Chapter 64 Benign Diseases
Definition and Classification of Benign Diseases
Many diseases that are pathologically benign (nonmalignant) but not clinically benign can be successfully treated with ionizing radiation. The use of irradiation for painful musculoskeletal diseases has a long tradition in Europe.1 Sokoloff reported positive results in radiotherapy of painful “rheumatoid diseases” as early as 1898.2 The traditional classification of benign diseases amenable to radiotherapy as inflammatory, degenerative, hyperproliferative, functional, and other types of disorders is currently outdated. Worldwide, irradiation of benign diseases has become more important, although indications and treatment concepts have changed considerably and there are clear differences between countries owing to clinical traditions and differences in organization and training.*
Long-Term Risk for Tumor Induction
Considering international data about the emergence of tumors and leukemias after whole-body exposure to ionizing radiation (United Nations Scientific Committee on the Effects of Atomic Radiation [UNSCEAR], Biological Effects of Ionizing Radiation [BEIR]), the risk for tumor induction can be calculated on a gender- and age-related basis.9,10 The average lifetime risk for exposure to radiation is lower in men (9.5%) than in women (11.5%). Table 64-1 summarizes the age- and gender-specific risks for tumor induction.
TABLE 64-1 Tumor Induction Depending on Age and Gender: Relative Lifetime Risk
| Age-group | Men (%/Sv) | Women (%/Sv) |
|---|---|---|
| ≤10 yr | 25.0-26.0 | 32.0-33.0 |
| 11-20 yr | 15.0 | 19.0 |
| 21-30 yr | 13.0-14.0 | 17.0 |
| 31-40 yr | 7.0 | 8.0 |
| 41-50 yr | 5.0 | 6.0 |
| 51-60 yr | 4.5 | 5.0 |
| 61-70 yr | 3.5 | 4.5 |
| 71-80 yr | 2.5 | 3.0 |
| >80 yr | 1.0 | 1.5 |
From Jansen JTM, Broerse J, Zotelief J, et al: Assessment of carcinogenic risk in the treatment of benign disease of knee and shoulder joint. In Seegenschmiedt MH, Makoski H-B, editors: Kolloquium Radioonkologie/Strahlentherapie, Radiotherapie bei gutartigen Erkrankungen, Vol 15, Altenberge, 2001, Diplodocus Verlag, pp 13-15.
Principles of Irradiation of Benign Diseases
The principles of irradiation of benign diseases can be summarized in 10 statements (Table 64-2). These should be carefully considered for each patient in whom irradiation is being evaluated.
Data from Seegenschmiedt MH, Katalinic A, Makoski H, et al: Radiation therapy for benign diseases. Patterns of care study in Germany, Int J Radiat Oncol Biol Phys 47:195-202, 2000; Micke O, Seegenschmiedt MH: The German Working Group guidelines for radiation therapy of benign diseases. A multicenter approach in Germany, Int J Radiat Oncol Biol Phys 52:496-513, 2002.
Radiobiologic Aspects
Reactions in Connective Tissue
Several mechanisms are triggered by ionizing radiation in connective tissues. Following any trauma or acute or chronic inflammation, several cell systems regulate the repair process where fibroblasts play a central role, particularly during the reparative phase, which is characterized by high cell production and stimulation of specific growth factors. Furthermore, ionizing radiation also has a pivotal influence on cellular differentiation.11,12
Reactions in the Vascular System
The endothelial cells of the capillaries and the larger arterial and venous blood vessels are the origins of various cytokine-mediated cellular reactions and possess a high proliferative potential. Intercellular adhesion molecule 1 (ICAM-1), a mediator of the leukocyte-endothelial interaction, is induced by low radiation doses.13 Similarly, selectins mediate the penetration of mononuclear blood cells into the interstitial tissue. Endothelial prostaglandin release is also modulated by ionizing radiation.14 Cellular and membrane functions can be modified when they are exposed to radiation.
Mechanism of Action
To know individual target cells and potential pathogenic mechanisms of the various benign diseases also means to coordinate the radiation therapy concepts accordingly and consistently. The dose concepts applied in benign diseases differ greatly from each other due to other potential mechanisms of action (Table 64-3).
| Mechanisms of Action | Single Dose (Gy) | Total Dose (Gy) |
|---|---|---|
| Cellular gene and protein expression (e.g., eczemas) | <2 | <2 |
| Inhibition of inflammation in lymphocytes (e.g., in pseudotumor orbitae) | 0.3-1 | 2-6 |
| Inhibition of fibroblast proliferation (e.g., in keloids) | 1.5-3 | 8-12 |
| Inhibition of proliferation in benign tumors (e.g., in desmoids) | 1.8-3 | 45-60 |
Benign Disorders of the Head and Neck and Central Nervous System
Pituitary adenoma, meningioma, vestibular schwannoma, and chordoma are important benign CNS tumors treated with irradiation. These are covered fully in Chapters 17, 26, 27, and 28 and will not be discussed here.
Craniopharyngioma
Definition and Clinical Features
Craniopharyngiomas are rare dysontogenetic midline tumors originating from Rathke’s pocket or the ductus craniopharyngicus. They make up 6% to 10% of CNS tumors in children and appear mostly between ages 5 and 15 years. They are located near the sella, with a close connection to the pituitary gland, hypothalamus, chiasma opticum, and visual nerves. Intrasellar tumors are rare; some are also located suprasellarly or intrasellarly. Main symptoms are visual impairment or loss of vision, visual field impairment (bitemporal hemianopia), and endocrine dysfunctions such as dwarfism, fat tissue disturbance, or adrenal cortex insufficiency. Signs of intracranial pressure can appear as well. Diagnosis is made from skull radiographs (sella expansion), computed tomography (CT) (typical calcifications), and magnetic resonance imaging (MRI) (cystic or mixed solid and cystic tumor components).15
Surgical Treatment
Primary therapy consists of complete resection, which is equivalent to permanent cure. Ten-year control rates after complete tumor resection are 60% to 93%.16,17 Because radical neurosurgical procedures can result in relatively high postoperative toxicities, such as visual impairment (20%) and panhypopituitarism (≤95%),16 less radical surgery plus adjuvant three-dimensional conformal RT (3DCRT) is the preferred treatment in many patients.
Irradiation Options
In primary inoperable tumors or after subtotal resection, RT is indicated because otherwise the local progression rate after 2 to 3 years is 70% to 90%.18 After subtotal resection alone, the local recurrence rate is 30% or more; when subtotal resection is combined with postoperative RT, the rate is reduced to 5% to 15% after 5 to 20 years.18–20 Long-term local control after primary RT or after subtotal resection or cyst punctuation plus adjuvant RT, respectively, with a total dose of 50 to 54 Gy (1.8 to 2 Gy per fraction) is comparable to those of complete resection.21,22
Stereotactic RT places less stress on normal tissue. Due to the proximity of the tumor to the chiasm and visual nerves, fractionated stereotactic RT (FSRT) is preferred to single-dose irradiation. Using FSRT, a local 10-year control rate of 100% was reached in Heidelberg.23 With the use of MRI follow-up imaging, complete remission was noted in 4 of 26 patients and partial remission in 14; 8 had a stable MRI finding. In five patients, visual improvement was reached. The hypophyseal hormonal situation deteriorated in seven patients. There were no radionecroses or secondary malignant tumors, and visual deterioration did not occur.
After conformal RT, the rate of visual deterioration is up to 10%. Severe side effects such as radionecroses, cognitive changes, and secondary malignant diseases occur with an incidence of less than 2%.14
Arteriovenous Malformation
Definition and Clinical Features
Intracranial AVMs are rare vascular abnormalities consisting of widened arteries with connections to the normal capillary bed; this enables oxygenated blood to enter directly into the venous system. About 80% of AVMs are located supratentorially. The incidence of AVM is unknown. Its prevalence is below 0.01% (≈18 : 100,000) in the Western hemisphere. Most AVMs are discovered at the age of 20 to 40 years. AVM can extend to aneurysms and rupture (2% to 5% per year).24 Neurologic symptoms characterize the clinical course (headaches, hemorrhage, cramp attacks), which may culminate in sudden death through bleeding. Diagnosis is made with special imaging techniques (angiography, MRI).
Untreated AVMs have a bleeding risk of 2% to 4% per year, which increases after a rupture. Large AVMs with deep arterial feeders or those located at the basal ganglia or thalamus (9%) have an increased bleeding risk.25 Lethality after the first bleeding episode occurs in up to 30%; 10% to 20% of survivors have long-term neurologic defects. Spontaneous regression is very rare.
Irradiation Options
AVMs are irradiated with SRT/SRS with a linear accelerator or Gamma Knife* (see Chapter 17). Fractionated RT with total doses of up to 60 Gy produced inadequate results.28–31 Depending on the size and location of the AVM, a single dose of 15 to 30 Gy is required in the periphery of the nidus. If the therapy is successful, complete obliteration of the nidus will occur within a few years. However, the bleeding risk continues to exist during the interval between SRT/SRS and complete obliteration. The obliteration rate after SRT or SRS is 65% to 95%† (Table 64-4).
TABLE 64-4 Arteriovenous Malformations: Obliteration Rate and Rate of Radiation Side Effects after Radiosurgery
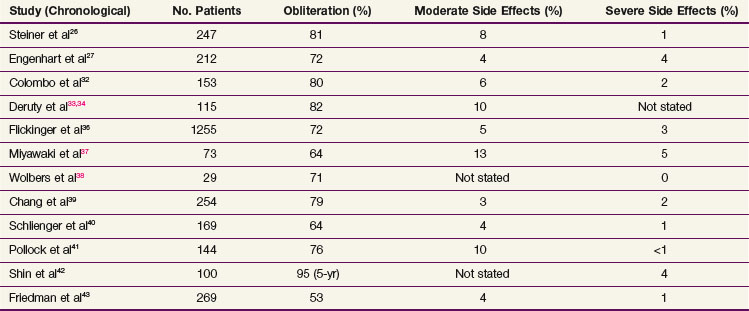
The side effects of SRT/SRS are mostly chronic and follow the time course of AVM obliteration: focal radionecroses or leukoencephalopathies occur 9 to 36 months after SRT,48,49 but they may also appear after several weeks.50 The risk correlates strongly with the irradiated brain volume and the total dose51–53: the brain volume irradiated with more than 10 Gy is an important predictive factor.54,55
Glomus Tumor or Chemodectoma
Definition and Clinical Features
About 50% of tumors are located near the skull base in the jugular fossa. The age peak is 45 years. The tumors are usually unilateral; only 10% to 20% are bilateral or multiple.56 They grow slowly, rarely have endocrinologic activity, and degenerate into malignant forms in 5% to 10% of patients. They can also infiltrate bone, vessels, the middle ear, and cranial nerves (CN). The main symptoms are headaches, CN failure (CN V to XII), dysphagia, pulsatile tinnitus, vertigo, and hypacusis. Without therapy, there is the risk for CN injury; the swelling can be so extreme as to be life-threatening. The diagnosis is made clinically and with high-resolution CT and MRI.
Irradiation Options
Irradiation of paragangliomas produces control rates as good as or even better than surgery.* Even in large, diffusely growing or multiple tumors, RT produces a local control rate of 88% to 93%.57–61 Kim and associates57 noticed a recurrence rate of 22% with doses of less than 40 Gy, whereas recurrences occurred in only 1.4% with doses of more than 40 Gy. Frequently, tumor rests are detectable on imaging for several years. Therapeutic success is usually assessed in terms of the regression of CN failures and the lack of tumor progression. A dose of 45 to 50 Gy does not complicate surgery that might become necessary later.
During the past decade, stereotactic single-dose RT and Gamma Knife therapy have been used for the treatment of paragangliomas.62–64 Although the observation time after surgery is short, the results are very favorable.
Irradiation of paragangliomas of the carotid glomus can acutely cause pharyngeal mucositis and chronically may lead to skin fibrosis and dryness of the pharyngeal mucosa on the irradiated side. Irradiation of jugular or tympanic paragangliomas can lead to acute skin reactions in the external acoustic canal; tube ventilation dysfunction, reduced sound conduction, and salivary retention may occur in the middle ear. Long-term sequelae are rare.58–60,61
Juvenile Nasopharyngeal Fibroma
Definition and Clinical Features
Intracranial spread occurs in about 25% of cases. Typical symptoms are epistaxis and impaired nose breathing. Depending on the pattern of spread, facial swelling as well as orbital (e.g., blindness) and intracranial symptoms (e.g., CN failure) may occur. A biopsy can cause massive bleeding so that histologic confirmation of diagnosis is often not performed. The presence of hormone receptors shows the influence of androgynous hormones. Spontaneous remission after puberty is possible, but therapy can hardly be delayed when the symptoms increase and when complications are threatening.65
Surgical Treatment
In JNF, the main emphasis is placed on surgery combined with embolization to decrease the size of the tumor.66–68 Small tumors that are restricted to the posterior nasal cavity and the nasopharynx can be completely removed after embolization. A JNF with lateral spread is also an indication for surgery. Through surgery, the local control rates for most JNFs without intracranial spread range up to 100% with minimal toxicity.66,67
Irradiation Options
Radiotherapy is a very effective treatment for JNF.* In locally advanced disease, complete resection is often not possible, and tumors with intracranial spread should receive primary irradiation. Other indications for 3DCRT are tumor rests, inoperability, or local recurrence after initial surgical resection. With modern CT-based treatment planning, high control rates are achieved in locally advanced JNF as well. FSRT is often recommended.68
Total doses of 30 to 55 Gy (1.8 to 2 Gy per fraction) are said to be effective,69 but for large tumors, doses of 40 to 46 Gy are currently recommended.70 With conventional fractionated RT, control rates of 80% to 100% can be reached.* Remission of JNFs after RT often requires several months71; sometimes, complete remission, as detected by imaging techniques, does not occur even after years, although there is no further growth.
Benign Disorders of the Eye and Orbit
Macular degeneration and endocrine orbitopathy (Graves’ disease) are important benign diseases of the eye or orbit that are treated with irradiation. They are discussed in detail in Chapter 29 and will not be covered here.
Pterygium
Definition and Clinical Features
Pterygium is a wing-shaped fibrovascular proliferating tissue originating at the lens epithelium at the border between the conjunctiva and the cornea. It normally extends from the medial (nasal) corner of the eye to the cornea and beyond. The highest incidence occurs in hot, dusty, dry, and sun-exposed regions (desert belts); in such areas, even people in their 20s and 30s are affected.76,77 Typical symptoms are the sensation of having a foreign body in the eye and tearing; motility problems are sometimes present. If the cornea is affected, vision may be impaired.
Surgical Treatment
Therapy is indicated if vision is threatened by the pterygium growing toward the pupil and if aesthetics are subjectively affected. Complete surgical excision is the therapy of choice; the several alternatives include open-wound defect (bare sclera technique), primary conjunctival occlusion, rotation flap, keratoplasties, and free transplant. The local control rate is 50% to 70%.76
In patients with local recurrence, additional treatments are indicated postoperatively. In those cases, local cytostatics (e.g., mitomycin C) are administered, which may lead to severe local complications such as scleral ulceration, secondary glaucoma, corneal edema, corneal perforation, iritis, or cataracts.78–80
Irradiation Options
Radiotherapy is indicated after local resection of a recurrent pterygium, but some centers also report success with primary and/or preoperative RT of the pterygium.81 Besides rare orthovoltage therapy,82 brachytherapy with beta radiators and eye applicators is usually employed.* Normally, radionuclide strontium-90, a fission product of uranium-235 (half-life period, 28 years), which decays to yttrium-90 (half-life period, 64 days) is used. Strontium-90 radiation has a maximum energy of 0.546 MeV; for yttrium-90 it reaches 2.27 MeV.86 The eye applicators have an effective diameter of 8 to 12 mm. The affected lesion is generously covered by the applicator for a certain time; if lesions are very large, they are treated with a circular motion toward the corneal limbus.87
Most clinical studies have used postoperative RT for recurrence prophylaxis with subsequent relapse rates of 1.5% to 11%.† Van den Brenk83 observed only 1.4% recurrences in 1300 treated pterygia (1064 patients); irradiation was carried out once a week (days 0, 7, and 14 postoperatively). Paryani and others87 achieved a recurrence rate of only 1.7% in 825 eyes with 60 Gy in six fractions of 10 Gy (once a week). Wilder and associates76 report a recurrence rate of more than 11% in 244 eyes after 24 Gy in three fractions of 8 Gy (once a week). In comparison to placebo irradiation, a Dutch double-blind randomized study with one fraction of 25 Gy showed significantly lower recurrence rates (local relapse in the irradiation arm in only 3 of 44 tumors and in the placebo arm in 28 of 42 tumors).88
Radiogenic consequences such as severe scleromalacia and corneal ulcerations occur in up to 4% to 5% of cases after application of higher total doses and after single-dose RT of 20 to 22 Gy.84,85
Choroidal Hemangioma
Definition and Clinical Features
Choroid membrane hemangiomas are slowly growing benign tumors originating from the vessels of the choroid. They can also occur in congenital Sturge-Weber syndrome. The diffuse type (ages 5 to 10 years) and the local type (ages 30 to 50 years) can be distinguished.94 Symptoms are determined by the size and location of the tumor. If the hemangioma is located close to the papilla or macula, “fuzzy or blurred vision,” metamorphopsia, and secondary retinal detachment are observed. In case of direct macular involvement, chronic glaucoma frequently develops. Sometimes there is complete loss of vision. On ophthalmoscopic examination, hemangiomas appear as red-orange swelling with concomitant clinical phenomena (e.g., glaucoma, retinal detachment). Further diagnostic procedures are ultrasound, fluorescence angiography, CT, MRI, and scintigraphy (phosphorus-32).95
Nonradiotherapeutic Treatment
Indications for therapy depend on the progression of the lesion and the severity of symptoms (visual impairment, retinal detachment, or secondary glaucoma). Small lesions outside the field of central vision are treated with photodynamic therapy, photocoagulation, or transpapillary thermotherapy (e.g., to prevent retinal detachment).96,97 Lesions near the macula or papilla are not coagulated because there is a risk for central scotoma; the same holds for incomplete retinal detachment and for the diffuse type (Sturge-Weber syndrome). Overall, ophthalmologists currently favor photodynamic therapy.
Irradiation Options
Irradiation can be done with photons, protons, or brachytherapy. It is indicated in cases of no response to photocoagulation and particularly in cases of critical proximity to the macula or papilla because invasive measures threaten vision.98 After successful irradiation, the retina reattaches partially or, perhaps, completely; the lesion becomes flatter, the eye and vision are maintained, and visual acuity is often improved. Reduction of visual acuity affects almost exclusively eyes with existing location-dependent maculopathy. The earlier RT starts, the better the long-term results.99,100
Schilling and associates101 irradiated 36 localized and 15 diffuse hemangiomas with 20 Gy in ten fractions of 2 Gy. After 5 years, 23 eyes (64%) of the localized type achieved complete retinal reattachment; visual acuity was stable in 50% and improved in 50%. Favorable results were also achieved for the diffuse type. In locally advanced cases, irradiation of the hemangioma cannot conserve visual acuity but it can often maintain the eye as a whole.
Brachytherapy is carried out in localized hemangiomas with eye plaques; iodine-125 seeds are preferred. Doses from the apex to the base of the lesion vary between 30 and 240 Gy. Results are excellent in the sense of a permanent resorption of the subretinal edema, complete retinal attachment, and maintenance of vision.100,102–104
Reactive Lymphoid Hyperplasia or Pseudotumor Orbitae
Definition and Clinical Features
Lymphoid diseases of the orbit are rare and have a broad range, including pseudotumor orbitae (PO) and malignant lymphomas.105 There are three possible causes: (1) an infectious process, for example, in transmitted sinusitis; (2) an autoimmune process; or (3) a fibroproliferative process. Experience shows that corticosteroids or immune suppressants can cause remission.
In differential diagnosis, other causes of orbital space requirement such as granulomatous diseases (e.g., sarcoidosis, Wegener’s granulomatosis), local infections, or autoimmune diseases have to be excluded. Frequently, the acute onset of symptoms, unilateral disease, and impaired eye motility point to pseudotumor. On imaging, the infiltrates appear in retrobulbar adipose tissue (≤80%), enlarged eye muscles (≤60%), thickening of the optic nerve (≤40%), and proptosis (≤70%). On the basis of clinical diagnosis and diagnostic imaging, it is hard to differentiate between benign and malignant changes; therefore, biopsy is essential.106
Nonradiotherapeutic Treatment
Surgical excision can be used in accessible lesions, but recurrences are frequent.107 Corticosteroids are the most important component of medical therapy, but up to 50% do not respond adequately.108 Some patients have to discontinue medication because of side effects.109 Without therapy, visual acuity can deteriorate seriously and permanently. There is no correlation between duration of progression and irreversible loss of visual acuity. The potential for malignant transformation of orbital pseudotumors is unclear.
Irradiation Options
As noted by Lambo and coworkers, radiotherapy has response rates of 70% to 100%.105,106,109–112 Recommended doses vary between 0.5 and 3 Gy per fraction and total doses of 20 to 35 Gy. Careful treatment planning helps to keep radiation side effects low.
Initially, a treatment attempt with a low dose of two fractions of 0.5 Gy per week up to a total dose of 5 Gy (first series) can be used. In acute or chronic inflammation, a gradual dose increase can initiate an early response on the one hand112; in case of nonresponse after 4 weeks, irradiation is changed to daily fractionation with 1.5 to 2 Gy as a single dose and up to 30 to 40 Gy as a total dose (second series). In the United States, most patients are treated with initial doses of approximately 30 Gy at standard fractionation.
Irradiation Technique
After CT planning, patients are treated via anterior and lateral fields with 1 : 3 weighting and wedged filters for dose homogenization while the patient’s eyes are open. In bilateral disease, parallel opposing lateral fields with a half-block technique107 or two anterior electron fields are used.
Benign Diseases of Joints and Tendons
Irradiation Options
In Germany, guidelines and dose concepts for radiotherapy of benign diseases were developed during the past 10 years where a dose per fraction of 0.5 Gy to a total dose of 6 Gy can be used.5,6,8 Conditions that have been treated include bursitis, tendonitis, subacromial syndrome (rotator cuff syndrome), tennis elbow (epicondylopathia humeri), calcaneodynia (heel spur), and degenerative joints with cartilaginous destruction (osteoarthritis).
Benign Diseases of Connective Tissue and Skin
Desmoid (Aggressive Fibromatosis)
Definition and Clinical Features
Desmoids are differentiated into extra-abdominal (≈70%) and intra-abdominal (≈10%) desmoids and those located in the abdominal wall (≈20%). Extra-abdominal forms tend to recur locally. Intra-abdominal forms are associated with the autosomal dominantly inherited Gardner syndrome. Histologically, desmoids are similar to low-grade (G1), highly differentiated fibrosarcomas. Mitotic activity is low, and cellular atypias are rare. Locally infiltrating growth has earned the name of “aggressive fibromatosis” for this disease. Local recurrences after resection alone are quite common.113–115 Pretreatment imaging with MRI is used to estimate the size and infiltration into other organs and incision biopsies are performed to distinguish benign from malignant lesions.
Nonradiotherapeutic Treatment
Desmoids can regress spontaneously or they can grow to a huge size, but they rarely cause death.116 Surgery with a safety margin of 2 to 5 cm is considered the “gold standard.” After R0 resection, no adjuvant therapy is usually required. After R1 resection, treatment options include observation if the lesion is in a site where re-resection is feasible; if not, postoperative RT is reasonable. Good long-term control can be achieved by resection alone, but up to 50% of patients develop local recurrence, which requires surgical and other measures subsequently.117 Tamoxifen and progesterone can exert growth inhibitory effects.118 Nonsteroidal antirheumatics, vitamin C,119 and alkylating substances (vincristine, methotrexate) have been tested.
Irradiation Options
Radiotherapy is indicated in cases of local inoperability and after R2 resection and in R1 resection if repeated surgery would not be feasible or has already been performed for local recurrence.* Radiotherapy is often used adjuvantly or as primary treatment. Adjuvant radiotherapy significantly reduces local recurrence rates compared with surgery alone. With total RT doses of more than 50 Gy, the local recurrence rate decreases from 60% to 80% with surgery alone to 10% to 30% after adjuvant RT. With normal fractionation and single doses of 1.8 to 2 Gy, a total dose of 50 to 55 Gy is recommended postoperatively. For inoperable or recurring desmoids, the recommended total dose is 60 to 65 Gy. After primary radiotherapy, the local control rate does not differ a lot from that after adjuvant irradiation.†
Irradiation Results
In most studies, tumor size has no prognostic influence on local control rates.126 According to a meta-analysis (698 cases in 13 studies),120 the local control rate after R0 resection and radiotherapy was improved by 17% compared with that of surgery alone; for macroscopic (R2) and microscopic (R1) tumor residual, patients treated with adjuvant radiotherapy had even better results.
In 2001 to 2002, the patterns of care study on the use of radiotherapy for treatment of desmoids was carried out in Germany; 345 patients were subjected to evaluation.130 The desmoids were distributed in the extremities (81.2%; 280 tumors), the trunk (13.9%; 48 tumors), and the head and neck region (4.9%; 17 tumors). A total of 204 patients (59%) were irradiated for locally recurrent or unresectable desmoids: 141 (40.8%) for high-risk situations postoperatively, 44 for unclear resection status, 49 after R1 resection, and 28 after R2 resection. Most patients were intensively pretreated, on average with two (range, one to five) operations.
Peyronie’s Disease
Definition and Clinical Features
Peyronie’s disease is a chronic and mostly progressive inflammation and connective tissue excrescence of the tunica albuginea in the cavernous bodies of the penis.137 It usually affects men at the age of 40 to 60 years. Its cause is unknown. Strands of scar lead to the typical bending of the penis, which may cause severe pain during erection. Spontaneous remission is described only very rarely.
Nonradiotherapeutic Treatment
There is no simple and successful standard treatment. Vitamin E, para-aminobenzoate, and steroids are said to have a favorable influence during the early phase. There are also local therapeutic attempts with ultrasound or shock waves as well as with corticoid, procaine, and hyaluronic acid injections. Resection and plastic surgery, for example, after Nesbit,138 is associated with complications and is only carried out in locally advanced stages. After radical resection, inflatable implants are used to maintain erection ability.
Irradiation Options
Ionizing radiation can delay further induration and lead to softening of lumps and strands that cause pain, bending, and functional problems of the penis.* Radiotherapy can be used during the early stages of Peyronie’s disease, but in the later stages, there are hardly any radiosensitive fibroblasts and inflammatory cells left. Irradiation is carried out with gonadal protection (lead apron or capsule), and the glans penis is spared. The nonerect penis is pulled forward manually by the patient and is irradiated via a dorsal stationary field with orthovoltage or electrons up to 6 MeV with a 5- to 10-mm bolus.
Irradiation Results
Within 12 to 24 months, radiotherapy leads to an improvement of symptoms in two thirds of all early-stage patients. Local pain and associated clinical symptoms decrease in up to 75%. Angulation (25% to 30%) and dysfunction of the penis (30% to 50%) show less response because these symptoms often indicate that the disease is already in a more locally advanced stage.*
Dupuytren’s Contracture (Morbus Dupuytren) and Morbus Ledderhose
Irradiation Results
Many studies showed a very good response to radiotherapy in the form of stabilized disease (70% to 80%). Only a small number of early-stage patients, however, experience degeneration of lumps and strands (20% to 30%). Only a few studies have controlled long-term observation for more than 2 years.153,154
Keloids and Hypertrophic Scars
Irradiation Options
Indications for irradiation are either demonstrated recurrences postoperatively or a high recurrence risk (e.g., marginal resection borders, wider spread, unfavorable location).* Fibroblasts, mesenchymal cells, and inflammatory cells are the target cells. Prophylactic irradiation immediately after excision of the recurrence is most effective. The local recurrence rate after postoperative irradiation is 20% to 25%.
Irradiation is initiated 24 hours after surgery at the latest. Orthovoltage (70 to 150 kV), electrons (<6 MeV), and brachytherapy with iridium-192 implants155,156,162 or with strontium-90 dermaplate157 are used. The target volume is limited to the scar plus a 1-cm safety margin. The recommended dose is 12 to 25 Gy, typically with 3- to 4-Gy fractions.158,163,164,166 Single-dose irradiation with 7.5 to 10 Gy is effective.159,160
Other Diseases of Connective Tissue and Skin or Cutaneous Appendages
Gynecomastia
Recently, there has been an increased interest in the use of radiotherapy of the male mammary gland as a prophylactic measure for gynecomastia or the use of therapeutic irradiation as a treatment for painful gynecomastia in patients who are undergoing hormone therapy for prostate carcinoma. Bilateral radiotherapy of the mammary region is performed with 8- to 12-MeV electrons. Usually, four to five fractions of 3 Gy, up to a total dose of 12 to 15 Gy, is used. This treatment can prevent pain or further growth of the mammary gland in 70% of male patients.168,169 However, radiotherapy cannot reverse gynecomastia.
Benign Disorders of Bony Tissues
Aneurysmal Bone Cysts
Definition and Clinical Features
Aneurysmal bone cysts are benign, vascular cystic lesions in the metaphase of bones, which can cause functional impairment, pathologic fractures, and damage of neighboring structures. They can infiltrate the surrounding soft tissue. Despite their nonmalignant character, cysts can lead to bone destruction and thus lead to serious problems, which is why treatment is recommended once a cyst has been diagnosed, particularly if the vertebral column is affected.170
Surgical Treatment
Therapy is primarily surgical (resection or curettage) as long as this does not lead to a considerable functional impairment. Following curettage, cysts recur in up to 60% of patients.171 After complete resection, there is normally no recurrence.170
Irradiation Options
Radiotherapy can be used in patients with cysts that cannot be treated by surgery or if curettage is difficult due to the size or location of cysts. Cyst progression or repeated recurrences are also indications for radiotherapy. Because more than 50% of patients are 10 to 19 years old, radiation doses should be kept as low as possible. Nobler and associates172 reported one recurrence in a total of 11 patients who were irradiated with total doses of 12 to 31.6 Gy. A dose of 10 to 20 Gy in 1.8- to 2- Gy fractions over 1 to 2 weeks seems to be adequate to control aneurysmal bone cysts reliably.
Pigmented Villonodular Synovitis
Definition and Clinical Features
Pigmented villonodular synovitis (PVNS) is a rare proliferative disease affecting the synovia of joints and the tendon sheaths.173 There are two types of disease: the strictly localized and the diffuse involvement of synovial membranes.174 In most cases, the lesion is restricted to one joint and can spread to muscles, tendons, and skin.
Surgical Measures
Surgical excision normally consists of synovectomy, which is rarely complete, particularly in large joints such as the knee.175 Recurrence is seen in up to 45% of patients,176 therefore, and the addition of perioperative or postoperative irradiation is appropriate.*
Irradiation Options
O’Sullivan and co-workers174 report on 14 patients who were irradiated with 30 to 50 Gy in 15 to 35 fractions at Princess Margaret Hospital. The patients had different risk factors: microscopic residual tumor (7), macroscopic tumor (7), tumor more than 10 cm (5), tumor 5 to 10 cm (7), recurrences (8), and skin infiltration with ulceration (2). During an average observation period of 69 months (13 to 250 months), one patient had persistant tumor 8 months after radiotherapy with 30 Gy in 15 fractions. All but two patients had tumor control. On the basis of the Princess Margaret Hospital results, RT with a total dose of 40 Gy in 20 fractions has been recommended.
Other institutions have evaluated their results with adjuvant irradiation after surgical resection and have achieved similar results.* The German Cooperative Group on Radiotherapy in Benign Diseases (GCG-BD) conducted a pattern-of-care analysis of the results of postoperative RT for PVNS in 41 patients from 14 institutions.181 The RT dose ranged from 30 to 50 Gy (median, 36 Gy; median single dose, 2 Gy). Local control was achieved in 95.1% of patients.
Vertebral Hemangiomas
Definition and Clinical Features
Vertebral hemangiomas are benign lesions that can lead to a resorption of the affected bone.183–185 Normally, only one vertebral body is affected. Hemangiomas are usually diagnosed by their radiologic picture of rarefaction with vertical, dense trabeculas of a honeycomb pattern. Most lesions require no therapy. In most cases, symptoms occur during the fourth or fifth decade of life.186–189 Women are affected more frequently than men.190 Spread of the tumor into the extradural space, hemorrhage, or rare compression fractures can lead to bone marrow compression.
Surgical Treatment
Surgical relief can become necessary, but it is difficult owing to the danger of hemorrhage.191–194 In most cases, only partial resection is possible, and postoperative irradiation can be considered.183,184
Irradiation Options (see also Chapter 28)
Rades and associates195 analyzed data from 339 patients with symptomatic vertebral hemangiomas from publications of the past 50 years. There were 222 patients who had to be excluded, either because surgery was part of the treatment (98 patients) or because the data were incomplete (124 patients). Of the remaining 117 patients, 54 patients received 36 to 44 Gy (group A) and 62 patients 20 to 34 Gy (group B). After a median observation period of 36 months (range, 6 to 312 months), 39% of group A and 82% of group B patients had complete pain relief. The researchers recommend a total dose of 40 Gy.
The GCG-BD evaluated the results of RT for vertebral hemangiomas in 84 patients from seven German institutions.196 The RT dose ranged from 4.5 to 45 Gy (median, 34 Gy; median single dose, 2 Gy). The overall response (complete plus partial response) was 90.5%, with long-term local control of 80.9%.
Heterotopic Ossifications
Definition and Clinical Features
Heterotopic ossifications (HOs) appear following trauma or surgery of the hip (total hip replacement) in 10% to 80% of cases, and with varying degrees of severity. HOs consist of real bone and are located in the periarticular soft tissue.197 Ten percent develop extensive HO, causing pain and functional impairment. Patients with HO frequently complain of pain only a few days after surgery. Radiologic studies detect calcified structures with blurred contours 3 to 6 weeks postoperatively.
The etiology of HO is only partially known. It is assumed that the pluripotent mesenchymal cells, which are present ubiquitously in periarticular soft tissue, develop into osteoblastic stem cells under certain conditions.198
For all patients with an indication for a hip replacement, there should be an individual estimation of the risk of HO before carrying out surgery. Patients who already have ipsilateral or contralateral HO carry the greatest risk. After a second surgery, 90% to 100% of those patients redevelop HO.199–202 Patients with moderate or severe osteophytes at the femoral head and socket also have a high risk for HO, with an incidence of more than 50%.199,203,204 After acetabular fractures, HOs appear in 90% of hips. Fifty percent of those patients develop HO with clinical pathology.205,206 Ankylosing spondylitis and the (rare) idiopathic hyperostosis of the skeleton are other influencing factors.
Diagnosis and Classification
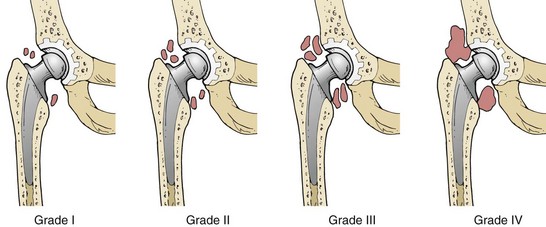
Extensive ossifications lead to mobility impairment of the hip joint and cause pain. If HO is suspected, radiographs of the hip should be taken. Discrete changes in the radiograph can be seen at 2 weeks after surgery at the earliest. The literature provides a multitude of staging approaches. The most frequent one is the classification of HO according to Brooker and colleagues197 (Fig. 64-1). To keep it simple, HO grades III and IV, according to Brooker and colleagues, are designated as severe or clinically relevant, although there may not be any pain or mobility impairment.
Nonradiotherapeutic Treatment
Medical Therapy
A number of medical options have been evaluated for the treatment of HO. Although ethane hydroxy-diphosphates (EHDPs) have been used as prophylaxis, the treatment results are not convincing.207–209 In contrast, some studies show that indomethacin, a prostaglandin synthesis inhibitor, is also effective in patients with high risk.210–215 Like indomethacin, ibuprofen inhibits prostaglandin synthesis. After treatment with indomethacin and ibuprofen, the incidence of HO was significantly lower than in the placebo group203,216; this was not the case after EHDP treatment.207,217
Irradiation Options
Radiotherapy for prophylaxis of HO has been employed since the late 1970s and has proved to be effective.* The initial dose was 20 Gy in 10 fractions.218–220,221,222,223 Multiple dose schedules have been used. Various authors pointed out that radiotherapy should be started no later than day 4 after surgery.†
In three randomized studies, a dose of 10 Gy was compared with 20 Gy,221 or with 17.5 Gy in five fractions,224 and a single fraction of 8 versus 10 Gy.225 There was no significant difference between the effectiveness of high and low doses. Severe ossifications occurred in 7% of patients with low and 5% of patients with high irradiation doses. No difference could be observed between fractionated and single-dose irradiation.
Preoperative radiation treatment with 7 to 8 Gy in one fraction was used successfully in high-risk patients.228 There was no significant difference in clinically relevant HO compared with patients who were irradiated postoperatively.
The relative roles of RT, indomethacin, and other nonsteroidal anti-inflammatory drugs (NSAIDs) for HO prophylaxis have been discussed by Pakos and others.237,238,239,240 In a 2004 meta-analysis of seven randomized trials by Pakos and colleagues,237 irradiation was demonstrated to be more effective than NSAIDs for HO prophylaxis in preventing grade III to IV ossification, but the absolute risk difference was only 1.2%. Pakos and associates239 subsequently reported a randomized trial in 96 patients comparing postoperative RT (7 Gy in a single fraction) plus indomethacin (first 15 days postoperatively) to indomethacin alone (same 15 days) for HO prophylaxis. Patients receiving the combined treatment of RT plus indomethacin had a lower rate of subsequent HO (4 versus 13 patients; p <.05).
Irradiation Technique and Procedures
The effect of radiotherapy on the ingrowth of bone and fixation of noncemented implants was investigated in dogs228 and rabbits.229 After irradiation with 10 Gy (in five or four fractions), the fixation was significantly decreased within 2 to 6 weeks.229,230 Sumner and associates231 were also able to show that irradiation initially decreases the grade of fixation during the early postoperative phase, but after 4 weeks, the implants in irradiated and nonirradiated bone had the same strength.
In clinical application, the protection of the hip prosthesis with absorbers as recommended by Jasty and co-workers232 and the use of smaller blocks restricted to acetabular and femoral parts of the prosthesis can lead to ossifications underneath the block. Inadequate irradiation fields led to HO in 13 of 18 hips (76%) after irradiation with 7 Gy.233 An open irradiation field covers the entire periarticular risk region more completely.
Nonfixation of cementless implants was not observed after 6 Gy in one fraction,234 after 7 Gy in one fraction,227 or after 17.5 Gy in five fractions.224,226,235 Because of the animal experimental and clinical studies, there appears to be no objection to irradiating hips with noncemented prostheses without an absorber.
5 Seegenschmiedt MH, Katalinic A, Makoski H, et al. Radiation therapy for benign diseases. Patterns of care study in Germany. Int J Radiat Oncol Biol Phys. 2000;47:195-202.
8 Seegenschmiedt MH, Makoski HB, Trott KR, et al. Radiotherapy for non-malignant Disorders. New York: Springer, Merlin Heidelberg; 2008.
23 Schulz-Ertner D, Frank C, Herfarth KK, et al. Fractionated stereotactic radiotherapy for craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2002;54:1114-1120.
26 Steiner L, Lindquist C, Adler JR, et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1-8.
27 Engenhart R, Wowra B, Debus J, et al. The role of high-dose, single-fraction irradiation in small and large intracranial AVMs. Int J Radiat Oncol Biol Phys. 1994;30:521-529.
32 Colombo F, Pozza F, Chierego G, et al. Linear accelerator radiosurgery of cerebral arteriovenous malformations. An update. Neurosurgery. 1994;34:14-21.
36 Flickinger JC, Pollock BE, Kondziolka D, et al. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
39 Chang JH, Chang JW, Park YG, Chung S. Factors related to complete occlusion of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 2000;93(Suppl 3):96-101.
40 Schlienger M, Atlan D, Lefkopoulos D, et al. Linac radiosurgery for cerebral arteriovenous malformations. Results in 169 patients. Int J Radiat Oncol Biol Phys. 2000;46:1135-1142.
41 Pollock BE, Kline RW, Stafford SL. The rationale and technique of staged-volume arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 2000;48:817-824.
42 Shin M, Kawamoto S, Kurita H, et al. Retrospective analysis of a 10-yr experience of stereotactic radiosurgery for arteriovenous malformations in children and adolescents. J Neurosurg. 2002;97:779-784.
43 Friedman WA, Bova FJ, Bollampally S, et al. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296-308.
57 Kim JA, Elkon D, Lim ML, Constable WC. Optimum dose of radiotherapy for chemodectomas in the middle ear. Int J Radiat Oncol Biol Phys. 1980;6:815ff.
61 Hinerman RW, Amdur RJ, Morris CG, et al. Definitive radiotherapy in the manangement of paragangliomas arising in the head and neck. A 35-year experience. Head Neck. 2008;30:1431.
70 McGahan RA, Durrance FY, Parke RBJr, et al. The treatment of advanced juvenile nasopharyngeal angiofibroma. Int J Radiat Oncol Biol Phys. 1989;17:1067-1072.
71 Reddy KA, Mendenhall WM, Amdur RJ, et al. Long-term results of radiation therapy for juvenile nasopharyngeal angiofibroma. Am J Otolaryngol. 2001;22:172-175.
83 Van den Brenk HA. Results of prophylactic postoperative irradiation on 1300 cases of pterygium. Am J Radiol. 1968;103:723-733.
87 Paryani SB, Scott WP, Wells JWJr, et al. Management of pterygium with surgery and radiation therapy. The North Florida Pterygium Study Group. Int J Radiat Oncol Biol Phys. 1994;28:101-103.
88 Jurgenliemk-Schulz IM, Hartman LJ, Tersteeg RJ, et al. Prevention of pterygium recurrence by postoperative single-dose beta-irradiation. A prospective randomized clinical double-blind trial. Int J Radiat Oncol Biol Phys. 2004;59:1138-1147.
90 Viani GA, Stefano EJ, De Fendi LL, Foseca EC. Long-term results and prognostic factors of fractionated strontium-90 eye applicator for pterygium. Int J Radiat Oncol Biol Phys. 2008;72:1174-1179.
100 Augsburger JJ, Freire J, Brady LW. Radiation therapy for choroidal and retinal hemangiomas. In: Wiegel T, Bornfeld N, Foerster MH, Hinkelbein W, editors. Radiotherapy of Ocular Disease, Vol 30. Basel: Karger; 1997:265-280.
101 Schilling H, Sauerwein W, Lommatzsch A, et al. Long-term results after low dose ocular irradiation for choroidal hemangiomas. Br J Ophthalmol. 1997;81:267-273.
106 Lambo MJ, Brady LW, Shields CL. Lymphoid tumors of the orbit. In: Alberti WE, Sagerman RH, editors. Radiotherapy of Intraocular and Orbital Tumors. Berlin: Springer (1. Auflage); 1993:205-216.
124 Ballo MT, Zagars GK, Pollack A. Desmoid tumor. Prognostic factors and outcome after surgery, radiation therapy or combined surgery and radiation therapy. J Clin Oncol. 1999;17:158-167.
128 Suit HD. Radiation dose and response of desmoid tumors. Int J Radiat Oncol Biol Phys. 1990;9:225-227.
130 Seegenschmiedt MH, Micke O, Willich N. Radiation therapy for nonmalignant diseases in Germany. Current concepts and future perspectives. GCG-BD]. Strahlenther Onkol. 2004;180:718-730.
133 Jabarri S, Andolino D, Weinberg V, et al. Successful treatment of high risk and recurrent pediatric desmoids using radiation as a component of multimodality therapy. Int J Radiat Oncol Biol Phys. 2009;75:177-182.
136 Roeder F, Timke C, Oertel S, et al. Intraoperative electron radiotherapy for the management of aggressive fibromatosis. Int J Radiat Oncol Biol Phys. 2010;76:1154-1160.
145 Mira JG, Chahbazian CM, del Regato JA. The value of radiotherapy for Peyronie’s disease. Presentation of 56 new case studies and review of the literature. Int J Radiat Oncol Biol Phys. 1989;6:161-166.
150 Incrocci L, Wijnmaalen A, Slob AK, et al. Low-dose radiotherapy in 179 patients with Peyronie’s disease. Treatment outcome and current sexual function. Int J Radiat Oncol Biol Phys. 2000;47:1353-1356.
151 Niewald M, Wenzlawowicz KV, Fleckenstein J, et al. Results of radiotherapy for Peyronie’s disease. Int J Radiat Oncol Biol Phys. 2006;64:258-262.
152 Incroni L, Hop WE, Seegenschmiedt HM. Radiotherapy for Peyronie’s disease. A European survey. Acta Oncol. 2008;47:110-112.
153 Keilholz L, Seegenschmiedt MH, Sauer R. Radiotherapy in early stage Dupuytren’s contracture. Initial and long-term results. Int J Radiat Oncol Biol Phys. 1996;36:891-897.
155 Escarmant P, Zimmermann S, Amar A, et al. The treatment of 783 keloid scars by Iridium 192 interstitial irradiation after surgical excision. Int J Radiat Oncol Biol Phys. 1993;26:245-251.
156 Guix B, Henriquez I, Andres A, et al. Treatment of keloids by high-dose-rate brachytherapy. A seven-year-study. Int J Radiat Oncol Biol Phys. 2001;50:167-172.
158 Micke O, Seegenschmiedt MH. The German Working Group guidelines for radiation therapy of benign diseases. A multicenter approach in Germany. Int J Radiat Oncol Biol Phys. 2002;52:496-513.
163 Ogawa R, Miyashita T, Hyakusoku H, et al. Postoperative radiation protocol for keloids and hypertrophic scars. Statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg. 2007;59:688.
164 Sakamoto T, Oya N, Shibuya K, et al. Dose-response relationship and dose optimization in radiotherapy of postoperative keloids. Radiother Oncol. 2009;91:271-276.
166 Flickinger JC. A radiobiological analysis of multicenter data for postoperative keloid radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1164-1170.
172 Nobler MP, Higinbotham ML, Phillips RF. The cure of aneurysmal bone cyst. Irradiation superior to surgery in analysis of 33 cases. Radiology. 1968;90:1185.
174 O’Sullivan B, Cummings B, Catton C, et al. Outcome following radiation treatment for high-risk pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys. 1995;32:777-786.
179 Horoschak M, Tran PT, Bachireddy P, et al. External beam radiation therapy enhances local control in pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys. 2009;75:183-187.
181 Heyd R, Micke O, Berger B, et al. Radiation therapy for treatment of pigmented villonodular synovitis. Results of a national patterns of care study. Int J Radiat Oncol Biol Phys. 2010;78:199-204.
195 Rades D, Bajrovic A, Alberti A, Rudat V. Is there a dose-effect relationship for the treatment of symptomatic vertebral hemangioma? Int J Radiat Oncol Biol Phys. 2002;55:178-181.
196 Heyd R, Seegenschmiedt MH, Rades D, et al. Radiotherapy for symptomatic vertebral hemangionas. Results of a multicenter study and literature review. Int J Radiat Oncol Biol Phys. 2010;77:217-225.
221 Anthony P, Keys H, McCollister-Evarts C, et al. Prevention of heterotopic bone formation with early postoperative irradiation in high risk patients undergoing total hip arthroplasty. Comparison of 10 Gy versus 20 Gy schedules. Int J Radiat Oncol Biol Phys. 1987;13:365-369.
224 Seegenschmiedt MH, Goldmann AR, Wölfel R, et al. Prevention of heterotopic ossification (HO) after total hip replacement. Randomized high versus low dose radiotherapy. Radiother Oncol. 1993;26:271-274.
225 Konski A, Pellegrini V, Poulter C, et al. Randomized trial comparing single dose versus fractionated irradiation for prevention of heterotopic bone. Int J Radiat Oncol Biol Phys. 1990;18:1139-1142.
228 Gregoritch S, Chadha M, Pellegrini V, et al. Preoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement. Preliminary results. Int J Radiat Oncol Biol Phys. 1993;27(Suppl 1):157-158.
232 Jasty M, Schutzer S, Tepper J, et al. Radiation-blocking shields to localize periarticular radiation precisely for prevention of heterotopic bone formation around uncemented total hip arthroplasties. Clin Orthop. 1990;257:138-145.
237 Pakos EE, Ioannidis JP. Radiotherapy vs nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures. A meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004;60:888.
239 Pakos EE, Stafilas KS, Tsekeris PG, et al. Combined radiotherapy and indomethacin for the prevention of heterotopic ossification after total hip arthroplasty. Strahlenther Onkol. 2009;185:500-505.
1 Von Pannewitz G. Degenerative Erkrankungen. In: Zuppinger A, Ruckensteiner E, editors. Handbuch der medizinischen Radiologie. Berlin-Heidelberg-New York: Springer; 1970:96-98.
2 Sokoloff N. Röntgenstrahlen gegen Gelenkrheumatismus. Fortschr Röntgenstr. 1898;1:209-213.
3 Leer JWH, van Houtte P, Davelaar J. Indications and treatment schedules for irradiation of benign diseases. A survey. Radiother Oncol. 1998;48:249-257.
4 Order EO, Donaldson SS, editors. Radiation Therapy of Benign Diseases, ed 2, Berlin, Heidelberg, New York: Springer, 1998.
5 Seegenschmiedt MH, Katalinic A, Makoski H, et al. Radiation therapy for benign diseases. Patterns of care study in Germany. Int J Radiat Oncol Biol Phys. 2000;47:195-202.
6 Seegenschmiedt MH, Micke O, Willich N. Radiation therapy for nonmalignant diseases in Germany. Current concepts and future perspectives. Strahlenther Onkol. 2004;180:718-730.
7 Eng TY, Boersma MK, Fuller CD, et al. The role of radiation therapy in benign diseases. Hematol Oncol Clin North Am. 2006;20:523-557.
8 Seegenschmiedt MH, Makoski HB, Trott KR, et al. Radiotherapy for Non-malignant Disorders. New York: Springer, Merlin Heidelberg; 2008.
9 Jansen JTM, Broerse J, Zoetelief J, et al. Assessment of carcinogenic risk in the treatment of benign disease of knee and shoulder joint. In: Seegenschmiedt MH, Makoski H-B, editors. Kolloquium Radioonkologie/Strahlentherapie, Radiotherapie bei gutartigen Erkrankungen, Vol 15. Altenberge: Diplodocus- Verlag; 2001:13-15.
10 Trott KR, Kamprad F. Estimation of cancer risks from radiotherapy of benign diseases. Strahlenter Onkol. 2006;182:431-436.
11 Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35:83-90.
12 Von Wangenheim KH, Petersen HP, Schwenke K. A major component of radiation action. Interference with intracellular control of differentiation. Int J Radiat Biol. 1995;68:369-388.
13 Behrends U, Peter RU, Hintermeier-Knabe R, et al. Ionizing radiation induces human intercellular adhesion molecule 1 in vitro. J Invest Dermatol. 1994;103:726-730.
14 Hopewell JW, Robbins ME, Van den Aardweg GJ, et al. The modulation of radiation-induced damage to pig skin by essential fatty acids. Br J Cancer. 1993;68:1-7.
15 Sanford RA, Muhlbauer MS. Craniopharyngioma in children. Neurol Clin. 1991;9:453-465.
16 Hoffmann HJ, DeSilva M, Humphreys RP, et al. Aggressive surgical management of craniopharyngiomas in children. J Neurosurg. 1992;76:47-52.
17 Tomita T, McLone D. Radical resection of childhood craniopharyngiomas. Pediatr Neurosurg. 1993;19:6-14.
18 Sung DI, Chang CH, Harisiadis L, et al. Treatment results of craniopharyngiomas. Cancer. 1981;47:847-852.
19 Rajan B, Ashley S, Gorman C, et al. Craniopharyngioma. Long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26:1-10.
20 Bloom HJ, Glees J, Bell J. The treatment and long-term prognosis of children with intracranial tumors. A study of 610 cases, 1950-1981. Int J Radiat Oncol Biol Phys. 1990;18:723-745.
21 Becker G, Kortmann RD, Skaley M, et al. The role of radiotherapy in the treatment of craniopharyngioma. Indications, results, side effects. Front Radiat Ther Oncol. 1999;33:100-113.
22 Habrand JL, Ganry O, Couanet D, et al. The role of radiation therapy in the management of craniopharyngioma. A 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys. 1999;44:255-263.
23 Schulz-Ertner D, Frank C, Herfarth KK, et al. Fractionated stereotactic radiotherapy for craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2002;54:1114-1120.
24 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
25 Stefani MA, Porter PJ, terBrugge KG, et al. Large and deep brain arteriovenous malformations are associated with risk of future hemorrhage. Stroke. 2002;33:1220-1224.
26 Steiner L, Lindquist C, Adler JR, et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1-8.
27 Engenhart R, Wowra B, Debus J, et al. The role of high-dose, single-fraction irradiation in small and large intracranial AVMs. Int J Radiat Oncol Biol Phys. 1994;30:521-529.
28 Lindquist C, Steiner L, Blomgren H, et al. Stereotactic radiation therapy of intracranial arteriovenous malformations. Acta Radiol. 1986;368(Suppl):610-613.
29 Laing RW, Childs J, Brada M. Failure of conventionally fractionated radiotherapy to decrease the risk of hemorrhage in inoperable AVMs. Neurosurgery. 1992;30:872-875.
30 Poulsen MG. Arteriovenous malformation. A summary of 6 cases treated with radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13:1553-1557.
31 Wilms M, Kocher M, Makoski H-B, et al. Langzeitergebnisse der semistereotaktischen konventionell fraktionierten Strahlenbehandlung arteriovenöser Malformationen des Gehirns. Strahlenther Onkol. 2003;179(Suppl):69.
32 Colombo F, Pozza F, Chierego G, et al. Linear accelerator radiosurgery of cerebral arteriovenous malformations. An update. Neurosurgery. 1994;34:14-21.
33 Deruty R, Pelissou-Guyotat I, Amat D, et al. Complications after multidisciplinary treatment of cerebral arteriovenous malformations. Acta Neurochir (Wien). 1996;138:119-131.
34 Deruty R, Pelissou-Guyotat I, Morel C, et al. Reflections on the management of cerebral arteriovenous malformations. Surg Neurol. 1998;50:245-255.
35 Fleetwood IG, Marcellus ML, Levy RP, et al. Deep arteriovenous malformations of the basal ganglia and thalamus. Natural history. J Neurosurg. 2003;98:747-750.
36 Flickinger JC, Pollock BE, Kondziolka D, et al. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
37 Miyawaki L, Dowd C, Wara W, et al. Five year results of linac radiosurgery for arteriovenous malformations. Outcome for large AVMs. Int J Radiat Oncol Biol Phys. 1999;44:1089-1106.
38 Wolbers JG, Mol HC, Kralendonk JH, et al. Stereotactic radiosurgery with adjusted linear accelerator for cerebral arteriovenous malformations. Preliminary results in the Netherlands. Ned Tijdschr Geneeskd. 1999;143:1215-1221.
39 Chang JH, Chang JW, Park YG, Chung S. Factors related to complete occlusion of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 2000;93(Suppl 3):96-101.
40 Schlienger M, Atlan D, Lefkopoulos D, et al. Linac radiosurgery for cerebral arteriovenous malformations. Results in 169 patients. Int J Radiat Oncol Biol Phys. 2000;46:1135-1142.
41 Pollock BE, Kline RW, Stafford SL. The rationale and technique of staged-volume arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 2000;48:817-824.
42 Shin M, Kawamoto S, Kurita H, et al. Retrospective analysis of a 10-yr experience of stereotactic radiosurgery for arteriovenous malformations in children and adolescents. J Neurosurg. 2002;97:779-784.
43 Friedman WA, Bova FJ, Bollampally S, et al. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296-308.
44 Orio P, Stelzer KJ, Goodkin R, Douglas JG. Treatment of arteriovenous malformations with linear accelerator-based radiosurgery compared with Gamma Knife surgery. J Neurosurg. 2006;105S:58-63.
45 Moreno-Jimenez S, Celis MA, Larranga-Gutierrez JM, et al. Intracranial arteriovenous malformations treated with linear accelerator-based conformal radiosurgery. Clinical outcome and prediction of obliteration. Surg Neurol. 2007;67:487-492.
46 Yen CP, Varadey P, Sheehan J, et al. Subtotal obliteration of cerebral arteriovenous malformations after gamma knife surgery. J Neurosurg. 2007;106:361-369.
47 Abu-Salma Z, Nataf F, Ghossoub M, et al. The protective status of subtotal obliteration of arteriovenous malformations after radiosurgery. Significance and risk of hemorrhage. Neurosurgery. 2009;65:709-718.
48 van der Kogel AJ. Central nervous system radiation injury in small animal models. In: Gutin PH, Leibel SA, Sheline GE, editors. Radiation Injury to the Nervous System. New York: Raven Press, 1991.
49 Nakata H, Yoshimine T, Murasawa A, et al. Early blood-brain barrier disruption after high-dose single-fraction irradiation in rats. Acta Neurochir (Wien). 1995;136:82-86.
50 Kocher M, Voges J, Mueller R-P, et al. Linac radiosurgery for patients with a limited number of brain metastases. J Radiosurg. 1998;1:9-15.
51 Flickinger JC, Schell MC, Larson DA. Estimation of complications for linear accelerator radiosurgery with the integrated logistic formula. Int J Radiat Oncol Biol Phys. 1990;19:143-148.
52 Flickinger JC, Kondziolka D, Maitz AH, et al. Analysis of neurological sequelae from radiosurgery of AVMs. How location affects outcome. Int J Radiat Oncol Biol Phys. 1998;40:273-278.
53 Flickinger JC, Kondziolka D, Maitz AH, et al. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002;63:347-354.
54 Voges J, Treuer H, Sturm V, et al. Risk analysis of linear accelerator radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:1055-1063.
55 Voges J, Treuer H, Lehrke R, et al. Risk analysis of LINAC radiosurgery in patients with arteriovenous malformation (AVM). Acta Neurochir (Wien). 1997;68(Suppl):118-123.
56 Million RR, Cassisi NJ, Mancuso AA, Stringer SP. Chemodectomas (glomus body tumors. In: Million RR, Cassisi NJ, editors. Management of Head and Neck Cancer. A Multidisciplinary Approach. ed 2. Philadelphia: Lippincott; 1994:765-783.
57 Kim JA, Elkon D, Lim ML, Constable WC. Optimum dose of radiotherapy for chemodectomas in the middle ear. Int J Radiat Oncol Biol Phys. 1980;6:815ff.
58 Springate SC, Weichselbaum RR. Radiation or surgery for chemodectomas of the temporal bone. A review of local control and complications. Head Neck. 1990;12:303ff.
59 Li G, Chang S, Adler JR, et al. Irradiation of glomus jugulare tumors. A historical perspective. J Neurosurg. 2007;23:E13.
60 Henzel M, Hamm K, Gross MW, et al. Fractionated stereotactic radiotherapy of glomus jugulare tumors. Local control, toxicity, symptomatology and quality of life. Strahlenther Onkol. 2007;183:557.
61 Hinerman RW, Amdur RJ, Morris CG, et al. Definitive radiotherapy in the manangement of paragangliomas arising in the head and neck. A 35-year experience. Head Neck. 2008;30:1431.
62 Foote RL, Pollock BE, Gorman DA, et al. Glomus jugulare tumors. Tumor control and complications after stereotactic radiosurgery. Head Neck. 2002;24:332.
63 Lim M, Gibbs IC, Adler JR, et al. Efficacy and safety of stereotactic radiosurgery for glomus jugulare tumors. Neurosurg Focus. 2004;17:2E11.
64 Sharma MS, Gupta A, Kale SS, et al. Gamma knife radiosurgery for glomus jugulare tumors. Therapeutic advantages of minimalism in the skull base. Neurol India. 2008;56:57.
65 Spector JG. Management of juvenile angiofibromata. Laryngoscope. 1998;98:1016-1026.
66 Antonelli AR, Cappiello J, Donajo CA, et al. Diagnosis, staging and treatment of juvenile nasopharyngeal angiofibroma. Laryngoscope. 1997;97:1319-1325.
67 Waldman SR, Levine HL, Astor F, et al. Surgical experience with nasopharyngeal angiofibroma. Arch Otolaryngol. 1981;107:677-682.
68 Kuppersmith RB, Teh BS, Donovan DT, et al. The use of intensity modulated radiotherapy for the treatment of extensive and recurrent juvenile angiofibroma. Int J Pediatr Otorhinolaryngol. 2000;52:261-268.
69 Million RR, Cassisi NJ, Mancuso AA, Stringer SP. Juvenile angiofibroma. In: Million RR, Cassisi NJ, editors. Management of Head and Neck Cancer: A Multidisciplinary Approach. ed 2. Philadelphia: Lippincott; 1994:627-641.
70 McGahan RA, Durrance FY, Parke RBJr, et al. The treatment of advanced juvenile nasopharyngeal angiofibroma. Int J Radiat Oncol Biol Phys. 1989;17:1067-1072.
71 Reddy KA, Mendenhall WM, Amdur RJ, et al. Long-term results of radiation therapy for juvenile nasopharyngeal angiofibroma. Am J Otolaryngol. 2001;22:172-175.
72 Roche PH, Paris J, Regis J, et al. Management of invasive juvenile nasopharyngeal angiofibromas. The role of a multimodality approach. Neurosurgery. 2007;61:768-777.
73 Lee JT, Chen P, Safa A, et al. The role of radiation in the treatment of advanced juvenile angiofibroma. Lanyngoscope. 2002;112:1213-1230.
74 McAfee WJ, Morric CG, Amdur RJ, et al. Definitive radiotherapy for juvenile nasopharyngeal angiofibroma. Am J Clin Oncol. 2006;29:168-170.
75 Chakraborty S, Ghoshal S, Patil VM, et al. Conformal radiotherapy in the treatment of advanced juvenile nasopharyngeal angiofibroma with intracranial extension. An institutional experience. Int J Radiat Oncol Biol Phys. 2010 Jun 30. [Epub ahead of print]
76 Wilder RB, Buatti JM, Kittelson JM, et al. Pterygium treated with excision and postoperative beta-irradiation. Int J Radiat Oncol Biol Phys. 1992;23:533-537.
77 Monteiro-Grillo I, Gaspar L, Monteiro-Grillo M, et al. Postoperative irradiation of primary or recurrent pterygium. Results and sequelae. Int J Radiat Oncol Biol Phys. 2000;48:865-869.
78 Chen PO, Ariyasu RG, Kaza V, et al. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol. 1995;12:151-160.
79 Mahar PS, Nwokora GE. Role of mitomycin C in pterygium surgery. Br J Ophthalmol. 1993;77:433-435.
80 Rubinfield RS, Pfister RR, Stein RM, et al. Serious complication of topical mitomycin C after pterygium surgery. Ophthalmology. 1992;99:1647-1654.
81 Vastardis I, Pajic B, Rh Greiner, et al. Prospective study of exclusive strontium-yttrium-90 beta irradiation of primary and recurrent pterygia with no prior surgical excision. Clinical outcome of long-term follow-up. Strahlenther Onkol. 2009;185:808-814.
82 Willner J, Flentje M, Lieb W, et al. Soft X-ray therapy of recurrent pterygium. An alternative to Sr-90 eye applicators. Strahlenther Oncol. 2001;177:404-409.
83 Van den Brenk HA. Results of prophylactic postoperative irradiation on 1300 cases of pterygium. Am J Radiol. 1968;103:723-733.
84 Aswad M, Baum J. Optimal time for postoperative irradiation of pterygia. Ophthalmology. 1987;94:1450-1451.
85 MacKenzie FS, Hirst LW, Kynaston B, Bain C. Recurrence rate and complications after beta irradiation for ptergyia. Ophthalmology. 1991;98:1776-1780.
86 Jaakkola A, Heikkonen J, Tommula P, et al. Strontium plaque irradiation of subfoveal neovascular membranes image-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1998;236:24-30.
87 Paryani SB, Scott WP, Wells JWJr, et al. Management of pterygium with surgery and radiation therapy. The North Florida Pterygium Study Group. Int J Radiat Oncol Biol Phys. 1994;28:101-103.
88 Jurgenliemk-Schulz IM, Hartman LJ, Tersteeg RJ, et al. Prevention of pterygium recurrence by postoperative single-dose beta-irradiation. A prospective randomized clinical double-blind trial. Int J Radiat Oncol Biol Phys. 2004;59:1138-1147.
89 Isohashi F, Inoue T, Xing S, et al. Postoperative irradiation for pterygium. Retrospective analysis of 1,253 patients from the Osaka University Hospital. Strahlenther Onkol. 2006;182:437-442.
90 Viani GA, Stefano EJ, De Fendi LL, Foseca EC. Long-term results and prognostic factors of fractionated strontium-90 eye applicator for pterygium. Int J Radiat Oncol Biol Phys. 2008;72:1174-1179.
91 Kal HB, Veen RE, Jurgenliemk-Schulz IM. Dose-effect relationships for recurrence of keloid and pterygium after surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:245-251.
92 Ali AM, Thariat J, Bensadoun RJ, et al. The role of radiotherapy in the treatment of pterygium. A review of the literature including more than 6000 treated lesions. Cancer Radiother. 2011;15:140-147.
93 Yamada T, Mochizuki H, Ue T, et al. Comparative study of different β-radiation doses for preventing pterygium recurrence. Int J Radiat Oncol Biol Phys. 2010 Oct 1. [Epub ahead of print]
94 Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20:415-431.
95 Shields JA, Shields CL. Atlas of Orbital Tumors. Philadelphia: Lippincott, Williams & Wilkins; 1999.
96 Mashayekhi A, Shields CL. Circumscribed choroidal hemangioma. Curr Opin Ophthalmol. 2003;14:142-149.
97 Shields CL, Honavar SC, Shields JA, et al. Circumscribed choroidal hemangioma. Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237-2248.
98 Shields JA, Shields CL, Materin MA, et al. Changing concepts in management of circumscribed choroidal hemangioma. The 2003. J. Howard Stokes Lecture (Part 1). Ophthalmol Surg Lasers. 2004;35:383-393.
99 Madreperla SA. Choroidal hemangioma treated with photodynamic therapy using verteporfin. Arch Ophthalmol. 2001;119:1606-1610.
100 Augsburger JJ, Freire J, Brady LW. Radiation therapy for choroidal and retinal hemangiomas. In: Wiegel T, Bornfeld N, Foerster MH, Hinkelbein W, editors. Radiotherapy of Ocular Disease, Vol 30. Basel: Karger; 1997:265-280.
101 Schilling H, Sauerwein W, Lommatzsch A, et al. Long-term results after low dose ocular irradiation for choroidal hemangiomas. Br J Ophthalmol. 1997;81:267-273.
102 Kreusel KM, Bornfeld N, Lommatzsch A, et al. Ruthenium-106 brachytherapy for peripheral retinal capillary hemangioma. Ophthalmology. 1998;105:1386-1392.
103 Madreperla SA. Choroidal hemangioma treated with photodynamic therapy using verteporfin. Arch Ophthalmol. 2001;119:1606-1610.
104 Zografos L, Bercher L, Chamot L, et al. Cobalt-60 treatment of choroidal hemangiomas. Am J Ophthalmol. 1996;121:190-199.
105 Austin-Seymour MM, Donaldson SS, Egbert PR, et al. Radiotherapy of lymphoid diseases of the orbit. Int J Radiat Oncol Biol Phys. 1985;11:371-379.
106 Lambo MJ, Brady LW, Shields CL. Lymphoid tumors of the orbit. In: Alberti WE, Sagerman RH, editors. Radiotherapy of Intraocular and Orbital Tumors. Berlin: Springer (1. Auflage); 1993:205-216.
107 Donaldson SS, McDougall IR, Kriss JP. Graves’ disease. In: Alberti WE, Sagerman RH, editors. Radiotherapy of Intraocular and Orbital Tumors. Berlin: Springer (1 Auflage); 1993:191-197.
108 Leone C, Lloyd T. Treatment protocol for orbital inflammatory disease. Ophthalmology. 1985;92:1325-1331.
109 Barthold HJ, Harvey A, Markoe AM, et al. Treatment of orbital pseudotumors and lymphoma. Am J Clin Oncol. 1986;9:527-532.
110 Fritzpatrick PI, Macko SL. Lymphoreticular tumors of orbit. Int J Radiat Oncol Biol Phys. 1984;10:333-340.
111 Lanciano R, Fowble B, Sergott R, et al. The results of radiotherapy for orbital pseudotumor. Int J Radiat Oncol Biol Phys. 1989;18:407-411.
112 Notter M. Strahlentherapie bei pseudotumor orbitae. In: Seegenschmiedt MH, Makoski HB, editors. Radiotherapie gutartiger Erkrankungen, Symposium 5-6. März 2000. Altenberge, Germany: Diplodocus Verlag; 2000:123-136.
113 Gocht H. Therapeutische Verwendung der Röntgenstrahlen. Fortschr Röntgenstr. 1897;1:14.
114 Atahan I, Lale F, Akyol F, et al. Radiotherapy in the management of aggressive fibromatosis. Br J Radiol. 1989;62:854-856.
115 Goy BW, Lee SP, Eilber F, et al. The role of adjuvant radiotherapy in the treatment of resectable desmoid tumors. Int J Radiat Oncol Biol Phys. 1997;39:659-665.
116 Posner MC, Shiu MH, Newsome JL. The desmoid tumor. Not a benign disease. Arch Surg. 1989;124:191-196.
117 Suit HD, Spiro I. Radiation treatment of benign mesenchymal disease. Semin Radiat Oncol. 1999;9:171-178.
118 Wilcken N, Tattersall MH. Endocrine therapy for desmoid tumors. Cancer. 1991;68:1384-1388.
119 Wadell WR, Gerner RE. Indomethacin and ascorbate inhibit desmoid tumors. J Surg Oncol. 1980;15:85-90.
120 Kirschner MJ, Sauer R. Die Rolle der Radiotherapie bei der Behandlung von Desmoidtumoren. Strahlenther Onkol. 1993;169:77-82.
121 Kamath SS, Parsons JT, Marcus RB. Radiotherapy for local control of aggressive fibromatosis. Int J Radiat Oncol Biol Phys. 1996;36:325-328.
122 Assad WA, Nori D, Hilaris BS, et al. Role of brachytherapy in the management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1986;12:901-906.
123 Bataini JP, Belloir C, Mazabraud A, et al. Desmoid tumors in adults. The role of radiotherapy in their management. Am J Surg. 1988;155:754-760.
124 Ballo MT, Zagars GK, Pollack A. Desmoid tumor. Prognostic factors and outcome after surgery, radiation therapy or combined surgery and radiation therapy. J Clin Oncol. 1999;17:158-167.
125 Enzinger FM, Shiraki M. Musculoaponeurotic fibromatosis of the shoulder girdle (extra-abdominal desmoid). Cancer. 1967;20:1131-1140.
126 Kiel KD. Radiation therapy in the treatment of aggressive fibromatoses (desmoid tumors). Cancer. 1984;54:2051-2055.
127 Leibel SA, Wara WM, Hill D, et al. Desmoid tumors. Local control and patterns of relapse following radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:1167-1171.
128 Suit HD. Radiation dose and response of desmoid tumors. Int J Radiat Oncol Biol Phys. 1990;9:225-227.
129 Walther E, Hünig R, Zalad S. Behandlung der aggressiven. Fibromatose. Orthopädie. 1998;17:193-200.
130 Seegenschmiedt MH, Micke O, Willich N. Radiation therapy for nonmalignant diseases in Germany. Current concepts and future perspectives. GCG-BD]. Strahlenther Onkol. 2004;180:718-730.
131 Fontanesi J, Mott MP, Draut MJ, et al. The role of postoperative irradiation in the treatment of locally recurrent incompletely resected extra-abdominal desmoid tumors. Sarcoma. 2004;8:83-86.
132 El Haddad M, El-Sebaie M, Ahmad R, et al. Treatment of aggressive fibromatosis. The experience of a single institution. Clin Oncol. 2009;21:775-780.
133 Jabarri S, Andolino D, Weinberg V, et al. Successful treatment of high risk and recurrent pediatric desmoids using radiation as a component of multimodality therapy. Int J Radiat Oncol Biol Phys. 2009;75:177-182.
134 Rudiger HA, Sy Ngan, Ng M, et al. Radiation therapy in the treatment of desmoid tumours reduces surgical indications. Eur J Surg Oncol. 2010;36:84-88.
135 Gluck I, Griffith KA, Biermann JS, et al. Role of radiotherapy in the management of desmoid tumors. Int J Radiat Oncol Biol Phys. 2011;80:787-792.
136 Roeder F, Timke C, Oertel S, et al. Intraoperative electron radiotherapy for the management of aggressive fibromatosis. Int J Radiat Oncol Biol Phys. 2010;76:1154-1160.
137 Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie’s disease. J Urol. 2006;175:2115-2118.
138 Nesbit RM. Congenital curvature of the phallus. Report of three cases with description of corrective operation. J Urol. 1950;93:230-232.
139 Feder BH. Peyronie’s disease. J Am Geriatr Soc. 1971;19:947-951.
140 Helvie WW, Ochsner SF. Radiation therapy in Peyronie’s disease. South Med J. 1972;65:1192-1196.
141 Martin CL. Long time study of patients with Peyronie’s disease treated with irradiation. Am J Roentgenol. 1972;114:492-495.
142 Wagenknecht LV, Meyer WH, Kiskemann A. Wertigkeit ver-schiedener Therapieverfahren bei Induratio penis plastica. Urol Int. 1982;37:335-348.
143 Pambor M, Schmidt W, Wiesner M, Jahr U. Induratio penis plastica. Ergebnisse nach kombinierter Behandlung mit Röntgenbestrahlung und Tokopherol. Z Klin Med. 1985;40:1425-1427.
144 Weisser GW, Schmidt B, Hübener KH, et al. Die Strahlenbehandlung der Induratio penis plastica. Strahlenther Onkol. 1987;163:23-28.
145 Mira JG, Chahbazian CM, del Regato JA. The value of radiotherapy for Peyronie’s disease. Presentation of 56 new case studies and review of the literature. Int J Radiat Oncol Biol Phys. 1989;6:161-166.
146 Viljoen IM, Goedhals L, Doman MJ. Peyronie’s disease. A perspective on the disease and the long-term results of radiotherapy. S Afr Med J. 1993;83:19-20.
147 Rodrigues CI, Njo Hian, Karim AB. Results of radiotherapy and vitamin E in the treatment of Peyronie’s disease. Int J Radiat Oncol Biol Phys. 1995;31:571-576.
148 Williams JL, Thomas CG. The natural history of Peyronie’s disease. J Urol. 1970;103:75-76.
149 Bruns F, Kardels B, Schäfer U, et al. Strahlentherapie bei Induratio penis plastica. Röntgenpraxis. 1999;52:33-37.
150 Incrocci L, Wijnmaalen A, Slob AK, et al. Low-dose radiotherapy in 179 patients with Peyronie’s disease. Treatment outcome and current sexual function. Int J Radiat Oncol Biol Phys. 2000;47:1353-1356.
151 Niewald M, Wenzlawowicz KV, Fleckenstein J, et al. Results of radiotherapy for Peyronie’s disease. Int J Radiat Oncol Biol Phys. 2006;64:258-262.
152 Incroni L, Hop WE, Seegenschmiedt HM. Radiotherapy for Peyronie’s disease. A European survey. Acta Oncol. 2008;47:110-112.
153 Keilholz L, Seegenschmiedt MH, Sauer R. Radiotherapy in early stage Dupuytren.s contracture. Initial and long-term results. Int J Radiat Oncol Biol Phys. 1996;36:891-897.
154 Adamietz B, Keilholz L, Grünert J, Sauer R. Die Radiotherapie des Morbus Dupuytren im Frühstadium. Langzeitresuktate nach einer medianen Nachbeobachtungszeit von 10 Jahren. Strahlenther Onkol. 2001;177:604-610.
155 Escarmant P, Zimmermann S, Amar A, et al. The treatment of 783 keloid scars by Iridium 192 interstitial irradiation after surgical excision. Int J Radiat Oncol Biol Phys. 1993;26:245-251.
156 Guix B, Henriquez I, Andres A, et al. Treatment of keloids by high-dose-rate brachytherapy. A seven-year-study. Int J Radiat Oncol Biol Phys. 2001;50:167-172.
157 Prott FJ, Micke O, Wagner W, et al. Narbenkeloidprophylaxe durch Bestrahlung mit Strontium-90. MTA. 1997;12:425-428.
158 Micke O, Seegenschmiedt MH. The German Working Group guidelines for radiation therapy of benign diseases. A multicenter approach in Germany. Int J Radiat Oncol Biol Phys. 2002;52:496-513.
159 Lo TCM, Seckel BR, Salzman FA, Wright KA. Single-dose electron beam irradiation in treatment and prevention of keloids and hypertrophic scars. Radiother Oncol. 1990;19:267-272.
160 Janssen de Limpens MP. Comparison of the treatment of keloids and hypertrophic scars. Eur J Plast Surg. 1986;9:18-21.
161 Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Strahlenther Onkol. 2005;11:717-723.
162 De Lorenzi F, Teilemans HJP, van dre Hulst RRWJ, et al. Is the treatment of keloid scars still a challenge in 2006? Ann Plast Surg. 2007;58:186.
163 Ogawa R, Miyashita T, Hyakusoku H, et al. Postoperative radiation protocol for keloids and hypertrophic scars. Statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg. 2007;59:688.
164 Sakamoto T, Oya N, Shibuya K, et al. Dose-response relationship and dose optimization in radiotherapy of postoperative keloids. Radiother Oncol. 2009;91:271-276.
165 Emad M, Omidvari S, Dastgheib L, et al. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids. A prospective clinical trial. Med Princ Pract. 2010;19:402-405.
166 Flickinger JC. A radiobiological analysis of multicenter data for postoperative keloid radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1164-1170.
167 Stahl S, Barnea Y, Weiss J, et al. Treatment of earlobe keloids by extralesional excision combined with preoperative and postoperative “Sandwich” radiotherapy. Plast Reconstr Surg. 2010;125:135-141.
168 Metzger H, Junker A, Voss AC. Die Bestrahlung der Brustdrüsen ais Prophylaxe der östrogen-induzierten Gynäkomastie beim Prostatakarzinom. Strahlenther Onkol. 1980;156:102-104.
169 Chou JL, Easley JD, Feldmeier JJ. Effective radiotherapy in palliating mammalgia associated with gynecomastia after DES therapy. Int J Radiat Oncol Biol Phys. 1988;15:749-751.
170 Clough JR, Price CGH. Aneurysmal bone cyst. Pathogenesis and long term results of treatment. Clin Orthop Relat Res. 1973;97:52-63.
171 Marcove RC, Sheth DS, Takemoto S, et al. The treatment of aneurysmal bone cyst. Clin Orthop Relat Res. 1995;311:157.
172 Nobler MP, Higinbotham ML, Phillips RF. The cure of aneurysmal bone cyst. Irradiation superior to surgery in analysis of 33 cases. Radiology. 1968;90:1185.
173 Goldman AB, DiCarlo EF. Pigmented villonodular synovitis. Diagnosis and differential diagnosis. Radiol Clin North Am. 1988;266:1327-1347.
174 O’Sullivan B, Cummings B, Catton C, et al. Outcome following radiation treatment for high-risk pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys. 1995;32:777-786.
175 Wiss DA. Recurrent villonodular synovitis of the knee. Successful treatment with yttrium-90. Clin Orthop Relat Res. 1982;169:139-144.
176 Granowitz SP, D’Antonio J, Mankin HL. The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin Orthop Relat Res. 1976;114:335-351.
177 Mendenhall WM, Mendenhall CM, Reith JD, et al. Pigmented villonodular synovitis. Am J Clin Oncol. 2006;29:548-550.
178 Berger B, Ganswindt U, Bamberg M, Hehr T. External beam radiotherapy as postoperative treatment of diffuse pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys. 2007;67:1130-1134.
179 Horoschak M, Tran PT, Bachireddy P, et al. External beam radiation therapy enhances local control in pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys. 2009;75:183-187.
180 Kramer DE, Frassica FJ, Frassica DA, Cosgarea AJ. Pigmented villonodular synovitis of the knee. Diagnosis and treatment. J Knee Surg. 2009;22:243-254.
181 Heyd R, Micke O, Berger B, et al. Radiation therapy for treatment of pigmented villonodular synovitis. Results of a national patterns of care study. Int J Radiat Oncol Biol Phys. 2010;78:199-204.
182 Zook JE, Wurtz DL, Cummings JE, Cardenes HR. Intra-articular chromic phosphate (32P) in the treatment of diffuse pigmented villonodular synovitis. Brachytherapy. 2011;10:190-194.
183 Unni KK, Ivins JC, Beabout JW, et al. Hemangioma, hemangiopericytoma and hemangioendothelioma (angiosarcoma) of bone. Cancer. 1971;27:1403-1414.
184 McAllister VL, Kendall BE, Bull JWD. Symptomatic vertebral hemangiomas. Brain. 1975;98:71-80.
185 Raco A, Ciappetta P, Artico M, et al. Vertebral hemangiomas with cord compression. The role of embolization in five cases. Surg Neurol. 1990;34:164-168.
186 Laredo JD, Reizine D, Bard M, et al. Vertebral hemangiomas. Radiologic evaluation. Radiology. 1986;161:183-189.
187 Bremnes RM, Hauge HN, Sagsveen R. Radiotherapy in the treatment of symptomatic vertebral hemangiomas. Technical case report. Neurosurgery. 1996;39:1054-1058.
188 Doppman JL, Oldfield EH, Heiss JD. Symptomatic vertebral hemangiomas. Treatment by means of direct intralesional injection of ethanol. Radiology. 2000;214:341-348.
189 Kleinert H. Über die Telekobalttherapie der Wirbelhämangiome. Strahlenther Onkol. 1967;134:504-510.
190 Winkler C, Dornfeld S, Baumann M, et al. Effizienz der Strahlentherapie bei Wirbelhämangiomen. Strahlenther Onkol. 1996;172:681-684.
191 Pastushyn AI, Slinko EI, Mirzoyeva GM. Vertebral hemangiomas. Diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998;50:535-547.
192 Padovani R, Acciarri N, Giulioni M, et al. Cavernous angiomas of the spinal district. Surgical treatment of 11 patients. Eur Spine J. 1997;6:298-303.
193 Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78:36-45.
194 Harrison MJ, Eisenberg MB, Ullman JS, et al. Symptomatic cavernous malformations affecting the spine and spinal cord. Neurosurgery. 1995;37:195-205.
195 Rades D, Bajrovic A, Alberti A, Rudat V. Is there a dose-effect relationship for the treatment of symptomatic vertebral hemangioma? Int J Radiat Oncol Biol Phys. 2002;55:178-181.
196 Heyd R, Seegenschmiedt MH, Rades D, et al. Radiotherapy for symptomatic vertebral hemangionas. Results of a multicenter study and literature review. Int J Radiat Oncol Biol Phys. 2010;77:217-225.
197 Brooker AF, Bowerman JW, Robinson RA, Riley LH. Ectopic ossification following total hip replacement. J Bone Joint Surg. 1973;55A:1629-1632.
198 Ayers DC, Pellegrini VD, Evarts CM. Prevention of heterotopic ossification in high-risk patients of radiation therapy. Clin Orthop Relat Res. 1991;263:87-93.
199 De Lee J, Ferrari A, Charnley J. Ectopic bone formation following low friction arthroplasty of the hip. Clin Orthop Relat Res. 1976;121:53.
200 Ritter MA, Vaughan RB. Ectopic ossifications after total hip arthroplasty. Predisposing factors, frequency and effect on results. J Bone Joint Surg. 1977;59A:345-351.
201 Ayers DC, Evarts CM, Parkinson JR. The prevention of heterotopic ossification in high-risk patients by low-dose radiation therapy after total hip arthroplasty. J Bone Joint Surg. 1986;68A:1423-1430.
202 Pedersen NW, Kristensen SS, Schmidt SA, et al. Factors associated with heterotopic bone formation following total hip replacement. Arch Orthop Trauma Surg. 1989;108:92-95.
203 Schmidt SA, Kjaersgaard-Andersen P, Pedersen NW, et al. The use of indomethacin to prevent the formation of heterotopic bone after total hip replacement. J Bone Joint Surg. 1988;70A:834-838.
204 Goel A, Sharp DJ. Heterotopic bone formation after hip replacement. J Bone Joint Surg. 1991;73B:255-257.
205 Bosse MJ, Poka A, Reinert CM, et al. Heterotopic bone formation as a complication of acetabular fractures. J Bone Joint Surg. 1988;70A:1231-1237.
206 Slawson RG, Poka A, Bathon H, et al. The role of postoperative radiation in the prevention of heterotrophic ossification in patients with posttraumatic acetabular fracture. Int J Radiat Oncol Biol Phys. 1989;17:669-672.
207 Bijvoet OLM, Nollen AJG, Sloof TJJH, Feith R. Effect of diphosphonate on para-articular ossification after total hip replacement. Acta Orthop Scand. 1974;45:926-934.
208 Garland DE, Betzabe A, Kenneth GV, Vogt JC. Diphosphonate treatment for heterotopic ossification in spinal cord injury patients. Clin Orthop Relat Res. 1983;176:197-200.
209 Thomas BJ, Amstutz HC. Results of administration of diphosphonate for prevention of heterotopic ossification after total hip arthroplasty. J Bone Joint Surg. 1985;67A:400-403.
210 Almasbakk K, Roysiand P. Does indomethacin prevent postoperative ectopic ossification in total hip replacement? Acta Orthop Scand. 1977;48:556.
211 Kjaersgaard-Andersen P, Schmidt SA. Indomethacin for the prevention of ectopic ossification after hip arthroplasty. Acta Orthop Scand. 1986;57:12-14.
212 Ritter MA, Sieber JM. Prophylactic indomethacin for the prevention of heterotopic bone formation following total hip arthroplasty. Clin Orthop Relat Res. 1985;196:217-225.
213 Cella JP, Salvati EA, Sculco TP. Indomethacin for the prevention of heterotopic ossification following total hip arthroplasty. J Arthroplasty. 1988;3:229-234.
214 Sodemann B, Persson PE, Nilsson OS. Prevention of heterotopic ossification by nonsteroid anti-inflammatory drugs after total hip arthroplasty. Clin Orthop Relat Res. 1988;237:158-237.
215 McLaren AC. Prophylaxis with indomethacin for heterotopic bone. J Bone Joint Surg. 1990;72A:245-247.
216 Elmstedt E, Lindholm TS, Nilsson OS, Tornkvist H. Effect of ibuprofen on heterotopic ossification after hip replacement. Acta Orthop Scand. 1985;56:25-27.
217 Finerman GA, Stover S. Ossification following hip replacement or spinal cord injury. Two clinical studies with EHDP. Metab Bone Dis Relat Res. 1981;3:337-342.
218 Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J Bone Joint Surg. 1981;63A:201-208.
219 Van der Werf GJLM, van Hasselt NGM, Tonino AJ. Radiotherapy in the prevention of recurrence of paraarticular ossification in total hip prosthesis. Arch Orthop Trauma Surg. 1985;104:85-88.
220 McLennan I, Keys HM, Evarts CM, Rubin P. Usefulness of postoperative hip irradiation in the prevention of bone formation in a high risk group of patients. Int J Radiat Oncol Biol Phys. 1984;10:49-53.
221 Anthony P, Keys H, McCollister-Evarts C, et al. Prevention of heterotopic bone formation with early postoperative irradiation in high risk patients undergoing total hip arthroplasty. Comparison of 10 Gy versus 20 Gy schedules. Int J Radiat Oncol Biol Phys. 1987;13:365-369.
222 Sylvester JE, Greenberg P, Selch MT, et al. The use of postoperative irradiation for the prevention of heterotopic bone formation after total hip replacement. Int J Radiat Oncol Biol Phys. 1988;14:471-476.
223 Blount LH, Thomas BJ, Tran L, et al. Postoperative irradiation for prevention of heterotopic bone. Analysis of different dose schedules and shielding considerations. Int J Radiat Oncol Biol Phys. 1990;19:577-581.
224 Seegenschmiedt MH, Goldmann AR, Wölfel R, et al. Prevention of heterotopic ossification (HO) after total hip replacement. Randomized high versus low dose radiotherapy. Radiother Oncol. 1993;26:271-274.
225 Konski A, Pellegrini V, Poulter C, et al. Randomized trial comparing single dose versus fractionated irradiation for prevention of heterotopic bone. Int J Radiat Oncol Biol Phys. 1990;18:1139-1142.
226 Seegenschmiedt MH, Goldmann AR, Martus P, et al. Prophylactic radiation therapy for prevention of heterotopic ossification after hip arthroplasty. Results in 141 high-risk hips. Radiology. 1993;188:257-264.
227 Alberti W, Quack G, Krischke W, et al. Verhinderung ektoper Ossifikationen nach Totalendoprothese des Hüftgelenks durch Strahlentherapie. Dtsch Med Wochenschr. 1995;120:983-989.
228 Gregoritch S, Chadha M, Pellegrini V, et al. Preoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement. Preliminary results. Int J Radiat Oncol Biol Phys. 1993;27(Suppl 1):157-158.
229 Konski A, Weiss C, Rosier R, et al. The use of postoperative irradiation for prevention of heterotopic bone after total hip replacement with biological fixation (porous coated) prosthesis. An animal model. Int J Radiat Oncol Biol Phys. 1990;18:861-865.
230 Wise MW3rd, Robertson ID, Lachiewicz PF, et al. The effect of radiation therapy on the fixation strength of an experimental porous-coated implant in dogs. Clin Orthop Relat Res. 1990;261:276-280.
231 Summer DR, Turner TM, Pierson RH, et al. Effects of radiation on fixation of noncemented porous-coated implants in a canine model. J Bone Joint Surg. 1990;72A:1527-1533.
232 Jasty M, Schutzer S, Tepper J, et al. Radiation-blocking shields to localize periarticular radiation precisely for prevention of heterotopic bone formation around uncemented total hip arthroplasties. Clin Orthop Relat Res. 1990;257:138-145.
233 De Flitch DJ, Stryker JA. Postoperative hip irradiation in prevention of heterotopic ossification. Causes of treatment failure. Radiology. 1993;188:265-270.
234 Hedley AK, Leon PM, Douglas HH. The prevention of heterotopic bone formation following total hip arthroplasty using 600 rad in a single dose. Arthroplasty. 1989;4:319-325.
235 Sauer R, Seegenschmiedt MH, Goldmann A, et al. Prophylaxe periartikulärer Verknöcherungen nach endoprothetischen Hüftgelenksersatz durch postoperative Bestrahlung. Strahlenther Onkol. 1992;16:889-899.
236 Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification. Pathophysiology, clinical features and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006;65:1289.
237 Pakos EE, Ioannidis JP. Radiotherapy vs nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures. A meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004;60:888.
238 Karunaker MA, Sen A, Bosse MJ, et al. Indomethacin as prophylaxis for heterotopic ossification after the operative treatment of fractures of the acetabulum. J Bone Joint Surg. 2006;88B:1613.
239 Pakos EE, Stafilas KS, Tsekeris PG, et al. Combined radiotherapy and indomethacin for the prevention of heterotopic ossification after total hip arthroplasty. Strahlenther Onkol. 2009;185:500-505.
240 Vavken P, Castellani L, Sculco TP. Prophylaxis of heterotopic ossification of the hip. Systematic review and meta-analysis. Clin Orthop Relat Res. 2009;467:3283-3289.
241 Jensen AW, Viozzi CF, Foote RL. Long-term results of radiation prophylaxis for heterotopic ossification in the temporomandibular joint. J Oral Maxillofac Surg. 2010;68:1100-1105.
242 Robinson CG, Polster JM, Reddy CA, et al. Postoperative single-fraction radiation for prevention of heterotopic ossification of the elbow. Int J Radiat Oncol Biol Phys. 2010;77:1493-1499.
* See references 3, 4, 5, 6, 7, 8.
* See references 26, 27, 28–31, 32, 33–35, 36, 37, 38, 39–43, 44–47.
† See references 26, 27, 32, 33, 34, 36, 37, 38, 39–43.
* See references 57, 58–60, 61, 62–64.
* See references 68, 69, 70, 71, 72–75.
* See references 68, 69, 70, 71, 72–74.
* See references 83, 84–86, 87, 88, 89, 90, 91–93.
† See references 83, 84–86, 87, 88, 89, 90, 91–93.
* See references 115, 117, 120–123, 124, 125–127, 128, 129, 130, 131, 132, 133, 134, 135, 136.
† See references 115, 117, 120–123, 124, 125–127, 128, 129, 130, 131, 132, 133, 134, 135, 136.
* See references 139–144, 145, 146, 149, 150–153.
* See references 139–144, 145, 146–149, 150–153.
* See references 155, 156, 157, 158, 159–162, 163, 164, 165, 166, 167.
* See references 174, 177, 178, 179, 180, 181, 182.
* See references 177, 178, 179, 180, 181, 182.
* See references 198, 218–220, 221, 222, 223, 224, 225, 226, 227, 228, 229–231, 232, 233–236, 237, 238, 239, 240–242.