Chapter 8 Behavior and Personality Disturbances
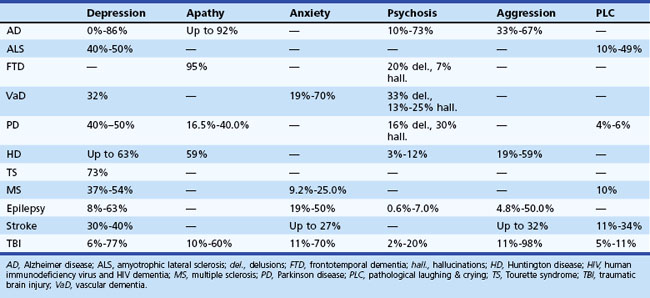
Behavioral and personality disturbances commonly occur in individuals with neurological disease or injury (Table 8.1). Identification and treatment of behavioral disturbances are critical because they are frequently associated with reduced functional capacity, decreased quality of life, and greater economic cost, caregiver burden, and morbidity. Dysfunction of various brain circuits, most notably the frontosubcortical and amygdaloid circuits, as well as psychological factors may contribute to increased rates of disturbances.
Frontosubcortical Circuitry
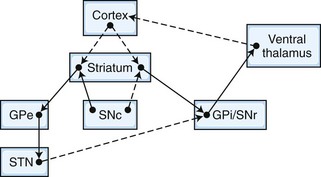
The frontosubcortical circuits provide a unifying framework for understanding the behavioral changes that accompany cortical and subcortical brain dysfunction. In the past 3 decades, a number of significant advances have been made in our understanding of the neuroanatomy, neurophysiology, and chemoarchitecture of the frontosubcortical circuits. An increasingly broad spectrum of neuropsychiatric phenomenology is now being interpreted in the context of dysfunction in this region. A brief overview of the frontosubcortical circuits and their signature behavioral syndromes is offered as a strategy to better understand the behavior and personality changes that accompany neurological conditions. Alexander and colleagues described five discrete parallel circuits linking regions of the frontal cortex to the striatum, the globus pallidus and substantia nigra, and the thalamus. These circuits consist of “direct” and “indirect” pathways. In general, the direct pathway facilitates the flow of information, and the indirect pathway inhibits it. The overall model for the frontosubcortical circuits can be observed in Fig. 8.1.
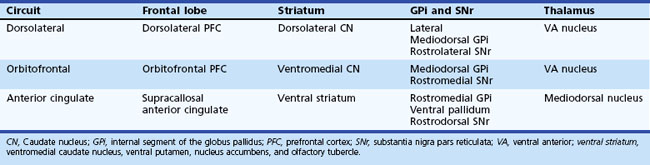
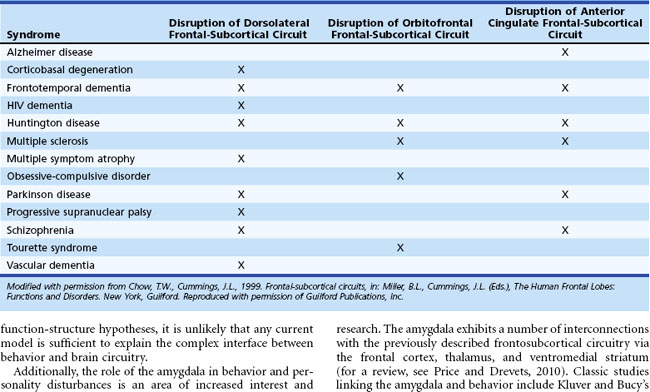
Five frontosubcortical circuits were initially described as motor, oculomotor, dorsolateral prefrontal, lateral orbitofrontal, and anterior cingulate gyrus. Table 8.2 gives descriptions of specific neuroanatomic pathways for these circuits. Efforts to link functional domains to this brain circuitry have been developed and revised over the past few decades. Disruption of dorsolateral prefrontal, lateral orbitofrontal, and anterior cingulate gyrus circuits is associated with behavioral and personality disruptions. Specific behavioral syndromes have been attributed to dysfunction in these circuits (Box 8.1) (Mega and Cummings, 2001). Disruptions at any point in the circuit (e.g., the frontal cortex, corpus striatum, globus pallidus) may result in alterations of behavior.
Box 8.1 Behavioral Syndromes Associated with Dysfunction of the Motor Circuits
Disruption of the dorsolateral circuit (Fig. 8.2) is associated with executive dysfunction, including poor planning and organization skills, memory retrieval deficits, and poor set shifting. Table 8.3 lists neurological disorders associated with disruption of this circuit. The orbitofrontal circuit (see Fig. 8.2) is associated with increased irritability, impulsivity, mood lability, tactlessness, and socially inappropriate behavior, whereas disruptions of the latter part of the orbitofrontal circuit may also result in mood disorder or obsessive-compulsive disorder (OCD) or both. See Table 8.3 for disorders associated with disruption of this circuit. Finally, the anterior cingulate gyrus circuit is associated with decreased motivation, apathy, decreased speech, and akinesia. See Table 8.3 for disorders associated with disruption of this circuit. Although these models may be heuristic in developing function-structure hypotheses, it is unlikely that any current model is sufficient to explain the complex interface between behavior and brain circuitry.
Additionally, the role of the amygdala in behavior and personality disturbances is an area of increased interest and research. The amygdala exhibits a number of interconnections with the previously described frontosubcortical circuitry via the frontal cortex, thalamus, and ventromedial striatum (for a review, see Price and Drevets, 2010). Classic studies linking the amygdala and behavior include Kluver and Bucy’s early work with monkeys with bitemporal lesions. Following selective lesions to the amygdala, monkeys exhibited less caution and fear when exposed to unfamiliar stimuli. Human case studies of individuals with amygdala lesions have also been described and have revealed similar findings. One individual with bilateral amygdala damage exhibited difficulty in recognition of fear and exhibited increased social interactions with features of disinhibition (Adolphs, 2010). Researchers have long implemented the amygdala in anxiety and fear. For example, changes in amygdala volume and functioning have been observed in posttraumatic stress disorder (PTSD) (Shin, Rauch, and Pitman, 2006). More recently, research has implemented the amygdala in a wider range of emotional and behavioral responses and syndromes. Although findings have been somewhat mixed, the amygdala has been implicated in mood disorders including depression and bipolar disorder (Hamidi, Drevets, and Price, 2004).
The current chapter focuses on behavioral and psychiatric changes in neurological disease and injury. Please see Chapter 34 for detailed information on assessment and description of common cognitive changes observed in neurological disease and injury.
Assessing Behavior and Personality Disturbances in Patients with Cerebral Dysfunction
Several factors present challenges in the identification and diagnosis of behavior and personality disturbances in neurological samples. First, there is significant overlap between other symptoms of cerebral dysfunction and symptoms of behavior and personality disturbances. For example, psychomotor retardation or reduced energy, libido, or appetite might reflect the underlying illness or injury (i.e., TBI, Parkinson disease [PD]) or they may reflect a depressive disorder. Second, cognitive symptoms may confound the detection of behavioral changes. For example, language and cognitive deficits occurring in individuals with cerebral dysfunction can affect the ability to fully assess for changes in mood or insight. In these situations, observed behavior and collateral report must be heavily considered when assessing for behavioral or personality disturbances. Third, the validity of the behavioral dysfunction assessed can vary depending upon the source. Ample research shows that clinical ratings acquired from the patient, a collateral or spouse, and a healthcare worker can vary widely (see, for example, Hoth et al., 2007). Patients with cerebral dysfunction may have impaired insight; thus, they may underreport behavioral difficulties. Similarly, caregivers may also provide biased information, as their current mood or degree of caregiver burden may influence their reporting of behavioral symptoms.
Assessment of Depression
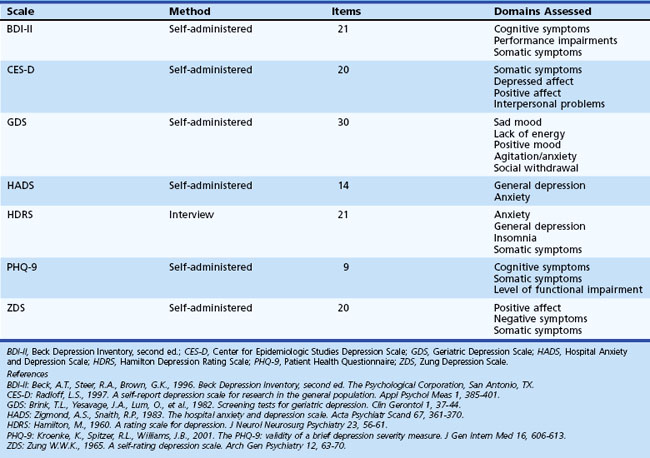
Symptoms of neurological illness or injury may manifest as depression. In fact, depression is frequently a very early symptom or precedes onset of illness in Huntington disease (HD) (Berrios et al., 2001), PD (Ishihara and Brayne, 2006), and Alzheimer disease (AD) (Green et al., 2003). There are several scales available for the assessment of mood disorders that might be useful in patients with acquired cerebral dysfunction. Although clinicians may not have enough time to thoroughly assess altered mood, self-report scales can be helpful in determining which symptoms are present and how bothersome or severe each symptom is. Table 8.4 offers additional information regarding these scales. Individuals scoring highly on these self-report measures may benefit from referral for additional evaluation and possible intervention by mental health professionals.
Domains assessed by the different measures vary such that certain scales may not detect some symptoms of depression. Two of the most commonly used measures are the Beck Depression Inventory (BDI) and the Hamilton Depression Rating Scale (HDRS). Research suggests that the BDI may be a useful screening tool in PD and Tourette syndrome, and the HDRS may be an appropriate screening tool in PD. However, these measures assess several symptoms such as psychomotor retardation and reduced energy that are common in neurological illness and injury. Thus, care must be taken to be certain that these measures do not suggest the person is depressed based on symptoms of neurological syndrome or injury. The Geriatric Depression Scale (GDS) was developed to be used in elderly populations and may be a useful screening tool for patients with early dementia and PD. The Patient Health Questionnaire (PHQ-9) is a self-report measure designed to be used in primary care settings and may be appropriate in neurological settings. See Chapter 9 for more detailed information on depression in neurological settings.
Assessment of Other Behavioral and Personality Disturbances
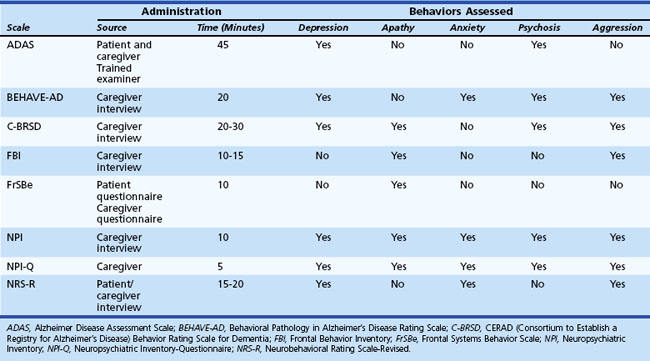
In addition to depression, other behavioral and personality disturbances occur in patients with cerebral dysfunction, and several measures have been created to assess them (Table 8.5). These measures were specifically designed to assess behavioral symptoms in AD: Alzheimer’s Disease Assessment Scale (ADAS); Behavioral Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD); CERAD Behavior Rating Scale for Dementia (C-BRSD); general dementia: Neuropsychiatric Inventory (NPI); frontal lobe dementia: Frontal Behavior Inventory (FBI); TBI: Neurobehavioral Rating Scale-Revised (NRS-R); and damage to frontal regions: Frontal Systems Behavior Scale (FrSBe). Some measures such as the NPI and the FrSBe have been implemented in diverse conditions including AD, PD, HD, and multiple sclerosis (MS). In addition, the NPI, which is available in an interview and a questionnaire format, has been frequently used as an outcome measure in clinical trials. These measures might be useful ways to screen for a wide variety of potential behavioral disruptions among patients with neurological illness or injury.
Behavior and Personality Disturbances Associated with Cerebral Dysfunction
Alzheimer Disease
Based on 2000 census data, it is estimated that AD affects 5.1 million individuals in the United States (Hebert et al., 2003). Patients with AD experience a wide range of behavioral disturbances, including affective symptoms, agitation, aggression, and psychosis. Behavioral disturbances in AD are associated with increased caregiver burden, patient and caregiver abuse, greater use of psychotropic medications, more rapid cognitive decline, and earlier institutionalization. The relationship between behavioral changes in AD and neuropathological markers is equivocal. Some researchers report a correlation between behavioral changes in AD and increased white matter hyperintensities (WMH) (Berlow et al., 2009), while others have not observed this relationship (Staekenborg et al., 2008). Many studies do not document a correlation between the presence or absence of behavioral symptoms and whole brain or hippocampal volume (Berlow et al., 2009; Staekenborg et al., 2008). In contrast to frontotemporal dementia (FTD), social comportment is relatively spared in AD.
Use of atypical antipsychotic medications have historically been the preferred method of treatment for behavioral disturbances in AD including irritability, aggression, and psychosis. However, use of atypical antipsychotic medications in elderly adults may be associated with a nearly twofold increase in risk for mortality (Kuehn, 2005). Additionally, a multisite study of atypical antipsychotics (olanzapine, quetiapine, and risperidone) showed no significant difference in Clinical Global Impression Scale scores for any antipsychotic medication over a placebo group (Schneider et al., 2006). Moreover, significantly more participants found the side effects of the atypical antipsychotic medications to be intolerable compared to the placebo group (Schneider et al., 2006). In a retrospective observational study, behavioral symptoms were reduced in one-fifth to one-fourth of patients following treatment with antipsychotics, while a full half of participants exhibited worsening of symptoms (Kleijer et al., 2009). However, other retrospective observational studies have reported improvements in 33% to 43% of individuals with AD and behavioral disturbances treated with atypical antipsychotics (Rocca et al., 2007). The U.S. Food and Drug Administration (FDA) has issued a black-box warning on the use of antipsychotics in elderly persons with dementia. Antipsychotics may be beneficial in a small subgroup of individuals, but care must be taken in prescribing such medications, owing to the potential side effects in the context of questionable effectiveness. A review of the clinical trial literature for cholinesterase inhibitors and memantine suggests that individuals treated with these pharmaceuticals typically do experience a reduction in behavioral symptoms, including improved mood and abatement of apathy (Cummings, Mackell, and Kaufer, 2008).
Although the neurodegenerative process itself can be the cause of behavioral disturbances in AD, other causes must be explored: premorbid psychiatric diagnoses, medication side effects, or medical comorbidities to name a few. In many situations, behavioral disturbances may reflect an individual with impaired cognitive and language abilities attempting to communicate information to their providers (Sutor, Nykamp, and Smith, 2006). Given the nature of these behavioral disturbances and the limited availability of pharmacological interventions, behavioral interventions and environmental modifications may be among the most helpful strategies in managing undesired behaviors. Detailed discussion of such behavioral interventions is beyond the scope of this chapter, but for more detailed information, readers may wish to review Sutor and colleagues (2006).
Clinicians may wish to refer patients to geriatric psychiatry and/or neuropsychology providers for identification and implementation of behavioral and environmental interventions. Common environmental interventions include use of familiar and personal belongings readily viewable in the environment to reduce confusion and agitation. Similarly, minimizing background distracters and establishing a standard predictable routine may also be helpful in reducing confusion and agitation. It is not uncommon for undesired behaviors (e.g., aggression) to receive significant attention while preferred behaviors (e.g., working on quiet activity) receive no reinforcement. To successfully reduce undesired activities, individuals need to increase desired activities through reinforcing preferred behavior, offering desired activities, and reducing reinforcement of undesired behavior. Finally, redirection is frequently attempted in individuals with cognitive impairment who are engaging in undesired activities. Redirection is likely to be most successful if done in a multistep process involving validation of emotion, joining of behavior, distraction, and only then followed by redirection (Sutor, Nykamp, and Smith, 2006). Table 8.6 lists more detailed information.
Table 8.6 Multistep Approach for Redirecting Patients with Dementia
| Step | Description and Example |
|---|---|
| Validate | Validation of the individual’s emotional state to establish rapport Example: “You look worried.” |
| Join | Join the patient’s behavior. Example: “You’re looking for your children? Well, I’m trying to find something too. Let’s look together.” |
| Distract | Distraction is easier after establishing a common goal. This works best when individuals have significant cognitive impairment. Example: “Let’s look over there where they are having coffee.” |
| Redirect | At this stage, redirection may be possible. Example: “That coffee smells good; do you want a cup?” |
Reprinted with permission from Sutor, B., Nykamp, L.J., Smith, G.E., 2006. Get creative to manage dementia-related behaviors. Curr Psychiatry 5, 81-96.
Depression
The true prevalence of depression in AD is controversial, with estimates up to 86%. One reason for the mixed findings lies in the different methods employed to assess depression in AD, such as family interviews and patient self-report. Some symptoms of depression are confounded with components of AD (e.g., concentration, energy, interest). The probability of depression in AD appears to be greater if there is a history of depression either in the patient or in the family. Table 8.7 suggests differences between the signs of depression and possibly confounding signs of dementia. Interestingly, there does not appear to be a clear relationship between depressive symptoms and severity of AD (Verkaik et al., 2007). Depression is associated with greater social and functional impairments in patients with AD (Starkstein et al., 2005), although others have not observed a correlation between depression and functional impairment (Landes, Sperry, and Strauss, 2005).
Table 8.7 Clinical Aspects Differentiating Dementia from Depression
| Major Depression | Dementia |
|---|---|
| Acute, nonprogressive | Insidious and progressive |
| Affective before cognitive | Cognitive before affective |
| Attention impaired | Memory impaired |
| Orientation intact | Orientation impaired |
| Complains of memory | Minimizes/normalizes memory |
| Gives up on testing | Obvious effort on testing |
| Language intact | Aphasic errors |
| Better at night | Sundowning |
| Self-referred | Referred by others |
Selective serotonin reuptake inhibitors (SSRIs) are the preferred mode of treatment for depression in AD. Although clinical trials are relatively few, sertraline and citalopram have been shown to be effective (Lyketsos et al., 2000).
Apathy
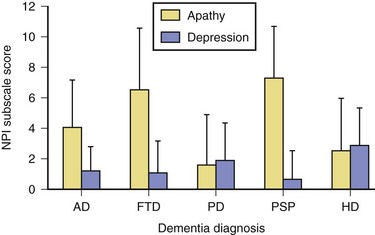
Apathy, defined as diminished motivation not attributable to decreased level of consciousness, cognitive impairment, or emotional distress, is among the most common behavioral changes noted in AD. Assessment of apathy in AD may be difficult because it may be unclear whether decreased activity is due to apathy or inability to perform activities. Consistent with expectations based on frontal-subcortical circuitry, apathy in AD has been shown to be associated with bilateral reductions in gray matter volume in the anterior cingulate cortex, orbitofrontal cortex, dorsolateral prefrontal cortex, and putamen (Bruen et al., 2008). Apathy in AD is associated with greater functional and cognitive impairment (Landes et al., 2005) as well as lower quality of life (Hurt et al., 2008).
Aggression
Aggressive verbalizations and acts are common in AD. Reported prevalence rates range from 25% to 67%, and studies have indicated that verbal aggression is more common in men and in individuals with delusions or agitation (Eustace et al., 2001) and is associated with increased placement in skilled nursing facilities. Sertraline has been associated with a 38% response rate for the treatment of aggression and irritability in AD (Lanctot et al., 2002).
Psychosis
Prevalence rates of psychotic symptoms in AD range from 10% to 73%, with rates in clinical populations exceeding community-based samples. Interestingly, hallucinations and delusions are significantly less common among individuals with early-onset AD (Toyota et al., 2007). Once present, delusions recur or persist for several years in most patients with AD (Fig. 8.3). The presence of hallucinations is associated with increased placement in skilled nursing centers.
Previously it was believed that individuals with AD experienced delusions secondary to significant cognitive difficulties. However, more recent research has identified additional correlates and biological markers of psychosis. Evidence from neuropsychological investigations suggests more executive and frontal dysfunction in AD with psychotic symptoms than AD without these symptoms. For example, delusions have been associated with reduced gray matter volume in the inferior right frontal gyrus and the inferior parietal lobule (Bruen et al., 2008). The presence of delusions in AD is associated with poorer performance on the Frontal Assessment Battery (FAB) but was not related to global measures of cognitive impairment (i.e., MMSE) (Nagata et al., 2009). Persons with AD and hallucinations are at significantly increased risk for mortality; however, delusions alone are not associated with increased mortality (Wilson et al., 2005)
The most common psychotic symptoms reported in patients with AD are delusions and hallucinations. The delusions are typically paranoid-type, non-bizarre, and simple. Complex or bizarre delusions seen in patients with schizophrenia are conspicuously absent in patients with AD. Misidentification phenomena, however, are common in AD. Whereas hallucinations in AD are more often visual than auditory, the reverse is true for schizophrenia (Table 8.8).
Table 8.8 Psychotic Symptoms in Alzheimer Disease versus Schizophrenia in Elderly Patients
| Psychosis in Alzheimer Disease | Schizophrenia in the Elderly | |
|---|---|---|
| Incidence | 30%-50% | <1% |
| Bizarre or complex delusions | Rare | Common |
| Misidentification of caregivers | Common | Rare |
| Common form of hallucinations | Visual | Auditory |
| Schneiderian first-rank symptoms | Rare | Common |
| Active suicidal ideation | Rare | Common |
| History of psychosis | Rare | Very common |
| Eventual remission of psychosis | Common | Uncommon |
| Need for many years of maintenance on antipsychotics | Uncommon | Very common |
| Average optimal daily dose of an antipsychotic | 15%-25% of that in young adult with schizophrenia |
40%-60% of that in a young adult with schizophrenia |
Reprinted with permission from Jeste, D.V., Finkel, S.I., 2000. Psychosis of Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry 8, 29-34.
Frontotemporal Dementia
Frontotemporal lobar degeneration (FTLD) is a heterogeneous group of syndromes including semantic dementia (SD), progressive nonfluent aphasia (PNFA), and behavioral variant frontotemporal dementia (bvFTD). Consensus criteria for diagnosis of FTD have been described, with presence of behavioral change an important feature. While changes in personality and behavior are most commonly described in bvFTD, behavioral change has been reported across all FTLD syndromes to varying degrees. Caregiver distress is greater among individuals with FTLD and behavioral changes, particularly apathy and disinhibition, versus those with primarily aphasic difficulties (Massimo et al., 2009).
Behavioral Disruption
Atrophy within the frontal lobes leads to disruption of the frontosubcortical circuits and the characteristic behavioral syndromes in FTLD. Two classic behavioral syndromes have been described among individuals with FTD: an apathetic and a disinhibited subtype. Apathy is a very common symptom in individuals with FTD. Individuals may show little concern for personal hygiene and may appear unkempt. Moreover, symptoms of orbitofrontal syndrome, such as disinhibition, poor impulse control, tactlessness, and poor judgment are common. Loss of empathy, mental inflexibility, and stereotyped behaviors are also common. Symptoms similar to those observed in Klüver-Bucy syndrome, such as hyperorality and hypersexuality, may occur in late stages. Frequently the family members and caregivers are the ones who report these behavioral disturbances, as many patients with FTD experience reduced insight into their current difficulties. Behavioral change to varying degrees has been described in all FTLD syndromes, including SD and PNFA (Kertesz et al., 2010), although they frequently are less severe and/or occur later in the progression of the illness.
No curative treatments exist for FTLD. However, there has been some success with pharmacological intervention for behavioral dyscontrol. Although few large-scale studies have been completed, evidence suggests that behavioral disturbances such as disinhibition, overeating, and compulsions may show some response to treatment with SSRIs (Huey, Putnam, and Grafman, 2006).
Anosognosia
As noted in the consensus criteria, individuals with FTD frequently exhibit anosognosia. This loss of insight may reflect psychological denial of illness, inability to perceive symptoms, or lack of concern for their current difficulties. Among individuals with FTLD, individuals with bvFTD exhibit greater anosognosia than individuals with the aphasic subtypes of FTLD (Zamboni et al., 2010). Patients with FTD frequently describe significantly fewer problems with cognition and behavior than what their caregivers describe. Moreover, this observed discrepancy between patient and caregiver report is greater among individuals with FTD than in individuals with AD, particularly for language, behavior, and functioning difficulties (Salmon et al., 2008). Severity of anosognosia is not typically associated with severity of dementia (Zamboni et al., 2010). The relationship between impaired awareness and specific neuropathology is somewhat unclear. Some studies have shown an association between impaired awareness and right frontal disruptions (Mendez and Shapira, 2005) while others have shown a link between anosognosia and involvement of the right temporoparietal cortex (Zamboni et al., 2010).
Relationship to Primary Pathology
From a pathological perspective, individuals with FTLD vary with regard to the degree to which the frontal versus temporal lobes and right versus left hemispheres are affected. Significant research has looked at the relationship between patterns of behavioral syndromes and underlying neuropathology (see Josephs, 2007 for a review). Individuals with bvFTD typically exhibit greater frontal versus temporal atrophy, which is typically symmetrical. There is emerging evidence to suggest that individuals with bvFTD and primarily apathetic behavioral changes show greater frontal involvement, particularly right dorsolateral prefrontal cortex (Zamboni et al., 2008; Massimo et al., 2009). Individuals with primarily disinhibited behavioral change show greater involvement of the right mediotemporal limbic and temporal lobe (Zamboni et al., 2008), although others have described increased atrophy within the left dorsolateral prefrontal cortex (Massimo et al., 2009). Individuals with SD most typically exhibit atrophy and dysfunction within the left anterior temporal lobe, while individuals with SD and behavioral changes are more likely to also exhibit changes in the ventromedial and superior frontal lobes. Individuals with PNFA are most likely to show changes in left frontal and perisylvian areas.
Vascular Dementia
Depression
The mean reported prevalence of depression in VaD is 32%, although rates vary widely between studies (Ballard and O’Brien, 2002). Sample source likely influences the reported prevalence rates, with community samples endorsing lower rates of depression than clinic samples. Individuals with VaD and depression are less likely to have had a stroke and are more likely to have a prior history of depression and impairments in memory or attention than patients with VaD without depression. The relationship between age and depression in VaD is unclear, with increased rates of depression being reported in both younger and older samples.
Additional Behavioral and Psychiatric Disorders
Apathy in VaD is associated with increased impairment in both basic and instrumental activities of daily living (Zawacki et al., 2002). This relationship is particularly apparent in patients with VaD who have also experienced a stroke. Rates of psychotic symptoms are similar in AD and VaD. Delusions (33%) and visual hallucinations (13% to 25%) are reported in VaD and are associated with impaired cognitive functioning (Ballard and O’Brien, 2002). Care must be taken in the assessment of delusions in VaD and in dementia in general. It is important to differentiate delusions from confabulation or thought processes based on impaired cognitive functioning.
Parkinson Disease
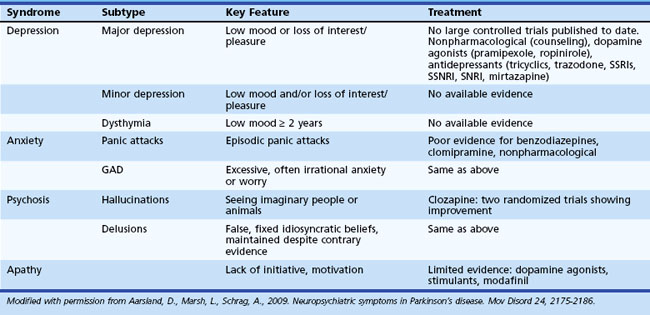
Behavioral changes are common in PD, and while research has adequately characterized these difficulties, little controlled research has assessed the effectiveness of various interventions. Table 8.9 offers more detailed information regarding characteristics of behavioral change observed in PD.
Depression
Depression is the most common psychiatric disturbance in persons with PD. Depending on threshold for diagnosis and sample assessed, reported rates vary depending on the threshold for diagnosis of depression that is used. Depression may predate the onset of motor symptoms in PD (Ishihara and Brayne, 2006). Risk factors for depression in PD include greater cognitive impairment, earlier disease onset, and family history of depression. Depression is not associated with increased motor symptom severity (Holroyd et al., 2005). The correlation between depression and disability is equivocal. Although the precise etiology is unknown, it is believed that depression in PD results from disruptions in dopamine (D2), noradrenaline, and serotonin pathways (Veazey et al., 2005).
Very few well-controlled studies have assessed antidepressant therapy in PD. Available research suggests that SSRIs are well tolerated and likely effective in the treatment of depression in PD (see McDonald et al., 2003 for a review). SSRIs are frequently implemented as a first-line therapy for depression in patients with PD, although SSRIs may worsen motor symptoms. In such cases, tricyclic antidepressants may be an effective alternative. Successful treatment of depressive symptoms with an SSRI may also result in reductions in anxiety and decreased disability.
Psychosis
Hallucinations, typically visual, occur in up to 40% of patients with PD, with 16% reporting delusions (Fenelon et al., 2000). Psychotic symptoms are very uncommon early in the course of PD. Other diagnoses such as dementia with Lewy bodies (DLB) should be considered in patients exhibiting hallucinations early in the course of the disease. Table 8.10 summarizes important distinctions between psychosis in PD and DLB. Psychotic symptoms are more common in PD patients with greater cognitive impairment, longer duration of illness, greater daytime somnolence, and older age and in those who are institutionalized. Psychotic symptoms are strong predictors of nursing home placement and mortality in PD (Fenelon et al., 2000).
Table 8.10 Differentiating Psychosis in Parkinson Disease and Dementia with Lewy Bodies
| Psychosis in Parkinson Disease | Psychosis in Dementia with Lewy Bodies |
|---|---|
| • Psychosis occurs in many but not all patients. | • Psychosis is a core feature for diagnosis. |
| • Visual hallucinations are more common than delusions. | • Visual hallucinations and delusions occur at similar frequencies. |
| • Psychosis is generally medication induced. | • Psychosis occurs in the absence of antiparkinsonian medications. |
| • Hallucinations are usually fleeting and nocturnal. | • Hallucinations are generally persistent/recurrent. |
| • Fluctuating level of consciousness represents onset of delirium. | • Fluctuating level of consciousness can be a core feature. |
| • Dementia may or may not accompany psychosis. | • Presence of dementia is required for a diagnosis of DLB. |
| • Motor impairment virtually always precedes psychosis. | • Motor impairment may occur after psychosis. |
| • Neuroleptics worsen motor function, but “neuroleptic sensitivity” associated with DLB is not a feature of PD. | • Neuroleptic sensitivity characterized by dramatic motor and cognitive worsening and associated with increased morbidity and mortality has been reported. |
| • Disordered dopaminergic (and possibly serotonergic) transmission are the most frequently hypothesized underlying mechanisms. | • Disordered cholinergic transmission may be the most important underlying mechanism. |
DLB, Dementia with Lewy bodies; PD, Parkinson disease.
From Ismail, M.S., Richard, I.H., 2004. A reality test: how well do we understand psychosis in Parkinson’s disease? J Neuropsychiatry Clin Neurosci 16, 8–18. Copyright 2004 by American Psychiatric Press Inc. Reproduced with permission.
Historical accounts of PD rarely described psychotic symptoms, and it has been postulated that psychosis occurred secondary to dopamine agonist use. While dopamine agonists may contribute to the development of psychosis, additional factors are also important. For example, individuals with psychosis are more likely to exhibit cholinergic deficits and have Lewy bodies in the temporal lobe observed at autopsy (Aarsland et al., 2009).
Intervention for remediation of psychotic symptoms in PD can involve several processes. Discontinuation of anticholinergics, selegiline, and amantadine before reducing l-dopa is recommended. Following these discontinuations, reduction and simplification of dopamine agonists may be beneficial. Atypical antipsychotics are added only when a reduction of other medications has not resulted in improvement, as even atypical antipsychotics have been associated with worsening of PD motor symptoms (Goetz et al., 2000).
Apathy
Individuals with PD often experience increased rates of apathy. Estimates of apathy in PD have ranged from 16.5% to 40.0%. Individuals with apathy exhibit greater cognitive impairment (Dujardin et al., 2007). Controlled clinical trials for apathy in PD are very limited. Environmental and other behavioral interventions including establishment of a routine, structured schedule, and cuing from others can be helpful in some settings. Dopamine agonists, psychostimulants, modafinil, dopamine agonists, and testosterone have been reported to be helpful in decreasing apathy (see Aarsland et al., 2009 for more detailed information).
Dementia with Lewy Bodies
Dementia with lewy bodies is increasingly being recognized as a common cause of dementia in older adults. DLB is associated with fluctuating cognitive difficulties, parkinsonism, and hallucinations. Clinical presentation overlap occurs between the presentation of DLB with AD and PD. Research has observed greater overall behavioral symptoms among individuals with DLB compared to individuals with AD, particularly with regard to hallucinations and apathy (Ricci et al., 2009).
Psychosis
Psychotic symptoms, particularly hallucinations, are a hallmark feature of DLB. Insight is typically poor. Unlike patients with AD or PD, patients with DLB exhibit hallucinations early in the course of the illness. Delusions are also common in DLB. The neuropathological correlates of hallucinations in DLB are somewhat unclear. It has been suggested that hallucinations are likely due to decreased acetylcholine as well as changes in the basal forebrain and the ventral temporal lobe (Ferman and Boeve, 2007).
Hallucinations are correlated with poorer functioning with regard to instrumental activities of daily living (Ricci et al., 2009). Typical neuroleptics are avoided in DLB, because patients exhibit high sensitivity to these drugs and may experience severe parkinsonian symptoms and other side effects. In contrast, atypical neuroleptics such as clozapine and quetiapine, as well as cholinesterase inhibitors, are associated with improved cognition and decreased psychotic symptoms (McKeith, 2002).
Huntington Disease
Up to 79% of individuals with HD report psychiatric and behavioral symptoms as the presenting manifestation of the disease. Symptom presentation varies across stage of illness in HD (Table 8.11). Behavioral symptoms are commonly observed among institutionalized patients with HD (Table 8.12). The behavioral difficulties can lead to placement difficulties in these patients.
Table 8.11 Percentage of Patients with Huntington Disease Endorsing Psychiatric Symptoms by TFC Stage
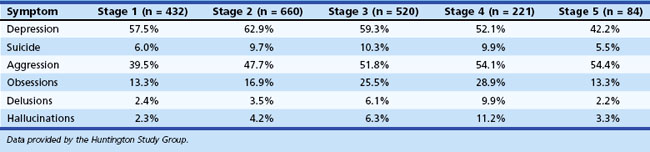
Table 8.12 Ratings by Nursing Home Staff of Problematic Behaviors in Patients with Huntington Disease
| Behavior Problem | Percentage | Rank |
|---|---|---|
| Agitation | 76 | 2.0 |
| Irritability | 72 | 2.9 |
| Disinhibition | 59 | 3.3 |
| Depression | 51 | 4.2 |
| Anxiety | 50 | 4.4 |
| Appetite | 54 | 5.1 |
| Delusions | 43 | 5.5 |
| Sleep disorders | 50 | 5.5 |
| Apathy | 32 | 6.8 |
| Euphoria | 40 | 6.9 |
From Paulsen and Hamilton, unpublished data.
Depression
Depression is one of the most common concerns for individuals and families with HD, occurring in up to 63% of patients. Depression may precede the onset of neurological symptoms in HD by 2 to 20 years, although large-scale empirical research has been minimal. Depression is common immediately before diagnosis, when neurological soft signs and other subtle abnormalities become evident. Following a definite diagnosis of HD, however, depression is most prevalent in the middle stages of the disease (i.e., Shoulson-Fahn stages 2 and 3) and may diminish in the later stages (Paulsen et al., 2005b). Positron emission tomography (PET) studies indicate that patients with HD with depression have hypermetabolism in the inferior frontal cortex and thalamus relative to nondepressed patients with HD or normal age-matched controls.
Suicide
Suicide is more common in HD than in other neurological disorders with high rates of depression such as stroke and PD. Most studies have found a four- to sixfold increase of suicide in HD, with reports as high as 8 to 20 times greater than the general population. Two “critical periods” during which suicidal ideation in HD increased dramatically have been identified. First, frequency of suicidal ideation doubles from 10.4% in at-risk persons with a normal neurological examination to 20.5% in at-risk persons with soft neurological signs. Second, in persons with a diagnosis of HD, 16% had suicidal ideation in stage 1, whereas nearly 21% had suicidal ideation in stage 2. Although the underlying mechanisms for suicidal risk in HD are poorly understood, it may be beneficial for healthcare providers to be aware of periods during which patients may be at an increased risk of suicide (Paulsen et al., 2005a).
Psychosis
Psychosis occurs with increased frequency in HD, with estimates ranging from 3% to 12%. Psychosis is more common among early adulthood-onset cases than among those whose disease begins in middle or late adulthood. Psychosis in HD is more resistant to treatment than psychosis in schizophrenia. Huntington Study Group data suggest that psychosis may increase as the disease progresses (see Table 8.11), although psychosis can become difficult to measure in the later stages of disease.
Tourette Syndrome
Tourette syndrome (TS) is associated with disinhibition of frontosubcortical circuitry; as a result, it is not surprising that increased rates of psychiatric and behavioral symptoms are observed. These behavioral difficulties are more strongly associated with problems with psychosocial functioning than the presence of tics (Zinner and Coffey, 2009). Rates of psychiatric disorders vary widely; significantly higher rates of psychiatric disorders are reported when samples are drawn from psychiatric clinics than from movement disorder clinics. Given the correlation between psychiatric symptoms and changes in psychosocial functioning, treatments in TS that consider psychiatric and behavioral symptoms are encouraged.
Approximately 20% to 40% of individuals with TS meet criteria for OCD, while up to 90% of individuals in a clinic referred sample may exhibit subthreshold levels of obsessive-compulsive symptoms (Zinner and Coffey, 2009). The frequency and severity of tics often decrease as individuals enter adulthood, but the comorbid obsessive-compulsive symptoms are more likely to continue into adulthood and are associated with difficulties with psychosocial functioning (Cheung, Shahed, and Jankovic, 2007). Mood and anxiety symptoms are common in TS. The relationship between severity of depression and tics is unclear. The comorbid presence of obsessive-compulsive symptoms is associated with increased risk for depressive symptoms (Zinner and Coffey, 2009).
Multiple Sclerosis
Depression
Depression is the most common behavioral symptom in MS, occurring at rates of 37% to 54%. Patients with MS may report symptoms of depression even with outward signs of euphoria. While depression is frequently associated with reduced quality of life, the correlation between depressive symptoms and disability in MS is debated with equivocal research. Depression in MS is not consistently associated with increased rates of stressful events, disease duration, sex, age, or socioeconomic status. Among the subtypes of MS, depression may be most common in those with relapsing-remitting MS (Beiske et al., 2008). Fatigue is a strong predictor of depression among individuals with MS (Beiske et al., 2008).
Increased rates of suicidal ideation, suicide attempts, and completed suicides have been observed in individuals with MS. Suicide rates in MS are between two and seven times higher than in the general population (Bronnum-Hansen et al., 2005). Risk factors for suicidal ideation in MS include social isolation, current depression, and lifetime diagnosis of alcohol abuse disorder. Although suicide attempts occur throughout the progression of the disease, some have suggested that increased risk may be particularly high in the year following diagnosis (Bronnum-Hansen et al., 2005).
Biological factors likely contribute to depressive symptoms in MS. It has been hypothesized that the inflammatory process associated with MS may directly lead to depressive symptoms. Similarly, demyelination lesions in MS may directly contribute to the etiology of depression. Imaging studies in MS, however, have failed to show clear neuropathological correlates of depression. Disruptions have been observed in right parietal, right temporal, and right frontal areas (Zorzon et al., 2001) as well as the limbic cortex, implying disruption of frontosubcortical circuitry. It is likely that depression in MS results from a combination of psychosocial and biological factors.
Although controversial, depression may be a side effect for some individuals treated with interferon beta-1b (IFN-β-1b) (Feinstein, 2000). Patients with severe depression should be closely monitored while receiving IFN-β-1b. The relationship between depression and IFN-β-1a and interferon alfa (IFN-α) is equivocal, as conflicting results have been reported. In contrast, glatiramer acetate has not been associated with increased depressive symptoms (Feinstein, 2000). Because of the potential relationship between depression and treatment for MS, as well as the high rates of depression in MS, it is critical that physicians take care to thoroughly assess a patient’s current and past history of depression. This may be particularly important prior to beginning IFN interventions, as patients with histories of depression may be more likely to experience symptoms of depression following IFN treatment.
Few randomly assigned clinical trials have been conducted for the treatment of depression in MS. Several open-label trials of SSRIs have been conducted, which suggest that SSRIs may be effective in the treatment of depression in MS (Siegert and Abernethy, 2005). In addition, psychotherapy, particularly that focusing on coping skills, is efficacious in the reduction of depressive symptoms.
Anxiety
Although common, anxiety is often overlooked because anxiety symptoms may be viewed as a result of poor coping skills. Some strategies to minimize anxiety in individuals with MS are described in Box 8.2. Comorbid anxiety and depression are associated with greater somatic complaints, social difficulties, and suicidal ideation than either anxiety or depression alone. Predictors of anxiety in individuals with MS include fatigue, pain, and younger age of onset (Beiske et al., 2008).
Box 8.2
Strategies to Minimize Anxiety in Patients with Multiple Sclerosis
 Respect adaptive denial as a useful coping mechanism.
Respect adaptive denial as a useful coping mechanism.
 Provide referrals to the National Multiple Sclerosis Society (1-800-Fight-MS) early in disease.
Provide referrals to the National Multiple Sclerosis Society (1-800-Fight-MS) early in disease.
 Help patients to live “one day at a time,” and restrict predictions regarding the future.
Help patients to live “one day at a time,” and restrict predictions regarding the future.
 Help patients manage stress with relaxation techniques.
Help patients manage stress with relaxation techniques.
 Involve occupational therapists for energy conservation techniques.
Involve occupational therapists for energy conservation techniques.
 Focus on the patient’s abilities, not disabilities.
Focus on the patient’s abilities, not disabilities.
 Consider patient’s educational and financial background when giving explanations and referrals.
Consider patient’s educational and financial background when giving explanations and referrals.
 Realize that patients have access to the Internet, self-help groups, and medical journals, and may ask “difficult” questions.
Realize that patients have access to the Internet, self-help groups, and medical journals, and may ask “difficult” questions.
 Expect grief reactions to losses.
Expect grief reactions to losses.
 Deal with losses one at a time.
Deal with losses one at a time.
 Attend to the mental health needs of patients’ families and caregivers.
Attend to the mental health needs of patients’ families and caregivers.
 Respect the patient’s symptoms as real.
Respect the patient’s symptoms as real.
 Focus supportive psychotherapy on concrete, reality-based cognitive and educational issues related to multiple sclerosis.
Focus supportive psychotherapy on concrete, reality-based cognitive and educational issues related to multiple sclerosis.
 Provide targeted pharmacotherapy.
Provide targeted pharmacotherapy.
 Refer appropriate patients for cognitive remediation training.
Refer appropriate patients for cognitive remediation training.
 Ask about sexual problems, as well as bowel and bladder dysfunction.
Ask about sexual problems, as well as bowel and bladder dysfunction.
 Keep an open dialogue with the patient about suicidal thoughts.
Keep an open dialogue with the patient about suicidal thoughts.
Modified with permission from Riether, A.M., 1999. Anxiety in patients with multiple sclerosis. Semin Neuropsychiatry 4, 103-113.
Pathological Laughing and Crying
Pathological laughing and crying (PLC) occurs when there is disparity between an individual’s emotional experience and his or her emotional expression; affected individuals are unable to control laughter or crying. Approximately 10% of individuals with MS exhibit periods of PLC. (Parvizi et al., 2009). PLC is more common in MS patients who have entered the chronic-progressive disease course, have high levels of disability, and cognitive dysfunction. The neuropathological substrate for PLC is believed to involve several aspects of the frontosubcortical circuits as well as the cerebellum (Parvizi et al., 2009). Table 8.13 gives more detailed information. Dextromethorphan/quinidine may be effective in treating such symptoms (Panitch et al., 2006). Additionally, tricyclic and SSRI antidepressant medications may be helpful in reducing PLC symptoms (Parvizi et al., 2009).
Table 8.13 Neuroanatomical Structures and Pathological Laughing and Crying
| Structure | Neuroanatomical Significance |
|---|---|
| Prefrontal cortex and anterior cingulate | A major component of the limbic lobe, with motor efferents to the brainstem structures involved in emotional expression |
| Internal capsule | A white matter structure consisting of pathways descending from the brain to the brainstem and spinal cord. Some of these pathways are related to the brainstem nuclei, some to the cerebellum (via basis pontis), and some reach the spinal cord. |
| Thalamus | A node in the pathways to the cortex originated from the brainstem, cerebellum, and basal ganglia |
| Subthalamic nucleus | A crucial node in the indirect pathways that carry signals from the striatum to the frontal lobe via the thalamus |
| Basis pontis | Relay center for pathways entering the cerebellum |
| Cerebellar white and gray matter | Receives inputs from many parts of the nervous system and sends its signals to the spinal cord, brainstem, and cerebral cortex (mostly frontal lobe and some to somatomotor parietal cortical areas) through the thalamus |
Modified with permission from Parvizi, J., Coburn, K.L., Shillcutt, S.D., et al., 2009. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry. Clin Neurosci 21, 75-87. Copyright 2009, American Psychiatric Association.
Amyotrophic Lateral Sclerosis
Historically, amyotrophic lateral sclerosis (ALS) was largely viewed as a pure motor neuron disease. More recently, however, researchers have recognized that cognitive abilities in individuals with ALS can vary widely from normal cognition to dementia. In particular, a relationship between ALS and FTD has been described (Lomen-Hoerth, Anderson, and Miller, 2002).
Depression
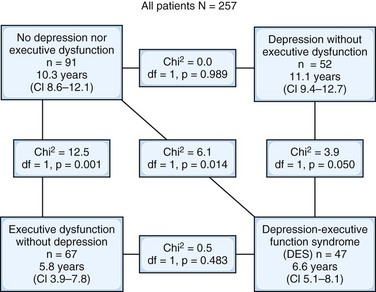
Depressive symptoms occur in 40% to 50% of individuals with ALS (Kubler et al., 2005), although most individuals exhibit subsyndromal depression. Depression in ALS is associated with increased physical impairment, although these results are not always replicated. Individuals with low psychological well-being were at increased risk of mortality (Fig. 8.4). Mortality risk was more strongly associated with psychological distress than age and was similar to the association of risk associated with severity of illness. Depression is correlated with duration of illness; however, depression is not associated with ventilator use or tube feeding (Kubler et al., 2005).
Pathological Laughing and Crying
Up to 50% of individuals with ALS, most often those with pseudobulbar syndrome, report PLC (Parvizi et al., 2009). Individuals with PLC may be more likely to exhibit behavioral changes similar to those observed among individuals with FTD (Gibbons et al., 2008). Little research has assessed treatment of pseudobulbar affect. Potential pharmacological interventions include use of tricyclic and SSRI antidepressant medications (Parvizi et al., 2009). Dextromethorphan/quinidine may also be an effective treatment for PLC (Parvizi et al., 2009). Reduction in PLC symptoms was associated with improved quality of life and quality of relationships.
Personality Change
With recognition of the correlation between ALS and FTD, increased interest has been placed on assessing for potential behavioral changes in ALS. Minimal research has fully explored this question. Gibbons and colleagues (2008) assessed behavioral changes among a small group of individuals with ALS by using a structured interview of close family members of those with ALS. In this small study, 14/16 individuals with ALS exhibited behavioral changes. Of those with behavioral changes, 69% exhibited reduced concern for others, 63% exhibited increased irritability, and 38% exhibited increased apathy.
Epilepsy
Behavioral and personality disturbances occur in up to 50% of individuals with epilepsy. Identification and treatment of these behavioral disturbances remains inadequate, with less than half of individuals with epilepsy and major depressive disorder (MDD) being treated for depression. Presence of a psychiatric disorder is an independent predictor of quality of life in individuals with epilepsy (Kanner et al., 2010). In epilepsy, psychiatric disturbances are classified based on their chronological relationship to seizures. Ictal disturbances occur during the seizure. Peri-ictal disturbances occur immediately before (preictal) or after (postictal) a seizure. Finally, interictal disturbances are those that occur independent of seizure states (Table 8.14). To facilitate the patient’s understanding and for accurate treatment of symptoms, it is important to recognize that behavioral and personality disturbances can occur during the ictal state. Individuals in the ictal period may experience episodes of anxiety, depression, psychosis, and aggression. However, because much of the research regarding psychiatric disturbances in epilepsy has focused on interictal behavioral and personality disturbances, these disturbances will be the focus of this section.
Table 8.14 Psychiatric Disturbances in Ictal, Postictal, and Interictal States
| Ictal | Postictal | Interictal |
|---|---|---|
| Anxiety | Agitation | Panic disorder |
| Intense feelings of horror | Generalized anxiety disorder | |
| Panic attacks | Phobias | |
| Depressed mood | Depression | Major depressive disorder |
| Tearfulness | Dysthymic disorder | |
| Atypical depressive syndromes | ||
| Medication-induced mood changes | ||
| Adjustment disorder | ||
| Paranoia | Paranoia | Psychotic syndromes |
| Hallucinations | Hallucinations | |
| Illusions | ||
| Forced thoughts resembling obsessions | Obsessive-compulsive disorder | |
| Obsessions | ||
| Aggression/violence | Aggression/violence | Aggression/violence |
| Confusion | Confusion | |
| Sexual Excitement | ||
| Laughter | Mania | |
| Déjà vu and other memory experiences | ||
| Conversion disorder | ||
| Medication-induced conditions |
Reprinted with permission from Marsh, L., Rao, V., 2002. Psychiatric complications in patients with epilepsy: a review. Epilepsy Res 49, 11-33.
Depression
Depression is the most common psychiatric disorder in epilepsy. Rates of depression vary as a function of the sample assessed (clinical samples report higher rates of depression than population samples) and the measures used to diagnosis depression. Depression often goes undiagnosed in patients with epilepsy, because symptoms of depression may be viewed as a normal reaction to illness. However, accurate diagnosis of depression is critical because depression is associated with poorer quality of life, employment, and family functioning (Ettinger et al., 2004). Interestingly, presurgical depression is associated with poorer postsurgical seizure outcomes (Metternich et al., 2009).
Attempted and completed suicides are common in epilepsy. The suicide rate in epilepsy is two or more times greater than in the general population (Stefanello et al., 2010). Rates of suicide are even higher in temporal lobe epilepsy. Risk factors include history of self-harm, family history of suicide, stressful life situations, poor morale, stigma, and psychiatric disorders. Individuals with difficulties with comorbid anxiety and depression are at greater risk for suicidal ideation than individuals with only one syndrome (Stefanello et al., 2010).
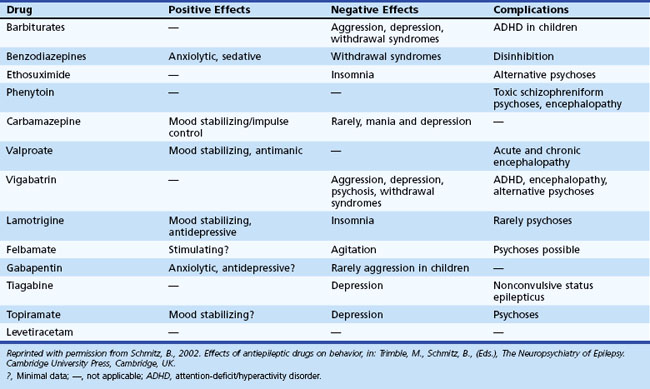
Pharmacological treatment of epilepsy may contribute to depression and psychiatric symptoms in general. Table 8.15 notes commonly used antiepileptic drugs and their psychotropic effects. Medications associated with sedation (e.g., barbiturates, benzodiazepines) may lead to depression, fatigue, and mental slowing.
Although the phenomenology of depression in epilepsy may prove dissimilar from that in patients with general depression, similar treatments are efficacious in the treatment of depression. Supportive psychotherapy may prove beneficial, particularly after initial diagnosis as patients begin to adapt to their illness. Few clinical trials have assessed the efficacy of antidepressant medications in patients with epilepsy. Older antidepressants and the antidepressant, bupropion, have been associated with increased seizures and thus should be avoided. Prueter and Norra (2005) suggest that citalopram and sertraline be considered first-line antidepressant medications in epilepsy because of their limited interactions with antiepileptic medication.
Anxiety
Increased rates of anxiety disorders occur in patients with epilepsy. Between 19% and 50% of individuals with epilepsy meet criteria for one or more DSM-IV anxiety disorder (Beyenburg et al., 2005). Individuals with comorbid anxiety and depressive disorders report lower quality of life than individuals with either disorder alone (Kanner et al., 2010). Common anxiety disorders include agoraphobia, generalized anxiety disorder, and social phobia. Fear of having a seizure and anticipatory anxiety are quite common. Care must be taken to distinguish between panic attacks and fear occurring in the context of a seizure (“ictal fear”). Fear is the most common psychiatric symptom to manifest during a seizure.
The relationship between antiepileptic drugs (AEDs) and anxiety is complex. Some AEDs appear to exacerbate anxiety symptoms, and others are associated with reduction in anxiety symptoms. Antidepressant medication, particularly the SSRIs, is the most common pharmacological treatment for anxiety in epilepsy. See the review by Beyenburg and colleagues (2005) for a more detailed discussion of treatment of anxiety in epilepsy.
Psychosis
The association between epilepsy and psychosis has been debated throughout the past century. Individuals with epilepsy onset before age 20 years, duration of illness greater than 10 years, history of complex partial seizures, and temporal lobe epilepsy are at increased risk of psychotic disturbances. Postictal and interictal psychosis are most commonly reported. Postictal psychosis most commonly develops after many years of epilepsy (Devinsky, 2003). Episodes of postictal psychosis are short in duration, lasting from a few hours to a few months. Postictal psychosis is more common with limbic lesions (Devinsky, 2003). In interictal psychosis, episodes of psychosis are not temporally tied to seizure onset and typically last for more than 6 months.
Aggression
The relationship between epilepsy and aggression remains controversial. Early research suggested that the prevalence of aggression in epilepsy ranged from 4.8% to 50.0%. Aggression occurring in the context of a seizure is quite rare (Devinsky, 2003). Rates of aggression are believed to be higher in individuals with temporal lobe epilepsy. Results vary owing to the definition of aggression used and the method of group selection. Interictal aggression may be described as episodic dyscontrol or, as in the DSM-IV nosology, intermittent explosive disorder (IED), which is characterized by periods of largely unprovoked anger, rage, severe aggression, and violent behavior. Hippocampal sclerosis is less common in individuals with epilepsy and aggression (Tebartz van Elst et al., 2000). A subgroup of individuals with epilepsy and aggression have significant amygdala atrophy (Tebartz van Elst, 2002).
Stroke
Depression
Within the first year following a stroke, 30% to 40% of patients experience depression, with most developing depression within the first month (Ballard and O’Brien, 2002). Interestingly, rates appear to be similar for individuals in early, middle, and late stages following stroke. Depression after a stroke is associated with age, time since stroke, cognitive impairment, and social support. Significantly higher rates (5 to 6 times more likely) of poststroke depression have been reported among individuals with a premorbid diagnosis of depression (Ried et al., 2010).
Depression is associated with longer hospital stays, suggesting that it affects rehabilitation efforts. Depression is associated with poorer recovery of activities of daily living and increased morbidity. Depression and executive dysfunction commonly co-occur following a stroke. The presence of executive dysfunction with or without co-occurring depressive symptoms may be the strongest predictor of morbidity following stroke (Melkas et al., 2010) (see Fig. 8.4). Studies assessing the relationship between disability and depression in stroke patients have been equivocal. Depression is associated with poorer quality of life in individuals who have had a stroke, even when neurological symptoms and disability are held constant.
The relationship between depression and lesion location has been the focus of significant research and controversy. Early research by Robinson and Price showed that left anterior lesions were associated with increased rates and severity of depression. Lesions nearer the left frontal pole or left caudate nucleus were associated with increased rates of depression. Some researchers have replicated these findings, but others have failed to do so. More recent review articles have not supported a relationship between lesion location and depression in poststroke patients (Bhogal et al., 2004). Of note, there is significant heterogeneity in previous studies, particularly between different sample sources.
If more homogeneous groups of patients are considered, some relationships emerge. Depression is associated with left-sided lesions in studies using hospital samples, whereas depression is associated with right-sided lesions in community samples (Bhogal et al., 2004). Time since stroke is an additional important variable to consider. Poststroke depression is associated with left-sided lesions in individuals in the first month following stroke (Bhogal et al., 2004). However, poststroke depression is associated with right-sided lesions in individuals more than 6 months after the stroke (Bhogal et al., 2004). Other differences in previous research, such as method of depression diagnosis, may contribute to the mixed results.
Few studies have assessed the effectiveness of various treatments for depression in these patients. A recent review suggests that there is no clear evidence that standard antidepressant medications are effective in the treatment of poststroke depression (Hackett et al., 2005). Although such interventions may not lead to effective cessation of depressive disorders, they may result in overall reductions in depressive severity. One study suggests that nortriptyline was more effective in the treatment of depression than either placebo or fluoxetine (Robinson et al., 2000). In this study, response to treatment with nortriptyline was associated with improvement in cognitive and functional abilities. This improvement in cognition and functional abilities following reduction in depressive symptoms has not always been replicated (Hackett et al., 2005).
Pathological Laughing and Crying
A portion of individuals experience PLC after a stroke. Between 11% and 35% of individuals experience emotional incontinence after stroke (Parvizi et al., 2009). Emotional incontinence is associated with lesions of the brainstem and cerebellar lesions (see Parvizi et al., 2009 for a review). Research assessing treatment for PLC has been largely limited to case studies. Preliminary evidence suggests that tricyclic and SSRI antidepressants may be helpful in alleviating symptoms of PLC (Parvizi et al., 2009).
Aggression
Reports have suggested that individuals have difficulty controlling aggression and anger following a stroke. Inability to control anger or aggression was associated with increased motor dysfunction and dysarthria. Aggression following stroke is associated with increased rates of MDD and generalized anxiety disorder. There is some evidence that lesions in the area supplied by the subcortical middle cerebral artery are associated with inability to control anger or aggression. Poststroke irritability and aggression are associated with lesions nearer to the frontal pole. Fluoxetine has been shown to successfully reduce levels of poststroke anger (Choi-Kwon et al., 2006). Similarly, reductions in irritability and aggression have been associated with reductions in depression following pharmacological intervention (Chan et al., 2006).
Traumatic Brain Injury
Traumatic brain injury is a significant public health concern, affecting approximately 1.4 million individuals, with 235,000 individuals hospitalized each year in the United States. Significant behavioral and psychiatric disturbances can be observed in individuals following TBI (Table 8.16). Kim and colleagues (2007) provide a comprehensive summary of recent literature regarding psychiatric symptoms following brain injury. Behavioral and psychiatric changes are common after TBI and can remain for decades following injury. Behavioral or mood disturbances are associated with decreased quality of life, increased caregiver burden, more challenges to the treating physician, and can significantly affect daily functioning including management of close relationships and employment. Psychiatric diagnoses following TBI are more common in individuals with a history of psychiatric illness, poor social functioning, alcoholism, arteriosclerosis, lower MMSE score, and fewer years of education. Many behavioral changes such as increased disinhibition are associated with dysfunction within the frontal cortex.
Table 8.16 Lifetime Prevalence of Major Psychiatric Disorders by Head Injury Status from the New Haven Epidemiologic Catchment Area Study (N = 5034)
| Head Injury (%) | No Head Injury (%) | |
|---|---|---|
| Major depression (n = 242) | 11.1 | 5.2 |
| Dysthymia (n = 172) | 5.5 | 2.9 |
| Bipolar disorder (n = 45) | 1.6 | 1.1 |
| Panic disorder (n = 60) | 3.2 | 1.3 |
| Obsessive-compulsive disorder (n = 102) | 4.7 | 2.3 |
| Phobic disorder (n = 361) | 11.2 | 7.4 |
| Alcohol abuse/dependence (n = 412) | 24.5 | 10.1 |
| Drug abuse/dependence (n = 175) | 10.9 | 5.2 |
| Schizophrenia (n = 73) | 3.4 | 1.9 |
Note: Adjusted for age, sex, marital status, socioeconomic status, alcohol abuse, and quality of life.
Reprinted with permission from Silver, J.M., Kramer, R., Greenwald, S., et al., 2001. The association between head injuries and psychiatric disorders: findings from the New Haven NIMH epidemiologic catchment area study. Brain Inj 15, 935-945.
Depression
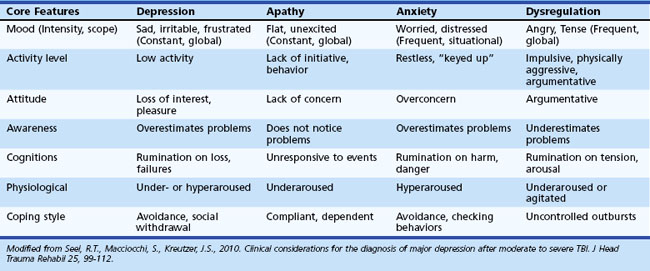
Depression following TBI is common. Diagnosis of depression in TBI is complicated, because symptoms of depression (e.g., fatigue, concentration difficulties, sleep disturbances) are common following TBI. For further discussion regarding the diagnosis of depression in TBI, see Seel and colleagues (2010). MDD occurs in up to 60% of individuals who have suffered TBI (Kim et al., 2007). Rates of depression in TBI vary as a function of severity of TBI assessed, method of depression diagnosis, and sample source. The best predictor of depression after TBI is the presence of premorbid depression; however, some have failed to replicate this finding. Other factors associated with post-TBI depression include poor coping styles, social isolation, and increased stress (Kim et al., 2007). Depression in TBI is associated with increased suicidality, increased cognitive problems, greater disability, and aggression. See Table 8.17 for additional information regarding differentiating features associated with depression in TBI.
Suicidal ideation (65%) and attempts (8.1%) are common following TBI (Silver et al., 2001). In contrast to sex differences reported in the general population, women with TBI are more likely to commit suicide than men with TBI. Furthermore, suicide was more common in individuals with more severe injury and those younger than 21 years or older than 60 years at the time of injury.
No large class I studies of use of antidepressant medications, particularly SSRIs, in TBI have been completed, but small studies provide preliminary support for their use to treat depressive symptoms following TBI. Care must be taken in certain situations, because some antidepressants (i.e., bupropion) are associated with increased risk of seizures. Close monitoring following the beginning of a trial of antidepressant medication is encouraged; in some settings, such medications can increase agitation or anxiety in individuals with TBI. Please see Alderfer and colleagues (2005) for more details regarding recommendations for treatment of depression following TBI.
Anxiety
Less research has assessed the prevalence of anxiety disorders in TBI; however, studies suggest that 11% to 70% of individuals meet criteria for anxiety. A meta-analysis suggested that the mean prevalence of anxiety disorders following TBI is 29%. Panic disorder occurs in 3.2% to 9.0% of individuals with a TBI (Silver et al., 2001).
Apathy
Symptoms of apathy are reported in 10% to 60% of individuals with a TBI. Among individuals with TBI referred to a behavioral management program, lack of initiation was among the most common reported problems, occurring in approximately 60% of the sample (Kelly et al., 2008). Apathy in TBI is associated with depressive symptoms, although a significant number of individuals (28%) report experiencing apathy but not depression. Lesions affecting the right hemisphere and subcortical regions are more strongly associated with apathy than lesions affecting the left hemisphere.
Personality Change
Aggression within 6 months of TBI has been reported in up to 60% of individuals with TBI (Baguley et al., 2006). Among individuals referred to a TBI behavior management service, verbal aggression along with inappropriate social behavior were among the most commonly reported behavioral difficulties and occurred in more than 80% of individuals (Kelly et al., 2008). Aggression following TBI is associated with onset of depression following TBI, poorer psychosocial functioning, and greater disability (Rao et al., 2009).
A number of pharmacological interventions have been used to reduce and remediate behavioral changes following brain injury. See Nicholl and LaFrance (2009) for a review. One class of medication used in these settings is AEDs, now routinely used to treat aggression, disinhibition, and mania following TBI. Again, few large-scale studies have assessed the effectiveness of AEDs in the treatment of behavioral change following TBI. Historically, neuroleptic drugs were used in high doses to treat behavioral dyscontrol in individuals with cognitive impairment. More recently, there has been increased interest in the use of atypical neuroleptics to treat both psychosis and behavioral changes following TBI.
In addition to pharmacological interventions, behavioral and environmental interventions have been shown to be effective at remediating behavioral dyscontrol following TBI. The discussion of behavioral and environmental techniques aimed at decreasing behavioral dyscontrol, including aggression and irritability, is beyond the scope of this chapter (see Sohlberg and Mateer, 2001 for more information). Providers may find referrals for such interventions within rehabilitation programs. Briefly, interventions may seek to reduce stimulation in the environment, increase structure and predictability, reinforce good behavior with limited response to undesired behavior, and use of structured problem-solving strategies.
Aarsland D., Marsh L., Schrag A. Neuropsychiatric Symptoms in Parkinson’s disease. Mov Disord. 2009;24:2175-2186.
Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42-61.
Alderfer B.S., Arciniegas D.B., Silver J.M. Treatment of depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:544-562.
Baguley I.J., Cooper J., Felmingham K. Aggressive behavior following traumatic brain injury: how common is common? J Head Trauma Rehabil. 2006;21:45-56.
Ballard C.G., O’Brien J.T. Behavioural and psychological symptoms. In: Erkinjuntti T., Gauthier S. Vascular Cognitive Impairment. London: Taylor and Francis; 2002:237-252.
Beiske A.G., Svensson E., Sandanger I., et al. Depression and anxiety amongst multiple sclerosis patients. Eur J Neurol. 2008;15:239-245.
Berlow Y.A., Wells W.M., Ellison J.M., et al. Neuropsychiatric correlates of white matter hyperintensities in Alzheimer’s disease. Int J Geriatr Psychiatry. 2009. Available at http://dx.doi.org/10.1002/gps.2418
Berrios G.E., Wagle A.C., Markova I.S., et al. Psychiatric symptoms and CAG repeats in neurologically asymptomatic Huntington’s disease gene carriers. Psychiatry Res. 2001;102:217-225.
Beyenburg S., Mitchell A.J., Schmidt D., et al. Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav. 2005;7:161-171.
Bhogal S.K., Teasell R., Foley N., et al. Lesion location and poststroke depression: Systematic review of the methodological limitations in the literature. Stroke. 2004;35:794-802.
Bronnum-Hansen H., Stenager E., Nylev Stenager E., et al. Suicide among Danes with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1457-1459.
Bruen P.D., McGeown W.J., Shanks M.F., et al. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131:2455-2463.
Chan K.L., Campayo A., Moser D.J., et al. Aggressive behavior in patients with stroke: Association with psychopathology and results of antidepressant treatment on aggression. Arch Phys Med Rehabil. 2006;87:793-798.
Cheung M.Y., Shahed J., Jankovic J. Malignant Tourette syndrome. Mov Disord. 2007;22:1743-1750.
Choi-Kwon S., Han S.W., Kwon S.U., et al. Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness: A double-blind, placebo-controlled study. Stroke. 2006;37:156-161.
Cummings J.L., Mackell J., Kaufer D. Behavioral effects of current Alzheimer’s disease treatments: A descriptive review. Alzheimers Dement. 2008;4:49-60.
Devinsky O. Psychiatric comorbidity in patients with epilepsy: implications for diagnosis and treatment. Epilepsy Behav. 2003;4:S2-S10.
Dujardin K., Sockeel P., Devos D., et al. Characteristics of apathy in Parkinson’s disease. Mov Disord. 2007;22:778-784.
Ettinger A., Reed M., Cramer J. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004;63:1008-1014.
Eustace A., Kidd N., Greene E., et al. Verbal aggression in Alzheimer’s disease. Clinical, functional and neuropsychological correlates. Int J Geriatr Psychiatry. 2001;16:858-861.
Feinstein A. Multiple sclerosis, disease modifying treatments and depression: a critical methodological review. Mult Scler. 2000;6:343-348.
Ferman T.J., Boeve B.F. Dementia with Lewy Bodies. Neurol Clin. 2007;25:741-760.
Fenelon G., Mahieux F., Huon R., et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123:733-745.
Gibbons Z.C., Richardson A., Neary D., et al. Behavior in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:67-74.
Goetz C.G., Blasucci L.M., Leurgans S., et al. Olanzapine and Clozapine comparative effects on motor function in hallucinating PD patients. Neurology. 2000;55:789-794.
Green R.C., Cupples L.C., Kurz A., et al. Depression as a risk factor for Alzheimer’s disease. Arch Neurol. 2003;60:753-759.
Hackett M.L., Anderson C.S., House A.O. Management of depression after stroke: a systematic review of pharmacological therapies. Stroke. 2005;36:1098-1103.
Hamidi M., Drevets W.C., Price J.L. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563-569.
Hebert L.E., Scherr P.A., Bienias J.L., et al. Alzheimer disease in the US population prevalence estimates using the 2000 Census. Arch Neurol. 2003;60:1119-1122.
Holroyd S., Currie L.J., Wooten G.F. Depression is associated with impairment of ADL, not motor function in Parkinson disease. Neurology. 2005;64:2134-2135.
Hoth K.F., Paulsen J.S., Moser D.J., et al. Patients with Huntington’s disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol. 2007;29:365-376.
Huey E.D., Putnam K.T., Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17-22.
Hurt C., Bhattacharyya S., Burns A., et al. Patient and caregiver perspectives of quality of life in dementia. Dement Ger Cogn Disord. 2008;26:138-146.
Ishihara L., Brayne C. A systemic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol Scand. 2006;113:211-220.
Josephs K.A. Frontotemporal Lobar Degeneration. Neurol Clin. 2007;25:683-696.
Kanner A.M., Barry J.J., Gilliam F., et al. Anxiety disorders, subsyndromic depressive episodes, and major depressive episodes: Do they differ on their impact on the quality of life of patients with epilepsy? Epilepsia. 2010. Available at http://dx.doi.org/10.1111/j.1528-1167.2010.02582.x
Kelly G., Brown S., Todd J., et al. Challenging behavior profiles of people with acquired brain injury living in community settings. Brain Inj. 2008;22:457-470.
Kertesz A., Jesso S., Harciarek M., et al. What is semantic dementia? A cohort study of diagnostic features and clinical boundaries. Arch Neurol. 2010;67:483-489.
Kim E., Lauterbach E.C., Reeve A., et al. Neuropsychiatric complications of traumatic brain injury: A critical review of the literature (A report by the ANPA Committee on Research). J Neuropsychiatry Clin Neurosci. 2007;19:106-127.
Kleijer B.C., van Marum R.J., Egberts A.C.G., et al. The course of behavioral problems in elderly nursing home patients with dementia when treated with antipsychotics. Int Psychogeriatr. 2009;21:931-940.
Kubler A., Winter S., Ludolph A.C., et al. Severity of depressive symptoms and quality of life in patients with amyotrophic lateral sclerosis. Neurorehabil Neural Repair. 2005;19:182-193.
Kuehn B.M. FDA warns antipsychotic drugs may be risky for elderly. JAMA. 2005;293:2462.
Lanctot K.L., Herrmann N., van Reekum R., et al. Gender, aggression and serotonergic function are associated with response to sertraline for behavioral disturbances in Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17:531-541.
Landes A.M., Sperry S.D., Strauss M.E. Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2005;17:342-349.
Lomen-Hoerth C., Anderson T., Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077-1079.
Lyketsos C.G., Sheppard J.M.E., Steele C.S., et al. Randomized, placebo-controlled, double-blind clinical trial of sertraline in the treatment of depression complicating Alzheimer’s disease: initial results from the depression in Alzheimer’s disease study. Am J Psychiatry. 2000;157:1686-1689.
Massimo L., Powers C., Moore P., et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;37:96-104.
Melkas S., Vataja R., Oksala N.K.J., et al. Depression-executive dysfunction syndrome relates to poor poststroke survival. Am J Geriatr Psychiatry. 2010;18:1007-1016. Available at http://dx.doi.org/10.1097/JGP.0b013e3181d695d7
Mendez M.F., Shapira J.S. Loss of insight and functional neuroimaging in frontotermporal dementia. J Neuropsychiatry Clin Neurosci. 2005;17:413-416.
McDonald W.M., Richard I.H., DeLong M. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry. 2003;54:363-375.
McKeith I. Dementia with Lewy bodies. Br J Psychiatry. 2002;180:144-147.
Mega M.S., Cummings J.L. Frontal subcortical circuits: Anatomy and function. In: Salloway S.P., Malloy P.F., Duffy D. The Frontal Lobes and Neuropsychiatric Illness. Washington, DC: American Psychiatric Publishing; 2001:15-32.
Metternich B., Wagner K., Brandt A., et al. Preoperative depressive symptoms predict postoperative seizure outcome in temporal and frontal lobe epilepsy. Epilepsy Behav. 2009;16:622-628.
Nagata T., Ishii K., Ito T., et al. Correlation between a reduction in Frontal Assessment Battery scores and delusional thoughts in patients with Alzheimer’s disease. Psychiatry Clin Neurosci. 2009;63:449-454.
Nicholl J., LaFrance W.C. Neuropsychiatric sequelae of traumatic brain injury. Semin Neurol. 2009;29:247-255.
Panitch H.S., Thisted R.A., Smith R.A., et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006;59:780-787.
Parvizi J., Coburn K.L., Shillcutt S.D., et al. Neuroanatomy of pathological laughing and crying: A report of the American neuropsychiatric committee on research. J Neuropsychiatry Clin Neurosci. 2009;21:75-87.
Paulsen J.S., Hoth K.F., Nehl C., et al. Critical periods of suicide risk in Huntington’s disease. Am J Psychiatry. 2005;162:725-731.
Paulsen J.S., Nehl C., Hoth K.F., et al. Depression and stages of Huntington’s disease. J Neuropsychiatry Clin Neurosci. 2005;17:496-502.
Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacol Rev. 2010;35:192-216.
Prueter C., Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17:20-28.
Rao V., Rosenberg P., Bertrand M., et al. Aggression after traumatic brain injury: prevalence and correlates. J Neuropsychiatry Clin Neurosci. 21, 2009. 420–239
Ricci M., Guidoni S.V., Sepe-Monti M., et al. Clinical findings, functional abilities and caregiver distress in the early stage of dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD). Arch Gerontol Geriatr. 2009;49:e101-e104.
Ried L.D., Jia H., Cameon R., et al. Does prestroke depression impact poststroke depression and treatment? Am J Geriatr Psychiatry. 2010;18:624-633. Available at http://dx.doi.org/10.1097/JGP.0b013e3181ca822b
Robinson R.G., Schultz S.K., Castillo C., et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double blind study. Am J Psychiatry. 2000;158:351-359.
Rocca P., Marino F., Montemagni C., et al. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer’s disease: Preliminary findings from a naturalistic, retrospective study. Psychiatry Clin Neurosci. 2007;61:622-629.
Salmon E., Perani D., Collette F., et al. A comparison of unawareness in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2008;79:176-179.
Schneider L.S., Tariot P.N., Dagerman K.S., et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
Seel R.T., Macciocchi S., Kreutzer J.S. Clinical considerations for the diagnosis of major depression after moderate to severe TBI. J Head Trauma Rehabil. 2010;25:99-112.
Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67-79.
Siegert R.J., Abernethy D.A. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76:469-475.
Silver J.M., Kramer R., Greenwald S., et al. The association between head injuries and psychiatric disorders: findings from the New Haven NIMH epidemiologic catchment area study. Brain Inj. 2001;15:935-945.
Sohlberg M.M., Mateer C.A. Cognitive Rehabilitation: an Integrative Neuropsychological Approach. New York, London: The Guilford Press; 2001.
Staekenborg S.S., Gillissen F., Romkes R., et al. Behavioural and psychological symptoms are not related to white matter hyperintensities and medial temporal lobe atrophy in Alzheimer’s disease. Int J Geriatr Psychiatry. 2008;23:387-392.
Starkstein S.E., Jorge R., Mizrahi R., et al. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry. 2005;162:2086-2093.
Stefanello S., Marin-Leon L., Fernandes P.T., et al. Psychiatric comorbidity and suicidal behavior in epilepsy: A community-based case-control study. Epilepsia. 2010;51:1120-1125. Available at http://dx.doi.org/10.1111/j.1528-1167.2009.02386.x
Sutor B., Nykamp L.J., Smith G.E. Get creative to manage dementia-related behaviors. Heed “unspoken messages” before weighing antipsychotics. Curr Psychiatr. 2006;5:81-96.
Toyota Y., Ikeda M., Shinagawa S., et al. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22:896-901.
Tebartz van Elst L. Aggression and epilepsy. In: Trimble M., Schmitz B. The Neuropsychiatry of Epilepsy. Cambridge, UK: Cambridge University Press; 2002:81-106.
Tebartz van Elst L., Woermann F.G., Lemieux L., et al. Affective aggression in patients with temporal lobe epilepsy: a quantitative MRI study of the amygdala. Brain. 2000;123:234-243.
Veazey C., Aki S.O., Cook K.F., et al. Prevalence and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2005;17:310-323.
Verkaik R., Nuyen J., Schellevis F., et al. The relationship between severity of Alzheimer’s disease and prevalence of comorbid depressive symptoms and depression: a systematic review. Int J Geriatr Psychiatry. 2007;22:1063-1086.
Wilson R.S., Krueger K.R., Kamenetsky J.M., et al. Hallucinations and mortality in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:984-990.
Zamboni G., Grafman J., Krueger F., et al. Anosognosia for behavioral disturbances in frontotemporal dementia and corticobasal syndrome: A voxel-based morphometry study. Dement Cogn Disord. 2010;29:88-96.
Zamboni G., Huey E.D., Krueger F., et al. Apathy and disinhibition in frontotemporal dementia. Insights into their neural correlates. Neurology. 2008;71:736-742.
Zawacki T.M., Grace J., Paul R., et al. Behavioral problems as predictors of functional abilities of vascular dementia patients. J Neuropsychiatry Clin Neurosci. 2002;14:296-302.
Zinner S.H., Coffey B.J. Developmental and behavioral disorders grown up: Tourette’s disorder. J Dev Behav Pediatr. 2009;30:560-573.
Zorzon M., de Masi R., Nasuelli D., et al. Depression and anxiety in multiple sclerosis. A clinical and MRI study in 95 subjects. J Neurol. 2001;248:416-421.