Balloon Tamponade of Gastroesophageal Varices
Introduction
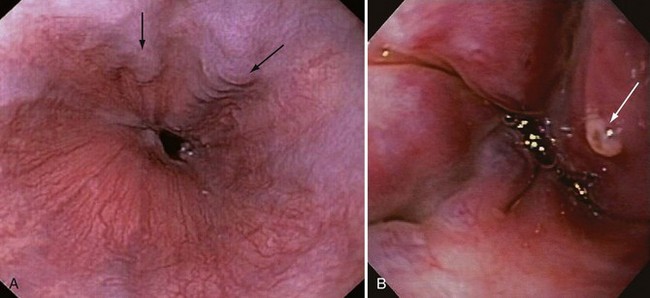
Managing patients with acute gastrointestinal bleeding from gastroesophageal varices can be one of the most challenging scenarios in emergency medicine. These patients often have advanced liver disease and can arrive at the emergency department (ED) with massive hematemesis, airway compromise, hemodynamic instability, critical anemia, thrombocytopenia, and coagulopathy. Gastroesophageal varices are the fourth most common cause of upper gastrointestinal bleeding (UGIB) and account for almost 12% of cases (Fig. 41-1).1 In patients with cirrhosis, varices account for up to 80% of cases of UGIB.2,3 In patients with established gastric or esophageal varices, the annual incidence of acute hemorrhage ranges from 4% to 15%.2,4
Over the past 3 decades, advances in resuscitation, critical care, pharmacology, and endoscopy have significantly reduced the mortality rate associated with acute variceal bleeding. In fact, mortality rates in patients with acute variceal bleeding currently range from 15% to 20%.1,5–7 Despite advances in management, a small number of patients with acute variceal bleeding fail standard therapy. Rescue therapies for this group of patients are limited and include balloon tamponade, surgery, and placement of a transjugular intrahepatic portosystemic shunt. This chapter details the indications and contraindications for balloon tamponade in patients with acute variceal bleeding, the techniques for placement of the various devices, and the potential complications of this intervention. Although this procedure is rarely needed and placement in the ED is not considered a standard intervention, emergency physicians with knowledge of the technique can attempt to place these critical and potentially lifesaving devices.
Background
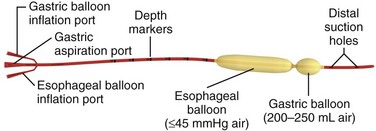
In 1950, Sengstaken and Blakemore developed and described the use of a double-balloon device to control variceal hemorrhage.8,9 Since that time, the Sengstaken-Blakemore tube has become the most widely known balloon tamponade device. The Sengstaken-Blakemore tube has an esophageal and a gastric balloon, along with a gastric aspiration port that allows continuous suction of stomach contents (Fig. 41-2). In 1968, Edlich and colleagues, from the University of Minnesota, modified the Sengstaken-Blakemore tube by adding an esophageal aspiration port and increasing the capacity of the gastric balloon.10
Currently, three balloon tamponade devices are commercially available: the Linton-Nachlas, the Sengstaken-Blakemore, and the Minnesota tubes. In contrast to the Sengstaken-Blakemore and Minnesota tubes, the Linton-Nachlas tube is a single-balloon device that consists of a gastric balloon and two ports (esophageal and gastric) for aspiration and lavage. Because placement of these tubes remains a relatively rare procedure, most hospitals stock only one type of device. Regardless of the type of device, success rates for the control of hemorrhage with balloon tamponade tubes range from 60% to 90%.11
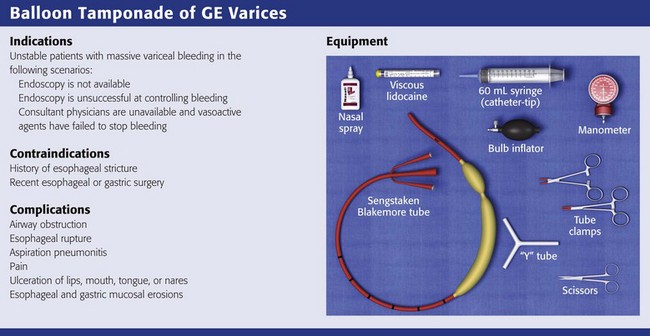
Indications
The general management of unstable patients with acute variceal bleeding is described in detail elsewhere. In brief, initial resuscitation should focus on early endotracheal intubation; circulatory resuscitation, including blood transfusion and administration of vasoactive agents and antibiotics; and early endoscopy. Vasoactive agents should be given as soon as possible in cases of confirmed or suspected variceal hemorrhage. Vasoactive medications reduce portal pressure and have been shown to decrease or stop variceal bleeding.12–17 Somatostatin and its synthetic analogue octreotide decrease release of the vasodilator hormone glucagon, thereby indirectly resulting in splanchnic vasoconstriction and reduced portal blood flow. Vasopressin and its synthetic analogue terlipressin are direct vasoconstrictors and can be given systemically or locally during angiography. These two medications, however, can cause significant coronary, cerebral, and splanchnic ischemia and are typically used in patients who fail somatostatin or octreotide therapy.
Endoscopy by a gastroenterologist remains the “gold standard” for the diagnosis and treatment of acute variceal hemorrhage.2 Sclerotherapy and band ligation are the two endoscopic techniques used to control bleeding esophageal or gastric varices. Endoscopic band ligation has been shown to be superior to sclerotherapy in initially controlling hemorrhage and improving survival.18 In fact, endoscopic band ligation is considered the treatment of choice for esophageal varices.2,18,19
It is important to recognize that balloon tamponade is only a temporizing measure. Even though success rates in controlling the initial hemorrhage are high, up to 50% of patients rebleed when the device is deflated.20 Although rebleeding rates can be reduced with the concomitant use of vasoactive agents, arrangements must be made for more definitive control of varices in patients with a balloon tamponade device in place.
Procedure
Patients with an acute variceal hemorrhage that requires a balloon tamponade device are critically ill. Because these patients are at high risk for vomiting, aspiration, and airway compromise, endotracheal intubation should be strongly considered in all patients before placement of a balloon tamponade tube.21 For the rare patient who is not intubated, use of soft restraints and administration of appropriate analgesia and sedation are critical for a successful procedure.
For placement of a balloon tamponade tube, begin by testing the esophageal and gastric balloons for air leaks (Fig. 41-3, step 1). If there is any concern about a leak, submerge the balloons in water during inflation. If time permits, inflate the gastric balloon in 100-mL increments to the maximal recommended volume while measuring the pressure. Importantly, do not exceed a pressure of 15 mm Hg within the gastric balloon with each successive instillation of 100 mL. Note the pressure at full inflation of the gastric balloon. If no air leaks are detected, fully deflate the esophageal and gastric balloons and clamp the inflation ports. If the kit comes with plastic plugs, they may be used in lieu of clamps to occlude the ports and maintain deflation of the balloon during insertion (see Fig. 41-3, step 2). Once fully deflated, coat the balloons with a thin layer of water-soluble lubricating jelly.
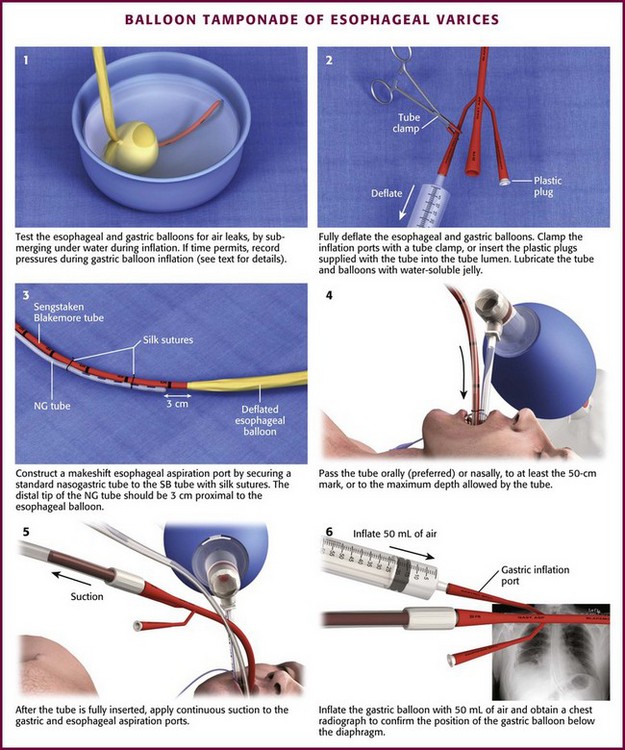
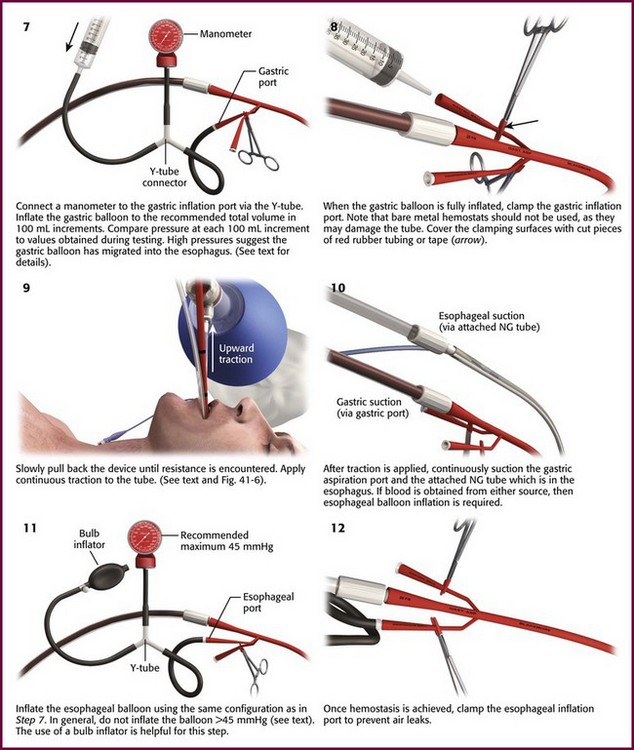
Figure 41-3 Balloon tamponade of gastroesophageal varices with the Sengstaken-Blakemore (SB) tube. NG, nasogastric.
When using the Sengstaken-Blakemore tube, it is important to recall that the device does not have an esophageal aspiration port. To construct a makeshift aspiration port, secure a nasogastric tube (NGT) to the tamponade tube with silk sutures such that the distal tip of the NGT is placed approximately 3 cm proximal to the esophageal balloon (see Fig. 41-3, step 3).
Orogastric passage of the tamponade tube is the preferred route of insertion, especially in intubated patients. Nasogastric insertion can be attempted in nonintubated patients. Pass the tube at least to the 50-cm mark and preferably to the maximum depth allowed by the length of the tube (see Fig. 41-3, step 4). After the tube is inserted, apply continuous suction to its gastric and esophageal aspiration ports (see Fig. 41-3, step 5). Inflate the gastric balloon with approximately 50 mL of air and obtain a chest radiograph to confirm that the gastric balloon is below the diaphragm (Fig. 41-4; also see Fig. 41-3, step 6). Confirm the location of the gastric balloon, which is essential to reduce the risk for esophageal rupture from inflation of a misplaced gastric balloon.
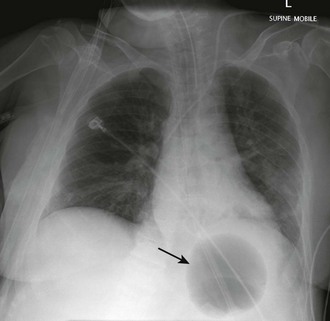
Figure 41-4 Chest radiograph showing a fully inflated gastric balloon (arrow) of a Sengstaken-Blakemore tube properly positioned under the diaphragm and in the stomach. Before inflation, obtain a radiograph to confirm that the gastric balloon is indeed in the stomach. Inflation of the gastric balloon in the esophagus can lead to esophageal rupture. (Courtesy of Dr. Frank Gaillard, www.radiopaedia.org.)
Once the location of the gastric balloon is confirmed, connect a manometer to the pressure-monitoring outlet of the gastric balloon (see Fig. 41-3, step 7). Inflate the gastric balloon to the recommended total volume of air in 100-mL increments. Compare the pressure at each 100-mL increment with the values obtained during testing of the gastric balloon. If the pressure during inflation is more than 15 mm Hg higher than the testing pressure at an equivalent volume, it is likely that the gastric balloon has migrated to the esophagus. At this point deflate the gastric balloon and advance it further into the stomach. Obtain another chest radiograph before reinflation.
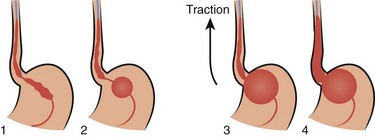
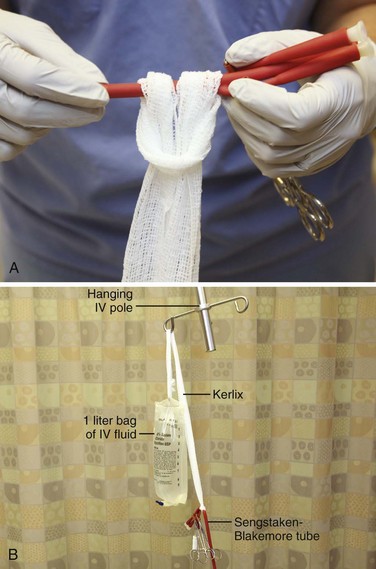
When the gastric balloon is fully inflated, clamp the inflation and pressure-monitoring ports (see Fig. 41-3, step 8). Slowly pull the device back until resistance is encountered (see Fig. 41-3, step 9). Resistance indicates that the gastric balloon has engaged the cardia and fundus of the stomach (Fig. 41-5). To maintain proper position of the gastric balloon, apply continuous traction. Accomplish this by using an overhead frame and pulley system, a football helmet or catcher’s mask, or the sponge rubber cuff (provided in most kits) for patients who underwent nasogastric insertion. Of these methods, the pulley system is preferred to deliver the recommended 0.5 to 1.0 kg of traction. If the emergency physician does not have the weights required for a pulley system, a 1-L bag of crystalloid solution conveniently provides 1 kg of traction (Fig. 41-6).
After traction is applied, connect the esophageal and gastric aspiration ports to continuous suction (see Fig. 41-3, step 10). If blood is obtained from either port, inflate the esophageal balloon to approximately 35 to 40 mm Hg. Similar to the gastric balloon, monitor inflation of the esophageal balloon with a manometer connected to the esophageal pressure–monitoring outlet (see Fig. 41-3, step 11). In general, do not inflate the esophageal balloon to more than 45 mm Hg. Keep esophageal balloon pressure at the lowest inflation pressure that achieves hemostasis. Occasionally, esophageal pressure may transiently spike to values approaching 70 mm Hg. This can occur with respiratory variation or esophageal contraction and is not indicative of overinflation. Once hemostasis is achieved, clamp the esophageal inflation port to prevent air leaks (Fig. 41-3, step 12).
Complications
Complications from balloon tamponade can be severe and occur in up to 14% of patients.20 Major complications include airway obstruction, esophageal rupture, and aspiration pneumonitis. Airway obstruction can be catastrophic and usually results from migration of a dislodged esophageal balloon into the oropharynx.10,22 Prevent proximal migration of the tube by maintaining adequate inflation of the gastric balloon, radiographic confirmation, and periodic monitoring of inflation pressure. Treat respiratory distress in nonintubated patients with a balloon tamponade device as airway obstruction until proved otherwise. In these patients use surgical scissors to cut across the lumen of the tube just distal to the inflation and aspiration ports. This will result in deflation of both balloons and allow immediate extraction of the device. Given the risk for airway obstruction, always keep surgical scissors at the bedside of patients who have a balloon tamponade device in place.
Aspiration pneumonitis can result from the aspiration of blood, oral secretions, and gastric contents and is a frequent complication of balloon tamponade.23 The incidence of pneumonitis can be decreased by evacuating the stomach and intubating the patient before placement of the tamponade device.21
Additional complications of balloon tamponade include pain; ulceration of the lips, mouth, tongue, and nares; and esophageal and gastric mucosal erosions.23 As discussed, patients with a tamponade device should receive adequate analgesia and sedation. Frequent monitoring of tube placement and pressure can decrease the incidence of esophageal or gastric mucosal erosions.
References
1. Peura, DA, Lanza, FL, Gostout, CJ, et al. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997;92:924.
2. Cardenas, A. Management of acute variceal bleeding: emphasis on endoscopic therapy. Clin Liver Dis. 2010;14:251.
3. D’Amico, G, De Franchis, R. Upper digestive bleeding in cirrhosis: post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599.
4. de Franchis, R, Primignani, M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645.
5. Chalasani, N, Kahi, C, Francois, F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653.
6. Carbonell, N, Pauwels, A, Serfaty, L, et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652.
7. Bambha, K, Kim, WR, Pedersen, R, et al. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut. 2008;57:814.
8. Westphal, K. Uber eine Kompressionsbehandlung der Blutungen aus Osophagusvarizen. Dtsch Med Wochenschr. 1930;56:1135.
9. Sengstaken, RW, Blakemore, AH. Balloon tamponage for the control of hemorrhage from esophageal varices. Ann Surg. 1950;131:781.
10. Edlich, RF, Lande, AJ, Goodale, RL, et al. Prevention of aspiration pneumonia by continuous esophageal aspiration during esophagogastric tamponade and gastric cooling. Surgery. 1968;64:405.
11. Feneyrou, B, Hanana, J, Daures, JP, et al. Initial control of bleeding from esophageal varices with the Sengstaken-Blakemore tube: experience in 82 patients. Am J Surg. 1988;155:509.
12. Gotzsche, PC, Gjorup, I, Bonnen, H, et al. Somatostatin v placebo in bleeding oesophageal varices: randomised trial and meta-analysis. BMJ. 1995;310:1495.
13. Planas, R, Quer, JC, Boix, J, et al. A prospective randomized trial comparing somatostatin and sclerotherapy in the treatment of acute variceal bleeding. Hepatology. 1994;20:370.
14. Sung, JJ, Chung, SC, Lai, CW, et al. Octreotide infusion or emergency sclerotherapy for variceal haemorrhage. Lancet. 1993;342:637.
15. Levacher, S, Letoumelin, P, Pateron, D, et al. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet. 1995;346:865.
16. Ioannou, G, Doust, J, Rockey, DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev. (1):2003. [CD002147].
17. Escorsell, A, Ruiz del Arbol, L, Planas, R, et al. Multicenter randomized controlled trial of terlipressin versus sclerotherapy in the treatment of acute variceal bleeding: the TEST study. Hepatology. 2000;32:471.
18. Laine, L, el-Newihi, HM, Migikovsky, B, et al. Endoscopic ligation compared with sclerotherapy for the treatment of bleeding esophageal varices. Ann Intern Med. 1993;119:1.
19. Garcia-Tsao, G, Sanyal, AJ, Grace, ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922.
20. Haddock, G, Garden, OJ, McKee, RF, et al. Esophageal tamponade in the management of acute variceal hemorrhage. Dig Dis Sci. 1989;34:913.
21. Mandelstam, P, Zeppa, R. Endotracheal intubation should precede esophagogastric balloon tamponade for control of variceal bleeding. J Clin Gastroenterol. 1983;5:493.
22. Butler, ML. Variceal hemorrhage: a review. Mil Med. 1980;145:766.
23. Panes, J, Teres, J, Bosch, J, et al. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices: results in 151 consecutive episodes. Dig Dis Sci. 1988;33:454.