8 Biomechanics of the Senescent Spine
KEY POINTS
Introduction
Biomechanical measurements can be affected by numerous indicators, and it is important to distinguish which are related to global measures, for example, body mass index, and which may be relevant specifically to the local spinal elements, e.g., friability of a vertebral body. Two distinct but related indicators should be evaluated with spinal pathologies: the advancement of age and degenerative changes resulting in anatomical transmutation that potentially leads to abnormal loading of the spine. Anatomical changes may be attributed to the primary degenerative conditions associated with age. Miller et al reported an approximate 10% occurrence of severely degenerated intervertebral discs in 50-year-old males, with an increase to 60% in 70-year-olds.1 The degenerative conditions result in several anatomical changes and, of particular importance to an aging population, is the potential for constriction of the spinal canal diameter. The cause of the constriction may be from a single specific etiology or from a combination of factors, including spinal canal stenosis, disc herniation, osteophyte growth into the canal, hypertrophy of the ligamentum flavum, and calcification of the posterior longitudinal ligament and the ligamentum flavum.
Aging and Degenerative Changes on the Effects of Biomechanical Range of Motion
The relationship between age, degeneration, and RoM has been studied both in human cadaveric FSU testing and in clinical studies. The instability of the lumbar spine was proposed by Kirkaldy-Willis and Farfan to be categorized into three diskrete stages of degenerative change. In order of progression, the clinical assessment of the lumbar spine categorized pathologic changes as temporary dysfunction, the unstable phase, and finally, stabilization.2 Well-defined, controlled, biomechanical testing and clinical studies involving well-documented patient profiles have tested various aspects of this initial hypothesis on spinal instability.
Traditional methods of comparing the effects of age, degeneration, or subsequent treatments have been subjected to biomechanical characterization through the flexibility test method. The methodology of flexibility testing has been well described in the literature, originating with Panjabi’s early description of load input utilizing pure moments.3 Subsequent comparisons, particularly relevant in fixation instrumentation via flexibility testing, have described the performance of these devices relative to the intact spine. often with high mean age donor specimen. Additionally, comparisons between fixation treatments, as well as comparison of fixation treatments from laboratory to laboratory, have been possible. The standardization of the pure moment test protocol by Goel et al has contributed to the repeatability despite biologic variability inherent in cadaveric testing.4
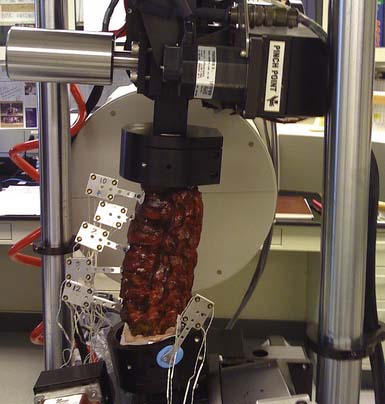
It is important to understand the rationale of the test methodology when considering clinically relevant biomechanical studies. The basis of the traditional flexibility test, or pure moment testing, is to apply a uniform moment across all FSUs in a given specimen. Figure 8-1 is an example of a mounted lumbar specimen that will be subjected to flexion-extension bending. The ability to extrapolate the biomechanical effects to clinical outcomes is dependent on study design and successful interpretation of the resulting data. Clinically relevant biomechanical testing in the appropriate form is an important parameter for clinicians to consider in the triage of patients with spinal pathologies.
In a cadaveric human lumbar study by Mimura et al, the authors were able to demonstrate a statistically significant difference between RoM in lateral bending, but not in flexion-extension bending, for intervertebral discs with degenerative ratings in whole lumbar specimens under a flexibility protocol.5 Biomechanical studies involving age as a variable in the analysis are often shown to be correlated to RoM. Board et al reported on the results of a human cadaveric cervical biomechanical study. Their results suggest that biomechanical flexion-extension in pure moment loading decreases the RoM as a function of the age of the specimen.6 These findings agreed with published articles, when extrapolated and compared to equivalent test parameters. In a similar clinical evaluation on bending in the cervical spine involving only males, Sforza et al concluded that young adult males exhibited statistically significant larger flexion-extension RoM compared to their middle-aged counterparts who participated in the study.7 Similarly, in a clinical cervical study involving multiple factors including both age and degeneration, Simpson et al determined age to be the most significant factor on RoM.8
Confounding these results are clinical considerations in which surgical treatment may be warranted, but subsequent conditions and outcomes related to the specific implant or procedure for the elderly patient may not be clear. For example, symptomatic spine pathology resulting in instability of a FSU and suitable for an instrumented fusion procedure must consider the interaction of the hardware and the patient’s local host tissue. In addition to global metrics of bone quality, the local bone purchase dependent upon the microstructural integrity of bony trabeculation at the index FSU may have undergone severe anatomical changes. These differences affect the load response, exacerbate degenerative pathologies, and require additional considerations for the type of instrumentation suitable for the patient preoperatively. Intraoperatively, additional factors may further alter the structural integrity of the FSU, for example, endplate preparation or pilot hole drilling combined with tapping.
Osteoporosis, Aging, and Biomechanical Properties
The use of clinical guidelines based primarily on BMD results has been widely accepted. The ability to identify patients with high risk of fracture via low BMD measurements, defined by T-scores of −2.5 or lower, and to subsequently provide effective pharmacological treatments, has been proved through large double-blinded placebo-controlled trials. Several challenges remain in identifying low-risk population and ultimately a means in cost effectively managing fracture risk. In an examination of 149,524 postmenopausal women 50 years of age and older with fractures, 82% had T-scores above the threshold criterion of −2.5.9 Thus, it has been suggested that the value of BMD would be enhanced with additional risk factors for improved diagnostic capabilities.
BMD and Implications on Instrumented Procedures
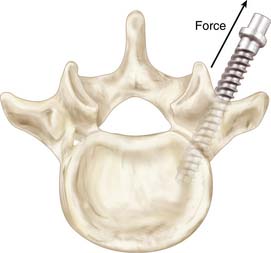
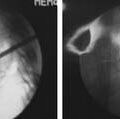
Biomechanical measures used to test screw-bone interfaces have been evaluated in a number of different ways. Axial pull-out strength has been frequently reported in the literature, including in human cadaveric spines that would be considered osteoporotic. Figure 8-2 illustrates a common test method for determining axial screw-bone interface strength. However, cyclical loading has been suggested to mimic more realistic modes of failure for implanted constructs. Studies have examined bending failure as an appropriate method of loading.10
The limitation of any test protocol is the ability to directly compare against native human conditions. Several of the published studies have considered various test materials including both cadaveric and synthetic test specimens. The utility of such tests should still be recognized but it must be tempered with an appropriate understanding of the clinical ramifications. Testing on cadaveric animal models is a consideration that should be taken into account when evaluating screw-bone interface results. Bending modes of failures are considered more realistic complications, but test protocols are more difficult to execute. This is often due to the difficulty in defining the appropriate test methodology.
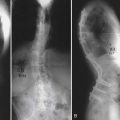
The bending moment and the associated load levels are one set of test parameters. A depiction of testing the effects of the screw-bone interface through bending moments in vertebrae is shown in Figure 8-3. The construct configuration is another study design consideration with implications for unilateral versus bilateral constructs with and without crosslinks. Fatigue is also another major factor difficult to mimic in a cadaveric test environment during biomechanical testing. Screw pull-out tests can be performed along the bone screw axis, but the flexion-extension type of bending should be executed under a cyclical protocol that eventually fails the screw-bone interface through off-bone screw axis loading. This results in a markedly different biomechanical response at the FSU and, in turn, may have different complications, for example, screw loosening. Gau et al reported modes of radiological failure in a clinical radiographic study that examined implanted constructs that exhibited “windshield-wipering,” which may be an indication of bending fatigue at the screw-bone interface, and classified them accordingly.11 Interestingly, these were not symptomatic complications.
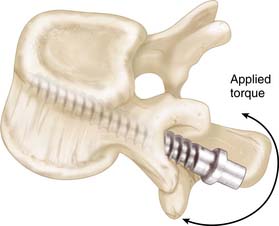
FIGURE 8-3 Application of cyclical bending moments necessary for creating “windshield-wiper” failures.
The ability to derive a specific BMD measurement has been published in a study by Wittenberg et al12 The authors hypothesized an equivalent mineral density of 90 mg/ml from quantitative computed tomography (qCT) as a threshold level to expect complications associated with screw loosening and 120 mg/ml as a threshold for fewer problems. This has not been validated in a clinical outcomes trial. Often, it is surgeon perception on the adequacy of bony purchase that governs the decision to instrument a patient with hardware. Additional data to provide a validated standardized DXA metric with positively correlated clinical outcomes for specific threshold levels would provide a higher confidence in BMD measurements as a preoperative indicator for instrumented procedures.
Dual Energy x-ray Absorptiometry and Mechanical Strength
Studies have shown the failure strength of vertebral bodies as measured by indentation testing differs between superior and inferior endplates; and also between locations on the same vertebral body endplate; for example, posterolateral regions tend to have the highest relative strength. With exceptions, the authors concluded from their study that a decrease in BMC correlated to a decrease in strength. In addition, the same research group13 later reported removal of the endplate resulted in a significant decrease in compressive failure strength. However, it was not clear if removal of the endplate affected DXA measurements.
The consistency of DXA measurements, particularly as it relates to strength, is dependent upon a number of factors, including artifacts from soft tissue. The correlations are especially problematic with higher BMD content. In a study utilizing DXA and cadaveric spine positioning, Myers et al suggested clinical studies to confirm supine lateral patient positioning would be more effective in determining BMD measurements.14 The aging phenomenon that occurs within every human body may potentially cause global osteoarthritic changes, including BMC and BMD within the spine, that subsequently affect local DXA measurements. Utilizing animal models to control the homogeneity of specimens has not resulted in more significant correlations between BMD and strength. Contrarily, in a study involving porcine cervical spines,15 the investigators reported no significant correlation between BMC or BMD with compressive failure strength. Furthermore, large animal models rarely exhibit vertebral body fractures even with reduced BMD levels, and thus would not be characterized into high risk for low-trauma fracture categories.
Modic Classification of Vertebral Endplate Change
Degenerative changes of the lumbar spine have been observed with MRI techniques. Specific signal changes from vertebral body endplates and marrow have been differentiated through imaging techniques that increased tissue contrast. A classification system of MRI scans using two different pulse sequences was published by Modic et al16 Optimizing T1 and T2 relaxation times in pulse sequences during MRI studies helped define and characterize the imaged tissues. Three different types of change were recognized from T1-weighted and T2-weighted MRI scans of the same spine segment. The following is the accepted classification used for Modic changes:
The interobserver and intraobserver error in a clinical study has been documented and the consistency of this imaging classification system was confirmed.17 The study involved five independent observers of various clinical spine experience who graded 50 sagittal T1-weighted and T2-weighted MRI scans. The evaluation of the same scans was repeated by each participant following a 3-week interval with no reference to the first assessment. The intraobserver agreement, or consistency between the first and second evaluations by the same observer, was assessed based on Landis and Koch’s use of the kappa statistic,18 which was equal to 0.71. Additionally, interobserver agreement or consistency among all the observers was calculated to be 0.85 for the study. This study demonstrated the intraobserver agreement was substantial while interobserver agreement was excellent for the Modic classifications.
Magnetic Resonance Imaging and Modic Changes in 40-Year-Old Men and Women
A 5-year prospective study was conducted on a large sample of 40-year-old men and women drawn from the general population.19 In this study, every ninth person born in the county of Funen, Denmark between May 27, 1959 and May 26, 1960 was selected by the Central Office of Civil Registration. Of the 625 selected study subjects, 412 agreed to participate (66%). The study included 199 males and 213 females.
1. Miller J.A., Schmatz C., Schultz A.B. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13:173-178.
2. Kirkaldy-Willis W.H., Farfan H.F. Instability of the lumbar spine. Clin. Orthop. Relat. Res. 165. 1982:110-123.
3. Panjabi M.M. Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine. 1988;13:1129-1134.
4. Goel V.K., Panjabi M.M., Patwardhan A.G., et al. Test protocols for evaluation of spinal implants. J. Bone Joint Surg. Am.. 2006;2(88 Suppl):103-109.
5. Mimura M., Panjabi M.M., Oxland T.R., et al. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371-1380.
6. Board D., Stemper B.D., Yoganandan N., et al. Biomechanics of the aging spine. Biomed. Sci. Instrum.. 2006;42:1-6.
7. Sforza C., Grassi G., Fragnito N., et al. Three-dimensional analysis of active head and cervical spine range of motion: effect of age in healthy male subjects. Clin. Biomech. (Bristol, Avon). 2002;17:611-614.
8. Simpson A.K., Biswas D., Emerson J.W., et al. Quantifying the effects of age, gender, degeneration, and adjacent level degeneration on cervical spine range of motion using multivariate analyses. Spine. 2008;33:183-186.
9. Siris E.S., Chen Y.T., Abbott T.A., et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch. Intern. Med.. 2004;164:1108-1112.
10. McLain R.F., McKinley T.O., Yerby S.A., et al. The effect of bone quality on pedicle screw loading in axial instability: a synthetic model. Spine. 1997;22:1454-1460.
11. Gau Y.L., Lonstein J.E., Winter R.B., et al. Luque-Galveston procedure for correction and stabilization of neuromuscular scoliosis and pelvic obliquity: a review of 68 patients. J. Spinal Disord.. 1991;4:399-410.
12. Wittenberg R.H., Shea M., Swartz D.E., et al. Importance of bone mineral density in instrumented spine fusions. Spine. 1991;16:647-652.
13. Oxland T.R., Grant J.P., Dvorak M.F., et al. Effects of endplate removal on the structural properties of the lower lumbar vertebral bodies. Spine. 2003;28:771-777.
14. Myers B.S., Arbogast K.B., Lobaugh B., et al. Improved assessment of lumbar vertebral body strength using supine lateral dual-energy x-ray absorptiometry. J. Bone Miner. Res.. 1994;9:687-693.
15. Parkinson R.J., Durkin J.L., Callaghan J.P. Estimating the compressive strength of the porcine cervical spine: an examination of the utility of DXA. Spine. 2005;30:E492-E498.
16. Modic M.T., Steinberg P.M., Ross J.S., et al. Degenerative disc disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193-199.
17. Jones A., Clarke A., Freeman B.J., et al. The Modic classification: inter- and intraobserver error in clinical practice. Spine. 2005;30:1867-1869.
18. Landis J.R., Koch G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363-374.
19. Kjaer P., Leboeuf-Yde C., Korsholm L., et al. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine. 2005;30:1173-1180.