Chapter 79A Benign liver lesions
Overview
One of the consequences of extensive use of imaging for an ever-increasing number of indications is the detection of asymptomatic tumors. In the absence of underlying chronic liver disease or a history of cancer, the vast majority of these lesions correspond to benign liver tumors, including cystic and solid lesions. Cystic tumors will be covered in Chapter69A, 69B . Solid benign lesions include a broad spectrum of regenerative and true neoplastic processes (Table 79A.1). Based on the cell of origin, most solid, benign tumors can be classified into two groups: epithelial lesions include hepatocellular (focal nodular hyperplasia [FNH] and adenoma) and cholangiocellular tumors (bile duct adenoma); mesenchymal tumors include those lesions that originate from blood vessels (hemangioma), adipose tissue (angiolipoma), and muscle (leiomyoma). Except hepatocellular adenoma (HA), which may be associated with serious complications that may require surgery, the vast majority of solid, benign liver lesions remain asymptomatic and do not increase in volume; therefore they do not require any treatment or follow-up.
Table 79A.1 Histologic Classification of Benign Liver Lesions and Main Clinical Data
| Lesion | Type | Comments |
|---|---|---|
| Epithelial Lesions | ||
| Hepatocytes | Hepatocellular adenoma | |
The understanding of clinical, biologic, radiologic, and pathologic characteristics of each tumor is important for accurate diagnosis and appropriate management. Advances in imaging studies allow an accurate diagnosis in the majority of cases, reducing the use of percutaneous biopsy to only a few patients and limiting the number of resections for final diagnosis to exceptional cases. The most frequent benign liver cell lesions include hemangioma, FNH, and HA. Because radiologic imaging is of critical importance in diagnosis and management, there is considerable emphasis on the role of radiologic study, and this section should be read in conjunction with Chapters 13, 16, and 17.
Cavernous Hemangioma
Hepatic hemangiomas are probably the most common of all liver tumors. These tumors occur at all ages, however, because of the difference of histologic structure between the adult and the infantile forms (see Chapter 82) and the different clinical presentation, they must be regarded as separate entities (Hobbs, 1990). Only hemangioma of the liver in adults will be considered in this chapter.
Pathogenesis and Pathology
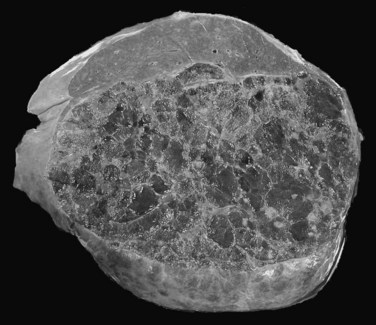
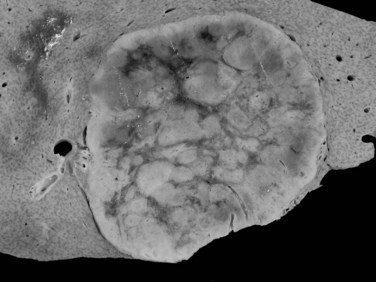
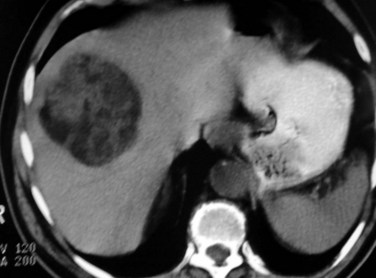
The prevalence of hemangioma in the general population ranges from 1% to 20% (Semelka & Sofka, 1997), but it is predominant in women by a 5 : 1 ratio (Mergo & Ros, 1998; Trotter & Everson 2001; Biecker et al, 2003). In adults, hemangiomas are usually found in patients at a mean age of 50 years and equally in the left and right lobes of the liver; the majority are less than 5 cm in diameter and are rarely pediculated, but hemangiomas measuring 10 cm or more are referred to as giant hemangiomas and can present with fibrosis, thrombosis, and calcifications (Fig. 79A.1).
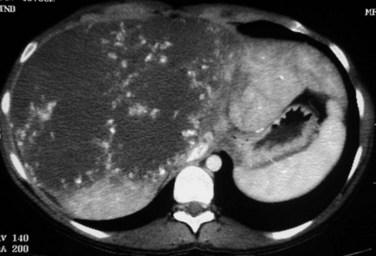
FIGURE 79A.1 Giant hemangioma. Portal-venous phase helical computed tomographic and centripetal contrast enhancement.
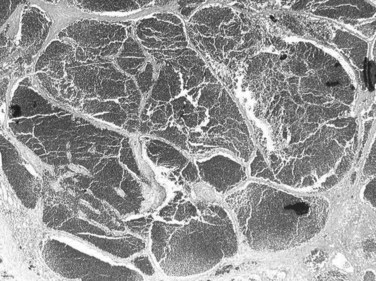
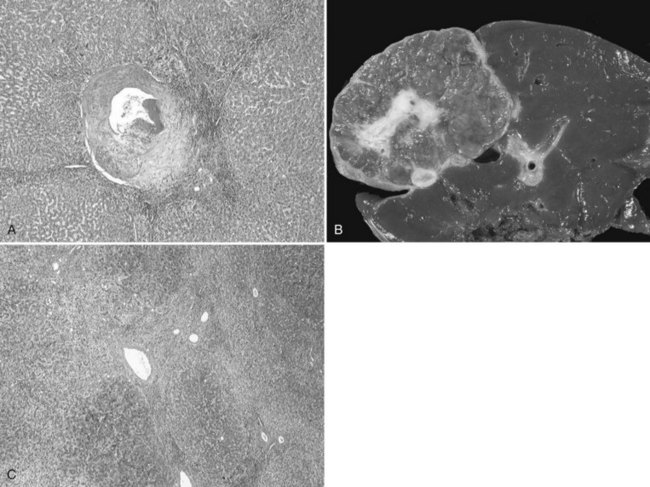
The pathogenesis of hemangioma is not well understood. Some of these tumors have estrogen receptors, and accelerated growth has been observed with high-estrogen states, such as those associated with puberty, pregnancy, oral contraceptive use, and with androgen treatment. These findings suggest that hormonal effect may be one of the pathogenic mechanisms (Trotter & Everson, 2001; Cobey & Salem, 2004). Macroscopic examination demonstrates well-delineated, flat lesions of red-blue color that may partially collapse on sectioning. Some degree of fibrosis, calcification, and thrombosis may be observed, most commonly in the largest lesions (Fig. 79A.2). Microscopically, hemangiomas are made of cavernous vascular spaces lined by flattened endothelium underlying fibrous septa of various widths (Fig. 79A.3). Small hemangiomas may become entirely fibrous, appearing as a solitary fibrous nodule.
Clinical and Biologic Data
The vast majority of hemangiomas are less than 5 cm and are found incidentally during ultrasound (US) or computed tomography (CT) examination of the abdomen for unrelated reasons (Trotter & Everson, 2001). Hemangiomas remain stable in size (Takayasu et al, 1990) or demonstrate minimal increase in diameter over time (Nghiem et al, 1997). Pain related to an uncomplicated hemangioma is most probably due to associated disorders, such as gallbladder disease, liver cysts, gastroduodenal ulcers, or a hiatal hernia (Farges et al, 1995). Large hemangiomas may be asymptomatic or may manifest with an abdominal mass or pain (Erdogan et al, 2007). Large lesions located in the left lobe of the liver may cause pressure effects on adjacent structures with resulting symptoms. Jaundice as a result of compression of bile ducts by hemangioma has also been observed, but this is rare (Losanoff & Millis, 2008).
Some cases of inflammatory processes complicating giant hemangioma have been reported (Bornman et al, 1987; Takayasu et al, 1990), and the prevalence is probably underestimated. Signs and symptoms of an inflammatory process include low-grade fever, weight loss, abdominal pain, accelerated erythrocyte sedimentation rate, normal white blood cell count, anemia, thrombocytosis, and increased fibrinogen level. The imaging features are those of giant hemangioma. Clinical and laboratory abnormalities disappear after surgical excision of the mass (Bornman et al, 1987; Pateron et al, 1991; Pol et al, 1998).
Kasabach-Merritt syndrome is an exceptional complication of hepatic hemangioma in adults (Hall, 2001; see Chapter 82). This coagulopathy consists of intravascular coagulation, clotting, and fibrinolysis within the hemangioma, and it may progress to secondary increased systemic fibrinolysis and thrombocytopenia (Concejero et al, 2009); the syndrome is reversible after removal of the hemangioma. Although rarely encountered in hepatic hemangioma, intratumoral hemorrhage can occur spontaneously or after anticoagulation therapy. Spontaneous rupture of a hemangioma is exceptional, and very few indisputable cases have been reported (Corigliano et al, 2003); taking into account the high prevalence of these tumors, the extremely low incidence of this complication should not interfere with the therapeutic management, nor should it influence the specific advice to patients, who are often worried by the presence of a vascular liver tumor (Plackett & Lin-Hurtubise, 2008).
Imaging
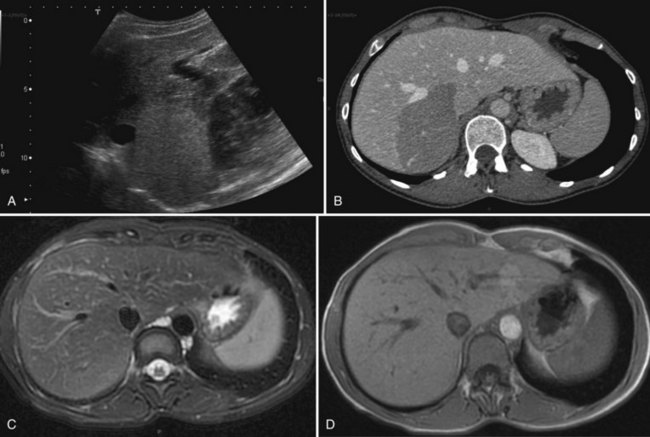
The vast majority of hemangiomas are diagnosed accurately by imaging studies alone because of characteristic imaging features. On US the classic appearance of hemangioma is that of a homogeneous hyperechoic mass less than 3 cm in diameter with acoustic enhancement and sharp margins (Fig. 79A.4; see Chapter 13). No vascular pattern is usually identified on color Doppler. Other investigations are required when US does not show typical patterns. Contrast-enhanced US reveals peripheral globular enhancement in the portal phase and isoechoic pattern on the late phase in most atypical hemangiomas (Quaia et al, 2002).
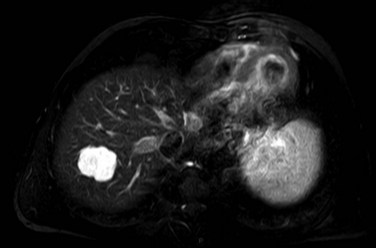
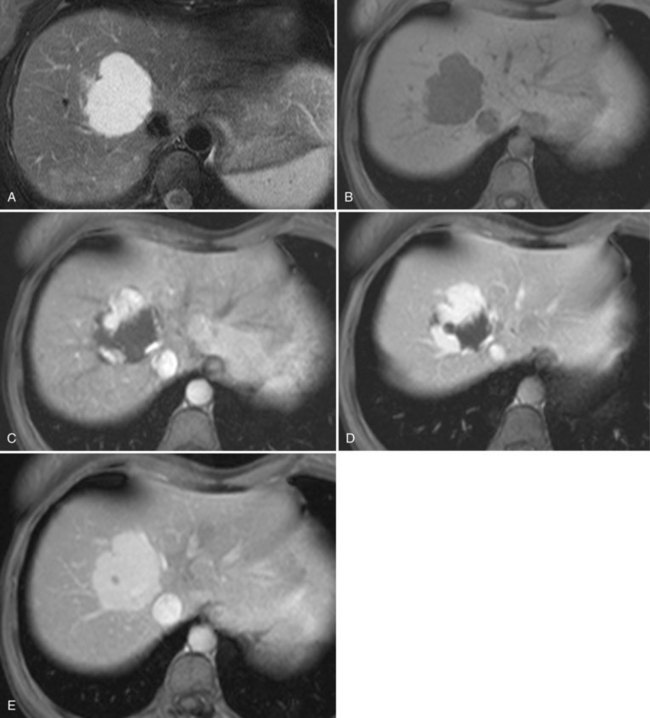
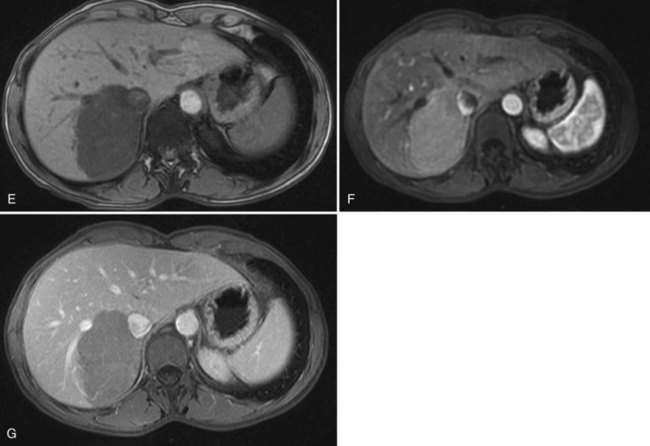
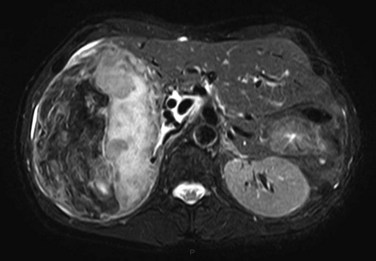
On CT, the criteria for the diagnosis of hemangioma are the following: 1) low attenuation on noncontrast CT, 2) peripheral and globular enhancement of the lesion followed by a central enhancement on contrast CT, and 3) contrast enhancement of the lesion on delayed scans (Freeny & Marks, 1986; see Chapter 16). Among these criteria, the presence of peripheral puddles on the arterial phase has a sensitivity of 67%, a specificity of 99%, and a positive predictive value of 86% for hemangioma (Nino-Murcia et al, 2000). Magnetic resonance (MR) imaging is the key imaging modality in the characterization of liver hemangiomas (Itai et al, 1985; Starck et al, 1985; see Chapter 17). The classic appearance of a liver hemangioma is that of a hypointense lesion on T1-weighted sequences, a strongly hyperintense lesion on heavily T2-weighted sequences, with a “lightbulb” pattern (Fig. 79A.5). Dynamic multiphasic T1-weighted sequences after gadolinium chelate administration show findings similar to that of contrast-enhanced CT phases (Fig. 79A.6; Semelka et al, 1994). Hemangiomas can be multiple in 10% of cases, and in exceptional cases, the presence of innumerable hemangiomas are called hemangiomatosis (Ishak & Rabin, 1975; Keegan et al, 2001).
The two most common imaging atypias are found in giant hemangiomas and in rapidly filling hemangiomas. Giant hemangiomas, which are defined when they exceed 6 or 12 cm in diameter, are often heterogeneous with marked central areas that correspond to thrombosis, extensive hyalinization, and fibrosis (Danet et al 2003; Coumbaras et al, 2002). However, usually the typical early, peripheral, nodular enhancement is observed along with strong hyperintensity on T2-weighted MR images at the periphery. Although present, the progressive centripetal enhancement of the lesion does not lead to complete filling. Rapidly filling hemangiomas are not uncommon and occur significantly more often in small lesions (42% of hemangiomas are <2 cm in diameter) (Hanafusa et al, 1995). CT and MR imaging show an immediate homogeneous enhancement in the arterial phase, which makes differentiation from other hypervascular tumors difficult. Their diagnosis is based on strong hyperintensity on T2-weighted images, the parallel enhancement with arterial structures, and the persistent enhancement on delayed-phase imaging; it is important to note that these rapidly filling hemangiomas may induce arterial–portal venous shunts (Kim et al, 2001). The other atypical hemangiomas are very uncommon and include very slow filling, calcified, hyalinized cystic and pedunculated hemangiomas, those with fluid-fluid levels, and hemangiomas with capsular retraction.
Hemangiomas associated with inflammatory response syndrome are usually due to acute thrombosis of a part or all the hemangioma. These can be diagnosed by showing spontaneous hyperattenuation on unenhanced CT scans. Hemangiomas developing in abnormal liver are difficult to diagnose, and those in fatty liver usually appear isoechoic or hypoechoic at US and hyperattenuating at unenhanced CT (Marsh et al, 1989). MR imaging with fat-suppressed, in-phase and opposed-phase T1-weighted sequences is the key to the diagnosis and is more helpful than CT; strong hyperintensity on T2-weighted images is also a reliable finding.
With progressive cirrhosis, hemangiomas are likely to decrease in size and become more fibrotic and difficult to diagnose radiologically (Brancatelli et al, 2001). In this clinical setting, US cannot be used to confidently make the diagnosis of hemangioma, as half of the hyperechoic lesions are hepatocellular carcinomas (HCCs) (Caturelli et al, 2001). The association of hepatic hemangioma and FNH can be observed in about 25% of cases and is not fortuitous (Mathieu et al, 1989; Vilgrain et al, 2003). FNH is considered to be a hyperplastic response as a result of focal increased arterial flow in the hepatic parenchyma and, like hemangioma, it is thought to have a vascular origin.
Imaging, and especially MR imaging, is therefore able to diagnose liver hemangiomas in nearly all cases, and liver biopsy should be restricted to exceptional cases. Liver hemangioma, which has been considered a contraindication to needle biopsy for many years, is possible without significant risk of hemorrhage (Heilo & Stenwig, 1997; Caldironi et al, 1998). However, as in other tumors, a cuff of normal hepatic parenchyma should be interposed between the capsule and the margin of the hemangioma.
Management
Indications for treatment include severe symptoms, complications, and inability to exclude malignancy (Yoon et al, 2003; Duxbury & Garden, 2010). Symptoms are more frequently encountered with huge hemangioma (Erdogan et al, 2007). Surgical resection remains the definitive treatment, but other less effective options include hepatic artery ligation and radiation therapy; radiation therapy can reduce the size of the lesion (Gaspar et al, 1993), but long-term effects of radiation therapy on the liver and adjacent structures can be deleterious. In addition, relief of symptoms is not well documented. Arterial embolization, which may be considered for the temporary control of hemorrhage, has limited success and is occasionally associated with morbidity (Reading et al, 1988). Arterial ligation may be considered during surgical procedures to allow manual decompression of large hemangiomas and facilitate their manipulation and enucleation (Yoon et al, 2003).
When indicated, the only treatment to be considered is resection (Herman et al, 2005; Erdogan et al, 2007), and it should be performed in specialized units, where a variety of techniques and approaches can be used. The choice between enucleation and resection requires consideration of the size and anatomic location of the lesion. Hemangioma located in the peripheral liver area is preferably treated by enucleation, whereas tumors deeply located are more safely resected with a formal anatomic liver resection (Kuo et al, 1994; Gedaly et al, 1999; Fu et al, 2009). In the majority of cases, patients showed symptoms relief after resection of a symptomatic hemangioma (Erdogan et al, 2007). Although laparoscopic resection is safe for peripheral hemangioma (Karahasanoglu et al, 2001; Jiang et al, 2007), this approach should not extend the rare indication for surgery. Liver transplantation has also been used successfully to treat symptomatic patients with a technically unresectable, complicated giant hemangioma or hemangiomatosis with cardiopulmonary complications (Browers et al, 1997; Tapetes et al, 1995; Ferraz et al, 2004; Keegan et al, 2001; Ercolani et al, 2010).
Focal Nodular Hyperplasia
Pathogenesis and Pathology
FNH accounts for the second most common benign liver process, after hemangioma. It is a benign, tumor-like condition predominantly diagnosed in women 30 to 50 years of age, and it is not influenced by oral contraceptives (International Working Party, 1995; Ishak, 1979). FNH is considered a hyperplastic reaction resulting from arterial malformation (Wanless et al, 1985). This hypothesis, suggesting that increased arterial flow hyperperfuses the local liver parenchyma and leads to secondary hepatocellular hyperplasia, has been reinforced by molecular data showing that FNH is a polyclonal regenerative process (Gaffey et al, 1996; Paradis et al, 1997; Rebouissou et al, 2008). This regenerative process induced in a specific vascular territory could explain the absence of size changes in the vast majority of cases. However, an involution of this lesion is possible after menopause (Kuo et al, 2009), which explains the occurrence of this lesion in patients with vascular disorders of the liver, including Budd-Chiari syndrome (Cazals-Hatem et al, 2003), hereditary hemorrhagic telangiectasia (Gincul et al, 2008), congenital absence of portal flow (Kim T, et al, 2004), or portal thrombosis with subsequent hepatic arterialization (Bureau et al, 2004).
Such focal regenerative processes are also described in cirrhotic tissue with the so-called FNH-like macronodules. FNH displays a typical pathologic pattern for both radiologists and pathologists. Grossly, it is a well-circumscribed, unencapsulated, usually solitary mass characterized by a central fibrous scar that radiates into the liver parenchyma (Fig. 79A.7). Histologically, FNH is composed of benign-appearing hepatocytes arranged in nodules, usually partially delineated by fibrous septa that originate from the central scar. The main diagnostic feature is the presence of large and dystrophic vessels in the fibrous septa, which can be accompanied by several degrees of ductular proliferation and inflammatory cells. The hepatocytes are hyperplastic, arranged in liver plates of normal or slightly increased thickness (see Chapter 78). Hepatocytes may be hydropic, related to cholestatic changes, or they may display some degree of steatosis.
Performance of biopsy in the diagnosis of FNH is often a challenge, because fibrous septa and thick abnormal arteries are usually missing on biopsy specimen (Makhlouf et al, 2005). In that context, glutamine synthetase showing focal positive hepatocellular areas, usually centered by hepatic veins and described as a maplike pattern, is a helpful surrogate marker (Bioulac-Sage et al, 2009a).
Besides this classic form of FNH, several variant lesions are described with increased frequency and commonly classified as nontypical FNH by radiologists. This group of lesions is somewhat heterogeneous, including FNH without a central fibrous scar and FNH containing fat (Nguyen et al, 1999). However, molecular studies have demonstrated that in this group of atypical FNH, lesions that display telangiectatic changes, the so-called telangiectatic form of FNH, are clonal processes that should be rather regarded as a variant form of liver cell adenoma; it is classified as a specific subgroup of adenoma other than FNH (Paradis et al, 2004; Bioulac-Sage et al, 2005).
Clinical and Biologic Data
FNH represents the second most frequent benign lesion, with an estimated prevalence around 1% (Vilgrain, 2006); it is seen predominantly in women (9 : 1) and often is discovered in patients aged 30 to 40 years, although men with FNH are usually older than 40 years (Nguyen et al, 1999). The vast majority of FNH is asymptomatic, usually discovered incidentally during liver US examination. In a few patients with large tumors, FNH can be discovered by abdominal pain or discomfort. Large lesions located in the left lobe of the liver may cause pressure effects on adjacent structures with resulting symptoms. The lesion may be felt when it is pedunculated, and it can be responsible for acute episodes of pain because of torsion on the pedicle. Large FNH below the Glisson capsule could also be responsible for pain (Buetow et al, 1996), but in most cases pain related to FNH is probably due to associated disorders.
Complications of FNH, such as rupture or bleeding, are exceptional and are usually related to atypical forms of FNH with specific imaging and biologic features, the so-called telangiectatic FNH, which should be classified as an adenoma. No malignant transformation of FNH has been demonstrated. A reduction in size after menopause may be observed, but in the vast majority of cases, FNH remains stable in size, even after interruption of oral contraception or after pregnancy (Mathieu et al, 2000).
Liver biologic test results are normal in nearly 80% of cases (Belghiti et al, 1993). Abnormalities include mild elevation of GGT and alkaline phosphatase in patients with a large FNH that caused extrinsic compression of intrahepatic biliary ducts. The presence of a slight elevation of serum transaminase levels could be due to the presence of associated steatosis in the underlying liver parenchyma.
Imaging
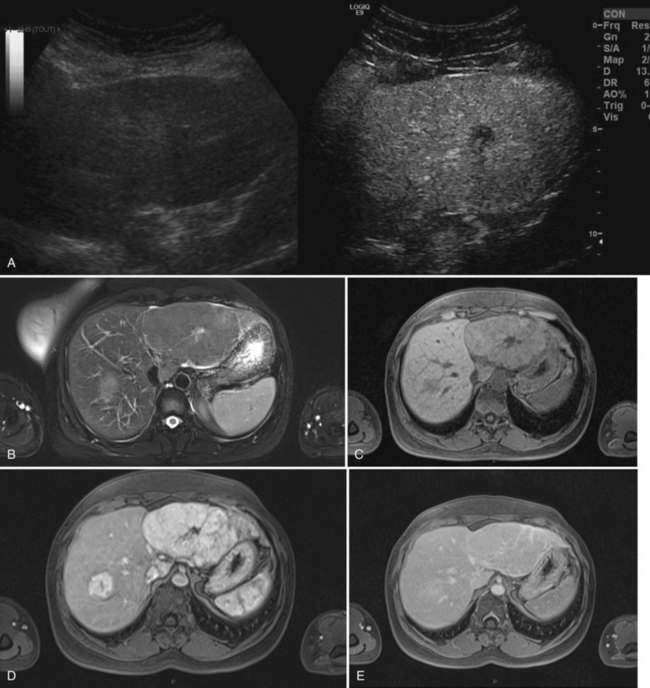
FNH is usually slightly hypoechoic or isoechoic at US (see Chapter 13). Some lesions are only detected because they displace the surrounding vessels. Lobulated contours and a hypoechoic halo are often observed; the central scar is slightly hyperechoic but is often difficult to visualize at US (20% of cases; Shamsi et al, 1993). Typical findings at color Doppler include the presence of a central feeding artery with a stellate or spoke-wheel pattern that corresponds to the artery running from the central scar to fibrous septa (Fig. 79A.8). Doppler spectral analysis shows, in most cases, an intralesional pulsatile waveform with high diastolic flow and low resistive index (Uggowitzer et al, 1997). Large draining vessels may be identified in the periphery at the tumor margins. US contrast agents and nonlinear continuous imaging modes at a low mechanical index are very interesting tools to better characterize FNH. Most lesions enhance at the arterial phase (Kim MJ, et al, 2004; Dietrich et al, 2005) with a central vascular supply and a centrifugal filling to the periphery. The lesion becomes homogeneously isoechoic in the late portal and delayed phases in the vast majority of the cases (Kim MJ, et al, 2004), and very little FNH is evident with washout; when the central scar is detected, it appears hypoechoic on both arterial and portal phases. By showing centrifugal enhancement with radiated vascularization, differentiation from hepatocellular adenoma is possible in most cases (Kim T, et al, 2008).
On precontrast CT scans, FNH is demonstrated as a focal hypodense or isodense mass. A central hypodense scar is depicted in only one third of the patients (Shamsi et al, 1993). Calcifications within the central scar are very rare and are observed in only about 1% (Caseiro-Alves et al, 1996). Because of the prominent arterial supply to the FNH, the lesion enhances rapidly at the arterial phase of contrast-enhanced CT in most cases (Brancatelli, et al 2001). Lesion enhancement decreases at the portal venous phase, and the lesion may be either isodense or slightly hyperdense relative to normal liver. Additional findings are lesion homogeneity, a lobulated contour, and the absence of a capsule. A central scar is observed more often in large lesions than in small ones, and it enhances over time (Carlson et al, 2000). It has been shown that the enhancement of FNH in the arterial phase is significantly higher than in HA (Ruppert-Kohlmayr et al, 2001).
On MRI, typical FNH is isointense or hypointense on T1-weighted images and isointense or slightly hyperintense on T2-weighted images (Vilgrain et al, 1992; Buetow et al, 1996; see Chapter 17). The central scar is hypointense on T1-weighted images and strongly hyperintense on T2-weighted images (Mortelé et al, 2000; Kehagias et al, 2001). Another key finding is the lesion homogeneity apart from the central scar. After administration of gadolinium chelates, the enhancement is similar to that observed on contrast-enhanced CT. On delayed-phase imaging, 5 to 10 minutes after injection, the central scar shows high signal intensity because of the accumulation of contrast material in the fibrous tissue. Hepatobiliary contrast agents can be used to highlight the hepatocellular origin of the lesions and to differentiate FNH from hepatocellular adenomas; however, the biokinetics are different among those specific contrast agents (Ba-Ssalamah et al, 2002; Grazioli et al, 2005). On diffusion-weighted MR sequences, FNH is often hyperintense on high b values, but their apparent diffusion coefficient (ADC) values are close to that of the liver.
Atypical forms of FNH that are difficult to diagnose include FNH without a scar, especially in lesions measuring less than 3 cm in diameter. In some cases, scars do not enhance on delayed imaging, or they are hypointense on T2-weighted images; this leads the clinician to suspect the diagnosis of fibrolamellar carcinoma. Other atypical findings include hyperintensity on T1-weighted imaging, which may be caused by various pathologic changes that include fat deposition (mostly in patients with steatosis; Stanley et al, 2002). FNH heterogeneity is exceptional and should make the diagnosis doubtful.
Although FNH is mainly a solitary lesion, multiple FNH lesions may be observed in 20% of cases and may be associated with hemangiomas or more seldom with HA (Mathieu et al, 1989; Vilgrain et al, 2003; Laurent et al, 2003). Accuracy of contrast-enhanced US has been established, and MR imaging has the higher sensitivity (70%) and specificity (98%) (Soussan et al, 2010). However, the diagnosis of FNH is based on a combination of features, and none of them are specific for FNH. When imaging cannot establish a firm diagnosis, liver biopsy plays a role in diagnosing FNH (Fabre et al, 2002; Makhlouf et al, 2005).
Management
Surgical resection is restricted to symptomatic patients. Symptomatic patients with confirmed FNH should be thoroughly investigated to exclude other pathology before attributing symptoms to the liver lesion. The development of a liver laparoscopic approach should not extend the indication for resection, which should be performed in specialist hepatobiliary units, as it is clearly indicated in patients with large, symptomatic FNH located in the left liver and in those with a pedunculated lesion. The safer resection procedure is liver resection with a surgical margin, rather than enucleation, because large veins often surround FNH, which renders enucleation difficult. In some symptomatic patients with large FNH located in the right liver or in segment I and necessitating difficult or risky resection, transarterial embolization has been advocated to determine the relationship between the lesion and symptoms (De Rave & Hussain, 2002; Kehagias et al, 2001). Data in the literature (Amesur et al, 2009) and our experience have shown that this is an attractive treatment with reduction of size in conjunction with relief of symptoms (Fig. 79A.9). Percutaneous radiofrequency ablation (RFA) has also been described as an effective treatment modality for symptomatic FNH (Hedayati et al, 2010).
Hepatocellular Adenoma
Pathogenesis and Pathology
Hepatocellular adenoma (HA) is a rare, benign liver neoplasm strongly associated with oral contraceptive (OC) use (Rosenberg, 1991). The most important complications of HA are hemorrhage and malignant transformation into HCC. HA is mainly observed in women of childbearing age, and it is rare in men (sex ratio 9 : 1). Its incidence is estimated to be 0.1 per year per 100,000 in non-OC users, and it reaches 3 to 4 per 100,000 in long-term OC users (Wittekind, 1995). HA is directly related to OC use, and the risk for developing adenoma increases with the duration and estrogen content of the medication (Rosenberg, 1991; Dokmak et al, 2009). This tumor can also occur in men receiving anabolic steroids (Socas, et al 2005), or it may be associated with underlying metabolic disease, including type 1 glycogen storage disease, and iron overload related to β-thalassemia and hemochromatosis (Barthelemes & Tait, 2005; Bioulac-Sage et al, 2008). The prevalence of obesity was high in patients with HA (Dokmak et al, 2009), and it was recently described in patients with nonalcoholic fatty liver disease (Watkins et al, 2009). HA can also occur in patients with portacaval shunt or portal deprivation, and familial cases have been reported in patients with mellitus onset diabetes type 3 (Chiche et al, 2000; Bioulac-Sage et al, 2008).
HA is usually solitary, sometimes pedunculated, with a diameter that can vary from a few millimeters up to 30 cm. The widespread use and technical progress in imaging techniques has revealed that one third of patients have multiple HAs (Dokmak et al, 2009; Deneve et al, 2009; Bioulac-Sage et al, 2009b). Patients with more than 10 HAs are subclassified as having liver adenomatosis, considered to be more common in men and to have an increased risk of complications (Fléjou et al, 1985). However, review of the literature and our experience do not support this arbitrary classification based on clinical and imaging characteristics (Ribeiro et al, 1998; Jovine et al, 2004; Barthelemes & Tait, 2005; Dokmak et al, 2009; Bioulac-Sage et al, 2008). The risk of bleeding and of malignant transformation is similar to that in patients with solitary adenoma and is mainly related to the size and subtype of tumors (Dokmak et al, 2009; Bioulac-Sage et al, 2008; Deneve et al, 2009; Stoot et al, 2010). It also appears that the prevalence of HA is rising in men, mainly as a result of the rising incidence of metabolic syndrome (Farges et al, 2011). Such HA displays a significantly higher risk of malignant transformation to HCC, reaching 47% in the last few years in our experience (Farges et al, 2011); therefore patients should not be managed according to the number of HAs, but rather according to the risk of complications, which is related to sex, the histologic subtype, and mainly to the size of the HA.
Large subcapsular vessels are commonly found on macroscopic examination. On cut sections, the tumor is well delineated, sometimes encapsulated, and has a fleshy appearance; it ranges in color from white to brown (Fig. 79A.10). HA frequently displays heterogeneous areas of necrosis and hemorrhage. Histologically HA consists of a proliferation of benign hepatocytes arranged in a trabecular pattern; however, the normal liver architecture organization is absent (Chapter 78). Hepatocytes may have intracellular fat or increased glycogen. Malignant transformation to HCC may be difficult to assess, especially on biopsy specimen (Deneve et al, 2009; Stoot et al, 2010), but it is observed in 4% to 10% of cases in published series (Barthelemes & Tait, 2005; Bioulac-Sage, 2008; Deneve et al, 2009; Zucman-Rossi et al, 2006).
Four subtypes of HA could be named according to genotype/phenotype classification (Bioulac-Sage et al, 2009). Group 1 includes HA with mutation in the hepatocyte nuclear factor (HNF)-1α gene (H-HA). Observed in approximately 35% to 40% of patients, almost exclusively in women and often with multiple HAs, this group corresponds to a histologically homogeneous group of tumors characterized by marked steatosis, no cytologic abnormalities, no inflammatory infiltrates, and a very low risk of complications. Group 2 includes HA with mutation of the β-catenin gene. Observed in approximately 10% of cases, mostly in men, this group is characterized by the presence of cytologic abnormalities with the greater risk of malignant transformation. Group 3 corresponds to telangiectatic/inflammatory HA. Observed in more than 50% of cases, these lesions usually occur in overweight women patients with inflammatory syndrome; tumors are characterized morphologically by the presence of inflammatory infiltrates, marked sinusoidal dilation or congestion, and numerous isolated or clustered arteries. The risk of complication in this group is high. The last group corresponds to HA that does not express any of the above-mentioned phenotypic or morphologic features. Besides the morphologic features associated with the different subtypes of HA, additional immunohistochemical markers have been described that provide significant assistance in the classification of HA, especially in biopsy specimens (Bioulac-Sage et al, 2009).
Clinical and Biological Data
Although patients with HA can come to medical attention with symptoms, the majority are asymptomatic, especially if they have small tumors (Trotter & Everson, 2001; Weimann et al, 2000; Bioulac-Sage et al, 2009; Dokmak et al, 2009). Tumors are discovered during diagnostic workup of elevated liver enzymes or during liver imaging monitoring in pregnancy or for other reasons. A large adenoma may cause a sensation of right upper quadrant fullness or discomfort.
Adenoma is of clinical importance because of its tendency to bleed spontaneously and result in hemorrhage and infarction in the tumor. The risk of spontaneous bleeding is likely to be between 20% and 40% (Barthelemes & Tait, 2005; Dokmak et al, 2009; Bioulac-Sage et al, 2009; Deneve et al, 2009). Although the risk of bleeding is increased in pregnant women and in those taking OC, this risk is quite exclusively observed in patients with lesions larger than 5 cm (Dokmak et al, 2009; Deneve et al, 2009). About 10% of patients are seen with acute, severe abdominal pain from intraperitoneal rupture and hemoperitoneum, which may be associated with hypovolemic shock. Hemorrhage results in acute pain and discomfort accompanied by anorexia, nausea, vomiting, and fever. Although tumor rupture with hemoperitoneum represents an alarming manifestation, hemodynamic instability is rare, except in patients treated with anticoagulation (Marini et al, 2002). The presence of sudden right upper quadrant pain with some respiratory manifestation leads some clinicians to erroneously suspect the diagnosis of pulmonary embolism; they then treat the patient with anticoagulant therapy, which can exacerbate the bleeding.
The risk of malignant transformation of HA in women is on the order of 5% (Barthelemes & Tait, 2005; Bioulac-Sage et al, 2009; Deneve et al, 2009; Dokmak et al, 2009; Stoot et al, 2010). No clinical or radiologic signs can predict preoperatively the diagnosis of degenerated HA; it is almost exclusively made on the pathologic specimen of resected HA. Malignancy displays well-differentiated HCC, and vascular extension or satellite nodules are exceptional. α-Fetoprotein (AFP) level is usually normal. Most cases of HCC develop at the site of the liver cell adenoma. The risk of malignancy is very high in men (50%), whatever the size of the tumor, and in women with a large HA (>8 cm) (Dokmak et al, 2009; Stoot et al, 2010; Farges et al, 2011). Moreover, HA with β-catenin activation carries a higher risk of malignant transformation (Bioulac-Sage et al, 2009; Farges et al, 2011). These cases of malignancy developed in HA should be differentiated from exceptional cases of multiple degenerated HA occurring in young women with symptomatic hepatomegaly associated with severe asthenia and high AFP level. The prognosis is poor, because surgical resection is not feasible, and liver transplantation is inevitably followed by rapid and fatal relapse of the disease.
In two thirds of cases, liver function tests are abnormal, including elevated cholestatic enzymes and mild elevation of transaminases (Dokmak et al, 2009). In case of malignancy, serum AFP is generally normal.
Imaging
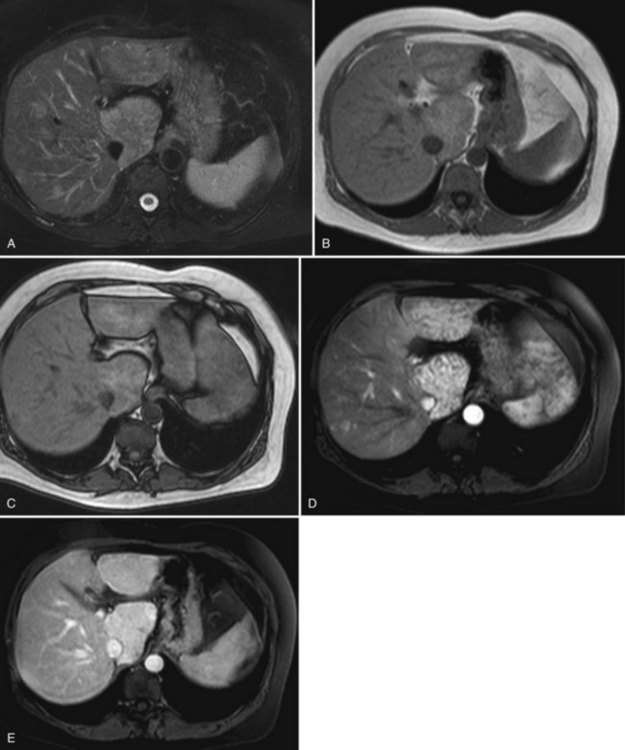
The key imaging feature of HNF-1α–inactivated HA is the presence of marked and diffuse fat within the lesion. Fat is responsible for lesion hyperechogenicity on US and strong hypoattenuation on unenhanced CT scan (see Chapter 16). Because of the fat content, the lesion does not enhance markedly and shows rapid washout on multiphasic examination. MR plays a crucial role in demonstrating the presence of diffuse fat (see Chapter 17). The striking finding is a diffuse and homogeneous signal dropout on chemical-shift T1-weighted sequences with a high specificity (Laumonier et al, 2008; Fig. 79A.11).
The key imaging feature of telangiectatic/inflammatory HA is the presence of telangiectatic components (Fig. 79A.12). The lesion is often hyperechoic at US but is also heterogeneous, and Doppler signals are commonly seen. Unlike other HAs, central arteries that could mimic FNH may be seen on contrast-enhanced US (Soussan et al, 2010). On unenhanced CT, telangiectatic/inflammatory HAs are hypoattenuating but often heterogeneous. The characteristic lesion behavior on multiphasic examination is a strong arterial enhancement and a persistent enhancement in the delayed phase (Laumonier et al, 2008). MR imaging is also very important because it shows a strong hyperintense signal on T2-weighted images. On T1-weighted sequences, lesion signal intensity is variable and is often hyperintense. This hyperintensity is not related to fat and persists on fat-suppressed and opposed-phase sequences (Attal et al, 2003; Lewin et al, 2006). Considering two imaging findings—a markedly hyperintense signal on T2-weighted images and persistent enhancement on the delayed phase—Laumonier and colleagues (2008) observed a positive predictive value for the diagnosis of telangiectatic/inflammatory hepatocellular adenoma of 88.5%, a negative predictive value of 84%, a sensitivity of 85.2%, and a specificity of 87.5%. Some of telangiectatic/inflammatory HAs may contain some fat deposition. The two other subtypes, β-catenin–activated and unclassified HA, are less characteristic on imaging. To note, some telangiectatic/inflammatory HA may display β-catenin activation and may therefore have imaging findings similar to classic telangiectatic/inflammatory HA. The others share findings of hepatocellular tumors, strong arterial enhancement with no delayed enhancement after gadolinium injection, and may have heterogeneous content.
Treatment
Whatever the size, HA in men represents an indication for resection because of the high risk of malignancy. Whatever the number of HAs, women with small lesions less than 5 cm in diameter have a low risk of complication and could be safely observed after cessation of OC; however, the influence of the discontinuation of OC on the regression of HA is a subject of debate (Gordon et al, 1986), justifying serial imaging examinations in patients with HA managed conservatively. Indeed, long-term follow-up evaluation for nearly 20 years of patients with small residual HAs after surgery showed that HA remains stable or involved in more than 90% of cases (Dokmak et al, 2009). Regression and even disappearance of HAs seem to occur more frequently in older patients. Reappearance or increase in size occurs in 15% of patients, but serious complications are rarely described in this subgroup (Dokmak et al, 2009). It was usually recommended that pregnancy be avoided in patients with a history of HA (Deneve et al, 2009); however, in our experience, pregnancies in several women treated for HA were not associated with reappearance or enlargement of residual HA; this led us to not contraindicate pregnancy in patients with HA (Dokmak et al, 2009).
Resection of HA greater than 5 cm is indicated. The operative approach should be tailored to the location and the size of the tumor. A large resection margin is not needed, allowing complete resection through a laparoscopic approach when feasible (Descottes et al, 2003). In some patients with large bilobar tumors, resection could be performed in two steps. Tumors located in one lobe are resected during a first procedure, followed some weeks later by a second procedure to resect tumors located in the remnant liver. Small tumors need not be resected, and liver transplantation is no longer indicated in patients with multiple HAs (Dokmak et al, 2009). Malignant transformation is often discovered on the pathologic analysis of the resected specimen, because no radiologic features are specific, and AFP level is almost always normal. Vascular invasion is absent, and lymph nodes are not involved. Complete resection of malignant HA is a safe and efficient therapeutic option.
Although surgical resection remains the standard of care of HA, other treatment modalities such as arterial embolization (Kobayashi et al, 2009; Stoot et al, 2007), percutaneous RFA (Atwell et al, 2005), or combinations of both are performed more and more with encouraging results in recent publications. Such modes of treatment are now proposed for tumors around 5 cm in diameter, in case of recurrence after surgical resection, and if the estimated risk of malignancy is very low (see Chapter 83, Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ).
Patients with ruptured HA should be treated electively. After stabilization of the patient and selective hepatic artery embolization, the patient can be observed for several months, until resorption of the hematoma surrounding the tumor (Terkivatan et al, 2001; Marini et al, 2002). The absorption of the hematoma surrounding the tumor allows an easier and parenchyma-sparing elective liver resection with a low risk of transfusion (Marini et al, 2002; Deneve et al, 2009). The involvement of these hemorrhagic HAs, which decrease dramatically in size and even disappear after embolization, may even bring into question the need for subsequent resection, because exceptional cases of hemorrhagic HA had a malignant component.
The impact of the genotype/phenotype classification on the management of HA remains unclear (Terkivatan & Ijzermans, 2009). We are in favor of preoperative biopsy in patients with large (>5 cm) steatotic HA, considering a conservative approach with observation if the HA belongs to group 1, with H-HA mutation. Patients with HA approximately 5 cm, which could be treated by transarterial embolization or RFA, should undergo a biopsy before employing a conservative approach, so that close follow-up or even systematic resection can be considered if the HA belongs to group 2 with β-catenin mutation.
Other Lesions
Fatty Lesions of the Liver
Angiomyolipoma
Hepatic angiomyolipoma is a rare, benign mesenchymal tumor composed of smooth muscle cells, adipose tissue, and proliferating blood vessels in various combinations. It belongs to the group of tumors derived from the perivascular epithelioid cell, referred to as perivascular epithelial cell tumors or PEComas (Tsui et al, 1999). Hepatic angiomyolipoma occurs frequently in the kidney but rarely in liver. This rare tumor may occur as a solitary mass (Zhou et al, 2008; Zeng et al, 2010) or as an associated finding with tuberous sclerosis (Hooper et al, 1994), which is present in about 15% of cases. In this situation, angiomyolipomas are usually multiple.
Hepatic angiomyolipoma predominantly affects women between 30 and 50 years of age, and lesions are often larger than 5 cm in diameter (Yang et al, 2007), and it can increase in size (Zeng et al, 2010). This tumor was once considered benign, but malignant transformation has been reported (Zhou et al, 2008; Rouquie et al, 2006; Dalle et al, 2000). Patients usually have no symptoms, and liver tests are normal; most of these tumors are found incidentally on routine imaging studies. The accuracy of preoperative diagnosis is low as a result of variable imaging appearance because of the varying content of the three components and the rarity of the lesion. The fat component of angiomyolipoma varies between 10% and 90% (Hooper et al, 1994).
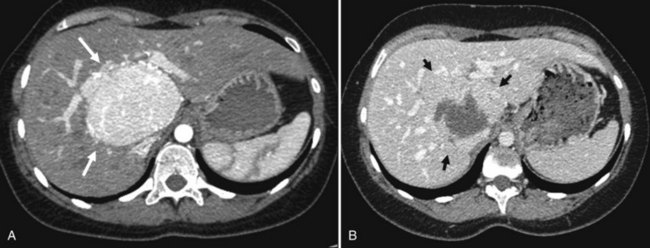
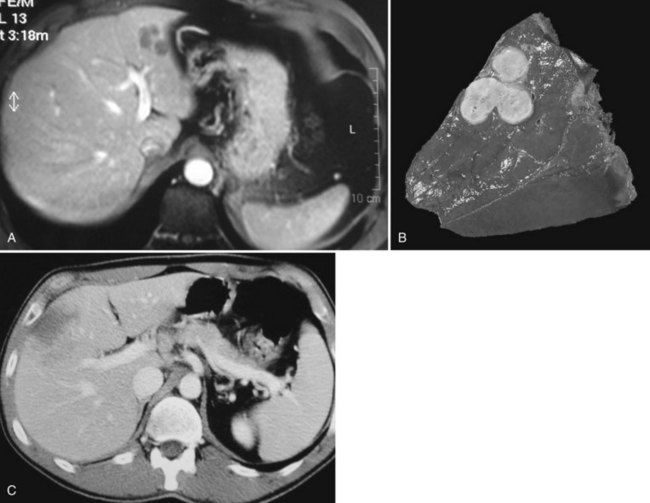
Typically, angiomyolipoma appears hyperechoic and hypoattenuated on unenhanced CT scans and markedly enhanced on the arterial phase with central vascular opacification (Fig. 79A.13). The presence of large central vessels, the so-called macroaneurisms, within the lesion is characteristic of angiomyolipoma. On contrast-enhanced US, the vast majority of angiomyolipomas enhance on the arterial phase without washout on the portal or delayed phase (Wang et al, 2010). MRI is also an important diagnostic technique that allows fat suppression and multiphase dynamic contrast-enhanced scanning. In general, MR shows a marked hypersignal in T1-weighted images, which drops on fat-suppressed sequences. However, the lesions have various imaging characteristics because of the different proportions of smooth muscle, fat, and vessels (Yan et al, 2002). The amount of fat may be too small to be detected (<5%), and some angiomyolipomas, especially those with a prominent epithelioid myoid component, may mimic hepatocellular lesions, mostly HA and HCC (Arblade et al, 1996). The accuracy of needle biopsy is reinforced by using the antibody anti–HMB-45, which stains the myoid component (Arblade et al, 1996); the positivity of this antibody in the cytoplasm of smooth cells is characteristic of angiomyolipoma (Arblade et al, 1996). When the diagnosis is established, careful observation with serial follow-up is recommended in asymptomatic patients with lesions less than 5 cm (Hoffman et al, 1997; Sawai et al, 1998). Because some degenerated cases have been reported in patients with large tumors, especially when major epithelioid content was present, liver resection is indicated in large (>5 cm) hepatic angiomyolipoma (Zeng et al, 2010; Fig. 79A.14).
Lipoma
Hepatic lipoma is a very rare liver tumor that can mimic angiomyolipoma. Lesions of hepatic lipoma are homogeneous and circumscribed, show fat attenuation on CT, and do not enhance after intravenous (IV) administration of contrast material. On MRI imaging, lipomas are hyperintense on T1-weighted images and moderately hyperintense on T2-weighted images. The key finding is the drop in signal intensity on fat-suppressed MR sequences (Horton et al, 1999). Unlike hepatocellular tumors that contain fat, lipomas do not drop in signal intensity on opposed-phase T1-weighted MR images. Microscopic analysis shows well-differentiated adipose tissue without any significant changes. There is no need to resect lipomas (Nakamura et al, 2009).
Bile Duct Adenoma
Bile duct adenoma, also called benign cholangioma, is a benign and asymptomatic mass that is typically discovered incidentally at imaging studies, surgery, or at autopsy (Kim et al, 2010). This rare benign lesion of the liver is usually a well-circumscribed, subcapsular nodule with a diameter of less than 1 cm consisting of a proliferation of noncystic biliary structures within a dense, fibrous stroma (Allaire et al, 1988). No relationship between this tumor and cholangiocarcinoma has been shown, and resection is unnecessary; its only clinical significance lies in the possible confusion with metastatic carcinoma during surgery (Hornick et al, 2005).
Biliary Hamartoma
This lesion, also known as a Von Meyenburg complex, is a development anomaly of small intrahepatic bile ducts composed of bile ductules, inflammatory cells, and fibrosis (Table 79A.1; Neri et al, 2004). The main practical problem raised by this tumor is for the pathologist, because its possible discovery during surgery for carcinoma of another abdominal organ leads the surgeon to perform a biopsy for frozen-section diagnosis (Guiu et al, 2009). The pathologist who is unaware of this rare entity may be puzzled and may be tempted to call the lesion metastatic carcinoma. Otherwise, hamartomas are easily recognized on heavily T2-weighted MR images and appear as multiple small lesions that are strongly hyperintense. The presentation is often mistaken for Caroli disease, but the lesions in biliary hamartoma do not communicate with bile ducts (Thomé-Noun et al, 2008). Treatment is not required.
Inflammatory Pseudotumors
Inflammatory pseudotumor of the liver (IPL) is a rare benign tumor that can mimic cholangiocarcinoma (Deng et al, 2010; see Chapter 48). Commonly found in the lung, IPL is a reactive fibroblastic proliferation infiltrated by polymorph inflammatory cells: plasmocytes, lymphocytes, and histiocytes. IPLs are also referred to as inflammatory myofibroblastic tumors, plasmocytic granuloma, histiocytoma, fibroxanthoma, pseudolymphoma, and necrotic tumor of the liver (Milias et al, 2009). They are more common in men than in women, are more frequently seen in the non-European population, and have an average age at presentation of 50 years (Goldsmith et al, 2009). The majority of patients with IPL typically experience vague constitutional symptoms such as fever (50%), abdominal pain (48%), weight loss (37%), and jaundice (Zamir et al, 1998; Goldsmith et al, 2009). Hematologic tests are generally normal, but one third of patients are seen with inflammatory syndrome. Liver function tests are abnormal in 15% of cases with mild elevations in cholestatic tests (alkaline phosphatase and GGT). The etiology of this pathology is not well known, but the predominantly inflammatory pattern and the associated systemic reaction suggest an underlying infectious agent (Koea et al, 2003) with microorganisms coming from the gut to the liver via the portal vein (Jover Díaz et al, 2009). This etiology can explain the response to antibiotics in some cases. Some authors have suggested an autoimmune etiology such as immunoglobulin G (IgG)-4 sclerosing cholangitis with histologic findings similar to autoimmune pancreatitis (Uchida et al, 2007), and in this case, treatment with steroids can be effective (Anthony, 1993; Kamisawa & Okamoto, 2008). In the tumoral hypothesis, authors suggest that some IPL may be of follicular dendritic cell origin (Shek et al, 1998).
Imaging studies often show diffuse and infiltrative large tumors, although multiple forms can be found (Jover Díaz et al, 2009). The majority of these lesions are located near the gallbladder or are related to the biliary tree, sometimes mimicking a malignant biliary stricture (Koea et al, 2003; Locke et al, 2005; Terada, 2010).
According to clinical and morphologic presentations, three different forms can be distinguished:
2) Encapsulated form: Clinically, this lesion is small and is usually discovered fortuitously. Inflammatory syndrome is absent. At CT, the lesion is hypoattenuating and presents with a peripheral capsule, and no enhancement is depicted within the lesion. On MRI, the lesion is well limited, hypointense on T1 imaging, isointense on T2 imaging, and surrounded by a capsule that enhances on delayed phase. This encapsulated form resembles a lesion called a solitary necrotic nodule of the liver (Abbey-Toby et al, 2003), and it can be a chronic sequela of an inflammatory process that has partially healed, circumscribed by a fibrotic capsule.
3) Infiltrating periportal form: Frequently revealed by jaundice (Venkataraman et al, 2003), this is a poorly demarcated lesion with periportal infiltration on imaging. The lesion is hypointense on T1-weighted sequences and moderately hyperintense on T2-weighted sequences with dilated bile ducts (Venkataraman et al, 2003). A progressive enhancement is seen after injection of gadolinium chelates. Lymphadenopathy can be observed along the hepatoduodenal ligament and in the retroperitoneum, mimicking cholangiocarcinoma (Fig. 79A.15; Chirica et al, 2005). On histology, periportal inflammation is associated with destruction of bile ducts and intrahepatic extension of inflammatory processes.
The optimal management has not been well established, with many types of treatment described, from simple observation to surgical resection and even liver transplantation (Milias et al, 2009). In the literature, most patients have been surgically treated, because the diagnosis was not made preoperatively because of the possible destructive nature of IPL, or because its malignant potential leads some authors to propose liver resection (Li et al, 1989). The outcome of patients treated surgically is very good (Goldsmith et al, 2009), but IPL can be associated with some risk, especially IPL that occurs as hilar lesions, necessitating major liver resection. Recurrence can be observed after resection (Lopez-Tomassetti Fernandez et al, 2006). However, IPLs are benign lesions with no malignant potential; once the diagnosis has been made, medical treatment by antibiotics, steroids (Maze et al, 1999), or observation can be undertaken with very good results. A number of reports exist that suggest the natural history of these lesions is of spontaneous complete resolution or regression (Levy et al, 2001; Koea et al, 2003; Goldsmith et al, 2009); therefore IPL can be safely managed nonoperatively.
Rare Tumors and Pseudotumors
Solitary Fibrotic Tumor
Also known as a solitary necrotic tumor, a solitary fibrotic tumor of the liver is rare with only about 100 cases reported in the literature. Slightly more frequent in men, these tumors are found at a mean age of 60 years, and the majority of patients are asymptomatic (Deniz & Coban, 2010). The solitary fibrotic tumor is usually a unique, well-circumscribed, small (<2 cm) lesion (Zhou et al, 2008). Histologically, it is composed in varying degrees of necrosis and fibrosis comprising abundant collagen fibers, and it can contain calcifications. This lesion can mimic liver malignancy, especially metastases (Delis et al, 2009; De Luca et al, 2000).
The etiology of this lesion is still uncertain, but possible causes included thrombi, hemangioma, trauma, and infection, especially parasitic infection such as with a hydatid cyst (Clouston et al, 1993; Deniz & Coban, 2010). On imaging, no findings are characteristic except a progressive enhancement that is related to abundant collagen (Moser et al, 2005), which can be very intense on delayed phase. It is assumed to be benign, but more than half the time, this lesion is associated with extrahepatic malignancy, especially colonic cancer and small metastatic adenocarcinoma; this justifies resection (Deniz & Coban, 2010).
Lymphangioma
Lymphangiomas are benign neoplasms located within the liver parenchyma, regarded as malformations that arise from sequestrations of lymphatic tissue that fail to communicate with the normal lymphatic system during embryogenesis (Weiss et al, 2008). Lymphangiomas are frequently found in the neonatal period and childhood but rarely in adulthood. The occurrence of a solitary lymphangioma in the liver is extremely rare, and very few reported cases—fewer than 10—have been described in the literature (Matsumoto et al, 2010). Contrary to neonatal (Shahi et al, 2009) or childhood cases with symptomatic, large tumors, lymphangiomas are generally small (<4 cm) and asymptomatic in adults. On imaging, this lesion has a solid and cystic component and can contain small peripheral calcifications; histologically, it is composed of vascular lymphatic spaces lined by regular cells. Immunohistochemistry using D2-40, LYVE-1, and Prox-1 are helpful to establish the diagnosis of lymphangioma, as they are able to discriminate between lymphatic and vascular endothelium (Matsumoto et al, 2010).
Leiomyoma
Leiomyomas are very rare, benign liver tumors developed from the smooth muscles of the vessels or bile ducts (Belli et al, 2001; Urizono et al, 2006) with lesions of variable size. It can be observed in patients with immunosuppression or AIDS (Prévot et al, 1994; Sclabas et al, 2002). On imaging, lesions appear hypoechoic on US and hypoattenuating on CT scan with variable enhancement after injection of contrast material. On MR imaging, leiomyoma is hypointense on T1-weighted sequences and strongly hyperintense on T2-weighted sequences (Santos et al, 2010). Histologic features include spindle-cell proliferation without nuclear atypia, hemorrhage, and necrosis. Immunoreactivity with mesenchymal markers shows positivity of the cell proliferation with vimentin and smooth muscular actin. Because radiologic findings are nonspecific, liver resection is usually recommended.
Heterotopic Tissue
The presence of heterotopic tissue within the liver has been described, and diagnosis is usually established after surgical excision (see Chapter 48). The most frequent is liver splenosis, which corresponds to autotransplantation of splenic tissue; it occurs after traumatic splenic rupture or, less frequently, after surgical rupture during splenectomy (Davidson & Reid, 1997; Labat-Debelleix et al, 2008). Splenosis is asymptomatic, most often localized in the left liver, with a mean size of approximately 4 cm. On imaging, the lesion shows features of a hypervascular tumor but with less enhancement on the delayed phase. These radiologic features make the differential diagnosis with another hypervascular liver tumor very difficult, and this can be misinterpreted in a standard preoperative evaluation as HCC or breast metastatic disease (d’Angelica et al, 1998; Foroudi et al, 1999; Labat-Debelleix et al, 2008; Abu Hilal et al, 2009). Diagnosis can be suspected with scintigraphy using technetium (Tc) 99m–labeled heat-denatured red blood, and it can be established with liver biopsy (Lu et al, 2008; Labat-Debelleix et al, 2008). Other heterotopic tissues include adrenal and pancreatic tissues located around the bile ducts (Suzuki et al, 1999; Heer et al, 2010).
Hepatic Pseudolesions
1) Perfusion disorders: These anomalies are the result of hyperarterialization of a liver segment as a result of decreased or absent portal vein flow. These anomalies are seen on CT after injection of contrast; they predominate on arterial or portal phase images and disappear or attenuate on the delayed phase. These perfusion disorders result from portal obstruction, compression, or arterioportal shunt (Tamura et al, 1997). In general, they have typical straight borders that correspond to segmental or subsegmental locations. The diagnosis can be more difficult in patients with small peripheral or central “pseudonodular” lesions, such as in cirrhotic patients as a result of arterioportal shunts. In patients with hepatic vein obstruction, these abnormalities are not systematized (mosaic form) because of the rapid development of interhepatic venous shunts (Vilgrain et al, 2007).
2) Localized parenchymal compression: This can display aspects of pseudolesion because of impaired enhancement in the compressed territory in the portal phase but without hyperarterialization on the arterial phase. In this case, the pseudolesion has no vascular topography and can be exacerbated by deep inspiration with compression by the ribs and diaphragm (Vilgrain et al, 2007).
3) Confluent fibrosis: This lesion can also mimic a tumor, and it is mainly observed in chronic liver disease; it corresponds to parenchymal extinction and is located in segments IV and VIII with capsular retraction (Vilgrain et al, 2007).
4) Radiation: Pseudolesion of liver parenchyma can be observed after radiation exposure. Previous history of radiation therapy and the appearance of straight borders without anatomic distribution are highly suggestive of this etiology. As a result of associated fibrosis, atrophy of the involved segment can be observed with compensatory hypertrophy (Vilgrain et al, 2007).
Peliosis
Peliosis is a rare condition characterized by multiple, small, blood-filled pools in liver parenchyma that vary from 1 mm to several centimeters without lobular systematization. This lesion results from focal rupture of sinusoidal walls and is mostly observed in adult patients after systemic chemotherapy for colorectal liver metastases using oxaliplatin (Rubbia-Brandt et al, 2010). It can also be associated with androgenic anabolic steroids (Tsirigotis et al, 2007), OC use (Gutiérrez Santiago et al, 2007), HIV infection (Radin & Kanel, 1991), or severe tuberculosis and malignancies such as Hodgkin’s lymphoma (Kleger et al, 2009).
Imaging findings often mimic those of true tumors, because lesions are well limited and strongly hyperintense on T2- weighted MR images, and they enhance with arterial phase CT or MR. The persistent contrast uptake within the lesion is due to the blood-pooled condition (Verswijvel et al, 2003). This lesion can lead to extensive fibrosis resulting from parenchymal destruction and collapse (Cavalcanti et al, 1994), which is revealed on histology.
In general, this lesion is asymptomatic, but severe complications such as liver rupture, subsequent bleeding (Choi et al, 2009; Wang et al, 2001; Fidelman et al, 2002), and hepatopulmonary syndrome have been reported (Kallel et al, 2008). Treatment includes withdrawal of the possible causative agents, which can lead to regression of the disease.
Focal Fatty Changes
Hepatic steatosis is generally a diffuse process; however, heterogeneous focal fat distribution seen as spared liver areas, without steatosis, or areas that are fattier than the rest of liver parenchyma may also be present (Wanless, 2002). Although the pathogenesis is not well understood, regional hypoxia of hepatic tissue is thought to play a role (Zeppa et al, 2002). The majority of patients have underlying disease such as diabetes, obesity, hepatitis C, nonalcoholic steatohepatitis, and malnutrition; lesions are often discovered incidentally on imaging studies. Focal steatosis is mainly localized in segment IV, around the gallbladder and the round ligament (Vilgrain et al, 2007). A nodular aspect can mimic liver metastasis (Tom, et al 2004; Rubaltelli et al, 2002).
Regenerative Processes
Nodular Regenerative Hyperplasia
Nodular regenerative hyperplasia (NRH) is a relatively rare, benign, diffuse micronodular transformation of the liver that has been referred to by many names in the literature, including nodular transformation, noncirrhotic nodulation, and partial nodular transformation. It is a distinct disease entity characterized by diffuse involvement of the liver with nodules composed of hyperplastic hepatocytes, and it should not be confused with the regenerative nodules of cirrhosis or FNH (Federle & Brancatelli, 2001).
The pathogenesis of NRH is not well known, but one of the proposed theories hypothesizes that a primary vascular process leads to obliteration of portal vein, which in turn induces ischemia, atrophy of hepatocytes in the central zone, and the proliferation of hepatocytes (Al-Mukhaizeem et al, 2004). The other theory proposes that a preneoplastic process leads to NRH in association with the reported high prevalence of hepatocyte dysplasia (20% to 42%) and HCC formed in livers of patients with NRH (Nzeako et al, 1996; Dogan et al, 2003). The prevalence of NRH was reported to be around 2% (Biecker et al, 2003).
NRH was classically associated with a wide spectrum of systemic diseases, processes, and drugs; these include myeloproliferative and lymphoproliferative disorders, chronic vascular disorders, rheumatologic and collagen vascular diseases (rheumatoid arthritis, Felty syndrome, polyarteritis nodosa, amyloidosis, and primary biliary cirrhosis) (Morris et al, 2010), primary hypogammaglobulinemia (Malamut et al, 2008), HIV infection (Tateo et al, 2008; Bihl et al 2010), hereditary hemorrhagic telangiectasia (Brenard et al, 2010), hepatoportal sclerosis, congenital absence of the portal vein (Peker et al, 2009), portal veinopathy (Hillaire et al, 2002), malignant disease (Al-Hamoudi et al, 2009), and after solid organ transplantation (Devarbhavi et al, 2007). NRH is also associated with the use of drugs such as steroids (Mortelé & Ros, 2002), azathioprine for inflammatory bowel disease (Vernier-Massouille et al, 2007; Seksik et al, 2010), and chemotherapeutic agents. This lesion is considered the most severe induced histologic liver lesion in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases, with an incidence up to 24% (Hubert et al, 2007; Wicherts et al, 2010; Rubbia-Brandt et al, 2010); it can be associated with high postoperative morbidity in cases of major liver resection (see Chapter 65).
Clinically, NRH usually causes no symptoms, and lesions are discovered incidentally, mainly on liver biopsy. More than two thirds of reported patients had abnormal liver test results, but liver function was preserved (Arvanitaki & Adler, 2001; Morris et al, 2010); the risk of liver insufficiency leading to liver transplantation is rare (Tateo et al, 2008). The main clinical presentation is portal hypertension, observed in 30% of patients, with subsequent esophageal varices, splenomegaly, and ascites (Arvanitaki & Adler, 2001; Dogan et al, 2003; Morris et al, 2010). Fatal bleeding from varices has been observed (Morris et al, 2010), but the risk of transformation to HCC is rare (Nzeako et al, 1996; Kataoka et al, 2006). Because of these clinical features, the diagnosis of NRH should be considered in patients with clinical signs of portal hypertension and normal or mildly abnormal liver function test results, in whom other causes of portal hypertension have been excluded.
Imaging findings are not specific for NRH. On imaging, three presentations are possible: first, liver imaging is unremarkable; second, imaging shows signs of noncirrhotic portal hypertension without any liver nodules, because the nodules are typically very small; third, NRH appears as multiple liver nodules with or without portal hypertension. In most cases, imaging shows normal hepatic parenchyma. When nodules are seen, they have a variable enhancement, and they are hyperintense on T1-weighted images and isointense or hypointense to normal liver on T2-weighted images (Clouet et al, 1999; Mortelé & Ros, 2002; Casillas et al, 1997; Horita et al, 2002). Contrary to FNH, the central scar is lacking. Differences between these two lesions are mainly based on the presence of multiple small nodules, signs of portal hypertension, and clinical context. Accurate diagnosis should be confirmed with histologic examination prior to treatment. In highly suspicious cases, percutaneous needle biopsy can establish the diagnosis (Hubert et al, 2007). In doubtful cases with negative percutaneous biopsy, open biopsy is indicated to obtain an adequate sample of hepatic tissue, because percutaneous needle biopsies may give falsely normal results (Biecker et al, 2003).
Management depends on the clinical symptoms. In asymptomatic patients, no treatment is recommended. The effect of the withdrawal or specific treatment of the possible causative disease or agents on the course of this entity is not well known. In patients with complications of portal hypertension, appropriate management includes drug therapy, endoscopic therapy, transjugular intrahepatic shunt, or portacaval shunt if necessary (Biecker et al, 2003). Rarely, patients may progress to liver failure and may ultimately require liver transplantation (Trotter & Everson, 2001; Tateo, 2008). Because of the high postoperative morbidity with major liver resection in cases of NRH associated with oxaliplatin-based chemotherapy (Wicherts et al, 2010), it is recommended in this group of patients, who underwent many cycles of preoperative chemotherapy, to perform a liver biopsy—either systematically or in case of portal hypertension—to search for NRH. If NRH is diagnosed, major liver resection should be avoided or prepared for by portal vein embolization.
FNH-like Lesions
FNH-like lesions histopathologically resemble FNH but are seen in patients with liver disease or liver vessel abnormalities. The most common causes of liver diseases responsible for the development of FNH-like lesions are Budd-Chiari syndrome (Cazals-Hatem et al, 2003), hereditary hemorrhagic telangiectasia (Gincul et al, 2008), and congenital hepatic fibrosis (Vilgrain et al, 1999; Zeitoun et al, 2004; Buscarini et al, 2004). All these diseases induce severe liver flow alterations with decreased portal vein blood flow and marked increased hepatic artery blood flow (Kim et al, 2004; Bureau et al, 2004). FNH-like lesions may be a hepatic response to increased arterial inflow. Similarly, FNH-like lesions have been reported in patients with cavernous transformation of the portal vein (Bureau et al, 2004); imaging findings of FNH-like lesions are similar to the FNH that arises on normal liver, and they may contain a central scar. Because the liver signal is abnormal in Budd-Chiari syndrome, the FNH-like lesions are mostly hyperintense on T1- and hypointense on T2-weighted images; however, they still enhance strongly at the arterial phase. Unlike most FNH, they may become more numerous and grow over time.
Abbey-Toby A, et al. Pseudotumeur inflammatoire du foie: le diagnostic pré-opératoire est-il possible? Gastroenterol Clin Biol. 2003;27:883-890.
Abu Hilal M, et al. Hepatic splenosis mimicking HCC in a patient with hepatitis C liver cirrhosis and mildly raised alphafetoprotein: the important role of explorative laparoscopy. World J Surg Oncol. 2009;5:7. 1
Al-Hamoudi WK, et al. Hepatic nodular regenerative hyperplasia in a patient with advanced carcinoid tumor. Eur J Gastroenterol Hepatol. 2009;21:1083-1085.
Allaire GS, et al. Bile duct adenoma: a study of 152 cases. Am J Surg Pathol. 1988;12:708-715.
Al-Mukhaizeem KA, et al. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol. 2004;75:225-230.
Amesur N, et al. Management of unresectable symptomatic focal nodular hyperplasia with arterial embolization. J Vasc Interv Radiol. 2009;20:543-547.
Anthony PP. Inflammatory pseudotumour (plasma cell granuloma) of the lung, liver and other organs. Histopathology. 1993;23:501-503.
Arblade S, et al. Angiomyolipome hépatique simulant une tumeur hépatocytaire. Gastroenterol Clin Biol. 1996;20:1022-1026.
Arvanitaki M, Adler M. Nodular regenerative hyperplasia of the liver: a review of 14 cases. Hepatogastroenterology. 2001;48:1425-1429.
Attal P, et al. Telangiectatic focal nodular hyperplasia: US, CT, and MR imaging findings with histopathologic correlation in 13 cases. Radiology. 2003;228:465-472.
Atwell TD, et al. Successful treatment of hepatocellular adenoma with percutaneous radiofrequency ablation. AJR Am J Roentgenol. 2005;184:828-831.
Ba-Ssalamah A, et al. Atypical focal nodular hyperplasia of the liver: imaging features of nonspecific and liver-specific MR contrast agents. AJR Am J Roentgenol. 2002;179:1447-1456.
Barthelemes L, Tait IS. Liver cell adenomas and liver cell adenomatosis. HBP (Oxford). 2005;7:186-196.
Belghiti J, et al. Resection of presumed benign liver tumours. Br J Surg. 1993;80:380-383.
Belli G, et al. Hepatic resection for primary giant leiomyoma of the liver. HPB (Oxford). 2001;3:11-12.
Biecker E, et al. Benign hepatic tumours. Z Gastroenterol. 2003;41:191-200.
Bihl F, et al. Anticoagulant therapy for nodular regenerative hyperplasia in an HIV-infected patient. BMC Gastroenterol. 2010;10:6.
Bioulac-Sage P, et al. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology. 2005;128:1211-1218.
Bioulac-Sage P, et al. Hepatocellular adenoma: what is new in 2008. Hepatol Int. 2008;2:316-321.
Bioulac-Sage P, et al. Over-expression of glutamine synthetase in focal nodular hyperplasia: a novel easy diagnostic tool in surgical pathology. Liver Int. 2009;29:459-465.
Bioulac-Sage P, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481-489.
Bornman PC, et al. Giant hepatic hemangiomas: diagnostic and therapeutic dilemmas. Surgery. 1987;101:445-449.
Brancatelli G, et al. Focal nodular hyperplasia: CT findings with emphasis on multiphasic helical CT in 78 patients. Radiology. 2001;219:61-68.
Brenard R, et al. Large spectrum of liver vascular lesions including high prevalence of focal nodular hyperplasia in patients with hereditary haemorrhagic telangiectasia: the Belgian Registry based on 30 patients. Eur J Gastroenterol Hepatol. 2010;22:1253-1259.
Browers MAM, et al. Surgical treatment of giant haemangioma of the liver. Br J Surg. 1997;84:314-316.
Buetow PC, et al. Focal nodular hyperplasia of the liver: radiologic-pathologic correlation. Radiographics. 1996;16:369-388.
Bureau C, et al. Liver nodules resembling focal nodular hyperplasia after portal vein thrombosis. J Hepatology. 2004;41:499-500.
Buscarini E, et al. High prevalence of hepatic focal nodular hyperplasia in subjects with hereditary hemorrhagic telangiectasia. Ultrasound Med Biol. 2004;30:1089-1097.
Caldironi MW, et al. Echo-guided fine-needle biopsy for the diagnosis of hepatic angioma: a report on 114 cases. Minerva Chir. 1998;53:505-509.
Carlson SK, et al. CT of focal nodular hyperplasia of the liver. AJR Am J Roentgenol. 2000;174:705-712.
Caseiro-Alves F, et al. Calcification in focal nodular hyperplasia: a new problem for differentiation from fibrolamellar hepatocellular carcinoma. Radiology. 1996;198:889-892.
Casillas C, et al. Pseudotumoral presentation of nodular regenerative hyperplasia of the liver: imaging in five patients including MR imaging. Eur Radiol. 1997;7:654-658.
Caturelli E, et al. Hemangioma-like lesions in chronic liver disease: diagnostic evaluation in patients. Radiology. 2001;220:337-342.
Cavalcanti R, et al. Impact and evolution of peliosis hepatis in renal transplant recipients. Transplantation. 1994;58:315-316.
Cazals-Hatem D, et al. Arterial and portal circulation and parenchymal changes in Budd-Chiari syndrome: a study in 17 explanted livers. Hepatology. 2003;37:510-519.
Choi SK, et al. Spontaneous liver rupture in a patient with peliosis hepatis: a case report. World J Gastroenterol. 2009;15:5493-5497.
Chiche L, et al. Liver adenomatosis: reappraisal, diagnosis and surgical management: eight new cases and review of the literature. Ann Surg. 2000;231:74-81.
Chirica M, et al. Major hepatectomy for peripheral papillary cholangiocarcinoma with hilar extension in a patient with situs ambiguous. Gastroenterol Clin Biol. 2005;29:456-460.
Clouet M, et al. Imaging features of nodular regenerative hyperplasia of the liver mimicking hepatic metastases. Abdom Imaging. 1999;24:258-261.
Clouston AD, et al. Parasitic origin of a solitary necrotic nodule of the liver. J Clin Pathol. 1993;46:578.
Cobey FC, Salem RR. A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg. 2004;187:181-191.
Concejero AM, et al. Giant cavernous hemangioma of the liver with coagulopathy: adult Kasabach-Merritt syndrome. Surgery. 2009;145:245-247.
Corigliano N, et al. Hemoperitoneum from a spontaneous rupture of a giant hemangioma of the liver: report of a case. Surg Today. 2003;33:459-463.
Coumbaras M, et al. CT and MR imaging features of pathologically proven atypical giant hemangioma of the liver. AJR Am J Roentgenol. 2002;179:1457-1463.
Dalle I, et al. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443-450.
Danet IM, et al. Giant hemangioma of the liver: MR imaging characteristics in 24 patients. Magn Reson Imaging. 2003;21:95-101.
D’Angelica M, et al. Isolated hepatic splenosis: first reported case. HPB Surg. 1998;11:39-42.
Davidson LA, Reid IN. Intrahepatic splenic tissue. J Clin Pathol. 1997;50:532-533.
Delis SG, et al. Solitary necrotic nodule of the liver mimicking hepatocellular carcinoma: a case report. Cases J. 2009;25:85.
De Luca M, et al. Solitary necrotic nodule of the liver misinterpreted as malignant lesion: considerations on two cases. J Surg Oncol. 2000;74:219-222.
De Rave S, Hussain SM. A liver tumour as an incidental finding: differential diagnosis and treatment. Scand J Gastroenterol. 2002;Suppl:81-86.
Deneve JL, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16:640-648.
Deng FT, et al. Hilar inflammatory pseudotumor mimicking hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2010;9:219-221.
Deniz K, Coban C. Solitary necrotic nodule of the liver: always benign? J Gastrointest Surg. 2010;14:536-540.
Descottes B, et al. Laparoscopic liver resection of benign liver tumors. Surg Endosc. 2003;17:23-30.
Devarbhavi H, et al. Significance of nodular regenerative hyperplasia occurring de novo following liver transplantation. Liver Transpl. 2007;13:1552-1556.
Dietrich CF, et al. Contrast-enhanced endoscopic ultrasound with low mechanical index: a new technique. Z Gastroenterol. 2005;43:1219-1223.
Dogan E, et al. Nodular regenerative hyperplasia of the liver: a case report. Turk J Gastroenterol. 2003;14:64-67.
Dokmak S, et al. A single center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698-1705.
Duxbury MS, Garden OJ. Giant haemangioma of the liver: observation or resection? Dig Surg. 2010;27:7-11.
Ercolani G, et al. Liver transplantation for benign hepatic tumors: a systematic review. Dig Surg. 2010;27:68-75.
Erdogan D, et al. Management of liver hemangiomas according to size and symptoms. J Gastroenterol Hepatol. 2007;22:1953-1958.
Fabre A, et al. Histological scoring of liver biopsy in focal nodular hyperplasia with atypical presentation. Hepatology. 2002;35:414-420.
Farges O, et al. Cavernous hemangiomas of the liver: are there any indications for resection? World J Surg. 1995;19:19-24.
Farges O, et al. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85-89.
Federle MP, Brancatelli G. Imaging of benign hepatic masses. Semin Liver Dis. 2001;21:237-249.
Ferraz AA, et al. Liver transplant for the treatment of giant hepatic hemangioma. Liver Transpl. 2004;10:1436-1437.
Fidelman N, et al. SCVIR 2002 Film Panel case 4: massive intraperitoneal hemorrhage caused by peliosis hepatis. J Vasc Interv Radiol. 2002;13:542-545.
Fléjou JF, et al. Liver adenomatosis: an entity distinct from liver adenoma? Gastroenterology. 1985;89:1132-1138.
Foroudi F, et al. splenosis mimicking metastases from breast carcinoma. Clin Oncol (R Coll Radiol). 1999;11:190-192.
Freeny PC, Marks WM. Hepatic hemangioma: dynamic bolus CT. AJR Am J Roentgenol. 1986;147:711-719.
Fu XH, et al. Enucleation of liver hemangiomas: is there a difference in surgical outcomes for centrally or peripherally located lesions? Am J Surg. 2009;198:184-187.
Gaffey MJ, et al. Clonal analysis of focal nodular hyperplasia of the liver. Am J Pathol. 1996;148:1089-1096.
Gaspar L, et al. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol. 1993;29:45-50.
Gedaly R, et al. Cavernous hemangioma of the liver: anatomic resection vs. enucleation. Arch Surg. 1999;134:407-411.
Gincul R, et al. Evaluation of previously nonscreened hereditary hemorrhagic telangiectasia patients shows frequent liver involvement and early cardiac consequences. Hepatology. 2008;48:1570-1576.
Goldsmith PJ, et al. Inflammatory pseudotumours of the liver: a spectrum of presentation and management options. Eur J Surg Oncol. 2009;35:1295-1298.
Gordon SC, et al. Resolution of a contraceptive-steroid–induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Intern Med. 1986;105:547-549.
Grazioli L, et al. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology. 2005;236:166-177.
Guiu B, et al. Multiple biliary hamartomas mimicking diffuse liver metastases. Dig Surg. 2009;26:209.
Gutiérrez Santiago M, et al. Hepatic lesions and prolonged use of oral contraceptive. Rev Clin Esp. 2007;207:257-258.
Hall GW. Kasabach-Merritt syndrome: pathogenesis and management. Br J Haematol. 2001;112:851-862.
Hanafusa K, et al. Hepatic hemangioma: findings with two-phase CT. Radiology. 1995;196:465-469.
Hedayati P, et al. Treatment of symptomatic focal nodular hyperplasia with percutaneous radiofrequency ablation. J Vasc Interv Radiol. 2010;21:582-585.
Heer C, et al. Heterotopic pancreatic tissue in the bifurcation of the bile duct: rare diagnosis mimicking a Klatskin tumour. Chirurg. 2010;81:151-154.
Heilo A, Stenwig AE. Liver hemangioma: US-guided 18-gauge core-needle biopsy. Radiology. 1997;204:719-722.
Herman P, et al. Management of hepatic hemangiomas: a 14-year experience. J Gastrointest Surg. 2005;9:853-859.
Hillaire S, et al. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51:275-280.
Hobbs KE. Hepatic hemangiomas. World J Surg. 1990;14:468-471.
Hoffman A, et al. Hepatic angiomyolipoma: two cases reports of caudate-based lesions and review of the literature. Liver Transpl Surg. 1997;3:46-53.
Hooper LD, Mergo PJ, Ros PR. Multiple hepatorenal angiomyolipomas: diagnosis with fat suppression, gadolinium-enhanced MRI. Abdom Imaging. 1994;19:549-551.
Horita T, et al. Significance of magnetic resonance imaging in the diagnosis of nodular regenerative hyperplasia of the liver complicated with systemic lupus erythematosus: a case report and review of the literature. Lupus. 2002;11:193-196.
Hornick JL, et al. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381-389.
Horton KM, et al. CT and MR imaging of benign hepatic and biliary tumors. Radiographics. 1999;19:431-451.
Hubert C, et al. Nodular regenerative hyperplasia: a deleterious consequence of chemotherapy for colorectal liver metastases? Liver Int. 2007;27:938-943.
International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983-993.
Ishak KG. Hepatic neoplasms associated with contraceptive and anabolic steroids: carcinogenesis hormones. In: Lingerman, CH, editor. Recent Results in Cancer Research. New York: Springer-Verlag; 1979:72-128.
Ishak KG, Rabin L. Benign tumors of the liver. Med Clin North Am. 1975;59:995-1013.
Itai Y, et al. Non-invasive diagnosis of small cavernous hemangioma of the liver: advantage of MRI. AJR Am J Roentgenol. 1985;145:1195-1199.
Jover Díaz F, et al. Multiple inflammatory liver pseudotumors and Enterococcus durans bacteremia. Gastroenterol Hepatol. 2009;32:97-100.
Jovine E, et al. Intrahepatic rupture of a caudate lobe adenoma in liver adenomatosis. J Hepatobiliary Pancreat Surg. 2004;11:324-329.
Jiang WS, et al. Laparoscope hepatectomy for hepatic hemangioma: a report of 18 cases. Zhonghua Wai Ke Za Zhi. 2007;45:1311-1313.
Kallel L, et al. Idiopathic peliosis hepatis complicated by a hepatopulmonary syndrome. Gastroenterol Clin Biol. 2008;32:88-90.
Kataoka TR, et al. Concomitant hepatocellular carcinoma and non-Hodgkin’s lymphoma in a patient with nodular regenerative hyperplasia. Pathol Int. 2006;56:279-282.
Kamisawa T, Okamota A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948-3955.
Karahasanoglu T, et al. Laparoscopic enucleation of giant liver hemangioma. Surg Endosc. 2001;15:1489.
Keegan MT, et al. Liver transplantation for massive hepatic haemangiomatosis causing restrictive lung disease. Br J Anaesth. 2001;86:431-434.
Kehagias D, et al. Focal nodular hyperplasia: imaging findings. Eur Radiol. 2001;11:202-212.
Kim MJ, et al. Evaluation of hepatic focal nodular hyperplasia with contrast-enhanced gray scale harmonic sonography: initial experience. J Ultrasound Med. 2004;23:297-305.
Kim T, et al. Discrimination of small hepatic hemangiomas from hypervascular malignant tumors smaller than 3 cm with three-phase helical CT. Radiology. 2001;219:699-706.
Kim T, et al. Hepatic nodular lesions associated with abnormal development of the portal vein. AJR Am J Roentgenol. 2004;183:1333-1338.
Kim TK, et al. Focal nodular hyperplasia and hepatic adenoma: differentiation with low-mechanical-index contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:58-66. 2008
Kim YS, et al. Imaging findings of intrahepatic bile duct adenoma (peribiliary gland hamartoma): a case report and literature review. Korean J Radiol. 2010;11:560-565.
Kleger A, et al. First reported case of disease: peliosis hepatis as cardinal symptom of Hodgkin’s lymphoma. Oncologist. 2009;14:1088-1094.
Kobayashi S, et al. Two cases of hepatocellular adenomatosis treated with transcatheter arterial embolization. Hepatol Int. 2009;3:416-420.
Koea JB, et al. Inflammatory pseudotumor of the liver: demographics, diagnosis, and the case for nonoperative management. J Am Coll Surg. 2003;196:226-235.
Kuo YH, et al. Natural course of hepatic focal nodular hyperplasia: a long-terme follow-up study with sonography. J Clin Ultrasound. 2009;37:132-137.
Kuo YT, et al. Imaging diagnosis of cavernous hemangioma of the rib: one case report and review of the literature. Gaoxiong Yi Xue Ke Xue Za Zhi. 1994;10:469-473.
Labat-Debelleix V, et al. Hepatic splenosis: a rare etiology of hepatic nodules. Gastroenterol Clin Biol. 2008;32:83-87.
Laumonier H, et al. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808-818.
Laurent C, et al. Association of adenoma and focal nodular hyperplasia: experience of a single French academic center. Comp Hepatol. 2003;2:6.
Levy S, et al. Spontaneous regression of an inflammatory pseudotumour of the liver presenting as an obstructing malignant biliary tumour. Gastrointest Endosc. 2001;53:371-374.
Lewin M, et al. Liver adenomatosis: classification and MR imaging features and comparison with pathologic findings. Radiology. 2006;241:433-440.
Li GH, et al. Inflammatory pseudotumor of the liver. J Surg Oncol. 1989;42:244-248.
Locke JE, et al. Inflammatory pseudotumor of the liver. J Hepatobiliary Pancreat Surg. 2005;12:314-316.
Lopez-Tomassetti Fernandez EM, et al. Recurrence of inflammatory pseudotumor in the distal bile duct: lessons learned from a single case and reported cases. World J Gastroenterol. 2006;12:3938-3943.
Losanoff JE, Millis JM. Liver hemangioma complicated by ostructive jaundice. Am J Surg. 2008;196:e3-44.
Lu HC, et al. Hepatic splenosis diagnosed by spleen scintigraphy. Am J Gastroenterol. 2008;103:1842-1844.
Makhlouf HR, et al. Diagnosis of focal nodular hyperplasia of the liver by needle biopsy. Hum Pathol. 2005;36:1210-1216.
Malamut G, et al. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol. 2008;48:74-82.
Marini P, et al. Management of spontaneous rupture of liver tumours. Dig Surg. 2002;19:109-113.
Marsh JI, et al. Hepatic hemangioma in the presence of fatty infiltration: an atypical sonographic appearance. Gastrointest Radiol. 1989;14:262-264.
Mathieu D, et al. Association of focal nodular hyperplasia and hepatic hemangioma. Gastroenterology. 1989;97:154-157.
Mathieu D, et al. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology. 2000;118:560-564.
Matsumoto T, et al. Solitary hepatic lymphangioma: report of a case. Surg Today. 2010;40:883-889.
Maze GL, et al. Inflammatory pseudotumor of the liver and pregnancy. Am J Gastroenterol. 1999;94:529-530.
Mergo PJ, Ros PR. Benign lesions of the liver. Radiol Clin North Am. 1998;36:319-331.
Milias K, et al. Inflammatory pseudotumors of the liver: experience of a specialist surgical unit. J Gastroenterol Hepatol. 2009;24:1562-1566.
Morris JM, et al. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010;22:1001-1005.
Mortelé KJ, Ros PR. Benign liver neoplasms. Clin Liver Dis. 2002;6:119-145.
Mortelé KJ, et al. CT and MR imaging findings in focal nodular hyperplasia of the liver: radiologic-pathologic correlation. Am J Roentgenol. 2000;175:687-692.
Moser T, et al. Delayed enhancement pattern in a localized fibrous tumor of the liver. AJR Am J Roentgenol. 2005;184:1578-1580.
Nakamura N, et al. A hepatic lipoma mimicking angiomyolipoma of the liver: report of a case. Surg Today. 2009;39:825-828.
Neri S, et al. Biliary hamartomas (Von Meyenburg complex): magnetic resonance imaging in a case report. Intern Med J. 2004;34:71-72.
Nghiem HV, et al. Cavernous hemangiomas of the liver: enlargement over time. AJR Am J Roentgenol. 1997;169:137-140.
Nguyen BN, et al. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999;23:1441-1454.
Nino-Murcia M, et al. Focal liver lesions: pattern-based classification scheme for enhancement at arterial phase CT. Radiology. 2000;215:746-751.
Nzeako UC, et al. Hepatocellular carcinoma and nodular regenerative hyperplasia: possible pathogenetic relationship. Am J Gastroenterol. 1996;91:879-884.
Paradis V, et al. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X chromosome inactivation. Hepatology. 1997;26:891-895.
Paradis V, et al. Telangiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology. 2004;126:1323-1329.
Pateron D, et al. Giant hemangioma of the liver with pain, fever, and abnormal liver tests: report of two cases. Dig Dis Sci. 1991;36:524-527.
Peker A, et al. Congenital absence of portal vein associated with nodular regenerative hyperplasia of the liver and pulmonary hypertension. Clin Imaging. 2009;33:322-325.
Plackett TP, Lin-Hurtubise KM. Hepatic hemangiomas and parachuting. Aviat Space Environ Med. 2008;79:986-988.
Pol B, et al. Inflammatory process complicating giant hemangioma of the liver: a report of three cases. Liver Transplant Surg. 1998;4:204-207.
Prévot S, et al. Detection of Epstein Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1–infected patient. Virchows Arch. 1994;425:321-325.
Quaia E, et al. Characterization of liver hemangiomas with pulse inversion harmonic imaging. Eur Radiol. 2002;12:537-544.
Radin DR, Kanel GC. Peliosis hepatis in a patient with human immunodeficiency virus infection. AJR Am J Roentgenol. 1991;156:91-92.
Reading NG, et al. Hepatic haemangioma: a critical review of diagnosis and management. Q J Med. 1988;67:431-445.
Rebouissou S, Bioulac-Sage P, Zucman-Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol. 2008;48:163-170.
Ribeiro A, et al. Management of liver adenomatosis: results with a conservative surgical approach. Liver Transpl Surg. 1998;4:388-398.
Rosenberg L. The risk of liver neoplasia in relation to combined oral contraceptive use. Contraception. 1991;43:643-652.
Rouquie D, et al. Malignant-like angiomyolipoma of the liver: report of one case and review of the literature. Ann Chir. 2006;131:338-341.
Rubaltelli L, et al. Targetlike appearance of pseudotumors in segment IV of the liver on sonography. AJR Am J Roentgenol. 2002;178:75-77.
Rubbia-Brandt L, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430-439.
Ruppert-Kohlmayr AJ, et al. Focal nodular hyperplasia and hepatocellular adenoma of the liver: differenciation with multiphasic helical CT. AJR Am J Roentgenol. 2001;176:1493-1498.
Santos I, et al. Primary hepatic leiomyoma: case report. Abdom Imaging. 2011;36:315-317.
Sawai J, et al. Angiomyolipoma of the liver: case report and collective review of cases diagnosed from fine needle aspiration biopsy specimens. J Hepatobiliary Pancreat Surg. 1998;5:333-338.
Sclabas GM, et al. Case report: hepatic leiomyoma in a renal transplant recipient. Transplant Proc. 2002;34:3200-3202.
Seksik P, et al. Incidence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with azathioprine. Inflamm Bowel Dis. 2010;17:565-572.
Semelka RC, Sofka CM. Hepatic hemangiomas. Magn Reson Imaging Clin N Am. 1997;5:241-253.
Semelka RC, et al. Hepatic hemangiomas: a multi-institutional study of appearance on T2-weighted and serial gadolinium-enhanced gradient-echo MR images. Radiology. 1994;192:401-406.
Shahi KS, et al. Solitary hepatic lymphangioma in a 22-day-old infant. J Pediatr Surg. 2009;44:E9-E11.
Shamsi K, et al. Focal nodular hyperplasia of the liver: radiologic findings. Abdom Imaging. 1993;18:32-38.
Shek TWH, et al. Intra-abdominal follicular dentritic cell tumour: a rare tumour in need of recognition. Histopathology. 1998;33:465.
Socas L, et al. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39:e27.
Soussan M, et al. Incidental focal solid liver lesions: diagnostic performance of contrast-enhanced ultrasound and MR imaging. Europ Radiol. 2010;20:1715-1725.
Stanley G, et al. CT findings and histopathology of intratumoral steatosis in focal nodular hyperplasia: case report and review of the literature. J Comput Assist Tomogr. 2002;26:815-817.
Starck DD, et al. Magnetic resonance imaging of cavernous hemangioma of the liver: tissue-specific characterization. AJR Am J Roentgenol. 1985;145:213-222.
Stoot JH, et al. Life-saving therapy for haemorrhaging liver adenomas using selective arterial embolization. Br J Surg. 2007;94:1249-1253.
Stoot JH, et al. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12:509-522.
Suzuki K, et al. Heterotopic pancreatic tissue associated with intra- and extrahepatic choledochal cyst. Pathol Int. 1999;49:759-762.
Takayasu K, et al. Computed tomography of a rapidly growing hepatic hemangioma. J Comput Assist Tomogr. 1990;14:143-145.
Tamura S, et al. Pseudo lesions on CTAP secondary to arterio-portal shunts. Clin Imaging. 1997;21:359-365.
Tapetes K, et al. Orthotopic liver transplantation for benign hepatic neoplasms. Arch Surg. 1995;130:153-156.
Tateo M, et al. A new indication for liver transplantation: nodular regenerative hyperplasia in human immunodeficiency virus–infected patients. Liver Transpl. 2008;14:1194-1198.
Terada T. Hepatic multiple minute inflammatory pseudotumors containing many biliary elements and hepatolithiasis: a suggestion of biliary origin. Scand J Gastroenterol. 2010;45:633-634.
Terkivatan T, Ijzermans JN. Hepatocellular adenoma: should phenotypic classification direct management? Nat Rev Gastroenterol Hepatol. 2009;6:697-698.
Terkivatan T, et al. Treatment of ruptured hepatocellular adenoma. Br J Surg. 2001;88:207-209.
Thomé-Noun C, et al. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. 2008;18:493-499.
Tom WW, et al. Hepatic pseudotumor due to nodular fatty sparing: the diagnosis role of opposed-phase MRI. AJR Am J Roentgenol. 2004;183:721-724.
Trotter JF, Everson GT. Benign focal lesions of the liver. Clin Liver Dis. 2001;5:17-42.
Tsirigotis P, et al. Peliosis hepatis following treatment with androgen-steroids in patients with bone marrow failure syndromes. Haematologica. 2007;92:e106-e110.
Tsui WM, et al. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol. 1999;23:34-48.
Uchida K, et al. Inflammatory pseudotumors of the pancreas and liver with infiltration of IgG4-positive plasma cells. Intern Med. 2007;17:1409-1412.
Uggowitzer M, et al. Power Doppler imaging and evaluation of the resistive index in focal nodular hyperplasia of the liver. Abdom Imaging. 1997;22:268-273.
Urizono Y, et al. Primary leiomyoma of the liver: report of a case. Surg Today. 2006;36:629-632.
Venkataraman S, et al. Inflammatory myofibroblastic tumor of the hepatobiliary system: report of MR imaging appearance in four patients. Radiology. 2003;227:758-763.
Vernier-Massouille G, et al. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut. 2007;56:1404-1409.
Verswijvel G, et al. Peliosis hepatis presenting as a multifocal hepatic pseudotumor: MR findings in two cases. Eur Radiol. 2003;13:40-44.
Vilgrain V. Focal nodular hyperplasia. Eur J Radiol. 2006;58:236-245.
Vilgrain V, et al. Focal nodular hyperplasia of the liver: MR imaging and pathologic correlation in 37 patients. Radiology. 1992;184:1-6.
Vilgrain V, et al. Hepatic nodules in Budd-Chiari syndrome: imaging features. Radiology. 1999;210:443-450.
Vilgrain V, et al. Prevalence of hepatic hemangioma in patients with focal nodular hyperplasia: MR imaging analysis. Radiology. 2003;229:75-79.
Vilgrain V, et al. Liver imaging: pitfalls, pseudolesions and pseudotumors. J Radiol. 2007;88:1104-1120.
Wang SY, et al. Hepatic rupture caused by peliosis hepatis. J Pediatr Surg. 2001;36:1456-1459.
Wang Z, et al. Imaging features of hepatic angiomyolipomas on real-time contrast-enhanced ultrasound. Br J Radiol. 2010;83:411-418.
Wanless IR. Benign liver tumors. Clin Liver Dis. 2002;6:513-552.
Wanless IR, et al. On the pathogenesis of focal nodular hyperplasia. Hepatology. 1985;5:1194-1200.
Watkins J, et al. Hepatocellular adenoma in advanced-stage fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:932-936.
Weimann A, et al. Critical issues in the diagnosis and treatment of hepatocellular adenoma. HPB (Oxford). 2000;2:25-32.
Weiss SW, et al. Benign tumors and tumorlike lesions of blood vessels: tumors of lymph vessels. In: Weiss, SW, Goldblum, JR, Enzinger, FM. Soft Tissue Tumors. 5th ed. Philadelphia: Elsevier Health Sciences; 2008:636-664. 734-743
Wicherts DA, et al. Regenerative nodular hyperplasia of the liver related to chemotherapy: impact on outcome of liver surgery for colorectal metastases. Ann Surg Oncol. 2010;18:659-669. Epub Oct 26 2010
Wittekind C. Hepatocellular carcinoma and cholangiocarcinoma. In: Hermanek, P, et al. Prognostic Factors in Cancer. Berlin: Springer; 1995:88-93.
Yan F, et al. Hepatic angiomyolipoma: various appearances on two-phase contrast scanning of spiral CT. Eur J Radiol. 2002;41:12-18.
Yang CY, et al. Management of hepatic angiomyolipoma. J Gastrointest Surg. 2007;11:452-457.
Yoon SS, et al. Diagnosis, management and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg. 2003;197:392-402.
Zamir D, et al. Inflammatory pseudotumor of the liver: a rare entity and a diagnostic challenge. Am J Gastroenterol. 1998;93:1538-1540.
Zeitoun D, et al. Congenital hepatic fibrosis: CT findings in 18 adults. Radiology. 2004;231:109-116.
Zeng JP, et al. Hepatic angiomyolipoma: a clinical experience in diagnosis and treatment. Dig Dis Sci. 2010;55:3235-3240. Epub Feb 18 2010
Zeppa P, et al. Fine needle aspiration biopsy of hepatic focal fatty change: a report of two cases. Acta Cytol. 2002;46:567-570.
Zhou YM, et al. Clinical features of solitary necrotic nodule of the liver. Hepatobiliary Pancreat Dis Int. 2008;7:485-489.
Zucman-Rossi J, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524.