Bladder and Cloacal Exstrophy
The exstrophic anomalies, often referred to as the exstrophy–epispadias complex,1 are considered a spectrum of embryological abnormalities including:
• Epispadias—the least severe anomaly, in which the urethra is a partial or complete open plate on the dorsal surface of the phallus
• Classic bladder exstrophy (BE)—the most common of these anomalies, in which the bladder is an open plate on the lower abdomen and always includes epispadias
• Cloacal exstrophy (CE)—the bladder and the ileocecal junction of the bowel are an open plate on the lower abdomen. This condition, commonly associated with other defects, is also known as the omphalocele/exstrophy/imperforate anus/spinal defect (OEIS) complex
• Exstrophy variants—partial manifestations are seen of the above anomalies, and commonly lack symmetry in the sagittal plane.
Bladder Exstrophy
Classic BE occurs in one per 10,000–50,000 live births,2,3 with a male to female ratio of 3–6 : 1.4,5 CE is even more rare with an incidence of 1 in 200,000–400,000,6 but is more common when stillborns are included in the data (1 in 10,000 to 1 in 50,000).7 The natural history of BE is well known; the anomaly is nonlethal although it is associated with significant morbidity. Since the 19th century, various efforts to manage BE have been described. As the condition is rare, these approaches were empiric and usually unsuccessful. Until the 20th century, there was no effective surgical technique.
BE results from an anterior herniation of the developing bladder and urethra with subsequent herniation of posterior developing structures, preventing the normal development of the lower abdominal wall and anterior fusion of the pelvis. The result is a flattened pelvis with wide diastasis of the symphysis pubis. This anomaly has been described as ‘if one blade of a pair of scissors were passed through the urethra of a normal person; the other blade were used to cut through the skin, abdominal wall, anterior wall of the bladder and urethra, and the symphysis pubis; and the cut edges were then folded laterally as if the pages of a book were being opened’ (Fig. 58-1).8 Children with BE typically have an anteriorly located anus. Also, the female genital anatomy is altered with a more vertically oriented vaginal opening following repair and a wider and shorter vagina than normal. The anterior component of the penis is also foreshortened in males compared to the general population. Classic BE, however, is rarely associated with other organ system malformation.
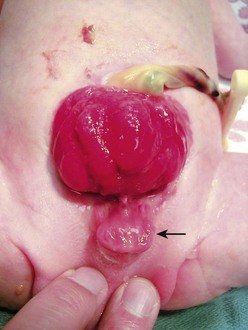
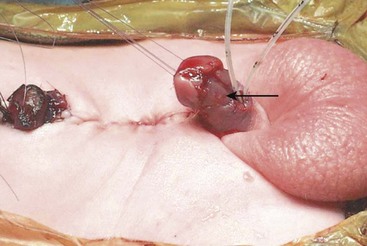
FIGURE 58-1 Bladder exstrophy in a male. The urethral mucosa is marked with the arrow. The corporeal bodies lie posterior to the urethral mucosa.
Diagnosis
BE can be diagnosed antenatally, although many affected fetuses are not identified before birth.9 Ultrasound (US) can reliably detect BE before the twentieth week of gestation.10,11 Absence of the bladder is the hallmark. Other ultrasound findings include:
• A semisolid mass protruding from the abdominal wall12
• A lower abdominal protrusion
• An anteriorly displaced scrotum with a small phallus in male fetuses
• Normal kidneys in association with a low-set umbilical cord13
Subtle findings such as low umbilical cord insertion and the location of the genitalia will only be seen if the fetus is examined in a sagittal alignment with the spine.14 As exstrophy affects the external genitalia, the diagnosis is easier to make in males than females. Iliac crest widening can also be seen during the routine prenatal evaluation of the lumbosacral spine to evaluate for myelomeningocele. The iliac angle will be about 110° rather than the usual 90°.14 Since urine production is normal in these fetuses, amniotic fluid levels are normal.
Prenatal diagnosis allows for prenatal counseling, optimal perinatal management, and the chance to be delivered near a pediatric center trained to treat these babies. This counseling should include the expertise of an experienced pediatric urologist or surgeon. The overall prognosis for these children can be good if treated at medical centers with experience with this disorder.15
In many areas of the developing world, early treatment remains problematic due to the lack of healthcare infrastructure and resources to care for these patients. Management of these patients often includes significantly delayed time to closure and adds another level of challenge to achieve optimum outcomes in this patient group. A recent overview from South Africa highlighted this problem: 58% of the patients presented in a delayed fashion and mortality rates approached 42% due to concomitant medical conditions and poor primary health care.16
Pathogenesis
In the prescientific era, the cause of BE was attributed to trauma to the unborn child causing ulceration of the abdominal wall and subsequent bladder herniation. Today, we know that the developing human embryo does not normally pass through a stage that corresponds to exstrophy. This knowledge excludes arrested development and implicates an error in embryogenesis involving the cloacal membrane.17 This membrane serves to separate the coelomic cavity from the amniotic space in early development and can be identified by two to three weeks of gestation. By the fourth gestational week, it forms the ventral wall of the urogenital sinus with the unfused primordia of the genital tubercles sitting cephalad and lateral to it. With further development, the primordia grow and fuse in the midline, and mesoderm grows toward the midline creating an infra-umbilical abdominal wall. Simultaneously, the urorectal septum develops medially and caudally to separate the cloaca into the urogenital sinus and rectum.18
One theory suggests a persistent cloacal membrane during fetal development.19 Persistence of this membrane could create a wedge effect that keeps the medially encroaching mesoderm from fusing in the midline.17 To further study this hypothesis, an animal model of CE was created using the developing chick embryo. By placing a plastic graft in the region of the tail bud, it was found that CE resulted, perhaps due to persistence of the cloacal membrane.17
Other experimental models implicate the cloacal membrane as well, but postulate that early disruption, rather than persistence, causes exstrophy. Another model of CE in the developing chick embryo, created by using a CO2 laser to create an early dehiscence in the tail bud caudal to the omphalomesenteric vessels, suggests that exstrophy may result from failure of the mesodermal ingrowth between the ectoderm and endoderm of the cloacal membrane which then later ruptures. It is hypothesized that such an event could be caused by early hypoxemic infarction in the region of the tail bud with subsequent cellular loss of the mesoderm followed by herniation of the developing bladder or cloaca.20 This type of ischemic injury has been implicated as the cause of gastroschisis and could explain the spectrum of the exstrophy/epispadias complex. Another possible mechanism resulting in a similar pathophysiology could be a defect in a genetic switch which results in premature senescence of the infra-umbilical membrane (analogous to an ischemic injury). This mechanism would imply an epigenetic basis for exstrophy.
Other proposed theories include caudal displacement of the paired primordia of the genital tubercles. Exstrophy is postulated to occur by this mechanism when the primordia of the genital tubercles fuse caudal to their usual location relative to where the urorectal fold divides the cloaca into the urogenital sinus and rectum.21 This theory readily explains the spectrum of variation seen in the exstrophy. However, it fails to explain the higher incidence of exstrophy compared to epispadias.8
A relatively new maldevelopment theory for CE is based on a suramin-exposed chick model.22 When chick embryos were examined at one or two days after pericardial injection of suramin, about 8% were noted to have a midline infra-umbilical opening into the cloaca and allantois, abnormal leg bud abduction, hypoplastic tail and allantois, a broad infra-umbilical pelvic region, and large aneurysmal dilation of the paired dorsal aorta at the level of the leg buds. The aortic dilation is transient, resolving on the second or third day after drug exposure. These authors implicate the aneurysmal paired dorsal aorta as the primary defects leading to CE in this animal model. Pelvic maldevelopment has also been implicated in the pathogenesis of exstrophy and explains both the bony abnormalities as well as soft tissue anomalies.23
The chick model to study BE has inherent limitations. Unlike primates, chickens possess a cloaca; thus this precludes the creation of BE versus CE. Other animal models to study BE have been difficult to create. In an exstrophy sheep model, a significant increase in the ratio of collagen-to-smooth muscle was noted in exstrophic versus normal control bladders (P < 0.05). These histological changes are similar in part to changes seen in human BE specimens.24,25
To date, the underlying cause of human exstrophy remains in question perhaps secondary to environmental exposure, an infectious pathogen, or other causes in genetically susceptible individuals. In fact, epidemiologic studies implicate a role for inherited susceptibility. As a result of the associated physical anomalies with this condition, patients with BE often have to overcome significant obstacles to reproduce. Men may need to resort to assisted reproductive techniques because of difficulty with sexual intercourse and ejaculation.26,27 Women with exstrophy are prone to uterine prolapse and miscarriage. Difficulty with conception and pregnancy are still a problem today despite in vitro fertilization and careful obstetric care.28,29 These issues combined may explain, in part, why familial patterns of inheritance of exstrophy–epispadias complex are noted infrequently. To date, 37 familial cases of BE have been reported, the most recent of which describes a mother and son with BE.30 Five cases of an affected parent–child pair have also been described.30–32 Another 18 cases with BE have been found in twins.30 However, in a study population of greater than six million births and 208 reported cases of exstrophy, no case had a positive family history for this anomaly.33
Current counseling recommendations about the risk of recurrence in a sibling of a patient with exstrophy cite an estimate of about 1% and a 1 : 70 chance of transmission to the progeny of an affected parent.31 A Florida population-based study found multiple births had a 46% increased risk of birth defects, with BE being the fifth highest adjusted relative risk.34 These findings support a multifactorial etiology with evidence for genetic predisposition. More recently, an epidemiologic survey of families with BE found no link between exstrophy and parental age, maternal reproductive history, or periconceptional maternal exposure to alcohol, drugs, chemical noxae, radiation, or infections. Periconceptional maternal exposure to smoking was noted to be significantly more common in patients with CE.35
Principles of Reconstruction
Modern objectives for exstrophy reconstruction are placed broadly into primary and secondary goals (Table 58-1).
While these goals are straightforward and often interconnected, their successful achievement can be elusive. Many of the secondary goals address complications that can arise from operations used to treat exstrophy. The goals have expanded since the first operations were attempted in the 19th century. These objectives address the primary pathophysiology of exstrophy and the problems associated with its management (Table 58-2).
TABLE 58-2
Primary Pathophysiology of Bladder Exstrophy and Complications that Occur in Relation to its Management
| Primary Pathophysiology (If Untreated) | Complications (Associated with Management of Exstrophy) |
| Malignancy (related to chronic exposure of the bladder plate) | Malignancy (related to the use of intestine in bladder reconstruction) |
| Pyelonephritis | Pyelonephritis |
| Kidney stones | Kidney and bladder stones |
| Total urinary incontinence | Stress or urge urinary incontinence |
| Chronic bladder irritation | Hydronephrosis |
| Pelvic floor insufficiency | Cystocele, uterine prolapse |
| Abnormal hip dynamics, back pain | Abnormal hip dynamics, back pain |
| Symphyseal diastasis, pelvic flattening | Urinary outlet obstruction |
| Abdominal wall defect | Absent umbilicus |
| Ventral and inguinal hernias | Incisional hernias |
| Severe penile shortening with dorsal chordee | Inadequate phallus in males with subsequent social and psychological sequelae |
Natural History and Early Attempts at Treatment
Children with BE can survive untreated and some untreated patients with BE have lived into their eighth decade.36 However, significant morbidity exists if these exstrophic conditions are left untreated, including total urinary incontinence, bladder and kidney infections, skin breakdown, and tumor development in the bladder plate. The surrounding skin around the exposed bladder is often inflamed secondary to urine contact dermatitis, loss of skin integrity from constant wetness, and secondary infection. Untreated inguinal hernias can be life-threatening and organ prolapse is especially challenging to manage later. In addition, these patients are often social pariahs because of odor and hygiene problems. In contrast, when these patients receive effective surgical and medical treatment, they can lead productive, healthy lives with manageable morbidity.
The morbidity associated with untreated BE led physicians to develop empiric operative approaches for this anomaly such as urinary reconstructive or diversion procedures. Initial efforts were directed at partial reconstruction of the abdominal wall to allow the application of a urinary receptacle to collect urine. Others performed urinary diversion through the creation of a ureterosigmoid fistula. Early results were poor.27 These early efforts were undertaken without an understanding of urinary tract and bladder physiology, or how these operations would affect urine storage and emptying, kidney function, electrolyte homeostasis, the propensity for urinary tract infection, or urinary calculus formation.
Current Operative Approaches
Urinary Diversion
Urinary diversion is not commonly used in the USA or most parts of Europe; this approach has been abandoned in favor of an anatomically based approach. However, continent urinary diversion techniques can produce a more consistent degree of urinary continence with less intervention than that achieved with anatomic reconstruction.37 Some urodynamic studies demonstrate low urine flow rates and poor contractility and continence in patients following primary bladder repair.38,39
Internal or incontinent urinary diversion avoids the potential complications associated with functional reconstruction such as urinary retention and subsequent kidney damage, and potential later dependence on clean intermittent catheterization (CIC) to empty the bladder. Advocates of early urinary diversion also cite a decreased risk of epididymitis and obstruction of the vas deferens by the creation of a receptacle with a suprapubic window at the level of the prostatic urethra.40 Diversion can also be combined with cosmetic and functional reconstructive procedures for the external genitalia. Because of the difficulties encountered with functional bladder reconstruction, advocates of early urinary diversion argue that their approach achieves the primary goals with fewer operations and higher success rates than are achieved with bladder closure and urethral reconstruction.
Given the successes of urinary diversion, why abandon it? Long-term complications associated with ureterosigmoidostomy (USO) are significant and include hyperchloremic metabolic acidosis, chronic pyelonephritis, bladder calculi, and a 250- to 300-fold increased risk of adenocarcinoma developing at the anastomosis.41–43 As a result of these complications, USO was subsequently replaced by incontinent urinary diversions such as colonic and ileal conduits. A significant disadvantage to these conduits is the incontinent abdominal stoma that is associated with them.
The Mainz II pouch and the Sigma pouch represent significant improvements to the USO.44–46 These rectal reservoirs permit urinary continence without reliance on CIC. In one study, renal preservation rates in children treated primarily with a urinary rectal reservoir (Mainz II pouch) approach 92% with continence rates up to 97%.37 The Heitz–Boyer–Hovelaque procedure involves isolation of a rectal segment for ureteral implantation followed by posterior sagittal pull-through of the sigmoid colon through the anal sphincter. A small series using this approach reported continence rates of 95% with an acceptable complication rate.47 Complications of this form of diversion include fecal-urinary incontinence in patients with impaired anorectal sphincter control. Metabolic electrolyte imbalances can be treated with frequent emptying of the rectal reservoir that reduces the contact time between urine and the absorptive rectal mucosa along with oral bicarbonate replacement. The significant risk of malignancy also remains a concern. Various modifications of the rectal reservoir to prevent admixture of feces and urine may decrease the incidence of adenocarcinoma if the risk is due to conversion of urinary nitrates into carcinogenic nitrites by fecal bacteria.48 Long-term results are not yet available. Techniques for constructing urinary diversions are listed in Table 58-3.
Anatomic Reconstruction
The first efforts at anatomic reconstruction for BE were unsuccessful, but set the stage for the current anatomic approach. In 1881, Trendelenberg described an exstrophy closure emphasizing the importance of pubic re-approximation in front of the reconstructed bladder in order to achieve continence and prevent dehiscence.49 However, because of discouraging results, anatomic reconstruction was largely replaced by urinary diversion in the early part of the 20th century. During this time there were scattered published reports of successful outcomes.50–53 However, several large series of patients who underwent single-stage anatomic reconstruction in the 1960s and 1970s reported continence rates of only 10–30%.54–59 Renal damage was as high as 90% in these series, generally because of bladder outlet obstruction.54
As a result of these complications and the low rate of urinary continence with single-stage approaches, reconstructive efforts were subsequently modified toward staged bladder reconstruction, an approach pioneered in the 1970s, and further refined to what is now known as the modern staged repair of exstrophy (MSRE).60–62 Recent advances in single-stage reconstruction for BE have been advocated and complete primary repair for exstrophy (CPRE) has gained favor.63
Preoperative Care
After delivery, to reduce trauma to the bladder plate, the umbilical cord should be ligated with silk suture rather than a plastic or metal clamp. A hydrated gel dressing or plastic wrap can be used to protect the exposed bladder from superficial trauma. Dressings should be changed daily, and the bladder should be irrigated with normal saline with each diaper change. A humidified air incubator may also minimize bladder injury.64
Operative Considerations
Ideally, the primary BE closure is performed in the newborn period. If the bladder template is amenable, early closure has several advantages. Early closure initiates the road toward anatomic normalcy as well as decreasing bladder exposure which can lead to histological changes such as acute and chronic inflammation, squamous metaplasia, cystitis glandularis and cystitis cystica, and muscular fibrosis which may adversely impact bladder capacity and compliance.65 Electron microscopy studies have shown that the newborn exstrophy bladder is ‘immature’ when compared to control newborns,66 and has fewer small nerve fibers,67 less smooth muscle, and a threefold increase in type III collagen content.68 While it is unclear whether these changes are part of the primary pathology or secondary to the lack of bladder cycling in utero, it is conceivable that early closure could ‘mature’ the bladder by restoring bladder cycling. Similarly, another study verified the importance of immediate postnatal exstrophy closure as infants closed before 7 days of age required fewer bladder augmentations to achieve eventual continence.69
Assessment of the adequacy of the bladder template is subjective. Even a small bladder template, if distensible and contractile, can enlarge to a useful size once closed. However, if the bladder template appears too small and stiff on initial assessment, some surgeons choose to defer early operation and re-evaluate the bladder at 4–6 months of age under anesthesia.70 If the bladder continues to appear inadequate for closure, a nonrefluxing colon conduit can be created in combination with an abdominal wall closure and epispadias repair. At a later age this can be converted to a continent diversion or anastomosed to the rectosigmoid.
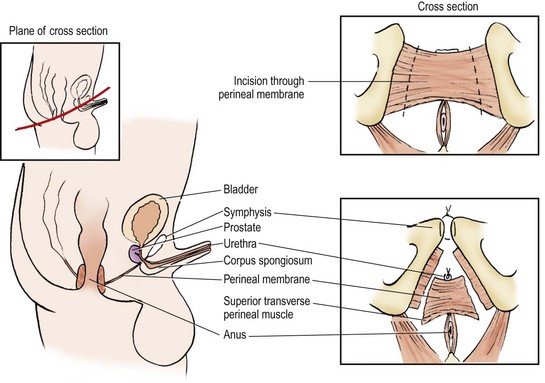
During general anesthesia, nitrous oxide should be avoided during primary closure as it can cause bowel distension, which decreases exposure during the operation and may increase the risk of wound dehiscence. Some advocate postoperative nasogastric tube drainage to decrease abdominal distension although we do not routinely use it.71 We prefer an epidural catheter to reduce the inhaled anesthetic requirement during the operation. Tunneling the epidural may reduce the risk of infection if it is left in for prolonged periods after repair.72
For patients older than 3 days, or newborns with a wide pubic diastasis, we perform anterior iliac osteotomies. Osteotomies are recommended in most patients unless the pelvis is very pliable. Osteotomies assist closure and enhance anterior pelvic floor support, which may improve future urinary continence.60,73
Factors that appear to be important in the perioperative period include:
Complete Primary Repair for Exstrophy
Clinical experience in babies with posterior urethral valves suggests that early restoration of nonobstructive emptying and filling of the bladder allows the bladder to regain some of its normal physiologic and developmental potential.76 This implies that the bladder progresses through developmental milestones in the first few months of life. Precedence for this form of organ development is found in the brain with the acquisition of language and visual perception, and this is also true for skeletal and neuromuscular development. Finally, early bladder reconstruction creates a more normal appearing baby, which may foster improved bonding between the parents and infant.
In the CPRE (or Mitchell) technique,63 the bladder, bladder neck, and urethra are relocated posteriorly within the pelvis. This shift positions the proximal urethra within the pelvic diaphragm in an anatomically more normal position to maximize the effect of the pelvic muscles and support structures needed for urinary continence. Posterior location of the bladder neck and urethra also facilitates reapproximation of the pubic symphysis which, in turn, helps prevent anterior migration of the urethra and bladder neck, and provides a more anatomically normal pelvic diaphragm.
CPRE Technique: Boys.
After sterile preparation and draping, the lines of dissection are marked (Fig. 58-2). When marking these lines, it is important to exclude dysplastic tissue at the edges of the exstrophic bladder and bladder neck. This is particularly important at the bladder neck where remaining dysplastic tissue may impair later bladder neck function. Catheters (3–5 French) are placed into both ureters and sutured with 5-0 chromic suture. Bladder polyps should be removed prior to beginning the dissection as these can act as space-occupying lesions after the bladder is reconstructed. Initial dissection begins superiorly and proceeds inferiorly to separate the bladder from the adjacent skin and fascia since it is usually easiest to identify tissue planes in these areas. The umbilical vessels are ligated as the umbilicus will be moved superiorly to a more anatomically normal location, and will be later used as the location to exteriorize the suprapubic (SP) catheter.
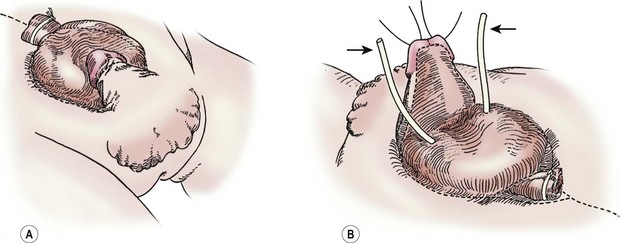
FIGURE 58-2 In a male undergoing complete primary repair for bladder exstrophy, the outlines of the planes of dissection are seen in these drawings. (A) Ventral view. (B) Dorsal view. The ureteral stents are marked with arrows.
Penile/Urethral Dissection.
Traction sutures are placed into each hemiglans of the penis to aid in dissection. These sutures will rotate to a parallel vertical orientation because the corporal bodies will naturally rotate medially after they are separated from the urethral wedge (urethral plate plus underlying corpora spongiosa). We begin the penile dissection along the ventral aspect of the penis with a circumscribing incision (Fig. 58-3A). This step precedes dissection of the urethral wedge from the corporal bodies because it is easier to identify the plane of dissection ventrally above Buck’s fascia. Buck’s fascia is deficient or absent around the corpora spongiosum. As the dissection progresses medially to separate the urethra from the corpora cavernosa, it is important to realize that the plane shifts subtly from above Buck’s fascia to just above the tunica albuginea. Failure to adjust the plane of dissection will carry the dissection into the corpora spongiosa which will result in excessive, difficult-to-control bleeding during the deep ventral dissection of the urethral wedge from the corporal bodies.
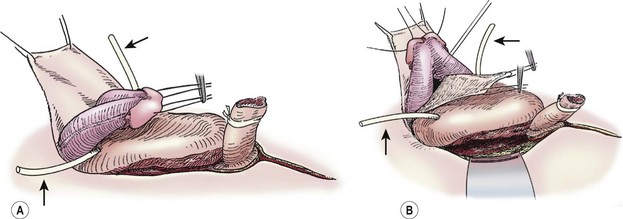
FIGURE 58-3 These two diagrams show early portions of the ventral subcoronal and corporeal dissection. (A) Initiation of the subcoronal dissection is seen. This is typically the easiest plane to initiate dissection. (B) Corporeal dissection proximally and dorsally is shown. Initiation of corporeal separation is easiest to establish proximally. Note the ureteral catheters (arrows), which have been introduced into the ureteral orifices.
Careful lateral dissection of the penile shaft skin and dartos fascia from the corporal bodies will avoid damaging the laterally located neurovascular bundles on the corpora of the epispadic penis. The lateral dissection on the penis should be superficial to Buck’s fascia because of the lateral location of the neurovascular bundles in the epispadic penis.
Complete Penile Disassembly and Deep Dissection.
Once a plane is established between the penis and the urethral wedge, the penis may be disassembled into three components: the right and left corporal bodies with their respective hemiglans, and the urethral wedge.77 This is performed in order to provide exposure to the intersymphyseal band and to allow adequate proximal dissection. We have found the easiest plane of dissection to completely isolate the corporal bodies is proximal and ventral. The plane of dissection should be carried out at the level of the tunica albuginea on the corpora (Fig. 58-3B). After a plane is established between the urethral wedge and the corporal bodies, this dissection is carried distally to separate the three components from each other (Fig. 58-4A). Complete separation of the corporal bodies increases exposure to the pelvic diaphragm for deeper dissection. The corporal bodies can be completely separated from each other since they are nourished via a separate blood supply. It is important to keep the underlying corpora spongiosa with the urethra as the vasculature to the urethra is based on this corporal tissue (which should appear wedge-shaped after its dissection from the adjacent corpora cavernosa). The urethral/corpora spongiosa component will later be tubularized and placed ventral to the corporal bodies. Para-exstrophy skin flaps should not be made with this technique because creating these flaps may injure the blood supply to the distal urethra. As the bladder and urethra are located posteriorly in the pelvis as a unit (with a common blood supply), division of the urethral wedge is counter-intuitive to the intent of the repair.
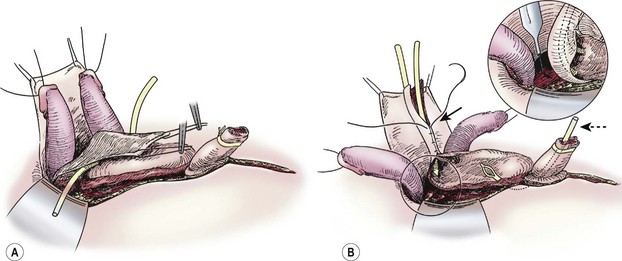
FIGURE 58-4 (A) Separation of corporeal bodies and urethra. (B) Deep pelvic dissection. Note the division of the intersymphyseal band (inset) is critical to allowing placement of the bladder in the pelvis. Also note the tubularization of the neourethra over the urethral catheter (solid arrow) and the suprapubic tube in the still-open bladder (dotted arrow).
After separating the components distally, the urethral dissection is carried proximally to the bladder neck. Exposure to the pelvic diaphragm is optimized by complete proximal separation of the urethra and corporal bodies (Fig. 58-4B). This creates the exposure to perform the deep incision of the intersymphyseal band (the condensation of anterior pelvic fascia and ligaments) which is necessary to move the bladder and urethra posteriorly (Fig. 58-5). When dissecting the urethral wedge from the corporal bodies medially, the dissection plane is along the tunica albuginea of the corpora cavernosa. This medial dissection should be carried down through the intersymphyseal band.
With a deep incision of the intersymphyseal band posterior and lateral to each side of the urethral wedge, the bladder and bladder neck can be moved to a posterior position in the pelvis. This dissection should be carried until the pelvic floor musculature becomes visible and the future bladder neck and proximal urethra lie deep in the pelvis with little anterior tension. Failure to adequately dissect the bladder and urethral wedge from these surrounding structures will prevent posterior movement of the bladder in the pelvis and will create anterior tension along the urethral plate, all of which can lead to failure of the closure (Fig. 58-5).
Primary Closure.
Once the above steps are completed, the bladder closure and urethral tubularization can be initiated. This portion of the repair is straightforward and anatomic (Fig. 58-6A). To provide urinary drainage, an SP tube is used. The bladder is closed in three layers with monofilament absorbable suture. The urethra is tubularized using a two-layer running closure with monofilament and braided absorbable suture. Because of the previous deep dissection, we can now position the tubularized urethra ventral to the corpora without tension. If the urethra cannot be positioned ventrally without creating tension and restricting the urine flow, it may be necessary to more aggressively incise the intersymphyseal band and pelvic diaphragm.
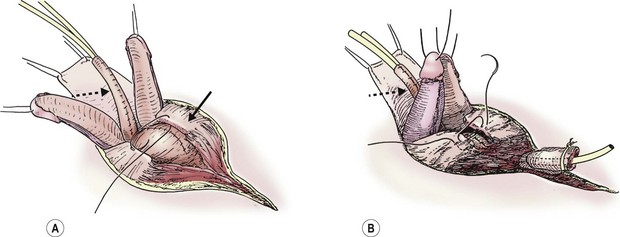
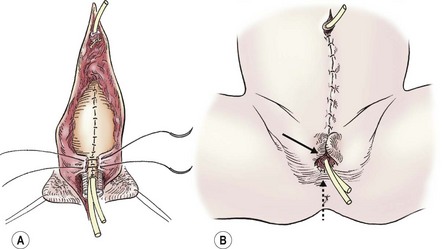
FIGURE 58-6 Once the intersymphyseal band is adequately incised and the bladder and urethral wedge are dissected from the surrounding tissues, bladder repair and urethral tubularization can be performed. (A) The bladder is being closed in three layers using monofilament absorbable suture (solid arrow). The urethra has been tubularized using a two-layer running closure with monofilament and braided absorbable suture (dotted arrow). (B) This schematic depicts closure of the pubic symphysis with two number 1 polydioxanone interrupted sutures placed in a figure-of-eight fashion. The knots are placed anteriorly to prevent suture erosion into the neck of the bladder. The urethra (dotted arrow) has been placed ventral to the corpora.
The pubic symphysis is reapproximated using two large polydiaxanone (PDS) interrupted sutures (Fig. 58-6B). Knots are left anteriorly to prevent erosion into the bladder neck. The rectus fascia is reapproximated using an interrupted or running 2-0 PDS. We also place interrupted 6-0 PDS sutures along the distal dorsal aspect of the corporal bodies to reapproximate them. Penile skin coverage is achieved with either a primary dorsal closure or reversed Byars flaps if needed. The skin covering the abdominal wall is reapproximated using a two-layer closure with absorbable monofilament suture.
The urethra lacks enough length to reach the glans in about half the cases. In this situation, it is matured along the ventral aspect of the penis to create a hypospadias (Fig. 58-7). Redundant shaft skin can be left ventrally in these patients to assist in later penile reconstruction.
CPRE Technique: Girls.
The principles of the single-stage technique are similar in boys and girls (Fig. 58-8). The planned lines of incision are marked with the bladder neck, urethra, and vagina mobilized as a unit (Fig. 58-9). The appropriate plane of dissection is found anteriorly along the medial aspect of the glans clitoris and proceeds posteriorly along the lateral aspect of the vaginal vault (Fig. 58-10A). The vagina is mobilized with the urethra and bladder neck. Dissection along the vaginal wall extends laterally. Insertion of a hemostat in the vaginal vault will help with retraction to identify the plane of dissection. The urethra and bladder neck should not be dissected from the anterior vaginal wall as this dissection will compromise the vascular supply to the urethra. During the posterior–lateral dissection, the intersymphyseal band will be encountered and should be incised deeply to allow the urethra and bladder neck to be located posteriorly. The posterior limit of the dissection is the pelvic floor musculature, and the bladder, bladder neck, and urethra are able to be moved into the pelvis without tension.
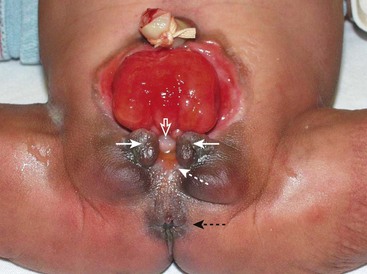
FIGURE 58-8 Bladder exstrophy in a female infant. The epispadial urethra is marked with an open white arrow. The bifid clitoris is marked with solid white arrows, and the vagina is marked with a dotted white arrow. The anus is noted with a dotted black arrow.
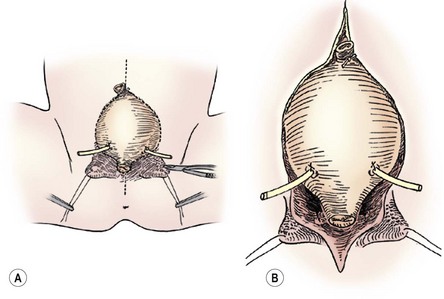
FIGURE 58-9 In a female infant, the bladder neck, urethra, and vagina are mobilized as a unit. (A) The lines of incision are seen. (B) The dissection is carried down dividing the intersymphyseal band (arrows). Note the ureteral catheters in both drawings.
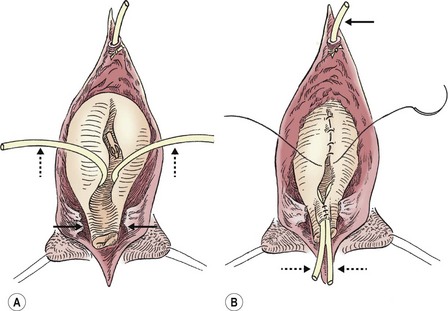
FIGURE 58-10 (A) The appropriate plane of dissection is found anteriorly along the medial aspect of the glans clitoris and proceeds posteriorly along the lateral aspect of the vaginal vault. The vagina is mobilized with the urethra and bladder neck (solid arrows). (B) The bladder and urethra are closed in multiple layers, and the suprapubic tube (arrow) is brought out superiorly. Note the ureteral catheters (dotted arrows) in both drawings.
Following adequate dissection, the vagina, urethra, and bladder neck are moved posteriorly using a Y–V plasty if the vagina is anteriorly located. The bladder neck is lengthened by making proximal parallel incisions in the bladder to define the proximal urethra and these incisions are extended to the trigone. The urethra and bladder neck are then tubularized in two layers with absorbable suture (Fig. 58-10B). The lengthening of the proximal urethra to form the bladder neck is critical. Two 3 French ureteral catheters are left in place. Prior to the urethral closure, we place a suprapubic tube for postoperative drainage. The pubis symphysis is reapproximated using two large PDS sutures (Fig. 58-11A). Iliac osteotomies may be necessary when a wide pubic diastasis prevents a low-tension reapproximation of the pubic symphysis, or if the patient is older than 72 hours. The rectus fascia can then be closed in the midline. We mature the neourethra with 5-0 polyglycolate sutures and reapproximate the bifid clitoris with 7-0 PDS by denuding each half medially. The labia majora should be advanced to the perineum at this time as well (Fig. 58-11B). A Z-plasty technique aids in skin closure. A monsplasty provides a satisfactory aesthetic result to the introital area and can be performed at the time of the primary repair.78
Other Primary Reconstructive Techniques.
Other approaches for primary BE repair include the radical soft tissue mobilization technique (Kelly technique) which has been used at some institutions for decades. This approach avoids the need for osteotomies as the periosteum is mobilized to allow the soft tissues of the bladder, bladder neck and urethra to be reconstructed in the midline. In a recent study, long-term continence rates were reported at 70% for complete or partial urinary control.79 Over half did not require bladder augmentation or CIC. Prolapse rates may be lower in adult females closed in this fashion. The abdominal wall was perceived as abnormal in adults repaired with this technique. Others have also reported successful short-term outcomes with the use of this approach. Complications such as bladder neck-cutaneous fistulas and penile loss have been described.80
Delayed Primary Closure.
Several recent studies have advocated for delayed primary closure, especially in regard to the CPRE approach. Proponents of delayed repair argue that it is safer for the child, allows a more coordinated surgical effort, and allows an elective operation so that the most expert team of clinicians and personnel are available.81 Others have shown it is possible to wait months after birth.82 Another group used the delay to stimulate the penis with testosterone with the goal of reducing the chance for glans injury, and reported good short-term outcomes with this approach in six patients.83 The successful use of a delayed closure approach is predicated on adequate protection of the exposed tissue. One series showed that the CPRE technique can be used in delayed or previous failed exstrophy closures.84 Another study found an increased cost of as much as 50% with delayed closure.85 Long-term outcomes will take years to mature.
Modern Staged Repair of Exstrophy
A staged approach for exstrophy repair developed in response to high rates of renal damage seen in single-stage approaches in the 1970s. The sequence for staged reconstruction initially consisted of bladder and posterior urethral closure at birth, BNR at 2–3 years of life, and epispadias repair later.86 This sequence later shifted when bladder capacity was noted to increase with added resistance from early epispadias repair.87 Currently, as described by the Johns Hopkins group, MSRE consists of bladder and posterior urethral closure at birth or soon thereafter (stage 1), epispadias repair at 6–12 months of life (stage 2), and BNR when the bladder capacity is adequate and the child is showing interest in toilet training (usually at 4–5 years of life) (stage 3).88 Occasionally, epispadias repair and BNR ± ureteral reimplantation can be combined.89,90
MSRE Stage 1: Initial Early Bladder Exstrophy Closure.
Male MSRE Bladder and Posterior Urethral Closure Technique.
At this point, the posterior urethra is assessed. If the child has severe dorsal chordee and a short urethra that inhibits attempts at penile lengthening, an incision in the urethral plate may be necessary, but only after extensive mobilization of the bladder and prostatic plate from the rectus fascia, the pubic bones, and the inferior ramus of the pubis. After complete division of the urogenital diaphragm from the posterior urethra/bladder neck area, the prostatic urethra can be displaced into the pelvis. Lack of an adequate urethral plate length can be managed by dividing the prostatic plate distal to the verumontanum in a V-shaped fashion. After corporal mobilization and midline reapproximation, the penile urethral plate is fashioned from a lateral rotational flap which is generated after a single lateral incision into the paraexstrophy skin. Paraexstrophy skin flaps should be used with great caution because of a high complication rate.55,91,92
After an SP tube has been placed, the umbilicus is excised and the bladder is closed in the midline in multiple layers with a running 2-0 polyglycolate suture. The posterior urethra is also closed onto the base of the penis, sizing it to accept a 12- to 14 French sound. It is important to perform a horizontal mattress closure of the pubic bones with heavy suture, and place the knot on the anterior surface of the pubis. Once the suture is placed but not tied, three sterile personnel are used to approximate the pubic symphysis. One individual applies pressure over each greater trochanter to push the pubic rami to the midline. A second individual depresses the posterior urethra and bladder neck. The third individual ties the suture. If the rectus fascia is strong, a second suture is placed just above the symphysis. The abdominal wall, subcutaneous tissues, and skin are then closed in multiple layers with construction of a neoumbilicus at or above the level of the iliac crests.
Female MSRE Bladder and Posterior Urethral Closure Technique.
In stage 1 of the MSRE in the female child, the technique of bladder, pelvic ring, and abdominal wall closure are identical to that described for the male. However, the female closure differs from the male closure in that the female urethra is completely reconstructed at stage 1.1,93 The incision in the urethral plate mucosa is 2 cm wide, traversing from the distal trigone to the vaginal orifice, in the female. Paraexstrophy skin flaps are not necessary in females.94 The medial aspect of each hemiclitoris is deepithelialized to permit approximation of the two glans clitori and reconstruction of the mons. The bladder and female urethra are closed in two layers with polyglycolate suture and the pubic bones, abdominal wall, subcutaneous tissue, and skin are closed as outlined in the male. The monsplasty and genitoplasty are then performed.
MSRE Stage 2: Epispadias Repair.
Preoperative Considerations.
Prior to performing the epispadias repair, several surgeons have described topical or intramuscular testosterone to increase the size of the phallus and the phallic skin.71,95 Intramuscular testosterone enanthate in oil (2 mg/kg) can be administered at five and two weeks prior to epispadias closure. Penile lengthening can usually be achieved at this time by division of all remnants of the suspensory ligament and scar tissue as well as further release of the crura from the inferior pubic rami. In some cases, further urethral lengthening may require free skin grafts, buccal mucosal grafts, ureteral grafts, or pedicle skin flaps. These same maneuvers can be used to repair the scarred or stenosed posterior urethra as well.
The Modified Cantwell–Ransley Technique.
Cantwell first described mobilization of the urethra and moving it ventrally for epispadias repair in the late nineteenth century. The technique has subsequently been modified.92,96 To begin this procedure, a stay suture is placed into the glans. A reverse MAGPI or IPGAM procedure at the distal urethral plate allows advancement of the urethral meatus onto the glans. Skin incisions are then made on the lateral edges of the urethral plate and around the epispadic meatus. This plate is dissected from the corporal bodies to the level of the glans distally and to the prostatic urethra proximally. Glans wings should be developed distally as well. The corporal bodies are then separated from each other to allow medial rotation. The urethra is then tubularized over a 6 or 8 French urethral catheter using running 6-0 absorbable suture. The corporal bodies are rotated over the urethra and reapproximated using interrupted 5-0 absorbable suture. Cavernocavernosotomies may be performed prior to reapproximating the corporal bodies to help correct persistent chordee. These are performed at the point of maximal angulation. The neurovascular bundles may require mobilization to avoid injuring them if cavernosotomies are performed. The glans wings are then closed over the urethra using interrupted 5-0 absorbable suture. The penile shaft skin can be trimmed and tailored to cover the penis using interrupted 5-0 or 6-0 absorbable sutures. Z-plasties at the level of the pubis may decrease the chance of a dorsal retractile scar at the base of the penis. Postoperative care includes bladder antispasmodics, broad-spectrum antibiotics, and removal of the urethral catheter at two weeks. In the event of a significantly delayed primary closure or failure of an initial bladder closure, a simultaneous closure of the exstrophy bladder and the epispadias can be performed.
Bladder Neck Reconstruction.
In incontinent children after CPRE or MSRE, a continence procedure is appropriate when (1) the urethra is stricture-free and capable of catheterization if necessary; (2) under anesthesia, the bladder capacity has achieved a minimum volume of 60–85 mL; and (3) the child is mature enough to participate in the postoperative voiding program.97 This is typically around 4–5 years of age at the earliest. Cystoscopy and gravity cystography are helpful for providing information regarding bladder capacity and the status of any previous repairs. Advocates of the staged reconstruction approach emphasize the importance of achieving adequate bladder capacity prior to performing BNR. A bladder capacity less than 60 mL under anesthesia or during urodynamic evaluation reduces the likelihood of continence after BNR. BNR requires an adequate bladder capacity because some volume is lost during construction of the bladder neck. Factors that increase the potential for the bladder to achieve an adequate capacity prior to BNR include:
• Avoidance of urinary tract infections
• Complete bladder emptying with institution of CIC if bladder emptying is incomplete
Ureteroneocystostomy may be required at the time of BNR to correct VUR and to relocate the ureters away from the lower bladder where the bladder neck will be constructed. Although the Cohen technique is often employed, others have described a cephalotrigonal technique which is particularly applicable to exstrophy patients because of the angle of ureteral entry into the bladder.98 The Marshall–Marchetti–Kranz (MMK) bladder neck suspension, or a bladder neck wrap using rectus or gracilis muscle or fascia, may be combined with BNR as well. Osteotomies may also be necessary to stabilize the intersymphyseal bar and improve continence at the time of BNR.
MSRE Stage 3: Modified YDL-BNR.
The modified YDL-BNR and the transtrigonal/cephalotrigonal bilateral ureteral reimplantation are the techniques employed in the MSRE Stage 3.98,99 The combined thoughts of several surgeons spanning an 80-year period has led to the modern YDL-BNR.97 To perform a modified YDL procedure, the bladder neck is extensively dissected and a vertical cystotomy is made. Occasionally, a portion of the intersymphyseal band needs to be divided completely for visualization. After transtrigonal/cephalotrigonal bilateral ureteral reimplantation, a strip of bladder mucosa, approximately 1.5–1.8 cm wide and 3–4 cm, long is generated, and the lateral bladder triangles are demucosalized. Use of an epinephrine-soaked sponge during this dissection aids in hemostasis. The bladder neck may be funneled further with small vertical incisions along the cut edge of the lateral bladder walls. The neourethra is tubularized over an 8 French catheter using interrupted or running polyglycolate suture (4-0 or 5-0). The two triangular regions of demucosalized detrusor muscle are then closed over the mucosal tube in a two-layer ‘vest over pants’ double-breasted technique using 3-0 polyglycolic acid suture. This reinforces the neobladder neck, decreases the risk of fistula, and augments the outlet resistance.100 The sutures in the third layer are not cut since they are used in the subsequent MMK bladder neck suspension.97
BNR: The Mitchell Repair.
This repair employs a modification of the YDL technique described previously.99 In this modification, a full-thickness incision is made in the anterior urethra and parallel incisions are extended cephalad to define the proximal urethral plate and bladder neck. After cross trigonal ureteral reimplantation, the urethral strip is tubularized in two layers using absorbable or monocryl suture (4-0 or 5-0) over an 8 to 10 French urethral catheter, depending on the size of the patient. The bladder may be closed in continuity with the urethral closure. This procedure effectively narrows and lengthens the proximal urethra. It also moves the anterior fibrotic tissue at the level of the original bladder neck to a more cephalad position, away from the new bladder neck. This anterior redundant tissue can be used as a fibromuscular flap to encircle and support the new bladder neck. If a fibromuscular bladder wrap is not possible, then a fascial sling or wrap may be performed following the bladder and urethral closure.
Occasionally, trigonal tubularization must be combined with bladder augmentation because of a small bladder capacity. Most BNRs decrease bladder capacity since the bladder is used to create the continence mechanism.101 The stomach offers the best potential to preserve spontaneous volitional voiding in this group, but places these children at risk of hematuria–dysuria syndrome which can be especially troubling in patients with persistent urinary incontinence and normal sensory innervation.102 Other intestinal segments may also be used depending on surgeon preference. If augmentation is needed and if the urethra is difficult to catheterize, appendicovesicostomy or another form of the Mitrofanoff operation is also performed because of the likelihood that they will require CIC to empty the bladder after BNR and augmentation.
Outcomes
Exstrophy
If a child develops chronic bladder and kidney infections following BE closure, he or she should be evaluated for possible outlet obstruction. Early intervention with CIC for several months will often protect the patient during this period. We routinely maintain our patients on suppressive antibiotic therapy because of the high incidence of VUR.
Epispadias
The urethrocutaneous fistula rate varies widely from 5–40%. In one study, complications were more common in those patients who underwent epispadius repair at the same time as BE closure versus an isolated epispadias repair.103 Other complications include atrophy of the corpora cavernosa and urethra. These complications can occur if the vascular supply to the corporal bodies or urethral wedge is damaged during dissection or during closure.104 Similar complications have been found following the initial stage of a staged reconstruction as well as after the use of the complete penile disassembly technique.105 In experienced hands, these complications are unusual and underscore the importance of involving surgeons experienced in the management of these patients.
Bladder Neck Reconstruction
After BNR, a good outcome is defined as a dry interval >2–3 hours accompanied by spontaneous voiding without catheterization. YDL-BNR and its variants have yielded urinary continence rates of 30% to 80% for patients with BE.99,106–108 Many factors influence these outcomes. An initial failed bladder closure or prior failed BNR reduces the chance for achieving subsequent urinary continence.109 A preoperative bladder capacity of >85 mL portends a greater continence rate after YDL-BNR.110 Use of osteotomies and patient immobilization in the postoperative period increases the success of bladder closure and subsequent continence. Delayed bladder closure increases the likelihood of the eventual need for bladder augmentation due to inadequate bladder capacity that, in turn, reduces the chance for volitional voiding. One long-term study found that eight of 13 patients with an initially successful bladder closure and BNR required further surgery in their second decade of life because of poorly compliant, low-capacity bladders that caused urinary incontinence.111
Adjunctive Aspects of the Repair
Indirect inguinal hernias are commonly associated with BE in both male and female patients.112,113 They arise as a consequence of enlarged internal and external inguinal rings combined with compromised fascial support and lack of obliquity of the inguinal canal.114 In one study 56% of classic male exstrophy patients and 15% of classic female exstrophy patients developed inguinal hernias over a 10-year period.64 The authors recommended these hernias be repaired at the time of primary bladder closure to prevent incarceration.
Osteotomies
Approximation of the open pelvic ring eases abdominal wall closure, diminishes the rate of bladder and abdominal wall dehiscence,74,115 allows the construction of an intrapelvic urethra, and reapproximates the pelvic floor musculature likely contributing to long-term continence.60,115 Therefore, pelvic ring reapproximation is useful at the initial bladder closure or at later stages of reconstruction. If the pelvis is not sufficiently malleable, osteotomies are performed.
Several operative approaches are available. Posterior iliac osteotomies are performed with the patient in a prone position, after which the patient is then repositioned for the bladder closure. Increased blood loss and occasional malunion of the ileum have led to the pursuit of other approaches. Anterior iliac osteotomies offer the advantage of not having to turn the patient. Compared to posterior iliac osteotomies, an anterior approach also has been shown to result in less blood loss and better apposition and mobility of the pubic rami. Anterior approaches include an anterior diagonal technique or a combination of bilateral combined anterior innominate and vertical iliac osteotomies which have had excellent initial and long-term results compared to anterior iliac osteotomies alone in some series.73,116 Both osteotomies can be performed through the same anterior skin incision on each side and with minimal blood loss and 4% complication rate.117,118 A diagonal mid-iliac osteotomy performed through the same incision as the exstrophy closure has also been described.119 Division of the superior pubic ramus is not as effective as the other methods described, but can be used in the newborn period.120,121
After the primary reconstructive procedure for exstrophy, the patient must be immobilized to decrease lateral stresses on the closure. Choice of immobilization remains controversial with a variety of techniques available. Current options include: (1) Bryant’s traction; (2) use of an external fixator; (3) spica casting; and (4) mummy or mermaid wrapping. The most important variable with these techniques is familiarity of the user as they all have learning curves. We prefer a spica cast for 3 weeks to prevent external hip rotation and optimize pubic apposition. It can facilitate early discharge and home care (Fig. 58-12). Modified Buck’s traction has also been used by many groups with success. A posterior lightweight splint can be used in newborns when the child is out of traction to maintain hip adduction. We have stopped using Buck’s traction because spica casts are easier for the families at home. External fixation devices have also been used with success.116 Fixator pins for these devices should be cleaned several times a day to reduce infection. Internal fixation may be necessary in older patients. Femoral nerve palsy is a possible complication with fixation which must be monitored and can be reduced by gradually tightening the fixator.
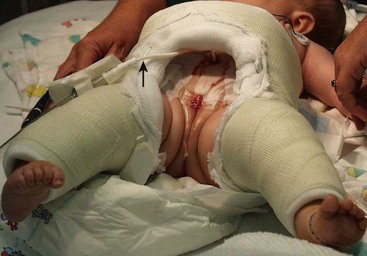
FIGURE 58-12 In order to immobilize the patient to decrease lateral stresses on the closure after the primary reconstructive procedure, a number of immobilization techniques are possible. This photograph shows a spica cast that was applied to prevent external hip rotation and optimize pubic apposition in the early postoperative period. Note the suprapubic bladder catheter (arrow).
To avoid osteotomies, a technique has been developed that secures the pubic symphyseal closure with deeply placed polyglycolate sutures through the bone followed by placement of a miniature metal plate which may be removed later. Initial results are promising.122 Osteotomies are not usually needed with the Kelly technique.79
Long-Term Considerations
Psychosocial Concerns
Children with exstrophy can experience marked difficulty in the development of their psychosocial identity. Adolescent males with exstrophy are psychosexually delayed for 2 to 4 years compared to their peers, and teenage girls with exstrophy struggle with sexual self-esteem issues such as body image and genital perception/appearance.123 We recommend regular assessment of social development in this patient group including psychiatric evaluation and parental education.124 Assessment can begin as early as 12 to 18 months of age and then as needed. Evaluation and intervention is also useful prior to reconstructive procedures. In these patients, anxiety and even suicidal ideation is greater than the general population.125 Fortunately, adolescents with exstrophy are resilient and often develop creative strategies for coping with the chronic aspects of their condition.126 Most adolescents with exstrophy express a strong desire to be normal and resent arrangements that emphasize their differences.127
Sexual Function
Although sexual function is of little relevance to the newborn or prepubertal child born with exstrophy, it is an issue of considerable importance to the parents of the child who will worry and wonder what future sexual function their children will experience, and also for the adolescents with exstrophy for whom sexual function or the potential for sexual function is an area of focus. Erectile function and sensation is usually intact for most male BE patients.128,129 However, persistent or recurrent chordee and small penile size can create difficulty in achieving intercourse for some males.129 Libido is usually present with or without operative correction. Studies of adult men with exstrophy have found that approximately one-third choose not to or cannot engage in intercourse.130 In one series, 33 of 43 BE patients were married or living with a partner. Sexual counseling in these patients is important because of the difficulties they may encounter.
Exceedingly small penile size can severely limit the potential of some men to have sexual intercourse. In this setting, reconstruction with a radial forearm flap to construct a penis analogue has shown an improved quality of life.131
Ejaculation is often possible despite the extensive reconstructive procedures that are performed. However, seminal emission may be slow and continue several hours after orgasm. Sperm quality and quantity is often diminished which may be due to partial obstruction after surgery, epididymitis, or recurrent urinary infections. In one series, 63% had antegrade ejaculation.132 Long-term studies in adults with BE found that a minority were able to conceive without assisted reproductive techniques.130,133,134
For women with exstrophy, sexual intercourse is possible and sexual function is often intact.128 In one series, 14 of 23 patients were able to have typical intercourse.135 Narrowing at the vaginal introitus can be problematic. This can be addressed with serial calibration and dilation as a first option, or with vaginal advancement skin flaps as another option. Fertility is unimpaired in female patients with BE. However, prolapse occurs more commonly because of the lack of pelvic floor support structures and bed-rest may be necessary in the later stages of pregnancy. Pregnancy in these patients is considered a high risk event.28,136 Pregnancy complications such as hydronephrosis and bacteriuira are common, and may necessitate antimicrobial prophylaxis.137 Cesarean section is the recommended mode of delivery as vaginal delivery can be problematic.138 Vaginal and uterine prolapse is more common in women with BE and treatment occurs at a younger age than in the general population.139
Cloacal Exstrophy
Although CE has been recognized for several hundred years, Spencer was the first surgeon to report successful repair and survival in 1965.140 Mortality rates of CE infants remained high for years following this initial success. Affected infants routinely died of malnutrition and sepsis. With continuing improvement in total parenteral nutrition and neonatal management, mortality rates currently are less than 10%.141 Quality of life issues are now paramount in this patient group.
The bladder plate associated with CE is divided in half by the hindgut plate, which represents the development anomaly of the colon that occurs with CE. Ileum enters and intussuscepts into the middle of the hindgut creating the ‘trunk of an elephant’s face’ appearance with appendiceal appendages located laterally to give the impression of ‘tusks on the face of the elephant’ (Fig. 58-13).142
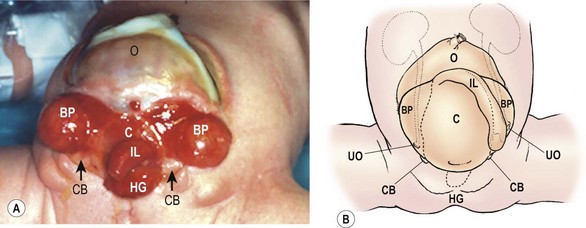
FIGURE 58-13 Photograph and schematic drawing of a neonate with cloacal exstrophy. BP, bladder plate; C, cecal plate; CB, corporeal body; HG, hindgut; IL, ileum; O, omphalocele; UO, ureteral orifice.
Associated Anomalies
In addition to the features seen with BE, up to one-half of patients with CE can have other skeletal abnormalities. These anomalies include congenital hip dislocation, talipes equinovarus, and a variety of limb deficiencies.143
The fascial anomalies associated with CE include those previously described for BE. In addition, omphaloceles are often found as well.144,145 The omphalocele can be repaired during the initial bladder closure if it is small. If the omphalocele is large, it may require closure with the reapproximated bladder halves acting as a silo to reduce the intra-abdominal pressure. Alternatively, the omphalocele can be treated with antiseptic paint to promote epithelialization. The intra-abdominal pressure following omphalocele closure determines whether we can proceed with primary bladder closure at the same time or whether bladder closure is performed later. Too aggressive an attempt at one-stage CE repair can lead to organ ischemia from the increased intra-abdominal pressure. On the other hand, rupture of an omphalocele clearly requires immediate attention and takes priority over other considerations.
Neurologic involvement of the lower spinal cord is reported to occur in 50–100% of patients.144,146,147 Most patients have lumbar or sacral cord involvement, but thoracic level myelodysplasia has also been reported.144 CE management must be coordinated with closure of the neural tube defect.
Cloacal Exstrophy Closure Techniques
Each CE patient is unique, requiring an individualized operative plan. In general, this plan would include: (1) closure of the omphalocele with reapproximation of the posterior bladder halves, tubularization of the cecum and incorporation of the hindgut into the gastrointestinal tract (functionally this creates the anatomy of classic BE); and (2) repair of the exstrophic bladder and genitalia. If the baby is medically stable, the initial reconstructive procedures can be performed within the first 72 hours of life, thus taking advantage of the pliable bony pelvis if closure of the entire defect is possible. However, in up to 75% of these children, spina bifida is present and may need urgent closure, delaying the ventral abdominal wall closure. Also, the omphalocele, seen in almost 90% of the patients,148 may require immediate care to prevent rupture. The size of the omphalocele, the size of the hindgut plate and bladder plates, the extent of the pubic diastasis, and the extent of comorbidities largely dictate the timing and staging of the initial closure. If the large omphalocele cannot be closed because of increased abdominal pressure, a silo can be placed or a biosynthetic patch used to bridge the abdominal wall fascial defect.
In the past, an ileostomy was routinely performed, but the metabolic consequences can be significant. Currently, management decisions focus around the use of the exstrophic cecal plate and the terminal blind-ending hindgut. The cecal plate can be retained in the bladder closure as a bladder augmentation or can be separated from the bladder plates and used in the gastrointestinal tract (Fig. 58-14A). However, to improve bowel length and water resorption, most feel the cecal plate should be tubularized, making the terminal ileum, cecum, and the blind-ending hindgut all in continuity. The hindgut segment can then be exteriorized as a colostomy.
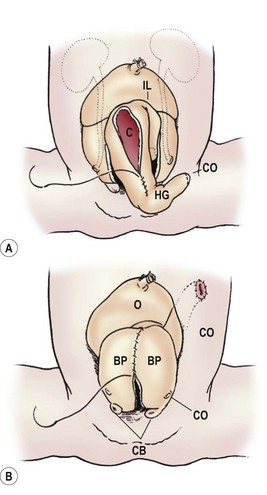
FIGURE 58-14 (A) Depiction of removal and tubularization of the cecal plate. (B) Reapproximation of the bladder plates. BP, bladder plate; C, cecum; CB, corporeal body; CO, colostomy site; HG, hindgut; IL, ileum; O, omphalocele; UO, ureteral orifice.
At initial repair, ureteral catheters are positioned in the ureteral orifices and secured with 5-0 chromic suture. For the omphalocele repair, the dissection begins superiorly. Next, the umbilical vessels are ligated and the bladder plates separated from the adjacent skin as described in the CPRE and MSRE techniques. The medial cecal hindgut plate is separated from the paired, separated bladder plates, and tubularized. The bladder halves are then reapproximated in the midline (Fig. 58-14B). Inguinal hernias should be repaired if found. Bladder repair can be performed if the intra-abdominal pressure remains low after omphalocele closure. This is determined clinically by assessing a change in ventilatory parameters. A one-stage CE closure, including closure of the omphalocele, cecal plate tubularization and hindgut terminal colostomy, bladder and urethral closure, reconstruction of the external genitalia and osteotomies, should be performed only under optimal anatomical conditions. The decision to proceed with one-stage closure versus staged reconstruction spanning a period of months must be weighed carefully. Following tight closure, increased abdominal pressure can cause organ ischemia, excessive tension on the midline closure, or compromised lower extremity blood flow. If necessary, CE repair can be staged at this point by converting the CE into a classic BE. A colostomy is then created.
If staging is indicated, once the baby has recovered sufficiently to tolerate another operation, reconstruction of the bladder, bladder neck and genitalia is performed using either the CPRE or MSRE approach.149
Osteotomies are almost always necessary to assist in closure and posterior positioning of the urinary tract, given the pubic diastasis is usually more severe than in BE. Prior to the initial incision, the need for osteotomies is assessed by watching for ischemia of the lower extremities and external genitalia with reapproximation of the pubic symphysis. The Hopkins group recommends bilateral anterior innominate and vertical iliac osteotomies with gradual approximation of the bones over one to two weeks via external fixture and interfragmentary pins.150,151 The Seattle group prefers iliac osteotomies. Other authors prefer to avoid osteotomies because they believe that osteotomies make the abdominal closure more difficult,152 but this has not been our experience. Fewer wound dehiscences and abdominal wall hernias have been reported with osteotomies.151
Secondary procedures may be required on the urinary tract to achieve continence, including BNR, bladder neck closure, bladder augmentation with stomach, ileum or hindgut, and creation of a continent catheterizable stoma.144,153–156 As most of these children will need CIC to achieve dryness, urinary reconstruction must be individualized to meet their needs.
Gender of Rearing
Gender assignment for patients with CE is currently debated both by the medical profession and by the lay public. Traditionally, male patients underwent gender conversion in infancy because of concerns that the small, paired male hemi-phalluses were inadequate in size. This approach was supported by anecdotal data of unsatisfied patients following genital reconstruction.144 However, these observations, in conjunction with the prevailing notion that humans are gender neutral at birth and can undergo gender conversion safely in infancy, have recently come into question.157 Gender identity now appears to be a much more complex issue than previously imagined.
Currently we reconstruct males with CE as males whenever technically possible. Many gender assigned individuals will later identify themselves in a male gender role in adolescence and adulthood.157 Technically, however, reconstruction of external male genitalia in these patients can be quite challenging. The wide pubic diastasis and small phallic size add to the technical difficulty because it is more difficult to join the two phallic halves in the midline. In some cases, if one phallic half is diminutive, the small one is removed, and the reconstruction is performed using only the larger half.
Gastrointestinal Reconstruction
Short-gut syndrome is usually present at birth in babies with CE, even those with a normal length of bowel. The effects of malabsorption and fluid loss appear to be most clinically significant early in life.158 Many such children require parenteral nutritional support in early infancy.159,160 As a result of this problem, we feel the hindgut should be constructed and placed in continuity with the intestine during the initial operation. This may improve intestinal absorption and nutrition, and will also preserve intestinal tissue that can be used in later reconstruction of the urinary tract or vagina if needed.
References
1. Gearhart, JP. The exstrophy-epispadias complex in the new millennium–science, practice and policy. J Urol. 1999; 162:1421–1423.
2. Lattimer, JK, Smith, MK. Exstrophy closure: A followup on 70 cases. J Urol. 1966; 95:356–359.
3. Engel, RM. Exstrophy of the bladder and associated anomalies. Birth Defects Orig Artic Ser. 1974; 10:146–149.
4. Epidemiology of bladder exstrophy and epispadias: A communication from the International Clearinghouse for Birth Defects Monitoring Systems. Teratology. 1987; 36:221–227.
5. Ives, E, Coffey, R, Carter, CO. A family study of bladder exstrophy. J Med Genet. 1980; 17:139–141.
6. Ziegler, M, Duckett, JW, Howell, JG. Cloacal exstrophy. In: Welch J, ed. Pediatric Surgery. Chicago: Year Book Publishers, 1986.
7. Keppler-Noreuil, KM. OEIS complex (omphalocele-exstrophy-imperforate anus-spinal defects): A review of 14 cases. Am J Med Genet. 2001; 99:271–279.
8. Brock, J, III., O’Neill, J, Jr. Bladder exstrophy. In: O’Neill J, Jr., eds. Pediatric Surgery. Philadelphia: Lippincott; 1998:1709–1732.
9. Skari, H, Bjornland, K, Bjornstad-Ostensen, A, et al. Consequences of prenatal ultrasound diagnosis: A preliminary report on neonates with congenital malformations. Acta Obstet Gynecol Scand. 1998; 77:635–642.
10. Gearhart, JP, Ben-Chaim, J, Jeffs, RD, et al. Criteria for the prenatal diagnosis of classic bladder exstrophy. Obstet Gynecol. 1995; 85:961–964.
11. Mirk, P, Calisti, A, Fileni, A. Prenatal sonographic diagnosis of bladder extrophy. J Ultrasound Med. 1986; 5:291–293.
12. Barth, RA, Filly, RA, Sondheimer, FK. Prenatal sonographic findings in bladder exstrophy. J Ultrasound Med. 1990; 9:359–361.
13. Jaffe, R, Schoenfeld, A, Ovadia, J. Sonographic findings in the prenatal diagnosis of bladder exstrophy. Am J Obstet Gynecol. 1990; 162:675–678.
14. Lee, EH, Shim, JY. New sonographic finding for the prenatal diagnosis of bladder exstrophy: A case report. Ultrasound Obstet Gynecol. 2003; 21:498–500.
15. Cacciari, A, Pilu, GL, Modenti, M, et al. Prenatal diagnosis of bladder exstrophy: What counseling? J Urol. 1999; 161:259–262.
16. Wiersma, R. Overview of bladder exstrophy: A third world perspective. J Pediatr Surg. 2008; 43:1520–1523.
17. Muecke, EC. The Role of the Cloacal Membrane in Exstrophy: The First Successful Experimental Study. J Urol. 1964; 92:659–667.
18. Klimberg, I. The Development of Voiding Control. AUA Update Series. 1988; 7:162–167.
19. Marshall, FF. Embryology of the lower genitourinary tract. Urol Clin North Am. 1978; 5:3–15.
20. Thomalla, JV, Rudolph, RA, Rink, RC, et al. Induction of cloacal exstrophy in the chick embryo using the CO2 laser. J Urol. 1985; 134:991–995.
21. Patten, BM, Barry, A. The genesis of exstrophy of the bladder and epispadias. Am J Anat. 1952; 90:35–57.
22. Manner, J, Kluth, D. A chicken model to study the embryology of cloacal exstrophy. J Pediatr Surg. 2003; 38:678–681.
23. Beaudoin, S, Barbet, P, Bargy, F. Pelvic development in the rabbit embryo: implications in the organogenesis of bladder exstrophy. Anat Embryol (Berl). 2004; 208:425–430.
24. Slaughenhoupt, BL, Mathews, RI, Peppas, DS, et al. A large animal model of bladder exstrophy: Observations of bladder smooth muscle and collagen content. J Urol. 1999; 162:2119–2122.
25. Slaughenhoupt, BL, Chen, CJ, Gearhart, JP. Creation of a model of bladder exstrophy in the fetal lamb. J Urol. 1996; 156:816–818.
26. Verpoest, W, Platteau, P, Van Steirteghem, A, et al. Pregnancy in a couple with a male partner born with severe bladder exstrophy. Reprod Biomed Online. 2004; 8:240–242.
27. Gross, SD. A Practical Treatise on the Diseases, Injuries, and Malformations of the Urinary Bladder, the Prostate Gland, and the Urethra, 3rd ed. Philadelphia: Henry C. Lea; 1876.
28. Burbige, KA, Henslel, TW, Chambers, WJ, et al. Pregnancy and sexual function in women with bladder exstrophy. Urology. 1986; 28:12–14.
29. Mathews, R, Gan, M, Gearhart, JP. Urogynaecological and obstetric issues in women with the exstrophy-epispadias complex. BJU Int. 2003; 91:845–849.
30. Froster, UG, Heinritz, W, Bennek, J, et al. Another case of autosomal dominant exstrophy of the bladder. Prenat Diagn. 2004; 24:375–377.
31. Messelink, EJ, Aronson, DC, Knuist, M, et al. Four cases of bladder exstrophy in two families. J Med Genet. 1994; 31:490–492.
32. Shapiro, E, Lepor, H, Jeffs, RD. The inheritance of the exstrophy-epispadias complex. J Urol. 1984; 132:308–310.
33. Paidas, MJ, Crombleholme, TM, Robertson, FM. Prenatal diagnosis and management of the fetus with an abdominal wall defect. Semin Perinatol. 1994; 18:196–214.
34. Tang, Y, Ma, CX, Cui, W, et al. The risk of birth defects in multiple births: A population-based study. Matern Child Health J. 2006; 10:75–81.
35. Gambhir, L, Höller, T, Müller, M, et al. Epidemiological survey of 214 families with bladder exstrophy-epispadias complex. J Urol. 2008; 179:1539–1543.
36. O’Kane, HO, Megaw, JM. Carcinoma in the exstrophic bladder. Br J Surg. 1968; 55:631–635.
37. Hohenfellner, R, Stein, R. Primary urinary diversion in patients with bladder exstrophy. Urology. 1996; 48:28–830.
38. Hollowell, JG, Hill, PD, Duffy, PG, et al. Lower urinary tractfunction after exstrophy closure. Pediatr Nephrol. 1992; 6:428–432.
39. Nisonson, I, Lattimer, JK. How well can the exstrophied bladder work? J Urol. 1972; 107:664–666.
40. Stein, R, Fisch, M, Stöckle, M, et al. Colonic conduit in children: Protection of the upper urinary tract 16 years later? J Urol. 1996; 156:1146–1150.
41. Husmann, DA, Rathbun, SR. Long-term follow up of enteric bladder augmentations: The risk for malignancy. J Pediatr Urol. 2008; 4:381–386.
42. Vemulakonda, VM, Lendvay, TS, Shnorhavorian, M, et al. Metastatic adenocarcinoma after augmentation gastrocystoplasty. J Urol. 2008; 179:1094–1097.
43. Husmann, DA, Spence, HM. Current status of tumor of the bowel following ureterosigmoidostomy: A review. J Urol. 1990; 144:607–610.
44. Fisch, M, Wammack, R, Hohenfellner, R. The sigma rectum pouch (Mainz pouch II). World J Urol. 1996; 14:68–72.
45. Hohenfellner, R. Continent urinary diversion in childhood: European experience. Scand J Urol Nephrol Suppl. 1992; 142:82–85.
46. Ghoneim, MA. The modified rectal bladder: A bladder substitute controlled by the anal sphincter. Scand J Urol Nephrol Suppl. 1992; 142:89–91.
47. Tacciuoli, M, Laurenti, C, Racheli, T. Sixteen years’ experience with the Heitz Boyer-Hovelacque procedure for exstrophy of the bladder. Br J Urol. 1977; 49:385–390.
48. Crissey, MM, Steele, GD, Gittes, RF. Rat model for carcinogenesis in ureterosigmoidostomy. Science. 1980; 207:1079–1080.
49. Trendelenberg, F. The treatment of ectopia vesicae. Ann Surg. 1906; 44:981–989.
50. Ansell, JS. Surgical treatment of exstrophy of the bladder with emphasis on neonatal primary closure: Personal experience with 28 consecutive cases treated at the University of Washington hospitals from 1962 to 1977: Techniques and results. J Urol. 1979; 121:650–653.
51. Young, H. Exstrophy of the bladder: The first case in which a normal bladder and urinary control have been obtained by plastic operations. Surg Gynecol Obstet. 1942; 74:729–737.
52. Montagnani, CA. One stage functional reconstruction of exstrophied bladder: Report of two cases with six-year follow-up. Z Kinderchir. 1982; 37:23–27.
53. Fuchs, J, Gluer, S, Mildenberger, H. One-stage reconstruction of bladder exstrophy. Eur J Pediatr Surg. 1996; 6:212–215.
54. King, L, Wendel, E. Primary cystectomy and permanent urinary diversion in the treatment of exstrophy of the urinary bladder. In: Scott JR, Gordon H, Carlton C, Beach PD, eds. Current Controversies in Urologic management. Philadelphia: WB Saunders Co; 1972:242–250.
55. Johnston, JH, Kogan, SJ. The exstrophic anomalies and their surgical reconstruction. Curr Probl Surg. 1974; 1–39.
56. Williams, DI, Keeton, JE. Further progress with reconstruction of the exstrophied bladder. Br J Surg. 1973; 60:203–207.
57. Megall, M, Lattimer, JK. Review of the management of 140 cases of exstrophy of the bladder. J Urol. 1973; 109:246–248.
58. Engel, RM. Bladder exstrophy: Vesicoplasty or urinary diversion? Urology. 1973; 2:20–24.
59. Ezell, WW, Carlson, HE. A realistic look at exstrophy of the bladder. Br J Urol. 1970; 42:197–202.
60. Aadalen, RJ, O’Phelan, EH, Chisholm, TC, et al. Exstrophy of the bladder: Long-term results of bilateral posterior iliac osteotomies and two-stage anatomic repair. Clin Orthop Relat Res. 1980; 151:193–200.
61. Jeffs, RD. Functional closure of bladder exstrophy. Birth Defects Orig Artic Ser. 1977; 13:171–173.
62. Saltzman, B, Mininberg, DT, Muecke, EC. Exstrophy of bladder: Evolution of management. Urology. 1985; 26:383–388.
63. Grady, RW, Mitchell, ME. Complete primary repair of exstrophy. J Urol. 1999; 162:1415–1420.
64. Churchill, B, Merguerian, PA, Khoury, AE, et al. Bladder exstrophy and epispadias. In: O’Donnel B, Koff S, eds. Pediatric Urology. Oxford, England: Reed Elsevier; 1997:495–508.
65. Gearhart, JP, Jeffs, RD. Bladder exstrophy: Increase in capacity following epispadias repair. J Urol. 1989; 142:525–526.
66. Mathews, R, Gosling, JA, Gearhart, JP. Ultrastructure of the bladder in classic exstrophy: Correlation with development of continence. J Urol. 2004; 172:1446–1449.
67. Mathews, R, Willis, M, Perlman, E, et al. Neural innervation of the newborn exstrophic bladder: An immunohistochemical study. J Urol. 1999; 162:506–508.
68. Lee, BR, Perlman, EJ, Partin, AW, et al. Evaluation of smooth muscle and collagen subtypes in normal newborns and those with bladder exstrophy. J Urol. 1996; 156:2034–2036.
69. McMahon, DR, Cain, MP, Husmann, DA, et al. Vesical neck reconstruction in patients with the exstrophy-epispadias complex. J Urol. 1996; 155:1411–1413.
70. Dodson, JL, Surer, I, Baker, LA, et al. The newborn exstrophy bladder inadequate for primary closure: evaluation, management and outcome. J Urol. 2001; 165:1656–1659.
71. Gearhart, JP. Bladder exstrophy: Staged reconstruction. Curr Opin Urol. 1999; 9:499–506.
72. Kost-Byerly, S, Jackson, EV, Yaster, M, et al. Perioperative anesthetic and analgesic management of newborn bladder exstrophy repair. J Pediatr Urol. 2008; 4:280–285.
73. Gearhart, JP, Forschner, DC, Jeffs, RD, et al. A combined vertical and horizontal pelvic osteotomy approach for primary and secondary repair of bladder exstrophy. J Urol. 1996; 155:689–693.
74. Husmann, DA, McLorie, GA, Churchill, BM. Closure of the exstrophic bladder: An evaluation of the factors leading to its success and its importance on urinary continence. J Urol. 1989; 142:522–524.
75. Gearhart, JP, Peppas, DS, Jeffs, DS. The failed exstrophy closure: Strategy for management. Br J Urol. 1993; 71:217–220.
76. Close, CE, Carr, MC, Burns, MW, et al. Lower urinary tract changes after early valve ablation in neonates and infants: Is early diversion warranted? J Urol. 1997; 157:984–988.
77. Mitchell, ME, Bagli, DJ. Complete penile disassembly for epispadias repair: The Mitchell technique. J Urol. 1996; 155:300–304.
78. Cook, AJ, Farhat, WA, Cartwright, LM, et al. Simplified mons plasty: A new technique to improve cosmesis in females with the exstrophy-epispadias complex. J Urol. 2005; 173:2117–2120.
79. Jarzebowski, AC, McMullin, ND, Grover, SR, et al. The Kelly technique of bladder exstrophy repair: Continence, cosmesis and pelvic organ prolapse outcomes. J Urol. 2009; 182:1802–1806.
80. Berrettini, A, Castagnetti, M, Rigamonti, W. Radical soft tissue mobilization and reconstruction (Kelly procedure) for bladder extrophy [correction of exstrophy] repair in males: Initial experience with nine cases. Pediatr Surg Int. 2009; 25:427–431.
81. Canning, D. Vesical Exstrophy Complete primary closure commentary. In: Hinman JaLBF, ed. Hinman’s Atlas of Pediatric Urologic Surgery. Philadelphia, PA: Saunders Elsevier; 2009:388.
82. Baradaran, N, Stec, AA, Schaeffer, AJ, et al. Delayed primary closure of bladder exstrophy: Immediate postoperative management leading to successful outcomes. Urology. 2012; 79:415–419.
83. Zaccara, A, Stec, AA, Schaeffer, AJ, et al. Delayed complete repair of exstrophy with testosterone treatment: An alternative to avoid glans complications? Pediatr Surg Int. 2011; 27:417–421.
84. Hafez, AT, De Gennaro, M, Di Lazzaro, A, et al. Complete primary repair of bladder exstrophy in children presenting late and those with failed initial closure: single center experience. J Urol. 2005; 174:1549–1552.
85. Nelson, CP, North, AC, Ward, MK, et al. Economic impact of failed or delayed primary repair of bladder exstrophy: Differences in cost of hospitalization. J Urol. 2008; 179:680–683.
86. Jeffs, RD. Exstrophy and cloacal exstrophy. Urol Clin North Am. 1978; 5:127–140.
87. Gearhart, JP, Jeffs, RD. State-of-the-art reconstructive surgery for bladder exstrophy at the Johns Hopkins Hospital. Am J Dis Child. 1989; 143:1475–1478.
88. Baker, LA, Gearhart, JP. The staged approach to bladder exstrophy closure and the role of osteotomies. World J Urol. 1998; 16:205–211.
89. Baka-Jakubiak, M. Combined bladder neck, urethral and penile reconstruction in boys with the exstrophy-epispadias complex. BJU Int. 2000; 86:513–518.
90. Baird, AD, Mathews, RI, Gearhart, JP. The use of combined bladder and epispadias repair in boys with classic bladder exstrophy: Outcomes, complications and consequences. J Urol. 2005; 174:1421–1424.
91. Duckett, JW. Use of paraexstrophy skin pedicle grafts for correction of exstrophy and epispadias repair. Birth Defects Orig Artic Ser. 1977; 13:175–179.
92. Gearhart, JP, Sciortino, C, Ben-Chaim, J, et al. The Cantwell-Ransley epispadias repair in exstrophy and epispadias: Lessons learned. Urology. 1995; 46:92–95.
93. Gearhart, JP, Ben-Chaim, J, Sciortino, C, et al. The multiple reoperative bladder exstrophy closure: What affects the potential of the bladder? Urology. 1996; 47:240–243.
94. Ben-Chaim, J, Jeffs, RD, Gearhart, JP. Loss of urethrovaginal septum as a complication of exstrophy closure in girls. Eur Urol. 1998; 33:206–208.
95. Perovi ;, S, Scepanovi
;, S, Scepanovi ;, D, Sremcevi
;, D, Sremcevi ;, D, et al. Epispadias surgery–Belgrade experience. Br J Urol. 1992; 70:674–677.
;, D, et al. Epispadias surgery–Belgrade experience. Br J Urol. 1992; 70:674–677.
96. Borzi, PA, Thomas, DF. Cantwell-Ransley epispadias repair in male epispadias and bladder exstrophy. J Urol. 1994; 151:457–459.
97. Ferrer, FA, Tadros, YE, Gearhart, J. Modified Young-Dees-Leadbetter bladder neck reconstruction: New concepts about old ideas. Urology. 2001; 58:791–796.
98. Canning, DA, Gearhart, JP, Peppas, DS, et al. The cephalotrigonal reimplant in bladder neck reconstruction for patients with exstrophy or epispadias. J Urol. 1993; 150:156–158.
99. Jones, JA, Mitchell, ME, Rink, RC. Improved results using a modification of the Young-Dees-Leadbetter bladder neck repair. Br J Urol. 1993; 71:555–561.
100. Ben-Chaim, J, Gearhart, JP. Current management of bladder exstrophy. Tech Urol. 1996; 2:2–33.
101. Mansi, M, Ahmed, S. Young-Dees-Leadbetter bladder neck reconstruction for sphincteric urinary incontinence: The value of augmentation cystoplasty. Scand J Urol Nephrol. 1993; 27:509–517.
102. Ganesan, GS, Nguyen, DH, Adams, MC, et al. Lower urinary tract reconstruction using stomach and the artificial sphincter. J Urol. 1993; 149:1107–1109.
103. Lottmann, HB, Yaqouti, M, Melin, Y. Male epispadias repair: Surgical and functional results with the Cantwell-Ransley procedure in 40 patients. J Urol. 1999; 162:1176–1180.
104. Gearhart, JP. Complete repair of bladder exstrophy in the newborn: Complications and management. J Urol. 2001; 165:2431–2433.
105. Gearhart, JP, Mathews, R. Penile reconstruction combined with bladder closure in the management of classic bladder exstrophy: Illustration of technique. Urology. 2000; 55:764–770.
106. Mollard, P. [Bladder and urethral reconstruction for bladder exstrophy]. Bull Soc Sci Med Grand Duche Luxemb. 1987; 124(Spec No):225–230.
107. Surer, I, Baker, LA, Jeffs, RD, et al. Modified Young-Dees-Leadbetter bladder neck reconstruction in patients with successful primary bladder closure elsewhere: A single institution experience. J Urol. 2001; 165:2438–2440.
108. Shaw, MB, Rink, RC, Kaefer, M, et al. Continence and classic bladder exstrophy treated with staged repair. J Urol. 2004; 172:1450–1453.
109. Gearhart, JP, Canning, DA, Peppas, DS, et al. Techniques to create continence in the failed bladder exstrophy closure patient. J Urol. 1993; 150:441–443.
110. Chan, DY, Jeffs, RD, Gearhart, JP. Determinants of continence in the bladder exstrophy population: predictors of success? Urology. 2001; 57:774–777.
111. Woodhouse, CR, Redgrave, NG. Late failure of the reconstructed exstrophy bladder. Br J Urol. 1996; 77:590–592.
112. Stringer, MD, Duffy, PG, Ransley, PG. Inguinal hernias associated with bladder exstrophy. Br J Urol. 1994; 73:308–309.
113. Connolly, JA, Peppas, DS, Jeffs, RD, et al. Prevalence and repair of inguinal hernias in children with bladder exstrophy. J Urol. 1995; 154:1900–1901.
114. Husmann, DA, McLorie, GA, CHurchill, BM, et al. Inguinal pathology and its association with classical bladder exstrophy. J Pediatr Surg. 1990; 25:332–334.
115. Oesterling, JE, Jeffs, RD. The importance of a successful initial bladder closure in the surgical management of classical bladder exstrophy: Analysis of 144 patients treated at the Johns Hopkins Hospital between 1975 and 1985. J Urol. 1987; 137:258–262.
116. Sponseller, PD, Jani, MM, Jeffs, RD, et al. Anterior innominate osteotomy in repair of bladder exstrophy. J Bone Joint Surg Am. 2001; 83:184–193.
117. Okubadejo, GO, Sponseller, PD, Gearhart, JP. Complications in orthopedic management of exstrophy. J Pediatr Orthop. 2003; 23:522–528.
118. Sponseller, PD, Gearhart, JP, Jeffs, RD. Anterior innominate osteotomies for failure or late closure of bladder exstrophy. J Urol. 1991; 146:137–140.
119. McKenna, PH, Khoury, AE, McLorie, GA, et al. Iliac osteotomy: A model to compare the options in bladder and cloacal exstrophy reconstruction. J Urol. 1994; 151:182–187.
120. Schmidt, AH, Keenen, TL, Tank, ES, et al. Pelvic osteotomy for bladder exstrophy. J Pediatr Orthop. 1993; 13:214–219.
121. Frey, P. Bilateral anterior pubic osteotomy in bladder exstrophy closure. J Urol. 1996; 156:812–815.
122. Kajbafzadeh, AM, Tajik, P. A novel technique for approximation of the symphysis pubis in bladder exstrophy without pelvic osteotomy. J Urol. 2006; 175:692–698.
123. Lee, C, Reutter, HM, Grässer, MF, et al. Gender-associated differences in the psychosocial and developmental outcome in patients affected with the bladder exstrophy-epispadias complex. BJU Int. 2006; 97:49–353.
124. Reiner, WG, Gearhart, JP, Jeffs, R. Psychosexual dysfunction in males with genital anomalies: Late adolescence, Tanner stages IV to VI. J Am Acad Child Adolesc Psychiatry. 1999; 38:865–872.
125. Reiner, WG, Gearhart, JP, Kropp, B. Suicide and suicidal ideation in classic exstrophy. J Urol. 2008; 180:1661–1664.
126. Wilson, CJ, Pistrang, N, Woodhouse, CR, et al. The psychosocial impact of bladder exstrophy in adolescence. J Adolesc Health. 2007; 41:504–508.
127. Wilson, C, Christie, D, Woodhouse, CR. The ambitions of adolescents born with exstrophy: a structured survey. BJU Int. 2004; 94:607–612.
128. Woodhouse, CR, Ransley, PG, Williams, DI. The patient with exstrophy in adult life. Br J Urol. 1983; 55:632–635.
129. Woodhouse, CR. The management of erectile deformity in adults with exstrophy and epispadias. J Urol. 1986; 135:932–935.
130. Gobet, R, Weber, D, Horst, M, et al. Long-term followup (37 to 69 years) in patients with bladder exstrophy treated with ureterosigmoidostomy: Psychosocial and psychosexual outcomes. J Urol. 2009; 182:1819–1823.
131. Ricketts, S, Hunter-Smith, DJ, Coombs, CJ. Quality of life after penile reconstruction using the radial forearm flap in adult bladder exstrophy patients – technique and outcomes. ANZ J Surg. 2011; 81:52–55.
132. Ben-Chaim, J, Jeffs, RD, Reiner, WG, et al. The outcome of patients with classic bladder exstrophy in adult life. J Urol. 1996; 155:1251–1252.
133. Gambhir, L, Reutter, H, Ludwig, M. Successful assisted reproduction in adult males with bladder extrophy-epispadias complex. Eur J Obstet Gynecol Reprod Biol. 2008; 139:259–260.
134. D’Hauwers, KW, Feitz, WF, Kremer, JA. Bladder exstrophy and male fertility: pregnancies after ICSI with ejaculated or epididymal sperm. Fertil Steril. 2008; 89:387–389.
135. Woodhouse, CR, Hinsch, R. The anatomy and reconstruction of the adult female genitalia in classical exstrophy. Br J Urol. 1997; 79:618–622.
136. Woodhouse, CR. The gynaecology of exstrophy. BJU Int. 1999; 83:34–38.
137. Ebert, AK, Falkert, A, Hofstädter, A, et al. Pregnancy management in women within the bladder-exstrophy-epispadias complex (BEEC) after continent urinary diversion. Arch Gynecol Obstet. 2011; 284:1043–1046.
138. Nikolov, A, Markov, P, Chanachev, S. [Pregnancy and delivery in patients with surgically corrected congenital extrophy of the bladder, through the operations of the continent urinary derivation]. Akush Ginekol (Sofiia). 2011; 50:60–63.
139. Rose, CH, Rowe, TF, Cox, SM, et al. Uterine prolapse associated with bladder exstrophy: surgical management and subsequent pregnancy. J Matern Fetal Med. 2000; 9:150–152.
140. Spencer, R. Exstrophia Splanchnica (Exstrophy of the Cloaca). Surgery. 1965; 57:751–766.
141. Davidoff, AM, Hebra, A, Balmer, D, et al. Management of the gastrointestinal tract and nutrition in patients with cloacal exstrophy. J Pediatr Surg. 1996; 31:771–773.
142. Warren, B. Exstrophy of the Cloaca. In: Ashcraft K, Holder T, eds. Pediatric Surgery. Philadelphia: W.B. Saunders Co.; 1993:402–412.
143. Sponseller, PD, Bisson, LJ, Gearhart, JP, et al. The anatomy of the pelvis in the exstrophy complex. J Bone Joint Surg Am. 1995; 77:177–189.
144. Lund, DP, Hendren, WH. Cloacal exstrophy: Experience with 20 cases. J Pediatr Surg. 1993; 28:1360–1369.
145. Hesser, JW, Murata, Y, Swalwell, CI. Exstrophy of cloaca with omphalocele: Two cases. Am J Obstet Gynecol. 1984; 150:1004–1006.
146. Howell, C, Caldamone, A, Snyder, H, et al. Optimal management of cloacal exstrophy. J Pediatr Surg. 1983; 18:365–369.
147. Hurwitz, RS, Manzoni, GA, Ransley, PG, et al. Cloacal exstrophy: A report of 34 cases. J Urol. 1987; 138:1060–1064.
148. Diamond, DA, Jeffs, RD. Cloacal exstrophy: A 22-year experience. J Urol. 1985; 133:779–782.
149. Lee, RS, Grady, R, Joyner, B, et al. Can a complete primary repair approach be applied to cloacal exstrophy? J Urol. 2006; 176:2643–2648.
150. Silver, RI, Sponseller, PD, Gearhart, JP. Staged closure of the pelvis in cloacal exstrophy: first description of a new approach. J Urol. 1999; 161:263–266.
151. Ben-Chaim, J, Peppas, DS, Spnseller, PD, et al. Applications of osteotomy in the cloacal exstrophy patient. J Urol. 1995; 154:865–867.
152. Husmann, DA, Vandersteen, DR, McLorie, GA, et al. Urinary continence after staged bladder reconstruction for cloacal exstrophy: The effect of coexisting neurological abnormalities on urinary continence. J Urol. 1999; 161:1598–1602.
153. Gearhart, JP, Mathews, R, Taylor, S, et al. Combined bladder closure and epispadias repair in the reconstruction of bladder exstrophy. J Urol. 1998; 160:1182–1190.
154. Mathews, R, Jeffs, RD, Reiner, WG, et al. Cloacal exstrophy–improving the quality of life: The Johns Hopkins experience. J Urol. 1998; 160:2452–2456.
155. Gearhart, JP, Jeffs, RD. Techniques to create urinary continence in the cloacal exstrophy patient. J Urol. 1991; 146:616–618.
156. Mitchell, ME, Brito, CG, Rink, RC. Cloacal exstrophy reconstruction for urinary continence. J Urol. 1990; 144:554–558.
157. Reiner, WG, Gearhart, JP. Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. N Engl J Med. 2004; 350:333–341.
158. Husmann, DA, McLorie, GA, Churchill, BM, et al. Management of the hindgut in cloacal exstrophy: Terminal ileostomy versus colostomy. J Pediatr Surg. 1988; 23:1107–1113.
159. Georgeson, KE, Breaux, CW, Jr. Outcome and intestinal adaptation in neonatal short-bowel syndrome. J Pediatr Surg. 1992; 27:344–350.
160. Figueroa-Colon, R, Harris, PR, Birdsong, E, et al. Impact of intestinal lengthening on the nutritional outcome for children with short bowel syndrome. J Pediatr Surg. 1996; 31:912–916.