Chapter 5 Behavioural and developmental paediatrics
Long Cases
Anorexia nervosa
The eating disorders anorexia nervosa (AN) and bulimia nervosa (BN) are potentially lethal conditions, and two of the leading bio-psychosocial developmental disorders amongst adolescents, particularly females, in countries with Western culture. They occur predominantly in countries where there is abundant food, where the ultimate sought-after physique is a slim one and where society is orientated to achievement. Eating disorders are about 10 times more common in females than males. AN affects around 0.5–1% of adolescent females in the USA, while BN affects around 1–5%.
The aims of this long-case section are as follows:
• To give an overview of current management strategies employed in treating children and adolescents diagnosed as having AN (by correctly interpreted criteria of ICD-10 [World Health Organization: ICD-10 Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines, 1992] or DSM-IV [American Psychiatric Association: Diagnostic and standard manual of mental disorders, 4th edn, 1994]). This section predominantly follows the DSM-IV manual.
• To suggest a directed examination technique for detecting salient physical findings, and to guide the ordering of appropriate investigations that will enable accurate diagnosis of AN and its common complications.
• To give guidelines regarding need for hospitalisation and a review of the mechanisms of the irreversible, life-threatening complications of AN.
This long case deals exclusively with AN. The long case can be challenging, but offers a wide discussion base regarding management. As AN is the third most common chronic illness in adolescent girls in Australia, the UK and the USA, there are always long-term patients available in hospitals where examinations are held.
Background information
Risk factors that create susceptibility for the development of eating disorders include:
• Occurrence of eating disorders, substance abuse or affective disorders, in other family members.
• Obsessional, perfectionist personalities with negative self-evaluation.
• Co-morbid clinical problems such as depression, anxiety, obsessive–compulsive disorder, post-traumatic stress disorder and substance use disorders.
Inadvertent positive reinforcement can occur if the patient was initially overweight, and the initial weight loss was greeted with support for dieting efforts. Parents’ accidental tacit agreement by complying with demands of the eating-disordered child (buying diet foods, allowing vigorous exercise schedules) can reinforce the evolving ‘anorexic identity’.
AN is very much a misnomer. These patients are not anorectic; they have normal appetites. They just exert their control, as mentioned above, by refusing to take notice of their (initially) ordered physiology. If a child or adolescent labelled as having AN does complain of anorexia, and does not have body image distortion, then that patient needs to be assessed for an organic problem such as inflammatory bowel disease or occult malignancy (for the differential diagnosis of AN, see the short-case approach to weight loss in Chapter 8, Gastroenterology).
History
Current status
Behavioural symptoms: the A to F of AN
1. Activity: exercise (compulsive, at unusual hours, solitary, not part of competitive sport, prolonged duration, long-distance running, sit-ups or stomach crunches a favourite), constant movement, standing rather than sitting. Ask ‘How long do you exercise each day?’
2. Body image: negative comments (unhappy with thighs, abdomen, hips), frequent weighing of self, not happy with new lower weight, chooses baggy clothes to hide weight loss. Ask ‘What do you think about your weight? Are you average, skinny or overweight? What would be a healthy weight for you?’ Document how large the patient judges his or her body to be. Body checking: repeated weighing, pinching, measuring body parts, checking protrusion of particular bones, checking certain clothes fit, mirror gazing, comparison with others’ bodies. Body avoidance: the opposite to body checking, avoiding above, refusal to weigh, avoidance of mirrors. Minimising symptom severity. Shape concerns. Overvaluation of weight and shape in determining self-worth. Fear of weight gain. Disturbance in how their own body is experienced. Note that many young people choose not to share these concerns with a candidate because they are shameful emotions. Younger adolescents do not commonly express such concerns even to health professionals they know well.
3. Cognitive aspects: rigid thought processes, impaired judgement, obsessive thoughts, rigid beliefs (e.g. cannot eat this food with that food; cannot eat after specified time, such as 6 p.m., as will not digest food), ritualised behaviour associated with purchase, preparation and consumption of food.
4. Drug aspects: use of laxatives, diet pills, diuretics, stimulants, thyroxine, cigarette smoking, diabetic (T1DM) patients withholding insulin. Ask: ‘What [medicines] do you have?’
5. Eating behaviour: missing meals, eating very slowly (e.g. cuts peas in half), hiding food, feeding her or his food to pets, feigning eating (e.g. pushing food around plate), avoiding social events involving eating, self-induced vomiting after meals, spitting, prolonged fasting (over eight waking hours), strict rules about eating, avoidance of social eating, little variety in foods (extreme vegan diets, avoidance of fat), secret eating, cooking for others but not eating themslves.
6. Food intake: types of eating—restrictive (eating less), selective (limiting intake to a range of preferred foods), restrained (controlling type and amount of foods); refusal to eat foods perceived as fattening (e.g. meat, dairy products, fat, carbohydrates); high intake of water, diet drinks, vegetarian foods; claims ‘allergy’ to various foods; immediate guilt after eating (may induce vomiting, or exercise excessively).
Physical symptoms of AN
1. Current weight, height, age-appropriate BMI centile, %BMI calculated from matching centiles on growth charts or 50% centile BMI for age, rate of weight loss, fluctuations in weight, lowest weight, highest weight.
2. Amenorrhoea, oligomenorrhoea. Absence of at least three consecutive menstrual cycles. Ask: ‘Are you having regular periods?’ ‘When was your last period?’
3. Pubertal delay—Tanner staging required.
4. Lethargy, weakness, fatigue. Ask: ‘How are your energy levels now compared to when you were at a healthier weight?’
5. Constipation (including pretending to have this to obtain laxatives). Ask: ‘Have you had any laxatives for this?’ ‘How often?’
6. Hair and skin: hair loss, dry skin, purplish skin peripherally, oedema.
7. Circulation: dizziness, lightheadedness, near-syncope, palpitations. Ask: ‘Have you had any dizziness, fainting, shortness of breath, palpitations/feeling your heart going really fast, or any chest pain?
8. Sensitivity to cold. Ask: ‘Does cold weather bother you?’ ‘Do you feel the cold more easily than other people?’
9. Symptoms in post-pubertal adolescents: reduced libido, reduced hair and beard growth, and reduced waking erections in adolescent males.
Complications of AN
1. Acute: refeeding syndrome (the key issue is hypophosphataemia, hence the need for daily bloods to check for low phosphate and to check electrolytes).
2. Chronic: growth deceleration, pubertal arrest, bone loss (osteopaenia or osteoporosis) or failure of normal bone accretion. Depression can be part of the biological effects on the brain from loss of weight, as well as part of the pre-morbid personality issues. Always ask about suicidal ideation; the two commonest causes of death from AN in adulthood are complications of malnutrition per se and suicide.
3. Potentially fatal: severe hypokalaemia, hypophosphataemia leading to arrhythmias, congestive heart failure.
Current management of AN for inpatients
1. Multidisciplinary list of team: members (e.g. paediatrician or physician specialising in adolescents, psychiatrist, psychologist, dietician, occupational therapist, physiotherapist, social worker, nurse, teacher, art therapist, music therapist) together, with clarity about case management role; degree of engagement with patient (against wishes, perhaps) and parents; degree of success so far; whether the team addresses issues adequately in the opinion of patient/parents.
2. Procedures of management program: usual measurements made (how patient is weighed—e.g. in underwear with gown; frequency of weighing), whether bladder scan is done (to subtract volume of urine from weight—note that this is inaccurate if patient drinks just prior to scan) or urine specific gravity checked (to detect water loading) unless salt loaded; whether there is a target weight known by patient; how height is checked; how often blood tests are performed; whether bone density is checked and how often; whether calcium supplements are given, whether the team has a target weight for the patient.
3. Approach to weight gain: eating meals and snacks provided; need for nasogastric tube, now or earlier in the admission; prescription of psychotropic medication during this admission.
Social history
Disease impact on siblings
Sibling rivalry, hostility, support, similar disordered thoughts developing.
Examination for anorexia nervosa
The approach given in Table 5.1 assesses patients with AN for disease severity and current status. It omits the various negative findings that would be relevant in a patient in whom the diagnosis was only suspected, but not yet proven. For the differential diagnosis for AN, see the short case on weight loss in Chapter 8 (Gastroenterology).
| 1. Introduce self |
| 2. General inspection |
| Parameters |
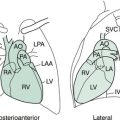
PCM = protein calorie malnutrition; SVC = superior vena cava.
Monitoring of disease
Not many routine tests are done in AN.
2 Documentation of disease progression
Progression is documented at intervals of 6 months to 1 year. The following may be done: bone density checked using DEXA (dual energy X-ray absorptiometry) machine (at 12 monthly intervals; Medicare only reimburses annually); gonadotropin levels (low); follicle-stimulating hormone (FSH); luteinising hormone (LH); oestradiol (low); pelvic ultrasound (ovarian shrinkage, few follicular cysts).
Management
Hospitalisation
Indications for admission to hospital are as follows:
(mnemonic: POLICE WT, police weight)
O. Obvious (over 5%) dehydration/Oxygen saturation < 85% (exceedingly rare)
Monitoring parameters
A target weight must be calculated for each patient, based on their height (measured with a stadiometer), their pre-morbid weight, their current weight (many units weigh patients in their underwear, wearing a gown, post-passing urine in the toilet, and before eating/drinking, checking for hidden weights and performing an ultrasound of the bladder to subtract the volume of urine), their previous maximum weight and their minimum weight, and their Tanner staging. The target weight may need ongoing revision, until epiphyseal fusion. Some patients, when they become aware of their target weight, have the uncanny ability to produce that number when on the scales. For this reason, many units do not specify the target weight, but merely indicate whether there has been a gain or loss, and the degree of that change.
Prognosis
In comparison to adults with established AN, adolescents with AN generally have a more optimistic course with about two thirds making a complete recovery. Those with a shorter duration of illness at the time of accessing clinical services (e.g. less than 1 year) generally have a better outcome.
Attention deficit hyperactivity disorder (ADHD)
Introduction
The aims of this long-case section, as well as of the subsequent short-case section, are as follows:
• To give a practical guide to the management of children diagnosed as having ADHD (by correctly interpreted criteria of ICD-10 [World Health Organization: ICD-10 Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines, 1992] or DSM-IV [American Psychiatric Association: Diagnostic and standard manual of mental disorders, 4th edn, 1994]). This section predominantly follows the DSM-IV manual.
• To enable diagnosis of conditions that can be misinterpreted as ADHD.
• To enable the candidate to define not only that symptoms are present (to fulfil criteria) and to exclude relevant differential diagnoses, but also to define the developmental predicament—which areas of development are ‘handicapped’ because of ADHD self-control problems.
• To provide clear guidelines regarding the use of stimulants, including dosage and timing recommendations, side effects, contraindications and appropriate follow-up.
• To enable the candidate to articulate a long-term management program based on routine regular visits to the paediatrician, developmental and mental health optimisation, and a plan to eventually get the child off medication (akin to epilepsy management). The candidate needs to understand that ADHD is usually lifelong neuropathology; hence there needs to be a long-term management structure such as we would regard as routine for other chronic conditions such as diabetes or CF.
Background information
Few diagnostic labels have caused as much media controversy and divergence of opinion as ADHD. Despite different rates of diagnosis in different countries, similar prevalence rates are reported across different cultures when standard diagnostic criteria are applied rigorously. Traditionally, ADHD is very commonly diagnosed in the USA but remains less frequently diagnosed in the UK. Diagnosis rates within Australia are somewhere in between. There is still uncertainty as to whether ADHD represents the dysfunctional end of a continuum of normal temperamental characteristics or whether it represents a discrete qualitatively different biological or psychological condition. The name for this behavioural syndrome has changed over the years, from labels that were unpopular with parents (‘minimal brain damage’) to ‘minimal brain dysfunction’, ‘hyperkinetic child syndrome’, ‘minimal cerebral dysfunction’, ‘psycho-organic syndrome of childhood’, to attention deficit disorder (ADD) and finally ADHD, which has been the most widely accepted term.
• Symptoms of inattention that are maladaptive and inconsistent with developmental level.
• Symptoms of hyperactivity–impulsivity that are maladaptive and inconsistent with developmental level.
• Age of onset criteria that specify that symptoms need to be present before age 7 (school age in the USA).
• Some impairment from the symptoms present in at least two settings (e.g. at school and at home).
• Clear evidence of clinically significant impairment in social or academic functioning.
• The symptoms must not occur exclusively during, or be better accounted for by, a pervasive developmental disorder, a psychosis or another mental disorder.
The aetiology of ADHD is likely to be multifactorial, a combination of genetic, biological and environmental aspects. Twin studies suggest that 75% of the cause of ADHD is genetic. Parental ADHD increases the risk of ADHD in the child eightfold. Researchers have been trying to find a candidate gene for ADHD. The most recognised gene association is the 7-repeat allele of the D4 dopamine receptor gene (DRD4∗7); agonists at the D4 receptor site include dopamine, adrenaline and noradrenaline. Independent of parental ADHD, exposure to cigarettes and alcohol in utero increases the risk of ADHD.
Co-morbid diagnoses include the following:
• Specific learning difficulties (SLD).
• Conduct disorder (repetitive, persistent pattern of behaviour, where the basic rights of others or major age-appropriate societal norms or rules are violated; see DSM-IV).
• Oppositional defiant disorder (ODD, a pattern of negativistic, hostile, defiant behaviour; see DSM-IV).
The frequency of diagnosis varies between countries. In some states of the USA, over 25% of boys are taking stimulant medication for ADHD. In Australia, it has been estimated to affect between 1 and 5% of the population. Difficulty arises in diagnosis because the condition is considered as a delay in maturation of normal learning processes. Poor short-term memory, lack of concentration, impulsiveness and hyperactivity are all normal in a child at 3 years of age. By age 5–7, however, these characteristics are usually controlled. This is the reason why many kindergarten and primary school teachers describe these children as immature. Approximately 95% of children will be capable of controlling these characteristics by 5–7 years of age. Although parents, teachers and health professionals seem quite comfortable with the concept of a spectrum of developmental motor function (some normal children walk at 10 months, others at 18 months), any apparent delay within the spectrum of the (normal) development of learning engenders great concern. It should help to remember that the brain is continually growing, doubling in size in the first 12 months of life, with neuronal growth occurring throughout adolescence and adult life. Obviously, this growth and development will not occur at the same rate in all children.
Recent developments in ADHD management
Recent evidence-based data has emerged that is challenging the way this condition has been managed previously. Important studies have been released, which have called into question several aspects of typical stimulant prescribing and use. There has been debate for years as to whether short-term effects of stimulants lead to any long-term benefits; so far there has been a paucity of well-designed studies to answer this issue. Some studies show academic improvement in the short term, but benefits gone by 3 years; some show no effect of stimulant use on academic outcomes, and some have found long-term academic gains, particularly in mathematics.
The spread of this disquieting new information, that stimulant medications, specifically methylphenidate, are associated with a six- to seven-times increased risk for sudden death in children and adolescents, was not slowed by the FDA’s statement. The study’s authors noted that sudden death was a rare event even among children who take stimulants; however, they concluded that careful assessment is necessary when prescribing these medications.
H. Hypertrophic cardiomyopathy (HCM)
E. Ex-cardiac surgery (esp. TGA, TOF, HLHS, AS, ASD, AV Canal)
P. Pulmonary hypertension/Primary ventricular fibrillation or tachycardia
See Chapter 6 (Cardiology) for more details. Candidates need to be informed of these controversies around the potential cardiovascular adverse side effects of stimulants.
The Royal Australasian College of Physicians (RACP) and the National Health and Medical Research Council (NHMRC) made available draft Australian ADHD Guidelines on 30 November 2009. These draft guidelines contain evidence-based information covering all aspects of ADHD. In particular, this draft pointed out that there was insufficient positive evidence to support the use of pharmacological therapeutic agents in preschool children diagnosed with ADHD, except in severe cases. Two short-term studies were identified that addressed the use of stimulants in preschoolers; one showed improvements in core ADHD symptoms, but no significant improvement in measures of compliance and attention tasks; the other produced reductions in ADHD symptoms, but an increased number and type of side effects compared to older children. No studies met inclusion criteria that addressed the long-term safety and efficacy of stimulants medications in preschoolers. Two studies were noted that reported on adverse outcomes in preschoolers receiving methylphenidate; these included decreased appetite, nightmares, feeling sad/unhappy, trouble sleeping, weight loss, emotional outbursts and social withdrawal. Candidates are advised to obtain a copy of this draft, and use it as the main reference for the management of ADHD, as it is very comprehensive and well written. It is available at www.health.gov.au/internet/ministers/publishing.nsf/Content/mr-yr09-nr-nr222.htm
History
Current symptoms
1. Inattention: failure to attend to details, careless mistakes in school work, difficulty sustaining attention in tasks or play activities, not seeming to listen, not following through on instructions, failing to finish school work, difficulty organising tasks, avoiding tasks requiring sustained mental effort, losing things necessary for tasks, easily distracted, forgetful in daily activities. Note duration.
2. Hyperactivity: fidgeting, squirming in seat, leaving seat when should be seated, running or climbing excessively, ‘on the go’, talking excessively.
3. Impulsivity: blurting out answers before questions completed, difficulty awaiting turn, interrupting or intruding on others.
Current management
Usual medication at home, stimulants (e.g. dexamphetamine, methylphenidate) used currently or previously, other drugs (e.g. antidepressants, clonidine), dosage regimen, side effects noted (e.g. stimulants may cause decreased appetite, insomnia, rebound moodiness, irritability or hyperactivity), any therapies (e.g. occupational therapy), school issues (e.g. any school assistance, where the desk is positioned in the classroom—hopefully not next to a hyperactive twin, or at the back where the teacher cannot see the child), psychosocial interventions (e.g. behavioural therapies, educational tutoring), professionals seen (e.g. local doctor, psychologists, paediatricians, psychiatrists), activities to promote concentration and self-discipline (e.g. martial arts), alternative therapies tried (e.g. homeopathic preparations), educational CD-ROMs.
Diagnosis of ADHD
Educational audiology assessment
An educational audiology assessment allows documentation of auditory processing, and may reveal difficulties with short-term auditory processing and hearing in association with competing background noise. A standard audiology assessment is of limited assistance.
Management
Correct diagnosis is essential for appropriate management. It is important to establish that drug therapy is not the only option for treating this condition, although stimulant medication has been proved more effective than any form of behavioural therapy. Adolescent patients may gain sufficient understanding of their difficulties that they do not require drug treatment, but rather develop strategies to cope. There are children who will not respond to basic behavioural management/assisted teaching techniques without the use of medication.
School strategies (educational management principles)
1. Inattention. Maximise attention by: (a) seating the child at front of class, near a good role model; (b) breaking longer assignments into smaller sections, so the child can see an endpoint to the work, and mastering material within his or her attention span; (c) pairing written instructions with oral instructions.
2. Impulsiveness. Counter this by: (a) complementing positive behaviour and increasing immediacy of rewards; (b) using time out for misbehaviour, avoiding lecturing or criticism; (c) supervising carefully at transition times.
3. Motor activity. Reduce overactivity by: (a) allowing the child to run errands that involve movement; (b) allowing the child to stand at times during schoolwork; (c) providing breaks between academic tasks.
4. Socialisation. Improve this by: (a) giving frequent praise to appropriate behaviour; (b) encouraging cooperative learning tasks with other children; (c) encouraging the child to take up an activity with supervised socialisation (e.g. sporting groups, scouts).
5. Academic skills. Overcome learning difficulties by: (a) organising remedial assistance for specific problem areas; (b) if mathematics is a problem, allowing a calculator, and providing additional time for maths; (c) if written language is weak, allowing non-written project submissions (using a tape recorder or a word processor).
6. Mood. Increase self-esteem by: (a) setting easily obtainable goals initially, then increasing the difficulty of tasks; (b) assisting the child to feel his or her contributions to the class are significant; (c) providing reassurance and encouragement.
7. Compliance. Increase compliance by: (a) seating the child near the teacher; (b) ignoring minor misbehaviour and reinforcing good behaviour; (c) recognising the positive behaviour of a child seated nearby.
8. Organisational planning. Help the child to follow instructions by: (a) checking homework assignments written down in a homework diary; (b) encouraging neatness but not criticising sloppiness; (c) encouraging the child to use notebooks with dividers and folders to organise schoolwork.
Medication
Stimulants
Short-acting: dexamphetamine, methylphenidate (MPH)
The most widely used are dexamphetamine and MPH. Dexmethylphenidate is the d-isomer of MPH and a newer preparation; it is more active than the l-isomer, has fewer side effects, requires half the standard MPH dose and lasts 4–6 hours. Dexamphetamine’s mechanism of action involves increasing extracellular synaptic dopamine, inhibiting noradrenaline reuptake and exerting weak effects on the serotonin system. Its average duration of action is 5 hours and its half-life is 3–6 hours. MPH’s mechanism is thought to involve selective binding of the presynaptic dopamine transporter (DAT) in striatal and prefrontal areas, with the effect of increasing extracellular dopamine levels, as does dexamphetamine. MPH also acts on the noradrenaline system by blocking the noradrenaline transporter; its average duration of action is around 3 hours and its half-life is 2–4 hours. The stimulant effect commences between 30 and 60 minutes after taking the dose. Both medications have similar side-effect profiles, and in head-to-head trials neither appears to have an advantage over the other; each works in around 70% of children diagnosed with ADHD. In children with co-morbid psychiatric disorders, stimulants may worsen the symptoms of the condition, especially in those with tic and mood disorders.
Dexamphetamine: dose range 0.2–1.0 mg/kg/day (usually 0.3–0.7 mg/kg/day; maximum 40 mg/day).
MPH: dose range 0.25–2.0 mg/kg/day (usually 0.3–0.7 mg/kg/day; maximum 60 mg/day).
Non-stimulants
Atomoxetine
This is a highly selective inhibitor of the presynaptic noradrenaline transporter, and is considered a first-line drug, as it is as effective as the stimulants. Its efficacy is based on three double-blind, placebo-controlled studies. It can be used when parents are opposed to stimulant medication. Animal studies show that it increases extracellular noradrenaline and dopamine levels in the prefrontal cortex. No serious adverse effects have been reported. It is metabolised by cytochrome P450 2D6; drugs that inhibit this (such as SSRIs) can increase levels and side effects of atomoxetine. Adverse effects include decrease in appetite, nausea, vomiting, fatigue, sedation, dizziness, mood swings and decreased growth velocity. The manufacturers have added a warning that atomoxetine can increase suicidality. Parents must be fully informed of all potential side effects, including this.
Tricyclic antidepressants (TCAs): imipramine, desipramine
These have been recommended as second-line options in the past, but now are rarely used. The likely mechanism of action of TCAs is on catecholamine reuptake. They may have potential benefits in children with ADHD and co-morbid mood disorders, anxiety disorders and tic disorders, but they have major potential cardiotoxicity. Children who have not been coping well with ADHD can develop low self-esteem and depression. Rather than initiate antidepressant medication, it is preferable to address the specific problems of the child. If co-existing depression, sadness or anxiety are severe, then antidepressants may be warranted, and they may clarify thinking and improve concentration. For ADHD without depression, antidepressants are rarely justified. As with clonidine, overdosages may be cardiotoxic (heart block and rhythm disturbances that, rarely, can be fatal). There have been reports of sudden, unexpected death in four children treated with desipramine. The author has encountered children admitted to paediatric intensive care units after an overdose of (inadvertent poisoning by) antidepressants prescribed for ‘ADHD’ in children as young as 3 years old has been taken by the patients themselves or by their friends or siblings, because no drug safety precautions were taken in the home. Antidepressants are much less popular with patients and parents than stimulants. Parents must be fully informed as to all the potential side effects of TCAs, including the cardiotoxicity.
Alternative treatments
The management of children diagnosed as having ADHD can be an emotional minefield. Parents at the end of their tether will try many and varied treatments, and any suggested ‘cure’ or quick fix will invariably lead some parents to try unorthodox treatments suggested by ‘practitioners’ of no fixed ability, representing a range of ‘therapies’. Challenging this approach may damage the doctor–patient/parent relationship. While unusual ‘therapies’ can ‘work’ in children who do not really have ADHD, the fact remains that stimulants are the most effective treatment, but as long as there are desperate parents, there will be charlatans who will make money from treatments that have no scientific basis. Most alternative treatments are ‘justified’ by the suggestion that drug therapy will not be necessary; universally, their theoretical justification is inconsistent with current scientific knowledge. Included within the many non-traditional approaches are: optometric training, tinted lenses, megavitamins, patterning, kinaesthesiology, homeopathic substances to counter ‘allergens’, and sound therapy. Most of these therapies have not undergone controlled clinical trials, and their use is based on anecdotal testimony. The disadvantages to these approaches include high cost, wasting valuable time, delay in commencing recognised treatments, and damage to self-esteem caused by suggestions that the child’s eyes, brain or ability to handle food are abnormal.
Autistic disorder (autism)
There are few medications that are useful in autism. Exceptions are methylphenidate (MPH), risperidone and selective serotonin reuptake inhibitors (SSRIs). MPH can decrease hyperactivity, but this can be at the expense of increased social withdrawal and irritability. Risperadone can improve behaviour, but its use is limited by adverse effects (weight gain, drowsiness, prolactinaemia and tremor). There is consensus that SSRIs may improve symptoms in ASD, although there are no RCTs, but this can be at the expense of increase in agitation, hostility and even suicidal ideation.
The aim of this long-case section is as follows:
• To give a practical guide to the management of children diagnosed as having autistic disorder/autism (by correctly interpreted criteria of ICD-10 [World Health Organization: ICD-10 Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines, 1992] or DSM-IV [American Psychiatric Association: Diagnostic and standard manual of mental disorders, 4th edn, 1994]). This section predominantly follows the DSM-IV manual, and discusses the classic autistic disorder. It does not cover the milder ‘variants’ of autistic disorder, such as Asperger’s disorder/syndrome (intellectual ability within the average range and, superficially at least, normal early language development: there are a multiplicity of varying criteria for this depending on which ‘authoritative’ source is read) or pervasive developmental disorders (PDD) in the DSM-IV. The term ‘autistic spectrum disorder’ (ASD) is widely used now to encompass autistic disorder, Asperger’s disorder, atypical autism and pervasive developmental disorder—not otherwise specified (PDD-NOS), but it does not appear in the DSM-IV. Within the PDD classification in DSM-IV are included Rett disorder and childhood disintegrative disorder.
• To enable diagnosis of conditions that can be misinterpreted as autism (which can be a pitfall in the environment of increased diagnoses of ‘ASD’).
• To clarify which potential aetiological factors have some evidence, and which do not but receive adverse publicity, to counter much misinformation in the lay press.
• To give clear guidelines regarding therapies for which there is evidence of efficacy and therapies for which there is insufficient evidence to support their use. Autism is an area in which ‘complementary’ and ‘alternative’ medical therapies (CAM) are widely used. While parents appreciate their doctor’s support for their use of their chosen CAM, it is also important to caution them against therapies that could have significant detrimental effects on their child’s health (and on their finances).
Background information
• Symptoms of impaired social interaction. These can include impaired non-verbal behaviours (e.g. eye-to-eye gaze), failure to develop peer relationships, lack of seeking to share (e.g. interests) and lack of emotional reciprocity.
• Symptoms of impairment in communication. These can include delay in development of spoken language, impaired ability to initiate or sustain a conversation (in those with adequate speech), stereotyped use of language and lack of make-believe play.
• Symptoms of repetitive behaviours and stereotyped behaviour patterns. These can include preoccupation with restricted patterns of interest, inflexible adherence to specific rituals, stereotyped repetitive motor mannerisms and persistent preoccupation with parts of objects.
• Delays of abnormal functioning in at least one of the following areas, with onset before 3 years: (a) social interaction, (b) language as used in social communication or (c) symbolic or imaginative play.
• Disturbance not better accounted for by Rett disorder or childhood disintegrative disorder.
The criteria set out in DSM-IV should be fulfilled before a diagnosis of autism is entertained.
• at 6–9 months, poor eye contact, absent facial expression, decreased social smiling, delayed babbling;
• at 9–12 months, less turning to name, not following a point when adult indicates and points to an object, tuning into environmental sounds more than human voice, infrequent babbling;
• at 12–15 months, absence of any words, lack of pointing, lack of showing objects of interest to others, failure to follow simple verbal command with a gesture, failure to wave bye-bye;
• at 15–18 months, lack of imitation, lack of engagement with other people, loss of previously acquired words, decreased variety of play, limited symbolic play.
• no pointing, no pretending, no perception (of others);
• no taking turns, no taking interest (in others), no taking part (in games);
• no speech, no sharing (pleasure), no starting games, no social play;
• unable to communicate non-verbally, unusual mannerisms, unusual reactions to sensory stimuli.
• cannot follow games, cannot follow trends (classroom ‘norms’; overly critical of teachers), cannot follow social cues (in relation to adults, too intense or no relationship at all), cannot follow social ‘norms’ (overwhelmed by social situations);
• cannot cope with altered environments (unstructured situations such as school camps), or large/open environments (not sure how to organise self in unstructured space, hugging perimeter of sports fields), or a totally new environment (cannot cope with change).
• difficulty making/keeping friends in peer group (gets on more easily with adults or younger children), difficulty ‘getting’ jokes (taking things literally, does not ‘get’ sarcasm or metaphor), difficulty adapting style of communication (can be too formal or, conversely, too familiar);
• rigid thinking (lacking imagination), rigid behaviour (ritualistic, repetitive);
• unaware of personal space invasion, unaware of peer group ‘norms’;
• talking ‘at’ rather than with, talking with unmodulated (flat), repetitive speech pattern, talking about very limited range of topics of own interest only.
Aetiology
Autism can be the endpoint of a number of disorders: fragile X syndrome, tuberous sclerosis, Angelman syndrome and Down syndrome can cause children to have symptomatology that fulfils the criteria of DSM-IV. Under 25% of autistic children have a demonstrable recognisable aetiological disorder. Twin studies demonstrate a significant genetic component. Monozygotic twins have 60% concordance for classic autistic disorder, and up to 90% for ASD. Dizygotic twins and siblings have a 10% concordance for classic autism, and 30% for other developmental issues, including personal/social issues as well as speech and language delays. Over the decades, many theories have attempted to explain the thought processes apparent in autism. In terms of cognitive explanations, there are currently three main theories, discussion of which is beyond the scope of this section: ‘executive dysfunction’ (inability to plan, inability to inhibit socially inappropriate responses, inability to flexibly shift attention), ‘weak central coherence’ (inability to integrate pieces of information into a meaningful whole) and ‘lack of theory of mind’ (lack of the ability to attribute feelings and belief systems to others and appreciate they are different to one’s own).
History
Current symptoms
1. Impaired social interaction: impaired non-verbal behaviours (level of eye contact, cuddling family members, hugging, facial expressions, body posture, gestures used), any friends of similar age, any attempts at sharing of interests, enjoyment, achievements, show-and-tell at preschool, showing toys to friends and family, bringing or pointing out objects of interest, any demonstration of empathy, social or emotional.
2. Impaired communication: current level of spoken language, ability to converse with another, quality of language, any unusual patterns of speech quality and content, including echolalia, repetition, pronoun reversal and semantic pragmatic language disorder.
3. Behaviour patterns: preferring own company, tendency to be a loner, ‘in his own world’, wandering aimlessly, preoccupation with specific areas of interest (e.g. able to talk only about Star Wars). Depends not on the presence of special interests, but on the level of handicap from the time spent with and intrusion on routines and family members. For the less able, may involve primary sensory stimulation; for example, fascination with noises, lights, patterns, and movements of objects or part objects. The underlying deficit is a lack of reciprocity in social interaction, communication and imagination.
4. Most problematic behaviours at present (e.g. rituals, anxiety, aggression, self-injury) and the impact of these on family, educational facility (e.g. special school), therapists and carers.
Past history
• Language delay: for example, not responding to name, no babbling, no pointing or waving by 12 months, no sharing of interest in objects, no single words by 16 months, appearing deaf, no spontaneous (non-echolalic) phrases/word combinations by 24 months, loss of words, language.
• Lack of social interaction: for example, not smiling socially, very ‘independent’, treating others as object rather than person, lacking or only superficial interest in other children, lack of or restricted eye contact, seeming to be in ‘own world’, seeming to ‘tune out’, lack of cuddling, no development of expected social skills.
• Behavioural issues: for example, temper tantrums, uncooperative, overactive, lining things up, obsessions with certain things (such as water, wheels, mechanical devices), odd motor movements, not playing with toys, unusual attachments to objects, refusal to wear anything but favourite outfit (e.g. dressing exclusively as Batman every day for many months), preoccupations, unusual sensory reactions, toe walking.
Family history
Any family history of ASD (recurrence is 10–20% in subsequent siblings). The genetic risk for a second child with autism is 5%. The genetic risk for a broader spectrum of ASD is greater.
Diagnosis of autism
Several evaluation tools can be used. Autism may be suspected by routine developmental surveillance (see below). The next step involves a number of autism-specific screening tests (see below). The traditional tool for developmental screening, the Denver-II (DDST-II, formerly the Denver Developmental Screening Test—Revised), is regarded as being insensitive and lacking in specificity. Similarly, the Revised Denver Pre-Screening Developmental Questionnaire (R-DPSDQ) is not recommended for primary-care developmental surveillance.
The differential diagnoses are listed in the short-case section.
Screening tests and diagnostic instruments
Autism-specific screening tests
• Checklist for Autism in Toddlers (CHAT; administer at 18 months).
• Modified Checklist for Autism in Toddlers (M-CHAT; administer at 24 months).
• Autism Screening Questionnaire (for children age 4 and over).
• Social Communication Questionnaire (SCQ; there are two of these, one for those under 6 years, the other for those 6 years or over).
• Developmental Behaviour Checklist (DBCL; administer at age 4 or older).
• Australian Scale for Asperger’s Syndrome (for older verbal children).
• Social Responsiveness Scale, a questionnaire to aid diagnosing the broader spectrum of ASD, including Pervasive Developmental Disorder.
Investigations
Blood tests
1. High-resolution chromosomal analysis (karyotype).
2. DNA probe for fragile X syndrome (3–25% of fragile X syndrome children have ASD). Molecular testing shows a trinucleotide repeat (CGG repeats) expansion and methylation pattern in the FMR1 gene.
3. Specific genetic testing for:
4. Serum lead levels for lead toxicity (especially if there is a history of pica, or living in an old house with lead-containing paint).
5. Serum amino acids to detect phenylketonuria (PKU).
6. Serum 7-dehydrocholesterol to detect Smith–Lemli–Opitz, if this is suspected.
Management
Management can be divided into seven groups of headings, with the mnemonic SPECIAL:
S. School-based special education (for children over 3 years)
P. Pharmacotherapeutic intervention
Pharmacotherapeutic intervention
Four main groups of behaviours may show a positive response to medication, as follows.
1 ADHD-like behaviours
Here, the same medications may be tried as are used in ADHD:
• The stimulants dexamphetamine and methylphenidate have poorer response rates and greater side effects in those with intellectual disability and autism. Side-effect profiles are similar, with anorexia, weight loss (often 1–2 kg in the first 3 months), emotional lability, tics, insomnia, social and emotional withdrawal, and occasional psychotic reaction. Most side effects are dose-related. Both drugs have a good safety record. Neither is recommended in patients with marked anxiety, motor tics or a family history of Tourette syndrome (for a discussion, see the long case on ADHD).
• Atomoxetine and amitriptyline have a noradrenergic pathway effect and are often better for impulsiveness and aggression. These drugs have a different side-effect profile to that of stimulants.
• The antihypertensive clonidine, to reduce impulsive behaviour, aggressive tendencies and oppositional-defiant symptoms. Low doses are used in autism, as in ADHD (1–3 micrograms/kg/dose), and rarely affect blood pressure. Parents must be told all the significant side effects of clonidine, including the cardiotoxicity of overdosage, and the uncommon but recognised complication of sudden discontinuation, which results in rebound hypertension (again, for a discussion, see the long case on ADHD).
2 Ritualistic/compulsive behaviours
• Selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, sertraline, fluvoxamine and paroxetine, have been used in treating repetitive behaviours and aggression. Abnormalities of serotonin function have been noted in patients with ASD, and there is dysregulation of serotonin in obsessive–compulsive disorder. Although these drugs are selective, they can still cause serotonin syndrome, from an excess of serotonin. Symptoms may include aggression, agitation, autonomic dysfunction, tremor, sweating, chills, restlessness, confusion, impaired coordination, fever, hyperreflexia, myoclonus, coma and, very rarely, death. SSRIs are much safer than tricyclic antidepressants as regards cardiac side effects; tricyclics can cause prolongation of corrected QT interval, which can lead to fatal arrhythmias, but SSRIs have lower cardiotoxicity. SSRIs may cause behavioural activation (which can lead to an increase in impulsive self-harm) or a switch to hypomania.
4 Difficult behaviours (e.g. aggression, self-injury, temper tantrums)
• If aggression is related to impulsivity, then stimulants may be useful.
• If aggression is related to anxiety, then SSRIs or mood stabilisers (e.g. carbamazepine, sodium valproate) may be useful.
• If aggression is intractable, then the newer atypical antipsychotic drugs (e.g. risperidone) may be useful. Risperidone blocks postsynaptic dopamine and serotonin receptors. Side effects may include increased appetite, mild fatigue (usually short-lived), tremors and, upon discontinuing this drug after long-term use (over 6 months), mild, reversible withdrawal dyskinesias. Children receiving risperidone may show improvements in disruptive behaviours (including aggression, self-abuse, temper tantrums), plus improvements in degree of irritability, social withdrawal, hyperactivity and inappropriate speech.
Intervention
There is adequate evidence to support the short-term and medium-term benefits of early intervention. Intervention should begin as early as possible. Early interventions may take place in the child’s home, in preschools with support, in special playgroups and in child-care facilities, and are individualised. The time required is around 15–25 hours of specific therapy per week: the National Initiative for Autism Plan in the UK recommends that 15 hours a week should be available to all; some have suggested that 40 hours is required, but this adds financial constraints without definite benefit. Early intervention may include behaviour management strategies, early developmental education, communication therapy, speech therapy, occupational therapy, physiotherapy, structured social play and much parent training. Once the child is old enough to enter the school system, it is essential to find the best educational facility available for that child’s intellectual ability, language, communication and socialisation skills, and safety (many parents wish to avoid schools on main or busy roads, or near bodies of water).
Alternative treatments
• Chelation with dimercaptosuccinic acid (DMSA): oral preparation for lead poisoning. The hypothesis is that exposure to heavy metal accumulation from vaccines or the environment may be causing autism. No randomised controlled trials exist investigating chelation and autism.
• Vitamin B6 with magnesium. Vitamin deficiencies were found in disturbed children in one study in 1979. B6 is a coenzyme in several metabolic processes, including neurotransmitter synthesis. Early studies in the 1980s suggested a positive behavioural response; there are methodological flaws in all studies.
• Gluten-free diet. The hypothesis is that gluten (and casein) are absorbed through a ‘leaky gut’ and form exorphins, thus mimicking opioids that affect the brain; constant exposure leads to less endogenous opioid released during social interaction. Studies show no association between autism and coeliac disease or gluten challenge and behaviour. A gluten-free diet can further isolate already socially handicapped patients and families.
Learning/Links: useful websites
The following may be useful both for candidates and for parents of children with autism:
<www.autism-society.org>, <www.mrc.ac.uk>, <www.firstsigns.org>, <www.dbpeds.org>, <www.autismaus.com.au>, <www.health.state.ny.us/nysdoh/eip/autism>, <www.nas.org.uk>, <www.rcpsych.ac.uk/college/faculty/niasa.htm>.
Short Cases
Child suspected of having ADHD
A. Autistic spectrum disorder (ASD)/Anorexia nervosa (overactive to lose weight)
C. Cerebral palsy/Cerebral tumour (frontal lobe)/Cerebrovascular accident (CVA)
C. Conduct disorder/Congenital heart disease (cyanotic, 22q11, TAPVD, HLHS)
R. Reaction to social problems: marital discord/separation/divorce/new partner
A. Acquired brain injury (especially frontal lobe damage, e.g. post-MVA)/Abuse/Attachment/Adjustment/Anxiety disorders
T. Teratogens in utero (crack, cocaine, heroin, methadone)/Tourette syndrome/Twins (‘hype’ each other when together)/Tuberous Sclerosis Complex
E. Expressive language disorder/Encephalitis/meningitis sequelae
D. Deafness/Drug ingestion (illicit)/Diencephalic syndrome (hypothalamic tumour)
I. Impaired vision/Impaired hearing/Impaired speech/language/Impaired reading
A. Alcohol: fetal alcohol syndrome (FAS)/Alcoholic parents
G. Global developmental delay (intellectual impairment; various causes)
 Neurofibromatosis type 1 (NF-1)/NICU graduate (ex-premmie, esp. < 1500 g)
Neurofibromatosis type 1 (NF-1)/NICU graduate (ex-premmie, esp. < 1500 g)
O. Obstructive sleep apnoea/Obsessive–compulsive/Oppositional defiant disorders
S. Small for gestational age (SGA)/Smoking in pregnancy/Schizophrenia
I. Iron deficiency/Inborn errors of metabolism (e.g. phenylketonuria, PKU)
H. Hydrocephalus/Head injury/Haemorrhage (IVH/ICH in ex-premmie)
I. Intrauterine disorders: infections (TORCH)/teratogens (crack, cocaine, heroin)
G. Graves’ disease (hyperthyroidism)/Government benefits: faking ADHD to obtain
(parent instructs child to trash paediatrician’s office to prove he needs stimulants)
H. Hypothyroidism (‘dreamy’ inattention)/Hypoxic insult (drowning, strangulation)
• Lead intoxication/Leukaemia survivor (cerebral irradiation)/Lack of stable family
Y. Young/inexperienced parents (unrealistic beliefs: e.g. 12-month-old referred!)
D. Depression/Drug acquisition (faking ADHD to get dexamphetamine)
E. Epilepsy: childhood absence epilepsy (‘petit mal’) or temporal lobe absences
S. Sickness in sibling (handicap)/parent/caregiver; reactions to psychiatric/medical
diagnoses (e.g. mimicking father with bipolar disorder or schizophrenia)
I. Iatrogenic (phenobarbitone/beta-2-sympathomimetics/antihistamines)
R. Reaction to trauma (post-traumatic stress disorder)/Reaction to food/food additives
(e.g. caffeine, red colourings, certain preservatives, cheeses)
A. Attention seeking/Acting out/Absent dad: ‘Acute Dad Deficiency’
B. Bright but bored (i.e. gifted and curious)/Bipolar disorder
• Learning disability (e.g. semantic pragmatic disorder)
E. Environmental stressors at school or home (doing poorly in class; chaotic house)
Examination
3 Dysmorphology examination (head and neck at this stage, remainder of dysmorphic assessment as proceeding through neurological examination)
4 Head
Look for shunt (hydrocephalus), scars (surgery for trauma/tumours), bony defects (encephalocoele).
12 Developmental assessment
Mention to the examiners that there are standardised tests that may be useful, both to identify ADHD and to note co-morbid pathology. These include behaviour rating scales, psychometric profiles (e.g. Wechsler scales), reading test booklets, drawing tests such as the draw-a-person test (which gives an indication of general intelligence and also of any graphomotor disability) and the kinetic family drawing test (a drawing of the child’s family with each person doing something, which can reveal underlying psychopathology).
Child with possible autism/autistic spectrum disorder (ASD)
D. Deafness (hearing loss alone, or combined with visual impairment [can cause poor eye contact, stereotypic head nodding, hand/finger flapping])/Disordered mood (bipolar or unipolar mood disorders)/Down syndrome
I. Intellectual impairment (any cause)
F. Fetal (intrauterine) TORCH infections/Fetal alcohol syndrome (FAS)
E. Expressive language disorder
R. Rett syndrome (females almost exclusively)
 Neurodegenerative conditions/Neurocutaneous conditions (other than tuberous sclerosis, which has the strongest association, ASD also has been associated with neurofibromatosis type 1 [NF-1] and hypomelanosis of Ito)/Neglect (or abuse)
Neurodegenerative conditions/Neurocutaneous conditions (other than tuberous sclerosis, which has the strongest association, ASD also has been associated with neurofibromatosis type 1 [NF-1] and hypomelanosis of Ito)/Neglect (or abuse)
C. Cerebral palsy/Cerebral tumour/Childhood schizophrenia/Chromosomal syndromes (Smith–Lemli–Opitz, Smith–Magenis, Cohen, Joubert, Timothy, 15q and 16p duplication)
H. Hyperactivity with attention deficit disorder/‘Happy puppet’ syndrome (Angelman syndrome)
I. Inborn errors of metabolism (presenting with encephalopathy) (PKU)
• Landau–Kleffner syndrome (acquired epileptic aphasia)/Lead intoxication
Examination
A suggested order for the examination is as follows.
1 Introduce self to parent and patient
Note the child’s degree of eye contact (ASD children classically avoid eye contact), gain an impression of hearing ability, note quality of speech. Decreased vocalisation occurs in severe ASD, hearing impairment and Rett syndrome. In girls, look for any unusual pattern of respiration, such as periods of hyperventilation or breath-holding and midline hand wringing (Rett). Observe how the child interacts with the surroundings. Note any compulsive replacement of any objects moved by the child to their exact original positions (ASD).
4 Dysmorphology examination (head and neck at this stage, remainder of dysmorphic assessment as proceeding through neurological examination)
7 Speech
Evaluate quality and content (impression of whether age-appropriate). Return to this later.
10 Developmental assessment
Gross motor, personal–social, fine motor adaptive. Be aware that the Denver-II lacks specificity (see the long case). Check spoken language (chat about an area of interest for that child, such as trains, wheels). The draw-a-person test is a good clinical measure of IQ/conceptual development. Reading age can be clinically estimated by testing the length of word the child can read. In autism, reading recognition is frequently better than comprehension. A specimen of handwriting can be useful, as poor legibility may indicate poor fine motor organisation.
1. Mention that there are three main areas—(a) impairment in social interactions, (b) impairment in communication and (c) repetitive behaviours and stereotyped behaviour patterns—with a total of at least six items from (a), (b) and (c); at least two from (a) and one each from (b) and (c).
2. Mention the other criteria of delays in abnormal functioning before the age of 3, in at least one of the following areas: (a) social interaction, (b) language used as social communication or (c) symbolic or imaginative play.
3. Mention that the disturbance is not better accounted for by Rett syndrome or childhood disintegrative disorder.