Basic Structure and Function of Human Joints
CLASSIFICATION OF JOINTS BASED ON MOVEMENT POTENTIAL
CLASSIFICATION OF SYNOVIAL JOINTS BASED ON MECHANICAL ANALOGY
HISTOLOGIC ORGANIZATION OF PERIARTICULAR CONNECTIVE TISSUES
TYPES OF PERIARTICULAR CONNECTIVE TISSUES
SOME EFFECTS OF IMMOBILIZATION ON THE STRENGTH OF PERIARTICULAR CONNECTIVE TISSUE AND BONE
This chapter focuses on the general anatomic structure and function of joints. The chapters contained in Sections II to IV of this text describe the specific anatomy and detailed function of the individual joints throughout the body. This detailed information is a prerequisite for understanding impairments of joints as well as employing the most effective rehabilitation of persons with joint dysfunction.
CLASSIFICATION OF JOINTS BASED ON MOVEMENT POTENTIAL
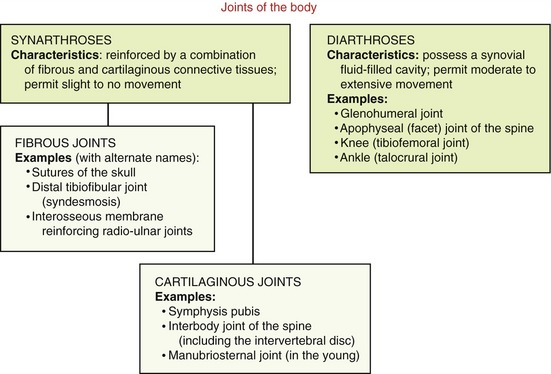
One method to classify joints focuses primarily on their movement potential. Based on this scheme, two major types of joints exist within the body: synarthroses and diarthroses (Figure 2-1).
Synarthroses
A synarthrosis is a junction between bones that allows slight to essentially no movement. Based on the dominant type of periarticular connective tissue that reinforces the articulation, synarthrodial joints can be further classified as fibrous or cartilaginous.63
Fibrous joints are stabilized by specialized dense connective tissues, usually with a high concentration of collagen. Examples of fibrous joints include the sutures of the skull, the distal tibiofibular joint (often referred to as a syndesmosis), and other joints reinforced by an interosseous membrane. Cartilaginous joints, in contrast, are stabilized by varying forms of flexible fibrocartilage or hyaline cartilage, often mixed with collagen. Cartilaginous joints generally exist in the midline of the body, such as the symphysis pubis, the interbody joints of the spine, and the manubriosternal joint.63
Diarthroses: Synovial Joints
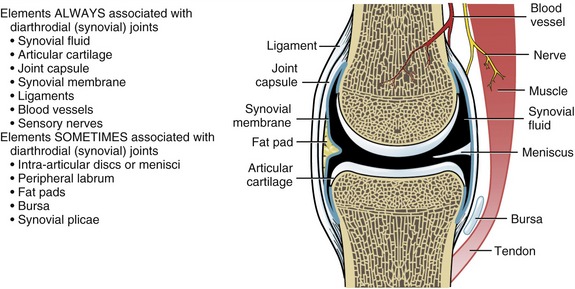
Diarthrodial or synovial joints are specialized for movement and always exhibit seven elements (Figure 2-2). Articular cartilage covers the ends and other articular surfaces of bones. The joint is enclosed by a peripheral curtain of connective tissue that forms the joint (or articular) capsule. The joint capsule is composed of two histologically distinct layers. The external, or fibrous, layer is composed of dense connective tissue. This part of the joint capsule provides support between the bones and containment of the joint contents. The internal layer of the joint capsule consists of a synovial membrane, which averages 3 to 10 cell layers thick. The cells within this specialized connective tissue manufacture a synovial fluid that is usually clear or pale yellow, with a slightly viscous consistency.63 The synovial fluid contains many of the proteins found in blood plasma, including hyaluronan and other lubricating glycoproteins.63,75 The synovial fluid coats the articular surfaces of the joint. This fluid reduces the friction between the joint surfaces as well as providing some nourishment to the articular cartilage.
To accommodate the wide spectrum of joint shapes and functional demands, other elements may sometimes appear in synovial joints (see Figure 2-2). Intra-articular discs, or menisci, are pads of fibrocartilage imposed between articular surfaces. These structures increase articular congruency and improve force dispersion. Intra-articular discs and menisci are found in several joints of the body (see box).
Synovial plicae (i.e., synovial folds, synovial redundancies, or synovial fringes) are slack, overlapped pleats of tissue composed of the innermost layers of the joint capsule. They occur normally in joints with large capsular surface areas such as the knee and elbow. Plicae increase synovial surface area and allow full joint motion without undue tension on the synovial lining. If these folds are too extensive or become thickened or adherent because of inflammation, they can produce pain and altered joint mechanics. The plicae of the knee are further described in Chapter 13.
CLASSIFICATION OF SYNOVIAL JOINTS BASED ON MECHANICAL ANALOGY
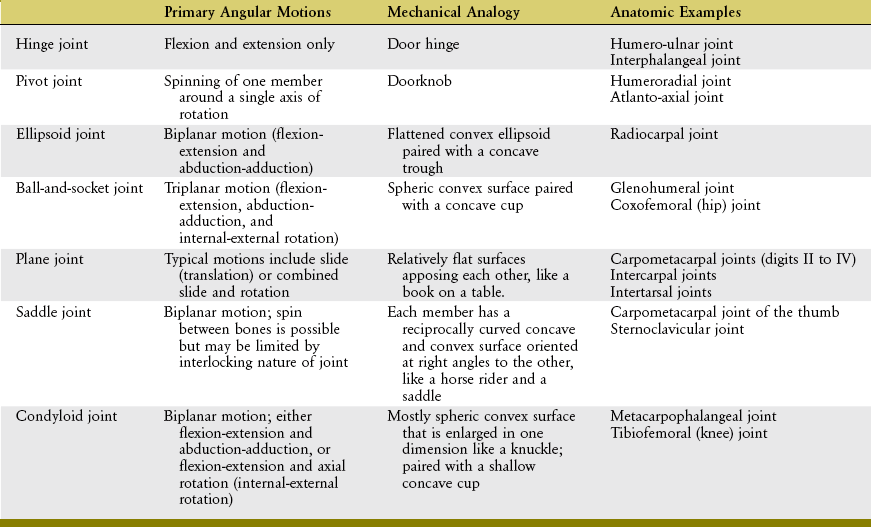
Thus far in this chapter, joints have been classified into two broad categories based primarily on movement potential. Because an in-depth understanding of synovial joints is so crucial to an understanding of the mechanics of movement, they are here further classified using an analogy to familiar mechanical objects or shapes (Table 2-1).
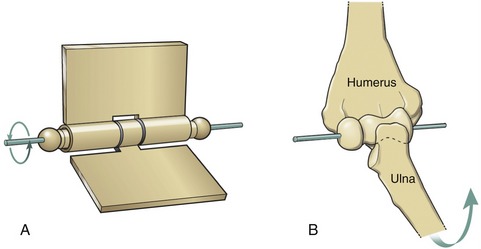
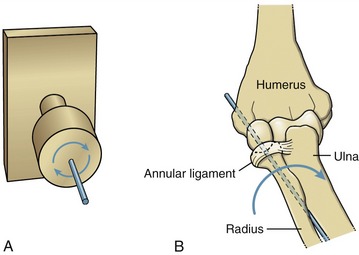
A hinge joint is generally analogous to the hinge of a door, formed by a central pin surrounded by a larger hollow cylinder (Figure 2-3, A). Angular motion at hinge joints occurs primarily in a plane located at right angles to the hinge, or axis of rotation. The humero-ulnar joint is a clear example of a hinge joint (see Figure 2-3, B). As in all synovial joints, slight translation (i.e., sliding) is allowed in addition to the rotation. Although the mechanical similarity is less complete, the interphalangeal joints of the digits are also classified as hinge joints.
A pivot joint is formed by a central pin surrounded by a larger cylinder. Unlike a hinge, the mobile member of a pivot joint is oriented parallel to the axis of rotation. This mechanical orientation produces the primary angular motion of spin, similar to a doorknob’s spin around a central axis (Figure 2-4, A). Two examples of pivot joints are the humeroradial joint, shown in Figure 2-4, B, and the atlanto-axial joint in the craniocervical region.
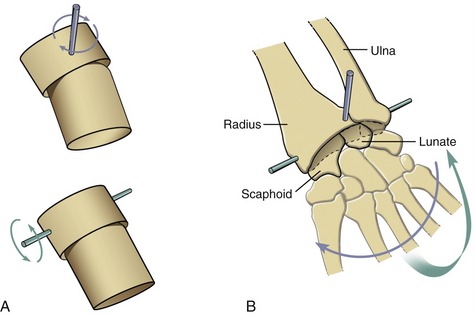
An ellipsoid joint has one partner with a convex elongated surface in one dimension that is mated with a similarly elongated concave surface on the second partner (Figure 2-5, A). The elliptic mating surfaces severely restrict the spin between the two surfaces but allow biplanar motions, usually defined as flexion-extension and abduction-adduction. The radiocarpal joint is an example of an ellipsoid joint (see Figure 2-5, B). The flattened convex member of the joint (i.e., carpal bones) significantly limits the spin within the matching concavity (i.e., distal radius).
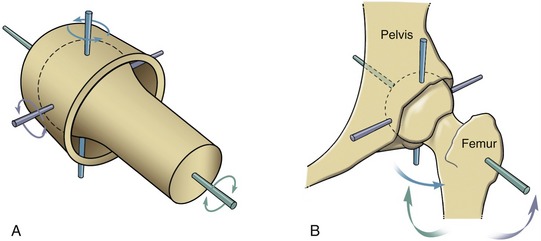
A ball-and-socket joint has a spheric convex surface that is paired with a cuplike socket (Figure 2-6, A). This joint provides motion in three planes. Unlike the ellipsoid joint, the symmetry of the curves of the two mating surfaces of the ball-and-socket joint allows spin without dislocation. Ball-and-socket joints within the body include the glenohumeral joint and the hip joint. As will be described further in Chapter 5, most of the concavity of the glenohumeral joint is formed not only by the glenoid fossa, but also by the surrounding muscle, labrum, joint capsule, and capsular ligaments.
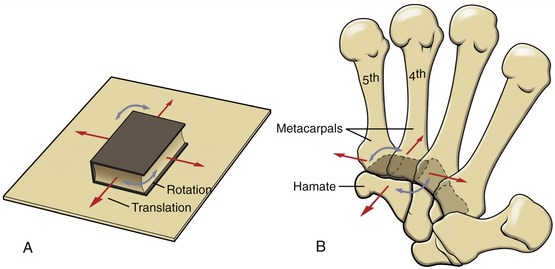
A plane joint is the pairing of two flat or relatively flat surfaces. Movements combine sliding and some rotation of one partner with respect to the other, much as a book can slide or rotate across a tabletop (Figure 2-7, A). As depicted in Figure 2-7, B, the carpometacarpal joints within digits II to V are often considered as plane, or modified plane, joints. Most intercarpal and intertarsal joints are also considered plane joints. The forces that cause or restrict movement between the bones are supplied by tension in muscles or ligaments.
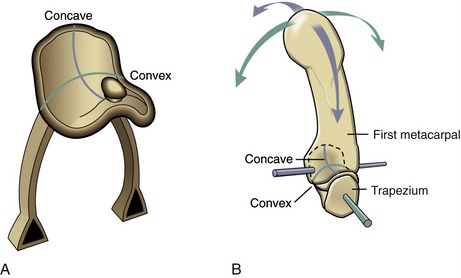
Each partner of a saddle joint has two surfaces: one surface is concave, and the other is convex. These surfaces are oriented at approximate right angles to each other and are reciprocally curved. The shape of a saddle joint is best visualized using the analogy of a horse’s saddle and rider (Figure 2-8, A). From front to back, the saddle presents a concave surface reaching from the saddle pommel in front to the back of the saddle. From side to side, the saddle is convex, stretching from one stirrup across the back of the horse to the other stirrup. The rider has reciprocal convex and concave curves to complement the shape of the saddle. The carpometacarpal joint of the thumb is the clearest example of a saddle joint (see Figure 2-8, B). The reciprocal, interlocking nature of this joint allows ample motion in two planes but limited spin between the trapezium and the first metacarpal.
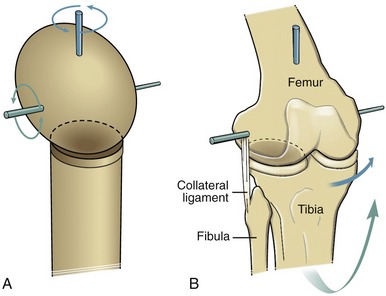
A condyloid joint is much like a ball-and-socket articulation except that the concave member of the joint is relatively shallow (Figure 2-9, A). Condyloid joints usually allow 2 degrees of freedom. Ligaments or bony incongruities often restrain the third degree. Condyloid joints often occur in pairs, such as the knees (see Figure 2-9, B) and the atlanto-occipital joints (i.e., articulation between the occipital condyles and the first cervical vertebra). The metacarpophalangeal joint of the finger is another example of a condyloid joint. The root of the word condyle actually means “knuckle.”
Simplifying the Classification of Synovial Joints: Ovoid and Saddle Joints
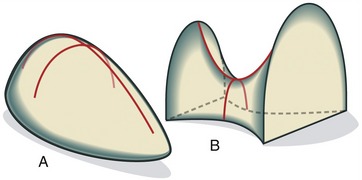
It is often difficult to classify synovial joints based on an analogy to mechanics alone. The metacarpophalangeal joint (condyloid) and the glenohumeral joint (ball-and-socket), for example, have similar shapes but differ considerably in the relative magnitude of movement and overall function. Joints always display subtle variations that make simple mechanical descriptions less applicable. A good example of the difference between mechanical classification and true function is seen in the gentle undulations that characterize the intercarpal and intertarsal joints. Several of these joints produce complex multiplanar movements that are inconsistent with their simple “planar” mechanical classification. To circumvent this difficulty, a simplified classification scheme recognizes only two articular forms: the ovoid joint and the saddle joint (Figure 2-10). Essentially all synovial joints of the body with the notable exception of planar joints can be categorized under this scheme.
An ovoid joint has paired mating surfaces that are imperfectly spheric, or egg-shaped, with adjacent parts possessing a changing surface curvature. In each case the articular surface of one bone is convex and of the other is concave. Most joints in the body fit this scheme. A saddle joint has been previously described. Each member presents paired convex and concave surfaces oriented at approximately 90 degrees to each other. This simplified classification system is functionally associated with the arthrokinematics of roll, slide, or spin (see Chapter 1).
AXIS OF ROTATION
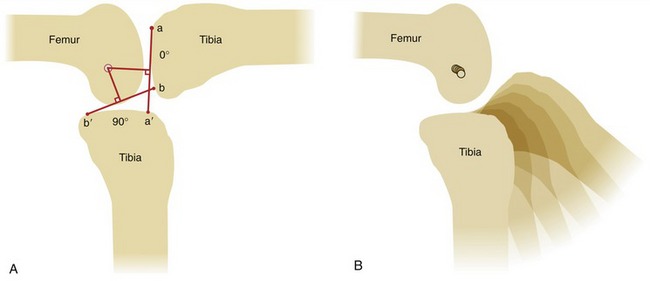
In the analogy of a door hinge (see Figure 2-3, A), the axis of rotation (i.e., the pin through the hinge) is fixed because it remains stationary as the hinge opens and closes. With the axis of rotation fixed, all points on the door experience equal arcs of rotation. In anatomic joints, however, the axis of rotation is rarely, if ever, fixed during bony rotation. Determining the exact position of the axis of rotation in anatomic joints is therefore not a simple task. A method of estimating the position of the axis of rotation in anatomic joints is shown in Figure 2-11, A. The intersection of the two perpendicular lines bisecting a to a′ and b to b′ defines the instantaneous axis of rotation for the 90-degree arc of knee flexion.70 The word instantaneous indicates that the location of the axis holds true only for the specified arc of motion. The smaller the angular range used to calculate the instantaneous axis, the more accurate the estimate. If a series of line drawings is made for a sequence of small angular arcs of motion, the location of the instantaneous axes can be plotted for each portion within the arc of motion (see Figure 2-11, B). The path of the serial locations of the instantaneous axes of rotation is called the evolute. The path of the evolute is longer and more complex when the mating joint surfaces are less congruent or have greater differences in their radii of curvature, such as in the knee.
HISTOLOGIC ORGANIZATION OF PERIARTICULAR CONNECTIVE TISSUES
Fibrous Proteins
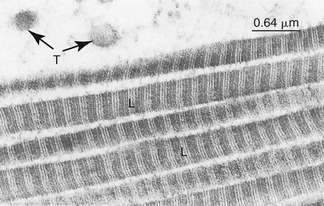
Collagen and elastin fibrous proteins are present in varying proportions in all periarticular connective tissues. Collagen is the most ubiquitous protein in the body, accounting for 30% of all proteins.30 At the most basic level, collagen consists of amino acids wound in a triple helical fashion. These spiraled molecular threads, called tropocollagen, are placed together in a strand, several of which are cross-linked into ropelike fibrils. A collagen fibril may be 20 to 200 nm in diameter.75 Many fibrils interconnect to form bundles or fibers. Although up to 28 specific types of collagen have been described based primarily on their amino acid sequences,67 two types make up the majority of collagen found in periarticular connective tissues: type I and type II.75 Type I collagen consists of thick fibers that elongate little (i.e., stretch) when placed under tension. Being relatively stiff and strong, type I collagen is ideal for binding and supporting the articulations between bones. Type I collagen is therefore the primary protein found in ligaments and fibrous joint capsules. This type of collagen also makes up the parallel fibrous bundles that comprise tendons—the structures that transmit forces between muscle and bone. Figure 2-12 shows a high resolution and magnified image of type I collagen fibrils.
Ground Substance
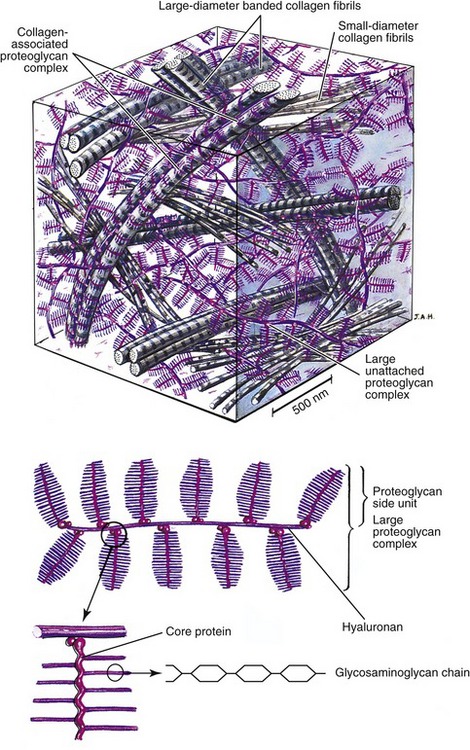
Collagen and elastin fibers within periarticular connective tissues are embedded within a water-saturated matrix or gel known as ground substance. The ground substance of periarticular connective tissues consists primarily of glycosaminoglycans (GAGs), water, and solutes.38,49,63 The GAGs are a family of large polymers of repeating polysaccharides that confer physical resilience to the ground substance. Figure 2-13 shows a stylized illustration of the ground substance within articular cartilage. Depicted at the bottom of Figure 2-13 are individual GAG chains attached to a core protein, forming a large complex proteoglycan side unit. Structurally, each proteoglycan side unit resembles a bottle brush—the wire stem of the brush being the core protein, and the bristles being the GAG chains. Many proteoglycan side units, in turn, are bonded to a central hyaluronan (hyaluronic acid), forming a large proteoglycan complex.30,63,75
Because the GAGs are highly negatively charged, the individual chains (or bristles on the brush) repel one another, greatly increasing three-dimensional volume of the proteoglycan complex. The negatively charged GAGs also make the proteoglycan complexes extremely hydrophilic, able to capture water equivalent to 50 times their weight.38 The attracted water provides a fluid medium for diffusion of nutrients within the matrix. In addition, water and other positive ions confer a unique mechanical property to the tissue. The tendency of proteoglycans to imbibe and hold water causes the tissue to swell.16 Swelling is limited by the embedded and entangled network of collagen (and elastin) fibers within the ground substance (see Figure 2-13, top). The interaction between the restraining fibers and the swelling proteoglycans provides a turgid, semifluid structure that resists compression, much like a balloon or a water-filled mattress. The tissue shown in Figure 2-13 depicts the ground substance that is unique to articular cartilage. This important tissue provides an ideal surface covering for joints and is capable of dispersing millions of repetitive forces that likely affect joints throughout a lifetime.7,8,38
Cells
The primary cells within ligaments, tendons, and other supportive periarticular connective tissues are called fibroblasts. Chondrocytes, in contrast, are the primary cells within hyaline articular cartilage and fibrocartilage.30,43,63 Both types of cells are responsible for synthesizing the specialized ground substance and fibrous proteins unique to the tissue, as well as conducting maintenance and repair. Damaged or aged components of periarticular connective tissues are constantly being removed, as new components are manufactured and remodeled. Cells of periarticular connective tissues are generally sparse and interspersed between the strands of fibers or embedded deeply in regions of high proteoglycan content. This sparseness of cells in conjunction with limited blood supply often results in poor or incomplete healing of damaged or injured joint tissues. In contrast to muscle cells, fibroblasts and chondrocytes do not confer significant mechanical properties on the tissue.
TYPES OF PERIARTICULAR CONNECTIVE TISSUES
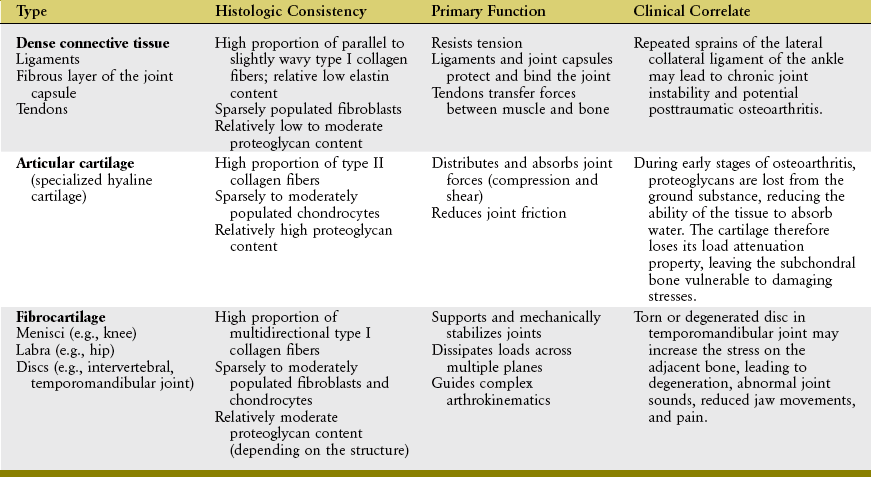
Three types of periarticular connective tissues exist to varying degrees in all joints: dense connective tissue, articular cartilage, and fibrocartilage (Table 2-2).
Dense Connective Tissue
Dense connective tissue includes most of the nonmuscular “soft tissues” surrounding a joint: the fibrous (external) layer of the joint capsule, ligaments, and tendons. These tissues have few cells (fibroblasts), relatively low to moderate proportions of proteoglycan and elastin, and an abundance of tightly packed type I collagen fibers. As with most periarticular connective tissues, ligaments, tendons, and capsules possess a limited blood supply and therefore have a relatively low metabolism.38 When physically loaded or stressed, however, the metabolism of these tissues can increase, often as a means of functionally adapting to a physical stimuli.36,58,69,71 Such adaption has been well documented at the histologic level in tendons.41,61 Strain placed on fibroblasts within the ground substance is believed to stimulate increased synthesis of collagen and GAGs, which can alter the tissue’s structure and thereby modify its material properties, such as stiffness or ultimate failure point.1,31,55,73
Most anatomic or histologic texts38,63,64 describe dense connective tissues as having two subsets, irregular and regular, based on the spatial orientation of the collagen fibers. The fibrous layer of the joint capsule is considered irregular dense connective tissue because of its irregular and often haphazard orientation of collagen fibers within its ground substance.63 This type of tissue is well suited to resist tensile forces from multiple directions, such as what is often required by the spiraled nature of the joint capsules at the glenohumeral or hip joints. Ligaments and tendons are considered regular dense connective tissue because of the more orderly or near parallel orientation of their collagen fibers. The collagen fibers in most ligaments function most effectively when they are stretched nearly parallel to the long axis of the ligament. After the initial slack is pulled tight, the tissues provide immediate tension that restrains undesirable motion between bony partners.
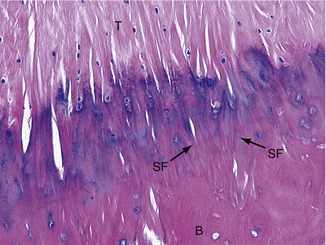
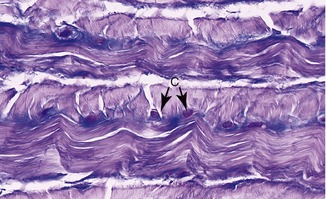
Tendons are designed to transfer large tensile loads between an activated muscle and the bone into which it inserts. The type I collagen fibers within tendons provide high tensile strength once they are fully elongated. Figure 2-14 illustrates a microscopic image of a tendon (T) as it inserts into bone (B). Note the near-parallel arranged collagen fibers, many of which are blending with the collagen of the periosteum. Some collagen fibers can be seen extending deeper into the bone material, often referred to as Sharpey’s fibers (SF).75
Although structurally strong, tendons experience varying amounts of elongation when subjected to a high tensile force. The human Achilles tendon, for example, elongates up to 8% of its resting length after a maximal contraction of the calf muscle.40 This elastic property provides a mechanism to store and release energy during walking or jumping.33,34,37 The property also allows the Achilles tendon to partially dissipate large or rapidly produced tensile force, which may offer some protection against injury.41
Articular Cartilage
Articular cartilage is a specialized type of hyaline cartilage that forms the load-bearing surface of joints. Articular cartilage covering the ends of the articulating bones has a thickness that ranges from 1 to 4 mm in areas of low compression and 5 to 7 mm in areas of high compression.35 The tissue is avascular and aneural.63,75 Unlike most hyaline cartilage throughout the body, articular cartilage lacks a perichondrium. This modification allows the opposing surfaces of the cartilage to form ideal load-bearing surfaces. Similar to periosteum on bone, perichondrium is a layer of connective tissue that covers most cartilage. It contains blood vessels and a ready supply of primitive cells that maintain and repair underlying tissue. This is an advantage not available to articular cartilage.
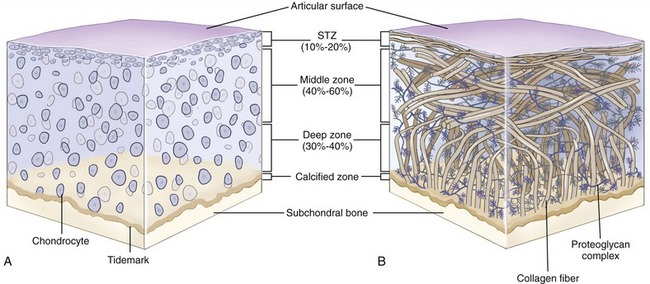
Chondrocytes of various shapes are located within the ground substance of different layers or zones of articular cartilage (Figure 2-15, A). These cells are bathed and nourished by nutrients contained within synovial fluid. Nourishment is facilitated by the “milking” action of articular surface deformation during intermittent joint loading. The chondrocytes are surrounded by predominantly type II collagen fibers. These fibers are arranged to form a restraining network or “scaffolding” that adds structural stability to the tissue (see Figure 2-15, B).49 The deepest fibers in the calcified zone are firmly anchored to the subchondral bone. These fibers are linked to the vertically oriented fibers in the adjacent deep zone, which in turn are linked to the obliquely oriented fibers of the middle zone and finally to the transversely oriented fibers of the superficial tangential zone. The series of chemically interlinked fibers form a netlike fibrous structure that entraps the large proteoglycan complexes beneath the articular surface. The large amounts of proteoglycans, in turn, attract water, which provides a unique element of rigidity to articular cartilage. The rigidity increases the ability of cartilage to adequately withstand loads.38
Articular cartilage distributes and disperses compressive forces to the subchondral bone. It also reduces friction between joint surfaces. The coefficient of friction between two surfaces covered by articular cartilage and wet with synovial fluid is extremely low, ranging from 0.005 to 0.02 in the human knee, for example. This is 5 to 20 times lower and more slippery than ice on ice, which has a friction coefficient of 0.1.45 The forces of normal weight-bearing activities therefore are reduced to a load level that typically can be absorbed without damaging the skeletal system.
Fibrocartilage
As its name implies, fibrocartilage is a mixture of dense connective tissue and articular cartilage (Figure 2-16).75 As such, fibrocartilage provides the resilience and shock absorption of articular cartilage and the tensile strength of ligaments and tendons. Dense bundles of type I collagen exist along with moderate amounts of proteoglycans. Depending on the tissue, fibrocartilage has varying numbers of chondrocytes and fibroblasts, located within a dense and often multidirectional collagen network.30
Fibrocartilage forms much of the substance of the intervertebral discs, the labra, and the discs located within the pubic symphysis, temporomandibular joint, and some joints of the extremities (e.g., the menisci of the knee). These structures help support and stabilize the joints, guide complex arthrokinematics, and help dissipate forces. Fibrocartilage is also found in some ligaments and tendons, especially at the point of insertion into bone.63,75 The dense interwoven collagen fibers of fibrocartilage allow the tissue to resist multidirectional tensile, shear, and compressive forces. Fibrocartilage is therefore an ideal tissue to dissipate loads.
Like articular cartilage, fibrocartilage typically lacks a perichondrium.18,30 Fibrocartilage is also largely aneural and therefore does not produce pain or participate in proprioception, although a few neural receptors may be found at the periphery where fibrocartilage abuts a ligament or joint capsule. Most fibrocartilaginous tissues have a limited blood supply and are largely dependent on diffusion of nutrients from synovial fluid or from adjacent blood vessels. The diffusion of nutrients and removal of metabolic wastes in most fibrocartilaginous discs is assisted by the “milking” action of intermittent weight bearing.26 This principle is readily apparent in adult intervertebral discs that are insufficiently nourished when the spine is held in fixed postures for extended periods. Without proper nutrition, the discs may partially degenerate and lose part of their protective function.3
A direct blood supply penetrates the outer rim of some fibrocartilaginous structures where they attach to joint capsules or ligaments, such as menisci in the knee or intervertebral discs. In adult joints, some repair of damaged fibrocartilage can occur near the vascularized periphery, such as the outer one third of menisci of the knee and the outermost lamellae of intervertebral discs. The innermost regions of fibrocartilage structures, much like articular cartilage, demonstrate poor or negligible healing as a result of the lack of a ready source of undifferentiated fibroblastic cells.6,38,63
BONE
Bone is a very specialized connective tissue, sharing several fundamental histologic characteristics with other periarticular connective tissues. Bone tissue consists of a highly cross-linked type 1 collagen, cells (such as osteoblasts), and a hard ground substance rich in mineral salts. The proteoglycans within the ground substance contain glycoproteins (such as osteocalcin) that strongly bind to calcium and phosphorous rich mineral salts—calcium hydroxyapatite (Ca10[PO4]6[OH]2).49,63,75
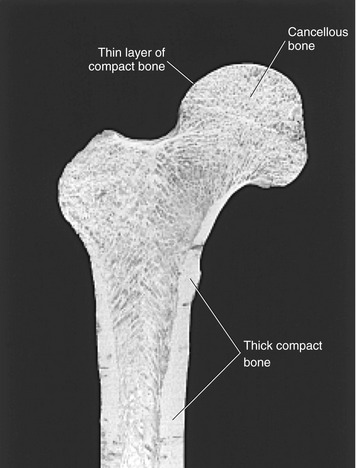
Bone gives rigid support to the body and provides the muscles with a system of levers. The outer cortex of the long bones of the adult skeleton has a shaft composed of thick, compact bone (Figure 2-17). The ends of long bones, however, are formed of a thinner layer of compact bone that surrounds a network of cancellous bone. Bones of the adult axial skeleton, such as the vertebral body, possess an outer shell of relatively thick compact bone that is filled with a supporting core of cancellous bone. As described earlier, articular cartilage covers the diarthrodial articular surfaces of all bones throughout the musculoskeletal system.
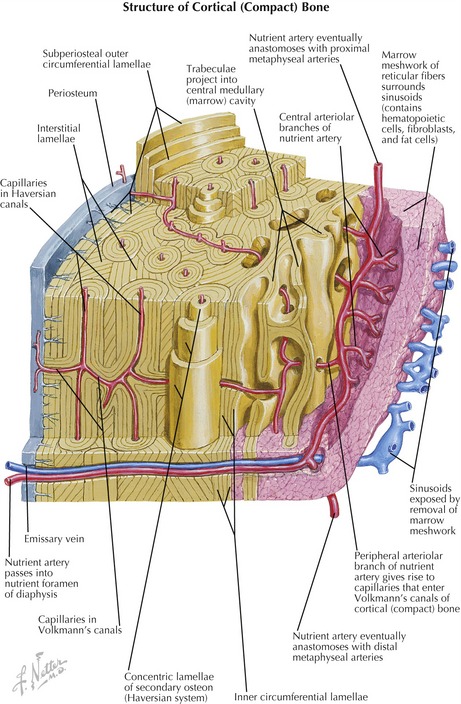
The structural subunit of compact bone is the osteon (Haversian system), which organizes the collagen fibers and mineralized ground substance into a unique series of concentric spirals that form lamellae (Figure 2-18).63,64,75 This infrastructure, made rigid by the calcium phosphate crystals, allows cortical bone to accept tremendous compressive loads. The osteoblasts eventually become surrounded by their secreted ground substance and become confined within narrow lacunae (i.e., spaces) positioned between the lamellae of the osteon.49 (The confined osteoblasts are technically referred to as osteocytes.) Because bone deforms very little, blood vessels can pass into its substance from the outer periosteal and the inner endosteal surfaces. The blood vessels can then turn to travel along the long axis of the bone in a tunnel at the center of the Haversian canals (see Figure 2-18). This system allows a rich source of blood to reach the cells deep within the cortex. Furthermore, the connective tissue comprising the periosteum and endosteum of bone is also richly vascularized, as well as innervated with sensory receptors for pressure and pain.
SOME EFFECTS OF IMMOBILIZATION ON THE STRENGTH OF PERIARTICULAR CONNECTIVE TISSUE AND BONE
The amount and arrangement of the fibrous proteins, ground substance, and water that constitute periarticular connective tissues are influenced by physical activity.9,41,72 At a normal level of physical activity, the composition of the tissues is typically strong enough to adequately resist the natural range of forces imposed on the musculoskeletal system. A joint immobilized for an extended period demonstrates marked changes in the structure and function of its associated connective tissues. The mechanical strength of the tissue is reduced in accordance with the decreased forces of the immobilized condition. This is a normal response to an abnormal condition. Placing a body part in a cast and confining a person to a bed are examples in which immobilization dramatically reduces the level of force imposed on the musculoskeletal system. Although for different reasons, muscular paralysis or weakness also reduces the force on the musculoskeletal system.
The rate of decline in the strength of periarticular connective tissue is somewhat dependent on the normal metabolic activity of the specific tissue.41,53 Chronic immobilization produces a marked decrease in tensile strength of the ligaments of the knee in a period of weeks.47,72 The earliest biochemical markers of this remodeling can be detected within days after immobilization.25,46 Even after the cessation of the immobilization and after the completion of an extended postimmobilization exercise program, the ligaments continue to have lower tensile strength than ligaments that were never subjected to immobilization.25,72 Other tissues such as bone and articular cartilage also show a loss of mass, volume, and strength after immobilization.9,10,21,28 The results from experimental studies imply that tissues rapidly lose strength in response to reduced loading. Full recovery of strength after restoration of loading is much slower and often incomplete.
BRIEF OVERVIEW OF JOINT PATHOLOGY
The repair of damaged fibrocartilaginous joint structures depends on the proximity and adequacy of blood supply. A tear of the outermost region of the meniscus of the knee adjacent to blood vessels embedded within the joint capsule may completely heal.19,56 In contrast, tears of the innermost circumference of a meniscus do not typically heal. This is also the case in the inner lamellae of the adult intervertebral disc, which does not have the capacity to heal after significant damage.3,20
Recurring instability may cause abnormal loading conditions on the joint tissues, which can lead to their mechanical failure. The surfaces of articular cartilage and fibrocartilage may become fragmented, with a concurrent loss of proteoglycans and subsequent lowered resistance to compressive and shear forces.13 Early stages of degeneration often demonstrate a roughened or “fibrillated” surface of the articular cartilage.2 A fibrillated region of articular cartilage may later develop cracks, or clefts, that extend from the surface into the middle or deepest layers of the tissue. These changes reduce the shock absorption quality of the tissue.
Two disease states that commonly cause joint dysfunction are osteoarthritis (OA) and rheumatoid arthritis (RA). Osteoarthritis is characterized by a gradual erosion of articular cartilage with a low inflammatory component.5,24,32 Some clinicians and researchers refer to OA as “osteoarthrosis” to emphasize the lack of a distinctive inflammatory component.11 As erosion of articular cartilage progresses, the underlying subchondral bone becomes more mineralized and in severe cases becomes the weight-bearing surface when the articular cartilage pad is completely worn. The fibrous joint capsule and synovium become distended and thickened. The severely involved joint may be completely unstable and dislocate or may fuse, allowing no motion.
The frequency of OA increases with age, and the disease has several manifestations.12,16 Idiopathic OA occurs in the absence of a specific cause; it affects only one or a few joints, particularly those that are subjected to the highest weight-bearing loads: hip, knee, and lumbar spine. Familial OA or generalized OA affects joints of the hand and is more frequent in women. Posttraumatic OA may affect any synovial joint that has been exposed to a trauma of sufficient severity.
REFERENCES
1. Arnoczky, SP, Lavagnino, M, Whallon, JH, Hoonjan, A. In situ cell nucleus deformation in tendons under tensile load: A morphological analysis using confocal laser microscopy. J Orthop Res. 2002;20:29–35.
2. Bae, WC, Wong, VW, Hwang, J, et al. Wear-lines and split-lines of human patellar cartilage: Relation to tensile biomechanical properties. Osteoarthritis Cartilage. 2008;16:841–845.
3. Beattie, PF. Current understanding of lumbar intervertebral disc degeneration: A review with emphasis upon etiology, pathophysiology, and lumbar magnetic resonance imaging findings. J Orthop Sports Phys Ther. 2008;38:329–340.
4. Begg, RK, Sparrow, WA. Aging effects on knee and ankle joint angles at key events and phases of the gait cycle. J Med Eng Technol. 2006;30:382–389.
5. Brandt, KD, Dieppe, P, Radin, EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34:531–559.
6. Buckwalter, JA, Brown, TD. Joint injury, repair, and remodeling: Roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7–16.
7. Buckwalter, JA, Kuettner, KE, Thonar, EJ. Age-related changes in articular cartilage proteoglycans: Electron microscopic studies. J Orthop Res. 1985;3:251–257.
8. Buckwalter, JA, Mankin, HJ, Grodzinsky, AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480.
9. Chen, JS, Cameron, ID, Cumming, RG, et al. Effect of age-related chronic immobility on markers of bone turnover. J Bone Miner Res. 2006;21:324–331.
10. Demirbag, D, Ozdemir, F, Kokino, S, Berkarda, S. The relationship between bone mineral density and immobilization duration in hemiplegic limbs. Ann Nucl Med. 2005;19:695–700.
11. Dequeker, J, Luyten, FP. The history of osteoarthritis-osteoarthrosis. Ann Rheum Dis. 2008;67:5–10.
12. Ding, C, Cicuttini, F, Blizzard, L, et al. A longitudinal study of the effect of sex and age on rate of change in knee cartilage volume in adults. Rheumatology. 2007;46:273–279.
13. Ding, C, Cicuttini, F, Scott, F, et al. Association between age and knee structural change: A cross sectional MRI based study. Ann Rheum Dis. 2005;64:549–555.
14. Dudhia, J. Aggrecan, aging and assembly in articular cartilage. Cell Mol Life Sci. 2005;62:2241–2256.
15. Dudley-Javoroski, S, Shields, RK. Dose estimation and surveillance of mechanical loading interventions for bone loss after spinal cord injury. Phys Ther. 2008;88:387–396.
16. Freemont, AJ, Hoyland, JA. Morphology, mechanisms and pathology of musculoskeletal aging. J Pathol. 2007;211:252–259.
17. Frost, HM. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004;74:3–15.
18. Gartner, LP, Hiatt, JL. Color textbook of histology, ed 3. Philadelphia: Saunders; 2007.
19. Greis, PE, Bardana, DD, Holmstrom, MC, Burks, RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10:168–176.
20. Grunhagen, T, Wilde, G, Soukane, DM, et al. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30–35.
21. Haapala, J, Arokoski, J, Pirttimaki, J, et al. Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int J Sports Med. 2000;21:76–81.
22. Hamerman, D. Aging and the musculoskeletal system. Ann Rheum Dis. 1997;56:578–585.
23. Hanna, F, Teichtahl, AJ, Bell, R, et al. The cross-sectional relationship between fortnightly exercise and knee cartilage properties in healthy adult women in midlife. Menopause. 2007;14:830–834.
24. Hardingham, T. Extracellular matrix and pathogenic mechanisms in osteoarthritis. Curr Rheumatol Rep. 2008;10:30–36.
25. Hayashi, K. Biomechanical studies of the remodeling of knee joint tendons and ligaments. J Biomech. 1996;29:707–716.
26. Humzah, MD, Soames, RW. Human intervertebral disc: Structure and function. Anat Rec. 1988;220:337–356.
27. Iannone, F, Lapadula, G. The pathophysiology of osteoarthritis. Aging Clin Exp Res. 2003;15:364–372.
28. Jortikka, MO, Inkinen, RI, Tammi, MI, et al. Immobilisation causes longlasting matrix changes both in the immobilised and contralateral joint cartilage. Ann Rheum Dis. 1997;56:255–261.
29. Khosla, S, Riggs, BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34:1015–1030.
30. Kierszenbaum, AL. Histology and cell biology: An introduction to pathology, ed 2. Philadelphia: Mosby; 2007.
31. Kjaer, M, Magnusson, P, Krogsgaard, M, et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006;208:445–450.
32. Krasnokutsky, S, Samuels, J, Abramson, SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 2007;65:222–228.
33. Kubo, K, Kanehisa, H, Takeshita, D, et al. In vivo dynamics of human medial gastrocnemius muscle-tendon complex during stretch-shortening cycle exercise. Acta Physiol Scand. 2000;170:127–135.
34. Kurokawa, S, Fukunaga, T, Nagano, A, Fukashiro, S. Interaction between fascicles and tendinous structures during counter movement jumping investigated in vivo. J Appl Physiol. 2003;95:2306–2314.
35. Kurrat, HJ, Oberlander, W. The thickness of the cartilage in the hip joint. J Anat. 1978;126:145–155.
36. Langberg, H, Skovgaard, D, Petersen, LJ, et al. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521(Pt 1):299–306.
37. Lieber, RL, Leonard, ME, Brown-Maupin, CG. Effects of muscle contraction on the load-strain properties of frog aponeurosis and tendon. Cells Tissues Organs. 2000;166:48–54.
38. Lundon, K. Orthopaedic rehabilitation science: principles for clinical management of nonmineralized connective tissue. St Louis: Butterworth-Heinemann; 2003.
39. Maganaris, CN, Reeves, ND, Rittweger, J, et al. Adaptive response of human tendon to paralysis. Muscle Nerve. 2006;33:85–92.
40. Magnusson, SP, Hansen, P, Aagaard, P, et al. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003;177:185–195.
41. Magnusson, SP, Narici, MV, Maganaris, CN, Kjaer, M. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008;586:71–81.
42. Martin, JA, Brown, TD, Heiner, AD, Buckwalter, JA. Chondrocyte senescence, joint loading and osteoarthritis. Clin Orthop Relat Res. 2004;427(Suppl):S96–S103.
43. Martin, JA, Buckwalter, JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85(Suppl 2):106–110.
44. Mikesky, AE, Mazzuca, SA, Brandt, KD, et al. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthr Rheumat. 2006;55:690–699.
45. Mow, VC, Hayes, WC. Basic orthopaedic biomechanics. New York: Raven Press; 1991.
46. Muller, FJ, Setton, LA, Manicourt, DH, et al. Centrifugal and biochemical comparison of proteoglycan aggregates from articular cartilage in experimental joint disuse and joint instability. J Orthop Res. 1994;12:498–508.
47. Noyes, FR. Functional properties of knee ligaments and alterations induced by immobilization: A correlative biomechanical and histological study in primates. Clin Orthop Relat Res. 1977;123:210–242.
48. Onambele, GL, Narici, MV, Maganaris, CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100:2048–2056.
49. Ovalle, WK, Nahirney, PC. Netter’s essential histology. Philadelphia: Saunders; 2008.
50. Pearson, OM, Lieberman, DE. The aging of Wolff’s “law”: Ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol. 2004;39(Suppl):63–99.
51. Podichetty, VK. The aging spine: The role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol. 2007;53:4–18.
52. Racunica, TL, Teichtahl, AJ, Wang, Y, et al. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis Rheum. 2007;57:1261–1268.
53. Reeves, ND. Adaptation of the tendon to mechanical usage. J Musculoskelet Neuronal Interact. 2006;6:174–180.
54. Reeves, ND, Narici, MV, Maganaris, CN. Strength training alters the viscoelastic properties of tendons in elderly humans. Muscle Nerve. 2003;28:74–81.
55. Rosager, S, Aagaard, P, Dyhre-Poulsen, P, et al. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12:90–98.
56. Rubman, MH, Noyes, FR, Barber-Westin, SD. Arthroscopic repair of meniscal tears that extend into the avascular zone. A review of 198 single and complex tears. Am J Sports Med. 1998;26:87–95.
57. Sargon, MF, Doral, MN, Atay, OA. Age-related changes in human PCLs: A light and electron microscopic study. Knee Surg Sports Traumatol Arthrosc. 2004;12:280–284.
58. Setton, LA, Chen, J. Cell mechanics and mechanobiology in the intervertebral disc. Spine. 2004;29:2710–2723.
59. Shields, RK, Dudley-Javoroski, S. Musculoskeletal adaptations in chronic spinal cord injury: Effects of long-term soleus electrical stimulation training. Neurorehabil Neural Repair. 2007;21:169–179.
60. Shields, RK, Dudley-Javoroski, S, Law, LA. Electrically induced muscle contractions influence bone density decline after spinal cord injury. Spine. 2006;31:548–553.
61. Smith, K, Rennie, MJ. New approaches and recent results concerning human-tissue collagen synthesis. Curr Opin Clin Nutr Metab Care. 2007;10:582–590.
62. Squires, GR, Okouneff, S, Ionescu, M, Poole, AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum. 2003;48:1261–1270.
63. Standring, S. Gray’s anatomy: the anatomical basis of clinical practice, ed 40. St Louis: Elsevier; 2009.
64. Stevens, A, Lowe, JS. Human histology. Philadelphia: Mosby; 2005.
65. Troke, M, Moore, AP, Maillardet, FJ, Cheek, E. A normative database of lumbar spine ranges of motion. Man Ther. 2005;10:198–206.
66. van Weeren, PR, Firth, EC, Brommer, B, et al. Early exercise advances the maturation of glycosaminoglycans and collagen in the extracellular matrix of articular cartilage in the horse. Equine Vet J. 2008;40:128–135.
67. Veit, G, Kobbe, B, Keene, DR, et al. Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J Biol Chem. 2006;281:3494–3504.
68. von, SS, Kemmler, W, Kalender, WA, et al. Differential effects of strength versus power training on bone mineral density in postmenopausal women: A 2-year longitudinal study. Br J Sports Med. 2007;41:649–655.
69. Wackerhage, H, Rennie, MJ. How nutrition and exercise maintain the human musculoskeletal mass. J Anat. 2006;208:451–458.
70. Winter, DA. Biomechanics and motor control of human movement. New Jersey: John Wiley & Sons; 2005.
71. Woo, SL, Abramowitch, SD, Kilger, R, Liang, R. Biomechanics of knee ligaments: Injury, healing, and repair. J Biomech. 2006;39:1–20.
72. Woo, SL, Gomez, MA, Sites, TJ, et al. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987;69:1200–1211.
73. Woo, SL, Gomez, MA, Woo, YK, Akeson, WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397–408.
74. Yamaguchi, K, Sher, JS, Andersen, WK, et al. Glenohumeral motion in patients with rotator cuff tears: A comparison of asymptomatic and symptomatic shoulders. J Shoulder Elbow Surg. 2000;9:6–11.
75. Young, B, Lowe, JS, Stevens, A. Wheater’s functional histology: a text and colour atlas, ed 5. Philadelphia: Churchill Livingstone; 2006.
STUDY QUESTIONS
1. Describe the morphologic differences between ovoid and saddle joints. Provide an anatomic example of each type of joint.
2. Cite the major distinguishing structural and functional differences between a synarthrodial and a diarthrodial (synovial) joint.
3. Intra-articular discs (or menisci) are sometimes found in diarthrodial joints. Name three joints in the body that contain intra-articular discs. Describe the most likely function(s) of these structures at these joints.
4. List the four primary types of tissues that exist throughout the body.
5. Which of the joints illustrated in Figures 2-3 through 2-9 have (a) the greatest and (b) the least degrees of freedom?
6. Cite the major functional differences between type I collagen and elastin. Cite tissues that contain a high proportion of each protein.
7. What is the difference between an evolute and an instantaneous axis of rotation? Cite one biomechanical or practical consequence of a joint that possesses a significantly large, although normal, evolute.
8. Define (a) perichondrium and (b) periosteum. What is the primary function of these tissues?
9. Describe the fundamental mechanism used by articular cartilage to repeatedly disperse compression forces across joints.
10. Describe the primary reasons why bone possesses a far superior healing potential than articular cartilage.
11. Describe the natural effects of advanced aging on periarticular connective tissues. In extreme cases, how could these changes manifest themselves clinically?
12. List three histologic features that are common to articular cartilage, tendon, and bone.
13. Briefly contrast osteoarthritis and rheumatoid arthritis.
14. List three structures always found in synovial joints. Cite common pathologies that may affect these structures, and comment on the nature of the resulting impairment.
 Answers to the study questions can be found on the Evolve website.
Answers to the study questions can be found on the Evolve website.