Benign and Malignant Tumors of the Gallbladder and Extrahepatic Biliary Tract
N. Volkan Adsay
David S. Klimstra
Introduction
Gallstones and inflammatory disorders constitute most biliary tract pathology. Neoplasms and tumorlike lesions are uncommon but form an important category with challenging diagnostic issues. Because of advances in radiographic modalities and widespread use of laparoscopic cholecystectomy, the frequency of gallbladder and biliary tumors that have come to clinical attention has increased. These techniques have also led to an increase in the diagnosis of mass-forming lesions, including “incidentalomas” such as cholesterol polyps, that would have otherwise gone undetected.
The terminology used for gallbladder and bile duct tumors and for preinvasive neoplasms has been reevaluated. This evaluation was done to standardize the approach to tumors across the entire pancreatobiliary system.
Sampling Gallbladder Specimens
A significant proportion of gallbladder carcinomas are unapparent both clinically and grossly,4a discovered only after microscopic examination of cholecystectomy specimens performed and processed with the clinical and gross impressions of cholecystitis.1–9 Therefore, a sampling protocol addressing this possibility ought to be utilized routinely.1,7 Our approach is the following: The cystic duct margin is sampled en-face unopened. The serosal and hepatic surfaces of the GB are inked in different colors and the gallbladder is opened from the serosal aspect. If no overt lesions are noted, a through-and-through section, going from the fundus to the cystic duct, is taken, rolled up, and submitted in the same cassette along with the cystic duct margin. If a lymph node is present, this is also included in the same cassette.
If focal epithelial atypia (undetermined nature) or low-grade dysplasia are identified in this random sample, 4 additional blocks are obtained. If high-grade dysplasia is identified, then the entire gallbladder needs to be examined because grossly invisible invasive carcinoma is commonly associated with this and ought to be documented since the prognosis changes dramatically. Along the same lines, if a Tis or T1a or T1b carcinoma is identified, then the entire gallbladder must be examined to rule out a T2 carcinoma (invading through the tunica muscularis) since the clinical outcome of the latter is starkly worse, and it is typically undetectable grossly.9
If there is a polyp or papillary lesion, it is submitted entirely for examination. If this proves neoplastic, several (a minimum of 5) sections of the uninvolved gallbladder ought to also be examined for other dysplastic lesions. If the polyp shows high-grade dysplasia, then the entire gallbladder ought to be examined because these can be associated with invasive carcinoma in other segments of gallbladder.10
For a carcinoma of T2 or beyond, the documentation should include, in addition to the cystic duct margin, the spread to the serosal versus hepatic surfaces, respectively. Typically proper documentation of the deepest region requires at least 12 cassettes because foci of carcinoma are often grossly unapparent, unless the first set of slides already documents the highest possible stages (T3 with surface involvement).
For a high-risk lesion, such as established examples of hyalinizing cholecystitis and xanthogranulomatous cholecystitis, which are notorious for hiding grossly unapparent carcinomas, extensive sampling ought to be performed (preferably entirely) from the beginning. Cases with a clinical history of a high risk disease for carcinoma, such as primary sclerosing cholangitis cases, may also have to be sampled more from the beginning, preferably with 3 to 4 cassettes with multiple strips during the initial random sampling.
Gallbladder
Tumoral Intraepithelial Neoplasms
Mass-forming, preinvasive neoplastic lesions (i.e., tumoral intraepithelial neoplasms) of the gallbladder occur in two forms. Papillary or polypoid, preinvasive neoplasms known as intracholecystic papillary-tubular neoplasms, or ICPNs (i.e., adenomas, papillary neoplasms, papillomatosis, and papillary adenocarcinomas) compose the first type.10 The second is referred to as mucinous cystic neoplasms with ovarian-like stroma, and these tumors are rare.11–13
These lesions represent one end of an adenoma-carcinoma sequence and can progress to more advanced degrees of dysplasia or invasive carcinoma. Although most invasive carcinomas of the gallbladder arise in the setting of nonpolypoid (flat) dysplasia, approximately 6% arise from tumoral intraepithelial neoplasms.10 The incidence of malignant transformation varies. The frequency is less for certain subsets of tumors such as pyloric gland adenomas and significantly higher for entities with high-grade dysplasia (i.e., papillary adenocarcinoma).10,14,15
Intracholecystic Papillary-Tubular Neoplasms
Terminology
Included in this category of tumors are all discrete, mass-forming, preinvasive neoplasms that arise in gallbladder mucosa. The ICPNs represent the gallbladder counterpart of gastrointestinal (GI) adenomas and pancreatic and biliary intraductal neoplasms (i.e., intraductal papillary mucinous neoplasms of the pancreas, pyloric gland adenomas of the pancreas, intraductal tubulopapillary neoplasms of the pancreas, and intraductal tubular and tubulopapillary neoplasms of the bile ducts), including those associated with biliary papillomatosis and tumors that once were classified as noninvasive papillary adenocarcinomas. In the 2010 World Health Organization (WHO) classification of tumors of the digestive system,16 these lesions are classified as adenoma or intracystic papillary neoplasm (Box 38.1). This distinction is presumably based on the degree of papilla formation or dysplastic transformation (both higher in the latter), although no specific criteria were established to help distinguish one from the other, which is problematic even for experts.17
The clinical and pathologic features of these lesions were not fully appreciated until recently because of the differences in terminology. For instance, many studies on adenomas discuss solely the pyloric gland type,18–20 which includes small lesions that represent metaplastic micronodules of pyloric glands. In the largest studies, the mean size of adenomas ranged between 0.6 and 0.9 cm and included cases as small as 2 mm in the largest dimension. In contrast, many truly preinvasive papillary lesions with extensive high-grade dysplasia were referred to as papillary variants of adenocarcinoma.14
Pyloric gland metaplasia is common in the gallbladder, and in some cases, it may form a polypoid nodule.21 We think that most of these cases are best regarded as polypoid metaplasia. We reserve the term neoplasm for lesions that can form large (>1 cm) tumors that are distinguishable from the background mucosa or a convincingly dysplastic epithelium.10 The 1-cm cutoff point is arbitrary, and is used by clinicians as a cutoff point for cholecystectomy and for pancreatic lesions (i.e., intraductal neoplasms).16
Clinical Features
The clinical features of ICPNs depend mainly on their size. The clinically more meaningful (>1 cm) tumors have certain recognized characteristics.10 They occur in older adults, who are typically in their early 60s at the time of diagnosis. At clinical presentation, patients may have pain, or the tumor may be detected incidentally. Gallstones are less common than in other types of gallbladder tumors.
Radiologically, almost 50% of cases are perceived as cancer, whereas the others are interpreted as polypoid tumor. Approximately 10% are not detected clinically, presumably because they are mistaken for stones or sludge. Rarely, patients have Peutz-Jeghers22,23 or Gardner syndrome24,25 or an anomalous union of pancreatobiliary ducts. Some cases are associated with a cholesterol-type polyp or, rarely, with a Brunner gland hamartoma of the duodenum. There are geographic differences in the incidence, histologic types, and association with invasive carcinoma. For instance, these tumors are more common in Asia.26
Pathologic Features
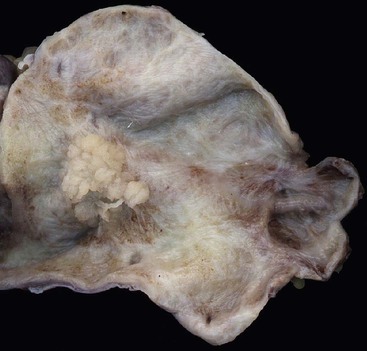
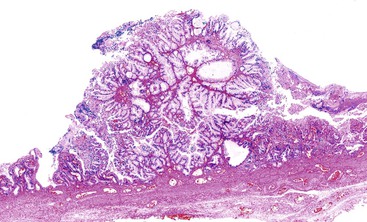
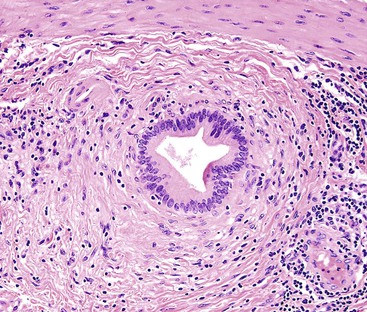
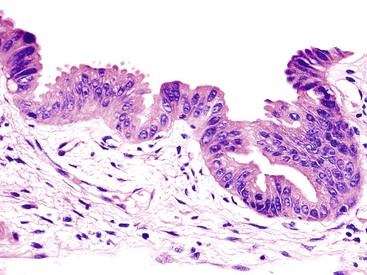
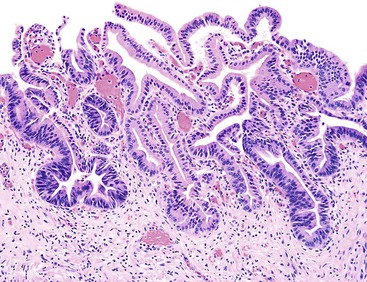
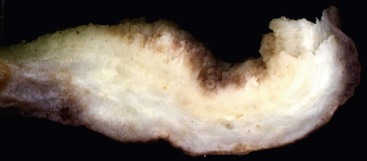
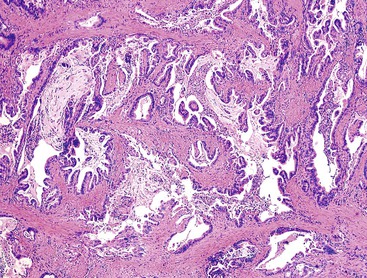
Macroscopically, ICPNs are characterized by large, villous or papillary growths with a feathery or cauliflower-like pattern, or by smooth-surfaced polypoid projections that may be pedunculated or sessile (Fig. 38.1).10 The lesions can be multifocal. They occur more commonly in the body or fundus of the gallbladder and may grow as large as 7 cm in the maximum dimension.
These lesions are often friable, and by the time they reach the dissection table, pedunculated cases may become detached and may be dismissed by prosectors as debris, necrosis, or sludge. If there is a radiologic or pathologic suggestion of a polyp in a gallbladder, it is important to sample all free-floating fragments in addition to the remaining mucosa of the gallbladder.
By definition, the tumors discussed in this section are premalignant. However, some, particularly those with pyloric gland differentiation, may lack significant cytologic atypia that is characteristic of conventional dysplasia.
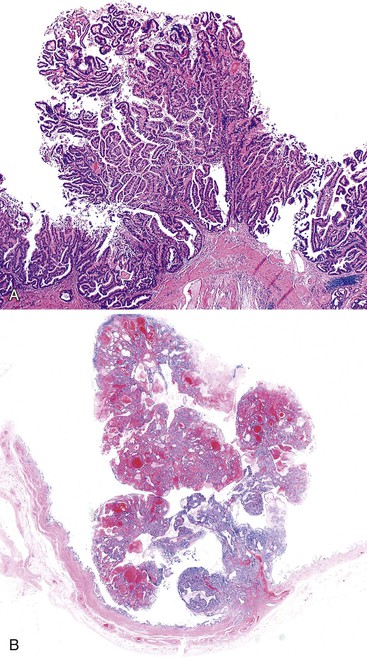
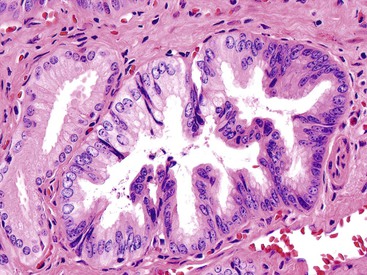
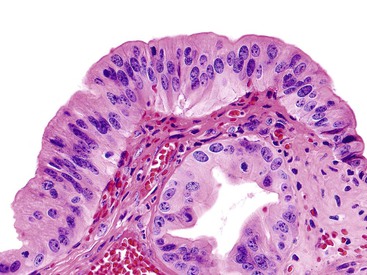
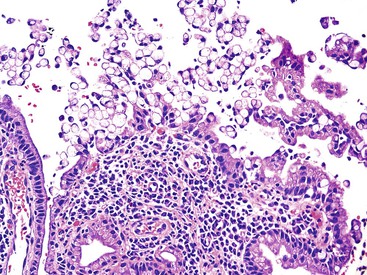
Microscopically, the lesions may be predominantly tubular or predominantly papillary (or villous), but a significant proportion shows a mixed tubulopapillary pattern (Fig. 38.2). The risk of malignancy is proportional to the amount of papilla formation. Various cell lineages that recapitulate the normal cell types in the GI tract (i.e., pyloric, foveolar, intestinal, and biliary) can be observed in the tumors. Although one cell type may be predominant in some cases, most exhibit a mixture of cell types.
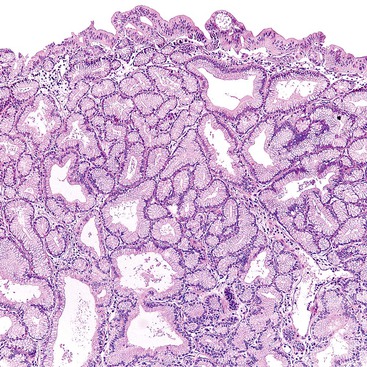
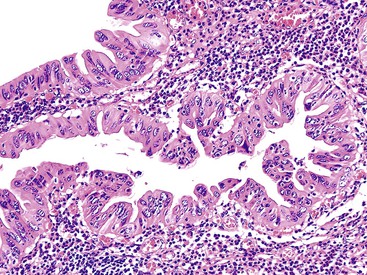
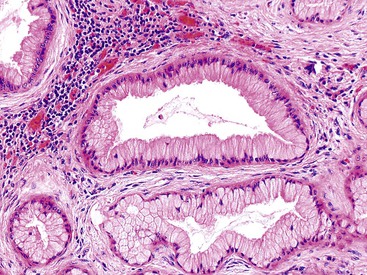
The pyloric gland pattern constitutes an overwhelming percentage of lesions that are less than 1 cm in the largest dimension (Fig. 38.3). The literature on so-called adenomas of the gallbladder18–20 is mostly based on small pyloric gland lesions, some of which may represent polypoid metaplasia rather than true neoplasms.10 Small lesions are often associated with extensive pyloric gland metaplasia in the background mucosa, further supporting the impression that they are exuberant polypoid examples of metaplasia.
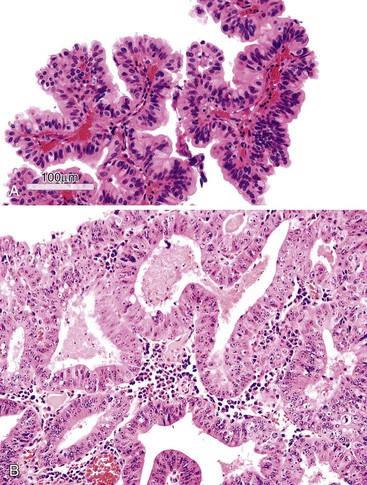
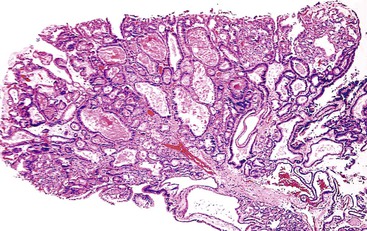
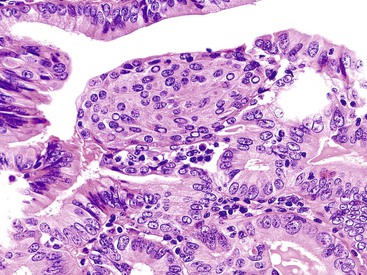
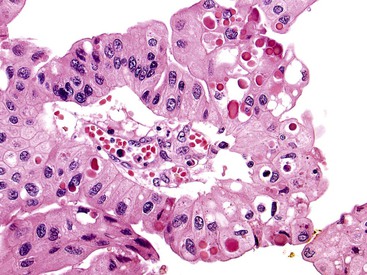
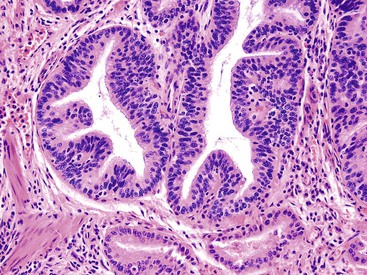
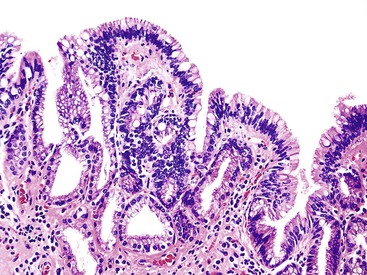
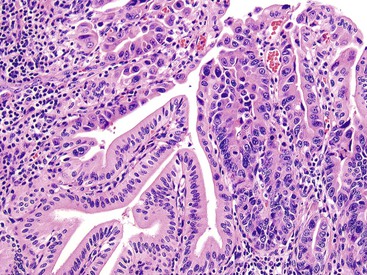
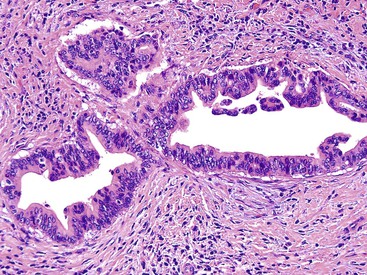
ICPNs larger than 1 cm in size are more commonly of the biliary type, showing cuboidal cells with prominent nucleoli and MUC1 positivity (Fig. 38.4, A). These tumors grow in a pattern known in the past as noninvasive papillary adenocarcinoma. They are often associated with extensive high-grade dysplasia (see Fig. 38.4, B) and invasive carcinoma. Prominent lymphoplasmacytic and neutrophilic inflammation may involve the papillary cores and the epithelium. Follicular cholecystitis occurs in patients with this type of tumor. It is evident in the background mucosa in 30% of cases.
Another tumor phenotype resembles gastric foveolar or endocervical epithelium (Fig. 38.5). It is composed of tall columnar cells with abundant, pale, mucinous cytoplasm, and it shows MUC5AC positivity. Although these tumors do not seem overtly atypical, their association with invasive carcinoma is fairly high. Among the cases that are larger than 1 cm in the largest dimension, it is uncommon to see tumors composed exclusively of bland-appearing pyloric (see Fig. 38.3) or Brunner-like glands. The incidence of associated invasive carcinoma in these cases is less than 15%.
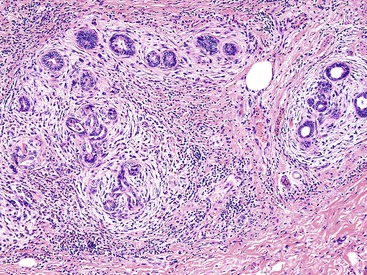
A distinctive and more common variant is characterized by a well-lobulated, complex tubular pattern of small, back-to-back, round or compressed glandular units with different degrees of lumen formation and with no intervening stroma. The glands are lined by cuboidal cells without overt intracytoplasmic mucin formation. These tumors have been referred to as pyloric gland adenoma, and the architecture of the polyps, along with MUC6-positivity, supports this designation. They may represent pyloric gland adenomas with high-grade dysplasia. However, they are seldom associated with a more conventional mucinous pyloric gland pattern within the lesion or in the uninvolved gallbladder. The overall morphology and immunophenotype of these tumors more closely resembles intraductal tubulopapillary or tubular neoplasms of the pancreas and bile ducts, and in one study, many examples of this variant were designated as such.27 We regard them as tumors with a complex pyloric gland phenotype (Fig. 38.6).10 Interestingly this variant is seldom if ever associated with invasive carcinoma. In addition many examples of this variant are associated with cholesterolosis, both within the lesion and away, and give the impression of arising in association with a cholesterol polyp.
In some examples of tumors with a complex pyloric gland phenotype, the nuclei are more elongated and show overlapping features and chromatin clearing, creating a pattern reminiscent of papillary thyroid carcinomas. Morular foci composed of squamoid spindle cells in a vague whorled pattern similar to the type of squamoid morules found in pancreatoblastoma, pulmonary blastoma, cribriform-morular thyroid carcinoma, or endometrioid adenocarcinoma are commonly seen in this variant. The nuclei in these morular areas have biotin-containing, optically clear pseudoinclusions (Fig. 38.7). Paneth cells and neuroendocrine cells are also commonly found in this variant.
Tumors with a complex pyloric gland phenotype are often large, have a thin stalk, and display a distinctive lobulated pattern in which the lobules are often covered by unremarkable gallbladder epithelium. The surrounding gallbladder is usually surprisingly free of abnormalities, including flat dysplasia or inflammation. Within the lobules, cystically dilated glands with acidophilic granular secretions are often evident. Some show amyloid-like hyalinization in the stroma.
A small percentage of ICPNs resemble intestinal adenomas (Fig. 38.8). They are characterized by pseudostratified columnar, cigar-shaped nuclei with conspicuous nucleoli, and tubular or villous architecture (often in combination). Goblet cells can be found in any type of ICPN, and their presence should not be considered evidence of intestinal differentiation. Many cases with columnar nuclei and basophilic cytoplasm lack true intestinal differentiation on immunohistochemical analysis.
Rare ICPNs may be oncocytic (Fig. 38.9), showing features similar to intraductal oncocytic papillary neoplasms of the pancreas and biliary tract. Even less common are transitional cell (urothelial-like) lesions that do not fit into any of the subtypes described previously. The clinical and biologic relevance of these subgroups has not been determined.
The 2010 WHO classification16 advocates use of one of two diagnostic categories: adenoma or intracystic papillary neoplasm. However, a study focusing on the reproducibility of international experts assigning cases to one of these two diagnostic categories revealed a very low level of agreement.17 We prefer to classify these tumors as ICPNs.10
Regardless of the term used, the most important aspect of tumor pathology is to rule out invasive carcinoma. Because invasive carcinoma is difficult to detect in the gallbladder grossly, extensive sampling is warranted. If an invasive carcinoma is identified, its type, size, and extent should be documented separately from the noninvasive component. Most invasive carcinomas that arise in these tumors are ordinary tubule-forming adenocarcinomas (pancreatobiliary type), although unusual types such as mucinous, squamous, and neuroendocrine tumors also occur.10 These unusual variants may be more common than those that arise from nontumoral (flat) dysplasia. If invasive carcinoma is not found, it is helpful to document as much as possible the amount of papillary growth, the cell types (especially biliary and foveolar types), and the extent of dysplasia, because these parameters are associated with a higher incidence of invasive carcinoma and disease progression.
Molecular Aspects
The immunophenotype of ICPNs corresponds to their line of differentiation. Most are cytokeratin 7 (CK7) positive, and many express mucin-related glycoproteins and oncoproteins, including carcinoembryonic antigen (CEA). The MUC1 response is typically confined to the apical membrane of cells in areas of biliary differentiation or high-grade dysplasia. MUC2 is expressed in intestinal, MUC5AC in foveolar, and MUC6 in pyloric or Brunner gland–like areas.10 The complex pyloric gland phenotype, which is a distinct group, also shows pyloric differentiation with diffuse MUC6 expression. Similar to other morule-forming tumors (i.e., biotin-rich, optically clear nuclei [BROCNs]–forming tumors28,29), the complex pyloric gland phenotype is commonly associated with mutations in β-catenin and expression of estrogen receptors.30 Mutation in KRAS is uncommon (<25%), similar to other gallbladder neoplasms.31,32 Tumor protein p53 (TP53) is expressed in approximately one third of cases, mostly in the biliary subtype with high-grade dysplasia.
The GNAS codon 201 mutation, which is seen in approximately 66% of pancreatic intraductal papillary mucinous neoplasms33 is rarely seen in ICPNs.27 This disparity is presumably related to the rarity of the intestinal variant, which appears to be linked with this mutation.
Differential Diagnosis
Metaplastic changes, particularly nodular pyloric gland metaplasia, can be indistinguishable from ICPNs with a pyloric or Brunner gland–like phenotype if the glandular units are interpreted solely at high-power magnification. An arbitrary size of 1 cm has been proposed as the minimum size for a tumor. Rarely, dysplastic cells that form a compact nodule less than 1 cm in diameter may be detected incidentally. We refer to these as incipient ICPNs, similar to incipient intraductal papillary mucinous neoplasms in the pancreas.
Ordinary nontumoral (flat) dysplasia of the gallbladder often has papillary structures. In some cases, these papillae may be tall and exuberant, creating a papillary adenocarcinoma pattern (Fig. 38.10, D), but these cases are not assigned to the ICPN category unless they form a distinct mass.
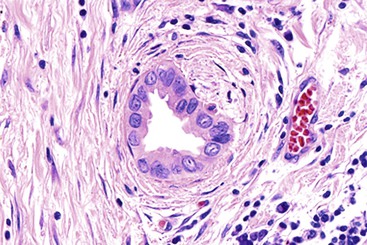
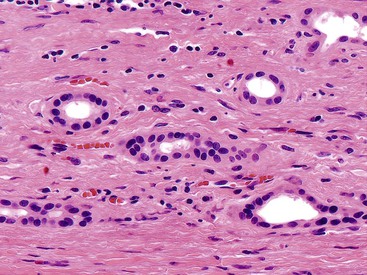
Recognition of the presence or absence of invasive carcinoma in or underneath polypoid tumors may be problematic because of architectural complexity and tangential sectioning. Features that favor carcinoma include wide separation of glandular units, their irregular contours (Fig. 38.11), cytologic differences from the surface lesion (including paradoxical differentiation with more abundant cytoplasm and tubule formation), foamy gland features,10,34 and cell clusters that reside in clefts. Extension of dysplastic epithelium into Rokitansky-Aschoff sinuses creates a pseudoinvasive appearance (Table 38.1).35 The distribution of the units, their spatial connection to the overlying mucosa, and the finding of remnants of normal epithelium are helpful clues in determining that the lesion is not invasive. Unlike invasive adenocarcinomas, Rokitansky-Aschoff sinuses are often flask shaped or elongated with the longitudinal axis oriented perpendicular to the surface (Fig. 38.12). Paradoxically, these sinuses often have concentric bands of loose stroma that resembles desmoplasia (Fig. 38.13). Most gallbladder adenocarcinomas do not exhibit significantly desmoplastic stroma.
Table 38.1
Invasive Carcinoma Compared with Involvement of Rokitansky-Aschoff Sinuses by Neoplastic Epithelium
| Invasive Carcinoma | Involvement of Rokitansky-Aschoff Sinuses |
| Small to medium-sized, round, dispersed glands | Long tubular units; flask shaped |
| Perineural invasion | Absence of perineural invasion |
| Longitudinal axis parallel to surface | Longitudinal axis perpendicular to surface |
| Cuboidal epithelium with attenuated or foamy cells | Columnar epithelium with basally located nuclei and acidophilic cytoplasm |
| Glands without a connection to the surface | Invaginations reveal connection to surface |
| Absence of a mixture of normal and neoplastic cells within glands | Mixture of normal and neoplastic cells within invaginations |
| Absence of inspissated bile within long, dilated spaces | Inspissated bile within long, dilated spaces |
Natural History and Prognosis
Polypoid neoplasms smaller than 1 cm in the greatest dimension (e.g., pyloric gland adenomas) are clinically inconsequential.18–20 However, when larger tumors (>1 cm) are analyzed separately, it is evident that even pyloric or Brunner gland tumors that were previously thought to be innocuous may be associated with or progress to invasive carcinoma.10 In our experience, invasive carcinomas occur in about 65% of ICPNs larger than 1 cm in diameter. Six percent of invasive gallbladder carcinomas arise in association with tumoral intraepithelial neoplasms. Although biliary and foveolar phenotypes have a higher frequency of invasive carcinoma (60% to 70%), the incidence of invasion is fairly low in those with pyloric and Brunner gland subtypes (approximately 15%). In fact, invasion is seldom encountered in complex, non-mucinous examples of pyloric gland lineage (MUCC6 positive, see above), although they are often highly atypical.27 Similar to lower GI tract lesions, papillary architecture signifies a stronger association with carcinoma.
The 3-year survival rate for noninvasive cases is 90%, compared with 60% for invasive cases. Lesions initially classified as noninvasive may recur, possibly because of missed invasive carcinoma. Invasive carcinomas that arise from ICPNs have a better survival rate than those that arise from flat dysplasia (3-year survival: 60% versus 30%). This survival advantage is maintained even in stage-matched studies.10
Some noninvasive cases are found to have recurrences and metastases on long-term follow-up that cannot be explained by undersampling or missed invasion. This finding suggests that a field effect may lead to the development of new biliary tract carcinomas years after the original gallbladder tumor was diagnosed. Long-term follow-up of these patients is advisable.
Mucinous Cystic Neoplasms
Hepatobiliary mucinous cystic neoplasms with ovarian-type stroma that arise in the bile ducts and liver (discussed later) can secondarily involve the gallbladder. However, convincing cases that originate in gallbladder mucosa are uncommon.12,13 A few examples were reported in the wall of the gallbladder, but they most likely represented adenomyomatous hyperplasia with hypercellular stroma rather than true mucinous cystic neoplasms.
Nontumoral Preinvasive Lesions: Dysplasia and Carcinoma In Situ
Microscopic, incidental preinvasive neoplasms of the gallbladder are categorized as nontumoral (flat) lesions, in contrast to mass-forming preinvasive neoplasms (i.e., tumoral intraepithelial neoplasms) discussed previously. Flat lesions often have a papillary microscopic configuration, but by definition, they do not form a grossly or radiographically recognizable mass lesion. In the past, these lesions were called epithelial atypia or adenomatous hyperplasia, but the current preferred terminology is dysplasia or intraepithelial neoplasia. Biliary intraepithelial neoplasia, a term originally proposed for the bile ducts,32 is now used synonymously for gallbladder dysplasia, as in the WHO classification.16 However, we and many other authorities prefer the term dysplasia for the gallbladder.
Dysplasia in the gallbladder is graded as low or high (Figs. 38.14 and 38.15). The terms high-grade dysplasia and carcinoma in situ (CIS) are interchangeable, but the former is strongly recommended. The 2010 WHO classification of tumors of the digestive system advocates use of “high-grade dysplasia” instead of CIS.16
Clinical Features
Dysplastic lesions are detected incidentally in gallbladder specimens removed for other disorders because dysplasia is clinically unapparent.36–39 The overall incidence of dysplasia varies greatly among different populations, but it parallels the incidence of adenocarcinoma. Dysplasia is more likely to be detected when multiple sections of the gallbladder are examined. In one North American population, routine cholecystectomies (for gallstones and morbid obesity), contained low-grade dysplasia in less than 5%, high-grade dysplasia in less than 1%, and frank carcinoma in less than 0.2% of cases.40 The rate approaches 15% in high-risk regions such as Mexico and Chile. Dysplasia is associated with 40% to 60% of invasive gallbladder carcinomas. However, it is often difficult to distinguish true dysplasia from colonization of the surface epithelium by invasive carcinoma cells. This makes analysis of dysplasia in invasive carcinoma cases more difficult.41
Patients with dysplasia are identified mostly in their early 60s. Dysplasia cases without invasive carcinoma are diagnosed at a mean age of 57 years, compared with 66 years for patients with invasive carcinoma.42
All risk factors that have been established for gallbladder adenocarcinoma (see section below) including geography, gallstones, sclerosing cholangitis, anomalous union of pancreatobiliary ducts are also considered risk factors for dysplasia.
Pathologic Features
By definition, nontumoral (flat) intraepithelial neoplasia (dysplasia) is difficult to appreciate grossly.43 Some cases, especially those with prominent papillary configuration, may reveal granularity or a feathery change in the mucosa, but most appear hyperemic or entirely normal by macroscopic examination.
Microscopically, gallbladder dysplasia is categorized as negative, indefinite, low grade, or high grade, similar to the grading system used in other portions of the GI tract. Negative refers to non-neoplastic (regenerating) epithelium. Cases are categorized as indefinite if the cytologic features approach but do not quite reach those of definite dysplasia or the epithelium shows atypical features suggesting dysplasia but because of ulceration, inflammation, or technical reasons, a definite diagnosis of dysplasia cannot be made.
Low-grade dysplasia is characterized by columnar cells that show mild pseudostratification, nuclear enlargement, an increased nucleus-to-cytoplasm (N : C) ratio, and nuclear irregularity. In low-grade dysplasia, the nuclei are usually limited to the basal portion of the cell cytoplasm, and there is only a minimal amount of architectural distortion (see Fig. 38.10) and loss of nuclear polarity. Mitoses are increased in number, but atypical mitoses are rare. It should be kept in mind, however, that mitotic activity can be brisk in some examples of reparative atypia. High-grade dysplasia is characterized by cells with a markedly increased N : C ratio, marked nuclear enlargement, more significant nuclear irregularity, hyperchromasia, and loss of polarity. High-grade dysplasia is most commonly characterized by nuclei that appear round, but some examples have more elongated and pencillate appearance (Fig. 38.16).
Because of a lack of prospective (outcome) studies, it is difficult to determine the biologic and clinical significance of low- versus high-grade dysplasia in the gallbladder. However, It should be kept in mind that high-grade dysplasia is often associated with invasive carcinoma, so thorough sampling is crucial. Recurrences and metastasis that are seen in some patients with high-grade dysplasia are attributable to missed invasion and field effect phenomenon. Gallbladder dysplasia may be composed of several cell types and growth patterns, which are often difficult to subcategorize as low or high grade. Similarly, the prognostic significance of specific cell types of dysplasia and certain growth patterns is unknown.
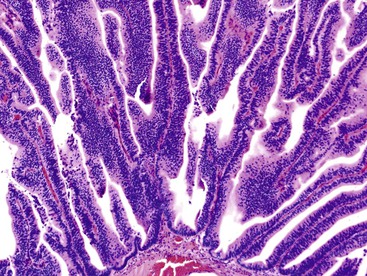
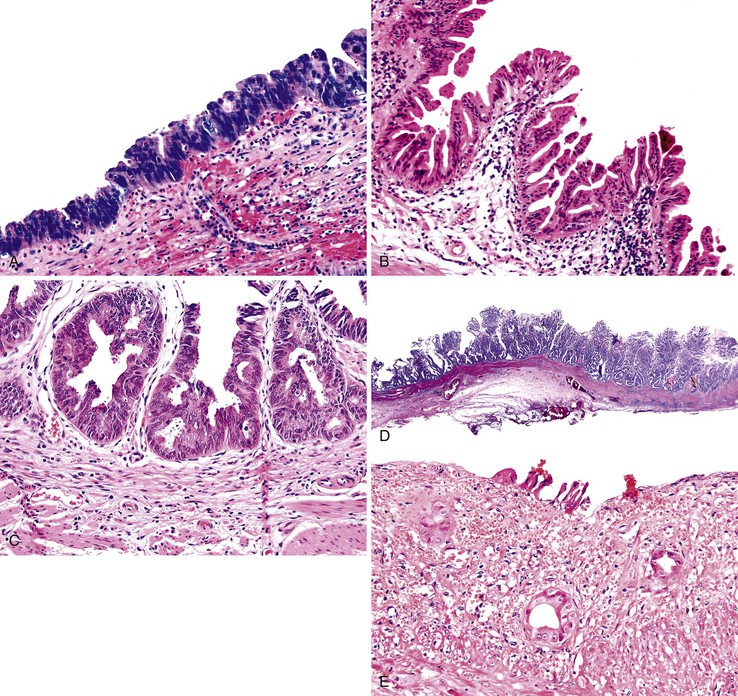
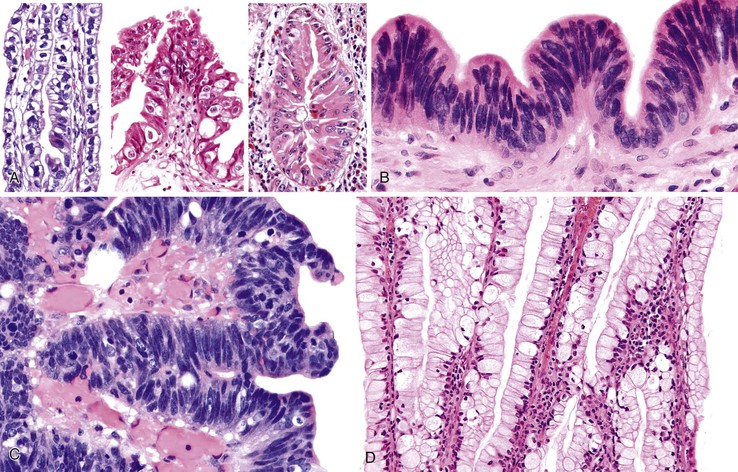
Dysplasia has various growth patterns,44 and any one case may have multiple growth patterns (see Fig. 38.10). The most common is the flat or undulating pattern, in which the mucosa is flat (see Fig. 38.10, A). In these cases, the diagnosis is established solely on the basis of cytologic findings rather than architecture.
The second most common patterns are micropapillary or tufting (see Fig. 38.10, B) and tubular (see Fig. 38.10, C), which are seen in more than one half of cases. The tubular pattern can be difficult to distinguish from invasive well-differentiated adenocarcinoma. In some cases, the glands reveal partially preserved but normal epithelium, indicating that the dysplasia represents pagetoid involvement of metaplastic pyloric glands by dysplastic cells. In other cases, the glands are formed entirely by the dysplastic epithelium itself.
The tall papillary (see Fig. 38.10, D) pattern is seen in approximately 25% of cases. If the papillae form a compact polypoid mass, we prefer to classify it as an ICPN (discussed earlier) instead of flat dysplasia.
The denuding or clinging pattern is seen in less than 25% of cases. It occurs commonly in cases with ulceration and hemorrhage and can be difficult to distinguish from reactive atypia (see Fig. 38.10, E). The denuding or clinging pattern is important in cases with hyalinizing cholecystitis (i.e., incomplete porcelain gallbladder).8
Other rare patterns of dysplasia include transitional epithelial (urothelial-like) or acantholytic.42 Occasionally, the cells are dyshesive and show signet ring cell features.45 Unusual types of carcinoma such as adenosquamous, squamous cell, neuroendocrine, and mucinous also can have intramucosal components.
Gallbladder dysplasia consists mainly of four types of cells, alone or in combination (Fig. 38.16).46 Most cases are biliary type (see Fig. 38.16, A), characterized by relatively monotonous-appearing, large, cuboidal cells with abundant cytoplasm, centrally or suprabasally located nuclei, cherry-red macronucleoli, and relatively fine chromatin. The cytoplasm may reveal clear cell (i.e., centrally located nuclei and nuclear contour irregularities), chromophobe-like (i.e., perinuclear cytoplasmic clearing), or oncocytoid (i.e., abundant acidophilic granular cytoplasm) features. The latter two often contain smoother nuclear contours.
Biliary-pencillate dysplasia closely resembles the pencillate cell of the normal gallbladder epithelium. It is characterized by crowded, thin, elongated, hyperchromatic, pencillate nuclei without nucleoli and with only a minimal amount of cytoplasm (see Fig. 38.16, B). This type and the intestinal type discussed below often appear more organized and give the impression of a low-grade dysplasia, but they often have more atypical areas in close inspection, prove to have high-grade dysplastic foci (see Fig. 38.16, C), and may be associated with invasive carcinoma.
Intestinal-type dysplasia has features similar to those of colonic adenomas, such as basophilia and pseudostratified, cigar-shaped nuclei (see Fig. 38.16, C). Goblet cells can be seen in any type of dysplasia and is not regarded as a sign of intestinal differentiation.
Gastric-foveolar dysplasia (see Fig. 38.16, D) shows abundant, apical, pale cytoplasm, basally located nuclei that are compressed at the base and hyperchromatic, are mildly enlarged with marked nuclear irregularity, or show prominent nucleoli. The degree of cytologic atypia is minimal. The dysplastic nature of the process is difficult to appreciate. However, this variant is biologically more aggressive.42 The clinical significance of these patterns has not been determined.
Differential Diagnosis
Dysplasia versus Reactive Atypia
The differential diagnosis of dysplasia and reactive atypia is a well-known problem in gallbladder pathology (Figs. 38.17 and 38.18; Table 38.2).47 Dysplasia in the gallbladder has the added challenge of having considerable overlap with reactive changes associated with chronic inflammatory conditions. Molecular studies to document abnormalities in cancer-associated genes—findings that could help to establish the neoplastic nature of specific morphologically defined lesions—have been limited. Dysplasia is defined and distinguished from other epithelial lesions based primarily on morphologic principles, drawing in part from experience with early neoplastic changes in the pancreatobiliary tract.
Table 38.2
Features Helpful in Distinguishing Reactive Atypia from Dysplasia
| Feature | Reactive Atypia | Dysplasia |
| Architectural growth pattern, such as tall papillary, micropapillary or tufting, cribriform arrangements | − | +++ |
| Nuclear chromasia | Hyperchromatic/hypochromatic | Hyperchromatic |
| Nuclear enlargement with very large, prominent, cherry-red nucleoli | − | + |
| Surface maturation | + | − |
| Dislodged stone–related changes | + | ± |
| Inflammatory cells | ++ | ± |
| Stromal hemorrhage and edema | ++ | ± |
| Goblet cells | ± | ++ |
| Mitotic figures | ++ | ++ |
+, Present, −, not present, ±, may be present.
The architectural pattern of growth, such as a tall papillary configuration with cytologic atypia, micropapillary or tufting pattern, piled cells, cribriform arrangements, fused units, and severe dispolarity of cells, is helpful in diagnosing dysplasia, because these patterns of growth are unusual in reactive lesions (see Fig. 38.10). In the biliary type, cells typically lose their columnar shape and often acquire a monotonous appearance. Nuclear enlargement (two or three times normal size) and prominent, cherry-red nucleoli (>2.7 µm) are characteristic features not seen in reactive lesions (see Fig. 38.16, A).46 Some cases are associated with abundant neutrophils, so inflammatory cells should not always be regarded as evidence of reactive atypia (Fig. 38.19). There is often dispolarity of cells, and in many instances, the cells pile up and become stratified.
Unlike the biliary type, pencillate cells have a lower degree of cytologic atypia and nuclear enlargement, but nucleoli are conspicuous. However, the nuclei in this type are typically hyperchromatic, and the N : C ratio is usually quite high, which creates a basophilic appearance to the epithelium. The gastric-foveolar type of dysplasia is typically composed of a single layer of innocuous-appearing cells, with pale cytoplasm and eccentric nuclei. Some cases have foamy or microvesicular cytoplasm. Nuclei, if compressed at the periphery, are hyperchromatic, whereas if they are suprabasal, they typically have a raisinoid appearance or prominent nucleoli. Goblet cells are more commonly encountered in dysplastic than reactive or normal epithelium (Fig. 38.20). Mitotic figures, including atypical forms, can be prominent in areas of regeneration and are therefore not helpful in this differential diagnosis. Detached reactive cells can also exhibit signet ring cell–like features (Fig. 38.21).45
High-grade dysplasia may show a wild-fire phenomenon in the gallbladder, which means it is typically extensive at the time it is detected, involving a significant proportion of epithelium.42,44,46 Focal epithelial atypia in a background of well-preserved nondysplastic epithelium is more likely to be reactive atypia. Reactive atypia shows surface maturation (Fig. 38.22). Deeper aspects of the epithelium show atypical basophilic cells with an attenuated appearance, molding of slightly disorganized nuclei, and cells with a high N : C ratio, but the surface is mature. Another feature that favors reactive atypia is the finding of denuded epithelium with compressed, flattened stroma or stroma with unnatural shapes that correspond to dislodged stones.
Reactive atypia in areas of ulceration can be even more challenging. In the setting of hemorrhage in adjacent stroma, epithelial atypia should be evaluated with caution. Atypical cells in this setting often have washed-out chromatin and nucleoli, but they are not as large as in high-grade dysplasia (see Fig. 38.18). Nuclear enlargement is also not as striking. Reactive changes tend to wax and wane throughout the epithelium, gradually changing from areas with marked atypia to areas without atypia. Dysplasia shows more abrupt transitions to non-neoplastic or metaplastic epithelium.
In cases with hyalinizing cholecystitis8 with minimal or no calcifications (i.e., incomplete porcelain gallbladder in which the entire gallbladder wall transforms into a thin, uniform, paucicellular, fibrotic band with distinctive clefting), any type of clinging epithelium on the surface should be regarded as highly suspicious for dysplasia. In well-preserved (nonhyalinized) gallbladders, the pathologist should refrain from rendering a diagnosis of dysplasia for detached epithelial cells because they often acquire degenerative changes that resemble dysplasia.32
Cancerization of Mucosa versus Dysplasia
In gallbladders with invasive carcinoma, secondary involvement of the epithelium, referred to as cancerization or colonization, may be difficult or impossible to distinguish from true dysplasia (Fig. 38.23). If the process has direct continuity with and morphologic similarity to an underlying invasive carcinoma, it is best regarded as cancerization rather than dysplasia. Abrupt transition from normal epithelium to atypical cells also favors cancerization.
High-Grade Dysplasia versus Early Invasive Carcinoma
For early gallbladder carcinomas (i.e., Tis, T1a, or early T1b), it may be difficult to distinguish in situ changes from true early invasive carcinomas if dysplastic changes occur in areas of the gallbladder with peribiliary mucous glands (i.e., neck and cystic duct region), in areas with pyloric gland metaplasia (i.e., common in injured gallbladders and in as many as 60% of cholecystectomy specimens), or in areas with Rokitansky-Aschoff sinuses.9 The gallbladder epithelium shows undulations, and there is no muscularis mucosa to separate the mucosa from submucosa. More importantly, the tunica muscularis (there is no muscularis propria in the gallbladder) is highly irregular and porous.48 Dysplastic glands can often be seen lying within or deep to the muscularis (Fig. 38.24). There are no basal or myoepithelial cells to distinguish native epithelium from invasive carcinoma.
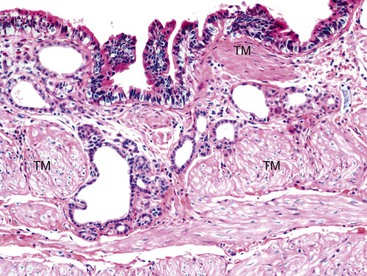
Features that favor true invasive carcinoma include invasion of nerves or blood vessels, a monotonous population of atypical glands (rather than a mixture of dysplastic and benign or metaplastic glands), lack of luminal bile, and lack of a connection to benign epithelium at the surface or adjacent submucosal glands (see Table 38.1). Fortunately, this distinction may not be important clinically because data from high-risk regions have led researchers to conclude that cases classified as early gallbladder cancer (i.e., Tis, T1a with lamina propria invasion, and T1b with invasion to tunica muscularis) have a very good long-term survival rate, much better than advanced carcinomas.9
It is far more important to exclude an advanced carcinoma than to distinguish high-grade dysplasia from early carcinoma. In fact a case should not be classified as Tis, T1a, or T1b unless complete sampling of the gallbladder is performed. The reported differences in survival rates of T1 cases in Western populations compared with high-risk regions such as Chile and Southeast Asia may result from undersampling in the West, resulting in downstaging of T2 carcinomas. For cases of early gallbladder cancer identified by thorough sampling of the gallbladder with the protocol established by Roa and colleagues, in which advanced carcinomas are definitely ruled out, the 10-year survival rate approaches 90%.7,9
Molecular Aspects
Immunohistochemically, CEA and MUC1 labeling shows a linear pattern at the apical border of dysplastic cells. In high-grade dysplasia, intercellular membranes may also be accentuated by CEA. Intracytoplasmic labeling with CEA and MUC1 is uncommon in high-grade dysplasia. Dense intracytoplasmic staining favors cancerization rather than dysplasia. TP53 nuclear staining occurs in more than 30% of cases of dysplasia and tends to be more common in high-grade lesions.31 Although TP53 expression is significantly less common in non-neoplastic mucosa, it can be seen in areas of regenerative atypia.49 Similarly, although the Ki67 labeling index is high in cases of dysplastic lesions and increases by grade, it can also be high in areas of regenerative change.
Analysis of normal and dysplastic gallbladder epithelium has shown that allelic losses of chromosome 8p occur in normal mucosa.31 In low-grade dysplastic lesions, allelic loss of chromosome 3p is common and regarded as an intermediate change, which also corresponds to progressive loss of fragile histidine triad (FHIT) protein in dysplastic lesions with increasing grade. Loss of heterozygosity of 5q has been found in various grades of dysplasia.50 Oncogenic mutations of the KRAS gene is uncommon in upper biliary (gallbladder and proximal bile duct) dysplasia.51,52 Telomere shortening is very common in dysplastic lesions, similar to invasive carcinomas, and it is typically absent in normal epithelium.53 However, metaplastic changes also commonly show telomere shortening. Various other molecular alterations are being evaluated.33
Natural History and Prognosis
Compared with a tumoral dysplastic neoplasm (i.e., ICPN), carcinomas arising in flat dysplasia may be biologically and prognostically distinct.10 Prognostic data are limited for dysplasia cases not accompanied by invasive carcinoma. The only information available is from the Surveillance, Epidemiology, and End Results (SEER) database sponsored by the National Cancer Institute (NCI), which is itself limited by the lack of pathologic review or standardized sectioning of specimens. Nevertheless, a recent analysis of the cases recorded as “carcinoma in-situ” in the SEER data revealed age-adjusted survival estimates of 5- and 10-years as 87% and 79%.54 It is presumed that the early mortalities represent missed invasive carcinomas and the late ones represent field effect phenomenon (development of new cancers in the biliary tract).54,54a
Data from high-risk geographic regions such as Chile and Southeast Asia have shed light on this topic. Cases classified as early gallbladder cancer by entire sampling of the gallbladder (which encompasses minimally and superficially invasive carcinomas) have a very good prognosis, with 10-year survival rates close to 90%.9
Rokitansky-Aschoff sinus involvement is a strong predictor of recurrence. Some groups advocate a more radical operation for cases with early cancer involving Rokitansky-Aschoff sinuses.9 Recurrences have developed in patients several years after cholecystectomy, which suggests a field effect involving the remainder of the biliary tract. Overall, dysplastic lesions are mostly clinically inconsequential, but they may be early evidence of a field effect that places the remainder of the biliary tract at risk for neoplasia.54,54a Appropriate clinical management for patients with a cholecystectomy specimen that reveals dysplasia without invasive carcinoma has not been defined. Involvement of the cystic duct margin by high-grade dysplasia raises concern about the status of the distal cystic duct and other extrahepatic bile ducts.
Adenocarcinoma
Most adenocarcinomas of the gallbladder are the pancreatobiliary type, showing morphologic, immunophenotypic, and biologic similarities to pancreatic ductal adenocarcinomas and carcinomas of the bile ducts.
Clinical Features
Most adenocarcinomas of the biliary tract occur in the gallbladder; their incidence ranks fifth among all GI cancers.55 Gallbladder carcinomas occur predominantly in elderly patients, with a mean age of 65 years, and they are three times more common in women than men.8,55a,56–58 They are uncommon in children; the youngest age reported is 9 years.59 In patients younger than 20 years, the possibility of hyperimmunoglobulin M syndrome should be considered.
Gallbladder carcinoma is significantly more common in Latin American countries (i.e., Chile, Mexico, and Bolivia) and among Native Americans. The highest incidence rates in the United States are found in the southwestern, north central, and Appalachian regions. The association of gallbladder adenocarcinoma with prior chronic inflammation has been well established, mostly by epidemiologic data showing a high incidence of gallbladder cancer in populations with a very high incidence of gallstones (particularly cholesterol stones) or cholecystitis, such as Native Americans.55a,60 This association is strong enough to indicate elective cholecystectomy as a preventive measure for some patients. However, the overall incidence of gallbladder cancer among patients with gallstones is 0.2%.
A long-term Salmonella carrier state has been implicated as a risk factor for gallbladder cancer, but this issue is still being debated. The highest risk is for patients with an incomplete porcelain gallbladder (i.e., hyalinizing cholecystitis with minimal or no calcifications).8 Primary sclerosing cholangitis and anomalous union of the pancreatobiliary ducts are also risk factors.61,62
The most common symptoms are upper quadrant pain and fever. Laboratory abnormalities include increased serum alkaline phosphatase levels. At clinical presentation, many patients with gallbladder adenocarcinoma have cholecystitis. Because their gallbladders usually contain gallstones, the organ is typically removed through a routine cholecystectomy (including laparoscopic cholecystectomy) with the clinical intent of treating cholecystitis and cholelithiasis. In fact, more than one third of gallbladder carcinomas are “clinically unapparent” (i.e., detected incidentally).4a
Many of these cases are hard to identify on gross examination,1–3,63 and therefore, if any evidence of dysplasia or carcinoma is detected in random sampling of the gallbladder, extensive sampling has to follow for proper identification and staging of the cancer. Routine sampling of the cystic duct margin is important, especially considering that clinically unsuspected gallbladder carcinomas tend to be located more often in the neck region.64
Pathologic Features
Between 50% and 75% of gallbladder adenocarcinomas arise in the fundus, with a smaller proportion occurring in the body (30%) and neck (10%). They are firm, white, gritty, and ill-defined tumors that typically grow diffusely and have a propensity to infiltrate surrounding tissues in an insidious fashion (Fig. 38.25). It is often difficult to distinguish carcinoma from chronic cholecystitis. Even on careful macroscopic evaluation of the gallbladder, more than one third of advanced gallbladder carcinomas (pathologic [p] T2 stage or higher) are missed at the time of gross examination. This figure increases to 70% for superficial cancers.1–3
Some cases have a more polypoid appearance and are often associated with a noninvasive intraepithelial component (i.e., ICPN component) (see Fig. 38.11). They often appear tan, granular, soft, and friable. Tumors often become detached from the surface, and because they are often admixed with necrotic material, they may be disregarded as debris by the prosector.
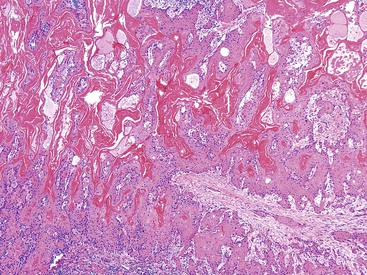
Carcinomas that arise from hyalinizing cholecystitis warrant special attention. Most invasive carcinomas in this setting are difficult to recognize grossly because they often are hidden within the thin, uniform band of the fibrotic wall of the gallbladder. However, some form subserosal or subhepatic nodular infiltrates, whereas the gallbladder wall remains a thin, rigid band (Figs. 38.26 and 38.27).8
Uncommon carcinoma types exhibit subtle differences in their gross appearances. In some cases, mucinous carcinomas are associated with acute cholecystitis and have a gelatinous appearance.56 Sarcomatoid and undifferentiated tumors often have a more fleshy appearance and may have a polypoid contour. Some signet ring (poorly cohesive cell) tumors have a linitis plastica pattern, in which the wall of the gallbladder and its layers are thickened but relatively well preserved.
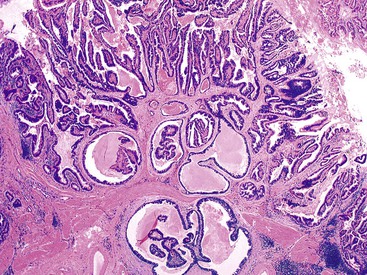
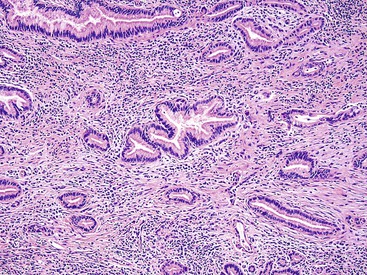
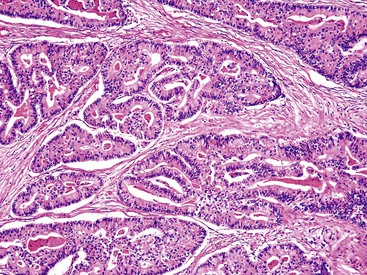
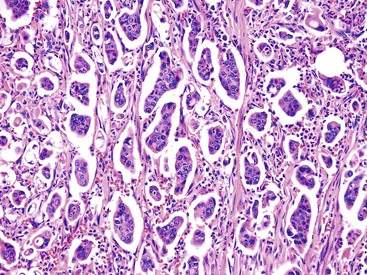
Microscopically, most gallbladder adenocarcinomas display features typical of pancreatobiliary adenocarcinomas (Figs. 38.28 through 38.30), such as widely separated, well-formed glands and small clusters of cells embedded in a densely fibrotic stroma (see Fig. 38.28).55a,65,66 Hyalinizing cholecystitis (i.e., incomplete porcelain gallbladder) often shows plaquelike lesions with glands embedded within thin bands of paucicellular sclerotic stroma (see Fig. 38.27).8 In most cases, the glands are simple, lined by cuboidal cells, and have dilated, round lumina. More complex structures, with cribriforming and papilla formation, may be found (see Figs. 38.29 and 38.30).
Architecturally, the glands are arranged in a random or haphazard pattern, which contrasts sharply with the organoid clusters of benign ductules normally located in the wall of the bile ducts. Often, the nuclear grade is high compared with the degree of glandular differentiation. The cytoplasm may be acidophilic and granular in some cases or pale to clear in others. Various amounts of intracytoplasmic and intraluminal mucin may be seen. In some cases, this is evident by routine histologic examination, and in others, it is demonstrable only by special stains. Goblet, Paneth and neuroendocrine cells may occur randomly in some tumors and are more common in carcinomas with an intestinal appearance.55a,67
Perineural and vascular invasion are common and can be prominent in some cases. Extensive intravascular spread through the subserosal or subhepatic vasculature can cause a plaquelike growth in perimuscular tissue. Tumor-infiltrating inflammatory cells may consist of various amounts of lymphocytes, plasma cells, neutrophils, and eosinophils. Follicular cholecystitis is associated with some tumors.
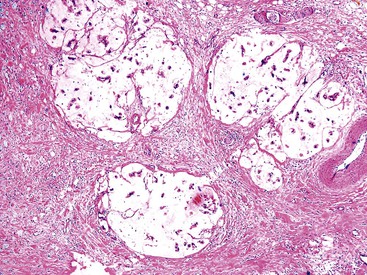
In addition to the characteristics previously described, adenocarcinomas of the gallbladder may exhibit unusual patterns of growth (Figs. 38.31 and 38.32) that typically are mixed with the conventional pattern. Some tumors have a fair amount of cytoplasm that may appear clear, foamy, or microvesicular (see Fig. 38.32).68 This pattern is typically associated with distinct cytoplasmic borders, an apical cuticle-like cytoplasmic condensation, and raisinoid or round nuclei with prominent nucleoli. Conversely, some well-differentiated tumors exhibit atrophic-appearing glands consisting of attenuated cells with a minimal amount of cytoplasm and small, bland-appearing nuclei, resembling a tubular carcinoma of the breast (Fig. 38.33). However, these benign-appearing cells often have nuclear grooves.
The well-differentiated appearance is commonly retained in metastatic sites. The degree of differentiation may become even more pronounced in some sites, such as the ovaries, where metastatic biliary carcinomas may form cystic tumors that are often mistaken as a primary ovarian mucinous tumor that is benign or borderline. Some metastatic biliary carcinomas in the lung resemble primary mucinous bronchioloalveolar carcinoma. Examination of some adenocarcinomas reveals substantial intraglandular architectural complexity, including papillary units and cribriform arrangements (see Figs. 38.29 and 38.30).
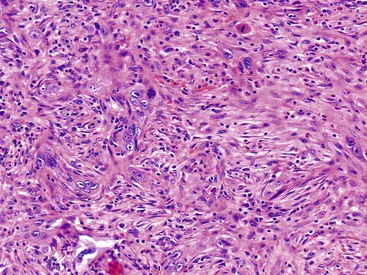
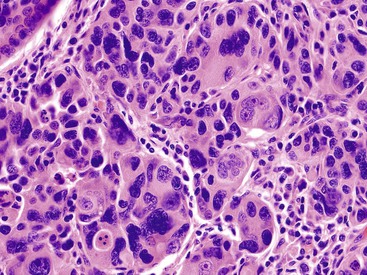
Poorly differentiated carcinomas may exhibit various patterns of growth. In some, carcinoma cells form cords or nests or may infiltrate as single cells (Fig. 38.34). A diffuse sheetlike arrangement may be seen in other tumors. Some are undifferentiated. Occasionally, carcinoma cells form compressed units with central clefts and acantholysis in a pseudoangiomatous pattern. Some cases have a characteristic micropapillary pattern (Fig. 38.35). Poorly differentiated adenocarcinomas may show significant pleomorphism, bizarre nuclei, and even multinucleated giant cells, which are true tumor giant cells (Fig. 38.36). Focal choriocarcinoma-like areas may be seen.
The histologic grading system used for gallbladder adenocarcinomas, as advocated in the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) classification system, is based on the degree of glandular differentiation. More than 95% is well differentiated, between 50% and 95% is moderately differentiated, and less than 49% is poorly differentiated.55a
Histochemical, Immunohistochemical, and Molecular Aspects
Adenocarcinomas of the gallbladder have various amounts of mucin that range from pale shades detectable only by mucin stains or molecular analysis of glycoproteins to scattered intraluminal globules that are easily identified by light microscopy. Stromal mucin deposition occurs in some cases. The mucin type more commonly associated with neoplasia is sialomucin, whereas benign reactive conditions tend to produce sulfomucin. In some studies, mucin core protein expression has been associated with a higher rate of metastasis and a worse prognosis.69
The immunoprofile of these carcinomas indicates a foregut type. Tumors are typically positive for CK7.70 CK20 may also be positive, but staining is usually focal. The latter pattern contrasts with intrahepatic (peripheral) cholangiocarcinomas, which tend to be negative for CK20. Several mucin-related glycoproteins and oncoproteins, such as CEA, MUC1, MUC5AC, TAG72 (B72.3), and CA 19-9, are expressed, although focally in some specimens.55a,65,66,71 For some markers, such as CEA and MUC1, there is a progressive increase in the level of expression from preinvasive to well-differentiated to poorly differentiated carcinomas, with dense intracytoplasmic expression detected mostly in advanced tumors.
Among oncogenic pathways, mitogen-activated protein (MAP) kinase pathway alterations (e.g., RAS, RAF, MAPKK, MAPK) are the most common, detected in two thirds of cases of adenocarcinoma.33 A BRAF mutation is identified in approximately one third of cases. The incidences of KRAS oncogenic mutation and loss of SMAD4 (DPC4) protein expression are far less common than in pancreatic tumors72 HER2 (ERBB2 or HER2/NEU) gene amplification is found in 70% of biliary tract cancers, but there is no correlation with prognosis. The potential role of HER2 in humans is supported by observations of transgenic mice that overexpress the Erbb2-encoded protein in the gallbladder epithelium and later develop cancer.
Among the tumor suppressor genes, TP53 alterations occur in almost one half of cases, and mutations in CDKN2A (also known as INK4, CDKN, or p16) occur in approximately one third. Decreased expression of the fragile histidine triad gene (FHIT) is very common, and upregulation of the maspin protease inhibitor gene (SERPINB5) appears to be an early event. Microsatellite instability is reported in as many as 10% of cases.
Alterations in other molecular pathways involved in tumor suppression, such as mitogen-activated protein kinase 3 (MAPK3, formerly ERK1), mitogen-activated protein kinase 1 (MAPK1, formerly ERK2), and phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3CA, formerly PI3K), and the cyclin D1 (CCND1)/CDKN2A/RB1 pathways have also been detected. Other molecular changes are being studied,33 and some of these alterations show geographic differences.60,73
Differential Diagnosis
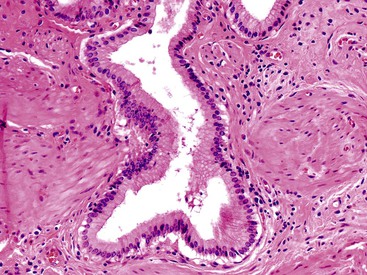
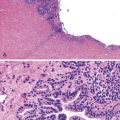
Most of the difficulties in distinguishing dysplasia from reactive atypia at the cytologic level also apply to differentiating invasive carcinoma from benign processes, because carcinoma cells can appear deceptively benign. Architecturally, well-differentiated adenocarcinomas often show exceedingly well-organized glandular formations, which can be difficult to distinguish from Rokitansky-Aschoff sinuses (see Figs. 38.12 and 38.13), peribiliary mucous glands in the neck and cystic duct region, or Luschka ducts (Fig. 38.37) in the subhepatic or subserosal tissues.74–76 Peribiliary mucous glands normally have a lobular architecture and are composed of uniform and evenly sized tubules (Fig. 38.38), whereas invasive carcinomas are typically composed of dispersed, haphazardly arranged tubular units (Table 38.3).
Table 38.3
Features Helpful to Distinguish Malignant Glands from Benign Peribiliary (Normal) Glands in Gallbladder and Bile Duct Specimens
| Feature | Benign Glands | Malignant Glands |
| Lobular arrangement of glands | ++ | − |
| Haphazard infiltrative pattern | − | + |
| Irregular glands, various sizes and shapes, angulated contours | − | + |
| Uniformity of glands | ++ | ± |
| Mitoses | ± | + |
| Necrosis (intraluminal) | − | + |
| Cytologic atypia (pleomorphism, hyperchromicity, loss of polarity) | − to + | + to ++ |
| Infiltrative single cells or clusters of cells | − | ± |
| Perineural invasion | − | + |
| Concentric periglandular fibrosis | ± | + |
| Carcinoembryonic antigen (CEA) positivity | ± (apical) | + (cytoplasmic) |
| TP53 positivity | ± (weak) | ± (strong) |
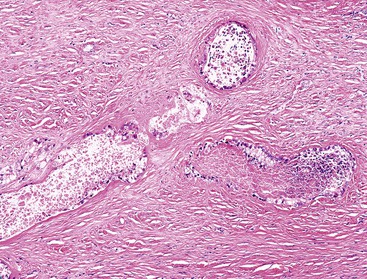
Rokitansky-Aschoff sinuses and adenomyomatous changes can be more difficult to distinguish from carcinoma, because they do not have a lobular arrangement and can be found in any portion of the gallbladder wall and surrounding adipose tissue.76 The epithelium of Rokitansky-Aschoff sinuses may be continuous with the surface epithelium, the sinuses are usually oriented perpendicular to the surface, they have an undulating contour, and they may appear flask shaped in the muscularis layer. Luminal bile can be seen. In contrast, malignant glands are usually smaller and often have open, round lumina or angulated contours, and they may be oriented parallel to the mucosal surface (see Figs. 38.31 through 38.33). Carcinomas are usually more densely packed than Rokitansky-Aschoff sinuses or adenomyomatous hyperplasia.
Stromal changes may not be helpful because adenomyomatous hyperplasia and Rokitansky-Aschoff sinuses are often associated with fibrotic, desmoplastic-like stroma (see Fig. 38.13). If these glands rupture because of increased pressure, the mucin in the stroma may induce reactive changes and an infiltrative appearance to the glands. Clear cell change and dense cytoplasmic chromophilia are more commonly seen in malignant glands. Nuclear enlargement, nuclear irregularities, hyperchromatism, loss of polarity, mitotic figures, apoptotic cells, intraglandular necrosis with neutrophils, open lumina, perineural or vascular invasion, and microvesicular cytoplasm with a distinct, thin apical chromophilic band are findings that favor carcinoma. Direct contact of glands with the muscularis points to invasive carcinoma, because Rokitansky-Aschoff sinuses remain surrounded by a layer of fibrotic stroma within the muscle layer. Subtle nuclear grooves can be helpful in recognizing extremely well-differentiated adenocarcinomas (see Fig. 38.33).
In some cases, proliferating metaplastic glands can exhibit dispersion accompanied by intervening stroma, creating a pseudoinfiltrative appearance, and they may impinge on nerves, mimicking perineural invasion. However, metaplastic glands typically form a band confined to the mucosa and superficial muscularis, rather than showing vertical spread. Luschka ducts (i.e., remnants of hepatic biliary ductules located on the outside of the gallbladder wall) (see Fig. 38.37) may have a highly proliferative atypical and pseudoinfiltrative appearance, especially in the setting of acute cholecystitis.75 However, this process often forms a discernible band confined to the subhepatic or subserosal surface of the gallbladder. Another challenging differential diagnosis is encountered in cases with hyalinizing cholecystitis (discussed later).
No molecular markers have been reliably useful in differentiating carcinoma from nonneoplastic changes in the biliary tract. TP53 protein overexpression, a high Ki67 labeling index, and dense cytoplasmic positivity for glycoproteins such as CEA and MUC1 are significantly more common in carcinomas than in benign conditions.55a,65,66 However, the high degree of overlap prevents effective use of these markers in daily practice.
Natural History and Prognosis
Gallbladder adenocarcinomas are highly aggressive neoplasms, with a prognosis only slightly better than for pancreatic cancer.55 The overall 5-year survival rate is approximately 10% to 20%.55 Stage is the best predictor of outcome. Unfortunately, most patients have high-stage tumors at clinical presentation, and the tumor has already invaded neighboring organs or has metastasized to the liver or regional lymph nodes. In studies in which T2 carcinoma (invasion through tunica muscularis) has been ruled out by total sampling, early gallbladder cancer (Tis, T1a or early T1b) has a very good prognosis, with 10-year survival rates approaching 90% (Table 38.4).9,77
Table 38.4
American Joint Committee on Cancer Staging for Gallbladder and Cystic Duct Cancers*
| Stage | Definition |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1a T1b |
Tumor invades lamina propria Tumor invades muscle layer |
| T2 | Tumor invades perimuscular connective tissue; no extension beyond serosa or into liver |
| T3 | Tumor perforates serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts |
| T4 | Tumor invades main portal vein or hepatic artery or invades two or more extrahepatic organs or structures |
* Sarcomas and well-differentiated neuroendocrine neoplasms (carcinoid tumors) are not included.
Data from Fritz AG, Compton CC, Edge SB, Byrd DR, eds. AJCC Cancer Staging Manual. Part III, 7th ed. New York: Springer; 2011.
Management of early gallbladder cancer is controversial. Data suggest that if there is Rokitansky-Aschoff involvement, the risk of recurrence is higher,9 and this feature may warrant additional surgery. Some evidence indicates that gallbladder carcinoma may be a marker of involvement in the remainder of the biliary tract.
The 5-year survival of T2 carcinoma is reported to be 30% to 50% in the West, and >70% in Japan and Korea, presumably related to definitional differences. Surgery is the only hope for cure, which is unfortunately uncommon for advanced-stage carcinomas.78 Radical surgery involving lymphadenectomy with right hepatic lobectomy is performed in resectable cases.79–82 However, most gallbladder carcinomas are incidental (clinically inapparent), diagnosed in cholecystectomies performed with the diagnosis of cholecystitis or cholelithiasis.4a Hepatic resection with lymphadenectomy may still benefit these patients if the tumor is relatively localized (pT1 or pT2). This method is controversial for more advanced (pT3) cancers.83 The type of surgery also is an issue of debate, with substantial differences in approach between Eastern and Western societies.83–85 Part of the controversy stems from different outcomes, which may be related to understaging resulting from a poor undersampling of the gallbladder.
There is a growing consensus regarding the role of adjuvant chemotherapy in the management of biliary tract cancer,86 but the chemotherapy protocol that is most beneficial is a subject of debate.87,88 Most major centers administer a chemotherapy protocol similar to that for pancreatic adenocarcinoma.87 The role of radiotherapy is also disputed.
Papillary Carcinomas
A papillary configuration can be seen in a variety of preinvasive and invasive neoplasms in the gallbladder. Historically, cases previously classified as papillary adenocarcinoma encompass a spectrum of tumors ranging from noninvasive lesions (now classified as ICPNs) to invasive cancers with a papillary configuration (see Figs. 38.2, 38.10, D, 38.29, and 38.30).10,14 An ICPN may contain invasive carcinoma (see Fig. 38.11).10 Preinvasive papillary neoplasms were discussed previously.
For ICPNs with invasive carcinoma, the prognosis depends on the amount of invasion. Tumors with minimal invasion have a very good prognosis, with 5-year survival rates of more than 90%.55a For any papillary tumor of the gallbladder, it is important to document the amount and characteristics of the invasive component separately (see Intracholecystic Papillary-Tubular Neoplasms). In some cases, the transition from the preinvasive ICPN component to invasive carcinoma is subtle, because the two components have overlapping cytoarchitectural features. It may be challenging to determine the proportion of the carcinoma that is invasive.
Invasive adenocarcinoma with papilla formation in the invasive elements should be classified as a well-differentiated invasive adenocarcinoma. This should not be confused with a carcinoma with a micropapillary pattern (see Fig. 38.35), which is composed of clusters of cells with cleft formation around them.
Mucinous Carcinoma
Gallbladder carcinomas often have intracytoplasmic or intraluminal mucin in the invasive glands. Stromal mucin deposition is seen in approximately 7% of gallbladder carcinomas.56 Approximately one third of these tumors qualify as mucinous carcinoma as defined by the WHO classification (>50% of the tumor has stromal mucin).16 Tumors are often large and manifest with an acute cholecystitis-like illness. Most are mixed mucinous types; they are mixed with other nonmucinous adenocarcinoma types or show abundant cells clinging to the stroma. Pure colloid-type mucinous carcinoma is exceedingly uncommon in the gallbladder (Fig. 38.39). Some are mixed mucinous–signet ring cell carcinomas, in which the cells floating in the mucin have a signet ring cell appearance, and there is individual signet ring cell infiltration in the stroma without stromal mucin. Calcifications may be prominent.89
Immunophenotypically, mucinous carcinomas show MUC2 positivity, which differentiates them from conventional gallbladder adenocarcinomas.56 They differ from intestinal carcinomas by an inverse CK7/20 profile and from pancreatic mucinous carcinomas by showing CDX2 negativity. Mucinous carcinomas also show a lack of MUC6 staining. Unlike GI mucinous carcinomas, they are microsatellite stable.
Mucinous carcinomas are typically large and advanced at the time of diagnosis. They exhibit more aggressive behavior than ordinary gallbladder carcinomas.56
Adenosquamous and Squamous Cell Carcinomas
Focal squamous differentiation is found in approximately 5% of gallbladder carcinomas.57 Unless the squamous component is substantial (>25% of the lesion), it is not necessary to include it in the diagnosis. If squamous elements constitute a substantial part of the tumor, the neoplasm is best classified as an adenosquamous carcinoma.57,90 Squamous elements may form separate foci, or they may be admixed with the glandular elements, in which case, squamous areas may appear benign (i.e., adenoacanthoma-like). Glandular and squamous components of the tumors have immunophenotypes that correspond to their respective areas of differentiation. For example, squamous areas often show nuclear tumor protein p63 (TP63) expression and positivity with high-molecular-weight keratins, whereas glandular areas express CEA and tumor-associated glycoprotein 72 (TAG72 [clone B72.3]).
Pure squamous cell carcinomas constitute less than 1% of gallbladder carcinomas.57 They often show overt and abundant keratinization (Fig. 38.40). They are usually associated with gallstones. Intramucosal components of the carcinoma may also be entirely squamous, or there may be squamous metaplasia. Mucin stains to identify foci of glandular differentiation may be performed to exclude an adenosquamous carcinoma with predominant squamous differentiation. Any glandular differentiation is considered sufficient for a diagnosis of adenosquamous carcinoma.
Similar to their counterpart in the breast, some squamous cell carcinomas have a spindle cell pattern and may be difficult to distinguish from high-grade sarcoma. In these cases, the finding of more conventional squamous patterns may be helpful. Immunostains may not be helpful because many spindle cell carcinomas are keratin negative, and conversely, some sarcomas may show weak keratin expression.
Squamous cell carcinomas tend to manifest at a higher stage.57 They have more adverse outcomes than ordinary adenocarcinomas, when compared stage by stage.