Chapter 94
Biologic Grafts
Jeffrey Kalish, Alik Farber
Based on a chapter in the seventh edition by Alik Farber
In the field of vascular surgery, the use of surgical bypass is fundamental to the treatment of a wide variety of arterial and venous disorders. In turn, the technical conduct and success of surgical bypass are directly dependent on the conduit used. The ideal conduit should be readily available, easy to handle, resistant to thrombosis and infection, durable, inexpensive, and should have characteristics similar to the vessel that it is replacing.
Although the perfect conduit does not exist, autogenous blood vessels are closest to the ideal. Autogenous arterial conduits, such as the internal mammary, radial, and gastroepiploic arteries, have been used with great success in the coronary circulation.1–3 The internal iliac and radial arteries have been used in the visceral vascular bed,4,5 and the superficial temporal artery has been used for extracranial-intracranial bypass.6 Unfortunately, short conduit length and invasive harvest have limited the use of autogenous arterial grafts to a relatively small number of clinical scenarios.
Autogenous vein has been the preferred conduit for infrainguinal bypass because long lengths of vein can be harvested, removal is inconsequential, and harvest complexity is minimal.7 Autogenous veins have been used for the bypass of upper extremity,8 carotid,9 coronary,1 and visceral10 arterial beds. They have also been preferentially used in the construction of arteriovenous fistulae (AVFs) for hemodialysis.11
Although autogenous vascular grafts perform well, there are multiple clinical situations in which these conduits are inadequate, unavailable, or improperly matched to the recipient vascular bed. These unmet demands led to the development of artificial grafts. Although multiple materials have been tried, polyethylene terephthalate (Dacron, DuPont, Wilmington, Del) and polytetrafluoroethylene (PTFE) have emerged as the standard materials for prosthetic vascular grafts. These grafts have been used with excellent success for the bypass of large vessels, such as the aorta and iliac arteries,12 and medium-sized vessels, such as the subclavian artery.9 Prosthetic grafts have also been used extensively for dialysis access11 and with mixed results for infrainguinal revascularization.13 Dacron and PTFE grafts offer “off-the-shelf” availability and a variety of sizes to permit replacement of even the largest vessels. In general, however, they cannot be used in infected fields, and compared with autogenous conduits, are at increased risk for infection, structural deterioration, and occlusion. Although patency rates are acceptable for aorto-iliac reconstruction because of high flow rates and low outflow resistance, bypass to smaller targets, such as the tibial arteries, is associated with low graft patency.
Limitations of autogenous and prosthetic grafts have fueled exploration for other potential conduits, and this investigative effort has led to the evaluation of biologic grafts for bypass. Biologic grafts, or biografts, are bypass conduits made of nonautogenous biologic vessels modified for use in clinical practice. Allografts or homografts refer to arteries or veins that are transplanted from one individual to another within the same species. Xenografts or heterografts are vessels transplanted from an individual of one species to an individual of another species.
Carrel14 was first to experiment with fresh allografts and xenografts in dogs during the first decade of the 20th century. The first recorded human use of allografts, obtained from casualties, occurred during World War I.15 In 1948, Gross et al16 described the first clinical series of fresh arterial allografts, and less than a decade later, Linton17 published his series of fresh venous allografts. Various methods of allograft cryopreservation were developed in the 1950s,17 refined in the 1970s,18,19 and finally standardized and commercialized in the late 1980s.20 In parallel, enzymatically treated and tanned bovine carotid artery (BCA) xenografts were evaluated and first described in a clinical setting in 1966.21 Application of similar techniques to human vessels led to development of the human umbilical vein (HUV) graft by Dardik and Dardik in 1976.22
Theoretically, biografts promise to be the optimal vascular conduit. They can potentially offer “off-the-shelf” availability, a wide variety of sizes, excellent handling characteristics, and patency rates similar to those of autogenous vessels. These attractive features prompted scientific investigation and clinical use of these conduits that has spanned the course of almost a century. Although this collective experience with an assortment of biografts in a variety of clinical settings led to specific clinical indications for their use, biologic grafts have failed to become the “Holy Grail” of vascular surgery.
Graft Properties
Fresh Vascular Allografts
Fresh arterial and venous allografts have been studied in animal experiments.14,23,24 In one canine model, fresh venous allografts had a patency rate of 69% at 20 months. Pathologic analysis of explanted veins revealed intimal proliferation, medial inflammation, medial degeneration, and periadventitial fibrosis.23 In another canine venous allograft experiment, dogs that were immunosuppressed with azathioprine demonstrated slightly better graft patency than did those that were not.24 Conversely, in a murine model, fresh venous allografts implanted in rats had excellent patency rates and minimal intimal thickening on histologic analysis.24
In humans, fresh venous allografts used for infrainguinal bypass had a failure rate of 55% in one series; failed grafts either occluded or became aneurysmal. Patency rates of allografts appeared to be higher in patients whose grafts were harvested from blood types ABO compatible donors.25 In another study, fresh arterial allografts placed in the aortic position were noted to be highly immunogenic, with evidence of both a humoral and cellular immune response.26 These animal and human data suggest that fresh vessel allografts initiate a host immune response. Furthermore, the patency of these grafts appears to vary among species.
Aside from their immunogenicity, the use of fresh vascular allografts in the clinical setting has been hampered by logistic factors. Scarce availability of fresh arteries and veins and a need to successfully store such vessels for future use have led to the development of a number of preservation and modification techniques. These techniques can be divided into those that involve preservation without a planned significant change in graft integrity and those in which the graft is intentionally chemically altered. Cryopreservation is the most common example of the preservation technique, whereas proteolytic enzymatic digestion and dialdehyde starch tanning are examples of the modification technique. In addition to creating a conduit that would be more readily available, there it is hoped that these techniques will inhibit the host immune response and thereby increase graft patency.27
Over the course of the past century, multiple vessel preservation techniques have been tested. Grafts were stored in a number of solutions, including nutrient broths,16 glycerol,28 and plasminate.29 A variety of storage temperatures ranging from room temperature to −70°C were tried.17,28 Finally, a number of adjunctive sterilization techniques, including ethyl dioxide and irradiation, were attempted.17 Early techniques focused on preservation without much regard to viability of the vascular tissue. Initial results with preserved vascular grafts were inconsistent, probably because significant cellular and structural damage occurred in many of these vessels and made them nonviable.25,30
Cryopreserved Allografts
Methods of Preparation
There is evidence that cryopreservation can result in significant cellular damage unless appropriate precautions are taken.31 During the cryopreservation process, the extracellular matrix freezes at a higher temperature than cellular cytoplasm. This leads to a vapor pressure gradient between the intracellular and extracellular components. When cooling occurs slowly, this gradient can result in cellular dehydration, whereas rapid cooling can lead to plasma membrane rupture. Work with cell suspensions, such as blood and semen, has revealed that certain substances, when added during the freezing process, can significantly improve cell viability.32 These substances, called cryoprotectants, include dimethylsulfoxide and glycerol. Their mechanism of action is to enter cellular cytoplasm and decrease the vapor pressure gradient that exists between the intracellular and extracellular components.20
Over the last 20 years, cryopreservation techniques have been optimized and commercialized. Important variables inherent in modern cryopreservation processes include the type and amount of cryoprotectant used, freezing rate, storage temperature, duration of storage, and additives used.20 The most common cryoprotectant in use today is dimethylsulfoxide at 10% to 20% dilution. The freezing rate varies among protocols, and there is some evidence that rapid freezing at 5°C/s may work best. Storage temperature may vary from −102°C to −196°C. The duration of cryopreservation may be important, and longer duration has been shown to have an adverse influence on vessel wall morphology but not on graft patency in one animal model.33 Finally, there is evidence that the addition of certain additives such as chondroitin sulfate to the storage solution enhances vein viability and function.34
Histology and Physiology
Cryopreserved arteries and veins are affected by both cryopreservation and immune rejection; a large body of research has been performed to define and dissect these processes from one another.
Cryopreservation has effects on the mechanical properties, histology, and physiology of the treated vessel. Elasticity and compliance of a vessel are important mechanical characteristics that affect its performance as a conduit. Changes in these properties lead to an increased difference in compliance between the conduit and host vessel, which can adversely affect graft patency. In vitro models comparing the mechanical properties of cryopreserved and freshly harvested arteries and veins reveal that cryopreservation does not significantly affect elasticity, contractility, compliance, and the mechanical buffering function of the treated vessel.19,35,36
Cryopreservation of blood vessels leads to changes in the intima, media, and adventitia. Although appropriate cryopreservation does not affect the gross morphology of the endothelial layer, histologic changes such as focal microvillous projection, cytoplasmic vacuolization, nuclear prominence, and interruption of tight junctions have been visualized.33,37 These changes increase with the duration of cryopreservation33,37 and lead to partial endothelial cell loss.37–39 Endothelial loss is significant when the cryopreserved graft is exposed to arterial flow. Although autogenous grafts re-establish an endothelial layer, only minimal re-endothelialization is observed in allografts.20,29 Because of a compromise in intimal integrity, cryopreserved grafts accumulate low-density lipoprotein cholesterol at an accelerated rate as measured in an ex-vivo organ perfusion system.40
Endothelial vasodilatory function, as measured by response to acetylcholine, thrombin, and calcium ionophore, appears to be retained, but is somewhat diminished, with cryopreservation.41 With regard to coagulation homeostasis, although cryopreservation of vein grafts is not associated with increased platelet deposition,41 it does cause decreased thrombomodulin activity.38 Fibrinolytic activity appears to be similar in both fresh and cryopreserved canine jugular veins, but this activity may be adversely affected by the duration of cryopreservation.37
The medial layer of cryopreserved vascular grafts appears to have grossly normal smooth muscle cells, although slight lysis and minimal mitochondrial edema were observed in a rabbit model. In that model, implantation of autologous veins into an arterial circuit led to the preservation of both smooth muscle cells and the elastic lamina. The smooth muscle cells displayed a synthetic, rather than a contractile, phenotype characterized by dilatation of the endoplasmic reticulum.33 Despite these findings, collagen synthesis in cryopreserved veins, but not fresh veins, was diminished in a canine model.39 Smooth muscle cells in cryopreserved canine saphenous autografts were noted to have a diminished relaxation response to nitric oxide,41 although contraction induced by norepinephrine, potassium chloride, and serotonin was unaltered.20
Immunology
Allogeneic implantation of cryopreserved vessels leads to a different histologic and physiologic picture than that seen with cryopreserved autologous grafts. These observed changes are caused by immune mechanisms. Endothelial loss, encountered when an allograft is exposed to arterial flow, is not appreciably reversed, and exposed subendothelial elements are noted on electron microscopy.20,29 Smooth muscle cell viability is lost,23,42 severe medial fibrosis and disruption of elastic fibers occur,23,29,42 and medial necrosis has been described.43 Significant lymphocytic infiltration of the media and adventitia has been observed.29 These alterations in vessel wall biology are not routinely observed with autologous conduits.43
Although it is well known that transplanted allograft and xenograft organs elicit an immune response, it was initially believed that the host-mediated immune response of transplanted vessel allografts was minor44,45 and could be successfully blunted by the cryopreservation process.27 Recent literature, however, suggests that vascular allografts do trigger a significant immune response.20,26 Endothelial cells present surface antigens that stimulate a cell-mediated immune response46 against the donor graft. An immunoglobulin-G–mediated humoral immune response to donor-specific antigens has been described.26,47 Transplanted canine venous allografts, but not autografts, demonstrated extensive medial fibrosis and lymphocytic infiltration consistent with immunologic rejection.48 In a human model, analysis of 22 explanted cryopreserved saphenous vein (CSV) allografts revealed moderate to severe intimal, medial, and adventitial inflammatory infiltrates. Immunohistochemical analysis demonstrated an abundance of activated T lymphocytes containing cytotoxic granules.49 In another experiment, cryopreservation did not alter antigenic expression and the immunologic response of a murine host to allograft transplantation in a number of studies.50,51 Chronic immunologic rejection clearly plays a role in allograft biology and appears to be responsible for both diminished patency of cryopreserved vascular grafts and the predilection of these grafts to aneurysmal degeneration.43
A number of investigators hypothesized that manipulating the host immune response to vascular allografts may attenuate immune rejection and improve graft patency. Matching of ABO blood groups was suggested by Ochsner et al,25 who noted improved patency of allografts transplanted to ABO-matched patients. In animal models, immunosuppression with cyclosporine has been demonstrated to diminish immunologic rejection of aortic43 and venous allografts.52 Azathioprine has likewise been shown to decrease the effects of rejection in venous allografts.24
Based on these findings, attempts were made to improve the results of allograft use in humans by modulating the host immune response. Carpenter and Tomaszewski,53 in a prospective, randomized trial of 40 CSV allografts implanted in patients treated with low-dose azathioprine, failed to show a significant improvement in graft patency at 1 year. Azathioprine immunosuppression, however, was associated with a decreased presence of T-lymphocyte cytotoxic granules in that study.49 In another small human trial, a combination of low-dose cyclosporine, azathioprine, prednisone, warfarin, aspirin, and vasodilators was used in patients who underwent CSV bypass. Grafts treated with this immunosuppressive regimen demonstrated increased patency rates. This regimen, however, was associated with an increased incidence of complications and graft aneurysmal degeneration.54 In one series of patients with prosthetic aortic infection, 10 of 30 patients who underwent aortic allograft replacement were concomitantly treated with cyclosporine. Although the measured humoral immune response was blunted in patients who received cyclosporine, no differences in graft patency or graft complication rates were appreciated.26 In contrast, Randon et al55 contended that a low-dose cyclosporine immunosuppressive regimen for lower extremity bypass using CSV was effective at reducing the risk of rejection while facilitating host cell repopulation of biograft endothelium.
Furthermore, an immunologic response evoked by a cryopreserved allograft can induce allosensitization, which may interfere with future organ transplantation. This mostly affects the use of cryopreserved femoral vein (CFV) allografts in hemodialysis access. A case-matched series of 20 patients who underwent creation of hemodialysis access with this graft demonstrated host allosensitization in all patients as measured by the panel-reactive antibody assay.56 Allosensitization, however, did not occur when the CFV graft was processed to remove cellular elements.57 Diminution of the immune response by removal of antigenic epitopes has led to multiple attempts to structurally modify biologic grafts.
Structurally Modified Biologic Grafts
In parallel with the development of cryopreservation techniques, further research was conducted to modify blood vessels so that an acceptable vascular substitute could be developed. The goal was to transform a harvested blood vessel into a durable nonimmunogenic graft that could be easily produced and stored. During early experiments in the 1950s, animal arteries were modified by enzymatic digestion of the musculoelastic portion of the vessel wall with ficin, a proteolytic enzyme isolated from figs, to remove immunologically reactive proteins. The resultant collagenous vascular skeleton was strengthened by collagen cross-linking through subsequent tanning with dialdehyde starch.21,58 This modified graft was then sterilized and stored in a 1% propylene oxide–50% ethanol solution.21
In the earliest experiments, modified BCA grafts were implanted as xenografts first in dogs and then in patients with symptomatic lower extremity occlusive disease. Although no graft ruptures had occurred at 3 years of follow-up, early neointimal hyperplasia and diminished patency were observed.21 An unacceptable late rate of graft infection and aneurysmal degeneration led to a change to glutaraldehyde-based tanning protocols.58,59
Bovine mesenteric veins (BMVs) have also been modified by a patented process of glutaraldehyde cross-linking and sterilized by γ radiation.60 Both BCAs and BMVs have been used as xenografts in a number of clinical applications.
HUV is a modified biologic conduit that was first evaluated in baboons22 in the early 1970s and subsequently used in humans22,61 in 1975. Umbilical vessels are uniform in caliber, valveless, and branchless. The umbilical vein was removed from the umbilical cord by a variety of techniques, including enzymatic digestion and mechanical stripping. After a rinsing process with a cold isotonic solution, this vein was tanned with glutaraldehyde. A polyester fiber mesh was then sutured in place about the length and outside circumference of the graft for added support.22
Although thrombosis and aneurysm formation were common in early experiments, tanning and external support modifications significantly reduced these complications. Increased adherence of platelets to the luminal surface of these grafts has been observed in a canine model.62 Histologic analysis of modified HUV grafts explanted from baboons revealed an early neutrophil and late macrophage response in the vicinity of the surrounding polyester mesh. The inner collagen layer appeared thickened and dense, but was free of significant inflammation.22 The reduced immunogenicity of this graft was hypothesized to be secondary to pretreatment with glutaraldehyde, which was thought to bind to graft histocompatibility antigen sites and thereby shielded them from the host immune response.63
Other Grafts
The search for an ideal blood vessel substitute led to the investigation of a number of nonconventional biologic grafts in animal models. Vascular prostheses fashioned from pericardium64 and small intestinal mucosa65 have been evaluated. Chemically modified human66 and bovine67 ureters have been used as vascular conduits with some success. Modified bovine ureters have been used clinically with acceptable patency rates in femoral to popliteal bypass in one small Australian series.68 In addition, a small randomized trial claimed clinical equivalence between bovine ureters and PTFE used for hemodialysis access in patients with no vein options.69
Clinical Use in Peripheral Vascular Surgery
Indication
Biologic grafts have been used in modern peripheral vascular surgery mostly in three distinct clinical settings: extremity bypass in the absence of suitable autogenous conduit, arteriovenous (AV) access for hemodialysis, and replacement of infected prosthetic grafts.
Extremity Bypass
Acute or chronic ischemia of an extremity is caused by a number of conditions, including atherosclerosis, trauma, embolization, and in-situ thrombosis. Treatment of extremity ischemia involves revascularization by endovascular or surgical techniques. During infrainguinal surgical bypass, the choice of conduit is crucial to success of the operation. The autogenous great saphenous vein has proven to be the preferred conduit for infrainguinal revascularization.70,71 When the autogenous great saphenous vein is not available, alternative autogenous conduits, such as an arm vein,72 the small saphenous vein,73 and the composite autogenous vein,74 have been used with good results. The ever-increasing age and complexity of patients with infrainguinal arterial occlusive disease has brought about increasingly frequent clinical scenarios in which the autogenous vein is not available and an alternative conduit must be found. Although prosthetic grafts have been used with moderate success above the knee, they have been disappointing when used for infrageniculate bypass.75,76 Distal modification of prosthetic grafts with a vein cuff or distal AVF may improve patency rates,77–79 but is more cumbersome.
Given the absence of reliable conduit options for infrageniculate bypass when a suitable autogenous vein is lacking, the feasibility of biologic grafts has been evaluated. In this setting, CSV allografts,80 cryopreserved femoro-popliteal artery (CFA) allografts,42 HUV grafts,81 BCA xenografts,58 and BMV xenografts82 have been used with varying degrees of success.
Arteriovenous Access
End-stage renal disease is a significant public health problem in the United States; its prevalence is increasing steadily, and it is forecast that by the year 2030, more than 2 million patients will be undergoing hemodialysis.83,84 Long-term hemodialysis is best performed through a surgically created AVF that connects the arterial and venous circulations via a conduit. The ideal AV conduit carries high flow for efficient dialysis, is superficial enough for easy access, is sufficiently durable to withstand multiple cannulations, allows rapid sealing of cannulation sites, and is resistant to infection, stenosis, and thrombosis. A mature, native vein AVF comes closest to the ideal, and its use is strongly encouraged.11 Unfortunately, many individuals lack suitable veins for native AVF construction because of small vein size, previous access procedures, or vein harvest for peripheral or coronary bypass. Furthermore, up to 60% of native AVFs fail to mature, and therefore, cannot be used successfully.85,86 Although prosthetic AV grafts are widely used, they have lower patency rates, require more frequent revision, and are at higher risk than vein AVFs for infection.87 The search for optimal hemodialysis access in patients who are not candidates for a native vein AVF has led to the use of biologic grafts. CFV allografts,88 BCA xenografts,89 bovine ureter xenografts,69 and BMV xenografts60 have been used in a variety of settings with variable results.
Replacement of Infected Prosthetic Grafts
Prosthetic graft infection, particularly when the aorta is involved, is one of the most dreaded complications in vascular surgery and is associated with high morbidity and mortality.90 Treatment of an infected aortic prosthesis includes excision of the infected segment and extra-anatomic prosthetic bypass90 or reconstruction with an antibiotic-soaked or antibiotic-bonded prosthetic graft,91 femoral vein,92 or aortic allograft.93 Extra-anatomic bypass and aortic ligation are associated with long operative times, risk of remote infection, bypass thrombosis, and aortic stump rupture.90,94 Antibiotic-soaked prosthetic grafts may work well for infections caused by relatively indolent Staphylococcus epidermidis, but are much less effective against more virulent organisms.91 Finally, use of the femoral vein for aortic reconstruction is tedious and associated with harvest-related complications.92
Cryopreserved aortic allografts offer “off-the-shelf” availability, good handling properties, and the potential for expeditious in-situ repair. Cryopreserved aortic allografts were more resistant than prosthetic grafts to S. epidermidis infection in a canine model.95 Resistance of vascular allografts to infection has led to the wide use of arterial allografts to treat aortoiliac infection,96 CFV allografts to treat infection involving prosthetic AV grafts,97 and CSV allografts to replace infected infrainguinal prosthetic bypass grafts.98
Biologic Graft Preparation
Cryopreserved Allografts
A number of tissue banks and commercial companies prepare, store, and supply cryopreserved blood vessels. Despite similarities in conduit preparation, many have proprietary cryopreservation protocols.99 The great saphenous vein, femoral vein, and arterial segments are harvested from multiorgan donors who are screened for an array of viral, bacterial, and fungal infections. Branches are suture ligated, and the allografts are sized with calibrated dilators. They are tested for presence of pathogens, rinsed in an antibiotic solution, placed in a proprietary cryoprotectant solution, and stored in the vapor phase of liquid nitrogen at −110°C to −196°C. Allografts are shipped and stored in a solution of dimethylsulfoxide at −96°C until needed. The vein, but not artery, is usually matched for ABO/Rh compatibility with the recipient to decrease the risk of rejection. At the start of the procedure, the allograft is rapidly thawed by submersion in a warm water bath at 37°C to 42°C for 20 minutes. After rinsing in a series of solutions provided by the manufacturer, it is ready for use.
CSV allografts are available in a number of lengths and diameters. Most commonly, the vein measures 3 to 5 mm in diameter. These grafts look, feel, and handle like the autogenous saphenous vein. During an infrainguinal bypass, the allograft is usually reversed and placed in a superficial tunnel for easy access.80 Postoperative surveillance was not considered useful in one large series.80
CFV allografts are usually less than 25 cm in length and have a diameter between 5 and 7 mm. They have most commonly been used in hemodialysis, particularly in the setting of prosthetic AV graft infection. When used for dialysis access, this allograft is appropriately reversed and tapered to a 5 mm diameter at the arterial anastomosis to decrease the incidence of ischemic steal syndrome.88 It is allowed to mature for 3 to 4 weeks before it is accessed for hemodialysis. Revision of these grafts is very difficult because of their thin wall and surrounding fibrosis.97
Arterial allografts have most frequently been used for aortic replacement in the setting of primary or prosthetic aortic infection. Given this clinical setting and the need to replace a large artery such as the aorta, these allografts have to withstand particularly hostile conditions. Technical modifications for the use of these allografts have been developed, including vigilance in following thawing instructions, use of appropriately long grafts, and construction of tension-free anastomoses, taking great care that suture ligation of branches is performed with polypropylene sutures that include the graft wall along with the branches, aggressive excision of infected tissue and wound drainage, circumferential anastomotic reinforcement with allograft strips, use of gentamicin-impregnated fibrin glue, and coverage of the graft with viable tissue, such as a pedicled omental or muscle flap.93,100
Structurally Modified Allografts
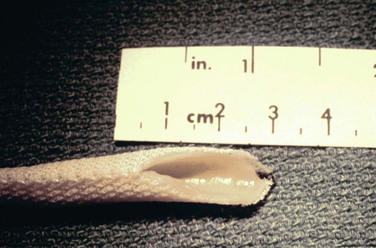
The HUV graft (Fig. 94-1) is shipped and stored in 50% ethanol and provided on a glass mandril. It is irrigated up to 10 times with a low-molecular-weight dextran–containing solution and rinsed with a high-concentration heparin solution (10,000 U/L). This graft does not tolerate traction or the application of standard vascular clamps. To avoid injury, it needs to be passed through a metal or plastic conduit during tunneling and preferably controlled with a tourniquet. To decrease the risk of pseudoaneurysm formation during suturing, both the vein and the Dacron mesh need to be incorporated into the suture line. Finally, infusion of low-molecular-weight dextran has been recommended in the early postoperative period by the manufacturer to decrease the risk of early thrombosis.
Manufacturing of the umbilical vein graft by Synovis Life Technologies, Inc. (St. Paul, Minn) stopped in May 2005 in compliance with new U.S. Food and Drug Administration (FDA) guidelines governing combination tissue–medical devices.101 To date, a next-generation HUV graft that will fulfill FDA regulations is not yet available, although developers are actively seeking corporate industry support for such a venture (Herbert Dardik, personal communication, December 13, 2012).
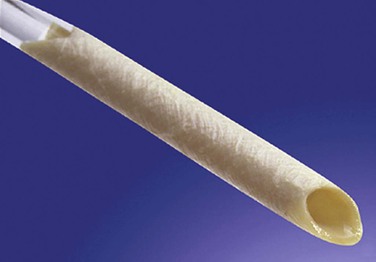
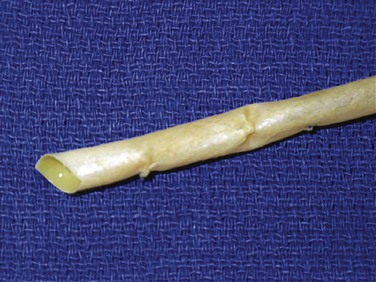
The BCA graft (Fig. 94-2) is supplied in a specially designed tube containing a proprietary solution of 1% propylene oxide in 40% aqueous ethyl alcohol. It is naturally compliant, soft, and relatively easy to use. It is presently available in 6-, 7-, and 8-mm diameters and 15- to 50-cm lengths (Artegraft; Artegraft, Inc., New Brunswick, NJ). The BMV graft (Fig. 94-3) is shipped in a sterile saline solution and is available in 6-mm diameters and 10- to 40-cm lengths (ProCol Vascular Bioprosthesis, Hancock Jaffe Laboratories, Irvine, Calif). This graft is compliant and handles much like a saphenous vein.60 Both xenografts require a series of rinsing steps in the operating room before use.
Clinical Outcomes
Cryopreserved Saphenous Vein Allografts
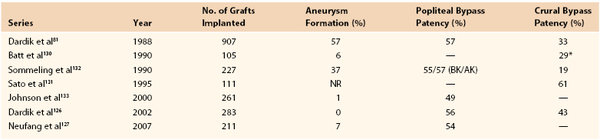
Numerous reports on the utility of CSV allografts for infrainguinal revascularization have been published (Table 94-1).53,54,55,80,102–109 However, this literature has confounding factors that may affect the interpretation of outcomes. CSV has been distributed by a number of vendors who use similar, but not identical, cryopreservation techniques. There is significant variability pertaining to patients, location of the proximal and distal anastomoses, use of anticoagulation, and use of immunosuppressive agents. The majority of these reports are retrospective, and the two largest studies contain 115105 and 24080 grafts. The most recently published report spanned a 15-year period of implantation.55 Four prospective studies have been published, but they include small numbers of patients.53,54,80,104
Table 94-1
Summary of Published Cryopreserved Saphenous Vein Allograft Series Containing More than 20 Grafts
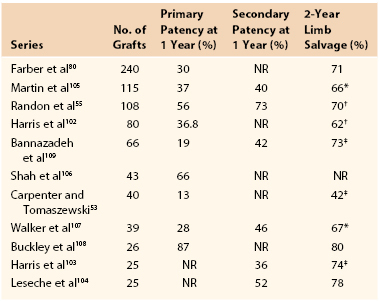
* At last follow-up.
† At 3 years.
‡ At 12 months.
NR, Not reported.
Graft Patency
Although use of CSV allografts has been reported in a number of settings, these grafts are generally used for the treatment of limb-threatening ischemia.80,105 The primary patency rate of CSV grafts has been noted to be relatively low in most retrospective series (see Table 94-1).80,102–108 The largest case series reported a 30% primary patency rate at 1 year,80 similar to the 37% primary patency rate noted in the second largest series.105 Although Buckley et al108 published an impressive 87% primary patency rate in their prospective study of 26 patients, Carpenter and Tomaszewski53 found a dismal 13% primary patency rate in their prospectively monitored patient cohort. Randon et al55 showed slightly better than average patency and limb salvage rates in CSV recipients treated with low-dose cyclosporine.
Secondary procedures on failing or failed grafts generally seem to yield little gain. Primary-assisted and secondary patency of CSV grafts were not significantly higher than primary patency in the two largest allograft series.80,105 Once the graft failed, it was abandoned, and secondary grafting was performed when indicated.80 Other authors, however, almost doubled their secondary patency rates by adopting an aggressive posture toward allograft thrombectomy and revision.107,109
Multiple patient and procedural variables were evaluated for their influence on allograft patency. In the largest published series, multivariate analysis identified that diabetes negatively affected graft patency. Age, gender, hypertension, smoking, renal dysfunction, indication for surgery, history of bypass grafting, and site of distal anastomosis did not have an effect.80 A recent series likewise noted a negative effect of diabetes on graft patency,55 although others found no significant effect.105,107 Two separate investigations found that secondary and composite allograft reconstructions adversely affected graft patency.106,109 Lastly, in a recent study, statin use was noted to improve graft patency.55
Role of Anticoagulation and Immunosuppression
A number of studies have evaluated the effect of anticoagulation on allograft patency. Aspirin and warfarin, alone or in combination, did not improve graft patency in most series.53,80,103,105,106 Buckley et al,108 however, reported an impressive 87% primary patency rate in their prospective cohort of 26 patients who were treated with an intensive anticoagulation protocol consisting of preoperative aspirin, perioperative low-dose heparin and dextran, and postoperative warfarin, aspirin, and dipyridamole. Of note, 42% of grafts in that series underwent distal anastomotic modification with either vein cuffs or AVFs. A limitation of most retrospective studies is that the precise level of therapeutic anticoagulation was not rigorously followed for each individual patient. The true effect of an anticoagulation protocol on allograft patency awaits a prospective randomized study that will closely monitor the adequacy of postoperative oral warfarin therapy.
Immunosuppressive regimens have been evaluated clinically. In a prospective randomized trial of 40 grafts in patients treated with low-dose azathioprine, Carpenter and Tomaszewski53 failed to show a significant improvement in graft patency at 1 year. In contrast, Randon et al55 declared their low-dose cyclosporine immunosuppressive regimen to be effective at reducing the risk of graft rejection and thrombosis, and reported minimal side effects. Although immunosuppressive protocols may be effective, potentially serious side effects of therapy may not justify routine use in this patient population.54
Limb Salvage
Despite discouraging graft patency, use of CSV allografts has been associated with acceptable limb salvage rates (see Table 94-1). In the largest published series, a 71% 2-year limb salvage rate was achieved,80 whereas Randon et al55 reported a 5-year limb salvage rate of 64%. The discrepancy between graft patency and limb salvage can be explained, in part, by secondary bypass procedures performed after primary graft failure. Others have reported that repetitive bypass grafting significantly extends limb salvage.110 Another possibility is that the saphenous allografts remained patent long enough to enable healing of lower extremity ulceration in a large proportion of patients. The ulcers may not have recurred despite graft failure.80,104,105 Unfortunately, this hypothesis can be proven only in a trial in which ulcer healing is prospectively monitored, along with limb salvage.
Of the various clinical factors that could potentially influence limb salvage, multivariate analysis found the site of distal anastomosis to be significant.80 Patients who underwent allograft bypass to the popliteal artery had better limb salvage than did those who underwent tibial bypass. Martin et al105 found patient age to be inversely related to limb salvage.
Aneurysmal Degeneration
CSV allografts that remain open for a prolonged period are prone to aneurysmal degeneration,54,80,105 which is probably related to the immune response by the recipient against the graft. The true incidence of allograft aneurysm formation cannot be accurately determined because the majority of these grafts occlude long before a clinically detectable aneurysm can develop. In one series, aneurysmal degeneration developed in nine grafts, for a 2-year aneurysm incidence of 44%.80 Martin et al105 reported a 25% aneurysm formation rate at 2.5 years. The development of aneurysms in allografts necessitates close surveillance of those few patients whose graft remains open for a prolonged period. Because allograft aneurysm rupture has been reported,54,80 preemptive graft revision is recommended.
Summary and Indications for Use
Although CSV allografts look, feel, and handle like an autogenous vein, they are far from being the “Holy Grail” of conduits for infrainguinal reconstruction. Their poor patency rates are worsened by several risk factors, including diabetes, previous bypass, and composite reconstruction. Postoperative anticoagulation has not significantly improved graft patency,80 although some authors believe that an intensive peri- and postoperative anticoagulation regimen has merit.108 Given the immunologic mechanism of graft failure, it is unlikely that anticoagulation alone is sufficient to prevent graft occlusion. Grafts that stay open for extended periods, perhaps as a result of chance matching of important immunologic loci, are prone to aneurysmal degeneration. Finally, CSV allografts are expensive, costing approximately $7000 to $9000 (in 2012 dollars), depending on the length of the graft.
Given published clinical data, many authors conclude that the use of CSV allografts should be limited.54,80,102,104,105 Nevertheless, these grafts clearly have a place in the armamentarium of the modern vascular surgeon. Because they have been reported to be relatively resistant to graft infection,98 they have an advantage when revascularization needs to be performed in an infected field. They also have an advantage when distal bypass needs to be extended onto the foot because closure of the wound is considerably easier than if a prosthetic graft is used. Finally, CSV allografts typically remain patent long enough to allow healing of an ischemic ulcer or minor amputation. The final piece of evidence supporting the continued role of this graft is persistent demand for the product by the vascular surgery community as evidenced by the number of grafts that continue to be sold.
Cryopreserved Femoral Vein Allografts
Femoral vein allografts have been used for hemodialysis access in the setting of prosthetic AV graft infection, multiple graft failures, or compromised venous outflow sites. In one series of 48 allografts, 1-year primary and secondary patency rates of 49% and 75%, respectively, were achieved. No allograft infection or aneurysmal degeneration was noted.88 In another series of 45 allografts, a cumulative 1-year patency rate of 68% was reported. Although no infection was noted during follow-up, two pseudoaneurysms required repair.111 Madden et al112 compared the outcomes of 90 femoral allografts with 100 concurrent PTFE AV grafts and noted similar patency rates. No infections were seen in the allograft group, whereas 10% of the PTFE AV grafts became infected. In 18% of the allografts, however, aneurysmal degeneration developed.112 In a prospective, randomized trial between CFV and PTFE grafts (suspended by the FDA after enrollment of 27 patients into each group), need for fistulography and aneurysmal degeneration was higher in the CFV cohort.113 Others found that the use of CFV for AV access was associated with a 55% rate of infection, which was particularly common in thigh grafts. Allograft rupture occurred in 46% of infected grafts.114
CFV allografts do not have a primary role in hemodialysis access. They may have a secondary role in the setting of infected prosthetic access in a patient with limited reconstructive options. Because of allosensitization, they should not be used in patients who are candidates for future kidney transplantation,56 although de-cellularized femoral vein allografts appear be safer in that regard.57 Symptomatic pseudoaneurysms may develop in these allografts and should elicit a low threshold for repair. Finally, CFV placement in the thigh should be avoided.
Cryopreserved Arterial Allografts
Although most experience with arterial allografts has been gained with aortic replacement in the setting of prosthetic graft infection, CFAs have been used for infrainguinal revascularization. Cryopreserved femoropopliteal arterial allografts used for infrageniculate revascularization had a primary patency rate of 51% at 17 months of follow-up in one series of 17 bypasses,42 and 61% at 1 year of follow-up in a more recent series of 35 bypasses.115 Because of short conduit length, a composite bypass was necessary in 53% of cases. Another series of 35 allografts reported a 39% primary patency and 59% secondary patency rate at 18 months of follow-up. Two grafts required replacement as a result of aneurysmal degeneration.116 A 5-year primary patency rate of 16% was achieved in a retrospective multicenter trial of 165 fresh and cryopreserved arterial allografts.117 A more recent study compared 39 peripheral reconstructions using CFAs with 35 non-CFA bypasses, including extra-anatomically tunneled prosthetic grafts, autogenous veins, and endarterectomized superficial femoral artery grafts performed in the setting of infection. Total graft-related morbidity was 18% in the CFA group and 57% in the non-CFA group.118 In this study, mortality at 18 months of follow-up was high in both groups: 31% in the CFA group and 43% in the non-CFA group.118 These results suggest that in lower extremity bypass, cryopreserved arterial allografts have low patency rates, are predisposed to aneurysmal degeneration, do not offer any significant advantage over the use of saphenous vein allografts, and have the additional potential drawback of the need to connect two or more arterial segments together to create a conduit of sufficient length.
Arterial allografts have been used extensively for the management of primary and prosthetic aortic infection. To this end, fresh aortic allografts stored for less than 1 month at 4°C have been used with some success.93,119,120 An Italian study of 44 patients treated with 13 fresh and 31 cryopreserved aortic allografts did not find a difference in patient outcomes.119 A French study of 179 patients treated with 111 fresh and 68 cryopreserved grafts, however, did note long-term differences in graft behavior: fresh allografts were associated with allograft rupture and an increased incidence of late graft-related complications.93
There are seven published studies using more than 40 grafts that provide information about the outcomes of cryopreserved aortic allografts for the treatment of aortic infection (Table 94-2). Most of these represent multi-institutional registries with relatively short follow-up periods.96,121,122 Although many patients had polymicrobial aortic infections, staphylococcal species were the most common organisms cultured.96,100,119,122–124 As expected, perioperative mortality rates were high and ranged between 5% and 36%. Factors associated with increased mortality included emergent or urgent surgery and the presence of an aortoenteric fistula.100,122 Aortic allograft repair in patients with aortoenteric fistulae was associated with prohibitive long-term mortality rates of 80% in one recent series125 and 83% in an older series.122
Table 94-2
Summary of Published Cryopreserved Aortic Allograft Series Containing More Than 40 Grafts
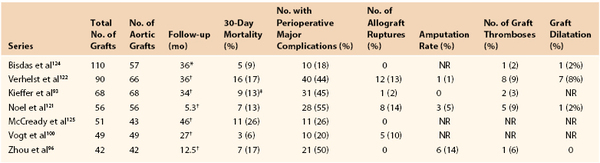
* Median follow-up.
† Mean follow-up.
‡ In-hospital mortality.
NR, Not reported.
As expected, these patients had very high perioperative complication rates, ranging between 18% and 55%. Allograft rupture was seen in the immediate postoperative period and up to 4 years of follow-up.100 This devastating complication occurred in 2% to 14% of cases and was associated with high mortality. Allograft aneurysmal dilatation was noted to occur in as many as 8% of patients in one series.122 Graft stenosis and thrombosis were more often associated with grafts extending to the iliac or femoral arteries.93 Amputation rates ranged between 1% and 14%. Despite these sobering statistics, 87% and 60% of patients were free of aortic and iliofemoral complications or interventions, respectively, at 7 years in one large single-institution series.93
Aortic infection is one of the gravest conditions in peripheral vascular surgery. Therefore, allograft performance needs to be viewed against the results of other treatment options for the management of infected aortic grafts. Graft excision with extra-anatomic bypass was associated with a 30-day mortality of 13% and an amputation rate of 10%.90 Likewise, in-situ aortic graft replacement with a rifampicin-bonded prosthetic graft had a perioperative mortality rate of 18%.91 Although cryopreserved aortic allografts clearly have a place in the management of prosthetic aortic graft infection, their precise role has yet to be clearly defined. They are associated with allograft dilatation and rupture, probably because of the previously discussed immunologic mechanisms.20,23,29,43,42 Graft surveillance protocols have yet to be standardized and validated. Although the use of current immunosuppressive regimens in these very ill patients is not practical, the development of more focused immunosuppressive therapy in the future may better define the role of aortic allografts in the armamentarium of vascular surgeons.
Human Umbilical Vein Grafts
Patency and Limb Salvage
The first large clinical experience with the use of HUV grafts was reported in 1988 by Dardik et al.81 Nine hundred seven lower limb bypass procedures were performed in 799 limbs of 715 patients. The 5-year primary-assisted patency rates were 57% and 32% for femoropopliteal and femorotibial bypasses, respectively. The 5-year limb salvage rate ranged between 70% and 80%. Fifty-seven percent of the grafts exhibited aneurysmal dilatation at a mean follow-up of 5 years. In 1989, ownership and manufacture of the HUV graft changed hands. In an attempt to address the issue of time-dependent graft degradation, efforts were made to improve the graft manufacturing process. Improved cross-linking with glutaraldehyde and upgraded quality control procedures, including time, temperature, and pressure determinants during manufacture, led to the development of a second-generation graft that was resistant to aneurysmal degeneration.101 In parallel, Dardik et al79 attempted to improve the patency of femorotibial HUV grafts by using adjunctive distal AVFs. These investigators published an updated experience with 283 second-generation HUV grafts in 2002. Five-year primary patency rates for this graft were 60% and 50% for below-knee popliteal and tibial bypass, respectively. Five-year limb salvage rates were 80% and 65% for below-knee popliteal and tibial bypass, respectively. No graft aneurysmal degeneration was noted on duplex surveillance of these grafts.126
Neufang et al published three series of patients treated with the HUV graft for popliteal bypass,127 composite bypass,128 and composite sequential bypass.129 Two hundred eleven patients treated by HUV femoropopliteal bypass had 5-year primary and secondary patency rates of 54% and 76%, respectively. Reported complications included early graft thrombosis in 17% and aneurysmal degeneration in 7%. This group did not use adjunctive distal AVFs, but they did recommend an aggressive anticoagulation protocol with peri- and postoperative aspirin and clopidogrel, early postoperative heparin, and long-term warfarin to keep the international normalized ratio at 2.5.127
Fifty-four patients with critical limb ischemia treated by a HUV–autologous vein composite bypass to tibial targets had 4-year primary patency, secondary patency, and limb salvage rates of 53%, 67%, and 88%, respectively. Patients who did not undergo anticoagulation had a significantly higher incidence of early graft thrombosis.128 HUV–autologous vein composite sequential femoral-tibial bypasses demonstrated lower long-term patency rates.129 Patency and limb salvage results in the most recent (and largest) studies using HUV grafts are listed in Table 94-3.81,126,127,130–133
Comparison of Human Umbilical Vein With Other Grafts
A number of studies compared the utility of HUV grafts with other conduits for infrainguinal revascularization. Cranley et al134 reported a 3-year cumulative patency rate of 74% for umbilical vein grafts, 41% for PTFE grafts, and 76% for saphenous vein grafts when used for bypass to the popliteal artery in the setting of critical limb ischemia. HUV patency was comparable to PTFE graft patency when tibial targets were evaluated (31% versus 35%, respectively). A small Scandinavian multicenter, prospective randomized trial compared outcomes of HUV grafts with PTFE for below-knee femoropopliteal bypass. The 4-year primary-assisted patency rate was 42% for umbilical vein grafts versus 22% for PTFE grafts.135 The New England Society for Vascular Surgery Registry revealed HUV to have improved 5-year patency rates in comparison to PTFE grafts for bypass to the below-knee popliteal artery (45% versus 20%, respectively).136
Another small, prospective randomized trial evaluated the outcomes of first-generation HUV grafts and PTFE grafts in above-knee femoropopliteal bypass. At 6 years, the primary patency rate of HUV was significantly higher than that of PTFE grafts (71.4% versus 38.7%, respectively). Thirty percent of HUV grafts demonstrated aneurysmal dilatation.137 The largest randomized trial of HUV grafts was a Veterans Administration–sponsored trial in the United States in which the outcomes of HUV, saphenous vein, and PTFE grafts were evaluated in 752 patients who underwent above-knee femoropopliteal bypass. At 5 years, primary-assisted patency rates were 73%, 53%, and 39% for saphenous, umbilical vein, and PTFE grafts, respectively. Although HUV grafts outperformed PTFE grafts, they were associated with a higher incidence of early graft thrombosis and amputation.133 This study prompted the manufacturer of this graft to recommend low-molecular-weight dextran in the postoperative period. Some investigators routinely recommend antiplatelet agents, postoperative heparin, and long-term warfarin therapy.126,127
Aside from thrombosis, umbilical vein grafts are associated with a number of complications, including infection, stenosis, dissection, and pseudoaneurysm. These complications vary between the first- and second-generation HUV grafts (Table 94-4).126
Table 94-4
Nonthrombotic Complications of Umbilical Vein Grafts
| First-Generation Grafts (1975-1985) | Second-Generation Grafts (1990-2000) | |
| Total no. of grafts | 907 | 283 |
| Failure without thrombosis | 49 (5.4%) | 2 (0.7%) |
| Infection | 39 (4.3%) | 9 (3.2%) |
| Stenosis | 19 (2.1%) | 5 (1.8%) |
| Dissection | 1 (0.1%) | 1 (0.4%) |
| Pseudoaneurysm | 13 (1.4%) | 1 (0.4%) |
| Aneurysm (surgical repair) | 26 (2.9%) | 0 |
Adapted from Dardik H, et al: Comparative decades of experience with glutaraldehyde-tanned human umbilical cord vein graft for lower limb revascularization: an analysis of 1275 cases. J Vasc Surg 35:64, 2002.
Although HUV grafts have demonstrated adequate outcomes and improved patency rates compared with PTFE grafts, they have never made it into the mainstream of vascular practice. Many factors, including perceived handling difficulty, complexity of adjunctive distal AVF creation, and industry bias, partially account for this observation. Nevertheless, vascular surgeons must be aware of the HUV graft and its unique complexities and complications given the likelihood of encountering a patient with this type of bypass in clinical practice. Currently, the HUV graft is not commercially available.
Bovine Carotid Artery Xenografts
The BCA xenograft was first used for dialysis access by Chinitz et al,138 who found that the graft tolerated frequent cannulation and maintained flow sufficient for successful hemodialysis. Patency rates of this graft range from 21% to 86% at 1 year and 45% to 76% at 2 years.139–142 With the advent of PTFE and its use in hemodialysis access in the mid-1970s, multiple studies comparing BCA xenografts with PTFE grafts have been published (Table 94-5).139–148 A prospective, controlled, randomized trial of 140 BCA and PTFE AV grafts found no significant differences in patency and complication rates.146 Other studies, however, including a recently published small randomized trial, revealed PTFE grafts to have superior patency rates.139,141,144,148 BCA xenografts are associated with higher infection139,141 and aneurysmal degeneration139,144 rates than PTFE AV grafts. A 9% to 20% infection rate139,141,149 and a 1% to 8% aneurysmal degeneration rate139,142,144,149 have been observed.
Table 94-5
Published Reports Comparing the Cumulative Patency of Bovine Carotid Artery and Expanded Polytetrafluoroethylene Grafts
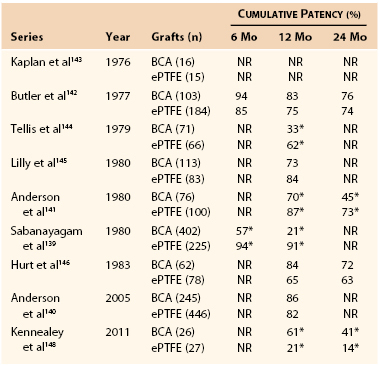
* Significant difference between conduits.
BCA, Bovine carotid artery; ePTFE, expanded polytetrafluoroethylene; NR, not reported.
Adapted from Scott EC, et al: Conduits for hemodialysis access. Semin Vasc Surg 20:158, 2007.
Although BCA xenografts elicit a dense desmoplastic reaction, they are predisposed to aneurysmal degeneration, which is exacerbated by repeated cannulation during hemodialysis. They are prone to infection, and when it occurs, they are very difficult to excise because of intense inflammation and the fragile nature of the graft.144 Finally, they are more expensive than PTFE grafts. These issues have limited widespread use of this graft for hemodialysis access.147
There have been a few published series on the use of different BCA xenografts for infrainguinal revascularization. In one series, 30% of the grafts underwent degeneration within 4 months of insertion.150 A study of 112 grafts used for femoropopliteal bypass yielded a 1-year primary patency rate of 90%.151 Another study of 58 grafts used for above-knee femoropopliteal bypass yielded a 56% 5-year primary-assisted patency rate. No graft infections or aneurysmal degeneration was noted.58 Short available lengths, wide availability of PTFE, and concern about graft degeneration have dampened enthusiasm for the use of BCA xenografts in lower extremity bypass.
Bovine Mesenteric Vein Xenografts
BMV xenografts have been successfully used for hemodialysis access. In one series of 50 grafts placed in 49 patients who had an average of 3.6 previous AVFs, a primary patency rate of 62% was noted at 30 months. Four infections but no aneurysmal degeneration developed.152 In one prospective, multicenter registry, 183 patients with previously failed synthetic grafts were treated with a BMV hemodialysis access. Outcomes were compared with a concomitant nonrandomized group of patients who received PTFE grafts. One-year primary and secondary BMV patency rates were 36% and 66%, respectively. Although primary rates were similar to those of PTFE AV grafts, secondary rates were significantly higher for bovine xenografts. Graft infection was less common in the BMV xenograft group, and the pseudoaneurysm formation rate was similar to that seen with PTFE grafts. However, significant dilatation occurred in six grafts.60 In another recent series of 62 BMV grafts used for hemodialysis access, 30% primary and 58% secondary patency rates were reported. Thirteen infections occurred, and six (10%) grafts required surgical excision. Significant graft dilatation was noted in two patients.153
BMV xenografts appear to have acceptable patency rates that are similar to those seen with PTFE grafts. Although data are limited, these grafts do not appear to be any more predisposed than PTFE grafts to infection or pseudoaneurysm formation. The significance of the dilatation that occurs in some of these grafts is not yet clear. More research will be required before the role of this graft for hemodialysis access is more precisely defined.
BMV xenografts have been used for infrainguinal revascularization. In one small trial involving six patients, all grafts failed within 4 months.82 In another trial of 32 patients with critical limb ischemia, a 16% primary patency rate was noted at 1 month. Most of the occlusions occurred within 1 day of the operation.154 Given these results, this conduit cannot be recommended for infrainguinal bypass.
Tissue-Engineered Vascular Grafts
Spurred by repeated failures of commercially available xenografts and allografts, numerous researchers started to focus on tissue engineering as a tool to improve functionality of current biologic grafts. These new graft concepts aim to utilize the patient’s own cells grown on a variety of supportive scaffolds. Such grafts hold promise to eliminate immunogenicity and propensity for infection that have plagued many biologic grafts. A number of researchers have experimented with different scaffold systems and have created both in vitro and animal models for such grafts.155–160 Despite these trends, the optimal tissue-engineered vascular graft has yet to be identified.
Cytograft Tissue Engineering, Inc. (Novato, Calif) has been actively involved in this line of research and has incorporated their novel sheet-based tissue engineering scaffold into the creation of the Lifeline vascular graft for use in humans.161 Sheets of living fibroblasts grown from cells extracted from patient biopsy samples (skin and superficial veins) are wrapped around a stainless steel mandrel and allowed to fuse in culture during a 10-week maturation phase.162 Seven days before surgery, the lumen of the new vessel is seeded with autologous endothelial cells and preconditioned to both flow and pressure; the total production time for these grafts ranges between 6 and 9 months (mean 7.5 months).163 In the first study of its kind, 10 patients in need of hemodialysis were implanted with their own autologous Lifeline grafts. The primary patency of these grafts was 78% and 60% at 1 and 6 months, respectively.163 There was one graft aneurysm, one graft dilation, and one immediate graft thrombosis that required replacement with a PTFE graft. The remaining eligible patients continued to undergo successful dialysis through their grafts, with only one intervention required to maintain patency at short-term follow-up. To circumvent the prolonged time period required to create these unique and personalized grafts, the manufacturer has begun experimenting with an “off-the-shelf” version of their Lifeline graft for hemodialysis.164 As Lifeline grafts continue to be evaluated in clinical trials for arteriovenous access in dialysis patients, in the near future, similar grafts will reportedly become available for patients with critical limb ischemia.165
Future Directions
Biologic grafts differ from one another in composition and method of preparation. They are useful in a number of clinical scenarios and have earned a place in the armamentarium of modern vascular surgeons. They have not, however, delivered on the expectations that many early vascular surgeons had for these conduits. Despite an enormous amount of basic and clinical investigation they have failed to become the ideal conduit. The search for such a conduit is still progressing.
Significant research is currently being conducted in an attempt to create a biologic nonimmunogenic graft, mainly through improvements using vascular tissue engineering. It is still conceivable that in the future a biologic graft with little or no immunogenicity and characteristics similar to that of a normal artery or vein can be developed, in an acceptable period of time and without prohibitive cost.
Selected Key References
Dardik H, Wengerter K, Qin F, Pangilinan A, Silvestri F, Wolodiger F, Kahn M, Sussman B, Ibrahim IM. Comparative decades of experience with glutaraldehyde-tanned human umbilical cord vein graft for lower limb revascularization: an analysis of 1275 cases. J Vasc Surg. 2002;35:64–71.
The largest single-center experience with HUV grafts..
Farber A, Major K, Wagner WH, Cohen JL, Cossman DV, Lauterbach SR, Levin PM. Cryopreserved saphenous vein allografts in infrainguinal revascularization: analysis of 240 grafts. J Vasc Surg. 2003;38:15–21.
Largest single-center series of CSV allografts for infrainguinal revascularization..
Katzman HE, Glickman MH, Schild AF, Fujitani RM, Lawson JH. Multicenter evaluation of the bovine mesenteric vein bioprosthesis for hemodialysis access in patients with an earlier failed prosthetic graft. J Am Coll Surg. 2005;201:223–230.
Largest multicenter registry of the use of BMV grafts for dialysis access..
Kieffer E, Gomes D, Chiche L, Fléron MH, Koskas F, Bahnini A. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004;39:1009–1017.
One of the largest single-center series of aortic allografts for the treatment of aortic infection..
Madden RL, Lipkowitz GS, Browne BJ, Kurbanov A. Experience with cryopreserved cadaveric femoral vein allografts used for hemodialysis access. Ann Vasc Surg. 2004;18:453–458.
One of the largest single-center series of CFV allografts for hemodialysis access..
McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L’heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446.
First series of patients implanted with tissue-engineered biologic graft for hemodialysis access..
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Loop FD, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6.
2. Hayward PA, et al. Which arterial conduit? Radial artery versus free right internal thoracic artery: six-year clinical results of a randomized controlled trial. Ann Thorac Surg. 2007;84:493–497.
3. Hirose H, et al. Coronary artery bypass grafting using the gastroepiploic artery in 1,000 patients. Ann Thorac Surg. 2002;73:1371–1379.
4. Piercy KT, et al. Renovascular disease in children and adolescents. J Vasc Surg. 2005;41:973–982.
5. Patterson R, et al. Radial artery as conduit for distal renal artery reconstruction. J Vasc Surg. 2003;38:609–612.
6. Amin-Hanjani S, et al. Extracranial-intracranial bypass in the treatment of occlusive cerebrovascular disease and intracranial aneurysms in the United States between 1992 and 2001: a population-based study. J Neurosurg. 2005;103:794–804.
7. Pomposelli FB, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37:307–315.
8. Hughes K, et al. Bypass for chronic ischemia of the upper extremity: results in 20 patients. J Vasc Surg. 2007;46:303–307.
9. Synn AY, et al. Is there a conduit of preference for a bypass between the carotid and subclavian arteries? Am J Surg. 1993;166:157–162.
10. Modrall JG, et al. Comparison of superficial femoral vein and saphenous vein as conduits for mesenteric arterial bypass. J Vasc Surg. 2003;37:362–366.
11. NKF-K/DOQI clinical practice guidelines for vascular access: update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S176.
12. Prager M, et al. Collagen versus gelatin coated Dacron versus stretch polytetrafluoroethylene in abdominal aortic bifurcation graft surgery: results of a seven year prospective randomized multicenter trial. Surgery. 2001;130:408–414.
13. Faries PL, et al. A comparative study of alternative conduits for lower extremity revascularization: all autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32:1080–1090.
14. Carrel A. Ultimate results of aortic transplantations. J Exp Med. 1912;15:389–392.
15. Jeger E. Zur Technik der Blutgefassnaht. Beitr Klin Chir. 1915;97:553.
16. Gross RE, et al. Preliminary observations of the use of human arterial grafts in the treatment of certain cardiovascular defects. N Engl J Med. 1948;239:578.
17. Linton RB. Some practical considerations in the surgery of blood vessel grafts. Surgery. 1955;38:817.
18. Boren CH, et al. Maintenance of viable arterial allografts by cryopreservation. Surgery. 1978;83:382–391.
19. L’Italien GJ, et al. The preservation of the mechanical properties of venous allografts by freezing. J Surg Res. 1979;27:239–243.
20. Faggioli GL, et al. The role of cryopreserved vein allografts in infrainguinal reconstructions. Adv Vasc Surg. 1995;3:173.
21. Rosenberg N, et al. Tanned collagen arterial prosthesis of bovine carotid origin in man. Ann Surg. 1966;164:247–256.
22. Dardik H, et al. Successful arterial substitution with modified human umbilical vein. Ann Surg. 1976;183:252–258.
23. Barner HB, et al. Fresh and frozen homologous venous grafts for arterial repair. Angiology. 1966;17:389–401.
24. Perloff LJ, et al. The venous homograft: an immunological question. Surgery. 1972;72:961–970.
25. Ochsner JL, et al. Experience with fresh venous allografts as arterial substitute. Ann Surg. 1971;173:933–939.
26. Mirelli M, et al. Fresh and cryopreserved arterial homografts: immunological and clinical results. Transplant Proc. 2005;37:2688–2691.
27. Weber TR, et al. Cryopreservation of venous homografts. Surg Forum. 1975;26:291–293.
28. Bortolotti U, et al. Coronary artery bypass with glycerol-preserved saphenous vein allografts. Bull Tex Heart Inst. 1981;8:250–258.
29. Balderman SC, et al. Preparation of venous allografts: a comparison of techniques. Ann Surg. 1984;200:117–130.
30. Stephen M, et al. Allograft vein arterial bypass. Arch Surg. 1978;113:591–593.
31. Livan GG. Mechanism of cryoinjury in biological systems. Cryobiology. 1972;9:182–191.
32. Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939–949.
33. Faggioli GL, et al. Long-term cryopreservation of autologous veins in rabbits. Cardiovasc Surg. 1994;2:259–265.
34. Brockbank KGM. Effects of cryopreservation upon vein function in vivo. Cryobiology. 1994;31:71–81.
35. Pukacki F, et al. The mechanical properties of fresh and cryopreserved arterial homografts. Eur J Vasc Endovasc Surg. 2000;20:21–24.
36. Muller-Schweinitzer E, et al. Impact of freezing/thawing procedures on the post-thaw viability of cryopreserved human saphenous vein conduits. Cryobiology. 2007;54:99–105.
37. Malone JM, et al. Venous cryopreservation: endothelial fibrinolytic activity and histology. J Surg Res. 1980;29:209–222.
38. Bambang LS, et al. Effects of cryopreservation on the proliferation and anticoagulant activity of human saphenous vein endothelial cells. J Thorac Cardiovasc Surg. 1995;110:998–1004.
39. Brockbank KGM, et al. Functional analysis of cryopreserved veins: preliminary report. J Vasc Surg. 1990;11:94–100.
40. Ligush J, et al. First results on the functional characteristics of cryopreserved human saphenous vein. Cells Materials. 1991;1:359.
41. Elmore JR, et al. Cryopreservation affects endothelial and smooth muscle function of canine saphenous vein grafts. J Vasc Surg. 1991;13:584–592.
42. Alonso M, et al. Cryopreserved arterial homografts: preliminary results in infrainguinal arterial reconstructions. Ann Vasc Surg. 1999;13:261–267.
43. Schmitz-Rixen T, et al. Immunosuppressive treatment of aortic allografts. J Vasc Surg. 1988;7:82–92.
44. Schwartz SI, et al. Antigenicity of homografted veins. Surgery. 1967;61:471–477.
45. Tice DA, et al. Clinical experience with preserved human allografts for vascular reconstruction. Surgery. 1972;72:260–267.
46. Pober JS, et al. Interactions of T lymphocytes with human vascular endothelial cells: role of endothelial cell surface antigens. Immunobiology. 1984;168:483–494.
47. Balzer KM, et al. Donor-specific sensitization by cadaveric venous allografts used for arterial occlusive vascular disease. Tissue Antigens. 2004;64:13–17.
48. Bank HL, et al. Transplantation of cryopreserved canine venous allografts. J Surg Res. 1991;50:57–64.
49. Carpenter JP, et al. Human saphenous vein allograft bypass grafts: immune response. J Vasc Surg. 1998;27:492–499.
50. Axthelm SC, et al. Antigenicity of venous allografts. Ann Surg. 1979;189:290–293.
51. Cochran RP, et al. Cryopreservation does not alter antigenic expression of aortic allografts. J Surg Res. 1989;46:597–599.
52. Miller VM, et al. Cryopreserved venous allografts: effects of immunosuppression and antiplatelet therapy on patency and function. J Vasc Surg. 1993;18:216–226.
53. Carpenter JP, et al. Immunosuppression for human saphenous allograft bypass surgery: a prospective randomized trial. J Vasc Surg. 1997;26:32–42.
54. Posner MP, et al. Early results of infrageniculate arterial reconstruction using cryopreserved homograft saphenous conduit (CADVEIN) and combination low-dose systemic immunosuppression. J Am Coll Surg. 1996;183:208–216.
55. Randon C, et al. Fifteen years of infrapopliteal arterial reconstruction with cryopreserved venous allografts for limb salvage. J Vasc Surg. 2010;51:869–877.
56. Benedetto B, et al. Use of cryopreserved cadaveric vein allograft for hemodialysis access precludes kidney transplantation because of allosensitization. J Vasc Surg. 2001;34:139–142.
57. Madden R, et al. Decellularized cadaver vein allografts used for hemodialysis access do not cause allosensitization or preclude kidney transplantation. Am J Kidney Dis. 2002;10:1240–1243.
59. Rosenberg N. The modified bovine arterial graft. Arch Surg. 1972;105:547–548.
60. Katzman HE, et al. Multicenter evaluation of the bovine mesenteric vein bioprosthesis for hemodialysis access in patients with an earlier failed prosthetic graft. J Am Coll Surg. 2005;201:223–230.
61. Dardik H, et al. Clinical experience with modified human umbilical cord vein for arterial bypass. Surgery. 1976;79:618–624.
62. Roedersheimer LR, et al. Comparison of platelet adherence and aggregation in modified human umbilical vein and autogenous vein grafts. Am J Surg. 1980;140:591–595.
63. Schechter I. Prolonged survival of glutaraldehyde treated skin homografts. Proc Natl Acad Sci U S A. 1971;68:1590–1593.
64. Love C, et al. Rapid intraoperative construction of autologous small caliber blood vessels. ASAIO J. 1998;44:M648–M652.
65. Lantz GC, et al. Small intestinal submucosa as a small-diameter arterial graft in a dog. J Invest Surg. 1990;3:217–227.
66. Uematsu M, et al. A modified human ureter graft tanned by a new crosslinking agent polyepoxy compound for small diameter arterial substitutions: an experimental preliminary study. Artif Organs. 1998;22:909–913.
67. Ketharanathan V, et al. Bovine ureter as a vascular prosthesis: a preliminary report of an experimental study in dogs. Aust N Z J Surg. 1982;52:590–593.
68. Field PL. The chemically treated bovine ureter—clinical performance of a novel biological vascular prosthesis. Cardiovasc Surg. 2003;11:30–34.
69. Chemla ES, et al. Randomized clinical trial comparing decellularized bovine ureter with expanded polytetrafluoroethylene for vascular access. Br J Surg. 2009;96:34–39.
70. Taylor LM, et al. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11:193–206.
71. Shah DM, et al. Long term results of in situ saphenous vein bypass. Analysis of 2058 cases. Ann Surg. 1995;222:438–448.
72. Faries PL, et al. The use of arm vein in lower-extremity revascularization: result of 520 procedures performed in eight years. J Vasc Surg. 2000;31:50–59.
73. Chang BB, et al. The lesser saphenous vein: an underappreciated source of autogenous vein. J Vasc Surg. 1992;15:152–157.
74. Londrey GL, et al. Infrainguinal reconstruction with arm vein, lesser saphenous vein and remnants of greater saphenous vein: a report of 257 cases. J Vasc Surg. 1994;20:451–457.
75. Veith FJ, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstruction. J Vasc Surg. 1986;3:104–114.
76. Hobson RW, et al. Results of revascularization and amputation in severe lower extremity ischemia: a five year clinical experience. J Vasc Surg. 1985;2:174–185.
77. Pappas PJ, et al. Patency of infrainguinal polytetrafluoroethylene grafts with distal interposition vein cuffs. Cardiovasc Surg. 1998;6:19–26.
78. Neville RF, et al. Tibial bypass for limb salvage using polytetrafluoroethylene and a distal vein patch. J Vasc Surg. 2001;33:266–272.
79. Dardik H, et al. Improved method to create the common ostium variant of the distal arteriovenous fistula for enhancing crural prosthetic graft patency. J Vasc Surg. 1996;24:240–248.
80. Farber A, et al. Cryopreserved saphenous vein allografts in infrainguinal revascularization: analysis of 240 grafts. J Vasc Surg. 2003;38:15–21.
81. Dardik H, et al. A decade of experience with the glutaraldehyde-tanned human umbilical cord vein graft for revascularization of the lower limb. J Vasc Surg. 1988;7:336–346.
82. Kovalic AJ, et al. Outcome of ProCol, a bovine mesenteric vein graft, in infrainguinal reconstruction. Eur J Vasc Endovasc Surg. 2002;24:533–544.
83. Szczech LA, et al. Projecting the United States ESRD population: issues regarding treatment of patients with ESRD. Kidney Int Suppl. 2004;90:S3–S7.
84. Xue JL, et al. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12:2753–2758.
85. Patel ST, et al. Failure of arteriovenous fistula maturation: an unintended consequence of exceeding Dialysis Outcome Quality Initiative guidelines for hemodialysis access. J Vasc Surg. 2003;38:439–445.
86. Dember LM, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299:2164–2171.
87. Woo K, et al. Evaluation of the efficacy of the transposed upper arm arteriovenous fistula: a single institutional review of 190 basilic and cephalic vein transposition procedures. J Vasc Surg. 2007;46:94–99.
88. Matsuura JH, et al. Cryopreserved femoral vein grafts for difficult hemodialysis access. Ann Vasc Surg. 2000;14:50–55.
89. Hutchin P, et al. Bovine graft arteriovenous fistulas for maintenance hemodialysis. Surg Gynecol Obstet. 1975;141:255–258.
90. O’Hara PJ, et al. Surgical management of abdominal aortic grafts: review of 25 year experience. J Vasc Surg. 1986;3:725–731.
91. Hayes PD, et al. In situ replacement of infected aortic grafts with rifampicin-bonded prostheses: the Leicester experience (1992-98). J Vasc Surg. 1999;30:92–98.
92. Clagett GP, et al. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg. 1997;25:255–270.
93. Kieffer E, et al. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004;39:1009–1017.
94. Quinones-Baldrich WJ, et al. Long-term results following surgical management of aortic graft infection. Arch Surg. 1991;126:507–511.
95. Knosalla C, et al. Treatment of vascular infection by in situ replacement with cryopreserved aortic allografts: an experimental study. J Vasc Surg. 1998;27:689–698.
96. Zhou W, et al. In situ reconstruction with cryopreserved arterial allografts for management of mycotic aneurysms or aortic prosthetic graft infections: a multi-institutional experience. Tex Heart Inst J. 2006;33:14–18.
97. Matsuura JH, et al. Hemodialysis graft infections treated with cryopreserved femoral vein. Cardiovasc Surg. 2002;10:561–565.
98. Fujitani RM, et al. Cryopreserved saphenous vein allogeneic homografts: an alternative conduit in lower extremity arterial reconstruction in infected fields. J Vasc Surg. 1992;15:519–526.
99. Buzzi M, et al. Vascular tissue banking: state of the art. Transplant Proc. 2005;37:2428–2429.
100. Vogt PR, et al. Technical details with the use of cryopreserved arterial allografts for aortic infection: influence on early and midterm mortality. J Vasc Surg. 2002;35:80–86.
101. Dardik H. A 30-year odyssey with the umbilical vein graft. J Am Coll Surg. 2006;203:582–583.
102. Harris L, et al. Long term assessment of cryopreserved bypass grafting success. J Vasc Surg. 2001;33:528–532.
103. Harris RW, et al. Allograft vein bypass: is it an acceptable alternative for infrapopliteal revascularization? J Vasc Surg. 1993;18:553–560.
104. Leseche G, et al. Femorodistal bypass using cryopreserved venous allografts for limb salvage. Ann Vasc Surg. 1997;11:230–236.
105. Martin RS, et al. Cryopreserved saphenous vein allografts for below-knee lower extremity revascularization. Ann Surg. 1994;219:664–672.
106. Shah RM, et al. Early results with cryopreserved saphenous vein allografts for infrainguinal bypass. J Vasc Surg. 1993;18:965–971.
107. Walker PJ, et al. Early experience with cryopreserved saphenous vein allografts as a conduit for complex limb-salvage procedures. J Vasc Surg. 1993;18:561–569.
108. Buckley CJ, et al. Suggested treatment protocol for improving patency of femoral-infrapopliteal cryopreserved saphenous vein allografts. J Vasc Surg. 2000;32:731–738.
109. Bannazadeh M, et al. Reoperative lower extremity revascularization with cadaver vein for limb salvage. Ann Vasc Surg. 2009;23:24–31.
110. Dalsing MC, et al. Infrapopliteal bypass for established gangrene of the forefoot or toes. J Vasc Surg. 1985;2:669–677.
112. Madden RL, et al. Experience with cryopreserved cadaveric femoral vein allografts used for hemodialysis access. Ann Vasc Surg. 2004;18:453–458.
113. Madden RL, et al. A comparison of cryopreserved vein allografts and prosthetic grafts for hemodialysis access. Ann Vasc Surg. 2005;19:686–691.
114. Bolton WD, et al. The use of cryopreserved femoral vein grafts for hemodialysis in patients at high risk for infection: a word of caution. J Vasc Surg. 2002;36:464–468.
115. Esperon A, et al. Uruguayan experience with cryopreserved arterial homografts. Transplant Proc. 2009;41:3500–3504.
116. Castier Y, et al. Early experience with cryopreserved arterial allografts in below-knee revascularization for limb salvage. Am J Surg. 1999;177:197–202.
117. Albertini JN, et al. Long-term results of arterial allograft below-knee bypass for limb salvage: a retrospective multicenter study. J Vasc Surg. 2000;31:426–435.
118. Brown KE, et al. Arterial reconstruction with cryopreserved human allografts in the setting of infection: a single-center experience with midterm follow-up. J Vasc Surg. 2009;49:660–666.
119. Chiesa R, et al. Fresh and cryopreserved arterial homografts in the treatment of prosthetic graft infections: experience of the Italian Collaborative Vascular Homograft Group. Ann Vasc Surg. 1998;12:457–462.
120. Locati P, et al. The use of arterial allografts in aortic graft infection: a three year experience on eighteen patients. J Cardiovasc Surg. 1998;39:735–741.
121. Noel AA, et al. Abdominal aortic reconstruction in infected fields: early results in the United States Cryopreserved Aortic Allograft Registry. J Vasc Surg. 2002;35:847–852.
122. Verhelst R, et al. Use of cryopreserved arterial homografts for management of infected prosthetic grafts: a multicenter study. Ann Vasc Surg. 2000;14:602–607.
123. Nevelsteen A, et al. Experience with cryopreserved arterial allografts in the treatment of prosthetic graft infections. Cardiovasc Surg. 1998;6:378–383.
124. Bisdas T, et al. Eight-year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010;52:323–330.
125. McCready RA, et al. Long-term results with cryopreserved arterial allografts (CPAs) in the treatment of graft or primary arterial infections. J Surg Res. 2011;168:149–153.
126. Dardik H, et al. Comparative decades of experience with glutaraldehyde-tanned human umbilical cord vein graft for lower limb revascularization: an analysis of 1275 cases. J Vasc Surg. 2002;35:64–71.
127. Neufang A, et al. Femoropopliteal prosthetic bypass with glutaraldehyde stabilized human umbilical vein (HUV). J Vasc Surg. 2007;46:280–288.
128. Neufang A, et al. Infrapopliteal composite bypass with autologous vein and second generation glutaraldehyde stabilized human umbilical vein (HUV) for critical limb ischemia. Eur J Vasc Endovasc Surg. 2007;34:583–589.
129. Neufang A, et al. Sequential femorodistal composite bypass with second generation glutaraldehyde stabilized human umbilical vein (HUV). Eur J Vasc Endovasc Surg. 2005;30:176–183.
130. Batt M, et al. Human umbilical vein grafts as infrainguinal bypasses: long-term clinical follow-up and pathological investigation of explanted grafts. Clin Invest Med. 1990;13:155–164.
131. Sato O, et al. Biodegradation of glutaraldehyde-tanned human umbilical vein grafts. Surg Today. 1995;25:901–905.
132. Sommeling CA, et al. Long-term behavior of modified human umbilical vein grafts: late aneurysmal degeneration established by colour-duplex scanning. Eur J Vasc Endovasc Surg. 1990;4:89–94.
133. Johnson WC, et al. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. J Vasc Surg. 2000;32:268–277.
134. Cranley JJ, et al. Revascularization of the femoropopliteal arteries using saphenous vein, polytetrafluoroethylene, and umbilical vein grafts. Arch Surg. 1982;117:1543–1550.
135. Eickhoff JH, et al. Four years’ results of a prospective, randomized clinical trial comparing polytetrafluoroethylene and modified human umbilical vein for below-knee femoropopliteal bypass. J Vasc Surg. 1987;6:506–511.
136. Johnson WC, et al. Axillofemoral (PTFE) and infrainguinal revascularization (PTFE and umbilical vein). J Cardiovasc Surg. 1991;32:344–349.
137. Aalders GJ, et al. Polytetrafluoroethylene versus human umbilical vein in above knee femoropopliteal bypass: six-year results of a randomized clinical trial. J Vasc Surg. 1992;16:816–824.
138. Chinitz JL, et al. Self-sealing prosthesis for arteriovenous fistula in man. Trans Am Soc Artif Intern Organs. 1972;18:452–457.
139. Sabanayagam P, et al. A comparative study of 402 bovine heterografts and 225 reinforced expanded PTFE grafts as AVF in the ESRD patient. Trans Am Soc Artif Intern Organs. 1980;26:88–92.
140. Anderson C, et al. Renewed interest in bovine heterograft for vascular access: a comparison between polytetrafluoroethylene and bovine. Henry ML. Vascular Access for Hemodialysis IX. Bonus Books: Los Angeles, CA; 2005:185–193.
141. Anderson CB, et al. Bovine carotid artery and expanded polytetrafluoroethylene grafts for hemodialysis vascular access. J Surg Res. 1980;29:184–188.
142. Butler HG, et al. Vascular access for chronic hemodialysis: polytetrafluoroethylene versus bovine heterograft. Am J Surg. 1977;134:791–793.
143. Kaplan MS, et al. Comparison of PTFE and bovine grafts for blood access in dialysis patients. Trans Am Soc Artif Intern Organs. 1976;22:388–393.
144. Tellis VA, et al. Expanded polytetrafluoroethylene graft fistula for chronic hemodialysis. Ann Surg. 1979;189:101–105.
145. Lilly L, et al. Comparison between bovine heterograft and expanded PTFE grafts for dialysis access. Am Surg. 1980;46:694–696.
146. Hurt AV, et al. Bovine carotid artery heterografts versus polytetrafluoroethylene grafts: a prospective, randomized study. Am J Surg. 1983;146:844–847.
147. Scott EC, et al. Conduits for hemodialysis access. Semin Vasc Surg. 2007;20:158–163.
148. Kennealey PT, et al. A prospective, randomized comparison of bovine carotid artery and expanded polytetrafluoroethylene for permanent hemodialysis access. J Vasc Surg. 2011;53:1640–1648.
149. Hertzer NR, et al. Venous access using the bovine heterograft: techniques, results, and complications in 75 patients. Arch Surg. 1978;113:696–700.
150. Broyn T, et al. Early complications with a new bovine arterial graft (Solcograft P). Acta Chir Scand. 1986;152:263–266.
151. Wagner WH, et al. Early results of infrainguinal arterial reconstruction with a modified biological conduit. Ann Vasc Surg. 1992;6:325–333.
152. Bourquelot P. ProCol bioprosthetic vascular grafts for dialysis access. Henry ML. Vascular Access for Hemodialysis VI. Precept Press: Chicago, IL; 1999:223–229.
153. Widmer MK, et al. Intermediate outcome and risk factor assessment of bovine vascular heterografts used as AV-fistulas for hemodialysis access. Eur J Vasc Endovasc Surg. 2004;27:660–665.
154. Schmidli J, et al. Bovine mesenteric graft (ProCol) in critical limb ischaemia with tissue loss and infection. Eur J Vasc Endovasc Surg. 2004;27:251–253.
155. Tillman BW, et al. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J Vasc Surg. 2012;56:789–793.
156. Torikai K, et al. A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J Thorac Cardiovasc Surg. 2008;136:37–45.
157. Zhou Y, et al. The development of a tissue-engineered artery using decellularized scaffold and autologous bovine mesenchymal stem cells. Biomaterials. 2010;31:296–307.
158. Quint C, et al. Allogeneic human tissue engineered blood vessel. J Vasc Surg. 2012;55:790–798.
160. Hoenicka M, et al. Properties of the human umbilical vein as a living scaffold for a tissue-engineered vessel graft. Tissue Eng. 2007;13:219–229.
161. L’Heureux N, et al. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–1453.
162. L’Heureux N, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365.
163. McAllister TN, et al. Effectiveness of hemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446.
164. Wystrychowski W, et al. Case study: first implantation of a frozen, devitalized tissue-engineered vascular graft for urgent hemodialysis access. J Vasc Access. 2011;12:67–70.
165. Cytograft Tissue Engineering. [Available at:] http://www.cytograft.com/clinicaltrials.html [Accessed December 20, 2012] .