CHAPTER 372 Basilar Trunk Aneurysms
Aneurysms of the basilar artery trunk are among the most challenging vascular lesions to treat surgically because of their structural relationship to the brainstem and cranial base. The intricate neurovascular anatomy of the anterior brainstem makes the potential for surgical morbidity relatively high, necessitating the surgeon’s familiarity with surgical approaches to this region. Basilar trunk aneurysms (BTAs) are those located between the vertebrobasilar junction (VBJ) and the take-off of the superior cerebellar arteries (SCAs), frequently arising at the origin of one of many perforating branch vessels of the region. According to a series of nearly 1200 posterior circulation aneurysms treated by Peerless and colleagues, 8% were reported to be BTAs.1
Preoperative consideration of the superior-inferior origin of aneurysms along the basilar artery is imperative and is often characterized in relation to segments of the clivus or expressed as a distance from the dorsum sellae.2 The origin of the anterior inferior cerebellar artery (AICA) is a common site for many of these aneurysms and is therefore also frequently referred to in order to distinguish upper BTAs (those located between AICA and SCA) from lower BTAs (those located between the AICA and VBJ). The origin and size of the aneurysm will in part dictate the surgical approach required to achieve adequate exposure of the aneurysm neck and surrounding neurovascular structures, based primarily on the bony structures that impede the trajectory to the region of interest. Because minimization of brain retraction is a priority in any approach to the basilar trunk, the surgical management of BTAs traditionally employs one of a variety of cranial base approaches with standard microneurosurgical clipping strategies. As with any neurosurgical procedure, the extent of each surgical approach must be weighed against the potential morbidity incurred.
In recent years, endovascular coil embolization has proved to be an increasingly effective and minimally invasive alternative to the surgical clipping of posterior circulation aneurysms and has become the first-line treatment for such lesions at several major institutions.3–5 As a higher proportion of BTAs are successfully coiled by endovascular techniques, however, the subset of aneurysms not amenable to coil embolization that require operative intervention are therefore increasingly complex and challenging to treat from a surgical standpoint. More specifically, this group includes giant, partially thrombosed, and fusiform aneurysms, those with unfavorable neck-to-dome ratios, and those that have failed attempted endovascular coiling.4,6 An ability to perform complex cranial base approaches, in addition to a variety of adjunctive surgical measures that will be discussed, must be at the surgeon’s disposal to safely and efficiently treat these complex aneurysms.
Clinical Presentation
Aneurysms of the posterior circulation frequently present secondary to subarachnoid hemorrhage resulting from aneurysmal rupture. Clinical symptoms such as severe headache, photophobia, nausea and vomiting, and meningismus are typically encountered. More severe clinical sequelae are frequently observed after aneurysm rupture in this region and include syncope, coma, and death.7 The mortality rate associated with ruptured BTAs remains high, nearing 30% in some series.7–11 A variety of cranial nerve palsies and hydrocephalus may additionally be encountered secondary to SAH in the brainstem region.12
Unruptured BTAs frequently present secondary to mass effect on brainstem structures and the related cranial nerves.7 Traditionally, aneurysms of the basilar apex, SCA, and upper basilar trunk may result in oculomotor nerve paresis. Middle BTAs in the region of the AICA may result in abducens nerve paresis, facial nerve paresis, or hearing loss. Those located at the VBJ may present secondary to mass effect on the 9th, 10th, and 11th cranial nerves. Signs of brainstem compression, such as hemiparesis, hemisensory deficits, and gait imbalance, may result from mass effect from unruptured BTAs.11,13 Signs and symptoms of brainstem ischemia or transient ischemic attacks (TIAs) may also be noted.7,14 Finally, BTAs are sometimes discovered incidentally during cranial imaging for a variety of other reasons. As in any patient with an unruptured cerebral aneurysm, the risks of treating the aneurysm are to be weighed carefully against the cumulative risks of conservative management anticipated over the duration of the patient’s life. Our discussion is restricted to saccular lesions. Giant dolichoectasias are considered a separate pathology with nonanalogous natural history and treatment.
Anatomy of Basilar Trunk Aneurysms and Surrounding Structures
The basilar artery originates at the VBJ and courses anterior to the brainstem until it bifurcates in the interpeduncular cistern. The length of the basilar artery varies between 20 and 40 mm, with a mean length of 30 mm.15 Along its course posterior to the clivus, the basilar trunk supplies multiple arteries that are responsible for the perfusion of the brainstem and cerebellum.16 Identification and preservation of the perforating arteries of this region is axiomatic in reducing surgical morbidity when treating BTAs.
The AICA originates several millimeters above the VBJ near the abducens nerve rootlets and courses through the cerebellopontine angle adjacent to the foramen of Luschka to ultimately supply the inferior aspect of the cerebellum, pons, and eighth cranial nerve.15 The SCAs originate within 2.5 mm of the basilar apex and course below the tentorium and adjacent to the superior cerebellar peduncle in the cerebellomesencephalic cistern to supply the superior aspect of the cerebellum.15 Multiple intermediate perforating arteries also arise from the basilar artery, the most proximal being the pontomedullary artery, which arises between the VBJ and AICA.16 Multiple perforating vessels typically arise from the midbasilar trunk, at the level of the pons. These include the superolateral and inferolateral pontine arteries, which supply the paramedian and lateral pontine surface, as well as numerous additional pontine perforators.16 Additionally, several smaller groups of microperforators are found in subgroups known as the caudal, middle, and rostral groups.16 Each group may include up to 10 perforating vessels, with a significant degree of anastomosis within each subtype.
Endovascular Treatment of Basilar Trunk Aneurysms
During the past decade, the ability to treat increasingly complex aneurysms using endovascular coiling procedures has progressed significantly. Some endovascular series have reported excellent outcomes and safety profiles in patients with basilar artery aneurysms.3,5,8,14,17,18 In the largest series to date reporting BTAs treated by endovascular coil embolization, 39 patients had 41 basilar trunk aneurysms treated with a coiling procedure.18 The authors reported excellent or good outcomes in nearly 90% of these patients, with sacrifice of the parent artery in 5 patients.
Complex or fusiform BTAs may not be amenable to clip ligation or direct aneurysm coiling, and may instead require endovascular occlusion of the parent basilar artery.8,19 A balloon occlusion test is performed before complete occlusion, in which multiple modalities are used to demonstrate that the basilar artery can be safely occluded without evidence of brainstem ischemia. These include neuroleptic anesthesia with direct neurological examination, brainstem auditory evoked response (BAER) monitoring, single-photon emission computed tomography (SPECT), and cerebral angiography.8 Furthermore, the size of the posterior communicating arteries is an important prognostic factor in determining a patient’s ability to tolerate complete parent artery occlusion.20
Even more recently, stent-assisted coiling for BTAs has been reported.21 Additionally, the placement of one or multiple porous stents, independent of coiling, has been described as a sole treatment modality for these aneurysms. However, the long-term efficacy of this treatment remains to be determined.
Surgical Treatment of Basilar Trunk Aneurysms
Selection of Surgical Approach to Aneurysms of the Basilar Trunk
Selection of the appropriate surgical approach for a BTA depends on the superior-inferior location of its origin along the basilar artery and its relationship to the clivus and petrous bone. Aneurysms originating from the upper portion of the basilar trunk, frequently at or directly below the bifurcation of the SCA, can be exposed and treated in a manner similar to basilar apex aneurysms. The relationship of the basilar apex to the dorsum sella is a major factor in the preoperative planning of such aneurysms.15 Standard surgical approaches to the upper basilar trunk have included various modifications of the standard transsylvian approach (such as the orbitozygomatic approach), the extradural temporopolar approach, subtemporal transtentorial approach, and anterior transpetrosal approach.2,22–28 Because of the relatively high morbidity rates associated with transpetrosal approaches, these more extensive cranial base procedures have fallen out of favor during the past decade or so. Extended pterional-based approaches, such as the orbitozygomatic (OZ) craniotomy, in conjunction with surgical drilling of the anterior and posterior clinoid processes, is frequently sufficient in achieving adequate exposure of the upper basilar region.11
Aneurysms located in the region of the midbasilar trunk are among the most difficult to approach surgically, because of their location posterior to the midportion of the clivus, distance below the dorsum sellae, and interposed petrous bone. Midbasilar trunk aneurysms are commonly located at the origin of AICA, although less frequently, they may originate from smaller pontine arteries or arise as fusiform lesions. In general, one of several potential transpetrosal approaches is required for the surgical exposure of midbasilar trunk aneurysms. Typical approaches to this region include the combined supratentorial and infratentorial transpetrosal approach, retrolabyrinthine-transsigmoid approach, and anterior transpetrosal (Kawase) approach.2,29–33 Depending on the author, the term transpetrosal implies either removal of varying degrees of the petromastoid bone or ligation and division of the superior petrosal sinus. The retrolabyrinthine, translabyrinthine, and transcochlear approaches are increasingly extensive transpetrosal approaches that can be used in conjunction with various supratentorial trajectories, yet carry higher risks for cranial nerve injury and cerebrospinal fluid (CSF) leakage. In the translabyrinthine and transcochlear exposures, hearing is necessarily sacrificed, whereas the transcochlear approach additionally endangers function of the facial nerve.11
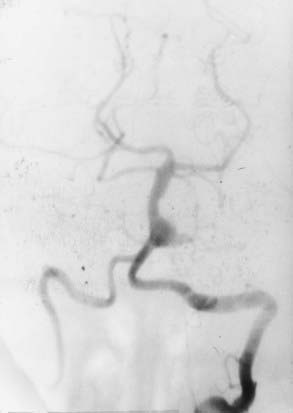
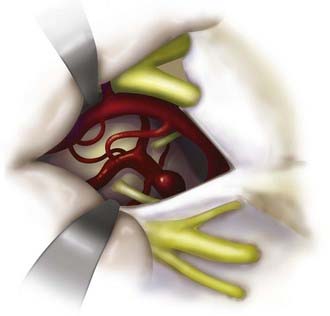
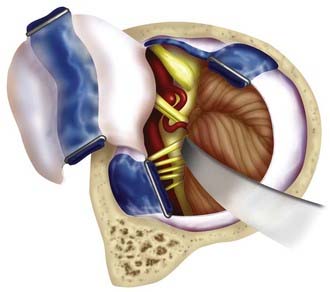
Aneurysms of the lower basilar trunk and VBJ have traditionally been accessed surgically through the retrolabyrinthine-transsigmoid, far-lateral, or extreme lateral inferior transtubercular (ELITE) approaches (Fig. 372-1).4,29–32,34–36 The transoral-transclival approach has also been used to treat aneurysms of this region, yet carries with it greater risks for CSF leakage and meningitis.37,38 In recent years, however, there has been a resurgence of transclival approaches to the anterior brainstem, based on endoscopic exposures with subsequent reconstruction of the skull base using vascularized nasoseptal-based flaps.39–41 Significantly reduced rates of CSF leakage have been achieved using such flaps in the reconstruction of large skull base defects.
Approaches Oriented Parallel to the Basilar Artery
Modifications of the Transsylvian Approach for Basilar Trunk Aneurysms
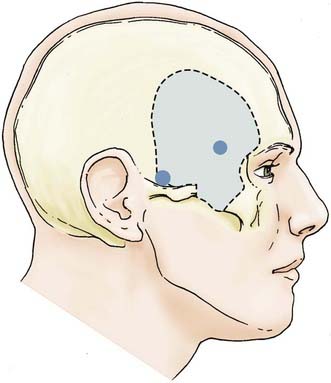
Various modifications of the original transsylvian approach have been used to treat aneurysms of the upper basilar trunk and basilar apex. Although standard pterional and supraorbital craniotomies can be used to gain access to this region, wider cranial exposures are often required to safely improve visualization of the upper basilar artery region. These modifications have included the addition of an orbitopterional or OZ craniotomy.25,42 The major benefits of these modifications include a widened exposure, redirection of the bulky temporalis muscle, and an improved approach angle that is more parallel to the basilar artery complex. Despite these benefits, the OZ craniotomy is generally limited in reaching basilar artery aneurysms originating below the origin of the SCA and are usually reserved for accessing aneurysms limited to the upper two fifths of the basilar artery.11
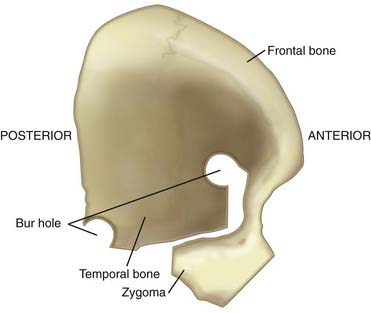
The OZ construct can be removed in a one-piece or two-piece fashion. Osteotomies are required in three locations: (1) at the lateral orbital rim extending to the frontozygomatic suture, (2) at the root of the zygoma parallel to the temporal squama, and (3) at the malar eminence. A frontotemporal craniotomy is performed, with bur holes placed in the frontal and temporal regions (Figs. 372-2 and 372-3). The dura is tacked up to the periphery of the craniotomy, and the sphenoid wing is flattened extradurally using a high-speed drill. The dura is opened in a semilunar fashion and tacked up to the anterior face of the exposure. Standard transsylvian dissection is carried out with the use of the operating microscope, using frontal and temporal retractors as needed for gentle retraction. Additional maneuvers to widen the exposure to the upper basilar region include drilling down the anterior and posterior clinoid processes and dorsum sella as well as retraction of the temporal tip following the coagulation of temporal tip veins. Mobilizing the oculomotor nerve and resecting or retracting the edge of the tentorium further increases the aperture to the upper basilar artery, posterior cerebral arteries, and SCAs.
Extradural Temporopolar Approach
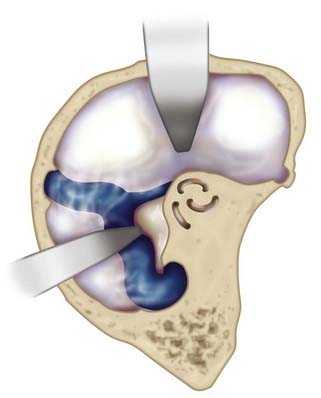
A more expanded approach to the upper basilar artery region is the extradural temporopolar approach. Often used in conjunction with an OZ craniotomy, this approach improves parallel visualization of the basilar artery complex above the origin of the SCA. A significant degree of drilling of the middle skull base is required to untether and retract the temporal and frontal lobes and expose the posterior petroclival region. The major benefit offered by this approach is extradural retraction of the temporal and frontal lobes, which provides a significant margin of brain protection compared with intradural retraction. After initial exposure, a sequential progression of extradural drilling, untethering, and retraction is carried out, including (1) drilling of the sphenoid wing and lateral aspect of the superior orbital fissure (SOF), (2) drilling of the anterior clinoid process, (3) unroofing of the optic canal, (4) mobilization and retraction of the temporal tip, (5) transcavernous mobilization of the internal carotid artery (ICA) and cranial nerves, and (6) drilling of the posterior clinoid process.22,23
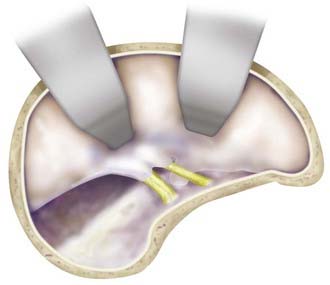
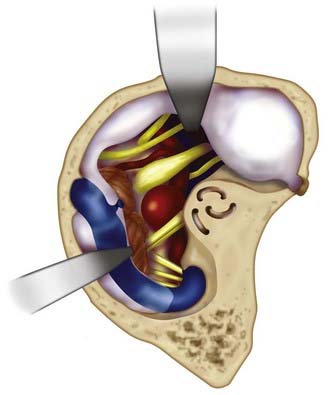
Starting with a standard OZ craniotomy, as previously described, the temporal lobe is elevated extradurally to expose the SOF and foramen rotundum (Fig. 372-4). The limitations of dural retraction at this point include the superior ethmoidal artery and foramen ovale. The sphenoid wing is drilled down, followed by drilling of the lateral wall of the SOF, which is skeletonized to gain access to the junction of the dural sleeve and the dura propria of the temporal lobe. The foramen rotundum and optic canal are unroofed for the same reason. The anterior clinoid process is drilled down extradurally. The temporal tip is retracted to expose the junction of the dura propria of the temporal lobe and the true inner cavernous membrane. The meningo-orbital vessels are coagulated and divided. This dural plane is then carefully developed with special attention directed to preserving the sheaths over the third, fourth, and fifth cranial nerves. As the outer layer of the cavernous sinus is dissected, hemostasis is maintained with gentle packing of Surgicel.
After exposure of the ICA and the oculomotor and trochlear nerves, the opticocarotid and Liliequist’s membranes are opened. The porus oculomotorius is sharply unroofed, and the edge of the tentorium is excised to gain further exposure of the aperture to the upper basilar trunk. The cavernous sinus triangle between the oculomotor and trochlear nerves is opened, exposing the oculomotor nerve along its entire course and allowing gentle retraction of the nerve as required. The posterior clinoid process is drilled down to facilitate exposure of the upper basilar artery region. This and the aforementioned approach are used when the aneurysm is strongly lateralized and resides on the upper basilar trunk. The natural trajectory allows for readily applying a clip parallel to the course of the parent artery (Fig. 372-5).
Transverse Approaches to the Basilar Trunk
Subtemporal (Transtentorial) Approach
The subtemporal approach was initially developed by Drake to treat aneurysms of the basilar apex.13 This approach offers a transverse trajectory to the upper basilar artery region, yet requires intradural retraction of the temporal lobe.28 It is limited with respect to its applicability in the case of BTAs and is only useful as a stand-alone approach if the aneurysm is within 1 to 2 cm of the dorsum sella. The subtemporal approach can additionally be combined with various transpetrosal exposures to improve exposure to the midbasilar region.
Anterior Transpetrosal Approach
This strategy emerged from a combination of the extended middle fossa approach used for petroclival tumors and the work of Kawase.30,43 As a strictly extradural approach, it is restricted to midtrunk lesions that are relatively small. By alternating between an extradural strategy to remove the apex and then resuming the intradural trajectory as described by Day and coworkers, a wider view of the upper and middle trunk can be accessed.2,30,43–46 A significant degree of caution must be used to identify and preserve the cochlea, abducens nerve, and ICA. This approach begins with a standard subtemporal or OZ exposure, as described previously. Extradural retraction of the temporal lobe exposes the middle meningeal artery (MMA), arcuate eminence, and greater superficial petrosal nerve. The MMA, exiting the foramen spinosum, is coagulated and divided. The petrous apex is identified, bounded anteriorly by the petrous carotid artery, the cochlea laterally, and Meckel’s cave superiorly and medially. The roof of the internal acoustic canal and petrous apex are drilled down. A complete anterior petrosectomy is performed, and the superior petrosal sinus is identified, coagulated, and divided. The dura is opened to a point below the tentorial margin.
Combined Supratentorial-Infratentorial Transpetrosal Approach
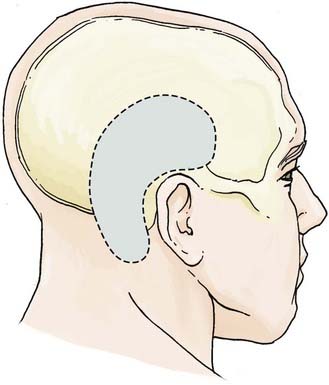
The combined transpetrosal approach uses an extended subtemporal craniotomy in conjunction with a retrolabyrinthine posterior transpetrosal approach, providing exposure that is based both superior and inferior to the tentorium. This approach can be modified accordingly to expose any region of the basilar trunk, with a trajectory transverse to the basilar artery.11,22,27,29,32,33,46,47 Both inferior and superior vascular control of the basilar artery can be obtained with the use of this approach, making it the optimal approach for large or giant aneurysms of the basilar artery trunk. Although some degree of drilling of the mastoid bone is mandated, both hearing and the sigmoid sinus can be preserved if a retrolabyrinthine approach is used. Key features of the approach include (1) a subtemporal craniotomy extended to the level of the mastoid process, (2) partial or minimal mastoidectomy, (3) ligation of the superior petrosal sinus, (4) division of the tentorium, and (5) skeletonization and retraction of the sigmoid sinus.11,22,32,46
The patient is placed in a lateral decubitus position. An incision is made starting at the level of the root of the zygoma and extending around the ear, about 5 cm above the external auditory canal, and inferiorly to 1 cm posterior and inferior to the mastoid process. The galeocutaneous flap is reflected inferiorly. The temporalis fascia and muscle are divided into a superior and inferior flap, and subsequently reflected. An L-shaped craniotomy is performed consisting of a temporal (supratentorial) and posterior fossa (infratentorial) component, with exposure and preservation of the transverse sinus (Fig. 372-6). The squamous temporal bone is drilled flush with the floor of the middle fossa. A partial mastoidectomy is undertaken, completely exposing the sigmoid sinus and entry point of the superior petrosal sinus. In most cases, a posterior petrosectomy is performed with care taken to preserve the semicircular canals. In the event that the posterior semicircular canal is inadvertently breached, it should be waxed off. For increased exposure, a translabyrinthine or transcochlear approach can be used, although both sacrifice hearing, and the latter risks injury to the facial nerve. The dura is opened along the inferior temporal lobe and inferiorly to the transverse-sigmoid junction (Fig. 372-7). The superior petrosal sinus is ligated at its entry to the transverse-sigmoid junction, yet the entry point of the vein of Labbé is preserved. The dural opening is continued inferiorly at the anterior border of the sigmoid sinus. The tentorium is incised parallel to the petrous ridge in the direction of the trochlear nerve. A self-retaining retractor is introduced, with gentle upward elevation of the posterior temporal lobe. This provides wide exposure of the retroclival region and entire course of the basilar trunk, with facilitation of proximal and distal control of the basilar artery (Fig. 372-8).
Retrolabyrinthine Transsigmoid Approach
The retrolabyrinthine transsigmoid approach offers widened exposure of the lower basilar artery and VBJ, when a standard lateral suboccipital is insufficient owing to demands for retraction of the cerebellum and brainstem. Since developing this approach in 1990, we have used it for many small and medium lower basilar trunk and AICA aneurysms.34 This approach is frequently used for aneurysms less than 1.5 cm in diameter. Careful analysis of preoperative venous-phase angiographic images must be done to identify the side of the dominant sigmoid sinus. For midline lesions, the approach is made from the side of the nondominant sigmoid sinus. Large aneurysms pointing to one side or another are approached from the side of the aneurysm neck. Temporary occlusion of the sigmoid sinus with direct puncture manometry can be used to predict tolerance to permanent occlusion. A rise in venous pressure of less than 5 mm Hg predicts acceptable tolerance to permanent sigmoid sinus ligation. When a rise of greater than 5 mm Hg is observed, an alternative approach should be performed to preserve the sigmoid sinus.34
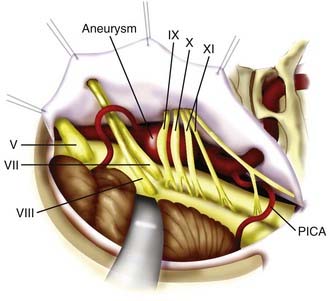
The patient is positioned in lateral or supine position, with the lateral aspect of the head parallel to the floor. Facial nerve electromyography and BAER are used. A curvilinear retroauricular incision is made from the superior nuchal line to the level of C1. The scalp is opened in two layers, and the fascia and muscle are retracted laterally with fishhooks. A partial mastoidectomy is performed with retrolabyrinthine exposure and preservation of the sigmoid sinus and superior petrosal sinus. The sigmoid sinus is exposed from the level of the superior petrosal sinus down to the jugular bulb. The margins of the craniotomy are the middle fossa dura (superiorly), jugular bulb (inferiorly), posterior semicircular canal (anteriorly), and 2 cm of retrosigmoid dura (posteriorly). The sigmoid sinus is ligated superiorly at the superior petrosal sinus and inferiorly at the jugular bulb. The dura of Trautmann’s triangle is opened across the divided sinus and reflected anteriorly. The retrolabyrinthine transsigmoid exposure provides excellent exposure of cranial nerves V to XI, and most of the middle and lower basilar trunk to the VBJ (Fig. 372-9). Because of the proximity of the cranial nerves to one another in the subarachnoid space, access to the aneurysm neck may be limited, and low-profile aneurysm clips are usually required.
Extreme Lateral Inferior Transtubercular Approach
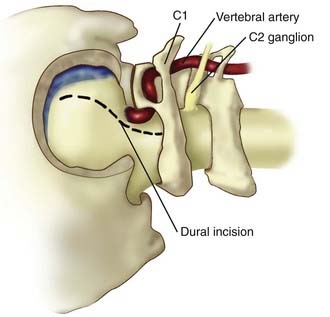
The ELITE approach is a modified far-lateral suboccipital approach that offers a more expanded exposure of the lower basilar trunk, VBJ, and upper vertebral arteries.35 This approach provides a caudal to rostral and lateral to medial trajectory at the medullary region and foramen magnum with minimal brainstem retraction, by removing the mastoid process, foramen magnum, occipital condyle, and jugular tubercle.35,48 Proximal control can be obtained at the level of the vertebral arteries near the foramen magnum. This approach is useful for large or giant lower basilar trunk and VBJ aneurysms, particularly in the setting of a dominant sigmoid sinus.
A retromastoid craniectomy is then performed and extended down to the rim of foramen magnum laterally, with the anterior margin based at the sigmoid sinus. The remainder of the foramen magnum ring and posterior one third of the occipital condyle are drilled and removed. The jugular tubercle is then removed by drilling the bone between the hypoglossal canal and jugular bulb (Fig. 372-10). The dura is opened from the transverse-sigmoid sinus junction, along the posterior wall of the sigmoid sinus, to the level of C1. The dura is incised posterior to the entry point of the vertebral artery and is preserved around the artery’s ring to facilitate dural closure. The dura is reflected anteriorly, along with the inferior portion of the sigmoid sinus. Cranial nerves IX, X, and XI are identified along with the vertebral arteries (Fig. 372-11). The line of sight can be adjusted to look superiorly to the VBJ and lower basilar trunk. For widened exposure inferiorly, a C1 hemilaminectomy can be performed and the dural opening extended. For widened exposure superiorly, a more expanded retrolabyrinthine mastoidectomy can be performed. The intradural vertebral artery is then followed to its junction with the basilar artery, thereby providing proximal control of both vertebral arteries.
Adjunctive Measures to Surgical Clipping of Basilar Artery Aneurysms
Temporary Proximal Balloon Occlusion
Although the surgical approaches previously described are often sufficient in providing exposure of a basilar trunk aneurysm, achieving proximal control of the basilar artery through temporary clip placement can be a more difficult undertaking. Previous series have described the role of temporary endovascular intraoperative balloon occlusion of the basilar artery to achieve proximal control with excellent results.49 This adjunctive measure has been used along with a variety of skull base approaches to aneurysms of the upper and lower basilar trunk, with no reported ischemic or embolic complications related to transient occlusion in most cases. This technique offers the benefits of proximal control before initiation of the exposure, avoidance of obstruction of the operative field by the temporary clip, and ability to confirm occlusion of the aneurysm sac with digital subtraction angiography.49
Profound Hypothermia with Circulatory Arrest
The use of cardiopulmonary bypass with profound hypothermia has been reported for complex basilar artery aneurysms.50,51 This adjunctive measure facilitates manipulation and collapse of the aneurysm sac, provides cerebroprotective benefits associated with hypothermia, and eliminates the risk for intraoperative rupture.50 Lawton and associates reported on the use of hypothermic arrest for a series of 60 patients, of which 17 midbasilar aneurysms were treated.50 Major morbidity was observed in 37% of patients after hypothermic arrest procedures, and a surgical mortality rate of 8% was reported. Among the most frequent complication associated with this procedure is the increased risk for postoperative hematoma, owing to an induced hypocoagulable state and administration of heparin. Because of restricted working space, this adjunct and proximal balloon occlusion should be considered for any lesion 1.5 cm or greater.
Hunterian Ligation and Revascularization Procedures
A small proportion of BTAs are not amenable to treatment with endovascular coil embolization or direct surgical clipping. These include giant, fusiform, or severely atherosclerotic aneurysms of the basilar artery. In such cases, hunterian ligation or trapping of the basilar artery at the level of the aneurysm may be required, often in combination with revascularization procedures.6,20,52,53 When necessary, complete aneurysmectomy can be performed in conjunction with trapping and revascularization procedures.53 Complete occlusion of the basilar artery is tolerated in a small proportion of patients, yet the risk for brainstem injury due to intraoperative ischemia or embolic events associated with such cases poses a significant source of morbidity and mortality.20,52,54 In the largest series reported of deliberate occlusion of the basilar artery trunk, 76% of these cases were reported to have an excellent outcome.20 The risk for brainstem ischemia after surgical occlusion of the basilar artery for aneurysm ligation has been reported to be 13% to 30%.20,52
Revascularization procedures may be used to bypass arterial flow beyond the occluded segment of the parent artery.6,52,53 In 2002, Terasaka and associates reported the use of an external carotid artery–to–posterior cerebral artery high-flow bypass in conjunction with surgical obliteration of a BTA in a patient who failed balloon test occlusion.6
Aziz KM, van Loveren HR, Tew JMJr, et al. The Kawase approach to retrosellar and upper clival basilar aneurysms. Neurosurgery. 1999;44:1225.
Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997;87:544.
Day JD, Giannotta SL, Fukushima T. Extradural temporopolar approach to lesions of the upper basilar artery and infrachiasmatic region. J Neurosurg. 1994;81:230.
Dolenc VV, Skrap M, Sustersic J, et al. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987;1:251.
Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96.
Giannotta SL, Maceri DR. Retrolabyrinthine transsigmoid approach to basilar trunk and vertebrobasilar artery junction aneurysms: technical note. J Neurosurg. 1988;69:461.
Hakuba A, Liu S, Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271.
Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559.
Hopkins LN, Budny JL, Castellani D. Extracranial-intracranial arterial bypass and basilar artery ligation in the treatment of giant basilar artery aneurysms. Neurosurgery. 1983;13:189.
Kasdon DL, Stein BM. Combined supratentorial and infratentorial exposure for low-lying basilar aneurysms. Neurosurgery. 1979;4:422.
Kawase T, Toya S, Shiobara R, et al. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857.
Lawton MT, Daspit CP, Spetzler RF. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997;41:513.
Lawton MT, Raudzens PA, Zabramski JM, et al. Hypothermic circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. Neurosurgery. 1998;43:10.
MacDonald JD, Day AL. Surgical approaches to aneurysms of the upper basilar artery. Clin Neurosurg. 1996;43:127.
Miller CG, van Loveren HR, Keller JT, et al. Transpetrosal approach: surgical anatomy and technique. Neurosurgery. 1993;33:461.
Mizoi K, Yoshimoto T, Takahashi A, et al. Direct clipping of basilar trunk aneurysms using temporary balloon occlusion. J Neurosurg. 1994;80:230.
Peerless SJ, Hernesniemi JA, Gutman FB, et al. Early surgery for ruptured vertebrobasilar aneurysms. J Neurosurg. 1994;80:643.
Sano K. Temporo-polar approach to aneurysms of the basilar artery at and around the distal bifurcation: technical note. Neurol Res. 1980;2:361.
Seifert V, Raabe A, Stolke D. Management-related morbidity and mortality in unselected aneurysms of the basilar trunk and vertebrobasilar junction. Acta Neurochir (Wien). 2001;143:343.
Seifert V, Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85:373.
Solomon RA, Stein BM. Surgical approaches to aneurysms of the vertebral and basilar arteries. Neurosurgery. 1988;23:203.
Spetzler RF, Daspit CP, Pappas CT. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76:588.
Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms: immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161.
Sugita K, Kobayashi S, Takemae T, et al. Aneurysms of the basilar artery trunk. J Neurosurg. 1987;66:500.
1 Peerless SJ, Hernesniemi JA, Gutman FB, et al. Early surgery for ruptured vertebrobasilar aneurysms. J Neurosurg. 1994;80:643.
2 Aziz KM, van Loveren HR, Tew JMJr, et al. The Kawase approach to retrosellar and upper clival basilar aneurysms. Neurosurgery. 1999;44:1225.
3 Bavinzski G, Killer M, Gruber A, et al. Treatment of basilar artery bifurcation aneurysms by using Guglielmi detachable coils: a 6-year experience. J Neurosurg. 1999;90:843.
4 Collice M, Arena O, D’Aliberti G, et al. Aneurysms of the vertebro-basilar junction area: preliminary experience in endovascular and surgical management. Acta Neurochir (Wien). 1997;139:124.
5 Van Rooij WJ, Sluzewski M, Menovsky T, et al. Coiling of saccular basilar trunk aneurysms. Neuroradiology. 2003;45:19.
6 Terasaka S, Itamoto K, Houkin K. Basilar trunk aneurysm surgically treated with anterior petrosectomy and external carotid artery-to-posterior cerebral artery bypass: technical note. Neurosurgery. 2002;51:1083.
7 Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141.
8 Hassan T, Ezura M, Takahashi A. Treatment of giant fusiform aneurysms of the basilar trunk with intra-aneurysmal and basilar artery coil embolization. Surg Neurol. 2004;62:455.
9 Hernesniemi J, Vapalahti M, Niskanen M, et al. Management outcome for vertebrobasilar artery aneurysms by early surgery. Neurosurgery. 1992;31:857.
10 Hillman J, Saveland H, Jakobsson KE, et al. Overall management outcome of ruptured posterior fossa aneurysms. J Neurosurg. 1996;85:33.
11 Lawton MT, Daspit CP, Spetzler RF. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997;41:513.
12 Seifert V, Raabe A, Stolke D. Management-related morbidity and mortality in unselected aneurysms of the basilar trunk and vertebrobasilar junction. Acta Neurochir (Wien). 2001;143:343.
13 Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96.
14 Pierot L, Boulin A, Castaings L, et al. Selective occlusion of basilar artery aneurysms using controlled detachable coils: report of 35 cases. Neurosurgery. 1996;38:948.
15 Rhoton ALJr. The cerebellar arteries. Neurosurgery. 2000;47:S29.
16 Marinkovic SV, Gibo H. The surgical anatomy of the perforating branches of the basilar artery. Neurosurgery. 1993;33:80.
17 Nichols DA, Brown RDJr, Thielen KR, et al. Endovascular treatment of ruptured posterior circulation aneurysms using electrolytically detachable coils. J Neurosurg. 1997;87:374.
18 Uda K, Murayama Y, Gobin YP, et al. Endovascular treatment of basilar artery trunk aneurysms with Guglielmi detachable coils: clinical experience with 41 aneurysms in 39 patients. J Neurosurg. 2001;95:624.
19 Wenderoth JD, Khangure MS, Phatouros CC, et al. Basilar trunk occlusion during endovascular treatment of giant and fusiform aneurysms of the basilar artery. AJNR Am J Neuroradiol. 2003;24:1226.
20 Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161.
21 Kim M, Levy EI, Meng H, et al. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery. 2007;61:1305.
22 Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997;87:544.
23 Day JD, Giannotta SL, Fukushima T. Extradural temporopolar approach to lesions of the upper basilar artery and infrachiasmatic region. J Neurosurg. 1994;81:230.
24 Dolenc VV, Skrap M, Sustersic J, et al. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987;1:251.
25 Hakuba A, Liu S, Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271.
26 Sano K. Temporo-polar approach to aneurysms of the basilar artery at and around the distal bifurcation: technical note. Neurol Res. 1980;2:361.
27 Solomon RA, Stein BM. Surgical approaches to aneurysms of the vertebral and basilar arteries. Neurosurgery. 1988;23:203.
28 Sugita K, Kobayashi S, Takemae T, et al. Aneurysms of the basilar artery trunk. J Neurosurg. 1987;66:500.
29 Kasdon DL, Stein BM. Combined supratentorial and infratentorial exposure for low-lying basilar aneurysms. Neurosurgery. 1979;4:422.
30 Kawase T, Toya S, Shiobara R, et al. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857.
31 Miller CG, van Loveren HR, Keller JT, et al. Transpetrosal approach: surgical anatomy and technique. Neurosurgery. 1993;33:461.
32 Seifert V, Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85:373.
33 Spetzler RF, Daspit CP, Pappas CT. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76:588.
34 Giannotta SL, Maceri DR. Retrolabyrinthine transsigmoid approach to basilar trunk and vertebrobasilar artery junction aneurysms: technical note. J Neurosurg. 1988;69:461.
35 Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559.
36 Rosenberg SI, Flamm ES, Hoffer ME, et al. The retrolabyrinthine transsigmoid approach to midbasilar artery aneurysms. Laryngoscope. 1992;102:100.
37 Ogilvy CS, Barker FG2nd, Joseph MP, et al. Transfacial transclival approach for midline posterior circulation aneurysms. Neurosurgery. 1996;39:736.
38 Saito I, Takahashi H, Joshita H, et al. Clipping of vertebro-basilar aneurysms by the transoral transclival approach. Neurol Med Chir (Tokyo). 1980;20:753.
39 Eloy JA, Carai A, Patel AB, et al. Combined endoscope-assisted transclival clipping and endovascular stenting of a basilar trunk aneurysm: case report. Neurosurgery. 2008;62:142.
40 Fortes FS, Carrau RL, Snyderman CH, et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117:1329.
41 Pinheiro-Neto CD, Prevedello DM, Carrau RL, et al. Improving the design of the pedicled nasoseptal flap for skull base reconstruction: a radioanatomic study. Laryngoscope. 2007;117:1560.
42 Fujitsu K, Kuwabara T. Zygomatic approach for lesions in the interpeduncular cistern. J Neurosurg. 1985;62:340.
43 Kawase T, Shiobara R, Toya S. Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28:869.
44 MacDonald JD, Antonelli P, Day AL. The anterior subtemporal, medial transpetrosal approach to the upper basilar artery and ponto-mesencephalic junction. Neurosurgery. 1998;43:84.
45 MacDonald JD, Day AL. Surgical approaches to aneurysms of the upper basilar artery. Clin Neurosurg. 1996;43:127.
46 Rhoton ALJr. The temporal bone and transtemporal approaches. Neurosurgery. 2000;47:S211.
47 Sekhar LN, Estonillo R. Transtemporal approach to the skull base: an anatomical study. Neurosurgery. 1986;19:799.
48 Bertalanffy H, Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991;29:815.
49 Mizoi K, Yoshimoto T, Takahashi A, et al. Direct clipping of basilar trunk aneurysms using temporary balloon occlusion. J Neurosurg. 1994;80:230.
50 Lawton MT, Raudzens PA, Zabramski JM, et al. Hypothermic circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. Neurosurgery. 1998;43:10.
51 Sullivan BJ, Sekhar LN, Duong DH, et al. Profound hypothermia and circulatory arrest with skull base approaches for treatment of complex posterior circulation aneurysms. Acta Neurochir (Wien). 1999;141:1.
52 Hopkins LN, Budny JL, Castellani D. Extracranial-intracranial arterial bypass and basilar artery ligation in the treatment of giant basilar artery aneurysms. Neurosurgery. 1983;13:189.
53 Sekhar LN, Chandler JP, Alyono D. Saphenous vein graft reconstruction of an unclippable giant basilar artery aneurysm performed with the patient under deep hypothermic circulatory arrest: technical case report. Neurosurgery. 1998;42:667.
54 Caplan LR. Occlusion of the vertebral or basilar artery: follow up analysis of some patients with benign outcome. Stroke. 1979;10:277.