CHAPTER 91 BACTERIAL MENINGITIS
Meningitis has long been known as a clinical disorder. Unequivocal descriptions of meningitis date to the seventeenth century,1 and tuberculous meningitis and syphilitic meningitis have been known as discreet entities for nearly as long.2 In contrast, Lyme disease is a much more recently recognized entity. Prior to the advent of antibiotics, bacterial and tuberculous meningitis were, with very rare exceptions, fatal disorders, and even today, despite antibiotic therapy, both entities carry significant mortality and appreciable morbidity. The spirochetal meningitides, in contrast, usually pursue a more protracted course, and although both syphilitic or Lyme meningitis may result in significant neurological impairment, death from either condition is unusual.
BACTERIAL MENINGITIS
Epidemiology
Approximately 15,000 cases of acute bacterial meningitis occur in the United States each year.3 The overall incidence of acute bacterial meningitis has fallen over time, however, largely due to the sharp decline in early childhood cases of H. influenzae meningitis. Schlech,4 in a review of cases of meningitis in 27 states from 1978 through 1981, noted an incidence of three cases per 100,000 of population. In contrast, Shuchat and colleagues,1 in a study encompassing acute care hospitals in 22 counties of four states, found a decline in cases of meningitis from 2.9 cases per 100,000 population in 1986 to 0.2 case in 1995. In earlier studies, the most common organisms, in descending order, were H. influenzae, Neisseria meningitidis, and Streptococcus pneumoniae. By the time of Shuchat’s report, H. influenzae accounted for only 0.2% of cases. Prior to the advent of conjugated vaccines for H. influenzae type B (Hib), the median age of meningitis cases was 15 months, whereas by 1995 the median age had risen to 25 years.1 Meningitis in developed countries has thus changed from being a disease of infants to a disease of older adults. This is in sharp contrast to developing countries in which immunization against Hib has not been used: there, as in Western countries before the advent of Hib vaccination, meningitis remains a disease of early childhood, with the greatest number of cases occurring in children from 6 months to 2 years of age.5–7 A trend worldwide over this same period of time has been the emergence of clusters of meningococcal meningitis and meningococcemia, in particular within sub-Saharan Africa, with over 300,000 cases and 30,000 deaths.8 A key event in these outbreaks has been the introduction of virulent strains of meningococci into populations without strain-specific antibody.
Causative Agents
The agents causing bacterial meningitis vary with both the age of the patient (Table 91-1) and with the route by which infection is acquired (Table 91-2).
TABLE 91-1 Agents of Acute Bacterial Meningitis According to Patient Age
| Age | Agent |
|---|---|
| Neonates | Streptococcus agalactiae: in particular type III |
| E. coli and other gramnegative organisms (Proteus mirabilis, Klebsiella, Enterobacter sp., Pseudomonas aeruginosa, Citrobacter diversus, Salmonella sp.) | |
| Listeria monocytogenes | |
| Staphylococcus epidermidis | |
| Staphylococcus aureus | |
| S. pneumoniae | |
| Childhood-early adulthood | N. meningitidis |
| S. pneumoniae | |
| S. aureus | |
| Mid-adulthood | S. pneumoniae |
| S. aureus | |
| Old age | S. pneumoniae |
| S. aureus | |
| L. monocytogenes | |
| Gram-negatives |
TABLE 91-2 Agents of Acute Bacterial Meningitis According to Route of Acquisition
| Condition | Probable Organism(s) |
|---|---|
| Sinusitis or otitis | S. pneumoniae, H. influenzae, microaerophilic and anaerobic streptococci, Bacteroides, S. aureus |
| Penetrating head trauma | S. aureus |
| Shunt infections | S. epidermidis |
| Complications of neurosurgery | Gramnegative bacteria (Klebsiella pneumonia, Acinetobacter calcoaceticus-baumannii complex, E. coli) |
Causative Agents of Meningitis by Patient Age
The most common cause of bacterial meningitis in neonates and infants is Streptococcus agalactiae (group B streptococci), followed in order of frequency by E. coli, other gram-negatives, and L. monocytogenes1,9 (see Table 91-1). Meningitis due to S. agalactiae occurs at two points in time: within the first 48 hours of the postnatal period or between 7 days and 6 weeks of age.10 Cases occurring in the immediate postnatal period represent acquisition of the agent from the mother at the time of birth, and meningitis often occurs as part of a systemic infection; cases in older infants more frequently occur as an isolated meningitis. Less common agents include Listeria monocytogenes, Staphylococcus epidermidis, Staphylococcus aureus, and S. pneumoniae. Listeria and S. aureus are discussed later. Prior to the development of an effective vaccine, H. influenzae type B was the most common cause of meningitis below the age of 6 and the most common organism overall in total number of cases. Today, H. influenzae is rarely associated with meningitis in the United States or western Europe.1,9 In areas where vaccination is not yet widely used, however, H. influenzae remains a major cause of disease. Meningitis in older children and young adults is usually caused by N. meningitidis or, less frequently, S. pneumoniae. In older adults, S. pneumoniae causes over 50% of cases, with the incidence of meningitis due to gram-negatives, Listeria, and S. aureus rising in later life.
Causative Agents According to Route of Infection
Here, meningitis is the result of extension or introduction of organisms into the subarachnoid space, by spread through emissary veins from infected sinuses or other cranial structures, penetrating trauma, or neurosurgical procedures (see Table 91-2). The most common agent associated with cases of sinusitis or otitis is S. pneumoniae. In this setting, H. influenzae may cause meningitis in adults as well as children, and infection may also be caused by anaerobic or microaerophilic organisms or by S. aureus.11 S. aureus is particularly associated with penetrating trauma. S. epidermidis tends to be associated with infection of ventricular shunts.12 S. aureus, S. epidermidis, and gram-negatives may cause meningitis in the setting of neurosurgical procedures.
Causative Agents in Immunocompromised Patients
The organisms associated with bacterial meningitis in immunocompromised patients vary with the type of immune deficiency. Individuals with defects of cell-mediated immunity, as in AIDS or Hodgkin disease, have an increased prevalence of meningitis due to L. monocytogenes.13–15 Patients with defects of humoral immune response—and patients who have undergone splenectomy—are prey to fulminant meningitis with S. pneumoniae, H. influenzae type B, and, less frequently N. meningitidis. Patients with neutropenia are susceptible to meningitis caused by Pseudomonas aeruginosa and by gramnegative enteric bacteria.16
Meningitis Due to L. monocytogenes and S. aureus
These agents deserve specific comment. L. monocytogenes differs from the other organisms previously listed in that it is an obligate intracellular parasite whose control depends on T cell–mediated immunity as well as opsonization. For this reason, L. monocytogenes appears as a cause of meningitis in three groups of patients: very young infants, especially in the setting of prematurity; patients who are immunocompromised; and the very elderly.17 S. aureus is the major agent associated with meningitis in patients with penetrating head trauma.16 In addition, however, S. aureus is a cause of meningitis in a minority of patients of all ages. Conditions in which S. aureus meningitis is particularly likely include known meningitis in the setting of endocarditis, intravenous drug abuse, burns, or superficial or deep abscesses or decubiti.
Pathogenesis and Pathology
The pathogenesis of bacterial meningitis involves three separate steps: colonization of the host, entry of bacteria across the blood-brain barrier, and development of infection within the subarachnoid space and ventricular system. Once established, bacteria may invade the central nervous system to cause meningitis via one of three routes. In the majority of cases of meningitis, colonization of the nasopharynx is a key initial event.18 Studies by Kim and coworkers19 indicate that entry of E. coli across the blood-brain barrier requires a high level of bacteremia, followed by entry of the organism into brain microvascular endothelial cells and movement across these cells into the subarachnoid space. Less frequently, meningitis may be caused by direct spread of agents from infected pericranial structures such as sinuses, middle ear, or mastoid via emissary veins. Bacteria may also enter the subarachnoid space directly, either through introduction of bacteria during penetrating trauma or, occasionally, via entry of organisms through congenital or acquired defects in the skull or spinal column.20–22
The ability of blood-borne bacteria to invade the meninges is determined to a considerable degree by properties of the bacterium itself, and many of the organisms associated with meningitis have a particular ability to adhere to mucous membranes, promoting their entry into the host’s bloodstream.20,23 Thus, most cases of S. agalactiae meningitis are associated with type III organisms; the majority of cases of E. coli meningitis are caused by those bearing the K1 capsular antigen24; virtually all cases of H. influenzae meningitis are caused by type b strains; and many cases of meningococcal meningitis are associated with those bearing groups B and C antigens. The reasons for the association of these serotypes with meningitis are not completely understood, but in each of these cases, the bacterial capsule contains sialic acid, making the organisms more closely resemble host cell surfaces; furthermore, there is antigenic cross-reactivity between the K100 antigen of E. coli, the capsular antigens of H. influenzae b, and those of group B N. meningitidis.20,23,25 Although more than 90 strains of S. pneumoniae are known to exist, only 18 of these are commonly associated with meningitis.
Despite pathogenesis studies extending back over 50 years, the actual routes by which bacteria penetrate the blood-brain barrier are still not fully defined. S. pneumoniae has been shown in vitro to bind to isolated human endothelial cells and to be detected in vacuoles within cells, suggesting that the organism may cross the blood-brain barrier via transcellular spread. Work in experimental animals with both E. coli and N. meningitidis suggests that binding of bacterial fimbriae is important in adherence of organisms to the luminal surfaces of brain microvascular endothelial cells. Transformation of organisms to nonfimbriated forms may then enhance transmural spread into the subarachnoid space.19,20,23 In vitro data suggest that neuroinvasion by E. coli involves interaction of bacterial proteins with cellular proteins present in brain but not systemic endothelia.19,26,27 In addition, certain brain regions, such as the choroid plexus, lack tight junctions, and meningitis of hematogenous origin might also begin with the entry of bacteria into brain ventricles across these structures. This hypothesis is supported both by morphological studies and by the frequent occurrence of ventriculitis as a concomitant of meningitis.28
Once within the subarachnoid space, bacteria multiply in an environment that is devoid of complement or leukocytes, and the initial stage of meningitis is essentially noninflammatory.28 Endotoxin, teichoic acid, and other products released from bacteria, however, elicit a brisk inflammatory response. This response includes not only leukocytes but also tumor necrosis factor and other chemical mediators of inflammation that alter blood-brain barrier permeability. Cerebrospinal fluid (CSF) protein concentrations rise and glucose characteristically falls, due not only to consumption of glucose by bacteria but also to altered transport of glucose across brain capillaries. Inflammation within the subarachnoid space is often accompanied by cortical encephalitis and ventriculitis, at times with hydrocephalus. Spread of inflammation into superficial areas of the brain may result in thrombosis of superficial cerebral vessels.29,30 This combination of factors results in vasogenic as well as cytotoxic cerebral edema, often complicated by arterial, venous, and capillary thrombosis, resulting in focal infarction, hemorrhage, or both. Death during the acute stages of bacterial meningitis is almost always due to brain herniation, resulting from cerebral edema and, in some cases, accompanying hydrocephalus. Delayed death or neurological disability in meningitis results from the combined effects of direct brain involvement, vascular compromise, and elevated intracranial pressure. In the process, any of several cranial nerves may be affected, in particular cranial nerve VIII. A major challenge in bacterial meningitis is that antibiotic therapy has no immediate effect on this cascade of events.
Meningitis is often viewed as a disease involving solely the meninges and, in some cases, the ventricular ependyma. The disease, however, is considerably more complex and destructive and may include communicating or obstructive hydrocephalus, cerebritis, vasculitis with axonal injury and/or arterial or venous infarcts, and actual myelitis.30–32 Actual cortical abscesses have been described in gramnegative meningitis and also may occur in cases of meningitis due to other disorders. Suppurative labyrinthitis has been described in experimental S. pneumoniae meningitis and may account for the hearing loss seen in meningitis. S. pneumoniae meningitis may also be accompanied by myelitis.30
Clinical Features
Bacterial meningitis typically presents in one of three ways.33,34 Most commonly, meningitis is preceded by 3 to 5 days of insidiously progressive symptoms of fever, malaise, irritability, or vomiting. In a smaller number of cases, meningitis develops over 1 to 2 days. In a minority of cases, bacterial meningitis begins fulminantly and may be so rapid that it remains one of the few neurological conditions capable of causing death within hours in an otherwise healthy young person. Typical symptoms are fever, headache, photophobia, and changes in mental status. Patients may or may not complain of neck stiffness, and some patients, in particular those with meningitis due to S. aureus, may complain of back pain. Seizures may occur early in meningitis in up to 40% of affected children and may also occur in adults. Presentation with focal seizures or focal neurological symptoms, however, should raise concern of brain abscess or other localized process.
Physical examination typically reveals fever, tachycardia, and nuchal rigidity.16,33 Many patients will also have altered mental status. Presentation in coma, seen in up to 12% of patients, is an ominous prognostic sign. Papilledema may be present in severe cases. Papilledema develops over time (often over 24 hours), however, and the rapid progression of meningeal infection may result in severely increased intracranial pressure before papilledema has had a chance to appear. In many patients, evidence of systemic infection is also present. Particular attention should be paid to identifying cutaneous rashes, petechiae, or purpura suggestive of meningococcemia, pulmonary consolidation, which may be present in S. pneumoniae meningitis, or cardiac murmurs suggesting endocarditis.
Tests of Meningeal Irritation
The classic tests for bacterial meningitis are resistance to passive flexion of the neck (nuchal rigidity), Kernig’s sign, and Brudzinski’s sign. Kernig’s sign represents resistance to passive extension of the leg at the knee. Brudzinski developed several tests of meningeal irritation, but the maneuver most commonly referred to as Brudzinski’s sign involves spontaneous flexion of the hips and knees when the neck is passively flexed. Both signs are strongly suggestive of meningeal irritation. Brudzinski’s sign is the more sensitive of the two, in particular where the observer takes pain to note even a slight degree of spontaneous flexion. Both signs, however, were developed long prior to the advent of antibiotic therapy, when meningitis was frequently advanced at the time of presentation, and both may be absent early in the course of illness. In awake patients, a more sensitive test is to ask the patient to put the chin on the chest with the mouth closed, because patients experiencing pain on flexion may hold the neck still but touch the chin to the chest by opening the jaw widely. Perhaps the most sensitive test of nuchal rigidity is a test developed during days of epidemic polio and involves asking the patient either to kiss the knee or to touch his or her forehead to the knee: this test will often detect meningeal irritation at a time when the other tests are negative. One should keep in mind that very elderly patients with extensive cervical spine disease may have neck stiffness, and occasional patients with influenza and severe myalgias may also complain of neck pain. In both cases, pain and resistance to movement occur not only on flexion but also on turning the head, whereas in meningitis, one can usually turn the head even if neck stiffness to flexion is present.
Atypical Presentations of Meningitis
Signs of meningeal irritation are often absent in four groups of patients: neonates, immunocompromised patients, the elderly, and patients with meningitis related to neurosurgical procedures.16 Neonates often do not exhibit nuchal rigidity, and the presence of meningitis may be signaled only by tachypnea, apneic spells, changes in heart rate, atypical seizures, or simply vague decline.10 The typical high-pitched “meningeal cry” may or may not be present. Similarly, the presence of a bulging fontanel is a late sign, indicating significantly increased intracranial pressure. Because the presentation of meningitis in neonates and infants may be so atypical, the threshold for lumbar puncture must be very low. Immunocompromised individuals, like neonates, may not develop fever or nuchal rigidity. Alcoholics, in particular those presenting in the setting of severe inebriation, may also have meningitis without clearly detectable signs. Meningitis may also be deceptively asymptomatic in the elderly, and the only sign of meningitis may be confusion in a previously alert older patient or altered responsiveness in a patient who is already demented.35,36 In these patients, threshold for lumbar puncture should also be low. However, alcoholics and elderly patients are also at risk for falls and subdural hematomas; and immunosuppressed patients may also have brain abscesses or other space-occupying lesions. In such patients, it may thus be prudent to begin appropriate antibiotics presumptively and obtain appropriate head imaging (magnetic resonance imaging [MRI] or head computed tomography [CT] scanning) before performing the lumbar puncture. The onset of bacterial meningitis following neurosurgical procedures may also be insidious, developing over hours or days. Patients in this setting are at increased risk, because alteration of consciousness or neck stiffness may be attributed to the preceding surgical intervention, and CSF cell count and chemistries are often altered by the preceding surgery.
Diagnosis
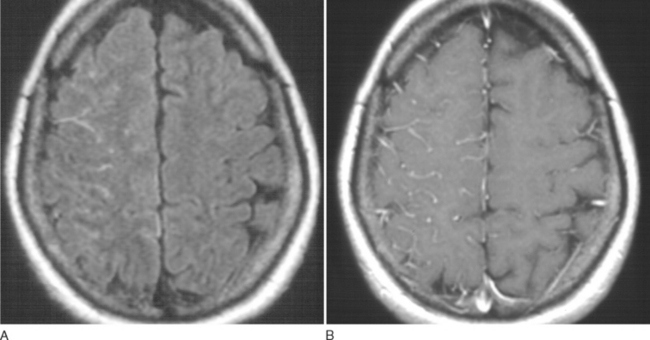
Bacterial meningitis is suspected on the basis of clinical presentation and physical findings. MRI may show cortical meningeal or meningovascular enhancement (Fig. 91-1). Definitive diagnosis, however, is almost always made on the basis of lumbar puncture. Bacterial meningitis usually produces diffuse meningeal involvement, so that relatively little brain shift may occur, even when intracranial pressure is significantly increased. Brain herniation may occur, however, if intracranial pressure is greatly increased, and the likelihood of fatal herniation cannot be predicted from CT.37,38 In severely ill patients, in whom very high intracranial pressure is suspected, the most prudent course may be to begin empirical treatment and wait until CSF pressure has been controlled before performing lumbar puncture.
Typical CSF findings are shown in Table 91-3. These include elevated pressure, fluid which is often turbid, elevated protein, depressed glucose, and elevated white blood cell count, consisting predominantly of polymorphonuclear leukocytes.39 The degree to which glucose is depressed in bacterial meningitis has been a matter of debate. Silver and Todd, in a study of 181 pediatric patients with CSF glucose levels under 50 mg/dL identified 35 patients with bacterial meningitis.40 Of these, 22 (77%) had glucose levels of 20 or below, and of 37 patients with glucose levels under 20 mg/dL, 22 (73%) had bacterial meningitis. A CSF : blood glucose ratio of less than 0.3 was highly correlated with bacterial meningitis. Bacterial meningitis was less likely if the CSF glucose levels of 20 to 50 or if the CSF/blood glucose ratio was greater than 0.30. Spanos and colleagues41 found that a CSF glucose of 18 mg/dL or less or a CSF/blood glucose ratio of less than 0.23 was associated with bacterial rather than viral meningitis in 99% of patients studied. It should be remembered, in evaluating blood glucose, that CSF glucose values will be higher in severely hyperglycemic patients and that changes in CSF glucose levels may lag 30 to 120 minutes behind those in blood. Protein levels in meningitis are a reflection of blood-brain barrier injury and usually range between 100 and 500 mg/dL.39
TABLE 91-3 Typical Cerebrospinal Fluid Findings in Bacterial Meningitis
| Opening pressure | Usually elevated |
| Fluid | Turbid |
| Cells | >100/mm3; often >1000 cells/mm2 |
| Cell type | Polymorphonuclear leukocytes |
| Protein | Elevated: usually >100 |
| Glucose | Depressed: usually <50% of blood glucose |
| Gram’s stain | Positive in 60-80% of cases |
Not all patients with bacterial meningitis exhibit the characteristic findings listed earlier.39 CSF, especially early in the course of meningitis, may have normal cell count and chemistries yet contain bacteria. Severely immunosuppressed patients may also fail to develop CSF leukocytosis. Approximately 14% of patients with bacterial meningitis will have a CSF cellular response that is predominantly lymphocytic; CSF lymphocytosis is particularly likely in neonatal meningitis and in infections caused by L. monocytogenes.39 Although a high CSF cell count, in particular, with a large percentage of polymorphonuclear leukocytes, suggests a bacterial infection, similar CSF leukocytosis with polymorphonuclear predominance may be seen in tuberculous or fungal meningitis, spirochetal meningitis, or viral meningitis. Approximately 9% of patients with bacterial meningitis have normal CSF glucose. Prior antibiotic treatment of bacterial meningitis may have little effect on CSF cell count, glucose, and protein within the first 2 to 3 days but will reduce the yield on Gram’s stain and culture.39 In some instances, prior antibiotic treatment will cause a shift from a polymorphonuclear to a lymphocytic CSF pleocytosis.
Specific identification of the infecting organism has traditionally involved Gram’s stain and bacterial culture.39 Gram’s stain provides the most rapid initial identification of the organism. Gram’s stain will be positive in approximately 25% of cases in which the CSF contains 103 colony-forming units (CFU)/mL and 97% of cases with greater than 105CFU. Errors in Gram’s stain may result from inadequate efforts to resuspend bacteria if CSF has been allowed to settle and errors in decolorization or reading of the slide. Bacterial culture and determination of antibiotic sensitivity are routine in virtually all hospital laboratories. It may be useful, however, to review culture requirements with the laboratory in advance if anaerobic infection or other unusual organisms or culture requirements are anticipated. Yield on culture can be reduced by prior antibiotic therapy.
Several adjunctive tests exist for the diagnosis of bacterial meningitis.39 Determination of lactic acid levels, in particular, D-lactate, and C-reactive protein may help differentiate bacterial from viral meningitis. Tests for bacterial antigens have proved disappointing as diagnostic tools and have been abandoned by many laboratories. Polymerase chain reaction is a promising tool in the diagnosis of meningitis.39,42 This test, which involves amplification of bacterial DNA sequences from CSF, has far greater sensitivity than do conventional culture methods and is also much less readily affected by prior antibiotic treatment. To date, the major use of polymerase chain reaction has been in the diagnosis of viral meningoencephalitides (e.g., herpes simplex encephalitis, enterovirus meningitis, or polymorphonuclear leukocytes) or of tuberculous meningitis. Several promising reports, however, have demonstrated rapid and successful use of polymerase chain reaction in meningitis due to N. meningitis, S. pneumoniae, β-hemolytic streptococci, and E. coli, including detection of penicillin-resistant strains of S. pneumoniae.42
Treatment of Acute Bacterial Meningitis
Antibiotic Therapy
Antimicrobial agents used for the treatment of bacterial meningitis must meet two requirements.16,43,44 First, they must be bactericidal for the causative agent. Second, they must be able to penetrate the blood-brain barrier to reach the infected meninges. Penetration across the blood-brain barrier is a function of three factors: the degree to which the antibiotic is protein bound, the degree to which it is lipid soluble, and the degree to which the blood-brain barrier has been disrupted by the meningitis. The first two of these factors, protein binding and lipid solubility of the antimicrobial agent, are constants for the given drug. Blood-brain permeability, however, is a function of inflammation and decreases as inflammation resolves, so that CSF antibiotic levels will often fall to some degree during treatment; as discussed later, this becomes a theoretical concern in the use of corticosteroids, because these agents may diminish antibiotic penetration into CSF at a time when high levels of antibiotics are particularly important. Blood-brain barrier integrity is also different in the neonatal period than it is in later infancy or thereafter, so that systemically administered aminoglycosides such as gentamicin will reach therapeutically effective levels in neonatal meningitis, whereas the drug will not reach bactericidal CSF levels in older infants, children, or adults.
For many years, five major groups of antibiotics have been used to treat bacterial meningitis: penicillins for infections due to S. pneumoniae and N. meningitidis; ampicillin or trimethoprim-sulfamethoxazole for L. monocytogenes; nafcillin, oxacillin, and vancomycin for infections due to S. aureus; third-generation cephalosporins such as cefotaxime and ceftazidime for gramnegative meningitis; and metronidazole for anaerobic organisms such as Bacteroides fragilis. Currently, however, the increasing prevalence of S. pneumoniae resistant to both penicillin and cephalosporins dictates that selection of antibiotics for the treatment of meningitis take into account local prevalence of resistant organisms.45 Thus, where the incidence of penicillin-resistant S. pneumoniae is known to be low, one might safely use ceftriaxone or cefotaxime. In general, however, and in particular if there is any suspicion that one may be dealing with penicillin- or cephalosporin-resistant organisms, vancomycin should be added to the regimen as a first-line agent.46 Similarly, vancomycin, rather than nafcillin or oxacillin, should be used if methicillin-resistant S. aureus is at all a consideration. Recommendations for provisional antibiotic treatment of bacterial meningitis are shown in Tables 91-4 and 91-5.
TABLE 91-4 Provisional Antibiotic Therapy of Bacterial Meningitis According to Gram’s Stain
| Gram’s Stain | Probable Organism | Provisional Antibiotic Therapy |
|---|---|---|
| Gram-positive diplococci | S. pneumoniae | Vancomycin |
| Plus | ||
| Cefotaxime or ceftriaxone | ||
| Gram-positive cocci | S. aureus | Vancomycin |
| S. epidermidis | ||
| Streptococci | ||
| Gramnegative intracellular diplococci | N. meningitidis | Penicillin G, or ampicillin |
| Cefotaxime or ceftriaxone is also effective | ||
| Gramnegative bacilli | Enterobacteraciae (P. aeruginosa) | Cefotaxime or ceftriaxone (ceftazidime if clinical setting suggests P. aeruginosa) |
TABLE 91-5 Provisional Antibiotic Therapy of Bacterial Meningitis When Organisms Are Not Seen on Gram’s Stain
| Setting | Probable Organism | Provisional Antibiotic Therapy |
|---|---|---|
| Preterm infants | S. aureus (nosocomial) | Vancomycin plus ceftazidime |
| Gram-negatives | ||
| Infants <3 mo | Group B streptococci | Ampicillin plus |
| E. coli | ||
| Other gram-negatives | Cefotaxime or ceftriaxone: | |
| L. monocytogenes (S. aureus)* | ||
| Age 3 mo-18 yr | N. meningitidis | Cefotaxime |
| S. pneumoniae | or | |
| (H. influenzae) | ceftriaxone (vancomycin‡) | |
| (S. aureus) | ||
| Age 18-50 yr | S. pneumoniae | Cefotaxime |
| N. meningtidis | or | |
| (S. aureus) | ceftriaxone (vancomycin‡) | |
| Adults >50yr | S. pneumoniae | Ampicillin |
| L. monocytogenes§ | plus | |
| Gram-negatives (S. aureus) | cefotaxime or ceftriaxone (vancomycin‡) | |
| Meningitis in the setting of sinusitis, otitis, or known CSF leak† | S. pneumoniae | Vancomycin plus ceftazidime plus metronidazole |
| H. influenzae | ||
| Gram-negatives, including P. aeruginosa | ||
| Anaerobic or microaerophilic streptococci | ||
| B. fragilis (S. aureus*) | ||
| Head trauma, neurosurgical procedures, shunt infections | S. aureus | Vancomycin plus ceftazidime |
| S. epidermidis | ||
| Gram-negatives, including P. aeruginosa | ||
| S. pneumoniae | ||
| AIDS or other states of impaired cellular immunity | L. monocytogene§ | Ampicillin plus ceftazidime |
| Gram-negatives, including | ||
| P. aeruginosa | ||
| S. pneumoniae | ||
| S. aureus |
* S. aureus is an uncommon cause of meningitis in every group of patients except those with penetrating head trauma or neurosurgical procedures. Nonetheless, the organism causes meningitis in all patient groups, and anti-staphylococcal coverage should be added if any possibility of the organism is suspected.
† S. pneumoniae is the most common causative agent in patients with CSF leaks and with acute otitis, and in these cases one might treat simply with vancomycin and ceftriaxone or cefotaxime. In more chronic infections, however, or in cholesteatoma, there is increased likelihood of other organisms, including P. aeruginosa; here, initial treatment should include vancomycin plus ceftazidime plus metronidazole.
‡ Vancomycin should be added to the regimen of empirical therapy in regions where there is significant occurrence of S. pneumoniae resistant to third-generation cephalosporins. Rifampin should be considered if corticosteroids are used.
§ Trimethoprim-sulfamethoxazole should be used where Listeria is suspected and the patient is allergic to penicillin.
Corticosteroid Therapy in Bacterial Meningitis
The realization that neurological injury in bacterial meningitis was due in part to tumor necrosis factor and other mediators of host inflammation led to attempts to control this aspect of meningitis with intravenous corticosteroids. Work by Lebel and coworkers in 198847 and Odio and colleagues in 199148 demonstrated more rapid control of CSF inflammatory response and decreased incidence of deafness in children with H. influenzae meningitis who were treated with cefotaxime plus dexamethasone compared with cefotaxime alone. In contrast, several studies failed to detect a beneficial effect of dexamethasone in infants with S. agalactiae meningitis.49,50 However, a European cooperative trial has demonstrated the effectiveness of dexamethasone in reducing both mortality and overall unfavorable outcome in adults with acute bacterial meningitis.51 The regimen used in this study was dexamethasone, 10 mg, administered intravenously 15 to 20 minutes before or with the first dose of antibiotic and every 6 hours for 4 days. Outside of the neonatal period at least, early treatment with dexamethasone should be strongly considered in the initial treatment of acute bacterial meningitis. Although dexamethasone treatment, with its resultant influence on meningeal inflammation and blood-brain barrier permeability, may theoretically reduce antibiotic penetration into CSF, this does not seem to have been of actual clinical importance.
Other Complications of Bacterial Meningitis Requiring Treatment
Bacterial meningitis may be accompanied by a variety of neurological and systemic complications. Meningitis accompanying sinusitis or otitis may be accompanied by epidural abscess, subdural empyema, brain abscess, or venous sinus thrombosis, any of which may require emergent surgery. Hydrocephalus is common in bacterial meningitis. It is often transient but may require shunting in some cases. Seizures may require emergent treatment with lorazepam, phenytoin (fosphenytoin) or more aggressive therapy such as midazolam, propofol, phenobarbital or pentobarbital coma in patients who fail to respond. Hypothalamic involvement may lead to either diabetes insipidus or, more commonly, the syndrome of inappropriate antidiuretic hormone.52 Subdural effusions are common in children with meningitis; these do not usually require drainage and may be followed by CT or MRI. Bacterial sepsis and shock may be present, as may disseminated intravascular coagulation and, in the case of N. meningitidis, Waterhouse-Friederichsen syndrome with widespread hemorrhage and adrenal failure. Cases of meningitis associated with S. aureus and, less often, S. pneumoniae may be accompanied by bacterial endocarditis. Meningitis in the presence of S. pneumoniae endocarditis may be accompanied by pneumonia and by rapid destruction of the aortic valve (Austrian syndrome).
Prophylaxis for Bacterial Meningitis
The occurrence of meningitis in a given patient always raises concern about the risk of meningitis in family members or other close contacts, and although outbreaks of meningitis within families are uncommon, temporary nasal carriage occurs frequently with S. pneumoniae, N. meningitidis, and H. influenza. Prophylaxis is not usually an issue with close contacts of patients with S. pneumoniae meningitis. In cases of meningitis due to N. meningitidis or H. influenzae, however, consideration should be given to antimicrobial chemoprophylaxis, aimed at eliminating nasal carriage.53 As a rule, chemoprophylaxis should be administered only to individuals who frequently eat and sleep in the same dwelling as the index case, that is, family members, close associates, girlfriends, or boyfriends. The most frequently used regimen for prophylaxis involves rifampin or ceftriaxone (Table 91-6). Ciprofloxacin, ofloxacin, and azithromycin have also been used in adults but are not used in children. Although not used for immediate prophylaxis, the role of vaccination in the prevention of H. influenzae meningitis is well established. Initial work suggests that immunization against S. pneumoniae or N. meningitidis are as effective in reducing the incidence of meningitis.50
TABLE 91-6 Regimens of Antimicrobial Prophylaxis for Contacts of Meningitis Cases Caused by N. meningitidis or Haemophilus influenzae
| Antimicrobial | Adults | Children |
|---|---|---|
| Rifampin | 600 mg twice daily × 2 days | N. meningitidis: 10 mg/kg twice daily for 2 days |
| H. influenzae: 20 mg/kg once daily for 4 days | ||
| Ceftriaxone (intramuscular) | 250 mg intramuscular; one dose | 125 mg intramuscular; one dose |
| Ciprofloxacin | 500-750 mg by mouth; one dose | Not recommended for children |
| Azithromycin | 500 mg by mouth; one dose | Not recommended for children |
Adapted from Peltola et al.: Infect Dis Clin N Am 1999; 13:684-710.
Prognosis
Bacterial meningitis remains a disease with significant mortality and morbidity.21,30,54 Mortality in several studies has been in the range of 17% for individuals under 60 years of age but up to 37% in individuals above 60. In the study by Kastenbauer and Pfister,30 21 of 87 adult patients with pneumococcal meningitis died (24.1% mortality), 9 of these because of brain herniation and 12 because of cardiocirculatory or multiorgan failure, often in the setting of neuroradiological evidence of severe, irreversible cerebral injury. Fewer than one half of the patients studied (48.3%) made a good recovery. Level of consciousness is a major prognostic factor.30 Prognosis is worse in the elderly.30,54 In many studies, seizures have been associated with increased mortality. Elevated CSF pressure or, more accurately, brain perfusion pressure (brain perfusion pressure = systemic blood pressure—intracranial pressure) is an adverse prognostic sign.55,56 Other factors associated with adverse outcome have included immunosuppression, the presence of a high CSF protein level or cell count, severely depressed glucose, and, in some series, failure to generate a CSF leukocyte response.54 Asplenic patients have a worse prognosis in pneumococcal meningitis, as they do in invasive pneumococcal disease overall.30 Although meningococcal meningitis may pursue a fulminantly lethal course, mortality in most series is worse for pneumococcal meningitis than for meningococcal meningitis.
MENINGITIS ASSOCIATED WITH TUBERCULOSIS, SYPHILIS, AND LYME DISEASE
Tuberculous Meningitis
Pathogenesis
The pathogenesis of tuberculous meningitis was elucidated in 1933 by Rich and McCordock,57 who found that the initial event in tuberculous meningitis was rupture of a subpial or subependymal granuloma into the subarachnoid space or ventricles. Classically, tuberculosis meningitis was a disease of children and occurred as a complication of miliary tuberculosis, having its onset during the initial weeks or months after primary infection, before effective containment of organisms.58–61 At present, tuberculosis is more frequently a complication of reactivated infection. Reactivated infection most often begins in the lungs or systemic organs; in such cases, the diagnosis of tuberculosis may be made by chest radiography, detection of M. tuberculosis in urine, or (as was done historically) detection of the agent in gastric washings. In a minority of cases, however, reactivation may occur within the central nervous system itself; in this setting, tuberculous meningitis may occur with no systemic evidence of infection.62
Clinical Features
As mentioned, the onset of tuberculous meningitis is usually subacute and may also be as fulminant as that of acute bacterial meningitis. Most patients present within 2 to 3 weeks of onset. In children, the condition typically manifests with nausea, vomiting, headache, and change in mental status. Initial misdiagnoses include acute otitis media and a variety of abdominal complaints. Seizures, although occurring in up to 50% of children during their clinical course, are a presenting feature in only 10% to 20% of cases. Adults more frequently present with headaches, malaise, and, at times, behavioral changes. A system of staging introduced by the British Medical Research Council in 1947 has been widely used. In stage I, individuals exhibit nonspecific symptoms, without clouding of consciousness or neurological deficits. Patients in stage II exhibit clouding of consciousness, signs of meningeal irritation, and minor neurological deficits, including cranial nerve palsies. Stage III is characterized by coma, seizures, abnormal movements, and/or severe neurological deficits.63 Many patients present with stage II disease, however, and in some patients, the course of illness is so rapid that presentation is at stage III. Patients presenting with tuberculous meningitis may or may not exhibit signs of meningeal irritation. In most cases, a prior history of tuberculosis is not obtained, nor may patients always give a history of prior exposure.
Diagnosis
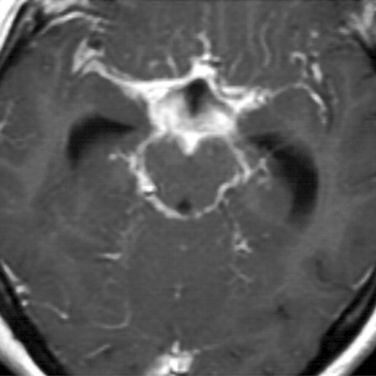
Diagnosis of tuberculous meningitis may be difficult.39,64 Evidence of systemic tuberculosis may or may not be present. MRI in some but not all cases may show a characteristic basilar meningitis (Fig. 91-2). The tuberculin skin test, where both first- and second-strength reagents are used, may be positive in up to 85% to 90% of children but is positive in only 35% to 65% of adults. CSF findings vary greatly but characteristically show a mixed pleocytosis with lymphocytic predominance, low glucose (which may develop before cells are present), and elevated protein. Mean glucose values in most studies have ranged between 18 and 40 mg/dL; protein levels have usually been in the range of 150 to 250 mg/dL.39 Detection of organisms may also prove difficult. Although some laboratories have reported success rates as high as 58%, most experienced laboratories have found acid fast stains to be positive in only about 30% of cases.39,65 In most community hospitals, the yield is well below 10% and may be totally unsuccessful.39 Mycobacterial cultures are positive in only about 70% cases and may require up to 6 weeks. Determination of adenosine deaminase levels in CSF has been highly diagnostic in a few series, but other studies have found the test lacking in both sensitivity and specificity.39,66 Polymerase chain reaction is emerging as a valuable tool in rapid diagnosis. At present, however, the yield of polymerase chain reaction is 50% to 70%.39,67,68
Treatment
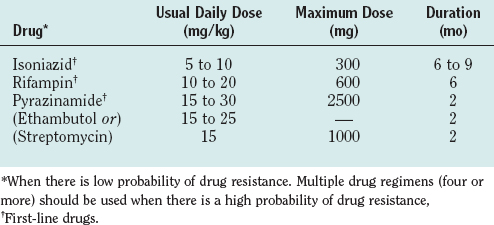
Because of its rapid and destructive course and because diagnostic tests are limited, tuberculous meningitis should be treated on suspicion. Current World Health Organization recommendations are that cases be treated with INH, rifampin, pyrazinamide, and ethambutol for 2 months followed by 6 to 7 months of INH and rifampin. Others, however, use considerably longer periods of treatment (12 to 24 months) depending on the severity of infection at the time of presentation (Table 91-7). When multidrug resistance is suspected, a four- to seven-drug regimen is used, and streptomycin may be added.
Corticosteroids (prednisone or dexamethasone) may be added in cases manifesting with stupor, coma, or neurological deficits. Their use may be lifesaving; paradoxically, however, steroid-treated survivors may have a higher incidence of neurological deficits.69 Surgery, including ventricular shunting, may be required to treat obstructive hydrocephalus or to manage intraparenchymal tuberculomas. Earlier work by Schoeman and colleagues70 suggests that nonobstructive hydrocephalus may be managed by the addition of furosemide and acetazolamide to the antituberculous regimen.
Prognosis
Mortality in tuberculous meningitis is influenced heavily by clinical status at presentation. Both are low in patients presenting with stage I disease; in patients presenting with stage III disease, however, mortality is 30%.71 Mortality is higher in the very young, the very old, and patients with miliary disease. Individual studies have also associated higher mortality with pregnancy, positive CSF cultures, markedly elevated protein concentrations, and very low CSF glucose levels. Long-term cognitive changes have been observed in children with tuberculous meningitis, correlating with the clinical stage at presentation.72 The course of disease in patients with HIV infection is similar to that in non–HIV-infected individuals; in this setting, however, duration of illness for more than 14 days is a poor prognostic sign, as is a CD4+ cell count of less than 200/mm3.73
Syphilitic Meningitis
The neurological complications of syphilis acquired after birth have traditionally been divided into three major categories: syphilitic meningitis (meningovascular syphilis), parenchymal neurosyphilis (general paresis), and tabes dorsalis.74,75 The clinical presentation of the disease has been highly variable, however, and in days prior to penicillin therapy, neurosyphilis was considered to account for roughly 10% of neurological consultations and 25% of psychiatric hospitalizations. The observed clinical features of patients presenting with syphilis changed greatly following the introduction of penicillin therapy76 and with the advent of AIDS.77,78
Central nervous system invasion is common in systemic syphilis and, in the preantibiotic era, occurred in 60% to 70% of patients with secondary syphilis. In contrast, true, symptomatic syphilitic meningitis—as opposed to meningovascular or parenchymatous neurosyphilis—is an uncommon disorder, ranging from 0.2% to 1.6% of cases of primary or secondary syphilis.79 Syphilitic meningitis most commonly occurs within 1 year of initial infection, often during secondary syphilis, but has also been observed to develop many years after primary infection.79 Symptoms and signs have usually resembled those seen in viral meningitis, with the exception that patients may also develop optic neuritis or perineuritis, chorioretinitis, or retinal vasculitis.78 CSF may at times be normal. More usually, however, CSF contains a predominantly lymphocytic pleocytosis that is usually less than 300 cells/mm3 but may be as high as 1500 cells/mm3. Protein may be elevated to as high as 250 to 300 mg/dL. Glucose is usually normal but may be depressed.75,79 These values may be significantly blunted in patients with AIDS. On occasion, in non-AIDS patients, syphilitic meningitis may manifest acutely, with symptoms and signs resembling acute bacterial meningitis, with neutrophilic predominance, and with depressed glucose.80 There is no universally successful test for the diagnosis of syphilitic meningitis. Diagnosis is suggested by positive serum rapid plasma reagin (RPR) and fluorescent treponemal antibody-absorption (FTA-ABS) determinations and by the presence of a positive Venereal Disease Research Laboratory (VDRL) test on CSF. VDRL is positive in approximately 70% of patients.81 In patients with negative CSF VDRL, diagnosis may be made by the presence of CSF-FTA-ABS or serum FTA-ABS in the setting of a CSF pleocytosis.81,82 Treatment is usually with aqueous crystalline penicillin G; 18 to 24 million units intravenously per day for 10 to 14 days is most commonly used. Intramuscular procaine penicillin plus probenecid has also been used in adults but is contraindicated in individuals severely allergic to sulfa. Limited experience exists with ceftriaxone, 2 g intravenously per day. Patients with HIV are at greater risk of relapse and may warrant more careful follow-up.
Lyme Meningitis
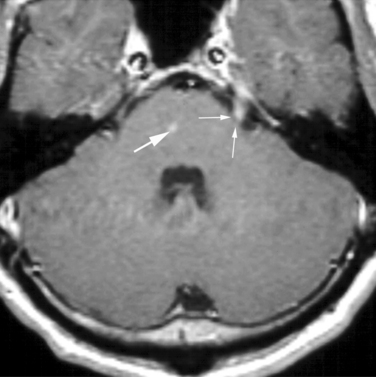
The agent of Lyme disease, Borrelia bergdorferi, is endemic in North America, Europe, and Asia, its distribution corresponding with that of ticks of the Idoxes genus. The organism produces a highly variable systemic illness83,84 as well as protean reported neurological manifestations, which include encephalitis, encephalopathy, myelopathy, cranial neuritis, radiculitis, plexopathies, and Guillain-Barré syndrome.83,85 Neurological involvement has been reported in 5% to 20% of North American patients and a higher percentage of patients in Europe. The frequency of meningeal symptoms has been reported to range from 30% to 90% of patients and is more common in children; the meningitis may occur alone or in combination with the other complications of neuroborreliosis listed earlier. Symptoms include headache, myalgias, athralgias, and weight loss. Lyme meningitis may spontaneously remit but may also recur. Gadolinium-enhanced MRI may show enhancement of meninges or of cranial or spinal nerve roots (Fig. 91-3). CSF findings in Lyme meningitis are similar to those in viral meningitis, with lymphocytic pleocytosis, mild to moderate elevation of protein, and normal glucose. Presentation as an acute purulent meningitis has also been reported but is rare.86 Diagnosis is made by positive serum serology (enzyme-linked immunosorbent assay, confirmed by Western blot analysis). Detection of CSF antibody and of intrathecal antibody production is occasionally helpful, but the yield of CSF antibodies in individuals who have negative serum studies is less than 1%.87 Direct tests for Borrelia DNA or proteins have not proved reliable. Treatment of choice for Lyme meningitis is ceftriaxone, 2 g intravenously daily for 2 to 4 weeks. Cefotaxime, 2 g intravenously every 8 hours for 2 to 4 weeks has also been used.
Cadavid D. Lyme disease and relapsing fever. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. Philadelphia: Lippincott Williams & Wilkins; 2004:659-690.
de Gans J, van de BD. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-1556.
Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain. 2003;126:1015-1025.
Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4:435-440.
Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741-1751.
van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-1859.
1 Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337:970-976.
2 Whytt R. Observations on the nature, causes, and cure of those disorders which are commonly called nervous, hypoochondriac, or hysteric. In: Robinson DN, editor. Significant Contributions to the History of Psychiatry. Washington, DC: University Publications of America; 1978:551.
3 Roos KL, Tunkel AR, Scheld WM. Acute bacterial meningitis. In: Scheld WM, Whitney CG, Marra CM, editors. Infections of the Central Nervous System. Philadelphia: Lippincott Williams & Wilkins; 2004:347-422.
4 Schlech WFIII. The epidemiology of bacterial meningitis. Antibiot Chemother. 1992;45:5-17.
5 Asturias EJ, Soto M, Menendez R, et al. Meningitis and pneumonia in Guatemalan children: the importance of Haemophilus influenzae type b and Streptococcus pneumoniae. Rev Panam Salud Publica. 2003;14:377-384.
6 Kojouharova M, Gatcheva N, Setchanova L, et al. Epidemiology of meningitis due to Haemophilus influenzae type b in children in Bulgaria: a prospective, population-based surveillance study. Bull World Health Organ. 2002;80:690-695.
7 Mwangi I, Berkley J, Lowe B, et al. Acute bacterial meningitis in children admitted to a rural Kenyan hospital: increasing antibiotic resistance and outcome. Pediatr Infect Dis J. 2002;21:1042-1048.
8 Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2:721-736.
9 Gold R. Epidemiology of bacterial meningitis. Infect Dis Clin North Am. 1999;13:515-525.
10 Pong A, BradLey JS. Bacterial meningitis and the newborn infant. Infect Dis Clin North Am. 1999;13:711-1734.
11 Giannoni C, Sulek M, Friedman EM. Intracranial complications of sinusitis: a pediatric series. Am J Rhinol. 1998;12:173-178.
12 Bayston R. Hydrocephalus shunt infections. J Antimicrob Chemother. 1994;34(Suppl A):75-84.
13 Levidiotou S, Charalabopoulos K, Vrioni G, et al. Fatal meningitis due to Listeria monocytogenes in elderly patients with underlying malignancy. Int J Clin Pract. 2004;58:292-296.
14 Rivero GA, Torres HA, Rolston KV, et al. Listeria monocytogenes infection in patients with cancer. Diagn Microbiol Infect Dis. 2003;47:393-398.
15 Singh N, Husain S. Infections of the central nervous system in transplant recipients. Transpl Infect Dis. 2000;2:101-111.
16 Roos KL, Tunkel AR, Scheld WM. Acute bacterial meningitis in children and adults. In: Scheld WM, Durack DT, Whitley RJ, editors. Infections of the Central Nervous System. Philadelphia: Lippincott-Raven; 1997:335-401.
17 Pollock SS, Pollock TM, Harrison MJG. Infection of the central nervous system by Listeria moncytogenes: a review of 54 adult and juvenile cases. Q J Med. 1984;211:331-340.
18 Scheld WM, Koedel U, Nathan B, Pfister HW. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis. 2002;186(Suppl 2):S225-S233.
19 Kim KS. E. coli invasion of brain microvascular endothelial cells as a pathogenetic basis of meningitis. Subcell Biochem. 2000;33:47-59.
20 Leib SL, Tauber MC. Pathogenesis of bacterial meningitis. Infect Dis Clin North Am. 1999;13:527-547.
21 Hosoglu S, Ayaz C, Geyik MF, et al. Acute bacterial meningitis in adults: analysis of 218 episodes. Ir J Med Sci. 1997;166:231-234.
22 Osma U, Cureoglu S, Hosoglu S. The complications of chronic otitis media: report of 93 cases. J Laryngol Otol. 2000;114:97-100.
23 Kim KS. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci. 2003;4:376-385.
24 Schiffer MS, Oliveira E, Glode MP, et al. A review: relation between invasiveness and the K1 capsular polysaccharide of Escherichia coli. Pediatr Res. 1976;10:82-87.
25 Alkmin MG, Shimizu SH, Landgraf IM, et al. Production and immunochemical characterization of Neisseria meningitidis group B antiserum for the diagnosis of purulent meningitis. Braz J Med Biol Res. 1994;27:1627-1634.
26 Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102:347-360.
27 Cundell DR, Gerard C, Idanpaan-Heikkila I, et al. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv Exp Med Biol. 1996;416:89-94.
28 Koedel U, Pfister H-W. Models of experimental bacterial meningitis: role and limitations. Infect Dis Clin North Am. 1999;13:549-577.
29 Pfister HW, Feiden W, Einhaupl KM. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol. 1993;50:575-581.
30 Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain. 2003;126:1015-1025.
31 Kaiser AB, McGee ZA. Aminoglycoside therapy of gramnegative bacillary meningitis. N Engl J Med. 1975;293:1215-1220.
32 Nau R, Gerber J, Bunkowski S, et al. Axonal injury: a neglected cause of CNS damage in bacterial meningitis. Neurology. 2004;62:509-511.
33 Kaplan SL. Clinical presentation, diagnosis, and prognostic factors of bacterial meningitis. Infect Dis Clin North Am. 1999;13:570-594.
34 Radetsky M. Duration of symptoms and outcome in bacterial meningitis: an analysis of causation and the implications of a delay in diagnosis. Pediatr Infect Dis J. 1992;11:694.
35 Proulx N, Frechette D, Toye B, et al. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98:291-298.
36 Choi C. Bacterial meningitis in aging adults. Clin Infect Dis. 2001;33:1380-1385.
37 Rennick G, Shann F, de Campo J. Cerebral herniation during bacterial meningitis in children. Brit Med J. 1993;306:953-955.
38 Winkler F, Kastenbauer S, Yousry TA, et al. Discrepancies between brain CT imaging and severely raised intracranial pressure proven by ventriculostomy in adults with pneumococcal meningitis. J Neurol. 2002;249:1292-1297.
39 Greenlee JE, Carroll KC. Cerebrospinal fluid in central nervous system infections. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. Philadelphia: Lippincott Williams & Wilkins; 2004:6-30.
40 Silver TS, Todd JK. Hypoglychorrhachia in pediatric patients. Pediatrics. 1976;58:67-71.
41 Spanos A, Harrell FE, Durack DT. Differential diagnosis of acute meningitis, an analysis of the predictive value of initial observations. JAMA. 1989;262:2700-2707.
42 du Plessis M, Smith AM, Klugman KP. Rapid detection of penicillin-resistant Streptococcus pneumoniae in CSF by a seminested-PCR strategy. J Clin Microbiol. 1998;36:453-457.
43 Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708-716.
44 Tunkel AR, Scheld WM. Treatment of Bacterial Meningitis. Curr Infect Dis Rep. 2002;4:7-16.
45 Spach DH. New issues in bacterial meningitis in adults. Antibiotic resistance has complicated treatment. Postgrad Med. 2003;114:43-50.
46 Chang WN, Lu CH, Wu JJ, et al. Staphylococcus aureus meningitis in adults: a clinical comparison of infections caused by methicillin-resistant and methicillin-sensitive strains. Infection. 2001;29:245-250.
47 Lebel MH, Freij BJ, Syrogiannopoulos GA, et al. Dexamethasone therapy for bacterial meningitis. Results of two doubleblind, placebo-controlled trials. N Engl J Med. 1988;319:964-971.
48 Odio CM, Faingezicht I, Paris M, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324:1525-1531.
49 Daoud AS, Batieha A, Al Sheyyab M, et al. Lack of effectiveness of dexamethasone in neonatal bacterial meningitis. Eur J Pediatr. 1999;158:230-233.
50 Williams AJ, Nadel S. Bacterial meningitis: current controversies in approaches to treatment. CNS Drugs. 2001;15:909-919.
51 de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-1556.
52 Kaplan SL, Fishman MA. Supportive therapy for bacterial meningitis. Pediatr Infect Dis J. 1987;6:670-677.
53 Peltola H. Prophylaxis of bacterial meningitis. Infect Dis Clin North Am. 1999;13:685-710.
54 van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-1859.
55 Goh D, Minns RA. Cerebral blood flow velocity monitoring in pyogenic meningitis. Arch Dis Child. 1993;68:111-119.
56 Lindvall P, Ahlm C, Ericsson M, et al. Reducing intracranial pressure may increase survival among patients with bacterial meningitis. Clin Infect Dis. 2004;38:384-390.
57 Rich AR, McCordock HA. The pathogenesis of tuberculous meningitis. Bull Johns Hopkins Hosp. 1933;52:5-37.
58 Dube MP, Holtom PD, Larsen RA. Tuberculous meningitis in patients with and without human immunodeficiency virus infection. Am J Med. 1992;93:520-524.
59 Kennedy DH, Fallon RJ. Tuberculous meningitis. JAMA. 1979;241:264-268.
60 Lincoln EM, Sordillo SVR, Davies PA. Tuberculous meningitis in children: a review of 167 untreated and 74 treated patients with special reference ot early diagnosis. J Pediatr. 1960;57:807-823.
61 Sutlas PN, Unal A, Forta H, et al. Tuberculous meningitis in adults: review of 61 cases. Infection. 2003;31:387-391.
62 Slavin RE, Walsh TJ, Pollack AD. Late generalized tuberculosis: a clinical pathologic analysis and comparison of 100 cases in the preantibiotic and antibiotic eras. Medicine (Baltimore). 1980;59:352-366.
63 British Medical Research Council. Streptomycin treatment of tuberculous meningitis. Lancet. 1947;1:582-596.
64 Davis LE, Rastogi KR, Lambert LC, et al. Tuberculous meningitis in the southwest United States: a community-based study. Neurology. 1993;43:1775-1778.
65 Thwaites GE, Chau TT, Farrar JJ. Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol. 2004;42:378-379.
66 Corral I, Quereda C, Navas E, et al. Adenosine deaminase activity in CSF of HIV-infected patients: limited value for diagnosis of tuberculous meningitis. Eur J Clin Microbiol Infect Dis. 2004;23:471-476.
67 Narayanan S, Parandaman V, Narayanan PR, et al. Evaluation of PCR using TRC and IS6110 primers in detection of tuberculous meningitis. J Clin Microbiol. 2001;39:2006-2008.
68 Caws M, Wilson SM, Clough C, et al. Role of IS6110-targeted PCR, culture, biochemical, clinical, and immunological criteria for diagnosis of tuberculous meningitis. J Clin Microbiol. 2000;38:3150-3155.
69 Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741-1751.
70 Schoeman J, Donald P, van ZL, Keet M, et al. Tuberculous hydrocephalus: comparison of different treatments with regard to ICP, ventricular size and clinical outcome. Dev Med Child Neurol. 1991;33:396-405.
71 Kent SJ, Crowe SM, Yung A, et al. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993;17:987-994.
72 Schoeman CJ, Herbst I, Nienkemper DC. The effect of tuberculous meningitis on the cognitive and motor development of children. S Afr Med J. 1997;87:70-72.
73 Berenguer J, Moreno S, Laguna F, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326:668-672.
74 Merritt HH, Adams RD, Solomon HC. Neurosyphilis. New York: Oxford University Press, 1946.
75 Marra CM. Neurosyphilis. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. Philadelphia: Lippincott Williams & Wilkins; 2004:649-657.
76 Hooshmand H, Escobar RM, Kopf SW. Neurosyphilis. A study of 241 patients. JAMA. 1972;219:726-729.
77 Johns DR, Tierney M, Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987;316:1569-1572.
78 Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4:435-440.
79 Merritt HH, Moore M. Acute syphilitic menngitis. Medicine. 1935;14:119-183.
80 Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System, 2nd ed. Philadelphia: WB Saunders, 1992.
81 Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75:1727-1730.
82 Marra CM, Tantalo LC, Maxwell CL, et al. Alternative CSF tests to diagnose neurosyphilis in HIV-infected individuals. Neurology. 2004;63:85-88.
83 Coyle PK, Schutzer SE. Neurologic aspects of Lyme disease. Med Clin North Am. 2002;86:261-284.
84 Steere A. Lyme disease. N Engl J Med. 1989;321:586-595.
85 Halperin JJ. Nervous system Lyme disease. J Neurol Sci. 1998;153:182-191.
86 Bourke SJ, Baird A, Bone FJ, et al. Lyme disease with acute purulent meningitis. Brit Med J. 1988;297:460-461.
87 Cadavid D. Lyme disease and relapsing fever. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. Philadelphia: Lippincott Williams & Wilkins; 2004:659-690.