CHAPTER 266 Biomaterials and Biomechanics of Spinal Arthroplasty
The success associated with replacement of a joint of the appendicular skeleton, such as the hip joint or knee joint, has led to the assumption that joints of the spine may also be amenable to arthroplasty. The functional spinal unit (FSU) is composed of the intervertebral disk and paired facet joints and may be partially augmented (nuclear replacement) or completely replaced (disk arthroplasty, facet joint replacement). Unfortunately, the significant stress placed on the axial skeleton, in combination with the complex biomechanical properties of both the cervical and lumbar spine, such as coupled motion, makes both design of the arthroplasty device and selection of the ideal composite biomaterial a complicated undertaking to say the least. In 1955, Cleveland reported 14 patients in whom he implanted a methyl-acrylic device into the intervertebral space at the time of diskectomy.1 This was followed by Harmon’s use of Vitallium spheres in 13 patients from 1959 to 1961.2 These implants, which were inserted into the lumbar spine through an anterior retroperitoneal approach, led to spontaneous fusion rather than preservation of motion. Another pioneer was Fernstrom, who in 1966 published the outcomes of patients undergoing implantation of a solid stainless steel sphere into the lumbar disk space through a posterior approach at the time of lumbar diskectomy.3 These were important steps that unfortunately met with profound failure. Inadequate surface area resulted in subsidence. Poor design resulted in disk space collapse without restoration of motion. With the advent of more forgiving designs of arthroplasty devices, the challenge has become creation of a device that can withstand hundreds of thousands of wear cycles while allowing ease of insertion and uncomplicated revision.
Historically, anterior cervical decompression with arthrodesis has been a very successful operation for treating both neck pain and cervical spinal cord or root compression syndromes. With the use of interbody graft material and plate fixation, high rates of fusion and high rates of clinical success have been well documented. The surgical goal is solid bony arthrodesis and decompression with a clinical end point of relief of neck pain and neural compression. It has been estimated that failure or pseudarthrosis after attempted anterior cervical fusion may develop in up to 20% of patients.4 Pseudarthrosis may be associated with increasing neck pain, progressive neurological deficit, or the development of spinal deformity. Even in cases of successful fusion, loss of the FSU may transfer biomechanical stress to adjacent levels and presumably lead to more rapid deterioration of affected levels.5,6 Clearly, successful arthroplasty would eliminate the possibility of pseudarthrosis while maintaining neural decompression. This would achieve the ultimate goal of minimizing degeneration of adjacent segments while curing both neck pain and radicular symptoms.
Arthroplasty Biomaterials
Spinal disk arthroplasty designs have clearly been influenced by progress made in the field of hip and knee joint arthroplasty. A number of the designs use a combination of metals and polymers. The polymers or plastics essentially provide some measure of shock absorption for the joint while also providing a low-friction surface for joint articulation. Polymers themselves are not strong enough to tolerate the stress of normal joints and are therefore always supported by a metal base. Articular surfaces may involve a metal-on-metal, metal-on-polymer, ceramic-on-polymer, or ceramic-on-ceramic interface. Two polymers that have been used successfully are polyurethane and the derived ultra-high-molecular-weight polyethylene (UHMWPE). Metallic devices have been constructed from solitary metals such as stainless steel, titanium, and cobalt. Newer devices take advantage of metallic alloys. Alloys are homogeneous mixtures or solid solutions of two or more metals. For example, cobalt-chromium alloy, cobalt-chromium-molybdenum alloy, and titanium-aluminum-vanadium7 have characteristics that make them uniquely suited for use in arthroplasty. The alloys seem to wear more slowly than polymers and may resist corrosion better than single-metal implants.
Use of a metallic alloy such as cobalt-chromium-molybdenum to minimize the effects of oxidative corrosion unfortunately does not eliminate the concern for wear of the articular surface. Any articulating surface will generate debris secondary to friction. The greater the physiologic load on the device, as well as the greater the ROM, the greater the generation of wear debris. In particular, Hellier and coauthors published the results of determining the absolute and relative wear volume rates of various metal alloys through simulation of an intervertebral disk prosthesis.8 It was found that among all the available alloys, a cobalt-chromium-molybdenum (CoCrMo) alloy generated the least amount of wear debris. It had an average wear volume rate of 0.093 mm3 per million cycles from an arthroplasty device, whereas a titanium alloy containing 6% aluminum and 4% vanadium (Ti6Al4V) had an average wear volume rate of 2.9 mm3 per million cycles. Schmiedberg and colleagues in 1994 used scanning electron microscopy to further define the size and shape of the wear debris fragments generated from an arthroplasty articular surface.7 The fragments from a titanium-aluminum-vanadium surface are extremely rough and irregularly shaped. The size of the fragments ranges from less than 1.0 µm to greater than 30 µm. Fragments from the cobalt-chromium-molybdenum alloy have an irregular polyhedral shape when the alloy was formed from a forged process, but the fragments have a spherical shape ranging between 5.0 and 30 µm when the alloy was formed from a hot isostatically pressed process. Catelas and associates published the results of their study investigating wear debris in metal-on-metal total hip arthroplasty devices.9 The study, published in 2003, demonstrated a significant number of wear debris particles composed predominantly of chromium oxide particles, with estimated loss rates as high as 100 mm3/yr for hip arthroplasty.
Basic Terms for Biomaterials
Goals for Biomaterials Used for Disk Replacement
Alloys Available for Total Disk Arthroplasty
There are three principal metal alloys used in arthroplasty:
Imaging Properties of Biomaterials
The imaging characteristics of various materials have been mentioned briefly. Although the specific radiographic characteristics are beyond the scope of this work, it nonetheless remains critically important to visualize both the bony and soft tissue structures of the spine at the level of operated disease, as well as at adjacent levels. CT and MRI are often used to evaluate the adequacy of decompression, determine the completeness of arthrodesis, or investigate the cause of continued radicular complaints. Biomaterials clearly have different imaging properties. For example, polymers such as PEEK are radiolucent and typically contain embedded radiopaque markers. Titanium and ceramic have good MRI qualities. Cobalt-chromium and stainless steel do not image well on MRI because of extensive artifact. As a result, myelography with postmyelography CT is recommended for adequate visualization. A direct comparison of the clarity of cervical arthroplasty devices on MRI concluded that titanium devices, with or without polyethylene, allow satisfactory monitoring of the index and adjacent levels with MRI whereas devices containing metals other than titanium prevent accurate postoperative assessment of the index and adjacent levels by MRI (Tables 266-1 and 266-2).10
TABLE 266-1 Cervical Devices and Materials
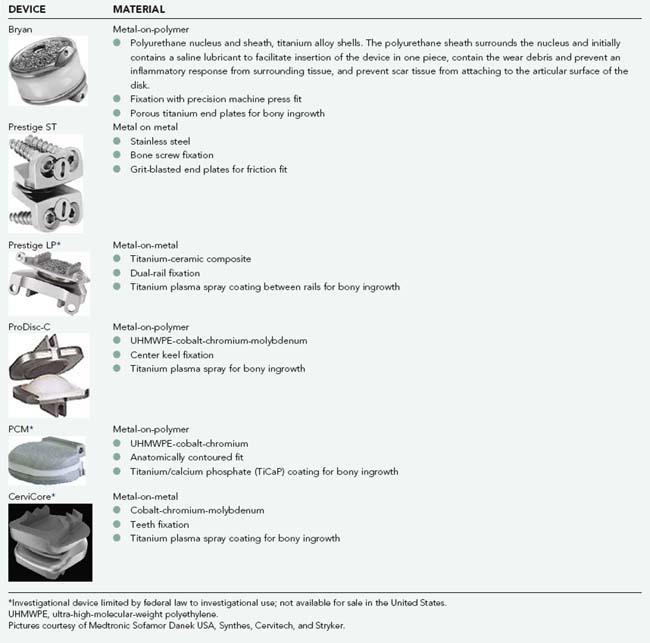
TABLE 266-2 Lumbar Devices and Materials
Pictures courtesy of Depuy, Synthes, Medtronic Sofamor Danek USA, and Stryker.
| DEVICE | MATERIAL |
|---|---|
| Charité |
UHMWPE, ultra-high-molecular-weight polyethylene.
* Investigational device limited by federal law to investigational use; not available for sale in the United States.
Normal Biomechanics of the Spine
Rotation and Translation
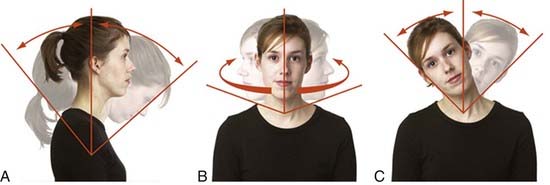
Rotational movements are movement of the vertebra around an axis. All rotations produce a change in orientation of the vertebra (Fig. 266-1).11
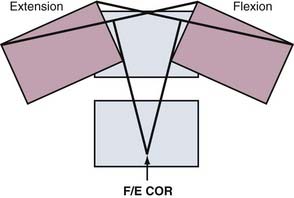
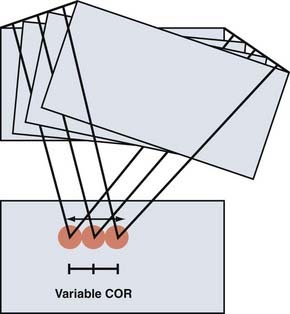
The COR for flexion-extension has been found to be located in the posterior third of the inferior vertebral body in both the cervical12 and lumbar13 spine (Fig. 266-2). The COR for axial rotation is in the midline, posterior to the disk but anterior to the facet joints for the lumbar spine14 and aligned with the facet joints in the cervical spine.12 When both translation and rotation occur in the same plane, the COR is variable. Because motions of the spine are typically coupled, they also have a variable COR. In flexion, the COR moves to the anterior side of the spinal column; in extension, to the posterior side; and during lateral bending and axial rotation, to the opposite side of the spinal column.15 A variable COR decreases loads because the working distance of the spinal muscles and ligaments is increased during the motions (Figs 266-3 and 266-4).16,17
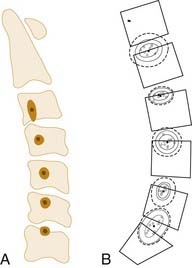
FIGURE 266-2 Locations of the centers of rotation for the cervical spine as described by Bogduk and Mercer.12 (A) and for the lumbar spine as described by Pearcy and Bogduk (B).13
(A, Reprinted with permission from Elsevier. B, Reprinted with permission from Lippincott Williams & Wilkins.)
Range of Motion and Its Limits
The FSU is a three-joint complex consisting of the intervertebral disk and the two posterior facet joints. These three joints act together to provide normal ROM and resist supraphysiologic stress. Lumbar facet joints provide resistance to excessive extension and experience high compressive loads in extension. In flexion, they experience minimal load. The facets resist anterior translation because of their orientation, and hence, anterior shear loads increase facet pressure. The posterior ligaments (supraspinous, interspinous, facet capsules) resist posterior translation; in posterior shear loading, the posterior ligaments are placed under tension, whereas the facets are unloaded. Stiffness is the measure of resistance to displacement. In the center of the ROM, the FSU offers little resistance to movement, and large displacement may be caused by small forces. When the FSU gets closer to the limit of its ROM, its stiffness increases and the resistance to further displacement is quite high.18,19 The typical cervical motion segment allows 10 degrees of flexion and extension, 11 degrees of lateral bending, 7 degrees of axial rotation, and 2 mm of translation. A lumbar motion segment typically allows 12 degrees of flexion and extension, 8 degrees of lateral bending, and 7 degrees of axial rotation.
Biomechanics of Total Disk Arthroplasty
Total disk arthroplasty (TDA) refers to devices that replace the majority of the disk while conserving a small portion of the anulus for ligamentous stability.20 Surgical goals are conservation of ROM, restoration of sagittal balance and disk height, and preservation of the facet joints. Because the intervertebral disk consists of concentric layers of interwoven collagen with a proteoglycan gel-filled center, a plausible design for a TDA device would be a thick-walled balloon or a flexible elastomeric device attached to each end plate. A TDA device mimicking the natural disk was indeed designed and underwent clinical trials (Acroflex Lumbar Disc, Depuy Spine, Raynham, MA). However, clinical trials were aborted because of premature mechanical failure of the elastomer and osteolysis caused by debris released from the failed elastomer. In contrast, the majority of currently available TDA devices do not mimic the normal intervertebral viscoelastic disk. They use various forms of low-friction, sliding, gliding, and rotational joints in an attempt to restore normal function to the motion segment.
Limits to Range of Motion
A TDA device with independent translation does not resist a shear force. The TDA device translates and the shear force must be resisted by the facet joints. In contrast, a TDA device without independent translation resists a shear force and the shear is instead experienced at the device-bone interface. All current TDA designs lack inherent stiffness. Hence, all TDA devices do not provide an inherent “brake” toward the limits of ROM. However, most TDA devices do have a built-in mechanical stop at the end of their ROM to resist supraphysiologic stress (Tables 266-3 and 266-4).
TABLE 266-3 Biomechanical Properties of Cervical Devices
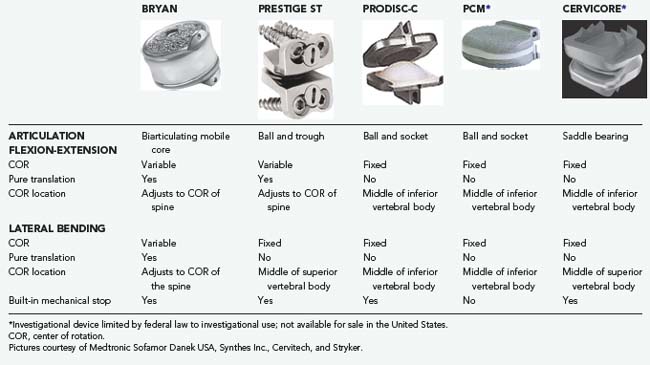
TABLE 266-4 Biomechanical Properties of Lumbar Devices
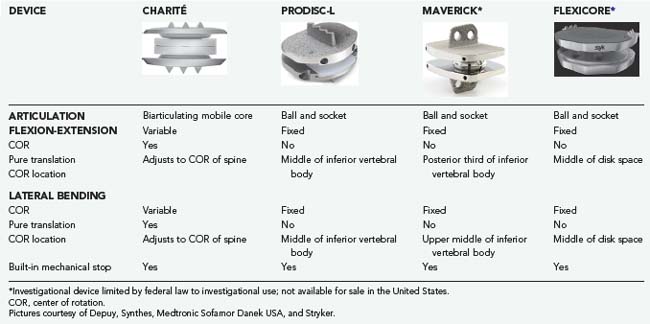
Consistency between the Natural Center of Rotation and the Center of Rotation of a Total Disk Arthroplasty Device
If the COR of a TDA device does not match the natural COR, soft tissues may be made to lengthen or shorten and facet joints to impinge or distract. The exact location of the COR will depend on both the specific design of the device and positioning of the implant within the disk space during surgery. A variable COR device is less sensitive than a fixed COR device to surgical placement.21 Although spinal movement follows a variable COR, a fixed COR device may be preferred in some situations in which the ability to resist shear is important, such as with a steeply angled disk space or when pars defects are present.20 To this point, spondylolysis has been considered a contraindication to implantation of TDA devices.
The location of the COR is crucial for proper biomechanics, but its measurement remains difficult. In vitro studies are able to measure COR with high precision but they rely on simplified loads and loading influences the location of the COR. In vivo measurements are approximations taken from two-dimensional radiographic studies.22 These difficulties may explain divergent COR localizations.
Adjacent Segments
The conservation of motion allowed by TDA devices is theorized to prevent degeneration of the adjacent spinal segments. The adjacent segments have normal kinematics after TDA, in contrast to the situation after fusion.23 However, there is inconclusive evidence concerning the relative rates of degeneration of adjacent segments in nonoperated and operated patients.24,25
Shock Absorption Properties
A major function of the natural intervertebral disk is shock absorption. The intervertebral disk takes up most of the axial weight of the trunk and upper extremities and distributes it between the vertebral bodies. “Soft” biomaterials (polymers) are believed to have greater shock absorption capacity than “hard” biomaterials (metals). In vitro studies demonstrate some energy absorption ability with a polyurethane sliding core26 but not with UHMWPE ball-and-socket designs.27 The ability of current TDA devices to absorb shock adequately at physiologically significant levels remains a matter of current debate.
Bogduk N, Mercer S. Biomechanics of the cervical spine. I: Normal kinematics. Clin Biomech. 2000;15:633-648.
Catelas I, Bobyn JD, Medley JB, et al. Size, shape, and composition of wear particles from metal-metal hip simulator testing: effects of alloy and number of loading cycles. J Biomed Mater Res A. 2003;67:312-327.
Cleveland DA. Management of cervical disk and cervical arthritis syndromes. Postgrad Med. 1955;18:99-105.
Crawford NR. Biomechanics of lumbar arthroplasty. Neurosurg Clin N Am. 2005;16:595-602.
Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg Spine. 1998;88:943-948.
Dahl MC, Rouleau JP, Papadopoulos S, et al. Dynamic characteristics of the intact, fused, and prosthetic-replaced cervical disk. J Biomech Eng. 2006;128:809-814.
Delamarter RB, Bae HW, Pradhan BB. Clinical results of ProDisc-II lumbar total disc replacement: report from the United States clinical trial. Orthop Clin North Am. 2005;36:301-313.
Farfan HF. The biomechanical advantage of lordosis and hip extension for upright activity. Man as compared with other anthropoids. Spine. 1978;3:336-342.
Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand. 1966;357(suppl):154-159.
Gracovetsky S, Farfan HF. The optimum spine. Spine. 1986;11:543-573.
Grieve GP. Common Vertebral Joint Problems. Edinburgh: Churchill Livingstone; 1988.
Haher TR, O’Brien M, Felmy WT, et al. Instantaneous axis of rotation as a function of the three columns of the spine. Spine. 1992;17(6 suppl):S149-S154.
Hambly MF, Wiltse LL, Raghavan N, et al. The transition zone above a lumbosacral fusion. Spine. 1998;23:1785-1792.
Harmon HP. Subtotal anterior lumbar disc excision and vertebral body fusion. III. Application to complicated and recurrent multilevel degenerations. Am J Surg. 1959;97:649-659.
Hellier WG, Hedman TP, Kostuit JP. Wear studies for the development of an intervertebral disc prosthesis. Spine. 1992;17(6 suppl):S86-S96.
LeHuec J-C, Kiaer T, Friesen T, et al. Shock absorption in lumbar disc prosthesis. J Spinal Disord Tech. 2003;16:346-351.
Lunsford LD, Bissonnette DJ, Jannetta PJ, et al. Anterior surgery for cervical disc disease, Part 1: treatment of lateral cervical disc herniation in 253 cases. J Neurosurg Spine. 1980;53:1-11.
Moumene M, Geisler FH. Comparison of biomechanical function at ideal and varied surgical placement for two lumbar artificial disc implant designs. Mobile-core versus fixed-core. Spine. 2007;12:1840-1851.
Panjabi MM, Crisco JJ, Vasavada A, et al. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine. 2001;26:2692-2700.
Panjabi MM, Macolmson G, Teng E, et al. Hybrid testing of lumbar Charite discs versus fusion. Spine. 2007;32:959-966.
Panjabi MM, Oxland TR, Yamamoto I, et al. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J Bone Joint Surg Am. 1994;76:413-423.
Pearcy MJ, Bogduk N. Instantaneous axes of rotation of the lumbar intervertebral joints. Spine. 1988;13:1033-1041.
Phillips FM, Carlson G, Emery SE, et al. Anterior cervical pseudarthrosis. Natural history and treatment. Spine. 1997;22:1585-1589.
Schmiedberg SK, Chang DH, Frondoza CG, et al. Isolation and characterization of metallic wear debris from a dynamic intervertebral disc prosthesis. J Biomed Mater Res. 1994;28:1277-1288.
Sears WR, McCombe PF, Sasso RC. Kinematics of cervical and lumbar total disc replacement. Semin Spine Surg. 2006;18:117-129.
Sekhon LHS, Duggal N, Lynch JJ, et al. Magnetic resonance imaging clarity of the Bryan, Prodisc-C, Prestige LP, and PCM cervical arthroplasty devices. Spine. 2007;32:673-680.
White AA, Panjabi MM. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1990.
1 Cleveland DA. Management of cervical disk and cervical arthritis syndromes. Postgrad Med. 1955;18:99-105.
2 Harmon HP. Subtotal anterior lumbar disc excision and vertebral body fusion. III. Application to complicated and recurrent multilevel degenerations. Am J Surg. 1959;97:649-659.
3 Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand. 1966;357(suppl):154-159.
4 Phillips FM, Carlson G, Emery SE, et al. Anterior cervical pseudarthrosis. Natural history and treatment. Spine. 1997;22:1585-1589.
5 Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg Spine. 1998;88:943-948.
6 Delamarter RB, Bae HW, Pradhan BB. Clinical results of ProDisc-II lumbar total disc replacement: report from the United States clinical trial. Orthop Clin North Am. 2005;36:301-313.
7 Schmiedberg SK, Chang DH, Frondoza CG, et al. Isolation and characterization of metallic wear debris from a dynamic intervertebral disc prosthesis. J Biomed Mater Res. 1994;28:1277-1288.
8 Hellier WG, Hedman TP, Kostuit JP. Wear studies for the development of an intervertebral disc prosthesis. Spine. 1992;17(6 suppl):S86-S96.
9 Catelas I, Bobyn JD, Medley JB, et al. Size, shape, and composition of wear particles from metal-metal hip simulator testing: effects of alloy and number of loading cycles. J Biomed Mater Res A. 2003;67:312-327.
10 Sekhon LHS, Duggal N, Lynch JJ, et al. Magnetic resonance imaging clarity of the Bryan, Prodisc-C, Prestige LP, and PCM cervical arthroplasty devices. Spine. 2007;32:673-680.
11 Grieve GP. Common Vertebral Joint Problems. Edinburgh: Churchill Livingstone; 1988.
12 Bogduk N, Mercer S. Biomechanics of the cervical spine. I: Normal kinematics. Clin Biomech. 2000;15:633-648.
13 Pearcy MJ, Bogduk N. Instantaneous axes of rotation of the lumbar intervertebral joints. Spine. 1988;13:1033-1041.
14 Haher TR, O’Brien M, Felmy WT, et al. Instantaneous axis of rotation as a function of the three columns of the spine. Spine. 1992;17(6 suppl):S149-S154.
15 White AA, Panjabi MM. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1990.
16 Farfan HF. The biomechanical advantage of lordosis and hip extension for upright activity. Man as compared with other anthropoids. Spine. 1978;3:336-342.
17 Gracovetsky S, Farfan HF. The optimum spine. Spine. 1986;11:543-573.
18 Panjabi MM, Oxland TR, Yamamoto I, et al. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J Bone Joint Surg Am. 1994;76:413-423.
19 Panjabi MM, Crisco JJ, Vasavada A, et al. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine. 2001;26:2692-2700.
20 Sears WR, McCombe PF, Sasso RC. Kinematics of cervical and lumbar total disc replacement. Semin Spine Surg. 2006;18:117-129.
21 Moumene M, Geisler FH. Comparison of biomechanical function at ideal and varied surgical placement for two lumbar artificial disc implant designs. Mobile-core versus fixed-core. Spine. 2007;12:1840-1851.
22 Crawford NR. Biomechanics of lumbar arthroplasty. Neurosurg Clin N Am. 2005;16:595-602.
23 Panjabi MM, Macolmson G, Teng E, et al. Hybrid testing of lumbar Charite discs versus fusion. Spine. 2007;32:959-966.
24 Lunsford LD, Bissonnette DJ, Jannetta PJ, et al. Anterior surgery for cervical disc disease, Part 1: treatment of lateral cervical disc herniation in 253 cases. J Neurosurg Spine. 1980;53:1-11.
25 Hambly MF, Wiltse LL, Raghavan N, et al. The transition zone above a lumbosacral fusion. Spine. 1998;23:1785-1792.
26 Dahl MC, Rouleau JP, Papadopoulos S, et al. Dynamic characteristics of the intact, fused, and prosthetic-replaced cervical disk. J Biomech Eng. 2006;128:809-814.
27 LeHuec J-C, Kiaer T, Friesen T, et al. Shock absorption in lumbar disc prosthesis. J Spinal Disord Tech. 2003;16:346-351.