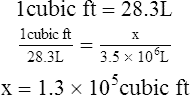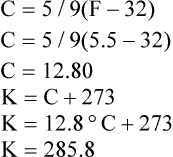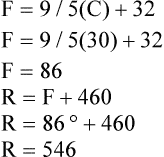Basic Chemistry
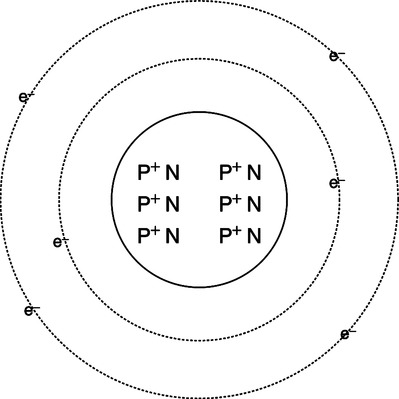
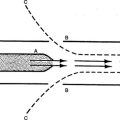
A Atom: The smallest subdivision of a substance that still maintains the properties of that substance, frequently referred to as the building blocks of the universe. An atom is composed of the following (Figure 1-1):
1. Nucleus: Central portion of an atom, which contains protons and neutrons.
a. Proton: Positively charged particle with a mass of one atomic mass unit.
b. Neutron: Neutral particle with a mass of one atomic mass unit.
2. Electron: Negatively charged particle that revolves around the nucleus of the atom with a mass of approximately 1/1000 of an atomic mass unit. Electrons exist in well-defined orbitals that establish the chemical reactivity of an atom.
3. Normally, in its nonreactive state, an atom contains the same number of protons and electrons. The number of neutrons in the nucleus of a substance varies among atoms (isotopes).
B Element: General term applied to each of the 109 specifically named different types of atoms.
C Isotope: Atom of a substance with the same number of protons but with a varying number of neutrons. All elements have at least two isotopes. The following are the three primary isotopes of oxygen:
D Atomic weight: Average weight of an atom of a particular substance based on its comparison with the atomic weight of the carbon 12 isotope. The atomic weight is approximately equal to the sum of the number of protons and neutrons in the nucleus of an atom but is not a whole number because of the presence of isotopes (Table 1-1).
TABLE 1-1
| Element | Symbol | Atomic No. | Atomic Weight | Valence |
| Elements Commonly Seen in the Body | ||||
| Aluminum | Al | 13 | 26.98 | +3 |
| Boron | B | 5 | 10.83 | +3 |
| Calcium | Ca | 20 | 40.08 | +2 |
| Carbon | C | 6 | 12.0 | + or − 4 |
| Chlorine | Cl | 17 | 35.5 | − 1 |
| Chromium | Cr | 24 | 51.99 | − 1 or − 2 |
| Cobalt | Co | 27 | 58.93 | +2 |
| Copper | Cu | 29 | 63.55 | + 1 or +2 |
| Fluorine | F | 9 | 18.99 | − 1 |
| Hydrogen | H | 1 | 1.00 | +1 |
| Iodine | I | 53 | 126.9 | − 1 |
| Iron | Fe | 26 | 55.84 | + 1 or +2 |
| Magnesium | Mg | 12 | 24.31 | +2 |
| Manganese | Mn | 25 | 54.94 | − 2 or − 3 |
| Molybdenum | Mo | 42 | 95.94 | − 1 or − 2 |
| Nitrogen | N | 7 | 14.01 | − 3 |
| Oxygen | O | 8 | 15.99 | − 2 |
| Phosphorus | P | 15 | 30.97 | − 3 |
| Potassium | K | 19 | 39.09 | +1 |
| Selenium | Se | 34 | 78.96 | − 2 |
| Silicone | Si | 14 | 28.09 | + or − 4 |
| Sodium | Na | 11 | 22.98 | +1 |
| Sulfur | S | 16 | 32.06 | − 2 |
| Tin | Sn | 50 | 118.7 | + or − 4 |
| Vanadium | V | 23 | 50.94 | − 2 or − 3 |
| Zinc | Zn | 40 | 91.22 | + 1 or +2 |
| Other Elements Commonly Seen in Medicine | ||||
| Barium | Ba | 56 | 137.34 | +2 |
| Gallium | Ga | 31 | 69.72 | +3 |
| Helium | He | 2 | 4.00 | + or − 2 |
| Lead | Pb | 82 | 207.19 | + 1 or +2 |
| Lithium | Li | 3 | 6.94 | +1 |
| Mercury | Hg | 80 | 200.59 | + 1 or +2 |
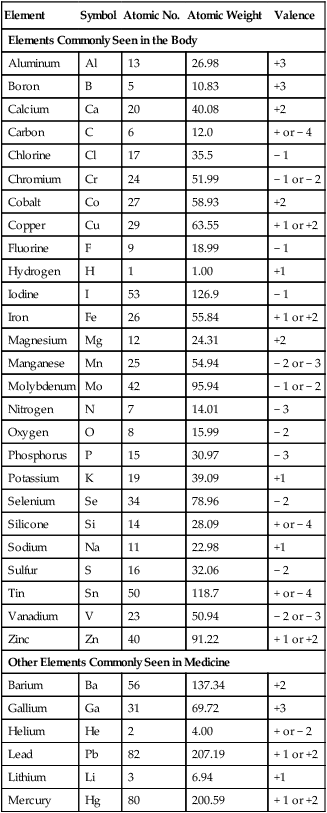
E Gram atomic weight: Mass in grams of an element equal to its atomic weight (see Table 1-1).
F Atomic number: Equal to the number of protons in the nucleus of an atom (see Table 1-1).
G Ion: Charged species of a particular atom; occurs as a result of the loss or gain of electrons from an atom.
A Molecule: Particle that results from the chemical combination of two or more atoms normally having a neutral charge but may be positively or negatively charged.
B Compound: Molecule formed from two or more elements.
C Free radical: A charged compound, reacting as any other ion reacts.
D Molecular formula: Chemical expression indicating the types and number of atoms in a molecule. The particle that is positively charged is usually listed first.
NaCl = 1 sodium atom and 1 chloride atom contained in the molecule.
H2SO4 = 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms contained in the molecule.
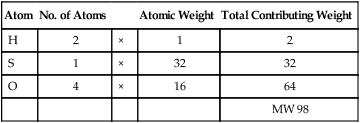
E Molecular weight (MW): Sum total of all individual atomic weights of atoms that make up a molecule.
| Atom | No. of Atoms | Atomic Weight | Total Contributing Weight | |
| H | 2 | × | 1 | 2 |
| S | 1 | × | 32 | 32 |
| O | 4 | × | 16 | 64 |
| MW 98 |
| Atom | No. of Atoms | Atomic Weight | Total Contributing Weight | |
| C | 1 | × | 12 | 12 |
| O | 2 | × | 16 | 32 |
| MW 44 |
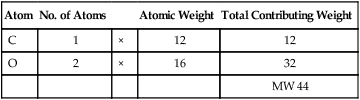
F Gram molecular weight (GMW): Mass in grams of a molecule equal to its MW.
G One mole of a substance is equal to one GMW of the substance.
A Valence: Number given to an atom that indicates its tendency to gain or lose electrons in a chemical reaction.
| Na+1 (sodium): | Valence of +1 indicates that in a chemical reaction it will react by losing one electrons. |
| Ca+2 (calcium): | Valence of +2 indicates that in a chemical reaction it will react by losing two electrons. |
| F−1 (fluorine): | Valence of − 1 indicates that in a chemical reaction it will react by gaining one electron. |
B Generally, valences of elements allow predictions of their chemical reactivity with each other.
C Inert gases (noble gases) have an electron distribution that has full outer orbitals. These elements (e.g., helium, neon, argon, krypton, and xenon) do not react with other elements under normal atmospheric conditions.
IV Types of Chemical Compounds
A Ionic compound: A compound formed by atoms in the compound transferring electrons, one atom gaining and the other losing electrons. Ionic compounds form ions when dissolved in solution.
| NaCl: | Na+1 has a valence of +1, and Cl−1 has a valence of − 1. The Na+1 atom has lost an electron, and the Cl− 1 atom has gained an electron during the formation of NaCl. |
| CaF2: | Ca+2 has a valence of +2, and each F−1 atom has a valence of − 1. The Ca+2 atom has lost two electrons, and each F− 1 atom has gained one electron during the formation of CaF2. |
1. Properties of ionic compounds:
c. Dissolve readily in polar solvents (solvents formed by hydrogen bonding).
d. Strong electrolytes: dissociate readily in polar solvents:
B Covalent compound: A compound formed by the sharing of electrons between the various atoms in the compound. In solution the molecule does not disassociate into its component parts.
C Hydrogen bonding (polar covalent compound): An intermediate compound between a pure covalent compound and an ionic compound characterized by an incomplete (partial) sharing of electrons. In solution the molecule only partially disassociates into its component parts.
A Synthesis reactions: Two substances react to form a third releasing energy
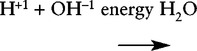
B Decomposition reaction: A substance breaks up into its component parts with the release of energy, normally in the form of heat.
C Exchange reaction: Two substances exchange parts to form two new substances, such as the reaction of an acid and base to form a salt and water.
VI Volume Percent and Gram Percent
A Volume percent (vol%): Method of indicating the number of milliliters of a substance in 100 ml of solution.
B Gram percent (g%): Method of indicating the number of grams of a substance in 100 ml of solution.
A Solution: Homogeneous mixture of two substances.
B Solute: Substance dissolved in a solution.
C Solvent: Substance that is the dissolving agent.
D Effects of a solute on the physical characteristics of water:
1. Solutes cause the boiling point of water to increase.
2. Solutes cause the freezing point of water to decrease.
3. The osmotic pressure of a solution containing a solute is higher than that of pure water.
E As the temperature of the solvent increases, the volume of solute that can be dissolved in the solvent also increases.
F Dilute solution: A solution with a small amount of solute dissolved in each unit of solvent at a particular temperature.
G Saturated solution: A solution with the maximum amount of solute dissolved in each unit of solvent at a particular temperature. In a saturated solution a precipitate is seen at the bottom of the solution.
H Supersaturated solution: A solution with a greater amount of solute than the solvent would normally hold, dissolved at a particular temperature. However, any physical disturbance of this solution causes the excess solute to precipitate.
I Precipitate: A crystallized solid formed at the bottom of a saturated solution.
A Ratio solution: Solution concentration represented as a ratio (1:100) between solute and solvent in number of grams to number of milliliters.
1. How many milligrams of solute are there in 1 ml of a 1:200 solution?
< ?xml:namespace prefix = "mml" />
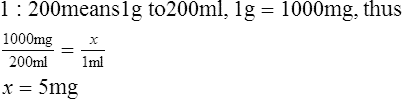
2. How many milligrams are there in 5 ml of a 1:500 solution?
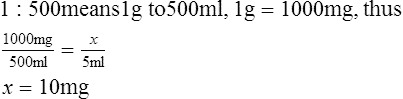
B Percent weight/volume (w/v): Solution concentration in which the actual percentage indicates the number of grams of solute per 100 ml of solution.
1% w/v solution means 1 g of solute is contained in 100 ml of solution.
1. How many milligrams are there in 10 ml of a 3% w/v solution?
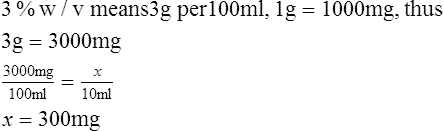
2. How many milligrams are there in 3 ml of a 0.5% w/v solution?

C True percent solution: Solution concentration in which solute and solvent are expressed in either weight (% w/w) or volume (%v/v). The solute is expressed as a true percentage of the solution.
1. How many grams of solute are there in 250 g of a 5% w/w solution.
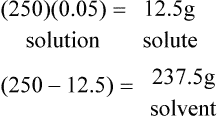
2. How many milliliters of solute are there in 500 ml of a 10%v/v solution.
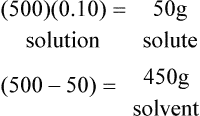
D Molal solution: Solution concentration in which the solute is expressed in moles, and the solvent is expressed in kilograms, or millimoles per gram (mmol/g).
2.5 molal solution contains 2.5 moles of solute in 1 kg of solvent.
1. What is the molality of a solution with 117 g of NaCl in 1000 g of water?
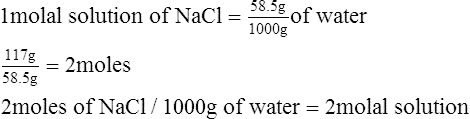
2. How much H2SO4 must be dissolved in 500 g of H2O to make a 0.5 molal solution?
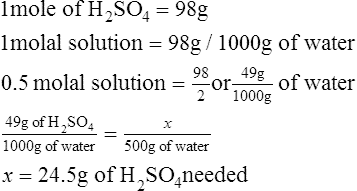
E Molar solution (m): Solution containing 1 mole of solute per liter of solution (or mmol/ml).
1.75 m solution contains 1.75 moles of solute per liter (L) of solution.
2.0 m solution of NaOH contains 2 moles, or 80 g of NaOH/L of solution (2 × MW of NaOH).
1. What is the molarity of 5.85 g of NaCl in 1 L of solution?
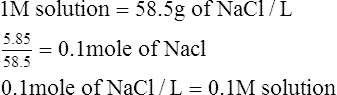
2. In what volume of solution must 149.2 g of KCl be dissolved to make a 4 m solution?
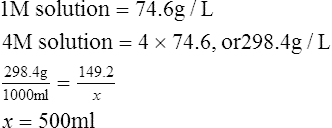
F Normal solution (N): Solution concentration containing 1 g equivalent weight (GEW; see Section X, A, Gram Equivalent Weights) of solute/L of solution (or 1 mg equivalent weight/ml).
1.25 N solution contains 1.25 GEW/L of solution.
2.00 N solution of HCl contains 2 GEWs, or 73 g of HCl/L of solution (1 GEW of HCl = 36.5 g).
1. What is the normality of a solution containing 42 g of NaHCO3?
2. How many grams of NH3Cl must be dissolved in 250 ml of solution to make a 2 N solution?
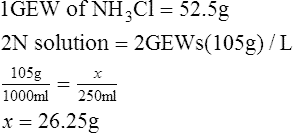
A The following formula is used to determine the concentration that will result when a solution is diluted:
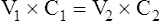 (1)
(1)V1 is the volume before dilution
C1 is the concentration before dilution
V2 is the volume after dilution
C2 is the concentration after dilution
B Before equation 1 can be used, three of the four variables must be known.
1. What volume of water should be added to 50 ml of a 40% v/v solution of alcohol to dilute it to a 20% v/v solution?
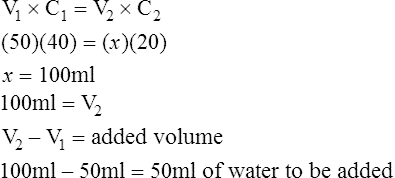
2. If 4 ml is added to 0.5 ml of a 15% v/v solution, what is the solution’s final concentration?
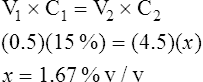
A GEW: Amount of a substance that will react completely with 1 mole of H+1 or OH−1 or 1 mole of any monovalent substance.
B The GEW of an element is determined by dividing the gram atomic weight of the substance by its valence. The charge of the valence is disregarded.

C The GEW of an acid is determined by dividing its GMW by the number of replaceable hydrogen ions in the molecular formula. Normally all H+1 are replaceable. However, H2CO3 is an exception: only 1 H+1 is replaceable.

D The GEW of a base is determined by dividing its GMW by the number of replaceable hydroxyl ions (OH−1) in the molecular formula. Normally all OH−1are replaceable.
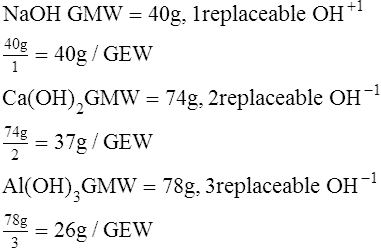
E The GEW of a salt is determined by dividing its GMW by the total valence of the positive ions or free radicals in the molecule.
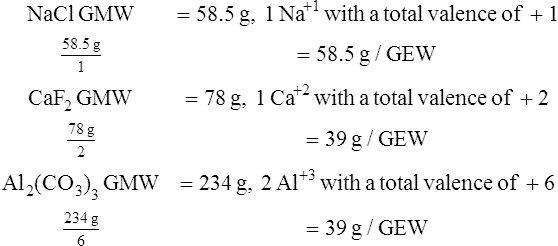
F The GEW of a free radical is determined by dividing its GMW by its valence, disregarding the charge of the valence.
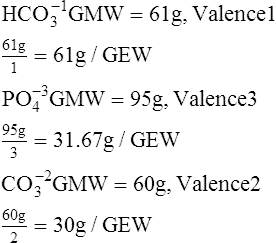
G Milliequivalent weight (mEq): Weight of a substance that will react with 1 mmole of H+1, OH−1, or any monovalent substance. Numerically, the mEq of a substance is equal to its GEW.
H Equivalent weights are used to determine the precise quantity of a substance that reacts completely with a given quantity of another substance.
XI Other Types of Liquid Mixtures
A Suspension: A mixture formed by placing a solid unable to dissolve (dissociate into its component parts) into a liquid. Particles suspended are large (>100 μm). A suspension is characterized by:
1. An insoluble substance dispersed in a liquid
2. The dispersion is heterogenous
3. The solid settles out over time
4. The solid does not pass through filter paper or membranes
B Colloid: A mixture formed the same way as a suspension except the particles are between 1 and 100 μm in size. A colloid is characterized by:
1. The solid in the mixture does not settle over time
2. Colloids can pass through filters
3. Colloids do not pass through membranes
4. Colloids possess an electrical charge
5. Colloids exhibit the Tyndall effect or the ability to disperse or scatter a beam of light passing through the mixture
6. Colloids exhibit Brownian movement, the constant movement of the colloid particles by bombardment from the liquid medium
7. Colloid dispersions (mixtures) can be either hydrophilic (attracting water) or hydrophobic (repelling water)
8. Gels: Systems in which there is a strong attraction between the colloid particles and water
9. Sols: Systems in which there is little attraction between the colloid particles and water
XII Inorganic Molecules and Compounds
A Compounds generally formed by ionic or hydrogen bonds that do not contain C-C or C-H covalent bonds
B Acids, bases, and salts are inorganic compounds or molecules
XIII Organic Compounds or Molecules
A Temperature scales (in degrees) in general use:
B The Rankine and Kelvin scales are absolute zero scales (i.e., zero on their scales represents the point where all molecular activity stops).
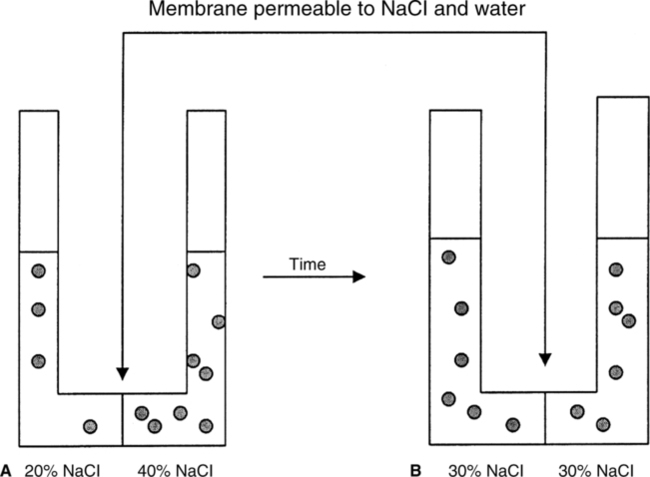
A The movement of water and the dissolved solute from an area of high concentration of each to an area of low concentration of each across a membrane permeable to both (Figure 1-2).
B When complete, the concentration of water and the solute is equal on each side of the membrane.
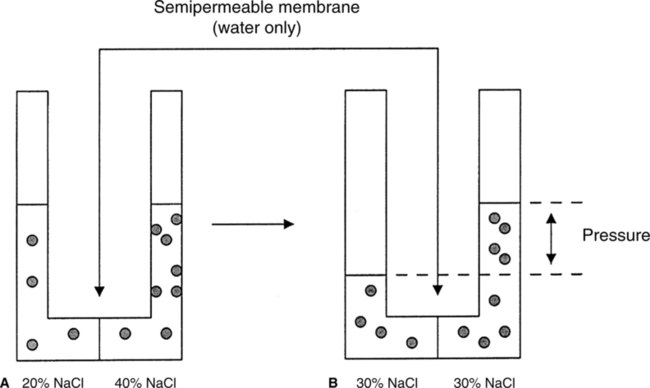
A Osmosis is the movement of water from an area of high concentration of water to an area of low concentration of water.
B Osmosis occurs when a membrane that is selectively permeable only to water separates two compartments of fluid (Figure 1-3).
C Osmosis will proceed in a system until the concentration of water in the involved compartments is equal. When concentrations are equal, no net movement of fluid occurs; however, molecules of water still move back and forth across the membrane.
D Osmosis occurs between two solutions as a result of osmotic pressure differences in the solutions.
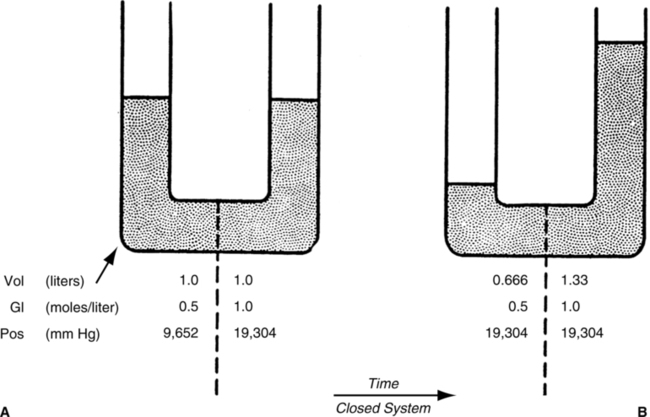
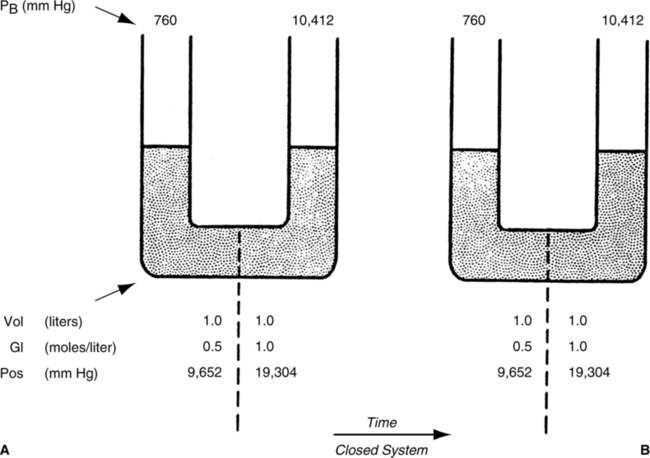
1. The potential pressure of the molecules of pure H2O is approximately 1,073,000 mm Hg.
2. When a solute is dissolved in H2O, the potential pressure of the H2O is decreased.
3. The osmotic pressure of a solution is equal to the potential pressure of pure water minus the potential pressure of the solution.

4. Osmotic pressure is a force drawing water into the solution.
5. Osmosis can be stopped by exerting a force on a solution equal to the osmotic pressure of the solution (Figures 1-4 and 1-5).
XVII Starling’s Law of Fluid Exchange
A Fluid movement across capillaries is controlled by the interaction of hydrostatic and osmotic pressures (colloid osmotic pressure caused as a result of protein being the only nondiffusible substance).
B As a result of this interaction there is a net movement of a small amount of fluid from capillaries into the interstitial space.
C An imbalance in the forces controlling fluid exchange can result in edema.
D Edema can occur either in the lungs (pulmonary edema) or in the legs (systemic edema).
XIX Expressions of H+ Ion Concentration
A pH: Negative log of the H+ concentration per liter of solution; [ ] is used to symbolize molar concentration.
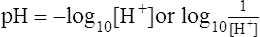
3. pH >7.0: Basic or alkalotic
4. pH <7.0: Acidic or acidotic
5. pH scale: 1 to 14, equivalent to an [+] of 10−1 to 10−14 mol/L
B Nanomoles per liter (nmol/L): + concentration in number of billionths of moles of + per liter.
A Acid: A compound that donates + when placed into solution.
1. The active compound responsible for the properties of acids is the hydronium ion (H3O+1).
2. In solution the liberated + reacts with H2O to form the H3O+1 ion:
B Base: A compound that accepts + when placed into solution. The active compound responsible for the properties of most bases is the OH−1 (hydroxyl ion).
C Neutralization reaction: The reaction between an acid and a base where the results are a salt plus water:
A Oxidation: Process in a chemical reaction whereby a substance loses electrons.
B Reduction: Process in a chemical reaction whereby a substance gains electrons.
1. The basic unit of length is the meter (m). One meter is equal to 39.37 inches (in.).
2. One meter is equal to all of the following, and they are thus equal to each other:
3. Basic factors used to convert the metric to the British system or the British to the metric system:
1. How many angstroms are equal to 2.2 × 102 cm?
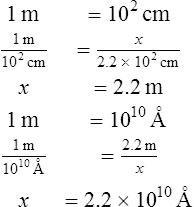
2. How many inches are equal to 5.3 × 106 mm?
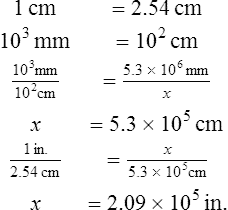
1. The basic unit of weight in the metric system is the kilogram (kg). One kilogram is equal to 2.2 pounds (lb).
2. One kilogram is equal to all of the following, and they are thus equal to each other:
3. Basic factors used to convert from metric to British system or from British to metric system:
1. How many milligrams are there in 1.5 × 102 kg?
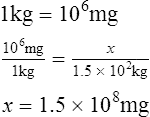
2. How many grams are equal to 0.6 lb?
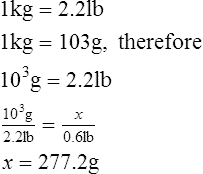
1. The basic unit of volume in the metric system is the liter, which is equal to 1.057 quarts (qt).
2. One liter is equal to 1000 ml (103 ml) and also to 1000 cc (cubic centimeters) (103 cc).
3. One cubic meter contains 103 L.
4. Basic factors used to convert from the metric to British system or from British to metric system:
1. How many liters are equal to 2.5 × 109 m?
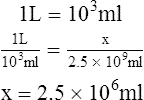
2. How many cubic feet are equal to 3.5 × 106 L?