CHAPTER 213 Birth Brachial Plexus Injury
Pediatric obstetric brachial plexus palsy results from injury to one or more cervical and thoracic nerve roots (C5-T1) before, during, or after the birth process. The incidence of this injury has been estimated to range from 0.19 to 2.5 per 1000 live births1,2 but has declined significantly over the past century as a result of early recognition of risk factors, improved obstetric technique, and an increased rate of cesarean section.1,3–5 A recent 3-year review of more than 11 million births in the United States revealed an incidence of 1.51 per 1000 live births.6
Most injuries are mild and improve after conservative management,3,7 and some authors have concluded that complete recovery occurs in 70% to 95% of cases.3,5,8,9 However, several current studies have portrayed a more dismal spontaneous recovery rate, with close to 60% of patients experiencing permanent impairment.10,11
In the past, injuries that failed to improve were thought not to be amenable to surgical treatment.12 Although many controversies remain, it is clear that with proper patient selection, timing of surgical intervention, and implementation of microsurgical techniques, pediatric brachial plexus palsies can be treated surgically.
Surgical repair will result in improved function of the arm, not return of complete motor strength. Even though only a few hundred axons seem to be sufficient to reinnervate poorly differentiated muscles such as the biceps, thousands are required to restore the delicate balance of the intrinsics of the hand.13 It is thus unlikely that the hand will regain complete motor function in patients with severe brachial plexus injury.
History
Smellie, a London obstetrician, is credited with the first medical description of a brachial plexus injury in 1768.14 In 1872, Duchenne in his book Traite de l’Eelectrisation Localisee et de son Application à la Pathologie et à la Thérapeutique described traction during birth as the mechanism of injury.15 Erb in 1874 reported that weakness of the deltoid, biceps, coracobrachialis, and brachioradialis muscles was caused by disruption of the C5 and C6 nerve roots.16 The location where the C5 and C6 nerve roots join to form the upper trunk is thus called Erb’s point. The much less common lower brachial plexus palsy (C8-T1) was described by Klumpke in 1885.17
Surgery for obstetric brachial plexus injury was introduced in 1903 by Kennedy, who reported on three patients. At 2 months of age, his patients underwent resection of C5-6 neuromas with direct suturing of the ends, and favorable results were achieved.18 Additional reports during the early 1900s resulted in many surgeons advocating early brachial plexus surgery in children who did not improve spontaneously.19–21
In 1925, Sever concluded that there was no difference in outcome between surgically treated and conservatively managed patients after examining 1100 cases of obstetric brachial plexus palsy.12 Sever’s results, coupled with the relatively high morbidity and mortality rates associated with surgery during the first part of the century, led to almost complete abandonment of brachial plexus surgery for nearly 5 decades.
With the advent of microsurgical techniques, the operating microscope, and modern pediatric anesthesia, resurgence of interest in the surgical treatment of brachial plexus injuries ensued in the 1970s. The reports of Gilbert and Tassin demonstrating favorable outcomes after direct repair of obstetric brachial plexus injury22,23 are largely responsible for the increasing use of surgical treatment in the past 3 decades.
Pathophysiology
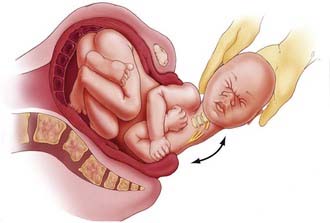
The traumatic origin of birth brachial plexus injury is considered to be a result of excessive traction applied to nerves secondary to shoulder dystocia, the use of extreme or improper traction, and hyperextension of the arms during breech delivery.7 Damage to the upper roots of the brachial plexus usually occurs during vertex delivery when shoulder dystocia necessitates excessive lateral flexion of the neck with the arm in adduction to free the shoulder from the pubic arch (Fig. 213-1). The right arm is involved more frequently because of the more common left occiput–anterior position of the descending fetus.24 During breech deliveries, the upper roots are also more commonly affected.25,26 One percent of obstetric palsies have occurred after cesarean section.27
A number of studies have identified risk factors for these injuries, including high birth weight (>4 kg), shoulder dystocia, prolonged labor, primiparity, grand multiparity, instrument deliveries, breech presentation, siblings with obstetric palsy, maternal age (>35 years), maternal pelvic anatomy, and maternal gestational diabetes.28–33 Although some patients with birth brachial plexus injury will have one or more risk factors, other will not have any. The last point has led many to raise questions regarding the etiology of this condition.
There appears to be a lack of evidence of identifiable risk factors for neonatal brachial plexus palsy.34–38 Although shoulder dystocia is the most common risk factor identified, only 17% of patients with neonatal brachial plexus palsy in the largest series to date had this risk factor.6 The same study found that only 46% of infants with brachial plexus palsy had one or more identifiable risk factors.6 An example of patients born without risk factors include uncomplicated vaginal deliveries of infants with smaller than average birth weight.39,40 These reports demonstrate that not all permanent brachial plexus injury is due to physician traction.39 Some authors speculate that individual infants have inherent biologic variability in susceptibility to brachial plexus palsy.41
Bilateral injuries are rare (4%) and occur more commonly with breech presentation.25,42 Neonatal brachial plexus injuries can be associated with clavicle and humerus fractures, facial nerve injury, cephalohematoma, and torticollis, but these conditions do not appear to portend a worse prognosis.
Natural History
As mentioned previously, recent studies indicate a good prognosis for patients, with 70% to 100% achieving complete spontaneous recovery (Table 213-1).3,5,9,43–46 Over the past 3 to 4 decades, the rate of recovery has been used to predict which patients will have full spontaneous recovery and which will have incomplete recovery, thus identifying patients who could benefit from surgical reconstruction of the brachial plexus. Poor prognostic features after obstetric brachial plexus injury include total plexopathy and lower plexus injury, typically characterized by Horner’s syndrome and increased root avulsion.26,45,47,48 These injuries usually result in incomplete recovery. In general, injuries to the upper plexus are thought to be less severe and carry a better prognosis.
Infants who have complete recovery of motor function will show improvement in strength as early as 2 weeks of age, with normalization of strength complete by 12 to 18 months.49 Assessment of strength in the biceps, triceps, and deltoid muscle groups by 6 months of age was an excellent predictor of outcome in our series of patients who experienced complete recovery.46 Each child recovered to 3/5 strength in all three muscle groups by 5.5 months. Infants who show no signs of recovery and have a flaccid upper extremity at 2 months of age have suffered severe total brachial plexus palsy, and their long-term outcome is poor.21,22,50–52 Some investigators have noted that if an infant is to have functional use of an extremity, some recovery must be seen by 4 months of age.8,9,26,49 We found that patients who experience persistent moderate weakness (less than antigravity strength of the biceps at 6 months of age) have unsatisfactory outcomes with observation.46 This study and others have concluded that biceps muscle function at the end of the third month is the most important indicator of functional recovery of the affected limb.45,46,53 Michelow and colleagues reported that favorable outcome was correctly predicted by biceps function at 3 months in 87% of patients.45 The prediction rate was improved to 95% if biceps function along with extensor function of the elbow, wrist, thumb, or finger was used in the clinical assessment. Tassin’s study monitored 20 patients prospectively and found that if infants do not have biceps function by 3 months of age, limited shoulder movement is a probable consequence.53 Recently, this particular finding has come into question inasmuch as Smith and coauthors reported good long-term shoulder function in patients with absent biceps function.54 However, the prognostic value of absence of biceps function on improvement in shoulder function has yet to be determined.
Clinical Findings
Brachial plexus injury patterns vary and can be classified by two distinct schemes: anatomic localization and severity of pathology. Anatomic localization is used most by clinicians and allows surgical planning. Pathologic severity is difficult to apply clinically and is based on Seddon and colleagues’ original work, which defined the terms neurapraxia (stretch injury without axonal disruption), axonotmesis (physical disruption of axons or nerve fascicles), and neurotmesis (complete disruption of a nerve trunk or root).55
There are four major anatomic patterns of injury. The first is a purely upper brachial plexus lesion involving only C5 and C6; it results in weakness of the deltoid and biceps, with sparing of the triceps and distal musculature. It has been suggested that such patients typically demonstrate good spontaneous recovery.56,57 The second and most common form is classic Erb’s palsy involving C5-7. This pattern is characterized by an extended arm, internally rotated shoulder, volar-flexed wrist, and extended fingers resulting in the “waiter’s tip” posture. The third type of injury is a total plexopathy involving C5-T1, which accounts for 20% of obstetric palsies.57 Physical examination reveals a flaccid, insensate arm with marbled skin secondary to vasomotor disturbance. Damage to the spinal cord may have occurred. The last type of injury pattern seen is Klumpke’s palsy, which involves only the lower (C8-T1) brachial plexus. Clinically, it is manifested as an inability to pronate the forearm, hand paralysis with a “clawhand” posture, and Horner’s syndrome. Isolated lower brachial plexus injury is exceedingly rare and reportedly accounts for less than 1% of obstetric palsies.56 We have not encountered such a case in our own patients. Patients with injury to the lower brachial plexus are less likely to recover useful function of the arm with conservative management than are those who suffer upper brachial plexus injury.
Other causes of arm immobility or paralysis, both reversible and irreversible, should be considered in the differential diagnosis, including epiphyseal separation of the humeral head, fracture of the clavicle or humerus, septic arthritis of the upper limb, birth-related spinal cord injury, and congenital varicella of the upper limb.58
Patient Evaluation
Since 1991, evaluation of patients with obstetric brachial plexus palsy at our institution has been performed in a multidisciplinary brachial plexus clinic by a team consisting of a pediatric neurosurgeon, pediatric neurologist, orthopedic surgeon, physical and occupational therapists, electrophysiologist, and nurse coordinator. A detailed obstetric and birth history is taken, with attention to the predisposing factors discussed previously. Associated injuries such as rib, spinal, clavicle, or humeral fractures are noted on plain radiographs. Clinically observed asymmetry of chest wall expansion and chest x-ray evidence of an elevated hemidiaphragm denote phrenic nerve injury. The patient is assessed for evidence of facial paresis or Horner’s syndrome. Passive range of motion of the involved arm, especially the shoulder, is evaluated. Motor strength is assessed with the simplified British Medical Council scale for muscle testing (0 to 5 scale). The Mallet score, which documents functional changes in the shoulder and arm, requires patient cooperation and is used additionally in patients older than 2 years.59 Sensory examination is difficult, although reaction to pinch is helpful. Sensory loss is sometimes evidenced by self-mutilation (i.e., biting) of fingers.
The infant is re-examined at 4 weeks of age. Frequently, recovery is complete or nearly complete, which signifies a neurapraxic injury. At this point, if there is still total palsy associated with Horner’s sign, the outlook is poor, and parents are informed of the possible need for surgical intervention. If hand function is improving without evidence of shoulder recovery, the patient is managed conservatively because there is still opportunity for recovery.60 Patients are examined on a monthly basis. Infants who make progressive recovery and have antigravity or greater than antigravity strength in the biceps, triceps, or deltoid muscles by 3 to 4 months of age continue to be monitored expectantly.46 Infants displaying less than antigravity strength in each of these muscles are referred for neuroimaging and electrophysiologic studies in anticipation of surgery. If the deltoid, biceps, or triceps muscle still has less than antigravity strength at 6 months of age, we recommend brachial plexus surgery (Table 213-2). Braces are no longer advocated because of the propensity for contracture.25
| Flaccid upper extremity (≥2 months of age) |
| Less than 3/5 motor strength in the deltoid, biceps, and triceps, with the presence of Horner’s syndrome on physical examination or pseudomeningocele on magnetic resonance imaging (≥3 months of age) |
| Less than 3/5 strength in the deltoid, biceps, and triceps in the affected extremity (≥6 months of age) |
When surgery is being considered, electromyography (EMG) with nerve conduction studies is delayed until the third month. If an intrauterine lesion is suspected, EMG is performed within a week after delivery to document denervation.61,62 EMG can provide useful information on the extent and distribution of brachial plexus injury and the recovery pattern. In addition, nerve root avulsion can be confirmed by normal sensory conduction in the presence of severe motor weakness. The usefulness of EMG in postoperative follow-up of patients is unclear. In infants, it takes only a small number of intact axons to provoke an electrical response that may not be associated with clinical recovery.
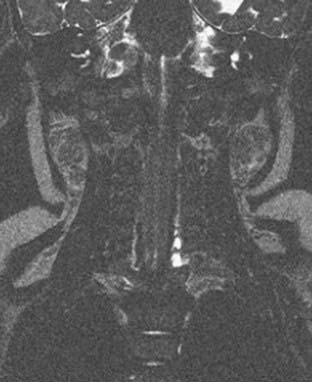
Preoperative cervical magnetic resonance imaging (MRI) evaluates nerve root avulsion.63–65 The presence of pseudomeningoceles has been associated with nerve root avulsion; however, this finding is not pathognomonic and should instead be considered a sign of root damage inasmuch as 15% of pseudomeningoceles are not associated with complete nerve root avulsion.66 In addition, 20% of avulsed nerve roots may not have an associated pseudomeningocele.67 We use fast spin echo MRI for preoperative neuroimaging because it is less invasive and provides data comparable to that of computed tomographic myelography for operative planning (Fig. 213-2).47 MRI does not clearly delineate nerve roots within the pseudomeningocele. In cases of total palsy, MRI allows imaging of the spinal cord.
Surgical Treatment
Exposure of the Brachial Plexus
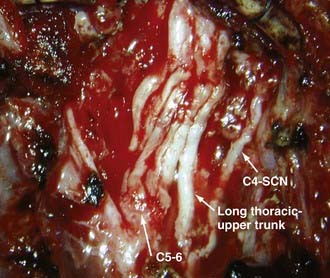
Under general anesthesia with a short-acting neuromuscular agent, the patient is placed supine with a shoulder roll to elevate the clavicular region, and the head is turned to the contralateral side. The neck, chest, affected upper extremity, and both lower extremities are prepared, the incision marked, and drapes applied. In an infant, an arm board is not usually necessary. The entire affected upper extremity is kept available for visual inspection of muscle contraction in response to electrical stimulation during surgery. The supraclavicular portion of the incision begins a fingerbreadth beneath the mastoid tip and courses along the posterior border of the sternocleidomastoid muscle to the clavicle. For infraclavicular exposure, the incision then turns laterally along the superior border of the clavicle to reach the deltopectoral groove. At this point, it curves inferiorly along the groove and anterior axillary fold (Fig. 213-3). Repair of the upper roots is accomplished purely through a supraclavicular approach, whereas total palsy may require a combined approach.
Resection of Neuromas
Surgical techniques used for birth brachial plexus injury include neurolysis, neurotization, and nerve grafting, either alone or in combination. External neurolysis alone is performed when resection of neuroma is deemed unnecessary. We do not perform internal neurolysis because of its unproven efficacy. A critical issue involves indications for resection of neuromas. The feasibility of using intraoperative nerve action potentials as a prognostic study has been determined in the adult population but not in obstetrically injured infants.68,69 Laurent and colleagues use the compound muscle action potential in response to stimulation both proximal and distal to the neuroma to determine whether to perform neurolysis or a grafting procedure. Neuromas are resected and a graft performed if the amplitude of the compound muscle action potential drops more than 50% across the neuroma. Conversely, neurolysis is performed if the decrease is less than 50%.26,42,57 However, the validity of this approach remains to be determined. Alternatively, other authors advocate a more aggressive approach consisting of resection of all neuromas-in-continuity regardless of the presence of conducting fibers.50–52,70,71 At present, we base our decisions regarding resection of neuromas on intraoperative inspection, semiquantitative assessment of muscle contraction in response to electrical stimulation of the nerve root proximal to the neuroma, preoperative muscle strength, and MRI findings. If a ruptured root or trunk is present and electrical stimulation (up to 10 mA) elicits no or weak muscle contraction, neuromas are resected. If nerve root avulsion is present and there is minimal conduction through the remaining nerve root as assessed by the degree of muscle contraction in response to electrical stimulation of the proximal nerve root, the remaining nerve root sheath is divided. If the brachial plexus is in continuity but elicited muscle contraction is weak, neuromas are resected. If the brachial plexus is in continuity, strong muscle contraction is elicited, and the neuromas are not extensive, no resection is performed. If the infant has some hand and wrist function, the C8-T1 roots and lower trunk are left alone.
Repair Procedures
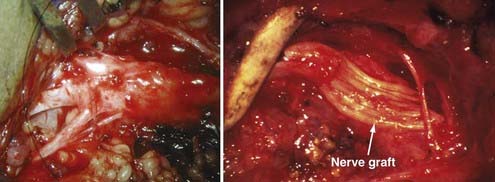
Various grafting and neurotization procedures are needed to repair the brachial plexus. The main goal of surgery for primarily upper palsy is to restore shoulder and biceps muscle function. This goal can be achieved by nerve grafting to all or part of the upper trunk, supraclavicular nerve, or axillary nerve arising from the posterior cord. The accessory nerve, stumps of the C5 or C6 roots, or both, or the C7 nerve root can be used for grafting (Fig. 213-4). In patients with total plexus injury, various combinations of nerve grafting are needed. If two or three nerve root stumps are found, the nerve roots are divided and used for grafting all trunks or cords of the brachial plexus. If only one nerve root stump is available, it is grafted to the musculocutaneous nerve, and three to four intercostal nerves are connected to the ulnar nerve. Nerve grafts should be prepared 10% to 15% longer than the measured length to account for later contraction. Anastomosis can be completed with 9-0 Prolene epineural sutures or fibrin glue. We prefer to combine suture with fibrin glue because the results of repair with glue are equivalent or possibly slightly better than the use of suture alone. The pectoralis major is reinserted with nonabsorbable suture, and the wound is closed in layers, including the platysma muscle, in routine fashion. The shoulder is held in an adducted position over the trunk with a large elastic bandage.
Neurotization is required in avulsion injuries. It involves nerve crossover or nerve transfer such that an uninjured neighboring donor nerve is connected directly or by grafts to a distal portion of a nonfunctioning nerve. The spinal accessory, phrenic, intercostal, medial pectoral, thoracodorsal, long thoracic, and subscapular nerves have been used for neurotization. When the spinal accessory nerve is used, one should use the nerve distal to the first branch to the trapezius muscle to avoid massive denervation of the trapezius. Partial denervation of the trapezius does not cause another deficit in the patient because it usually alleviates the elevated shoulder, which most adult patients consider an aesthetic benefit.13,72 Although phrenic nerve transfer is safe in adult patients with normal diaphragmatic function, it is not recommended in infants because of their immature respiratory system and increased risk for fatal pulmonary complications. The efficacy of neurotization of the long thoracic nerve and the thoracodorsal, subscapularis, and pectoral nerves in infants is unknown.
Complications
Potential complications include loss of existing motor function, phrenic nerve injury, leakage of cerebrospinal fluid, pneumothorax, thoracic duct injury (left side), vascular injury to the carotid artery or the jugular or subclavian veins, pseudoarthrosis of the clavicle associated with osteotomy of the clavicle, and wound infection. Airway compromise secondary to hematoma or edema is a potential problem. The infant should be monitored for ability to clear secretions and maintain adequate airway exchange. The patient’s ability to swallow and the presence of a gag reflex should be checked preoperatively because vagal and laryngeal nerve function may be altered after surgical manipulation.73
Surgical Outcome
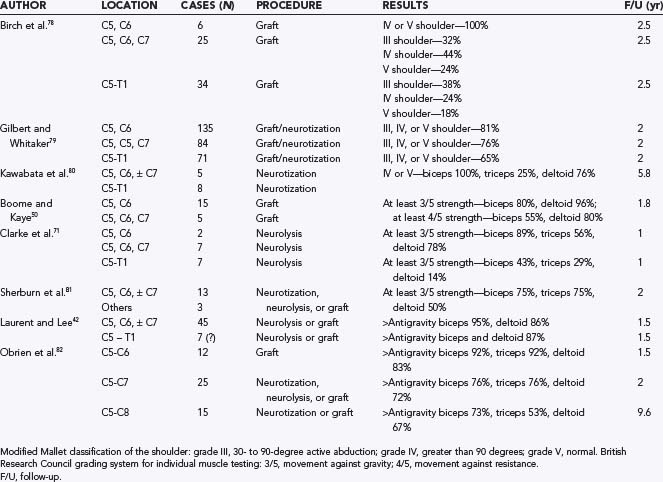
Surgical reinnervation is advocated to avoid permanent changes in the denervated muscle and developing skeletal system that can result in long-term orthopedic sequelae.74,75 Surgical treatment aims to achieve (1) stabilization of the shoulder by reactivation of the supraspinatus and deltoid, (2) establishment of elbow flexion with reactivation of the biceps, and (3) in patients with a nonfunctional hand, restoration of median nerve sensory function for future secondary reconstruction procedures.76 Some surgeons believe that primary reinnervation should be considered up to 18 months of age, after which time secondary procedures should be performed.57 For infants who are appropriate surgical candidates, surgery offers a chance for better long-term functional recovery. A number of authors have shown that neurologic improvement occurs in 75% to 95% of patients undergoing surgical reconstruction of the brachial plexus, with the majority attaining antigravity strength in the shoulder or elbow, or both.23,42,71,76,77 Boome and Kaye reported that greater than 95% of deltoid and 80% of biceps muscles attain antigravity strength after surgery.50 Laurent and colleagues found that 85% to 95% of patients recover antigravity strength above the elbow, along with 50% to 70% recovery below the elbow.42,57 In our most recent series, results after surgery for upper plexus lesion were better than those for lesions of the lower plexus (Table 213-3).42,50,71,78–82
Secondary Reconstruction
Secondary reconstructions are performed in patients with chronic obstetric brachial plexus lesions that are not amenable or fail to respond to microsurgical reconstruction. It is required because of the muscle imbalance secondary to unopposed contraction of innervated muscle groups. Abnormal stress placed on the bones and joints of the upper limb, with resultant progressive shoulder and elbow bony deformities, will result and require operative intervention to maximize upper extremity function. Frequently, patients will have an internally rotated and adducted shoulder after upper plexus lesions.83 Joint contractures are common in patients after neonatal brachial plexus injury. Secondary procedures to treat the affected limb include arthrodesis, tenodesis, muscle releases, pedicled muscle/tendon transfers, and free neurovascular tissue transfer.
Conclusion
The incidence of birth brachial plexus injury has remained constant over the past several decades. Patients should be given a trial of conservative therapy with frequent and close follow-up. Motor strength examination at 3 months of age is predictive of good outcome if antigravity strength of the deltoid, biceps, and triceps is present. Strength testing at 6 months of age is predictive of poor outcome if antigravity strength in these muscles is not attained, and surgical treatment is recommended. Only careful clinical examination and detailed understanding of the anatomy of the brachial plexus will allow a carefully planned operation. MRI is recommended over computed tomographic myelography for preoperative evaluation. Intraoperative electrophysiology can help determine repair strategies, and knowledge of historically successful donor graft sites is important in determining surgical repair options. Each operation must be tailored to the individual patient’s needs. This approach avoids operating unnecessarily on patients who are destined to recover spontaneously, and yet it is early enough to avoid permanent changes, such as contractures, in denervated and developing skeletal muscle.46,84,85
al-Qattan MM, Clarke HM, Curtis CG. Klumpke’s birth palsy. Does it really exist? J Hand Surg Br. 1995;20:19.
al-Qattan MM, el-Sayed AA, al-Kharfy TM, et al. Obstetrical brachial plexus injury in newborn babies delivered by caesarean section. J Hand Surg Br. 1996;21:263.
Ashley WW, Baty JD, Hollander T, et al. Long-term motor outcome analysis using a motor score composite following surgical brachial plexus repair. J Neurosurg. 2007;106(4 suppl):276-281.
Belzberg AJ, Dorsi MJ, Storm PB, et al. Surgical repair of brachial plexus injury: a multinational survey of experienced peripheral nerve surgeons. J Neurosurg. 2004;101:365-376.
Birch R, Bonney G, Wynn Parry CB. Birth lesions of the brachial plexus. In: Birch R, Bonney G, Wynn Parry CB, editors. Surgical Disorders of the Peripheral Nerves. Edinburgh: Churchill Livingstone; 1998:209-233.
Boome RS, Kaye JC. Obstetric traction injuries of the brachial plexus. Natural history, indications for surgical repair and results. J Bone Joint Surg Br. 1988;70:571.
Clarke HM, Al-Qattan MM, Curtis CG, et al. Obstetrical brachial plexus palsy: results following neurolysis of conducting neuromas-in-continuity. Plast Reconstr Surg. 1996;97:974.
Foad SL, Mehlman CT, Ying J. The epidemiology of neonatal brachial plexus palsy in the United States. J Bone Joint Surg Am. 2008;90:1258.
Francel PC, Koby M, Park TS, et al. Fast spin-echo magnetic resonance imaging for radiological assessment of neonatal brachial plexus injury. J Neurosurg. 1995;83:461.
Gilbert A, Razaboni R, Amar-Khodja S. Indications and results of brachial plexus surgery in obstetrical palsy. Orthop Clin North Am. 1988;19:91.
Gilbert A, Whitaker I. Obstetrical brachial plexus lesions. J Hand Surg Br. 1991;16:489.
Hoeksma AF, ter Steeg AM, Nelissen RG, et al. Neurological recovery in obstetric brachial plexus injuries: an historical cohort study. Dev Med Child Neurol. 2004;46:76.
Kawabata H, Kawai H, Masatomi T, et al. Accessory nerve neurotization in infants with brachial plexus birth palsy. Microsurgery. 1994;15:768.
Laurent JP, Lee RT. Birth-related upper brachial plexus injuries in infants: operative and nonoperative approaches. J Child Neurol. 1994;9:111.
Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008:198.
McNeely PD, Drake JM. A systematic review of brachial plexus surgery for birth-related brachial plexus injury. Pediatr Neurosurg. 2003;38:57.
Michelow BJ, Clarke HM, Curtis CG, et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675.
Noetzel MJ, Park TS, Robinson S, et al. Prospective study of recovery following neonatal brachial plexus injury. J Child Neurol. 2001;16:488.
O’Brien DF, Park TS, Noetzel MJ, et al. Management of birth brachial plexus palsy. Childs Nerv Syst. 2006;22:103.
Sherburn EW, Kaplan SS, Kaufman BA, et al. Outcome of surgically treated birth-related brachial plexus injuries in twenty cases. Pediatr Neurosurg. 1997;27:19.
1 Adler JB, Patterson RLJr. Erb’s palsy. Long-term results of treatment in eighty-eight cases. J Bone Joint Surg Am. 1967;49:1052.
2 Pollack RN, Buchman AS, Yaffe H, et al. Obstetrical brachial palsy: pathogenesis, risk factors, and prevention. Clin Obstet Gynecol. 2000;43:236.
3 Greenwald AG, Schute PC, Shiveley JL. Brachial plexus birth palsy: a 10-year report on the incidence and prognosis. J Pediatr Orthop. 1984;4:689.
4 Painter MJ, Bergman I. Obstetrical trauma to the neonatal central and peripheral nervous system. Semin Perinatol. 1982;6:89.
5 Specht EE. Brachial plexus palsy in the newborn. Incidence and prognosis. Clin Orthop Relat Res. 1975;110:32.
6 Foad SL, Mehlman CT, Ying J. The epidemiology of neonatal brachial plexus palsy in the United States. J Bone Joint Surg Am. 2008;90:1258.
7 Sjoberg I, Erichs K, Bjerre I. Cause and effect of obstetric (neonatal) brachial plexus palsy. Acta Paediatr Scand. 1988;77:357.
8 Gordon M, Rich H, Deutschberger J, et al. The immediate and long-term outcome of obstetric birth trauma. I. Brachial plexus paralysis. Am J Obstet Gynecol. 1973;117:51.
9 Hardy AE. Birth injuries of the brachial plexus: incidence and prognosis. J Bone Joint Surg Br. 1981;63:98.
10 Hoeksma AF, ter Steeg AM, Nelissen RG, et al. Neurological recovery in obstetric brachial plexus injuries: an historical cohort study. Dev Med Child Neurol. 2004;46:76.
11 Pondaag W, Malessy MJ, van Dijk JG, et al. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46:138.
12 Sever JW. Obstetric paralysis: report of eleven hundred cases. JAMA. 1925;85:1862.
13 Narakas A. Surgical treatment of traction injuries of the brachial plexus. Clin Orthop Relat Res. 1978;133:71.
14 Smellie W. Collection of Cases and Observations in Mid Wifery. Vol 2, 4th ed, 1768.
15 Duchenne G. Traité de l’électrisation localisée et de son application à la pathologie et à la thérapeutique, 3rd ed. Paris; 1872.
16 Erb W. Über eine eigentumliche Localisation von Lähmungen im Plexus brachialis. Verh Naturhist-Med Heidelberg. 1874:130.
17 Klumpke A. Contribution à l’étude des paralysies readiculaires du plexus brachial. Rev Med Paris. 1885;5:591.
18 Kennedy R. Suture of the brachial plexus in birth paralysis of the upper extremity. BMJ. 1903;11:298.
19 Clark LP, Taylor AS, Prout TP. A study on brachial birth palsy. BMJ. 1905;130:670.
20 Taylor AS. Brachial birth palsy and injuries of similar type in adults. Surg Gynecol Obstet. 1920;30:494.
21 Wyeth JA, Sharpe W. The field of neurological surgery in a general hospital. Surg Gynecol Obstet. 1917;24:29.
22 Gilbert A, Tassin JL. Obstetrical palsy: a clinical pathologic and surgical review. In: Microreconstruction of Nerve Injuries. Philadelphia: WB Saunders; 1987.
23 Gilbert A, Tassin JL. [Surgical repair of the brachial plexus in obstetric paralysis.]. Chirurgie. 1984;110:70.
24 Brown KL. Review of obstetrical palsies. Nonoperative treatment. Clin Plast Surg. 1984;11:181.
25 Eng GD, Koch B, Smokvina MD. Brachial plexus palsy in neonates and children. Arch Phys Med Rehabil. 1978;59:458.
26 Laurent JP, Lee R, Shenaq S, et al. Neurosurgical correction of upper brachial plexus birth injuries. J Neurosurg. 1993;79:197.
27 al-Qattan MM, el-Sayed AA, al-Kharfy TM, et al. Obstetrical brachial plexus injury in newborn babies delivered by caesarean section. J Hand Surg Br. 1996;21:263.
28 Marcus JR, Clarke HM. Management of obstetrical brachial plexus palsy evaluation, prognosis, and primary surgical treatment. Clin Plast Surg. 2003;30:289.
29 McNeely PD, Drake JM. A systematic review of brachial plexus surgery for birth-related brachial plexus injury. Pediatr Neurosurg. 2003;38:57.
30 Nehme A, Kany J, Sales-De-Gauzy J, et al. Obstetrical brachial plexus palsy. Prediction of outcome in upper root injuries. J Hand Surg Br. 2002;27:9.
31 Shenaq SM, Bullocks JM, Dhillon G, et al. Management of infant brachial plexus injuries. Clin Plast Surg. 2005;32:79.
32 van Ouwerkerk WJ, van der Sluijs JA, Nollet F, et al. Management of obstetric brachial plexus lesions: state of the art and future developments. Childs Nerv Syst. 2000;16:638.
33 Waters PM. Obstetric brachial plexus injuries: evaluation and management. J Am Acad Orthop Surg. 1997;5:205.
34 Dunn DW, Engle WA. Brachial plexus palsy: intrauterine onset. Pediatr Neurol. 1985;1:367.
35 Gherman RB, Ouzounian JG, Miller DA, et al. Spontaneous vaginal delivery: a risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423.
36 Ouzounian JG, Korst LM, Phelan JP. Permanent Erb palsy: a traction-related injury? Obstet Gynecol. 1997;89:139.
37 Paradiso G, Granana N, Maza E. Prenatal brachial plexus paralysis. Neurology. 1997;49:261.
38 Peterson GW, Bohr TW. Neonatal obstetric palsy a preexisting condition: two case reports. Muscle Nerve. 1995;18:1031.
39 Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198:e7.
40 Nocon JJ, McKenzie DK, Thomas LJ, et al. Shoulder dystocia: an analysis of risks and obstetric maneuvers. Am J Obstet Gynecol. 1993;168:1732.
41 Allen RH, Gurewitsch ED. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet Gynecol. 2005;105:1210.
42 Laurent JP, Lee RT. Birth-related upper brachial plexus injuries in infants: operative and nonoperative approaches. J Child Neurol. 1994;9:111.
43 Tan KL. Brachial palsy. J Obstet Gynaecol Br Commonw. 1973;80:60.
44 Jackson ST, Hoffer MM, Parrish N. Brachial-plexus palsy in the newborn. J Bone Joint Surg Am. 1988;70:1217.
45 Michelow BJ, Clarke HM, Curtis CG, et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675.
46 Noetzel MJ, Park TS, Robinson S, et al. Prospective study of recovery following neonatal brachial plexus injury. J Child Neurol. 2001;16:488.
47 Francel PC, Koby M, Park TS, et al. Fast spin-echo magnetic resonance imaging for radiological assessment of neonatal brachial plexus injury. J Neurosurg. 1995;83:461.
48 Strombeck C, Krumlinde-Sundholm L, Forssberg H. Functional outcome at 5 years in children with obstetrical brachial plexus palsy with and without microsurgical reconstruction. Dev Med Child Neurol. 2000;42:148.
49 Bennet GC, Harrold AJ. Prognosis and early management of birth injuries to the brachial plexus. Br Med J. 1976;1:1520.
50 Boome RS, Kaye JC. Obstetric traction injuries of the brachial plexus. Natural history, indications for surgical repair and results. J Bone Joint Surg Br. 1988;70:571.
51 Gilbert A, Brockman R, Carlioz H. Surgical treatment of brachial plexus birth palsy. Clin Orthop Relat Res. 1991;264:39.
52 Gilbert A, Razaboni R, Amar-Khodja S. Indications and results of brachial plexus surgery in obstetrical palsy. Orthop Clin North Am. 1988;19:91.
53 Tassin JL. Paralysies obstetricales du plexus brachial. Evolution spontanee, resultats des interventions reparatrices precoces. Paris: Universite Paris; 1983.
54 Smith NC, Rowan P, Benson LJ, et al. Neonatal brachial plexus palsy. Outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004;86:2163.
55 Seddon HJ, Medawar PB, Smith H. Rate of regeneration of peripheral nerves in man. J Physiol. 1943;102:191.
56 al-Qattan MM, Clarke HM, Curtis CG. Klumpke’s birth palsy. Does it really exist? J Hand Surg Br. 1995;20:19.
57 Shenaq SM, Berzin E, Lee R, et al. Brachial plexus birth injuries and current management. Clin Plast Surg. 1998;25:527.
58 al-Qattan MM, Thomson HG. Congenital varicella of the upper limb. A preventable disaster. J Hand Surg Br. 1995;20:115.
59 Mallet J. [Obstetrical paralysis of the brachial plexus. 3. Conclusions.]. Rev Chir Orthop Reparatrice Appar Mot. 1972;58(suppl 1):201.
60 Hentz VR, Meyer RD. Brachial plexus microsurgery in children. Microsurgery. 1991;12:175.
61 Koenigsberger MR. Brachial plexus palsy at birth: intrauterine or due to delivery trauma. Ann Neurol. 1980;8:228.
62 Mancias P, Slopis JM, Yeakley JW, et al. Combined brachial plexus injury and root avulsion after complicated delivery. Muscle Nerve. 1994;17:1237.
63 Miller SF, Glasier CM, Griebel ML, et al. Brachial plexopathy in infants after traumatic delivery: evaluation with MR imaging. Radiology. 1993;189:481.
64 Popovich MJ, Taylor FC, Helmer E. MR imaging of birth-related brachial plexus avulsion. AJNR Am J Neuroradiol. 1989;10:S98.
65 Walker AT, Chaloupka JC, de Lotbiniere AC, et al. Detection of nerve rootlet avulsion on CT myelography in patients with birth palsy and brachial plexus injury after trauma. AJR Am J Roentgenol. 1996;167:1283.
66 Carvalho GA, Nikkhah G, Matthies C, et al. Diagnosis of root avulsions in traumatic brachial plexus injuries: value of computerized tomography myelography and magnetic resonance imaging. J Neurosurg. 1997;86:69.
67 Hashimoto T, Mitomo M, Hirabuki N, et al. Nerve root avulsion of birth palsy: comparison of myelography with CT myelography and somatosensory evoked potential. Radiology. 1991;178:841.
68 Kline DG, Judice DJ. Operative management of selected brachial plexus lesions. J Neurosurg. 1983;58:631.
69 Tiel RL, Happel LTJr, Kline DG. Nerve action potential recording method and equipment. Neurosurgery. 1996;39:103.
70 Capek L, Clarke HM, Zuker RM. Endoscopic sural nerve harvest in the pediatric patient. Plast Reconstr Surg. 1996;98:884.
71 Clarke HM, Al-Qattan MM, Curtis CG, et al. Obstetrical brachial plexus palsy: results following neurolysis of conducting neuromas-in-continuity. Plast Reconstr Surg. 1996;97:974.
72 Allieu Y, Cenac P. Is surgical intervention justifiable for total paralysis secondary to multiple avulsion injuries of the brachial plexus? Hand Clin. 1988;4:609.
73 Brucker J, Laurent JP, Lee R, et al. Brachial plexus birth injury. J Neurosci Nurs. 1991;23:374.
74 Akasaka Y, Hara T, Takahashi M. Free muscle transplantation combined with intercostal nerve crossing for reconstruction of elbow flexion and wrist extension in brachial plexus injuries. Microsurgery. 1991;12:346.
75 Pollock AN, Reed MH. Shoulder deformities from obstetrical brachial plexus paralysis. Skeletal Radiol. 1989;18:295.
76 Terzis JK, Liberson WT, Levine R. Obstetric brachial plexus palsy. Hand Clin. 1986;2:773.
77 Piatt JHJr, Hudson AR, Hoffman HJ. Preliminary experiences with brachial plexus exploration in children: birth injury and vehicular trauma. Neurosurgery. 1988;22:715.
78 Birch R, Bonney G, Wynn Parry CB. Birth lesions of the brachial plexus. In: Birch R, Bonney G, Wynn Parry CB, editors. Surgical Disorders of the Peripheral Nerves. Edinburgh: Churchill Livingstone; 1998:209-233.
79 Gilbert A, Whitaker I. Obstetrical brachial plexus lesions. J Hand Surg Br. 1991;16:489.
80 Kawabata H, Kawai H, Masatomi T, et al. Accessory nerve neurotization in infants with brachial plexus birth palsy. Microsurgery. 1994;15:768.
81 Sherburn EW, Kaplan SS, Kaufman BA, et al. Outcome of surgically treated birth-related brachial plexus injuries in twenty cases. Pediatr Neurosurg. 1997;27:19.
82 O’Brien DF, Park TS, Noetzel MJ, et al. Management of birth brachial plexus palsy. Childs Nerv Syst. 2006;22:103.
83 Al-Zahrani S. Combined Sever’s release of the shoulder and osteotomy of the humerus for Erb’s palsy. J Hand Surg Br. 1997;22:591.
84 Carlstedt T, Cullheim S. Spinal cord motoneuron maintenance, injury and repair. Prog Brain Res. 2000;127:501.
85 Jahnke AHJr, Bovill DF, McCarroll HRJr, et al. Persistent brachial plexus birth palsies. J Pediatr Orthop. 1991;11:533.