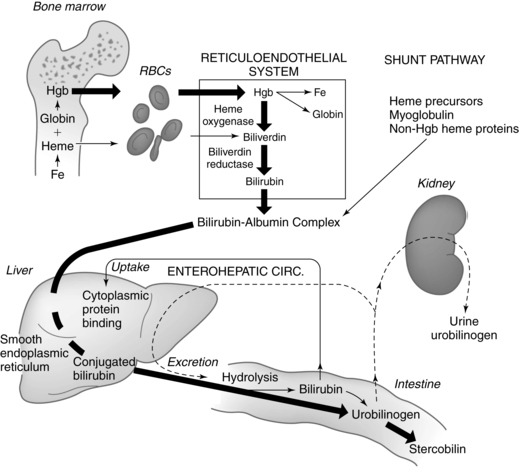
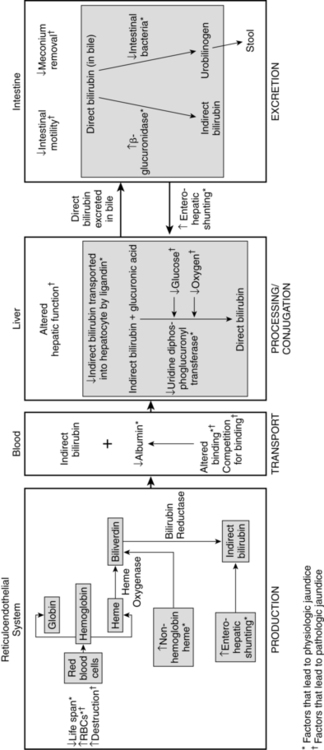
Bilirubin metabolism
Physiologic jaundice is a common problem in term and preterm infants during the first week after birth. For most of these infants, this phenomenon is mild and resolves without treatment. A small group of infants develop neonatal hyperbilirubinemia, which may be an exaggeration of normal physiologic processes or may herald underlying disorders such as hemolytic disease of the newborn or sepsis. When any infant develops significant hyperbilirubinemia, concerns arise about possible sequelae in the form of bilirubin encephalopathy. This chapter focuses on bilirubin metabolism in the fetus and neonate along with issues related to neonatal hyperbilirubinemia and its management. Maternal adaptations are discussed only briefly, because bilirubin metabolism is not normally significantly altered in pregnancy. Bilirubin synthesis, transport, and metabolism are summarized in Figure 18-1 and in Box 18-1 on page 609.
Maternal physiologic adaptations
Alterations in liver and hepatic function during pregnancy are described in Chapter 12. Bilirubin metabolism is not significantly altered in the pregnant woman, with bilirubin levels generally reported to be similar or slightly lower than those in nonpregnant women; i.e., values of 0.3 to 1 mg/dL (5.3 to 17.1 μmol/L) for total bilirubin and an upper limit of 0.2 mg/dL (3.4 μmol/L) for direct bilirubin.102 However, others report that both total and free bilirubin levels are slightly lower across all three trimesters and direct is lower in the second and third trimesters, due to hemodilution and decreased albumin.9,52 This discrepancy may be because of differences in the reference ranges used. Reference ranges for liver function tests during pregnancy have been proposed, with the upper limit of normal values lower than previous values for both pregnant and nonpregnant women of childbearing age.9,40,52 Activity of phase II enzymes involved in bilirubin metabolism is increased in pregnant women.82 Elevated serum bilirubin levels in pregnancy warrant further evaluation for liver or hematologic dysfunction.72,102,133
Clinical implications for the pregnant woman and her fetus
A major difference between fetal and adult handling of bilirubin is that the fetus uses the placenta rather than its own intestines as the major elimination pathway. Most of the bilirubin produced by the fetus remains in the indirect state, a form that can be readily cleared by the placenta. The indirect fetal bilirubin eliminated across the placenta is conjugated and excreted by the maternal liver. Even with severe hemolysis, infants are rarely born jaundiced because the placenta efficiently clears excess fetal indirect bilirubin.59 However, these infants may have an accumulation of direct bilirubin and are often severely anemic due to the ongoing hemolysis. The maternal system efficiently handles the fetal bilirubin load and has sufficient reserve so maternal hyperbilirubinemia secondary to fetal hemolysis is rare.102 Immunologic aspects of hemolytic disorders secondary to blood group incompatibility are discussed in Chapter 13.
Maternal hyperbilirubinemia
Elevated total and direct serum bilirubin levels during pregnancy may occur with viral hepatitis, hyperemesis gravidarum (usually less than 5 mg/dL [85.5 μmol/L]), intrahepatic cholestasis of pregnancy (usually less than 4 to 5 mg/dL [68.4 to 85.8 μmol/L]), preeclampsia/eclampsia (often normal but if increased levels are generally less than 5 mg/dL [85.5 μmol/L]), acute fatty liver of pregnancy (usually less than 10 mg/dL [171 μmol/L]), cholelithiasis, and hepatic rupture.52,102 The most common cause of jaundice in the first two trimesters of pregnancy is viral hepatitis.102 Other causes of jaundice in early pregnancy include drug-induced hepatotoxicity, septicemia, or cholelithiasis, with biliary tract disease becoming more prominent in the second trimester.99 Causes of jaundice in the third trimester include intrahepatic cholestasis of pregnancy (see Table 14-2), viral hepatitis, gallstone disease, HELLP syndrome (characterized by hemolysis [H], elevated liver enzymes [EL], and a low platelet [LP] count), acute fatty liver of pregnancy, and disseminated herpes. Postpartum jaundice is most often a result of septicemia, drug use, viral hepatitis, or cholelithiasis.52,72 Liver function and hepatic disorders during pregnancy are discussed further in Chapter 12.
The effects of excessive maternal bilirubin production on the fetus depend on whether the woman has direct or indirect hyperbilirubinemia. Direct (conjugated) bilirubin is not transferred across the placenta in either direction.71,102 Therefore the fetus of a woman with direct hyperbilirubinemia and jaundice secondary to hepatitis or other functional liver disorders does not have an elevated direct bilirubin level. Indirect (unconjugated) bilirubin has been reported to be transferred across the placenta from mother to fetus (as well as the usual transfer from fetus to mother) and prolonged exposure to elevated levels may increase the risk of neurologic complications in the infant.74,102 HELLP syndrome is associated with an increase in indirect bilirubin, although levels in the mother are usually less than 5 mg/dL (85.5 μmol/L) and the mother usually does not appear jaundiced.102 Neonatal hyperbilirubinemia is seen in about half of the infants in pregnancies complicated by this syndrome. Isolated indirect hyperbilirubinemia is rare in adults; however, several cases of elevated indirect bilirubin levels in cord blood have been reported in infants of women with end-stage cirrhosis.102 It is unclear whether this increase results from maternal-to-fetal transfer or whether the elevated maternal bilirubin levels prevented the normal fetal-to-maternal transfer of indirect bilirubin. Intrahepatic cholestasis of pregnancy can alter the removal of bile acids and bilirubin from the fetus across the placenta. If this occurs, these substances may accumulate in the maternal liver and eventually in the placenta and fetal liver.8,71,82
Development of bilirubin metabolism in the fetus
In early pregnancy, the fetus begins producing bile acids and bile pigments (the most prominent being biliverdin and bilirubin). Elimination of these cholephilic organic anions requires a complex interplay of the mother, placenta, and fetus.71,82 The placenta contains detoxifying enzymes and organic anion carrier transport systems, which facilitate this removal and prevent fetal accumulation of these potentially harmful substances.71,82 For example, expression and activity of phase II enzymes are increased in the placenta.82 As a result, bile acids and bilirubin levels remain low in the fetus.71 Biliverdin crosses the placenta poorly; thus conversion to bilirubin facilitates carrier-mediated transport across the placenta.71 The early production of bile acids may have a role in development that is as yet incompletely understood, since bile acids act as signaling molecules with both endocrine and paracrine functions.62 Most fetal bilirubin remains in an unconjugated state.22,71 This is facilitated by immaturity of the liver and intestine, decreased hepatic blood flow as a result of shunting of some placental blood away from the fetal liver by the ductus venosus, and increased recirculation of bilirubin by the enterohepatic shunt (see Figure 18-1 and Box 18-1). Bilirubin production from biliverdin before liver maturation may play a role in protecting the fetus from oxidative stress and in inducing expression of antioxidant systems.71,108
Hepatic uptake is reduced by very low levels of the intracellular carrier protein ligandin.74 Conjugation of indirect bilirubin by the fetal liver is reduced because of immaturity of a hepatic microsomal enzyme 1A1 isoform of uridine diphosphate-glucuronosyltransferase (UGT) and other liver enzyme systems, and decreased hepatocyte uptake and excretion of bilirubin. UGT1A1 can be detected by 16 weeks’ gestation.61 Activity of this enzyme remains low in the fetus and is 0.1% of adult activity at 17 to 30 weeks, increasing to 1% by term.61,74,129
Elevated concentrations of β-glucuronidase in the fetal small intestine lead to increased deconjugation of direct bilirubin with recirculation to the blood for removal by the placenta. Limited intestinal motility also promotes intestinal reabsorption of bilirubin by lengthening the time available for β-glucuronidase to act. Fetal total bilirubin levels are slightly higher than maternal values (averaging 1.5 ± 0.3 mg/L [25.6 ± 5.1 μmol/L]), which may facilitate diffusion across the placenta.101 The fetus demonstrates little hepatobiliary elimination of bilirubin. Unconjugated bilirubin levels are also greater in the fetus than the mother.71 Bilirubin and its conjugates can be detected in fetal bile by 22 to 24 weeks’ gestation.41 Fetal total and direct bilirubin increases with increasing gestational age, probably due to increasing numbers of red blood cells (RBCs) that then undergo physiologic hemolysis. Bilirubin production increases about 150% per unit of body weight in late gestation as RBCs formed earlier in gestation undergo normal degradation.
Indirect bilirubin can be found in the amniotic fluid beginning at about 12 weeks’ gestation.59 Amniotic fluid bilirubin levels initially rise, plateau between 16 and 25 weeks, and then decrease, essentially disappearing by about 36 weeks.59,101 This pattern has been plotted on graphs used to monitor and manage the fetus in pregnancies complicated by Rh sensitization and other blood group incompatibilities. The increased bilirubin reflects increased RBC destruction by maternal antibodies (see Chapter 13).
The mechanisms by which bilirubin reaches amniotic fluid are uncertain.59 The fetal kidney excretes small amounts of organic anions into amniotic fluid.22 Bilirubin may be transferred directly across placental tissue from the mother or across the amnion or umbilical cord from fetal blood vessels.59,61 The lipid-soluble indirect bilirubin may enter the amniotic fluid dissolved in phospholipids from tracheobronchial secretions.59 Failure of amniotic fluid bilirubin levels to decrease during gestation is associated with hemolytic disease or disorders that interfere with the normal production and turnover of amniotic fluid (see Chapter 3), such as high intestinal obstruction or anencephaly with decreased fetal swallowing.
Heme oxygenase, an enzyme involved in bilirubin production, is found in the placenta. Heme oxygenase catabolizes heme into carbon monoxide and biliverdin, which is subsequently catabolized to bilirubin (see Figure 18-1 and Box 18-1). Carbon monoxide is a potent vasodilator and bilirubin a potent antioxidant (see Benefits of Bilirubin). Thus, these substances may have a local role in control of placental vascular tone and protection of fetal-placental endothelium and syncytiotrophoblast from oxidative injury.24,115,118
Neonatal physiology
Transitional events
Cord blood bilirubin levels are normally less than 2 mg/dL (34.2 μmol/L) and range from 1.4 to 1.9 mg/dL (23.9 to 32.5 μmol/L).74 With clamping of the umbilical cord, blood flow and pressure in the venous circulation decrease, the ductus venosus constricts, and flow of relatively desaturated blood to the liver increases. Persistent or fluctuating patency of the ductus venosus, which occurs in some immature or ill infants, results in shunting of portal blood past the liver sinusoidal circulation (reducing the amount of blood perfusing the liver) and may interfere with normal clearance of bilirubin from the plasma.84
At birth the meconium in the intestines may contain 100 to 200 mg of bilirubin.41 About half of this is deconjugated bilirubin and equals 5 to 10 times the daily bilirubin production rate from heme catabolism in the term neonate. Meconium passage usually occurs within 6 to 12 hours (69% of infants); it occurs in 94% of term infants by 24 hours of age (see Chapter 12). Any delay in passage of meconium through the intestinal tract increases the likelihood that conjugated bilirubin will be deconjugated (see Box 18-1 on page 609) and returned to the circulation.
Benefits of bilirubin
The by-products of heme degradation (CO, iron, and bilirubin) have both toxic and beneficial effects.24,118 Unconjugated bilirubin can diffuse into any cell. While high levels of bilirubin are toxic, at low levels, bilirubin acts as a potent intracellular antioxidant by binding to membranes to prevent their peroxidation and scavenging reactive oxygen species.10,24,45,59,71,74,85,108,109 Levels of bilirubin correlate with total blood oxidative capacity in the newborn.108 Highly reactive metabolites of oxygen (free radicals) are a by-product of oxidative phosphorylation. Cellular enzymes normally scavenge for and destroy these radicals before they can interfere with cell metabolic functions and destroy cell lipid membranes. Bilirubin accumulation after birth may augment other antioxidant systems and help protect the fetus in moving from the lower oxygenation of the intrauterine environment to the oxygen-rich extrauterine environment.21,71,74,108 Infants with neonatal disorders associated with free radical production (e.g., respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and retinopathy of prematurity) have been found to have lower serum bilirubin levels than those of similar gestational ages with non-oxidative disorders, which is suggestive that bilirubin is being used to cope with oxidative stress.108 Bilirubin may also have a protective role against cardiovascular disease in adults.71
Bilirubin production in the neonate
The usual destruction of circulating red blood cells (RBCs) accounts for about 75% of the bilirubin produced in the healthy term newborn. Catabolism of nonhemoglobin heme, ineffective erythropoiesis, and enterohepatic recirculation (enterohepatic shunt) account for 20% of the bilirubin produced in the term and 30% in the preterm infant.74 In newborns the amount of nonhemoglobin heme is increased by heme from the large pool of hematopoietic tissue that ceases to function after birth.59 Bilirubin produced by catabolism of nonhemoglobin heme and immature RBCs is sometimes referred to as early bilirubin.
The newborn produces up to 8 to 10 mg/kg/day of bilirubin (more than twice as much as adults).74 Bilirubin production is inversely correlated with gestational age and remains higher (per kilogram) for 3 to 6 weeks.59 Increased bilirubin production in the newborn is due to a greater circulating RBC volume per kilogram (and subsequent breakdown of senescent cells), decreased RBC life span (80 to 100 days in term, 60 to 80 days in preterm, and 35 to 50 days in extremely low–birth weight [ELBW] infants, versus 120 days in adults), increased numbers of immature or fragile cells, and an increase in early bilirubin.51,74
Levels of unbound bilirubin are also higher in the newborn, and more so in the preterm infant, because of lower albumin concentrations, decreased albumin-binding capacity, and decreased affinity of albumin for bilirubin.4,23,21,59 This may be due to a maturational defect in albumin structure, or endogenous metabolic products produced during periods of stress or abnormal metabolism may block or interfere with albumin-binding sites.22 Bilirubin processing (conjugation) by the liver and excretion are also altered in the newborn (see Causes of Physiologic Jaundice).
Physiologic jaundice
Physiologic jaundice in the newborn is seen in 50% to 60% of term infants and up to 80% of preterm infants during the first days after birth.59,74 Visible jaundice in neonates usually appears as the bilirubin levels reach 5 to 6 mg/dL (85.5 to 103 μmol/L).59 Almost all term infants develop bilirubin levels over 2 mg/dL (34.2 μmol/L) in the first week and two thirds or more develop clinical jaundice.74 Neonatal jaundice accounts for 75% of hospital readmissions in the first week after birth.86
Patterns of physiologic jaundice
The usual pattern of bilirubin change is a two-phase process.38,39,59 These general phases are seen in term and preterm infants and in breastfed and formula-fed infants, although characteristics of the phases vary depending on gestation, ethnicity and method of feeding. Phase I is primarily secondary to the reduced hepatic UGT1A1 activity and phase II to low levels of ligandin binding.51 During phase I in white and African-American term infants, total bilirubin levels peak at 48 to 120 hours (most infants peak at 72 to 96 hours) at 5 to 6 mg/dL (86 to 103 μmol/L) and then decrease to less than 3 mg/dL (51 μmol/L) by day 5.59 Infant of East Asian ethnicity usually peak slightly later (72 to 120 hours) at 10 to 14 mg/dL (171 to 239 μmol/L), and fall to less than 3 mg/dL (51 μmol/L) by 7 to 10 days.59 All groups gradually fall to adult values of less than 2 mg/dL (34 μmol/L) over the first 1 to 2 weeks (phase II).
The reason for the increased bilirubin levels in infants of East Asian ethnicity is not known, although there is some evidence to suggest that their rate of bilirubin synthesis may be slightly elevated.59 These infants are also more likely to have glucose-6-phosphate dehydrogenase (G6PD) deficiency. In addition, genomic polymorphisms (variations in gene sequencing) in the genes involved in bilirubin production and conjugation are seen with greater frequency.59,129 Polymorphisms in these genes increase the risk of hyperbilirubinemia and are seen in other infants as well. For example, alterations in UGT1A1, which is involved in bilirubin conjugation, and soluble carrier organic anion transporter polypeptide (SLCO1B1), which is involved in uptake of unconjugated bilirubin by the hepatocyte, alone or in conjunction with environmental influences can alter bilirubin clearance and increase the risk of hyperbilirubinemia.129
Patterns of physiologic jaundice in term breastfed infants are similar to phases in term formula-fed infants, except that the peak is higher (may be up to 7 to 14 mg/dL [119.7 to 239.4 μmol/L]) and later and phases I and II are longer. This difference is due in part to a delay in UGT1A1 maturation. In these infants, bilirubin levels generally decrease over 2 to 4 weeks, although it may take up to 6 weeks.51,59,74
Preterm infants also have two phases with a higher peak and longer phase I and II. The mean peak total bilirubin is 10 to 12 mg/dL (171 to 205 μmol/L) by day 5.59 These patterns are only seen if early prophylactic phototherapy is not used. The greater the immaturity of the infant, the greater the alterations in phases and the risk of hyperbilirubinemia. Maisels suggests that the term physiologic jaundice has little usefulness with preterm and especially very low–birth weight (VLBW) infants since these infants are treated with phototherapy even at physiologic levels.74
Causes of physiologic jaundice
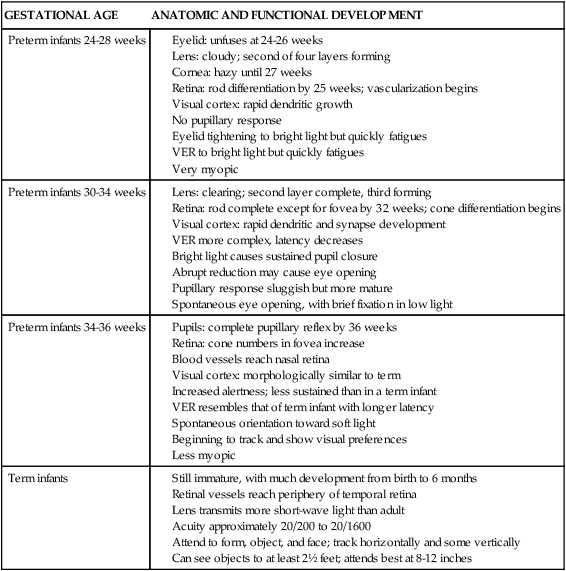
Physiologic jaundice is not caused by a single factor, but rather reflects a combination of factors related to the newborn’s physiologic maturity (Table 18-1 and Figure 18-2). The increased levels of circulating indirect bilirubin in the newborn are due to the combination of increased bilirubin availability and decreased clearance. Phase I bilirubin elevations are primarily due to a sixfold increase in bilirubin load, decreased bilirubin-specific hepatic UGT1A1 activity, and increased enterohepatic shunting.59 Phase II elevations are primarily due to the continuing high bilirubin load from increased reabsorption of bilirubin by the enterohepatic shunt (see Figure 18-1), increased bilirubin production, and decreased hepatic uptake of bilirubin.39 Table 18-2 lists other factors associated with the development of increased bilirubin levels in newborns.
Table 18-1
Factors Associated with the Development of Physiologic Jaundice
| BASIS | CAUSES |
| Increased bilirubin availability | |
| Increased bilirubin production |
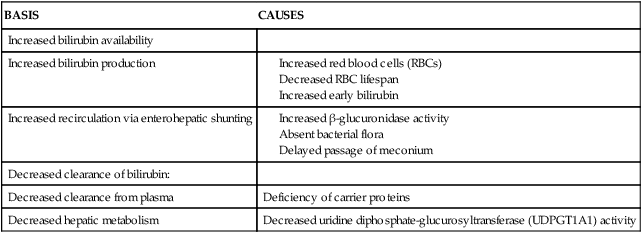
Table 18-2
Causes of Neonatal Indirect Hyperbilirubinemia
| BASIS | CAUSES |
| INCREASED PRODUCTION OF BILIRUBIN | |
| Increased hemoglobin destruction | |
| Increased amount of hemoglobin | |
| Increased enterohepatic circulation | |
| ALTERED HEPATIC CLEARANCE OF BILIRUBIN | |
| Alteration in UDT1A1 production or activity | |
| Alteration in hepatic function and perfusion (and thus conjugating ability) | |
| Hepatic obstruction (associated with direct hyperbilirubinemia) |
G6PD, Glucose-6-phosphate dehydrogenase; SGA, small for gestational age.
Increased bilirubin availability results from greater bilirubin production (with more RBCs per kilogram), decreased RBC life span, and greater early bilirubin. Active recirculation of bilirubin by the enterohepatic shunt also raises serum indirect bilirubin levels (see Figure 18-1 and Box 18-1 on page 609). Increased recirculation of bilirubin is promoted by reduced bacterial flora (which normally further metabolizes direct bilirubin for excretion in feces), high levels of β-glucuronidase activity (which deconjugates direct bilirubin back into indirect bilirubin), and decreased intestinal motility. Concentrations of β-glucuronidase, an intestinal brush border enzyme, are 10 times higher in newborns than in adults, in whom little bilirubin is normally reabsorbed from the small intestine.51
The longer direct bilirubin remains in the small intestine, the greater the likelihood it will be deconjugated. Thus infants who are fed earlier (before 4 hours of age versus after 24 hours) or fed more frequently, and infants with meconium staining or early passage of meconium tend to have a lower incidence of physiologic jaundice.17,38 Formula-fed infants tend to excrete more bilirubin in their meconium during the first 3 days after birth than breastfed infants. Among breastfed infants, bilirubin levels tend to be lower in those who defecate more frequently.28 Infants with delayed passage of meconium, meconium ileus, or intestinal obstructions are more likely to develop physiologic jaundice. Recirculated bilirubin puts an additional load on an already stressed and functionally immature liver.
Clearance of bilirubin from the plasma and metabolism by the liver are impaired in the newborn because of deficient ligandin (the main hepatocyte intracellular bilirubin-binding protein), reduced UGT1A1 activity, and diminished excretion by a liver overloaded with bilirubin. Levels of ligandin reach adult values by 5 days of age.51,74
UGT1A1 activity is 0.1% at 17 to 30 weeks, gestation and remains minimal (less than 1% of adult activity) from 30 to 40 weeks and during the first 24 hours after birth.74,129 Activity of this enzyme increases rapidly after the first 24 hours, due to a gradual up-regulation of UGT1A1 activity. This up-regulation is seen across all gestational ages.129 Activity is lower in preterm than term infants; however, increases in activity after birth are related more to postbirth age than to gestational age. The increase in bilirubin levels in the newborn infant help induce UGT1A1 activity and bilirubin conjugation in the liver after birth.21 UGT1A1 activity does not reach adult levels for 6 to 14 weeks.59,61,74,129 The major bilirubin conjugate formed in the newborn infant is monoglucuronide rather than diglucuronide, as in older individuals (see Box 18-1 on page 609). Monoglucuronide is more easily hydrolyzed to indirect bilirubin than diglucuronide, and only indirect bilirubin is reabsorbed across the intestinal mucosa via enterohepatic shunting.39
Hypoglycemia or hypoxemia may interfere with bilirubin conjugation. Decreased liver perfusion due to persistent patency of the ductus venosus may also impede clearance of bilirubin from plasma, particularly in preterm infants.74 Hypoxemia further reduces blood flow to the liver and alters hepatocyte function. The ability of the newborn’s liver to excrete conjugated bilirubin may also be decreased. This may be critical in disorders with large bilirubin loads (e.g., severe erythroblastosis fetalis) and leads to the direct hyperbilirubinemia seen in these infants.59,74
The lower serum albumin levels in the ELBW infant may limit extracellular binding and transport of bilirubin when concentrations are high.21 These infants often have delayed feeding, with a low caloric intake initially and slower intestinal transit time. Feeding provides a substrate for intestinal flow and bacterial colonization. A major factor contributing to the increased risk in the preterm infant is decreased UGT1A1 activity. Postnatal maturation of UGT1A1 and ligandin and canalicular transportation pathways for bile may be slower in ELBW infants.21
Clinical implications for neonatal care
Alteration in bilirubin metabolism is a relatively common event during the first week after birth. Neonatal jaundice results from either physiologic or pathologic causes. Physiologic jaundice is a normal process in the first days after birth and is due to normal physiologic adaptations. Physiologic jaundice is seen in 50% to 60% of term infants and up to 80% of preterm infants. Pathologic jaundice is due to pathologic factors, such as Rh or ABO incompatibility, polycythemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency, intestinal obstruction, sepsis, and other factors that alter normal bilirubin metabolism. Neonatal jaundice reflects an increase in bilirubin. At times these increases may reach levels that characterize hyperbilirubinemia. Readmission rates for treatment of jaundice have increased twofold to threefold in recent years and are higher in late preterm infants (born between 35 and 366/7 weeks’ gestation).14,22,74 In addition, although still rare, bilirubin encephalopathy and kernicterus continues to occur with breastfed and late preterm infants at higher risk.22,42,53 This section addresses these issues and discusses the basis for phototherapy and other methods of managing hyperbilirubinemia. Guidelines for the management of neonatal hyperbilirubinemia are available from professional groups in several countries, including the American Academy of Pediatrics, the Canadian Pediatric Society, the United Kingdom’s National Institute for Health and Clinical Excellence, and others.7,19,29
Neonatal hyperbilirubinemia
Hyperbilirubinemia can be due to physiologic or pathologic causes or may be due to a combination of physiologic and pathologic causes. The risk of this disorder is increased in breastfed, late preterm, and preterm infants. Neonatal hyperbilirubinemia is of concern because it may be a sign of an underlying pathologic problem, such as hemolysis or sepsis, and because of its association with bilirubin encephalopathy and kernicterus.16
Neonatal hyperbilirubinemia is due to increased production or decreased hepatic clearance of bilirubin (see Table 18-2 and Figure 18-2). Significant hyperbilirubinemia within the first 36 hours after birth is usually due to increased production (primarily from hemolysis), because hepatic clearance is rarely altered enough during this period to produce bilirubin values greater than 10 mg/dL (171 μmol/L).59,74 An increase in the hemoglobin destruction rate by 1% leads to a fourfold increase in the bilirubin production rate.
According to the American Academy of Pediatrics (AAP), the major risk factors for developing severe hyperbilirubinemia in late preterm and term infants are total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) level in the high-risk zone on the bilirubin nomogram,7 jaundice in the first 24 hours postbirth, blood group incompatibility with a positive direct Coombs test, other known hemolytic disease (e.g., G6PD deficiency), gestational age of 35 to 36 weeks, previous sibling who received phototherapy, cephalohematoma or significant bruising, East Asian ethnicity, and exclusive breastfeeding, particularly in infants with difficulties with nursing and excessive infant weight loss.7 Any infant born prematurely is also at greater risk for hyperbilirubinemia, with the risk increasing with decreasing gestational age and the factors listed previously.
There is no consistent definition for neonatal hyperbilirubinemia; what is considered to be in that range varies with population characteristics and postbirth age. One definition is “an elevation of plasma bilirubin two standard deviations or greater than the expected mean values for the infant’s age” or greater than 90th percentile values on the bilirubin nomogram.7,22 Kaplan et al. define unconjugated (indirect) hyperbilirubinemia in the newborn as bilirubin levels greater than 20 mg/dL (34 μmol/L) and conjugated (direct) hyperbilirubinemia as bilirubin levels greater than 1.5 mg/dL (26 μmol/L).59 With more diverse populations and increases in breastfeeding, total serum bilirubin (TSB) levels are higher than have been previously reported, and may approach values of 15 to 18 mg/dL (256.5 to 307.8 μmol/L).22,56,74 Bhutani et al. calculated that about 1:10 infants will have TSB levels equal to or greater than 17 mg/dL (290.7 μmol/L); 1:70 will have levels equal to or greater than 20 mg/dL (342 μmol/L); 1:700 will have levels equal to or greater than 25 mg/dL (425 μmol/L); and 1:10,000 will have levels equal to or greater than 30 mg/dL (513 μmol/L).14 Jaundice within the first 24 hours after birth; increases of more than 0.25 mg/dL/hr (4.3 μmol/L/hr), which is equivalent to greater than 6 mg/dL/day; and jaundice associated with other abnormal findings such as feeding problems, G6PD deficiency, irritability, hepatosplenomegaly, acidosis, or metabolic abnormalities are also of concern.7,55,56,59 Infants with G6PD deficiency are at high risk for both hyperbilirubinemia and kernicterus. G6PD deficiency is an inherited X-linked metabolic disorder that is one of the most common genetic diseases in the world. G6PD is an enzyme that normally protects the RBC and other cells from oxidative injury and hemolysis.55,116
Investigators have attempted to predict the risk of later significant hyperbilirubinemia in healthy term infants before early hospital discharge.5,7,12,110,124 For example, Alpay reported that a serum bilirubin level of 6 mg/dL (102.6 μmol/L) or greater in the first 24 hours after birth predicted nearly all healthy term infants who later developed significant hyperbilirubinemia and all who required phototherapy for bilirubin levels greater than 20 mg/dL (342 μmol/L).5 Bhutani and colleagues developed an hour-specific nomogram by plotting total serum bilirubin levels with age in hours to identify infants at high, intermediate, and low risk of later requiring phototherapy.12 They reported that no infant who fell into the low-risk area of the graph later required phototherapy. This nomogram has been incorporated into the revised AAP guidelines for management of hyperbilirubinemia in newborns of 35 weeks’ gestational age and older.7 In a comparison of the predictive value of predischarge bilirubin measurement with this nomogram and clinical risk factor assessment, Keren and colleagues reported that the predischarge bilirubin measurement was more accurate in identifying infants at risk for severe hyperbilirubinemia (TSB greater than 95th percentile) and generated a wider risk stratification.63 Several investigators have noted that the factors most predictive of readmission for phototherapy are infants with a peak transcutaneous bilirubin greater than 75th percentile (or high-intermediate to high zone) on the nomogram, exclusive breastfeeding, and less than 38 weeks’ gestation.64,80 Concerns have been raised about methodological flaws in the development of the nomogram and the risks of false positives and, particularly, false negatives.33
Direct hyperbilirubinemia
Direct or conjugated hyperbilirubinemia (obstructive jaundice), which is common in adults with jaundice, is rare in the neonate. A direct bilirubin greater than 1 mg/dL (17.1 μmol/L) with TSB levels ≤5 mg/dL (85.5 μmol/L), or TSB levels greater than 5 mg/dL (85.5 μmol/L) with the direct bilirubin equal to 20% of the TSB, are abnormal.7 Elevations of direct bilirubin involve cholestasis and are associated with alterations in hepatic function and interference with excretion of bilirubin into bile or obstruction of bile flow in the biliary tree. In neonates this can occur with hepatitis, severe erythroblastosis, sepsis, biliary atresia, and inborn errors of metabolism, (including galactosemia, α1-antitrypsin deficiency, tyrosinemia, and cystic fibrosis), prolonged parenteral alimentation.
Breastfeeding and neonatal jaundice
The increased incidence of hyperbilirubinemia in the United States over the past 25 years has been attributed primarily to the increase in breastfeeding. The majority of infants with hyperbilirubinemia for which no specific cause can be found are breastfed.86 Two forms or phases of neonatal jaundice are described in breastfed infants: the more common early (breastfeeding-associated) jaundice, and late (breast milk) jaundice. However, these forms overlap and may not be readily distinguishable from each other.40,42,51,59,74
The early-onset form, often referred to as breastfeeding-associated jaundice, is believed to be related primarily to the process of feeding.38,42 The major factor leading to breastfeeding-associated jaundice is increased enterohepatic shunting due to lower fluid and caloric intake, less frequent feedings, stooling patterns, increased β-glucuronidase activity, and decreased formation of urobilins.42,74,81 Decreased caloric intake results in increased fat breakdown for energy and fatty acid production that increases intestinal fat absorption and may indirectly interfere with UGT1A1 and ligandin.74 When fatty acids reach the liver, they may inhibit UGT1A1 activity or saturate the hepatic protein carrier system.27 The increased absorption of fat from breast milk may also increase intestinal bilirubin absorption. Breastfed infants produce lower weight individual stools, have a lower initial stool output, and have stools that contain less bilirubin than formula-fed infants.74 Greater weight loss after birth and less stooling are associated with higher bilirubin production. These infants also have slower urobilinogen formation, possibly due to different intestinal colonization patterns after birth (see Chapter 13). Breastfed infants excrete less bilirubin in stools than formula-fed infants because more conjugated direct bilirubin is changed back to indirect bilirubin by β-glucuronidase, which has greater activity in breastfed infants.28,42,43 Breastfeeding-associated jaundice is not associated with increased new bilirubin production or abnormal hepatic uptake or conjugation of bilirubin, suggesting that the most likely mechanism is increased enterohepatic shunting.39
Breastfed infants also develop a later onset, prolonged hyperbilirubinemia. The late-onset form is less common and is believed to be related primarily to attributes of breast milk that interfere with normal conjugation and excretion.39,59 Many of these infants also have a history of the early-onset form of jaundice. Late-onset jaundice is characterized by increasing bilirubin levels after 3 to 5 days, peaking at 5 to 10 mg/dL (86-171 μmol/L) by 2 weeks, followed by a slow decrease in bilirubin values to normal limits over the next 3 to 12 weeks.38,59 These infants do not have any signs of hemolysis or abnormal liver function.
The cause of late-onset jaundice in breastfed infants is unknown but has been attributed to the presence of specific factors in breast milk that appear to be minimal or absent in colostrum but appear in transitional and mature milk.39 Although the specific factor or factors have not been identified, these may include the following: 3α-20β-pregnanediol (which may interfere with UGT1A1 activity or release of conjugated bilirubin from the hepatocyte); increased lipoprotein lipase activity with subsequent release of free fatty acids in the intestines; inhibition of conjugation by the increased amounts of unsaturated fatty acids found in breast milk; or β-glucuronidase or other factors in breast milk that may increase enterohepatic shunting.39,41,59,74
Prevention of hyperbilirubinemia in breastfed infants
Hyperbilirubinemia in breastfed infants may be reduced by preventive interventions. Encouraging feeding soon after delivery will increase intestinal activity and begin establishing gut flora. Frequent feeding stimulates intestinal activity and meconium removal (less bilirubin for enzymes to convert back to the indirect form), and reduces enterohepatic shunting, and stimulates maternal milk production.7,16,59 Infants fed in the first 1 to 3 hours after birth pass meconium sooner than infants fed after 4 hours.17 Feeding stimulates the gastrocolonic reflex, increases intestinal motility, and stimulates meconium passage (colostrum acts as a laxative). This removes conjugated bilirubin from the small intestine, thus reducing the likelihood that this bilirubin will be recirculated by the enterohepatic shunt.
A critical factor in reducing the risk of jaundice in breastfed infants seems to be enhancing breast milk intake.7,37,38,42,59 Bilirubin levels in these infants tend to correlate negatively with breast milk intake; that is, as intake decreases, bilirubin levels tend to rise. Supplements should be avoided as they can interfere with establishment of breastfeeding.7 If supplementation is required for medical reasons, supplementation with formula provides more calories. Supplementation of breastfeeding with water or dextrose water does not lower bilirubin levels in healthy breastfeeding infants. Supplemental feedings with dextrose water have been found to decrease breast milk intake, increase bilirubin levels, and possibly increase the risk of hyponatremia.26,69 Dextrose water supplementation may satiate the infant but lead to inadequate caloric intake; caloric deprivation increases bilirubin levels.38 Use of any form of supplementation can alter intake of breast milk and establishment of the mother’s milk supply (see Chapter 5). Use of supplementation is associated with a significant decrease in the number of infants still breastfeeding at 3 months.47
An inverse relationship between the number of feedings per day and bilirubin levels has been reported.27,28 Bilirubin levels were lowest in infants who were breastfed more than eight or nine times in 24 hours during the first 3 days after birth.135 Increasing the frequency of feedings may stimulate gut motility and decrease intestinal absorption of bilirubin.27 Current AAP recommendations are to breastfeed at least 8 to 10 times per 24 hours initially to ensure adequate milk intake.7 Signs of inadequate milk intake include delayed meconium passage, fewer bowel movements (less than 3 to 4 stools per day by day 4), decreased urine output (less than 4 to 6 thoroughly wet diapers in 24 hours), and weight loss greater than 7%.7,39,69
Initial and continuing support of the mother and other family members is essential to enhance breastfeeding success.73 The American Academy of Pediatrics (AAP) recommends that all newborns be seen by 72 hours if discharged before 24 hours of age, at 96 hours if discharged at 24 to 49.9 hours of age, and at 120 hours if discharged at 48 to 72 hours of age with earlier and more frequent follow-up for infants at risk for hyperbilirubinemia.7 Some infants discharged before 48 hours may need multiple follow-up visits at 24 to 72 hours depending on their clinical status.7 Several studies examining compliance with these recommendations have reported that only about one third of all newborns were seen in a timely manner after hospital discharge.34,96,100
Management of hyperbilirubinemia in breastfed infants
Management of hyperbilirubinemia in breastfed infants is often challenging. Multiple issues and concerns, including maternal desire to breastfeed, advantages of breastfeeding to both mother and infant, effects on maternal-infant interaction, parental stress with the potential for bilirubin toxicity, and legal implications related to “safe” bilirubin values must be balanced.59 This has been further complicated by the continuing reports of kernicterus (although still rare) in breastfed term and late preterm infants.15,23,56,64,70,73,86,93 In the early 1990s, less aggressive treatment of hyperbilirubinemia in term infants was advocated and reflected in guidelines released by the AAP in 1994. One of the bases for these guidelines was the estimate that the risk of kernicterus in well term infants with bilirubin levels of 20 to 25 mg/dL (342 to 428 μmol/L) was lower than the risks associated with exchange transfusion.93
The AAP guidelines recommend that breastfeeding be continued whenever possible, but that supplementation with expressed breast milk or formula be considered if “the infant’s intake is inadequate, weight loss is excessive, or the infant seems dehydrated.”7 Amato and colleagues report no difference in the time needed to reduce bilirubin levels with jaundice managed by discontinuing breastfeeding versus the use of phototherapy and continued breastfeeding.6 Martinez and associates compared four interventions (continue breastfeeding and observe; discontinue breastfeeding and begin formula feeding; discontinue breastfeeding, begin formula feeding, and start phototherapy; and continue breastfeeding and start phototherapy) once bilirubin levels reached 17 mg/dL (291 μmol/L).83 They found that if an adequate dosage of phototherapy was provided, there was no significant advantage to stopping breastfeeding.83
Maisels recommends that any interruption of breastfeeding be avoided, unless the infant develops bilirubin levels above 25 mg/dL (428 μmol/L), and rather to continue frequent breastfeeding (every 2 to 3 hours) while using intensive phototherapy, unless the infant’s weight loss from birth is greater than 12% or there is clinical evidence of dehydration.7,74 Lawrence emphasizes an approach, focusing on prevention and modifying factors (particularly inadequate frequency of feeding) that are associated with early-onset jaundice in breastfed infants (Table 18-3).69 Any interruption of breastfeeding must be accompanied by parental emotional support and facilitation of breast pumping or manual expression of milk.
Table 18-3
Management Outline for Early Jaundice While Breastfeeding

AAP, American Academy of Pediatrics.
Modified from Lawrence, R.A., & Lawrence, R.M. (2005). Breastfeeding: A guide for the medical profession (6th ed.). Philadelphia: Mosby.
Measurement of serum bilirubin
Serum bilirubin levels are measured by laboratory and transcutaneous methods. Clinical estimations of serum bilirubin levels by the cephalopedal progression of jaundice are correlated with serum bilirubin concentrations in most but not all studies.66,67,89 Cephalopedal progression is most useful at low bilirubin levels and is less reliable at levels greater than 12 mg/dL (205 μmol/L).74 Knudsen suggests that the basis for this progression may be due to conformational changes in the bilirubin-albumin complex and in the binding affinity of bilirubin for albumin.66,67 Indirect bilirubin leaving the reticuloendothelial system, where it is produced, binds to albumin. Initially binding affinity is lower with less effective binding, so bilirubin is more likely to be deposited in this area (i.e., in more proximal tissues). By the time bilirubin reaches more distal areas, it is more tightly bound and less likely to be deposited in the peripheral tissues unless bilirubin levels are high (overwhelming the available albumin-binding capacity).66,67 Several studies suggest that visual assessment may be useful for deciding about which infants need a transcutaneous bilirubin assessment.20,58,65,76,103 These assessments are not predictive of who will develop severe hyperbilirubinemia.64 Thus laboratory assessments are also necessary.56
Laboratory methods involve measurement of total and direct bilirubin and calculation of indirect values. Total serum bilirubin (TSB) is the gold standard for assessment of neonatal jaundice.70 Peak total bilirubin concentrations are poorly associated with development of bilirubin toxicity.3 Plasma unbound bilirubin is a better predictor of abnormal outcomes, but more difficult to measure.2,3,132
Transcutaneous bilirubinometry is an alternative method that is most appropriate for screening and monitoring healthy term infants with physiologic jaundice because it avoids repeated heel sticks. These devices work by either calculating changes in light optical density between reflected light sources or measuring the amount of light reflected from light transmitted into the skin.70 TcB is linearly related to laboratory measurements of TSB, especially in infants of more than 30 weeks’ gestation.7,13,20,32,50,70,74,121 TcB levels have been reported to also correlate with TSB in preterm infants but more study is needed.107 Newer TcB instruments have a correlation that is within 2 to 3 mg/dL (34.2 to 51.3 μmol/L) of TSB if the TSB is less than 15 mg/dL (356.5 μmol/L).56,59 This correlation is poorer once infants are under phototherapy. Other studies report that TcB levels significantly underestimate TSB in both term and late preterm infants, especially at higher bilirubin levels.35,36,48,54,60,75 Measurement of TcB at the sternum has been reported to more closely approximate TSB than forehead measurements.95 If therapy is being considered, a TSB should be obtained.79 Universal bilirubin screening has been recommended by some, whereas others have noted the lack of evidence that this will prevent acute bilirubin encephalopathy and concerns about excessive use of phototherapy.68,79,94,122,123 Implementation of universal screening has been associated with a lower incidence of hyperbilirubinemia, but increased phototherapy use.122,123
Serum albumin and bilirubin/albumin levels may also be measured in addition to the TSB and may be useful as additional data in those infants at risk for an exchange transfusion.7 Lower albumin levels or low albumin binding of bilirubin suggests that these infants may be at increased risk for deposition of bilirubin in the brain. However, the bilirubin/albumin ratio does not correlate well with unbound bilirubin and significant differences can occur between newborns.7 End-tidal carbon monoxide (CO) monitors have been evaluated to assess excessive bilirubin production due to hemolysis.24,59,116,117,118 The breakdown of heme by heme oxygenase produces biliverdin and CO. CO binds to RBCs and is circulated to the lungs where the CO detaches and is exhaled. The amount of CO exhaled can be measured. These monitors have a low positive but strong negative predictive value, so they are not often used clinically.56
Management of neonatal hyperbilirubinemia
Pharmacologic agents
Pharmacologic agents have been used in the management of hyperbilirubinemia to stimulate the induction of hepatic enzymes and carrier proteins, to interfere with heme degradation, or to bind bilirubin in the intestines to decrease enterohepatic reabsorption. Inert nonabsorbable substances such as charcoal and agar have been tried for the latter purpose with equivocal results and are not recommended.74 Intravenous immunoglobulin has been used with infants with severe Rh and ABO incompatibility to suppress isoimmune hemolysis and decrease the number of exchange transfusions.7,51 Phenobarbital has also been used, although generally only with some rare forms of congenital hyperbilirubinemia.59 Phenobarbital stimulates activity and concentrations of UGT1A1 and ligandin and may increase the number of bilirubin-binding sites.74 β-glucuronidase inhibitors, such as L-aspartic acid and enzymatically hydrolyzed casein, and other nonabsorbable substances that bind bilirubin in the intestines (and thus possibly reduce enterohepatic shunting) have also been examined with further investigation needed.43,59 Kaplan suggests that frequent breastfeeding may be as effective as these interventions.59
Prevention of hyperbilirubinemia with the use of synthetic metalloprotoporphyrins has also been investigated.51,118,119,134 These substances are synthetic heme analogues. Protoporphyrin has been shown to be an effective competitive inhibitor of heme oxygenase, the enzyme necessary for catabolism of heme to biliverdin (see Figure 18-1), and the rate-limiting step in the formation of bilirubin. With use of these substances, the heme that is prevented from being catabolized does not accumulate but is excreted intact in bile.51 In studies with both term and preterm infants, and in infants with and without hemolytic diseases, tin-protoporphyrin (Sn-PP) and tin-mesoporphyrin (Sn-MP) have decreased serum bilirubin levels and the need for phototherapy.24,30,51,118,119,134 Use of phototherapy after the administration of Sn-PP has been associated with phototoxic erythema. Sn-MP is a less toxic variant, especially when used in conjunction with phototherapy. Studies continue with use of Sn-MP and other metalloprotoporphyrins such as chromium mesoporphyrin and zinc deuteropophyrin.24,59,134 Long-term outcomes have not been established.
Exchange transfusion
Exchange transfusions are used in the management of indirect hyperbilirubinemia and hemolytic disease of the newborn. An exchange transfusion removes antibody-coated blood cells and bilirubin and helps to correct the anemia associated with hemolytic disease. A two-volume exchange replaces 85% of the circulating red blood cell (RBC) volume and reduces the bilirubin by approximately 50%. Post exchange, bilirubin rebounds by up to 60% of pre-exchange values as bilirubin diffuses into the vascular space from extravascular tissues. The frequency of exchange transfusions has been significantly reduced with the availability of Rho(D) immune globulin (see Chapter 13) and phototherapy.
Phototherapy
Phototherapy was first introduced in 1958 and has been used extensively and effectively in treating neonatal indirect hyperbilirubinemia since the late 1960s.25 Multiple studies have documented the effectiveness of phototherapy in preventing and treating neonatal hyperbilirubinemia.74,77,91 Protocols are available to guide initiation of phototherapy in healthy term and late preterm infants (greater than or equal to 35 weeks’ gestational age) and in preterm infants.7,19,29,59,74
Physics of phototherapy.
Absorbance of light by bilirubin is strongest in the blue light spectrum at about 460-nm wavelength.104 Absorption of a photon of light excites the bilirubin molecule due to the accumulation of excess energy. In order to lose this excess energy, the bilirubin molecule can reemit the light photon (rare), use the excess energy to produce heat (accounts for about 80% of the excess energy), or the energy can alter the bilirubin molecule (about 20% of the excess energy) by photochemical reactions.22 These photochemical reactions are photoisomerization and photo-oxidization. Indirect bilirubin is composed of four pyrrole rings. Photoisomerization involves conversion of poorly soluble indirect bilirubin into water-soluble reversible configurational (rearrangement of chemical groups in the molecule by temporarily disrupting the chemical bonds between carbon atoms in the molecule, resulting in a 180-degree rotation of the pyrrole rings) or irreversible structural (rearrangement of the atoms) photoisomers (i.e., photobilirubin, lumirubin).51,59,104,125 The photoisomers can be excreted into bile without conjugation. Since these photoisomers are polar and need transporters to reach neurons, Hansen suggested that they are less likely to cross the blood-brain barrier than unconjugated bilirubin.104 Formation of configurational isomers is rapid (nanoseconds), but these isomers are excreted slowly in bile, with a serum half-life of 12 to 21 hours.51,85,90,105 By 6 to 12 hours after phototherapy has been initiated, 20% of TSB has been converted to configurational isomers.105 These isomers are unstable and may be changed back into unconjugated bilirubin in the intestines and recirculated via enterohepatic shunting.
Lumirubin, a structural nonreversible isomer, is formed at a slower rate but is excreted in bile more rapidly with a serum half-life of 2 hours. Lumirubin is the major pathway through which bilirubin is eliminated during phototherapy. Lumirubin accounts for 2% to 6% of the TSB under steady-state conditions during phototherapy.51,104,105,125 There is a dose-response relationship between lumirubin formation and phototherapy irradiance.51 Formation of lumirubin is irreversible; it is excreted in bile or, to a lesser extent, in urine. Excretion of these isomers increases bile flow, which may stimulate intestinal activity and more rapid removal of bilirubin. As a result, phototherapy is often more effective in infants being fed and less effective in infants who are not being fed or infants with bowel obstruction.
Photo-oxidization has a minor role in elimination of bilirubin with phototherapy.59,105 In this process the bilirubin molecule absorbs light energy from the phototherapy lights. Some of this energy is transferred to oxygen, leading to the formation of a highly reactive oxygen molecule (singlet oxygen). This molecule aids in oxidation and breakdown of bilirubin into water-soluble breakdown products (e.g., monopyrroles, dipyrroles) that are excreted primarily in urine. Decomposition of bilirubin under phototherapy occurs in the superficial capillaries of the and in interstitial spaces.
Side effects of phototherapy.
Although many concerns have been raised about the safety of phototherapy and possible short-term and long-term effects, significant side effects are rare. Concerns focus on complications of photoisomerization and photo-oxidization; long-term concerns focus on irradiation damage, retinal damage (eye protection is always needed), and neurodevelopmental issues.7,59 Investigations have generally failed to demonstrate any significant long-term problems with phototherapy usage in human infants and side effects are usually transient.7,50,59 Transient effects include thermal and metabolic changes, hemodynamic changes, increased insensible water loss (IWL) and stool water loss, altered physiologic function and weight gain, skin and ocular effects, behavioral alterations, and hormonal changes (Table 18-4).1,43,50,74 IWL loss may increase up to 25%, increasing the needs for fluids and monitoring. IWL is less with light-emitting diode (LED) units and slightly less with fiberoptic blankets.59 Some infants with an elevated direct bilirubin and cholestasis develop bronze baby syndrome, which is characterized by a dark gray-brown discoloration of the skin, serum, and urine. This syndrome may be due to retention of bile pigments with accumulation of porphyrins and other metabolites secondary to impaired bile excretion due to cholestasis.7,59
Table 18-4
| SIDE EFFECT | SPECIFIC CHANGES | IMPLICATIONS |
| Thermal and other metabolic changes |
Due to increases in evaporative water loss, metabolic rate, and possibly respiratory rate
Influenced by environment (e.g., air flow, humidity, temperature); characteristics of phototherapy unit (e.g., heat dissipation, distance from infant); ambient temperature alteration; infant alterations in skin and core temperature, heart rate, respiratory rate, metabolic rate, caloric intake; type of bed (increased with radiant warmer and incubator)
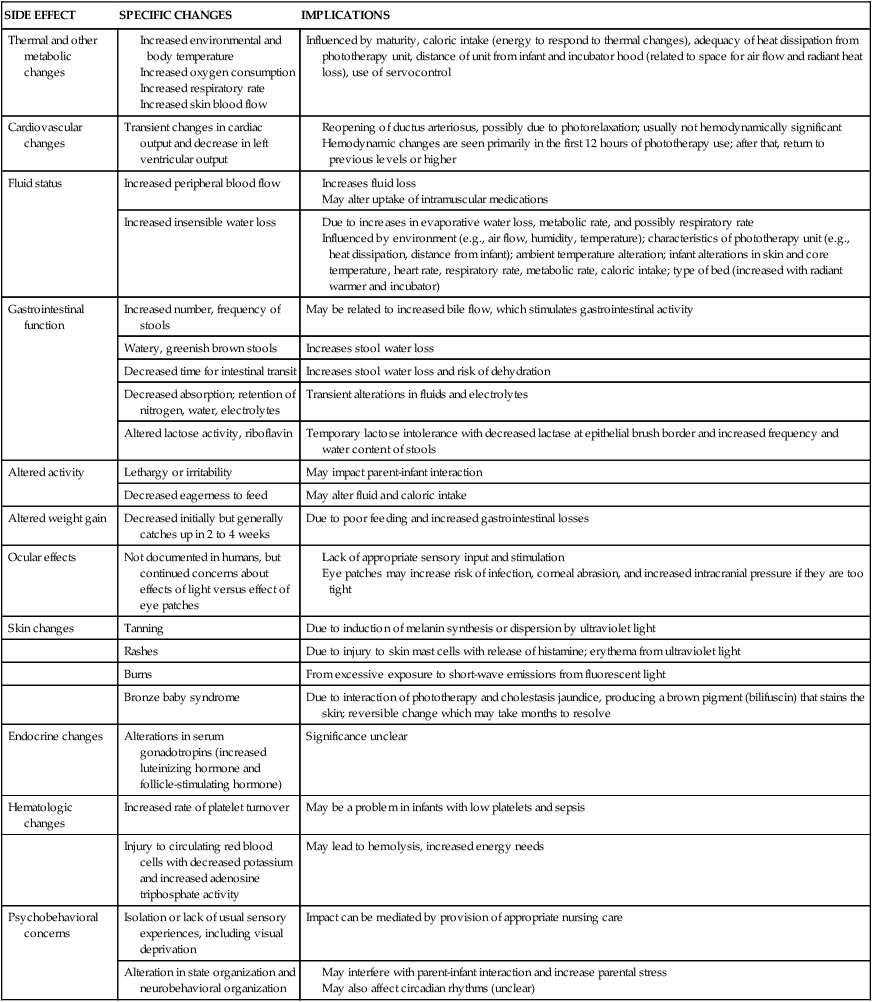
Psychobehavioral concerns associated with the use of phototherapy include the potential effect of isolation and the lack of usual sensory experiences, behavioral and activity changes (including lethargy, irritability, and altered feeding behavior), and alterations in state organization and biologic rhythms as well as effects of parental stress (see Table 18-4). These concerns may influence parental perceptions of their infant and early parent-infant interactions.
Methods of providing phototherapy.
Phototherapy can be provided using a bank of fluorescent lights, tungsten-halogen or quartz halide spotlights, high-intensity blue gallium nitride light-emitting diodes (LEDs), and fiberoptic blankets. Plexiglas covering lights protects against ultraviolet irradiation.59 A bassinet system is also available that provides simultaneous phototherapy above and below the infant. Bilirubin blankets are woven fiberoptic pads. Halogen light beams are transmitted through a cord of fiberoptic filaments to a flexible pad that is placed beneath or wrapped around the infant. These pads remain at room temperature and deliver light within the 425- to 475-nm wavelength. In most studies, the fiberoptic pads have been shown to be somewhat less effective than single conventional phototherapy (in both term and preterm infants) in reducing bilirubin levels in infants with mild to moderate hyperbilirubinemia but more effective than no treatment.59,74,87,125 A disadvantage of these pads is lower spectral power due to small surface area, making them less useful in treating infants with severe hyperbilirubinemia, unless used in conjunction with overhead bililights to provide intensive phototherapy. Tungsten-halogen spotlights cover a smaller infant surface area; therefore they also provide less spectral power than fluorescent bank lights.74 High-intensity blue gallium nitride LEDs produce less ultraviolet light, infrared radiation, and heat, and the amount of blue-green light can be customized.59,116,126 Because these units generate little heat, the unit can be placed a shorter distance from the infant to deliver high irradiances, thus enhancing lumirubin formation.59,111,116,126
An issue in caring for infants under phototherapy is whether to turn off the “lights” or remove the infant from under them during feeding and other caregiving. The benefits to both the infant and the parents of removing the eye shields and holding the infant during feeding seem to outweigh concerns regarding the effectiveness of bilirubin reduction in most situations.7 The most rapid catabolism of bilirubin appears to take place within the first few hours after the start of each phototherapy period. It takes bilirubin about 3 hours to return to the skin after removal of photoisomers.51 Thus for infants with mild to moderate hyperbilirubinemia, the irradiance, area of skin exposed, and initial effects of phototherapy on bilirubin in the skin seem to have more influence than whether the infant is removed for short periods of feeding or holding.22,74 A significant postphototherapy bilirubin rebound has been reported in infants who are preterm, have a positive direct Coombs test, or are under phototherapy for less than 72 hours.57 Alternating positions from supine to prone while under phototherapy has not been reported to affect the pattern of decreased TSB.31
Effective phototherapy requires sufficient illumination over an adequate area of exposed skin at a sufficiently short distance to produce the desired effect of light on bilirubin molecules.7,59 Therefore, the effectiveness of phototherapy depends on factors such as the spectrum of light delivered by the phototherapy unit, intensity of the energy output (power output) or irradiance, peak wavelength of the light delivered, and the surface area of the infant exposed to the light.74,104 The surface area of exposure can be increased with the use of intensive phototherapy.
Irradiance is the radiant power of light on a surface per unit of surface area (μW/cm2/nm). Irradiance is directly related to the distance of the light source from the infant.74 Spectral irradiance—the irradiance of the light source (radiant power per unit area) within the therapeutic wavelength of maximum light absorbance by the bilirubin molecule (425 to 550 nm), not the intensity (i.e., illumination or brightness)—determines effectiveness.74 Maximal absorbance of albumin-bound bilirubin is about 460-nm wavelength and 440-nm wavelength for unbound bilirubin.59 The intensity of the light is inversely related to the distance between the light source and the skin surface.7,86 There is a significant linear relationship between the spectral irradiance received by the infant and the decrease in serum bilirubin levels over a 24-hour period.7,46,74
Phototherapy units vary in effectiveness, and light emission may decrease over time. Irradiance levels can be monitored with a radiometer using the instructions in equipment manuals or unit protocol. The radiometer has a wide bandwidth so that the effective irradiance of the phototherapy unit (as amount of blue light) can be measured. Measurement is in μW/cm2/nm and evaluates the spectral irradiance. A spectral irradiance of 6 μW/cm2/nm in the 425- to 475-nm waveband is sufficient for production of configurational isomers; however, for production of the structural isomer lumirubin, a higher spectral irradiance of 8 to 10 μW/cm2/nm is required.7,22 A spectral radiance of 10 μW/cm2/nm should reduce the TSB 20% to 50% during the first 24 hours of therapy.104 Intensive phototherapy requires a spectral irradiance of greater than 30 μW/cm2/nm.7,59 In comparing different devices, spectral power may be a more useful measure since it measures the average spectral irradiance across the skin surface area of the infant.74
For phototherapy to be effective, light photons must penetrate the skin and be absorbed by bilirubin molecules. Only certain wavelengths are absorbed by bilirubin; longer waves penetrate deeper into the skin. Light wavelengths in the blue-green spectrum (425 to 550 nm) are most effective in reducing bilirubin levels. Daylight white, blue, green, and special blue (super blue) fluorescent bulbs have been used in conventional phototherapy devices. Special blue (narrow spectrum) bulbs are the most effective because they provide more irradiance at 450 nm (maximal blue wavelength absorbance).74 Green light favors formation of lumirubin, and its longer waves penetrate farther into the skin.51,86 Blue-green lights found in LED devices may thus be advantageous.111
Intensive phototherapy is used for infants with severe hyperbilirubinemia to produce irradiance in the 430- to 490-nm wavelength with an irradiance of greater than 30 μW/cm2/nm.7 By using multiple (fluorescent or halogen) phototherapy units or combining overhead phototherapy with fiberoptic blankets or using special blue lights on an uninterrupted schedule, over as much of the infant’s body as possible, the intensity and thus effectiveness of phototherapy can be increased.59,74 Simultaneous use of overhead phototherapy and fiberoptic blankets increases the surface area exposed to light and spectral irradiance, which can also increase effectiveness. As compared to single phototherapy, double phototherapy has been reported to be twice as effective in preterm infants and 50% more effective in term infants.106,120 Although use of special blue fluorescent bulbs is most effective in reducing bilirubin levels, these lights can interfere with skin color assessment and are associated with reports of nausea and dizziness in caregivers.59 Exposure can be increased by using a reflective surface (such as a white sheet) around the incubator or bassinet or two to three fiberoptic blankets to cover more surface area.59,74 The spectral irradiance can also be increased by placing the lights at the minimum safe distance from the infant’s surface.59 Care must be taken to keep all phototherapy lights, halogen lights in particular, at the manufacturer’s recommended distance from the infant’s skin, because these lights can cause burns if too close.51,74
Competition for albumin binding
Most indirect bilirubin is transported in plasma bound to albumin (1 g albumin binds 8.5 to 10 mg of bilirubin). Two terms used to describe albumin binding are capacity and affinity. Each molecule of albumin has a certain number of binding sites available (the binding capacity). The tightness by which bilirubin is bound to sites available for binding is the affinity. Avidity increases with increasing plasma albumin concentration as well as the ability of the albumin to bind bilirubin.3
In adults, each molecule of albumin can bind at least two molecules of bilirubin, with the first molecule bound more tightly.59 Binding sites may be primary (tight or high affinity) or secondary (weak affinity). Each albumin molecule has one primary binding site and one or more secondary sites. If the primary site is saturated, there is a rapid increase in loosely bound or free bilirubin.74 Albumin-binding capacity is lower in the neonate due to lower albumin levels and decreased albumin-binding capacities.4,59 Albumin-binding capacity increases with both gestational and postnatal age and is impaired in sick infants.11,130 Unbound bilirubin can leave the vascular system and enter the skin, brain, and other organs. Albumin-bound bilirubin does not enter the brain.104 The unbound fragment of bilirubin is thought to be more closely associated with bilirubin levels in the central nervous system and risk of bilirubin encephalopathy than TSB.2,59,131 An infant with a greater binding capacity may have a greater TSB, but lower risk of toxicity since more of the bilirubin is bound to albumin.3 Various techniques have been developed to measure albumin binding of bilirubin, but to date none of them has been acceptable for widespread clinical management in terms of either application or interpretation.2,22,59,74,85
Many substances can influence bilirubin binding to albumin.11 Competing substances easily displace bilirubin bound to secondary sites. Drugs such as sulfisoxazole and other sulfonamides, salicylate, chlorothiazide, ceftriaxone, rifampicin, indomethacin, certain x-ray contrast substances, and sodium benzoate (a preservative once used in some multiple-injection vials) may displace bilirubin.22,51,59 Combinations of drugs may exacerbate these effects. Bilirubin also displaces some drugs (including ampicillin, phenobarbital, and phenytoin) from albumin (see Chapter 7).
Albumin binding of bilirubin can be altered by pathologic events. Plasma free fatty acids compete with bilirubin for albumin-binding sites.51 Hypothermia increases metabolism and catabolism of fatty acids, which may displace bilirubin from albumin. However, the risk is minimal if molar concentrations of free fatty acids to albumin are less than 4:1.51 Fatty acids are also elevated with sepsis and hypoxemia.59,130 Although concerns have been raised about risks with the use of emulsified lipid solutions (e.g., Intralipid) in neonates, dosages of 2 to 3 g/kg every 15 hours produce fatty acid to albumin ratios of only 0.1 to 1.8.74 The amount of unbound indirect bilirubin may also be increased if there is more bilirubin than available albumin because of excess production of bilirubin or decreased albumin (e.g., with malnourishment). Serum pH per se may not alter binding but may influence deposition of unbound bilirubin in the central nervous system.74,104 Thus there may be an advantage to monitoring bilirubin to albumin ratios as well as TSB levels in determining the need for exchange transfusions.7
Bilirubin encephalopathy and kernicterus
Development of bilirubin encephalopathy and kernicterus is a concern for any infant with elevated bilirubin levels. These complications, although rare, still occur.3,23,78,81 Early discharge, breastfeeding, and lack of adequate early follow-up may increase the risk.3,23,78,81 Other infants at higher risk are late preterm infants, other preterm infants, male infants, and infants with low albumin levels and other factors that alter the blood-brain barrier, such as asphyxia, hypoxia, infection, and hypothermia.7,22,78,79,97,125 Although the terms acute bilirubin encephalopathy and kernicterus are sometimes used interchangeably, acute bilirubin encephalopathy usually refers to early, acute clinical central nervous system symptoms of bilirubin toxicity, whereas kernicterus (or chronic bilirubin encephalopathy) refers to the chronic, permanent changes to the brain secondary to deposition of bilirubin in brain cells, with yellow staining and neuronal necrosis.51,53,56,73,78,113 Another term used is bilirubin-induced neurologic dysfunction (BIND).112,113 The risk of neurotoxicity is related to the amount of unbound bilirubin, blood pH, duration of exposure, and albumin-binding capacity; co-morbidities such as infection, hemolysis, and hypoxic-ischemic injury may increase the risk.7,50,79,112,117
Early signs of acute bilirubin encephalopathy, which is reversible in early stages, include progressive lethargy, poor feeding, vomiting, temperature instability, hypotonia, alternating hypotonia and hypertonia of the extensor muscles, and a high-pitched cry.85,113,114 Many infants, especially VLBW infants, are asymptomatic in the neonatal period.113 Signs of kernicterus include ataxia, opisthotonos, extrapyramidal disturbances, dental enamel hypoplasia of the primary teeth, deafness, seizures, and developmental and motor abnormalities. The neurotoxic effects of bilirubin are exacerbated by other pathologic conditions such as hypoxia, asphyxia, and hypercapnia.51,55,74,113,114
Bilirubin has a high affinity for phospholipids in cell membranes.22 Although the specific effects of bilirubin on the brain are still unclear, acidic forms of bilirubin act as mitochondrial poisons that uncouple oxidative phosphorylation in the mitochondria, interfering with cellular respiration, blocking adenosine triphosphate (ATP) production, inhibiting cellular enzymes, and altering cerebral glucose metabolism, water and electrolyte transport, and protein synthesis, and damaging or interfering with DNA.22,51,55,59,74,104,113 Some bilirubin may be produced in the brain and interference with its transport out of the brain (by alterations in the blood-brain barrier) may increase the risk of kernicterus.59 Areas of the brain most often affected include the basal ganglia, brainstem auditory pathways, and oculomotor nuclei. The reason for the increased risk in these area may be related to increased blood flow and metabolic activity in these areas.51 Some infants also have extraneural lesions, primarily in the renal tubular cells, lungs, adrenal glands, or gastrointestinal system.59,74 Bilirubin toxicity may be prevented in individual cells by specific transporter proteins that maintain intracellular bilirubin levels at nontoxic levels.92 Genetic differences in the expression and function of these proteins might lead to differences in susceptibility to the effects of unbound bilirubin and risk of kernicterus.127,129
In order for bilirubin to pass into neural tissue, it must cross the blood-brain barrier.18 The blood-brain barrier has tight junctions between endothelial cells of the cerebral blood vessels. These junctions are permeable to lipid-soluble substances but usually are impermeable to water-soluble substances, proteins, and other large molecules (see Chapter 15).51 Unbound (free) indirect bilirubin crosses the blood-brain barrier in both directions, although its passage is slowed by this barrier. Factors that influence the passage of bilirubin include the amount of free bilirubin, blood flow, the bilirubin-albumin dissociation rate, transit time in the capillary bed, permeability and surface area of the capillary epithelium, and perhaps energy-dependent and multidrug-resistant transporters such as P-glycoprotein.131 Because indirect bilirubin is fat soluble, at high levels it accumulates in the brain, especially in the basal ganglia and in other areas with a high lipid content. The intact blood-brain barrier is normally impermeable to albumin-bound bilirubin.56 However, if the blood-brain barrier is damaged, reversible alterations (“openings”) in the blood-brain barrier (caused by infection, dehydration, hyperosmolality, severe respiratory acidosis, hypoxemia, or other injury) allow entry of albumin-bound bilirubin as well as increased movement of unbound bilirubin.22,56,74,131
The critical level of bilirubin beyond which brain damage occurs is not certain.55 In addition, the role of small elevations of TSB in neurologic damage in the preterm infant is uncertain.88 Most healthy term infants with kernicterus in the recent resurgence have had bilirubin levels greater than 30 mg/dL (513 μmol/L).74 Ip and colleagues examined reports of outcomes of infants with kernicterus over 30 years and concluded that “kernicterus, although infrequent, has at least 10% mortality and at least 70% long-term morbidity. It is evident that the preponderance of cases of kernicterus occurred in infants with bilirubin levels higher than 20 mg/dL (342 μmol/L).”50
Reanalysis of data from the Collaborative Perinatal Project as well as follow-up studies of bilirubin in healthy term infants shows no consistent association between bilirubin levels, hearing loss, and neurologic abnormalities.50,74,92,98 Most infants with hyperbilirubinemia do not develop neurodevelopment problems.49 Low bilirubin values are not safe for all infants, because pathologic changes associated with bilirubin encephalopathy have been clearly demonstrated on autopsy at much lower values.74,130 Factors associated with greater risk for developing kernicterus at lower bilirubin levels are prematurity, respiratory distress syndrome, G6PD deficiency, hypoxia, and acidosis.7,21,22,79 However, there has not been a consistent pattern of hazardous bilirubin levels related to birth weight or gestational age with low-bilirubin-level kernicterus nor have serum bilirubin concentrations or treatment been significant independent variables affecting long-term neurodevelopmental outcome.21,88,130 As a group, peak bilirubin levels in VLBW infants (less than 1500 g), are associated with an increased risk of hearing loss, altered psychomotor development, and possibly neurodevelopmental problems, although these findings are confounded by many co-morbidities in this group of infants.74,88,128,130
Maturational changes during infancy and childhood
The bilirubin load tends to remain higher in infants for 3 to 6 weeks after birth. In older children and adults, visible jaundice may be noticed at bilirubin levels as low as 2 mg/dL (34.2 μmol/L). Bilirubin-specific UGT1A1) activity increases to adult values by 3 months of age.59 Plasma levels of indirect bilirubin reach adult values by 2 to 4 weeks postbirth in most healthy infants.22 δ-bilirubin, absent in the first two weeks, is seen in older neonates and children and is increased in children with congenital hyperbilirubinemia associated with liver problems.59
Summary
Alterations in bilirubin metabolism that occur with birth result in one of the more frequent problems seen in the neonate: physiologic jaundice. These changes also interact with pathologic factors in the development of hyperbilirubinemia. Hyperbilirubinemia is of concern because it may be a sign of underlying pathologic processes, such as hemolysis or sepsis, and may lead to adverse consequences—namely, kernicterus. Therefore it is essential for caregivers to appreciate the processes involved in bilirubin metabolism and its maturation, to understand the basis for physiologic jaundice and hyperbilirubinemia, and to recognize infants at risk for these disorders. Clinical recommendations are summarized in Table 18-5.
Table 18-5
Recommendations for Clinical Practice Related to Bilirubin Metabolism in Neonates
Know the usual cord blood values for serum bilirubin (p. 610).
Know the usual patterns for serum bilirubin in term and preterm neonates, as well as breastfed infants (p. 611).
Recognize infants at risk for physiologic jaundice (pp. 611, 613-614, Figure 18-2, and Table 18-1).
Monitor fluid intake and stooling patterns (pp. 613, 615-616).
Assess and monitor infants at risk for physiologic jaundice (pp. 611, 613-614).
Recognize infants at risk for hyperbilirubinemia (pp. 614-615 and Table 18-2).
Know the substances and pathophysiologic events that may compete with bilirubin for albumin-binding sites and monitor status of exposed infants (p. 621).
Counsel and support the family of a jaundiced infant (p. 619 and Table 18-4).
Monitor breastfed infants for early and late hyperbilirubinemia (pp. 615-616).
Institute interventions to prevent early-onset jaundice in breastfed infants (pp. 615-616 and Table 18-3).
Counsel and support parents of a jaundiced breastfed infant (pp. 615-616).
Teach parents to monitor for hyperbilirubinemia and importance of health care following early hospital discharge (pp. 615-616, 621-622).
Plan with parents of term infants for follow-up care with the primary care provider after discharge (pp. 614-616).
Assess and monitor infants at risk for hyperbilirubinemia (pp. 614-615, 621-622 and Table 18-2).
Know the methods of monitoring serum bilirubin levels and factors that can alter accuracy of measurement (pp. 616-617).
Monitor infants during and after exchange transfusion for alterations in fluid, electrolyte, and acid-base status (p. 618).
Recognize and monitor for physiologic and psychobehavioral side effects associated with phototherapy (pp. 618-619 and Table 18-4).
Provide a safe environment for the infant under phototherapy (pp. 618-621 and Table 18-4).
Recognize infants at risk for bilirubin encephalopathy and kernicterus (pp. 621-622).
Monitor for signs of bilirubin encephalopathy and kernicterus (pp. 621-622).