Benign and Malignant Tumors of the Liver
Linda D. Ferrell
Introduction
Primary tumors of the liver are classified as benign and malignant epithelial and nonepithelial neoplasms. The most common are epithelial neoplasms, which are predominantly hepatocellular and bile ductular types. Other non-neoplastic tumor-like lesions are of clinical significance. The 2010 classification scheme of the World Health Organization (WHO), which includes neoplasms and tumor-like lesions, is provided in Table 55.1.1 The nomenclature used in this chapter follows these recommendations.
Table 55.1
World Health Organization Classification of Primary Liver Tumors and Tumor-Like Lesions
| Epithelial Tumors or Tumor-Like Lesions | Nonepithelial Tumors or Tumor-Like Lesions |
| Benign | |
| Hepatocellular adenoma | Cavernous hemangioma |
| Focal nodular hyperplasia | Angiomyolipoma |
| Bile duct adenoma | Infantile hemangioma |
| Bile duct hamartoma | Lymphangioma |
| Microcystic adenoma | Mesenchymal hamartoma |
| Biliary adenofibroma | Inflammatory pseudotumor |
| Solitary necrotic nodule | |
| Premalignant or Precursor Lesions | |
| Dysplastic nodule | |
| Low-grade | |
| High-grade | |
| Mucinous cystic neoplasms, low and high grade | |
| Intraductal papillary neoplasms, low and high grade | |
| Malignant | |
| Hepatocellular carcinoma, including fibrolamellar variant | Angiosarcoma Epithelioid hemangioendothelioma Embryonal (undifferentiated) sarcoma Lymphoma and other hematopoietic tumors |
| Cholangiocarcinoma, peripheral, hilar, and extrahepatic types | |
| Mucinous cystic neoplasm with invasive carcinoma | |
| Intraductal papillary neoplasm with invasive carcinoma | |
| Malignancies of Mixed or Uncertain Origin | |
| Combined hepatocellular carcinoma and cholangiocarcinoma | Kaposi sarcoma Leiomyosarcoma Rhabdomyosarcoma Synovial sarcoma Germ cell tumors (teratoma, yolk sac tumor) |
| Hepatoblastoma | |
| Carcinosarcoma | |
| Calcifying nested epithelial stromal tumor | |
| Malignant rhabdoid tumor | |
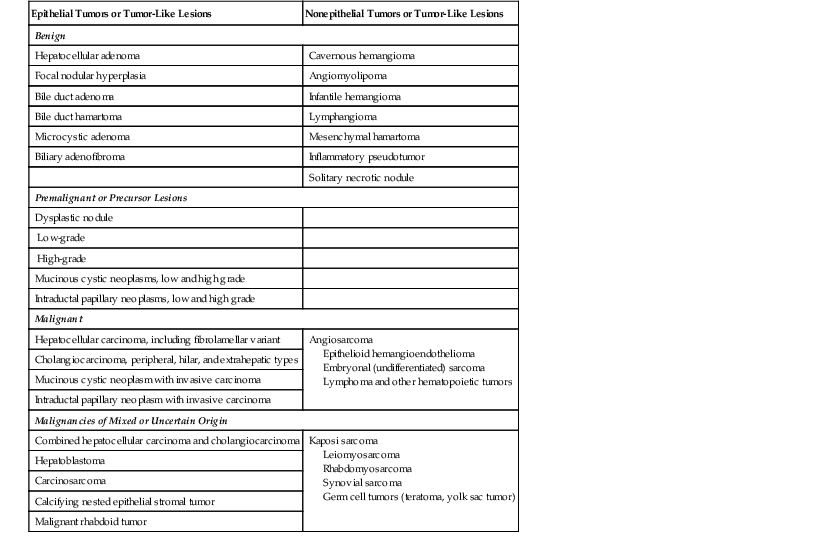
Data from Bioulac-Sage P, Balabaud C, Wanless I, et al. Tumours of the liver and intrahepatic bile ducts. In: Bosman F, Carneiro F, Hruban R, Theise N, eds. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC; 2010:195-261.
Specimen Analysis
Documentation
Verification of tumor type and status as a primary or metastatic neoplasm or as a non-neoplastic tumor is the most important consideration in the diagnosis. Descriptive comments should focus on information important for tumor staging, such as tumor size (smaller or larger than 5 cm for hepatocellular carcinoma [HCC]), number of lesions (single or multiple), specific lobes or segments involved, local extension of the lesion outside of the liver, and presence or absence of gross vascular invasion, and it should include notation regarding which of the major vessels (portal or hepatic vein) are involved.
Microscopic evaluation should involve adequate numbers of sections relative to tumor size to assess vascular invasion or extension outside of the Glisson capsule.2,3 Sections near the edge of the tumor are recommended for detection of vascular invasion if gross invasion is not seen. Gross observation of possible large vessel invasion should be documented microscopically. A section of non-neoplastic liver should be included for identification of the background pathology. Table 55.2 delineates tumor (T) staging for HCC by the Union for International Cancer Control and American Joint Committee on Cancer (UICC/AJCC) recommendations (http://www.uicc.org). Metastasis (M) and node (N) staging consist of X for not known, 0 for absent, and 1 for present.
Table 55.2
Primary Tumor Staging for Hepatocellular Carcinoma
| Tumor Stage | Definition |
| TX | Primary tumor cannot be determined |
| T0 | No evidence of primary tumor |
| T1 | Solitary tumor without vascular invasion |
| T2 | Solitary tumor with vascular invasion or multiple tumors, none >5 cm |
| T3a | Multiple tumors, any >5 cm |
| T3b | Single or multiple tumors of any size involving a major branch of hepatic or portal vein |
| T4 | Tumor invading adjacent organs (other than gallbladder) or perforating visceral peritoneum |
In typical situations, the surgical margins of the resection specimen should be inked before the tumor is sectioned for definitive evaluation of the distance from the margin to the tumor, and the status of the resection margins should be included in the report. Some studies suggest that a tumor-free margin of at least 1 cm is directly related to a better prognosis for some types of malignant tumors.4 Other studies have shown that a wide resection is preferable to a limited excision when the HCC is small.3 The distance from the tumor to the closest margin of resection should be documented clearly in the pathology report.
Biopsy Methods
Small-core and fine-needle aspiration biopsies (FNABs) may be used to diagnose specific tumor types (see Chapter 45). The accuracy of FNABs is enhanced by the use of extra tissue preparations, such as cell buttons, for evaluation of the histologic features of tissue sections obtained from the FNAB material.5 This method is especially helpful to evaluate well-differentiated hepatocellular lesions, in which subtle cytologic features such as cell size, crowding of nuclei, and increased nucleus-to-cytoplasm ratio or architectural features such as formation of trabeculae or width of cell plates may be difficult to assess with smears alone. In these instances, use of histologic sections in combination with well-prepared smears can be successful to distinguish well-differentiated HCC from other lesions such as hepatocellular adenoma, focal nodular hyperplasia, macroregenerative nodules, or high-grade dysplastic nodules. Preparation of cell buttons, particularly in cases of poorly differentiated neoplasms or tumors with unusual morphology, may result in a more accurate diagnosis through the application of immunohistochemical techniques.
Because the amount of biopsy material is often limited, slides of unstained material should be prepared when the tissue block is initially sectioned to ensure that sufficient material is available for additional levels or stains. The usual panel of stains, which includes trichrome, reticulin, periodic acid–Schiff with diastase digestion (d-PAS), and iron stain, should not be ordered automatically at the time of gross examination, because excess sectioning (before examination of the hematoxylin and eosin–stained slides) may deplete the tissue block of diagnostic material that should be available for other diagnostic stains that are determined later.
Hepatocellular Tumors
Hepatocellular Adenoma
Clinical Features
In the past, hepatocellular adenomas (HCAs) were considered rare tumors that developed almost exclusively in young women during their reproductive years and only rarely occurred in men6,7 or children.8 However, the incidence is increasing, mostly because of the obesity epidemic, and lesions are seen in older women (40 to 50 years) and in men.1
Most patients have an abdominal mass, but some also have abdominal pain, discomfort, or nausea. Radiographically, most HCAs exhibit an enhanced vascular pattern. A significant proportion manifest with hemoperitoneum.9 Lesions larger than 5 cm in diameter usually are associated with a higher risk of hemorrhagic complications, and resection is typically recommended for these lesions. The serum alkaline phosphatase level may be elevated, but serum α-fetoprotein (AFP) levels are usually normal or only minimally elevated.
Pathogenesis
HCAs have historically developed as a result of oral contraceptive10 or anabolic steroid use. However, low-dose estrogens are now widely used, and the incidence of HCAs related to oral contraceptives may be decreasing. Some HCAs associated with oral contraceptive use regress after cessation of the drugs, but others do not.
Other risk factors for the development of adenomas include obesity (and probably the metabolic syndrome) and metabolic disorders, especially the carbohydrate metabolic disorders such as glycogen storage diseases I and IV, galactosemia, tyrosinemia, and familial diabetes mellitus.11 The consensus of the International Working Party is that a diagnosis of HCA should not be assigned for a lesion that arises in a cirrhotic liver unless regression is evident when the stimulus is removed or unless one of the risk factors is discernable.12 HCCs may arise within HCAs (see Chapter 44).10,13
Genetic and molecular analyses have resulted in the subclassification of HCA into four major groups (Table 55.3) (see Chapter 44).14 The first group demonstrates hepatocyte nuclear factor 1α (HNF1A) inactivation mutations that are somatic or germline. These tumors are characterized by steatosis, and they tend to occur in younger patients, some of whom have diabetes. Patients frequently have a history of familial adenomatous polyposis. The second major group of HCAs has β-catenin mutations. Tumors in this group tend to occur more in males, may have more cytologic abnormalities, exhibit a focal acinar pattern, and are less steatotic. This group is more frequently associated with the development of HCC.15 The third group known as inflammatory HCAs (previously known as telangiectatic or variant HCA and originally described as telangiectatic focal nodular hyperplasia16,17) is characterized by sinusoidal dilation, inflammatory infiltrates, and a focal ductular reaction. The fourth group consists of the remaining HCAs, which are heterogeneous, lack HNF1A or β-catenin mutations, atypical histologic features, and a telangiectatic pattern of growth.
Table 55.3
Hepatocellular Adenoma Variants
| HCA Variant Type | Routine Histologic Features | Immunohistochemical Staining | Clinical Associations |
| HNF1A inactivated | Diffuse fatty change; irregular borders | LFABP1 negative | Maturity onset diabetes of the young (type 3). Adenomatosis is common; risk for HCC low |
| β-Catenin mutated | Focal features overlapping those of well-differentiated HCC such as pseudogland (acinar) formation and/or cytologic atypia | β-Catenin nuclear staining and diffuse GS staining | Male gender and anabolic steroid more common. Variant with most risk for HCC |
| Inflammatory | Dilated sinusoids, fatty change may be focal; stromal/vascular zones with clusters of small arteries, inflammatory cells and peripheral ductular reaction | CRP and SAA demonstrate diffuse cytoplasmic staining; about 10% may have β-catenin mutations; CK7 highlights ductular reaction but often will not stain as intensely as normal ducts | Obesity very typical association; can be seen in men or women; adenomatosis also common; more difficult to identify by gross appearance |
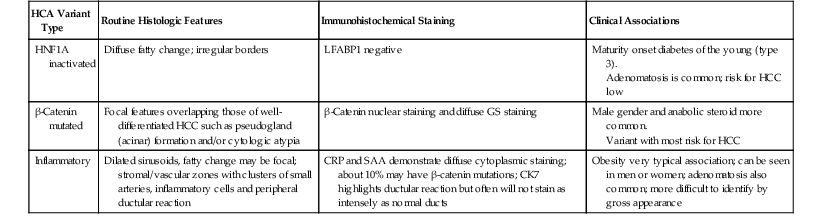
CK, Cytokeratin; CRP, C-reactive protein; LFABP1, liver-type fatty acid binding protein 1; GS, glutamine synthetase; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; HNF1A, hepatocyte nuclear factor 1α; SAA, serum amyloid A.
Pathologic Features
Adenomas may be single or multifocal, and when they are multifocal (>10 lesions),1,14 the condition is referred to as hepatocellular adenomatosis. In most cases, these tumors occur in histologically normal or almost normal livers.12 Adenomas usually are round tumors that tend to bulge on cut section. They are soft and typically somewhat lighter in color than the surrounding liver parenchyma, but their appearance may vary if necrosis or hemorrhage exists. The inflammatory variants can be very ill defined and are notable only in some cases by an increased congestive appearance, some bulging, or bogginess of the parenchyma. HCAs usually lack significant fibrosis or nodularity, although these features are rarely present.13 Normally, HCAs are not encapsulated and can have an irregular, ill-defined border, especially in the inflammatory forms. Rarely, HCAs may have a slate gray to black color caused by large amounts of lipofuscin pigment, which is sometimes referred to as black adenoma.11
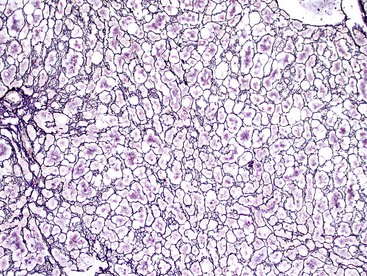
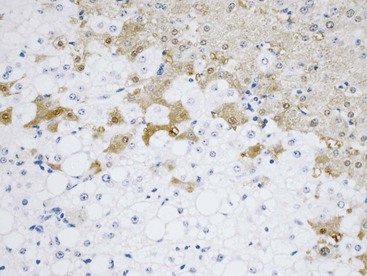
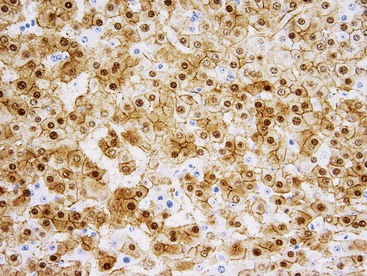
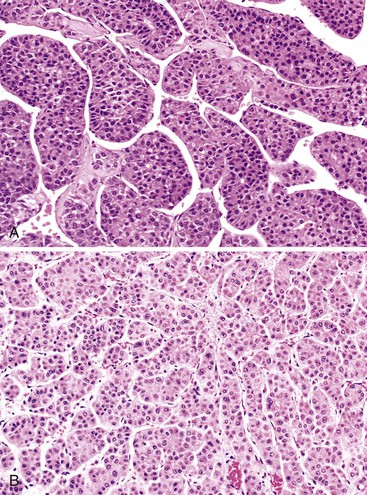
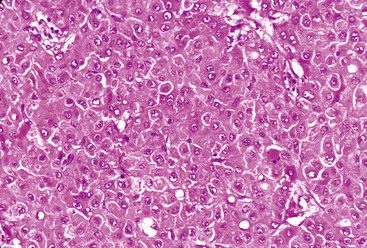
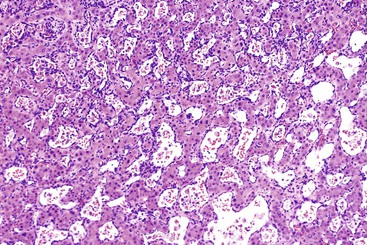
Microscopically, HCAs are composed of a relatively uniform population of hepatocytes arranged in cell plates one to three cells thick (Fig. 55.1). The cell plates usually have a slightly more irregular pattern than those in normal liver. A key feature of HCAs is that the reticulin framework of the cell plates is intact or only focally decreased (Fig. 55.2). The tumor cells are usually similar in size to normal hepatocytes, although they may be slightly smaller or larger. However, the nucleus-to-cytoplasm ratio of adenoma cells is usually the same as for normal hepatocytes. The cytoplasm of tumor cells may be eosinophilic or clear, or it may contain fat droplets, bile stasis, lipofuscin pigment, or Mallory hyaline (rare). The HNF1A-inactivated HCA has a diffuse fatty change, and these lesions also show lack of staining for liver-type fatty acid binding protein 1 (LFABP1) (Fig. 55.3). Other mild variations in cellular morphology, such as multinucleated tumor cells, or rare atypical or pleomorphic hepatocytes may also be seen, but they are usually focal and few in number. Regardless of the cellular morphology, mitotic figures are typically absent or rare in HCAs.
Variations in architecture, such as the formation of acini (i.e., pseudoglands or glandlike structures composed of hepatocytes) may be seen but are typically limited in number. However, this can be a more prominent feature in the HNF1A-inactivated variant and acinar structures may contain bile. Alterations in the sinusoids may appear compressed, resulting in a somewhat uniform and solid appearance of the tumor. Alternatively, peliosis hepatis and sinusoidal dilation may be identified in some adenomas, particularly the inflammatory variant (see Fig. 55.1). Large arterial vessels often are prominent in all forms, and multiple, small arteries in clusters surrounded by mild inflammatory cell infiltrates are common features of the inflammatory variant. The inflammatory variant exhibits diffuse immunostaining for inflammatory proteins, including C-reactive protein (CRP) and serum amyloid A (SAA) (Fig. 55.4).
Kupffer cells may be seen in these adenomas, but fewer are identified than in normal liver. Rarely, a partial capsule exists, showing foci of tumor cells that merge with adjacent liver parenchyma at sites where the capsule is absent (see Fig. 55.3). A bile ductular reaction is typically absent, but in the inflammatory type, a ductular reaction at the periphery of the arterial foci may simulate portal tracts. Portal tracts are occasionally found within HCAs at the border of the lesions. Areas of infarction and hemorrhage are relatively frequent findings, especially in larger lesions.
The mutated β-catenin variant demonstrates focal positive hepatocellular nuclear immunostaining for β-catenin (Fig. 55.5) and diffuse staining for glutamine synthetase. This type is most likely associated with anabolic steroid use and is more likely to show nuclear atypia, peliosis hepatis, or a prominent acinar (pseudoglandular) pattern.11,12 However, it is controversial whether some of these lesions represent well-differentiated HCCs. It is prudent to consider anabolic steroid–related lesions with atypia as well-differentiated hepatocellular neoplasms of uncertain malignant potential, and most authorities recommend resection of all β-catenin–mutated lesions, regardless of size.
Differential Diagnosis
The main differential diagnoses for HCA are focal nodular hyperplasia (FNH) and well-differentiated HCC (see Table 55.4). Adenomas normally have a relatively uniform cell population that resemble normal liver, lack mitotic activity, reveal cell plates less than three cells thick, and have an intact reticulin framework lining the cell plates. These features help to distinguish this lesion from well-differentiated HCC. If a lesion shows more than rare cytologic atypia or focal loss of reticulin pattern, particularly in a male patient or older woman, a diagnosis of well-differentiated HCC or well-differentiated hepatocellular neoplasm of uncertain malignant potential should be considered.
HCAs stain positively for hepatocellular markers, such as the cytokeratin marker CAM 5.2 and the canalicular marker polyclonal carcinoembryonic antigen (CEA). Adenomas often show CD34 positivity in the endothelial cells that line the cell plates, similar to that seen in HCC. This marker cannot be used reliably to distinguish HCA from HCC.18 Staining for AFP is typically negative in HCAs, but because it is often negative in well-differentiated HCC, use of this marker is not typically helpful. Staining for glypican 3 (GPC3), an oncofetal protein, is negative in typical HCAs, and it is more often positive in HCCs than AFP staining.19,20 However, GPC3 is less useful in distinguishing very-well-differentiated HCCs from HCA, because the two may have similar (negative) staining patterns.21,22
For differentiation of HCA from FNH, isolated arteries in small clusters with limited periarterial stroma, the lack of fibrous bands or central fibrous zone, and lack of nodularity are the most helpful features supporting a diagnosis of adenoma. Ductular reaction at the edge of the fibrous or hepatocyte zones is more prominent in FNH, but some degree of ductular reaction is typically seen in inflammatory HCAs. Acinar formation in HCA must not be confused with bile ductules.
Immunohistochemistry can be helpful to distinguish HNF1A-inactivated HCAs and inflammatory variants of HCA from FNH. Staining for LFABP1 is negative in the former (see Fig. 55.3), and staining for SAA or CRP is diffusely positive in the latter (see Fig. 55.4). Staining of SAA and CRP can be seen in FNH, but it should be focal rather than diffuse. Glutamine synthetase immunostaining in FNH produces a different pattern of staining (discussed later) than that seen in HCAs, which show no significant staining, limited perivenular staining, or diffuse strong staining (e.g., in β-catenin–mutated HCAs).22
Treatment
Surgical excision is justified, particularly for large lesions (>5 cm), because of the risks of tumor growth, life-threatening hemorrhage, the rare development of HCC, and because it is difficult to differentiate an HCA from a well-differentiated HCC. Liver transplantation may be considered for patients with adenomatosis.13
Focal Nodular Hyperplasia
Clinical Features
FNH is a benign, non-neoplastic lesion most commonly encountered in young women,7,9 although significant numbers are seen in men. Classic FNH is often an incidental finding, but it may also come to clinical attention because of its large size (which rarely results from hemorrhage).23 Some patients have abnormal liver function test results, such as elevated γ-glutamyltranspeptidase activity.23
Nodules similar to FNH nodules have been described adjacent to hemangiomas. Some patients with multiple FNH syndrome have at least two FNH lesions associated with one or more other lesions, such as hepatic hemangioma, an arterial structural defect, vascular malformation, meningioma, or astrocytoma.12 Nodules with FNH-like changes are commonly seen in Budd-Chiari syndrome24,25 and in hereditary hemorrhagic telangiectasia (i.e., Rendu-Osler-Weber disease). Many refer to these lesions as “regenerative nodules”6 or as FNH-like lesions.
Pathogenesis
Unlike HCA, FNH does not develop as a result of oral contraceptive use; however, evidence suggests that FNH lesions may increase in size with contraceptive use or regress after cessation of therapy. The exact pathogenesis is not established for FNH, but FNH is thought to represent a hyperplastic or altered growth response to alterations in parenchymal blood flow that occur around preexisting vascular malformations.26 The presence of numerous abnormal muscular vessels in FNH and the association of some of these lesions or similar hyperplastic foci with hemangiomas and the Budd-Chiari syndrome lend support to this theory. Another theory suggests that the lesions result from vascular occlusive events with the formation of collaterals and secondary reactive or ischemic changes.27 Lesions caused by vascular occlusive events may be referred as “FNH-like lesions” (my preferred nomenclature) instead of FNH because they have different etiologic factors and because FNH-like lesions may not have all the typical features of FNH.
FNH has no known malignant potential, and despite its rare association with fibrolamellar HCC, most authorities think that the lesion does not progress to carcinoma. Instead, the association of FNH with fibrolamellar HCC may represent a hyperplastic response to increased vascularity, which is typical of carcinomas. The lesion formerly known as the “telangiectatic” type of FNH, which was originally associated with the multiple FNH syndrome,11 is now recognized as the inflammatory variant of HCA (see Hepatocellular Adenoma, earlier).14
Pathologic Features
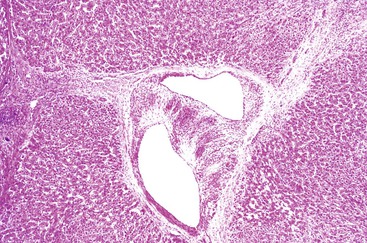
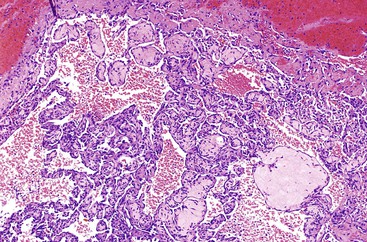
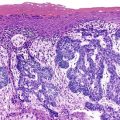
Similar to HCAs, FNH usually involves a solitary nodule, but it may be multifocal.12,23 FNH has a nodular appearance, which may simulate the appearance of macronodular cirrhosis, and it tends to be lighter in color than the surrounding liver parenchyma. These lesions are often located near the liver capsule, which may impart a localized nodular appearance mimicking cirrhosis. Occasionally, FNH may be pedunculated. The edges of FNH are normally well demarcated from the normal liver parenchyma, but a fibrous capsule is usually absent. FNH may vary considerably in size. Most FNH lesions have a central fibrous scar, which consists of fibrovascular tissue, not dense scar tissue.
Microscopically, the classic type of FNH is composed of normal-appearing hepatocytes arranged in incomplete nodules and partially separated by fibrous tissue that extends outward from the central fibrous zone of the tumor. Variable numbers of bile ductular structures are normally seen in the fibrous stroma and particularly at the edge of the nodules. The cell plate architecture is similar to that of normal liver. However, the cell plates are usually wider (two to three cells thick), similar to regenerative nodules. The reticulin framework should be intact, but in some lesions, cell plates may have a more acinar appearance, may be wider than three cells (usually not thicker than five cells), and be separated by thin collagen bands. The hepatocytes in FNH may demonstrate increased levels of glycogen, fatty change, bile stasis, lipofuscin, iron pigment, copper or copper-associated protein, and Mallory bodies.12 Foci of atypical hepatocytes containing larger nuclei and mild hyperchromasia, with or without conspicuous nucleoli, may be seen in some cases.
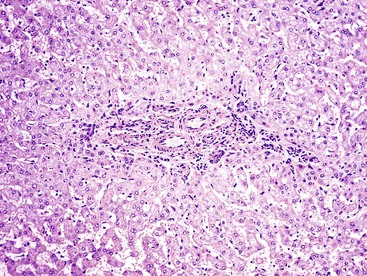
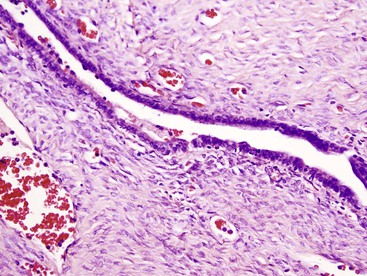
Another important diagnostic feature is the presence of medium- to large-sized, thick-walled muscular vessels, which often exhibit myointimal myxoid or fibromuscular hyperplastic changes (Figs. 55.6 and 55.7). These vessels are not considered components of a portal tract, because large ducts of similar caliber and portal veins are not associated with them. Portal tracts are absent in FNH except at the periphery of the lesion, although a bile duct of intermediate or large caliber may be found in the central fibrous zone in rare cases.23 The sinusoids may be dilated, and Kupffer cells may be evident. Inflammatory cell infiltrates are relatively common and consist mainly of lymphocytes, although neutrophils and eosinophils may be seen, particularly around bile ductular structures. Rarely, granulomas are identified.
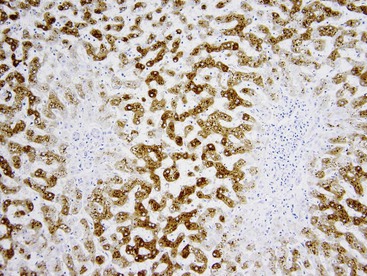
Immunohistochemistry demonstrates intense but patchy staining (i.e., maplike pattern) for glutamine synthetase1,14 (Fig. 55.8), which is distinctly different from the centrizonal hepatocyte staining pattern of normal liver. For FNH-like lesions, portal zones may be seen within the lesion, the ductular reaction may be decreased or lacking, and the central scarlike zone may be absent.
Differential Diagnosis
FNH may resemble HCA or normal liver in small biopsy samples. One of the most important distinguishing features of FNH is the bile ductular reaction (Table 55.4), but it must not be mistaken for the ductular reaction in the inflammatory variant of HCA. Because of the patchiness of the bile ductular reaction, a large-core needle biopsy or a wedge biopsy may be necessary for this feature to be identified. The finding of large blood vessels with abnormal hyperplastic features and usually surrounded by at least a moderate amount of connective tissue may be helpful, because the blood vessels in HCA tend to have a more normal configuration and lack significant perivascular connective tissue stroma.
Table 55.4
Comparison of Hepatocellular Adenoma and Focal Nodular Hyperplasia
| Morphologic Feature | Hepatocellular Adenoma | Focal Nodular Hyperplasia |
| Hepatocytes | Similar in size, slightly larger or smaller than in normal liver; rare tumors show pleomorphism; cytoplasmic glycogen or fat may be seen | Usually normal in size; no significant cytologic abnormalities but possible foci of cellular pleomorphism; glycogen or fat may be seen |
| Bile ductules | None (except in inflammatory HCA) | Present in fibrovascular zone, typically at edges of hepatocytic nodules |
| Vessels or other blood-filled spaces | Large vessels often seen but without surrounding connective tissue zone; small to medium-sized, isolated hepatic arteries; peliosis hepatis and sinusoidal dilation | Abnormal, large, muscular vessels surrounded by connective tissue stroma |
| Connective tissue component | Occasional fibrous septa | Fibrovascular central zone; myxoid or dense collagen; chronic inflammatory infiltrates; possible focal sinusoidal fibrosis |
| Encapsulation | Occasionally occurs, often discontinuous | None |
| Cell plate architecture | 1 to 3 cells wide; regenerative appearance | 1 to 3 cells wide; plates may be compressed; foci of plates > 3 cells wide may be seen |
| Reticulin stain | Normal or slightly decreased; may have acinar-like pattern | Normal staining pattern but can show irregular architecture with wider cell plates and small acinar change |
| Immunohistochemistry | β-Catenin, SAA, CRP staining and lack of LFABP1 staining define variants | GS maplike staining |
| Other features | Patient age: between 10 and 50 yr; typically female; normal serum AFP level and possible increased alkaline phosphatase level, large lesions prone to hemorrhage | Young adults, female > male; normal AFP level; radiographic imaging often demonstrates central fibrous zone |
AFP, α-Fetoprotein CRP, C-reactive protein; LFABP1, liver-type fatty acid binding protein 1; GS, glutamine synthetase; HCA, hepatocellular adenoma; SAA, serum amyloid A.
The use of immunohistochemical stains can help to differentiate FNH from HCA. SAA and CRP typically are diffusely positive in inflammatory HCA but show very limited or no focal staining in FNH. Staining for glutamine synthetase in FNH should be intense and extensive compared with the patchy perivenular staining or lack of staining in HCA. The exception is the β-catenin–mutated variant of HCA, which may show diffuse intense staining of glutamine synthetase.
Similar to HCA, FNH normally stains positively for hepatocellular markers, including the cytokeratin (CK) marker CAM 5.2 and the canalicular marker polyclonal CEA. The reticulin stain typically demonstrates an intact reticulin framework in FNH, as in HCA, but rare foci may also show cell plates three to five cells thick, and care must be taken not to mistake this finding for HCC.28 A copper stain may demonstrate deposits in hepatocytes adjacent to fibrous bands.28 CD34 often is positive in endothelial cells that line cell plates, although to a lesser extent than in many HCAs and HCCs, but in any one case, this marker is not reliable for distinguishing FNH from HCA or HCC.18 However, CD34 may still be useful for demonstrating lesional tissue in the sample. Staining for AFP is typically negative in FNH.
Treatment
Surgical resection is usually unnecessary unless the lesion is pedunculated or very large, in which case there is a risk for torsion and hemorrhage, or for abdominal pain and distress. Excision of FNH is justified if the lesion cannot be differentiated from HCC or HCA with confidence.
Benign and Dysplastic Lesions in the Cirrhotic Liver
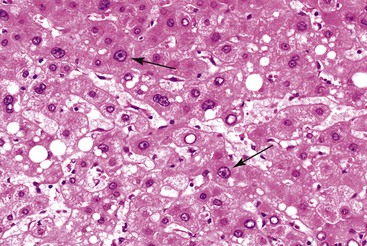
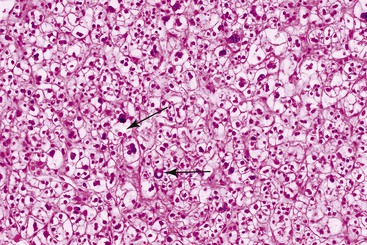
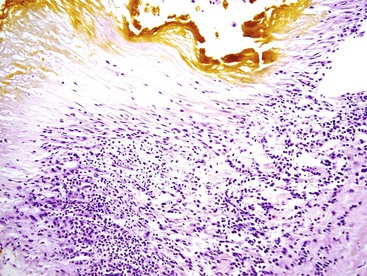
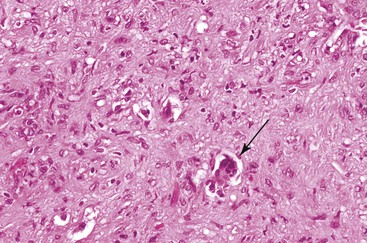
A review of the terminology recommended for cytologic changes is necessary to discuss the various types of nodules that occur in cirrhosis. Cirrhotic nodules frequently contain scattered, enlarged hepatocytes with abundant cytoplasm and atypical, enlarged nuclei with a preserved nucleus-to-cytoplasm ratio (Fig. 55.9). This cytologic feature was previously designated large cell dysplasia. However, the International Working Party recommends the term large cell change12 for this finding because the alteration in this typical pattern of scattered cells in cirrhosis is not definitely dysplastic and more likely a degenerative change.29 This type of cellular change is quite prevalent.29
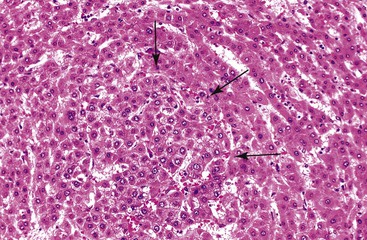
Cirrhotic nodules may contain small hepatocytes with normal, slightly smaller, or slightly larger nuclei and scant cytoplasm, which results in an overall increase in the nucleus-to-cytoplasm ratio (Fig. 55.10). This cytologic feature was previously designated small cell dysplasia, but the International Working Party recommends the term small cell change for the process.12 The consensus is that small cell features in cirrhotic livers may be regenerative rather than dysplastic and may be preneoplastic only in certain instances, such as when the cells are arranged in clusters in a cirrhotic nodule.
Clusters of small cells may be seen in any type of cirrhotic nodule and may be only 1 to 2 mm in diameter, which imparts the appearance of a nodule within a nodule (see Fig. 55.10). This type of change has been referred to as a dysplastic focus, without further classifying it as low or high grade.12 Dysplastic foci have a high prevalence among diseases such as chronic hepatitis B virus (HBV) and chronic hepatitis C virus (HCV) infection, α1-antitrypsin deficiency, and tyrosinemia.12 Dysplastic foci may also contain cells with enlarged nuclei and hyperchromasia and with minimal to severe nuclear atypia. Cytoplasmic fat (i.e., glycogen) often is different from that seen in the surrounding liver. The International Working Party recommends that the term dysplasia be used only for dysplastic foci or for bona fide dysplastic nodules and not for scattered cytologic changes in a cirrhotic liver.
The whole nodule may be abnormally large or dysplastic, and these nodules have been designated as large regenerative nodules and as low and high-grade dysplastic nodules. The dysplastic nodules are thought to be part of the pathogenetic pathway to HCC.30
Macroregenerative Nodules and Low-Grade Dysplastic Nodules
Nomenclature
In cirrhosis, benign nodules larger than typical cirrhotic nodules have been referred to by various names, including large regenerative nodule12 and macroregenerative nodule. Low-grade dysplastic nodules are thought to represent a clonal proliferation of hepatocytes. However, because the gross and microscopic features of large regenerative nodules and low-grade dysplastic nodules are often indistinguishable, the following description applies equally to both lesions.
Many clinicians do not try to distinguish large regenerative nodules from low-grade dysplastic nodules, because clonality studies may be the only valid method for doing so. In clinical practice, these terms are used interchangeably to describe a nodule that is distinct from the surrounding cirrhotic liver but lacks the cytologic or architectural abnormalities characteristic of high-grade dysplastic nodules. I prefer the term macroregenerative nodule for these lesions and reserve the dysplastic label for high-grade lesions.
For similar-appearing nodules in noncirrhotic livers, the International Working Party recommends the term multiacinar regenerative nodules.12 They tend to occur in the setting of Budd-Chiari syndrome or portal vein thrombosis or as a sequela of hepatocyte necrosis with regeneration. These lesions may be similar to FNH and be designated as FNH-like lesions, (see Focal Nodular Hyperplasia). Large regenerative nodules and multiacinar regenerative nodules represent a reactive process rather than a clonal preneoplastic lesion.
Clinical Features
Macroregenerative nodules and low-grade dysplastic nodules occur in the setting of cirrhosis, with only a few exceptions, such as in the setting of chronic liver disease without fully developed cirrhosis.31 They are usually an incidental finding at autopsy or at the time of transplantation, but they may also be identified on radiographic studies. The serum AFP concentration is typically normal or within the low range expected for the underlying chronic liver disease or cirrhosis.
Pathogenesis
These nodules usually are considered benign lesions, but nodules with this histologic appearance have been associated with an increased incidence of HCC.32 Almost no previous studies of macroregenerative nodules have attempted to define the lesions as regenerative or clonal but instead have defined them as lacking features of high-grade dysplastic nodules or HCC on routine histologic examination. The literature may be somewhat contradictory, and it cannot be definitively stated whether regenerative nodules and clonal, low-grade dysplastic nodules have similar risks for HCC. These nodules are typically seen in cirrhosis related to HBV or HCV infection, alcohol consumption, or hemochromatosis, and they are less likely to be seen in primary biliary cirrhosis. The risk factors for development of macroregenerative nodules are similar to those for HCC.
Pathologic Features
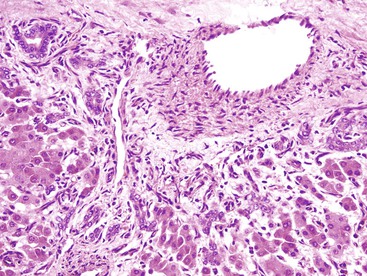
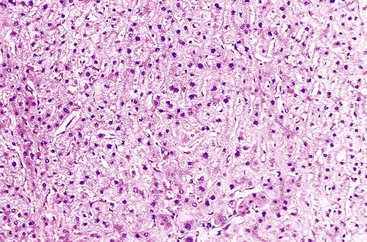
Macroregenerative nodules or low-grade dysplastic nodules are larger than typical cirrhotic nodules. The generally accepted lower limit is between 0.8 and 1 cm in diameter, and the higher limit is less than 3 cm in the greatest diameter. These nodules tend to bulge on cut section. The edges of the nodules are usually round and sharply circumscribed, and they may be more deeply bile stained or paler than typical cirrhotic nodules.
Histologically, these nodules resemble cirrhotic nodules. They have an intact reticulin framework similar to that of normal liver, and the cell plates are usually one or two cells thick (Figs. 55.11 and 55.12). The hepatocytes typically have unremarkable cytology, although some focal variation in cell size and scattered large cell change similar to that seen in other cirrhotic nodules may be seen in large regenerative nodules. Low-grade dysplastic nodules are expected to have a more uniform population of hepatocytes because of their clonal nature, but many specific features of this type of clonal nodule have not been definitively established. Findings such as Mallory bodies, bile stasis, clear cell cytoplasmic changes, iron or copper deposits, a slight decrease in cell size,33 and focal or diffuse fatty changes may be seen. Portal tracts are usually seen in the nodule, and bile ductular reaction may be prominent. Fibrous septa that extend into the nodule without a complete triad of duct, vein, and artery may be identified.34
Differential Diagnosis
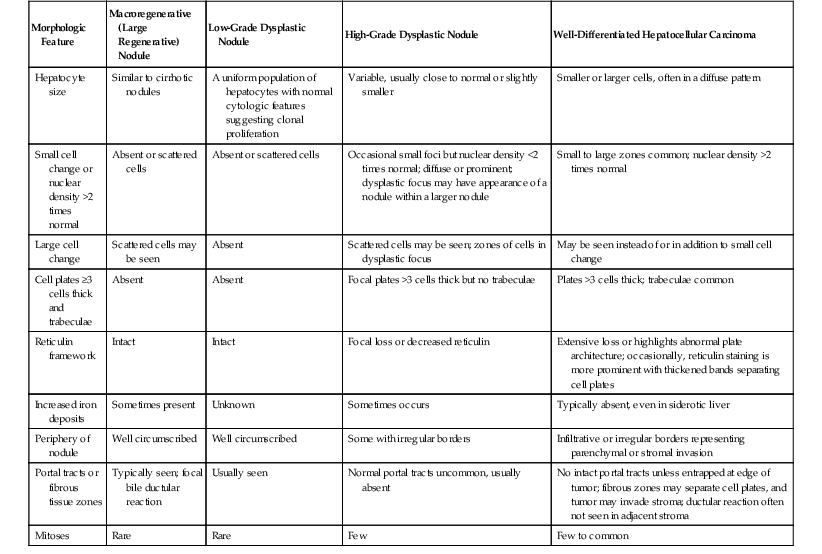
The size of this nodule is important for differentiating it from typical cirrhotic nodules. Rarely, a nodule with this histologic pattern lacks portal structures, but this does not warrant a diagnosis of adenoma in a cirrhotic liver without one of the risk factors for adenoma. Features that differentiate these nodules from high-grade dysplastic nodules and small, well-differentiated HCCs are outlined in Table 55.5.
Table 55.5
Differentiating Features of Macroregenerative and Dysplastic Nodules and Well-Differentiated Hepatocellular Carcinoma
| Morphologic Feature | Macroregenerative (Large Regenerative) Nodule | Low-Grade Dysplastic Nodule | High-Grade Dysplastic Nodule | Well-Differentiated Hepatocellular Carcinoma |
| Hepatocyte size | Similar to cirrhotic nodules | A uniform population of hepatocytes with normal cytologic features suggesting clonal proliferation | Variable, usually close to normal or slightly smaller | Smaller or larger cells, often in a diffuse pattern |
| Small cell change or nuclear density >2 times normal | Absent or scattered cells | Absent or scattered cells | Occasional small foci but nuclear density <2 times normal; diffuse or prominent; dysplastic focus may have appearance of a nodule within a larger nodule | Small to large zones common; nuclear density >2 times normal |
| Large cell change | Scattered cells may be seen | Absent | Scattered cells may be seen; zones of cells in dysplastic focus | May be seen instead of or in addition to small cell change |
| Cell plates ≥3 cells thick and trabeculae | Absent | Absent | Focal plates >3 cells thick but no trabeculae | Plates >3 cells thick; trabeculae common |
| Reticulin framework | Intact | Intact | Focal loss or decreased reticulin | Extensive loss or highlights abnormal plate architecture; occasionally, reticulin staining is more prominent with thickened bands separating cell plates |
| Increased iron deposits | Sometimes present | Unknown | Sometimes occurs | Typically absent, even in siderotic liver |
| Periphery of nodule | Well circumscribed | Well circumscribed | Some with irregular borders | Infiltrative or irregular borders representing parenchymal or stromal invasion |
| Portal tracts or fibrous tissue zones | Typically seen; focal bile ductular reaction | Usually seen | Normal portal tracts uncommon, usually absent | No intact portal tracts unless entrapped at edge of tumor; fibrous zones may separate cell plates, and tumor may invade stroma; ductular reaction often not seen in adjacent stroma |
| Mitoses | Rare | Rare | Few | Few to common |
Studies of the vascularity of these nodules have shown that they have a large number of arteries unassociated with other components of a normal portal space. They are referred to as unpaired arteries. However, staining for CD34 or CD31, as a marker for sinusoidal capillarization, is usually similar to that seen in cirrhotic nodules; some peripheral staining is normally seen at the edge of the nodules.35,36 The nodules are usually negative for AFP37 and otherwise show a staining pattern similar to that expected in normal liver for cytokeratin and polyclonal CEA.
Treatment
Therapy for macroregenerative nodules varies because most lesions are found incidentally on excision or explantation. For lesions that have not been excised, clinical follow-up for an increase in size or other features that suggest malignant transformation is recommended.
High-Grade Dysplastic Nodules
Clinical Features
High-grade dysplastic nodules,12,34 previously known as borderline nodules,33 almost always occur in cirrhotic livers. The serum AFP level is usually normal or within the range typical of chronic liver disease or cirrhosis. The lesions may enhance on radiographic imaging, but lesions less than 1 cm in diameter tend to fall below the limits of radiographic resolution.
Pathogenesis
High-grade dysplastic nodules, which typically occur in the setting of cirrhosis, are considered a premalignant change in the carcinogenic pathway to HCC. Various pathologic features and molecular features support this theory (see Chapter 44).
Pathologic Features
High-grade dysplastic nodules essentially have the same gross features as large regenerative or low-grade dysplastic nodules, although some may appear less circumscribed or have an irregular border.
Microscopically, when dysplastic changes are observed uniformly throughout a nodule, a designation of high-grade dysplastic nodule is warranted. A nodule containing one or more dysplastic foci is referred to as a dysplastic nodule (see Fig. 55.10). The atypical features of these lesions are not overtly severe enough to be diagnostic of HCC, but they are more atypical than expected in usual cirrhotic nodules. The nodule is often recognized by zones of small cell change (see Fig. 55.10) with an increased nucleus-to-cytoplasm ratio (i.e., increased nuclear density), which is defined as an increased number of hepatocyte nuclei per microscopic field compared with normal liver.33 Large cell change, which is rarely a feature of high-grade dysplastic nodules, may be identified in a discrete zone of atypical cells rather than as enlarged nuclei scattered singly throughout the nodule.
Other common features of high-grade dysplastic nodules include focal zones of cell plates that measure as much as three cells thick, a focal decrease in the reticulin framework, and mild dilation of sinusoids. These nodules may also contain foci of Mallory bodies, fat, clear cell change, cytoplasmic basophilia, rare mitoses, bile, and portal tracts. The nodules may contain iron deposits. High-grade dysplastic lesions tend to lack iron deposits, which are more common in low-grade nodules. The borders of these nodules may be irregular, and focal acini (pseudoglands) may be seen.
Differential Diagnosis
High-grade dysplastic nodules are differentiated from overt HCC by several features (see Table 55.5). Those particularly helpful for diagnosing HCC include the mitotic figures in moderate numbers, trabeculae, cell plates greater than three cells thick, nuclear density more than two times normal, marked reduction in the reticulin framework, numerous unpaired arteries, and absence of portal tracts. High-grade dysplastic nodules are more likely to show an immunostaining pattern similar to that of HCC, such as sinusoidal staining with CD34 and rare positivity with GPC3 (see Hepatocellular Carcinoma and Its Variants).19,38
Treatment
Most authorities recommend excision or ablation of high-grade lesions because they are thought to represent the early stage of a malignant process. Sampling by small-core biopsies may miss the areas of tissue diagnostic of HCC, which may be found focally in high-grade dysplastic nodules.
Hepatocellular Carcinoma and Its Variants
Clinical Features
HCC is the most common primary malignant tumor in the liver. In approximately 85% of cases in the United States, HCC occurs in the setting of cirrhosis.39 The incidence of HCC varies by geographic location, with a low incidence in Europe and North America (2 to 7 cases per 100,000 persons) and a high incidence in eastern Asia and southern Africa (30 cases per 100,000 persons). The incidence of HCC generally increases with age, but the mean age of occurrence depends on the specific geographic location. For example, in North America, the mean age at diagnosis is approximately 60 years, but in Africa, the average age is approximately 35 years. In Taiwan, the mean age of occurrence ranges from 40 to 60 years.40 HCC is three times more common among men than women, and worldwide, it is the fifth most common type of malignancy in men and the eighth most common in women.
The presenting symptoms vary, but many patients are completely asymptomatic or have mild abdominal pain. Other signs of HCC include weight loss, jaundice, ascites, malaise, and fever. Late serious complications of large HCC tumors include rupture with hemorrhage and disease that is metastatic most often to the abdominal cavity or lungs.
Many patients with cirrhosis are diagnosed by imaging or an elevated serum AFP level. Lesions smaller than 1.5 cm in diameter usually do not enhance on radiographic angiography, and HCC may be more echogenic or less echogenic than adjacent normal liver parenchyma. Almost two thirds of patients with large HCC tumors have a high serum AFP level (>1000 ng/mL).39 Tumors smaller than 2 to 3 cm in diameter are unlikely to be associated with an elevated serum AFP level.37 A serum AFP level of less than 500 ng/mL may be seen in many types of liver disorders. Elevation of AFP levels to between 500 to 1000 ng/mL suggests HCC but is not specific. Other types of malignancies, such as some types of tubal gut adenocarcinomas, ovarian tumors, and germ cell tumors, may also have markedly elevated AFP levels.
Pathogenesis
HCC develops during a long period and is probably a multistep process that involves various risk factors (e.g. chronic hepatitis), exposure to certain toxic or viral agents, and genetic alterations (see Chapter 44).41 The major etiologic association is cirrhosis, but HBV or HCV infection is also an independent predisposing factor. Patients with cirrhosis resulting from hemochromatosis or α1-antitrypsin disease have an increased risk of HCC compared with patients with cirrhosis related to other causes (excluding HBV and HCV). Other risk factors include exposure to thorium dioxide (Thorotrast), aflatoxins, and estrogenic steroids.9 Stem or progenitor cells in the liver have the potential to differentiate into hepatocytic and biliary cell types, and they may play a role in the pathogenesis of primary liver tumors, particularly HCC, cholangiocarcinoma, or mixed HCC and cholangiocarcinoma,42,43 but their exact role remains unclear.
Pathologic Features
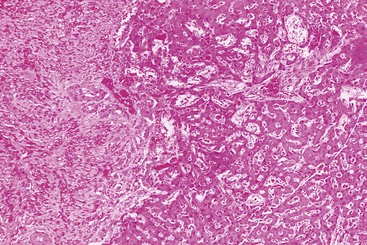
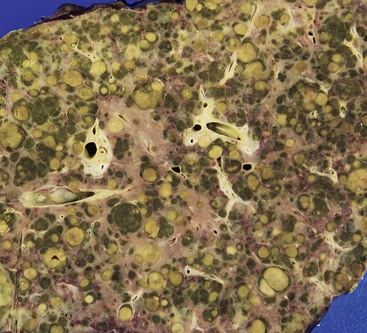
In the United States, most HCC tumors arise in cirrhotic livers. The liver of a patient with HCC may be more bile stained or paler than a nontumorous liver, and it may have irregular borders or satellite nodules. Rarely, the entire lesion is composed of multiple nodules similar in size to typical cirrhotic nodules (Fig. 55.13). Large vein invasion and a fibrous capsule may be identified, particularly in association with large tumors. Small HCCs (<2 cm in diameter) usually lack vascular invasion, necrosis, and hemorrhage.
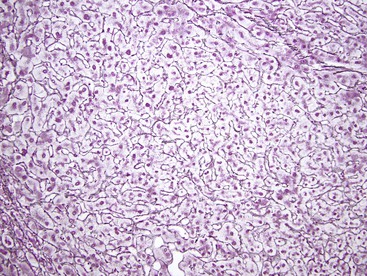
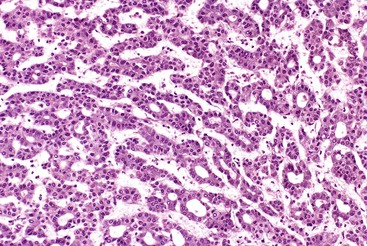
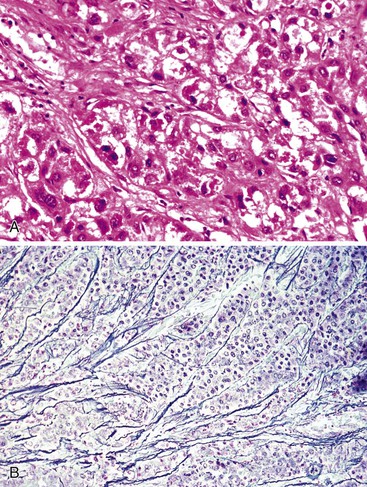
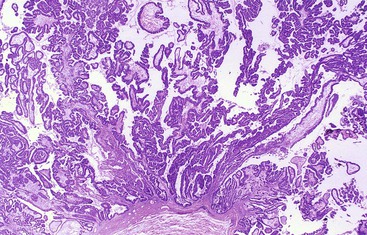
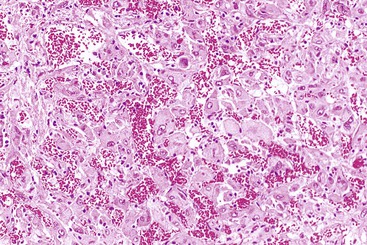
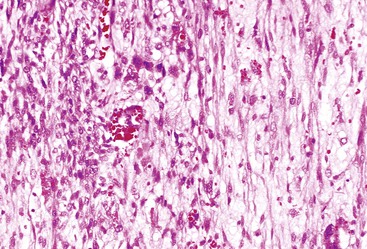
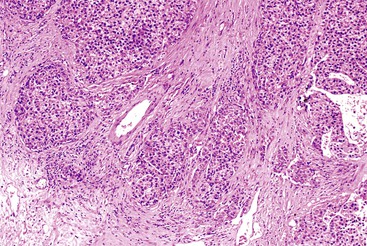
The WHO1 has described the four typical histologic patterns of HCC: trabecular, acinar, compact, and scirrhous. The trabecular (platelike) pattern is the most common (Fig. 55.14). In this variant, tumor morphology mimics the cell plate architecture of normal liver but has important differences. The cell plates in trabecular HCC are typically three or more cells thick, whereas the cell plates in normal or regenerative liver are normally only one or two cells thick. As in normal liver, tumor cell plates are lined by endothelial cells, but reticulin staining usually shows that the reticulin framework is absent or markedly decreased or distorted, with irregular or absent staining of the borders of the trabeculae (Fig. 55.15). Tumor cells often have features of a small cell change (see Figs. 55.14 and 55.15). Large cell change may be seen infrequently, except in high-grade tumors. Foci of small and large cell changes often are admixed. Kupffer cells are typically absent in HCC. Occasionally, the tumor cell plates (or trabeculae) may be separated by an increased amount of connective tissue instead of by endothelial cells (Fig. 55.16), and reticulin staining is usually increased in the bands of connective tissue. The thickened cell plates, which are separated by a prominent reticulin framework, often have a linear or ribbon-like arrangement. This pattern has been subclassified as an early form of scirrhous HCC, although the overall amount of fibrous tissue may not be as high as previously described.
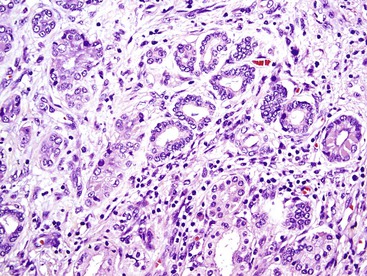
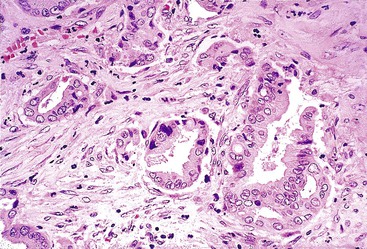
The acinar (pseudoglandular) pattern of HCC is less common than the trabecular pattern. This variant is defined by glandlike spaces (i.e., acini) that are lined by hepatocytic tumor cells (Fig. 55.17). The acinar structures are formed by dilation or expansion of bile canaliculi, and they often contain bile or proteinaceous material. Less frequently, the spaces develop as a result of central necrosis of trabeculae and contain protein, cellular debris, or macrophages. The formation of glandlike spaces can lead to a misdiagnosis of adenocarcinoma. The acinar pattern is frequently admixed as a minor component with the trabecular pattern (see Fig. 55.15).
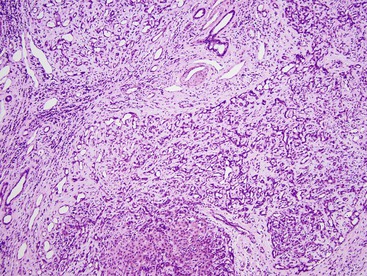
The compact (solid) pattern of HCC is a relatively uncommon variant characterized by dense aggregates of tumor cells that seem to lack endothelial cell–lined trabeculae or cell plates (Fig. 55.18), and it is more common in poorly differentiated HCC. However, careful examination with endothelial cell markers often reveals compressed trabeculae. Loss of the reticulin framework is typically seen in solid and crowded cellular zones.
The scirrhous pattern contains prominent, focal or diffuse areas of fibrosis and may be associated with any of the patterns previously described (Fig. 55.19). Differentiation of scirrhous HCC from fibrolamellar HCC (see Fibrolamellar Variant of Hepatocellular Carcinoma) is based on identification of the characteristic cytologic features of the latter and on the different clinical settings in which these two tumors occur. The term sclerosing hepatic carcinoma has been used for some of the HCCs and cholangiocarcinomas associated with hypercalcemia, but this form is not recognized as a distinct variant.44
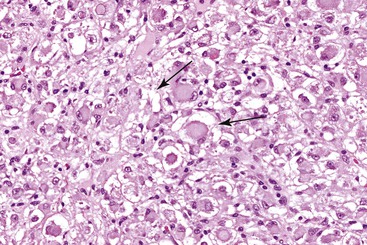
In all of these patterns, the cytologic features of HCC tend to resemble those of normal hepatocytes, but the degree of nuclear atypia varies, ranging from near normal to markedly abnormal (i.e., angular nuclei with clumped chromatin). The tumor cells often maintain their polygonal shape and have round vesicular nuclei and prominent nucleoli that exhibit features typical of hepatocytic differentiation. Intranuclear vacuoles (composed of cytoplasmic invaginations) and glycogenation of nuclei (another feature seen in normal liver) are fairly common in HCC tumors (Fig. 55.20). Small cell change is probably the most common atypical cytologic change, but a large cell change and giant or pleomorphic cells may be seen focally or diffusely (see Fig. 55.20). The amount of cytoplasm may vary, and it is often slightly more basophilic than in normal hepatocytes. The cytoplasm may have a granular appearance, or it may be exceptionally oxyphilic because of a large number of mitochondria. Cytoplasmic inclusions (e.g., Mallory bodies, globular acidophilic bodies) composed of proteins (i.e., albumin, fibrinogen, α1-antitrypsin, or ferritin) may be seen (see Fig. 55.20).
Fat (Fig. 55.21), glycogen, or possibly water may be prominent in HCCs, providing the cells with a clear appearance. This has been described as the clear cell variant of HCC. If the entire tumor shows this type of clear cell change and it occurs in a noncirrhotic liver, it may be difficult to differentiate HCC from other types of clear cell tumors, such as metastatic renal cell carcinoma (see Differential Diagnosis). Other cytoplasmic changes seen much less frequently include pale bodies, which are round to oval, lightly eosinophilic or clear cytoplasmic structures most frequently seen in the fibrolamellar variant of HCC; ground-glass cells containing hepatitis B surface antigen, which is found in some patients with HBV infection45; and dark brown to black pigment similar to that seen in the Dubin-Johnson syndrome. Rare forms of HCC that show a prominent spindle cell component or diffuse small cells (i.e., small cell type) have been described.
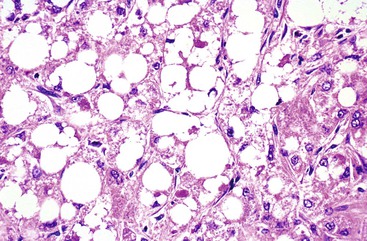
Histologic Grading of Hepatocellular Carcinoma
Grading of HCC is based on the system developed by Edmondson and Steiner in 1954.46 These investigators originally defined four grades that were distinguished by proportional increases in the nucleus-to-cytoplasm ratio, variability in nuclear shape, hyperchromasia, and loss of cell plate architecture from low- to high-grade tumors. This grading system is still used with some minor modifications,6 but descriptive rather than numeric designations are used.1 Methods that use nucleus-to-cytoplasm ratios and proliferative rates are not typically applied.
Some of the Edmondson-Steiner well-differentiated (grade 1) tumors with minimal cytologic atypia and architectural distortion were recognized as malignant only by their association with an admixture of high-grade HCC or by the finding of metastatic lesions.39,46 With the current criteria established by the International Working Party6 and the International Consensus Group for Hepatocellular Neoplasia,30 the stricter definition of grade 1 lesions may help to reclassify some of these lesions as early HCC or as low- or high-grade dysplastic nodules. Ideally, a true grade 1 HCC exhibits definitive architectural features of malignancy or invasion (see Table 55.5), even though it shows little or no cytologic atypia.
Grade 2 tumors are well differentiated. They typically have a trabecular pattern, but they have larger nuclei than grade 1 tumors. Grade 2 lesions may also contain acinar structures and bile.
Grade 3 tumors are moderately differentiated, and they have greater cytologic and architectural variability than grade 2 lesions. Multinucleated (giant) cells are often seen focally, and unlike grade 2 lesions, they usually do not contain bile. When identified, trabeculae typically are wider or have a more varied structure than in grade 2 tumors.
Grade 4 tumors are poorly differentiated or anaplastic tumors in which classification of the lesion as HCC is difficult without knowledge of other clinical or serologic parameters, such as the presence or absence of cirrhosis or an increase in serum level of AFP. Grade 4 lesions include spindle cell and small cell HCC tumors.
An alternative three-grade system is also used. With the three-tier system, grade 1 represents well-differentiated lesions (combined grades 1 and 2 from the previously described system), grade 2 represents moderately differentiated lesions, and grade 3 comprises poorly differentiated and anaplastic lesions. Regardless of which grading system is used, vascular invasion and poor differentiation are considered independent risk factors for a poor prognosis. The current WHO system adapts these criteria and recommends the terminology of well, moderately, and poorly differentiated and undifferentiated for grades of HCC.1
Early or Small Hepatocellular Carcinoma
Small HCC, defined as those less than 2 cm in diameter, are typically well-differentiated tumors with histologic features that often include fat, small acinar changes, intratumoral portal zones, thin cell plates (less than three cells thick), and unpaired arteries.1,30 Another helpful diagnostic marker for possible early HCC is the relative lack of ductular reaction surrounding the edges of the lesions.47 Molecular aspects of transformation to early HCC and concepts of progression are discussed further in Chapter 42. These lesions can be so well differentiated that the primary means of making the diagnosis of HCC may depend on identifying tumor invasion into adjacent stroma or parenchyma.
Rare Variants of Hepatocellular Carcinoma
Variants of HCC that have been selected by the WHO1 for special consideration include the types known as lymphoepithelioma-like carcinoma and sarcomatoid HCC. The lymphoepithelioma-like variant of HCC has pleomorphic tumor cells admixed with a prominent lymphoid infiltrate, and the tumor cells are notably positivity for Epstein-Barr virus. The sarcomatoid variant is composed of spindled tumor cells, but most of these lesions have areas within them that are typical of HCC. This change may be more commonly seen in lesions that have undergone therapy.
Differential Diagnosis
Immunostains may help to distinguish HCC from other tumors in the liver. Most diagnostic problems consist of differentiating adenoma or FNH from well-differentiated HCC, poorly differentiated adenocarcinoma (metastatic or primary) from HCC, and HCC from other primary or metastatic neoplasms. Well-differentiated HCC may be differentiated from large regenerative or low-grade dysplastic nodules and high-grade dysplastic nodules by the features listed in Table 55.5.
AFP is a reasonably specific marker for HCC. However, the staining tends to be patchy or absent in as many as 50% of HCCs. It is typically absent in small, well-differentiated HCCs. Polyclonal CEA, which highlights bile canaliculi, is another highly specific marker for hepatocellular differentiation, but it tends to stain only moderately differentiated to well-differentiated lesions. This stain also delineates the outer cell membranes of adenocarcinomas, which may mimic a canalicular pattern on tangential sectioning.
Hepatocyte antibody (i.e., clone OCH1E5.2.10 or HepPar-1 antibody) is relatively specific for hepatocellular differentiation. This antibody demonstrates a granular cytoplasmic pattern of some degree among tumors with hepatocytic differentiation.48,49 Similar to polyclonal CEA, HepPar-1 is less sensitive for poorly differentiated HCCs, and it may also stain hepatoid variants of adenocarcinoma, many of which metastasize to the liver.50,51 Another relatively specific hepatocyte marker is arginase 1, which may stain a slightly broader spectrum that extends to moderately or poorly differentiated HCCs.52
CK8 and CK18 (in the CAM 5.2 stain) occur in tumors with hepatocellular differentiation. Staining with the AE1/AE3 antibody, which recognizes most keratin types except CK8 and CK18, is usually negative. However, because both of these cytokeratin stains react in most adenocarcinomas, CAM 5.2 should not be used in isolation. CK7 staining is commonly negative in HCCs, but it often focally stains small ductular-like hepatocytes or acinar structures in HCC and can be diffusely positive, especially in tumors with fibrotic stromal backgrounds. CK7 staining has also shown a relative lack of ductular reaction at the borders of HCC.47 CK20 staining is usually negative (>95% of cases) except for rare isolated cellular staining, some of which may be caused by Mallory hyaline staining.
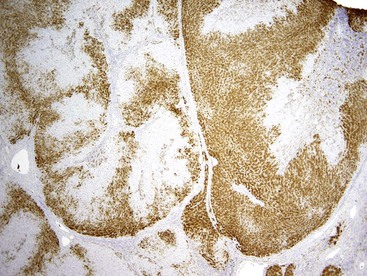
CD10 stains the bile canalicular cytoplasmic surface of hepatocytes. CD34 stains endothelium-lined trabeculae in HCC and can be used to highlight increased vascularity typical of this tumor type. GPC3, an oncofetal protein the concentration of which is elevated in the sera of many patients with HCC, can be helpful to distinguish benign from malignant hepatocellular neoplasms (Fig. 55.22) and HCC from many other tumor types, but it is not entirely specific for HCC.19,20,22,53 Use of a combination of immunostains has been advocated to increase diagnostic accuracy of HCC, including use of GPC3 and CD3454 and the triple combination of GPC3, heat shock protein (HSP70), and glutamine synthetase.55
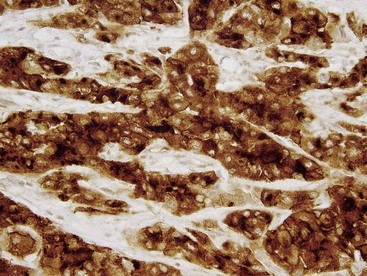
Hepatocellular Carcinoma versus Adenocarcinoma
Differentiating primary or metastatic adenocarcinoma from HCC in noncirrhotic livers is a common problem in tumor pathology (Table 55.6). MOC (monoclonal antibody)-31 is one of the more reliable and consistent markers of adenocarcinoma. It stains most adenocarcinomas (membranous pattern) and stains HCC in less than 20% of cases.56,57 Because MOC-31 may also stain benign hepatocytes in cirrhosis, a routine morphology investigation should be correlated with interpretation of this marker.
Table 55.6
Differentiating Features of Hepatocellular Carcinoma, Cholangiocarcinoma, and Metastatic Adenocarcinoma
| Morphologic Feature or Immunostain | Hepatocellular Carcinoma | Cholangiocarcinoma | Metastatic Adenocarcinoma |
| Acinar pattern | Pseudoglands: tumor cells have hepatocytic appearance; lumen contains bile, pink material, or debris | Glands have nuclei with side-by-side, low columnar arrangement or apical nuclei; mucin in lumina | Glands have nuclei with side-by-side, columnar arrangement or apical nuclei; mucin in lumina |
| Solid pattern | No intracellular mucin but possible intracellular fat droplets | May have intracellular vacuoles containing mucin | May have intracellular vacuoles containing mucin |
| Scirrhous pattern | Uncommon; clusters of tumor cells may have polygonal shape, eosinophilic cytoplasm | Common, glands show basophilic cytoplasm, mucin production | Glands show columnar cytoplasm, mucin production |
| MOC-31 | Negative in ≥80% HCCs (also positive in mixed HCC and cholangiocarcinoma) | Positive | Positive |
| Hepatocyte antibody (HepPar-1) | Positive granular cytoplasmic staining in well and moderately differentiated HCCs | Typically negative | May be positive in hepatoid adenocarcinomas, particularly from stomach, colon, lung, ovary |
| Polyclonal CEA | Positive in canalicular pattern in well and moderately differentiated HCCs | Negative for canalicular pattern; positive for cytoplasmic or membranous pattern | Negative for canalicular pattern; positive for cytoplasmic or membranous pattern |
| Cytokeratin (CK) profile | May be positive for CK7, especially in pseudoglands; usually negative for CK19 and CK20 | Almost all positive for CK7 and CK19; CK20 staining varies | CK7 positive in many tumor types; CK19 variably positive (breast and GI primaries, including stomach, pancreas, colon); combination of CK20 positive, CK7 negative in colonic adenocarcinoma |
| Other immunostaining features | May be positive for ARG1, GPC3 in moderate to poorly differentiated HCC; negative for AFP in most cases | Rarely GPC3 and AFP positive | Site-related markers (see Table 55.16) |
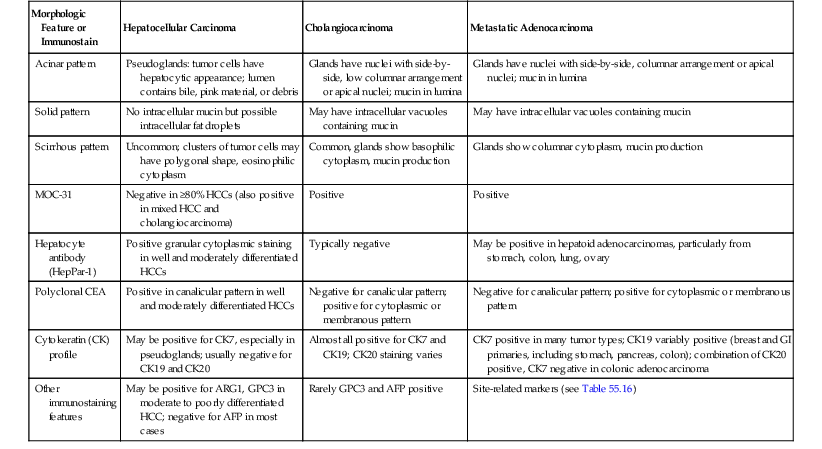
AFP, α-Fetoprotein; ARG1, arginase 1; GI, gastrointestinal; GPC3, glypican 3; HCC, hepatocellular carcinoma; HepPar-1, hepatocyte paraffin 1 antigen; MOC-31, monoclonal antibody.
Monoclonal CEA may be useful, because it is often diffusely positive in some types of adenocarcinoma and is normally absent in hepatocellular tumors. MOC-31 or monoclonal CEA, when used in combination with the hepatocyte antibody (i.e., polyclonal CEA) and arginase 1, may help in distinguishing poorly differentiated adenocarcinoma from HCC. GPC3 may prove helpful in this process, but this stain is also a marker for germ cell tumors, and staining has been positive in other tumor types, including squamous cell carcinoma of lung, liposarcoma, and ovarian adenocarcinomas.38,53 Focal staining has been positive in neuroendocrine tumors.
Routine histochemical stains for epithelial mucins, such as mucicarmine or d-PAS, are often helpful, because mucins are not present in HCC, except in rare cases such as mixed HCC and cholangiocarcinoma and in some cases of the fibrolamellar variant of HCC. The d-PAS stain also reacts with many hepatocyte cytoplasmic glycoproteins, which may result in false positives. A combination of CK7, CK19, and CK20 can be used to differentiate cholangiocarcinoma (CK7 and CK19 typically are positive in cholangiocarcinoma) or metastatic carcinoma58–60 from HCC.59,61 Positivity for CK7 or CK19 does not confirm cholangiocarcinoma differentiation unless there is definite glandular formation or mucin production in the regions of CK7 and CK19 positivity.
Hepatocellular Carcinoma versus Neuroendocrine Tumor
Rarely, HCC may be difficult to distinguish from a metastatic neuroendocrine neoplasm (Table 55.7) because both tumors may form acinar or trabecular-like structures and may be composed of tumor cells with abundant eosinophilic cytoplasm and round nuclei. Features that favor a diagnosis of neuroendocrine tumor include a prominent vascular or capillary network and stromal hyalinization. Neuroendocrine tumors only very rarely arise in the liver; they are almost always metastatic.39
Table 55.7
Comparison of Hepatocellular Carcinoma and Neuroendocrine Tumors
| Morphologic Feature or Immunostain | Hepatocellular Carcinoma | Well-Differentiated Neuroendocrine Tumor |
| Trabecular pattern with endothelial lining cells | Common | Common |
| Small, uniform cells | Common in well-differentiated tumors | Common |
| Pleomorphic or large tumor cells with variable nuclear features | Common in moderately to poorly differentiated HCCs | Uncommon |
| Acinar pattern | Pseudoglands may contain bile, pink proteinaceous material, or debris (no mucin) | Common; no bile and no mucin |
| Hepatocyte antibody | Commonly positive in well and moderately differentiated HCCs | Usually negative, but when positive, tends to be patchy |
| Glypican 3 | Commonly positive in HCC | May be focally positive |
| Chromogranin, synaptophysin | Focally positive in occasional HCCs | One or both stains strongly and diffusely positive |
| MOC-31 | Negative in > 80% | Diffusely and strongly positive |
| CK profile | CK20 usually negative, Mallory hyaline may be positive, CK7 variable | CK20 usually negative (positive finding may indicate pancreatic primary), CK7 variable |
CK, Cytokeratin; HCC, hepatocellular carcinoma; MOC, monoclonal antibody.
Focal neuroendocrine differentiation has been observed in HCC, including the fibrolamellar variant, and in hepatoblastoma with the use of neuron-specific enolase, protein gene product 9.5 (PGP9.5), vasoactive intestinal peptide, calcitonin, and S100 protein.62–64 Diffuse staining with chromogranin or synaptophysin strongly supports a diagnosis of neuroendocrine tumor. Because MOC-31 may also produce membranous staining of neuroendocrine tumors, care must be taken to not mistake a neuroendocrine tumor for an adenocarcinoma.
If a metastatic tumor is suspected, CDX2 and thyroid transcription factor 1 (TTF1) can help in distinguishing GI and pulmonary primary tumors, respectively,65 but the absence of staining does not preclude these primary sites. Isolated GPC3 staining has also been observed in neuroendocrine tumors. When a neuroendocrine tumor is diagnosed in the liver, the lesion should be assigned a grade primarily based on mitotic activity or Ki67 (MIB1) proliferative rate in manner similar to that described for gastrointestinal primaries (see Chapter 29).
Hepatocellular Carcinoma versus Other Tumor Types
Metastatic clear cell renal carcinoma may be difficult to distinguish from the clear cell variant of HCC (Table 55.8).49 In cirrhosis, a diagnosis of the clear cell variant of HCC is far more likely. However, in noncirrhotic livers without significant elevation of AFP levels, the likelihood of a metastatic lesion is increased.
Table 55.8
Differentiation of Hepatocellular Carcinoma from Other Clear and Eosinophilic Cell Tumors
| Morphologic Feature or Immunostain | Hepatocellular Carcinoma | Renal Cell Carcinoma, Clear Cell Type | Adrenal Cortical Carcinoma | Melanoma | Angiomyolipoma |
| Clear cell change | Common variant | Common variant | Common variant | Uncommon variant | Uncommon |
| Vascular prominence | Common | Common | Variable | Variable | Not typical in liver |
| Epithelial membrane antigen | Negative | Usually positive | Negative | Negative | Negative |
| CK7, CK20 | CK7 variably positive, CK20 usually negative | Negative for both | Negative for both | Negative for both | Negative |
| S100, HMB-45 | Negative | Negative | Negative | Usually positive for one or both | HMB-45–positive epithelioid cells |
| PCEA, CD10 | Positive in canalicular pattern | Negative for PCEA, positive for CD10 | Negative | Negative | Negative |
| Inhibin | Negative | Negative | Usually positive | Negative | Negative |
| Other markers | HepPar-1, arginase 1, glypican 3 variably positive | PAX2 or PAX8 nuclear staining, vimentin positive | Melan-A positive | Vimentin, tyrosinase, melan-A, SOX10 positive | SMA-positive spindle cells |
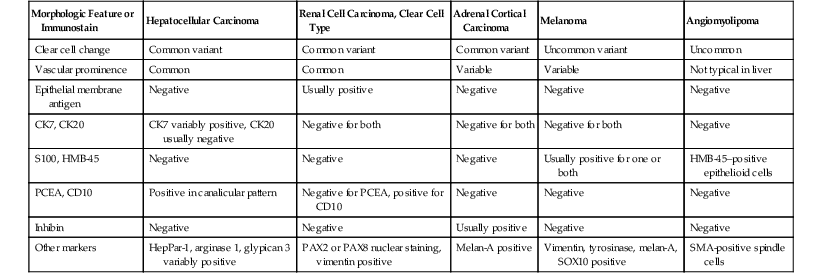
Cytokeratin profiles are not helpful, because these tumors have similar staining profiles (i.e., positive for CAM 5.2 and negative for CK7 and CK20). However, clear cell HCC often demonstrates polyclonal CEA canalicular staining, whereas renal cell carcinomas do not. Other useful markers include epithelial membrane antigen (EMA), which tends to be positive in renal cell carcinoma and negative in HCC, and the hepatocyte antibody (discussed earlier), which does not usually stain renal cell carcinomas. Arginase 1 positivity in HCC and PAX2 or PAX8 nuclear positivity in renal cell carcinoma also may be helpful.
Melanoma may mimic HCC tumors, but S100, human melanoma black (HMB)-45, and other markers used for the diagnosis for melanoma (see Table 55.8) are typically negative in hepatocellular tumors. Rarely, an adrenocortical tumor may need to be distinguished from a primary hepatocellular tumor. Positive staining for inhibin or melan-A (i.e., MLANA or MART1) may be helpful,66,67 because they have a high rate of positivity in adrenal tumors but not in hepatocytic lesions. The hepatocyte markers previously discussed are useful in these cases. Use of immunohistochemistry to distinguish HCC from mesenchymal tumors is described later.
Prognosis and Treatment
The prognosis of patients with HCC is primarily related to the pathologic stage, including the presence or absence of lymph node and distant metastases, and to certain histologic features, such as vascular invasion, adequacy of the surgical resection margins (at least 1 cm), number and location of lesions, presence or absence of cirrhosis, and tumor size. The relation of histologic tumor grade, or subtype, of HCC to prognosis is not considered highly important by some authorities.6 However, almost all small, early HCCs are well differentiated and have a good prognosis, and HCCs with CK19 positivity have a poorer prognosis.68,69 HCC tumors that arise in a noncirrhotic liver are associated with a better prognosis than those that arise in the setting of cirrhosis, as observed for the fibrolamellar variant of HCC.
Liver transplantation is an effective form of therapy for HCC tumors smaller than 5 cm (including multifocal, small HCCs no greater than 5 cm in aggregate diameter) and with no evidence of large vessel invasion. Alternative therapies, such as cryoablation, percutaneous ethanol injection, and transarterial chemoembolization, have been used in inoperable cases and may help to improve survival time.
Fibrolamellar Variant of Hepatocellular Carcinoma
Clinical Features
Fibrolamellar HCC occurs mainly in young adults without cirrhosis (mean age, 26 years), and the incidence is higher among women than men.70–72 The most common clinical symptoms include abdominal pain or swelling, anorexia, weight loss, jaundice, and hemoperitoneum (rare).
No definitive risk factors have been identified. Nodular hyperplastic changes have been observed adjacent to fibrolamellar HCC,73,74 but most authorities think that this is a secondary effect caused by vascular changes adjacent to the tumor and that it does not implicate FNH as the precursor lesion. Except in rare cases, serum AFP levels are usually normal in patients with fibrolamellar HCC.70,71
Pathologic Features
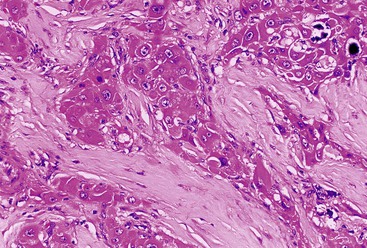
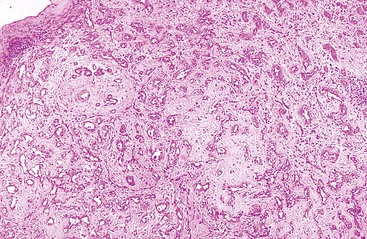
Fibrolamellar HCC tumors are typically well circumscribed, nodular, yellow to brown tumors with extensive fibrosis. Rarely, a prominent central fibrous zone, as seen with FNH,70 may be identified. Larger tumors may show foci of hemorrhage and necrosis. Satellite lesions are rare.
Microscopically, the cellular component of these tumors consists of clusters or sheets of tumor cells separated by dense bands of lamellar fibrous tissue (Fig. 55.23). The tumor cells are polygonal and exhibit eosinophilic and granular cytoplasm. The nuclei are usually large and contain prominent nucleoli. Other cytoplasmic features include pale bodies, which may contain fibrinogen and albumin, and d-PAS–positive bodies, which probably represent glycoprotein secretions. Other features that may be seen focally in the tumor include acinar structures, bile, multinucleated tumor cells, copper, fat, epithelioid granulomas, and peliosis hepatis.
Neuroendocrine markers (see Hepatocellular Carcinoma versus Neuroendocrine Tumor) may be focally positive, but they do not have any known clinical significance.64 CD68, a marker for a transmembrane protein in lysosomes and endosomes that is used to highlight macrophages, is positive to a significant degree in fibrolamellar HCC compared with less intense, less frequent positivity in HCC in cirrhotic or noncirrhotic livers.75 Fibrolamellar HCCs are typically positive for CK7.76,77 Glandular differentiation (with mucin secretion) and areas of trabecular HCC have also been observed.78 Stem cell–like staining has been described that is consistent with CK7 positivity and with CD133 positivity.79 Staining for CK19 and epithelial cell adhesion molecule (EpCAM) is positive in approximately 30% of cases.77
Differential Diagnosis
Clinically, fibrolamellar HCC should be differentiated from benign hepatocellular tumors (e.g., adenoma, FNH) that arise in patients of a similar young age group without cirrhosis, but the morphologic differences are usually easily distinguishable. Fibrolamellar carcinomas may be difficult to differentiate from other variants of HCC, particularly the scirrhous type (Table 55.9), but strict reliance on the characteristic cytologic and stromal features of fibrolamellar HCC tumors helps in this differential, as does the fact that fibrolamellar HCC should arise in a noncirrhotic liver.
Table 55.9
Differentiating Fibrolamellar from Scirrhous Hepatocellular Carcinoma
| Pathologic Feature | Fibrolamellar HCC | Scirrhous HCC |
| Lamellar fibrosis | Found diffusely throughout lesion | May be seen but not prominent; stroma tends to be loose, not lamellar |
| Large, polygonal tumor cells with prominent nucleoli | Diffuse | Focal, not diffuse |
| Cytoplasmic features | Eosinophilic, granular | Variable cytoplasmic changes, including clear cell and basophilic changes |
| Serum α-fetoprotein level | Within normal range | May be elevated in larger tumors |
| Background liver | No cirrhosis | Cirrhosis in 80-85% of cases |
HCC, Hepatocellular carcinoma.
Prognosis and Treatment
Some studies have suggested that patients with fibrolamellar HCC have a better prognosis than those with typical HCC tumors of a similar size when associated with cirrhosis.70–73 However, later findings demonstrated that patients with HCC but without cirrhosis have a prognosis that is similar, stage for stage, to that of patients with fibrolamellar HCC.78
Complete excision of the involved lobe is the preferred therapy. When tumor location precludes surgical resection, liver transplantation is an option. However, the outcome of this type of therapy is not considered favorable.
Combined Hepatocellular Carcinoma and Cholangiocarcinoma
Combined (mixed) hepatocellular carcinoma and cholangiocarcinoma (combined HCC-CC) accounts for as many as 5% of all primary carcinomas of the liver.80 The risk factors are similar to those for pure HCC, as evidenced by its frequent association with HCV or HBV infection and cirrhosis in Asia. The pathogenesis is uncertain, but there is evidence that progenitor or stem cells play a role in the cause of these lesions (see Chapter 44).
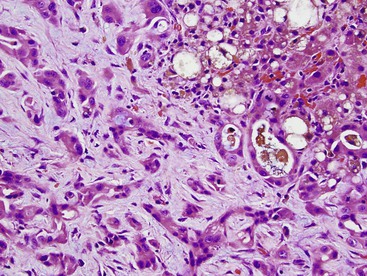
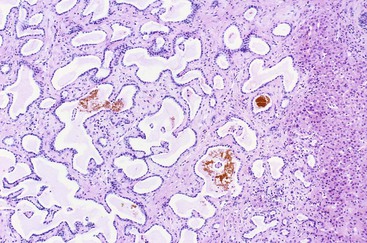
By definition, classic combined HCC-CC demonstrates morphologic and histochemical evidence of a mixture of hepatocellular and ductular (or glandular) elements throughout the tumor (Fig. 55.24).1 The WHO defines the hepatic component by unequivocal hepatocellular carcinoma elements that are well to poorly differentiated. Hepatic differentiation can be confirmed by conventional findings such as bile production or Mallory hyaline formation and immunostaining positivity for HepPar1, AFP, or polyclonal CEA (i.e., canalicular pattern). GPC3 is not included in this list because it may be not specific enough for HCC.
The glandular component is defined by glandular formation with luminal or cytoplasmic mucin production. Positivity for CK7, CK19, or MOC-31 can help to identify the cholangiocarcinoma component, but positivity for these markers alone (without glandular forms or mucin) is not sufficient to define this component as HCC-CC, and an HCC that is positive for CK19 or MOC-31 is not classified as combined HCC-CC. Collision tumors, in which these elements are clearly separated in the tumor mass or are situated side by side, are not considered true HCC-CC tumors according to the WHO.1
Findings on percutaneous needle biopsies of combined HCC-CC may be confusing, because a glandular or ductular malignancy may be sampled in a patient who has strong risk factors for HCC. Combined HCC-CC tumors should be kept in mind in the differential diagnosis of primary liver malignancies sampled by needle biopsy.
Subtypes of combined HCC-CC with stem cell features have been classified by the WHO1 (see Chapter 44). In summary, three variants were described. First, the typical subtype has features of mature-appearing hepatocytes with peripheral clusters of small cells positive for stem cell markers such as CK7, CK19, nuclear cell adhesion molecule 1 (NCAM1 or CD56), KIT, or EpCAM.). Second, the intermediate-cell subtype consists of small cells in trabeculae, strands, nests, or ill-defined glandlike structures (without mucin) embedded in stroma, with differentiation seen between hepatocytes and cholangiocytes. Third, the cholangiolocellular carcinoma subtype has small cells arranged in cordlike to tubular patterns in fibrous stroma, reminiscent of the canals of Hering or cholangiocytes.
Bile Duct Tumors
Bile Duct Hamartoma
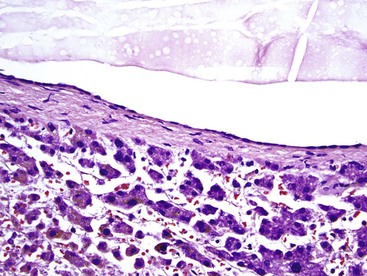
Bile duct hamartoma, also called von Meyenburg complex, is a type of ductal plate malformation. These lesions often occur as part of the spectrum of polycystic disease of the liver or other organs, but they also occur sporadically.
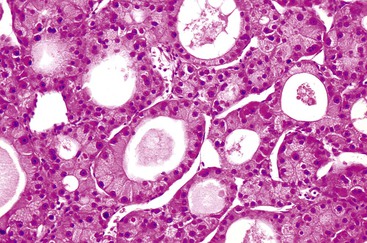
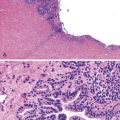
Bile duct hamartomas typically are small (<0.5 cm), gray to white, irregularly shaped lesions. Multifocality is common. Microscopically, bile duct hamartomas consist of numerous, small to medium-sized ductules, which are usually more dilated than normal ducts and separated by dense collagen (Fig. 55.25). These lesions are usually located in and at the periphery of portal tracts. The ductules are lined by small, cuboidal or flattened cells with round to oval nuclei. Bile duct hamartomas contain cysts that are more irregularly shaped than normal ducts, and they may contain eosinophilic debris or inspissated bile. Bile duct hamartomas are benign lesions with no significant degree of malignant potential. No specific treatment is necessary for these lesions.
Bile Duct Adenoma
Clinical Features
Bile duct adenomas are less common than hamartomas. The designation of this lesion as an adenoma may be biologically incorrect, because evidence suggests that this lesion is not a true neoplasm but a localized ductular reaction that develops as a result of previous injury.81 Another theory suggests that bile duct adenomas are a type of peribiliary gland hamartoma.82 I favor the concept that these are reactive lesions. These lesions are typically discovered incidentally at the time of laparotomy, and they are usually biopsied to exclude metastatic disease.
Bile duct adenomas occur in patients of any age group but are more common after age 20 years and more common in men than women. They are also commonly seen in the setting of cirrhosis.
Pathologic Features
Bile duct adenomas are small (<2 cm in diameter), firm, white to gray-tan, well-circumscribed lesions. They are usually located directly underneath the liver capsule, but they may develop deep in the liver parenchyma, particularly in the setting of cirrhosis. They may be single or multiple.
Histologically, the ductules are usually uniform in size, are not dilated, and usually contain less intervening fibrous stroma than bile duct hamartomas (Figs. 55.26 and 55.27). However, the amount of collagen may vary considerably, depending on the age or size of the lesion, and focal, abundant, dense collagen may be seen in the center of the lesion. The ductules have a tubular or curvilinear shape and are lined by cuboidal cells with bland, round to oval nuclei. Most importantly, they do not show mitotic activity. Mucinous metaplasia of the epithelium is a common feature, and α1-antitrypsin droplets and neuroendocrine differentiation are occasionally seen focally in the ductal cells. Residual portal tracts are often preserved in or very near the borders of the lesion, and small aggregates of lymphocytes may be seen, most often at the periphery.
Differential Diagnosis
Bile duct adenomas may be difficult to distinguish from metastatic adenocarcinoma, particularly on frozen section analysis. Bland cytologic features combined with the uniformity of the shape of the ductules and the lack of mitoses help to distinguish a bile duct adenoma from a metastatic adenocarcinoma (Tables 55.10 and 55.11).83
Table 55.10
Bile Duct Adenoma Compared with Cholangiocarcinoma and Metastatic Adenocarcinoma
| Morphologic Feature or Immunostain | Bile Duct Adenoma | Cholangiocarcinoma | Metastatic Adenocarcinoma |
| Size | <1-2 cm | Varies, typically > 1 cm | Varies |
| Location | Subcapsular, but may be deep, particularly in cirrhosis | Hilar or peripheral | Varies |
| Number of lesions | Single or multiple; no dominant nodule | Mostly single, but when multifocal, a dominant mass often seen | Often numerous |
| Occurrence in cirrhosis | Common | Uncommon, increased in PSC; can be combined with HCC | Very rare |
| Glandular pattern | Curvilinear or tubular glands, uniform size; uniform spacing | Variation in glandular shapes and sizes; irregular spacing | Variation in glandular shapes and sizes; irregular spacing |
| Cell type | Cuboidal | Cuboidal to columnar, piling of cells variable | Cuboidal to columnar, piling of cells variable |
| Cytologic atypia, including increased N : C ratio, other features | Round to oval nuclei with minimal atypia | Variable nuclear features | Variable nuclear features |
| Mitotic activity | Typically absent | May be seen, as may atypical forms | May be seen, as may atypical forms |
| Mucinous metaplasia or change | May be seen | May be seen | May be seen |
| Portal tracts in lesion | Common | Uncommon except at invasive edge | Uncommon except at invasive edge |
| Dense fibrosis surrounding glands, inflammatory foci | Common | Common | Common |
| α1-Antitrypsin globules | May be seen | Absent | Absent |
| CK7 and CK19 | Positive | Both typically positive | Varies |
| MOC-31 | Positive | Positive | Positive |
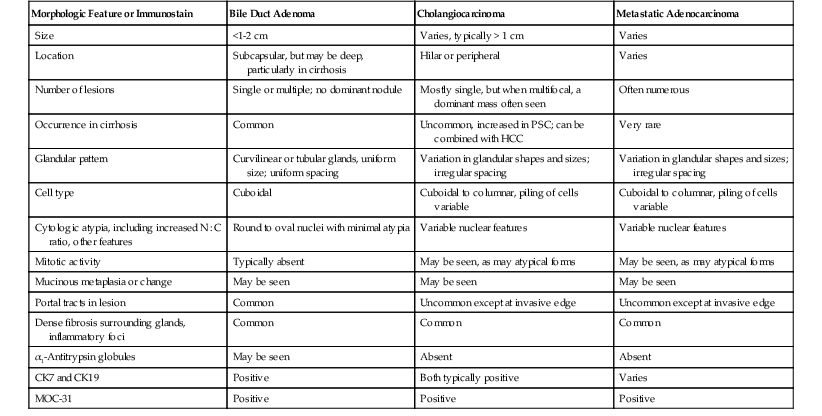
CK, Cytokeratin; HCC, hepatocellular carcinoma; MOC, monoclonal antibody; N : C, nucleus-to-cytoplasm ratio; PSC, primary sclerosing cholangitis.
Table 55.11
Comparison of Bile Duct Adenoma and Metastatic Pancreatic Adenocarcinoma
| Immunostain* | Bile Duct Adenoma or Hamartoma | Metastatic Pancreatic Adenocarcinoma* |
| SMAD4 (DPC4) | Preservation of staining | Absence of staining in 44-55% |
| TAG72 (B72.3) | Negative | Positive in 88% |
| Mesothelin | Negative | Positive in 64% |
| Monoclonal CEA | Adenoma negative, hamartoma positive (10%) | Positive in 92% |
* SMAD4 loss is highly specific for pancreatic adenocarcinoma. TAG72, mesothelin, and monoclonal CEA also stain other adenocarcinomas to some degree.
CEA, Carcinoembryonic antigen; SMAD4, SMAD family member 4; TAG72, tumor-associated glycoprotein 72.
Data from Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381-389.
On permanent sections, immunohistochemistry is quite helpful. For example, bile duct adenomas normally show preservation of SMAD4 (DPC4) staining and negativity for TAG72 (B72.3), mesothelin, and monoclonal CEA, in contrast to metastatic pancreatic adenocarcinomas, which show an absence of SMAD4 staining in as many as 55% of cases and positivity for TAG72, mesothelin, and monoclonal CEA.83
Mucinous Cystic Neoplasms
Clinical Features
Mucinous cystic neoplasms (MCNs), formerly known as “hepatobiliary cystadenomas,” are rare lesions and occur almost exclusively in women. Their morphology is similar to that of neoplasms of the pancreas, including the ovarian type of stroma (see Chapter 40).1
These cystic tumors have been subdivided into two categories: those with low- or intermediate-grade intraepithelial neoplasia and those with high-grade intraepithelial neoplasia. They may progress to adenocarcinoma with time. Adenocarcinomas that develop in MCNs are usually low grade.
The mean ages of patients with MCN or adenocarcinoma are 45 and 59 years, respectively. Presenting symptoms and signs include a palpable mass, abdominal pain or discomfort, and, less commonly, jaundice, complications of tumor rupture, and infection. Some patients have elevated serum levels of CA 19-9.
Most MCNs are clinically indolent and treated by surgical resection. However, thorough examination of all MCNs is highly recommended for grading the lesion and to exclude the possibility of focal malignant transformation, which is more likely in the high-grade lesions.
Pathologic Features
MCNs typically are multilocular and contain a smooth or somewhat trabeculated epithelial lining. The cysts may contain serous, mucinous, gelatinous, and bloody or purulent material. MCNs do not communicate with the normal biliary tract. Large, polypoid projections or dense masses in the wall of the cysts often indicate areas of malignant transformation.
Microscopically, the cysts are typically lined by a single layer of epithelial cells, usually of the mucinous type, and the cells stain similar to biliary-type epithelium (i.e., CK7 and CK19 positive). The cells may be flat, cuboidal, or columnar (Fig. 55.28), and small papillary tufts may be seen along the inner surface of the cysts. Epithelial nuclei normally appear bland, are basally located, and have no mitotic activity. Features such as marked nuclear pleomorphism, loss of polarity, mitotic figures, and multilayering of the epithelium support the diagnosis of high-grade dysplasia and warrant extensive examination for invasive adenocarcinoma. Invasion of tumor cells into the stroma is the only reliable evidence of invasive carcinoma. The changes designating low-, intermediate-, or high-grade dysplasia that are detailed in Chapter 40 are essentially those described for MCN in the pancreas and pancreatic intraepithelial neoplasia.
Rarely, gastric, intestinal, and squamous metaplasia can be seen, and many of the lesions contain a component of chromogranin- or synaptophysin-positive endocrine cells.1 The underlying stroma resembles that of the ovary in women, but this pattern may not be uniformly seen in the lesions. A layer of dense hyalinized stroma often separates or can replace the ovarian-like stroma from adjacent liver. The cyst walls may be lined focally by macrophages, calcifications, or scar tissue. Adenocarcinomas that arise in MCNs may have tubulopapillary histology and other typical features of invasive adenocarcinoma of the pancreatobiliary tree.84
Differential Diagnosis
The primary cystic lesions in the differential diagnosis are simple biliary cysts. Simple cysts are often resected as a large, single cystic lesion that is not multiloculated, but these cysts can still be multifocal. All lack the ovarian-like stroma and typically have flatter, less mucinous-appearing epithelium. Differentiation from intraductal papillary neoplasia is based on the configuration of the papillary lesions and evidence of communication with the biliary tree in cases of intraductal papillary neoplasia. Other rare lesions in the differential include endometriosis, which has an endometrial stroma that may mimic the ovarian-like stroma of MCN but should also have significant evidence of intralesional hemorrhage and mural changes of pigmented macrophages. MCNs should not be confused with microcystic adenoma (i.e., serous cystadenoma). This lesion is most commonly seen in the pancreas (see Chapter 40), but it rarely can be seen in the liver. The cysts are much smaller in microcystic adenoma and are lined by nonmucinous, glycogen-containing (PAS-positive) epithelial cells.
Simple (Biliary) Cyst
Simple cysts usually are an incidental finding and occur in patients older than 40 years.40 Multiple cysts often represent a component of polycystic disease and are accompanied by von Meyenburg complexes (i.e., biliary hamartomas). Simple cysts have no or only slight premalignant potential.
Simple cysts are usually located directly underneath the liver capsule, but some may occur deeper in the liver parenchyma. They typically contain clear, light yellow fluid. These cysts are lined by biliary-type cuboidal to low columnar epithelium with a fibrous wall (Fig. 55.29). The epithelium may be disrupted or flattened, and the wall may be thickened. Evidence of reactive changes and recent or remote hemorrhage may be evident in the cyst wall. CK7 and CK19 immunostaining of the epithelium is positive, identical to that of biliary epithelium.
Other Benign Biliary Tumors or Lesions
Biliary adenofibroma is a rare entity considered to be biologically benign.85 The lesion consists of ductular and stromal elements with solid and microcystic regions. The ductular components may be similar to those in bile duct adenomas, and they often have tortuous or branching configurations. Ducts may be dilated and may form microcysts. The cuboidal or low columnar epithelium lining the ducts is often flattened in microcysts. The cytoplasm of the ductal cells is amphophilic, and foci of apocrine change may be seen. Occasionally, epithelial tufts and mitotic figures are seen. These lesions do not produce mucin, but some cysts may contain eosinophilic fluid. The stroma, which is composed of spindle-shaped fibroblasts and patchy, moderately chronic inflammation, forms well-defined septa between the glands.
Microcystic adenomas, which are grossly and microscopically similar to microcystic adenomas (i.e., serous cystadenoma) of the pancreas, have only rarely been described in the liver.84 Similar to its pancreatic counterpart, this tumor consists of numerous microcysts lined by cuboidal epithelial cells that contain abundant cytoplasmic glycogen (i.e., PAS positive), as discussed earlier.
The term biloma describes a large collection of bile located outside of the bile ducts and often found in perihepatic tissues related spatially to the gallbladder or larger hepatic ducts. Large collections of bile often occur after injury to a large duct wall or gallbladder that results in rupture or loss of functionality due to necrosis. A biloma can result from trauma, iatrogenic injury, or ischemic damage or severe infections of the biliary tree. The lesion consists of bilious debris admixed with and surrounded by inflammatory cells and macrophages, which often have a xanthomatous appearance. An exuberant fibrous connective tissue reaction may occur at the periphery of the lesion and impart an encapsulated appearance. The preferred treatment is surgical excision.
Cholangiocarcinoma
Clinical Features
Cholangiocarcinoma is primarily a malignant tumor of older adults, with both sexes affected equally. A marked association with cirrhosis has not been identified, although patients with primary sclerosing cholangitis have a significantly increased risk of developing cholangiocarcinoma.86,87 These tumors are most prevalent in Southeast Asia, where liver fluke (Clonorchis and Opisthorchis species) infestation is high. Other risk factors include congenital anomalies of the biliary tree, such as von Meyenburg complex,88 choledochal cyst,89 Caroli disease,90 anomalous arrangements of the pancreatic and common bile ducts,91 hepatolithiasis,92 and use of Thorotrast.93 Presenting symptoms vary with the anatomic location of the tumor, which may be peripheral (intrahepatic), hilar,94 extrahepatic, or intraductal. Extrahepatic lesions are discussed in greater detail in Chapter 38. Peripheral cholangiocarcinomas usually remain asymptomatic until the tumor is in its late stage; the hilar, extrahepatic, and intraductal types usually manifest with signs of bile duct obstruction.
The intraductal papillary type, previously known as “intraductal papillomatosis”6 but designated as a distinct variant form of intraductal papillary neoplasm by the WHO 2010,1 often involves extensive areas of intrahepatic or extrahepatic bile ducts, with a predilection for the latter. Similar to the grading of MCNs, this neoplasm is divided into two grades: low-to-intermediate grade and high grade. Men are affected more than women (about 2.4 to 1), and patients are usually middle-aged or older (mean age, 60 years).
Pathologic Features
The peripheral, hilar, and extrahepatic variants of cholangiocarcinoma are usually firm, white-tan lesions because of the amount of dense fibrous tissue in the tumor. In contrast, the intraductal papillary neoplasm variant is typically soft and polypoid or cauliflower-like, causing ductal dilation; they are typically multifocal.
Microscopically, the peripheral, hilar, and extrahepatic variants often have a significant amount of dense fibrous stroma. These tumors are usually well-differentiated adenocarcinomas, formed of tubular glands with only mild cytologic atypia. However, foci of marked atypia with an increased nucleus-to-cytoplasm ratio, prominent nucleoli, increased variation in nuclear size, and loss of polarity are often seen at least focally in the tumor (Fig. 55.30).
Features that support a diagnosis of carcinoma rather than a benign hyperplastic or reactive process include the formation of intracytoplasmic lumina and a cribriform pattern of growth. Multilayering of nuclei and intraluminal cellular debris are also features of malignancy, as are destruction and infiltration of portal tracts. Some cholangiocarcinomas spread into the portal bile ducts, which presumably is the result of pagetoid spread of tumor cells along the biliary system. This may be difficult to differentiate from high-grade intraepithelial neoplasia of the bile ducts (BilIN-3).95 Some types of adenocarcinomas metastatic to the liver may show spread of tumor cells into the biliary system, mimicking BilIN-3.
Intraductal papillary neoplasms grow into the bile duct lumina as multifocal, papillary lesions. The papillae are lined by columnar epithelial cells and supported by a delicate fibrovascular stroma (Fig. 55.31). The tumor cell nuclei are round to oval and basally located without significant multilayering in the low-grade lesions. Cytoplasm is abundant and mucinous, but clear or oncocytic differentiation and intestinal metaplasia with goblet cell change may also be seen. Mitotic figures are typically uncommon. High-grade intraductal papillary neoplasias show evidence of cytologic features typical of BilIN-3.95 Frank invasion of the stalk and underlying periductular tissues is a requisite for a diagnosis of invasive adenocarcinoma.
Differential Diagnosis
Immunoperoxidase and mucin histochemical staining may be used to differentiate intrahepatic cholangiocarcinoma from HCC (see Table 55.6). However, differentiation from metastatic adenocarcinoma may be difficult, particularly if there is no evidence of a primary tumor at another anatomic site. Adenocarcinomas composed of tall columnar cells with an adenomatous pattern, cribriforming, lack of intraepithelial mucin, and luminal necrotic debris suggest metastatic (colonic) carcinoma rather than primary cholangiocarcinoma, which usually shows a greater degree of intracellular mucin differentiation and is composed of glands lined by low columnar to cuboidal cells. CK7, CK19, and CK20 are useful to help differentiate primary from metastatic lesions. Cholangiocarcinomas are usually CK7 and CK19 positive, but most are CK20 negative. In contrast, metastatic colorectal adenocarcinomas are CK7 negative in as many as 90% of cases but are usually positive for CK19 and CK20.58,59
Differentiation from benign ductular reactions may be difficult. Localized ductular reactions may occur in cirrhotic and noncirrhotic livers (e.g., bile duct adenomas) (see Table 55.10), but extensive ductular reactions may develop in cirrhotic livers that contain large zones of fibrosis. An extensive ductular reaction may form as a sequela of tumor ablation (Fig. 55.32). In this case, the changes are thought to represent a reaction to ischemic necrosis of cirrhotic nodules. Zones of parenchymal extinction maintain for the most part the background architecture of cirrhotic nodules, and a somewhat nodular grouping of ductules can usually be identified on microscopic examination at low magnification. Reactive ductules may appear atrophic (with flattened epithelium), they have a more uniform pattern of growth (e.g., in bile duct adenomas), and the native portal tracts are usually well preserved.
TP53 staining is not a good method for differentiating benign from malignant ductular lesions, because weak TP53 staining is a common feature of benign lesions. However, Ki67 (MIB1) staining that demonstrates a high proliferative rate of more than 30% offers a marker favoring malignancy.96 For intraductal papillary neoplasias, the typical differential diagnostic consideration may be an MCN (discussed earlier).
Prognosis and Treatment
The prognosis for peripheral, hilar, and extrahepatic cholangiocarcinoma is dismal because the disease is usually advanced at the time of diagnosis, rendering surgical removal difficult or impossible. However, survival time may be increased when tumor-free surgical margins are attained.4
Intraductal papillary neoplasms, although histologically benign in most cases, are regarded clinically as borderline or low-grade malignant tumors because of their tendency to recur, their multicentricity, and their ability to undergo malignant transformation and rarely metastasize.39 The tumor is associated with significant morbidity and mortality. Morbidity most often results from recurrent bouts of cholangitis, obstructive jaundice, sepsis, and hemobilia. The incidence of metastasis is much lower than for other forms of cholangiocarcinoma. Although most intraductal papillary neoplasms do not metastasize, the probability of achieving cure without liver transplantation is low because of the multicentric nature of the lesion. Tumor recurrence has been observed in the extrahepatic ducts.
Mesenchymal Tumors
Hemangioma
Clinical Features
Hemangioma (i.e., cavernous hemangioma [CH]) is the most common primary tumor of the liver. The benign vascular neoplasm usually is an incidental finding at the time of surgery or autopsy. CHs often require surgical excision because of their large size or the possibility of hemorrhage.97 Some studies have shown that estrogen therapy may lead to enlargement of the tumor.97 Rarely, thrombosis in a CH may lead to thrombocytopenia.98 These tumors may be isolated or multiple, or they may be associated with CHs in other organs as part of von Hippel-Lindau disease or skeletal-systemic hemangiomatosis syndrome.99
Pathologic Features
CHs are well-circumscribed, red or red-brown tumors. They usually have a spongy texture and a honeycombed surface, which is an expression of the cavernous vascular component. Many lesions undergo thrombosis and sclerosis, which results in a firm, white to white-tan appearance. Larger lesions may also show small satellite foci in the liver parenchyma adjacent to the main tumor mass.100
The hallmark of this tumor is cavernous vascular channels. The channel walls consist mainly of fibrous stromal bands lined by a single layer of flattened endothelial cells without cytologic atypia or mitotic activity (Fig. 55.33). Sclerotic zones may be extensive, and vascular channels may be thrombosed. Extensively sclerotic hemangiomas may have only a few remaining vascular channels and mimic a localized scar reaction. Multiple, dilated vascular channels (with morphologic features identical to those of hemangioma) may be seen in the liver parenchyma adjacent to primary CHs.100
Rarely, atypical small vessel variant forms of hemangiomas may be seen in the liver.101 They have bland-appearing endothelial cell morphology and irregular infiltrating patterns of growth along sinusoids surrounding the lesions. The benign or malignant nature of these rare lesions is unclear, but because they have lower proliferative rates than angiosarcomas, they likely are low-grade lesions.
Angiomyolipoma
Clinical Features
Angiomyolipomas may occur in the liver but are rare. Patients are usually 30 to 40 years of age, and distribution is equal between the sexes.102,103 Most angiomyolipomas are sporadic, but some occur in patients with tuberous sclerosis.102,103 Patients may have abdominal pain or discomfort, malaise, fever, or anorexia. Many cases are asymptomatic, diagnosed on imaging studies performed for other reasons. Angiomyolipomas are thought to arise from a perivascular epithelioid cell. Related lesions in other organs include clear cell “sugar” tumor and lymphangioleiomyomatosis of the lung.104
Pathologic Features
Angiomyolipomas usually appear as variably colored tumors because of hemorrhage, necrosis, and adipose tissue in the lesions.102,103 The size of these tumors varies considerably, but they are often large at the time of clinical presentation.
Angiomyolipomas are composed of a variety of tissue elements, including smooth muscle–like cells, blood vessels, and adipose and hematopoietic tissue. Smooth muscle–like differentiation is more prominent in liver angiomyolipomas than in renal tumors. The tumor consists of epithelioid or spindle-shaped cells, often surrounding blood vessels. Epithelioid cells have a round or polygonal shape with abundant eosinophilic cytoplasm (Fig. 55.34). Nuclei are typically large and round with prominent nucleoli, but their appearance can vary. The cytoplasmic contents are usually oncocytic and often condensed around the nucleus, leaving a clear zone directly underneath the cell membrane, resulting in an appearance like that of a spider web (Fig. 55.35).103
Spindle cells have eosinophilic cytoplasm and small, oval nuclei. Trabeculae, usually composed of epithelioid cells, may be seen. The epithelioid or spindle cell component may predominate in any angiomyolipoma. Spindle cells often stain more strongly for smooth muscle actin, whereas epithelioid cells stain for HMB-45.103 Desmin positivity has been observed in spindle cells.103 Stains for keratin are typically negative. S100 staining is focally positive in angiomyolipomas, and it usually stains epithelioid and fat cells.103
The vascular component is typically composed of thick-walled arterial or venous-like channels mixed with thin-walled, venous-like spaces. Adipose tissue consists of mature fat cells scattered throughout the tumor singly or in clusters or sheets of cells. However, in the liver, the fat component of angiomyolipomas may be scant or absent. Foam cells, containing fine droplets of lipid, often are seen. Peliotic spaces closely associated with areas of hemorrhage are common, and these spaces typically lack an endothelial lining. Dense lymphoid aggregates, composed of a mixture of T and B cells, may be prominent in some cases. Hemosiderin and melanin pigments are rare. Some hematopoietic elements, including megakaryocytes and erythroid and myeloid precursors, may be scattered in the tumor.
Differential Diagnosis
Most diagnostic problems arise when epithelioid smooth muscle–like cells predominate in the tumor. Epithelial cells with large, round nuclei and prominent nucleoli, particularly when there is abundant eosinophilic cytoplasm, may mimic HCC or HCA (Table 55.12). The formation of trabeculae containing epithelioid cells may closely mimic HCC.
Table 55.12
Epithelioid Variant of Angiomyolipoma Compared with Hepatocellular Carcinoma and Hepatocellular Adenoma
| Morphologic Feature or Immunostain | Angiomyolipoma (Epithelioid Variant) | Hepatocellular Carcinoma | Hepatocellular Adenoma |
| Background liver | No cirrhosis | Cirrhosis in 80-85% | Cirrhosis very rare |
| Gross appearance | Tan, may have zones of hemorrhage and necrosis | Tan, yellow-tan, or greenish; foci of hemorrhage; necrosis may occur | Tan, yellow-tan; not likely bile-stained; hemorrhage and necrosis may be seen |
| Tumor cell cytoplasm | Eosinophilic or oncocytic cytoplasm; condensation around nucleus (spider-web pattern); no fatty tumor cells in this variant | Eosinophilic or oncocytic cytoplasm, fatty change common | Eosinophilic cytoplasm, fatty change common |
| Tumor cell nuclei | Round to oval, can vary; small to large; no nucleoli to prominent nucleoli | Vary | Small, round nuclei with no nucleoli or small nucleoli |
| Mitotic activity | Absent | Often present | Absent |
| Trabecular pattern | Notable in variant | Common pattern | Cell plates intact; typically not 3 cells thick |
| Immunostains | Human melanoma black-45 and smooth muscle actin positive; cytokeratin negative | CAM 5.2 positive; many are HepPar-1 positive | CAM 5.2 and HepPar-1 positive |
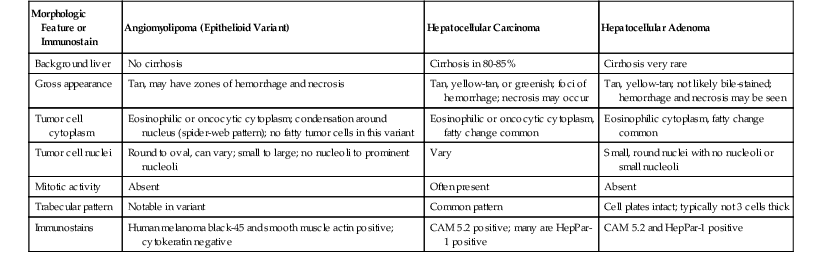
Immunohistochemistry is helpful in identifying the tumor as an angiomyolipoma rather than HCC or adenoma by demonstrating positivity for HMB-45 and smooth muscle actin in the smooth muscle–like cells. A combination of spindle and epithelioid cells mimics metastatic melanoma, and HMB-45 positivity may add to diagnostic confusion. In this case, other melanoma markers may be useful. Rarely, inflammatory cells (usually mononuclear cells) may be prominent, mimicking inflammatory pseudotumor. Features that differentiate angiomyolipoma from other mesenchymal tumors, such as epithelioid hemangioendothelioma and angiosarcoma, are listed in Table 55.13.
Table 55.13
Differentiating Histologic and Clinical Features of Mesenchymal Tumors in Adults
| Morphologic Feature or Immunostain | Angiomyolipoma | Epithelioid Hemangioendothelioma | Angiosarcoma |
| Risk factors | Many not associated with tuberous sclerosis | Unknown | Exposure to vinyl chloride, Thorotrast |
| Gross appearance | Tan, yellow-tan; hemorrhage and necrosis common | Firm, white to tan; calcifications may be present | Dark red to brown, hemorrhagic appearance, with irregular, spongy texture; may be cystic and necrotic |
| Satellite nodules | Absent | May be present | Common |
| Vascular invasion | Absent | Common | May be present |
| Cellular morphology | Spindle, epithelioid, and fat cells; epithelioid variant common in liver | Spindle (dendritic), epithelioid cells common | Spindle cells predominate |
| Growth pattern | Solid with foci of necrosis; variants with trabecular growth | Small nests or single cells in myxoid or fibrous stroma; papillary tufts in vascular spaces | Sinusoidal growth (scaffolding pattern) most common; solid to papillary growth at tumor border, cystic areas in larger tumors |
| Immunostains | Positive for smooth muscle actin, human melanoma black-45 | Positive for vascular markers (CD31, CD34, factor VIII, FLI1) | Usually positive for vascular markers (CD31, CD34, factor VIII, FLI1) |
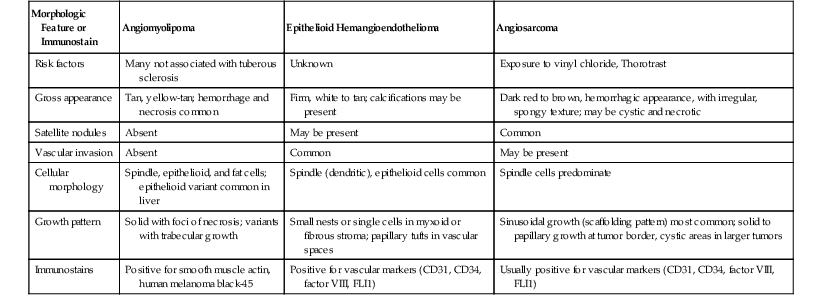
Inflammatory Pseudotumor
Clinical Features
Inflammatory pseudotumors of the liver may be divided into two types. The first type, commonly referred to as an inflammatory myofibroblastic tumor, is a rare, inflammatory, fibrosing lesion that may be found in organs other than the liver.105–107 Patients may have abdominal pain, fever, chills, jaundice, vomiting, and weight loss. Evidence for Epstein-Barr virus has been found in these tumors from various sites, including the liver.108 Some authorities have suggested that an inflammatory pseudotumor represents a proliferation of follicular dendritic reticulum cells.108
The other type of inflammatory pseudotumor, which is the most common type in the liver, is a heterogeneous group of true inflammatory lesions resulting from chronic injury, such as masslike infiltrates of inflammatory cells associated with chronic cholangitis.105 This lesion may be clinically mistaken for cholangiocarcinoma if it is located in the hilar region of the liver.
Pathologic Features
The gross appearance of inflammatory pseudotumors varies considerably, especially if the lesion is large and contains foci of fibrosis, hemorrhage, and necrosis.107 These tumors may vary considerably in size and may be solitary or multiple. They develop anywhere in the liver parenchyma but occur particularly in the porta hepatis.98
Microscopically, inflammatory pseudotumors consist of a mixture of inflammatory and fibrous tissue, but the relative amounts of these components vary (Fig. 55.36). In inflammatory myofibroblastic tumors, the spindle cells are myofibroblasts and typically immunostain positively for smooth muscle actin.
The inflammatory component of both pseudotumor types often contains a polyclonal population of plasma cells,105,106 but neutrophils, eosinophils, and lymphocytes (predominantly T cells) also may be seen. Some macrophages (often xanthomatous) may be seen, particularly in lesions related to cholangitis and bile duct injury. Sclerotic foci are common. Mitotic figures may be seen but are not normally numerous,107 and abnormal mitotic figures are typically absent. Occasionally, granulomas or phlebitis (or some degree of venous obstruction) may be observed in patients with inflammatory pseudotumors.105,106
Differential Diagnosis
Inflammatory pseudotumors should be differentiated from other types of sarcomas, such as angiosarcoma and metastatic GI stromal tumor. The sarcomas usually have cellular atypia and frequent mitotic figures and lack inflammatory cells.
Immunohistochemistry may be helpful. Angiosarcomas normally stain positively for vascular endothelial markers (including CD34), and metastatic GI tumors typically react with CD117 or CD34. Differentiation of an inflammatory myofibroblastic tumor from a follicular dendritic cell tumor may be difficult. However, the absence of plasma cells and the presence of pleomorphic tumor cells, as well as positive immunostaining for CD21, CD35, and R4/23 in the latter, help to distinguish between these two types of lesions.109 Rarely, the inflammatory form of angiomyolipoma may mimic an inflammatory pseudotumor, but staining for HMB-45 in the background tumor cells can help to differentiate the former from the latter.103
Angiosarcoma
Clinical Features
Angiosarcoma is a rare primary malignant tumor that usually occurs in middle-aged adults but rarely has been diagnosed in children, sometimes in the setting of infantile hemangioendothelioma.110 Other risk factors include Thorotrast and vinyl chloride exposure,111 but many angiosarcomas have no apparent cause. Presenting signs include hepatomegaly, ascites, jaundice, thrombocytopenia, hemoperitoneum, and liver failure.
The mean survival of patients with typical forms of angiosarcoma is only 6 months. No effective therapy is available.
Pathologic Features
Angiosarcomas are often large, hemorrhagic tumors with indistinct borders and solid and cystic areas; the latter usually contain blood. Satellite nodules may be seen. They typically have a mixed histologic pattern showing sinusoidal, solid, papillary, and cavernous types of growth. The sinusoidal pattern is the most distinctive pattern in the liver. In this pattern, endothelial cells line both sides of the hepatic cell plates in a scaffold-like arrangement that dissects the plates, often resulting in sinusoidal dilation (Fig. 55.37). The tumor cells that line the cell plates are more numerous, more hyperchromatic, and larger than seen in normal endothelial cells. The sinusoidal pattern is more likely to be identified at the periphery of the tumor and may represent an early outgrowth of the tumor, which later transforms into the solid or papillary form. The solid pattern may have a fascicular, or whorled, appearance, or it may resemble fibrosarcoma. The papillary pattern consists of nodules of stroma lined by tumor cells that protrude into a lumen (Fig. 55.38). The cavernous pattern consists of large, blood-filled spaces and is most commonly seen in combination with the other patterns.
Differential Diagnosis
Key features that help differentiate angiosarcoma from other mesenchymal tumors are listed in Table 55.13. Endothelial markers, such as factor VIII, CD31, CD34, and FLI1 are typically positive in tumor cells, but the staining may be focal or patchy. Factor VIII may be the most sensitive and specific marker for this type of tumor.
Epithelioid Hemangioendothelioma
Clinical Features
Epithelioid hemangioendothelioma is a rare, low-grade vascular malignancy that occurs in adults of any age and tends to be more common among females.112 Most lesions are discovered as an incidental finding, but presenting symptoms and signs may include upper abdominal discomfort or a mass lesion. Serum alkaline phosphatase levels may be elevated in some cases.
Pathologic Features
Epithelioid hemangioendotheliomas are firm, white to yellow tumors that often have ill-defined borders. The tumor is usually multifocal, with involvement of the right and left liver lobes. Calcifications may add a gritty consistency to the tumor.
Microscopically, the tumor cells may be dendritic or epithelioid in appearance. The former are irregularly shaped, elongated, or stellate cells with long, branching processes. The cell cytoplasm may contain a vacuole that represents an intracellular “capillary” luminal space. Epithelioid tumor cells are rounder and contain more abundant cytoplasm than dendritic cells. These cells often form small papillations, or tufts, in thin-walled vascular spaces (Fig. 55.39). Both cell types are typically surrounded by a myxoid or fibrous stroma, and the dense stroma may be calcified.
The tumor tends to grow around and leave intact preexisting structures, such as portal tracts and residual hepatocytes. Bile ducts may be seen in the tumor, particularly at the periphery of the lesion. The tumor also has a predilection for invading large vascular structures, such as portal and central veins, mimicking the appearance of vascular thrombosis. Scattered inflammatory cells, such as lymphocytes and neutrophils, are often seen.
Differential Diagnosis
Differentiation of this lesion from adenocarcinoma (including cholangiocarcinoma) or HCC may be difficult on routine hematoxylin and eosin staining (Table 55.14). However, immunohistochemical staining for endothelial markers in tumor cells, such as CD34, CD31, factor VIII, and FLI1, helps to confirm the diagnosis. Care must be taken not to mistake focal keratin positivity in trapped hepatocytes or bile ducts as evidence of a carcinoma. Keratin may stain some tumor cells.113 Another diagnostic problem is differentiation of epithelial hemangioendothelioma from venous thrombosis or veno-occlusive disease, because tumor growth in large vessels may mimic an organizing thrombus. Table 55.13 compares this lesion with angiomyolipoma and angiosarcoma.
Table 55.14
Comparison of Epithelioid Hemangioendothelioma, Hepatocellular Carcinoma, and Adenocarcinoma
| Morphologic Feature or Immunostain | Epithelioid Hemangioendothelioma | Hepatocellular Carcinoma | Adenocarcinoma (Cholangiocarcinoma or Metastatic) |
| Gross appearance | Firm, white | Tan-brown or yellow-tan; usually not firm | Often firm, white to tan |
| Stromal reaction | Myxoid or fibrous stroma prominent | Most lesions lack significant fibrous stroma | Desmoplastic reaction common |
| Vascular invasion | Venous invasion common; can mimic organizing thrombosis | Venous invasion common | May occur |
| Entrapment of normal tissues | Residual portal tracts, hepatocytes, bile ducts common in tumor mass | Uncommon | Uncommon |
| Growth pattern | Small nests or single cells embedded in stroma; papillary tufts in vascular spaces | Trabecular growth most common; clusters of cells in scirrhous pattern | Glands or clusters of cells within fibrous stroma |
| Immunostains | Vascular markers positive; occasional CK positivity (e.g., CK7) | CD34 positive in endothelial cells lining trabeculae | Positive for cytokeratin markers, MOC-31 |
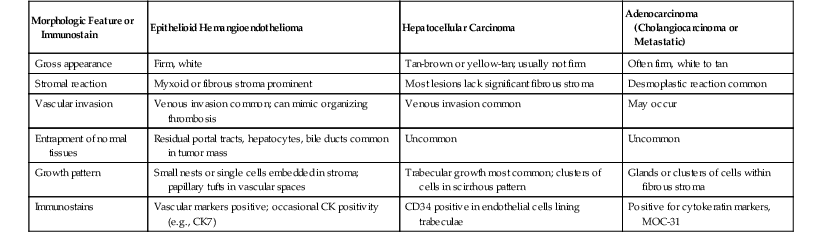
Prognosis and Treatment
Overall, the prognosis of patients with epithelioid hemangioendothelioma is better than for those with angiosarcoma, even if excision is incomplete or extrahepatic metastases are identified. Liver transplantation may be a reasonable treatment in unresectable cases.114
Kaposi Sarcoma
Clinical Features
Kaposi sarcoma occurs most often in the liver of patients with acquired immunodeficiency syndrome (AIDS), but tumors at other sites are typical. Kaposi sarcoma rarely occurs in immunosuppressed patients in the absence of AIDS or in nonimmunosuppressed older adult patients (more commonly in Europe and Africa), but liver involvement is highly unusual in these clinical settings. Human herpesvirus 8, which occurs in the semen of infected patients and is transmitted through sexual contact, is thought to play a causative role in the development of Kaposi sarcoma.115
Pathologic Features
The pathologic features of Kaposi sarcoma in the liver are similar to those that occur at other sites. The tumor may be fibrous, hemorrhagic, and multifocal, and it often centers on portal tracts. Microscopically, the tumor consists of a spindle cell proliferation that forms slitlike spaces or, in larger lesions, a solid, fibrosarcomatous growth pattern (Fig. 55.40). Cellular pleomorphism and mitotic activity are minimal, and extravasation of erythrocytes, hemosiderin deposits, and small eosinophilic globules are common. One pattern typical of Kaposi sarcoma of the liver is growth of tumor cells into sinusoidal spaces, usually at the periphery of tumor nodules. Growth results in dilated channels containing erythrocytes that replace normal sinusoids, a feature that imparts a peliotic appearance to the liver (see Fig. 55.40). The tumor also tends to surround and infiltrate portal tracts but does not involve the hepatic artery and interlobular bile ducts.
Tumor cells are positive for endothelial markers (CD31, CD34), which are useful in differentiating Kaposi sarcoma from fibroblastic tumors. Positivity for human herpesvirus 8 is pathognomonic.115
Pediatric Neoplasms
Several primary liver tumors occur mainly in the pediatric age group. Table 55.15 provides an overview of these lesions.
Table 55.15
Histologic Features of Pediatric Tumors
| Tumor Type | Clinical Features | Major Histologic Features | Differential Diagnosis |
| Mesenchymal hamartoma | Tends to become cystic with time; no risk of malignancy | Mixture of hepatocytes, cystic change, myxoid to fibrous stroma, ductules | Hepatoblastoma: more atypical cellular stromal and hepatocytic components; small samples may be mistaken for normal liver |
| Infantile hemangioma | Association with cardiac failure | Vascular channels of various sizes; infiltrative edges entrap ducts and hepatocytes | Angiosarcomas in children: rare, and tends to occur in older age group; more atypical cytologic features, more necrosis |
| Hepatoblastoma | Malignant; occurs at <5 yr of age and >90% in noncirrhotic liver; AFP elevation | Epithelial and mesenchymal components predominate; multinodularity common | HCC: occurs in setting of cirrhosis or underlying metabolic or storage disorder in older age group; more atypical cytologic features |
| Embryonal (undifferentiated) sarcoma | Malignant; aggressive tumor; poor prognosis; occurs at 6-10 yr | Anaplastic and pleomorphic tumor cells, necrosis common, eosinophilic globules | Angiosarcoma in children: vascular; scaffolding growth pattern, solid to papillary features; more spindle cell component; lacks globules |
AFP, α-Fetoprotein; HCC, hepatocellular carcinoma.
Mesenchymal Hamartoma
Clinical Features
Mesenchymal hamartoma is a benign tumor that occurs primarily in young children, particularly those younger than 2 years. It is the third most common tumor of the liver in this age group (after hepatoblastoma and infantile hemangioma).116 At presentation, patients often have a palpable liver mass, abdominal enlargement, or respiratory distress; the latter is caused by tumor compression. Mesenchymal hamartoma may be associated with high serum levels of AFP and arise in Beckwith-Wiedemann syndrome, associations that mimic hepatoblastoma.117,118 Mesenchymal hamartoma may be capable of transforming into undifferentiated (embryonal) sarcoma (discussed later).
Pathologic Features
Mesenchymal hamartomas may be solid or cystic. Tan cysts may contain translucent fluid or gelatinous material.116,119 Cysts probably develop from degeneration of loose tumor mesenchymal tissue, and the tumors may enlarge through progressive accumulation of fluid in the cysts.
Histologically, mesenchymal hamartoma contains a mixture of epithelial and stromal components. The former consists of relatively normal-appearing hepatocytes and bile ducts, both of which are usually surrounded by some amount of myxoid or fibrous stroma (Fig. 55.41). The hepatocytes are cytologically unremarkable. They are often arranged in small clusters or larger groups but retain the normal cell plate architecture of the liver (Fig. 55.42). Bile duct structures are typically arranged in a branching pattern and associated with an acute inflammatory infiltrate. Cystic spaces, when present, may be lined by flattened to cuboidal epithelial cells surrounded by fibrous tissue (see Fig. 55.41). Occasionally, the cysts lack a distinct epithelial lining. The stroma contains increased numbers of small vascular structures, spindle cells, and inflammatory cells. Normal-appearing portal tracts are typically absent. Extramedullary hematopoiesis is often observed.
Infantile Hemangioma
Clinical Features
Infantile hemangioma, formerly known as infantile hemangioendothelioma, is the second most common tumor (after hepatoblastoma) in children younger than 3 years. Most reported cases have been in infants younger than 6 months. The tumor is twice as common among female than male patients.120,121
The tumor is often multifocal, and in approximately 10% of patients, hemangiomas also are found in other organs.6 The tumors may be associated with other congenital anomalies, such as bilateral renal agenesis, Beckwith-Wiedemann syndrome, hemihypertrophy, and meningomyelocele.6,120
Patients have an abdominal mass or distention (with hepatomegaly), jaundice, diarrhea, constipation, vomiting,120 congestive heart failure, or failure to thrive. Other, less common presentations include thrombocytopenia resulting from sequestration of platelets in the tumor and rupture with hemoperitoneum.9
Pathologic Findings
Infantile hemangiomas are poorly circumscribed lesions that may be solid and cystic with hemorrhage. Hemorrhagic foci usually alternate with fibrotic (solid) areas. The tumors are typically multifocal.
The two histologic subtypes may be difficult to distinguish microscopically from one another. One type has a mixture of small vascular channels with irregularly shaped cavernous spaces (Fig. 55.43). Both types of vascular channels are lined by a single layer of endothelial cells. The vascular spaces are separated by a poorly developed stroma containing scattered collagen or reticulin fibers. Small bile ducts and hepatocytes may be identified in the stroma and are often accentuated near the periphery of the tumor (see Fig. 55.43). Focal areas of necrosis, hemorrhage, fibrosis, and calcifications are often seen. The second type contains endothelial cells with a more atypical cytologic appearance, including increased mitotic activity and more prominent nuclear hyperchromasia. Tumor cells in this type are arranged in a more complex budding or branching pattern.98
Tumor endothelial cells stain with vascular markers, including CD34, CD31, and factor VIII. Stromal cells underlying the basement membrane of capillary structures are positive for α-smooth muscle actin (SMA) and a muscle actin–specific monoclonal antibody (HHF35), and they are negative for desmin. This immunoprofile suggests pericytes.122
Prognosis and Treatment
Infantile hemangiomas are histologically benign tumors. However, because of the tumor’s multifocality and large size, the mortality rate is high, often as a result of cardiac or hepatic failure.120 These tumors may regress. More often, surgical resection, embolization, hepatic arterial ligation, or chemotherapy or irradiation (or both) are used to prolong patient survival.121 Angiosarcomas may rarely arise in this lesion.110
Hepatoblastoma
Clinical Features
Hepatoblastoma is the most common malignant liver neoplasm in children. Approximately 66% occur in children younger than 2 years, and 90% occur before age 5, although they occasionally arise in older children119,123 and rarely occur in adults.124 Males are affected twice as often as females. Hepatoblastomas are associated with other congenital conditions, such as Beckwith-Wiedemann syndrome,119 Down syndrome, familial polyposis coli,125 hemihypertrophy, renal malformation, and other chromosomal abnormalities,126 in as many as one third of reported cases.
Presenting symptoms such as abdominal mass, failure to thrive, and loss of weight are relatively common. Less common features include vomiting, diarrhea, and jaundice. Rarely, affected patients may have signs of precocious puberty, such as virilization, which is associated with human chorionic gonadotropin production by the tumor.127 The serum AFP level is usually elevated and has proved to be a useful marker for tumor recurrence or metastasis after therapy. Molecular changes include β-catenin mutations in more than 50% of cases,128 and chromosomal alterations have been described (see Chapter 44).41
Pathologic Features
Hepatoblastomas typically occur as a large, single mass in noncirrhotic livers. Their gross appearance varies, but the tumor is often multinodular and contains areas of hemorrhage and necrosis. Because distinct tumor nodules may contain different histologic components, generous tissue sampling is recommended. After chemotherapy, tumors often become quite necrotic, but the mesenchymal components, especially osteoid, remain prominent.129
Microscopically, there are two main subtypes of hepatoblastoma. One consists of purely epithelial components, and the other consists of mixed epithelial and mesenchymal elements. The pure epithelial type usually has a combination of the embryonal and fetal patterns (Fig. 55.44). Rarely, a pure fetal subtype occurs. The mixed epithelial and mesenchymal subtype is usually composed of epithelial patterns admixed with spindle cell mesenchyme, and osteoid is a common finding (Fig. 55.45).
In the epithelial subtype, the embryonal pattern represents the more immature form. It consists of small tumor cells with round to oval nuclei and scant basophilic cytoplasm. The cells tend to form tubular, acinar, or ribbon-like structures (see Fig. 55.44). The fetal pattern represents a more mature form that more closely resembles fetal liver and has tumor cells arranged in plates or cords (see Fig. 55.44). Fetal tumor cells are typically smaller than normal hepatocytes, but they are slightly larger than embryonal tumor cells. Fetal cells show the cytoplasmic features of hepatocellular differentiation. Fetal tumor cells have a moderate amount of eosinophilic cytoplasm or clear cytoplasm that is caused by the presence of lipid or glycogen. Eosinophilic and clear cytoplasmic features often occur in the same tumor, resulting in a distinctive alternating pattern of pink and white (Fig. 55.46). Fetal cell nuclei are small and round, similar to normal fetal liver cells. In the embryonal and fetal patterns, mitotic figures are rare. Extramedullary hematopoiesis often is associated with the fetal component.
Less common hepatoblastomas include a small cell undifferentiated type, a macrotrabecular type, and a mixed type with teratoid features. The small cell type consists of sheets of small tumor cells without evidence of hepatocellular differentiation. These cells have scant cytoplasm, similar to that of neuroblastoma. One of the other patterns of hepatoblastoma should be identified to differentiate it from neuroblastoma.
The macrotrabecular type consists of wide trabeculae greater than 10 cells thick. Fetal and embryonal tumor cells form trabeculae, but a much less common pattern consists of large cells with abundant cytoplasm. This pattern may mimic HCC. Other patterns of hepatoblastoma and their occurrence in a noncirrhotic liver helps to distinguish the macrotrabecular variant of hepatoblastoma from HCC. Tumors with a minor macrotrabecular component should be classified according to the most predominant patterns.130
The mixed-type of hepatoblastoma with teratoid features contains epithelial and mesenchymal components mixed with other tissue types, such as intestinal-type glands, squamous epithelium, melanin pigment, and other mesenchymal elements, including cartilage, skeletal muscle, and neural tissue.
Differential Diagnosis
Pure fetal hepatoblastoma may resemble HCA. However, fetal hepatoblastoma tumor cells tend to be smaller. Alternating pink and white cytoplasmic staining is not typically seen in HCAs. These tumor types also may be distinguished on the basis of clinical parameters. For example, HCAs do not generally occur before the patient is 5 years old, except in association with metabolic disorders such as glycogen storage disease. The serum AFP level is not normally elevated in adenoma cases. Clinical parameters also help to distinguish the macrotrabecular variant of hepatoblastoma from HCC, because the latter occurs primarily in the setting of preexisting liver disease or in association with an underlying metabolic disorder (usually in the setting of cirrhosis) in the pediatric population.
Hepatoblastomas immunostain positively for AFP in the embryonal component63 and hepatocyte antibody and polyclonal CEA in the epithelial component, especially in fetal cells.49 β-Catenin nuclear or cytoplasmic staining is often observed.131 Focal neuroendocrine staining with chromogranin A in the embryonal, fetal, and osteoid components has been reported.63,132
Prognosis and Treatment
Prognosis is directly related to completeness of surgical excision and tumor stage.130 The treatment of choice is complete surgical resection. However, chemotherapy is often used preoperatively to reduce tumor size or for treatment of residual and nonresectable tumors. Certain histologic subtypes (discussed earlier), such as the pure fetal type, have a better prognosis after complete resection123 than the fetal and embryonal or the mixed patterns. Other histologic subtypes, such as the small cell and macrotrabecular variants, have been associated with worse outcomes.130
Tumor-free margins are an important prognostic factor. However, vascular invasion has not been shown to have a significant effect on outcome.123 Additional indicators of a poor prognosis are young age at presentation (<1 year), large tumor size, and involvement of adjacent vital organs.
Embryonal (Undifferentiated) Sarcoma
Clinical Features
Embryonal sarcoma is a rare liver tumor that typically occurs in children between the ages of 6 and 10 years. Rarely, older children, teenagers (<20 years),133 and adults may be affected.134 Common presenting features include a mass lesion and abdominal pain.133,135
The prognosis has been considered poor,135 with complete surgical excision usually offering the best outcome. However, newer therapeutic modalities have contributed to improvements in survival.
The histogenesis of this tumor is unknown, and risk factors have not been reliably identified. Rare cases have been reported to arise from mesenchymal hamartomas.136 Molecular studies have suggested a possible relationship between these two tumors, because both lesions have 19q chromosomal abnormalities.136,137
Pathologic Features
Embryonal sarcomas are usually large, soft tumors with cystic (often multicystic) and solid areas and a white, shiny, or gelatinous mucoid surface. Areas of necrosis and hemorrhage are often observed.
Microscopically, embryonal sarcomas contain a mixture of stellate and spindle-shaped cells embedded in a myxoid stroma. The tumor cells have a granular or bubbly appearance and light pink cytoplasm and contain cytoplasmic globules that are d-PAS positive (Fig. 55.47). Globules may also be seen in the extracellular stroma. Other features include large, atypical tumor cells with hyperchromatic nuclei and multinucleation, dense collagen deposits, and numerous mitotic figures. Extramedullary hematopoiesis is often identified, and entrapped hepatocytes and ductules may be observed at the periphery of the tumor.
Embryonal sarcomas stain with vimentin, α1-antitrypsin, and α1-antichymotrypsin.135,138 Staining for GPC3 expression is positive in this tumor, including the giant tumor cells.139 The differential diagnosis includes angiosarcoma and rhabdomyosarcoma, but immunostaining for CD34 and myogenin is negative in embryonal sarcomas and helps to exclude these possibilities.138
Hematopoietic Malignancies
All types of leukemias and lymphomas (e.g., Hodgkin, non-Hodgkin) and myeloid lesions may secondarily involve the liver (see Chapter 31).140 Leukemic involvement of the liver typically exhibits a diffuse pattern of infiltration of the sinusoids by malignant cells, except chronic lymphocytic and acute lymphoblastic leukemias, which often involve the portal tracts, a pattern that is more typical of lymphoma. Hairy cell leukemia may be associated with the formation of a peliosis hepatis–like lesion consisting of dilated sinusoids lined by tumor cells. Immunostains for various types of hematopoietic cells are helpful in determining the diagnosis in these cases.
Hodgkin lymphoma usually involves the liver as nodular masses in the portal tracts. Reed-Sternberg cells should be identified for a definitive diagnosis, but an infiltrate composed of lymphocytes admixed with plasma cells, eosinophils, and few atypical cells is consistent with Hodgkin lymphoma if the patient has known disease elsewhere. Occasionally, epithelioid granulomas may be found in the parenchyma or in the portal tracts, but granulomas without the other features described here are not sufficient to confirm a diagnosis of liver involvement by Hodgkin lymphoma. Rarely, intrahepatic cholestasis, which may be associated with a paucity of bile ducts, may be seen in Hodgkin lymphoma.141
Non-Hodgkin lymphomas usually involve the liver as metastatic lesions by forming nodular masses in the portal tracts, and the high-grade B-cell lymphomas often form large, destructive lesions. In some cases, especially in T-cell lymphomas, sinusoidal infiltration by the tumor cells is similar to that seen in leukemia.140 Liver involvement by peripheral T-cell lymphomas occurs in as many as 50% of patients.142 Intrahepatic cholestasis, rarely with ductopenia143 and epithelioid granulomas similar to those seen in Hodgkin lymphoma, has been seen. Primary non-Hodgkin lymphoma only rarely occurs in the liver. Many primary lymphomas have been associated with AIDS and occur in the setting of liver transplantation as posttransplantation lymphoproliferative disorders related to Epstein-Barr virus infection.
Other Lesions Involving the Liver
Benign Neoplasms
Benign neoplasms that may involve the liver include chondroma,144 fibroma, leiomyoma, lipoma, lymphangioma, myxoma,90 schwannoma,145 solitary fibrous tumor,146 and adrenal and pancreatic rests.147 Granular cell tumors may involve the biliary tract.148
Focal fatty change is a localized zone of hepatocytes that contains abundant fat. This finding is often subcapsular and may be confused grossly or radiographically with a neoplasm, but this lesion should not be confused with the fatty nodules seen in adenomatosis, particularly of the HNF1A type. Focal fatty change may also be associated with diabetes or alcoholic hepatitis.149
Solitary necrotic nodules are rare non-neoplastic lesions that consist of a central zone of amorphous, eosinophilic debris rimmed by a hyalinized fibrotic capsule containing prominent elastic fibers. These lesions may be clinically mistaken for metastatic disease, and they rarely are associated with parasitic infection.150
Segmental atrophy of the liver can mimic a neoplasm. These atrophic liver lesions have some degree of elastosis, and when this change produces a nodular appearance, it is designated as nodular elastosis. The lesion shows some degree of hepatocyte atrophy and elastosis in a background of sclerotic vessels.151
Malignant Tumors
Carcinoid, fibrosarcoma, malignant fibrous histiocytoma, follicular dendritic cell tumor, leiomyosarcoma, liposarcoma, malignant mesenchymoma, malignant mixed tumor, osteosarcoma, pheochromocytoma, plasmacytoma, malignant rhabdoid tumor, rhabdomyosarcoma, malignant schwannoma, squamous carcinoma, malignant trophoblastic tumor, teratoma, and yolk sac tumor have been described as primary malignancies in the liver.109,111,147,152–160 These tumors are histologically similar to those that occur in other organs.
Metastatic Neoplasms
General Diagnostic Approach
Metastatic neoplasms are extremely uncommon in cirrhotic livers. In a patient with known cirrhosis, a malignant neoplasm usually is a primary hepatocellular tumor, with the caveat that many HCCs have a cholangiocarcinoma component.
In noncirrhotic livers, metastatic neoplasms are the most common type of malignant tumor. Immunohistochemical stains are very useful for determining the possible site of origin in this setting. Many of the most common metastatic tumors, such as adenocarcinomas of nonhepatobiliary origin, neuroendocrine tumors, renal cell carcinoma, adrenal carcinoma, and melanoma, must be differentiated from HCCs, cholangiocarcinomas, bile duct adenomas, or primary mesenchymal tumors (see Tables 55.6 through 55.8, 55.10, 55.11, and 55.14). HCCs may need to be considered in the differential diagnosis of metastatic tumors in patients without cirrhosis, cholangiocarcinoma, or unusual primary tumors (e.g., angiomyolipoma, epithelioid hemangioendothelioma, angiosarcoma) (see Tables 55.12 through 55.14).
Because many tumors from nonhepatobiliary sites may have a hepatoid, glandular, or neuroendocrine appearance and look like primary liver tumors, it may be difficult to determine the exact site of origin. Antibodies such as TTF1 (highly specific for lung and thyroid), CDX2 (positive in metastases from many colon and some other GI primaries), various prostatic markers, and SMAD4 (no staining in many pancreatic adenocarcinomas; see Table 55.11) may help to determine or exclude possible sites of origin.
Spindle cell lesions may warrant a somewhat different approach, including consideration of metastatic melanoma (see Table 55.8) or metastatic GI stromal tumor. The latter is expected to stain positively for CD117 (KIT), CD34, and ANO1 (formerly DOG1).161 An approach to differentiating primary from metastatic tumors in the liver is reviewed in Table 55.16.
Table 55.16
Immunohistochemical Analysis of Liver Tumors
| Differential Diagnostic Problems | Immunohistochemistry |
| Poorly differentiated tumor (not obviously carcinoma), including small cell tumor morphology | Pankeratin (CAM 5.2), leucocyte common antigen, vimentin, NSE |
| Carcinoma: HCC versus cholangiocarcinoma | HepPar-1, polyclonal CEA, glypican 3, CK7, CK19, CK20, MOC-31 |
| Clear cell tumors, such as HCC, renal cell carcinoma, melanoma | Pankeratin, EMA, vimentin, polyclonal CEA, HMB-45, S100, PAX2 and/or PAX8 |
| HCC versus well-differentiated NET | Synaptophysin, chromogranin (MOC-31 often positive in NET) |
| Nonepithelial tumors mimicking carcinoma (e.g., melanoma, angiomyolipoma, GI stromal tumor, angiosarcoma, epithelioid hemangioendothelioma) | HMB-45, S100, SMA, CD117, ANO1, CD34, CD31, FlI1, pankeratin; follow-up stains as indicated: tyrosinase, melan-A, SOX10 for melanoma |
| Carcinoma, poorly differentiated or glandular, primary or metastatic | CK7, CK10, CK20, MOC-31, CDX2, TTF1, SMAD4, PSA, ER, PR |
| Spindle cell tumors (e.g., primary or metastatic sarcomas, rare HCC, melanoma, angiomyolipoma) | Suggested primary stains: pankeratin, CD117, HMB-45; follow-up stains as indicated: SMA, S100, CD34, ANO1, tyrosinase, melan-A, SOX10 |
ANO1, Formerly DOG1; CDX2, intestine-specific transcription factor; CEA, carcinoembryonic antigen; CK, cytokeratin; EMA, epithelial membrane antigen; ER, estrogen receptor; GI, gastrointestinal; HCC, hepatocellular carcinoma; NET, neuroendocrine tumor; NSE, neuron-specific enolase; PR, progesterone receptor; PSA, prostate-specific antigen; SMA, smooth muscle actin; TTF1, thyroid transcription factor 1.
Frozen Section Technique
Frozen sections of liver tumors are often submitted to determine whether the tumor is a metastatic or a primary lesion. Features of HCC and other primary tumors that are relevant to analyzing frozen sections have been discussed (see Tables 55.4 through 55.9). The thickness of the tissue section can affect the apparent degree of cellularity of the lesion. An artificially increased width of the liver cell plates can lead to overdiagnosis of well-differentiated HCC.
Lesions discovered incidentally at the time of laparotomy are submitted as frozen sections. In this setting, problems arise when determining whether FNH, bile duct adenoma, and biliary hamartoma are primary or metastatic carcinomas. Because FNH can be easily mistaken for cirrhosis, it is wise to obtain a biopsy sample of the noninvolved liver adjacent to the tumor mass to help exclude cirrhosis. The features of bile duct adenoma that help differentiate primary from metastatic adenocarcinomas have been discussed (see Tables 55.10 and 55.11). Tissue samples from a cirrhotic liver may reveal a bile duct adenoma or a focal area of benign bile ductular reaction, both of which are common in cirrhosis, whereas metastatic adenocarcinoma in cirrhosis is rare. Cholangiocarcinoma is rare in cirrhosis, particularly compared with HCC, but combined HCC-CC is more common, and cholangiocarcinoma is commonly associated with primary sclerosing cholangitis.
Frozen sections are used to evaluate liver parenchymal or bile duct margins. Evaluation of liver margins usually is not challenging, but portal and central veins should be carefully examined for possible vascular spread of tumor. Examination of bile duct margins in the hilum or hepatic and common ducts may be more problematic, particularly if the pathologist is not familiar with the normal anatomy of this region. Periductal or peribiliary glands adjacent to large ducts are normal benign structures. These glands, which occur in clusters adjacent to large ducts, may undergo some degree of hyperplastic, atrophic, and reactive change, but they tend to retain a uniform configuration. Inflammatory changes surrounding large ducts, including acute or chronic cholangitis, may result in a considerable degree of cytologic atypia that should not be confused with carcinoma. Embolization or ablation of a neoplasm before the biopsy can result in ischemic changes in the liver parenchyma (e.g., necrosis, fibrosis) and marked ductular metaplasia of liver cell plates (hepatocytes) with epithelial atypia. These changes, which often suggest cholangiocarcinoma, epithelioid hemangioendothelioma, or angiosarcoma, can be easily misinterpreted as malignant (see Fig. 55.32). In ductular metaplasia, the size and shape of the ductules remain uniform, and there may be atrophy; neither of these features is normally seen in cholangiocarcinoma.