Bedside Laboratory and Microbiologic Procedures
Assessment of Urine
Several methods are available for obtaining a urine specimen. They can be found in Table 67-1 and are listed in order of increasingly precise collection techniques, which comes at the cost of increasing difficulty, patient discomfort, or both.
TABLE 67-1
Urine Collection Methods Listed in Order of Increasing Precision
| METHOD | DESCRIPTION AND COMMENTS |
| Random voided | Any specimen provided by the patient |
| Midstream voided | No skin preparation, container placed in the urinary stream 2-3 sec after initiation of micturition |
| Clean catch | Same as above, plus antiseptic cleansing of the urethral area. This is performed by retraction of the prepuce in males and the labia in females and then cleansing the meatus in an anterior-to-posterior direction. Use three swabs soaked in povidone-iodine (or some other antiseptic solution). For female patients who are physically capable, the ideal position is sitting astride a toilet, facing backward. This helps separate the labia and position the cup for collection of the specimen |
| Midstream clean catch | Cleansing as in “clean catch,” with midstream collection as in “midstream voided” |
| Catheterized | Obtained from a newly placed catheter after cleansing of the meatus |
| Suprapubic aspiration | See Chapter 55 |
General Considerations regarding Urine Collection
The advantages and disadvantages of each of the techniques listed in Table 67-1 can be determined only by the purpose of the urine test and the clinical context. The clinical context influences interpretation of the results. For the great majority of clinical scenarios, the basic dichotomy is between specimens obtained for infectious versus noninfectious reasons. With the exception of testing for red blood cells (RBCs) and white blood cells (WBCs), most of the noninfectious tests (e.g., ketones, glucose, bilirubin, protein) are not affected by the collection method. Urine specimens collected to diagnose infection can be contaminated in a number of ways, and the choice and interpretation of tests are intricately influenced by the clinical scenario.
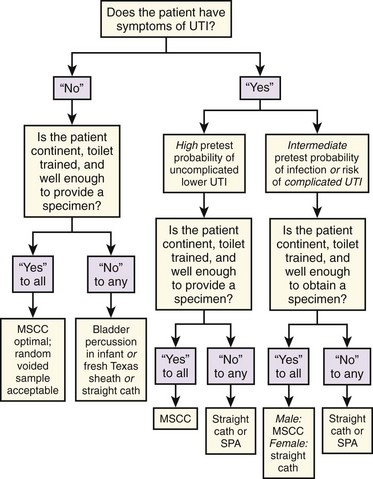
Urinary tract infections (UTIs) are either symptomatic or asymptomatic, and the symptoms determine which collection method is required (Fig. 67-1). With symptomatic UTI, extremely low levels of bacteriuria (102 colony-forming units [CFUs]/mL) and pyuria are of clinical significance.1,2 This may be obscured in some clinicians’ minds by an alternative, more widely promulgated fact: in asymptomatic patients the threshold for “significant bacteriuria” is 1000-fold higher at greater than 105 CFUs/mL.3,4 Symptomatic patients constitute a clinically distinct group who require a urine test that is much more sensitive and thus will render a false-positive result with much lower levels of contaminants. Although many studies do not show a statistically significant increase in contamination rates with less stringent urine collection techniques, most show increased accuracy with more meticulous or invasive collection methods.5–8 Because it takes only marginally longer, it makes sense to always strive for the highest-quality urine specimen available, especially in view of the delays, repeated testing, and additional cost entailed by false-positive results. A frequent misconception is that contaminated specimens are characterized by the isolation of multiple pathogens, but in fact, up to 50% of symptomatic women may have polymicrobial infections.1,9
The issue of whether a patient is “symptomatic” might appear trivial, but the clinical practice of checking for UTI in most patients with any type of abdominal pain has important implications. Studies of urine collection and testing in symptomatic patients focus on the classic signs and symptoms of UTI (e.g., urgency, frequency, dysuria, flank pain, costovertebral angle tenderness) and do not include patients with nonspecific abdominal pain. Whether undifferentiated abdominal pain or fever constitutes a “symptom” of UTI has never been studied, and how to apply the results of studies done on patients with classic symptoms to those with nonspecific symptoms is unclear.10 Patients with classic symptoms need the most careful urine collection method because they have the most riding on the outcome of the test. This group of patients should include those with systemic signs of infection (e.g., fever and chills) who are unable to accurately report their symptoms and patients in whom failure to diagnose asymptomatic bacteriuria would be potentially dangerous (e.g., the immunocompromised, neonates and infants, pregnant patients, diabetics) or for whom urine cultures are going to be necessary because of a history of relapsing, recurrent, complicated, or childhood UTI.9,11
In cooperative, motivated males and females with symptoms of uncomplicated lower UTI or pyelonephritis who are capable of diligently performing the necessary maneuvers, a midstream clean-catch (MSCC) specimen is as accurate as a catheterized specimen, especially when the possibility of urethral or prostatic trauma and patient discomfort are considered.12 In patients unable to provide an MSCC specimen, a catheterized specimen is usually warranted.
If no symptoms of UTI are present, the urine examination can be considered a “screening” test. In asymptomatic patients, routine screening for bacteriuria is unwarranted in all but two clinical situations: pregnant women and all patients scheduled for urologic surgery.13,14 If only a urine culture is to be performed, some would argue that any spontaneously voided specimen would suffice since the diagnosis of asymptomatic bacteriuria depends on 105 or more CFUs of a single pathogen per milliliter of urine. With such criteria, contaminants are usually easily identified.8 Because performing cultures on all such patients is prohibitively costly, dipstick testing, urinalysis (UA), or both are commonly used for screening.15,16 With these tests, contamination by bacteria, leukocytes, or erythrocytes results in diagnostic confusion. The advantages of a less contaminated specimen are worth the minimal, extra effort of asking the patient to provide an MSCC sample. The approach in Figure 67-1 covers the vast majority of situations. A few circumstances and techniques deserve special mention.
Bladder Percussion and the Midstream Specimen in Infants
The emergency clinician is familiar with how frequently a urine stream is generated in infants confronted by the alarming emergency department (ED) environment and a cold stethoscope. Rather than wasting a potentially perfect MSCC specimen on a laboratory coat with an ensuing delay in obtaining urine, the clinician can exploit the situation by approaching an infant with an open sterile urine container in hand in case the urine stream is spontaneously forthcoming. The process is facilitated by the application of cold povidone-iodine to the genitalia. Such an approach has been shown to generate a urine sample in a median time of 10 minutes.17 This is less than the typical time needed for straight catheterization or suprapubic aspiration (SPA), and it can be performed concomitantly with the history and physical examination and thereby circumvent an invasive procedure. If the urine specimen is not immediately forthcoming, a parent can be equipped with a sterile container and be instructed on collection of an ensuing specimen to free up ED staff for other tasks. Two techniques to actively induce voiding in infants have been described. The first, which is useful in newborns, exploits the Perez reflex.18 After cleansing the genitalia, hold the infant in one hand while stroking the paraspinal muscles in a cephalad to caudad direction. This causes extension of the back and flexion of the hips and induces micturition in less than 5 minutes in the majority of cases.18 The second technique is known as “bladder tapping.” After urethral cleansing, if there is still no urine, use two fingers to tap on the suprapubic area at a rate of approximately once per second for a full minute, followed by a minute’s rest. Repeat the cycle until urine is produced. The mean time before the production of a urine sample is about 5 minutes. This technique, though not practicable for the staff in a busy ED, can provide an infant’s parents with a task that invests them in the clinical process. This clinical pearl may expeditiously furnish a specimen with significantly less investment of staff time than required for more invasive techniques.19
Bag Collection in Non–Toilet-Trained Children
The incidence of unsuspected UTI in a febrile neonate or infant is about 5%.20 A true UTI in an infant or child requires subsequent evaluation for urinary tract pathology, and the disease may produce significant morbidity (e.g., hypertension, renal disease). One must be certain of the presence or absence of infection in this subgroup. Numerous studies have demonstrated the disutility of urine specimens obtained for culture from a collection bag stuck to an infant’s perineum.18,19,21–23 Bag specimens may be more sensitive than catheter specimens when used for UA or microscopy to identify infection in children at low or moderate risk for UTI.24 In this group it is acceptable to perform screening UA, microscopy, or both on a bag specimen.25,26 If negative, UTI is ruled out. If positive (leukocyte esterase or nitrite present or more than 5 WBCs/high-power field [HPF] on spun urine or bacteria on an unspun Gram-stained specimen), it is followed by catheterization and culture, with treatment usually pending the results of culture.21,25–27 If a urine specimen is needed solely for chemical analysis (e.g., glucose, ketones, specific gravity), a bag specimen will suffice.
Urine Specimens from Patients with Chronic Urinary Drainage Systems
Urine obtained from any part of a chronic urinary drainage system is highly inaccurate for bacteriologic purposes. If UTI is suspected, insert a new catheter and obtain a fresh bladder urine specimen.28 A small study advocating replacement of a chronically applied “Texas sheath” catheter with a fresh one was performed on subjects who did not have symptoms of UTI.29 Such a method might be sufficiently accurate for screening asymptomatic patients, but a Foley catheter should be used to obtain urine from patients with sheath catheters who have signs or symptoms of acute UTI and are unable to provide an MSCC specimen.
Catheterization and SPA
The low levels of bacteriuria found in 2% to 8% of patients after straight catheterization are generally below the threshold that defines the presence of UTI.30 Catheterization can cause minor local injury, as reflected by low-level hematuria in 15% of patients.30,31 SPA continues to be advocated by some for neonates in cases in which accurate diagnosis is essential and the risk for infection must be minimized. Both SPA (see Chapter 55) and catheterization have an approximately 25% “failure rate” as a result of an empty bladder.32 This problem and associated complications can be avoided by performing bedside ultrasound before the procedure.32–34 SPA may spuriously lower leukocyte or bacterial colony counts because of the necessity of filling the bladder before performing the procedure.35 A study in infants demonstrated that the discomfort associated with SPA is greater than that with catheterization.36 Surprisingly, however, older men who underwent both catheterization and SPA strongly preferred SPA.5
Urine Dipstick
Urine dipstick tests are available to test 10 separate parameters. The unassuming appearance and commonplace use of the urine dipstick might lead one to mistakenly underestimate its technical sophistication. Each colored square on a urine dipstick involves a biochemically complex assay, and therefore it is essential to meticulously follow the manufacturer’s instructions for storage and use.37 Even with optimal storage and testing conditions, the false-negative and false-positive rates of these tests are problematic. In addition, most of the tests are susceptible to interference from a variety of substances (Table 67-2).
TABLE 67-2
Overview of Urine Dipstick Tests
| SOURCES OF ERROR AND ARTIFACT | COMMENTS | |
| Glucose | False positive with peroxide, hypochlorite, ketonuria, levodopa, and the dipstick exposed to air False negative with ascorbate, ketones, uric acid, and high specific gravity |
Hypothermia may cause glycosuria despite hypoglycemia Glycosuria without hyperglycemia suggests renal tubular dysfunction |
| Ketones | False positive with ascorbate, low pH urine, high specific gravity, levodopa, valproate, phenazopyridine, N-acetylcysteine, high-protein diet, phenylketonuria, phthalein compounds | Very susceptible to deterioration with humidity and delay in analysis, which can cause false-negative results |
| Nitrites | False positive with phenazopyridine False negative high specific gravity, frequent urination, ascorbate, high urine pH, and urine standing in the specimen cup >2 hr |
75% false-negative rate when exposed to air for 15 days Does not detect reductase-negative bacteria |
| Protein | False positive with pH >7 and chlorhexidine False negative with low pH, very dilute urine |
Only reliable for albumin (glomerular proteinuria) Does not detect Bence-Jones protein Positive with pyuria, rarely with hematuria |
| Blood | False positive with povidone-iodine, certain (peroxidase-producing) bacteria, hypochlorite False negative with high specific gravity and high concentrations of urinary nitrites, ascorbate, or captopril |
Positive test with speckles or dots implies nonhemolyzed blood Positive test with a diffuse pattern implies hemolyzed red blood cells or high levels of myoglobin |
| Bilirubin | False positive with iodine, stool contamination, chlorpromazine, mefenamic acid False negative after prolonged standing |
Hard to read with agents causing marked urine discoloration |
| Urobilinogen | False positive with phenazopyridine, sulfisoxazole, sulfonamides, porphyrin, methyldopa, procaine, aminosalicylic acid, 5-hydroxyindolacetic acid False negative with sulfisoxazole and phenazopyridine |
Use a fresh specimen: rapidly broken down by light and in acidic urine |
| Leukocyte esterase | False positive with vaginal contamination, oxidizing agents, eosinophils in urine, Trichomonas False negative with high glucose, ketones, protein (especially albumin), pH, and specific gravity, as well as with cephalexin, tetracycline, oxalates, ascorbic acid, neutropenia |
Sterile pyuria seen with tuberculosis, nephrolithiasis, interstitial nephritis |
| pH | Urea-splitting bacteria elevate pH Run off from the protein strip can falsely lower pH |
Use a fresh specimen: standing raises pH by loss of CO2 |
| Specific gravity | Overestimates specific gravity with low pH, ketoacidosis, and protein Underestimates specific gravity with glucose, urea, or pH >7 |
Not reliable at specific gravity >1.025 Elevated with use of dextran, intravenous contrast material, proteinuria |
Method
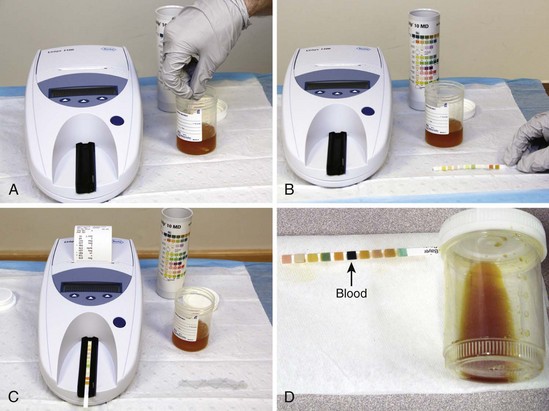
Test urine specimens as soon as possible after they are collected. If the urine has been standing, stir or shake the specimen well because cells sink rapidly in a container. Immerse the test strip completely for 1 second or less. Draw the edge of the strip along the rim of the specimen container and lightly tap it to remove excess urine, thus avoiding mixing the reagents between different test patches. Next, hold the strip horizontally or place it on a clean gauze pad until the recommended time has elapsed. Most strips are designed so that all the test results can be read together after 1 to 2 minutes (Fig. 67-2).
Interpretation
Glucose: The urine glucose test is normally negative. Urine glucose testing has limited usefulness in quantitative testing because the serum glucose level at which spillage occurs varies (although in most patients it starts at between 180 and 200 mg/dL).38 Changes in urine glucose lag behind changes in blood glucose by approximately half the interval between voids.39 Glycosuria in the absence of hyperglycemia suggests renal tubular dysfunction. Glycosuria may occur in hypothermic patients in the absence of hyperglycemia and indeed may actually occur with hypoglycemia in such patients.40
Ketones: Ketones are found in the urine of patients with starvation, inadequate carbohydrate intake, diabetic and alcoholic ketoacidosis, isopropyl alcohol poisoning, or glycogen storage disease. Tests for urine ketones are 5 to 10 times more sensitive to acetoacetate than to acetone, similar to the serum tests. Dipsticks do not detect 5-hydroxybutyrate, which accounts for 80% to 95% of the three “ketone bodies” and is the predominant form in the setting of ketoacidosis. Urine ketone testing is significantly more sensitive than serum ketone testing.41 There is generally no need to obtain “serum acetones” to diagnose or manage diabetic ketoacidosis when urine ketone monitoring is coupled with blood gas and anion gap analysis.
Leukocyte Esterase: This portion of the dipstick test is designed to detect enzymes from the azurophilic granules in neutrophils. Normally the test is negative. Studies report a wide and clinically important range of thresholds for the sensitivity of dipstick testing, from 10 to 100 WBCs/µL urine.15,16 Studies suggest that the test is between 50% and 96% sensitive in detecting infection.2,42,43 Its specificity for the presence of WBCs is between 91% and 99%. The most common cause of a false-positive leukocyte test is vaginal contamination.
Nitrites: Normally, urine does not contain nitrites. Nitrites are specific (≈95%), but not sensitive (≈45%) indicators of UTI.13,44,45 Urinary nitrates are converted to nitrites most strongly by enteric coliform bacteria, thus explaining the nitrite test’s 90% sensitivity in detecting UTI caused by Escherichia coli. Enterococcus, a moderately frequent urinary pathogen, Pseudomonas species, and Acinetobacter lack the reductase enzyme and are not detected. False-negative results also occur because of the lack of dietary nitrate, frequent voiding, and diuresis. Early morning–voided specimens are ideal because they allow time for conversion of nitrate to nitrite, but they are rarely available in the ED. If possible, a specimen obtained more than 4 hours after the last voiding is preferred.
Protein: Proteins with a molecular weight below 50,000 to 60,000 daltons can pass through the glomerulus to be reabsorbed in the proximal tubule. Normal passage of protein in urine is less than 150 mg/24 hours, or approximately 10 mg/dL of urine. About 10% to 33% of urinary protein is albumin, 33% is Tamm-Horsfall glycoprotein (secreted by renal tubular cells), and the balance is made up of a variety of immunoglobulins and other proteins. Proteinuria is a finding noted in about 5% of routine urine screens in men.46 This may represent a normal variant since 3% to 5% of healthy adults have postural proteinuria (proteinuria when standing but not when recumbent).38 Proteinuria is rarely clinically significant unless 3+ or greater on the dipstick, although lower levels are still indicative of some degree of renal dysfunction (see later). The dipstick detects negatively charged proteins more strongly than positively charged ones; it is therefore most sensitive to albumin. A study of ED patients with severe acute hypertension identified renal dysfunction, defined as elevated serum creatinine, with 100% sensitivity when using urine dipstick detection of 1+ proteinuria or hematuria.47 Along similar lines, in patients being considered for radiographic contrast-enhanced studies who do not have a serum creatinine measurement, a negative dipstick test for protein or blood combined with the absence of prior renal disease, hypertension, diabetes, congestive heart failure, and age younger than 60 years effectively excludes renal insufficiency.48 The urine dipstick test is positive for protein with pyuria of greater than 6 WBCs/HPF.49 This is a false-positive finding for protein but is helpful when the urine dipstick is being used to screen for UTI since the threshold for leukocyte esterase is often significantly higher. Hematuria only slightly elevates urine protein levels.
In assessing a patient with proteinuria, it is helpful to divide the list of causes into those that are and those that are not associated with hematuria. These are listed in Box 67-1. Elevated urinary protein is more commonly due to renal than to systemic causes. The source is either glomerular, with passage of normally unfiltered proteins, or tubular, with failure to reabsorb physiologically filtered, low-molecular-weight globulins. The former condition causes albuminuria. Renal tubular proteinuria is characterized by low levels of urinary albumin and is therefore more likely to be missed.
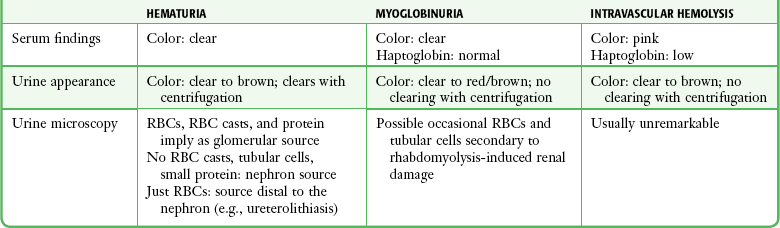
“Blood”: The blood section of the urine dipstick is positive if exposed to RBCs, hemoglobin (Hb), or myoglobin. Urine in healthy volunteers contains fewer than 7 RBCs/mL. Studies have shown that the urine dipstick is very sensitive to 10 RBCs/mL. False-negative results are confined to clinically insignificant hematuria.50 The dipstick pad should be inspected for discrete positive “dots,” indicative of nonhemolyzed RBCs. Moderate intravascular hemolysis does not cause hemoglobinuria because Hb is tightly bound to haptoglobin and is therefore not filtered. Massive intravascular hemolysis gives rise to free plasma Hb with a molecular weight of 32,000 daltons, which easily passes through the glomerulus. Myoglobin has a molecular weight of 17,000 daltons, which also allows easy glomerular passage, but the dipstick has been shown to have a sensitivity of only 14% with heat-induced rhabdomyolysis, as reflected by serum creatine phosphokinase levels of up to 1000 U/L.51 Guidelines for distinguishing hematuria, hemoglobinuria, and myoglobinuria are outlined in Table 67-3. In asymptomatic men older than 50 years, significant disease can be signaled by intermittent hematuria, thus mandating follow-up of patients with this incidental finding.52
Dipsticks are vitiated by humidity and air, which can cause false-negative results after improper storage.53 Because RBCs may lyse rapidly, delays in performing UA may misleadingly suggest myoglobinuria or hemoglobinuria. Microscopy or dipstick testing of a freshly obtained specimen can clarify this issue. Conversely, high specific gravity or low pH can inhibit lysis of erythrocytes, which is necessary for the dipstick chemical reaction to occur, thus causing false-negative results.53a A study reproducing clinical conditions has demonstrated that povidone-iodine does not cause false-positive results on the dipstick.53b Iatrogenically caused trace positive results may occur after catheterization in 15% of cases.31
Urine Bilirubin: Urine bilirubin represents the filtered, soluble, conjugated form of bilirubin. Unconjugated bilirubin is bound to protein and does not pass through the glomerulus. Bilirubinuria is therefore due to intrahepatic or extrahepatic cholestasis. Bilirubinuria will be detected significantly earlier than clinical jaundice. Urinary bilirubin excretion is enhanced by alkalosis. A fresh sample of urine should be tested because bilirubin glucuronide is hydrolyzed when exposed to light. Ascorbic acid and high levels of urinary nitrites decrease the sensitivity of the test to bilirubin.
Urobilinogen: In a healthy person, conjugated bilirubin is excreted in bile. In the colon it is broken down into a number of compounds, including urobilinogen. Most of these compounds are excreted in stool, which is the source of its characteristic color. A small amount of urobilinogen is absorbed from the colon, and if it is not taken up on the first pass through the liver, it enters the circulation. Ultimately, some of this urobilinogen may enter the urine, so it is normal to have zero to moderate levels of urinary urobilinogen on dipstick testing. Most diseases that cause hepatocyte dysfunction (hepatitis, cirrhosis, passive liver congestion, etc.) increase urinary urobilinogen excretion by impairing hepatic uptake of urobilinogen. As a qualitative test with a wide range of normal values, it is rarely helpful, but in evaluating a patient with jaundice, it can have diagnostic significance (Table 67-4).
pH: The average daily excretion of 50 to 100 mmol of H+ in urine gives rise to a typical urine pH of around 6 with a range from 4.5 to 8. Dietary protein lowers urinary pH, whereas fruit (especially citrus) and vegetables tend to raise it. The significance of pH testing is in the assessment of normal renal function. In most states of alkalosis and acidosis, healthy kidneys maintain homeostasis by conserving or excreting H+. Failure to do so suggests renal disease, especially renal tubular acidosis. An exception is the “paradoxical aciduria” of hypokalemic alkalosis secondary to volume contraction, hypercorticism, or diuretics, where the highest priority of the renal tubule is to conserve sodium. pH is elevated by the action of urea-splitting bacteria, especially Proteus species. This can occur with “stasis” of urine, either in the bladder or in specimen cups. A persistently alkaline urine is seen in patients with struvite (triple phosphate) urolithiasis.
Specific Gravity: The dipstick test for urine specific gravity assays for the primary urinary cations sodium and potassium. True specific gravity, which is also dependent on anions, albumin, proteins, urea, and glucose, is therefore not measured. Artifactually low specific gravity readings are obtained with alkaline urine, whereas acidic urine and albumin falsely elevate the specific gravity reading. Consequently, some investigators believe that these strips on the dipstick test are of marginal clinical utility.54 Other clinical indicators of a patient’s hydration status are probably more reliable. If necessary, a refractive specific gravitometer or a hygrometer should be used.
Microscopic UA
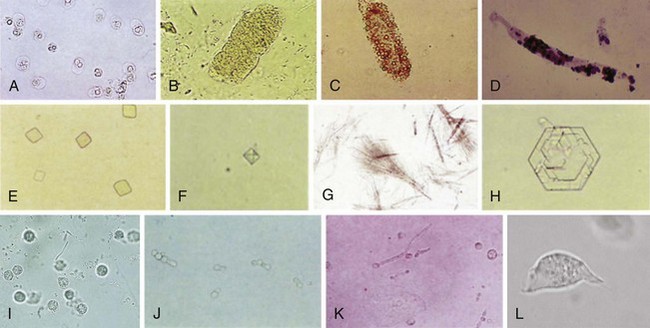
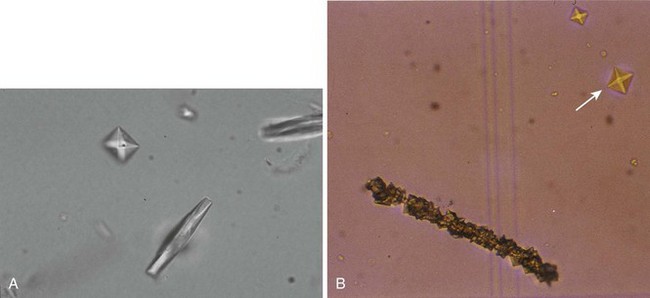
Microscopic UA is performed to identify cells, bacteria, and other microbes, as well as formed elements such as casts and crystals (Fig. 67-3). The following discussion focuses on the findings of significance in diagnosing UTI: WBCs, bacteria, and WBC casts. The presence of WBCs with bacteria distinguishes infection from colonization (bacteriuria without pyuria). Some authorities state that significant infection without pyuria occurs in less than 5% of cases, thus making pyuria alone a sensitive marker of infection.53,55 Other studies do not support reliance on pyuria by itself as an indicator of infection.2,3,5,27 The presence of WBC casts distinguishes pyelonephritis (“upper UTI”) from cystitis (“lower UTI”).
Microscopic UA is performed by one of five methods. Traditionally, the most common technique has been examination of unstained centrifuged urine. It has the advantage of concentrating formed elements that might otherwise be missed. Its disadvantage is that the presence and quantity of elements in a specimen will depend on many uncontrolled factors: the volume of the specimen, the duration and speed of centrifugation, the fragility of the formed elements, the volume of the “drop” in which the pellet is resuspended, and the size of the microscope’s HPF.14,56,57
1. Examination of unspun urine in a hemocytometer counting chamber. A hemocytometer is a precisely milled slide etched with measured squares, which allows exact enumeration of the cells in each square. Because the distance between the etched surface and the coverslip is known exactly, it is possible to determine the number of cells per unit volume of specimen. Enough fresh unspun urine is placed on the slide to fully cover the counting area, and the cells are counted. There should usually be less than 1 WBC/µL, although more than 10 WBCs/µL and more than 5 RBCs/µL are unequivocally abnormal.58 The threshold for diagnosing UTI is usually set at 8 or more WBCs/µL.9,22,56,59,60 Bacteria do not sink to the surface of the hemocytometer, so counting them through the many focal planes of the chamber is not possible, although methods to estimate the bacterial count per unit volume have been described.57
The hemocytometer is accurate, fast (time need not be spent on staining or centrifugation), and relatively easy to master. Its major drawbacks are cost (around $150 for the slide and cover slip) and fragility (easily destroyed if dropped). These characteristics are problematic given the conditions and resources in many EDs, although their use has been advocated by some.61 Formed elements other than WBCs and RBCs (e.g., casts) occur in such low concentrations that they are encountered in the hemocytometer only by chance, and therefore microscopy of spun urine is needed for their identification.
2. Examination of unspun, unstained urine placed on a regular microscope slide. This qualitative method is sometimes used for the diagnosis of UTI. Using 1 organism/HPF as a positive result, the sensitivity and specificity of detecting 105 CFUs/mL are between 60% and 90%.62 This method identifies only 1 WBC/HPF with the highly pyuric state of 250 WBCs/µL,60 which has led some to advocate the use of more than 1 WBC per low-power field as a criterion for infection.58
3. Examination of unstained, centrifuged urine. With this method, 10 mL of urine is centrifuged at approximately 450g (1000 to 4000 rpm) for 3 to 5 minutes. Roughly 9 mL of supernatant is poured off, and the pellet is resuspended in the remaining fluid. This suspension is placed on a slide with a coverslip and examined. The larger formed elements, especially casts, tend to migrate to the edge of the coverslip, and they can be seen with low magnification. One or two casts, depending on the clinical context, may be normal; more are not. The morphology of the more commonly encountered formed elements of urine sediment is shown in Figure 67-3. The significance of each is beyond the scope of this text, but this information can be found in a standard textbook of clinical laboratory procedures and diagnostic testing.38,39 When examining centrifuged urine, more than 5 WBCs/HPF in the middle of the coverslip has traditionally been taken as being indicative of abnormal pyuria. Most authors have estimated that 10 WBCs/µL is equivalent to approximately 1 WBC/HPF; thus, this oft-cited threshold for diagnosing UTI is actually equivalent to 50 WBCs/µL in unspun urine.56,58,59 Since the gold standard for the diagnosis of UTI is more than 8 WBCs/µL of unspun urine, many infections will escape detection with this method. Various numbers of bacteria per HPF have been used as criteria for the diagnosis of UTI. A threshold of 10 to 20 organisms/HPF has been recommended to rule out bacteriuria at the 105-CFU/mL level.62 As noted previously, this threshold would not exclude infection in symptomatic patients.
4. Examination of Gram-stained, uncentrifuged urine. Also a semiquantitative measurement, it is estimated that 1 bacterium/HPF is equivalent to 105 CFUs/mL in bacterial culture.38,62 A drop of urine is placed on the slide, allowed to air-dry, and then heat-fixed and Gram-stained.
5. Examination of Gram-stained, centrifuged urine. This method is probably the optimal technique, short of culture, for the assessment of bacteriuria. It is more than 95% sensitive and more than 60% specific at 104 CFUs/mL—an order of magnitude lower concentration of bacteriuria than the previously described methods.63 Detection of 1 organism per oil-immersion field constitutes a positive result. Specificity is increased to 95% if 5 organisms/HPF are seen.62
Summary of Tests Used in the Diagnosis of UTI
The three tests commonly used to evaluate a patient for the presence or absence of UTI are urine dipstick, microscopic UA, and urine culture. Each represents an increasing degree of expense, delay, and resources.64 A brief discussion of their relative strengths and weaknesses ensues.
Urine Dipstick
Dipstick testing of urine is faster than microscopic UA, is less labor-intensive and cheaper, and circumvents multiple sources of potential and proven error. Is it sufficiently accurate to replace UA, however? If either leukocyte esterase or nitrites are used to indicate infection, the dipstick is only 50% to 90% sensitive for culture-proven infection.15,43,44,63,65 This is not adequate to rule out infection in symptomatic patients, in whom the prevalence of disease is high, but may be acceptable in asymptomatic patients, in whom the test has a serviceably high negative predictive value of 95% to 99%. In symptomatic men, sensitivity is enhanced by taking a positive result in any one (or more) of either leukocyte esterase, nitrites, protein, or blood as an indication of UTI.15,66–68 This process can be augmented by allowing extra time before reading the strip. For women with symptoms of UTI, empirical treatment is recommended because no test can rule out infection.64 A negative dipstick test in a patient with a high pretest probability of UTI should prompt a search for an alternative source of the patient’s symptoms. Maneuvers to enhance dipstick sensitivity diminish its specificity, which may be as low as 26%.10 This has led some to advocate microscopic UA on all urine specimens that are found to be abnormal by dipstick,67 but this probably adds little to a carefully performed dipstick test.
Microscopic UA
If the urine dipstick has such poor specificity when it is used as a test with adequate sensitivity, should it be discarded altogether and microscopic UA be relied on instead? Apart from the hemocytometer, the most reliable method for identifying significant bacteriuria is oil-immersion microscopy of Gram-stained, centrifuged urine.62 Nonetheless, the practice in most hospital laboratories is to examine a resuspended pellet of unstained, centrifuged urine. The problems with this method have been discussed. In various studies a range between 1 and 10 organisms/HPF or 5 WBCs/HPF has been considered a “positive” test (as the threshold number rises, so does specificity, at the price of sensitivity). In aggregate, the accuracy of microscopy in the diagnosis of UTI is similar to that of the dipstick alone, with 22% false-positive and 23% false-negative rates when compared with culture.53,57,64 Microscopic UA, like the dipstick, cannot rule out infection in symptomatic patients. The specificity of pyuria will be improved when viewed as a marker of all genitourinary infections, including urethritis, prostatitis, epididymitis, vaginitis, and cervicitis. These diagnoses should always be entertained in patients with urinary symptoms, especially those with sterile pyuria.
The Bottom Line
It is well established that female patients with symptoms of uncomplicated lower UTI can be treated without culture since the prevalence of disease is 50% or greater in women with classic urinary symptoms.14,45,69,70 Treatment is generally benign, and 95% of urine cultures that are positive will yield a limited number of organisms with predictable antibiotic susceptibilities. Some authors recommend dipstick or microscopic UA (or both) because a negative test might prompt more careful consideration of alternative diagnoses in this group with a high incidence of sexually transmitted disease.14,45 If none is found, such patients can be treated empirically. Traditionally, trimethoprim-sulfamethoxazole was recommended, although increasing resistance to this drug has lead to levofloxacin (itself with unacceptably high resistance rates in many locations) being the current choice of empirical antibiotics.71,72
Urine testing is more likely to influence clinical management in patients with an intermediate pretest probability of infection.10 Urine microscopy may also help in distinguishing pyelonephritis (WBC casts), vaginitis (absence of pyuria or hematuria in a meticulous MSCC or catheterized specimen), and urethritis (pyuria, rarely hematuria) from cystitis (pyuria and often hematuria).14,45 In the ED, the ease of performing a dipstick test argues for its use without UA since it is equally sensitive unless formed elements such as casts, crystals, Trichomonas, or other parasites are suspected. In patients with pyelonephritis, urine cultures are warranted because they alter therapy in about 5% of cases.73 In most asymptomatic patients, a negative dipstick or UA result has sufficient negative predictive value to rule out disease unless the patient is pregnant or undergoing urologic surgery. Special clinical considerations in some patients (e.g., the immunocompromised, diabetic females at high risk for UTI) may mandate adjustments to this approach.15 Recommendations vary regarding infants. Cultures are recommended for all febrile infants younger than 2 months and for children who appear sick or have a high pretest probability of infection.21,23 In a recent study, combined microscopic and dipstick UA was only 64% sensitive (and 91% specific) for culture-proven UTI in febrile infants younger than 24 months, thus suggesting the use of culture unless an alternative source of infection is clear.74
Testing for Pregnancy
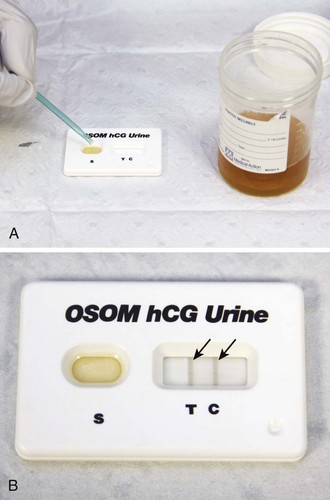
Some investigators have found female patients reliable in determining their own pregnancy status.75,76 Others have not.77 With respect to ordering of radiographic studies on women of childbearing age, clinicians should determine from their own practice experience the reliability of patient history in this regard. If in doubt and with the potentially lethal outcome of an ectopic pregnancy, it might be prudent to use an objective test. Pregnancy tests are based on the detection of β-human chorionic gonadotropin (β-hCG) in serum or urine. β-hCG is secreted by trophoblastic cells of the placenta starting from the time of implantation of the blastocyst. Qualitative serum and urine tests can detect β-hCG levels of between 15 and 25 mIU/mL (Fig. 67-4).78 The concentration of β-hCG is usually lower in urine than in serum, which accounts for the slight advantage of serum tests in detecting early pregnancy. Optimal results on urine pregnancy tests are obtained with first-voided, concentrated morning specimens. It has recently been established that whole blood is a reliable specimen for the bedside dipstick tests used in most EDs.79,80 This alternative may be lifesaving in timely recognition of an ectopic pregnancy in a hypotensive female with pelvic complaints and unknown pregnancy status when a urine specimen is unavailable or results in significant delay.
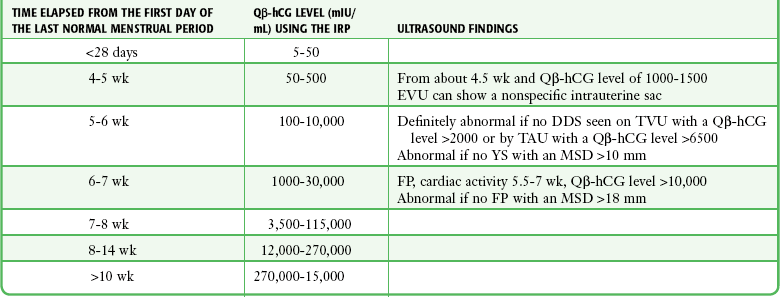
If fertilization has occurred, β-hCG levels of 5 to 8 mIU/mL (the threshold of the quantitative serum test) correspond to the 9th to 11th day after ovulation (23 to 25 days after the first day of the last normal menstrual period). In a viable intrauterine pregnancy (IUP), the β-hCG level doubles approximately every 2 days during the first 4 weeks of gestation and reaches a serum level of greater than 25 mIU/mL, which is detectable by virtually all pregnancy tests on the first day of the missed menstrual period. The doubling rate declines to every third day thereafter.81,82 β-hCG reaches a peak of between 100,000 and 200,000 mIU/mL between the 10th and 14th gestational weeks and declines to 10,000 to 20,000 mIU/mL for the rest of the pregnancy (Table 67-5). There is a wide range of β-hCG levels in different women at the same stage of gestation, thus making definite clinical determination on the basis of a single quantitative test impossible.83,84 False-positive test results have been described in association with molar pregnancy, choriocarcinoma, teratoma, occasional malignancies outside the genitourinary tract, and very high levels of proteinuria. There is one report of a positive urine β-hCG finding (but normal serum β-hCG) associated with a tuboovarian abscess.85
Although a previous quantitative β-hCG level is rarely available to the emergency clinician, doubling rates are an important part of the assessment of a healthy first-trimester pregnancy. Fetal nonviability, ectopic pregnancy, and intrauterine demise are signaled by abnormalities in the predicted rise in quantitative β-hCG.86,87 A serum quantitative β-hCG level that does not increase by 66% every 48 hours has a 75% chance of being a nonviable pregnancy.87,88 β-hCG levels in a healthy IUP and associated sonographic findings are listed in Table 67-5.
The rate of decline in quantitative β-hCG after gestation depends on the reason for the conclusion of the pregnancy. After a term delivery, β-hCG falls to zero in 2 weeks; after surgery for an ectopic pregnancy, the range is 1 to 31 days, with a median of 8.5 days; after a first-trimester spontaneous abortion, the range is 9 to 35 days (median, 19 days); and after a first-trimester elective abortion, the range is 16 to 60 days (median, 30 days).38
The association between β-hCG levels and gestational dates has led to the concept of the “discriminatory zone.” In a normal pregnancy, a quantitative β-hCG level of 1000 to 2000 mIU/mL should be reflected by the presence of a double decidual sac (at the least) on transvaginal ultrasound (and on transabdominal ultrasound with levels higher than 6500 mIU/mL).89 The clinician should beware of several potential pitfalls in applying this concept. First, it applies only to the double decidual sign, itself subject to interobserver variation among sonographers. The discriminatory zone cannot be extrapolated to the expected levels at which any of the more definitive sonographic signs of IUP such as yolk sac, fetal pole, or cardiac motion should be seen. Second, although it is true that if the double decidual sign is absent at these thresholds the pregnancy is almost certainly abnormal with a significant possibility of being ectopic, the converse—that it is pointless to perform ultrasonography if the quantitative β-hCG level is lower than 1000 mIU/mL—is not true. This is because the discriminatory zone does not preclude the possibility of identifying an ectopic pregnancy (or an IUP) before the quantitative β-hCG level reaches 1500 mIU/mL. Ectopic gestational sacs are pathological and therefore do not display β-hCG levels according to nomograms established for normal IUPs, and advanced ectopic pregnancies may have low β-hCG levels. One percent of ectopic pregnancies have a quantitative β-hCG level of less than 10 mIU/mL, and approximately a third of ectopic pregnancies are diagnosed in patients with a quantitative β-hCG level lower than 1000.90–93 Even if no definitive diagnosis can be made, the clinician should be aware that pregnant ED patients with pelvic complaints and a β-hCG level of 1000 mIU/mL or lower have a fourfold increased risk for ectopic pregnancy in comparison to those with the same symptoms and a β-hCG level higher than 1000 mIU/mL.94
In summary, the “discriminatory zone” provides a basis for interpreting the ultrasound image, but not a basis for deciding whether to perform it.89,90,95 Although the percentage of patients below the “discriminatory zone” who will receive a definitive diagnosis by ultrasound is much lower than the percentage in those above the discriminatory zone (25% versus 90%), even a 25% diagnosis rate would seem to merit pursuit for a potentially lethal condition, especially since follow-up for patients whose evaluation is “indeterminate” on their ED visit is time-consuming and resource-intensive.90,96
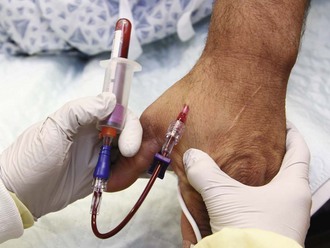
Blood Cultures in the ED
Blood cultures are indicated when the clinical findings suggest an otherwise unidentifiable bacteremic state (Box 67-2). Twenty-five percent of patients with documented bacteremia have periods without fever.97 In the elderly the proportion is even higher, with 50% of bacteremic patients older than 65 having a temperature between 97.1°F (36.2°C) and 100.9° F (38.3°C) and at least 13% with no documented temperature higher than 99.1°F (37.3°C) at any time.98–101 In the elderly, increasing age, vomiting, altered mental status, urinary incontinence, presence of a Foley catheter, or greater than 6% band forms is predictive of a positive blood culture.99,102 The subjective impression of “having fever” in adults is not a reliable indicator of the presence of fever, although the subjective impression of “no fever” is much more likely to be accurate.103 Prediction models to optimize the use of this costly test have been explored but are cumbersome, add little to educated clinical judgment, and lack widespread validation or acceptance.104–106 Many studies have shown that blood cultures in patients with uncomplicated pneumonia or pyelonephritis are of very limited clinical value,107–118 although a recent prospective investigation of patients with upper UTI found that malignancy, an indwelling urinary catheter, and ongoing antimicrobial treatment were clinical conditions that made it significantly more likely that blood cultures would be discordant with urine cultures and that they would reveal clinically important additional information.119 Blood cultures are obtained during 2.8% of all ED visits in the United States, and in a single recent 3-year period this rate increased by 33%.120 Since disease prevalence is unlikely to have changed so rapidly, this increase is probably due to changes in practice. These changes have been coincident with regulatory agency–mandated blood cultures in patients being evaluated for pneumonia and have led to calls for more stringent criteria for obtaining blood cultures.
In children, the traditional teaching that blood cultures are indicated for all patients younger than 2 years with fever higher than 38.6°C (>101.5°F) and without an obvious source is being modified by the widespread use of pneumococcal conjugate (PC) and Haemophilus influenzae type b (HIB) vaccines.121,122 In the post-PC and post-HIB vaccine era, the incidence of occult bacteremia in otherwise well-appearing febrile children is probably lower than 1%, thus making the false-positive rate at least four times as high.121–123 It seems reasonable to withhold blood cultures and empirical antibiotic coverage in otherwise well-appearing febrile children with reliable parents. The risk for bacteremia in a child is positively correlated with the degree of fever, WBC count, and rapidity of onset of the illness. It is inversely proportional to the patient’s age.124–127 In infants younger than 2 months with temperatures higher than 38°C (>100.5°F), some authorities would recommend blood cultures regardless of the presence or absence of a source, although this approach is subject to modification by experienced clinicians based on the patient’s age and clinical setting. A child with a normal temperature in the ED and a history from the parents of a tactile fever needs to be approached in the same way as a patient with fever documented on physical examination. Parents’ tactile impression of fever is reliable, and bacteremic children, like adults, have intermittent fever, with up to 50% afebrile rates in children with demonstrated bacteremia.128,129
The Controversy Regarding “Outpatient Blood Cultures”
There is a long-standing debate regarding the utility of outpatient blood cultures (i.e., blood cultures on patients who are discharged from the ED pending results). Arguments for and against outpatient blood cultures are summarized in Box 67-3. Opponents cite medicolegal issues, problems with follow-up, high contamination rates, low rates of positive cultures, and even lower rates of frequency in patients in whom therapy is changed because of culture results.130–133 Proponents also cite medicolegal concerns, positive rates similar to those seen with inpatient blood cultures, cost savings, and the benefit of diagnosing significant, yet subtle bacteremic states (such as endocarditis).134,135 They also point out that the high false-positive rates seen in many ED series should be an indictment of poor technique, not of the test itself.
On the basis of current data, it seems fiscally extravagant to admit all patients in whom a bacteremic state is possible and injudicious to deny blood cultures solely on the basis of a patient not appearing “toxic enough” to warrant admission. Societal and economic pressures to avoid hospital admission buttress these clinical considerations. Outpatient blood cultures, with due attention to collection technique, patient selection, and arrangements for follow-up, have a place in emergency practice. This approach is reflected by the fact that fully half of ED blood cultures are obtained on patients who are not admitted to the hospital.120
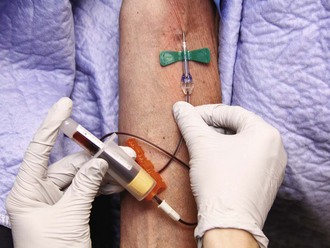
Technique for Obtaining Blood for Culture
Studies have demonstrated sources of contamination at every stage of the process of performing and processing blood cultures. In addition to obvious sources of contamination from the patient’s and phlebotomist’s skin, antiseptic agents and gloves have been implicated.136,137 Some authorities have argued that the primary source of contamination is in the laboratory processing of specimens.138 The consensus is that the most common source of contamination is the process of phlebotomy and inoculation of blood culture bottles.139 Obviously, this is the single step over which emergency clinicians have control, either directly or via protocols of technique for blood culture phlebotomy. Contamination rates are typically between 1.5% and 3%,121,140,141 although many ED series show much higher rates.132,142
A high degree of sensitivity is required for blood cultures. Many significant bacteremic illnesses have been documented with as little as 1 CFU/10 mL of blood.143,144 In a cadaver study, human skin has been shown to have a bacterial concentration of between 103 and 106 CFUs/mL on the forearm and groin, respectively.145 Designed to detect vanishingly low concentrations of bacteria, the test is clearly susceptible to false-positive results (impaired specificity) when blood must necessarily be obtained by passing a needle through the skin. Eighty percent of the skin flora is transient, superficial, and removable; 20% inhabit the sebaceous ducts and hair follicles and cannot be removed without destroying the skin.145,146 The former group consists of predominantly gram-positive and gram-negative aerobes and is the target of skin disinfectants.
The primary agents for skin disinfection are iodine compounds, alcohols, chlorhexidine, and hexachlorophene. Iodine solution remains a gold standard and kills bacteria, fungi, protozoa, and viruses but has been replaced in many institutions because of concern about skin burns and allergic reactions. Reports of the former were probably due to the use of 7% solution. The risk for a burn or an allergic reaction is thought to be negligible with the currently available 2% preparation.139 The most effective cleansing agent is tincture of iodine, which is a mixture of 2% iodine solution and 70% alcohol.147,148 Povidone-iodine 10% solution (Betadine) has a much lower free iodine concentration than iodine solution and is therefore less potent. Iodine is superior to hexachlorophene and chlorhexidine in killing gram-negative bacteria. Iodine, like other antiseptic agents, is inhibited by the presence of organic matter and thus requires thorough skin cleansing before the application of any skin disinfectant.
Ethyl or isopropyl alcohol should be used in a 60% to 80% solution. Alcohol prep pads, which generally contain 70% isopropanol, have solved traditional concerns regarding evaporation of alcohol from cotton balls stored in jars. Alcohol is a less powerful germicide than iodine in vitro and kills only 90% of surface bacteria after a full 2 minutes with reapplication to prevent drying.149 Alcohol foam applicators can avoid premature drying. Alcohol is inactive against fungi, spores, and viruses, but in vivo studies of blood culture contamination rates have shown it to compare favorably with iodine.147,150 Because iodine solution is often not available and iodophor solutions are less potent, alcohol still has an important place in skin antisepsis. In addition, alcohol is an excellent solvent, so alcohol pads may assist in skin preparation by removing dirt- and microbe-laden skin oils before the application of iodine compounds.
Chlorhexidine (Hibiclens) and hexachlorophene (pHisoHex) are antiseptics that are more effective against gram-positive than gram-negative bacteria. Both agents are absorbed intradermally, thereby offering prolonged antimicrobial activity, which is the basis of their popularity as surgical scrub and operative site preparations. This also makes them preferable agents when indwelling lines, especially central lines, are being placed.151 For routine blood culture phlebotomy, they are not as effective as alcohol and iodine combinations, although they are superior to povidone-iodine solution.141 Most studies show chlorhexidine to be more potent than hexachlorophene, and it has not been associated with induction of seizures in infants.
Box 67-4 presents a skin preparation protocol. Optimal results seem to be obtained with alcohol-iodine mixtures.145,148,152,153 The most important concept in skin disinfection is that bacteria do not die at the instant of contact with disinfectant agents. Iodine (2%), which is twice as potent as 10% povidone-iodine, requires at least 90 seconds in contact with the skin to kill 90% of surface bacteria.149 In many ED patients it will be necessary to use alcohol prep pads to remove gross dirt and debris from phlebotomy sites before initiating the steps of formal skin preparation.
Special Considerations in Obtaining Blood for Culture
“Changing the Needle” after Phlebotomy
In considering this issue it is important to emphasize the distinction between needle changing and needle recapping.154 The latter is a well-established risk to health care workers. It contravenes standard recommendations for universal precautions and should not be performed. Needle replacement using the standard needle removal device on “sharps” containers is an unquantified risk, but clearly much less dangerous than recapping.
Based on little scientific data it was long considered essential to change the phlebotomy needle before inoculating blood culture bottles. With increasing awareness of the risks associated with needlestick injuries, this practice has come under scrutiny. Studies generally show trends toward lower contamination rates with needle change, but without reaching statistical significance.155–157 Thus, not changing needles before inoculation of blood culture bottles is acceptable practice for routinely obtaining blood for culture. In situations in which the results of blood cultures are of paramount importance (e.g., suspected infectious endocarditis, where empirical antibiotics are to be started immediately), needles can be changed before inoculation of culture bottles (without recapping).
Special Access Sites
Most studies show that newly placed intravenous (IV) catheters are an acceptable source of blood specimens for culture, provided that the usual measures are taken in skin preparation.140,157 Chronically placed lines either trend toward or show statistically significant increases in contamination rates.158–161 An exception can be made for carefully tended central venous access ports in cancer patients. These cultures may have increased sensitivity in identifying bacteremia, possibly because of the fact that the catheters themselves are often a source of bacteremia in these patients.162
Heel Stick in Neonates
This technique resulted in recovery rates of bacteria equivalent to those with phlebotomy in two studies.163,164 Since approximately 25% of bacteremic infants have fewer than 5 CFUs/mL of blood, this proportion (25%) will be missed if less than 0.2 mL is obtained for culture. For this reason, heel stick should be considered a source of last resort for blood culture.
Timing of Blood Cultures
In most circumstances the timing of blood cultures is moot in the ED. Patients are sick enough to warrant the initiation of empirical antibiotics or are well enough for discharge, so two or more sets of blood need to be drawn immediately. The timing of blood cultures might become a consideration in a patient requiring admission but in whom the diagnosis of bacteremia is in doubt such that empirical antibiotic therapy is withheld. Contrary to medical lore, true-positive blood cultures are more likely if blood is drawn in the 12 hours before a fever spike.166,167 Furthermore, except for infectious endocarditis, most clinically significant bacteremia is thought to be intermittent, so multiple sets of specimens obtained at one time for culture would heighten the risk for missing the period of bacteremia.168 For patients admitted to the hospital with the tentative diagnosis of sepsis, it is theoretically advantageous to draw the three sets of blood for culture over the first 12 to 24 hours of admission.169 If immediate administration of antibiotics is indicated, the two or three sets of blood for culture should be obtained before initiation of antibiotic therapy.
Blood Culture Volumes
A large number of studies almost uniformly demonstrate that the sensitivity of blood cultures is directly related to the volume of blood cultured.170–175 In a representative study, Ilstrup and Washington showed that 20 mL and 30 mL of blood yielded, respectively, 38% and 62% more true-positive results than 10 mL did.172 Mermel and Maki showed that each additional milliliter of blood yields an average of 3% more true-positive results.175 This finding is also consistent with the fact that 40% of adults with bacteremia have less than 1 CFU/mL of blood and that 20% have less than 1 CFU/10 mL.176 Alternatively expressed, if 10 mL of blood is obtained for culture, 20% of patients with continuous bacteremia will be missed. Since most bacteremia is intermittent and endogenous factors in blood will cause some inhibition of bacterial growth even with modern lysis and filtration centrifugation techniques, the false-negative rate in clinical practice will always be significantly higher. On purely mathematical grounds, 10 mL per set of blood cultures is a bare minimum for culture. In adults, most authorities recommend at least 30 mL of blood per culture site or set.177–179 To ensure dilution of the blood’s antibacterial properties (e.g., immunoglobulins, complement, WBCs), culture bottles should contain a concentration of blood of less than 1 part blood to 10 parts medium.179 If 30 mL of blood is obtained from one site, it should be divided equally into three of the usual 100-mL broth bottles.
Volumes in Children
A blood volume of 30 mL from a 70-kg adult is equivalent to 0.5 mL of blood from a 3.5-kg neonate. Fortunately (for the utility of blood cultures), levels of bacteremia are typically 10-fold higher in neonates than in adults.180 The sicker the child, the greater the likelihood of a high level of bacteremia.180,181 As the immune system matures during infancy, levels of bacteremia might be expected to fall toward those seen in adults, so small culture volumes are at increased risk for false-negative results.182–184 As a rule of thumb, a similar volume of blood with respect to body mass should be drawn from children as is drawn from adults: approximately 1 mL/2.5 kg, or 4 mL blood per 10-kg body mass.185,186
How Many Sets of Blood Cultures Are Needed?
A set of blood cultures is the sample obtained from a single site. A 1-mL specimen from a neonate placed in an aerobic bottle and a 30 mL specimen from an adult divided between fungal, aerobic, and anaerobic bottles are both a single set of blood cultures (Fig. 67-5). Two or more sets of blood cultures make up a series.169 The information derived from the blood culture sets is pooled in such a way to make both the sensitivity and specificity of the series greater than that of the component sets. Sensitivity is enhanced because as discussed earlier, an individual set is typically not more than 80% sensitive.187 Specificity is improved for microbes that can act as both pathogens and contaminants by determining whether they appear in all sets (pathogen) or in only some (more likely a contaminant).
Although this conceptual process is applied to all blood culture series, the focus of inquiry depends on the infectious process being ruled in or out. For example, in an elderly patient with sepsis and a chronic indwelling Foley catheter, it is extremely unlikely that the causative organism is a typical skin contaminant. The usual causes of “false-positive” blood cultures will therefore be easily recognized, thus lowering the false-positive rate for the series and yielding a test with intrinsically higher specificity. At the same time, with the typical pathogens in this clinical context being nonfastidious organisms, sensitivity is typically around 99% with two sets consisting of 20 mL of blood per set.178 Conversely, in a patient with a prosthetic heart valve, fever, and signs of septic emboli, many probable pathogens are also skin contaminants, thereby lowering the specificity of each individual blood culture set. Thus, at least two sets of cultures must be positive with such organisms before the overall test (i.e., the series) is considered positive. At the same time, this clinical picture makes the pretest probability of disease very high (diminishing the negative predictive value of a negative set), so an extremely sensitive overall test (i.e., series) will be needed to adequately rule out disease. In this setting, most authorities would recommend four sets of blood culture bottles, with good volumes in each.168,187 Except in infants, single sets of blood for culture are of insufficient sensitivity or specificity to be of any utility and should not be drawn.167,168,187–189 The recommended numbers of sets of blood cultures as they relate to the pretest probability of disease and causative organism are summarized in Table 67-6.
TABLE 67-6
Numbers of Blood Culture Sets to Be Obtained in Various Clinical Situations in Adults
| NUMBER OF SETS (MINIMUM) | CLINICAL CONTEXT |
| Two sets | The cause is likely to easily be distinguished from contaminants and the pretest probability of bacteremia is low to moderate |
| Three sets | Skin contaminants are possible causes of the infectious process, the pretest probability of bacteremia is high, or infectious endocarditis is a consideration, but with a low to moderate pretest probability |
| Four sets | Infectious endocarditis AND either a moderate to a high pretest probability or the patient has recently been taking antibiotics |
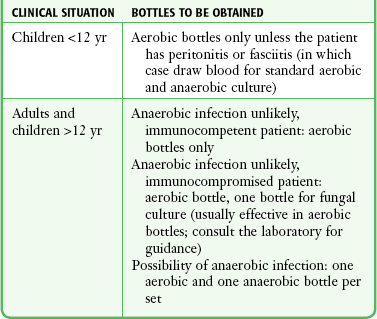
Aerobic versus Anaerobic (versus Other) Bottles
Anaerobic infections tend to occur in poorly perfused tissues or locations and frequently evolve into abscesses. Both characteristics mean that these infections tend to be isolated from the bloodstream, thereby decreasing the likelihood of bacteremia and detection by blood culture. For these reasons it is not surprising that anaerobic isolates account for 0.5% to 12% of positive blood cultures.168,190–192 Blood cultures in general have a typical true-positive rate of about 5%; with 5% or less of positive blood cultures being anaerobic, more than 400 patients need to have a complete series of blood drawn for culture to detect one case of anaerobic bacteremia.193–197 If more than 50% of anaerobic infections are clinically evident before culture, at least 800 blood culture series are needed to generate a single anaerobic result that would alter clinical management. Anaerobic cultures may also have the unintended consequence of compromising the sensitivity of aerobic cultures in situations in which less than the ideal 20 mL of blood is drawn for a blood culture set.175,192 Allocation of blood to anaerobic bottles will also diminish the likelihood of detecting fungal infections, which are increasingly common in the rising population of immunocompromised patients.198 In addition to these pathophysiologic considerations, there was a widely reported decrease in the positive anaerobic blood culture rate in the 1980s and 1990s, although some authors have recently suggested a resurgence of anaerobic bacteremia rates.190–192,194,196,199 At the current time, the arguments for selective use of anaerobic blood cultures are compelling, especially if working in an institution where there is a low rate of anaerobic bacteremia. Based on a review of this topic, Table 67-7 suggests a possible approach to the allocation of blood specimens to various blood culture media after phlebotomy.198–202 Clinical features that place a patient at high risk for anaerobic bacteremia are listed in Box 67-5.
Identifying Contaminants
The emergency clinician may receive calls from the laboratory about the results of positive blood cultures obtained on previous shifts. These may be “true positives” as a result of true contamination or may be caused by the intermittent bacteremia that occurs in normal, healthy people. This situation has been complicated by the increasingly common identification of Staphylococcus epidermidis, Streptococcus viridans, and fungi as real pathogens in blood culture series.203–206 The expense associated with false-positive blood cultures has been estimated to be $900 per episode for discharged patients and more than $5000 per episode for inpatients. These costs emphasize the importance of good technique in obtaining blood for culture.207,208 Distinguishing contaminants from clinically significant bacteremia is based on both microbiologic information and the patient’s clinical condition. Features of false-positive blood culture results are listed in Box 67-6.178,187,209 It would probably be prudent to contact discharged patients with positive blood cultures, even when contamination is suspected on a microbiologic basis, to ensure that their condition is improving.
Principles and Pitfalls in Phlebotomy for Blood Testing
A number of blood tests are ordered as a part of emergency practice, but by far the most common are the complete blood count (CBC) and serum chemistries. Many medications, substances, and diseases have been identified as causes of hematologic abnormalities besides the familiar categories of infectious, inflammatory, stress-related, neoplastic, and hematopoietic processes. These include antibiotics (especially sulfonamides), antineoplastic and therapeutic drugs, immunosuppressives, and toxins (mercury and black widow spider envenomation causing leukocytosis and arsenicals causing leukopenia). There are also substances and disease processes that cause purely artifactual errors by interfering with the equipment or procedures used to perform the tests. Examples include in vivo and in vitro hemolysis, cellular clumping, and markedly elevated platelet, leukocyte, or triglyceride levels, all of which can perturb proper functioning of the machinery used to perform blood assays. The less common causes of laboratory abnormalities (both pathophysiologic and artifactual) are legion. It is necessary for most clinicians, when encountering a confirmed laboratory abnormality, to resort to standard reference texts or online sources to review potential causes or sources of error. Some of the analytical causes of false laboratory values on the CBC are listed in (Table 67-8).
TABLE 67-8
Artifactual Causes of False Values of the CBC*
| ARTIFACTUAL INCREASE | ARTIFACTUAL DECREASE | |
| White blood cell count | Nucleated red blood cells | Multiple myeloma and other monoclonal gammopathies |
| Hemoglobin and hematocrit | Severe leukocytosis (>30,000), hyperlipemic serum, giant platelets, cryoproteins | Microcytic anemia, in vitro hemolysis |
*Improper collection techniques are the most common cause of errors in all categories.
Before checking for obscure or uncommon causes of laboratory abnormalities, one should bear in mind that the most common source of error in laboratory blood tests is in the “preanalytical phase.”210–213 This is the part of the laboratory process that actively involves the practicing clinician. Preanalytical errors have been broken down into problems with specimen loss and handling, clotting, hemolysis, inadequate volume, and patient identity.212 In one series, more than 90% of these errors related to specimen collection.214 These key components and their most common pitfalls are as follows:
1. Preparation of the site. The importance of aseptic technique in the preparation of a site to draw blood for culture has been discussed. For hematologic and serum analysis, enough time should be allowed for drying of the alcohol since trace amounts can cause hemolysis. Povidone-iodine can cause errors in several chemistry assays. When used, it should be cleaned off with alcohol, as described in the section on blood cultures.
2. Venous occlusion. Both intracellular and chemical changes start occurring in blood as soon as a tourniquet is applied, not—as is often thought—only after it has passed through a needle into a specimen container. Serum potassium levels may increase by 6% in a vessel that has been occluded for only 3 minutes.213 Venous access sites, if visually identifiable, should be prepared before placement of a tourniquet. After tourniquet application, phlebotomy and sample acquisition should proceed as rapidly as possible, ideally within 30 seconds.215 Pumping the fist should be avoided if possible because it increases serum concentrations of potassium, lactate, and phosphate.213
3. Routine phlebotomy. Smaller-gauge needles and higher suction pressure are associated with hemolysis. Overexuberant application of suction on a syringe is doubly counterproductive because in addition to causing hemolysis, the needle is likely to be occluded by the wall of the vein. This increases the likelihood of unsuccessful phlebotomy and iatrogenic injury to the patient. When applying negative or positive pressure to a syringe, a given amount of force on the plunger causes higher pressure within the chamber of a smaller-diameter syringe (pressure is proportional to 1/radius2). Blood drawn into either a standard Vacutainer or a syringe via a butterfly needle and tubing causes similar hemolysis rates (Fig. 67-6).215 If a phlebotomy site is tenuous, specimens are obtained in order of clinical priority. In most cases, tubes should be filled in the following order to avoid cross-contamination of chemicals: blood cultures, red, blue, speckled red, green, lavender, gray.213 Consistent with experience, the perception of difficult access or phlebotomy is associated with higher hemolysis rates.216,217
4. Phlebotomy through a freshly placed IV catheter. Routine phlebotomy typically causes a hemolysis rate of less than 2%.212 Reported hemolysis rates in ED patients are often 7% to 15%.217–219 This may be due to any or all of the reasons considered here, but one contributing factor is the number of blood specimens drawn in the ED through a catheter being placed for therapeutic use (Fig. 67-7). Several studies have shown that this arrangement significantly increases rates of hemolysis.217,219,220 The popularity of this technique in emergency care is most probably due to limitations of personnel and temporal resources and an attempt to enhance patient comfort by avoiding an extra needlestick. Since tourniquet times are probably longer with catheter placement than with simple phlebotomy, it is likely that this contributes to hemolysis, although it has never been experimentally verified. Perceived ease of blood aspiration, larger-bore catheters, and small aliquots drawn through the catheter are associated with lower rates of hemolysis.219,220 Laboratory results of specimens obtained in this manner are accurate within clinically acceptable margins of error.
5. Phlebotomy through an established IV catheter. An established IV line appears to be a reliable source of blood for analysis, although success rates are lower and hemolysis rates are higher than for phlebotomy from a fresh site.221,222 Box 67-7 lists common blood tests that are accurate when drawn through an established IV line.222,223 As with the use of a freshly placed catheter, results are accurate within clinical tolerances.221,224 If fluids are being administered through the catheter, it should be turned off for at least 3 minutes before the application of a tourniquet for phlebotomy. A typical 18-gauge 30-mm-long plastic IV catheter without a heparin well has a volume of 0.07 mL. The heparin well adds a volume of 0.05 mL to make a combined volume of 0.12 mL. Thus, when using a syringe directly attached to the heparin well (IV tubing detached), just 1 mL of aspirated fluid should have replaced the entire volume of the heparin well at least six times. Most studies have discarded at least three times that volume. Some have suggested a “push-pull” technique (aspirating a small volume into the syringe and reinjecting it before drawing a final 3 mL for discarding) to minimize the likelihood of small sequestered pockets of IV fluid in the heparin well.225 Use of the standard distal port of the IV tubing (usually 25 cm from the end) is not recommended. The IV tubing has a volume (combined with the heparin well and catheter) of about 1.6 mL, thus making it necessary to discard large volumes of blood, in addition to the risk of contamination from IV fluids entrained in the specimen from the main IV line during aspiration through the port.
6. Disposition of the specimen after phlebotomy. If blood has been drawn into a syringe, it should be promptly decanted into the appropriate containers for the laboratory. If it has already started to clot, it should not be forced since this can cause hemolysis of the specimen. Since cells and platelets are fragile, specimens requiring agitation (all except red and speckled red-topped tubes) should be rocked gently, not shaken. If specimens are sent to the laboratory in pneumatic tubes, they should be surrounded by shock-absorbing material. Artifactual increases in measured HCO3− occur fairly rapidly at room temperature.221 Progressive hemolysis of blood occurs with prolonged standing, and it is likely that specimens are of little utility after more than 2 to 3 hours of typical ED storage conditions. One study showed that unrefrigerated, nonagitated samples were reliable for up to 8 hours.226
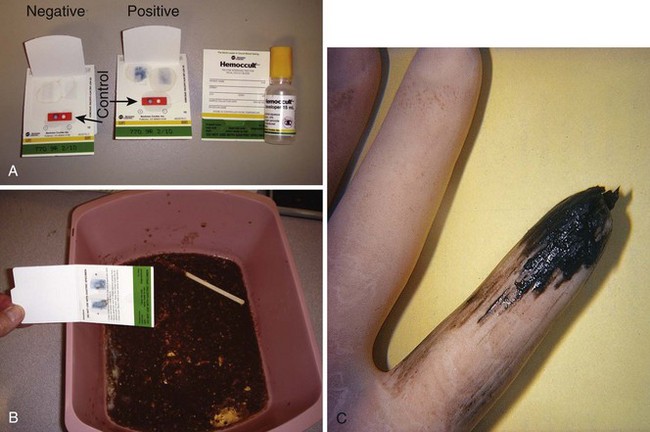
Bedside Tests for GI Hemorrhage
Testing for occult blood in stool is associated with false-positive and false-negative results, but in its primary role in emergency medical practice the test is usually reliable in detecting significant acute gastrointestinal (GI) hemorrhage (Fig. 67-8).39 Low pH, heat, dry stools, reducing substances (including antioxidants such as vitamin C), and antacids can cause false-negative findings.227–229 Slow bleeding in the upper GI tract, during which heme can be converted (denatured) to porphyrin during transit through the gut, may not be identified by stool testing. False-positive results have been attributed to the ingestion of partly cooked or large quantities of meat (dietary sources of myoglobin and Hb) and peroxidase-rich food.229,230 Most vegetables contain peroxidase, including (in decreasing order) broccoli, turnips, cantaloupe, red radishes, horseradish, cauliflower, parsnips, Jerusalem artichokes, bean sprouts, beans, lemon rind, mushrooms, parsley, and zucchini.230 A simple in vivo study convincingly called into question the possibility of peroxidase passing through the stomach without being denatured.231 False-positive test results can also be caused by the presence of povidone-iodine solution at concentrations of less than 0.1% (a 1% dilution of the 10% solutions commonly available at the bedside), but they are uncommon. A positive test should be considered evidence of the presence of blood until proved otherwise. Routine iron supplementation does cause black stool but does not a cause a positive Hemoccult test result despite early in vitro studies to the contrary.232,233
Normal GI blood loss is limited to less than 2.5 mL/day, which translates to less than 2 mg of Hb per gram of stool (0.2% by weight).234 The sensitivity of the Hemoccult test varies both with the concentration of Hb present in stool and with the extent of Hb exposure to the proteolytic effects of the digestive tract. The Hemoccult test is 37% sensitive to stool containing 2.5 mg Hb/g of stool but 95% sensitive when the concentration is 20 mg Hb/g of stool, thus indicating that low to moderate levels of blood may be missed.38 The test is much more likely to detect lower GI hemorrhage than an identical rate of upper GI bleeding because of the 100-fold diminution in the peroxidase activity of blood during transition through the GI tract.71 Impaired detection of Hb may also occur as a result of dilution because of diarrheal illness.38,39,235 Recent regulatory attempts in the United States to improve quality control in bedside occult blood testing seem to have had the unintended consequence of discouraging digital rectal examination in patients for whom they are indicated.236,237 In the event of a trace positive Hemoccult test that is not the source of an emergency illness, notification of the patient and advice regarding further outpatient evaluation should not be overlooked.228
Method
Smear the stool specimen onto the reagent area on the card and add a drop of developer. Because the reaction must occur in an aqueous medium, add a drop of water to very dry specimens and allow it to moisten the specimen before adding the developer. Adding water increases the false-positive rate.228,229 Formation of a blue color on the paper anywhere around or under the specimen within 60 seconds should be considered a positive result.
Testing for Gastric Blood
Heme tests designed for use on stool specimens can be unreliable when applied to gastric juices, with increasing inaccuracies being reported as the pH decreases.238,239 Although a positive test of gastric contents with a fecal Hemoccult card is likely to be accurate, a negative result with the fecal Hemoccult card does not rule out the presence of blood. The Gastroccult card uses a modified guaiac developer containing buffers to neutralize gastric acid, thereby facilitating accurate detection of Hb. The test works on the same basis as the fecal guaiac test in that it uses the properties of Hb as a peroxidase. In product testing, the Gastroccult card was 100% sensitive in detecting specimens with greater than 500 ppm of blood by volume, equivalent to 0.05% or 0.25 mL of blood in 500 mL of gastric contents.
Blood Glucose Meters
Bedside testing of capillary blood for glucose levels is a common procedure performed in the ED and is generally an excellent substitute for venous blood testing. A variety of meters are available and they are reasonably accurate (±10%) when a small drop of blood is electronically analyzed (Fig. 67-9). In patients with poor tissue perfusion, the accuracy of determining hypoglycemia is less precise and may vary up to about 5% from venous blood. In the setting of hypoperfusion, bedside measurement of whole blood is preferable. Bedside testing is also less accurate in patients with extreme hypoglycemia or hyperglycemia, but readings are sufficiently accurate to alert the clinician to very high or very low glucose levels. Fingertip capillary blood is the preferred specimen for bedside glucose meter testing. Blood from alternative sites, such as the skin of the forearm, may give slightly lower results than those taken at the fingertips since they may sample venous blood rather than capillary blood. When blood glucose concentrations are rising rapidly or falling rapidly (such as a hypoglycemic response secondary to rapidly acting insulin), blood glucose results from alternative sites may yield significantly delayed results (up to 30 minutes) when compared with finger stick readings, which are generally accurate at all time points.
Diagnostic and Therapeutic Toxicologic Bedside Procedures
Noninvasive Diagnostic Procedures
A simple colorimetric test for detecting amatoxins (the Meixner test) has been developed that can be used on gastric contents, stool, or actual mushroom samples. The basis of this test is the acid-catalyzed color reaction of amatoxins with lignin, a complex organic compound found in wood pulp (Fig. 67-10). Cheaper grades of paper (e.g., newsprint or the whiter pages of a telephone book) contain high amounts of lignin. Although there have been no extensive reports of in vivo studies, in vitro tests have shown this method to be somewhat sensitive and relatively specific for amatoxins, but it should be considered an adjunctive test only. Psilocybin-containing mushrooms can cause false-positive results for amatoxin.240

Figure 67-10 The Meixner test is a crude assay for amatoxins. Place portions of the unknown dried mushroom on low-grade newsprint and add 10-N hydrochloric acid. The dried-up mushroom (left) is Galerina marginata, which yields a blue reaction (positive = probably deadly poisonous); the little brown mushroom (right) does not (negative = toxicity uncertain). This test is the only one readily available but has varying accuracy and depends on the paper being used (regular newsprint is shown here). (Courtesy of Kathie T. Hodge and Kent Loeffler [photographer], Cornell University. Available at http://blog.mycology.cornell.edu.)
The procedure for the qualitative detection of amatoxin consists of squeezing a drop of liquid from a fresh mushroom sample or squashing a piece of fresh mushroom onto a piece of newspaper. If stool or gastric samples are the only specimens available, mix the sample with reagent-grade methanol (99.8%) to extract the amatoxin. If the samples are mixed with methanol, centrifuge and filter them. Then place a drop of the liquid extract on newspaper. Gently air-dry all specimens at room temperature and avoid direct sunlight. Add two to three drops of concentrated hydrochloric acid (37%) to the dried specimen. Use an adjacent area for control. High amounts of amatoxin in the dried samples produce a blue color in 1 to 2 minutes. Small amounts of amatoxin yield a blue color in the sampled area in 10 to 20 minutes. This procedure has not been proved to be effective with other bodily secretions, such as blood or urine.240
Mothball Identification
At present, commercial mothballs are composed of either nontoxic paradichlorobenzene or possibly toxic naphthalene. Naphthalene can cause a hemolytic reaction in neonates and patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.241 In the past, mothballs have also been produced from camphor, which can cause central nervous system depression and seizures. Fortunately, camphor mothballs are no longer commercially available, although they may still exist in older households. Rapid differentiation between these groups of mothballs can expedite patient management and disposition. Several bedside tests have been reported to facilitate this.
1. Paradichlorobenzene is heavier than naphthalene. In turn, naphthalene is heavier than camphor. In lukewarm tap water, camphor will float and naphthalene and paradichlorobenzene will sink. In a solution of 3 tbsp of table salt thoroughly dissolved in 4 oz of lukewarm water, camphor and naphthalene will float and paradichlorobenzene will sink.
2. Paradichlorobenzene has a lower melting point than naphthalene does. Paradichlorobenzene mothballs will melt in a water bath at 53°C, whereas naphthalene requires a water bath hotter than 80°C.
3. Paradichlorobenzene is described as “wet and oily,” whereas naphthalene is described as having a “dry” appearance. Paradichlorobenzene is familiar to many people as a cake of disinfectant used in urinals and diaper pails.
Body Secretion Analysis
Careful analysis of bodily secretions, the odor emanating from poisoned patients, and the color of their urine can help identify certain toxins. Some characteristic smells and urine colors are listed in Table 67-9 and Box 67-8.
TABLE 67-9
| CHARACTERISTIC ODOR | RESPONSIBLE DRUG OR TOXIN |
| Acetone (sweet, fruity; pearlike) | Lacquer, ethanol, isopropyl alcohol, chloroform, diabetic ketoacidosis, alcoholic ketoacidosis, trichloroethane, paraldehyde, chloral hydrate, methylbromide, Pseudomonas infection |
| Alcohols | Ethanol (congeners), isopropyl alcohol |
| Ammonia-like | Uremia |
| Automobile exhaust | Carbon monoxide (odorless, but associated with exhaust) |
| Beer (stale) | Scrofula |
| Bitter almond | Cyanide |
| Carrots | Cicutoxin (or water hemlock) |
| Coal gas (stove gas) | Carbon monoxide (odorless, but associated with coal gas) |
| Disinfectants | Phenol, creosote |
| Eggs (rotten) | Hydrogen sulfide, carbon disulfide, mercaptans, disulfiram, N-acetylcysteine |
| Feculent | Intestinal obstruction |
| Fish or raw liver (musty) | Hepatic failure, zinc phosphide, hypermethioninemia, trimethylaminuria |
| Fruitlike | Nitrites (e.g., amyl, butyl), ethanol (congeners), isopropyl alcohol |
| Garlic | Phosphorus, tellurium, arsenic, parathion, malathion, selenium, dimethyl sulfoxide, thallium |
| Halitosis | Acute illness, poor oral hygiene |
| Hay | Phosgene |
| Mothballs | Naphthalene, p-dichlorobenzene, camphor |
| Peanuts | N-3-pyridyl-methyl-N–p-nitrophenyl urea (Vacor) |
| Pepper-like | O-chlorobenzylidene malonitrile |
| Putrid | Anaerobic infections, esophageal diverticulum, lung abscess, scurvy |
| Rope (burned) | Marijuana, opium |
| Shoe polish | Nitrobenzene |
| Sweating feet | Isovaleric acid acidemia |
| Tobacco | Nicotine |
| Vinegar | Acetic acid |
| Vinyl-like | Ethchlorvynol (Placidyl) |
| Violets | Turpentine (metabolites excreted in urine) |
| Wintergreen | Methyl salicylate |
From Chiang WK. Otolaryngologic principles. In: Goldfrank LR, Flomenbaum NE, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 5th ed. East Norwalk, CT: Appleton & Lange; 1994:374.
Bedside Toxicologic Tests on Urine
Ethylene Glycol: Evaluation of the urine of patients who may have ingested ethylene glycol can be helpful. Urine should be tested for fluorescence (an additive in many commercial antifreeze products) under an ultraviolet light and for the presence of calcium oxalate crystals (a metabolic by-product of ethylene glycol metabolism).
The presence of calcium oxalate crystals (either envelope-shaped calcium dihydrate or needle-shaped calcium monohydrate) in urine on microscopic inspection is indicative of high oxalate levels in serum (Fig. 67-11; see also Fig. 67-3F). Calcium monohydrate crystals can easily be confused with sodium urate crystals; therefore, the presence of the dihydrate crystal tends to be more specific for ethylene glycol ingestion. Lack of these crystals does not rule out significant ethylene glycol ingestion because excretion of these crystals may occur late in the ingestion (>6 hours) and occasionally does not occur at all.242,243
Visual inspection of urine under a Wood lamp or ultraviolet light to ascertain fluorescence may also be helpful in the diagnosis of ethylene glycol exposure. Antifreeze is the most common source of ingested ethylene glycol. Fluorescein, the actual fluorescing material, is often placed in commercially available antifreeze to enable mechanics to detect radiator leaks with a Wood lamp or other ultraviolet light source. Fluorescein is a nontoxic inert vegetable dye that is eliminated unchanged in urine. Therefore, high levels of fluorescein in urine suggest significant ethylene glycol ingestion. However, lack of fluorescein does not rule out a significant exposure because not all antifreezes contain fluorescein or high concentrations of fluorescein in relation to ethylene glycol. False-positive findings can occur if certain plastic urine containers are used.244
To perform this procedure, place the test urine sample and control samples into separate glass test tubes. Inspect for fluorescence under a Wood lamp in a dark room. Always perform this test with controls that include urine that does not contain fluorescein and urine that does contain fluorescein. The use of controls may increase the sensitivity and specificity from 49% and 75%, respectively, to a sensitivity and specificity of 100%.245 Fluorescein is readily available because fluorescein-containing strips are commonly used in ophthalmologic procedures (see Chapter 62).
Salicylates: Several bedside tests have been developed to qualitatively detect salicylates in urine, including 10% ferric chloride solution, Trinder’s solution, and Phenistix reagent strips. All are rapid, inexpensive, sensitive tests that give a qualitative rather than a quantitative result.
Ferric chloride and Trinder’s solution both have sensitivities of 100% with serum salicylate levels of 5 mg/dL. False positives can occur with both tests. Acetoacetic acid, acetone, and phenylpyruvic acid will cause false-positive results. Thus, this test may be falsely positive in patients with diabetic, alcoholic, or starvation ketoacidosis. Phenol-containing drugs such as diflunisal, sulfasalazine, and salicylamide may also produce false positives. Any positive result requires a confirmatory quantitative serum salicylate assay.246
The ferric chloride test is a commonly used rapid, qualitative, urinary screening procedure. To perform this test, add several drops of 10% ferric chloride to 1 or 2 mL of urine that has been collected in a test tube. The immediate appearance of a bluish purple color signifies that salicylates are present in the urine (Fig. 67-12). This test is very sensitive, and just one aspirin tablet taken within 12 to 24 hours will give a positive result. It will require 60 to 120 minutes from the time of ingestion for this reaction to become positive in patients with normal renal function, so early test results may be misleading.
Bedside Toxicologic Tests on Oral Secretions and Breath: Ethyl Alcohol
Breath alcohol analyzers have been developed since the 1950s and are currently used in law enforcement. These devices typically use infrared spectral analysis to determine the concentration of alcohol in expired air. Almost all the alcohol found in expired air at the level of the mouth is secondary to alcohol diffused from the bronchial system rather than the alveolar system.247 Minor alterations in breathing patterns can cause large variations in readings. Thus, uncooperative patients who do not exhale properly will cause an inaccurate reading. Other causes of inaccurate readings include the recent ingestion of alcohol-containing products, belching or vomiting, use of inhalers, poor technique, or restrictive pulmonary pathology.
Alcohol concentrations in saliva have been shown to correlate with serum concentrations. Bedside measurement of salivary alcohol concentrations can also be obtained with a dipstick-like device. These devices use an enzymatic reaction involving alcohol dehydrogenase to measure alcohol concentrations.248 Patients who are dehydrated (a common occurrence in alcohol-intoxicated patients) are often unable to provide adequate saliva samples, and inaccurate readings have occurred in patients with high blood alcohol concentrations.249,250
Bedside Toxicologic Tests on Blood: Methemoglobinemia
To evaluate for methemoglobinemia, place a drop of sample blood on a white background (a white coffee filter is appropriate) in a well-lit environment. Next to this, place a drop of normal blood as a comparison control sample. Blood with methemoglobinemia appears “darker” or “chocolate brown.”251 This method relies on the ability of the examiner to distinguish changes in color and may therefore have a degree of interobserver variance. Methemoglobin levels of less than 10% may alter the color of blood only slightly and thereby cause a false-negative finding. Methemoglobin levels of between 12% and 14% may cause a false-negative reading 50% of the time. Methemoglobin levels greater than 15% are reported to cause a cyanotic appearance in patients. With levels of 35% or greater, identification of methemoglobinemia by visual inspection of the color of blood is quite accurate.251 At this level, almost all patients are obviously cyanotic and symptomatic.
Invasive Diagnostic Procedures
Naloxone
Naloxone hydrochloride (Narcan) is a µ-opioid receptor antagonist. A diagnostic challenge with IV naloxone has been recommended for all patients with central nervous system depression.252 Certain clinical findings such as miosis, decreased respiratory rate, and evidence of illicit drug use can predict many patients who will respond to a challenge dose of naloxone.253 If a patient’s mental status improves significantly after a dose of naloxone, the patient should be considered to have been exposed to an opioid substance. This is true even if a laboratory drug screen is negative for opioids. Furthermore, many of the synthetic opiates, such as fentanyl, propoxyphene, meperidine, methadone, and pentazocine, may not be detected by the routinely used immunoassay drug screen. Although cases have been reported of patients with nonopioid overdoses (such as alcohol or phencyclidine) responding to naloxone, these single observations have not been confirmed in controlled animal or human studies.
The traditional challenge dose of naloxone in an adult or child is 2 mg every 2 minutes intravenously until a response is achieved or 10 mg is given.254 Some clinicians prefer to use much smaller doses (0.1 to 0.2 mg) and titrate to effect. This may partially reverse the opioid overdose–related symptoms and confirm the diagnosis without precipitating the opioid withdrawal syndrome seen in patients with opioid dependency. Most patients with an opioid overdose will exhibit some response to 1 to 4 mg of naloxone, but some massive overdoses might require larger amounts. A patient who does not respond at all to 10 mg of naloxone probably does not have a pure opioid overdose. High doses of naloxone may be needed to reverse many synthetic opiates, such as propoxyphene and methadone. Lower doses can be given (0.4 to 0.8 mg in adults or 0.01 mg/kg in children) to reverse known opioid-induced respiratory depression without reversing the analgesia. Because naloxone has a half-life of between 30 and 60 minutes, a continuous drip of naloxone can be used to avoid resedation. A reasonable choice is to use two thirds of the initial bolus dose that achieved the desired reversal effect as the hourly IV dose. For example, a patient who satisfactorily responded to 1.5 mg of naloxone might receive a naloxone solution of 10 mg of naloxone in 500 mL of normal saline at a rate of 1 mg (50 mL) per hour intravenously. Nalmefene, a long-acting opioid receptor antagonist that has a terminal half-life of roughly 11 hours, can also be given to patients with suspected overdose. Theoretically, a single dose of nalmefene will be effective longer than the effects of heroin or most abused opiate substances. The initial recommended dose is 1.0 to 1.5 mg intravenously.
Flumazenil
Flumazenil (Romazicon) is a competitive benzodiazepine receptor antagonist that has the ability to reverse the central nervous system depression caused by all currently commercially available benzodiazepines. Its routine use in the setting of possible benzodiazepine overdose is controversial but is supported as a diagnostic and therapeutic agent in selected cases. Unlike naloxone, flumazenil can have significant side effects, but only in certain subsets of patients,253 and its downsides are probably exaggerated in many texts. Complications include precipitation of seizures or a withdrawal syndrome in benzodiazepine-dependent patients. To minimize the chance of seizures, flumazenil should be avoided in known benzodiazepine-dependent patients and those who may have ingested epileptogenic drugs (e.g., cyclic antidepressants, cocaine, theophylline, lithium, carbamazepine, or isoniazid).
If a patient responds to flumazenil with an improvement in depressed mental status, this suggests only that the patient is under the influence of a benzodiazepine. Flumazenil can partially reverse the effects of many of the newer nonbenzodiazepine sleeping agents that affect the γ-aminobutyric acid pathway, such as zolpidem, zopiclone, and eszopiclone. Flumazenil can improve mental status in patients with hepatic encephalopathy.255–257 It does not have any significant effect on alcohol, barbiturates, and other nonbenzodiazepine sedative-hypnotics.
Physostigmine
Physostigmine is an acetylcholinesterase inhibitor that can penetrate into the central nervous system and thus can reverse both the central and peripheral effects of anticholinergic agents. In the majority of patients with anticholinergic toxicity, no laboratory tests are available to rapidly confirm the diagnosis, and testing for specific drugs is limited or unavailable. A clinical picture that may consist of mydriasis, dry and flushed skin, dry mucous membranes, urinary incontinence, absent bowel sounds, tachycardia, hyperthermia, hallucinations, agitation, and seizures suggests an anticholinergic syndrome. In some cases (low-dose antihistamines and others), only a central nervous system syndrome characterized by hallucinations, agitation, and confusion exists. A rapid and dramatic response to physostigmine frequently confirms a diagnosis of anticholinergic toxicity. In these patients, physostigmine often decreases the degree of agitation and confusion.258–260 The use of physostigmine as a diagnostic challenge can be helpful in selected situations, but similar to flumazenil, routine use of physostigmine as a diagnostic bedside challenge in all obtunded patients, especially in those without anticholinergic findings, should be discouraged. In some cases the agitation produced by potent anticholinergics, such as scopolamine, is totally resistant to benzodiazepines, and judicious use of physostigmine is warranted to avoid excessive sedation, chemical paralysis, or impaired ventilation. Physostigmine has recently been used to reverse a curious anticholinergic syndrome precipitated by propofol sedation.
Deferoxamine
Deferoxamine is an organic compound derived from the bacterium Streptomyces pilosus and can chelate iron. It can be used as a therapy or as a diagnostic challenge in patients with iron overdoses. Patients who have unstable vital signs or significant GI or central nervous system symptoms usually require therapeutic doses of deferoxamine. Asymptomatic patients with a history of iron overdose typically require supportive care only. Patients with persistent but mild symptoms, such as vomiting and diarrhea, may be given a diagnostic challenge dose of deferoxamine. A diagnostic challenge is preferential over ancillary laboratory testing because tests such as iron levels and total iron-binding capacity in the setting of iron overdose can be inaccurate, misleading, and time-consuming.261
Invasive Therapeutic Procedures
Alkalinization of Urine and Blood
Alkalinization of urine consists of manipulating the pH of urine to enhance the excretion of certain drugs (Box 67-9). Weak acids remain in ionic form in a basic milieu. The ionic form often prevents reabsorption of that drug in the proximal tubule, and urinary alkalinization can therefore promote elimination in urine. For certain drugs, this can play a significant role in their elimination. For example, salicylate elimination increases proportionately to the urinary flow rate, but it increases exponentially with increases in urinary pH. The increased serum clearance attributed to alkalinization does not correlate well with outcome or length of hospitalization.
Urinary alkalinization can sometimes be difficult to achieve or maintain. Hypovolemia is probably the leading cause of an inability to achieve alkaline urine. Other theoretical causes are hypokalemia, hypomagnesemia, and hypochloremia. Several authors have suggested that in patients with severe salicylate poisoning, urinary alkalinization may be difficult, if not impossible to achieve.262 Some will empirically add potassium and magnesium to the diuresis fluid.
Ethanol Infusion
Fomepizole (4-methylpyrazole) has been approved by the U.S. Food and Drug Administration for the treatment of ethylene glycol poisoning. It has also been used successfully in treating methanol poisoning.263,264 When compared with the traditional treatment of toxic alcohol poisoning, namely, ethanol, fomepizole has the advantages of ease of use, fewer side effects (specifically hypoglycemia), and ability to maintain therapeutic levels.263,265 Fomepizole is considered the antidote of choice; however, because of the cost and the logistics of stocking this antidote, many hospitals might not have this drug readily available.
Ethanol can be used as a therapeutic intervention in patients with methanol or ethylene glycol poisoning because of ethanol’s much greater affinity for alcohol dehydrogenases. These enzymes metabolize methanol and ethylene glycol to toxic by-products. However, with serum ethanol levels of 100 mg/dL, minimal amounts of ethylene glycol or methanol are metabolized by alcohol dehydrogenases.116,118 Ethanol infusions are not useful in the treatment of isopropyl alcohol poisoning.
Ethanol can be administered orally or intravenously (Table 67-10). IV ethanol has the advantages of achieving therapeutic levels rapidly, ensuring complete absorption, limiting the chance of aspiration, and avoiding gastritis. A 5% concentration of ethanol, which can be given in a peripheral vein, requires the use of large fluid volumes. In a 70-kg patient, a loading dose requires 1.4 L of a 5% solution, with a maintenance dose of 700 mL/hr. If IV ethanol is given, maintain careful attention to cardiopulmonary status. In contrast, oral loading can be achieved with much lower volumes. However, oral loading can be difficult in an uncooperative or unconscious patient or if vomiting or GI hemorrhage is present. A therapeutic level is reached more slowly with oral loading.
TABLE 67-10
Use of Ethanol for Methanol or Ethylene Glycol Poisoning
*If a 5% ethanol solution is used, double the volume of the loading dose.
†Equivalent to a 40-proof solution.
‡If a 5% ethanol solution is used, double the volume rate; monitor closely for potential volume overload.
§Serum ethanol levels should be monitored closely.
 At higher infusion rates, it may be necessary to administer by volume rather than by milliliters per hour.
At higher infusion rates, it may be necessary to administer by volume rather than by milliliters per hour.
References
1. Stamm, WE, Counts, GW, Running, KR, et al. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463.
2. Kunin, CM, White, LV, Hua, TH. A reassessment of the importance of “low-count” bacteriuria in young women with acute urinary symptoms. Ann Intern Med. 1993;119:454.
3. Kunin, C, Southall, I, Paquin, AJ. Epidemiology of urinary tract infections. N Engl J Med. 1960;263:817.
4. Nicolle, LE, Bjornson, J, Harding, G, et al. Bacteriuria in elderly institutionalized men. N Engl J Med. 1983;309:1420.
5. Lipsky, BA, Ireton, RC, Fihn, SD, et al. Diagnosis of bacteriuria in men: specimen collection and culture interpretation. J Infect Dis. 1987;155:847.
6. Lohr, JA, Donowitz, LG, Dudley, SM. Bacterial contamination rates for non–clean-catch and clean-catch midstream urine collection in boys. J Pediatr. 1986;109:659.
7. Walter, FG, Knopp, RK. Urine sampling in ambulatory women: midstream clean catch versus catheterization. Ann Emerg Med. 1989;18:166.
8. Immergut, MA, Gilbert, EC, Frensilli, FJ, et al. The myth of the clean catch urine specimen. Urology. 1981;17:339.
9. Stamm, WE, Wagner, KF, Amsel, R, et al. Causes of the acute urethral syndrome in women. N Engl J Med. 1980;303:409.
10. Sultana, RV, Zalstein, S, Cameron, P, et al. Dipstick urinalysis and the accuracy of the clinical diagnosis of urinary tract infection. J Emerg Med. 2001;20:13.
11. Komaroff, AL. Urinalysis and urine culture in women with dysuria. Ann Intern Med. 1986;104:212.
12. Lipsky, BA, Ireton, RC, Fihn, SD, et al. Diagnosis of bacteriuria in men: specimen collection and culture interpretation. J Infect Dis. 1987;155:847.
13. Pels, RJ, Bor, DH, Woolhandler, S, et al. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders: II Bacteriuria. JAMA. 1989;262:1214.
14. Stamm, WE, Hooton, TM. Management of urinary tract infections in adults. N Engl J Med. 1993;329:1328.
15. Loo, SY, Scottolini, AG, Luangphinith, S, et al. Urine screening strategy employing dipstick analysis and selective culture: an evaluation. Am J Clin Pathol. 1984;81:634.
16. Mariani, AJ, Luangphinith, S, Loo, SY, et al. Dipstick chemical urinalysis: an accurate cost-effective screening test. J Urol. 1984;132:64.
17. Amir, J, Ginzburg, M, Straussberg, R, et al. The reliability of midstream urine cultures from circumcised male infants. Am J Dis Child. 1993;147:969.
18. Boehm, JJ, Haynes, JL. Bacteriology of “midstream catch” urines. Am J Dis Child. 1966;111:366.
19. Taylor, MR, Dillon, M, Keane, CT. Reduction of mixed growth rates in urine by using a finger tap method. Br Med J. 1986;292:990.
20. Shaw, KN, Gorelick, M, McGowan, KL, et al. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102(2):e16.
21. Crain, EF, Gershel, JC. Urinary tract infections in febrile infants younger than eight weeks of age. Pediatrics. 1990;86:363.
22. Hardy, JD, Furnell, PM, Brumfitt, W. Comparison of sterile bag, clean catch, and suprapubic aspiration in the diagnosis of urinary infection in early childhood. Br J Urol. 1976;48:279.
23. Aronson, AS, Gustafson, B, Svenningsen, NW. Combined suprapubic aspiration and clean voided urine examination in infants and children. Acta Paediatr Scand. 1973;62:396.
24. McGillivray, D, Mok, E, Mulrooney, E, et al. A head-to-head comparison: “clean-void” bag versus catheter urinalysis in the diagnosis of urinary tract infection in young children. J Pediatr. 2005;147:451–456.
25. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Pediatrics. 1999;103:843–852.
26. Whiting, P, Westwood, M, Bojke, L, et al. Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model. Health Technol Assess. 2006;10(36):iii–iv. [xi-xiii, 1-154].
27. Hoberman, A, Wald, ER, Penchansky, L, et al. Enhanced urinalysis as a screening test for urinary tract infection. Pediatrics. 1993;91:1196.
28. Grahn, D, Norman, DC, White, ML, et al. Validity of urinary catheter specimen for diagnosis of urinary tract infection in the elderly. Arch Intern Med. 1985;145:1858.
29. Ouslander, JG, Greengold, BA, Silverblatt, FJ, et al. An accurate method to obtain urine for culture in men with external catheters. Arch Intern Med. 1987;147:286.
30. Sullivan, NM, Sutter, VL, Carter, WT, et al. Bacteremia after genitourinary tract manipulation: bacteriological aspects and evaluation of various blood culture systems. Appl Microbiol Biotechnol. 1972;23:1101.
31. Hockberger, RS, Schwartz, B, Connor, J. Hematuria induced by urethral catheterization. Ann Emerg Med. 1987;16:550.
32. Chen, L, Hsiao, AL, Moore, CL, et al. Utility of bedside bladder ultrasound before urethral catheterization in young children. Pediatrics. 2005;115:108–111.
33. Milling, TJ, Jr., Van Amerongen, R, Melville, L, et al. Use of ultrasonography to identify infants for whom urinary catheterization will be unsuccessful because of insufficient urine volume: validation of the urinary bladder index. Ann Emerg Med. 2005;45:510–513.
34. Baumann, BM, McCans, K, Stahmer, SA, et al. Volumetric bladder ultrasound performed by trained nurses increases catheterization success in pediatric patients. Am J Emerg Med. 2008;26:18–23.
35. Pollock, HM. Laboratory techniques for detection of urinary tract infection and assessment of value. Am J Med. 1983;75:79.
36. Kozer, E, Rosenbloom, E, Goldman, D, et al. Pain in infants who are younger than 2 months during suprapubic aspiration and transurethral bladder catheterization: a randomized, controlled study. Pediatrics. 2006;118:e51–e56.
37. Gallagher, EJ, Schwartz, E, Weinstein, R. Performance characteristics of urine dipsticks stored in open containers. Am J Emerg Med. 1990;8:121.
38. Henry JB, ed. Clinical Diagnosis and Management by Laboratory Methods, 19th ed, Philadelphia: Saunders, 1996.
39. Jacobs DS, DeMott WR, Finley PR, et al, eds. Laboratory Test Handbook, 3rd ed, Cleveland, OH: Lexicomp, 1994.
40. Fitzgerald, FT. Hypoglycemia and accidental hypothermia in an alcoholic population. West J Med. 1980;133:105.
41. Fraser, K, Fretter, MC, Mast, RL, et al. Studies with a simplified nitroprusside test for ketone bodies in urine, serum, plasma, and milk. Clin Chim Acta. 1965;11:372.
42. Gelbart, SM, Chen, WT, Reid, R. Clinical trial of leukocyte test strips in routine use. Clin Chem. 1983;29:997.
43. Propp, DA, Weber, D, Ciesla, ML. Reliability of a urine dipstick in emergency department patients. Ann Emerg Med. 1989;18:560.
44. Pfaller, MA, Koontz, FP. Laboratory evaluation of leukocyte esterase and nitrite tests for the detection of bacteriuria. J Clin Microbiol. 1985;21:840.
45. Johnson, JR, Stamm, WE. Urinary tract infections in women. Ann Intern Med. 1989;111:906.
46. Woolhandler, S, Pels, RJ, Bor, DH, et al. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA. 1989;262:1214.
47. Karras, DJ, Heilpern, KL, Riley, L, et al. Urine dipstick as a screening test for serum creatinine elevation in emergency department patients with severe hypertension. Acad Emerg Med. 2002;9:27.
48. Firestone, DN, Band, RA, Hollander, JE, et al. Use of a urine dipstick and brief clinical questionnaire to predict an abnormal serum creatinine in the emergency department. Acad Emerg Med. 2009;16:699–703.
49. Wenz, B, Lampasso, JA. Eliminating unnecessary urine microscopy. Am J Clin Pathol. 1989;92:78.
50. Moore, GP, Robinson, M. Do urine dipsticks reliably predict micro hematuria? The bloody truth!. Ann Emerg Med. 1988;17:257.
51. Young, SE, Miller, MA, Docherty, M. Urine dipstick testing to rule out rhabdomyolysis in patients with suspected heat injury. Am J Emerg Med. 2009;27:875–877.
52. Messing, EM, Young, TB, Hunt, VB, et al. The significance of asymptomatic microhematuria in men 50 or more years old: findings of a home screening study using urinary dipsticks. J Urol. 1987;137:919.
53. Stamm, WE, Running, K, McKevitt, M, et al. Treatment of the acute urethral syndrome. N Engl J Med. 1981;304:956.
53a. Levin, K, Engström, I. Inadequate hemolysis of erythrocytes at low pH causes false negative readings. Clin Chem. 1984;30:1845.
53b. Niemi, TA, Fischer, RP, Gervin, AS. Povidone iodine: a cause for false-positive dipstick hematuria? Ann Emerg Med. 1984;13:984.
54. Assadi, FK, Fornell, L. Estimation of urine specific gravity in neonates with a reagent strip. J Pediatr. 1986;108:995.
55. Stamm, WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75:53.
56. Brumfitt, W. Urinary cell counts and their value. J Clin Pathol. 1965;18:550.
57. Vickers, D, Ahmad, T, Coulthard, MG. Diagnosis of urinary tract infection in children: fresh urine microscopy or culture? Lancet. 1991;338:767.
58. Musher, DM, Thorsteinson, SB, Airola, VM, II. Quantitative urinalysis: diagnosing urinary tract infection in men. JAMA. 1976;236:2069.
59. Gadeholt, H. Quantitative estimation of urinary sediment, with special regard to sources of error. Br Med J. 1964;15:47.
60. Stansfeld, JM. The measurement and meaning of pyuria. Arch Dis Child. 1962;37:257.
61. Shaw, KN, McGowan, KL, Gorelick, MH, et al. Screening for urinary tract infection in infants in the emergency department: which test is best? Pediatrics. 1998;101(6):E1.
62. Jenkins, RD, Fenn, JP, Matsen, JM. Review of urine microscopy for bacteriuria. JAMA. 1986;255:3397.
63. Lohr, JA, Portilla, MG, Geuder, TG, et al. Making a presumptive diagnosis of urinary tract infection by using urinalysis performed in an on-site laboratory. J Pediatr. 1993;122:22.
64. Goldsmith, BM, Campos, JM. Comparison of urine dipstick, microscopy, and culture for the detection of bacteriuria in children. Clin Pediatr (Phila). 1990;29:214.
65. Bonnardeaux, A, Somerville, P, Kaye, M. A study of the reliability of dipstick urinalysis. J Clin Nephol. 1994;41:167.
66. Sewell, DL, Burt, SP, Gabbert, NJ, et al. Evaluation of the Chemstrip 9 as a screening test for urinalysis and urine culture in men. Am J Clin Pathol. 1985;83:740.
67. Gutman, SI, Solomon, RR. The clinical significance of dipstick negative, culture positive urines in a veterans population. Am J Clin Pathol. 1987;88:204.
68. Morrison, MC, Lum, G. Dipstick testing of urine—can it replace urine microscopy? Am J Clin Pathol. 1986;85:590.
69. Kellogg, JA, Manzella, JP, Shaffer, S, et al. Clinical relevance of culture versus screens for the detection of pathogens in urine specimens. Am J Med. 1987;83:739.
70. Fihn, SD. Clinical practice. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259–266.
71. Hooton, TM, Besser, R, Foxman, B, et al. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin Infect Dis. 2004;39:75–80.
72. Guneysel, O, Onur, O, Erdede, M, et al. Trimethoprim/sulfamethoxazole resistance in urinary tract infections. J Emerg Med. 2009;36:338–341.
73. Thanassi, M. Utility of urine and blood cultures in pyelonephritis. Acad Emerg Med. 1997;4:797.
74. Reardon, JM, Carstairs, KL, Rudinsky, SL, et al. Urinalysis is not reliable to detect a urinary tract infection in febrile infants presenting to the ED. Am J Emerg Med. 2009;27:930–932.
75. Minnerop, MH, Garra, G, Chohan, JK, et al. Patient history and physician suspicion accurately exclude pregnancy. Am J Emerg Med. 2011;29:212–215.
76. Strote, J, Chen, G. Patient self assessment of pregnancy status in the emergency department. Emerg Med J. 2006;23:554–557.
77. Ramoska, EA, Sacchetti, AD, Nepp, M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18:48–50.
78. Mishalani, SH, Seliktar, J, Braunstein, GD. Four rapid serum-urine combination assays of choriogonadotropin compared and assessed. Clin Chem. 1994;40:1944.
79. Fromm, C, Likourezos, A, Haines, L, et al. Substituting whole blood for urine in a bedside pregnancy test. J Emerg Med. 2012;43:478–482.
80. Habboushe, JP, Walker, G. Novel use of a urine pregnancy test using whole blood. Am J Emerg Med. 2011;29:840. [e3-e4].
81. Maymon, R, Shulman, A, Maymon, BB, et al. Ectopic pregnancy, the new gynecologic epidemic disease: review of the modern work up and the non-surgical treatment option. Int J Fertil Womens Med. 1992;37:146.
82. Speroff, L, Glass, RH, Kase, NG. Clinical Gynecological Endocrinology and Infertility, 5th ed. Baltimore: Williams & Wilkins; 1994.
83. Cole, LA, Kardana, A. Discordant results in human gonadotropin assays. Clin Chem. 1992;38:263.
84. Creinin, MD, Meyn, L, Klimashko, T. Accuracy of serum beta-human chorionic gonadotropin cutoff values at 42 and 49 days’ gestation. Am J Obstet Gynecol. 2001;185:966.
85. Levsky, ME, Handler, JA, Suarez, RD, et al. False-positive urine beta-hCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407.
86. DeCherney AH. Ectopic pregnancy. American College of Obstetricians and Gynecologists Technical Bulletin No. 150, Dec 1990, p 2.
87. Kadar, N, Caldwell, BU, Romero, RA. A method for screening for ectopic pregnancy and its indications. Obstet Gynecol. 1981;58:162.
88. Kadar, N, Romero, R. Serial human chorionic gonadotropin measurements in ectopic pregnancy. Am J Obstet Gynecol. 1988;158:1239.
89. Bree, RL, Edwards, M, Bohm-Velez, M, et al. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol. 1989;153:75.
90. Counselman, FL, Shaar, GS, Heller, RA, et al. Quantitative β-hCG levels less than 1000 mIU/mL in patients with ectopic pregnancy: pelvic ultrasound still useful. J Emerg Med. 1998;16:699.
91. Dart, RG, Kaplan, B, Cox, C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: how often is the study diagnostic? Ann Emerg Med. 1997;30:135.
92. Dimarchi, JN, Kosasa, TS, Hale, RW. What is the significance of human gonadotropin value in ectopic pregnancy? Obstet Gynecol. 1989;74:851.
93. Romero, RA, Kadar, N, Copel, JA, et al. The effect of different human chorionic gonadotropin assay sensitivity on screening for ectopic pregnancy. Am J Obstet Gynecol. 1985;153:72.
94. Kaplan, BC, Dart, RG, Moskos, M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med. 1996;28:10.
95. Marill, KA, Ingmire, TE, Nelson, BK. Utility of a single beta HCG measurement to evaluate for absence of ectopic pregnancy. J Emerg Med. 1999;17:419.
96. Barnhart, KT, Simhan, H, Kamelle, SA. Diagnostic accuracy of ultrasound above and below the beta-hCG discriminatory zone. Obstet Gynecol. 1999;94:583.
97. Musher, DM, Fainstein, V, Young, EJ, et al. Fever patterns: their lack of clinical significance. Arch Intern Med. 1979;139:1225.
98. Castle, SC, Norman, DC, Yeh, M, et al. Fever response in elderly nursing home residents: are the older truly colder? J Am Geriatr Soc. 1991;39:853.
99. Fontanarosa, PB, Kaeberlein, FJ, Gerson, LW, et al. Difficulty in predicting bacteremia in elderly emergency department patients. Ann Emerg Med. 1992;2:842.
100. Gleckman, R, Hilbert, D. Afebrile bacteremia: a phenomenon in geriatric patients. JAMA. 1982;248:1478.
101. Sinclair, D, Svendsen, A, Marrie, T. Bacteremia in nursing home patients. Prevalence among patients presenting to an emergency department. Can Fam Physician. 1998;44:317.
102. Richardson, JP, Hricz, L. Risk factors for the development of bacteremia in nursing home patients. Arch Fam Med. 1995;4:785.
103. Buckley, RG, Conine, M. Reliability of subjective fever in triage of adult patients. Ann Emerg Med. 1996;27:693.
104. Bates, DW, Cook, EF, Goldman, L, et al. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113:495.
105. Yehezkelli, Y, Subah, S, Elhanan, G, et al. Two rules for early prediction of bacteremia: testing in a university and a community hospital. J Gen Intern Med. 1996;11:98.
106. Tokuda, Y, Miyasato, H, Stein, GH. A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98:813–820.
107. Hickey, RW, Bowman, MJ, Smith, GA. Utility of blood cultures in pediatric patients found to have pneumonia in the emergency department. Ann Emerg Med. 1996;27:721.
108. Paisley, JW, Lauer, BA. Pediatric blood cultures. Clin Lab Med. 1994;14:17.
109. Waterer, GW, Wunderink, RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95:78.
110. Wing, DA, Park, AS, Debuque, L, et al. Limited clinical utility of blood and urine cultures in the treatment of acute pyelonephritis during pregnancy. Am J Obstet Gynecol. 2000;182:1437.
111. Campbell, SG, Marrie, TJ, Anstey, R, et al. Utility of blood cultures in the management of adults with community acquired pneumonia discharged from the emergency department. Emerg Med J. 2003;20:521–523.
112. Shah, SS, Alpern, ER, Zwerling, L, et al. Risk of bacteremia in young children with pneumonia treated as outpatients. Arch Pediatr Adolesc Med. 2003;157:389–392.
113. Campbell, SG, Marrie, TJ, Anstey, R, et al. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest. 2003;123:1142–1150.
114. McMurray, BR, Wrenn, KD, Wright, SW. Usefulness of blood cultures in pyelonephritis. Am J Emerg Med. 1997;15:137–140.
115. Velasco, M, Martinez, JA, Moreno-Martinez, A, et al. Blood cultures for women with uncomplicated acute pyelonephritis: are they necessary? Clin Infect Dis. 2003;37:1127–1130.
116. Mills, AM, Barros, S. Are blood cultures necessary in adults with pyelonephritis? Ann Emerg Med. 2005;46:285–287.
117. Ramanujam, P, Rathlev, NK. Blood cultures do not change management in hospitalized patients with community-acquired pneumonia. Acad Emerg Med. 2006;13:740–745.
118. Kennedy, M, Bates, DW, Wright, SB, et al. Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005;46:393–400.
119. van Nieuwkoop, C, Bonten, TN, van’t Wout, JW, et al. Risk factors for bacteremia with uropathogen not cultured from urine in adults with febrile urinary tract infection. Clin Infect Dis. 2010;l50(11):e69–e72.
120. McCaig, LF, McDonald, LC, Cohen, AL, et al. Increasing blood culture use at US hospital emergency department visits, 2001 to 2004. Ann Emerg Med. 2007;50:42–48. [48.e1-e2].
121. Alpern, ER, Alessandrini, EA, Bell, LM, et al. Occult bacteremia from a pediatric emergency department: current prevalence, time to detection, and outcome. Pediatrics. 2000;106:505.
122. Sard, B, Bailey, MC, Vinci, R. An analysis of pediatric blood cultures in the postpneumococcal conjugate vaccine era in a community hospital emergency department. Pediatr Emerg Care. 2006;22:295–300.
123. Wilkinson, M, Bulloch, B, Smith, M. Prevalence of occult bacteremia in children aged 3 to 36 months presenting to the emergency department with fever in the postpneumococcal conjugate vaccine era. Acad Emerg Med. 2009;16:220–225.
124. Browne, GJ, Ryan, JM, McIntyre, P. Evaluation of a protocol for selective empiric treatment of fever without localising signs. Arch Dis Child. 1997;76:129.
125. Haddon, RA, Barnett, PL, Grimwood, K, et al. Bacteraemia in febrile children presenting to a paediatric emergency department. Med J Aust. 1999;170:475.
126. Isaacman, DJ, Shults, J, Gross, TK, et al. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics. 2000;106:977.
127. Kuppermann, N, Fleisher, GR, Jaffe, DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med. 1998;31:679.
128. Banco, VD. Ability of mothers to subjectively assess the presence of fever in their children. Am J Dis Child. 1984;138:976.
129. Kline, MW, Lorin, MI. Bacteremia in children afebrile at presentation to an emergency room. Pediatr Infect Dis J. 1987;6:197.
130. Eisenberg, JM, Rose, JD, Weinstein, AJ. Routine blood cultures from febrile outpatients: use in detecting bacteremia. JAMA. 1976;236:2863.
131. Stair, TO. Outpatient blood cultures: retrospective and prospective audits in one ED. Ann Emerg Med. 1984;13:986.
132. Sturmann, KM, Bopp, J, Molinari, D, et al. Blood cultures in adult patients released from an urban emergency department: a 15-month experience. Acad Emerg Med. 1996;3:768.
133. Howie, N, Gerstenmaier, JF, Munro, PT. Do peripheral blood cultures taken in the emergency department influence clinical management? Emerg Med J. 2007;24:213–214.
134. Epstein, D, Raveh, D, Schlesinger, Y. Adult patients with occult bacteremia discharged from the emergency department: epidemiological and clinical characteristics. Clin Infect Dis. 2001;32:559.
135. Sklar, DP, Rusnak, R. The value of outpatient blood cultures in the emergency department. Am J Emerg Med. 1987;5:95.
136. Berger, SA. Pseudobacteremia due to contaminated alcohol swabs. J Clin Microbiol. 1983;18:974.
137. York, MK. Bacillus species bacteremia traced to gloves used in the collection of blood from patients with acquired immune deficiency syndrome. J Clin Microbiol. 1990;28:2114.
138. Shahar, E, Wohl-Gottesman, B, Shenkman, L. Contamination of blood cultures during venepuncture: fact or myth? Postgrad Med J. 1990;66:1053.
139. Washington, JA. Collection, transport, and processing of blood cultures. Clin Lab Med. 1994;14:59.
140. Everts, RJ, Vinson, EN, Adholla, PO, et al. Contamination of catheter-drawn blood cultures. J Clin Microbiol. 2001;39:3393.
141. Mimoz, O, Karim, A, Mercat, A, et al. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture. A randomized, controlled trial. Ann Intern Med. 1999;131:834.
142. Stalnikowicz, R, Block, C. The yield of blood cultures in a department of emergency medicine. Eur J Emerg Med. 2001;8:93.
143. Henry, NK, McLimans, CA, Wright, AJ, et al. Microbiological and clinical evaluation of the isolator lysis-centrifugation blood culture tube. J Clin Microbiol. 1983;17:864.
144. Kellogg, JA, Manzella, JP, Bankert, DA. Frequency of low-level bacteremia in children from birth to fifteen years of age. J. Clin Microbiol Rev. 2000;38:2181.
145. Selwyn, S, Ellis, H. Skin bacteria and skin disinfection reconsidered. Br Med J. 1972;1:136.
146. Lovell, DL. Preoperative skin preparation with reference to surface bacteria contaminants, and resident flora. Surg Clin North Am. 1946;26:1053.
147. Story, P. Testing of skin disinfectants. Br Med J. 1952;2:1128.
148. Strand, CL, Wajsbort, RR, Sturmann, K. Effect of iodophor versus iodine tincture skin preparation on blood culture contamination rates. JAMA. 1993;269:1004.
149. Harvey, SC. Antiseptics and disinfectants; fungicides and ectoparasiticides. In: Gilman AG, Goodman LS, Rall TW, et al, eds. Goodman and Gilman’s The Pharmacologic Basis of Therapeutics. 7th ed. New York: Macmillan; 1980:959.
150. Lee, S, Schoen, I, Malkin, A. Comparison of use of alcohol with that of iodine for skin antisepsis in obtaining blood cultures. Am J Clin Pathol. 1967;47:646.
151. Maki, DG, Ringer, M, Alvarado, CJ. Prospective randomized trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991;338:339.
152. Little, JR, Murray, PR, Traynor, PS, et al. A randomized trial of povidone-iodine compared with iodine tincture for venipuncture site disinfection: effects on rates of blood culture contamination. Am J Med. 1999;107:119.
153. Schifman, RB, Pindur, A. The effect of skin disinfection materials on reducing blood culture contamination. Am J Clin Pathol. 1993;99:536.
154. Waters, BL. Inoculating blood cultures: recapping needles and contamination rates. JAMA. 1991;265:1685.
155. Leisure, MK, Moore, DM, Schwartzman, JD, et al. Changing the needle when inoculating blood cultures: a no-benefit and high-risk procedure. JAMA. 1990;264:2111.
156. Krumholz, HM, Cummings, S, York, M. Blood culture phlebotomy: switching needles does not prevent contamination. Ann Intern Med. 1990;113:290.
157. Smart, D, Baggoley, C, Head, J, et al. Effect of needle changing and intravenous cannula collection on blood culture contamination rates. Ann Emerg Med. 1993;22:65.
158. Bryant, JK, Strand, CL. Reliability of blood cultures collected from intravenous catheters versus venepuncture. Am J Clin Pathol. 1987;88:113.
159. Tonneson, A, Peuler, M, Lockwood, WR. Culture of blood drawn by catheter versus venepuncture. JAMA. 1976;235:1877.
160. Tafuro, P, Colbourn, D, Gurevich, I, et al. Comparison of blood culture obtained simultaneously by venepuncture and from vascular lines. J Hosp Infect. 1986;7:283.
161. Wormser, GP, Onorato, IM, Preminger, TJ, et al. Sensitivity and specificity of blood cultures obtained through intravenous catheters. Crit Care Med. 1990;18:152.
162. DesJardin, JA, Falagas, ME, Ruthazer, R, et al. Clinical utility of blood cultures drawn from indwelling central venous catheters in hospitalized patients with cancer. Ann Intern Med. 1999;131:641.
163. Mangurten, HH, LeBeau, LJ. Diagnosis of neonatal bacteremia by a microblood culture technique. J Pediatr. 1977;90:990.
164. Knudsen, RP, Alden, ER. Neonatal heel stick blood culture. Pediatrics. 1980;65:505.
165. Orlowski, JP, Porembka, DT, Gallagher, JM, et al. The bone marrow as a source of lab studies. Ann Emerg Med. 1989;18:1348.
166. Bennett, IL, Beeson, PB. Bacteremia: a consideration of some experimental bacteremias. Yale J Biol Med. 1954;226:241.
167. Washington, JA. Collection, transport, and processing of blood cultures. Clin Lab Med. 1994;14:59.
168. Washington, JA. Evolving concepts on the laboratory diagnosis of septicemia. Infect Dis Clin Pract. 1993;1:65.
169. Chandrasekar, PH, Brown, WJ. Clinical issues of blood cultures. Arch Intern Med. 1994;154:841.
170. Hall, MM, Ilstrup, DM, Washington, JA. Effect of volume of blood cultured on detection of bacteremia. J Clin Microbiol. 1976;3:643.
171. Tenney, JH, Reller, LB, Mirrett, S, et al. Controlled evaluation of the volume of blood cultured on detection of bacteremia. J Clin Microbiol. 1982;15:558.
172. Ilstrup, DM, Washington, JA. The importance of volume of blood cultured in the detection of bacteremia and fungemia. Diagn Microbiol Infect Dis. 1983;1:107.
173. Arpi, M, Bentzon, MW, Jenson, J, et al. Importance of blood volume cultured in the detection of bacteremia. Eur J Clin Microbiol Infect Dis. 1989;8:838.
174. Brown, DF, Warren, RE. Effect of sample volume on yield of positive blood cultures for adult patients with hematological malignancy. J Clin Pathol. 1990;43:777.
175. Mermel, LA, Maki, DG. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med. 1993;119:270.
176. Kellogg, JA, Manzella, JP, Bankert, DA. Frequency of low-level bacteremia in children from birth to fifteen years of age. J. Clin Microbiol Rev. 2000;38:2181.
177. Kellogg, JA. Selection of a clinically satisfactory blood culture system: the utility of anaerobic media. Clin Microbiol Newslett. 1995;17:121.
178. Weinstein, MP, Reller, LB, Murphy, JR, et al. The clinical significance of a positive blood culture: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults: I Laboratory and epidemiological observations. Rev Infect Dis. 1983;5:35.
179. Washington, JA, Ilstrup, DM. Blood cultures: issues and controversies. Rev Infect Dis. 1986;8:792.
180. Dietzman, DE, Fisher, GW, Schoenknecht, FD. Neonatal Escherichia coli septicemia—bacterial counts in blood. J Pediatr. 1974;85:128.
181. Mangurten, HH, LeBeau, LJ. Diagnosis of neonatal bacteremia by a microblood culture technique. J Pediatr. 1977;90:990.
182. Durbin, WA, Szymczak, EG, Goldman, DA. Quantitative blood cultures in childhood bacteremia. J Pediatr. 1978;92:778.
183. Neal, PR, Kleiman, MB, Reynolds, JK, et al. Volume of blood submitted for culture from neonates. J Clin Microbiol. 1986;24:353.
184. Szymczak, EG, Barr, JT, Durbin, WA, et al. Evaluation of blood culture procedures in a pediatric hospital. J Clin Microbiol. 1979;9:88.
185. Campos, JM. Detection of bloodstream infections in children. Eur J Clin Microbiol Infect Dis. 1989;8:815.
186. Paisley, JW, Lauer, BA. Pediatric blood cultures. Clin Lab Med. 1994;14:17.
187. Aronson, MD, Bor, DH. Blood cultures. Ann Intern Med. 1987;106:246.
188. Novis, DA, Dale, JC, Schifman, RB, et al. Solitary blood cultures: a College of American Pathologists Q-probes study of 132,778 blood culture sets in 333 small hospitals. Arch Pathol Lab Med. 2001;125:1290.
189. Roberts, FJ. The value of the second blood culture. J Infect Dis. 1993;168:795.
190. Dorsher, CW, Rossenblatt, JE, Wilson, WR, et al. Anaerobic bacteremia: decreasing rate over a 15-year period. Rev Infect Dis. 1991;13:633.
191. Murray, PR, Traynor, P, Hopson, D. Critical assessment of blood culture techniques: analysis of recovery of obligate and facultative anaerobes, strict aerobic bacteria, and fungi in aerobic and anaerobic culture bottles. J Clin Microbiol. 1992;30:142.
192. Sharp, SE. Routine anaerobic blood cultures: still appropriate today? Clin Microbiol Newslett. 1991;13:23.
193. Chandler, MT, Morton, ES, Byrd, RP, Jr., et al. Reevaluation of anaerobic blood cultures in a veteran population. South Med J. 2000;93:986.
194. Lombardi, DP, Engleberg, NC. Anaerobic bacteremia: incidence, patient characteristics, and clinical significance. Am J Med. 1992;92:53.
195. Salonen, JH, Eerola, E, Meurman, O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26:1413–1417.
196. Saito, T, Senda, K, Takakura, S, et al. Anaerobic bacteremia: the yield of positive anaerobic blood cultures: patient characteristics and potential risk factors. Clin Chem Lab Med. 2003;41:293–297.
197. Lassmann, B, Gustafson, DR, Wood, CM, et al. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44:895–900.
198. Mylotte, JM, Tayara, A. Blood cultures: clinical aspects and controversies. Eur J Clin Microbiol Infect Dis. 2000;19:157.
199. Geerdes, HF, Ziegler, D, Lode, H, et al. Septicemia in 980 patients at a university hospital in Berlin: prospective studies during four selected years between 1979 and 1989. Clin Infect Dis. 1992;15:991.
200. Zaidi, AK, Knaut, AL, Mirrett, S, et al. Value of routine anaerobic blood cultures for pediatric patients. J Pediatr. 1995;127:263–268.
201. Thomas, JG, Meda, BA. Anaerobic cultures: tailoring their selective use. Clin Microbiol Newslett. 1995;17:125.
202. Morris, AJ, Wilson, ML, Mirrett, S, et al. Rationale for the selective use of anaerobic blood cultures. J Clin Microbiol. 1993;31:2110.
203. DesJardin, JA, Falagas, ME, Ruthazer, R, et al. Clinical utility of blood cultures drawn from indwelling central venous catheters in hospitalized patients with cancer. Ann Intern Med. 1999;131:641.
204. Jarvis, WR, Martone, WJ. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29:19.
205. Silva, HL, Strabelli, TM, Cunha, ER, et al. Nosocomial coagulase negative staphylococci bacteremia: five-year prospective data collection. Braz J Infect Dis. 2000;4:271.
206. Zierdt, CH. Evidence for transient Staphylococcus epidermidis bacteremia in patients and in healthy humans. J Clin Microbiol. 1983;17:628.
207. Bates, DW, Goldman, L, Lee, TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265:365.
208. Segal, GS, Chamberlain, JM. Resource utilization and contaminated blood cultures in children at risk for occult bacteremia. Arch Pediatr Adolesc Med. 2000;154:469.
209. St Geme, JW, III., Bell, LM, Baumgart, S, et al. Distinguishing sepsis from blood culture contamination in young infants with blood cultures growing coagulase-positive staphylococci. Pediatrics. 1990;86:157.
210. Howanitz, PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med. 2005;129:1252–1261.
211. Young, DS. Conveying the importance of the preanalytical phase. Clin Chem Lab Med. 2003;41:884–887.
212. Romero, A, Munoz, M, Ramos, JR, et al. Identification of preanalytical mistakes in the stat section of the clinical laboratory. Clin Chem Lab Med. 2005;43:974–975.
213. Young, DS, Bermes, EW, Haverstick, DM. Specimen collection and processing. In: Burtis CA, Ashwood ER, Bruns DE, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. Philadelphia: Saunders, 2006.
214. Bonini, P, Plebani, M, Ceriotti, F, et al. Errors in laboratory medicine. Clin Chem. 2002;48:691–698.
215. Lippi, G, Salvagno, GL, Brocco, G, et al. Preanalytical variability in laboratory testing: influence of the blood drawing technique. Clin Chem Lab Med. 2005;43:319–325.
216. Dugan, L, Leech, L, Speroni, KG, et al. Factors affecting hemolysis rates in blood samples drawn from newly placed IV sites in the emergency department. J Emerg Nurs. 2005;31:338–345.
217. Dwyer, DG, Fry, M, Somerville, A, et al. Randomized, single blinded control trial comparing haemolysis rate between two cannula aspiration techniques. Emerg Med Australas. 2006;18:484–488.
218. Grant, MS. The effect of blood drawing techniques and equipment on the hemolysis of ED laboratory blood samples. J Emerg Nurs. 2003;29:116–121.
219. Cox, SR, Dages, JH, Jarjoura, D, et al. Blood samples drawn from IV catheters have less hemolysis when 5-mL (vs 10-mL) collection tubes are used. J Emerg Nurs. 2004;30:529–533.
220. Kennedy, C, Angermuller, S, King, R, et al. A comparison of hemolysis rates using intravenous catheters versus venipuncture tubes for obtaining blood samples. J Emerg Nurs. 1996;22:566–569.
221. Herr, RD, Bossart, PJ, Blaylock, RC, et al. Intravenous catheter aspiration for obtaining basic analytes during intravenous infusion. Ann Emerg Med. 1990;19:789–792.
222. Mohler, M, Sato, Y, Bobick, K, et al. The reliability of blood sampling from peripheral intravenous infusion lines. Complete blood cell counts, electrolyte panels, and survey panels. J Intraven Nurs. 1998;21:209–214.
223. Corbo, J, Fu, J, Silver, M, et al. Comparison of laboratory values obtained by phlebotomy versus saline lock devices. Acad Emerg Med. 2007;14:23.
224. Zlotowski, SJ, Kupas, DF, Wood, GC. Comparison of laboratory values obtained by means of routine venipuncture versus peripheral intravenous catheter after a normal saline solution bolus. Ann Emerg Med. 2001;38:497–504.
225. Holmes, KR. Comparison of push-pull versus discard method from central venous catheters for blood testing. J Intraven Nurs. 1998;21:282–285.
226. Stahl, M, Brandslund, I. Controlled storage conditions prolong stability of biochemical components in whole blood. Clin Chem Lab Med. 2005;43:210–215.
227. Jaffe, RM, Kasten, B, Young, DS, et al. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83:824.
228. Mandel, JS, Bond, JH, Church, TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365.
229. Ransohoff, DF, Lang, CA. Screening for colorectal cancer with the fecal occult blood test: a background paper. American College of Physicians. Ann Intern Med. 1997;126:811.
230. Gnauck, R, Macrae, FA, Fleisher, M. How to perform the fecal occult blood test. Cancer. 1984;34:134.
231. Meyer, GW, Komadina, K, Perucca, P. Vegetable peroxidase is denatured by gastric acid: fresh vegetables do not cause false-positive stool Hemoccults in normal subjects. Gastroenterology. 1991;101:871.
232. Anderson, GD, Yuellig, TR, Krone, RE, Jr. An investigation into the effects of oral iron supplementation on in vivo Hemoccult stool testing. Am J Gastroenterol. 1990;85:558.
233. McDonnell, WM, Ryan, JA, Seeger, DM, et al. Effect of iron on the guaiac reaction. Gastroenterology. 1989;96:74.
234. Ahlquist, DA, McGill, DB, Schwartz, S, et al. Fecal blood levels in health and disease. A study using Hemoquant. N Engl J Med. 1985;312:1422.
235. Morris, DW, Hansell, JR, Ostrow, JD, et al. Reliability of chemical tests for fecal occult blood in hospitalized patients. Dig Dis. 1976;21:845.
236. Adams, BD, McHugh, KA, Bryson, SA, et al. The law of unintended consequences: the Joint Commission regulations and the digital rectal examination. Ann Emerg Med. 2008;51:197–201.
237. Cleveland, NJ, Yaron, M, Ginde, AA. The effect of removal of point-of-care fecal occult blood testing on performance of digital rectal examinations in the emergency department. Ann Emerg Med. 2010;56:135–141.
238. Layne, EA, Mellow, MH, Lipman, TO. Insensitivity of guaiac slide tests for detection of blood in gastric juice. Ann Intern Med. 1981;94:774.
239. Holman, JS, Shwed, JA. Influence of sucralfate on the detection of occult blood in simulated gastric fluid by two screening tests. Clin Pharmacol Ther. 1992;11:625.
240. Beuhler, M, Lee, DC, Gerkin, R. The Meixner test in the detection of α-amanitin and false-positive reactions caused by psilocin and 5-substituted tryptamines. Ann Emerg Med. 2004;44:114.
241. Santucci, K, Shah, B. Association of naphthalene with acute hemolytic anemia. Acad Emerg Med. 2000;7:42.
242. Huhn, KM, Rosenberg, FM. Critical clue to ethylene glycol poisoning. CMAJ. 1995;152:193.
243. Hylander, B, Kjellstrand, CM. Prognostic factors and treatment of severe ethylene glycol intoxication. Intensive Care Med. 1996;22:546.
244. McStay, CM, Gordon, PE. Images in clinical medicine. Urine fluorescence in ethylene glycol poisoning. N Engl J Med. 2007;356:611.
245. Wallace, KL, Suchard, JR, Curry, SC, et al. Diagnostic use of physicians’ detection of urine fluorescence in a simulated ingestion of sodium fluorescein–containing antifreeze. Ann Emerg Med. 2001;38:49.
246. Weiner, AL, Ko, C, McKay, CA, Jr. A comparison of two bedside tests for the detection of salicylates in urine. Acad Emerg Med. 2000;7:834.
247. Hlastala, MP. The alcohol breath test—a review. J Appl Physiol. 1998;84:401.
248. Bates, ME, Martin, CS. Immediate, quantitative estimation of blood alcohol concentration from saliva. J Stud Alcohol. 1997;58:531.
249. Bendtsen, P, Hultberg, J, Carlsson, M, et al. Monitoring ethanol exposure in a clinical setting by analysis of blood, breath, saliva, and urine. Alcohol Clin Exp Res. 1999;23:1446.
250. Keim, ME, Bartfield, JM, Raccio-Robak, N. Blood ethanol estimation: a comparison of three methods. Acad Emerg Med. 1996;3:85.
251. Henretig, FM, Gribetz, B, Kearney, T, et al. Interpretation of color change in blood with varying degree of methemoglobinemia. J Toxicol Clin Toxicol. 1988;26:293.
252. Doyon, S, Roberts, JR. Reappraisal of the “coma cocktail.” Dextrose, flumazenil, naloxone, and thiamine. Emerg Med Clin North Am. 1994;12:301.
253. Hoffman, JR, Schriger, DL, Luo, JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20:246.
254. Hoffman, RS, Goldfrank, LR. The poisoned patient with altered consciousness. Controversies in the use of a “coma cocktail. JAMA. 1995;274:562.
255. Basile, AS, Hughes, RD, Harrison, PM, et al. Elevated brain concentrations of 1,4-benzodiazepines in fulminant hepatic failure. N Engl J Med. 1991;325:473.
256. Gyr, K, Meier, R, Haussler, J, et al. Evaluation of the efficacy and safety of flumazenil in the treatment of portal systemic encephalopathy: a double-blind, randomised, placebo-controlled multicentre study. Gut. 1996;39:319.
257. Laccetti, M, Manes, G, Uomo, G. Flumazenil in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized, placebo-controlled study. Dig Liver Dis. 2000;32:335.
258. Beaver, KM, Gavin, TJ. Treatment of acute anticholinergic poisoning with physostigmine. Am J Emerg Med. 1998;16:505.
259. Burns, MJ, Linden, CH, Graudins, A, et al. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35:374.
260. Oakley, P. Physostigmine versus diazepines for anticholinergic poisoning. Ann Emerg Med. 2001;37:239.
261. Siff, JE, Meldon, SW, Tomassoni, AJ. Usefulness of the total iron binding capacity in the evaluation and treatment of acute iron overdose. Ann Emerg Med. 1999;33:73.
262. Wax, P, Hoffman, R. Sodium bicarbonate. Contemp Manage Crit Care. 1991;1:81.
263. Brent, J, McMartin, K, Phillips, S, et al. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344:424.
264. Megarbane, B, Borron, SW, Trout, H, et al. Treatment of acute methanol poisoning with fomepizole. Intensive Care Med. 2001;27:1370.
265. Brent, J, McMartin, K, Phillips, S, et al. Fomepizole for the treatment of ethylene glycol poisoning. Methylpyrazole for Toxic Alcohols Study Group. N Engl J Med. 1999;340:832.