Chapter 324 Biomechanical Basis of Traumatic Brain Injury
The complex pathophysiologic phenomena encountered in patients with traumatic brain injuries (TBIs) can ultimately be viewed as a response of the brain to an external mechanical force. Therefore, preventing and treating the consequences of these injuries require an understanding of the causative mechanical factors that induce TBIs. We review the primary mechanical forces that contribute to these brain injuries, how these mechanical forces cause movement and damage within the brain, and the available data on how to prevent these types of injuries. This information is provided as an introduction only, and more detailed investigations of these principles can be found in other publications.1–4
Clinical Classification of Brain Injuries
Clinically, head injuries can be classified into five distinct categories: skull fracture, focal injury, diffuse brain injury, penetrating injury, and blast injury. Skull fracture may or may not involve damage to the underlying brain, but the fracture is often not a direct cause of neurological disability. Focal injuries are defined simply as visible damage that is generally limited to a well-circumscribed region; examples of focal injuries include contusions to the cortex and subdural, epidural, and intracerebral hematomas. Focal injuries occur in nearly half of all patients with severe brain injury and are responsible for approximately two thirds of brain injury–related deaths.5 Diffuse brain injury differs from focal brain injury and skull fracture in that it can often occur without macroscopic structural damage, is associated with widespread brain dysfunction, and affects approximately 40% of patients with severe brain injury.5,6 Although contributing to nearly a third of the deaths attributable to brain injury, the most important aspect of diffuse brain injury is that it is the most prevalent cause of disability in survivors of TBI. In its mildest form (concussion), diffuse brain damage may not necessarily be structural and may involve only alterations in neural excitability, neurotransmission, or long-term changes in receptor dysfunction and associated disabilities. In more severe cases, diffuse brain injury is manifested as prolonged coma without a mass lesion and involves some degree of structural derangement at the microscopic level. Diffuse brain injury may sometimes include secondary damage from both brain swelling and ischemic injury. However, the most commonly injured substrate in diffuse brain injury is the axons within the white matter or the neuronal cell body; for this reason, the prominent forms of diffuse brain injury are diffuse axonal injury (DAI) and ischemic brain damage.5
Mechanisms of Injury
Impact loading is the more frequent type of dynamic loading. Impact loading is complex and usually results in a combination of contact force and inertial (head motion) force. The response of the head to impact conditions depends on the object that strikes the head. For example, inertial effects may be minimal if the head is prevented from moving when it is struck. In this situation, the injuries that occur are the result of contact phenomena, or mechanical events that occur both near and distant from the point of impact. The effects of contact phenomena vary with the size of the impact object, the magnitude of force delivered, and the direction of the force. Factors contributing to the magnitude of the force include the mass, surface area, velocity, and hardness of the impacting object. For objects larger than approximately 2 square inches, localized skull bending occurs immediately beneath the impact point and peripheral to the impact sites. If the skull deformation exceeds the tolerance of the skull, a fracture occurs. Penetration, perforation, or localized depressed skull fractures are more likely if the object has a surface area of less than 2 square inches. Additionally, shock waves can travel through the skull and parenchyma from the point of impact; within the brain, these shock waves can cause localized changes in pressure, distortion, and injury in the form of small hemorrhages and contusion. Children are at greater risk for this type of injury because their skulls are more flexible than adult skulls. Fracture deformation is between 1.7 and 5 times greater in a child than in an adult, depending on the zone of the skull affected.7 The increase in deformability of the child’s skull can result in extensive diffuse brain damage if the child’s skull has received a significant impact, as well as in a greater propensity for the formation of epidural hematomas because of the dural stripping action of skull deformation.7 Children also appear to be at greater risk for diffuse brain injury because their skulls have a lower degree of calcification and thus a reduced capacity to absorb an impact. This results in greater transfer of the kinetic energy from the impact site to the brain tissue.7
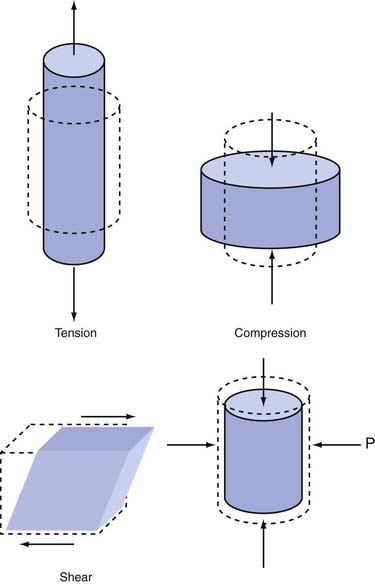
Although these definitions highlight the etiologic differences between impact and impulsive loading, the fundamental means of damaging the skull and brain are the same: distortion or straining of bone or soft tissues beyond their functional or structural tolerance. Strain, or deformation, is considered the proximal cause of tissue damage. This strain can cause alterations in the functioning of neural circuits and receptors and changes in the properties of neural tissue8,9 (for recent reviews, see Spaethling and colleagues10 and LaPlaca and coworkers11). In general, strain can be considered the amount of deformation that the tissue experiences as a result of applied mechanical force. Strain is often described as compressive, tensile, dilational, or shear in nature (Fig. 324-1). Compressive strain is the amount of contraction observed when the material is compressed. For instance, if a stiff cylinder is placed upright on a tabletop and a stack of books is placed on the top circular face of the cylinder, the cylinder would shorten with respect to its original, unloaded length. If the cylinder were originally 10 cm in length and became 8 cm when the books were placed on top, the material is said to have a 20% compressive strain. In comparison, tensile strain is the amount of elongation that occurs when the material is stretched. If a column 10 cm in length becomes 11 cm long when stretched, it undergoes 10% tensile strain (stretched length minus original length divided by original length). Dilational strain, also referred to as volumetric strain, describes the change in volume that occurs when pressure is applied to all exposed faces of a material. Most material will show either negative or positive dilational strain when positive or negative pressure, respectively, is applied to the material. Finally, shear strain can be considered the amount of distortion that occurs in response to forces applied all along the surface of the material. A common illustration of shear strain is the distortional change that occurs in a deck of playing cards when one hand is moved across the top of the deck. None of the cards are compressed or stretched as a result of this motion, but the side profile of the deck changes to a slanted rectangle. The amount that the side profile varies from a normal rectangle indicates the state of its shear strain.
Mechanistic Causes of Head Injuries
Contact Injuries
Local Contact Effects
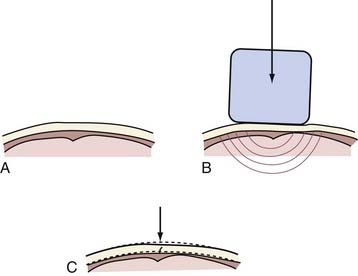
Examples of injuries caused by local contact effects include most linear and depressed skull fractures, some basilar skull fractures, epidural hematomas, and coup contusions. A linear skull fracture occurs as a result of local skull bending at the impact site that exceeds the local strain limit for the bony tissue (Fig. 324-2). Because strain tolerance is related to the inherent mechanical properties of the material, it is not surprising to find that skull fracture depends partly on the material properties of the skull and its thickness in the impact region. Additional factors include the magnitude and direction of impact and the size of the impacted area. Mechanistically, the local in-bending caused by the impact creates compressive strain on the outer skull surface and tensile strain on the inner surface (see Fig. 324-2). Bone, although naturally resistant to compressive force and strain, is less resistant to tensile force on the inner skull surface. Thus, the initial fracture begins at the inner table. Once initiated, the fracture follows the path of least resistance dictated by the geometric and strength characteristics of the surrounding skull. During the continuing fracture process, energy from the impacting object is transferred to the skull via the fracture. The linear fracture is complete when the impact energy is dissipated completely.
Head Motion (Inertial) Injuries
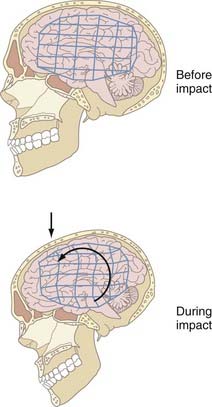
Similar to contact injuries, head motion results in strain within brain tissue that can cause either functional or structural damage (Fig. 324-3). First, differential movement of the skull and brain can be produced by head acceleration or motion. This relative movement occurs because the brain is free to move to some degree within the skull and the brain lags behind the skull for a brief moment after acceleration begins as a result of inertia. When combined, these factors allow the skull and dura to move relative to the brain surface, thereby potentially causing localized strain at the surface. Particularly susceptible in this situation are the parasagittal bridging veins between the brain surface and dura, which may tear if the strain exceeds the tolerance of the vessels, and such tears cause about 60% of acute subdural hematomas. Furthermore, movement of the brain away from the skull creates a region of low pressure that if sufficiently intense, causes contrecoup contusions. Second, head acceleration can produce strain within the brain parenchyma and therefore result in widespread disturbances in brain function or structures. Strain within the brain parenchyma can be manifested as classic “cerebral concussion,” DAI and associated hemorrhages from tearing of tissue, and intermediate coup contusions. In each type of injury, the severity and extent of damage are intimately linked to the magnitude, rate, duration, direction, and type of inertial loading.
Types of Head Acceleration
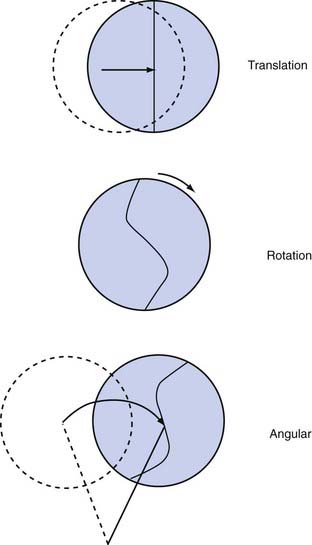
Three types of acceleration can occur: translational, rotational, and angular (Fig. 324-4). Translational acceleration occurs when the center of gravity of the brain (which is approximately in the pineal region) moves in a straight line. Purely translational acceleration is uncommon because the physiologic articulation of the head and neck limits this pure movement. Exceptions occur when the head moves in a translational manner for brief periods or the head becomes arrested with other motions. An exception may be vertex impact, during which superior to inferior motions can occur. The brain motions that take place during translational acceleration are primarily due to the relative brain skull motions previously described and not to strain produced deep within the brain. Concussive injuries do not occur when the head experiences a purely translational acceleration.12 For concussion or DAI to develop, the brain must undergo angular acceleration. Therefore, translational acceleration does not cause diffuse brain injuries, but it can produce focal injuries, including contrecoup contusions and intracerebral and subdural hematomas.
Angular acceleration occurs when components of translational and rotational acceleration are combined. In this situation, the center of gravity moves in an angular manner. Because of the neck’s anatomy, angular acceleration is the most common head motion encountered clinically. Frequently, the center of rotation occurs in the lower cervical region. The exact location of this rotation point, in conjunction with the magnitude of the impact force, determines the proportion of translation and rotation that the brain experiences. As the rotation point moves higher up the cervical spine, there is a proportionally greater rotational component; moving the rotation point lower introduces proportionally more translational acceleration. As might be expected, angular acceleration is the most damaging brain injury mechanism because it combines the injurious mechanism of both translational and rotational movements, especially the latter. Virtually every known type of head injury can be produced by angular acceleration, except for skull fracture and epidural hematoma. Several studies have now documented the in vivo motion of the brain during angular motions13,14 and have investigated the head motions associated with sports and concussive impacts.15–20
In helmeted sports, it is important to note that the helmet can and will accentuate angular accelerations when the head does not receive a direct impact because of the added mass of the helmet. Angular acceleration is greatly increased as a helmet’s mass increases and moves away from the center of rotation. This is especially important in children’s sports because of the inertial loading of a child’s head. If a child’s head is 1 kg lighter and 2 cm smaller in diameter than the typical 6.2-kg adult head, the inertia of a child’s head is approximately half that of an adult head, thereby allowing these greater accelerations.7,21 In a study conducted to determine a recommended standard for youth helmets, it was found that boys aged 6 to 11 years have an average head mass 0.9 kg lighter than that of an average adult man with a head circumference approximately 2.5 cm less than the adult average circumference.22 Thus, modern helmet design must take mass into consideration.
Determinants of Acceleration Injury
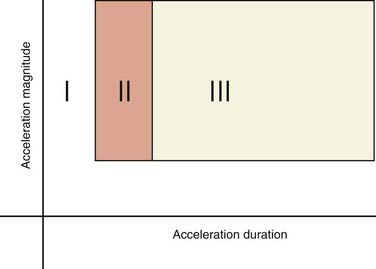
If one applies a constant amount of acceleration and varies only the duration over which the acceleration occurs, three zones of clinical interest are encountered (Fig. 324-5). The exact relationships between mechanical loading (magnitude, type, and direction of acceleration) and injury patterns are becoming increasingly more refined with computational models of the head/brain structure.23–26 First, with very brief accelerations, many of the inertial effects within the brain are damped, and as a result the brain actually experiences very little strain. Consequently, extremely high accelerations are required to produce injury. Second, if the duration of acceleration is slightly longer, strain begins to appear within the brain but is primarily restricted to the periphery. Moreover, the brain surface can slide relative to the skull/dura surface. Injuries produced in these circumstances are confined to the brain periphery and vessels (e.g., bridging veins). Third, as the duration of acceleration increases even more, strain propagates deeper within the brain and can cause DAI, which in its severe form is manifested as prolonged traumatic coma.
Blast-Induced Brain Injuries
The effects of blast loading on humans originated with the term “shell shock” from World War I, which was used to describe a collection of symptoms that included a temporary altered mental state or confusion immediately after the blast.27 In more severe forms, unconsciousness would occur. Despite being periodically addressed since its recognition as a clinical syndrome, only very recent studies have better described the conditions of blast-induced brain injury.28–30 Although blast injury is formally defined in four phases, the bulk of blast-induced traumatic brain injury (bTBI) occurs in the primary, secondary, and tertiary phases. The primary injury phase consists of the response of brain tissue to the blast wave (an intense overpressurization impulse component of the blast). The secondary injury phase results from penetration of shrapnel into the head. The tertiary injury phase is due to head contact/acceleration as the body is moved by the “blast wind” (forced super-heated airflow).
Primary blast injury is limited to injuries caused by the rapidly expanding blast wave.31 Past work identified typical pressure wave profiles generated during blast events32 and how this pressure wave propagates through air and biologic tissues.33–35 Shock waves from a blast are typically characterized as a rapid rise and fall of high pressures.36 The shock wave passes on the order of milliseconds, although the exact profile and range are dependent on the size, type, and shape of the explosive. In air, the shock wave oscillates between overpressure and underpressure segments, but these waves dampen out quickly. In water, the shock wave maintains a normal pressure profile over a longer range. The presence of structures and fluid interfaces complicates this pressure profile.
Most structural damage occurs when the shock wave travels from water to air. For this reason, a large number of studies have focused on the fluid-air interfaces in the body because they are highly vulnerable to the passing pressure waves. Studies in these areas have generated injury thresholds for both the pulmonary system and the gut/bowel.37 As this pressure wave travels through the organs, there are stress concentrations that arise at tissue-air interfaces, reflections of stress waves at tissue interfaces, and possibly constructive stress wave interference within internal points of tissue that can further complicate the pattern of injury.36 Models to study the primary injury phase should account for both the blast pressure transmitted through the organ of interest and shock wave reflection/transmission behavior at tissue interfaces.
Secondary blast injury covers both the penetrating and nonpenetrating injuries that occur when high-velocity projectiles/fragments impact the head.31 Certainly, these injuries share some common ground with the ballistic wounds in civilians (for recent publications, see elsewhere36–39 but are considerably more complex because of the nonuniform size and number of impacting fragments. Nevertheless, the mechanics of these penetrating lesions is becoming clearer, given the frequency of gunshot wounds in the civilian population.38 A projectile moving through soft tissue at high speed will cause rapid expansion and subsequent collapse of tissue along the penetration track. This induces cavitation damage along the path of the projectile, as well as primary laceration damage along the path of the fragment. At the mechanistic level, these injuries are best modeled as a tissue laceration, with the obvious complicating mechanisms related to blood in the extracellular space and the potential for secondary brain injuries such as hypoxia.
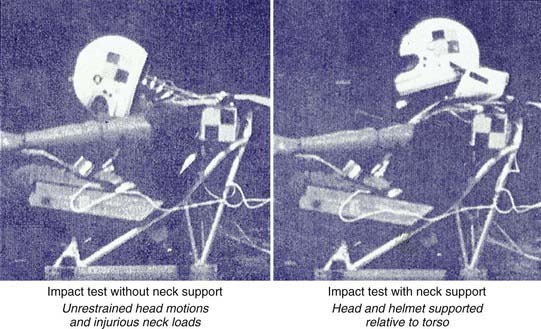
Tertiary blast injury occurs when the primary blast wind causes the victim to collide with fixed or mobile objects. These types of injuries share the most in common with contact/acceleration injuries in civilians, where both the contact and acceleration forces can contribute to the different intracranial injuries that occur. Helmets worn by troops reduce the likelihood of injuries from direct contact for most of the head. However, current military helmets are not designed to specifically reduce rotational acceleration after a helmeted impact.19,39 The Head Acceleration Neutralization System (HANS) worn by race car drivers has been dramatically successful in mitigating this rotational acceleration damage (Fig. 324-6). There is still high potential for inertially based injuries, including subdural hematoma and DAI. As a result, the predominant mechanism of tertiary blast injuries in helmeted troops is the intracranial deformations caused by the head striking an object or the head being struck by an object with sufficient mass to cause a significant inertial load. In nonhelmeted victims, tertiary blast injuries can also include skull fracture or contusions from focal contact forces when the unprotected head strikes an object or surface.
Injuries and Their Mechanisms
Skull Fracture
Basilar Fracture
Fatal basilar skull fracture can also occur when the torso of a driver or passenger is adequately restrained in a vehicular crash but the head is allowed to move as a result of the impulsive forces in such a manner that the cervical spine is distracted from the base of the skull. This injury has occurred after both frontal and rear impacts. The HANS device, developed in the early 1990s by Dr. Robert Hubbard and Jim Downing, was designed specifically to prevent this type of injury. By keeping the head, neck, and torso all moving in the same direction during a crash, the HANS device prevents a whiplash type of motion and thereby helps prevent distraction-type injuries. With use of the HANS or similar device, neck loads are decreased by 40% to 60% in all types of crashes (Fig. 324-7). The HANS device or similar approved devices are now mandatory in all major forms of professional motor sports and are under consideration by the military for pilots. The HANS device requires the use of seat belts to hold the device in place. Other similar restraining systems have been designed for use in motorcycle racing and equestrian events.
Focal Brain Injury
Diffuse Brain Injury
Cerebral Concussion
All gradations of concussion (transient reversible neurological dysfunction as a result of trauma) are produced entirely by inertial loading and not from contact phenomena effects. Experimentally, it is now possible to produce concussion in small animals with acceleration.40,41 It is probable, however, that concussion is observed in concert with injuries arising from the contact phenomena, simply because the contact loading will produce both contact effects and head acceleration. Unlike subdural hematoma, concussion does not occur with purely translational motion of the head. Angular rotational head motion causes the deeper structures within the brain to deform and results in the classic widespread disruption of brain function that underlies concussion. For a concussive injury, most of the strain is insufficient to cause structural damage. Instead, damage to the structures may be either partially or completely reversible, depending on the severity of the inertial loading. The precise location of the functional derangement in a concussion continues to be debated. It remains uncertain whether the effects of angular acceleration are principally seen in the brainstem, the cerebral hemispheres, or both regions.
Diffuse Axonal Injury
Axonal damage appears to be one of the two most important pathologic substrates producing prolonged traumatic coma not attributable to mass lesions and, like cerebral concussion, is caused only by angular rotational acceleration and not by contact phenomena (the second pathologic substrate being ischemic/hypoxic neuronal damage). DAI nearly always coincides with other forms of contact or inertial injuries.42 Furthermore, recent evidence has suggested that the magnitude of rotational acceleration needed to produce DAI requires the head to strike an object or surface, a requirement that raises the likelihood of superimposed contact injuries.24,43 In high-g vehicular crashes, as seen frequently in modern motor sports, DAI does occur without direct impact to the helmeted head. The amount and location of axonal damage as a consequence of rotational acceleration probably determine the severity (depth and duration) of the injury, as well as the quality of recovery. Critical factors in estimating the amount and extent of axonal damage include the magnitude, duration, and onset rate of the angular acceleration, in addition to the direction of motion and the role of the intracranial membranes.44–46 In particular, DAI is produced by a longer duration of acceleration loading, as opposed to the relatively brief loading duration that usually produces acute subdural hematoma. DAI is most likely to occur when the head is impulsively loaded or when the impact involves a relatively soft, broad object, as in an occupant involved in a motor vehicle accident. The former is not common clinically because of the levels of rotational acceleration needed for it; instead, the latter is the most frequent circumstance producing DAI.
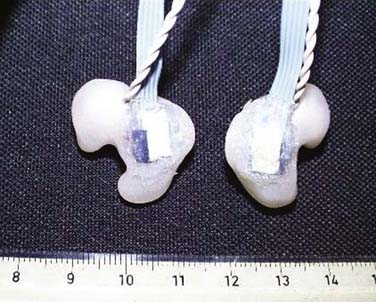
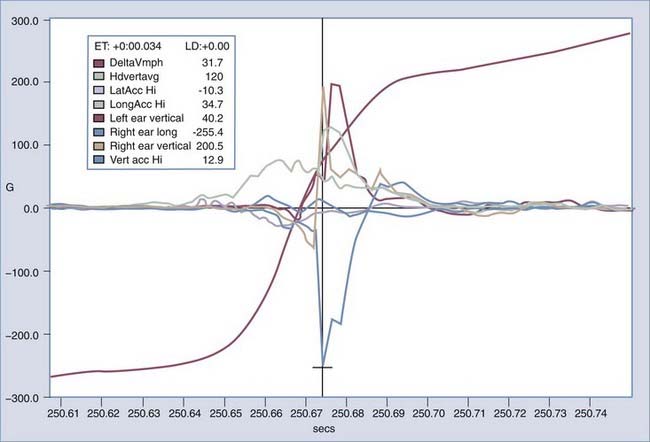
The direction that the head moves plays an important role in the amount and distribution of axonal damage in a given situation. For equivalent levels of angular acceleration, the brain is most vulnerable to axonal damage if it is moved laterally. The brain tolerates sagittal movement best, and horizontal motions are somewhere between lateral and sagittal movements. However, sagittal motions are the most effective in producing vascular injuries in the superior margin of the brain because the motion of the brain in this plane is not severely restricted by intracranial membranes. To this end, the full-blown picture of widely scattered damage to the cerebral hemispheres and brainstem, along with tissue tear hemorrhages, most likely occurs because of the spatial changes in the strain pattern induced by the falx and tentorium during lateral motions. Furthermore, the gyral geometry of the cerebrum and brainstem plays an important role in the response of the brain to rotational motions. In response to a lateral head motion, small centers of rotation occur in the superior frontal and temporal lobe. Although the induced clockwise motion is the same for all centers of rotation, at the periphery of the rotation, the brain tissue is moving in opposite directions. The combination of three main factors—the complex gyral geometry, the white/gray matter mechanical properties, and the intracranial membranes—leads to a complex deformation pattern within the brain that correlates well with areas showing damage. Until recently there has not been an effective way to measure head accelerations directly in real time in humans. A method using custom-fitted ear plugs with implanted triaxial accelerometers to measure head acceleration in the x-, y-, and z- axes with 5 degrees of freedom was developed in 2002 (Fig. 324-8).47 Current technology will allow these systems to record data and to transmit that data wirelessly to the sidelines or other remote location (Fig. 324-9). Studies including automobile racing drivers, football players, rodeo cowboys, and aerobatic pilots have currently been or are being contemplated. In-ear accelerometers have an advantage over other methods that instrument the helmet because they are not affected by the independent movement of the helmet on impact. This movement is usually in the direction opposite the force applied and not proportional to the resultant motion of the head (crash test results at Wayne State University, Melvin J, personal communications). It is anticipated that data from these studies will help gain a better understanding of what degree and direction of force will cause what specific types of severe TBI. Additionally, it is anticipated that a threshold level of acceleration will be recognized that if exceeded, will identify an athlete or combatant who should be immediately pulled out of competition or conflict because of a concussion. Having accurate head acceleration data should also help validate various head injury models.
Goldsmith W. The state of head injury biomechanics: past, present, and future: part 1. Crit Rev Biomed Eng. 2001;29:441-600.
Goldsmith W, Monson KL. The state of head injury biomechanics: past, present, and future part 2: physical experimentation. Crit Rev Biomed Eng. 2005;33:105-207.
King AI. Fundamentals of impact biomechanics: part I—biomechanics of the head, neck, and thorax. Annu Rev Biomed Eng. 2000;2:55-81.
Nahumand A, Melvin J, editors. Accidental Injury, Biomechanics and Prevention, 2nd ed, New York: Springer, 2002.
1 Goldsmith W. The state of head injury biomechanics: past, present, and future: part 1. Crit Rev Biomed Eng. 2001;29:441-600.
2 Goldsmith W, Monson KL. The state of head injury biomechanics: past, present, and future part 2: physical experimentation. Crit Rev Biomed Eng. 2005;33:105-207.
3 McIntosh TK, Smith DH, Meaney DF, et al. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74:315-342.
4 King AI. Fundamentals of impact biomechanics: part I—biomechanics of the head, neck, and thorax. Annu Rev Biomed Eng. 2000;2:55-81.
5 Gennarelli TA, Spielman GM, Langfitt TW, et al. Influence of the type of intracranial lesion on outcome from severe head injury: a multicenter study. J Neurosurg. 1982;56:26-32.
6 Adams JH, Graham DI, Murray LS, et al. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557-563.
7 Corner JP, Whitney CW, O’Rourke NO, et al. Motorcycle and Bicycle Protective Helmets: Requirement Resulting from a Post Crash Study and Experimental Research, Vol 3, 1979-1987. Australian Transport Safety Bureau, 1987.
8 Elkin BS, Morrison B3rd. Region-specific tolerance criteria for the living brain. Stapp Car Crash J. 2007;51:127-138.
9 Elkin BS, Azeloglu EU, Costa KD, et al. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma. 2007;24:812-822.
10 Spaethling JM, Geddes-Klein DM, Miller WJ, et al. Linking impact to cellular and molecular sequelae of CNS injury: modeling in vivo complexity with in vitro simplicity. Prog Brain Res. 2007;161:27-39.
11 LaPlaca MC, Simon CM, Prado GR, et al. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13-26.
12 Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness: correlation of experimental and clinical observations on blunt head injuries. Brain. 1974;97:633-654.
13 Bayly PV, Black EE, Pedersen RC, et al. In vivo imaging of rapid deformation and strain in an animal model of traumatic brain injury. J Biomech. 2006;39:1086-1095.
14 Bayly PV, Cohen TS, Leister EP, et al. Deformation of the human brain induced by mild acceleration. J Neurotrauma. 2005;22:845-856.
15 Kleiven S. Predictors for traumatic brain injuries evaluated through accident reconstructions. Stapp Car Crash J. 2007;51:81-114.
16 Funk JR, Duma SM, Manoogian SJ, et al. Biomechanical risk estimates for mild traumatic brain injury. Annu Proc Assoc Adv Automot Med. 2007;51:343-361.
17 Withnall C, Shewchenko N, Gittens R, et al. Biomechanical investigation of head impacts in football. Br J Sports Med. 2005;39(suppl 1):i49-57.
18 Brolinson PG, Manoogian S, McNeely D, et al. Analysis of linear head accelerations from collegiate football impacts. Curr Sports Med Rep. 2006;5:23-28.
19 Pellman EJ, Viano DC, Tucker AM, et al. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003;53:799-812.
20 Naunheim RS, Bayly PV, Standeven J, et al. Linear and angular head accelerations during heading of a soccer ball. Med Sci Sports Exerc. 2003;35:1406-1412.
21 Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and pediatric head injury. Br J Neurosurg. 2002;16:220-242.
22 Olvey SE, Trammell TR, Mellor A. Recommended Standards for Helmet Design in Children Based on Anthropometric and Head Mass Measurements in 223 Children Ages Six to Seventeen. Paper presented at the SAE International Motorsports Engineering Conference, December 5-7, 2006, Detroit, MI (Document Number 2006-01-3656).
23 Takhounts EG, Ridella SA, Hasija V, et al. Investigation of traumatic brain injuries using the next generation of simulated injury monitor (SIMon) finite element head model. Stapp Car Crash J. 2008;52:1-31.
24 Yoganandan N, Li J, Zhang J, et al. Influence of angular acceleration-deceleration pulse shapes on regional brain strains. J Biomech. 2008;41:2253-2262.
25 Cloots RJ, Gervaise HM, van Dommelen JA, et al. Biomechanics of traumatic brain injury: influences of the morphologic heterogeneities of the cerebral cortex. Ann Biomed Eng. 2008;36:1203-1215.
26 Zhang L, Yang KH, King AI. A proposed injury threshold for mild traumatic brain injury. J Biomech Eng. 2004;126:226-236.
27 Southborough L. Report of the War Office Committee of Inquiry into “Shell-Shock.”. London: Her Majesty’s Stationary Office; 1922.
28 Pary R, Lippmann SB, Turns DM, et al. Post-traumatic stress disorder in Vietnam veterans. Am Fam Physician. 1988;37:145-150.
29 Macleod AD. Shell shock, Gordon Holmes and the Great War. J R Soc Med. 2004;97:86-89.
30 Jones E, Thomas A, Ironside S. Shell shock: an outcome study of a First World War “PIE” unit. Psychol Med. 2007;37:215-223.
31 Scott SG, Belanger HG, Vanderploeg RD, et al. Mechanism-of-injury approach to evaluating patients with blast-related polytrauma. J Am Osteopath Assoc. 2006;106:265-270.
32 Wharton RK, Formby SA, Merrifield R. Airblast TNT equivalence for a range of commercial blasting explosives. J Hazard Mater. 2000;79:31-39.
33 Clemedson CJ. Shock wave transmission to the central nervous system. Acta Physiol Scand. 1956;37:204-214.
34 Clemedson CJ, Pettersson H. Propagation of a high explosive air shock wave through different parts of an animal body. Am J Physiol. 1956;184:119-126.
35 Clemedson CJ, Jonsson A, Pettersson H. Propagation of an air-transmitted shock wave in muscular tissue. Nature. 1956;177:380-381.
36 DePalma RG, Burris DG, Champion HR, et al. Blast injuries. N Engl J Med. 2005;352:1335-1342.
37 Yang Z, Wang Z, Tang C, et al. Biological effects of weak blast waves and safety limits for internal organ injury in the human body. J Trauma. 1996;40:S81-S84.
38 Zhang J, Guan Y, Yoganandan N, et al. Experimental model for civilian ballistic brain injury biomechanics quantification. J Biomech. 2007;40:2341-2346.
39 Richter M, Otte D, Lehmann U, et al. Head injury mechanisms in helmet-protected motorcyclists: prospective multicenter study. J Trauma. 2001;51:949-958.
40 Fijalkowski RJ, Stemper BD, Pintar FA, et al. New rat model for diffuse brain injury using coronal plane angular acceleration. J Neurotrauma. 2007;24:1387-1398.
41 Fijalkowski RJ, Stemper BD, Pintar FA, et al. Biomechanical correlates of mild diffuse brain injury in the rat. Biomed Sci Instrum. 2007;43:18-23.
42 Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18:307-316.
43 Yoganandan N, Gennarelli TA, Zhang J, et al. Association of contact loading in diffuse axonal injuries from motor vehicle crashes. J Trauma. 2009;66:309-315.
44 Meaney DF, Smith DH, Shreiber DI, et al. Biomechanical analysis of experimental diffuse axonal injury. J Neurotrauma. 1995;12:689-694.
45 Zhang J, Yoganandan N, Pintar FA, et al. Role of translational and rotational accelerations on brain strain in lateral head impact. Biomed Sci Instrum. 2006;42:501-506.
46 Li J, Zhang J, Yoganandan N, Pintar FA, et al. Regional brain strains and role of falx in lateral impact induced head rotational acceleration. Biomed Sci Instrum. 2007;43:24-29.
47 Olvey SE, Knox T, Cohn KA. The development of a method to measure head acceleration and motion in high-impact crashes. Neurosurgery. 2004;54:672-677.