Chapter 5 Biology of Anterior Cruciate Ligament Graft Healing
INTRODUCTION
Anterior cruciate ligament (ACL) tears are common among athletes and may become functionally disabling knee injuries. Reconstruction of a torn ACL in order to restore function and limit injury to the menisci has become a common orthopaedic procedure. Despite advances in surgical techniques and the ability to implant an anatomic, isometric graft, ACL reconstruction is not a universally successful procedure. Rates of recurrent laxity 1 year postoperatively have been reported to be as high as 17%.78
Failure of graft integration and tendon-to-bone healing may be an important cause of recurrent laxity. The healing of tendon to bone is the basic requirement for the long-term survival of the graft.12,24 Whether an autograft or an allograft tendon is used, biomechanical testing has shown that the initial strength of the graft material is superior to that of the intact ACL.12,24 Therefore, the weakest link after reconstruction is not the graft itself, but rather the fixation points until graft osteointegration occurs. The intra-articular portion of the graft must also undergo remodeling and a process of “ligamentization” to form a structure that resembles a native ligament.3,5,28,36,38
Current techniques of ACL reconstruction require tendon-to-bone healing in a surgically created bone tunnel. There are no native sites in humans at which a tendon passes through a bone tunnel and, therefore, no analogous situation to the healing that is required after reconstruction. When a bone-tendon-bone graft is used for ACL reconstruction, graft fixation initially depends on bone-to-bone healing. However, tendon-to-bone healing still remains critical regardless of whether a soft tissue graft or a bone-tendon-bone graft is selected. The length of the tendinous portion of the bone-tendon-bone graft is greater than the intra-articular length of the native ACL, resulting in substantial tendon in the bone tunnel. Aperture fixation to minimize graft micromotion and tunnel widening requires tendon-to-bone healing with any graft.38
NATIVE TENDON-BONE INSERTION
Critical Points NATIVE ACL INSERTION
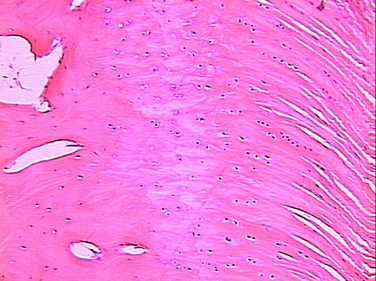
neural elements.4 The ACL inserts to bone via a direct type of insertion, similar to the transition seen from tendon to bone. Microscopic examination of the sites of bony attachment show interdigitation of the collagen fibers with bone through four distinct transition zones: tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone (Fig. 5-1).4,17,57,70 This graduated change in stiffness allows for transmission of complex mechanical loads from soft tissue to bone while minimizing peak stresses at any single point along the ligament. Cartilage-specific collagens including types II, IX, X, and XI are found in the fibrocartilage insertion site. Collagen X plays a key role in maintaining the interface between the mineralized and the unmineralized zones.4,17,57,70
TENDON-BONE INSERTION AFTER ACL RECONSTRUCTION
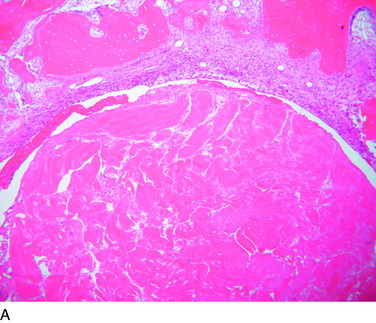
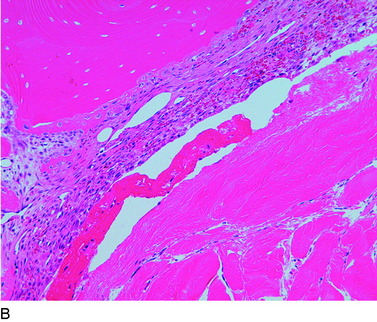
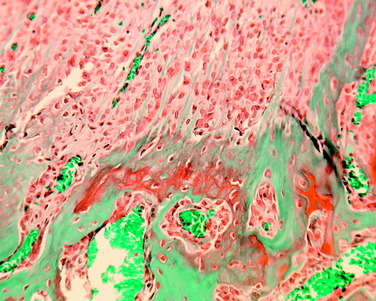
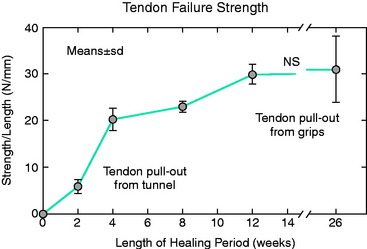
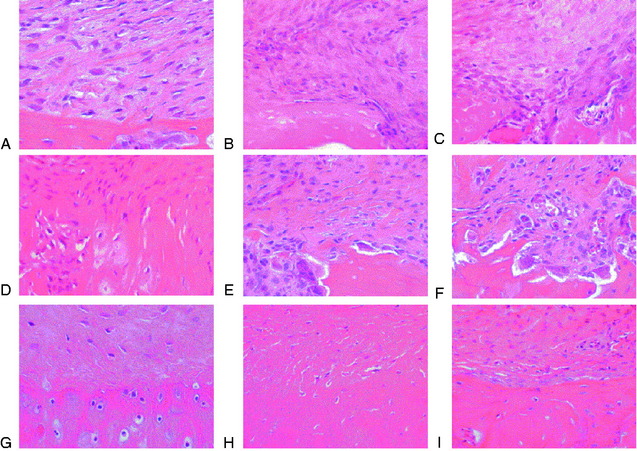
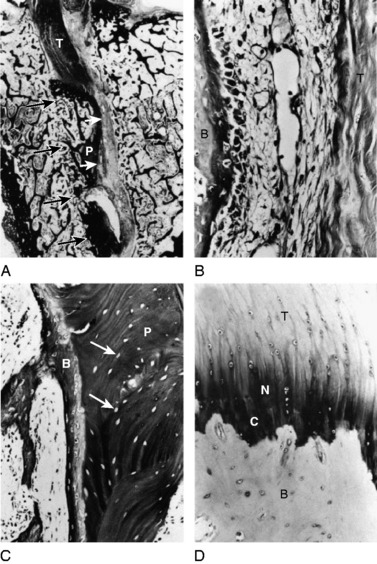
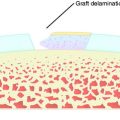
The overall structure, composition, and organization of a native ACL insertion site are not reproduced after ACL reconstruction and reflect an inability to recapitulate the events that occur during embryonic development with current surgical techniques. Rather than regenerating the four organized zones of direct insertion, the graft heals with an interposed zone of vascular, highly cellular granulation tissue between the graft and the tunnel wall (Fig. 5-2).20,21,65 After 3 to 4 weeks, this interface tissue undergoes a maturation process until its matrix consists of oriented, Sharpey-like collagen fibers that bridge the bone to the graft (Fig. 5-3). The number and size of these collagen fibers positively correlate with the pull-out strength of the graft (Fig. 5-4). Graft attachment strength further improves as bone grows into the interface tissue and outer portion of the graft.20,21,65
The maturation process of a tendon graft in a bone tunnel was defined by Kanazawa and coworkers in a rabbit ACL model.34 In the initial postoperative period, the graft-tunnel interface is filled with vascular granulation tissue containing type III collagen. Vascular endothelial growth factor (VEGF) and
Critical Points TENDON-BONE INSERTION AFTER ACL RECONSTRUCTION
fibroblast growth factor (FGF) are expressed, stimulating an influx of macrophages and enlarged fibroblasts. Chondroid cells accumulate along the walls of the bone tunnel and deposit type II collagen. The granulation tissue layer is degraded and becomes indistinct. The chondroid cells are gradually replaced with lamellar bone in a process similar to endochondral ossification.34 The Sharpey-like fibers are composed of type III collagen and extend into the surrounding bone to resist shear stresses. The time interval for this process has been variably reported in the literature, ranging from 8 to 30 weeks.34
CHALLENGES OF TENDON-BONE HEALING AFTER ACL RECONSTRUCTION
The biology of healing between a grafted tendon and a bone tunnel remains incompletely understood. The biologic and biomechanical environments result in the formation of an inferior attachment site that is different from the organized, direct-type ACL insertion. Current work suggests a number of fundamental challenges that are responsible for the suboptimal healing response between tendon and bone instead of regeneration of the insertion site.23 These factors include
MODULATION OF TENDON-BONE HEALING
Technical Factors
Adjustments can be made in the surgical technique of reconstruction to optimize tendon-to-bone healing. The fundamental principle is to maximize the surface area of the tendon-bone interface. Animal studies have shown that increasing the length of the bone tunnel positively correlates with the quality and strength of the reconstruction.80 Minimizing graft tunnel mismatch by achieving as tight a fit as possible also improves healing.22 In addition, maximizing circumferential contact area of the graft and tunnel (i.e., avoiding use of an interference screw) may improve healing.73
Critical Points MODULATION OF TENDON-BONE HEALING
Mechanical Factors
Relative graft-tunnel micromotion may preclude the formation of a firm attachment to the tunnel wall. Yu and Paessler83 compared “aggressive” versus “conservative” rehabilitation protocols in patients after ACL reconstruction with quadrupled hamstring grafts. Significantly greater tunnel widening was reported in the aggressive rehabilitation group, supporting the view that graft-tunnel micromotion is a cause of tunnel widening and that the mechanical environment in the bone tunnel influences tendon-bone healing.
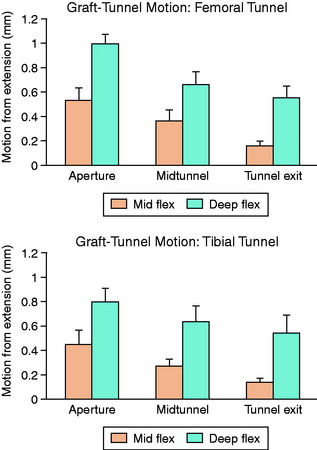
Animal studies have corroborated these clinical observations. Sakai and associates71 compared immediate motion with varying periods of immobilization for up to 6 weeks after ACL reconstruction in a rabbit model. Biomechanical analysis demonstrated a greater load-to-failure of the graft in immobilized animals, and histologic studies demonstrated closer tendon-bone apposition with less fibrovascular interface scar tissue. Rodeo and colleagues66 evaluated the effect of graft-tunnel motion on tendon-to-bone healing in a rabbit ACL reconstruction model. ACL reconstruction was performed in five cadaveric rabbit limbs with aluminum beads fixed to the tendon and bone tunnel. Three-dimensional graft-tunnel motion was quantified using micro–computed tomography (CT) analysis. Motion was observed to be greatest at the tunnel apertures and least at the tunnel exits, adjacent to the graft fixation at the tunnel exit (Fig. 5-5). Histomorphometric analysis demonstrated healing to be slowest at the apertures, with a wider fibrovascular interface tissue present between tendon and bone. An inverse relationship between graft-tunnel motion and healing in the femoral tunnel was demonstrated. In addition, osteoclasts were preferentially observed at the tunnel aperture, supporting the hypothesis that graft-tunnel motion may stimulate osteoclast-mediated bone resorption and secondary tunnel widening.66
Biologic Factors
Modulation of the Inflammatory Response
Immediately after ACL reconstruction and release of the tourniquet, the knee is filled with blood from the drilled bone tunnels. This initiates an acute inflammatory response marked by influx of neutrophils, macrophages, and mesenchymal cells. Important lessons about the effect of this inflammatory response on tissue healing can be learned from the study of fetal wound healing. Wounds in the embryo and early fetus heal by tissue regeneration and result in “scarless healing.” These healing events are characterized by the absence of an acute inflammatory response. Inflammation after trauma, although essential for healing in adults, is also responsible for healing by scar rather than regeneration of native tissue. The rapid inflammatory response that ensues after ACL reconstruction may trigger a cascade of events that ultimately leads to fibrosis rather than tissue regeneration and an anatomic tendon-to-bone insertion.19,39,72
The fibrin clot that forms after surgery allows for a controlled release of cytokines that drive the early response. Transforming growth factor-β (TGF-β) and platelet derived growth factor (PDGF) act together to modulate tissue healing and matrix deposition by recruiting neutrophils and macrophages to the local tissue.19,39,72 Neutrophil influx peaks at about 2 days after the operation and is followed by an influx of macrophages. These monocytes are essential to the early formation of granulation tissue from the clot, initiating the process of soft tissue adherence to bone.35 They also drive the angiogenic phase, providing nutrients and oxygen for the prolonged period of matrix synthesis and remodeling that ensues over the next several weeks. TGF-β secreted by macrophages recruits and stimulates fibroblasts to degrade matrix through matrix metalloproteinases (MMPs). Simultaneously, these fibroblasts synthesize new matrix proteins to replace the early granulation tissue with scar tissue. Tissue inhibitors of matrix metalloproteinases (TIMPs) inhibit MMPs and provide a check-and-balance regulation to this complex process of matrix degradation, synthesis, and remodeling.18
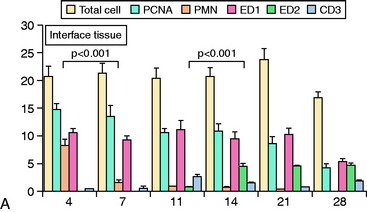
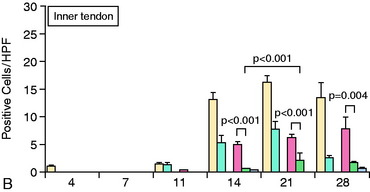
The accumulation of macrophages around the tendon graft in bone has been further characterized in a rat ACL reconstruction model.35 Two distinct subpopulations of macrophages were identified at the tendon-bone interface. ED1+ macrophages derived from the circulation act in a proinflammatory fashion in the early response with neutrophils to migrate into the tissues and remove debris after surgery. This is followed by the accumulation of proregenerative ED2+ macrophages from the local tissues, which promote anabolic tissue healing and scar formation by fibroblasts via TGF-β secretion (Fig. 5-6).35
Given the critical role of macrophages in scar tissue formation, animal studies have investigated the role of macrophage depletion on tendon-bone healing. It has been theorized that depletion may promote formation of a “scarless,” native insertion site. Hays and coworkers26 in the authors’ laboratory demonstrated less scar tissue formation, improved collagen organization, and superior biomechanical strength in a rat ACL reconstruction model when macrophages were depleted by administration of liposomal clodronate, a selective inducer of macrophage apoptosis. Other studies have identified gastric pentadecapeptide BPC 157, a novel anti-inflammatory used in the treatment of inflammatory bowel disease, to improve Achilles tendon-to-surface bone healing and tissue regeneration in a rat Achilles tendon model.37
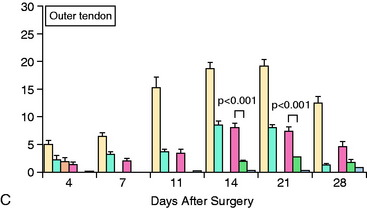
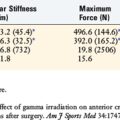
The impact of inflammatory response suppression, however, has not been uniformly favorable for tendon-bone healing. Cohen and associates11 evaluated the effect of the anti-inflammatory medications indomethacin and celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, on tendon-bone healing in a rat rotator cuff model. The treated group was found to have histologically and biomechanically inferior tendon-bone interfaces at both 4 and 8 weeks relative to controls (Fig. 5-7).
Modulation of Bone Ingrowth
Bone ingrowth plays a critical role in the later stages of tendon-bone healing after ACL reconstruction and is ultimately responsible for the improved biomechanical properties after healing is complete. Animal studies have provided strong evidence to support that increased bone ingrowth positively correlates with graft-bone fixation strength.67 The primary strategies that have been employed include use of osteoinductive agents, osteoconductive agents, and modulation of osteoclast activity.
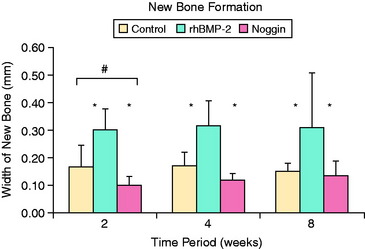
Osteoinductive agents, particularly from the bone morphogenic protein (BMP) family, have been shown to promote ingrowth of bone from the tunnel into the soft tissue graft. Rodeo and coworkers67 delivered recombinant human BMP-2 (rhBMP-2) on an absorbable type I collagen sponge in a tendon-bone canine model. At all time points, the rhBMP-2–treated limbs healed with increased bone formation around the tendon. Biomechanical testing demonstrated higher tendon pull-out strength in the treated limbs relative to controls at 2 weeks. Ma and colleagues41 delivered rhBMP-2 in an injectable calcium phosphate matrix to the bone tunnel in a rabbit ACL reconstruction model. Histologic analysis revealed a dose-dependent increase in bone formation at the tendon-bone interface and significantly narrower tunnel diameters (15%–45%) relative to the control group. Increased construct stiffness was also seen in the treatment group at 8 weeks postoperatively.41 Martinek and associates44 transected semitendinosus grafts in vitro with adenovirus-BMP-2 (Ad-BMP-2) and compared them with untreated controls in a rabbit ACL reconstruction model. These investigators demonstrated the formation of a fibrocartilaginous interface at the tendon-bone junction in the experimental group that was absent in the controls. Both stiffness and load-to-failure parameters were superior in the treatment group at 8 weeks. BMP-7 has also been shown to improve bone formation at the tendon-bone interface and load to failure at both 3 and 6 weeks postoperatively in an ovine reconstruction model.1,45
Further support for the role of BMPs in bone ingrowth around tendon graft comes from studies of BMP inhibition. Ma and colleagues41 delivered noggin, a potent inhibitor of all BMP activity, to the healing tendon-bone interface using an injectable calcium phosphate matrix in a rabbit ACL reconstruction model. Noggin significantly inhibited new bone formation at the tendon-bone interface. Furthermore, a significant increase in the width of the fibrous tissue interface between tendon and bone was found in the noggin-treated animals (Fig. 5-8).
The favorable effect of osteoinductive agents on tendon-bone healing in a tunnel is further supported by studies that have used tendons wrapped in periosteum. Transplantation of a long digital extensor tendon wrapped in periosteum into a tunnel in the proximal tibia was compared with controls in a rabbit model.10,61 Improved biomechanical strength and bone and fibrocartilage formation at the tendon-bone interface were shown in the treated animals at 8 and 12 weeks. Ohtera and colleagues61 completed further studies comparing fresh and frozen periosteum and demonstrated better histologic and biomechanical outcomes using fresh periosteal wraps. These findings support the hypothesis that the viable osteoinductive factors in the periosteum may contribute to the improved outcomes.
Osteoconductive agents have also been met with favorable outcomes in animal models. Calcium phosphate (CaP)–hybridized grafts used in a rabbit ACL reconstruction model demonstrated improved fibrocartilage and bone formation relative to animals treated with unhybridized grafts at 3 weeks postoperatively.56 Furthermore, studies by Tien and coworkers76 using injectable CaP cement in the femoral tunnel of a rabbit ACL reconstruction model showed improved bone formation and biomechanical strengths relative to controls at 1 and 2 weeks postoperatively.
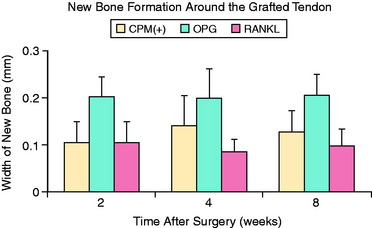
Modulation of osteoclast activity in the bone tunnel is another technique of promoting bone formation at the tendon-bone interface. Furthermore, inhibition of osteoclast-mediated bone resorption offers a potential means by which to limit the bone tunnel expansion commonly seen after ACL reconstruction. One study demonstrated that increased knee laxity correlated with radiographic tunnel widening after ACL reconstruction using hamstring tendon.25 The role of osteoclastic bone resorption on tendon-bone healing has been evaluated in a rabbit ACL reconstruction model.15 Osteoprotegerin (OPG), a potent inhibitor of osteoclast activity, or receptor activator of nuclear factor κB ligand (RANKL), a potent stimulant of osteoclast formation, were delivered to the bone tunnels around a tendon graft using a CaP carrier matrix. A significantly greater amount of bone surrounding the tendon at the interface was seen in the OPG-treated limbs relative to controls and RANKL-treated limbs at all time points (Fig. 5-9).15 Furthermore, biomechanical testing at 8 weeks demonstrated significantly increased stiffness of the femur-graft-tibia complex in the OPG-treated limbs compared with the RANKL-treated limbs.
Stem Cells
Undifferentiated, pluripotent mesenchymal cells, also termed stem cells, may be critical to stimulate tissue regeneration rather than scar formation at the tendon-bone interface. These cells retain the capacity to differentiate into various specialized cell types based on biologic signals in the local environment. Animal studies have tested the effects of local stem cell delivery on tendon-bone healing. Rabbit bone marrow stromal cells placed in a fibrin glue carrier were delivered to the tendon-bone interface of hallucis longus tendon in a calcaneal tunnel.62 Histologic analysis revealed improved healing with fibrocartilaginous attachment between tendon and bone in the experimental group. Lim and associates40 performed bilateral ACL reconstructions in a rabbit model and evaluated the role of mesenchymal stem cells (MSC) on the tendon-bone interface. The grafts coated with MSC demonstrated cartilage at the tendon-bone interface, whereas only fibrous tissue was observed at the interface in the contralateral control limbs. The interface stained positively for type II collagen in the MSC-treated grafts, and was similar in organization to a native, direct ligament insertion. Furthermore, biomechanical testing at 8 weeks demonstrated higher loads to failure and stiffness relative to controls.40
Modulation of Vascularity
Whereas vascularity is critical for the efficient delivery of oxygen and nutrients to support tissue healing, the precise role of local vascularity at the tendon-bone interface remains to be defined. Krivic and colleagues37 demonstrated improved vascularity at the healing Achilles tendon–bone interface after treatment with gastric pentadecapeptide BPC 157 in a rat model. This improved vascularity correlated with favorable histologic and biomechanical properties of the tendon-bone interface. In contrast, however, a recent study examined the effect of VEGF on graft healing in an ovine ACL reconstruction model.82 Although vascularity and cellularity were increased in the VEGF-soaked grafts relative to controls, the stiffness of the femur-graft-tibia complex was significantly inferior to controls at 3 months. Although only a single concentration of VEGF was utilized, the results present the possibility that excessive vascularity may adversely affect healing and the biomechanical properties of the tendon-bone interface.82
Modulation of MMPs
The MMPs are a family of zinc-dependent endoproteinases that play a critical role in tissue degradation, healing, and normal remodeling. They function both in an extracellular environment and through transmembrane and intracytoplasmic domains.14 Inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF) initiate the transcription and activation of MMPs from their zymogen form. Their catabolic, destructive activity is balanced, however, by TIMPs. TIMPs provide a check-and-balance mechanism to control the activity of these degradative enzymes and thereby maintain homeostasis of extracellular matrix formation and degradation that occurs with tissue remodeling.18
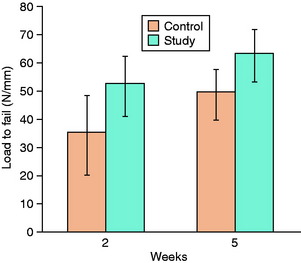
Synovial fluid tracking between the graft and the tunnel walls after ACL reconstruction is known to contain large amounts of collagenase and stromelysin. It has been theorized that these MMPs may have adverse effects on the tendon-bone interface, perhaps by limiting the formation of Sharpey-like collagen fibers. Demirag and coworkers14 used α2-macroglobulin, an endogenous inhibitor of MMPs, to study this hypothesis in a rabbit ACL reconstruction model. Each rabbit underwent bilateral ACL reconstruction with hamstring autograft. α2-Macroglobulin was injected into one knee postoperatively and compared with the contralateral control limb. The interface tissue in treated specimens was more mature with significantly greater Sharpey-like fibers. Biomechanical studies demonstrated greater load to failure compared with controls at 2 and 5 weeks14 (Fig. 5-10). Further studies are necessary to characterize the precise mechanism of action of MMPs at the tendon-bone interface. Nonetheless, this work provides preliminary evidence that modulation of MMP activity can improve graft-bone healing in a bone tunnel.
Modulation of Nitric Oxide
Nitric oxide is a free radical agent synthesized by nitric oxide synthase from l-arginine. It acts as a regulatory molecule both in cells and in the extracellular matrix. Studies have shown that it is induced during tendon healing in vitro, with a dose-dependent effect on fibroblast collagen production.54,55 ACL ligament fibroblasts are uniquely able to produce more nitric oxide compared with other local fibroblasts, including those derived from the medial collateral ligament (MCL).46 The influence of nitric oxide levels on tendon-bone healing after ACL reconstruction remains to be defined.
Effect of Hyperbaric Oxygen
Yeh and associates81 recently studied the influence of hyperbaric oxygen (HBO) therapy on the graft-bone tunnel interface in a rabbit model. The HBO group was exposed to 100% oxygen at 2.5 atm pressure for 2 hours daily, 5 consecutive days a week. The control group was exposed to normal air. The HBO group demonstrated increased neovascularization and an increased number of Sharpey fibers relative to controls. Furthermore, the HBO group achieved higher maximal pull-out strengths at 12 and 18 weeks relative to control specimens.81 Although the mechanism of action is unclear, these preliminary results suggest that HBO therapy may improve tendon-bone healing after ACL reconstruction.
BONE-TO-BONE HEALING IN ACL AUTOGRAFT RECONSTRUCTION
The sequence of bone-tendon-bone autograft incorporation has been defined in animal models. Tomita and colleagues77 compared healing of a soft tissue graft to a bone–patellar tendon–bone autograft in a canine model. The bone plug undergoes osteonecrosis and is gradually replaced by a process of creeping substitution (Fig. 5-11). Newly formed bone surrounding the plug is seen at 3 weeks. These investigators found pull-out strength of the bone–patellar tendon–bone graft to be superior to the soft tissue graft at 3 weeks, but not significantly different at 6 weeks. At 6 weeks, a change in the point of failure was noted from the graft-tunnel interface to the tendon-bone plug interface. Papageorgiou and coworkers64 directly compared tendon-bone with bone-bone healing in a goat model. Bone–patellar tendon autografts were harvested; the soft tissue graft was placed in the tibial tunnel while the bone plug was secured in the femoral tunnel. Biomechanical testing at 3 weeks demonstrated universal failure of the tendon-bone interface with
Critical Points BONE-TO-BONE HEALING AFTER ACL AUTOGRAFT RECONSTRUCTION
pull-out from the tibial tunnel. At 6 weeks, however, approximately 20% of cases demonstrated midsubstance failures. The remaining cases continued to be tibial tunnel graft pull-out. Histologic evaluation at 3 weeks confirmed creeping substitution with a necrotic bone plug surrounded by granulation tissue. Evaluation at 6 weeks revealed complete plug incorporation with bridging cancellous bone.64
Synovial fluid flows from the joint into the tunnels and can interfere with healing at the tendon-bone interface. The high quantities of MMPs and other proteolytic enzymes in synovial fluid can slow bone-tendon-bone and bone-bone healing. Berg and associates9 evaluated the role of synovial fluid by drilling femoral and tibial tunnels and leaving them empty in a rabbit ACL model. These authors found healing to occur most rapidly at points farthest away from the joint, whereas the slowest and most incomplete regions of healing were at the tunnel apertures. These findings are suggestive of a possible inhibitory effect of synovial fluid on healing in the tunnel.
INTRA-ARTICULAR GRAFT HEALING IN ACL AUTOGRAFT RECONSTRUCTION
Ligamentization refers to the complex process of biologic incorporation and remodeling that occurs to the tendon after ACL reconstruction. Animal studies have attempted to define the phases of intra-articular graft healing that occur postoperatively. The graft goes through an initial phase of avascular necrosis. At 2 weeks, the graft demonstrates patches of necrosis, although the collagen architecture and scaffold remain intact.2,3,36,63 By 4 weeks, the graft is almost entirely avascular and acellular. This phase, however, is followed by cellular repopulation from the host synovial cells. Biopsy at 3 months reveals extensive vascular proliferation and cellular repopulation. By 9 months, the graft histologically resembles the native ligament.2,3,36,63
Intra-articular healing has also been studied in other animal models. Oaks and colleagues60 demonstrated remodeling to occur from the periphery toward the graft center. This was associated with a change from large-diameter to small-diameter collagen fibrils. This pattern of remodeling supports the hypothesis that healing is dependent on gradual revascularization and cellular repopulation from the host synovium. Arnoczky and coworkers3 evaluated patellar tendon graft revascularization in a canine model. Initially the grafts were avascular, but by 6 weeks, they were completely ensheathed in a vascular synovial envelope. The soft tissues of the infrapatellar fat pad, the tibial remnant of the ACL, and the posterior synovial tissues contributed to this synovial vasculature. Intrinsic revascularization of the patellar tendon graft progressed from the proximal and distal portions of the graft centrally.3 The tibial attachment of the patellar tendon graft did not contribute any vessels to the revascularization process. The contribution of the soft tissues of the knee to the revascularization process of the graft emphasized the importance of their preservation in maintaining the graft’s viability.
Although animal models have provided insight into the process of ligamentization, the process has not been fully characterized in humans. Jackson and associates28 concluded that an incorporated graft never replicates the native ACL and rather functions like a checkrein of organized scar tissue. Delay and
Critical Points INTRA-ARTICULAR GRAFT HEALING IN ACL AUTOGRAFT RECONSTRUCTION
colleagues13 described a case report of a bone–patellar tendon–bone autograft with avascular, acellular regions in the deep, distal graft 18 months after reconstruction. Rougraff and Shelbourne69 performed patellar tendon graft biopsies on nine subjects 3 to 8 weeks after autogenous ACL reconstruction. Graft vascularity was present at 3 weeks and increased over the 8-week interval.
PRIMARY ACL HEALING AND REPAIR
Primary repair of the ACL after traumatic rupture was historically considered to be a viable surgical option. Outcome studies, however, have reported unacceptable rates of failure after primary surgical repair.16 In their classic study, Feagin and Curl16 reported a 94% rate of instability in patients at 5-year follow-up after primary suture and drill hole ACL repair. Marshall and coworkers43 reported a 20% to 40% failure rate despite repair using a sophisticated, multiple-depth suture technique. Zysk and Refior85 also reported modest outcomes in a study on middle-aged patients who underwent primary ACL repair and recommended that open primary repair be abandoned in favor of autogenous tissue reconstruction or augmentation. These results were recently corroborated by a Norwegian study that assessed long-term outcomes in a large series of patients who underwent primary ACL repair.75 An open procedure using the original Palmer technique with nonabsorbable Bunnell sutures was performed in all cases. At 15 to 23 years postoperatively, 57% had greater than 3 mm of anterior translation on KT-1000 testing. The estimated rate of total failure was 27%. These results are in sharp contradistinction to the MCL, in which failure to primarily heal is the exception rather than the rule.
The poor rate of primary healing observed after ACL rupture is believed to be multifactorial in nature. One of the most salient factors is the intra-articular environment and synovial fluid that surrounds the ACL.47–53,74 Studies by Murray and associates47–53,74 elegantly demonstrated the differences in intra-articular (i.e., ACL) versus extra-articular (i.e., MCL) healing in a canine, central ACL wound model. An empty wound persists at the defect of an intra-articular ligament wound, whereas these wounds are rapidly filled with a fibrin-platelet scaffold in an extra-articular injury. This scaffold is critical to allow subsequent cellular repopulation, revascularization, and ligament remodeling into mature scar tissue. The lack of a scaffold in the intra-articular ligament wounds was associated with decreased inflammatory cytokines needed for the healing response, including fibrinogen, PDGF, TGF-β, and FGF.47–53,74 Studies by Murray and associates47–53,74 demonstrated that replacement of the central intra-articular ligament void with a collagen-platelet–rich plasma scaffold resulted in increased filling of the wound with repair tissue that
had similar profiles of protein expression to matched, extra-articular ligament wounds. Biomechanical studies of suture ACL repair augmented with a collagen-platelet–rich scaffold in a porcine model have shown significant improvement in load to failure and linear stiffness at 4 weeks compared with unaugmented, control repairs.47–53,74
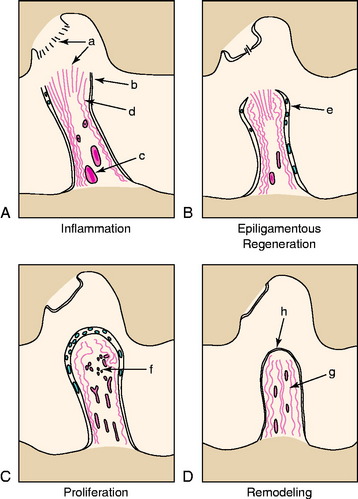
Other factors implicated in the poor ACL healing response include alterations in cellular metabolism after injury, cellular loss within the tissues after injury, and intrinsic deficiencies in ACL fibroblasts compared with fibroblasts in extra-articular ligaments. Murray and associates47–53,74 defined the histologic changes that occur after ACL rupture. The human ACL undergoes four histologic phases: inflammation, epiligamentous regeneration, proliferation, and remodeling (Fig. 5-12). Although similar to the response to injury in other dense connective tissues, major exceptions include (1) formation of an alpha-smooth muscle actin-expressing synovial cell layer on the surface of the ruptured ends, (2) the lack of any tissue bridging the rupture site, and (3) the presence of an epiligamentous reparative phase that lasts 8 to 12 weeks.47–53,74 The biology involved in intra-articular ligament healing will need to be further defined to overcome the obstacles to long-term success with primary ACL repair.
ALLOGRAFT HEALING IN ACL RECONSTRUCTION
An increasing desire to avoid the morbidity of graft harvest and to reduce operative times has led to a dramatic increase in the use of allografts as an alternative graft source in primary ACL reconstruction.7 Allografts are also frequently used in revision procedures or multiligament reconstruction procedures. Good clinical outcomes have been reported with allograft reconstructions at 2 to 5 years follow-up, and multiple studies have found no significant subjective or objective difference in knee function after allograft versus autograft ACL reconstructions.8 However, other studies have reported less favorable results after allograft reconstruction, particularly in the setting of chronic or revision knee surgery.58,59
Despite compatible clinical outcomes, animal studies have shown that allografts have a slower rate of incorporation, prolonged inflammatory response, and greater initial decrease in biomechanical properties compared with autografts.2–6,29–33,84 The phases of healing, although delayed, resemble those of the autograft. The tendon-bone interface develops a fibrovascular granulation tissue interface that eventually undergoes bone
ingrowth and develops Sharpey-like anchoring fibers.2–6,32,33,84 The intra-articular graft undergoes ligamentization with a phase of avascular necrosis followed by cellular repopulation and vascular proliferation from host synovium. Donor DNA was entirely replaced by host DNA within 4 weeks in a goat reconstruction model. Revascularization starts at 3 weeks and progresses gradually over the next several weeks. Jackson and colleagues compared healing of patellar tendon autografts with fresh allografts in a goat ACL reconstruction model.31–33 Although graft structural properties were similar at time zero, the allografts healed at a much slower rate. At 6 months, the autografts demonstrated a superior load to failure, larger increase in graft cross-sectional area, and better restraint to anteroposterior displacement. The allografts demonstrated a significantly greater decrease in their preimplantation structural properties.
Human studies have supported these findings of slower allograft incorporation. At 2 years postoperatively, biopsy studies have shown that the central portion of allografts can continue to be acellular.27 Cellular repopulation of the entire graft was often seen only after 3 or more years after allograft reconstruction.42
Because of the relative hypocellularity of tendon allografts and current sterilization techniques, the host immune response is relatively limited. Major histocompatibility complex (MHC) antigens that incite a potent immune response are largely depleted. Matrix antigens, however, persist and can elicit an immune response that may contribute to the delayed incorporation and the pronounced alteration in structural properties after surgery.68,79 The specific influences of such an immune response on the biology of ACL graft healing remains to be defined.
1 Anderson K., Seneviratne A.M., Izawa K., et al. Augmentation of tendon healing in an intra-articular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29:689-698.
2 Arnoczky S.P. Biology of ACL reconstructions: what happens to the graft? Instr Course Lect. 1996;45:229-233.
3 Arnoczky S.P., Tarvin G.B., Marshall J.L. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64:217-224.
4 Arnoczky S.P. Anatomy of the ACL. Clin Orthop. 1983;172:19-25.
5 Arnoczky S.P., Warren R.F., Ashlock M.A. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68:376-385.
6 Arnoczky S.P., Rubin R.M., Marshall J.L. Microvasculature of the cruciate ligaments and its response to injury. An experimental study in dogs. J Bone Joint Surg Am. 1979;61:1221-1229.
7 Bach B.R.Jr., Aadalen K.J., Dennis M.G., et al. Primary anterior cruciate ligament reconstruction using fresh-frozen, nonirradiated patellar tendon allograft: minimum 2-year follow-up. Am J Sports Med. 2005;33:284-292.
8 Barrett G., Stokes D., White M. Anterior cruciate ligament reconstruction in patients older than 40 years: allograft versus autograft patellar tendon. Am J Sports Med. 2005;33:1505-1512.
9 Berg E.E., Pollard M.E., Kang Q. Interarticular bone tunnel healing. Arthroscopy. 2001;17:189-195.
10 Chen C.H., Chen W.J., Shih C.H., et al. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: A biomechanical and histologic study in rabbits. Arthroscopy. 2003;19:290-296.
11 Cohen D.B., Kawamura S., Ehteshami J.R., Rodeo S.A. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362-369.
12 Cooper D.E., Deng X.H., Burstein A.L., Warren R.F. The strength of the central third patellar tendon graft. A biomechanical study. Am J Sports Med. 1993;21:818-823. discussion 823–824
13 Delay B.S., McGrath B.E., Mindell E.R. Observations on a retrieved patellar tendon autograft used to reconstruct the anterior cruciate ligament. J Bone Joint Surg Am. 2002;84:1433-1438.
14 Demirag B., Sarisozen B., Durak K., et al. The effect of alpha-2 macroglobulin on the healing of ruptured anterior cruciate ligament in rabbits. Connect Tissue Res. 2004;45:23-27.
15 Dynybil C., Kawamura S., Kim H.J., et al. [The effect of osteoprotegerin on tendon-bone healing after reconstruction of the anterior cruciate ligament: a histomorphological and radiographical study in the rabbit]. Z Orthop Ihre Grenzgeb. 2006;144:179-186.
16 Feagin J.A.Jr., Curl W.W. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Clin Orthop Relat Res. 1996;325:4-9.
17 Fujioka H., Thakur R., Wang G.J., et al. Comparison of surgically attached and non-attached repair of the rat Achilles tendon-bone interface. Cellular organization and type X collagen expression. Connect Tissue Res. 1998;37:205-218.
18 Gomez D.E., Alonso D.F., Yoshiji H., Thorgeirsson U.P. Tissue inhibitors of metalloproteinases: structures, regulation and biological functions. Eur J Cell Biol. 1997;74:111-122.
19 Goodman R.B., Pugin J., Lee J.S., Matthay M.A. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523-535.
20 Goradia V.K., Rochat M.C., Grana W.A., et al. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13:143-151.
21 Grana W.A., Egle D.M., Mahnken R., Goodhart C.W. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344-351.
22 Greis P.E., Burks R.T., Bachus K., Luker M.G. The influence of tendon length and fit on the strength of a tendon-bone tunnel complex. A biomechanical and histologic study in the dog. Am J Sports Med. 2001;29:493-497.
23 Gulotta L.V., Rodeo S.A. Biology of autograft and allograft healing in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:509-524.
24 Hamner D.L., Brown C.H.Jr., Steiner M.E., et al. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am. 1999;81:549-557.
25 Hantes M.E., Mastrokalos D.S., Yu J., Paessler H.H. The effect of early motion on tibial tunnel widening after anterior cruciate ligament replacement using hamstring tendon grafts. Arthroscopy. 2004;20:572-580.
26 Hays P., Kawamura S., Deng X., et al. The role of macrophages in early healing of a tendon graft in a bone tunnel: an experimental study in a rat anterior cruciate ligament reconstruction model. J Bone Joint Surg Am. 2008;90:565-579.
27 Hortsman J.K., Ahmadu-Suka F., Norrdin R.W. Anterior cruciate ligament fascia lata allograft reconstruction: progressive histological changes towards maturity. Arthroscopy. 1993;9:509-518.
28 Jackson D.W., Grood E.S., Goldstein J.D., et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176-185.
29 Jackson D.W., Simon T.M. Donor cell survival and repopulation after intra-articular transplantation of tendon and ligament allografts. Microsc Res Tech. 2002;58:25-33.
30 Jackson D.W., Grood E.S., Arnoczky S.P., et al. Freeze-dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med. 1987;5:295-303.
31 Jackson D.W., Grood E.S., Cohn B.T., et al. The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg Am. 1991;73:201-213.
32 Jackson D.W., Simon T.M., Kurzweil P.R., Rosen M.A. Survival of cells after intra-articular transplantation of fresh allografts of the patellar and anterior cruciate ligaments. DNA-probe analysis in a goat model. J Bone Joint Surg Am. 1992;74:112-118.
33 Jackson D.W., Corsetti J., Simon T.M. Biologic incorporation of allograft anterior cruciate ligament replacements. Clin Orthop Relat Res. 1996;324:126-133.
34 Kanazawa T., Soejima T., Murakami H., et al. An immunohistological study of the integration at the bone-tendon interface after reconstruction of the anterior cruciate ligament in rabbits. J Bone Joint Surg Br. 2006;88:682-687.
35 Kawamura S., Ying L., Kim H.J., et al. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425-1432.
36 Kleiner J.B., Amiel D., Roux R.D., Akeson W.H. Origin of replacement cells for the anterior cruciate ligament autograft. J Orthop Res. 1986;4:466-474.
37 Krivic A., Anic T., Seiwerth S., et al. Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: promoted tendon-to-bone healing and opposed corticosteroid aggravation. J Orthop Res. 2006;24:982-989.
38 Kurosaka M., Yoshiya S., Andrish J.T. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:225-229.
39 Leask A., Holmes A., Abraham D.J. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4:136-142.
40 Lim J.K., Hui J., Li L., et al. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899-910.
41 Ma C., Kawamura S., Deng X., et al. BMP-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med. 2007;35:597-604.
42 Malinin T.I., Levitt R.L., Bashore C., et al. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18:163-170.
43 Marshall J.L., Warren R.F., Wickiewicz T.L. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10:103-107.
44 Martinek V., Latterman C., Usas A., et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer. A histological and biomechanical study. J Bone Joint Surg Am. 2002;84:1123-1131.
45 Mihelic R., Pecina M., Jelic M., et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619-1625.
46 Murakami H., Shinomiya N., Kikuchi T., et al. Up-regulated expression of inducible nitric oxide synthase plays a key role in early apoptosis after anterior cruciate ligament injury. J Orthop Res. 2006;24:1521-1534.
47 Murray M.M., Spindler K.P., Ballard P., et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007-1017.
48 Murray M.M., Spindler K.P., Abreu E., et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81-91.
49 Murray M.M., Spindler K.P., Devin C., et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820-830.
50 Murray M.M., Bennett R., Zhang X., Spector M. Cell outgrowth from the human ACL in vitro: regional variation and response to TGF-beta1. J Orthop Res. 2002;20:875-880.
51 Murray M.M., Martin S.D., Martin T.L., Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82:1387-1397.
52 Murray M.M., Martin S.D., Spector M. Migration of cells from human anterior cruciate ligament explants into collagen-glycosaminoglycan scaffolds. J Orthop Res. 2000;18:557-564.
53 Murray M.M., Spector M. Fibroblast distribution in the anteromedial bundle of the human anterior cruciate ligament: the presence of alpha-smooth muscle actin-positive cells. J Orthop Res. 1999;17:18-27.
54 Murrell G.A., Szabo C., Hannafin J.A., et al. Modulation of tendon healing by nitric oxide. Inflamm Res. 1997;46:19-27.
55 Murrell G.A., Doland M.M., Jang D., et al. Nitric oxide: an important articular free radical. J Bone Joint Surg Am. 1996;78:265-274.
56 Mutsuzaki H., Sakane M., Nakajima H., et al. Calcium-phosphate-hybridized tendon directly promotes regeneration of tendon-bone insertion. J Biomed Mater Res A. 2004;70:319-327.
57 Niyibizi C., Sagarrigo Visconti C., Gibson G., Kavalkovich K. Identification and immunolocalization of type X collagen at the ligament-bone interface. Biochem Biophys Res Commun. 1996;222:584-589.
58 Noyes F.R., Barber-Westin S.D., Roberts C. Use of allografts after failed treatment of rupture of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:1019-1031.
59 Noyes F.R., Barber S.D. The effect of a ligament augmentation device on allograft reconstructions for chronic ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 1992;74:960-973.
60 Oaks B.W., Knight M., McLean I.D., et al. Goat ACL autograft collagen remodeling: quantitative collagen fibril analysis over one year. In: Transactions of the Combined Meeting of the Orthopaedic Research Societies of USA, Japan, and Canada. Alberta, Canada: Calgary; October 21–23, 1991:60.
61 Ohtera K., Yamada Y., Aoki M., et al. Effects of periosteum wrapped around tendon in a bone tunnel: a biomechanical and histological study in rabbits. Crit Rev Biomed Eng. 2000;28:115-118.
62 Ouyang H.W., Goh J.C., Lee E.H. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32:321-327.
63 Panni A.S., Milano G., Lucania L., Fabbriciani C. Graft healing after anterior cruciate ligament reconstruction in rabbits. Clin Orthop Relat Res. 1997;343:203-212.
64 Papageorgiou C.D., Ma C.B., Abramowitch S.D., et al. A multidisciplinary study of the healing of an intra-articular anterior cruciate ligament graft in a goat model. Am J Sports Med. 2001;29:620-626.
65 Rodeo S.A., Arnoczky S.P., Torzilli P.A., et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795-1803.
66 Rodeo S.A., Kawamura S., Kim H.J., et al. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34:1790-1800.
67 Rodeo S.A., Suzuki K., Deng X.H., et al. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476-488.
68 Rodrigo J., Jackson D., Simon T., Muto K. The immune response to freeze-dried bone-tendon-bone ACL allografts in humans. Am J Knee Surg. 1993;6:47-53.
69 Rougraff B.T., Shelbourne K.D. Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1999;7:9-14.
70 Sagarriga H., Visconti C., Kavalkovich K., et al. Biochemical analysis of collagens at the ligament-bone interface reveals presence of cartilage-specific collagens. Arch Biochem Biophys. 1996;328:135-142.
71 Sakai H., Fukui N., Kawakami A., Kurosawa H. Biological fixation of the graft within bone after anterior cruciate ligament reconstruction in rabbits: effects of the duration of postoperative immobilization. J Orthop Sci. 2000;5:43-51.
72 Singer A.J., Clark R.A. Cutaneous wound healing. N Engl J Med. 1999;341:738-746.
73 Singhatat W., Lawhorn K.W., Howell S.M., Hull M.L. How four weeks of implantation affect the strength and stiffness of a tendon graft in a bone tunnel: a study of two fixation devices in an extra-articular model in ovine. Am J Sports Med. 2002;30:506-513.
74 Spindler K.P., Murray M.M., Devin C., et al. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res. 2006;24:401-406.
75 Strand T., Molster A., Hordvik M., Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15–23 years postoperatively. Arch Orthop Trauma Surg. 2005;125:217-221.
76 Tien Y.C., Chih T.T., Lin J.H., et al. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J Bone Joint Surg Br. 2004;86:1072-1076.
77 Tomita F., Yasuda K., Mikami S., et al. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:461-476.
78 Tyler T.F., McHugh M.P., Gleim G.W., Nicholas S.J. Association of KT-1000 measurements with clinical tests of knee stability 1 year following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1999;29:540-542.
79 Xiao Y., Parry D.A., Li H., et al. Expression of extracellular matrix macromolecules around demineralized freeze-dried bone allografts. J Periodontol. 1996;67:1233-1244.
80 Yamazaki S., Yasuda K., Tomita F., et al. The effect of intraosseous graft length on tendon-bone healing in anterior cruciate ligament reconstruction using flexor tendon. Knee Surg Sports Traumatol Arthrosc. 2006;14:1086-1093.
81 Yeh W.L., Lin S.S., Yuan L.J., et al. Effects of hyperbaric oxygen treatment on tendon graft and tendon-bone integration in bone tunnel: biochemical and histological analysis in rabbits. J Orthop Res. 2007;25:636-645.
82 Yoshikawa T., Tohyama H., Katsura T., et al. Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2006;34:1918-1925.
83 Yu J.K., Paessler H.H. Relationship between tunnel widening and different rehabilitation procedures after anterior cruciate ligament reconstruction with quadrupled hamstring tendons. Chin Med J (Engl). 2005;118:320-326.
84 Zhang C.L., Fan H.B., Xu H., et al. Histological comparison of fate of ligamentous insertion after reconstruction of anterior cruciate ligament: autograft vs allograft. Chin J Traumatol. 2006;9:72-76.
85 Zysk S.P., Refior H.J. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120:59-64.