Chapter 53 Basic Principles of Deep Brain Stimulation for Movement Disorders, Neuropsychiatric Disorders, and New Frontiers
• The spectrum of movement disorders treated with deep brain stimulation (DBS) includes Parkinson’s disease (PD), essential tremor, and dystonia. The cardinal features of PD are resting tremor, bradykinesia, rigidity, and postural instability. Replacement of dopamine via dopamine agonists and L-dopa is the mainstay of medical treatment. DBS is the neurosurgical therapy of choice for movement disorders. DBS can improve motor symptoms, decrease medication usage, and decrease on/off motor fluctuations in patients with good response to L-dopa.
• In choosing an appropriate target for neuromodulation by DBS, it is important to note that multiple targets can be efficacious for a single disease. For example, Parkinson’s disease responds well to DBS of the globus pallidus interna (GPi) and subthalamic nucleus (STN).
• Deep brain stimulation of the ventralis intermedius nucleus of the thalamus can be used to treat patients with disabling essential tremor. Extremity tremors are more reliably treated than axial tremors.
• Primary generalized and focal dystonias can be treated with deep brain stimulation of the GPi. Some secondary dystonias may respond to deep brain stimulation; however, this is a heterogeneous group of disorders and response to deep brain stimulation may be the exception rather than the rule.
• Deep brain stimulation of the ventral capsule/ventral striatum is approved under a Food and Drug Administration (FDA) humanitarian device exemption for the treatment of severe, treatment-refractory obsessive-compulsive disorder. Active investigation of deep brain stimulation for neuropsychiatric disorders, pain, epilepsy, and others is currently ongoing.
• The pharmacological therapies available for dystonia are limited; therefore, surgical alternatives have been developed. These include intrathecal baclofen pumps, botulinum toxin injections, ablative procedures such as thalamotomy, and neuromodulation procedures such as DBS. The GPi is the DBS “target of choice” for “primary generalized dystonia.”
• Careful selection of appropriate patients is critical to good outcomes in DBS procedures.
• DBS for movement and neuropsychiatric disorders should be performed in the context of a multidisciplinary team.
• In common movement disorders, surgical targets are the STN and GPi for Parkinson’s disease, ventralis intermedius nucleus of the thalamus for essential tremor, GPi for dystonia, and the ventral capsule/ventral striatum (VC/VS) target for neuropsychiatric disorders such as depression and obsessive-compulsive disorder (OCD).
• Targeting of subcortical nuclei in DBS is based on stereotactic principles.
Historical Background
Deep brain stimulation (DBS) is now considered the neurosurgical therapy of choice for movement disorders, with over 80,000 patients having undergone DBS worldwide.1 DBS works through the delivery of therapeutic electrical energy to highly specific targets in the brain. The safety and efficacy of DBS have been validated in many high-quality studies in over 20 years of use. DBS is also the subject of intense intellectual study; a recent PubMed search for “deep brain stimulation” returned over 3500 published studies.2 A particularly exciting aspect of the ongoing research in DBS is evaluation of expanding indications for the therapy, which span neuropsychiatric disorders, epilepsy, and disorders of consciousness, among others. This chapter reviews the historical underpinnings of DBS, indications for use, and future horizons. Additionally, a brief description of DBS components and purported mechanisms of action will be provided.
Historical Beginnings
The modern era of deep brain stimulation has its historical roots in the development of stereotactic surgery, which has its own origins in the work of the French mathematician and philosopher René Descartes. In part II of his 1637 work, Discourse on the Method, Descartes describes a method for specifying the location of a point based on its relationship to two intersecting axes.3 (Of note, this work is best known for its famous quotation, “Je pense, donc je suis” [I think, therefore I am], which occurs in part IV.) This method, known as the Cartesian coordinate system, facilitated the development of calculus by Isaac Newton and Gottfried Wilhelm Leibniz as well as modern stereotactic neurosurgery.
The happy marriage of the fields of stereotaxy, movement disorder surgery, and psychosurgery gave rise to the underlying principles of modern DBS surgery. On the movement disorders front, much work had been done on cortical resections and subcortical lesioning to palliate these disorders. In 1909, Sir Victor Horsley, who described the first stereotactic apparatus in animals, relieved hemiathetosis in a 15-year-old boy by removing the precentral gyrus somatotopically associated with the upper extremity.4 In 1931, Bucy performed the same procedure for seizure relief and relieved the patient’s choreoathetosis in the process.5 He later, in 1937, performed this procedure with Theodore Case for the treatment of post-traumatic tremor.6 These patients had postoperative hemiparesis and dyspraxia that resolved to a great extent postoperatively.7 In 1931, Putnam began sectioning of the extrapyramidal tracts in the high cervical cord, with good results for choreoathetosis but not with parkinsonian tremor.8,9 Given that cortical ablation would relieve tremor, but extrapyramidal ablation would not, he reasoned that impulses in the pyramidal tract were responsible for the production of parkinsonian tremor, and in 1940, he reported the results of lateral pyramidotomy in the high cervical region in seven patients.10 Again, these patients had postoperative improvement of tremor but some resultant hemiplegia.
The search for a suitable procedure to maximize tremor control yet minimize postoperative morbidity led to cerebral pedunculotomy by Walker;11 open, transcortical, transventricular removal of portions of the basal ganglia by Meyers;12 and anterior choroidal artery ligation by Cooper.13–15 (As an aside, Cooper’s procedure was discovered accidentally when he tore the anterior choroidal artery during an aborted cerebral pedunculotomy.) These procedures, while therapeutic, were both variable in their outcomes and suffered from high perioperative morbidity rates, thereby necessitating more precise and less invasive targeting.
Furthermore, proponents of psychosurgery wanted a means for performing controlled prefrontal lobotomy.16 In the 1940s, there was concern among neurosurgeons regarding the high morbidity and mortality rates associated with open psychosurgical procedures including prefrontal leucotomy as introduced by Egas Moniz and prefrontal lobotomy as introduced by Walther Freeman and James Watts.17 There was a general feeling that the principles of psychosurgery were sound, but smaller and more specific structures should be targeted to achieve optimal therapeutic benefit while avoiding unnecessary morbidity and unwanted neurological deficit.
Initial attempts of stereotactic surgery began in the late nineteenth and early twentieth centuries. In 1889, D.N. Zernov, a Russian surgeon in Moscow, used a navigation system (called an encephalometer) based on polar coordinates and referenced to external landmarks to drain a cerebral abscess.18,19 The first truly modern stereotactic apparatus based on Cartesian coordinates and based on internal cerebral landmarks was first described in animals in the early part of the twentieth century by Sir Victor Horsley and Robert Clarke. In 1908, they described a stereotactic apparatus that could introduce a probe into subcortical structures defined in a Cartesian coordinate system.20
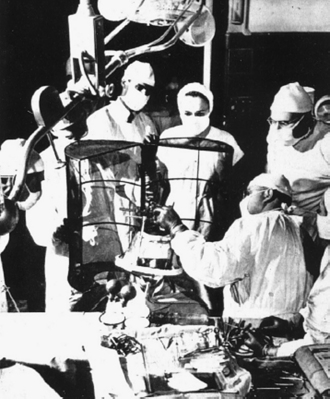
The first device developed for inserting an electrode accurately into a chosen structure in the human brain was described by Ernest A. Spiegel, Henry T. Wycis, and others in 194721 (Fig. 53.1). At that time, cerebral anatomical structures were targeted based on location relative to the third ventricle using ventriculogram x-rays. Electrode position in the apparatus of Spiegel and Wycis was adjusted by sliding the carrier along a base plate in the anteroposterior and lateral directions, with vertical adjustments made by a microdrive. Leksell introduced a semicircular arc apparatus in 1949, allowing for the introduction of an electode to a target along any trajectory, because the target now would lie in the center of the arc22 (Fig. 53.2).
The first lesioning procedures performed by Spiegel and Wycis were in the dorsomedial thalamic nucleus for agitation and psychosis, the medial thalamus for epilepsy, and mesencephalic pain pathways for intractable pain.7,23 With the advent of stereotactic lesioning procedures, many new techniques were developed for the management of psychiatric and movement disorders. These included pallidotomy and thalamotomy for the relief of Parkinson’s disease (PD)–associated tremor,24–29 as well as anterior cingulotomy,30,31 anterior capsulotomy,32 subcaudate tractotomy,33 and limbic leucotomy34 in the treatment of affective disorders7,17,35,36Fig. 53.3. However, with the advent of L-dopa in the 1960s for PD, there was a steep decrease in surgery for PD.37 Similarly, the introduction of lithium in the late 1940s and the antipsychotic drug chlorpromazine in the mid-1950s led to a nonsurgical bend in the management of psychiatric disorders.17,36,38 Therefore, the next paradigm shift in the treatment of these disorders did not come until the limitations of these medications with their side effects and lack of sustained benefits were realized, and DBS provided a safer, nondestructive, reversible, and adjustable approach for the treatment of these disorders.
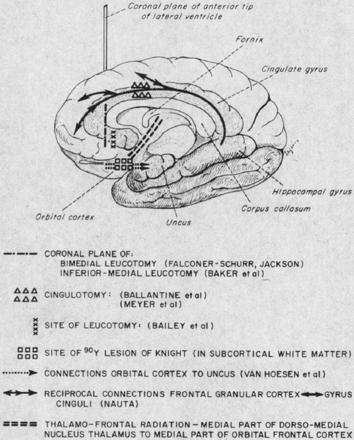
FIGURE 53.3 Classical drawing depicting locations of stereotactic lesioning procedures for psychosurgery.
(Reprinted from Sweet WH. Treatment of medically intractable mental disease by limited frontal leucotomy—justifiable? N Engl J Med. 1973;289(21):1117-1125; used with permission of the Massachusetts Medical Society.)
In 1809, Rolando first demonstrated that electrical impulses could modify the function of brain regions, and prior to that, Aldini attempted stimulation of the brains of criminals immediately following their executions.37,39 Hassler found, during stereotactic exploration of the brain for pallidotomy, that low-frequency stimulation would worsen tremor and high-frequency stimulation would ameliorate it.37,40 This was first put into practice in 1967 by Bechtereva and associates with chronic DBS of the thalamus, striatum, and pallidum for the treatment of movement disorders.41
It was not until the 1970s and 1980s, however, that the modern era of chronic stimulation was ushered in. Hosobuchi and co-workers reported on stimulation of the VPM thalamus for the control of facial anesthesia dolorosa following rhizotomy for trigeminal neuralgia in 1973.42 DBS of ventral periaqueductal gray matter in the midbrain for the treatment of cancer pain was reported by Richardson and Akil in the mid-1970s.43 Chronic DBS for the management of movement disorders was utilized by several groups including Benabid and colleagues,44,45 Blond and Siegfried,46 Siegfried and Shulman,47 and Brice and McLellan.48 DBS has similar efficacy to its corresponding lesioning procedures, but with an improved safety profile, inherent reversibility, and adjustment of the stimulation parameters over time to accommodate the patient’s needs. These factors have led to DBS being adopted as the neurosurgical standard of care for movement disorders as well as the expansion of DBS to the treatment of epilepsy, psychiatric disorders including obsessive-compulsive disorder (OCD) and depression, as well as chronic pain, Tourette’s syndrome, and even disorders of consciousness and addiction.
Basic Neurocircuitry Principles
Parallel cortico-striato-pallido-thalamo-cortical (CSPTC) circuits exist for limbic, associative, and motor function.49 The motor loop is the most commonly described loop and is important in the pathogenesis of PD and the therapeutic efficacy of DBS in this condition.37 Limbic and associative circuits have been implicated in the pathogenesis of neuropsychiatric conditions including OCD and major depressive disorder (MDD), which respond to DBS of critical nodes along the circuit, including the ventral capsule/ventral striatum (VC/VS), anterior limb of the internal capsule (ALIC), nucleus accumbens (NAc), subgenual cingulate cortex (SCC), and inferior thalamic peduncle.49–61 These circuits maintain some degree of topographic segregation, with motor circuitry more dorsolateral, limbic circuitry ventromedial, and associative circuitry in between.62
These circuits are not strictly “parallel.” Haber and associates demonstrated some interface between these circuits in primates with the more medial limbic circuits ultimately influencing more dorsolateral motor circuits.62 This unique arrangement allows for a link between emotion and motivation (limbic circuits) with cognition and planning (associative circuits), which finally manifests with a motor output and behavior (motor circuits).
Both direct and indirect loops exist for the associative and limbic circuits, as they do for the motor circuits.49 The direct loops connect from the striatum to the globus pallidus pars interna (GPi), substantia nigra pars reticulata (SNr), and ventral tegmental area (VTA). From there, the projections head to the thalamus. Indirect loops connect from the striatum to the globus pallidus pars externa (GPe), and then head to the subthalamic nucleus (STN) before going to the GPi, SNr, and VTA (Fig. 53.4).
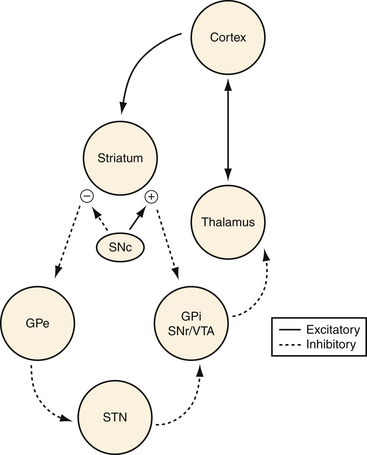
FIGURE 53.4 Schematic of relevant cortico-striato-pallido-thalamo-cortical (CSPTC) neural circuitry.
In choosing an appropriate target for neuromodulation by DBS, it is important to note that multiple targets can be efficacious for a single disease. For example, we know that PD responds well to DBS of the GPi and subthalamic nucleus.37,63 In psychosurgery, multiple targets have been used, for example, in OCD including the ALIC,52,59 VC/VS and NAc,51,57 inferior thalamic peduncle,56 and subthalamic nucleus.58 This is likely secondary to the rich connectivity of the modulated circuits and the mechanism of action of DBS. High-frequency stimulation (HFS) has a net inhibitory effect and low-frequency stimulation (LFS) has a net excitatory effect; however, the mechanism of DBS (high-frequency) is likely more complex with activation of nearby axons and suppression of abnormal impulses through the circuits involved.37
Deep Brain Stimulation Surgery: Indications, Target, and Outcomes
All patients should undergo detailed neuropsychological assessment. The aim is to identify patients who have underlying cognitive deficits or psychiatric conditions that may be worsened by DBS. Severe dementia or cognitive impairment is generally a contraindication to DBS, and patients with even mild deficits in frontal executive function or cognition need especially strong social support following surgery. Psychiatric conditions as depression, mania, and anxiety should be identified and optimized preoperatively, and patients with psychosis or personality disorders should generally be excluded from surgical intervention.37 These factors underscore the importance of a multidisciplinary team devoted to the care of DBS patients. (Selection criteria are summarized in Table 53.1.)
TABLE 53.1 General Principles of Patient Selection in Deep Brain Stimulation Surgery: Predictors of Successful Outcome
Surgical Technique
Preoperative Imaging and Planning
Stereotactic anatomical targeting may be by indirect or direct methods. Indirect targeting utilizes stereotactic atlases based on cadaveric sections. Known distances of the different nuclei from the midcommissural point (MCP), the halfway distance between the anterior commissure (AC) and posterior commissure (PC), are used in determining the position of the anatomical target. Typical anatomical coordinates for the common targets are as follows: STN (10-13 mm lateral to midline, 3-5 mm ventral to the AC-PC plane, and 2-4 mm posterior to the MCP), the GPi (19-21 mm lateral to the midline, 2-3 mm anterior to the MCP, and 4-5 mm ventral to the AC-PC plane), the ventralis intermedius (VIM) (11-12 mm lateral to the wall of the third ventricle, at the level of the AC-PC plane and between 2⁄12 and 3⁄12 of the AC-PC distance anterior to the PC). Table 53.2 shows the typical stereotactic targets for different indications as well as their stereotactic locations with reference to the AC, PC, and MCP.
TABLE 53.2 Deep Brain Stimulation Surgery: Targets, Indications, and Relationship to Anterior Commissure–Posterior Commissure (AC-PC) Line
| Target | Indication | Anatomical Coordinates∗ |
|---|---|---|
| STN | Parkinson’s disease | 11-13 mm lateral to midline 4-5 mm ventral to the AC-PC plane 3-4 mm posterior to the MCP |
| GPi | Dystonia | 19-21 mm lateral to the midline 2-3 mm anterior to the MCP 4-5 mm ventral to the AC-PC plane |
| VIM | Essential tremor | 11-12 mm lateral to the wall of the third ventricle At the level of the AC-PC plane Between 2⁄12 and 3⁄12 of the AC-PC distance anterior to the PC |
GPi, globus pallidus interna; MCP, midcommissural point; STN, subthalamic nucleus; VIM, ventralis intermedius nucleus of the thalamus.
∗ Relationship to AC-PC line and MCP.
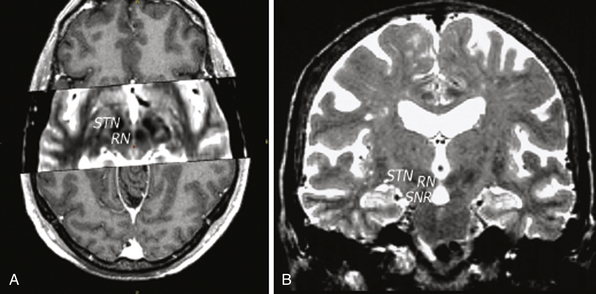
Direct targeting involves direct visualization of the deep nuclei using magnetic resonance imaging (MRI). Although computed tomography (CT) can be used in stereotactic targeting, it is difficult to visualize the various target nuclei or the AC and PC using CT alone. On the other hand, MRI offers multiplanar imaging capabilities with excellent anatomical delineation. The AC and PC can be identified on T1-weighted images while the STN can be identified on T2-weighted images (Fig. 53.5). The MRI studies may then be merged with stereotactically acquired CT images. The evident advantage of direct targeting is that discrete anatomical variation between individuals is taken into account because it is based on the individual’s anatomy as obtained from imaging. Generally, a combination of indirect and direct targeting methods is used. Following the targeting, localization of the target within the stereotactic space may be performed by frame-based or frameless systems.
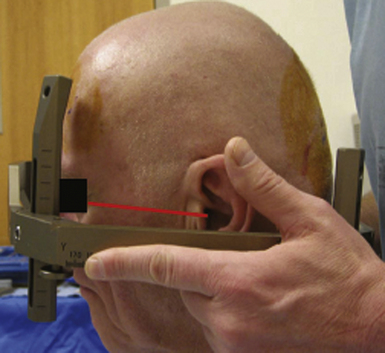
A variety of stereotactic head frames are available. Figure 53.2 shows the Leksell stereotactic head frame (Elekta, Stockholm, Sweden) that is used in our institution, and Figure 53.6 shows a frameless system. The accuracy of the various systems has been demonstrated in previous studies, and the use of any system depends on surgeon preference, familiarity, and what is available in a particular institution. In addition to stereotactic anatomical targeting, imaging is also used to plan an entry point and trajectory to the target. The entry point and trajectory are chosen with the aim of avoiding cortical, subcortical, and periventricular blood vessels, thus reducing the risk of hemorrhagic complications. Contrast-enhanced T1-weighted MRI or contrast-enhanced CT scans help to delineate vessels along the electrode path and assist with vessel avoidance during planning.
Surgical Procedure
The stereotactic frame is placed (Fig. 53.7) and the patient is positioned supine with the stereotactic head frame fixed to the operating room table after acquisition of a stereotactic CT scan. Typically, the stereotactic CT scan is then merged with the previously acquired MRI scan and the stereotactic coordinates are obtained. The stereotactic coordinates are set on the frame and used to determine the site for skin incision and bur hole based on earlier entry point and trajectory planning. Generally in the planning, a bur hole and entry point anterior to the coronal suture are chosen. After the bur hole is performed, the dura is opened and pia coagulated for corticotomy. The microelectrodes are then inserted with the tip of the electrode placed at a defined offset above the target. At this time microelectrode recording (MER) is commenced as the electrodes are advanced in sub-millimeter steps. All sedation should be stopped before the commencement of microelectrode recording as the sedation may affect the electrophysiological mapping. MER allows for definition and verification of the physiological target. The frequency and pattern of activity of the various nuclei and white matter tracts encountered in the path to the physiological target are noted and may help determine the relationship of the trajectory to the target. These characteristic electrophysiological signatures of the targeted nuclei help confirm the physiological target. Macrostimulation is then performed to determine benefits and side effects of the stimulation.
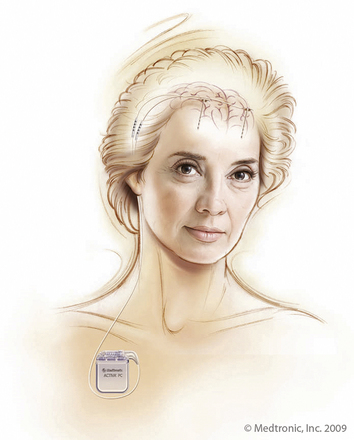
The main components of the DBS system are an intracranial electrode and an implantable pulse generator, by an extension wire (Fig. 53.8). After the physiological target is confirmed, the electrode is implanted at the target. The two currently available electrodes are the Medtronic 3389, which spans 7.5 mm, and the 3387, which spans 10.5 mm (each model has four contacts which are 1.5 mm in height with 0.5-mm spacing between the contacts in the 3389 and 1.5-mm spacing in the 3387 model) (Fig. 53.9). The implantation can be confirmed by fluoroscopy. After the implantation, intraoperative stimulation is then performed with the DBS lead to assess for clinical improvements and side effects of stimulation.
Complications
Complications associated with DBS may be related to the surgical implantation process, related to the hardware/device, or linked to stimulation. The most serious potential complication associated with DBS electrode implantation is intracranial hemorrhage (ICH), which may result in profound neurological deficits and even death (see Fig. 53.9). ICH is reported to occur between 0.2% and 12.5% of the time.37 Asymptomatic hemorrhage is more common than symptomatic cases and is detected only incidentally on postoperative brain CT imaging. In any case, intracerebral hemorrhage is usually related to direct damage to an arterial blood vessel. Occasionally, it may be due to injury to a cortical draining vein when it may present in a delayed manner with venous infarction. Careful preoperative planning of the entry point and trajectory of approach to the target that avoids evident cortical, subcortical, or periventricular blood vessels on a contrast-enhanced brain MRI scan is a first step in avoiding hemorrhagic complications. Other strategies to prevent intracerebral hemorrhagic complications include tight intraprocedural blood pressure control (maintenance of systolic blood pressure below 130 mm Hg), prevention of entry of large amounts of intracranial air by filling the subdural space with saline and occluding the bur hole with Gelfoam and tissue glue during microelectrode recording, avoiding repeated coughing or sneezing during the procedure, and avoiding the coagulation of cortical veins. There may also be increased risk of hemorrhage based on the choice of target. For example, the GPi is reported to have higher hemorrhage rates than the STN. Binder and co-workers showed a 7% risk of hemorrhage in targeting the GPi versus a 2.2% risk in targeting the STN.64 The DBS study group reported a 9.8% risk of hemorrhage in the GPi versus 2.9% risk in the STN.65
Elderly patients may develop transient episodes of confusion and agitation following DBS, especially with procedures. Staging of bilateral DBS procedures in these elderly patients may reduce the incidence of this complication. Infection of the hardware is an ever-present risk in these procedures. Risk of infection ranges between 1% and 15% in published studies, and most commonly affects the site of the IPG.37 The risk of infection may be reduced by adhering strictly to aseptic principles during the procedure as well as the judicious use of prophylactic antibiotics. Treatment of the infection may require removal of the DBS hardware and use of appropriate antibiotics. For superficial incisional site infections in which there is no evidence of direct hardware involvement, the patient may be treated only with the exhibition of appropriate antibiotics without the need for hardware removal.
The hardware-related complications have an incidence between 2.7% and 5%, may develop over the long term, and include lead fractures, lead migration, and hardware erosion.37 Lead migration and fracture may be prevented by placing the junction of the distal end of the DBS electrode wire and the proximal end of the extension wire (the connector) under the scalp and not in the cervical area where excessive motility of the head on the neck can tug and torque the leads and the connector, resulting in fractures. Wire fracture can usually be diagnosed with the use of x-rays. Hardware erosion may be prevented by utilizing low-profile hardware.
Specific Disorders: Indications and Efficacy
Movement Disorders
Parkinson’s Disease
The spectrum of movement disorders treated with DBS includes PD, essential tremor, and dystonia. PD is a progressive neurodegenerative disorder classically described as loss of dopaminergic neurons in the substantia nigra. The cardinal features of PD are resting tremor, bradykinesia, rigidity, and postural instability. Replacement of dopamine via dopamine agonists and L-dopa is the mainstay of medical treatment. Patients over time tend to require escalating doses of dopamine equivalents to achieve therapeutic response, and these dose increases can be associated with motor fluctuations (on/off periods) as well as dyskinesias.37,66–68 The circuitry implicated in the pathogenesis of PD is the direct and indirect dopaminergic pathways that begin with dopamine release in the substantia nigra and have downstream effects on the striatum, globus pallidus, subthalamic nucleus, and thalamocortical projections. The net result is hypokinesis resulting from a paucity of glutamatergic excitation from thalamocortical projections.
DBS can improve symptoms, especially those involving the extremities. In addition, DBS, of the STN in particular, can decrease the need for oral dopaminergic replacement, consequently improving dyskinesias.65,69–72 A recent, randomized study showed that in patients under 75 years old with severe motor complications from advanced PD, STN DBS was more effective than medical management alone.73 GPi stimulation is also effective in alleviating some of the motor symptoms of PD, and although some evidence suggests superiority of STN stimulation, other recent evidence suggests the results may be equivalent.63 In 2010, a multicenter study of 299 patients suggested that the primary motor outcome following GPi or STN DBS was equivalent; however, patients undergoing STN stimulation had worse visuomotor processing scores and depression ratings than those undergoing GPi stimulation. However, there was a trend toward increased ICH in the GPi group, and this has been suggested by other studies, as described previously.64,65
Regarding patient selection criteria, patients with classical PD, and not those with atypical parkinsonism, are candidates for DBS. Patients with progressive supranuclear palsy, Lewy body disease, and others do not respond well to and may even worsen after surgery.74 A corollary to this is that positive response of symptoms to L-dopa predicts outcome and benefit from stereotactic procedures including DBS, according to most authors,37,75,76 and this relationship was originally demonstrated in patients undergoing stereotactic pallidotomy.77 A 30% improvement of the Unified Parkinson’s Disease Rating Scale (Part III) score is a good indication of L-dopa responsiveness and is used as a cutoff point at our institution. An important exception to this is patients with tremor-predominant PD who still obtain good tremor control with STN DBS in spite of L-dopa unresponsiveness. Furthermore, surgical candidates tend to have one or more of the following features: troublesome dyskinesias and motor fluctuations despite optimal therapy, medication resistance, disabling tremor, freezing or rigidity, dystonia, or bradykinesia.
Surgery is more likely to improve symptoms involving the extremities than it does axial symptoms involving posture, balance, speech, and gait.37
Tremor
Essential tremor is a benign, common movement disorder with an estimated prevalence ranging from 0.4% to 5%.78 It is characterized by a 4- to 12-Hz tremor that is generally postural or intention associated and disappears at rest. It is often familial, exacerbated by anxiety, and commonly improved with alcohol intake. Generally, tremor can be managed medically with propranolol, a β-adrenergic blocker, or primidone, an anticonvulsant.79 In cases of disabling tremor despite optimal medical management, DBS of the VIM nucleus of the thalamus is a treatment option. Patients with distal, resting, or postural tremors of the upper extremities benefit most from surgery. Patients with intention tremor or tremors of the head, neck, voice, or lower extremities are more difficult to treat.80 In general, distal tremors respond better than proximal tremors. Furthermore, patients with axial, head, voice, or neck tremors are more challenging to manage and often require bilateral DBS.37 DBS for upper extremity tremor has excellent success rates, with reported ranges of 70% up to 90% of patients having significant resolution of the tremor.79 In a prospective single-blind, randomized trial, DBS of the VIM nucleus of the thalamus was as effective for the suppression of drug-resistant tremor with fewer adverse effects than thalamotomy.81 Again, DBS results are equivalent to those of lesioning procedures but with inherent reversibility of the side effect profile.
Dystonia
Primary dystonias are syndromes in which no clear etiological factor is responsible for its onset, and the dystonia is the only clinical sign. These patients have normal brain imaging, no history of brain injury, and normal cerebrospinal fluid and other laboratory results. A subset of these patients have a mutation denoted DYT-1, which encodes the TorsinA gene. TorsinA is a member of the AAA family of adenosine triphosphatases (ATPases) and related to the CLP protease/heat shock protein family. It has been localized to the 9q34 locus and is expressed prominently in the substantia nigra pars compacta.82 It is the most common mutation leading to childhood-onset primary dystonia, and although other mutations have been identified, they remain rare.83
Secondary dystonias represent a large, heterogeneous group of disorders in which the cause of the dystonia is secondary to other insults including drugs, stroke, tumors, trauma, perinatal anoxic injury, and infections. Dystonia plus syndromes are neurochemical, nondegenerative disorders associated with another movement disorder. These include dopa-responsive dystonia (Segawa syndrome), rapid onset dystonia-parkinsonism, and myoclonus dystonia syndrome. Finally, dystonias may be a part of a neurodegenerative disorder. These disorders include autosomal dominant disorders such as Huntington’s disease, autosomal recessive disorders such as Wilson’s disease and pantothenate kinase–associated neurodegeneration (PKAN), and other metabolic disorders including mitochondrial diseases and the X-linked recessive disorder Lesch-Nyhan syndrome.83
The pharmacological therapies available for dystonia are somewhat limited; therefore, surgical alternatives have been developed that include both peripheral procedures including intrathecal baclofen pumps, botulinum toxin injections, and denervation procedures as well as central procedures including thalamotomy and DBS. Historically, thalamotomy was the surgical treatment of choice for dystonia; however, results are variable and DBS, as mentioned before, provides a reversible, controlled therapy relative to ablative procedures.83
Bilateral DBS of the GPi is the surgical treatment of choice for generalized dystonia, of both DYT-1 and non-DYT-1 subtypes.37,83 Improvements in the 40% to 80% range of motor and disability scores of the Burke-Fahn-Marsden Dystonia Rating Scale (BFMRS) have been reported.84–90 In 2005, the French Stimulation du Pallidum Interne dans la Dystonie (SPIDY) Study Group published a study of 22 patients undergoing bilateral GPi DBS for primary, generalized dystonia. The BFMRS movement subscore improved from a mean 46.3 before surgery to 21.0 at 12 months. The disability subscore improved from 11.6 before surgery to 6.5 at 12 months. Similar results have been described in segmental dystonia as well.83,85,90–93 A 2006 study from the Deep Brain Stimulation for Dystonia Study Group evaluated 40 patients with primary generalized or segmental dystonia undergoing GPi DBS, and these patients had an average 15.8-point improvement in the movement subscore of the BFMRS.
In generalized dystonias, predictors of good outcome include age of onset older than 5 years and lack of multiple orthopedic deformities.94 Additionally, appendicular symptoms tend to be more responsive than axial symptoms.95 Regarding focal dystonias, bilateral and unilateral GPi DBS has also shown some efficacy in the management of cervical dystonia, with improvements on the Toronto Western Spasmodic Rating Scale (TWSTRS) ranging from 40% to 60%.88,95–97
Clinical results in secondary dystonia are more disappointing. Tardive dystonia, however, is the exception to this rule, and these patients do derive benefit from DBS.97–100 There is also a suggestion of benefit in postanoxic dystonia.83 Finally, data are sparse, but DBS may have some role in the palliation of dystonia in neurodegenerative disorders including Lesch-Nyhan syndrome, PKAN, Hallervorden-Spatz disease, and Huntington’s disease.101–106
Epilepsy
Despite advances in the medical management of epilepsy, a significant portion of patients remain refractory to maximal therapy. It is estimated that approximately 40% of newly diagnosed epilepsies are pharmacotherapy resistant. That is, approximately 47% of patients become seizure-free (no seizures for 1 year) after initiating a single antiepileptic drug (AED), and only an additional 13% become seizure-free after use of an additional agent.107 This leaves approximately 40% of patients that may benefit from surgical evaluation. Traditionally, surgical management of epilepsy has centered on lesional or seizure focus resection, but in difficult-to-manage cases, neuromodulation therapies are finding a promising role. These therapies include cerebellar cortex stimulation, vagal nerve stimulation, and DBS of targets including the anterior nucleus (AN) of the thalamus, centromedian (CM) thalamus, STN, and hippocampus.
There is evidence that thalamocortical interactions are important in the development and propagation in various types of seizures.108–112 Furthermore, the dorsomedian (DM) nucleus of the thalamus has been shown to be important in the propagation and maintenance of seizures, particularly in limbic epilepsy.113,114 The AN, as part of the Papez circuit and limbic system, has afferent connections from the hippocampus and mammillary bodies via the mammillothalamic tract and has efferent projections to the cingulate cortex and parahippocampal cortex, which in turn connect widely to the neocortex.115,116 Bilateral DBS of the AN recently was studied as a therapy for refractory epilepsy in the SANTE (stimulation of the anterior nucleus of the thalamus for epilepsy) trial.117 In this multicenter, double-blind, sham-controlled, randomized trial of 110 patients, subjects receiving stimulation experienced a median reduction in seizures of 56% at 2 years. A 50% responder rate was achieved in 54% of patients, and approximately one quarter of patients receiving stimulation were seizure-free. As a cautionary note, significant side effects of depression, confusion, and memory impairment were seen more commonly in the stimulation group.
Another intracranial stimulation paradigm, where electrical stimulation is automatically delivered to seizure foci, may also prove useful in the management of epilepsy. The NeuroPace RNS system is one such closed-loop system in which electrographic recordings from a patient’s brain are continuously monitored, and stimulation is applied to seizure foci when they are detected. The results of a randomized, double-blind, sham-controlled trial of 191 patients indicated a mean reduction in disabling seizures of 29% compared to 14% in the sham group.118
CM thalamic nucleus stimulation has shown some promise in the management of refractory epilepsy as well. Results currently seem most promising in the management of Lennox-Gastaut syndrome, where an 80% seizure reduction was seen.119 Chkhenkeli and colleagues reported a 40% to 80% reduction of seizure frequency with CM stimulation in patients with mesiotemporal epilepsy.120 Other groups report mixed results. Fisher and co-workers, in a study with blinded and open trial arms, did not demonstrate a overall reduction in seizure frequency, except in generalized, tonic-clonic seizures, in a trial of CM thalamus stimulation.121 Complications including lethargy, confusion, and anxiety have been reported,122 and the safety profile of CM stimulation in large numbers of patients has yet to be established.116
Hippocampal stimulation seems to be an obvious option, and early results from several groups seem promising in the management of temporal lobe onset epilepsy, especially those without mesiotemporal sclerosis.123–125 Stimulation of the STN is an appealing option because of its widespread use in the management of PD and its connections to the limbic system via the modulation of the substantia nigra, but results in open-label human trials have been mixed.126,127
Neuropsychiatric Disorders
Obsessive-Compulsive Disorder
OCD is characterized by intrusive thoughts (obsessions) and repetitive behaviors (compulsions) that significantly impact the lives of those afflicted. These obsessions and compulsions become time-consuming and impairing, leading patients to go to great lengths to avoid situations that provoke them. These behaviors are not desired or enjoyed by the patients, and are deleterious to social, scholastic, and occupational functioning. The prevalence in Western countries is approximately 2% of the population,128 and OCD is initially managed with pharmacotherapy and cognitive behavioral therapy. OCD is managed with selective serotonin reuptake inhibitors (SSRIs) as a first line, then clomipramine, a tricyclic antidepressant (TCA), as a second line, and finally atypical antipsychotics to augment therapy.129 Despite good response to pharmacotherapy in many patients, approximately 7% of patients are refractory to treatment.130
Although the details of the neurobiological basis of OCD have yet to be fully elucidated, much neuroimaging work has focused on the cortico-striato-thalamo-cortical circuitry. More specifically, some evidence points to dysfunction in the loops involving the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and basal ganglia.129 Specifically, the anterior limb of the internal capsule is the most widely used target for stimulation, and this consists of fiber tracts connecting the thalamus, frontal cortex, and basal ganglia.131 In movement disorder physiology, the cortico-subcortical loop connects the dorsal basal ganglia to the ventrolateral thalamus. However, as described earlier, a parallel loop involving ventral striatopallidal structures to the mediodorsal thalamus is believed to be the key structure that links motor function to motivational and emotional inputs, and this is further suggested by the presence of limbic cortical inputs to the striatum.62,132–134
Historically, attempts to modulate or disrupt this aberrant circuitry have consisted of lesioning procedures including subcaudate tractotomy, anterior cingulotomy, limbic leucotomy, and anterior capsulotomy (see Fig. 53.3). Geoffrey Knight first introduced the subcaudate tractotomy in the treatment of movement disorders in the early 1960s,135 and clinical improvement in about half of patients has been reported.136,137 Ballantine reported on the use of anterior cingulotomy in the 1980s, with improvements in the range of 25% to 50%.31,138 Limbic leucotomy was introduced by Kelly, which was in essence, a combination of anterior cingulotomy and subcaudate tractotomy. At 16 months in a series of 66, he reported significant improvements in 89% of patients.139,140 Anterior capsulotomy was introduced by Talairach32 and popularized by Leksell,22 and large series indicate that nearly half of patients achieve at least a 50% reduction of symptoms, with most others having at least a 33% improvement in symptoms as determined on the Yale-Brown obsessive-compulsive scale (YBOCS).141 Adverse effects for these procedures have similar profiles, with lethargy, confusion, transient incontinence, and personality changes being most common.129
The advent of DBS provides a reversible, nondestructive method to modulate these circuits. A series of patients undergoing bilateral stimulation of the ALIC was originally described by Nuttin and associates52 in The Lancet in 1999. Six patients received stimulation with quadripolar electrodes, and four patients continued with stimulation for 21 months; three of the four patients achieved significant improvement of symptoms. Three-year follow-up of a multicenter study of eight patients with OCD demonstrated significant improvement in 75% of patients.51 Hypomania was reported as an adverse event as was relapse of OCD symptoms with battery failure. STN stimulation has been attempted as well, and a 10-month, double-blind, crossover study published in 2008 demonstrated a mean reduction of symptoms of approximately 32% with active stimulation.58 Transient psychiatric and motor symptoms as well as one parenchymal brain hemorrhage were reported as adverse events.
Major Depressive Disorder
MDD is a common, debilitating psychiatric disorder with an estimated lifetime prevalence of approximately 9.5%.142 The primary medical treatment for MDD is SSRI medication, with other second-line antidepressant medications including monoamine oxidase (MAO) inhibitors, TCAs, and dual serotonin and norepinephrine reuptake inhibitors (SNRIs); however, up to a third of patients do not respond to any of these medications.143 The current neurocircuitry model of depression is based on neuroimaging studies that demonstrate abnormalities in regional metabolic brain activity that normalizes with successful treatment. Frontal abnormalities are most reproducibly found, including abnormalities in metabolism of the dorsolateral and ventrolateral prefrontal cortex, orbitofrontal cortex, and ventromedial frontal cortex. Anterior and subgenual cortical changes are also seen, in particular, changes in Brodmann areas (BA) 24 and 25.144
Similar to OCD, limbic leucotomy, anterior capsulotomy, cingulotomy, and subcaudate tractotomy have all been used to treat depression. Results were promising, with initial responder rates of around 67% for the procedures.145 Again, DBS provides an attractive option due to its nondestructive, reversible nature. Stimulation of the SCC has been described originally by Mayberg and co-workers in six patients,55 and then in a larger study of 20 patients out of Toronto.54 Approximately two thirds of patients in these studies had improvement of their MDD symptoms, and 35% of patients in the Toronto study have had resolution of symptoms.
Stimulation of the ALIC has also improved depressive symptoms in patients with comorbid OCD, as described by Greenberg and colleagues, who reported that four of the eight patients with comorbid MDD experienced improvements in mood and affect.51 The ventral striatum and nucleus accumbens target may also prove to be valuable. The nucleus accumbens projects to the limbic brain areas including the SCC, and as we will describe later, there may be farther-reaching, expanding indications with stimulation of this target in the management of addiction and disorders of brain reward circuitry. A recent study of 15 patients with stimulation of the VC/VS by Malone and colleagues demonstrated a 50% responder rate and 20% remission rate.61
Emerging Indications
Tourette’s Syndrome
The inherent ability of DBS to modulate neurobehavioral circuitry has opened the door for treatment of a number of otherwise intractable disorders. Tourette’s syndrome (TS) is characterized by chronic vocal and motor tics that have their onset typically in early school age and is often comorbid with attention-deficit hyperactivity disorder or OCD. TS is thought to affect 0.7% to 4.2% of individuals, though symptom severity typically lessens in adulthood.146
Vocal tics include coughing, clearing one’s throat, and coprolalia. Motor tics include blinking, grimacing, snapping, or movements of the face. These movements or gestures tend to resemble coordinated or repetitive fragments of normal behaviors. Tics are exacerbated by stress, fatigue, and boredom and are often present during sleep.146 Owing to the nature of tics, they can be disabling from a social, occupational, and overall functioning standpoint if they do not abate in adulthood.
It is thought that disruptions in CSPTC circuits mediate the behavioral disturbances in TS.147–149 Neuroimaging studies suggest abnormalities in the metabolism of the ventral striatum in TS.150 With this in mind, DBS may have efficacy in treatment of TS. Several reports indicate that both thalamic stimulation and stimulation of the GPi are effective in the reduction of tics.151–166
Disorders of Consciousness and Cognition
In 2007, Schiff and associates reported on the use of bilateral thalamic DBS in the management of a 38-year-old patient in a minimally conscious state (MCS).167 This patient had a severe traumatic brain injury (TBI) leading to inability to communicate or follow commands consistently over 6 years following his injury; however, MRI demonstrated preservation of bihemispheric language networks, but thalamic and midbrain injury. Bilateral DBS was targeted to the anterior interlaminar thalamic nuclei and paralaminar regions of the association nuclei, as these regions had maximal concentrations of calbindin-positive neurons that have projections to supragranular cortical regions and are believed to play a role in arousal. The patient had significant improvements in arousal, limb control, and oral feeding in the DBS on versus off state. Some results in larger trials have been mixed, however. For example, circa 1990, Medtronic initiated a trial for implantation of DBS into vegetative state (VS) patients. These patients received bilateral CM thalamus or cervical spinal cord dorsal columns in patients in a VS. Although some centers reported significant functional improvement,168,169 this result was not consistently identified.168,170,171 Some of the reasons for the mixed results may be that the group of patients enrolled were heterogeneous in terms of their injuries; that is, patients with widespread cortical or subcortical damage may have less potential for recovery than patients with intact networks and central arousal deficits.172 Clearly, careful patient selection and further trials must be done to determine the role in patients with TBI and disorders of consciousness.
DBS may find a role in the near future in the management of dementias. The group of Lozano reported on a patient who, during stimulation of the hypothalamus for obesity, began having autobiographical memories.173 This finding led to a phase 1 trial evaluating forniceal/hypothalamic DBS in the treatment of Alzheimer’s disease. The study suggested the potential slowing of cognitive decline in their cohort,174 opening the door to more research in this realm.
Addiction and Eating Disorders
Dysfunction in the reward circuitry underlying eating disorders and addiction is quickly becoming a target for DBS. Over 30 years ago, Quaade and co-workers stereotactically electrocoagulated portions of the lateral hypothalamus and safely achieved some weight reduction in three patients.175 Furthermore, animal studies have identified the lateral hypothalamus (LH), ventromedial hypothalamus (VMH), and NAc as potential targets for managing obesity.176 The NAc has overlaps in circuitry with the lateral hypothalamus and may be involved in food reward circuitry. Furthermore, the central role of the NAc in reward circuitry makes it an attractive target in treating addiction. Rat studies suggest that DBS of the NAc may ameliorate cocaine addiction.177 Preliminary studies in humans undergoing DBS of the NAc for other disorders (including Tourette’s syndrome and OCD) indicate that smoking cessation may be more feasible with NAc stimulation.178 A group from the Netherlands recently reported NAc stimulation in a single patient leading to both smoking cessation and weight loss.179 DBS of the bilateral NAc was also reported to reduce alcohol dependency in a patient undergoing the procedure for an anxiety disorder.180 On the flip side, DBS of the NAc/ventral striatum or subgenual cingulate cortex may show promise in the management of anorexia nervosa.181,182 With regard to eating disorders, some early results suggest DBS may be effective in the management of anorexia nervosa.181,182
Conclusion (Table 53.3)
DBS has transformed the management of more than 80,000 advanced and medication refractory patients with movement disorders. DBS is now being explored for the emerging indications in the management of neurobehavioral and neuropsychiatric disorders,183–185 epilepsy, obesity, Alzheimer’s disease, anorexia, traumatic brain injury, and disorders of consciousness. These emerging indications are enhanced by the intrinsic advantages of reversibility and adjustability of DBS, compared to stereotactic lesioning procedures. Efforts at expanding the clinical utility of DBS must, however, be tempered by ethical considerations and the need for rigorous scientific and evidence-based evaluations of these interventions.
TABLE 53.3 Evidence-Based Medicine Summary of Clinical Results for Deep Brain Stimulation (DBS) Surgery
| Evidence Class | Findings |
|---|---|
| 1 | DBS of the STN and GPi is effective in the management of the motor symptoms of PD.63,65,73 Bilateral GPi stimulation is effective in the treatment of primary generalized or segmental dystonia.84,85 DBS of the VIM nucleus of the thalamus is effective for the suppression of drug-resistant tremor with fewer adverse effects than with thalamotomy.81 Bilateral DBS of the AN of the thalamus reduces seizure frequency in medically resistant epilepsy.117 |
| 2 | DBS of the ALIC, VC/VS, NAc ameliorates the symptoms of OCD.50,51,184,185 DBS of the STN treats the symptoms of OCD.58 DBS of the subgenual cingulate gyrus can reduce the symptoms of treatment-resistant depression.54,55 DBS of the VC/VS can be effective in the management of treatment-resistant depression.61 |
| 3 | DBS of the bilateral thalamus reduces motor and sonic tics in treatment-refractory Tourette’s syndrome.158,162 |
ALIC, anterior limb of the internal capsule; AN, anterior nucleus; GPi, globus pallidus interna; MCP, midcommissural point; NAc, nucleus accumbens; OCD, obsessive-compulsive disorder; PD, Parkinson’s disease; STN, subthalamic nucleus; VC/VS, ventral capsule/ventral striatum; VIM, ventralis intermedius.
Deuschl G., Schade-Brittinger C., Krack P., et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896-908.
Follett K.A., Weaver F.M., Stern M., et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362:2077-2091.
Greenberg B.D., Gabriels L.A., Malone D.A.Jr., et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2008.
Kopell B.H., Greenberg B.D. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev. 2008;32(3):408-422.
Lozano A.M., Mayberg H.S., Giacobbe P., et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461-467.
Rezai A.R., Machado A.G., Deogaonkar M., et al. Surgery for movement disorders. Neurosurgery. 2008;62(Suppl 2):809-838. discussion 809-838
Schuurman P.R., Bosch D.A., Bossuyt P.M., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461-468.
Vidailhet M., Vercueil L., Houeto J.L., et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459-467.
Please go to expertconsult.com to view the complete list of references.
1. Medtronic. Parkinson’s Disease Treatment with Medtronic DBS Therapy. Accessed at http://www.medtronic.com/your-health/parkinsons-disease/therapy/ October 6, 2010
2. U.S. National Library of Medicine. National Institutes of Health. http://www.ncbi.nlm.nih.gov/pubmed. accessed October 5, 2010
3. Descartes R. Discourse on Method, Optics, Geometry, and Meteorology, Rev ed. Indianapolis, IN: Hackett; 2001.
4. Horsley V. The function of the so-called motor area of the brain. Br Med J. 1909;2:125-132.
5. Bucy P.C., Buchanan D.N. Athetosis. Brain. 1932;55:479-492.
6. Bucy P.C., Case T.J. Tremor, physiologic mechanism and abolition by surgical means. Arch Neurol Psychiatry. 1939;41:721-746.
7. Parrent A.G. History of surgery for movement disorders. Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 2. Berlin: Springer-Verlag; 2009:1467-1485.
8. Putnam T.J. Treatment of athetosis and dystonia by section of extrapyramidal motor tracts. Arch Neurol Psychiatry. 1933;29:504-521.
9. Putnam T.J. Results of treatment of athetosis by section of extrapyramidal tracts in the spinal cord. Arch Neurol Psychiatry. 1938;39:258-275.
10. Putnam T.J. Relief from unilateral paralysis agitans by section of the pyramidal tract. Arch Neurol Psychiatry. 1938;40:1049-1050.
11. Walker A.E. Cerebral pedunculotomy for the relief of involuntary movements; hemiballismus. Acta Psychiatr Neurol. 1949;24(3-4):723-729.
12. Meyers R. A surgical procedure for the alleviation of postencephalitic tremor, with notes on the physiology of the premotor fibres. Arch Neurol Psychiatry. 1940;44:455-459.
13. Cooper I.S. Anterior choroidal artery ligation for involuntary movements. Science. 1953;118(3059):193.
14. Cooper I.S. Ligation of the anterior choroidal artery for involuntary movements; parkinsonism. Psychiatr Q. 1953;27(2):317-319.
15. Cooper I.S. Effect of anterior choroidal artery ligation on involuntary movements and rigidity. Trans Am Neurol Assoc. 1953;3:6-7. discussion 8-9
16. Gildenberg P.L., Krauss J.K. History of stereotactic surgery. Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 1. Berlin: Springer-Verlag; 2009:3-34.
17. Bjarkam C.R., Sorensen J.C. Psychosurgery—a historical perspective. Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 2. Berlin: Springer-Verlag; 2009:2867-2886.
18. Kandel E.I., Schavinsky Y.V. Stereotaxic apparatus and operations in Russia in the 19th century. J Neurosurg. 1972;37(4):407-411.
19. Zonenshayn M., Rezai A. Stereotactic surgery. In: Rengachary S.S., Ellenbogen R.G., editors. Principles of Neurosurgery. 2nd ed. St. Louis: Elsevier; 2005:785-817.
20. Horsley V., Clarke R.H. The structure and functions of the cerebellum examined by a new method. Brain. 1908;31:45-124.
21. Spiegel E.A., Wycis H.T., Marks M., Lee A.J. Stereotaxic apparatus for operations on the human brain. Science. 1947;106(2754):349-350.
22. Leksell L. A stereotactic apparatus for intracerebral surgery. Acta Chir Scand. 1949;99:229-233.
23. Spiegel E.A., Wycis H.T., et al. Stereoencephalotomy. Proc Soc Exp Biol Med. 1948;69(1):175-177.
24. Cooper I.S., Bravo G. Chemopallidectomy and chemothalamectomy. J Neurosurg. 1958;15(3):244-250.
25. Hassler R. Extrapyramidal cortical systems and central regulation of motor function. Deutsch Z Nervenheilkd. 1956;175(3):233-258.
26. Hassler R., Riechert T. Indications and localization of stereotactic brain operations. Nervenarzt. 1954;25(11):441-447.
27. Housepian E.A., Pool J.L. An evaluation of pallido-ansal surgery. In: Houston Neurological Society, Fields W.S., Texas Medical Center. Pathogenesis and Treatment of Parkinsonism; Sixth Annual Scientific Meeting of the Houston Neurological Society, Texas Medical Center, Houston, Texas. Springfield, IL: Charles C Thomas; 1958:372.
28. Narabayashi H., Okuma T., Shikiba S. Procaine oil blocking of the globus pallidus. AMA Arch Neurol Psychiatry. 1956;75(1):36-48.
29. Svennilson E., Torvik A., Lowe R., Leksell L. Treatment of parkinsonism by stereotactic thermolesions in the pallidal region. A clinical evaluation of 81 cases. Acta Psychiatr Scand. 1960;35:358-377.
30. Whitty C.W., Duffield J.E., Tov P.M., Cairns H. Anterior cingulectomy in the treatment of mental disease. Lancet. 1952;1(6706):475-481.
31. Ballantine H.T.Jr., Cassidy W.L., Flanagan N.B., Marino R.Jr. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26(5):488-495.
32. Talairach J., Hecaen H., David M. Limited prefrontal lobotomy by electrocoagulation of the thalamofrontal fibers at the emergence of the anterior arm of the internal capsule. Rev Neurol (Paris). 1949;83:59.
33. Knight G. Stereotactic tractotomy in the surgical treatment of mental illness. J Neurol Neurosurg Psychiatry. 1965;28:304-310.
34. Kelly D., Richardson A., Mitchell-Heggs N. Stereotactic limbic leucotomy: neurophysiological aspects and operative technique. Br J Psychiatry. 1973;123(573):133-140.
35. Kopell B.H., Machado A.G., Rezai A.R. Not your father’s lobotomy: psychiatric surgery revisited. Clin Neurosurg. 2005;52:315-330.
36. Heller A.C., Amar A.P., Liu C.Y., Apuzzo M.L. Surgery of the mind and mood: a mosaic of issues in time and evolution. Neurosurgery. 2006;59(4):720-733. discussion 733-729
37. Rezai A.R., Machado A.G., Deogaonkar M., et al. Surgery for movement disorders. Neurosurgery. 2008;62(Suppl 2):809-838.
38. Rezai A.R., Kopell B.H., Gross R.E., et al. Deep brain stimulation for Parkinson’s disease: surgical issues. Mov Disord. 2006;21(Suppl 14):S197-S218.
39. Finger S. Origins of Neuroscience: a History of Explorations into Brain Function. New York: Oxford University Press; 1994.
40. Hassler R., Riechert T., Mundinger F., et al. Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain. 1960;83:337-350.
41. Bechtereva N.P., Bondartchuk A.N., Smirnov V.M., et al. Method of electrostimulation of the deep brain structures in treatment of some chronic diseases. Confin Neurol. 1975;37(1-3):136-140.
42. Hosobuchi Y., Adams J.E., Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 1973;29(3):158-161.
43. Richardson D.E., Akil H. Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47(2):178-183.
44. Benabid A.L., Pollak P., Gross C., et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62(1-4):76-84.
45. Benabid A.L., Pollak P., Hommel M., et al. Treatment of Parkinson tremor by chronic stimulation of the ventral intermediate nucleus of the thalamus. Rev Neurol (Paris). 1989;145(4):320-323.
46. Blond S., Siegfried J. Thalamic stimulation for the treatment of tremor and other movement disorders. Acta Neurochir Suppl (Wien). 1991;52:109-111.
47. Siegfried J., Shulman J. Deep brain stimulation. Pacing Clin Electrophysiol. 1987;10(1 Pt 2):271-272.
48. Brice J., McLellan L. Suppression of intention tremor by contingent deep-brain stimulation. Lancet. 1980;1(8180):1221-1222.
49. Kopell B.H., Greenberg B.D. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev. 2008;32(3):408-422.
50. Greenberg B.D., Gabriels L.A., Malone D.A.Jr., et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64-79. (Epub 2008 May 20)
51. Greenberg B.D., Malone D.A., Friehs G.M., et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
52. Nuttin B., Cosyns P., Demeulemeester H., et al. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354(9189):1526.
53. Van Laere K., Nuttin B., Gabriels L., et al. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47(5):740-747.
54. Lozano A.M., Mayberg H.S., Giacobbe P., et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461-467.
55. Mayberg H.S., Lozano A.M., Voon V., et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
56. Jimenez F., Velasco F., Salin-Pascual R., et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(Pt 2):393-398.
57. Sturm V., Lenartz D., Koulousakis A., et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive and anxiety disorders. J Chem Neuroanat. 2003;26(4):293-299.
58. Mallet L., Polosan M., Jaafari N., et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121-2134.
59. Gabriels L., Cosyns P., Nuttin B., et al. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003;107(4):275-282.
60. Abelson J.L., Curtis G.C., Sagher O., et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57(5):510-516.
61. Malone D.A.Jr., Dougherty D.D., Rezai A.R., et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
62. Haber S.N., Fudge J.L., McFarland N.R. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369-2382.
63. Follett K.A., Weaver F.M., Stern M., et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077-2091.
64. Binder D.K., Rau G.M., Starr P.A. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56(4):722-732. discussion 722-732
65. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345(13):956-963.
66. Obeso J.A., Grandas F., Vaamonde J., et al. Motor complications associated with chronic levodopa therapy in Parkinson’s disease. Neurology. 1989;39(11 Suppl 2):11-19.
67. Mouradian M.M., Heuser I.J., Baronti F., et al. Pathogenesis of dyskinesias in Parkinson’s disease. Ann Neurol. 1989;25(5):523-526.
68. Deogaonkar M., Subramanian T. Pathophysiological basis of drug-induced dyskinesias in Parkinson’s disease. Brain Res Brain Res Rev. 2005;50(1):156-168.
69. Rodriguez-Oroz M.C., Obeso J.A., Lang A.E., et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128(Pt 10):2240-2249.
70. Krause M., Fogel W., Heck A., et al. Deep brain stimulation for the treatment of Parkinson’s disease: subthalamic nucleus versus globus pallidus internus. J Neurol Neurosurg Psychiatry. 2001;70(4):464-470.
71. Krack P., Pollak P., Limousin P., et al. Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson’s disease. Brain. 1998;121(Pt 3):451-457.
72. Deuschl G., Fogel W., Hahne M., et al. Deep-brain stimulation for Parkinson’s disease. J Neurol. 249(Suppl 3), 2002. III/36–39
73. Deuschl G., Schade-Brittinger C., Krack P., et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896-908.
74. Shih L.C., Tarsy D. Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord. 2007;22(15):2149-2155.
75. Tan E.K., Jankovic J. Patient selection for surgery for Parkinson’s disease. Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 2. Berlin: Springer-Verlag; 2009:1529-1538.
76. Rodriguez R.L., Fernandez H.H., Haq I., Okun M.S. Pearls in patient selection for deep brain stimulation. Neurologist. 2007;13(5):253-260.
77. Kazumata K., Antonini A., Dhawan V., et al. Preoperative indicators of clinical outcome following stereotaxic pallidotomy. Neurology. 1997;49(4):1083-1090.
78. Louis E.D., Ottman R., Hauser W.A. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13(1):5-10.
79. Nazzaro J.M., Lyons K.E., Pahwa R. Management of essential tremor. Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 2. Berlin: Springer-Verlag; 2009:1743-1755.
80. Deuschl G., Bain P. Deep brain stimulation for tremor [correction of trauma]: patient selection and evaluation. Mov Disord. 2002;17(Suppl 3):S102-S111.
81. Schuurman P.R., Bosch D.A., Bossuyt P.M., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342(7):461-468.
82. TOR1A torsin family 1, member A (torsin A) [Homo sapiens]. http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1861#geneSummary. accessed June 6, 2010
83. Vasques X.A., Cif L., Biolsi B., Coubes P. Central procedures for primary dystonia. Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 2. Berlin: Springer-Verlag; 2009:1801-1833.
84. Vidailhet M., Vercueil L., Houeto J.L., et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352(5):459-467.
85. Kupsch A., Benecke R., Muller J., et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355(19):1978-1990.
86. Coubes P., Cif L., El Fertit H., et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg. 2004;101(2):189-194.
87. Coubes P., Roubertie A., Vayssiere N., et al. Treatment of DYT1-generalised dystonia by stimulation of the internal globus pallidus. Lancet. 2000;355(9222):2220-2221.
88. Hung S.W., Hamani C., Lozano A.M., et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68(6):457-459.
89. Diamond A., Shahed J., Azher S., et al. Globus pallidus deep brain stimulation in dystonia. Mov Disord. 2006;21(5):692-695.
90. Bereznai B., Steude U., Seelos K., Botzel K. Chronic high-frequency globus pallidus internus stimulation in different types of dystonia: a clinical, video, and MRI report of six patients presenting with segmental, cervical, and generalized dystonia. Mov Disord. 2002;17(1):138-144.
91. Foote K.D., Sanchez J.C., Okun M.S. Staged deep brain stimulation for refractory craniofacial dystonia with blepharospasm: case report and physiology. Neurosurgery. 2005;56(2):E415. discussion E415
92. Andaluz N., Taha J.M., Dalvi A. Bilateral pallidal deep brain stimulation for cervical and truncal dystonia. Neurology. 2001;57(3):557-558.
93. Houser M., Waltz T. Meige syndrome and pallidal deep brain stimulation. Mov Disord. 2005;20(9):1203-1205.
94. Parr J.R., Green A.L., Joint C., et al. Deep brain stimulation in childhood: an effective treatment for early onset idiopathic generalised dystonia. Arch Dis Child. 2007;92(8):708-711.
95. Lee J.Y., Deogaonkar M., Rezai A. Deep brain stimulation of globus pallidus internus for dystonia. Parkinsonism Relat Disord. 2007;13(5):261-265.
96. Kiss Z.H., Doig-Beyaert K., Eliasziw M., et al. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130(Pt 11):2879-2886.
97. Starr P.A., Turner R.S., Rau G., et al. Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. J Neurosurg. 2006;104(4):488-501.
98. Cohen O.S., Hassin-Baer S., Spiegelmann R. Deep brain stimulation of the internal globus pallidus for refractory tardive dystonia. Parkinsonism Relat Disord. 2007;13(8):541-544.
99. Zhang J.G., Zhang K., Wang Z.C. Deep brain stimulation in the treatment of tardive dystonia. Clin Med J (Engl). 2006;119(9):789-792.
100. Eltahawy H.A., Saint-Cyr J., Giladi N., et al. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery. 2004;54(3):613-619. discussion 619-621
101. Taira T., Kobayashi T., Hori T. Disappearance of self-mutilating behavior in a patient with Lesch-Nyhan syndrome after bilateral chronic stimulation of the globus pallidus internus. Case report. J Neurosurg. 2003;98(2):414-416.
102. Castelnau P., Cif L., Valente E.M., et al. Pallidal stimulation improves pantothenate kinase-associated neurodegeneration. Ann Neurol. 2005;57(5):738-741.
103. Hebb M.O., Garcia R., Gaudet P., Mendez I.M. Bilateral stimulation of the globus pallidus internus to treat choreathetosis in Huntington’s disease: technical case report. Neurosurgery. 2006;58(2):E383. discussion E383
104. Moro E., Lang A.E., Strafella A.P., et al. Bilateral globus pallidus stimulation for Huntington’s disease. Ann Neurol. 2004;56(2):290-294.
105. Cif L., Biolsi B., Gavarini S., et al. Antero-ventral internal pallidum stimulation improves behavioral disorders in Lesch-Nyhan disease. Mov Disord. 2007;22(14):2126-2129.
106. Umemura A., Jaggi J.L., Dolinskas C.A., et al. Pallidal deep brain stimulation for longstanding severe generalized dystonia in Hallervorden-Spatz syndrome. Case report. J Neurosurg. 2004;100(4):706-709.
107. Kwan P., Brodie M.J. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
108. Velasco F., Velasco A.L., Velasco M., et al. Centromedian thalamic stimulation for epilepsy. In: Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Berlin: Springer-Verlag; 2009:2777-2791.
109. Velasco M., Velasco F., Alcala H., et al. Epileptiform EEG activity of the centromedian thalamic nuclei in children with intractable generalized seizures of the Lennox-Gastaut syndrome. Epilepsia. 1991;32(3):310-321.
110. Steriade M. Spindling, incremental thalamo-cortical responses and spike-wave like epilepsy. In: Avoli M., Gloor P., Kustopoulus G., Niquet R., editors. Generalized Epilepsy: Neurobiological Approaches. Boston: Birkhouser; 1990:161-180.
111. Avoli M., Gloor P., Kostopoulos G., Gotman J. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 1983;50(4):819-837.
112. Quesney L.F., Gloor P., Kratzenberg E., Zumstein H. Pathophysiology of generalized penicillin epilepsy in the cat: the role of cortical and subcortical structures. I. Systemic application of penicillin. Electroencephalogr Clin Neurophysiol. 1977;42(5):640-655.
113. Bertram E.H., Mangan P.S., Zhang D., et al. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42(8):967-978.
114. Bertram E.H., Zhang D.X., Mangan P., et al. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res. 1998;32(1-2):194-205.
115. Hodaie M., Hamani C., Wennberg R., et al. Anterior nucleus DBS in epilepsy. In: Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Berlin: Springer-Verlag; 2009:2794-2800.
116. Lega B.C., Halpern C.H., Jaggi J.L., Baltuch G.H. Deep brain stimulation in the treatment of refractory epilepsy: update on current data and future directions. Neurobiol Dis. 2010;38(3):354-360.
117. Fisher R., Salanova V., Witt T., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
118. Morell M. Results of a multicenter double blinded randomized controlled pivotal investigation of the RNS system for treatment of intractable partial epilepsy in adults. American Epilepsy Society 63rd Annual Meeting. Boston: Massachusetts; 2009.
119. Velasco A.L., Velasco F., Jimenez F., et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47(7):1203-1212.
120. Chkhenkeli S.A., Sramka M., Lortkipanidze G.S., et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106(4):318-329.
121. Fisher R.S., Uematsu S., Krauss G.L., et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33(5):841-851.
122. Andrade D.M., Zumsteg D., Hamani C., et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology. 2006;66(10):1571-1573.
123. Boon P., Vonck K., De Herdt V., et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48(8):1551-1560.
124. Velasco F., Velasco M., Velasco A.L., et al. Electrical stimulation for epilepsy: stimulation of hippocampal foci. Stereotact Funct Neurosurg. 2001;77(1-4):223-227.
125. Tellez-Zenteno J.F., McLachlan R.S., Parrent A., et al. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. 2006;66(10):1490-1494.
126. Handforth A., DeSalles A.A., Krahl S.E. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. 2006;47(7):1239-1241.
127. Chabardes S., Kahane P., Minotti L., et al. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4(Suppl 3):S83-S93.
128. Rasmussen S.A., Eisen J.L. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15(4):743-758.
129. Gabriels L., Cosyns P., van Kuyck K., Nuttin B. DBS for OCD. In: Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Berlin: Springer-Verlag; 2009:2897-2924.
130. Zitterl W., Demal U., Aigner M., et al. Naturalistic course of obsessive compulsive disorder and comorbid depression. Longitudinal results of a prospective follow-up study of 74 actively treated patients. Psychopathology. 2000;33(2):75-80.
131. Axer H., Keyserlingk D.G. Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Methods. 2000;94(2):165-175.
132. Heimer L., Switzer R.C., Van Hoesen G.W. Ventral striatum and ventral pallidum. Components of the motor system? Trends Neurosci. 1982;5:83-87.
133. Parent A. Comparative Neurobiology of the Basal Ganglia. New York: Wiley; 1986.
134. Graybiel A.M. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70(1-2):119-136.
135. Knight G. The orbital cortex as an objective in the surgical treatment of mental illness. The results of 450 cases of open operation and the development of the stereotactic approach. Br J Surg. 1964;51:114-124.
136. Hodgkiss A.D., Malizia A.L., Bartlett J.R., Bridges P.K. Outcome after the psychosurgical operation of stereotactic subcaudate tractotomy, 1979-1991. J Neuropsychiatry Clin Neurosci. 1995;7(2):230-234.
137. Goktepe E.O., Young L.B., Bridges P.K. A further review of the results of stereotactic subcaudate tractotomy. Br J Psychiatry. 1975;126:270-280.
138. Ballantine H.T.Jr., Bouckoms A.J., Thomas E.K., Giriunas I.E. Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry. 1987;22(7):807-819.
139. Kelly D., Richardson A., Mitchell-Heggs N., et al. Stereotactic limbic leucotomy: a preliminary report on forty patients. Br J Psychiatry. 1973;123(573):141-148.
140. Mitchell-Heggs N., Kelly D., Richardson A. Stereotactic limbic leucotomy—a follow-up at 16 months. Br J Psychiatry. 1976;128:226-240.
141. Oliver B., Gascon J., Aparicio A., et al. Bilateral anterior capsulotomy for refractory obsessive-compulsive disorders. Stereotact Funct Neurosurg. 2003;81(1-4):90-95.
142. Angst J. The epidemiology of depressive disorders. Eur Neuropsychopharmacol. 1995;5(Suppl):95-98.
143. Giacobbe P., Kennedy S. Medical management and indications for surgery in depression. Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 2. Berlin: Springer-Verlag; 2009:2925-2941.
144. Mayberg H.S. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119(4):717-725.
145. Coenen V.A., Honey C.R. Ablative procedures for depression. In: Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Berlin: Springer-Verlag, 2009.
146. Jankovic J. Tourette’s syndrome. N Engl J Med. 2001;345(16):1184-1192.
147. Neurobiology Olson S. Making sense of Tourette’s. Science. 2004;305(5689):1390-1392.
148. Mink J.W. Neurobiology of basal ganglia and Tourette syndrome: basal ganglia circuits and thalamocortical outputs. Adv Neurol. 2006;99:89-98.
149. Mink J.W. Neurobiology of basal ganglia circuits in Tourette syndrome: faulty inhibition of unwanted motor patterns? Adv Neurol. 2001;85:113-122.
150. Albin R.L., Koeppe R.A., Bohnen N.I., et al. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology. 2003;61(3):310-315.
151. Visser-Vandewalle V., Ackermans L., van der Linden C., et al. Deep brain stimulation in Gilles de la Tourette’s syndrome. Neurosurgery. 2006;58(3):E590.
152. Ackermans L., Temel Y., Cath D., et al. Deep brain stimulation in Tourette’s syndrome: two targets? Mov Disord. 2006;21(5):709-713.
153. Kuhn J., Lenartz D., Huff W., et al. Transient manic-like episode following bilateral deep brain stimulation of the nucleus accumbens and the internal capsule in a patient with Tourette syndrome. Neuromodulation. 2008;11(2):128-131.
154. Kuhn J., Lenartz D., Heuckmann J., et al. Deep brain stimulation of different anatomic structures in therapeutically refractory Tourette-syndrome. Klin Neurophysiol. 2008;120:e81-e82.
155. Kuhn J., Lenartz D., Mai J.K., et al. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol. 2007;254(7):963-965.
156. Welter M.L., Mallet L., Houeto J.L., et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65(7):952-957.
157. Houeto J.L., Karachi C., Mallet L., et al. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76(7):992-995.
158. Maciunas R.J., Maddux B.N., Riley D.E., et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107(5):1004-1014.
159. Shahed J., Poysky J., Kenney C., et al. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology. 2007;68(2):159-160.
160. Bajwa R.J., de Lotbiniere A.J., King R.A., et al. Deep brain stimulation in Tourette’s syndrome. Mov Disord. 2007;22(9):1346-1350.
161. Flaherty A.W., Williams Z.M., Amirnovin R., et al. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery. 2005;57(Suppl 4):E403. discussion E403
162. Porta M., Brambilla A., Cavanna A.E., et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73(17):1375-1380.
163. Servello D., Porta M., Sassi M., et al. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79(2):136-142.
164. Diederich N.J., Kalteis K., Stamenkovic M., et al. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord. 2005;20(11):1496-1499.
165. Ackermans L., Duits A., Temel Y., et al. Long-term outcome of thalamic deep brain stimulation in two patients with Tourette syndrome. J Neurol Neurosurg Psychiatry. 2010;81(10):1068-1072. (Epub 2010 Jul 26)
166. Visser-Vandewalle V., Temel Y., Boon P., et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99(6):1094-1100.
167. Schiff N.D., Giacino J.T., Kalmar K., et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448(7153):600-603.
168. Hosobuchi Y., Yingling C. The treatment of prolonged coma with neurostimulation. In: Devinsky O., Beric A., Dogali M., editors. Electrical and Magnetic Stimulation of the Brain and Spinal Cord. New York: Raven Press; 1993:247-252.
169. Yamamoto T., Katayama Y. Deep brain stimulation therapy for the vegetative state. Neuropsychol Rehabil. 2005;15(3-4):406-413.
170. Tsubokawa T., Yamamoto T., Katayama Y., et al. Deep-brain stimulation in a persistent vegetative state: follow-up results and criteria for selection of candidates. Brain Inj. 1990;4(4):315-327.
171. Cohadon F., Richer E. Deep cerebral stimulation in patients with post-traumatic vegetative state. 25 cases. Neurochirurgie. 1993;39(5):281-292.
172. Schiff N.D. DBS disorders of consciousness. In: Lozano A.M., Gildenberg P.L., Tasker R.R., editors. Textbook of Stereotactic and Functional Neurosurgery. Berlin: Springer-Verlag, 2009.
173. Hamani C., McAndrews M.P., Cohn M., et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63(1):119-123.
174. Laxton A.W., Tang-Wai D.F., McAndrews M.P., et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68(4):521-534.
175. Quaade F., Vaernet K., Larsson S. Stereotaxic stimulation and electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir (Wien). 1974;30(1-2):111-117.
176. Halpern C.H., Wolf J.A., Bale T.L., et al. Deep brain stimulation in the treatment of obesity. J Neurosurg. 2008;109(4):625-634.
177. Vassoler F.M., Schmidt H.D., Gerard M.E., et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28(35):8735-8739.
178. Kuhn J., Bauer R., Pohl S., et al. Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res. 2009;15(4):196-201.
179. Mantione M., van de Brink W., Schuurman P.R., Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66(1):E218.
180. Kuhn J., Lenartz D., Huff W., et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78(10):1152-1153.
181. Sun B, Li D, Zhan S, et al. Surgical Treatment for Refractory Anorexia Nervosa. 8th World Congress of the International Neuromodulation Society; 11th Annual Meeting of the North American Neuromodulation Society. Acapulco, Mexico: 2007.
182. Israel M., Steiger H., Kolivakis T., et al. Deep brain stimulation in the subgenual cingulate cortex for an intractable eating disorder. Biol Psychiatry. 2010;67(9):e53-e54.
183. Sweet W.H. Treatment of medically intractable mental disease by limited frontal leucotomy—justifiable? N Engl J Med. 1973;289(21):1117-1125.
184. Goodman W.K., Foote K.D., Greenberg B.D., et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67(6):535-542.
185. Lakhan S.E., Callaway E. Deep brain stimulation for obsessive-compulsive disorder and treatment-resistant depression: systematic review. BMC Res Notes. 2010;3:60.