicate microscopic TCC led to its frequent use before cystectomy in the 1970s.146,147 In nonrandomized studies, these doses were found to reduce the frequency of metastases in the lymphadenectomy specimens by approximately 50%.148 By the 1980s, improvements in the technique of radical cystectomy, together with the introduction of more extensive therapeutic lymphadenectomies, made many question the need for radiation therapy.149 It has been argued that irradiation delays the time to definitive surgery, increases its morbidity, and may compromise the surgeon’s ability to perform continent urinary diversions. The question was addressed by an intergroup trial reported in 1997150 in which 140 patients with invasive or recurrent superficial bladder cancers were randomized to receive cystectomy alone or plus preoperative irradiation (20 Gy in five fractions). There was no significant survival advantage to either group at 5 years. The trial was small, the doses were low, and the predominant pattern of failure was distant, all factors that would make it practically impossible to detect any advantage to irradiation. Parsons and Million151 did, however, demonstrate an apparent survival advantage for patients with clinical T3 tumors who received preoperative irradiation.
Cole and colleagues152 reported on 133 patients with T3b disease treated at the M.D. Anderson Cancer Center (MDACC). This retrospective review documented that when pelvic wall disease recurs following modern radical cystectomy, it is usually only in patients who had clinical stage T3b or T4 tumors. The researchers report a 28% incidence of pelvic recurrence in stage T3b patients treated by radical cystectomy, with or without multidrug chemotherapy. When stage T3b patients were treated with preoperative radiation therapy (which this institution used extensively prior to 1980), the pelvic recurrence rate was only 10%, however. Before 1983, all patients received 50-Gy preoperative irradiation, followed 4 weeks later by cystectomy. The actuarial 5-year pelvic control rate was 91%. After 1983, radiation therapy was discontinued, and subsequent pelvic control rates have fallen to 73% despite improvements in surgical technique and staging and the addition of systemic chemotherapy. A survival decrement (52% to 42%) was also seen. The extent to which patient selection factors contributed to this observed difference is unknown. Others have reported lower rates of pelvic recurrence using cystectomy alone for this subset of patients, and it therefore remains uncertain as to whether these findings are widely applicable.149
The morbidity of preoperative irradiation is small. Whitmore148 reported comparable rates of wound healing and perioperative mortality for patients who received and those who did not receive irradiation before cystectomy. There is limited experience in the creation of continent urinary diversions after preoperative irradiation, although Housset and colleagues153 report that it is possible after the delivery of 44 Gy (24 Gy delivered in eight fractions over 17 days according to a modified bifractionated split-course schedule [3-Gy fractions twice daily on days 1, 3, 15, and 17] followed by a 20-Gy boost [2.5-Gy fractions twice daily on days 64, 66, 78, and 80]).
TCCs of the bladder have a high propensity to autotransplantation. Iatrogenic wound seeding after cystotomy was commonly reported by those who perfomed partial cystectomies or bladder brachytherapy. Low-dose preoperative irradiation (8.5 to 20 Gy) profoundly reduced the incidence of this problem.154 Low-dose irradiation is still occasionally given in the United States prior to a partial cystectomy. The partial cystectomy is, however, not commonly performed, because it is limited to small, unifocal tumors of the dome, an unusual location.
Postoperative Radiation Therapy
When the cystectomy specimen shows extensive extravesical disease or positive surgical margins, the risk for pelvic recurrence is high. Postoperative radiation therapy has been evaluated in only one randomized study. The National Cancer Institute of Egypt reported on 236 patients with T3 to T4 tumors (68% were SCCs) who received either no adjuvant therapy or postoperative irradiation (using three daily fractions of 1.25 Gy each, with 3 hours between fractions, up to a total dose of 37.5 Gy in 12 days [75 patients]; or conventional fractionation for a total dose of 50 Gy over 5 weeks [78 patients]).155 The 5-year rates of disease-free survival (DFS) (44% to 49% vs. 25%) and local control (87% to 93% vs. 50%) were improved and the incidence of pelvic recurrence was significantly reduced in the groups receiving postoperative irradiation. Whether this finding is as applicable to TCCs as to squamous cell tumors remains an unanswered question. Today, patients with such extensive disease commonly receive adjuvant chemotherapy, although the ability of this modality to prevent local recurrence has been called into question by Cole and colleagues.152 Radiation therapy rarely cures patients with pelvic wall recurrences following radical cystectomy.156 Some could perhaps be cured if given adjuvant radiotherapy.
The morbidity of postoperative irradiation is high because of the large volumes of small bowel that occupy the pelvis after cystectomy if the urologist does not use pelvic reconstruction techniques to displace small bowel loops from the tumor bed (e.g., omental pedicle flap or mesh). Two series reported an incidence of greater than 30% of small bowel obstruction when 50 Gy was delivered using conventional fractionation.157 If irradiation is envisaged as an accompaniment to cystectomy for advanced disease, it appears to be safer if delivered preoperatively. If this was not done, then the radiation volumes should be kept small, limited to the floor of the pelvis, and with a dose of no more than 45 Gy.
Salvage Radiation Therapy
Attempts to salvage patients with TCC of the bladder at the time of symptomatic or gross pelvic recurrence after radical cystectomy with radiation therapy (RT) or with combined chemotherapy and RT are usually unsuccessful. Some palliation can be achieved.156 In some centers, consideration is given to preoperative chemoradiation followed by attempted resection of the pelvic mass and intraoperative irradiation. There is no large published experience looking at this approach.
Bladder Preservation
Conservative Surgery
Partial Cystectomy
Surgical resection by partial cystectomy requires careful patient selection (i.e., the lesion is solitary and is located in a region of the bladder that allows for complete excision with a 2-cm tumor-free margin, such as the bladder dome). The portion of the bladder to be resected should be small enough to allow adequate bladder capacity. Contraindications include association with CIS in other sites of the bladder, prostatic urethral involvement, prior recurrent bladder or upper tract tumors, and bladder neck or trigone tumors in which ureteral reimplantation would be required to achieve an adequate margin. Given these constraints, only 6% to 19% of patients with primary, muscle-invading bladder cancer are potential candidates.158 Even for this select group of patients, local recurrence rates range from 38% to 78%; half of the recurrences appear in the first year and two thirds by 2 years.
Transurethral Resection of Bladder Tumor (TURBT)
Clinical complete response rates (assessed cystoscopically with repeat biopsy 3 weeks after initial TURBT) after TURBT for T2 and T3 cancers overall are in the 10% to 20% range.159,160 Five-year OS in 85 patients with grades 1 and 2 T2 cancers was an unacceptable 27%.161 Some studies have reported 10-year DSS as high as 76%,162 but these results included only patients who had no residual disease or no invasive disease on repeat biopsy and urine cytologic testing. Although potentially effective in a small proportion of favorable T2 tumors, TURBT is usually not sufficient as monotherapy in muscle-invading bladder cancer.
Radical External Beam Irradiation
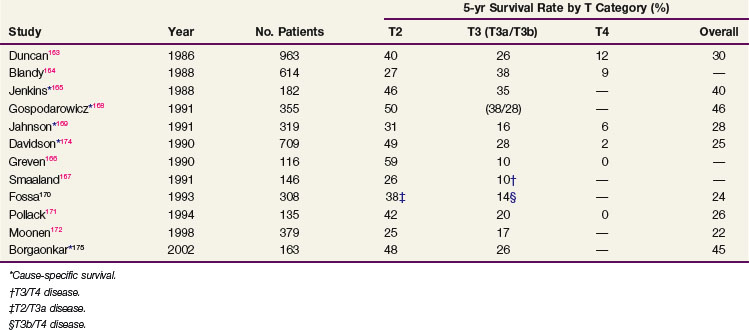
The total radiation dose used in modern series (Table 52-2) varied from 55 to 65 Gy, with 1.8 Gy to 2 Gy per fraction in North America, and from 50 to 55 Gy at 2.5 to 2.75 Gy per fraction in the United Kingdom.163–169,170,171–176 Most patients were treated with one fraction per day five times a week. Patient response was evaluated by cystoscopic examination and biopsy 3 to 6 months after completion of RT. Patients with residual tumor and no known metastatic disease underwent salvage surgery (range of salvage cystectomy rate, 13% to 24%) if they were suitable surgical candidates. Those who were unfit received palliative measures.
The 5-year local control rate ranged from 31% to 50% for the entire patient population and from 49% to 79% for the subgroup of patients with a complete response. Factors reported as having a significant favorable effect on local control176 with RT included:
The success of any bladder-conserving strategy rests upon the ability to rapidly recognize local recurrence and treat it promptly with salvage surgery. Salvage cystectomy can be undertaken safely without increased morbidity after a complete course of RT or chemoradiation regimens.177 After irradiation, an ileal conduit, rather than a neobladder, is the more likely method of urinary diversion. A Kock pouch urinary diversion may also be performed safely in patients who received prior RT to the pelvis.178
Interstitial Brachytherapy
Interstitial brachytherapy was developed as a technique in the early part of the twentieth century but has progressively declined in popularity and is mainly performed in specialist centers in France and the Netherlands. The early technique involved the implantation of permanent radon seeds or temporary radium or cobalt needles.179,180 Although effective, there were substantial problems with radiation safety and significant morbidity from urinary leakage. Hospital stays were protracted. As alternative techniques employing EBRT became available and as the morbidity and mortality of the cystectomy declined, these became preferred approaches. Over the last two decades, however, afterloading techniques and computer planning have meant that some centers are again looking at interstitial brachytherapy as an option for selected patients. It has been combined with EBRT to provide a radiation boost to the primary tumor as well. Appropriate candidates for brachytherapy are those with a solitary TCC with a diameter of less than 5 cm or stage T1 disease (with high grade) to T3a disease (muscle invasion but no extension through the wall).
Early experience made clear that TCC is a tumor that may be surgically seeded either into the wound or to the peritoneal cavity. Van der Werf-Messing154 demonstrated the value of small doses of preoperative irradiation to prevent iatrogenic scar implantation. In those centers using brachytherapy, low doses of EBRT are given preoperatively with fractionation schemes that fit the convenience and ideology of the center. These range from 1 fraction at 8.5 Gy or 3 fractions at 3.5 Gy to 15 fractions at 2 Gy. Historically, wound implants occurred in 10% to 20% of patients and are now rarely seen.
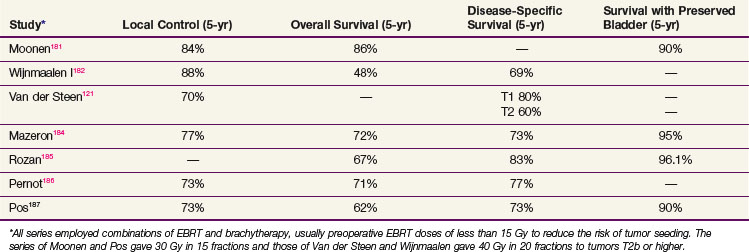
Five-year local control rates for selected patients treated with brachytherapy in combination with EBRT do appear to be excellent, varying between 70% and 90% with correspondingly high rates of bladder preservation181–186,187 (Table 52-3). In selected patients, survival rates appear to be similar to those of cystectomy.188
The most serious acute morbidity is fistula with wound leakage. This is most strongly predicted by tumor size, active length of radioactive sources, and use of a partial cystectomy. Serious late toxicity is rarely reported because the treated bladder volumes are relatively small. A study reported by Pos189 does, however, raise the possibility that there is more late morbidity using fractionated high-dose-rate therapy than using continuous low-dose-rate therapy.
Combined-Modality Therapy
Chemotherapy as monotherapy achieves a clinical complete response in only 25% to 37% of patients.190–197 This is more frequently reported in lower-stage disease or with small tumors (<5 cm) and papillary tumors. Two-year OS with chemotherapy alone is approximately 30% (cT3 to T4). Even in responders, chemotherapy alone spares the bladder in only 15% to 20% of patients. Chemotherapy is, therefore, rarely used alone for localized disease and almost invariably in combination with other therapies.
Chemotherapy and Conservative Surgery
Chemotherapy has been used with TURBT in an attempt to spare the bladder. In a highly selected patient population, 5-year local control rates of up to 48% have been reported. Complete response rates after chemotherapy and TURBT range from 45% to 54%.197–201,202,203 These findings suggest that the addition of TURBT to MVAC (methotrexate, vinblastine, doxorubicin [Adriamycin], and cisplatin) confers a considerable local control advantage over either alone. In a highly selected patient population, chemotherapy followed by partial cystectomy in conjunction with pelvic lymphadenectomy has been used in an attempt to spare the bladder.204 Patients should meet the following criteria to be considered for such treatment:
Chemotherapy and Radical Local Therapy
Neoadjuvant Chemotherapy before Definitive Local Therapy
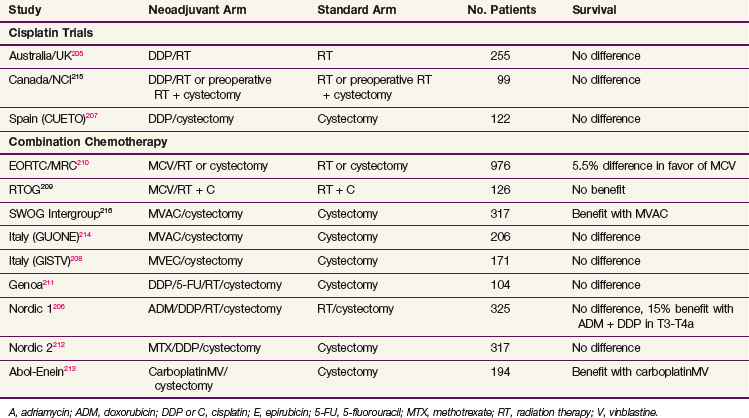
Neoadjuvant chemotherapy offers the potential to assess the response of the primary lesion and for tumor downstaging, though it may lead to a discordance between clinical and pathologic staging and delayed definitive local therapy. Chemotherapy before definitive local therapy has been well tested in invasive bladder cancer. The results of the major randomized controlled trials* are shown in Table 52-4.
Many of the earlier trials comparing neoadjuvant chemotherapy and definitive local therapy alone used single-drug (cisplatin) chemotherapy regimens. None of these studies detected a survival difference. The survival rates among both treatment arms were almost identical. The Spanish trial using neoadjuvant cisplatin reported a significant prolongation of disease-free interval with chemotherapy (mean time to progression, 13 months vs. 30 months; p = .03).207 It is important to note that in these trials, the definitive treatment of the primary bladder cancer was delayed for 3 months during the administration of chemotherapy but the ultimate survival time was not reduced.
A number of more recent trials* used cisplatin-based multidrug chemotherapy regimens. These studies, unfortunately, do not provide a clear and consistent indication of the efficacy of neoadjuvant cisplatin-based chemotherapy. The Nordic 1 Cooperative Bladder Cancer Study Group randomized T1G3 to T4a bladder cancer patients to two cycles of neoadjuvant cisplatin and doxorubicin or to no chemotherapy. Patients in both arms received preoperative irradiation (20 Gy in five fractions) and cystectomy as definitive local treatment. A significant survival advantage was seen in the chemotherapy arm for the subgroup of patients with cT3 to T4a disease (5-year OS rate of 52% vs. 37%; p = .03) but not for the entire patient population.206 The subsequent Nordic 2 trial evaluated cisplatin plus methotrexate followed by cystectomy versus cystectomy alone and did not show a statistically significant survival difference.212
The RTOG 89-03 trial evaluated two cycles of neoadjuvant MCV (methotrexate, cisplatin, and vinblastine) chemotherapy before concurrent cisplatin and definitive irradiation. Neoadjuvant chemotherapy did not confer any detectable survival advantage. Rates of distant metastases, local metastases, and complete responses were similar in the two arms.209
The Medical Research Council/European Organization for Research and Treatment of Cancer (MRC-EORTC) trial randomized 976 patients to three cycles of neoadjuvant MCV versus no chemotherapy.210,217 Definitive local treatment was cystectomy, radical RT, or preoperative RT followed by immediate cystectomy as determined by physician or patient preference. Final analysis, with a median follow-up of 7.4 years, reported a small but significant survival gain for neoadjuvant chemotherapy. At 8 years, OS for chemotherapy patients was 43% compared with 37% in the control arm (p = .048).
MVAC, the most aggressive regimen and most extensively evaluated, has been tested in several studies, including an intergroup trial led by the Southwest Oncology Group (SWOG).216 The SWOG trial, after slow accrual and very long follow-up, achieved a trend toward significance with a 25% reduction in the risk of death through the addition of MVAC (57% vs. 43% at 5 years; p = .06). One third of patients receiving neoadjuvant chemotherapy had a grade III or higher hematologic or gastrointestinal adverse effect. An almost identical study by the Italian GUONE group (Il Gruppo Uro-Oncologico del Nord Est) showed no significant difference.214
A meta-analysis that included data from 3005 patients enrolled in 11 randomized trials demonstrated a 5% absolute 5-year OS advantage (50% vs. 45%; hazard ratio [HR] = 0.86; 95% CI, 0.77 to 0.95; p = .003) for neoadjuvant cisplatin-based combination chemotherapy compared with local therapy alone.218,219 There was also a significant disease-specific survival benefit of 9% absolute improvement at 5 years (HR = 0.78; 95% CI, 0.71 to 0.86; p <.0001).
Adjuvant Chemotherapy after Definitive Local Therapy
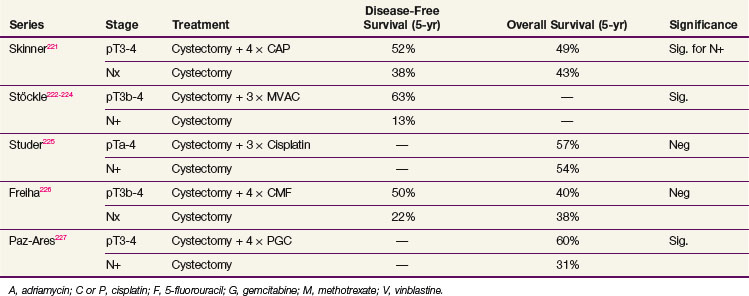
Adjuvant chemotherapy allows for pathologic staging and avoids delay in potentially curative local therapy. No randomized trials have compared neoadjuvant to adjuvant chemotherapy in patients undergoing definitive local therapy. A study from the MDACC attempted to address the optimal timing of perioperative chemotherapy by comparing neoadjuvant plus adjuvant chemotherapy to postoperative chemotherapy alone and did not show any difference.220 Several randomized, controlled trials provide information on the value of adjuvant chemotherapy after radical primary therapy221–227 (Table 52-5). All of these studies used three to four cycles of platinum-based multiagent chemotherapy. As with neoadjuvant therapy, the results have been conflicting, and most of these studies suffered from underpowering and poor accrual.
In three studies, a significant progression-free survival (PFS) benefit at 3 and 5 years was observed when patients were randomized to adjuvant chemotherapy compared with observation. OS was not significantly different, however. Patients in the observation group were treated with chemotherapy at relapse, except in the study reported by Stöckle and colleagues.222–224 The failure to provide salvage chemotherapy in this study may explain the low survival rates in the observation arm (5-year crude survival rate of 17%). The PFS benefit does not translate into an OS benefit if patients receive salvage chemotherapy. In other words, observation with salvage chemotherapy might be just as effective as adjuvant chemotherapy in terms of survival. A meta-analysis including 491 patients from six trials suggested a 25% relative reduction in the risk of death (HR = 0.75; 95% CI, 0.60 to 0.96; p = .019) for patients receiving cisplatin-based combination adjuvant chemotherapy; however, the power of this meta-analysis was clearly limited.228 A randomized phase III trial from the Spanish Oncology Genitourinary Group (SOGUG 99/01) comparing four cycles of adjuvant paclitaxel/gemcitabine/cisplatin to observation in patients with resected high-risk (pT3 to T4 or pN+) bladder cancer was prematurely closed due to poor accrual; however, preliminary results suggest improved 5-year OS (60% vs. 31%; p = .0009).227 Ongoing clinical trials will hopefully provide additional insight into the role of adjuvant chemotherapy.
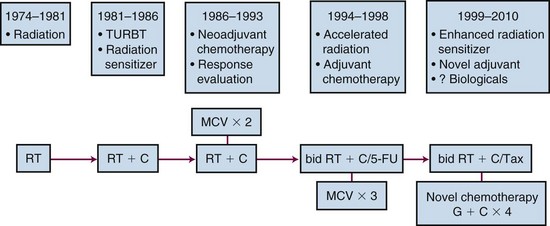
Trimodality Therapy Using Limited Resection, Chemotherapy, and Irradiation in Bladder Preservation
The rationale for combining chemotherapy with RT is twofold:
Trimodality therapy consisting of TURBT with concurrent chemoradiation may, therefore, increase the efficacy of bladder-sparing protocols. The evolution of approach over the last 3 decades is shown in Figure 52-3.
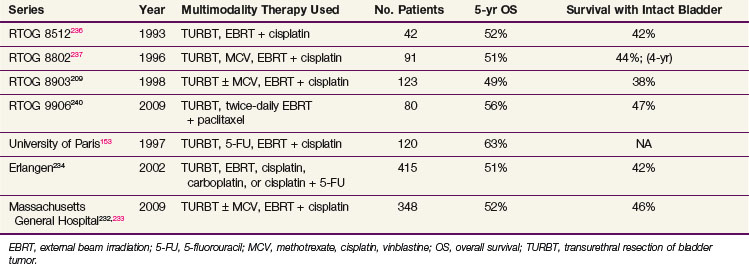
Many phase II trials have combined chemotherapy and RT in different sequences in patients with invasive bladder cancer. Although a variety of different drugs and radiation doses have been used, it is apparent that the highest clinical complete response rate (T0) was achieved in patients who received concurrent chemotherapy and RT compared with sequential regimens.123,215,153,229–231 Table 52-6 shows results from several modern series of multimodality bladder preservation therapy.*
A recently reported phase III randomized trial of 360 patients from the United Kingdom has suggested that concurrent chemoradiation using 5-FU and mitomycin C is well tolerated and significantly improves locoregional disease-free survival rates compared with irradiation alone (HR = 0.61; 95% CI, 0.42 to 0.90; p = .01). Bladder function was good, and no increase in late toxicity was found.241
Single-Institutional Experiences
One of the clearest indications of the potential of multimodality therapy for bladder preservation can be found in a study from the University of Paris.153 In this study, TURBT followed by concomitant cisplatin, 5-FU, and accelerated RT was initially used as a precystectomy regimen. The first 18 patients who had no residual tumor on cystoscopy and repeat biopsy (clinical complete response) underwent radical cystectomy as planned. None of these patients had any evidence of malignancy in the cystectomy specimens, a 100% pathologic complete response rate. Previous studies using TURBT and chemotherapy found residual tumor in as many as 50% of the cystectomy specimens in clinical complete responders.
At the University of Erlangen in Germany, a prospective bladder preservation study was started in 1982. The first 106 consecutive patients, however, were treated only with TURBT followed by irradiation to 50 to 56 Gy at 2 Gy daily. A multimodality approach was initiated in 1985 when chemotherapy (cisplatin or carboplatin) was added concurrently with the irradiation regimen in subsequent patients.123,234 This protocol differed in two aspects from the University of Paris study. All recruited patients underwent the entire course of chemoradiation. Restaging TURBT was performed at 6 to 8 weeks, only after completion of the entire protocol. In addition, only patients with invasive persistent disease or poorly differentiated residual superficial tumors underwent cystectomy. Patients with well-differentiated superficial disease (Ta, CIS) were treated further conservatively with TURBT and intravesical chemotherapy or immunotherapy. A complete response was achieved in 72%. The 10-year DSS was 42%, and bladder preservation was possible in more than 80% of survivors. Thirty-five percent developed distant metastases at 10 years. Concomitant chemotherapy was more effective than radiation therapy alone in terms of complete response and survival. The subgroup of patients who either had persistent invasive tumor (nonresponders) at first restaging or needed a cystectomy for early invasive recurrences within 9 months after completion of the treatment had dismal survival rates (5-year OS of 16%) because of early systemic spread. Patients who needed salvage cystectomy later than 9 months after completion of the treatment achieved 5-year survival comparable to that of the entire group.
In June 1986, investigators from Massachusetts General Hospital (MGH) implemented a selective bladder preservation protocol for operable patients with muscle-invading bladder cancer. Initially to improve on earlier results in terms of both local and distant control, two cycles of neoadjuvant MCV chemotherapy were added before the chemoradiation regimen.235 MCV was chosen because three- or four-drug (e.g., MVAC) chemotherapy had been shown to be more effective than single-agent cisplatin in metastatic bladder cancer. Five-year OS was 48% (68% for those with T2 disease).
Long-term follow-up data are available for 348 patients with T2 to T4aNXM0 bladder cancer treated successively at the MGH with combined-modality therapy using concurrent cisplatin-based chemotherapy and RT after maximal TURBT with or without neoadjuvant/adjuvant chemotherapy.233 The results demonstrate that a visibly complete TURBT was achieved in approximately two thirds of patients and a complete response rate to induction chemoradiation in 72% (≈40 Gy in 1.8- to 2-Gy fractions). Patients with a complete response and those medically unfit for cystectomy received boost chemoradiation to 64 to 65 Gy. Five- and 10-year OS was 52% and 35%, respectively (61% and 43% for T2 tumors), and 5-year and 10-year DSS was 64% and 59%, respectively (74% and 67% for T2 tumors). In this study, 102 patients (29%) ultimately underwent radical cystectomy, 17% for a response that was less than a complete response and 12% for recurrent invasive tumors. No patient required cystectomy due to treatment-related toxicity. Combined-modality therapy achieved a complete response and preserved the native bladder in approximately 70% of patients, while offering long-term survival rates comparable to those in contemporary radical cystectomy series. Use of neoadjuvant chemotherapy did not improve outcomes.
Cooperative Group Experience: The RTOG
In North America, most cooperative group, combined-modality therapy for bladder cancer has occurred within the Radiation Therapy Oncology Group (RTOG). RTOG 85-12 treated 42 patients who were candidates for cystectomy with induction once-daily radiation therapy (40 Gy in 2-Gy daily fractions) and concurrent cisplatin, with prompt cystectomy reserved for patients who responded incompletely.236 Complete responders received consolidation therapy, with an additional 24 Gy (in 2-Gy daily fractions) delivered with concurrent cisplatin. The approach was feasible and well-tolerated, and yielded a complete response of 66%, 5-year OS of 52%, and 5-year survival with an intact bladder of 42%.
The subsequent protocol, RTOG 88-02, looked at the role of neoadjuvant MCV given immediately following TURBT. It entered 91 patients and reported a 75% response rate and 51% 5-year OS rate.237 This was followed between 1989 through 1994 by RTOG 89-03, which was a phase III trial that directly tested the contribution of two cycles of neoadjuvant MCV chemotherapy before concurrent cisplatin and once-daily irradiation.209 This study fell short of its accrual goal and was prematurely closed because of an unexpectedly high rate of severe leukopenia in the MCV arm, which resulted in a 67% protocol completion rate (compared with 81% in the non-MCV arm) and three treatment-related deaths. Analysis of 123 patients showed no significant differences between the MCV arm and the non-MCV arm in complete response rates after induction (61% and 55%, respectively), 5-year OS rates (48% and 49%), 5-year survival rates with an intact functioning bladder (36% and 40%), or rates of distant metastasis (33% and 39%). Treatment-related morbidity and mortality rates, however, were significantly higher in the neoadjuvant chemotherapy arm. In summary, this trial suggested that two cycles of neoadjuvant MCV were not a necessary component of a bladder preservation strategy. Therefore, future studies abandoned neoadjuvant chemotherapy, though they continued to evaluate patient tolerance for newer chemotherapeutic agents and started to explore adjuvant regimens.
In 1995, the RTOG began phase I to II protocols to evaluate accelerated radiation fractionation schemes in combination with concurrent chemotherapy. RTOG 95-06 evaluated the regimen piloted by the University of Paris, using 5-FU plus cisplatin concurrent with accelerated but hypofractionated radiation therapy delivered over 17 days.238 Patients with tumor-associated hydronephrosis were excluded, based on findings that these patients had a significantly lower complete response rate and a higher cystectomy rate. Among 34 evaluable patients, 67% had a complete response on induction and an encouraging 3-year OS rate of 83%. Seven patients (21%), however, had grade III to IV hematologic toxicity on this regimen, though no deaths resulted and no patients required cystectomy for radiation toxicity.
In 1997, the RTOG began to study the role of adjuvant chemotherapy after treatment with either bladder preservation or cystectomy and turned to twice-daily hyperfractionation. In RTOG 97-06, induction and consolidation chemoradiation included twice-daily irradiation and outpatient cisplatin (30 mg/m2) as a radiation sensitizer given on the first 3 days of each week.239 Radiation doses of 1.8 Gy to the pelvis and 1.6 Gy to the bladder tumor were given daily for 12 days with a 4- to 6-hour interval between fractions during induction treatment. In the consolidation phase, 1.5-Gy fractions were given to both, twice daily for 8 days (total dose, 45.6 Gy to the pelvis and bladder and 64.8 Gy to the bladder tumor). After consolidation chemoradiation or cystectomy (depending on the response), patients received three cycles of adjuvant MCV chemotherapy. The rationale for this protocol was to reduce the duration of the induction treatment to 12 days, thereby decreasing the delay between onset of consolidation chemoradiation or cystectomy for those patients in whom induction therapy failed. The complete response rate was 74%, and only 11% of patients experienced grade III to IV toxicity during the induction and consolidation phases. Only 45% of patients went on to receive a full three cycles of MCV, however, and of those who did, 41% developed grade III toxicity. The potential benefit of adjuvant chemotherapy in delaying or preventing metastasis exists because the 2-year actuarial incidence of developing metastasis in this trial was only 18% (compared with ≈30% in trials without adjuvant systemic therapy).
RTOG 99-06 added paclitaxel as a radiation-sensitizing agent during the induction and consolidation schedules, along with twice-daily irradiation.240 The protocol also used the adjuvant chemotherapy regimen of cisplatin (70 mg/m2 on day 1) and gemcitabine (1000 mg/m2 on days 1, 8, and 15). There was a 70% protocol completion rate and an impressive 81% complete response rate. Five-year OS and DSS were 56% and 71%, respectively.
More recent RTOG trials have continued to address the question of the optimal concurrent chemotherapy regimen by evaluating different induction regimens in an effort to further enhance the radiation response and improve local control. RTOG 02-33 is a recently closed phase II study that finished accrual in 2008. In this trial, patients were randomized to twice-daily radiation therapy with either concurrent paclitaxel and cisplatin or concurrent 5-FU and cisplatin during the induction and consolidation phases. Adjuvantly, patients received a triplet regimen of gemcitabine (1000 mg/m2 on days 1 and 8), paclitaxel (50 mg/m2 on days 1 and 8), and cisplatin (35 mg/m2 on days 1 and 8). This is similar to the triplet regimen piloted by Bellmunt and co-workers242 and as tested in a phase III trial led by the (EORTC and SWOG. RTOG 07-12 is the currently open, phase II trial randomizing patients to twice-daily irradiation plus concurrent 5-FU and cisplatin or to daily irradiation plus gemcitabine (based on a regimen from the University of Michigan), followed by selective bladder preservation and gemcitabine/cisplatin adjuvant chemotherapy.
Another original way to combine chemotherapy and irradiation has been employed by Eapen and colleagues.243 This group has combined conventionally fractionated EBRT plus intra-arterial cisplatin. Their durable complete response rate (83%) is as high as any in the literature. The principal problem they have faced is a high level of sacral neuropathy, which, in recent years, they have reduced through reducing the dose of infused cisplatin.243
Another novel combination of therapy in advanced bladder cancer was undertaken by Hoskin and associates,244 who recently reported a phase III trial randomizing patients to irradiation alone versus irradiation plus radiosensitizing carbogen and nicotinamide. There was no indication of an increase in radiation-induced morbidity, though late morbidity and treatment outcome will ultimately determine if there is a therapeutic benefit.
The primary goal of bladder-preserving therapy, as with any therapy for muscle-invading TCC of the bladder, is optimizing patient survival. Bladder preservation in the interest of quality of life can only be considered a secondary objective. The long-term outcome data presented above from single-institution and cooperative group experience suggest that modern combined-modality therapy results in complete response rates of 60% to 80%, 5-year OS of 45% to 60%, and 5-year survival with an intact bladder of 40% to 45%. Although no randomized comparisons of cystectomy with combined-modality therapy exist, these long-term survival rates are encouraging and similar to those reported in contemporary radical cystectomy series (Table 52-7). Similarly, a retrospective review of a nonrandomized cohort of 458 patients undergoing radical radiotherapy or cystectomy in Yorkshire, United Kingdom, showed no significant difference in 10-year survival.245
TABLE 52-7 Muscle-Invasive Bladder Cancer: Survival Outcomes Following Curative Therapy in Contemporary Series
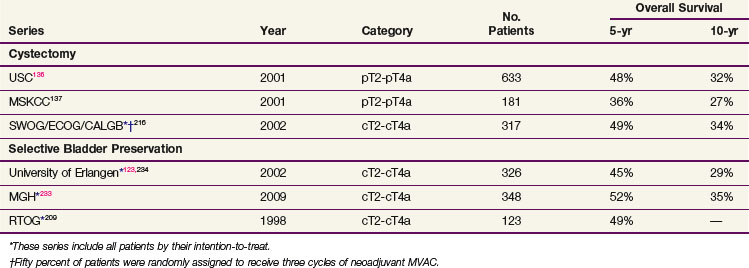
Selection bias of more favorable patients in the cystectomy studies makes a nonrandomized comparison very difficult. Surgical patients are generally younger, with a better performance status. Surgery allows for a more accurate pathologic staging and identification of only surgically identifiable metastatic disease, which leads to a discontinuation of approximately 15% of cystectomies (if node-positive, tumor-unresectable, distant disease), and, therefore, these series do not necessarily report by intention-to-treat. Furthermore, most cystectomy studies do not provide data on the initial clinical staging and only report outcomes by pathologic stage. The optimum way to compare outcomes of selective bladder-conserving strategies is to compare the outcomes of prospective protocols in which eligibility is based on clinical staging, all patients are cystectomy candidates, and all entered patients are reported for outcome whether or not they completed treatment. Comparison of survival outcomes from contemporary cystectomy and bladder-sparing therapy protocols in patients with muscle-invasive cancer of similar clinical stage, all of whom were potential candidates for cystectomy, are similar, with OS rates ranging from 45% to 54%.209,216,246,247 Martinez-Pineiro and associates207 also reported their cystectomy series by clinical stage. Five-year OS was 41% (48% for clinical T2 disease); this is similar to that of the bladder-preservation strategy (5-year OS range from 45% to 60%), in which all patients who initiated their therapy are reported, not only those whose tumor response was satisfactory to complete the organ-preservation program. It is important to remember that the appropriate strategy for bladder-preservation therapy includes cystectomy for nonresponders. Any comparison of cystectomy protocols with bladder-preservation protocols must include these nonresponders to avoid being biased by patient selection.
Prognostic Factors in Chemoradiation
Complete Response
A clinical complete response (or T0 response), evaluated by cystoscopy with tumor site biopsy with or without cytologic testing 2 to 8 weeks after completion of the induction chemoradiation, ranged from 70% to 80%. The complete response rate was significantly higher in patients in whom a macroscopically visibly complete TURBT could be achieved compared with patients with residual macroscopic disease after TURBT (80% to 90% vs. 60%; p <.001).153,232,233 Patients with clinical T2 to T3a disease achieved significantly more frequent complete responses than those with T3b to T4 disease (University of Paris, 83% vs. 58%; p <.001; MGH, T2 vs. T3 to T4, 81% vs. 64%). Patients who presented with hydronephrosis achieved a 66% complete response versus 83% in patients who had no hydronephrosis at presentation (University of Paris, p <.05; MGH, 52% vs. 77%). The Erlangen group has shown that those with Tcis disease in the bladder in addition to the invasive tumor are less likely to have a durable response and are more likely to require cystectomy.
Chemoradiation added significant improvement to the clinical complete response rates compared with the “early” complete response after induction chemotherapy alone. After two cycles of MCV, the complete response rate for patients with T2 disease was 62% and with T3 or T4 disease, 41%. After adding a course of concurrent chemoradiation, the complete response rates increased to 81% and 64%, respectively.232 Factors predicting for a complete response also predict local control and, ultimately, bladder preservation. For example, among patients who underwent a visibly complete TURBT, only 22% required cystectomy (vs. 42% of patients who underwent an incomplete TURBT; p <.001).233
Survival
Clinical complete responders generally have significantly higher survival rates than nonresponders. The amount of residual tumor after TURBT is also a prognostic factor for survival as well as for local control. The same is true for those with T2 clinical stage disease compared with T3 to T4 clinical stage disease and those presenting without hydronephrosis. In multivariate analyses, clinical T stage and complete response to induction therapy remained significantly associated with improved DSS and OS.232,233
The 5-year distant metastasis rate for all patients entered in the protocols ranged from 34% to 40%. In the MGH series, the 5-year distant metastasis rate in the T0 responder group was only 20%.232,233 Patients who did not respond rapidly (T > 0) and underwent immediate cystectomy had a 50% distant metastasis rate at 5 years. It was similar, however, to the 5-year rate of initial T0 responders who subsequently underwent salvage cystectomy for local failure (5-year distant metastasis rate, 54%). Delayed cystectomy for recurrent invasive disease did not confer worse outcomes when compared with immediate cystectomy for a noncomplete response.
Follow-up after Chemoradiation
All patients with bladder cancer (either superficial or invasive) treated with bladder-preserving therapies must be willing to return for regular, thorough clinical examinations, cystoscopic studies, biopsy of the tumor site, and urine cytologic follow-up so that transurethral surgery, intravesical therapy, or salvage cystectomy can be implemented at the earliest opportunity if necessary. The optimal timing of cystoscopy after RT is unclear but is usually first done at 3 months.167 At the MGH, our protocol mandates cystoscopy and urine cytologic testing every 3 months for the first 2 years, then every 6 months until 5 years, and annually thereafter. After 1 to 2 years, in patients with negative evaluations, the biopsy and examination under anesthesia are usually omitted if no worrisome office endoscopic findings are present.
Managing Recurrent Disease in the Bladder
Of patients who have complete responses, 84% remained free from invasive recurrence in the bladder at 10 years, and approximately 60% remained free from any noninvasive or invasive occurrence.232,233 Of patients whose invasive recurrence was cured, 20% to 30% subsequently experienced superficial TCC. Eighty-four percent of patients who experienced superficial recurrences (Ta or CIS) at MGH have been maintained in remission by TURBT and intravesical drug therapy, with 15 to 49 months of follow-up.
Although superficial tumors may be managed by TURBT and intravesical therapy,248,249 invasive recurrences are generally managed with salvage cystectomy. In selected series, salvage cystectomy results in a 40% to 50% survival rate at 5 years and a locoregional control rate of 60%.
Quality-of-Life Studies
Radical cystectomy causes changes in many areas of quality of life, including urinary, sexual, and social function, daily living activities, and satisfaction with body image.250–254 Sexual function has been particularly emphasized because of the high prevalence of erectile dysfunction. Researchers have, over the last decade, concentrated on the relative merits of continent and noncontinent diversions. Available data have been mixed, with some groups, surprisingly, reporting few differences between the quality of life of those with an ileal conduit and those with continent diversions.255 Until recently, few comparative data have been available on those who have neobladders. Hart and co-workers254 have compared outcomes in cystectomy patients who have ileal conduits, cutaneous Kock pouches, or urethral Kock pouches. Of 1074 patients undergoing cystectomy for bladder cancer at the University of Southern California, 368 were eligible for study because they were alive, spoke English, and had no major health issues that would affect global quality of life. Of these patients, 61% completed self-reporting questionnaires. Regardless of the type of urinary diversion, most patients reported good overall quality of life, little emotional distress, and few problems with social, physical, or functional activities. Problems with the diversions (in ≈60% of patients) and with sexual function were the most commonly reported. After controlling for age, no significant differences were seen among urinary diversion subgroups in any quality-of-life area. It might be anticipated that those receiving the urethral Kock diversions would be the most satisfied, and the explanation as to why this is not so is unclear. It may be that the subgroups were too small to detect differences, but perhaps it is more likely that each group adapted in time to the specific difficulties presented by each type of diversion.
Zietman and colleagues256 have performed a study on patients receiving TURBT, chemotherapy, and irradiation in the treatment of their bladder cancer at the MGH.256 Of 221 patients with clinical T2 to T4a cancer of the bladder treated at the MGH from 1986 to 2000, 71 were alive with their native bladders and disease free in 2001. These patients were asked to undergo a urodynamic study and to complete a quality-of-life questionnaire. Sixty-nine percent participated in some component of this study, with a median time from trimodality therapy of 6.3 years. This long follow-up is sufficient to capture the majority of late irradiation effects. Seventy-five percent of patients had normally functioning bladders by urodynamic study. Reduced bladder compliance, a recognized complication of irradiation, was seen in 22%, but in only one third of these was it reflected in distressing symptoms. Two of 12 women showed bladder hypersensitivity, involuntary detrusor contractions, and incontinence. The questionnaire showed that bladder symptoms were uncommon, especially among men, with the exception of control problems. These were reported by 19%, with 11% wearing pads (all women). Distress from urinary symptoms was half as common as their prevalence. Bowel symptoms (e.g., rectal urgency) occurred in 22%, with 14% recording any level of distress. The majority of men retained sexual function. The global health-related quality of life was high. The great majority of patients treated by trimodality therapy, therefore, retain good bladder function. It was concluded that there is a small but detectable level of lasting bowel dysfunction and distress and that this might be judged the additional price that these patients have had to pay to retain their bladders.
A prospective phase II multicenter study from France has recently been reported.257 It tracked voiding symptoms and quality of life in 53 patients from before their trimodality treatment. Sixty-seven percent retained their bladders, and these patients were interviewed 6, 12, and 24 months later. Levels of urinary symptoms were high, but only 5% reported any EORTC grade III symptoms. It was also notable that most patients experienced improvement of symptoms over the 2 years after treatment, presumably because of the eradication of a symptomatic primary tumor.
Two cross-sectional questionnaire studies, one from Sweden and one from Italy, have compared the outcome following irradiation with the outcome following cystectomy.258,259 The questionnaire results for urinary function following irradiation were very similar to those recorded in the MGH study. Over 74% of patients reported good urinary function. Both studies compared bowel function in irradiated patients with that seen in patients undergoing cystectomy. In both, the bowel symptoms were greater for those receiving irradiation than for those receiving cystectomy (10% vs. 3% and 32% vs. 24%, respectively), but in neither was this statistically significant.
In the assessment of sexual function, most women in the MGH study preferred not to answer the questions, and no data were therefore available for them. However, almost all the men did. In contrast to patients who have been irradiated for prostate cancer, most male patients who had bladder-sparing treatment reported adequate erectile function (full or sufficient for intercourse), and only 8% reported dissatisfaction with their sex lives.256 These are in line with the findings obtained in the Swedish and Italian series, in which 38% and 25% of men retained useful erections as compared with 13% and 8% of cystectomized controls. The MGH series allowed the use of sildenafil, probably contributing to the better outcome. Another study reported that 71% of women who underwent bladder-preservation therapy showed no decline in their subsequent satisfaction with sexual intercourse.260
A recent analysis of four prospective RTOG bladder-sparing protocols (89-03, 95-06, 97-06, 99-06) suggested that rates of significant late pelvic toxicity for patients completing combined-modality therapy for invasive bladder cancer and retaining their native bladder is low.261 Only 7% of patients experienced late grade III pelvic toxicity (5.7% genitourinary and 1.9% gastrointestinal), and in only one patient did the toxicity persist. Notably, there were no late grade IV toxicities reported and no treatment-related deaths.
Locally Advanced Disease and Palliation
For patients with locally advanced T4 primary tumors or locally recurrent cancer, there is a rationale for combining resection with intraoperative radiation therapy (IORT), but experience is limited. Resection and IORT would preferably be preceded by several cycles of multiagent chemotherapy and then pelvic irradiation (45 to 50.4 Gy in 1.8-Gy fractions) plus concurrent weekly cisplatin (see prior section). Trials have been published from colleagues in Lyon, France, and Pamplona, Spain, demonstrating the potential value of pursuing such approaches in other centers with IORT capabilities.262–264
If the patient is unfit for cystectomy, then irradiation may be given to the bladder for palliation. Duchesne and associates265 reported an MRC trial in the United Kingdom in which 500 patients were randomized to two palliative fractionation schedules: 35 Gy in 10 fractions and 21 Gy in three fractions (given weekly). When assessed at 3 months, 68% had had some symptomatic improvement, but there was no difference between the arms.265 There was no evidence for a difference in toxicity justifying the use of this very hypofractionated regimen for the palliation of patients at the end of their lives.
Irradiation Techniques and Tolerance
External Beam Irradiation
The two-dimensional technique is well established, and it is worth discussing the advantages and pitfalls in detail. At the MGH, the following technique has been used. The standard protocol for radical EBRT as well as combined-modality therapy uses a four-field isocentric technique, which consists of shaped anterior, posterior, right, and left lateral fields for the initial or induction treatment field (Fig. 52-4). This is followed by a boost or consolidation field (Fig. 52-5).
Figure 52-4 Sagittal digitally reconstructed radiograph (DRR) image showing a lateral view of a small pelvis field.
Simulation
Target Volumes
During the first phase of treatment, the bladder is treated along with a margin of 2 cm. If using a radiographic rather than a CT simulation, the contrast lines only the inner wall of the bladder and another 5 to 10 mm must be added to account for wall thickness, depending on the degree of bladder filling. In men, the entire prostate is also covered in this first phase because there may be occult stromal invasion or extension into the prostatic urethra. In women, the proximal 2 cm of urethra is also considered as part of the target field. Care must be taken to minimize the amount of vulva in the inferior border of the field, because this greatly limits tolerance. The internal and external iliac lymph nodes of the pelvis are also covered for this phase of therapy. The top border of the field usually lies around the midsacroiliac joint and only at the level of the bifurcation of the common iliac vessels and does not include the entire pelvis. This is because a significant number of irradiated patients will subsequently require salvage cystectomy with urinary diversion. It is therefore prudent to limit the amount of irradiation to bowel tissue that may subsequently be needed for a urinary diversion. Another good reason to limit the bowel volume is that quality-of-life studies have shown that the most troublesome consequences of irradiation for bladder cancer are related to the small bowel irradiation rather than the bladder irradiation.256,259
During the boost phase of treatment, MGH investigators treat the tumor alone with a 2-cm margin rather than the entire bladder (an alternative is to boost the entire bladder excluding the nodes and then give a further boost to the tumor alone). Defining the tumor may be difficult, but we employ a combination of a bladder map drawn by the urologist at the time of cystoscopy/TURBT and CT and MRI information from the staging workup. CT scanning after TURBT often overestimates the tumor residuum because of edema in the bladder wall. Using these sources of information, it is usually possible to exclude approximately one-third of the bladder from the boost volume (see Fig. 52-5). Although this may not seem like much, it does mean that less than the entire volume is treated to a full dose, a factor that may contribute to the good functional results seen after use of this technique.
Treatment Break to Evaluate Response
At the MGH, investigators have routinely built a 3-week break into the treatment program after the delivery of 39 to 42 Gy followed by repeat cystoscopy (see Fig. 52-7). Patients are selected to continue and complete radiation therapy (the consolidation) on the basis of their response at this point. Those who have had a complete response or in whom the tumor residuum is less than T1 by stage (Ta or Tis) continue. The others are recommended to undergo an immediate cystectomy, which is easier to perform before the patient has had full-dose radiation therapy. It also identifies patients responding poorly earlier so surgery can be performed promptly rather than waiting until an entire course of irradiation has been completed, by which time the cancer could have progressed, threatening the efficacy of salvage therapy. Concerns have been expressed on radiobiologic grounds that split-course treatment compromises efficacy.266,267 There is also the worry that those who have not been complete responders after 40 Gy might yet become complete responders after 65 Gy, and, therefore, some patients may be recommended for unnecessary cystectomy. This concern, however, probably affects only a tiny minority of patients.
Controversial Issues in EBRT Technique
Nodal Treatment
Lymph node involvement is common in this disease and is seen in approximately 25% of patients undergoing radical cystectomy.136 The number of lymph nodes involved is a well-established prognostic indicator that surgeons use to determine the extent of lymph node dissection and the need for adjuvant chemotherapy.140 This information is, however, unavailable to radiation oncologists. More interesting for them is the knowledge that some patients with positive lymph nodes may be cured by a lymphadenectomy, a situation different from prostate cancer and more akin to breast or head and neck cancers. The thoroughness of a lymph node dissection (>10 nodes removed) also correlates with the outcome independent of nodal status.268 Therefore radiation therapy to the pelvis might confer a survival advantage, although it comes at the price of additional morbidity. Centers that do irradiate the bladder alone do not report either an increased pelvic failure rate or a reduced survival rate, although direct comparisons are difficult and randomized data are not available.269 It may be either that chemotherapy does the job of the nodal irradiation or that nodal disease extends beyond the confines of the current pelvic template. Surgeons have started to perform extended lymphadenectomies to the level of the inferior mesenteric artery.125,270
Partial Bladder Treatment
Estimates of bladder tolerance have been made that suggest that up to 80 Gy could be given if one-third of the bladder is spared, whereas 65 Gy is the limit for whole-bladder treatment.271,272 In addition, the low rate of development of new invasive tumors elsewhere in the bladder after brachytherapy (5% to 7%) suggests that localized partial bladder irradiation is a reasonable strategy.181 Careful selection is, however, necessary, with such approaches being limited to patients with smaller tumors (<5 cm) or unifocal tumors or those without extensive Tis disease elsewhere in the bladder. A randomized trial in the United Kingdom has shown that partial bladder irradiation does allow for the delivery of a higher dose to the tumor than whole-bladder irradiation without an increase in morbidity.273 It did not, however, show improvement in either local control or survival rates. In one study, 149 patients were randomized to receive whole-bladder conformal RT to 52.5 Gy in 20 fractions or partial bladder conformal radiation therapy to either 57.5 Gy in 20 fractions or 55 Gy in 16 fractions. The 5-year local control rates for these three regimens were 58%, 59%, and 34%, respectively, without any significant differences between arms (p = .18).
Treatment Margins
At the MGH, in expanding the clinical target volume (CTV) to the planning tumor volume (PTV), we have adopted isotropic 2-cm margins around either the bladder (first phase of treatment) or tumor (boost) in all three dimensions. This is to account for setup errors and organ motion. Several studies employing serial CT scans have shown that margins less than this may be inadequate. Turner and colleagues274 demonstrated that bladder wall movement of more than 1.5 cm occurs at least once during a course of treatment in over 60% of patients. Several others have reported that the gross tumor volume (GTV) moves outside the PTV on at least one occasion in at least 20% of patients.275,276 Our own analysis showed that these variations are highly patient dependent and occurred even when strict instructions were given regarding bladder and rectal filling.277 The greatest degree of bladder wall positional change occurred in the cranial direction, with the least variation in the anteroinferior direction, limited by the pubic symphysis. In light of this information, it is clear that organ motion is the dominant source of error and that the magnitude of the error depends on the region of the bladder being treated. The solutions are clear but difficult to achieve. Graham and associates278 have recommended anisotropic margin widths of 1.6 cm anteriorly and posteriorly, 1.4 cm laterally, 3 cm superiorly, and 1.4 cm inferiorly. The problem is that these margins incorporate much normal tissue. Daily image-guided therapy is the only way to reduce these margins significantly. Daily CT or cone-beam therapy that can delineate the bladder is one option. There is also interest in the use of fiducial markers that could be placed by the urologist at the time of TURBT in the bladder wall around the tumor crater (Fig. 52-6). Roof and colleagues277 have shown that if the daily treatment is centered on the bladder centroid rather than referenced to the bony anatomy, margins of less than 1.5 cm could be feasible. None of these studies account for the fact that the bladder’s deformability causes it to change not only in size but also in shape. This will likely set a limit on our ability to shrink margins significantly at any time soon.
Radiation Dose
There is evidence for a dose-response curve in bladder cancer, with most studies showing that doses of less than 62 Gy (at 1.8 to 2 Gy per fraction) predict a poor outcome.279–281 Most of these studies have, however, employed irradiation alone, and the curve is likely to be greatly modified by effective debulking of the tumor or by radiation sensitization with concurrent chemotherapy. Increasing the dose may result in increased complete response rates but may add to the morbidity and may also be limited by the rapid proliferative index and short doubling time of these tumors.282 Interest recently has therefore concentrated on dose intensification using accelerated regimes rather than an increase in the total dose. Acceleration may be achieved through hypofractionation, but this is better limited to the palliative setting or through the use of conventional 2-Gy fractionation given twice a day.283 The Royal Marsden Hospital has reported on 85 patients with twice-daily fractions of 1.8 to 2 Gy 5 days a week to doses between 57.6 Gy and 64 Gy in 32 fractions over 26 days. Of 70 patients who had cystoscopic reevaluation 3 to 6 months after RT, 80% were found to be complete responders (i.e., cystoscopy and biopsy negative for tumor 3 to 6 months later).284 Plataniotis and colleagues285 reported a total response rate of 67% when twice-daily fractionation was used during the last (fifth) week of RT.
Unfortunately, acceleration with fraction sizes of 2 Gy seems to increase the acute toxicity. The RTOG has therefore explored acceleration with hyperfractionation, and recent protocols have employed fraction sizes of 1.2 to 1.8 Gy with a concomitant boost technique. No comparative studies have yet been performed, but morbidity rates appear to be low.239
Hyperfractionation has also been used to increase the total tumor dose. A randomized trial of 168 patients with T2 to T4 tumors, unsuited for cystectomy, compared hyperfractionated EBRT (1 Gy three times a day to a total dose of 84 Gy) with conventional fractionation (2 Gy every day to 64 Gy).286,287 Hyperfractionation achieved superior results with respect to survival at 5 years (27% vs. 18%), local control (12% vs. 7%), and clinical complete response rates (59% vs. 36%), with an increase in late toxicity. Both study arms were treated by a split course of RT with a 2-week gap after the first 3 weeks, the effect of which is unknown. The benefit of the hyperfractionated schedule persisted over a 10-year follow-up period, both for local control and survival rates. It should be noted, however, that patients in the conventional fractionation arm did poorly compared with those receiving similar treatment in other studies, largely because patients unfit for cystectomy were entered in this study.
Future improvements in the complete response rate may also come without any need to escalate the radiation dose; these would include better selection of patients, better radiation sensitization, and more aggressive TURBT. Others have argued that doses of 65 Gy at 1.8 to 2 Gy per fraction may actually be more than is needed, especially for those who have had visibly complete transurethral resections. The Erlangen group has delivered doses tailored to the estimated residuum following resection. These doses are in the range of 50 to 59 Gy (1.8 to 2 Gy per fraction), depending on whether the disease to be treated is microscopic or gross in volume.288
Summary, Treatment Algorithm, Challenges, and Future Possibilities
Selective bladder preservation is based on the response of the tumor to induction combined-modality therapy, which includes a TUR of as much of the primary bladder tumor as is judged safely possible combined with concurrent chemotherapy and EBRT; early salvage cystectomy is recommended for tumors that are not responding to combined-modality therapy or for an invasive recurrence to maximize the possibility of cure (Fig. 52-7). In appropriately selected patients, bladder-preserving treatment with chemoradiation offers a chance for long-term cure and for survival rates comparable to those of radical cystectomy, while affording a 70% chance of maintaining the native bladder (see Tables 52-6 and 52-7). Quality-of-life studies have demonstrated that the retained native bladder functions well and the long-term toxicity of chemoradiation to the pelvic organs is low. These results support the acceptance of modern bladder-sparing trimodality therapy for selected patients as a proven alternative to cystectomy. This approach is not suitable for patients with tumors obstructing a ureter. Twenty percent to 30% of patients cured of muscle-invading bladder cancer will subsequently acquire a new superficial tumor, but these tumors have generally responded well to the usual intravesical drug therapy. These patients require close urologic surveillance, as do any patients with superficial bladder cancer treated conservatively. Bladder-preserving treatment usually results in a normally functioning bladder for T2 and T3a patients; however, T3b to T4 patients experience local control less frequently using these techniques. No data exist, however, to suggest that patients with more advanced disease are in any way disadvantaged by preoperative chemoradiation as an attempt at bladder conservation. Further clinical practice guidelines are available through the updated National Comprehensive Cancer Network.289
The immediate therapeutic challenges for radiation oncologists include:
3 Pelucchi C, Bosetti C, Negri E, et al. Mechanisms of disease. The epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3:327-340.
48 Sidransky D, Frost P, Eschenbach AV, et al. Clonal origin of bladder cancer. N Engl J Med. 1992;326:737-740.
49 Bryan RT, Zeegers MP, James ND, et al. Biomarkers in bladder cancer. BJUI. 2010;105:608-613.
56 Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259-1264.
64 Hussain SA, Ganesan R, Hiller L, et al. BCL2 expression predicts survival in patients receiving synchronous chemo radiotherapy in muscle invasive transitional cell carcinoma of the bladder. Oncol Rep. 2003;10:571-576.
70 Rödel C, Grabenbauer GG, Rödel F, et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma. Possible predictors for response to radiochemotherapy and successful bladder preservation. Int J Radiat Oncol Biol Phys. 2000;46:1213-1221.
79 Hoskin PJ, Sibtain A, Daley FM, et al. GLUT1 and CA IX as intrinsic markers of hypoxia in bladder cancer. Relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer. 2003;89:1290-1297.
94 Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer. Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666-675.
115 Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical Bacillus Calmette-Guérin therapy prevents tumor progression and death from superficial bladder cancer. Ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;12:1404-1408.
124 Weiss C, Engehausen DG, Ott OJ, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer. An alternative to intravesical therapy or early cystectomy? J Clin Oncol. 2006;24:2318-2324.
133 Donat SM, Shabsigh A, Savage C, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy. Aa high volume tertiary cancer center experience. Eur Urol. 2009;55:177-186.
137 Dalbagni G, Genega E, Hashibe M, et al. Cystectomy for bladder cancer. A contemporary series. J Urol. 2001;165:1111-1116.
142 Herr HW, Bochner BH, Dalbagni G, et al. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295-1298.
152 Cole C, Pollack A, Zagars G, et al. Local control of muscle-invasive bladder cancer. Preoperative radiotherapy and cystectomy versus cystectomy alone. Int J Radiat Oncol Biol Phys. 1995;32:331-340.
162 Herr HW. Transurethral resection of muscle-invasive bladder cancer. 10-year outcome. J Clin Oncol. 2001;19:89-93.
170 Fossa SD, Waehre H, Aass N, et al. Bladder cancer definitive radiation therapy of muscle-invasive bladder cancer. A retrospective analysis of 317 patients. Cancer. 1993;72:3036-3043.
187 Pos F, Horenblas S, Dom P, et al. Organ preservation in invasive bladder cancer. Brachytherapy, an alternative to cystectomy and combined modality treatment? Int J Radiat Oncol Biol Phys. 2005;61:678-686.
202 Herr HW, Bajorin DF, Scher HI. Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer. Ten-year outcome. J Clin Oncol. 1998;16:1298-1301.
209 Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy. Initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576-3583.
215 Coppin C, Gospodarowicz M, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. J Clin Oncol. 1996;14:2901-2907.
216 Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859-866.
218 Neoadjuvant chemotherapy in invasive bladder cancer. A systematic review and meta-analysis. Lancet. 2003;361:1927.
219 Neoadjuvant chemotherapy in invasive bladder cancer. Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:202-205.
232 Shipley WU, Kaufman DS, Thakral HK, et al. Long-term outcome of patients treated for muscle-invasive bladder cancer by tri-modality therapy. Urology. 2002;60:62-68.
234 Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective bladder preservation in invasive bladder cancer. Long-term results. J Clin Oncol. 2002;20:3061-3071.
240 Kaufman DS, Winter KA, Shipley WU, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833-887.
243 Eapen L, Stewart D, Collins J, Peterson R. Effective bladder sparing therapy with intra-arterial cisplatin and radiotherapy for localized bladder cancer. J Urol. 2004;172:1276-1280.
244 Hoskin PJ, Rojas AM, Saunders MI, et al. Carbogen and nicotinamide in locally advanced bladder cancer. Early results of a phase III randomized trial. Radiother Oncol. 2009;91:120-125.
249 Zietman AL, Grocela J, Zehr E, et al. Selective bladder conservation using trans-urethral resection, chemotherapy, and radiation. The risk and consequences of superficial recurrences within the retained bladder. Urology. 2001;58:380-385.
256 Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy, and radiation. Results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772-1776.
261 Efstathiou JA, Bae K, Shipley WU, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer. RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol. 2009;27:4055-4061.
265 Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma. Results of Medical Research Council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47:379-388.
272 Marks LB, Carroll PR, Dugan TC, et al. The response of the urinary bladder, urethra and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257-1280.
273 Cowan RA, McBain CA, Ryder WDRR, et al. Radiotherapy for muscle invasive carcinoma of the bladder. Results of a randomised trial comparing whole bladder with dose-escalated partial bladder irradiation. Int J Radiat Oncol Biol Phys. 2004;59:197-207.
274 Turner SL, Swindell R, Bowl N. Bladder movement during radiation therapy for bladder cancer. Implications for treatment planning. Int J Radiat Oncol Biol Phys. 1997;39:355-360.
275 Harris SJ, Buchanan RB. An audit and evaluation of bladder movements during radical radiotherapy. Clin Oncol. 1998;10:262-264.
1 Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
2 Rubben H, Lutzeyer W, Wallace DMA. The epidemiology and aetiology of bladder cancer. In: Zingg EJ, Wallace DMA, editors. Bladder Cancer. New York: Springer-Verlag; 1985:1-21.
3 Pelucchi C, Bosetti C, Negri E, et al. Mechanisms of disease. The epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3:327-340.
4 Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33-64.
5 Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68-74.
6 Cohen SM, Johansson SL. Epidemiology and etiology of bladder cancer. Urol Clin North Am. 1992;19:421-428.
7 Nomura A, Kolonel LN, Yoshizawa CN. Smoking, alcohol, occupation and hair dye use in cancer of the lower urinary tract. Am J Epidemiol. 1989;130:1159-1163.
8 Schifflers E, Jamart J, Renard V. Tobacco and occupational risk factors in bladder cancer. A case-control study in southern Belgium. Int J Cancer. 1987;39:287-292.
9 Augustine A, Hebert JR, Kabat GC, et al. Bladder cancer in relation to cigarette smoking. Cancer Res. 1988;48:4405-4408.
10 Baris D, Karagas MR, Verrill C, et al. A case-control study of smoking and bladder cancer risk. Emergent patterns over time. J Natl Cancer Inst. 2009;101:1553-1561.
11 Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men. A pooled analysis of 11 case-control studies. Int J Cancer. 2000;86:289-294.
12 Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007;100:281-286.
13 Jiang X, Yuan JM, Skipper PL, et al. Environmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles County. Cancer Res. 2007;67:7540-7545.
14 Schulte PA, Ringen K, Hemstreet GP, et al. Risk factors for bladder cancer in a cohort exposed to aromatic amines. Cancer. 1986;58:2156-2162.
15 Clayson DB. Recent research into occupational bladder cancer. In: Connolly JG, editor. Carcinoma of the Bladder. Progress in Cancer Research and Therapy, vol 18. New York: Raven Press; 1981:13-24.
16 Cole P, Hoover R, Friedell G. Occupation and cancer of the lower urinary tract. Cancer. 1972;29:1250-1260.
17 Silverman DT, Levin LI, Hoover RN. Occupational risks of bladder cancer in the United States. I. White men. J Natl Cancer Inst. 1989;81:1472-1480.
18 Kogevinas M, Mannetje A, Cordier S, et al. Occupation and bladder cancer among men in Western Europe. Cancer Causes Control. 2003;14:907-914.
19 Silverman DT, Hoover RN, Mason TJ, et al. Motor exhaust-related occupations and bladder cancer. Cancer Res. 1986;46:2113-2116.
20 Morris RD, Audet AM, Angelillo IF, et al. Chlorination, chlorination by-products, and cancer. A meta-analysis. Am J Public Health. 1992;82:955-963.
21 Villanueva CM, Fernandez F, Malats N, et al. Meta-analysis of studies on individual consumption of chlorinated drinking water and bladder cancer. J Epidemiol Community Health. 2003;57:166-173.
22 King WD, Marrett LD. Case-control study of bladder cancer and water chlorination by-products in treated water (Ontario, Canada). Cancer Causes Control. 1996;7:596-604.
23 Marshall G, Ferreccio C, Yuan Y, et al. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst. 2007;99:920-928.
24 Evans DAP. Survey of the human acetylator polymorphism in spontaneous disorders. J Med Genet. 1984;21:243-253.
25 Bhagwandeen SB. Schistosomiasis and carcinoma of the bladder in Zambia. S Afr Med J. 1976;50:1616-1620.
26 McCredie M, Stewart JH, Ford JM, et al. Phenacetin-containing analgesics and cancer of the bladder or renal pelvis in women. Br J Urol. 1983;55:220-224.
27 O’Keane JC. Carcinoma of the urinary bladder after treatment with cyclophosphamide. N Engl J Med. 1988;319:871.
28 Travis LB, Curtis RE, Glimelius B, et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1995;87:524-530.
29 Chrouser K, Leibovich B, Bergstralh E, et al. Bladder cancer risk following primary and adjuvant external beam radiation for prostate cancer. J Urol. 2005;174:107-110.
30 Michaud DS, Spiegelman D, Clinton SK, et al. Fluid intake and the risk of bladder cancer in men. N Engl J Med. 1999;340:1390-1397.
31 Lai MN, Wang SM, Chen PC, et al. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst. 2010;102:179-186.
32 Johnson DE, Boileau MA. Bladder cancer overview. In: Johnson DE, Boileau MA, editors. Genitourinary Tumors. Fundamental Principles and Surgical Techniques. New York: Grune & Stratton; 1982:399-447.
33 Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology. Results of a comprehensive literature review and meta-analyses. Urology. 2003;61:109-118.
34 Messing EM, Young TB, Hunt VB, et al. The significance of asymptomatic microhematuria in men 50 or more years old. Findings of a home screening study using dipsticks. J Urol. 1987;137:919-922.
35 Messing EM, Young TB, Hunt VB, et al. Urinary tract cancers found by home screening with hematuria dipsticks in healthy men over 50 years of age. Cancer. 1989;64:2361-2367.
36 Joseph JV, Messing EM. Chemoprevention of bladder and prostate cancer. Cancer Control. 1997;4:136-141.
37 Michaud DS, Pietinen P, Taylor RR, et al. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer. 2002;87:960-965.
38 Virtamo J, Edwards BK, Virtanen M, et al. Effects of supplemental alpha-tocopherol and beta-carotene on urinary tract cancer. Incidence and mortality in a controlled trial (Finland). Cancer Causes Control. 2000;11:933-939.
39 Sabichi AL, Lerner SP, Atkinson EN, et al. Phase III prevention trial of fenretinide in patients with resected non-muscle-invasive bladder cancer. Clin Cancer Res. 2008;14:224-229.
40 Michaud DS, Speigelman D, Clinton SK, et al. Prospective study of dietary supplements, macronutrients, micronutrients, and risk of bladder cancer in US men. Am J Epidemiol. 2000;152:1145-1153.
41 Liang D, Lin J, Grossman HB, et al. Plasma vitamins E and A and risk of bladder cancer. A case-control analysis. Cancer Causes Control. 2008;19:981-992.
42 Messing EM, Kim KM, Sharky F, et al. Randomized prospective phase III trial of difluoromethylornithine vs placebo in preventing recurrence of completely resected low risk superficial bladder cancer. J Urol. 2006;176:500-504.
43 Bianchi GD, Cerhan JR, Parker AS, et al. Tea consumption and risk of bladder and kidney cancers in a population-based case-control study. Am J Epidemiol. 2000;151:377-383.
44 Dannenberg AJ, Lippman SM, Mann JR, et al. Cyclooxygenase-2 and epidermal growth factor receptor. Pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254-266.
45 Iida K, Itoh K, Kumagai Y, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424-6431.
46 Busby JE, Kamat AM. Chemoprevention for bladder cancer. J Urol. 2006;176:1914-1920.
47 Urinary bladder. In AJCC Cancer Staging Manual, 7th ed. Springer, New York, 2010, p 497.
48 Sidransky D, Frost P, Eschenbach AV, et al. Clonal origin of bladder cancer. N Engl J Med. 1992;326:737-740.
49 Bryan RT, Zeegers MP, James ND, et al. Biomarkers in bladder cancer. BJUI. 2010;105:608-613.
50 Knowles MA. What we could do now: molecular pathology of bladder cancer. Mol Pathol. 2001;54:215-221.
51 Pollack A, Wu CS, Czerniak B, et al. Abnormal bcl-2 and pRb expression are independent correlates of radiation response in muscle-invasive bladder cancer. Clin Cancer Res. 1997;3:1823-1829.
52 Cote RJ, Chatterjee SJ. Molecular determinants of outcome in bladder cancer. Cancer J Sci Am. 1999;5:2-15.
53 Shariat SF, Tokunaga H, Zhou J, et al. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004;22:1014-1024.
54 Cote RT, Dunn MD, Chatterjee SJ, et al. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998;58:1090-1094.
55 Cordon-Cardo C, Wartinger D, Petrylak D, et al. Altered expression of the retinoblastoma gene product. Prognostic indicator in bladder cancer. J Natl Cancer Inst. 1992;84:1251-1256.
56 Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259-1264.
57 Cote RJ, Esrig D, Groshen S, et al. p53 and treatment of bladder cancer. Nature. 1997;385:123-125.
58 Sidransky D, Von Eschenbach A, Tsai YC, et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991;252:706-709.
59 Shariat SF, Lotan Y, Karakiewicz PI, et al. p53 predictive value for pT1-2 N0 disease at radical cystectomy. J Urol. 2009;182:907-913.
60 Malats N, Bustos A, Nascimento CM, et al. P53 as a prognostic marker for bladder cancer. A meta-analysis and review. Lancet Oncol. 2005;6:678-686.
61 Chakravarti A, Winter K, Chin-Lee W, et al. Expression of the epidermal growth factor receptor and Her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy. A report from the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2005;62:309-317.
62 Hussain SA, Ganesan R, Hiller L, et al. Proapoptotic genes BAX and CD40L are predictors of survival in transitional cell carcinoma of the bladder. Br J Cancer. 2003;88:586-592.
63 Cooke PW, James ND, Ganesan R, et al. BCL2 expression identifies patients with advanced bladder cancer treated by radiotherapy who benefit from neoadjuvant chemotherapy. BJU I. 2000;85:829-835.
64 Hussain SA, Ganesan R, Hiller L, et al. BCL2 expression predicts survival in patients receiving synchronous chemo radiotherapy in muscle invasive transitional cell carcinoma of the bladder. Oncol Rep. 2003;10:571-576.
65 Qureshi KN, Griffiths TR, Robinson MC, et al. Combined p21WAF1/CIP1 and p53 overexpression predict improved survival in muscle-invasive bladder cancer treated by radical radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1234-1240.
66 Als AB, Dyrskjot L, von der Maase H, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407-4414.
67 Kader AK, Shao L, Dinney CP, et al. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006;66:11644-11648.
68 Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8:128-136.
69 Shariat SF, Karakiewicz PI, Ashfaq R, et al. Multiple biomarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer. 2008;112:315-325.
70 Rödel C, Grabenbauer GG, Rödel F, et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma. Possible predictors for response to radiochemotherapy and successful bladder preservation. Int J Radiat Oncol Biol Phys. 2000;46:1213-1221.
71 Cohen-Jonathan E, Muschel RJ, Gillies McKenna W, et al. Farnesyltransferase inhibitors potentiate the antitumor effect of radiation on a human tumor xenograft expressing activated HRAS. Radiat Res. 2000;154:125-132.
72 Latif Z, Watters AD, Dunn I, et al. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer. 2003;89:1305-1309.
73 Jimenez RE, Hussain M, Bianco FJJr, et al. Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder. Prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res. 2001;7:2440-2447.
74 Chow NH, Chan SH, Tzai TS, et al. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:1957-1962.
75 Underwood M, Bartlett J, Reeves J, et al. C-erbB-2 gene amplification. A molecular marker in recurrent bladder tumors? Cancer Res. 1995;55:2422-2430.
76 Bellmunt J, Hussain M, Dinney CP. Novel approaches with targeted therapies in bladder cancer. Therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit Rev Oncol Hematol. 2003;46:85-104.
77 Hernandez S, Lopez-Knowles E, Lloreta J, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24:3664-3671.
78 Turner KJ, Crew JP, Wykoff CC, et al. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial vs invasive bladder cancer. Br J Cancer. 2002;86:1276-1282.
79 Hoskin PJ, Sibtain A, Daley FM, et al. GLUT1 and CA IX as intrinsic markers of hypoxia in bladder cancer. Relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer. 2003;89:1290-1297.
80 Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1930 patients with hematuria to evaluate current diagnostic practice. J Urol. 2000;163:524-527.
81 Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria. 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol. 1989;141:350-355.
82 Denzinger S, Burger M, Walter B, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis. 8-year results of prospective randomized study. Urology. 2007;69:675-679.
83 Harving N, Wolf H, Melsen F. Positive urine cytology after tumor resection. An indicator for concomitant carcinoma in situ. J Urol. 1988;140:495-497.
84 Koss L, Dietch D, Ramanathan R, et al. Diagnostic value of cytology of voided urine. Acta Cytol. 1985;29:810-816.
85 Lin C, Young D, Kirley SD, et al. Detection of tumor cells in bladder washings by a monoclonal antibody to human bladder tumor-associated antigen. J Urol. 1988;140:672-677.
86 Boman H, Hedelin H. Holmang H: Four bladder tumor markers have a disappointingly low sensitivity for small size and low grade recurrence. J Urol. 2002;167:80-83.
87 Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293:810-816.
88 Lokeshwar VB, Selzer MG. Urinary bladder tumor markers. Urol Oncol. 2006;24:528-537.
89 Lokeshwar VB, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology. International Consensus Panel on bladder tumor markers. Urology. 2005;66:35-63.
90 Schlomer BJ, Ho R, Sagalowsky A, et al. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol. 2010;183:62-67.
91 Hillman B, Silvert M, Cook G, et al. Recognition of bladder tumors by excretory urography. Radiology. 1981;138:319-323.
92 Hatch TR, Berry JM. The value of excretory radiography in staging bladder cancer. J Urol. 1986;135:49.
93 Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder. A contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006;176:2414-2422.
94 Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer. Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666-675.
95 Voges G, Tauschke E, Stöckle M, et al. Computerized tomography. An unreliable method for accurate staging of bladder tumors in patients who are candidates for radical cystectomy. J Urol. 1989;142:972-974.
96 Nishimura K, Hida S, Nishio Y, et al. The validity of magnetic resonance imaging (MRI) in the staging of bladder cancer. Comparison with computed tomography (CT) and transurethral ultrasonography (US). Jpn J Clin Oncol. 1988;18:217-226.
97 Neuerburg J, Bohndorf K, Sohn M, et al. Urinary bladder neoplasms. Evaluation with contrast-enhanced MR imaging. Radiology. 1989;172:739-743.
98 Mallampati GK, Siegelman ES. MR imaging of the bladder. Magn Res Imaging Clin North Am. 2004;12:545-555.
99 Tekes A, Kamel I, Imam K, et al. Dynamic MRI of bladder cancer. Evaluation of staging accuracy. AJR Am J Roentgenol. 2005;184:121-127.
100 Deserno WM, Harisinghani MG, Taupitz M, et al. Urinary bladder cancer. Preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology. 2004;233:449-456.
101 Jewett H, Strong G. Infiltrating carcinoma of the bladder. Relation of depth of penetration of the bladder wall to incidence of local extension and metastases. J Urol. 1946;55:366-372.
102 Urinary bladder. In AJCC Cancer Staging Manual, 7th ed. Springer, New York, 2010.
103 Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage. Impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137-149.
104 Heney NH, Ahmad S, Flanagan MJ, et al. Superficial bladder cancer. Progression and recurrence. J Urol. 1983;130:1083-1086.
105 Fitzpatrick JM, West AB, Butler MR, et al. Superficial bladder tumors (stage, pTa, grades 1 and 2). The importance of recurrence pattern following initial resection. J Urol. 1986;135:920-922.
106 Malmstrom PU, Busch C, Norlen BJ. Recurrence, progression and survival in bladder cancer. A retrospective analysis of 232 patients with greater or equal to 5-year follow-up. Scand J Urol Nephrol. 1987;21:185-195.
107 Herr HW, Jakse G, Sheinfeld J. The T1 bladder tumor. Semin Urol. 1990;8:254-261.
108 Lamm DL. Carcinoma in situ. Urol Clin North Am. 1992;19:499-508.
109 Herr HW, Pinsky CM, Whitmore WF, et al. Experience with intravesical bacillus Calmette-Guérin therapy of superficial bladder tumors. Urology. 1985;25:119-123.
110 Pagano F, Bassi P, Milani C, et al. A low dose bacillus Calmette-Guérin regimen in superficial bladder cancer therapy. Is it effective? J Urol. 1991;146:32-35.
111 Melekos MD, Chionis H, Panatzakos A, et al. Intravesical bacillus Calmette-Guérin immunoprophylaxis of superficial bladder cancer. Results of a controlled prospective trial with modified treatment schedule. J Urol. 1993;149:744-748.
112 Shahin O, Thalmann GN, Rentsch C, et al. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guérin for primary state T1 grade 3 bladder cancer. Recurrence, progression and survival. J Urol. 2003;169:96-100.
113 Shelley MD, Court JB, Kynaston H, et al: Intravesical bacillus Calmette-Guérin in Ta and T1 bladder cancer. Cochrane Database Syst Rev 4:CD001986, 2000.
114 Davis JW, Sheth SI, Doviak MJ, et al. Superficial bladder carcinoma treated with bacillus Calmette-Guérin. Progression-free and disease specific survival with minimum 10-year followup. J Urol. 2002;167:494-500.
115 Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical Bacillus Calmette-Guérin therapy prevents tumor progression and death from superficial bladder cancer. Ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;12:1404-1408.
116 Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19:573-580.
117 Herr HW. Extravesical tumor relapse in patients with superficial bladder tumors. J Clin Oncol. 1998;16:1099-1102.
118 Sylvester RJ, van der Meijden AP, Witjes JA, et al. Bacillus Calmette-Guérin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder. A meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174:86-91.
119 Mulders PF, Hoekstra WJ, Heybrock RP. Prognosis and treatment of T1G3 bladder tumours. A prognostic factor analysis of 121 patients. Dutch South Eastern Bladder Cancer Study Group. Eur J Cancer. 1994;30:914-917.
120 Van der Werf-Messing B, Hop WJC. Carcinoma of the urinary bladder (T1N0M0) treated either by radium implant or transurethral resection only. Int J Radiat Oncol Biol Phys. 1981;7:299-303.
121 Van der Steen-Banasik EM, Visser AG, Reinders JG. Saving bladders with brachytherapy. Implantation technique and results. Int J Radiat Oncol Biol Phys. 2002;53:622-629.
122 Harland SJ, Kynaston H, Grigor K, et al. A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. J Urol. 2007;178:807-813.
123 Rodel C, Grabenbauer GG, Kuhn R, et al. Invasive bladder cancer. Organ preservation by radiochemotherapy. Front Radiat Ther Oncol.. 2002;36:118-130.
124 Weiss C, Engehausen DG, Ott OJ, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer. An alternative to intravesical therapy or early cystectomy? J Clin Oncol. 2006;24:2318-2324.
125 Dhar NB, Klein EA, Reuther AM, et al. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol. 2008;179:873-878.
126 Rowland RG, Mitchell ME, Bihrle R. The cecoileal continent urinary reservoir. World J Urol. 1985;3:185-190.
127 Kock NG. Continent ileostomy. Prog Surg. 1973;12:180-201.
128 Thuroff JW, Alken P, Riemiller H, et al. The Mainz pouch for augmentation or substitution of the bladder and continent diversion. Semin Urol. 1987;5:69-79.
129 Hautmann RE, Egghart G, Frohneberg D, et al. The ileal neobladder. J Urol. 1988;139:39-42.
130 Ghoneim MA. Orthotopic bladder substitution in women following cystectomy for bladder cancer. Urol Clin North Am. 1997;24:225-239.
131 Frazier HA, Robertson JE, Paulson DF. Complications of radical cystectomy and urinary diversion. A retrospective review of 675 cases in 2 decades. J Urol. 1992;148:1401-1405.
132 Chang SS, Cookson MS, Baumgartner RG, et al. Analysis of early complications after radical cystectomy. Results of a collaborative care pathway. J Urol. 2002;167:2012-2016.
133 Donat SM, Shabsigh A, Savage C, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy. A high volume tertiary cancer center experience. Eur Urol. 2009;55:177-186.
134 Novotny V, Hakenberg OW, Wiessner D, et al. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51:397-401.
135 Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol. 1987;138:1402-1406.
136 Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer. Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666-675.
137 Dalbagni G, Genega E, Hashibe M, et al. Cystectomy for bladder cancer. A contemporary series. J Urol. 2001;165:1111-1116.
138 Hautmann RE, Gschwend JE, de Petriconi RC, et al. Cystectomy for transitional cell carcinoma of the bladder. Results of a surgery only series in the neobladder era. J Urol. 2006;176:486-492.
139 Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder. A contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006;176:2414-2422.
140 Kassouf W, Agarwal PK, Herr HW, et al. Lymph node density is superior to TNM nodal status in predicting disease-specific survival after radical cystectomy for bladder cancer. Analysis of pooled data from MDACC and MSKCC. J Clin Oncol. 2008;26:121-126.
141 Lerner SP, Skinner DG, Lieskovsky G, et al. The rationale for en bloc pelvic lymph node dissection for bladder cancer patients with nodal metastases. Long-term results. J Urol. 1993;149:758-764.
142 Herr HW, Bochner BH, Dalbagni G, et al. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295-1298.
143 Poulsen AL, Horn T, Steven K. Radical cystectomy. Extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol. 1998;160:2015-2019.
144 Konety BR, Joslyn SA, O’Donnell MA. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer. Analysis of data from the Surveillance, Epidemiology and End Results Program data base. J Urol. 2003;169:946-950.
145 Karakiewicz PI, Shariat SF, Palapattu GS, et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol. 2006;176:1354-1361.
146 Whitmore WFJr, Batata MA, Ghoneim MA, et al. Radical cystectomy with or without prior irradiation in the treatment of bladder cancer. J Urol. 1977;118:184-187.
147 Shipley WU, Cummings KB, Coombs LJ, et al. 4,000 RAD preoperative irradiation followed by prompt radical cystectomy for invasive bladder carcinoma. A prospective study of patient tolerance and pathologic downstaging. J Urol. 1982;127:48-51.
148 Whitmore W. Integrated irradiation and cystectomy for bladder cancer. Br J Urol. 1980;52:1-9.
149 Skinner D, Lieskovsky G. Contemporary cystectomy with pelvic node dissection compared to preoperative radiation plus cystectomy in management of invasive bladder cancer. J Urol. 1984;131:1069-1072.
150 Smith JA, Crawford ED, Paradelo JC, et al. Treatment of advanced bladder cancer with combined preoperative irradiation and radical cystectomy versus radical cystectomy alone. A phase III intergroup study. J Urol. 1997;157:805-807.
151 Parsons J, Million R. Planned preoperative irradiation in the management of clinical stage B2-C (T3) bladder carcinoma. Int J Radiat Oncol Biol Phys. 1988;14:797-810.
152 Cole C, Pollack A, Zagars G, et al. Local control of muscle-invasive bladder cancer. Preoperative radiotherapy and cystectomy versus cystectomy alone. Int J Radiat Oncol Biol Phys. 1995;32:331-340.
153 Housset M, Maulard C, Chretien Y, et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder. A prospective study. J Clin Oncol. 1993;11:2150-2157.
154 van der Werf-Messing B. Carcinoma of the bladder treated by suprapubic radium implants. The value of additional external irradiation. Eur J Cancer. 1969;5:277-285.
155 Zaghloul M, Awaad H, Akoush H, et al. Post-operative radiotherapy of carcinoma in bilharzial bladder. Improved disease free survival through improving local control. Int J Radiat Oncol Biol Phys. 1992;23:511-517.
156 Fromenti SC. Management of patients with pelvic recurrence following radical cystectomy. In: Petrovich Z, Baert L, editors. Carcinoma of the Urinary Bladder. Innovation in Management. Berlin: Springer-Verlag; 1998:249-258.
157 Reisinger SA, Mohuiddin M, Mulholland SG. Combined pre- and post-operative adjuvant radiation therapy for bladder cancer—a ten-year experience. Int J Radiat Oncol Biol Phys. 1992;24:463-468.
158 Sweeny P, Kursh ED, Resnick MI. Partial cystectomy. Urol Clin North Am. 1992;19:701-711.
159 Herr HW. Conservative management of muscle-infiltrating bladder cancer. Prospective experience. J Urol. 1987;138:1162-1163.
160 Henry K, Miller J, Mori M, et al. Comparison of transurethral resection to radical therapies for stage B bladder tumors. J Urol. 1988;140:964-967.
161 Barnes RW, Dick AL, Hadley HL, et al. Survival following transurethral resection of bladder carcinoma. Cancer Res. 1977;37:2895-2898.
162 Herr HW. Transurethral resection of muscle-invasive bladder cancer. 10-year outcome. J Clin Oncol. 2001;19:89-93.
163 Duncan W, Quilty PM. The results of a series of 963 patients with transitional cell carcinoma of the urinary bladder primarily treated by radical megavoltage x-ray therapy. Radiother Oncol. 1986;7:299-310.
164 Blandy JP, Jenkins BJ, Fowler CG, et al. Radical radiotherapy and salvage cystectomy for T2/3 cancer of the bladder. Prog Clin Biol Res. 1988;260:447-451.
165 Jenkins BJ, Caulfield MJ, Fowler CG, et al. Reappraisal of the role of radical radiotherapy and salvage cystectomy in the treatment of invasive (T2/T3) bladder cancer. Br J Urol. 1988;62:342-346.
166 Greven KM, Solin LJ, Hanks GE, et al. Prognostic factors in patients with bladder carcinoma treated with definitive irradiation. Cancer. 1990;65:908-912.
167 Smaaland R, Akslen L, Tonder B, et al. Radical radiation treatment of invasive and locally advanced bladder cancer in elderly patients. Br J Urol. 1991;67:61-69.
168 Gospodarowicz MK, Rider WD, Keen CW, et al. Bladder cancer. Long term follow-up results of patients treated with radical radiation. Clin Oncol. 1991;3:155-161.
169 Jahnson S, Pedersen J, Westman G, et al. Bladder carcinoma—a 20-year review of radical irradiation therapy. Radiother Oncol. 1991;22:111-117.
170 Fossa SD, Waehre H, Aass N, et al. Bladder cancer definitive radiation therapy of muscle-invasive bladder cancer. A retrospective analysis of 317 patients. Cancer. 1993;72:3036-3043.
171 Pollack A, Zagars GK, Swanson DA. Muscle-invasive bladder cancer treated with external beam radiotherapy. Prognostic factors. Int J Radiat Oncol Biol Phys. 1994;30:267-277.
172 Moonen L van der Voet H, de Nijs R, et al. Muscle-invasive bladder cancer treated with external beam radiotherapy. Pretreatment prognostic factors and the predictive value of cystoscpic re-evaluation during treatment. Radiother Oncol. 1998;49:149-155.
173 Quilty PM, Duncan W, Chisholm GD, et al. Results of surgery following radical radiotherapy for invasive bladder cancer. Br J Urol. 1986;58:396-405.
174 Davidson SE, Symonds RP, Snee MP, et al. Assessment of factors influencing the outcome of radiotherapy for bladder cancer. Br J Urol. 1990;66:288-293.
175 Borgaonkar S, Jain A, Bollina P, et al. Radical radiotherapy and salvage cystectomy as the primary management of transitional cell carcinoma of the bladder. Results following the introduction of a CT planning technique. Clin Oncol. 2002;14:141-147.
176 Mameghan H, Fisher R, Mameghan J, et al. Analysis of failure following definitive radiotherapy for invasive transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys. 1995;31:247-254.
177 Sell A, Jakobsen A, Nerstrom B, et al. Treatment of advanced bladder cancer category T2, T3 and T4a. A randomized multicenter study of preoperative irradiation and cystectomy versus radical irradiation and early salvage cystectomy for residual tumor. Scand J Urol Nephrol Suppl. 1991;138:893-901.
178 Ahlering TE, Kanellos A, Boyd SD, et al. A comparative study of perioperative complications with Kock pouch urinary diversion in highly irradiated versus nonirradiated patients. J Urol. 1988;139:1202-1204.
179 Barringer BS. Twenty-five years of radon treatment of cancer of the bladder. JAMA. 1947;135:616-618.
180 Herger C, Sauer HR. Radium treatment of cancer of the bladder. Report of 267 cases. AJR. 1942;47:909-915.
181 Moonen LM, Horenblas S, van der Voet JC, et al. Bladder conservation in selected T1G3 and muscle-invasive T2-T3a bladder carcinoma using combination therapy of surgery and iridium-192 implantation. Br J Urol. 1994;74:322-327.
182 Wijnmaalen A, Helle PA, Koper PC, et al. Muscle invasive bladder cancer treated by transurethral resection, followed by external beam radiation and interstitial iridium-192. Int J Radiat Oncol Biol Phys. 1997;39:1043-1052.
183 Van der Steen-Banasik EM, Visser AG, Reinders JG, et al. Saving bladders with brachytherapy. Implantation technique and results. Int J Radiat Oncol Biol Phys. 2002;53:622-629.
184 Mazeron JJ, Crook J, Chopin D, et al. Conservative treatment of bladder carcinoma by partial cystectomy and interstitial iridium 192. Int J Radiat Oncol Biol Phys. 1988;15:1323-1330.
185 Rozan R, Albuisson E, Donnarieix D, et al. Interstitial iridium-192 for bladder cancer (a multicentric survey: 205 patients). Int J Radiat Oncol Biol Phys. 1992;24(3):469-477.
186 Pernot M, Hubert J, Guillemin F, et al. Combined surgery and brachytherapy in the treatment of some cancers of the bladder (partial cystectomy and interstitial iridium-192). Radiother Oncol. 1996;38:115-120.
187 Pos F, Horenblas S, Dom P, et al. Organ preservation in invasive bladder cancer. Brachytherapy, an alternative to cystectomy and combined modality treatment? Int J Radiat Oncol Biol Phys. 2005;61:678-686.
188 Nieuwenhuijzen JA, Pos F, Moonen LM, et al. Survival after bladder-preservation with brachytherapy versus radical cystectomy. A single institution experience. Eur Urol. 2005;48:239-245.
189 Pos FJ, Horenblas S, Lebesque J, et al. LDR is superior to HDR brachytherapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2004;59:696-705.
190 Rintala E, Hannisdal E, Fossa SD, et al. Neoadjuvant chemotherapy in bladder cancer. A randomized study. Scand J Nephrol. 1993;27:355-362.
191 Roberts JT, Fossa SP, Richards SB, et al. Results of Medical Research Council phase II study of low dose cisplatin and methotrexate in the primary treatment of locally advanced (T3 and T4) transitional cell carcinoma of the bladder. Br J Urol. 1991;68:162-168.
192 Farah R, Chodak GW, Vogelzang NI, et al. Curative radiotherapy following chemotherapy for invasive bladder carcinoma (a preliminary report). Int J Radiat Oncol Biol Phys. 1991;20:413-417.
193 Herr HW, Whitmore WF, Morse MJ, et al. Neoadjuvant chemotherapy in invasive bladder cancer. The evolving role of surgery. J Urol. 1990;144:1083-1088.
194 Sternberg C, Arena M, Calabresi F, et al. Neo-adjuvant M-VAC (methotrexate, vinblastine, Adriamycin, and cisplatin) for muscle infiltrating transitional cell carcinoma of the urothelium. Cancer. 1993;72:1975-1982.
195 Splinter TAW, Pavone-Macaluso M, Jacqmin D, et al. European Organization for Research and Treatment of Cancer-Genitourinary Group phase 2 study of chemotherapy in stage T3-4N0-XM0 transitional cell carcinoma of the bladder. J Urol. 1992;148:1793-1796.
196 Dreicer R, Messing EM, Loehrer PJ, et al. Perioperative methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) for poor risk transitional cell carcinoma of the bladder. An Eastern Cooperative Oncology Group Pilot Study. J Urol. 1990;144:1123-1127.
197 Scher H, Herr H, Sternberg C, et al. Neo-adjuvant chemotherapy for invasive bladder cancer. Experience with the M-VAC regimen. Br J Urol. 1989;64:250-256.
198 Prout GR, Shipley WU, Kaufman D, et al. Preliminary results in invasive bladder cancer with transurethral resection, neoadjuvant chemotherapy and combined pelvic irradiation plus cisplatin chemotherapy. J Urol. 1990;144:1128-1134.
199 Parsons JT, Million RR. Bladder cancer. In: Perez CA, Brady LW, editors. Principles and Practice of Radiation Oncology. Philadelphia: JB Lippincott; 1991:1036-1058.
200 Farah R, Chodak GW, Vogelzang NJ, et al. Therapy for invasive bladder carcinoma (a preliminary report). Int J Radiat Oncol Biol Phys. 1991;20:413-417.
201 Hall RR. Bladder preserving treatment. The role of transurethral surgery alone and with combined modality therapy for muscle-invading bladder cancer. In: Vogelzang NJ, Scardino PT, Shipley WU, Coffey DS, editors. Comprehensive Textbook of Genitourinary Oncology. Baltimore: Williams & Wilkins; 1995:509-513.
202 Herr HW, Bajorin DF, Scher HI. Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer. Ten-year outcome. J Clin Oncol. 1998;16:1298-1301.
203 deVere White RW, Lara PNJr, Goldman B, et al. A sequential treatment approach to myoinvasive urothelial cancer. A phase II Southwest Oncology Group trial (S0219). J Urol. 2009;181:2476-2480.
204 Herr HE, Scher HI. Neoadjuvant chemotherapy and partial cystectomy for invasive bladder cancer. J Clin Oncol. 1994;12:975-980.
205 Wallace DMA, Radhavan D, Kelly KA, et al. Neo-adjuvant (pre-emptive) cisplatin therapy in invasive transitional cell carcinoma of the bladder. Br J Urol. 1991;67:608-615.
206 Malmstrom Per-UnoRintala E. Members of the Nordic Cooperative Bladder Cancer Study Group: Five-year follow-up of a prospective trial of radical cystectomy and neoadjuvant chemotherapy. Nordic Cystectomy Trial 1. J Urol. 1996;115:1903.
207 Martinez-Pineiro JA, Martin MG, Arocena M, et al. Neoadjuvant cisplatin chemotherapy before radical cystectomy in invasive transitional cell carcinoma of the bladder. A prospective randomized phase III trial. J Urol. 1995;153:964-973.
208 GISTV (Italian Bladder Cancer Study Group). Neoadjuvant treatment for locally advanced bladder cancer. A randomized prospective clinical trial. J Chemo. 1996;8:345-346.
209 Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy. Initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576-3583.
210 Hall RR. Updated results of a randomized controlled trial of neoadjuvant cisplatin (C), methotrexate (M) and vinblastine (V) chemotherapy for muscle-invasive bladder cancer. Proc Ann Meeting Am Soc Clin Oncol. 2002;21(1):178a.
211 Orsatti M, Curotto A, Canobbio L. Alternating chemo-radiotherapy in bladder cancer. A conservative approach. Int J Radiat Oncol Biol Phys. 1995;33:173-178.
212 Sherif A, Rintala E, Mestad O, et al. Neoadjuvant cisplatin-methotrexate chemotherapy of invasive bladder cancer—Nordic cystectomy trial 2. Scand J Urol Nephrol. 2002;36:419-425.
213 Abol-Enein H, El Makresh M, El Baz M, et al. Neo-adjuvant chemotherapy in treatment of invasive transitional bladder cancer. A controlled, prospective randomised study. Br J Urol. 1997;80(Suppl. 2):49.
214 Bassi P, Pagano F, Pappagallo G, et al. Neo-adjuvant M-VAC of invasive bladder cancer. The G.U.O.N.E. multicenter phase III trial. Eur Urol. 1998;33(Suppl. 1):142.
215 Coppin C, Gospodarowicz M, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. J Clin Oncol. 1996;14:2901-2907.
216 Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859-866.
217 Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer. A randomized controlled trial. International Collaboration of Trialists. Lancet. 1999;354:533-540.
218 Neoadjuvant chemotherapy in invasive bladder cancer. A systematic review and meta-analysis. Lancet. 2003;361:1927.
219 Neoadjuvant chemotherapy in invasive bladder cancer. Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:202-205.
220 Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer. Final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;19:4005-4013.
221 Skinner DG, Daniels JR, Russell C, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer. A prospective comparative trial. J Urol. 1991;145:459-467.
222 Stöckle M, Meyenburg W, Wellek S, et al. Fortgeschrittenes Blasenkarzinom (Stadien pT3b, pT4a, pN1, pN2). Verbesserte Überlebensraten nach radikaler Zystektomie durch 3 adjuvante Zyklen M-VAC/M-VEC-Erste Ergebnisse einer kontrollierten Studie. Akt Urol. 1991;22:201.
223 Stockle M, Meyenburg W, Wellek S, et al. Advanced bladder cancer (stages pT3b, pT4a, pN1 and pN2). Improved survival after radical cystectomy and 3 adjuvant cycles of chemotherapy. Results of a controlled prospective study. J Urol. 1992;148:302-306.
224 Lehmann J, Franzaring L, Thuroff J, et al. Complete long-term survival data from a trial of adjuvant chemotherapy vs control after radical cystectomy for locally advanced bladder cancer. BJU Int. 2006;97:42-47.
225 Studer UE, Bacchi M, Biedermann C, et al. Adjuvant cisplatin chemotherapy following cystectomy for bladder cancer. Results of a prospective randomized trial. J Urol. 1994;152:81-84.
226 Freiha F, Reese J, Torti FM. A randomized trial of radical cystectomy versus radical cystectomy plus cisplatin, vinblastine and methotrexate chemotherapy for muscle invasive bladder cancer. J Urol. 1996;155:495-499.
227 Paz-Ares LG, Solsona E, Esteban E, et al: Randomized phase III trial comparing adjuvant paclitaxel/gemcitabine/cisplatin (PGC) to observation in patients with resected invasive bladder cancer. Results of the Spanish Oncology Genitourinary Group (SOGUG) 99/01 study. ASCO Meeting Abstracts Jun 20:LBA4518, 2010.
228 Adjuvant chemotherapy in invasive bladder cancer. A systematic review and meta-analysis of individual patient data. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:189-199.
229 Vogelzang NJ, Moormeier JA, Awan AM, et al. Methotrexate, vinblastine, doxorubicin and cisplatin followed by radiotherapy or surgery for muscle invasive bladder cancer. The University of Chicago experience. J Urol. 1993;149:753-757.
230 Cervak J, Cufer T, Kragelj B. Sequential transurethral surgery, multiple drug chemotherapy, and radiation therapy for invasive bladder carcinoma. Initial report. Int J Radiat Oncol Biol Phys. 1993;25:777-782.
231 Russell K, Boileau M, Higano C, et al. Combined 5-fluorouracil and irradiation for transitional cell carcinoma of the urinary bladder. Int J Radiat Oncol Biol Phys. 1990;19:693-699.
232 Shipley WU, Kaufman DS, Thakral HK, et al. Long-term outcome of patients treated for muscle-invasive bladder cancer by tri-modality therapy. Urology. 2002;60:62-68.
233 Efstathiou JA, Coen JJ, Spiegel DY, et al. Fifteen-year outcomes of selective bladder preservation by combined modality therapy for invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2009;75:S10-S11.
234 Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective bladder preservation in invasive bladder cancer. Long-term results. J Clin Oncol. 2002;20:3061-3071.
235 Kaufman DS, Shipley WU, Griffin PP, et al. Selective bladder preservation by combination treatment of invasive bladder cancer. N Engl J Med. 1993;329:1377-1382.
236 Tester W, Porter A, Asbell S, et al. Combined modality program with possible organ preservation for invasive bladder carcinoma. Results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys. 1993;25:783-790.
237 Tester W, Caplan R, Heaney J, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer. Results of RTOG phase II trial 88-02. J Clin Oncol. 1996;14:119-126.
238 Kaufman DS, Winter KA, Shipley WU, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06. A phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on initial response. Oncologist. 2000;5:471-476.
239 Hagan MP, Winter KA, Kaufman DS, et al. RTOG 97-06. Initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:665-672.
240 Kaufman DS, Winter KA, Shipley WU, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833-887.
241 James ND, Hussain SA, Hall E, et al. Results of a phase III randomized trial of synchronous chemoradiotherapy (CRT) compared to radiotherapy (RT) alone in muscle-invasive bladder cancer (MIBC) (BC2001 CRUK/01/004). J Clin Oncol. 2010;28:7s. abstract 4517
242 Bellmunt J, Guillem V, Paz-Ares L, et al. Phase I-II study of paclitaxel, cisplatin, and gemcitabine in advanced transitional cell carcinoma of the urothelium. Spanish Oncology Genitourinary Group. J Clin Oncol. 2000;18:3247-3255.
243 Eapen L, Stewart D, Collins J, Peterson R. Effective bladder sparing therapy with intra-arterial cisplatin and radiotherapy for localized bladder cancer. J Urol. 2004;172:1276-1280.
244 Hoskin PJ, Rojas AM, Saunders MI, et al. Carbogen and nicotinamide in locally advanced bladder cancer. Early results of a phase III randomized trial. Radiother Oncol. 2009;91:120-125.
245 Munro NP, Sundaram SK, Weston PMT, et al. A 10-year retrospective review of a nonrandomized cohort of 458 patients undergoing radical radiotherapy or cysetectomy in Yorkshire, UK. Int J Radiat Oncol Biol Phys. 2010;77:119-124.
246 Sternberg CN, Parmar MK. Neoadjuvant chemotherapy is not (yet) standard treatment for muscle-invasive bladder cancer. J Clin Oncol. 2001;19(Suppl 18):21S-26S.
247 Hussain MH, Glass TR, Forman J, et al. Combination cisplatin, 5-fluorouracil and radiation therapy for locally advanced unresectable or medically unfit bladder cancer cases. A Southwest Oncology Group Study. J Urol. 2001;165:56-60.
248 Pisters LL, Tykochinsky G, Wajsman Z. Intravesical bacillus Calmette-Guérin or mitomycin C in the treatment of carcinoma in situ of the bladder following prior pelvic radiation therapy. J Urol. 1991;146:1514-1517.
249 Zietman AL, Grocela J, Zehr E, et al. Selective bladder conservation using trans-urethral resection, chemotherapy, and radiation. The risk and consequences of superficial recurrences within the retained bladder. Urology. 2001;58:380-385.
250 Boyd SD, Feinberg SM, Skinner DG, et al. Quality of life survey of urinary diversion patients. Comparison of ileal conduits versus continent Koch urinary reservoirs. J Urol. 1987;138:1386-1389.
251 Mansson A, Johnson G, Mansson W. Quality of life after cystectomy: comparison between patients with conduit and those with caecal reservoir urinary diversion. Br J Urol. 1988;62:240-245.
252 Raleigh ED, Berry M, Monite JE. A comparison of adjustments to urinary diversions. A pilot study. J Wound Ostomy Continence Nurs. 1995;22:58-63.
253 Bjerre BD, Johansen C, Steven K. Health related quality of life after cystectomy. Bladder substitution compared with ileal conduit diversion. A questionnaire survey. Br J Urol. 1995;75:200-205.
254 Hart S, Skinner EC, Meyerowitz BE, et al. Quality of life after radical cystectomy for bladder cancer in patients with an ileal conduit, or cutaneous or urethral Kock pouch. J Urol. 1999;162:77-81.
255 Gerharz EW, Mansson A, Hunt S, et al. Quality of life after cystectomy and urinary diversion. An evidence based analysis. J Urol. 2005;174:1729-1736.
256 Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy, and radiation. Results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772-1776.
257 Lagrange JL, Bascoul-Mollevi C, Geoffrois L, et al. Quality of life assessment after concurrent chemoradiation for invasive bladder cancer. Results of a multicenter prospective study (GETUG 97-015). Int J Radiat Oncol Biol Phys. 2010. Epub ahead of print
258 Caffo O, Fellin G, Graffer U, Luciani L. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. Cancer. 1996;78:1089-1097.
259 Henningsohn L, Wijkstrom H, Dickman PW, et al. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother Oncol. 2002;60:215-225.
260 Kachnic LA, Shipley WU, Griffin PP, et al. Combined modality treatment with selective bladder conservation for invasive bladder cancer. Long-term tolerance in the female patient. Cancer J Sci Am. 1996;2:79.
261 Efstathiou JA, Bae K, Shipley WU, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer. RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol. 2009;27:4055-4061.
262 Gerard JP, Hulewicz G, Saleh M, et al. Pilot study of IORT for bladder carcinoma. Front Radiat Ther Oncol. 1997;31:250-252.
263 Calvo FA, Aristu J, Abuchaibe O, et al. Intraoperative and external preoperative radiotherapy in invasive bladder cancer. Effect of neoadjuvant chemotherapy in tumor downstaging. Am J Clin Oncol. 1993;16:61-66.
264 Calvo FC, Zincke H, Gunderson LL, et al. Genitourinary IORT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative Irradiation. Techniques and Results. Totowa. New Jersey: Humana Press; 1999:421-436.
265 Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma. Results of Medical Research Council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47:379-388.
266 Maciejewski B, Majewski S. Dose fractionation and tumour repopulation in radiotherapy for bladder cancer. Radiother Oncol. 1991;21:163-170.
267 Moonen L, van der Voet H, de Nijs R, et al. Muscle-invasive bladder cancer treated with external beam radiation. Influence of total dose, overall treatment time, and treatment interruption on local control. Int J Radiat Oncol Biol Phys. 1998;42:525-530.
268 Herr HW, Faulkner JR, Grossman MB, et al. Surgical factors influence bladder cancer outcomes. A co-operative group report. J Clin Oncol. 2004;22:2781-2789.
269 Sengelov L, von der Masse H. Radiotherapy in bladder cancer. Radiother Oncol. 1999;52:1-14.
270 Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer. Results of a prospective multicenter study. J Urol. 2004;171:139-144.
271 Emani B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122.
272 Marks LB, Carroll PR, Dugan TC, et al. The response of the urinary bladder, urethra and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257-1280.
273 Cowan RA, McBain CA, Ryder WDRR, et al. Radiotherapy for muscle invasive carcinoma of the bladder. Results of a randomised trial comparing whole bladder with dose-escalated partial bladder irradiation. Int J Radiat Oncol Biol Phys. 2004;59:197-207.
274 Turner SL, Swindell R, Bowl N. Bladder movement during radiation therapy for bladder cancer: implications for treatment planning. Int J Radiat Oncol Biol Phys. 1997;39:355-360.
275 Harris SJ, Buchanan RB. An audit and evaluation of bladder movements during radical radiotherapy. Clin Oncol. 1998;10:262-264.
276 Sur RK, Clinkard J, Jones WG, et al. Changes in target volume during radiotherapy treatment of invasive bladder carcinoma. Clin Oncol. 1993;5:30-33.
277 Roof KS, Mazal A, Sarkar S, et al. A three-dimensional CT based analysis of inter-fraction bladder motion during radiotherapeutic treatment of bladder cancer. Int J Radiat Oncol Biol Phys. 2004;60:S430.
278 Graham J, Gee A, Hilton S, et al. Geometric uncertainties in radiotherapy of the prostate and bladder. In: Geometric Uncertainties in Radiotherapy. London: British Institute of Radiology; 2003.
279 Shipley WU, Rose MA. Bladder cancer. The selection of patients for treatment by full-dose irradiation. Cancer. 1985;55:2278-2284.
280 Morrison R. The results of treatment of cancer of the bladder—a clinical contribution to radiobiology. Clin Radiol. 1975;26:67-75.
281 Maciejewski B, Majewski S. Dose fractionation and tumour repopulation in radiotherapy for bladder cancer. Radiother Oncol. 1991;21:163-170.
282 Wilson GD, McNally NJ, Dische S, et al. Cell proliferation in human tumours measured by in-vivo labelling with bromodeoxyuridine. Br J Radiol. 1988;61:419-422.
283 Cole DJ, Durrant KR, Roberts JT, et al. A pilot study of accelerated fractionation in the radiotherapy of invasive carcinoma of the bladder. Br J Radiol. 1992;65:792-798.
284 Horwich A, Pendlebury S, Dearnaley DP. Organ conservation in bladder cancer. Eur J Cancer. 1995;31(Suppl 5):208.
285 Plataniotis G, Michalopoulos E, Kouvaris J, et al. A feasibility study of partially accelerated radiotherapy for invasive bladder cancer. Radiother Oncol. 1994;33:84-87.
286 Edsmyr F, Andersson L, Espostl PL, et al. Irradiation therapy with multiple small fractions per day in urinary bladder cancer. Radiother Oncol. 1985;4:197-203.
287 Naslund I, Nilsson B, Littbrand B. Hyper-fractionated radiotherapy of bladder cancer. A 10 year follow up of a randomized clinical trial. Acta Oncol. 1994;33:397-402.
288 Sauer R, Birkenhake S, Kühn R. Efficacy of radiochemotherapy with platin derivates compared to radiotherapy alone in organ-sparing treatment of bladder cancer. Int J Radiat Biol Phys. 1998;40:121-127.
289 Montie JE, Clark PE, Eisenberger MA. The NCCN bladder cancer clinical practice guidelines in oncology. J Natl Comprehensive Cancer Network. 2009;7:8-39.