43 Biologic Treatment of Osteoporotic Compression Fractures: OptiMesh
KEY POINTS
Introduction
The Centers for Disease Control and Prevention (CDC) reports that there were 36.8 million U.S. residents older than 65 years in 2005. That number is predicted to grow to over 70 million by 2030.1 Presently, it is estimated that 25% of women older than 50 years, 40% of women older than 80 years, and 33% of men reaching 75 years of age will sustain an osteoporotic compression vertebral fracture (OCVF).2 Although many OCVFs appear to go clinically undetected, up to 30% of symptomatic fractures remain unresponsive to conservative management and are considered candidates for surgical intervention.3,4 Considering these statistics along with the general effectiveness of vertebral augmentation in treating chronic pain from OCVFs, it is easy to understand the expected steady increase in frequency of procedures performed to treat this disease process.
Originally developed in France during the mid-1980s to treat vertebral hemangiomas and later introduced in the United States in 1994 in the treatment of symptomatic OCVFs, the percutaneous injection of bone cement, polymethyl methacylate (PMMA), has gained widespread use by physicians who treat OCVFs. Vertebroplasty and kyphoplasty have consistently demonstrated the ability to improve patient function and quality of life through excellent pain control and rapid mobilization for this “at risk” group of patients.5 However, risks pertaining to cement toxicity, exothermic reactions and tissue damage, embolism, and the potential neurologically devastating complication of cement extravasation outside the vertebral body (spinal canal, neuroforamen) remain.2,6 Furthermore, concerns have been raised and debated about whether vertebral bodies augmented with PMMA (with or without intradiscal extravasation) are associated with a higher adjacent-level vertebral fracture (ALF) rate than would be expected because of the natural risk of additional fractures. Investigators have challenged the direct causal relationship between PMMA-augmented vertebral bodies and ALF, arguing that ALFs may simply be the result of the natural history of the underlying disease (osteoporosis). However, it is generally agreed that when fractures occur following PMMA augmentation, it is more common to see them occur within the first 3 months after the procedure, and they are more likely to occur at an adjacent level than elsewhere in the spinal column.7 In contrast, people with osteoporotic bones with compression fractures who have not had PMMA vertebral augmentation have subsequent vertebral fractures that are distributed more randomly throughout the spine and will occur sporadically throughout the following year. Data such as these do suggest that PMMA augmentation of vertebral bodies at least predisposes adjacent vertebral levels to fracture.8–12
It has been proposed that the ideal bone cement for vertebral augmentation should be biodegradable and nontoxic, have a low setting temperature, and have a biomechanical profile close to that of human bone.13 To avoid some of the drawbacks of PMMA noted previously, and to provide an “ideal” biological cement for vertebral augmentation, a new option was developed that involves the minimally invasive injection of morselized allograft bone into a polyester expandable mesh container (OptiMesh, Spineology Inc., Minneapolis, Minn.). The bone injection procedure generates lifting force for potential fracture reduction, and the resultant bone graft strut is immediately load sharing and has a modulus of elasticity closely approximating that of native bone.
Indications and Contraindications
The main contraindication, as with all vertebral augmentation procedures, is the presence of an unstable vertebral fracture with retropulsed fragments causing more than 20% canal compromise (i.e., true burst fracture pattern) or any fracture pattern with neurologic deficit. The author has successfully and safely used this technique to treat compression fractures with “bowing” of the posterior cortex (posterior longitudinal ligament intact) along with fractures demonstrating minimal retropulsion of the superior or inferior endplate into the canal causing less than 20% canal compromise. Of interest, the use of this device as a minimally invasive option to treat true burst fractures with the AO classification of A1 has even been shown effective when combined with short-segment pedicle screw fixation.14
Description of the Device
The basic concept behind this technology is that a cavity within a fractured vertebra is created percutaneously through a relatively small access portal via a fluoroscopically guided unilateral, extrapedicular approach. A deflated porous polyester mesh bag (OptiMesh) is inserted and subsequently filled with allograft morsels, allowing a large load-sharing graft and strut to be built within the vertebral body. OptiMesh is designed to contain and reinforce morselized bone graft. The pore size of the mesh is nominally 1500 μm, allowing the mesh to effectively constrain the morselized allograft while also allowing ingrowth of vessels and native bone elements for ultimate graft incorporation and remodeling.
Background of Scientific Testing and Clinical Outcomes
The nonresorbable mesh is knitted from yarn made of polyethylene terephthalate (PET) thread. Animal studies have shown that the mesh does not produce an adverse tissue reaction or create a barrier to bone growth from the host into the graft pack.15 In vitro biomechanical testing has shown that the mesh filled with bone graft restores intact vertebral body strength in compression. The final construct’s restored strength and stiffness is less than PMMA-augmented vertebral bodies, which may have a desired protective effect to reduce the potential for adjacent level fractures. As the reduction of the fracture (i.e., height restoration) occurs while the device is being deployed and filled, the author has found that this procedure more reliably restored and maintained vertebral body height. This is in contrast to loss of almost two thirds of potential restored height that occurs upon deflation of the kyphoplasty balloon in treating similar “mobile” compression fractures.16 Finally, the MTF bone mixture has been shown to generate new bone formation equivalent to autograft when placed in the vertebral bodies of sheep.17
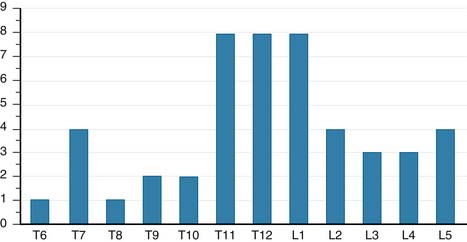
To assess clinical efficacy, the author conducted an INVESTIGATIONAL REVIEW BOARD (IRB)-approved retrospective study to review consecutive patients treated with OptiMesh from March, 2004, to December, 2007. Forty patients were enrolled under the protocol. There were 32 women and 8 men with an average age of 73.4 years (range: 44 to 95 years). Of the 40 patients, 29 patients had osteoporotic compression fractures, 10 patients had trauma, and 1 patient had breast cancer. A total of 48 levels were implanted. More than 50% of the fractures occurred at the thoracolumbar junction (Figure 43-1). Average follow-up was 16 months. There were 27 patients (67.5%) who had greater than 6-month follow-up, with an average of 23 months. Of the patients treated for osteoporotic VCFs, 19 patients had preoperative DUAL X-RAY ABSORPTIOMETRY(DEXA) scores with an average T-score of −2.4.
Clinical Presentation and Evaluation
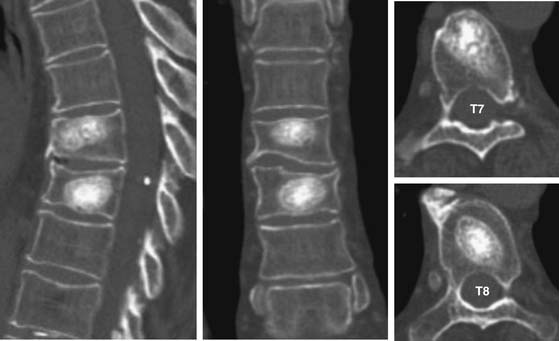
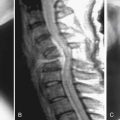
Patent MW: A 69-year-old woman with osteoporosis who suffered T7 and T8 VCFs (T-score: −2.5) secondary to a fall. Fractures were demonstrated by plain x-rays and MRI, and she had severe pain and disability unresponsive to conservative therapy. She underwent a T7 and T8 biologic vertebral augmentation procedure with OptiMesh and morselized allograft during a single session under IV conscious sedation. She reported complete pain relief at 24 hours, and within 48 hours was able to ambulate and perform activities of daily living without assistance. At the 3-year postoperative visit, she continued to be pain free and her 3-year follow-up CT scan showed osseous integration of the graft pack (Figure 43-2).
Operative Technique
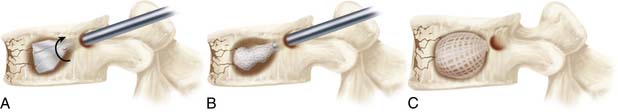
A cavity is then created within the vertebral body by first drilling obliquely across the vertebral body to within 5 to 6 mm of the contralateral vertebral body cortex with a 6-mm hand drill. An expandable shaper is then used to core out a cavity that leaves 2 to 3 mm of bone between the cavity and the endplates.
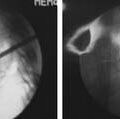
The mesh bag is attached to a mesh holder and passed into the cavity. The mesh is then filled using prefilled tubes prepared and provided by Musculoskeletal Transplant Foundation. The mesh bag is filled circumferentially utilizing initially the diverted (i.e., angled tip) tubes to fill the bag peripherally followed by a straight (i.e non-angled tip) tube which fills the mesh bag centrally ensuring an even packing of allograft throughout the construct. Upon completion of filling, the mesh is detached from its crimp tip, and then all instruments are removed from the patient (Figure 43-3).
Complications and Avoidance
The potential for retroperitoneal bleeding (for lumbar fracture repair) and for hemothorax or pneumothorax (with thoracic fracture repair) are valid concerns but in the author’s experience rarely occur. To avoid violating the pleural space in treating thoracic fractures, the guide pin should be walked along the dorsal side of the rib cage as it is guided from lateral to medial to the costovertebral junction, and then more ventrally along the lateral aspect of pedicle. Ipsilateral exiting nerve root injury at any level can be avoided by always keeping the guide pin (which defines the subsequent trajectory of all additional instrumentation) within the shadow of the pedicle on lateral fluoroscopic imaging.
ADVANTAGES AND DISADVANTAGES
The primary advantage of using a biologic material for VCF treatment is avoidance of complications associated with cement, which include containment, toxicity, and stiffness of final construct. With the biologic vertebral augmentation with OptiMesh and bone graft, the chances for extravasation and embolism of PMMA can be all but eliminated. The porous construct designed is entirely biocompatible and yet is strong enough to bear weight and provide equivalent pain relief. The final result may be that with biologic vertebral augmentation, a less stiff construct is less likely to cause or predispose to adjacent level fractures. Our early experience has demonstrated only a 5% rate of ALFs compared with the quoted literature average for new adjacent level fractures after treatment with vertebroplasty and kyphoplasty of 15% to 20%.18 Confirmation of these rates by other investigators will need to be performed to confirm these results.
1. Health, United States, 2007, Table 1. Retrieved 11/3/08 from http://www.cdc.gov/nchs/fastats/older_americans.htm.
2. Eicholz M., O’Toole J.E., Christie S.D., et al. Vertebroplasty and kyphoplasty. Neurosurg Clin. N. Am.. 2006;17:507-518.
3. Kado D.M., Duong T., Stone K.L., et al. Vertebral fractures and mortality in older women. Arch. Intern. Med.. 1999;159:1215-1220.
4. Jalava T., Sarna S., Pylkkanen L., et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J. Bone Miner. Res.. 2003;18:1254-1276.
5. Taylor R.S., Fritzell P., Taylor R.J. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur. Spine J.. 2007;16:1085-1100.
6. Patel A.A., Vaccaro A.R., Martyak G.G., et al. Neurologic deficit following percutaneous vertebral stabilization. Spine. 2007;32(16):1728-1734.
7. Fribourg D., Tang C., Sra P., Delamarter R., Bae H. Incidence of subsequent vertebral fracture after kyphoplasty. Clinical Case Series. Spine. October 15, 2004;29(20):2270-2276.
8. Faciszewski T., Kiernan F., Rao R. Treatment of osteoporotic vertebral compression fractures. In: Spivak J.M., Connolly P.J., editors. Orthopedic Knowledge Update, Spine 3. Rosemont, IL: American Academy of Orthopedic Surgeons, 2006.
9. Grados F., Hardy N. Treatment of vertebral compression fractures by vertebroplasty [abstract]. Rev. Rheum.. 1997;64:38.
10. Carlson SD, Smith JS, Gordon CD. Is there an increased risk of adjacent segment compression fracture after kyphoplasty. Poster presentation, Annual Meeting of the American Academy of Orthopaedic Surgeons, Feb 13-17, 2002, Dallas, TX.
11. Anselmetti GC. Long-term data confirm benefit of vertebroplasty for back pain relief after osteoporotic vertebral collapse. Presented at Society of Interventional Radiology 33rd Annual Scientific Meeting: Abstract 182, March 18, 2008, Washington, DC.
12. Lin E.P., Ekholm S., Hiwatashi A., Westesson P.L. Vertebroplasty:cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am. J. Neuroradiol.. 2004;25:175-180.
13. Berlemann U., Ferguson S.J., Nolte L.P., Heini P.F. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J. Bone Joint Surg. Br.. 2002;84:748-752.
14. Inamasu J., Guiot B.H., Uribe J.S. Flexion-distraction injury of the L1 vertebra treated with short-segment posterior fixation and OptiMesh. J. Clin. Neurosci.. 2008;15:214-218.
15. B.W. Cunningham, S.D. Kuslich, J.C. Sefter, et al., Interbody arthrodesis using a polyester surgical mesh (the BAG™ surgical mesh): an in-vivo and in-vitro assessment. Presented at the 3rd Annual Meeting of the Spine Society of Europe, Gotenburg, Sweden, Sept. 4-8, 2001.
16. L. Beckman, D. Giannitsios, T. Steffen, An evaluation of the height restoration performance of three vertebral body fracture repair procedures, ex-vivo. Presented at the 8th Annual Meeting of the Spine Society of Europe, Istanbul, Turkey, Oct. 25-28, 2006.
17. T. Fujishiro, T.W. Bauer, N. Kobayashi, et al., Histological evaluation of an impacted bone graft substitute composed of a combination of mineralized and demineralized allograft in a sheep vertebral bone defect. J Biomed Mat Res, published online 16 February 2007 in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/jbm.a.31056.
18. Lindsay R., Silverman S., Cooper C., et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320-323.