68 Benign Disease
The use of radiation in the treatment of benign diseases has a long and not entirely honorable history. Soon after the discovery of x-rays by Röntgen in 1895, the therapeutic potential of radiation was recognized. In the first half of the 20th century, radiation therapy was used empirically for a host of conditions, both benign and malignant. In many situations in which no effective therapeutic alternatives existed, radiation therapy may have been one of the few treatment options available. In the pre–World War II era, few controlled studies that would pass muster by today’s standards were performed. It is likely that the true efficacy of radiation treatment in relation to contemporary standard therapies for a host of conditions will never be known.1–3
Radiation treatment for benign conditions has declined for two major reasons. One is awareness of the deleterious late effects of radiation, including the potential for the induction of malignancy. In addition, advances in surgical technique and the development of effective medical therapies such as corticosteroids and antibiotics have provided effective alternatives to the use of ionizing radiation for benign conditions in most instances. With contemporary knowledge there is a tendency for prima facie condemnation of the prior widespread use of radiation for benign conditions. However, when critiquing past uses of radiation therapy for benign conditions, one must remember the limited alternative therapies available at the time the treatment was delivered, as well as the state of knowledge regarding the late effects of radiation. Recent evidence, however, has suggested that the selected use of radiotherapy for benign diseases may at least be stabilizing.4
Nonetheless, the radiation oncologist remains the clinician with primary expertise in the use of ionizing radiation to treat disease, both benign and malignant. There are situations in which ionizing radiation remains a commonly accepted therapeutic alternative or even the treatment of choice for nonmalignant conditions. In these situations, the radiation oncologist’s knowledge of the effects of radiation on normal tissues can assist the patient and referring clinician in selecting the treatment regimen with the highest therapeutic ratio for a particular individual. In many cases, the use of radiation therapy for benign disorders may be able to spare the patient morbidity associated with the progression of the disease, or additional medical or surgical therapy. Although radiation can have significant morbidity, in many cases the morbidity of radiation is low when compared with the morbidity (or even mortality) associated with alternative therapies, or with the complications associated with progressive or recurrent disease. Radiation carcinogenesis, although always a concern when ionizing radiation is used, appears to be a greater risk for pediatric patients and young adults than for older adults, and must be weighed in relation to the consequences of progressive disease and the side effects associated with alternative therapies.5,6
This chapter reviews the rationale, indications, and treatment of benign conditions commonly encountered in contemporary practice. Monographs and review articles written on the subject of radiation therapy for benign diseases provide more detailed and comprehensive reviews, as well as reference lists, than can be contained here.7–12 Literature reviews or computerized searches of medical databases may be useful in approaching a consultation on an unusual clinical problem.
The topics covered in this chapter include those that are most likely to be commonly encountered in contemporary radiation oncology practice and are not covered elsewhere in the book. They include eye and orbital conditions (pterygium, thyroid ophthalmopathy, orbital lymphoid hyperplasia, orbital pseudotumor, and macular degeneration), certain benign tumors of the head and neck (juvenile nasal angiofibroma, chemodectomas), proliferations of tissue in response to injury that cause functional or cosmetic impairment (pterygia, keloids, HO), and several miscellaneous uses of radiation for benign diseases (gynecomastia, Peyronie disease). The use of total lymphoid irradiation (TLI) in cardiac transplantation rejection is also discussed. In these situations, a role for radiation therapy in the contemporary management of a benign disorder continues to exist. More comprehensive lists of benign diseases for which radiation has been used therapeutically can be found in other sources.9,11,12
The need for informed consent exists everywhere in radiation oncology, and certainly in the use of radiation therapy for benign diseases. Patients should be informed of the rationale, potential side effects, and treatment alternatives to radiation treatment before it is delivered. Special caution should be used before radiation is delivered to pediatric patients and young adults for benign conditions. These young patients may be at increased risk of second malignancy induction and late atrophy, especially if the area to be treated is in proximity to the breasts or thyroid, tissues that may be sensitive to carcinogenesis. As in all aspects of radiation therapy, care should be taken to use beams of appropriate energy and to shield adjacent normal structures.3,7,9
Keloids
Keloids are hypertrophic scars in which collagenous tissue is overproduced and grows beyond the original dimensions of the wound. The causal factors of keloids are unknown, but increased rates of collagen metabolism have been noted. For unknown reasons, certain individuals are predisposed to keloid formation after surgery or other skin injury. Lesions appear to occur more frequently among African Americans and Asian people. In addition to cosmetic problems, keloids can become painful, pruritic, or fibrotic, and can produce significant morbidity.13
Various treatment options have been described. Surgical re-excision of the keloid alone is sufficient in some cases. The use of pressure postoperatively has been effective in some cases, but it is inconvenient because it must be applied for many months postoperatively to be effective. Injections of a corticosteroid (triamcinolone) have been used and can produce a reduction in the size of established keloids, although it is not effective in all cases.14 Pulsed-dye laser treatment has been reported to be successful in one series.15 Various other drugs and surgical methods have been used in small series of patients with variable results.
Radiation therapy has been employed successfully in the treatment of keloids since the early 20th century.16 In most series, external kilovoltage or electron-beam radiation is directed at the surgical bed within 24 to 72 hours after re-excision of the keloid. Interstitial iridium (Ir)-192 treatment has been reported in a large series from France.17 De Lorenzi and colleagues18 also reported favorable results using Ir-192 high-dose-rate brachytherapy after surgical excision of established keloids. A plastic catheter was placed parallel to the wound, and two fractions of 7 Gy were delivered daily to a 1.0-cm diameter cylindrical volume within 24 hours of surgery. All 30 patients had a significant difference in scar thickness before and after the treatment, with good patient satisfaction. Treatment of established lesions without re-excision may relieve symptoms in some cases, but results appear to be significantly less satisfactory than when radiation treatment is performed promptly after re-excision.19 Large lesions may be treated with a combination of re-excision and skin grafting followed by postoperative irradiation.20 There is little data regarding dose response for prevention of keloids with radiotherapy. Kal and Veen21 reported that for biologically effective dose (BED) values greater than 10 Gy, the keloid recurrence rate decreased as a function of BED. The authors gathered dose-response data from a literature search of 31 reports, and recommend a BED value of 30 Gy (single fraction 13 Gy, two fractions of 8 Gy, or three fractions of 6 Gy), which resulted in a recurrence rate less than 10%. Typical dose-fractionation schemes in the literature range from 9 to 16 Gy in 3- to 4-Gy fractions.
Results with the combination of re-excision and irradiation for keloids have been excellent. Borok and colleagues19 reported a recurrence rate of only 2% in a series of 393 lesions in 250 patients; doses in the range of 9 to 12 Gy were most commonly used. Doornbos and colleagues22 reported on 278 keloids treated with 120-kV x-rays and reported a 26% recurrence rate; an increased rate of recurrence has been reported when doses of 6 Gy or less have been used. Kovalic and colleagues23 analyzed results of 107 patients treated at the Mallinckrodt Institute of Radiology from 1966 to 1987. Of these, 74% were earlobe lesions and were most commonly treated with 12 Gy in three fractions of 4 Gy over 3 days, with initiation of radiation within 72 hours of surgery. With a mean follow-up of 117 months, 27% of lesions recurred, with a mean time to recurrence of 12.8 months. No advantage to beginning therapy within 24 hours was reported. Excellent cosmetic results were reported in 95% of the patients. Factors in this study associated with significantly increased risk of recurrence included male sex, lesion size greater than 2 cm, and previous antikeloid therapy. Ogawa and colleagues24 have suggested that recurrence rates are higher for keloids on the trunk compared with the face and neck because of differences in stretch tension. They customized doses of electron-beam irradiation for various keloid sites (10, 15, or 20 Gy), and reported an overall recurrence rate of 14% since 2003. They recommend high-risk sites (trunk, suprapubic region) be treated with 20 Gy in four fractions over 4 days, and that the earlobe should be treated with 10 Gy in two fractions over 2 days.
Pterygia
Radiation is applied postoperatively using a strontium-90 applicator, which replaced earlier generations of beta-ray applicators when strontium-90 became available in the 1950s. Strontium-90 (half-life, 28.1 years; maximal beta energy, 0.54 meV) is in secular equilibrium with yttrium-90 (half-life, 64 hours; maximal beta energy, 2.27 meV). The dose is rapidly attenuated, with approximately 38% of the dose at 2-mm depth, and 10% at the 4-mm depth of the lens.25
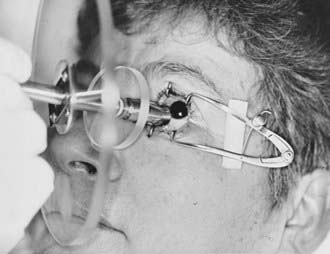
Radiation is delivered by the direct application of the strontium-90 applicator to the surgical bed, with a 1- to 2-mm margin. Lid retractors are often used to facilitate visualization and placement of the source (Fig. 68-1). The dose is determined by controlling the time the applicator is in contact with the eye. The time interval from surgery to the first strontium-90 application is important. Treatment is generally initiated within 48 hours of excision, with some authors recommending the initiation of radiation on the day of excision. Wilder and colleagues26 reported an improved local control rate (87% versus 62%; P = 0.005) for those patients in whom radiation began 1 to 8 versus 16 to 24 hours after excision.
Various dose-fractionation schemes have been used, ranging from 18 Gy in a single fraction27 to 60 Gy in 6 × 10 Gy fractions.28 A common dose-fractionation scheme is 3 × 8 Gy, with the first fraction delivered on the day of excision and the second and third doses 1 and 2 weeks later. Although there is not a clearly demonstrable dose-response relationship, a number of authors have reported improved results in patients who received three fractions (8 to 12 Gy) compared with only one or two fractions (8 to 12 Gy).26,29,30 Recurrence rates after the use of radiation have been low, ranging from approximately 2%28,29 to approximately 12%26,30–33 across a range of major reported series. Differences in results among series may be due to patient selection and the intensity and duration of follow-up, as well as the dose-fractionation schemes, time between surgery and radiotherapy, and technique employed.
Complications are reported after radiation treatment. It is not uncommon for patients to have conjunctival irritation, which can persist for several weeks to months after treatment in rare cases. Other complications, such as corneal ulceration, scleral thinning, granuloma formation, and telangiectasia formation, have been reported. The exact incidence of significant complications is difficult to define, but MacKenzie and colleagues32 estimated a 4.5% incidence of severe scleral thinning in a large Australian cohort that had been followed for at least 10 years.
Concern regarding cataractogenesis surrounds the role of radiation treatment for pterygia, but it is not clear that it occurs. Van den Brenk29 reported an absence of radiation-induced cataracts in a series of more than 1000 cases treated, and several studies26,34 have reported no difference in the number of cataracts in irradiated versus nonirradiated eyes if dose-fractionation schemes such as 8 Gy × 3 are used. In this fractionation scheme, the dose to the lens would be approximately 0.8 Gy × 3, below the threshold for cataractogenesis.
In patients who have undergone reirradiation of recurrent pterygia, higher recurrence rates and more severe complications have both been observed.26,35 Reirradiation of pterygia should be approached with caution, but the rate of serious complications after primary treatment is low.
Thyroid Ophthalmopathy
Thyroid ophthalmopathy occurs most frequently in association with Graves disease but can arise in association with other conditions such as Hashimoto thyroiditis. Euthyroid Graves ophthalmopathy may also occur. The cause of Graves disease is not certain, but thyroid ophthalmopathy arises when circulating T cells directed at an antigen on thyroid cells recognize a similar or closely related antigen on orbital fibroblasts or extraocular myocytes. These T cells then infiltrate orbital connective tissues, producing an autoimmune reaction whereby glycosaminoglycan production by fibroblasts is increased. This produces an increase in connective tissue volume and the resultant clinical manifestations of reduced extraocular muscle mobility, diplopia, exophthalmos, and periorbital edema. Histopathologically, these changes are characterized by lymphocytic infiltration of orbital tissues (primarily by T cells) and by the accumulation of glycosaminoglycans in orbital fat and extraocular muscles.36,37
Although most patients with Graves disease have some measure of exophthalmos or increased size of extraocular muscles when compared with normal subjects, clinically evident ophthalmopathy is present in approximately 40% of patients. Use of an exophthalmometer can assist in the assessment and follow-up of patients with thyroid ophthalmopathy. Clinical manifestations range from subtle changes of periorbital edema or exophthalmos, sometimes associated with lid retraction and stare, which may be primarily a cosmetic concern, to situations in which the eye or vision may be threatened. Severe exophthalmos can leave the cornea exposed to inflammation and potential injury. Decreased motion of extraocular muscles and orbital compression can lead to diplopia, pain, optic neuropathy, and the potential for permanent loss of vision.38
Except in circumstances in which there is the threat of impending visual loss, management of ophthalmopathy should begin with treatment of the underlying thyroid disorder by appropriate means (i.e., thyroidectomy, antithyroid drugs, radioactive iodine). For better or worse, there seems to be greater consensus and success surrounding the management of the thyroid condition than the associated ophthalmopathy.38 A wide range of therapeutic options have been proposed and advocated for the treatment of thyroid ophthalmopathy. For some patients with mild ophthalmopathy, no treatment at all or simple symptomatic remedies such as eye drops and sleeping with the head of the bed elevated to reduce periorbital edema may be sufficient, whereas for patients in whom there is rapid progression, neuropathy, and imminent loss of vision, emergency decompressive surgery may be appropriate.39 Between those extremes of therapy are the options for medical management with corticosteroids, cyclosporine, or other immunosuppressive agents40,41 and orbital radiation therapy.
Results of the use of orbital radiation therapy in the treatment of thyroid ophthalmopathy have been reported in many retrospective single-institution studies.42–46 The rationale for radiotherapy in this disorder is straightforward: Ophthalmopathy is produced by, or in association with, a local autoimmune infiltration of lymphocytes. Lymphocytes are sensitive to radiation; radiation beams can be collimated so that an adequate dose can be precisely delivered to the orbits without significant sequelae and without the potential side effects associated with systemic therapies.
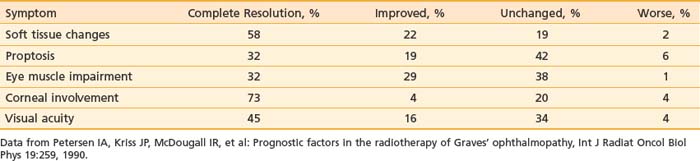
Petersen and colleagues45 reported on the Stanford experience in 311 patients treated with megavoltage radiation therapy from 1968 to 1988. The majority of patients were treated to doses of 20 Gy in 10 × 2 Gy fractions. A cohort of 69 patients were treated from 1979 to 1983 with a higher dose, 30 Gy in 15 × 2 Gy fractions, and although no increased complications were reported, there was no additional benefit to higher dose levels, and the treatment policy returned to 20 Gy in 10 fractions. Techniques were used to minimize the dose to the contralateral lens, initially using lateral fields angled 5 degrees posteriorly; later, a half-beam block (beam-split technique) was used to eliminate divergence anteriorly. Before treatment more than 90% of patients had soft tissue changes and involvement of extraocular muscles, approximately 66% had proptosis, approximately 50% had decreased visual acuity, and approximately 20% had corneal involvement.
Results obtained in the Stanford series showed a high rate of symptom response and a low rate of symptom progression after radiation therapy, as shown in Table 68-1.45 Worsening of symptoms occurred in only 1% to 6% of patients, with rates of complete resolution or improvement of symptoms ranging from 51% for proptosis, 61% for visual acuity and impairment of eye muscles, 77% for corneal involvement, and 80% for soft tissue changes. Of the one third of patients who were on steroids when beginning radiation, 76% were able to discontinue corticosteroid use within several months of completing radiation therapy.
Table 68-1 Response of Symptoms to Orbital Radiotherapy for Thyroid Ophthalmopathy: Stanford Experience with 311 Patients, 1968-1988
Side effects from radiation therapy for ophthalmopathy are typically minimal, and the time course to symptomatic improvement can be rapid. In the Stanford experience, about 10% of patients developed acute side effects, primarily transient soft-tissue inflammation.45 No long-term side effects were reported. In a series from the University of Pennsylvania, Sandler and colleagues44 reported acute symptoms of conjunctival irritation in 12 of 35 patients. Early symptomatic improvement often occurs, with objective signs of response to radiation occurring as early as 2 days into treatment, with a mean time to the first objective response of 14 days after the first fraction of radiation. However, the time to maximal clinical response is variable and can be prolonged, with a range of 1 to 24 months (mean 5.6) after the initiation of therapy.46
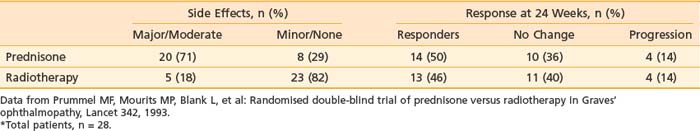
Although observational studies suggest that orbital radiation can lessen soft-tissue changes, reduce proptosis, and result in overall clinical improvement, randomized trial data indicate that the effect of orbital radiation may be limited to improving ocular dysmotiliy or halting its progression.47 Prummel and colleagues48 reported a double-blind, prospective, randomized study in which patients were assigned to receive either oral prednisone or radiotherapy (20 Gy using opposed lateral fields). Patients received either placebo or sham irradiation in addition to the prescribed therapy. Results are summarized in Table 68-2. The response rate to treatment was similar in both arms of the study (50% versus 46%). However, there was a much higher incidence of moderate to severe complications in the prednisone arm (e.g., weight gain, cushingoid facies, pyrosis requiring treatment with ranitidine, behavioral changes) than in the radiotherapy arm, favoring the use of radiotherapy by the authors.
Table 68-2 Double-Blind Randomized Trial of Prednisone Versus Radiotherapy for Thyroid Ophthalmopathy*
Orbital radiotherapy has also been evaluated in combination with systemic corticosteroid therapy.49–51 Ng and colleagues49 reported a randomized trial of combined orbital irradiation and systemic steroids compared with steroids alone in 16 patients with moderate to severe Graves ophthalmopathy and short duration of symptoms (3 months). The steroid therapy consisted of 3 days of intravenous (IV) methylprednisolone followed by oral therapy for 4 weeks, and radiotherapy followed within 2 weeks (20 Gy in 10 fractions). Both groups experienced improvement in soft-tissue swelling and ocular motility at 52-week follow-up, but the combined-modality group experienced earlier improvement of symptoms at 4 weeks. Proptosis was not improved in either group. Zoumalan and colleagues50 from Stanford evaluated the effectiveness of steroids combined with orbital irradiation in a literature review and found an overall response rate with corticosteroid treatment alone of 66.9% in a total of 834 patients with moderate or severe thyroid eye disease. IV corticosteroids in weekly pulses were more effective and had fewer side effects than daily oral steroid therapy (74.6% versus 55.5% response rate). The combination of IV steroid therapy and orbital irradiation resulted in the highest overall favorable response rate (52 out of 60 patients, or 86.7%). The authors caution that given the differences between the studies, there is inadequate evidence to ascertain reliably whether medical therapy with corticosteroids or radiation therapy improves long-term disability in patients with Graves ophthalmopathy.
Long-term complications are rare after radiation therapy for ophthalmopathy. Although cataractogenesis is a concern when orbital radiation is used and it is prudent to advise the patient that cataracts may develop, the threshold for cataractogenesis from fractionated radiation therapy is about 4 Gy,34 and in most cases with the use of either beam-split or posteriorly angled fields, the dose to the lens is about 5%.43 The thresholds for other optic complications of radiation, such as optic neuropathy, retinal injury, or severe dry eye resulting from lacrimal gland injury, are generally more than 20 Gy,52 although retinal injury has been reported after orbital irradiation for Graves ophthalmopathy.53,54 Wakelkamp and colleagues53 reported on the long-term complications of orbital irradiation in comparison with glucocorticoids in a cohort of 245 Graves ophthalmopathy patients treated with 20 Gy in 2 weeks between 1982 and 1993 in a single institution. At a median follow-up time of 11 years, mortality was similar in irradiated and nonirradiated patients, and no patient died from intracranial tumor. Of the 104 irradiated patients available for opthalmologic follow-up, 88 also received oral glucocorticoid therapy, and the prevalence and severity of cataract were similar in the radiotherapy group (29%) and the glucocorticoid-alone group (34%). Possible retinopathy (one or more hemorrhages or microaneurysms on retina photographs) was present in 15% of patients (22 of the irradiated, and one of the nonirradiated patients). In five irradiated patients, definite retinopathy was present (more than five retinal lesions). Diabetes was associated with both possible and definite retinopathy with a relative risk of 21, leading the authors to suggest that diabetes may be a contraindication to orbital radiation.53 Bradley and colleagues47 estimated the risk of retinopathy after orbital irradiation for Graves ophthalmopathy to be 1% to 2% within 10 years after treatment. As with all situations in which ionzing radiation is used, there is a theoretical risk of carcinogenesis that must be considered. However, patients treated with orbital radiation have not been demonstrated to have an increased risk of secondary malignancy or premature death.53,54
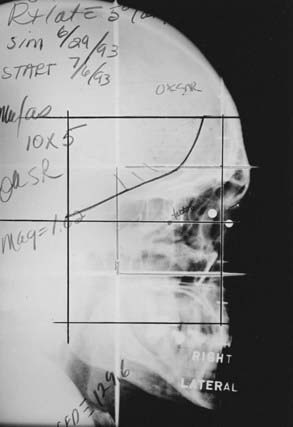
Two techniques to minimize divergence into the contralateral lens are commonly used. The lateral fields can be angled 5 degrees posteriorly. Alternatively, a nondivergent beam edge can be created using either a half-beam block or the asymmetrical collimation capabilities of certain linear accelerators, avoiding increased posterior divergence.55 In most cases, there is some degree of bilateral involvement, so both orbits are treated. A representative simulation film is shown in Fig. 68-2.
Orbital Pseudotumor (Orbital Lymphoid Hyperplasia; Orbital Pseudolymphoma)
Orbital pseudotumor, orbital lymphoid hyperplasia, and orbital pseudolymphoma are terms used to describe benign orbital mass lesions in which mature lymphocytes are noted to infiltrate orbital structures such as orbital fat, extraocular muscles, lacrimal glands, or other extraocular orbital structures. Some authors56 have attempted to distinguish orbital pseudotumor from orbital lymphoid hyperplasia, but these distinctions are difficult and of uncertain prognostic or therapeutic importance. However, in general, lymphoid hyperplasia arises in anterior ocular structures, and pseudotumor arises more posteriorly in the bony orbit. In practice and in many reports, the terms for these benign entities are used interchangeably and the cases intermingled. However, it is important to distinguish between the benign conditions, which are referred to as orbital pseudotumor in this section, and malignant orbital lymphomas, which can have similar clinical presentations and histopathologic appearance. Recent developments in immunology, electron microscopy, and flow cytometry have made it easier to differentiate benign (polyclonal) from malignant (generally monoclonal) orbital lymphoid disorders with the use of special studies.57 It is also important to rule out infectious causes of orbital inflammation (e.g., spread of an adjacent sinus infection, trichomonal infection), as well as endocrine and autoimmune disorders (e.g., thyroid ophthalmopathy, discussed previously), which can have similar clinical presentations. Because of the difficult differential diagnosis associated with this disorder, a thorough and careful complete history and physical examination, along with the use of appropriate laboratory and radiologic studies, is important before radiotherapy for orbital pseudotumor is initiated.
Clinical presentation of orbital pseudotumor is characterized by symptoms of soft tissue swelling, orbital pain, proptosis, extraocular muscle involvement, and, occasionally, decreased visual acuity (Table 68-3). Bilateral involvement is much less frequent than with thyroid ophthalmopathy. Computed tomography (CT) scans may demonstrate mass lesions anywhere in the orbit, often multiple, with the posterior globe a common site of involvement.58,59 Overall, orbital pseudotumor is quite rare. Although precise figures are not available, some authors estimate that the combination of orbital pseudotumor and primary orbital lymphomas make up approximately 1% of both primary orbital neoplasms and primary lymphoid neoplasms.60,61 Some controversy exists regarding the need for biopsy before therapy is initiated, with some authors believing that the diagnosis can be made on clinical grounds, after radiographic, ophthalmologic, and laboratory evaluation.59 However, given the unusual nature of this disorder and the similarity in clinical presentation to malignant orbital lymphomas, it is prudent to obtain a biopsy if this can be performed without significant morbidity.
Table 68-3 Symptoms of Orbital Pseudotumor and Response to Radiation Therapy
| Symptom | Percentage Presenting With Symptom | Percentage With Complete Response after RT |
|---|---|---|
| Soft tissue swelling | 92 | 87 |
| Pain | 92 | 75 |
| Proptosis | 85 | 82 |
| Extraocular muscle dysfunction | 69 | 78 |
| Decreased visual acuity | 19 | 60 |
RT, Radiotherapy.
Data from Lanciano RM, Fowble B, Sergott RC, et al: The results of radiotherapy for orbital pseudotumor, Int J Radiat Oncol Biol Phys 18:407, 1990.
In most cases, orbital pseudotumor responds to treatment with corticosteroids, with approximately 50% of patients having a durable complete response, and steroids are generally used as first-line therapy.62 However, radiation therapy has been used successfully and with low morbidity for those patients who have contraindications to steroid therapy, develop unacceptable complications while on steroids, or relapse after steroid therapy. Results of radiation therapy for orbital pseudotumor have often been combined with results of the radiation treatment of orbital lymphomas. As orbital lymphomas commonly occur localized to the orbit, the primary difference in management with radiation therapy is dose. Doses of 20 Gy in 10 fractions are commonly used in the treatment of pseudotumor, with higher doses generally used for malignant lymphomas. Overall durable complete response rates range from 75% to 100%.59–61,63–65
Lanciano and colleagues59 reported a series of 26 orbits in 23 patients treated with radiation for orbital pseudotumor. The mean follow-up was 53 months. In that series, 20 patients (87%) had a trial of corticosteroids before radiotherapy, with 15 (75%) having a complete response, 1 (5%) a partial response, and 4 (20%) no response. However, 16 patients (70%) were referred because clinical relapse was noted with steroid taper. Twelve patients (52%) developed significant morbidity to steroids, including aseptic necrosis, cushingoid facies, psychiatric disorders, gastritis, and pseudotumor cerebri. All patients were treated to 20 Gy in 10 × 2 Gy fractions. Symptomatic response is shown in Table 68-3. At the time of last follow-up, 66% had a durable complete response and required no further steroid treatment; 11% had local relapse 30 to 34 months after radiation therapy but subsequently achieved complete response again either spontaneously or with additional steroid treatment; 11% had partial responses to radiation; and three patients (11%) had no response to radiation. There were no cases of progression during or after radiotherapy. Acute side effects of irradiation were minimal, with 35% of patients having minor signs or symptoms; there were no radiation-related cataracts or other significant late morbidities related to the radiation treatment.
Various radiation treatment techniques have been used for orbital pseudotumor. In some cases, techniques similar to those used for thyroid ophthalmopathy are used, but as fewer patients have bilateral disease and the location of disease within the orbit is more variable than in thyroid ophthalmopathy, treatment must be individualized. CT or magnetic resonance imaging (MRI) of the orbit are necessary in most cases to adequately define the extent of disease, and CT planning can be helpful in evaluating and selecting treatment plans. Single and opposed lateral photon fields, anterior electron fields, wedged-pair techniques, and intensity-modulated radiation therapy (IMRT) have all been used. Patients with conjunctival or other anterior disease and no posterior disease may be well treated with an anterior electron field, whereas patients with disease in the retrobulbar region may be best treated with lateral photon fields. If anterior photon or electron fields are used, lens blocks may be added to reduce the dose to the lens and the chance of subsequent cataract formation.66
Progression to systemic lymphoma occurs in a small number of patients and must be a concern in continued follow-up of these patients. In individual series, progression has been noted in 0% to 25% of patients.67 In a literature review, Lanciano and colleagues59 reported that 12 of 101 (12%) patients subsequently developed systemic disease. In addition, a total of 14 of 101 (14%) had bilateral disease at presentation, with an additional 6 of 101 (6%) developing bilateral disease after therapy.59
In a small series, the anti-CD20 monoclonal antibody rituximab has shown activity in the treatment of benign orbital pseudolymphomas. Of 11 patients selected for treatment with rituximab at the Mayo Clinic, 10 (91%) responded. Of the 11 patients treated, 2 had previously been treated with radiotherapy, and 1 of these 2 patients responded to rituximab.68
Lymphoid Hyperplasia
Areas of benign lymphoid hyperplasia can arise outside the orbits, especially in the Waldeyer ring and the skin. Cases of cutaneous lymphoid hyperplasia have been reported in conjunction with acquired immunodeficiency syndrome, and progression to malignant lymphoma occurs in some cases. In pathologic examination, the disease is characterized by a cutaneous infiltrate of lymphocytes in the upper dermis associated with germinal centers underlying a typically normal epidermis. The appearance is similar to that of Spiegler-Fendt sarcoid and malignant lymphoreticular neoplasms. Treatment alternatives included corticosteroid treatment and excision. For cases refractory to conventional therapy, persistent local control has been reported with doses of 12 to 20 Gy with minimal morbidity.69–71
Heterotopic Ossification
HO, also known as heterotopic or ectopic bone formation, has long been recognized as a complication of THA. The use of THA began in the late 1960s, and by the 1970s a significant number of cases of HO had been described. The presence of HO can be demonstrated radiographically and can cause significant symptoms, such as pain, impaired joint mobility, or complete ankylosis of the joint. The exact incidence of HO is not known; incidence figures ranging from 8% to 90% have been reported, depending in large part on the risk factors (see later discussion) in each series, as well as the intensity of postoperative surveillance. Brooker and colleagues72 proposed an HO classification that is in general use (Table 68-4).
Table 68-4 Brooker Classification of Heterotopic Ossification
| Class | Findings |
|---|---|
| I | Isolated bone islands |
| II | Bone spurs; separated by >1 cm |
| III | Bone spurs; separated by <1 cm |
| IV | Apparent bony ankylosis |
Data from Brooker AF, Bowerman JW, Robinson RA, et al: Ectopic ossification following total hip replacement: Incidence and a method of classification, J Bone Joint Surg Am 55:1629, 1973.
A number of risk factors for HO have been described. These include male sex, age older than 60 years, previous HO in either hip, post-traumatic arthritis, hypertrophic osteoarthritis, or active ankylosing spondylitis. Surgical technique, or perioperative complications such as hemorrhage, do not appear to be a significant predisposing factors.73–75
As the variety of risk factors for HO suggests, the cause of this disorder is not well understood. However, it has been theorized that in the setting of significant trauma or surgery, pluripotent mesenchymal cells are induced to differentiate into osteogenic progenitor cells. These cells subsequently develop osteoblastic tissue, resulting in the production of heterotopic bone.76
The time course to formation of heterotopic bone after surgery has been characterized. Postoperatively, ectopic bone generally becomes visible radiographically by 3 to 6 weeks. By 8 weeks the bone mass may be complete, but not matured. Maturation of bone occurs within 6 to 12 months, with little change noted subsequently. Once the heterotopic bone has formed, there is little in the way of effective therapy short of reoperation.73,74
The difficulties presented by heterotopic bone formation led to an active interest in effective means of prophylaxis. In 1977, Ritter and Vaughan73 wrote, “To our knowledge, there is no way to prevent the formation of heterotopic bone or its recurrence after surgical resection.” Since that time, a wide variety of therapies have been proposed, including transplantation of fat into the operative bed and the use of diphosphonates, which may have significant systemic side effects on calcium and phosphate metabolism. In a recent review, the only methods of prophylaxis recognized as being effective were radiation therapy and the use of anti-inflammatory drugs.75
Nonsteroidal anti-inflammatory drugs (NSAIDs), and the drug indomethacin in particular, have been used effectively to prevent HO. The mechanism of action is not well defined, but initiation of therapy within the first 5 days of surgery may be important. In a randomized study, Schmidt and colleagues77 found that patients receiving 25 mg of indomethacin three times daily for 6 weeks postoperatively had decreased rates of HO compared with patients treated with placebo. However, indomethacin has the potential for significant gastrointestinal, hematologic, renal, and neurologic side effects, and there is some concern that it may promote loosening of the noncemented components of hip prostheses.75 In a series of 74 high-risk patients who received indomethacin after THA, Cella and colleagues78 reported that 37% were unable to complete the prescribed course of NSAID therapy. More recently, naproxen and diclofenac have been shown to be effective for HO prophylaxis after THA and can be considered as alternative first-line treatments.79
Radiation therapy has been shown to be a safe and effective prophylactic treatment for HO. In the 1970s, prophylactic treatment with radiation was first initiated. At the Mayo Clinic, 20 Gy in 10 fractions was chosen somewhat arbitrarily as a dose likely to impede bone formation (based on experience with radiation-induced bone-growth retardation in pediatric patients), yet not high enough to be associated with significant morbidity. From 1970 to 1977, 48 hips in 42 patients were treated in this manner, using megavoltage equipment and large (12 to 18 × 14 to 20 cm) radiation portals. In general, care was taken to exclude the lateral skin incision from the radiation portals. Patients who started radiation treatment early (within the first 2 to 10 postoperative days) had effective prophylaxis against HO. However, patients in whom the initiation of radiation was delayed until after radiographic evidence of heterotopic bone formation appeared (21 to 69 postoperative days) had regression noted in only 1 of 12 cases. No significant complications attributable to radiotherapy were noted.74
Since the initial report by Coventry and Scanlon,74 many reports documenting the efficacy of radiotherapy as a prophylactic measure have appeared. Select series are listed in Table 68-5. There has been an evolution in technique and doses in the past 10 to 15 years (see later discussion), but several general principles remain unchanged: (1) radiotherapy is effective if delivered prophylactically, but not once heterotopic bone has been established; (2) radiotherapy should be initiated early postoperatively (0 to 96 hours); and (3) radiotherapy is associated with a low level of complications.
Table 68-5 Select Series of Postoperative Radiation Therapy for Prophylaxis Against Heterotopic Ossification
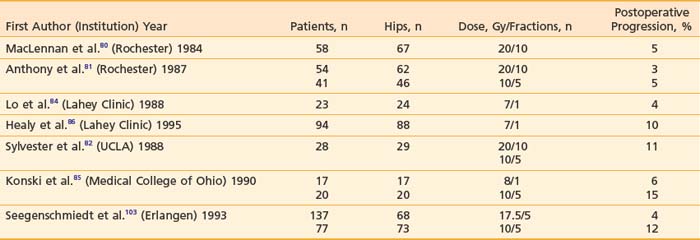
Radiation dose and fractionation schemes have changed since early reports. In an early series, MacLennan and colleagues80 from the University of Rochester obtained good results using 20 Gy in 10 × 2 Gy fractions. Subsequently, using the cohort of patients treated with 20 Gy as historical controls, Anthony and colleagues81 from the same institution demonstrated that 10 Gy in five fractions produced equivalent results. Similar findings have been reported by investigators from the University of California, Los Angeles, where no increase in failure rate was observed with 10 Gy compared with 20 Gy, and total doses of 7 to 8 Gy in one to two fractions were also found to be effective.82,83
Low-dose fractionated radiation therapy has since been replaced at many institutions with single-fraction radiotherapy. This has the main advantage of being easier logistically. Only one trip to the radiation oncology department may be required, and the number of times a patient has to be transported is dramatically reduced. Furthermore, with single-fraction therapy, the treatment can be completed while the patient remains hospitalized in the postoperative period. Doses used are generally 7 to 8 Gy in a single fraction. Lo and colleagues84 have reported good results with a single fraction of 7 Gy delivered within 72 hours postoperatively, with only 4% of patients developing recurrent disease. In a randomized trial, Konski and colleagues85 found no significant difference in recurrence rates between patients treated with 8 Gy in a single fraction and 10 Gy in five fractions. No significant side effects were reported in either patient group. The effectiveness of lower dose single fraction treatment was evaluated by Healy and colleagues86 comparing single-fraction 7 Gy with 5.5 Gy. This retrospective study of 107 hips treated after THA within 3 days of surgery demonstrated HO in 10% of those receiving 7 Gy and in 63% of those receiving 5.5 Gy in a single fraction. The authors concluded that 5.5 Gy is not a sufficient dose for HO prophylaxis. Although single-fraction doses of 7 to 8 Gy have been demonstrated to be effective, the lower limit of effective radiotherapy dose for HO prophylaxis remains to be determined.87
The role of preoperative prophylaxis for patients undergoing planned hip surgery at high risk of HO has also been studied. Kantorowitz and colleagues88 experimented with the use of preoperative radiation in a rat model. They found that 8 Gy given 1 hour preoperatively was as effective as a similar dose given 2 days postoperatively. This concept has subsequently been tested in a randomized clinical trial by Gregoritch and colleagues,89 with 122 patients randomized to receive a single 7 Gy fraction of radiation either within 4 hours before surgery or within 72 hours after surgery. The authors noted no significant difference between the treatment groups with clinically significant HO in 2% of the preoperative group and in 5% of the postoperative group. Preliminary results indicate that the preoperative approach may be as effective as postoperative radiotherapy, without increased risk of complications. Preoperative treatment would have potential advantages with respect to patient comfort, avoiding the need to transport the patient for radiation treatment in the immediate postoperative period.
Although most experience with the use of radiotherapy for heterotopic bone prophylaxis comes from older patients, there is evidence that prophylactic radiation is also of value in young adults who suffer traumatic hip fractures. Slawson and colleagues90 from the University of Maryland reported on 50 patients who presented to the trauma unit at their institution with acetabular fractures. Thirty consecutive patients (mean age 34 years) were treated with postoperative radiation (total dose of 10 Gy in five fractions), and 20 consecutive patients (mean age 28 years) treated immediately before the institution of postoperative radiation were selected as historical controls. There was a significant difference in the number of patients who developed severe (Brooker grade III to IV) HO in the two groups. Only 3 of 30 (10%) patients treated with radiotherapy developed significant HO compared with 10 of 28 (36%) who did not receive irradiation (P < 0.01). Complication rates were similar in both cohorts, and the authors concluded that radiation is useful and effective in the setting of traumatic acetabular fractures.
Multiple randomized trials have now been reported comparing single-fraction radiotherapy to 6 weeks of indomethacin for HO prophylaxis after surgical fixation of traumatic acetabular fractures. Burd and colleagues91 randomized 166 patients to receive 25 mg of indomethacin three times daily for 6 weeks or single-dose 8 Gy. Grade III to IV ossification was seen in 14% of indomethacin-treated patients and 7% of irradiated patients (P = 0.22). The control group received no prophylaxis and experienced a 38% rate of significant ossification. This study also demonstrated a significant increase in long-bone nonunion in patients receiving indomethacin compared with radiotherapy (26% versus 7%, P = 0.004). The authors concluded that indomethacin was not statistically inferior to radiotherapy for HO prevention. In contrast, Karunakar and colleagues92 from the University of Michigan compared 75-mg sustained-release indomethacin for 6 weeks to placebo in a randomized trial for HO prophylaxis after surgical treatment of acetabular fractures, and found no stastically significant reduction in severe HO with the use of indomethacin. Brooker grade III to IV ossification occurred in 9 of 59 patients (15.2%) in the indomethacin group and 12 of 62 patients (19.4%) in the control group. Of 63 patients in the indomethacin group, 13 withdrew during the 6-week treatment period because of side effects, indicating that compliance with this regimen was difficult. More recently, Pakos and colleagues93 performed a meta-analysis of seven randomized trials (1143 patients) comparing radiation therapy with NSAIDs for HO prophylaxis after surgical treatment for acetabular fractures or THA. They demonstrated irradiation to be more effective than drug treatment in preventing grade III or IV ossification. However, the absolute risk difference was only 1.2%. In contrast to indomethacin, the effectiveness, timing, and dose of radiotherapy for HO prophylaxis in patients treated for acetabular fractures has been demonstrated to be equivalent to patients treated with THA.94
The most concerning potential side effect of radiotherapy for HO prophylaxis, particularly in the younger trauma population, is carcinogenesis. To date, there have been no documented cases of radiation induced tumors after radiotherapy for HO prophylaxis.87 Kim and colleagues95 reported no cases of bone or soft-tissue sarcomas in their 50-year experience of radiation-induced sarcomas in patients exposed to doses lower than 30 Gy. With the latency for radiation-induced tumors typically 10 years or more, it is possible that the lack of second malignancy is due to the small number of young people treated in the past with radiotherapy and followed for many years. This potential risk is a consideration for young-adult trauma patients referred for HO prophylaxis with radiotherapy.
Radiation dose to the testis in men is also an issue of concern, given the potential for reduction in sperm counts and the theoretical risk of hereditary effects.94,96 Doses as low as 0.2 to 0.7 Gy can result in reversible oligospermia with recovery within 9 weeks, whereas permanent effects on fertility are not seen with less than 1.2 Gy.96 Oertel and colleagues94 calculated the average dose in the testis after HO prophylaxis using a Yale phantom to be 0.21 Gy. Testicular shielding can reduce the dose to 0.1 Gy and may be employed for young patients.94
The nature of radiation treatment fields used for prophylaxis of heterotopic bone formation has changed as the nature of hip prostheses has changed. In the 1970s and early 1980s, cemented hip prostheses were primarily used, and large fields were often treated. However, loosening of hip implants remained a significant indication for revision surgery, and alternative methods to cement were sought for securing implants. In recent years, porous, coated hip implants have been more frequently used. In these procedures, the porous implant is anchored physiologically by bone ingrowth (biologic fixation). This process has physiologic similarities to the process of heterotopic bone formation. This led to concern that postoperative radiation for HO would not only prevent ectopic bone formation but might also prevent secure fixation of the prostheses. This hypothesis was tested in a rabbit model by Konski and colleagues97 from Rochester, and decreased strength of prosthetic devices was demonstrated in the initial 2- to 3-week period after surgery, although not in the longer term. However, because of these concerns, treatment fields have been modified, and reduced field sizes have been successfully used in patients with porous prostheses. Although the fields used with cemented prostheses were large (about 14 × 16 cm),74 the fields used with porous prostheses are much smaller, with care taken to shield the porous components of both the femoral and the acetabular prosthesis. A typical field used in our department is on the order of 8 × 4 cm. An example is shown in Fig. 68-3. No significant complications or decreases in efficacy have been demonstrated with the use of restricted fields.81,82,85

FIGURE 68-3 • Megavoltage film of a treatment field for heterotopic ossification prophylaxis in a patient with a porous prosthesis.
Radiotherapy has been used as prophylactic treatment for HO in joints other than the hip. Use of radiation in the temporomandibular joint (TMJ) has been described by Durr and colleagues98 in a series of 15 TMJs in 10 patients who underwent arthroplasty or debridement of heterotopic bone in the TMJ. Patients received a modal dose of 10 Gy in five fractions, with irradiation generally beginning within 2 days of surgery. Treatment was effective, with improvement over preoperative levels of ectopic ossification noted in 13 of 15 (87%) TMJs. Acute toxicity was acute parotitis reported in three patients, with no long-term complications reported.
Radiation therapy to prevent HO of the elbow has been reported by Wolfson and colleagues.99 A series of 19 elbows at high risk for HO was treated with 10 Gy in five fractions postoperatively after excision of HO. Only 1 in 19 (5.3%) recurred, and no complications were observed. The successful use of radiation (10 Gy in five fractions) as prophylaxis against HO in the elbow, as well as in the wrist and shoulder, has also been reported by Rubenstein and colleagues.100 Recently, the use of single-fraction radiotherapy (7 to 8 Gy) has been used as prophylactic treatment for HO of the elbow.101,102
The role of radiation in HO prophylaxis continues to evolve. Work to optimize the dose-fractionation schemes continues, and other innovations are also developing. For example, the potential that radiation combined with NSAID use may be better than either modality alone as a prophylactic measure has been suggested by a group from Erlangen.103 They found a failure rate of 3% for patients treated with radiation and NSAIDs for 6 weeks postoperatively compared with 12% for patients treated with radiotherapy alone (p < 0.05). It is not certain if this approach will prove efficacious and safe in further studies.
Paragangliomas (Glomus Tumors; Chemodectomas)
Paragangliomas are tumors that arise from the paraganglia, collections of cells of neural crest origin dispersed throughout the body in association with the autonomic nervous system. They are histologically related to pheochromocytomas (which are chromaffin-positive). Nonchromaffin paragangliomas often arise from chemoreceptor tissues in the head and neck area and are also known as chemodectomas, or glomus tumors. The nomenclature can be confusing and imprecise, with the related terms often used interchangeably. Glomus jugulare tumors arise in the area of the jugular bulb, glomus tympanicum tumors in the middle ear, and glomus vagale tumors in the area of the ganglion nodosum adjacent to the vagal nerve. Paragangliomas that arise in the chemoreceptor cells near the carotid bifurcation are also called carotid body tumors. Although these sites in the neck, base of skull, and temporal bone are the most common, paragangliomas have been reported at a multitude of anatomic sites throughout the body. Paragangliomas arising in the head and neck are of the greatest concern to radiation oncologists.104–106
Glomus tumors are generally considered to be benign, although rare (<5%) cases of metastases, most commonly to the lungs, have been reported. Occasionally, patients may secrete catecholamines, serotonin, or similar substances, producing severe hypertension, flushing, and associated symptoms. If the history suggests, appropriate laboratory studies should be ordered. There may be some association with multiple endocrine neoplasia syndromes. A family history is present in approximately 10% of cases, and there is a significant incidence of bilaterality in familial cases.107,108 Glomus tumors are rare, but the peak incidence is between 40 and 60 years, with a higher incidence noted in patients suffering from chronic hypoxia or living at high altitudes. Although glomus tumors are generally highly vascular, they are made up of cells that are histologically similar to normal paraganglia associated with epithelioid tissue in a multitude of thin-walled blood vessels.104–106
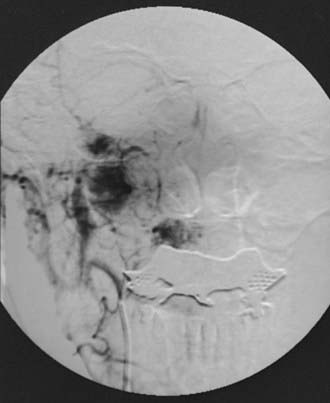
If the diagnosis of a glomus tumor is suspected, workup should proceed with a careful history and physical examination. CT or MRI imaging of the neck and base of skull should be performed. Angiography is helpful in establishing a diagnosis and is a recommended part of the preoperative evaluation if surgery is to be contemplated as a treatment option. An angiogram showing the characteristic appearance of a glomus jugulare tumor is shown in Fig. 68-4. The radiographic picture is generally sufficiently characteristic to establish a definitive diagnosis; histologic proof is not necessary except in unusual cases, especially as biopsy of these very vascular lesions can involve significant morbidity.
Optimal treatment of these histologically benign tumors remains controversial. The standard treatment option is surgical resection, which, based on the complex anatomy surrounding these tumors, can carry high morbidity and mortality rates. Despite improvements in microsurgery, complications from resection include stroke (8%-20%) and cranial nerve injury (33%-44%), with an overall mortality rate of 5% to 13% in the literature.109 The anatomy of cranial nerves and vessels within the jugular bulb and through the tumor often prevents a complete surgical resection.
Historically, external-beam radiation therapy has been used as an adjunct to surgery since the 1950s, and later was used alone for patients who were poor surgical candidates. Older radiation therapy techniques employed large fields of radiotherapy, often via wedge-pair or opposed lateral plans to doses in the 45 to 60 Gy range.110,111 Although local control after radiotherapy in these older studies exceeded 90%, serious sequelae of radiation therapy occurred in 2% to 3% of patients, including bone necrosis, brain necrosis, xerostomia, and rare second malignancy.112–114 The introduction of IMRT115 stereotactic fractionated radiotherapy115,116 and gamma knife radiosurgery (GKR)109,116,117 has allowed delivery of small-volume radiotherapy to these benign tumors in the sensitive head and neck region with greater sparing of surrounding normal tissue, and has essentially replaced the large-field radiotherapy of the past in the treatment of these tumors.
The 35-year experience of Hinerman and colleagues115 reflects the evolution of radiotherapy treatment for paragangliomas of the head and neck over time. A total of 121 paragangliomas in 104 patients were treated from 1968 to 2004 at the University of Florida. Of the 121 tumors, 89 (86%) were treated with conventional megavoltage techniques, 15 (14%) were treated with stereotactic fractionated radiation therapy (SFRT), 6 patients (6%) were treated with stereotactic radiosurgery, and 11 patients (11%) were treated with IMRT. In the series, there were six local recurrences with a reported 10-year actuarial local control of 94% and 10-year cause-specific survival rate of 95%. The incidence of treatment-related complications was low. Henzel and colleagues116 also reported good outcome treating glomus tumors with SFRT. Between 1999 and 2005, 17 patients were treated with SFRT, and 11 of the 17 (65%) underwent prior surgical resection. Median cumulative dose was 57 Gy. At a median follow-up time of 40 months, radiologic regression was seen in 5 of 16 cases, and 11 of 16 patients were stable. Freedom from progression and overall survival were 100% and 93.8% at 5 years, respectively. There was no incidence of grade 3 or 4 acute toxicity, and no late toxicity seen. These single-institution studies demonstrate excellent local control rates resulting from SFRT of paragangliomas of the temporal bone and neck, particularly in cases of large tumors or recurrences after incomplete resections.116
GKR was first used for glomus jugulare tumors in the 1990s. Glomus tumors were considered ideally suited for radiosurgery treatment because they are typically well defined, noninfiltrating, radiosensitive tumors.109 Because of the precision and the rapid fall-off of dose in GKR, a larger dose of radiation can be administered to the tumor without exceeding normal tissue tolerance of surrounding structures. Foote and colleagues118 from the Mayo Clinic reported their experience with GKR in 25 patients with glomus tumors who received 12 to 18 Gy and were followed with MRI imaging for a median of 35 months (10-113 months). None of the tumors increased in size, 17 were stable, and 8 decreased in size. No other new or progressive neuropathy of cranial nerves V through XII developed. The authors concluded that GKR resulted in excellent tumor control with low risk of morbidity in the treatment of glomus jugulare tumors. Sharma and colleagues117 supported these results in their series of 24 patients with paragangliomas in the jugular foramen treated from 1997 to 2006 with GKR. Fifteen patients were treated primarily and 9 patients as adjunctive therapy after surgery. Mean tumor size was 8.7 cc, and the mean tumor margin dose was 16.3 Gy (range 12-20 Gy). Imaging follow-up was available for 10 patients, and 7 patients demonstrated a decrease in size, with no patients demonstrating disease progression at a median follow-up time of 24 months (range 7-48 months). Six patients improved clinically, and one patient had transient trigeminal neuralgia, suggesting a low complication rate of GKR. Controversy still surrounds radiosurgical treatment of these lesions as some practitioners argue that at least 10-year follow-up time is needed to make predictions for long-term efficacy and safety of GKR in this population.109
Cyberknife radiosurgery has also been used in the treatment of glomus jugulare tumors. In some situations, glomus tumors may extend below the C2 level which may limit frame-based treatments such as GKR. Lim and colleagues119 reported the results of 15 patients with glomus tumors treated with Cyberknife and followed for a mean duration of 21 months (range, 4-114 months). Mean tumor size (maximum dimension) was 3.1 cm (range, 1.5-6.2 cm). Of 15 patients, 3 were treated with Cyberknife as postoperative adjunctive therapy; 4 of 15 patients (27%) experienced a regression of tumor size, with the remaining patients demonstrating stable tumor size; 2 of 15 patients (13%) experienced transient worsening of pre-existing cranial nerve deficits. These resolved within months, and there was no long-term worsening of pre-existing deficits reported, supporting the low rate of cranial nerve injury seen with other modalities.116,117
Juvenile Nasopharyngeal Angiofibroma
Juvenile nasopharyngeal angiofibroma (JNA) is a histologically benign vascular tumor that arises in boys between the ages of 12 and 15 years, with only rare cases reported in patients younger than 10 or older than 25. Although rare cases have been reported in females, the tumor has such a strong male predilection that the diagnosis has been questioned in those instances. The cell of origin is uncertain. Paraganglionic tissue, ectopic rests of hormone-sensitive vascular tissue, and others have been proposed as candidates. Pathologically, it is not certain if the tumor is of vascular or fibrous origin. JNA is characterized microscopically by dense fibrous tissue interspersed with thin-walled vasculature of differing caliber, often associated with metaplasia or ulceration, or both, of the overlying mucosa.120–122
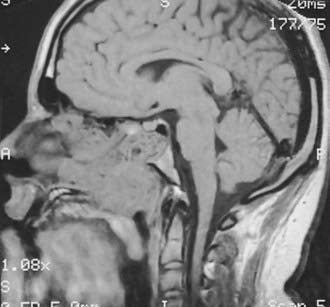
Clinically, JNA is rare, comprising less than 1% of head and neck neoplasms. The most common presenting symptoms are of nasal obstruction and recurrent or severe epistaxis, although other symptoms, such as headaches, facial deformities (“frog face”), and visual and neurologic disturbances, can be seen, especially with advanced lesions. On physical examination, a polypoid or lobulated red or reddish blue mass arising in the nasopharynx is evident. The diagnosis of JNA can be made by a combination of clinical and radiographic means. The radiographic picture obtained by contrast-enhanced CT, MRI, and angiography in the appropriate clinical setting is diagnostic. A sagittal MRI image of a typical JNA is shown in Fig. 68-5. Biopsy can produce severe hemorrhage and is not necessary to establish the diagnosis before therapy is begun.
There is controversy regarding the optimal initial therapy for JNA, with advocates for surgical resection and radiation as primary therapy. Both options have different risks.123,124 However, whenever possible, a single modality should be selected to minimize the risks. In general, early lesions limited to the nasopharynx or nasal cavity, or lesions with lateral extension, are more amenable to primary surgical therapy than are lesions with major intracranial extension. With surgical resection of extracranial tumors, cure rates ranging from 78% to 100% have been reported. Angiography is recommended for surgical planning, and preoperative embolization may be helpful in reducing intraoperative bleeding.120,122,125,126 Recent advances in surgical techniques, such as endoscopic surgical resection, have been reported to be effective and have been associated with significantly less morbidity than standard surgical approaches.127 Although successful resections of intracranial lesions have been reported, the risks of surgery can be significant, and radiotherapy may be preferred in some cases.
A number of authors have reported favorable results after radiation therapy for JNA, with local control rates ranging from 73% to 100% (Table 68-6). Many patients in these series were referred after surgical failure or because of advanced or intracranial lesions. Most authors suggest the use of doses between 30 and 36 Gy. However, in the Baylor experience, four of five patients treated with 32 Gy failed, whereas none of 11 patients treated with higher doses (36 to 46 Gy) failed.128 However, although other institutions have used doses greater than 36 Gy, the high success rate obtained with doses of 30 to 36 Gy in many series suggests that there is not a clear dose-response relationship above this level. Some failures are probably due to geographic miss of the tumor,124 underlying the need for CT-based, three-dimensional treatment planning for this disorder. Fortunately, recurrences after radiotherapy for JNA are uncommon, so experience with salvage therapy is limited. In some situations, the use of chemotherapy or re-irradiation has been reported.124,132,134
There is more recent limited experience using IMRT and radiosurgery for recurrent or advanced JNA. Kuppersmith and colleagues135 used highly conformal IMRT for the treatment of three patients (two patients with extensive postoperative recurrence and one unresectable patient). IMRT limited dose to the optic apparatus, brainstem, parotid, lens, and retina while delivering doses of 34 to 45 Gy to the tumor. The tumor shrank radiographically in all three cases, and there was no endoscopic evidence of disease in two patients at 15 and 40 months. The authors reported no acute toxicity, and late toxicity was limited to one episode of epistaxis and persistent rhinitis in one patient. Similarly, GKR has been evaluated in two patients with residual disease after surgery reported by Dare and colleagues.136 The diameter of treated residual tumor in the cavernous sinus and brain was 3.0 cm and 4.7 cm in the two patients respectively, and control of disease was documented by MRI more than 2 years after radiosurgery. These limited data suggest that highly conformal radiotherapy techniques may provide advantages over conventional radiotherapy in the treatment of these young patients with complex or recurrent angiofibroma. In summary, JNA is a unique neoplasm because of its peculiar predilection for a particular age, sex, and site of origin, as well as for the difficulties in management posed by this vascular neoplasm. Primary treatment with radiotherapy was historically employed, but advances in endoscopic surgical techniques have now made surgery a preferred initial approach for these young patients. IMRT and stereotactic radiosurgery have been used successfully for patients with advanced or recurent disease.
Peyronie Disease
Peyronie disease has been described since the 18th century, but the pathophysiology and natural history are still unclear. The prevalence of the disorder has been estimated at between 3.2% to 8.9%, and it is characterized by the development of penile plaques arising from fibrosis of the corpus cavernosum. The disorder is characterized by penile curvature during erection and the presence of penile plaques or induration, often leading to pain on erection or during intercourse or erectile dyfunction. Although the disorder is not life-threatening, it can have a significant negative effect on quality of life. Pathologically, the disorder is characterized in its early stages (<3 months’ duration) by an inflammatory cellular infiltrate and in its later stages by the development of fibrous connective tissue and rarely even ossification.137,138 Various therapeutic modalities have been used in the management of this disorder, including steroid injections, vitamin E, intralesional verapamil injections, surgery, and radiation therapy. A comparison of treatments is confounded by the fact that the condition is self-limited in many cases, with as many as 50% of cases resolving spontaneously within 12 to 18 months.139,140
Radiotherapy has been used as a treatment for Peyronie disease since at least the 1920s. Initially, radium molds were frequently used; however, external-beam radiotherapy has been used virtually exclusively in recent decades. Indications and rationale for radiotherapy, as reviewed and summarized by Mira and colleagues,141,142 include the following:
A recent European survey indicated that radiotherapy was still being used in approximately 20% of institutions, and confirmed that palliation of pain was obtained in 80% of patients.144
Gynecomastia
Gynecomastia (enlarged breasts) and mammalgia (painful breasts) are common side effects that may accompany estrogenic hormonal therapy for prostate cancer with agents such as diethylstilbestrol (DES). The risk of estrogen-associated breast changes has been estimated at about 70%. Physical changes in the male breast are associated with histologic proliferative changes in ductal epithelium and connective tissue stroma, associated with edema in the normally quiescent male breast. These changes can be tender, painful, and embarrassing for patients and can represent a significant source of morbidity.145 In patients treated with gonadotropin-releasing hormone (GnRH) agonists (e.g., leuprolide, goserelin), side effects of gynecomastia are significantly less common, occurring in less than 10% of patients.146 The incidence of gynecomastia decreased when GnRH agonists replaced DES for androgen suppression. However, some novel antiandrogenic regimens and some regimens of prolonged androgen suppression have been associated with significant incidences of gynecomastia, so it is likely to persist as a concern for some prostate cancer patients. In a study in which bicalutamide (Casodex) 150 mg daily was used for androgen suppression therapy, Wirth and colleagues noted a 47.5% incidence of gynecomastia with breast pain.147
Breast irradiation before the initiation of androgen suppression therapy is effective at preventing gynecomastia with minimal morbidity. For optimum efficacy, treatment should be delivered at least 2 days before androgen suppression therapy is intitiated. Doses of 9 to 15 Gy delivered in 3 to 4 Gy fractions have commonly been employed, with results indicating that approximately 80% of patients treated in this manner do not develop any significant gynecomastia. Circular 5- to 7-cm cones generally provide an adequate treatment volume. Treatment may be delivered with either kilovoltage units (100 to 300 kV; 2-mm Al to 1-mm Cu half-value layer) or with 6- to 9-meV electrons, although megavoltage photons and cobalt units have also been used. Morbidity is minimal, with an occasional patient developing erythema and a rarely desquamation. Significant late effects have not been reported.148–150
When patients who did not receive prophylactic breast irradiation subsequently develop breast symptoms, there is limited experience with the use of radiotherapy. Chou and colleagues151 found that total doses of 20 Gy in 5 fractions to 40 Gy in 20 fractions palliated symptoms of mammalgia in 11 of 11 patients. However, gynecomastia was palliated in only three of eight patients.
Tamoxifen has also been reported to be efficacious in some patients who have developed gynecomastia.152,153
Total Lymphoid Irradiation for Nonmalignant Conditions
TLI has been used in the treatment of conditions for which an autoimmune cause is hypothesized as an alternative to immunosuppressive drugs.154 Limited roles for TLI in benign conditions are still under investigation. The use of TLI has been reported also for the prevention or treatment or bone marrow or organ allograft rejection.155
TLI was found to produce improved cardiac allograft survival in animal models.156 TLI has played a role in cardiac transplantation, both as an adjunct to conventional immunosuppressive therapy and as salvage therapy before retransplantation for patients with persistent rejection on conventional immunosuppressive therapy.157–160 TLI doses in the range of 8 Gy in 10 twice-weekly 0.8 Gy fractions have been used.160,161 The potential for long-term cardiac sequelae or malignancy induction must be considered.160,162
Intravascular Brachytherapy for Prevention of Restenosis
The use of IVBT to reduce the risk of intravascular restenosis had its greatest clinical application in the coronary arteries, to reduce the risk of coronary artery restenosis following angioplasty. Restenosis following coronary angioplasty is a significant limitation to the long-term efficacy of the procedure. Pathologic correlates to restenosis include persistent and recurrent plaque formation, thrombus formation, and inflammatory cell infiltration and proliferation. After animal studies had shown that intravascular radiotherapy was technically feasible and produced reduced restenosis rates in animal models, clinical trials in human subjects were initiated.163
Both gamma-emitting and beta-emitting sources have been found effective at reducing the rates of restenosis in bare metal stents in randomized trials.164,165 However, IVBT has also been associated with an increased incidence of edge restenosis at the margins of the treatment, perhaps related to a geographic mismatch between the injury zone and the radiation coverage zone, especially when new metal stents are concurrently placed.166 In addition, some studies have shown a “late catch-up phenomenon” that eliminated the benefit of vascular brachytherapy (IVBT) within 3 to 5 years after treatment because of late thrombosis.164,167 Given these limitations, as well as the logistical complexity of brachytherapy, alternatives were sought to prevent in-stent restenosis.
Drug-eluting stents were introducted in the early 2000s and were shown to safely reduce restenosis compared with bare metal stents.168 Initially, immunosuppressant drugs such as sirolimus were delivered locally via eluting stents.169 More recently, paclitaxel-eluting stents have demonstrated efficacy in prevention of in-stent restenosis, and have been compared with IVBT in randomized trials.170–172 These trials have demonstrated superiority of drug-eluting stents compared with IVBT with lower rates of restenosis and similar rates of death, myocardial infarction, and target-vessel thrombosis.170–172 Since 2004, drug-eluting stents have undergone widespread adoption into routine practice and have largely replaced coronary IVBT to reduce revascularization procedures after coronary stent placement.
Macular Degeneration
Age-related macular degeneration (AMD) is the leading cause of blindness in older North Americans. The cause of AMD remains unknown, but recognized risk factors include age, white race, heredity, and smoking.173 AMD affects the macula, the posterior aspect of the retina responsible for central visual acuity. The early and intermediate forms of AMD account for 90% of all cases, and are characterized by discrete yellow deposits in the deep layers of the macula that can be associated with gradual visual loss.174 The most common subtype of advanced AMD is exudative or neovascular (wet AMD) characterized by proliferation of abnormal vessels in the choroid that can precipitate sudden visual loss. Despite the growth in treatment options for this disease, there is no curative therapy. Before 1999, thermal laser treatment was the only therapy available for the neovascular form of advanced AMD. Newer treatment options include verteporfin photodynamic therapy and vascular endothelial growth factor (VEGF)-targeted therapy such as pegaptanib sodium, an intraocular antiangiogenic agent, and ranibizumab, a recombinant humanized monoclonal antibody that neutralizes VEGF receptor interaction.174 Radiation therapy has been used to treat AMD because of its effect on the endothelium and inflammation modulation.
Most of the studies using external-beam radiation employed less than 25 Gy to the whole eye, which is below the dose of radiation that is toxic to the retina (approximately 50 Gy) and optic nerve (approximately 59 Gy). For example, Churei and colleagues from Kagoshima University Hospital in Japan treated 21 eyes of 18 patients with choroidal neovascularization in AMD with 20 Gy of 6-mV x-rays in 10 fractions and compared them with a control group of nontreated patients followed over the same 2-year period.175 The rate of improved or maintained visual acuity was 81% in the radiation therapy group and 40% in the control group at 24 months.
The optimal dose and fractionation of radiotherapy for AMD is unclear. Barak and colleagues176 at the University of California, Davis, performed a dose escalation study for patients with neovascular AMD using stereotactic fractionated external-beam radiation in incremental doses from 20 Gy to 40 Gy. Ninety-four eyes of 84 patients were treated from 1997 to 2000 and followed up to 12 months post-treatment. Visual acuity improved slightly postradiotherapy (0.82+/−0.35 before treatment and 0.89+/−0.33 at 12 months). However, no significant benefits were noted from increasing the doses of radiation in either visual acuity or membrane size on angiographic evaluation. There is limited experience with GKR for wet-type AMD from Indiana University.177 Seven patients with AMD received 12 Gy single fraction GKR with the majority maintaining stable vision at 2.2 years of follow-up time. These data support possible palliative benefits of radiotherapy in the treatment of neovascular AMD and merit further investigation of radiotherapy.
1 DiSantis DJ, DiSantis DM. Wrong turns on radiology’s road of progress. Radiographics. 1991;11:1121.
2 Berk LB, Hodes PJ. Roentgen therapy for infections: an historical review. Yale J Biol Med. 1991;64:155.
3 Kaplan II. Clinical radiation therapy, ed 2. New York: Paul B Hoeber; 1949.
4 Seegenschmiedt MH, Micke O, Willich N. Radiation therapy for nonmalignant diseases in Germany: current concepts and future perspectives. Strahlenter Onkol. 2006;180:718-730.
5 Hall EB. Radiation carcinogenesis. In: Hall EB, editor. Radiobiology for the radiologist. ed 4. Philadelphia: JB Lippincott; 1994:323.
6 Trott KR, Kamprad F. Estimation of cancer risks from radiotherapy of benign diseases. Strahlenter Onkol. 2006;182:431-436.
7 Bureau of Radiologic Health, US Department of Health, Education and Welfare (HEW), A Review of the Use of Ionizing Radiation for the treatment of benign Diseases, Vol I. (HEW Publication 78-8043); 1977; HEW, Rockville, Md.
8 Dewing SB. Radiotherapy of benign disease. Springfield, Ill: Charles C Thomas; 1965.
9 Order SE, Donaldson SS. Radiation therapy of benign diseases. A clinical guide. New York: Springer-Verlag; 1990.
10 Meyer JL. The radiation therapy of benign diseases: current indications and techniques. New York: Karger; 2001.
11 Eng TY, Boersma MK, Fuller CD, et al. The role of radiation therapy in benign diseases. Hematol Oncol Clin North Amer. 2006;20:523-557.
12 Seegenschmiedt MH, Makoski HB, Trott KR, et al. Radiotherapy for non-malignant Disorders. New York: Springer, Berlin Heidelberg; 2008.
13 Enzinger FM, Weiss SW. Benign tumors and tumorlike conditions of fibrous tissue. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors. ed 2. St Louis: CV Mosby; 1988:129.
14 Ketchum LD, Cohen IK, Masters FW. Hypertrophic scars and keloids. A collective review. Plast Reconstr Surg. 1974;31:140.
15 Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345:1198.
16 DeBeurmann R, Gougerot H. Cheloides des muqueuses. Ann Derm Syph. 1906;7:151.
17 Escarmant P, Zimmermann S, Amar A, et al. The treatment of 783 keloid scars by iridium-192 interstitial irradiation after surgical excision. Int J Radiat Oncol Biol Phys. 1993;26:245.
18 De Lorenzi F, Teilemans HJP, van dre Hulst RRWJ, et al. Is the treatment of keloid scars still a challenge in 2006? Ann Plast Surg. 2007;58(2):186.
19 Borok TL, Bray M, Sinclair I, et al. The role of ionizing radiation for 393 keloids. Int J Radiat Oncol Biol Phys. 1988;15:865.
20 Ship AG, Weiss PR, Mincer FR, et al. Sternal keloids: successful treatment employing surgery and adjunctive radiation. Ann Plast Surg. 1993;31:481.
21 Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Strahlenther Onkol. 2005;11:717-723.
22 Doornbos JF, Stoffel TJ, Hass AC, et al. The role of kilovoltage irradiation in the treatment of keloids. Int J Radiat Oncol Biol Phys. 1990;18:833.
23 Kovalic JJ, Perez CA. Radiation therapy following keloidectomy—a 20 year experience. Int J Radiat Oncol Biol Phys. 1989;17:77.
24 Ogawa R, Miyashita T, Hyakusoku H, et al. Postoperative radiation protocol for keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg. 2007;59(6):688.
25 Johns HE, Cunningham JR. The physics of radiology, ed 4. Springfield, Ill: Charles C Thomas; 1983. p. 493
26 Wilder RB, Buatti JM, Kittelson JM, et al. Pterygium treated with excision and postoperative beta irradiation. Int J Radiat Oncol Biol Phys. 1992;23:533.
27 Bahrassa F, Datta R. Postoperative beta radiation treatment of pterygium. Int J Radiat Oncol Biol Phys. 1983;9:679.
28 Paryani SB, Scott WP, Wells JW, et al. Management of pterygium with surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1994;28:101.
29 Van den Brenk HAS. Results of prophylactic postoperative irradiation in 1300 cases of pterygium. Am J Roentgenol. 1968;103:723.
30 Cooper JS. Postoperative irradiation of pterygia: ten more years of experience. Radiology. 1978;128:753.
31 Campbell OR, Amendola BE, Brady LW. Recurrent pterygia: results of postoperative treatment with Sr-90 applicators. Radiology. 1990;174:565.
32 MacKenzie FD, Hirst LW, Kynaston B, et al. Recurrence rate and complications after beta irradiation for pterygia. Ophthalmology. 1991;98:1776.
33 Viani GA, Stefano EJ, DeFendi LI, et al. Long-term results and prognostic factors of fractionated strontium-90 eye applicator for pterygium. Int J Radiat Oncol Biol Phys. 2008;72:1174.
34 Hall EB. Radiation cataractogenesis. In: Hall EB, editor. Radiobiology for the radiologist. ed 4. Philadelphia: JB Lippincott; 1994:379.
35 Dusenbery KE, Alul IH, Holland EJ, et al. Beta-irradiation of recurrent pterygia: Results and complications. Int J Radiat Oncol Biol Phys. 1992;24:315.
36 Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med. 1993;329:1468.
37 Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch Opthalmol. 1993;111:197.
38 Utiger RD. Treatment of Graves’ ophthalmopathy. N Engl J Med. 1989;321:1403.
39 Larkins R. Treatment of Graves’ ophthalmopathy. Lancet. 1993;342:941.
40 Bartalena L, Marcocci C, Bogazzi F, et al. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med. 1989;321:1349.
41 Prummel MF, Mourits MP, Berghout A, et al. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321:1353.
42 Palmer D, Greenberg P, Cornell P, et al. Radiation therapy for Graves’ ophthalmopathy: a retrospective analysis. Int J Radiat Oncol Biol Phys. 1987;13:1815.
43 Olivotto IA, Ludgate CM, Allen LH, et al. Supervoltage radiotherapy for Graves’ ophthalmopathy: CCABC technique and results. Int J Radiat Oncol Biol Phys. 1985;11:2085.
44 Sandler HM, Rubenstein JH, Fowble BL, et al. Results of radiotherapy for thyroid opthalmopathy. Int J Radiat Oncol Biol Phys. 1989;17:823.
45 Petersen IA, Kriss JP, McDougall IR, et al. Prognostic factors in the radiotherapy of Graves’ ophthalmopathy. Int J Radiat Oncol Biol Phys. 1990;19:259.
46 Hurbli T, Char DH, Harris J, et al. Radiation therapy for thyroid eye diseases. Am J Opthalmol. 1985;99:633.
47 Bradley EA, Gower EW, Bradley DJ, et al. Orbital radiation for Graves opthalmopathy. Opthalmology. 2008;115(2):398.
48 Prummel MF, Mourits MP, Blank L, et al. Randomised double-blind trial of prednisone versus radiotherapy in Graves’ ophthalmopathy. Lancet. 1993;342:949.
49 Ng CM, Yuen HKL, Choi KL, et al. Combined orbital irradiation and systemic steroids compared with systemic steroids alone in the management of moderate to severe Graves’ ophthalmopathy: a preliminary study. Hong Kong Med J. 2005;11(5):322.
50 Zoumalan CI, Cockerham KP, Turbin RE, et al. Efficacy of corticosteroids and external beam radiation in the management of moderate to severe thyroid eye disease. J Neuroophthalmol. 2007;27(3):205.
51 Wei RL, Cheng JW, Cai JP. The use of orbital radiotherapy for Graves’ ophthalmopathy: quantitative review of the evidence. Ophthalmologica. 2008;222:27.
52 Parsons JT, Fitzgerald CR, Hood CI, et al. The effects of irradiation on the eye and optic nerve. Int J Radiat Oncol Biol Phys. 1983;9:609.
53 Wakelkamp IMMJ, Tan H, Saeed P, et al. Orbital irradiation for Graves’ ophthalmopathy. Is it safe? A long term follow up study. Ophthalmology. 2004;111(8):1557.
54 Snijders-Keilholz A, De Keizer RJW, Goslings BM, et al. Probable risk of tumor induction after retro-orbital irradiation for Graves’ opthalmopathy. Radiother Oncol. 1996;38:69.
55 Stern RL, Rosenthal SA, Doggett EC, et al. Applications of asymmetric collimation on linear accelerators. Med Dos. 1995;20:95-98.
56 Knowles DM, Jakobiec FA. Orbital lymphoid neoplasms: a clinicopathologic study of 60 patients. Cancer. 1980;46:576.
57 Jakobiec FA, Neri A, Knowles DM. Genotypic monoclonality in immunophenotypical polyclonal orbital lymphoid tumors: a model for tumor progression in the lymphoid system. Ophthalmology. 1987;94:980.
58 Enzmann D, Donaldson SS, Marshall WH, et al. Computed tomography in orbital pseudotumor (idiopathic orbital inflammation). Radiology. 1976;120:597.
59 Lanciano RM, Fowble B, Sergott RC, et al. The results of radiotherapy for orbital pseudotumor. Int J Radiat Oncol Biol Phys. 1990;18:407.
60 Gordon PS, Juillard GJF, Selch MT, et al. Orbital lymphomas and pseudolymphomas: treatment with radiation therapy. Radiology. 1986;159:797.
61 Keleti D, Flickinger JC, Hobson SR, et al. Radiotherapy of lymphoproliferative diseases of the orbit. Am J Clin Oncol. 1992;15:422.
62 Leone C, Lloyd W. Treatment protocol for orbital inflammatory disease. Ophthalmology. 1985;92:1325.
63 Donaldson SS, McDougall IR, Egbert PR, et al. Treatment of orbital pseudotumor (idiopathic orbital inflammation) by radiation therapy. Int J Radiat Oncol Biol Phys. 1980;6:79.
64 Fizpatrick PJ, Macko S. Lymphoreticular tumors of the orbit. Int J Radiat Oncol Biol Phys. 1984;10:333.
65 Austin-Seymour M, Donaldson SS, Egbert PR, et al. Radiotherapy of lymphoid diseases of the orbit. Int J Radiat Oncol Biol Phys. 1985;11:371.
66 Smitt MC, Donaldson SS. Radiotherapy is successful treatment for orbital lymphoma. Int J Radiat Oncol Biol Phys. 1993;26:59.
67 Mittal BB, Deutsch M, Kennerdell J, et al. Paraocular lymphoid tumors. Radiology. 1986;159:793.
68 Witzig TE, Inwards DJ, Habermann TM, et al. Treatment of benign orbital pseudolymphomas with the monoclonal anti-CD20 antibody rituximab. Mayo Clin Proc. 2007;82:692-699.
69 Duray PH, Burgdorf WHC, Goltz RW. Persistent cutaneous lymphoid hyperplasia of the face with conjunctival involvement. Arch Ophthamol. 1985;103:555.
70 Olson LE, Wilson JF, Cox JD. Cutaneous lymphoid hyperplasia. Results of radiation therapy. Radiology. 1985;155:507.
71 Order SE, Donaldson SS. Lymphoid hyperplasia—pseudotumor in radiation therapy for benign diseases. New York: Springer-Verlag; 1990. p. 156
72 Brooker AF, Bowerman JW, Robinson RA, et al. Ectopic ossification following total hip replacement: Incidence and a method of classification. J Bone Joint Surg. 1973;55:1629.
73 Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty. Predisposing factors, frequency, and effect on results. J Bone Joint Surg Am. 1977;59:345.
74 Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. J Bone Joint Surg Am. 1981;63:201.
75 Kjaersgaard-Andersen P, Ritter MA. Prevention of formation of heterotopic bone after total hip arthroplasty. J Bone Joint Surg Am. 1991;73:942.
76 Thomas BJ. Heterotopic bone formation after total hip arthroplasty. Orthop Clin North Am. 1992;23:347.
77 Schmidt SA, Kjaersgaard-Andersen P, Pedersen NW, et al. The use of indomethacin to prevent the formation of heterotopic bone after total hip replacement. A randomized, double-blind, clinical trial. J Bone Joint Surg Am. 1988;70A:834.
78 Cella JP, Salvati EA, Sculco TP. Indomethacin for the prevention of heterotopic ossification following total hip arthroplasty: effectiveness, contraindications, and adverse effects. J Arthroplasty. 1988;3:229.
79 Macfarlane RJ, Ng BH, Gamie Z, et al. Pharmacological treatment of heterotopic ossification following hip and acetabular surgery. Expert Opin Pharmacother. 2008;9(5):767.
80 MacLennan I, Keys HM, Evarts CM, et al. Usefulness of postoperative hip irradiation in the prevention of heterotopic bone formation in a high risk group of patients. Int J Radiat Oncol Biol Phys. 1984;10:49.
81 Anthony P, Keys H, Evarts CM, et al. Prevention of heterotopic bone formation with early postoperative irradiation in high risk patients undergoing total hip arthroplasty: comparison of 10 Gy vs. 20 Gy schedules. Int J Radiat Oncol Biol Phys. 1987;13:365.
82 Sylvester JE, Greenberg P, Selch MT, et al. The use of postoperative irradiation for the prevention of heterotopic bone formation after total hip replacement. Int J Radiat Oncol Biol Phys. 1988;14:471.
83 Blount LH, Thomas BJ, Tran L, et al. Postoperative irradiation for the prevention of heterotopic bone: analysis of different dose schedules and shielding considerations. Int J Radiat Oncol Biol Phys. 1990;19:577.
84 Lo TC, Healy WL, Corall DJ, et al. Heterotopic bone formation after hip surgery: prevention with single dose postoperative hip irradiation. Radiology. 1988;168:851.
85 Konski A, Pellegrini V, Poulter C, et al. Randomized trial comparing single dose vs. fractionated irradiation for prevention of heterotopic bone: a preliminary report. Int J Radiat Oncol Biol Phys. 1990;18:1139.
86 Healy WL, Lo TC, DeSimone AA, et al. Single-dose irradiation for the prevention of heterotopic ossification after total hip arthroplasty. A comparison of doses of five hundred and fifty and seven hundred centigray. J Bone Joint Surg Am. 1995;77:590.
87 Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006;65(5):1289.
88 Kantorowitz DA, Miller GJ, Ferrara JA, et al. Preoperative versus postoperative irradiation in the prophylaxis of heterotopic bone formation in rats. Int J Radiat Oncol Biol Phys. 1990;19:1431.
89 Gregoritch SJ, Chadha M, Pelligrini VD, et al. Randomized trial comparing preoperative versus postoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement: preliminary results. Int J Radiat Oncol Biol Phys. 1994;30:55-62.
90 Slawson RG, Poka A, Bathon H, et al. The role of postoperative radiation in the prevention of heterotopic ossification in patients with traumatic acetabular fracture. Int J Radiat Oncol Biol Phys. 1989;17:669.
91 Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am. 2001;83A(12):1783.
92 Karunakar MA, Sen A, Bosse MJ, et al. Indometacin as prophylaxis for heterotopic ossification after the operative treatment of fractures of the acetabulum. J Bone Joint Surg Br. 2006;88B:1613.
93 Pakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004;60:888.
94 Oertel S, Schneider U, Keel M, et al. Prophylaxis of heterotopic ossification in patients sedated after polytrauma. Strahlenther Onkol. 2008;184:212.
95 Kim JH, Chu FC, Woodard HQ, et al. Radiation-induced soft-tissue and bone sarcoma. Radiology. 1978;129:501.
96 Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 12, 2005.
97 Konski A, Weiss C, Rosier R, et al. The use of postoperative irradiation for the prevention of heterotopic bone after total hip replacement with biologic fixation (porous coated) prosthesis: an animal model. Int J Radiat Oncol Biol Phys. 1990;18:861.
98 Durr ED, Turlington EG, Foote RL. Radiation treatment of heterotopic bone formation in the temporomandibular joint articulation. Int J Radiat Oncol Biol Phys. 1993;27:863.
99 Wolfson AH, McAuliffe JA, Ouelette EA, et al. Role of postoperative radiation therapy of the elbow as prophylaxis against heterotopic ossification. Radiology (RSNA Proceedings). 1993;189:267. Abstract
100 Rubenstein JH, Salenius SA, Blitzer PH, et al. Prevention of heterotopic bone formation with low dose radiation therapy. J Fla Med Assoc. 1992;79:828.
101 Poggi MM, Thomas BE, Johnstone PAS. Excision and radiotherapy for heterotopic ossification of the elbow. Orthopedics. 1999;22:1059.
102 Viola RW, Hastings H. Treatment of ectopic ossification about the elbow. Clin Orthop. 2000;370:65.
103 Seegenschmidt MH, Goldman AR, Martus P, et al. Prophylactic radiation therapy for prevention of heterotopic ossification after hip arthroplasty: results in 141 high-risk hips. Radiology. 1993;188:257.
104 Enzinger FM, Weiss SW. Paraganglioma. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors. ed 2. St Louis: CV Mosby; 1988:836.
105 Million RR, Cassisi NJ, Mancuso AA, et al. Chemodectomas (glomus body tumors. In: Million RR, Cassisi NJ, editors. Management of head and neck cancer. ed 2. Philadelphia: JB Lippincott; 1994:765.
106 Wang CC. Paraganglioma of the head and neck. In: Williams CJ, Krikorian JG, Green MR, et al, editors. Textbook of uncommon cancer. New York: John Wiley; 1988:725.
107 Coia LR, Fazekas JT, Farb SN. Familial chemodectoma. Int J Radiat Oncol Biol Phys. 1981;7:949.
108 Shedd DP, Arias JD, Glunk RP. Familial occurrence of carotid body tumors. Head Neck. 1990;12:496.
109 Li G, Chang S, Adler JR, et al. Irradiation of glomus jugulare tumors: a historical perspective. J Neurosurg. 2007;23(6):E13.
110 Kim J-A, Elkon D, Lim M-L, et al. Optimum dose of radiotherapy for chemodectomas of the middle ear. Int J Radiat Oncol Biol Phys. 1980;6:815.
111 Wang M-L, Hussey DH, Doornbos JF, et al. Chemodectoma of the temporal bone: a comparison of surgical and radiotherapeutic results. Int J Radiat Oncol Biol Phys. 1988;14:643.
112 Cummings BJ, Beale FA, Garrett PG, et al. The treatment of glomus tumors in the temporal bone by megavoltage radiation. Cancer. 1984;53:2635.
113 Springate SC, Weichselbaum RR. Radiation or surgery for chemo dectoma of the temporal bone: a review of local control and complications. Head Neck. 1990;12:303.
114 Springate SC, Haraf D, Weichselbaum RR. Temporal bone chemodectomas—comparing surgery and radiation therapy. Oncology. 1991;5:131.
115 Hinerman RW, Amdur RJ, Morris CG, et al. Definitive radiotherapy in the management of paragangliomas arising in the head and neck: a 35-year experience. Head Neck. 2008;30:1431.
116 Henzel M, Hamm K, Gross MW, et al. Fractionated stereotactic radiotherapy of glomus jugulare tumors. Local control, toxicity, symptomatology, and quality of life. Strahlenther Onkol. 2007;183(10):557.
117 Sharma MS, Gupta A, Kale SS, et al. Gamma knife radiosurgery for glomus jugulare tumors: therapeutic advantages of minimalism in the skull base. Neurol India. 2008;56(1):57.
118 Foote RL, Pollock BE, Gorman DA, et al. Glomus jugulare tumor: tumor control and complications after stereotactic radiosurgery. Head Neck. 2002;24(4):332.
119 Lim M, Gibbs IC, Adler JR, et al. Efficacy and safety of stereotactic radiosurgery for glomus jugulare tumors. Neurosurg Focus. 2004;17:2E11.
120 Grybauskas V, Parker J, Friedman M. Juvenile nasopharyngeal angiofibroma. Otolaryngol Clin North Am. 1986;19:647.
121 Enzinger FM, Weiss SW. Benign tumors and tumorlike lesions of fibrous tissue. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors. ed 2. St Louis: CV Mosby; 1988:127.
122 Million RR, Cassisi NJ, Stringer SP, et al. Juvenile angiofibroma. In Million RR, Cassisi NJ, editors: Management of head and neck cancer: a multidisciplinary approach, ed 2, Philadelphia: JB Lippincott, 1994.
123 Gantz B, Seid AB, Weber RS. Controversies: nasopharyngeal angiofibroma. Head Neck. 1992;14:67.
124 Cummings BJ, Blend R, Keane T, et al. Primary radiation therapy for juvenile nasopharyngeal angiofibroma. Laryngoscope. 1984;94:1599.
125 Ward PH, Thompson R, Calcaterra T, et al. Juvenile angiofibroma: a more rational therapeutic approach based upon clinical and experimental evidence. Laryngoscope. 1974;84:2181.
126 Krekorian EA, Kato RH. Surgical management of nasopharyngeal angiofibroma with intracranial extension. Laryngoscope. 1977;87:154.
127 Pryor SG, Moore EG, Kasperbauer JL. Endoscopic versus traditional approaches for excision of juvenile nasal angiofibroma. Laryngoscope. 2005;115:1201-1207.
128 McGahan RA, Durrance FY, Parke RB, et al. The treatment of advanced juvenile nasopharyngeal angiofibroma. Int J Radiat Oncol Biol Phys. 1989;17:1067.
129 Kasper ME, Parsons JT, Mancuso AA, et al. Radiation therapy for juvenile angiofibroma: evaluation by CT and MRI, analysis of tumor regression, and selection of patients. Int J Radiat Oncol Biol Phys. 1993;25:689.
130 Fields JN, Halverson KJ, Devineni VR, et al. Juvenile nasopharyngeal angiofibroma: efficacy of radiation therapy. Radiology. 1990;176:263.
131 Robinson ACR, Khoury GG, Ash DV, et al. Evaluation of response following irradiation of juvenile angiofibromas. Br J Radiol. 1989;62:245.
132 Goepfert H, Cangir A, Lee YY. Chemotherapy for aggressive juvenile nasopharyngeal angiofibroma. Arch Otolaryngol. 1985;111:285.
133 Cummings BJ. Relative risk factors in the treatment of juvenile nasopharyngeal angiofibroma. Head Neck Surg. 1980;3:21.
134 Fitzpatrick PJ, Rider WD. The radiotherapy of nasopharyngeal angiofibroma. Radiology. 1973;109:171.
135 Kuppersmith RB, Teh BS, Donovan DT, et al. The use of intensity modulated radiotherapy for the treatment of extensive and recurrent juvenile angiofibroma. Int J Pediatr Otorhinolaryngol. 2000;52(3):261.
136 Dare AO, Gibbons KJ, Proulx GM, et al. Resection followed by radiosurgery for advanced juvenile nasopharyngeal angiofibroma: report of two cases. Neurosurgery. 2003;52(5):1207.
137 Smith BH. Peyronie’s disease. Am J Clin Pathol. 1966;45:670.
138 Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie’s disease. J Urol. 2006;175:2115-2118.
139 Carson CC, Coughlin PWF. Radiation therapy for Peyronie’s disease: is there a place? J Urol. 1985;134:684.
140 Levine LA. Treatment of Peyronie’s disease with intralesional verapamil injection. J Urol. 1997;158:1395-1397.
141 Mira JG. Is it worthwhile to treat Peyronie’s disease? Urology. 1980;16:1.
142 Mira JG, Chabazian CM, del Regato JA. The value of radiotherapy for Peyronie’s disease: presentation of 56 new case studies and review of the literature. Int J Radiat Oncol Biol Phys. 1980;6:161.
143 Martin CL. Long-time study of patients with Peyronie’s disease treated with irradiation. Am J Roentgenol. 1972;114:492.
144 Incrocci L, Hop WC, Seegenschmiedt HM. Radiotherapy for Peyronie’s disease: a European survey. Acta Oncol. 2008;47:1110.
145 Gagnon JD, Moss WT, Stevens KR. Pre-estrogen breast irradiation for patients with carcinoma of the breast: A critical review. J Urol. 1979;121:182.
146 Physicians’ Desk Reference. ed 62. 2008; Thomson Healthcare, Montvale, NJ. pp 3197–3206
147 Wirth MA, Tyrrell C, Wallace M, et al. Bicalutamide (Casodex) 150 mg as immediate therapy in patients with localized or locally advanced prostate cancer significantly reduces the risk of disease progression. Urology. 2001;58:146-151.
148 Malis I, Cooper JF, Wolever THS. Breast radiation in patients with carcinoma of the prostate. J Urol. 1969;102:326.
149 Corvalan JG, Gill WM, Egelston TA, et al. Irradiation of the male breast to prevent hormone induced gynecomastia. Am J Roentgenol. 1969;106:839.
150 Fass D, Steinfeld A, Brown J, et al. Radiotherapeutic prophylaxis of estrogen-induced gynecomastia: a study of late sequela. Int J Radiat Oncol Biol Phys. 1986;12:407.
151 Chou L, Easley JD, Feldmeier JJ, et al. Effective radiotherapy in palliating mammalgia associated with gynecomastia after DES therapy. Int J Radiat Oncol Biol Phys. 1988;15:749.
152 Braunstein GD. Gynecomastia. N Engl J Med. 2007;357:1229-1237.
153 Sieber PR. Treatment of bicalutamide-induced breast events. Expert Rev Anticancer Ther. 2007;7:1773-1779.
154 Fuks Z, Slavin S. The use of total lymphoid irradiation (TLI) as immunosuppressive therapy for organ allotransplantation and autoimmune diseases. Int J Radiat Oncol Biol Phys. 1981;7:79.
155 Strober S, Lowsky RJ, Shizuru JA, et al. Approaches to transplantation tolerance in humans. Transplantation. 2004;77(6):932-936.
156 Slavin S, Reitz B, Bieber CP, et al. Transplantation tolerance in adult rats using total lymphoid irradiation: permanent survival of skin, heart, and marrow allografts. J Exp Med. 1978;147:700.
157 Salter MM, Kirklin JK, Bourge RC, et al. Total lymphoid irradiation in the treatment of early or recurrent heart rejection. J Heart Lung Transplant. 1992;11:902.
158 Kirklin JK, George JF, McGiffin DC, et al. Total lymphoid irradiation: is there a role in pediatric heart transplantation? J Heart Lung Transplant. 1993;12:S293.
159 Lim TS, O’Driscoll G, Freund J, et al. Short-course total lymphoid irradiation for refractory cardiac transplantation rejection. J Heart Lung Transplant. 2007;26(12):1249-1254.
160 Chin C, Hunt S, Robbins R, et al. Long-term follow-up after total lymphoid irradiation in pediatric heart transplant recipients. J Heart Lung Transplant. 2002;21(6):667-673.
161 Wolden S, Tate DJ, Hunt SA, et al. Long-term results of total lymphoid irradiation in the treatment of cardiac allograft rejection. Int J Radiat Oncol Biol Phys. 1997;39:953-960.
162 Pelletier MP, Coady M, Macha M, et al. Coronary atherosclerosis in cardiac transplant patients treated with total lymphoid irradiation. J Heart Lung Transplant. 2003;22(2):124-129.
163 Crocker I. Radiation therapy to prevent coronary artery restenosis. Semin Radiat Oncol. 1999;9:134.
164 Leon MB, Teirstein PS, Moses JW, et al. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Eng J Med. 2001;344:250.
165 Verin V, Popowski Y, De Bruyne B, et al. Endoluminal beta-radiation therapy for the prevention of coronary restenosis after balloon angioplasty. N Eng J Med. 2001;344:243.
166 Costa MA, Sabate M, van der Giessen WJ, et al. Late coronary occlusion aftr intracoronary brachytherapy. Circulation. 1999;100:789.
167 Baierl V, Baumgartner S, Pollinger B, et al. Three-year clinical follow-up after strontium-90/beta-irradiation for the treatment of in-stent coronary restenosis. Am J Cardiol. 2005;96:139.
168 Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent versus standard stent for coronary revascularization. N Engl J Med. 2002;346:1773.
169 Malenka DJ, Kaplan AV, Lucas FL, et al. Outcomes following coronary stenting in the era of bare-metal vs. the era of drug-eluting stents. JAMA. 2008;299(24):2868.
170 Stone GW, Ellis SG, O’Shaughnessy CD, et al. Paclitaxel-eluting stents vs. vascular brachytherapy for in-stent restenosis within bare-metal stents, The TAXUS V ISR randomized trial. JAMA. 2006;295(11):1253.
171 Ellis SG, O’Shaughnessy CD, Martin SL, et al. Two-year clinical outcomes after paclitaxel-eluting stent or brachytherapy treatment for bare metal stent restenosis; the TAXUS V ISR trial. Eur J Heart. 2008;29:1625.
172 Holmes DR, Teirstein P, Satler L, et al. Sirolimus-eluting stents vs. vascular brachytherapy for in-stent restenosis within bare-metal stents. The SISR randomized trial. JAMA. 2006;295(11):1264.
173 Smith W, Assinik J, Klein R, et al. Risk factors for age-related macular degeneration. Pooled findings from three continents. Ophthalmology. 2001;108:653.
174 Bourla DH, Young TA. Age-related macular degeneration: a practical approach to a challenging disease. J Am Geriatr Soc. 2006;54(7):1130.
175 Churei H, Ohkubo K, Nakajo M, et al. External-beam radiation therapy for age-related macular degeneration: two years follow-up results at a total dose of 20 Gy in 10 fractions. Radiat Med. 2004;22(6):398.
176 Barak A, Hauser D, Yipp P, et al. A phase I trial of stereotactic external beam radiation for subfoveal choroidal neovascular membranes in age-related macular degeneration. Br J Radiol. 2005 Sep;78(933):827.
177 Henderson MA, Valluri S, Lo SS, et al. Gamma knife radiosurgery in the treatment of choroidal neovascularization (wet-type macular degeneration). Stereotact Funct Neurosurg. 2007;85(1):11.