15 Basic Electromyography
Analysis of Motor Unit Action Potentials
Physiology
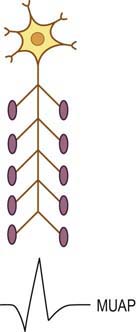
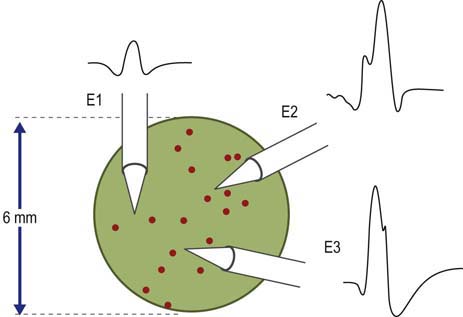
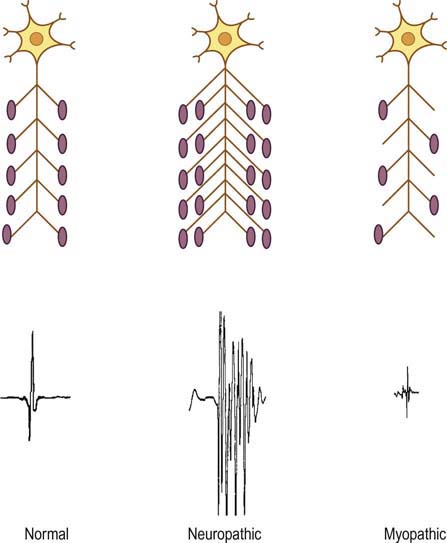
The basic component of the peripheral nervous system is the motor unit, defined as an individual motor neuron, its axon, and associated neuromuscular junctions (NMJs) and muscle fibers. The extracellular needle EMG recording of a motor unit is the MUAP (Figure 15–1). The number of muscle fibers per motor unit varies greatly, from 5 to 10 in laryngeal muscles to a couple of thousand in the soleus. The transverse territory of a motor unit usually ranges from 5 to 10 mm in adults, with many motor unit territories overlapping with one another. Because of this overlap, two muscle fibers from the same motor unit rarely lie adjacent to each other. Transverse motor unit territory increases greatly with age, doubling from birth to adulthood, mostly because of the increase in individual muscle fiber size.
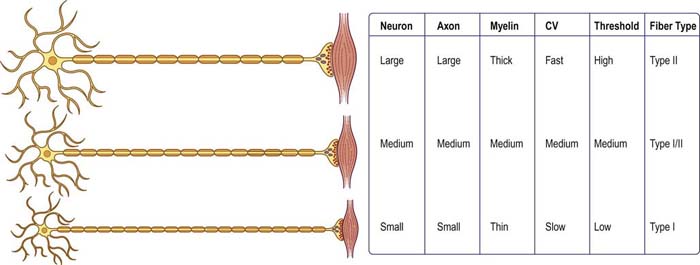
The “size principle” governs many of the properties of motor units (Figure 15–2). The size of the motor neuron is directly related to (1) the size of the axon, (2) the thickness of the myelin sheath, (3) the conduction velocity of the axon, (4) the threshold to depolarization, and (5) the metabolic type of muscle fibers that are innervated. The larger motor neurons have larger axons, with the thickest myelin sheath (hence, the fastest conduction velocity), highest threshold to depolarization, and connections to type II, fast twitch muscle fibers. Conversely, the smaller motor neurons have smaller axons, less myelin sheath, slower conduction velocity, lower threshold to depolarization, and, in general, connections to type I, slow twitch muscle fibers. Thus, with voluntary contraction, the smallest motor units with the lower thresholds fire first. As contraction increases, progressively larger motor units begin to fire. The largest type II motor units fire with maximum contraction. During routine needle EMG, most MUAPs analyzed are thus from the smaller motor units that innervate type I muscle fibers.
Morphology
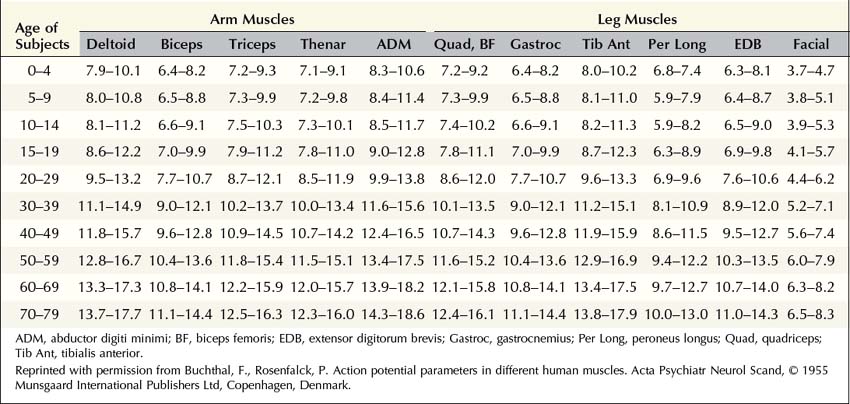
MUAP properties vary widely both within and between different muscles. Even within a muscle, there is a wide range of normal motor unit morphology, with MUAP size following a bell-shaped distribution curve (Figure 15–3). Due to this normal variability, normal values of MUAP morphology are based on the mean of many different MUAPs. The analysis of MUAP morphology can be performed on either a qualitative or a quantitative basis. To perform quantitative MUAP analysis, one must isolate 20 different MUAPs for each muscle being studied and measure their individual durations, amplitudes, and number of phases. From these values, the mean duration, amplitude, and number of phases are calculated and compared with a set of normal values for that particular muscle and age group. MUAP morphology varies depending on the muscle being studied and the patient’s age. This is particularly true of MUAP duration (Table 15–1). In general, MUAPs in proximal muscles tend to be shorter in duration than those in more distal muscles. MUAP size in adults is larger than in children, primarily because of an increase in the size of muscle fibers during development. In addition, MUAP size is generally larger in older individuals, probably as the result of dropout of motor units from the normal effects of aging, leading to some compensatory “normal” reinnervation. The loss of motor units has been estimated to be approximately 1% per year, beginning in the third decade of life, which then increases rapidly after age 60.
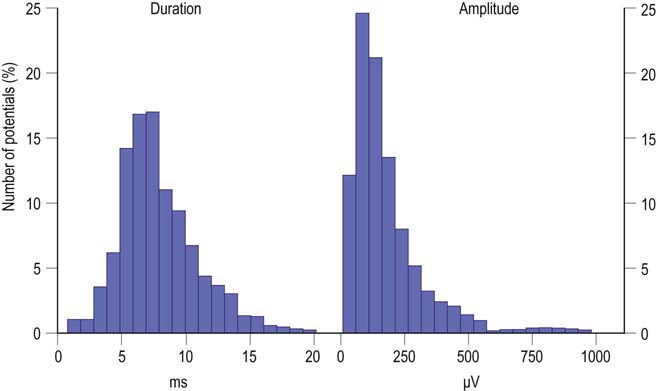
FIGURE 15–3 Range of normal motor unit action potential (MUAP) duration and amplitude.
(Reprinted with permission from Buchthal, F., Guld, C., Rosenfalck, P., 1954. Action potential parameters in normal human muscle and their dependence on physical variables. Acta Physiol Scand 32, 200.)
Duration
MUAP duration is the parameter that best reflects the number of muscle fibers within a motor unit (Figure 15–4). Typical MUAP duration is between 5 and 15 ms. Duration is defined as the time from the initial deflection from baseline to the final return of the MUAP to baseline. It depends primarily on the number of muscle fibers within the motor unit and the dispersion of their depolarizations over time. Dispersion in turn depends on the longitudinal and transverse scatter of endplates and on variations in terminal distances and conduction velocities. Duration lengthens as the number of fibers and the territory of a motor unit increase; it varies directly with age (increased age, increased duration) and inversely with temperature (decreased temperature, increased duration) and depends on the individual muscle being studied. Proximal and bulbofacial muscles in general have MUAPs of shorter duration. When performing EMG, it often is more rewarding to listen to the potential than to see it. This is especially true when evaluating MUAP duration, because duration correlates with pitch. Long-duration MUAPs (low frequencies) sound dull and thuddy, whereas short-duration MUAPs (higher frequencies) sound crisp and static-like. As the electromyographer gains experience, the sound of a long-duration versus a short-duration MUAP becomes unmistakable.
Polyphasia, Serrations, and Satellite Potentials
Polyphasia is a measure of synchrony, that is, the extent to which the muscle fibers within a motor unit fire more or less at the same time. This is a nonspecific measure and may be abnormal in both myopathic and neuropathic disorders. The number of phases can be easily calculated by counting the number of baseline crossings of the MUAP and adding one (Figure 15–4). Normally, MUAPs have two to four phases. However, increased polyphasia may be seen in up to 5 to 10% of the MUAPs in any muscle and is considered normal. The one exception is the deltoid, where up to 25% polyphasia may be normal. Increased polyphasia beyond 10% in most muscles and 25% in the deltoid is always abnormal. Through the speaker, polyphasic MUAPs are recognized as a high-frequency “clicking” sound.
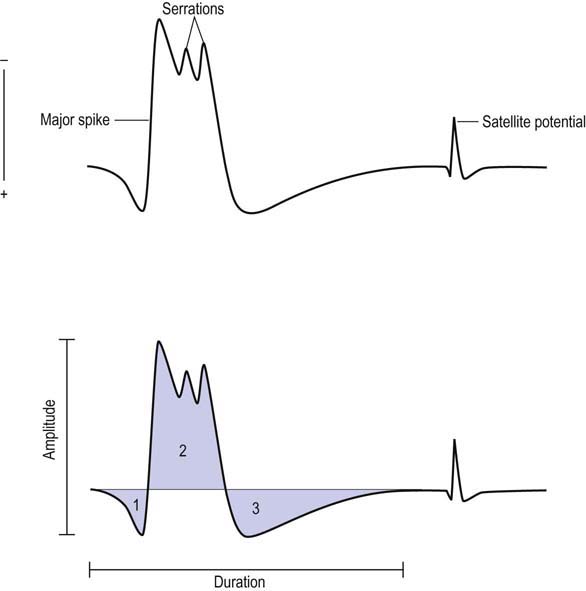
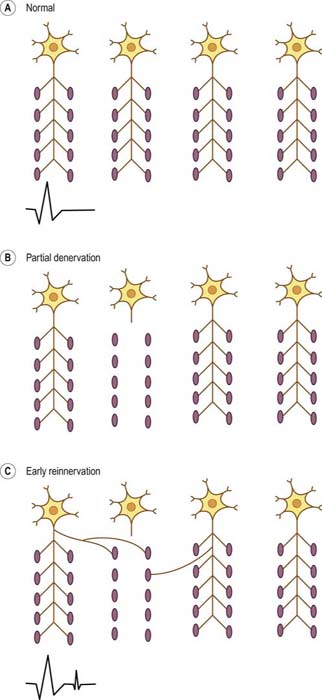
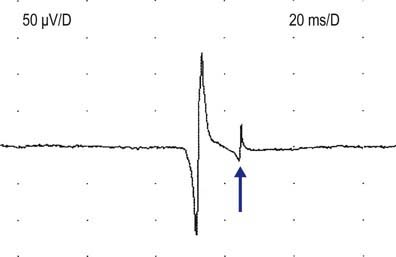
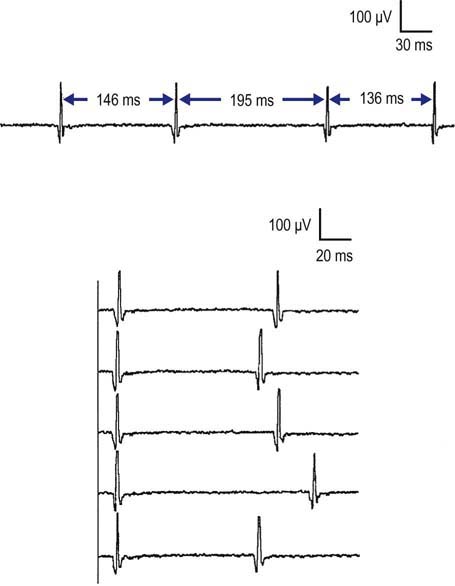
Satellite potentials (also known as linked potentials or parasite potentials) are interesting phenomena seen in early reinnervation. After denervation, muscle fibers often are reinnervated by collateral sprouts from adjacent intact motor units. The newly formed sprout often is small, unmyelinated or thinly myelinated, and therefore very slowly conducting. Because of the slow conduction time and increased distance, reinnervated muscle fibers are seen as time-locked potentials that trail the main MUAP (Figures 15–5 and 15–6). These satellite potentials are extremely unstable (see section on Stability) and may vary slightly in their firing rate or may block and not fire at all (Figure 15–7). Over time, the sprout matures, and the thickness of the myelin and consequently the conduction velocity increase. The satellite potential then fires more closely to the main potential and ultimately will become an additional phase or serration within the main complex. It is usually necessary to put the main MUAP on a delay line to appreciate a satellite potential and to demonstrate that it is time locked to the main potential.
Amplitude
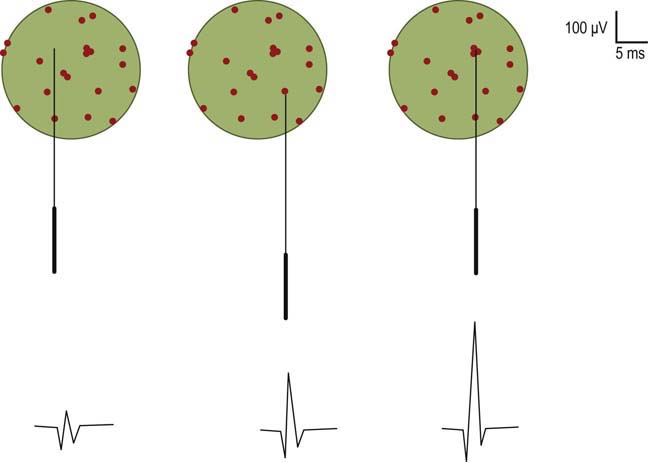
MUAP amplitude varies widely among normal subjects. Most MUAPs have an amplitude greater than 100 µV and less than 2 mV. Amplitude is generally measured from peak to peak of the MUAP (Figure 15–4). Amplitude is essentially a high-frequency response. Tissue between the needle and muscle fibers effectively acts as a high-frequency filter. Thus, unlike duration, most muscle fibers of a motor unit contribute little to the amplitude. MUAP amplitude reflects only those few fibers nearest to the needle (only 2–12 fibers). Hence, amplitude is not as helpful as duration in judging motor unit size. Several factors are associated with increased amplitude, including (1) the proximity of the needle to the motor unit (Figure 15–8), (2) increased number of muscle fibers in a motor unit, (3) increased diameter of muscle fibers (i.e., muscle fiber hypertrophy), and (4) more synchronized firing of the muscle fibers. Listening to the EMG, the amplitude of MUAPs is correlated not with pitch but with volume.
Major Spike
The major spike is the largest positive-to-negative component of the MUAP and usually occurs after the first positive peak (Figure 15–4). The major spike is the highest-frequency component of the MUAP. Because tissue acts as a high-frequency filter, as the needle is moved closer to the MUAP, the major spike increases in amplitude and its rise time shortens, indicating the proximity of the needle to the motor unit. MUAP parameters should be measured only when the needle is very close to the motor unit (Figure 15–9). When the needle is close to the motor unit, the MUAP becomes “sharp.” The sharp sound represents the high-frequency component of the major spike, occurring when the major spike rise time is less than 500 µs, indicating proper needle placement.
Stability
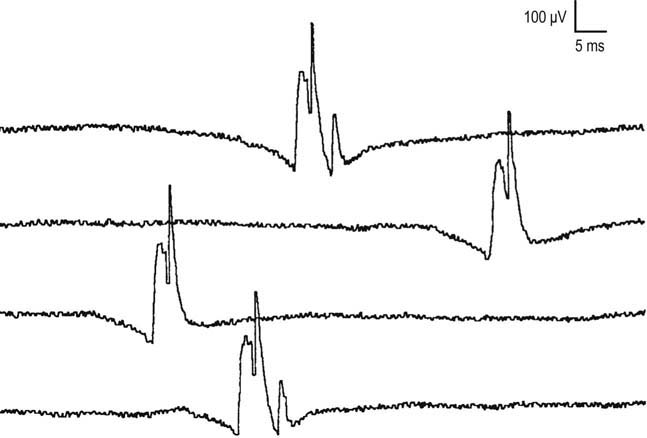
MUAPs usually are stable in morphology from potential to potential. This stability is due to the fact that each time a nerve action potential is generated, there is normally effective transmission across the NMJs, and all muscle fibers of the motor unit fire. If there is impaired NMJ transmission, unstable MUAPs may result (Figure 15–10). Unstable MUAPs occur when individual muscle fibers either are blocked or come to action potential at varying intervals, leading to an MUAP that changes in configuration from impulse to impulse. There is a change between potentials in either the amplitude or the number of phases (or serrations), or both. Although unstable MUAPs always indicate unstable NMJs, they occur not only in primary disorders of the NMJ (e.g., myasthenia gravis, Lambert–Eaton myasthenic syndrome) but are also often seen as secondary phenomena in both neuropathic and myopathic disorders. Any disorder associated with denervation may demonstrate unstable MUAPs. During the process of early reinnervation, newly formed, immature NMJs often fail to conduct NMJ transmission faithfully. The result is variability in endplate transmission or intermittent blocking of transmission across some of the muscle fibers within a motor unit (Figure 15–7).
Firing Pattern (Activation, Recruitment, Interference Pattern)
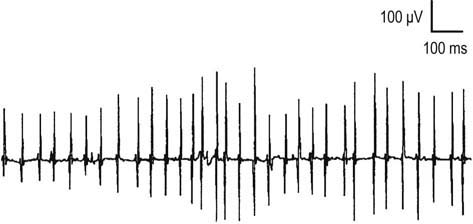
One of the most important and yet most difficult tasks for the electromyographer is the assessment of firing pattern and its relationship to the number of MUAPs. MUAPs normally fire in a semi-rhythmic pattern, that is, there is slight variation in the time interval between the same MUAP as it fires consecutively (Figure 15–11). This unique firing pattern helps to identify the potential as an MUAP under voluntary control, in contrast to various spontaneous waveforms that are not under voluntary control, and have other distinct firing patterns, such as fibrillation potentials and positive sharp waves, which are regular; complex repetitive discharges, which are perfectly regular or change abruptly; myotonic discharges, which have a waxing/waning amplitude; or fasciculation potentials, which are very slow and irregular.
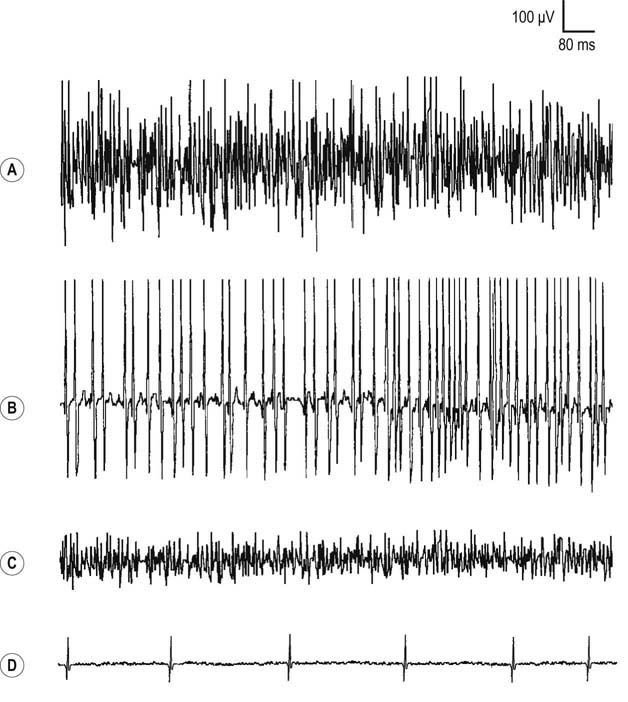
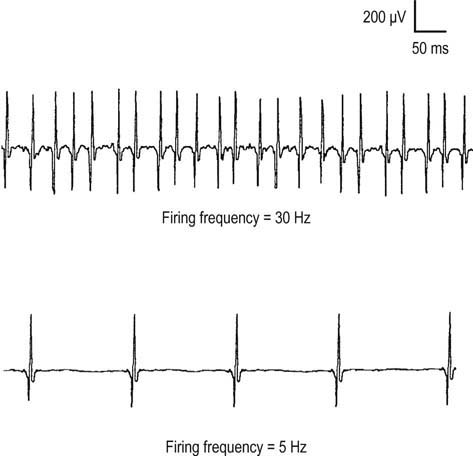
During muscle contraction, there are only two ways to increase muscle force: either motor units can increase their firing rate (up to tetanic fusion frequency which is approximately 50 Hz), or additional motor units can fire (Figure 15–12). Normally, one increases force using a combination of these two processes, resulting in an orderly recruitment of motor units. With the smallest contraction, a single motor unit action potential normally begins firing semi-rhythmically at 4 to 5 Hz. Any potential that fires more slowly than 4 to 5 Hz cannot be an MUAP under voluntary control and must be a spontaneous potential. As one increases force, the first motor unit action potential increases its firing rate, and then a second motor unit action potential begins to fire, and so forth. This process continues, with the firing rate increasing and additional motor unit action potentials being recruited as force is increased. Normally, the ratio of firing frequency to the number of different MUAPs firing is approximately 5 : 1. Thus, by the time the first MUAP firing frequency reaches 10 Hz, a second MUAP should begin to fire; by 15 Hz, a third motor unit action potential should fire, and so forth. During maximal contraction, multiple MUAPs normally overlap and create an interference pattern in which no single motor unit action potential can be distinguished (Figure 15–13A). For most muscles, the maximal firing frequency is 30 to 50 Hz. Important exceptions include quick ballistic contractions, in which the firing frequency may transiently reach 100 Hz, and muscles that are predominantly slow twitch (e.g., soleus), in which the maximal firing frequency is approximately 15 Hz.
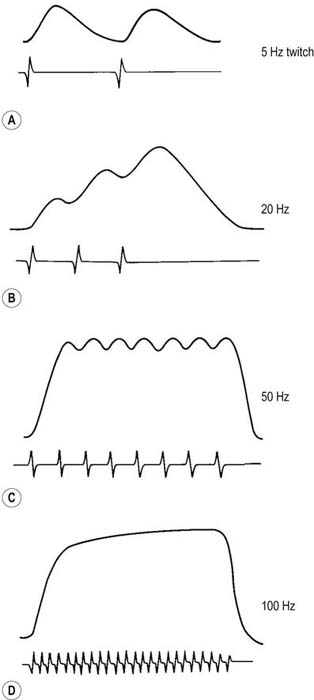
FIGURE 15–12 Relationship of force to firing frequency.
(Adapted with permission from Kandel, E.R., Schwartz, J.H., Jessell, T.M. (Eds.), 1991. Principles of neural science, third ed. Appleton & Lange, Norwalk, CT.)
An incomplete interference pattern may be due to either poor activation or poor recruitment. Consider the two different incomplete interference patterns shown in Figure 15–14. In both cases, the patient has been asked to maximally contract the muscle of interest. In the first case (top trace), note that the same MUAP is firing rapidly at 30 Hz. Thus, although the firing rate is maximal, only one MUAP is seen firing at 30 Hz (30 : 1 ratio). In a normal muscle, by the time the firing rate reaches 30 Hz, one should see five or six different MUAPs firing (a ratio of approximately 5 : 1). Thus, in this case, the interference pattern is reduced because of decreased recruitment, but activation (firing rate) is normal. Decreased recruitment occurs when there has been loss of MUAPs, usually through axonal loss or conduction block. In the unusual situation of end-stage myopathy, if every muscle fiber of an MUAP is lost, the number of MUAPs also will effectively decrease, leading to reduced recruitment.
Patterns of Motor Unit Abnormalities
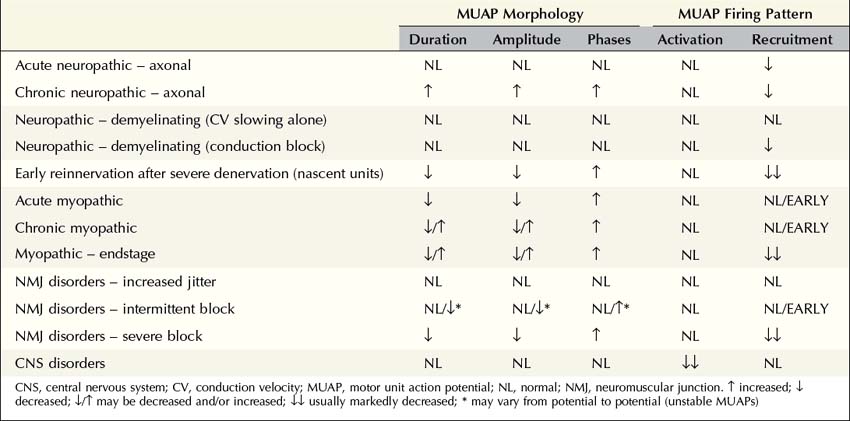
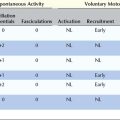
MUAP morphology and firing patterns usually can discriminate among the various disorders affecting the motor unit. No single parameter identifies an MUAP as myopathic, neuropathic, or associated with an NMJ disorder. Specific patterns of abnormalities in MUAP morphology and firing rate reflect whether the underlying disorder is (1) acute, chronic, or end stage; (2) neuropathic, myopathic, or associated with an NMJ transmission defect; and, if neuropathic, (3) whether the primary pathophysiology is axonal loss or demyelination (Table 15–2).
Neuropathic
Acute Axonal Loss
After an acute axonal injury to a nerve, the process of wallerian degeneration occurs in motor nerve fibers within the first 3 to 5 days, followed by denervation of the distal muscle fibers of the involved motor units. Reinnervation normally occurs as surviving nearby axons form sprouts that grow and eventually reinnervate the denervated fibers. When this occurs, the number of muscle fibers in the reinnervated MUAP is larger than normal, leading to an MUAP with increased duration, amplitude, and number of phases (Figure 15–15). However, this process takes time, usually many weeks to months. In the acute setting, MUAP morphology remains normal. The only abnormality seen on EMG in an acute neuropathic lesion is a decreased recruitment pattern in weak muscles due to the initial loss of motor units. Thus, in acute axonal loss lesions on needle EMG, there is a pattern of decreased recruitment of MUAPs with normal morphologies. This pattern does not occur in slowly progressive or chronic conditions (e.g., most polyneuropathies). In those conditions, changes in MUAP morphology are always present by the time the patient presents with symptoms. The acute neuropathic pattern associated with axonal loss characteristically occurs in the first several weeks after trauma, compression, or nerve infarction. The only other situation in which a similar pattern is seen is in pure demyelinating lesions with conduction block (discussed in section on Neuropathic: Demyelinating).
Chronic Axonal Loss
After axonal loss and denervation, the process of reinnervation can occur by one of two mechanisms. If there has been complete denervation, the only possible mechanism for reinnervation is axonal regrowth from the point of injury (see section on Early Reinnervation Following Severe or Complete Denervation). Typically, this regrowth is quite slow (no more than 1 mm per day) and may take months to years, depending on the length of the nerve. For the regrowth to occur, however, the anterior horn cells must remain intact. For example, the original nerve fibers can regrow following transection of a nerve, but not after poliomyelitis, which results in the death of anterior horn cells.
In contrast, in cases of partial or gradual denervation, reinnervation usually occurs through collateral sprouting by adjacent surviving motor units (Figure 15–5). As the number of muscle fibers per motor unit increases, MUAPs become prolonged in duration, with a high amplitude, and polyphasic. These MUAP changes, in conjunction with decreased recruitment, are the hallmarks of reinnervated motor unit action potentials and nearly always imply chronic neuropathic disease (i.e., disorders of the anterior horn cell, nerve root, or peripheral nerve). Similar to other neuropathic conditions, during maximal contraction, the interference pattern will not be full, secondary to decreased recruitment of MUAPs (Figure 15–13B). Long-duration, high-amplitude, polyphasic MUAPs are never seen in acute conditions. When present, they always imply that the process has been present for at least several weeks and more often for months or years.
Myopathic
Acute
In myopathies, the number of functioning muscle fibers in a motor unit decreases. Because there are fewer muscle fibers per motor unit, this results in MUAPs of shorter duration and smaller amplitude (Figure 15–15). In addition, there is less synchronous firing and consequently polyphasia of MUAPs due to dysfunction of the remaining muscle fibers. However, the actual number of functioning motor units (i.e., the number of anterior horn cells and axons) remains normal. Thus, the recruitment pattern remains normal for the level of activation. Because each motor unit contains fewer muscle fibers, however, it cannot generate as much force as a normal motor unit. To compensate, more MUAPs will fire than are normally needed for a certain level of force, resulting in early recruitment. The interference pattern will fill easily with a small amount of force from the patient (Figure 15–13C). Consequently, the pattern associated with an acute myopathy is short-duration, small-amplitude, polyphasic MUAPs with normal or early recruitment.
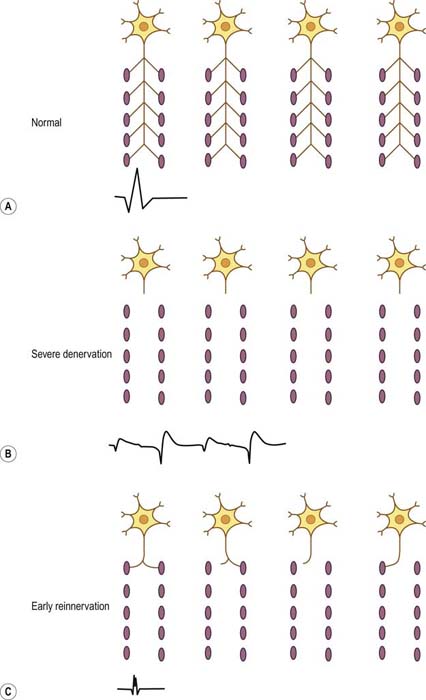
Early Reinnervation following Severe or Complete Denervation
Reinnervation most often occurs from collateral sprouting by adjacent surviving motor units. If there is severe or complete denervation, with no nearby surviving axons, the only possible mechanism for reinnervation is regrowth of the axon from the site of injury. As the axon regrows, at some point in time it will reinnervate some, but not all, of the original muscle fibers. At that point, the MUAP will be short duration, small amplitude, and polyphasic, similar to an acute myopathic motor unit action potential (Figure 15–16). Early reinnervated motor unit action potentials following severe denervation are known as nascent motor units. The key factor that differentiates nascent motor unit action potentials from myopathic motor unit action potentials is the recruitment pattern. Nascent MUAPs are always seen in the context of markedly reduced recruitment, whereas myopathic MUAPs are seen in the context of normal or early recruitment. Although nascent motor units are uncommon, they emphasize that not all short-duration, small-amplitude, polyphasic MUAPs are myopathic.
Central Nervous System Disorders
In CNS disorders, normally there is no loss of anterior horn cells and, accordingly, no denervation or reinnervation. MUAP morphology and recruitment remain normal. On needle EMG, weakness is demonstrated as the inability to fire motor unit action potentials rapidly (i.e., reduced activation). Thus, although the interference pattern is incomplete, with a reduced number of motor unit action potentials firing, the actual number of motor unit action potentials (i.e., recruitment) is appropriate for the reduced level of activation (Figure 15–13D).
Tremor may occur in some CNS disorders and can complicate the interpretation of both spontaneous activity (see Chapter 14) and MUAP morphology. Tremor is recognized as a bursting pattern of voluntary MUAPs separated by relative silence. When tremor occurs at rest (e.g., in Parkinson’s disease), the spontaneous bursting discharges may be mistaken for myokymic discharges. Although both tremor and myokymia result in a bursting pattern of MUAPs, the major difference is that in myokymia the same MUAP fires repetitively in a burst, whereas in tremor the burst is composed of many different MUAPs. In addition, most patients can voluntarily alter their tremor by changing their limb position or action, whereas myokymia cannot be voluntarily influenced by the patient. Most tremors, however, worsen with activation. Because multiple MUAPs fire simultaneously in tremor, the morphology of individual MUAPs may be difficult to assess, and polyphasia may appear to be increased. In general, it is very difficult to accurately judge MUAP morphology, stability, or recruitment if the patient has a tremor when activating their muscles.
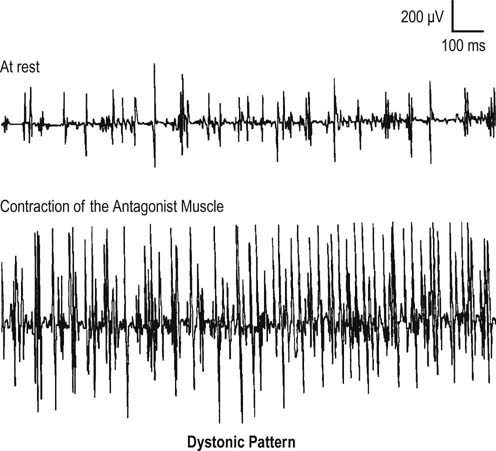
Lastly, persistent involuntary contraction can be seen during the needle EMG as the result of central disorders, including dystonia, stiff-person syndrome, and tetanus. In all of these disorders, MUAP morphology will be normal, and the EMG pattern will be one of involuntary persistent firing of MUAPs, characterized by delayed relaxation and co-contraction of muscles. Normally, individuals can easily relax their muscles and stop contracting. In these CNS disorders, however, this often is not possible. In addition, co-contraction of agonist and antagonist muscles occurs. Normally, antagonist muscles are quiet while agonists are contracting (e.g., the triceps is relaxed while the biceps is contracting and flexing the elbow). In dystonia, MUAP firing often actually increases in the antagonist muscle when the patient is instructed to move the agonist muscle (e.g., increased firing in the tibialis anterior when the patient plantar flexes the ankle) (Figure 15–17).
Brown W.F. The physiological and technical basis of electromyography. Boston: Butterworth; 1984.
Buchthal F., Guld C., Rosenfalck P. Action potential parameters in normal human muscle and their dependence on physical variables. Acta Physiol Scand. 1954;32:200.
Buchthal F., Rosenfalck P. Action potential parameters in different human muscles. Acta Psychiatr Neurol Scand. 1955;30:121.
Daube J.R. AAEM minimonograph #11: needle examination in clinical electromyography. Rochester, MN: American Association of Electrodiagnostic Medicine; 1991.
Dimitru D. Volume conduction: theory and application. In: Dimitru D., ed. Clinical electrophysiology: physical medicine and rehabilitation state of the art reviews. Philadelphia: Hanley Belfus; 1989:665.
Dimitru D., DeLisa J.A. AAEM minimonograph #10: volume conduction. Rochester, MN: American Association of Electrodiagnostic Medicine; 1991.
Kandel E.R., Schwartz J.H., Jessell T.M. Principles of neural science, third ed, Norwalk, CT: Appleton & Lange, 1991.
Mendell L.M. Essays on APS Classic Papers: the size principle: a rule describing the recruitment of motoneurons. J Neurophysiol. 2005;93:3024–3026.
Sacco G., Buchthal F., Rosenfalck P. Motor unit potentials at different ages. Arch Neurol. 1962;6:44.