chapter 12
Back Pain and Common Leg Problems With or Without Difficulty Walking
Patients experience difficulty walking as a result of pain in their legs, and the commonest cause of pain is arthritis of the joints in the lower limbs. In the absence of pain, altered strength (which may be due to a lower motor or upper motor neuron problem) or sensation (particularly proprioception) in the lower limbs or impaired balance resulting from either a cerebellar disturbance or vestibular problem may cause difficulty walking. In the absence of pain, weakness or altered sensation, patients may experience difficulty walking related to Parkinson’s syndrome or a condition often confused with Parkinson’s that is termed apraxia of gait.
Difficulty walking that is related to central nervous system problems will be discussed in Chapter 13, ‘Abnormal movements and difficulty walking due to central nervous system problems’.
BACK PAIN
The first is principle is to establish exactly where in the back the pain is:
• Most low back (lumbar) pain is non-specific and relates to problems affecting soft tissues or is related to degenerative disease of the lumbosacral spine.
• On the other hand thoracic back pain may be more sinister:
• Patients who subsequently develop malignant cord compression often experience thoracic back pain for days or weeks prior to the onset of the neurological symptoms.
• Pain related to osteoporotic vertebral fractures is commonly in the thoracic region.
• Rarely, thoracic back pain may be the presenting symptom of a ruptured aortic aneurysm.
• If there is an associated radiculopathy (sciatica), the degenerative disease or lumbar disc disease may be the cause.
• Routine imaging early on in the course of low back pain is of no benefit [1]. An MRI scan can detect spinal metastases in patients with normal plain X-rays or CT scan [2]. A nuclear bone scan can also detect metastases in the vertebral column before cord compression but MRI is superior [3].
• In most patients with low back pain, symptoms resolve without surgical intervention:
• Physical therapy and non-steroidal anti-inflammatory drugs (NSAIDs) are the cornerstones of non-surgical treatment [4].
• Superficial heat is the only therapy with good evidence of efficacy for treatment of acute low back pain.
• Bed rest is often advocated for the treatment of acute low back pain but continuing ordinary activity may lead to a more rapid recovery [5].
• There is conflicting evidence of efficacy for spinal manipulation in low back pain [6, 7].
• Massage and acupuncture are better than no treatment but have not been compared to conventional treatment.
PROBLEMS IN THE UPPER LEG
MERALGIA PARAESTHETICA
• Symptoms may be intermittent initially, but subsequently the patient may develop permanently altered sensation.
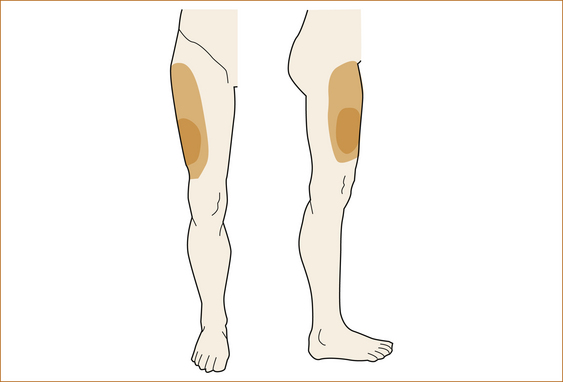
• Symptoms vary from a mildly altered sensation over the anterolateral aspect of the thigh, often only noticed when the patient touches the thigh or clothes brush up against the thigh, to a more marked alteration in sensation with persistent numbness within the distribution of the lateral cutaneous nerve of the thigh but not always involving the entire extent.
• Some patients complain of pain and others may experience dysaesthesia, an unpleasant sensation when the skin is touched. This condition is due to compression of the lateral cutaneous nerve of the thigh beneath the inguinal ligament just medial to the anterior superior iliac spine and pressure over this point is often associated with tenderness.
• The presence of weakness or an altered knee reflex excludes the diagnosis.
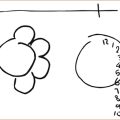
Examination: The simplest test is to stroke the skin over the lateral aspect of the thigh and, if there is altered sensation, test in all directions, mapping out the exact pattern and sensory loss. The patient is then shown Figure 12.1 and immediately identifies the problem.
Another diagnostic test is to inject local anaesthetic in the region of the lateral cutaneous nerve beneath the inguinal ligament; a transient resolution of symptoms is considered diagnostic [9].
Pain and weakness in the upper leg
DIABETIC AMYOTROPHY/FEMORAL AMYOTROPHY/LUMBOSACRAL NEURITIS
• Increasingly severe pain in the buttocks, hips and thighs developing over hours to days is the initial presenting symptom. It is most often unilateral but, as in brachial neuritis, the contralateral side may be involved, usually within 1–2 weeks.
• In addition to the pain, severe weakness and subsequently marked wasting of the quadriceps muscles occurs with or without parasthaesia in the anterior aspect of the thigh and at times the shin. The weakness can be so severe to render the patient unable to walk.
Examination: The examination reveals weakness of hip flexion and knee extension with an absent knee-jerk. Although it may occur at any age, lumbosacral neuritis is more common in the middle-aged to elderly [13].
The aetiology of these conditions is uncertain but it is thought to be based on an inflammatory vasculitis [12, 14].
POLYMYALGIA RHEUMATICA
Polymyalgia rheumatica is a condition seen in middle-aged to elderly patients.
Symptoms: It presents with aching and stiffness predominantly affecting the shoulders but in 50–70% of cases may also affect the hips [17]. The condition is thought to relate to synovitis of the proximal joints and extra-articular synovial structures. The pain is constant day and night, as opposed to osteoarthritis of the hips where the pain mainly occurs on weight bearing. It often radiates to the knee and is exacerbated by movement of the hip joints. Although initially it may be unilateral, almost invariably patients develop bilateral symptoms.
A low-grade fever, fatigue and anorexia may occur in as many as 40% of patients and the presence of high spiking fevers should alert the clinician to the possibility of an associated giant-cell arteritis affecting the aorta and major branches. This can result in constant headaches, scalp tenderness and jaw claudication (see Chapter 9, ‘Headache and facial pain’, for further discussion).
DRUG-INDUCED MUSCLE PAIN AND/OR WEAKNESS
A large number of pharmaceutical agents, including lipid lowering agents, NSAIDs, antineoplastic drugs and even over-the-counter essential amino acids, such as L-tryptophan, can result in myalgia or even myositis causing muscle pain, cramps, swelling, tenderness and weakness [19, 20]. This list is almost certain to enlarge with the advent of new therapeutic agents.
Weakness in the upper leg
Patients present with the insidious onset of proximal weakness in the legs in the absence of sensory disturbance. The weakness is bilateral but may be asymmetrical. Pain in the muscles occurs in approximately 50% of cases but, as opposed to the above two conditions, is rarely severe. Dysphagia, neck weakness and impaired respiratory function may occur [21]. Inclusion body myositis affects the long flexors of the fingers and, in the lower limbs, the extensor muscles of the knees and hip flexors, resulting in significant proximal weakness. In this latter condition the knee reflex is often absent, whereas in polymyositis and dermatomyositis the reflexes are preserved.
MUSCLE WEAKNESS DUE TO ENDOCRINE DYSFUNCTION
Hyperthyroidism, hypothyroidism and Addison’s disease may all present with muscle weakness. Usually there are associated manifestations to point to the underlying endocrine disorder. Hyperthyroidism may also present with periodic paralysis [24] or rhabdomyolysis [25] and hypothyroidism may present with muscle hypertrophy [26].
The weakness will resolve with correction of the endocrine disturbance.
PROBLEMS IN THE LOWER LEGS AND FEET
Unpleasant sensations in the feet
In the lower leg and feet patients can experience unpleasant sensations at rest that can cause difficulty walking or unpleasant sensation at rest improved by walking or, in fact, that can be improved by walking. Figure 12.2 shows an approach to patients with discomfort or unpleasant sensation in their feet. Erythromelalgia and painful legs moving toes are extremely rare.
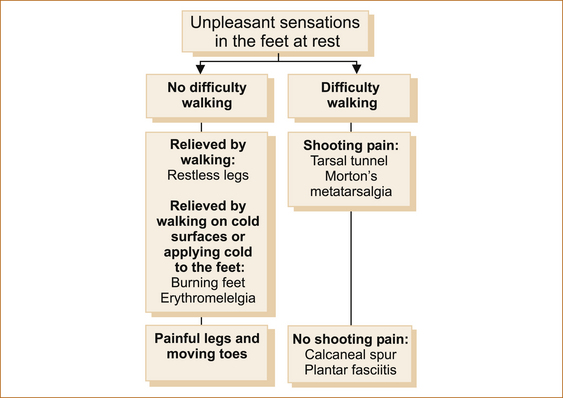
FIGURE 12.2 Suggested approach to pain in the feet
There are many non-neurological causes of pain in the feet where pain is either exacerbated or precipitated by weight bearing and/or walking. These include osteoarthritis in the ankle or joints of the feet, a calcaneal spur and plantar fasciitis. The neurological causes of pain in the feet that can result in difficulty walking due to pain include Morton’s neuroma or metatarsalgia and tarsal tunnel syndrome. All will have pain with weight bearing, but there are subtle differences in the nature of the pain and the presence of altered sensation that occurs with the neurological causes to help differentiate the various entities.
UNPLEASANT SENSATIONS AT REST THAT DO NOT CAUSE DIFFICULTY WALKING
Restless legs syndrome: Restless legs syndrome [27–31] is common, affecting 5% of the population. However, many patients complain that most doctors do not seem to be aware of the entity or know much about it. A more appropriate term is restless limbs syndrome because in more severe cases it can also affect the upper limbs and, rarely, symptoms may be confined to the upper limbs.
The essential features of RLS are:
• Patients develop symptoms when they are not moving, such as prolonged sitting at the dinner table, on an aeroplane or in a lecture or movie.
• Symptoms are particularly severe in bed at night.
• Patients find it very difficult to describe the nature of the symptoms except to say that they are unpleasant.2
• Patients cannot keep their limbs still because of this unpleasant sensation that feels deep inside the limb, not over the surface or affecting just the skin.
• The pain is in the feet, shins, calves and often seems to cross joints but is not confined to the joints.
• Symptoms persist on and off for hours until patients are forced to be up and about.
• Both legs are usually affected although the symptoms can be either confined to or more severe in one leg.
• Patients invariably pace the floor at night to obtain relief but, unlike burning feet syndrome, they do not need to walk on cold surfaces and the symptoms are not relieved by moving the feet to where the sheets are colder.
• There are no symptoms during the day unless the patient sits down.
• Sleep is disturbed whether the patient is sleeping at night or during the day.
• The neurological examination of the affected limbs should be completely normal, although sometimes this condition is associated with a peripheral neuropathy, so patients should be examined specifically looking for absent ankle reflexes, weakness of dorsiflexion of the toes and feet and possible peripheral sensory loss affecting the toes and distal aspect of the feet.
The characteristic feature of PVD is exercise-induced leg pain relieved by rest but occasionally it can cause pain at rest. The symptoms are confined to the lower limbs and upper limb symptoms would exclude this diagnosis. The pain is usually in the feet and calves; very rarely, buttock pain can occur due to involvement of the internal iliac artery. The peripheral pulses will be difficult to palpate with PVD.
Akathisia: Akathisia consists of an inner sense of restlessness with a desire to move not just the limbs but the entire body. It is only seen in patients who have been exposed to dopamine antagonists. Whereas the unpleasant sensations associated with RLS are alleviated by walking or moving the limbs, patients with akathisia cannot obtain relief with movement.
Painful legs and moving toes: Painful legs and moving toes may develop in the setting of spinal cord and cauda equina trauma, lumbar root lesions, injuries to bony or soft tissues of the feet and peripheral neuropathy; in some patients it is idiopathic [40]. It is not familial and is not thought to be related to RLS.
It consists of continuous or semi-continuous involuntary writhing movements of the toes associated with pain in the affected extremity [41]. Symptoms may begin on one side and become bilateral; movements may be momentarily suppressed by voluntary action or exacerbated by changing posture [40]. Pain preceding the movements was most commonly burning in nature. Movements consisted of flexion/extension, abduction/adduction and fanning or clawing of toes/fingers and sometimes the foot or hand [42]. Surface electromyography (EMG) showed movements suggestive of both chorea and dystonia. Movements are partially suppressible and diminished but still apparent during light sleep.
Gamma-aminobutyric acid (GABAergic) agents are most effective in controlling the pain and the movements [42].
Burning feet syndrome: Patients complain of an unpleasant burning sensation involving mainly the soles of the feet and occasionally the dorsal aspect of the feet and the legs below the knees that predominantly occurs in bed at night. They prefer to remove the bed covers, move their feet to where the sheets are colder or walk on cold surfaces to obtain some relief.
The aetiology of the syndrome is thought to be a small fibre neuropathy, and reduced intraepidermal nerve fibre density has been demonstrated on skin biopsy [43]. It has been reported in:
• diabetes mellitus or impaired glucose tolerance detected by oral glucose tolerance testing [44]
• vitamin B deficiency such as thiamine (B1), riboflavin (B2), nicotinic acid (B3), pantothenic acid (B5) and cyanocobalamin (B12)
Note: Vitamin B deficiency may cause burning feet syndrome that will respond to replacement therapy, but the evidence for this is poor [47].
Erythromelalgia: Erythromelalgia is a rare condition, of uncertain aetiology, characterised by episodic erythema, intense burning pain and warmth of the hands and/or feet. When chronic, it is associated with significant disability [50]. Severe erythromelalgia may spread up the legs and arms and even affect the ears and face. It may be unilateral or bilateral. Symptoms flare up late in the day and continue overnight.
This may not be a disease entity at all, but a syndrome of dysfunctional vascular dynamics [51]. Exposure to warmth can trigger flaring and increase its severity; symptoms are relieved by cooling in ice water. Sometimes the condition may be precipitated by immersing the affected area in hot water for 10–30 minutes [51]. Many patients have either small or large fibre neuropathy (quoted as unpublished data in the article by Kuhnert et al [52]).
Erythromelalgia can be primary or secondary. Primary erythromelalgia begins spontaneously at any age. Secondary erythromelalgia has been reported with many disorders but most often with polycythemia, thrombocythemia, neuropathies and autoimmune diseases.
UNPLEASANT SENSATIONS IN THE FEET AT REST THAT CAUSE DIFFICULTY WALKING
Tarsal tunnel syndrome: Tarsal tunnel syndrome (TTS) is a controversial entity in which the posterior tibial nerve is compressed under the flexor retinaculum in the tarsal tunnel, inferior and posterior to the medial malleolus. This is much rarer than most medical practitioners think.
• Tingling and numbness on the sole of the foot. If paraesthesia occurs it MUST be confined to the sole of the foot and within the distribution of either the medial and/or lateral plantar nerves (see Figure 1.21). Paraesthesia on the top of the foot excludes the diagnosis.
• Burning paraesthesia and pain described as sharp, shooting, shock-like or electric radiating either proximally or distally.
• The symptoms worsen after prolonged standing or walking [53].
• The symptoms are more intense at the end of the day.
• Symptoms do not awaken the patient from sleep and are unlike carpal tunnel.
• Rarely, pain confined to the soles of the foot may also occur at rest and in non-weight-bearing positions [54]. A positive Tinel’s sign with tapping of the nerve behind the medial malleolus producing tingling into the sole(s) would be diagnostic but is very rare. Altered sensation within the distribution of the medial and/or lateral plantar nerves is also rarely seen.
The diagnosis of TTS may be difficult as the symptoms are often vague, as are the physical findings and signs. Bilateral cases are seen occasionally. There is often a long delay in diagnosis of up to 2½ years [55].
Morton’s neuroma or metatarsalgia: Morton’s neuroma is entrapment of the interdigital nerve, most commonly between the 3rd and 4th and less commonly the 2nd and 3rd toes.
• Patients complain that it feels like there is a stone or pebble in their shoe.
• There is the gradual onset of pain while walking with sudden attacks of throbbing burning pain and paraesthesia, on the plantar surface of the foot localised to the web space that radiates to the corresponding toes.
• As opposed to pain of neurological origin it is not burning, shooting or associated with altered sensation.
• Pain is minimal or absent when sitting or lying as opposed to a calcaneal spur and plantar fasciitis.
• The clue to the diagnosis is that the pain is aggravated by wearing tight shoes and may be relieved by massage to the forefoot and toes [59].
• There is a localised area of reproducible tenderness between the metatarsal heads.
Calcaneal spur: A calcaneal spur causes:
• sharp, stabbing chronic heel pain, particularly in overweight patients [62]
• pain that is characteristically worse in the morning
• tenderness to firm palpation at the distal aspect of the heel where the tendon inserts into the calcaneus bone.
Calcaneal spurs are seen in some patients with plantar fasciitis.
Plantar fasciitis: Plantar fasciitis is the most common cause of plantar heel pain.
• Pain is felt in the sole of the foot.
• Pain is worse when standing after periods of rest or on taking the first steps in the morning.
• The pain improves only to worsen again later in the day after prolonged weight bearing.
• Nocturnal pain is not a feature of plantar fasciitis.
• The pain is searing, throbbing or piercing not burning or shooting.
• There is tenderness over the origin of the plantar fascia and the anteromedial aspect of the heel.
It is more common in runners who increase the distances they run, in obesity and in those who are on their feet most of the day. The condition is self-limiting in most patients and resolves within 12 months [63].
Weakness and/or sensory loss in the lower leg
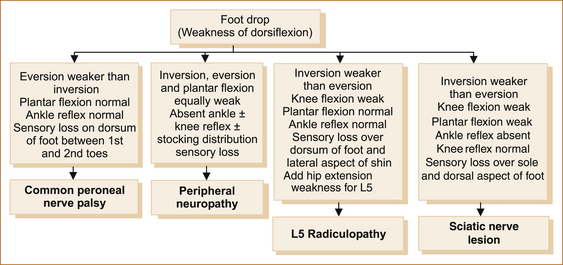
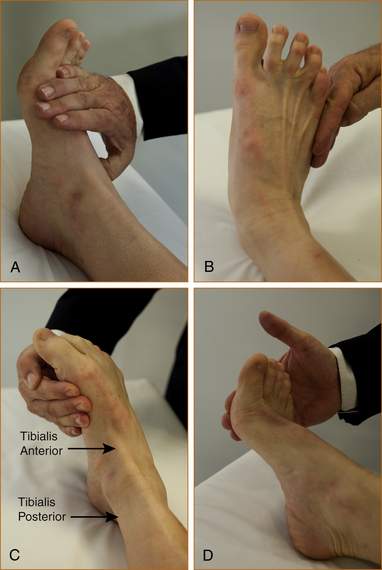
Figure 12.3 lists the features that help to differentiate the various causes of a foot drop. The crucial thing to note is that the tibialis posterior and tibialis anterior muscles invert the foot at the ankle. The tibialis posterior tendon can be seen and palpated behind the medial malleolus and the tibialis anterior tendon in front of the medial malleolus (see Figure 12.4).
Figure 12.4C shows both the tibialis anterior and tibialis posterior tendons contracting with inversion.
The neuroanatomy explains the signs:
• Foot drop or weakness of dorsiflexion of the foot relates to weakness of the tibialis anterior and extensor digitorum muscles. These muscles are supplied by the common peroneal nerve, the lateral branch of the sciatic nerve that arises in the popliteal fossa and traverses the neck of the fibula, a common site for compression. The 5th lumbar nerve root (L5) is the main innervation of these muscles.
• The peroneii muscles that are responsible for eversion of the foot are also innervated by the common peroneal nerve and the L5 nerve root.
• Inversion of the foot results from the combined action of the tibialis anterior and posterior muscles, both supplied by the L5 nerve root, but the tibialis posterior is supplied by the posterior tibial nerve and the tibialis anterior by the common peroneal nerve.
Common peroneal nerve lesion: This is the commonest cause of a foot drop and is related to compression of the common peroneal nerve at the neck of the fibula. Common peroneal nerve palsies are most often related to trauma or compression during surgery, in comatose patients or with prolonged squatting. Some occur for no obvious reason [68]. In the compressive group the prognosis for spontaneous recovery is excellent [68].
The patient invariably awakens, steps out of bed and almost falls because of weakness of dorsiflexion of the foot. The neurological signs are listed in Figure 12.3. The area of sensory loss is small (between the 1st and 2nd toes on the dorsal aspect of the foot) if only the deep peroneal nerve is affected and more extensive (dorsum of foot and lateral aspect of distal half of lower leg) if the superficial peroneal nerve is also affected (see Figure 1.19).
Sciatic nerve lesion: Sciatic nerve lesions are very rare and usually result from posterior dislocation fracture of the hip or as a complication of total hip joint replacement. It has also been reported as a complication following coronary artery bypass surgery when patients were sat upright while still unconscious [69].
The neurological signs are listed in Figure 12.3. As opposed to an L5 radiculopathy, hip extension and abduction are normal and the sensory loss involves the L5 and S1 dermatomes and is over the lateral aspect of the shin, dorsum of the foot, the lateral aspect of the foot on both the dorsal and plantar surfaces and also over the calf.
L5 radiculopathy: Lumbar nerve root compression is a common cause of leg pain with or without a foot drop and occasionally patients present with a painless radiculopathy [70]. The neurological signs are listed in Figure 12.3. If sensory loss occurs, it is within the distribution of the 5th lumbar nerve root (see Figure 1.22).
Peripheral neuropathy: The list of causes of peripheral neuropathy is long and well beyond the scope of this book. Readers are referred to more definitive texts [71].
There are a variety of ways to classify the peripheral neuropathies:
• One way is to differentiate between single nerves being affected, referred to as mononeuropathy, multiple single nerves, the mononeuropathy multiplex, or diffuse involvement of the peripheral nervous system. In this section the peripheral neuropathies with diffuse involvement are discussed.
• A second way to characterise these neuropathies is the rapidity with which they develop, differentiating acute with onset over days to weeks, subacute with onset over months and chronic with onset over years.
• Nerve conduction studies are used to classify whether the peripheral neuropathy is axonal (affecting the axons) or whether it is demyelinating (affecting the myelin) and, in the case of demyelinating neuropathies, whether there is uniform slowing or conduction block (see additional information in Table 12.1 below).
TABLE 12.1
Features on NCS and EMG of demyelinating versus axonal neuropathies
| Demyelinating | Axonal | |
| Amplitude of motor response | Normal or mildly reduced | Markedly reduced |
| Conduction velocity | Very slow | Normal or mildly reduced |
| Conduction block | Yes | No |
| Temporal dispersion∗ | Present | Absent |
| Distal motor latency | Prolonged | Not prolonged |
| Absent response | No | Possible |
| f-wave latencies | Prolonged | Not prolonged |
| EMG | No denervation | Denervation |
∗The term ‘temporal dispersion’ is where the proximal response (the site of stimulation is further up the arm or leg) is substantially lower in amplitude and of longer duration than the distal response.EMG – electromyography; NCS – nerve conduction studies.
Most neuropathies are mixed axonal and demyelinating.
DIABETES AND THE PERIPHERAL NERVOUS SYSTEM: There are a number of peripheral nerve lesions that occur in the setting of diabetes.
• Diabetic amyotrophy with proximal weakness and pain in the legs
• Third nerve palsy, usually not involving the pupil
• Truncal neuropathy with pain radiating around the lateral chest and abdominal wall toward the mid-line with sensory loss extending laterally from the mid-line anteriorly
• In clinical practice peripheral neuropathy most commonly relates to diabetes or alcohol in Western societies; the commonest cause worldwide is leprosy.
• The other more common causes include AIDP and CIDP, neuropathy related to mononclonal gammopathies such as IgG, IgM and IgA, drug-related neuropathy, uraemia, familial neuropathy and, although relatively rare, vitamin B12 deficiency should not be missed.
• There are many other causes including hereditary, toxic, metabolic, infectious (including HIV), inflammatory, ischaemic and paraneoplastic disorders. Occasionally, patients may present with a peripheral neuropathy in the setting of malignancy; more often the neuropathy appears after the diagnosis of the malignancy and the difficulty is to differentiate neuropathy related to drug therapy of the malignancy and a paraneoplastic neuropathy.
• In approximately 20% of patients a cause may not be established. A careful family history and examination of near relatives (including NCS) will detect a familial neuropathy in nearly half of the patients where the initial assessment fails to elucidate the cause.
Currently, peripheral neuropathies are classified as either demyelinating or axonal based largely on the results of NCS [72, 73].
The features on NCS and EMG of demyelinating versus axonal neuropathies are shown in Table 12.1 The term ‘temporal dispersion’ is where the proximal response (the site of stimulation is further up the arm or leg) is substantially lower in amplitude and of longer duration than the distal response and relates to differences in conduction of different fibres within the nerve. Conduction block (CB) refers to a marked reduction in the amplitude of the motor response between distal and proximal stimulation and/or prolongation of the distal motor latency, and is due to focal demyelination in the nerve. Occasionally, CB is so severe that no response can be obtained. CB cannot be diagnosed at sites where the nerve can be compressed, e.g. ulnar nerve at the elbow. The criteria for CB have varied over the years and are divided into definite or probable, based on the degree of reduction of amplitude and increase in duration of the motor response between distal versus proximal stimulation [74].
Pain in the lower leg
Lumbar nerve root compression is a common cause of leg pain, and L5 radiculopathy accounts for 75% and L4 for 15% of these cases.
• These patients present with pain radiating down the back of the thigh and lateral aspect of the shin to the dorsum of the foot with an L5 radiculopathy and to the medial aspect of the lower leg with an L4 radiculopathy.
• Pain related to disc herniation is exacerbated by bending forward, sitting, coughing or straining and is relieved by lying down or very rarely walking. Pain can occur in the absence of any weakness or sensory symptoms.
• If weakness does occur with an L5 radiculopathy, it affects dorsiflexion of the foot; inversion of the ankle is weaker than eversion as both the tibialis posterior and tibialis anterior muscles are affected; plantar flexion is normal.
• The area of sensory loss, if present, affects the lateral aspect of the shin and dorsal surface of the foot.
• A number of patients are seen with sciatic-like pain radiating from the buttock down to above the knee where no evidence of nerve root compression can be detected, and the aetiology of this pain is unclear.
Exercise-induced leg pain
When a patient says they have pain in the legs, it is important to establish whether the pain is in the joints or elsewhere. This seems an obvious thing to do and yet is rarely performed. Pain in the joints exacerbated by weight bearing or by movement of the affected joint clearly indicates local pathology within the joint such as arthritis. The diagnosis of acute arthritis is not difficult when there is associated swelling, tenderness, redness and heat emanating from the joint. In long-standing arthritis there may be associated deformity of the joint; on the other hand, the joints will look normal in most patients presenting for the first time with arthritis. If the pain relates to arthritis, it should be reproduced when the joint is moved during the examination. There may also be a crackling sensation termed crepitus in the joint on movement [79].
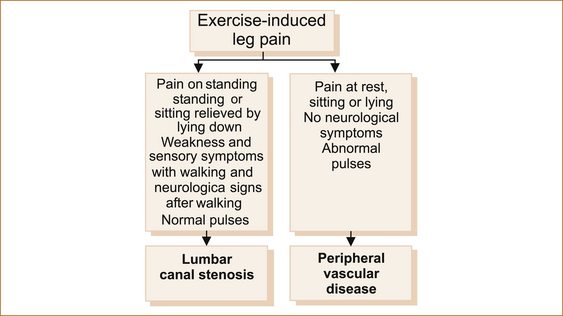
Pain symptoms: The pain resulting from PVD and the pain from lumbar canal stenosis (also termed cauda equina claudication) have many similarities.
• In both, the pain is predominantly in the calves, not in the joints, and is precipitated by walking and relieved by resting for as little as a few minutes.
• The pain from lumbar canal stenosis resolves when the patient lies flat and may be precipitated by simply standing or even sitting.
• Rarely, patients with PVD and severe ischaemia may experience leg pain at rest. The rest pain is present and of the same intensity lying, sitting and standing.
• Leg pain exacerbated by an alteration in posture indicates probable lumbar canal stenosis.
• When the pain in the legs is associated with neurological symptoms, clearly the exercise-induced leg pain is related to lumbar canal stenosis. The associated neurological symptoms may consist of paraesthesia or numbness in the feet and/or the development of weakness (a foot drop).
• Much less commonly the pain may be in the genital region, resulting in priapism or vulval pain. Once again the pain is exacerbated by sitting, standing or walking and relieved by lying flat or the cessation of walking. Symptoms in the genital region indicate involvement of the sacral nerve roots.
• The pain of lumbar canal stenosis is usually but not always associated with low back pain, whereas the pain of PVD is not. In both conditions there may the associated buttock pain and in patients with PVD this relates to disease in the internal iliac vessels.
Examination: Examination of the lower limbs can differentiate between these two entities:
• In patients with PVD the peripheral pulses, particularly in the feet, are diminished or absent.
• Often, but not invariably, in patients with lumbar canal stenosis the ankle reflexes are absent, although this is not always a useful sign as many elderly patients have absent ankle reflexes as a normal finding.
It is often useful to send the patient for a walk so that the symptoms are precipitated and examine them immediately while the symptoms are still present. The development of neurological signs with exercise, in particular sensory loss in the feet, loss of reflexes that were present prior to exercise and the development of weakness, in particular dorsiflexion of the feet, is strong evidence for a diagnosis of lumbar canal stenosis.
REFERENCES
1. Chou, R., et al. Imaging strategies for low-back pain: Systematic review and meta-analysis. Lancet. 2009;373(9662):463–472.
2. Avrahami, E., et al. Early MR demonstration of spinal metastases in patients with normal radiographs and CT and radionuclide bone scans. J Comput Assist Tomogr. 1989;13(4):598–602.
3. Algra, P.R., et al. Detection of vertebral metastases: Comparison between MR imaging and bone scintigraphy. RadioGraphics. 1991;11:219–232.
4. Madigan, L., et al. Management of symptomatic lumbar degenerative disk disease. J Am Acad Orthop Surg. 2009;17(2):102–111.
5. Malmivaara, A., et al. The treatment of acute low back pain – bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332(6):351–355.
6. Assendelft, W.J., et al. Spinal manipulative therapy for low back pain. Cochrane Database Syst Rev. (1):2004. [CD000447].
7. van Tulder, M.W., Gagnier, J.J., Furlan, A.D. Complementary and alternative therapies for low back pain. Best Pract Res Clin Rheumatol. 2005;19(4):639–654.
8. Waller, B., Lambeck, J., Daly, D. Therapeutic aquatic exercise in the treatment of low back pain: A systematic review. Clin Rehabil. 2009;23(1):3–14.
9. Haim, A., et al. Meralgia paresthetica: A retrospective analysis of 79 patients evaluated and treated according to a standard algorithm. Acta Orthop. 2006;77(3):482–486.
10. Khalil, N., Nicotra, A., Rakowicz, W. Treatment for meralgia paraesthetica. Cochrane Database Syst Rev. (3):2008. [CD004159].
11. Ducic, I., Dellon, A.L., Taylor, N.S. Decompression of the lateral femoral cutaneous nerve in the treatment of meralgia paresthetica. J Reconstr Microsurg. 2006;22(2):113–118.
12. Krendel, D.A., Zacharias, A., Younger, D.S. Autoimmune diabetic neuropathy. Neurol Clin. 1997;15(4):959–971.
13. Sander, H.W., Chokroverty, S. Diabetic amyotrophy: Current concepts. Semin Neurol. 1996;16(2):173–178.
14. Said, G., et al. Painful proximal diabetic neuropathy: Inflammatory nerve lesions and spontaneous favorable outcome. Ann Neurol. 1997;41(6):762–770.
15. Ogawa, T., et al. Intravenous immunoglobulin therapy for diabetic amyotrophy. Intern Med. 2001;40(4):349–352.
16. Courtney, A.E., McDonnell, G.V., Patterson, V.H. Human immunoglobulin for diabetic amyotrophy – a promising prospect? Postgrad Med J. 2001;77(907):326–328.
17. Salvarani, C., Cantini, F., Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372(9634):234–245.
18. Salvarani, C., et al. Polymyalgia rheumatica and giant cell arteritis: A 5-year epidemiologic and clinical study in Reggio Emilia,. Italy. Clin Exp Rheumatol. 1987;5(3):205–215.
19. Le Quintrec, J.S., Le Quintrec, J.L. Drug-induced myopathies. Baillieres Clin Rheumatol. 1991;5(1):21–38.
20. Kuncl, R.W., George, E.B. Toxic neuropathies and myopathies. Curr Opin Neurol. 1993;6(5):695–704.
21. Wiendl, H. Idiopathic inflammatory myopathies: Current and future therapeutic options. Neurotherapeutics. 2008;5(4):548–557.
22. Walker, U.A. Imaging tools for the clinical assessment of idiopathic inflammatory myositis. Curr Opin Rheumatol. 2008;20(6):656–661.
23. Choy, E.H., et al. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. (3):2005. [CD003643].
24. Tran, H.A. Thyrotoxic periodic paralysis. Mayo Clin Proc. 2005;80(7):960–961. [author reply 961].
25. Lichtstein, D.M., Arteaga, R.B. Rhabdomyolysis associated with hyperthyroidism. Am J Med Sci. 2006;332(2):103–105.
26. Tuncel, D., et al. Hoffmann’s syndrome: A case report. Med Princ Pract. 2008;17(4):346–348.
27. Ekbom, K.A. Restless legs; a report of 70 new cases. Acta Med Scand Suppl. 1950;246:64–68.
28. Satija, P., Ondo, W.G. Restless legs syndrome: Pathophysiology, diagnosis and treatment. CNS Drugs. 2008;22(6):497–518.
29. Benes, H., et al. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. 2007;22(Suppl18):S401–S408.
30. Oertel, W.H., et al. State of the art in restless legs syndrome therapy: Practice recommendations for treating restless legs syndrome. Mov Disord. 2007;22(Suppl18):S466–S475.
31. Hening, W.A. Current guidelines and standards of practice for restless legs syndrome. Am J Med. 2007;120(1 Suppl 1):S22–S27.
32. Silber, M.H., et al. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004;79(7):916–922.
33. Conti, C.F., et al. Levodopa for idiopathic restless legs syndrome: Evidence-based review. Mov Disord. 2007;22(13):1943–1951.
34. Wagner, M.L., et al. Randomized, double-blind, placebo-controlled study of clonidine in restless legs syndrome. Sleep. 1996;19(1):52–58.
35. Ferini-Strambi, L., et al. Effect of pramipexole on RLS symptoms and sleep: A randomized, double-blind, placebo-controlled trial. Sleep Med. 2008;9(8):874–881.
36. Bliwise, D.L., et al. Randomized, double-blind, placebo-controlled, short-term trial of ropinirole in restless legs syndrome. Sleep Med. 2005;6(2):141–147.
37. Trenkwalder, C., et al. Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2008;7(7):595–604.
38. Walters, A.S., et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16(4):327–332.
39. Ondo, W.G. Methadone for refractory restless legs syndrome. Mov Disord. 2005;20(3):345–348.
40. Dressler, D., et al. The syndrome of painful legs and moving toes. Mov Disord. 1994;9(1):13–21.
41. Walters, A.S., et al. Painless legs and moving toes: A syndrome related to painful legs and moving toes? Mov Disord. 1993;8(3):377–379.
42. Alvarez, M.V., et al. Case series of painful legs and moving toes: Clinical and electrophysiologic observations. Mov Disord. 2008;23(14):2062–2066.
43. Tavee, J., Zhou, L. Small fiber neuropathy: A burning problem. Cleve Clin J Med. 2009;76(5):297–305.
44. Singleton, J.R., Smith, A.G., Bromberg, M.B. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24(8):1448–1453.
45. Penza, P., et al. Painful neuropathy in subclinical hypothyroidism: Clinical and neuropathological recovery after hormone replacement therapy. Neurol Sci. 2009;30(2):149–151.
46. Gonzalez-Duarte, A., Robinson-Papp, J., Simpson, D.M. Diagnosis and management of HIV-associated neuropathy. Neurol Clin. 2008;26(3):821–832.
47. Makkar, R.P., et al. Burning feet syndrome: A clinical review. Aust Fam Physician. 2003;32(12):1006–1009.
48. Vinik, A.I. Diabetic neuropathy: Pathogenesis and therapy. Am J Med. 1999;107(2B):17S–26S.
49. Balakrishnan, S., et al. A randomized parallel trial of topical aspirin-moisturizer solution vs. oral aspirin for acute herpetic neuralgia. Int J Dermatol. 2001;40(8):535–538.
50. Buttaci, C.J. Erythromelalgia: A case report and literature review. Pain Med. 2006;7(6):534–538.
51. Cohen, J.S. Erythromelalgia: New theories and new therapies. J Am Acad Dermatol. 2000;43(5 Pt 1):841–847.
52. Kuhnert, S.M., Phillips, W.J., Davis, M.D. Lidocaine and mexiletine therapy for erythromelalgia. Arch Dermatol. 1999;135(12):1447–1449.
53. Goodgold, J., Kopell, H.P., Spielholz, N.I. The tarsal-tunnel syndrome: Objective diagnostic criteria. N Engl J Med. 1965;273(14):742–745.
54. Alshami, A.M., Souvlis, T., Coppieters, M.W. A review of plantar heel pain of neural origin: Differential diagnosis and management. Man Ther. 2008;13(2):103–111.
55. Sammarco, G.J., Chang, L. Outcome of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24(2):125–131.
56. Patel, A.T., et al. Usefulness of electrodiagnostic techniques in the evaluation of suspected tarsal tunnel syndrome: An evidence-based review. Muscle Nerve. 2005;32(2):236–240.
57. Erickson, S.J., et al. MR imaging of the tarsal tunnel and related spaces: Normal and abnormal findings with anatomic correlation. Am J Roentgenol. 1990;155(2):323–328.
58. Mullick, T., Dellon, A.L. Results of decompression of four medial ankle tunnels in the treatment of tarsal tunnel syndrome. J Reconstr Microsurg. 2008;24(2):119–126.
59. Hassouna, H., Singh, D. Morton’s metatarsalgia: Pathogenesis, aetiology and current management. Acta Orthop Belg. 2005;71(6):646–655.
60. Redd, R.A., et al. Morton neuroma: Sonographic evaluation. Radiology. 1989;171(2):415–417.
61. Thomson, C.E., Gibson, J.N., Martin, D. Interventions for the treatment of Morton’s neuroma. Cochrane Database Syst Rev. (3):2004. [CD003118].
62. Prichasuk, S., Subhadrabandhu, T., The relationship of pes planus and calcaneal spur to plantar heel pain. Clin Orthop Relat Res, 1994;306:192–196.
63. Buchbinder, R. Plantar fasciitis. N Engl J Med. 2004;350:2159.
64. Batt, M.E., Tanji, J.L., Skattum, N. Plantar fasciitis: A prospective randomized clinical trial of the tension night splint. Clin J Sport Med. 1996;6(3):158–162.
65. Kudo, P., et al. Randomized, placebo-controlled, double-blind clinical trial evaluating the treatment of plantar fasciitis with an extracorporeal shockwave therapy (ESWT) device: A North American confirmatory study. J Orthop Res. 2006;24(2):115–123.
66. Kalaci, A., et al. Treatment of plantar fasciitis using four different local injection modalities: A randomized prospective clinical trial. J Am Podiatr Med Assoc. 2009;99(2):108–113.
67. Cole, C., Seto, C., Gazewood, J. Plantar fasciitis: Evidence-based review of diagnosis and therapy. Am Fam Physician. 2005;72(11):2237–2242.
68. Berry, H., Richardson, P.M. Common peroneal nerve palsy: A clinical and electrophysiological review. J Neurol Neurosurg Psychiatry. 1976;39(12):1162–1171.
69. Kempster, P., et al. Painful sciatic neuropathy following cardiac surgery. Aust N Z J Med. 1991;21(5):732–735.
70. Lipetz, J.S., Misra, N., Silber, J.S. Resolution of pronounced painless weakness arising from radiculopathy and disk extrusion. Am J Phys Med Rehabil. 2005;84(7):528–537.
71. Dyck, P.J., Thomas, P.K. Peripheral neuropathy, 4th edn. Philadelphia: Saunders; 2005.
72. Donofrio, P.D., Albers, J.W. AAEM minimonograph No. 34: Polyneuropathy: Classification by nerve conduction studies and electromyography. Muscle Nerve. 1990;13(10):889–903.
73. Tankisi, H., et al. Pathophysiology inferred from electrodiagnostic nerve tests and classification of polyneuropathies: Suggested guidelines. Clin Neurophysiol. 2005;116(7):1571–1580.
74. Olney, R.K. Guidelines in electrodiagnostic medicine: Consensus criteria for the diagnosis of partial conduction block. Muscle Nerve Suppl. 1999;8:S225–S229.
75. Legrand, E., et al. Sciatica from disk herniation: Medical treatment or surgery? Joint Bone Spine. 2007;74(6):530–535.
76. Mirza, S.K., Deyo, R.A. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32(7):816–823.
77. Chou, R., et al. Correction: Diagnosis and treatment of low back pain. Ann Intern Med. 2008;148(3):247–248.
78. Chou, R., Huffman, L.H. Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–514.
79. Apley, A.G., Solomon, L., Osteoarthritis and related disorders. In: Concise system of orthopaedics and fractures. Oxford: Butterworth–Heinemann; 1994:36–41.