Autonomously Functioning Thyroid Nodules and Other Causes of Thyrotoxicosis
Autonomously Functioning Thyroid Nodules
Silent or Painless Thyroiditis
Thyrotoxicosis Caused by Pregnancy and Trophoblastic Disease
TSH-like Activity of Human Chorionic Gonadotropin
Gestational Transient Thyrotoxicosis
Hyperthyroidism Caused by Inappropriate TSH Secretion
Thyrotoxicosis Caused by Metastatic Differentiated Thyroid Carcinoma
The term thyrotoxicosis literally means “poisoning by thyroid hormone.” The term includes any situation in a patient who shows clinical and biochemical characteristics of overactivity of thyroid hormone. It involves not only hyperfunction of the thyroid gland, termed hyperthyroidism, but also any other condition with elevated thyroid hormone levels in combination with clinical characteristics of overactivity of thyroid hormones. For instance, when thyroid hormones leak from the thyroid gland because of infectious or other damaging processes or are produced outside the thyroid gland in toxic amounts (struma ovarii, thyroid carcinoma metastasis) or ingested in overdose, thyrotoxicosis may ensue. In thyrotoxicosis, free hormone levels are invariably increased. The reverse is not true in that increased free thyroid hormone levels do not always point to thyrotoxicosis. In illness or resistance to thyroid hormones, increased free hormone levels are present while the patients are clinically euthyroid or even sometimes hypothyroid.1
The following causes of thyrotoxicosis are distinguished and will be discussed in this chapter:
Subacute (de Quervain’s) thyroiditis
Autonomously functioning thyroid nodules (AFTNs)
Silent or painless thyroiditis
Thyrotoxicosis caused by pregnancy and trophoblastic disease
Iodine-induced thyrotoxicosis (IIT)
Hyperthyroidism caused by inappropriate thyroid-stimulating hormone (TSH) secretion
Autonomously Functioning Thyroid Nodules
Epidemiology and Natural History
About 5% to 10% of solitary thyroid nodules are toxic. This figure varies from country to country and is higher in Europe.2,3 In nodules with a diameter of less than 2.5 cm, the proportion that are toxic is only 1.9%, whereas in nodules of 2.5 cm or greater, this figure is 42.6%. In a study of patients 60 years and older with AFTNs, 57% were thyrotoxic. In patients younger than 60 years, thyrotoxicity was noted in only 13%. In patients younger than 40 years, only 19.5% had AFTNs with a diameter of 3 cm or larger, but in older patients, this figure was 45.9%.2 The proportion of AFTNs that are responsible for the hyperthyroidism of referred patients varies geographically between 1.5% and 44.5% (Table 11-1). About five times more women than men suffer from this disorder.4 The natural history of the growth of AFTNs varies. They may stay the same over the years, grow, or shrink. In a group of 159 patients who were observed for 1 to 15 years, an increase in size was seen in 10% and a decrease in 4%.2 Changes in function of the nodule over the years also occur. In an observation period of 6 years, 10% of patients with AFTNs became toxic, and loss of function due to degeneration was observed in 4%.2 Development of hyperthyroidism occurred predominantly in nodules greater than 3 cm in diameter,3 with a minimal volume of 16 mL by ultrasound.5
Table 11-1
Frequency of Toxic Adenoma in Various Countries
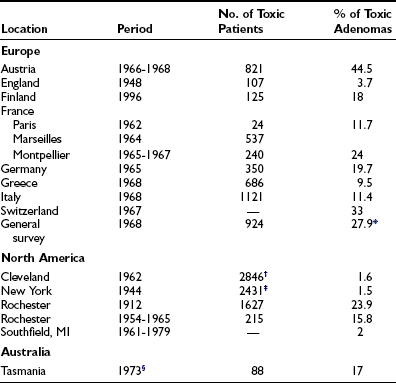
*Patients younger than 50 years.
†Graves’ disease plus toxic adenoma.
‡Thyrotoxicosis submitted to surgery.
§Six years after bread iodination.
From Orgiazzi JJ, Mornex R: Hyperthyroidism. In Greer MA (ed): The Thyroid Gland. New York: Raven, 1990, p. 442.
Pathogenesis
From a histologic point of view, two types of nodules (Figs. 11-1 and 11-2) may be discerned: a monoclonal and a polyclonal type. Studer and his group developed the concept that even if monoclonal at the molecular level, nodules may become polyclonal from a functional and histologic aspect during evolution. They suggest that individual follicular cells may acquire new qualities that were not present in the mother cells but become inheritable during further replication. Obviously this change supposes some sort of genetic event. This sequence of events may lead to loss of anatomic and functional integrity of the follicular cells. The process might be accelerated by stimulatory factors such as TSH (e.g., in iodine deficiency, by goitrogens) and by local stimulatory and growth factors.6
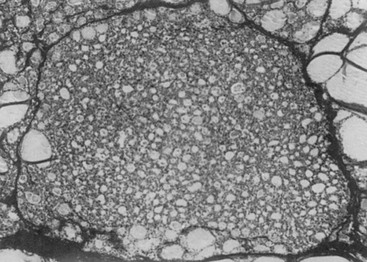
FIGURE 11-1 Uniform nature of cells formed in a nodule by proliferation of only one or a few clones of epithelial cells. (From Studer H, Ramelli F: Simple goiter and its variants: euthyroid and hyperthyroid multinodular goiters. Endocr Rev 3:40, © 1982, The Endocrine Society.)
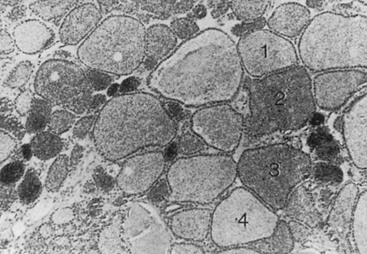
FIGURE 11-2 Autoradiograph of a hot nodule, illustrating areas with different capacity of uptake of radioiodine. (From Studer H, Gerber H, Peter HJ: Multinodular goiter. In DeGroot LJ [ed]: Endocrinology, 2nd ed, vol 1. Philadelphia: WB Saunders, 1989, p 722.)
At the genetic level, two types of monoclonal autonomously functioning nodules have been reported. Both are somatic mutations. One involves the TSH receptor (TSHR) gene and the other involves the Gsα protein gene. Both mutations lead to constitutive activation of the adenylate cyclase system, probably also activation of the inositol phosphates pathways7 in the follicular cell, and to autonomy of the follicle. Mutations of the TSHR gene, and with a much lower prevalence, the Gsα gene, play a major (principal) role in the pathogenesis of AFTN.8 For those monoclonal toxic adenomas (TA) in which no mutations are found in the TSHR or Gsα unit, probably other somatic mutations are involved.9 In a recent study of 75 hot thyroid nodules, somatic TSH receptor mutations were detected in 57% and Gsα mutations in 3% of the nodules.10 The same group studied females with AFTN, but without mutations in the TSH-R or Gsα unit, and detected a monoclonal origin in 10 out of 20 of cases when tested for X-chromosome inactivation. This strongly suggests a mutation at other location(s).10 Although some believe that iodine deficiency can increase mutation rate and functional expression of autonomy11 in AFTN, others12 consider the fundamental process of goitrogenesis in (multi)nodular goiter as independent from iodine deficiency but operating through mechanisms that are innate to the hereditary and acquired heterogeneity among the thyrocytes themselves. However, superimposed iodine deficiency may shift clinical expression to younger ages. Van Sande and co-workers13 hypothesized that as 16 different activating mutations were identified in the TSHR gene, this receptor is in a constrained conformation in its wild-type form. They also report that AFTA have a high level of Na+/iodide symporter gene expression, a high thyroperoxidase mRNA and protein content, and a low H2O2 generation. Inositol uptake was also increased, but inositol phosphates were not increased. TA secreted more thyroid hormone than the quiescent surrounding tissue. Other characteristics of TA were increased cycling of thyrocytes as compared to normal surrounding tissue, little apoptosis, and low expression of early immediate genes.14 An important study by Fuhrer and co-workers15 showed that a panel of different activating TSHR mutations caused different functional and morphologic responses in vitro in rat and human primary thyrocytes. Their data suggest that different biologic properties of the TSHR mutants may result in different in vivo phenotypes. Finally, other mutated genes causing AFTN may be located in the AMP cascade.
Pathology
On macroscopic examination, a solitary toxic nodule is surrounded by normal thyroid tissue that is functionally suppressed. Rarely, a microscopically monotonous picture is seen that consists of uniform follicular cells without signs of malignancy. Usually the picture is heterogeneous with cells of different size, sometimes with signs of fresh and old hemorrhage and calcification. This picture, however, does not exclude a monoclonal origin of the nodule but may be the result of stimulatory local growth factors (see the section entitled “Pathogenesis”). No information is available at present on the exact ratio between primarily monoclonal and polyclonal AFTNs of the thyroid. Sometimes, autonomously functioning micronodules are present in the surrounding thyroid tissue. This finding is in agreement with the thesis of Studer and co-workers6 that the true adenoma is one end of a large spectrum of thyroid nodules growing from single thyrocytes or tiny cell families, each replicating with an individual growth rate, whereas the grossly abnormal multinodular goiter is at the other end of the scale.
Clinical Features
The signs and symptoms of patients with toxic adenoma are those of thyrotoxicosis, without the specific signs of Graves’ disease, such as eye signs, pretibial myxedema, and acropachy. On palpation of the thyroid region, a nodule is found in one of the lobes, usually with a diameter of 3 cm or larger.2 The thyroid tissue surrounding the nodule and the thyroid lobe on the other side are not usually palpable because of TSH suppression.
Laboratory Diagnosis
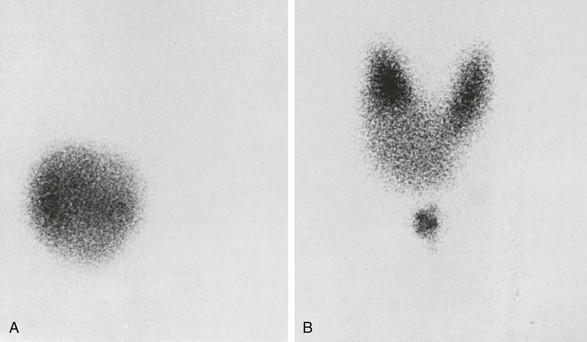
When a single nodule is found in the thyroid and the patient is clinically thyrotoxic, estimation of serum TSH is sufficient for the diagnosis of toxic adenoma. To have an idea about the severity of the thyrotoxicosis, estimation of serum free thyroxine (T4) is sufficient. If this parameter is normal, serum triiodothyronine (T3) or free T3 should be determined because it may be solely elevated in minimal thyrotoxicosis, that is, T3 toxicosis. If findings on palpation are inconclusive, it is wise to perform a scintiscan of the thyroid. In the case of a toxic nodule, activity is seen in the nodule, with minimal or no activity anywhere else in the thyroid region (Fig. 11-3). The presence of a thyroid carcinoma in an AFTN is rare.16 Although some authors are of the opinion that the occurrence of carcinoma in an AFTN may be more than coincidental,17 most thyroidologists believe that such is not the case. The differential diagnosis of a toxic nodule includes relapse of hyperthyroid Graves’ disease in remnant thyroid tissue after thyroid surgery or in thyroid dysgenesis. The latter possibility is especially remote. Theoretically, a TSH stimulation test would distinguish between an AFTN and the two other possibilities, but this test is hardly ever necessary in clinical practice. It is of no value to perform fine-needle aspiration cytology of an AFTN, because differentiation between a follicular adenoma and carcinoma is difficult if not impossible with this technique. When a hot nodule is present, that is, prominent uptake in the nodule but less in the surrounding tissue while serum TSH is normal, autonomous function can be tested by performing a T3 or T4 suppression test. In the case of autonomous function, uptake in the nodule is still present after the administration of 25 µg T3 three times daily for 10 days or 125 µg T4 for 14 days, whereas uptake is suppressed in the surrounding tissue. Sometimes uptake in a single nodule is indistinguishable from uptake in the surrounding tissue, a situation described as a “warm” nodule. This picture may be generated either by normal affinity of the nodular tissue for the isotope or because a nonfunctioning nodule is surrounded by normal thyroid tissue. Thus the possibility of the presence of a carcinoma in a warm nodule is certainly not excluded, which means that it should be evaluated as though it were a cold nodule.
Treatment
No treatment of a hot nodule is necessary as long as the patient remains euthyroid. Regular TSH measurement at intervals of a half to 1 year suffices. Most patients remain euthyroid. Occasionally, a hemorrhage in the nodule leads to spontaneous resolution. Treatment of toxic nodules with antithyroid drugs is useless because after discontinuation of medication, relapse invariably occurs. Three modes of treatment of toxic nodules include nodulectomy, administration of radioactive iodine, and percutaneous injection of alcohol into the nodule. Nodulectomy is very effective in rendering the patient euthyroid and has a low surgical complication rate. Surprisingly, permanent hypothyroidism develops in about 5% of patients, perhaps because of coexistent thyroid disease.2,18 Obviously, the disadvantages of surgery are its operative risks, the residual scar, and its cost, which is high in relation to the other two forms of treatment.
Treatment with radioactive iodine is safe, cheap, and effective. Two reports indicate no risk of posttreatment hypothyroidism 6 months after treatment in a total of 93 patients.19,20 However, when a longer period of follow-up was taken into account in 23 patients, such as 4 to 16.5 years after treatment, hypothyroidism developed in up to 36%.21 In another study of 126 patients with autonomously functioning nodules, the percentage of hypothyroidism after a mean period of 10 years was 9.7% when the nodules were hot and 1.5% when toxic.22 In this and the previous study, no relationship was found between the total dose administered, the size of the nodule, and the development of hypothyroidism. However, if thyroid autoantibodies were present, hypothyroidism occurred in 18% of patients and, when absent, in 1.4%.22 It is important that when 131I is administered, uptake be present only in the nodule and not in the surrounding tissue or in the other lobe, which could occur after pretreatment with antithyroid drugs. To avoid this complication, pretreatment with T3 or T4 might be considered. In some patients, typical Graves’ disease has developed months after treatment of a toxic nodule with 131I. Possibly, antigens released by the 131I therapy induce or exacerbate an autoimmune response.
The third treatment modality is of rather recent date and involves percutaneous alcohol injection into the nodule. Euthyroidism is achieved in 65% to 85% of patients by 12 months after treatment. Injections are repeated 2 to 12 times at weekly intervals. The treatment is usually well tolerated, with few side effects. When nodule volume exceeds 30 mL, results are less favorable.23,24 All three treatment modalities are acceptable, the choice depending on local circumstances and the patient’s preference. Laser photocoagulation of AFTN is the most recent introduced therapy.25 A recent study showed that its efficacy is comparable to that of ethanol injection, with possibly less ensuing hypothyroidism.24
Silent or Painless Thyroiditis
Incidence
The incidence varies geographically. In 1980, this syndrome accounted for 10% of cases of thyrotoxicosis in Japan but only 3% to 4% in New York City.22,26,27 A random poll showed that silent thyroiditis was uncommon in Argentina, Europe, and the east and west coasts of the United States but occurred more frequently around the Great Lakes and in Canada.28 Patients are usually between 30 and 60 years old, and the female-to-male ratio is 1.5 to 1. Apart from its association with pregnancy, known then as postpartum thyroiditis, the condition is currently rarely diagnosed. Postpartum thyroiditis occurs in 5% to 9% of women in the first year after delivery, especially in those who have circulating autoantibodies against thyroperoxidase.29
Etiology
Silent thyroiditis is an autoimmune lymphocytic thyroiditis, and many patients with this disease have a family history of thyroid disease. Postpartum thyroiditis has been significantly associated with HLA-D3 and HLA-D5.30 Exposure to iodine, such as in the form of amiodarone or lithium, interleukin (IL)-2, interferon, and etanercept has been suggested as an initiating event.31–33 Silent thyroiditis is associated with other autoimmune diseases such as rheumatoid arthritis, systemic sclerosis, Graves’ disease, primary adrenal insufficiency, systemic lupus erythematosus, idiopathic thrombocytopenic purpura, rubella, and seasonal allergies.33–38
Pathology
Microscopically, follicles are disrupted, and infiltration of lymphocytes and plasma cells occurs. The infiltration may be focal or diffuse, and sometimes formation of lymphoid follicles is seen. Follicular cells may be cuboidal or, when stimulated by TSH in the hypothyroid phase, columnar. Sometimes Hürthle or Askanazy cells are present. These cells are large and oxyphilic and contain many mitochondria. Thyroid tissue obtained during hypothyroidism or the recovery phase shows regenerating follicles with little colloid. Occasionally, persistent lymphocytic infiltration is observed. Extensive fibrosis may ultimately develop. A few multinucleated giant cells are regularly present.39
Clinical Features
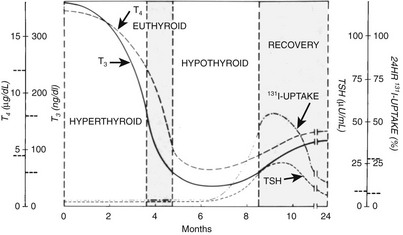
The initial symptoms in 112 patients with 122 episodes of silent thyroiditis have been reviewed.40 Characteristically, thyroid pain was absent in all cases. The female-to-male ratio was 1.3 : 1. The mean age (±SD) in females and males was 32 ± 8.5 and 24.9 ± 8.2 years, respectively. Recurrences were uncommon. The symptoms are similar to other causes of thyrotoxicosis and varied from mild to severe. Specific signs that are characteristic of Graves’ disease, such as eye signs, pretibial myxedema, and acropachy, were absent. The mean duration of the toxic phase in these patients was 3.6 ± 2.0 months. In most reports, the thyroid gland had a firm consistency. In about half the patients, a goiter was present. The course of the disease follows four sequential stages: thyrotoxicosis, euthyroidism, hypothyroidism, and euthyroidism. These stages need not be present in all patients. In one series, 57 of 112 patients became euthyroid, and hypothyroidism did not develop. Clinical hypothyroidism was present in only 32 patients. Hypothyroidism was transient in 24 patients but permanent in 8, who required thyroid hormone substitution. Ultimately, about half of the patients with silent thyroiditis become permanently hypothyroid.41
Laboratory Findings
The acute (first) phase of the disease is characterized by leakage of thyroid hormone and thyroglobulin from the damaged thyroid. This leakage results in elevated serum concentrations of thyroid hormones and thyroglobulin and suppression of serum TSH. Uptake of radioactive iodine by the thyroid is absent in this stage. The C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) are mostly, but not always, slightly elevated. The ESR was elevated in 34 of 53 episodes but higher than 40 mm only in 8.40 This result contrasts with subacute thyroiditis, in which the ESR is invariably much more elevated. T4 and T3 start to decline in the first phase and reach normal levels in the second (euthyroid) phase, but TSH remains suppressed. In the third (hypothyroid) phase, thyroid hormone levels are subnormal, and serum TSH starts to rise at the end of this stage. Because of TSH stimulation, the fourth stage is characterized by normalization of serum T4 and T3. Serum TSH ultimately normalizes, but normalization can take several months, so temporary subclinical hypothyroidism intervenes. For the mean period of the different phases, see Fig. 11-4.
Treatment
The degree of thyrotoxicosis is usually mild in silent thyroiditis, and treatment is not usually necessary. Prescription of α-adrenergic blocking agents may be considered. Antithyroid drugs have a very limited role because the thyrotoxicosis is not the result of increased thyroid hormone synthesis. Propylthiouracil or ipodate to block peripheral conversion of T4 to T3 may be of some value. In more serious cases, prednisone in a dose between 30 and 60 mg/day has a rapid ameliorating effect. The dose should be continued for 1 to 2 weeks and then be slowly tapered.42 In case of relapsing thyroiditis, prednisone can be reinstituted. It is seldom necessary to perform thyroidectomy. If necessary, radiochemical “thyroidectomy” may be contemplated during remission when sufficient thyroid uptake of radioactive iodine is present for effective treatment, often in the presence of prednisone administration. As was stated, thyrotoxicosis is usually mild, and “definitive” treatment is seldom necessary. After the thyrotoxic phase, temporary hypothyroidism develops in about 40% of patients. If needed, thyroid hormone treatment can be instituted in a dose that allows TSH to remain mildly elevated to promote resumption of thyroid hormone synthesis in the recovery phase. Only a small proportion of patients (see previous discussion) need permanent and full-dose substitution at this stage. Finally, however, permanent hypothyroidism develops in about half of the patients. This result is in contrast to subacute thyroiditis, after which patients almost always become permanently euthyroid. Thus patients who suffered from silent thyroiditis need lifelong follow-up because hypothyroidism may develop years later.43
Thyrotoxicosis Factitia
Other signs of Graves’ disease such as eye signs, pretibial edema, acropachy, and goiter are also absent. Differentiation from Graves’ disease is also feasible by color Doppler sonography, which shows absent thyroid vascularity and low-normal peak velocity, whereas these signs are increased in Graves’ disease.44 Because of TSH suppression, the thyroid shrinks and is often not palpable. Thyroid uptake of radioactive iodine is absent. Serum thyroglobulin is low or below detection limits in thyrotoxicosis factitia but elevated in silent thyroiditis. Factitious thyrotoxicosis is not difficult to distinguish from toxic multinodular goiter or toxic adenoma. Differentiation from subacute thyroiditis is easy on clinical grounds, because these patients suffer from frequent severe pain in the thyroid region. Furthermore, the CRP or ESR and serum thyroglobulin concentration are elevated in subacute thyroiditis. The diagnosis of thyrotoxicosis factitia should be considered when laboratory results are contradictory. Psychiatric help is urgently needed for such patients.
Thyrotoxicosis caused by accidental intake of excessive amounts of thyroid hormone has been observed in the “hamburger toxicosis patients.” Two epidemics were caused by the inclusion of bovine thyroid in hamburger.45,46
Thyrotoxicosis Caused by Pregnancy and Trophoblastic Disease
TSH-Like Activity of Human Chorionic Gonadotropin
The hCG from concentrated human pregnant urine has weak TSH-like activity when tested in a mouse bioassay.47 The hCG that is purified from molar tissue has intrinsic TSH bioactivity in the same bioassay, though 4000 times less than that of human TSH on a molar basis.48 However, when produced in sufficient amounts, it may induce clinical hyperthyroidism in humans, as shown in 2 of 20 patients with gestational trophoblastic neoplasia.49 These patients had extremely high serum (3,220,000 and 6,720,000 IU/L) and urine concentrations of hCG that correlated closely with TSH-like bioactivity. Other patients with moderately elevated serum hCG levels, between 110,000 and 310,000 IU/L, were euthyroid. When tested on human thyroid cell membranes, 1.0 IU hCG is biologically roughly equivalent to 0.27 µIU hTSH.50 Both hCG and human luteinizing hormone (HLH) compete with TSH for the TSHR, and hLH also has weak (10 to 100 times higher than hCG) TSH-like activity.50–52 The β subunit of hCG and hLH share 85% sequence identity in the first 114 amino acids but differ in the carboxyl-terminal peptide because hCG-β contains an extension of 31 amino acids.53 Carboxypeptidase digestion of hCG, with amino acid residues 142 to 145 cleaved from the β subunit, leads to an increase in its capacity to stimulate adenylate cyclase in human thyroid membranes.54 A variant of hCG that is lacking the C terminus of the β subunit because of enzymatic cleavage has been identified in pregnancy serum and molar tissue.55 In studies using human thyroid membranes56 or a cell line transfected with human TSHR,57 desialylated forms of hCG exhibited stronger inhibition of TSH-mediated cyclic adenosine monophosphate responses than did native hCG. Both TSH binding and TSH-induced adenylate cyclase stimulation were found to be more effectively inhibited by desialylated variants of hCG than unmodified hCG was.58 From these and other studies it seems that the biologic effect of hCG is predominantly confined to hCG containing little or no sialic acid. In cultured FRTL-5 cells, hCG has been found to increase iodide uptake, and it also causes a dose-related increment in adenylate cyclase activity and thymidine uptake.59,60
Gestational Transient Thyrotoxicosis
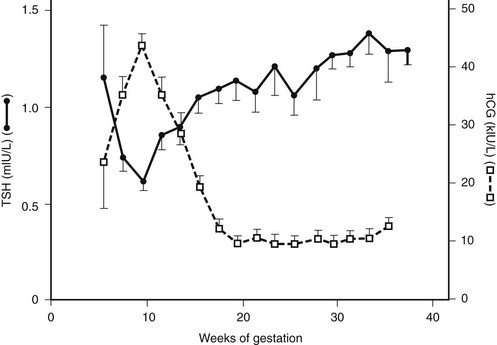
GTT occurs in 2% to 3% of pregnant women.61,62 It is diagnosed mostly between the 8th and the 14th weeks, when hCG levels peak. Fig. 11-5 shows the relationship between hCG and TSH during euthyroid pregnancy. Levels in gestational pregnancy are usually above 75,000 and 100,000 U/L and of sufficient duration to cause hyperthyroidism. About half of patients with GTT have clinical symptoms of hyperthyroidism. Differential diagnosis from Graves’ disease is important. GTT is a nonautoimmune type of hyperthyroidism, which means that circulating thyroid autoantibodies and the characteristic symptoms of Graves’ disease, such as eye signs, pretibial myxedema, and acropachy, are absent. Hyperthyroidism disappears spontaneously when serum hCG levels decrease. Treatment with antithyroid drugs is necessary only when hyperthyroidism is severe. In severe cases, hyperemesis is invariably present, and hospital admission is pertinent. In patients with hyperemesis, the β subunit of hCG, but not the α subunit, is elevated in serum.63 If treatment is necessary in milder cases, administration of β-adrenergic blocking agents is sufficient to suppress symptoms.
Familial Gestational Hyperthyroidism
Recently a mother and a daughter were described who suffered from recurrent gestational hyperthyroidism but in whom hCG serum levels were normal during pregnancy. Both patients were heterozygous for a missense mutation, guanine for adenine, at codon 183 in exon 7 in the TSHR gene. This mutation resulted in replacement of a lysine residue by arginine at position 138 of the receptor, which is in the middle of its extracellular N-terminal domain. Because the mutant receptor was about 3.5 times more sensitive to hCG than was the wild type, hyperthyroidism developed in these women during pregnancy. Both women had concomitant hyperemesis as well.64 No information is as yet available about the frequency of this syndrome.
Molar Pregnancy and Trophoblastic Disease
Several early reports describe molar pregnancy in combination with hyperthyroidism and disappearance of thyrotoxicosis after removal of a hydatidiform mole.65–67 Bioassayable serum TSH was decreased in parallel with normalization of thyroid function parameters and a decrease in serum hCG. From the parallelism of thyroid-stimulating and hCG activity, it was suggested that both are caused by the same molecule: hCG.68 The thyroid stimulator that is extracted from the serum of a patient with hyperthyroidism caused by a mole differed biologically and immunologically from TSH, from hCG found in normal placentas, and from thyroid-stimulating immunoglobulins. It contained less sialic acid and was biologically more active than normal pregnancy hCG.65,69 In patients with chorionic carcinoma, a similar relationship is present between thyroid-stimulating activity in serum and hCG serum concentrations, the β subunit of human hCG, and quantitation of tumor burden.70 Choriocarcinoma associated with hyperthyroidism in males is exceedingly rare. About four cases have been reported in the literature.71 The clinical picture of patients with trophoblastic hyperthyroidism is that of Graves’ disease without the specific features of the latter.
Iodine-Induced Thyrotoxicosis
IIT may be subdivided into different groups: (1) patients from endemic goiter areas, (2) patients with previous nonendemic goiter, (3) patients with previous or with actual Graves’ disease, and (4) patients without apparent previous thyroid disease.72
One of the best known studies on IIT is that by Connolly and co-workers.73 They found a steep rise in the incidence of thyrotoxicosis in the late months of 1966 in Tasmania (Australia), an area of iodine deficiency with a high prevalence of goiter. This increase was due to the addition of potassium iodide to bread in early 1966. The increased incidence occurred predominantly in subjects older than 40 years, in whom a rise in incidence from 50 per 100,000 to a maximum of 130 cases per 100,000 was seen in 1967 to 1968. By 1974, the incidence decreased to about the pre-epidemic level. Most thyrotoxic patients had nodular goiter, and few patients had Graves’ disease. Later it was recognized that a pre-epidemic increase in the incidence of thyrotoxicosis had been caused by the use of iodophor disinfectants on dairy farms.74 Despite the continued increase in the iodide supply, the prevalence of IIT decreased after its peak in 1967-1968. It was argued that this increase in thyrotoxicosis starting from 1964, in this area of relative iodine deficiency with a high prevalence of goiter, was due to autonomy in the nodular goiters. For a review of IIT caused by iodine prophylaxis in Tasmania and other countries, see Stanbury and co-workers.75
Many substances, such as iodinated drugs, radiographic contrast agents, iodochloroxyquinoline, iodine-containing contrast agents, disinfectants, and iodine-containing drugs, may also cause IIT.72,76 Precipitation of thyroid storm is rare. Subjects with longstanding goiter are especially susceptible to IIT. In a study of 85 consecutive patients with IIT, a preexisting thyroid disorder was present in at least 20%. Spontaneous reversal to euthyroidism occurred after a mean period of 6 months in 50 of 85 patients. Return to euthyroidism may be preceded by subclinical hypothyroidism.77 The recently developed nonionic contrast media do not prevent the development of IIT in elderly subjects.78
Evidence from human and animal studies suggests that chronic excess iodine intake may modulate thyroid autoimmunity and lead to thyrotoxicosis in genetically susceptible individuals.79 A necrotic effect of iodide excess has been demonstrated in vivo in various animal species and also in human thyroid follicles in vitro.80
The antiarrhythmic drug amiodarone is currently widely used. Because of its high iodine content (37.2%), it has been the cause of IIT in many patients from all parts of the world. Its basic molecular structure has some similarity to that of the iodothyronines. Amiodarone may also interfere with thyroid hormone transport into cells and with pathways of intracellular thyroid hormone metabolism and action.81 It interferes with 5′-monodeiodination of thyroid hormones, which leads to a decrease in intracellularly derived T3 from T4, thus inducing tissue hypothyroidism.82 Hypothyroidism occurs predominantly in patients with preexisting thyroid autoimmune disease and was recognized in 6% of 467 patients chronically treated with amiodarone.83 It is being used in many cardiac patients, particularly in France. The reported incidence of IIT caused by amiodarone varies between 0.003% and 11.5%. Estimation of serum total or free T4 in patients using amiodarone is not specific, because they may be elevated in hyperthyroid, euthyroid, and even hypothyroid patients. The two latter conditions are explained by a decrease in T4 metabolic clearance by amiodarone because of inhibition of T4 transport into tissues and subsequent T4 deiodination. This process results in high T4 plasma levels and may lead to subnormal tissue T3 concentrations. To differentiate between these possibilities, determination of serum TSH and T3 is useful.
Of the two forms of amiodarone-induced thyrotoxicosis, type 1 is due to an iodine-induced increase in thyroid hormone synthesis, and type 2 involves iodine- or amiodarone-induced cytotoxic damage of the thyroid gland, with subsequent leakage of iodothyronines into the circulation. The picture of type 2 on electron microscopy characteristically shows two types of damage: multilamellar lysosomal inclusions and intramitochondrial glycogen inclusions with a morphologic picture of thyrocyte hyperfunction.84 Thyroid radioactive uptake is usually low to normal in type 1 but low to suppressed in type 2. Serum IL-6 levels are normal to slightly elevated in type 1 and markedly elevated in type 2. Color flow sonography has been shown to differentiate between the two conditions. Type 1 shows normal vascularity (pattern 1) or increased vascularity (pattern 2) with a patchy distribution, whereas type 2 shows no vascularity (pattern 0).85 Amiodarone-associated IIT type 1 is a serious problem in many instances because of the coexisting heart disease of these patients, and treatment may often be difficult.86 Administration of a combination of methimazole and potassium perchlorate is reported to be effective in type 1.87 Treatment of type 2 thyrotoxicosis consists of administration of prednisone starting at a dose of 40 mg/day, for example. Normalization of thyroid hormone levels is achieved in about 1 week.86 Patients with previous type 2 thyrotoxicosis are at risk for hypothyroidism when given excessive iodine.88 (For review see Ref. 89.)
Radiographic contrast agents contain between 30% and 50% iodine and may also induce IIT. Patients who have multinodular goiter or live in countries where iodine intake is low are especially at risk.90 IIT often develops several weeks after the administration of radiographic contrast agents, so follow-up of such patients is advisable. In some instances, prophylactic administration of methimazole might be necessary. In view of the wide use of radiographic contrast agents, the probability of inducing IIT by these substances is probably low, but it might be inversely related to the iodine intake of the population.
Hyperthyroidism Caused by Inappropriate TSH Secretion
Selective Partial Tissue Resistance of The Pituitary to Thyroid Hormone
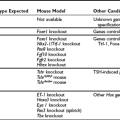
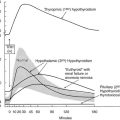
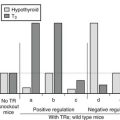
This syndrome is probably part of the spectrum of the syndromes of thyroid hormone resistance, with resistance predominant at the pituitary level. Because only the pituitary is partially resistant to thyroid hormone, the set point of the pituitary, that is, the specific TSH/thyroid hormone ratio needed to ensure normal thyroid gland activation, is set at a higher level of serum thyroid hormone concentration. Because the other body tissues appear to have a sensitivity to thyroid hormone that is at least higher than that of the pituitary but might be even normal, the clinical picture of thyrotoxicosis is present, although without eye symptoms and other characteristics specific for Graves’ disease. This syndrome may be inherited in an autosomal-dominant mode.91–94 Because no pituitary tumor is present, the ratio of TSH α subunits to total TSH is less than 1, whereas in the case of a TSH-producing pituitary tumor (see following), this ratio is usually above 1.
Hyperthyroidism Caused by A TSH-Secreting Pituitary Adenoma
A TSH-secreting pituitary tumor is a rare condition, although since the use of ultrasensitive TSH measurements, its detection has increased. In a 1993 publication, 2.8% of all pituitary tumors consisted of TSH-producing adenomas.95 More than 70% of these tumors are macroadenomas.96 Patients are usually mildly hyperthyroid, and specific symptoms of Graves’ disease are lacking. Serum levels of free T4 and/or free T3 are elevated, with a normal or increased serum TSH concentration. Visualization of the pituitary by magnetic resonance imaging shows a pituitary tumor. The concentration of TSH α subunits in blood is above normal, as is the ratio of TSH-α/ TSH.97 Pituitary TSH-secreting adenomas produce normal forms of TSH but secrete them in variable amounts with variable biologic activity, which explains the variable degree of hyperthyroidism in these patients.98 Treatment consists of surgery and postsurgical pituitary irradiation. About two thirds of patients have normal serum TSH after surgery alone or in combination with irradiation, but only one third of the patients are considered cured.99 Treatment with somatostatin analogues such as octreotide returns TSH levels to normal in 80% of patients, and tumor size shrinks in 50%. Vision improves in 75%, and euthyroidism returns in almost all patients.100 Results with long-acting somatostatin analogues have shown them to be effective as well.100 Somatostatin treatment is also effective in surgical failures.
Thyrotoxicosis Caused by Metastatic Differentiated Thyroid Carcinoma
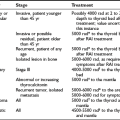
Thyrotoxicosis resulting from functioning metastasis of differentiated thyroid carcinoma is rare. Recently, 54 cases reported in the literature were analyzed.101 The age and sex distribution of these patients is no different from that of other patients with differentiated carcinoma but without thyrotoxicosis. About 85% of patients are older than 40 years, and the female-to-male ratio is 3 : 1. The clinical picture of this type of thyrotoxicosis is no different from other causes of thyrotoxicosis. Iodine uptake and thyroid hormone synthesis by the tumor are generally poor, and excessive hormone production is due to the large mass of metastatic tissue.102 The inefficient thyroid hormone synthesis is at least partly due to relative iodine deficiency in tumor tissue and the presence of abnormal thyroglobulin.103 Other deficiencies and abnormalities in the complicated process of thyroid hormone synthesis may, however, be present in carcinomatous tissue. For instance, evidence suggests that expression of the TSH receptor and Na+/I− symporter may be absent or low in carcinomatous thyroid tissue.104 In many cases, clinical symptoms are caused by T3 toxicosis, with suppressed serum TSH and normal (sometimes even low) serum T4.102,103 Recently, increased T4 to T3 conversion by deiodinases type I and type II in abundant metastatic mass has been suggested to often produce the T3 toxicosis in these cases.105 Uptake of radioactive iodine in metastatic tissue is often absent when the thyroid gland is still present. The metastatic pattern of this type of adenocarcinoma is the same as is usually found in patients with thyroid adenocarcinoma, predominantly in bone, lung, and the mediastinum.
Treatment of metastatic functioning thyroid carcinoma consists of the administration of radioactive iodine. The usual dose ranges between 3700 and 7400 MBq (100 to 200 mCi). Exacerbation of thyrotoxicosis, even precipitating thyroid storm, has been reported.106 For this reason, radioactive iodine for treatment of a functioning metastatic thyroid carcinoma should be administered with caution and only after adequate preparation of elderly patients with cardiovascular disease. If normal thyroid tissue is still present, it is appropriate to eradicate this tissue either by surgery or by radioactive iodine to ensure more efficient uptake of therapeutic doses of radioactive iodine in the metastatic tissue. Furthermore, it is dangerous to administer the above-mentioned doses of radioiodine in the presence of a normal thyroid gland, because severe radiation thyroiditis may ensue. The combination of Graves’ disease and follicular carcinoma may not be a coincidence,107,108 because recent knowledge suggests an association between Graves’ disease and thyroid carcinoma, possibly as a result of longstanding thyroid stimulation by immunoglobulins.109 In contrast, no significant association between Graves’ disease and papillary carcinoma could be detected.110 Although it has been postulated that thyroid carcinoma in patients with Graves’ disease behaves more aggressively,111 this statement has recently been denied.112
Struma Ovarii
Struma ovarii is a rare tumor that occurs in a teratoma or dermoid of the ovary. It constitutes about 1% of all ovarian tumors113 and may spread to the peritoneum.114 Often admixed with a carcinoid tumor,115 it has also been reported to occur in association with multiple endocrine neoplasia type IIA.116 Ovarian strumal carcinoid tumors have been found to synthesize different peptide hormones, including calcitonin, adrenocorticotropic hormone, somatotropin release–inhibiting factor, neuron-specific enolase, chromogranin, synaptophysin, serotonin, and other peptides.116,117 Struma ovarii is unilaterally localized in about 90% of patients, and about 80% or more are benign.118,119 Because differentiation between carcinoid and struma tissue is sometimes difficult, electron microscopic studies in combination with specific immunochemistry are sometimes necessary. Struma ovarii seldom causes hyperthyroidism. In thyrotoxicosis caused by struma ovarii, uptake of radioactive iodine by the thyroid gland is low in the presence of elevated serum thyroid hormones and suppressed TSH. Uptake of radioactive iodine over the ovarian tumor confirms the diagnosis.120 Although one would suspect that the thyroid gland would be reduced in size in thyrotoxic cases resulting from struma ovarii, in several reports, the thyroid was enlarged.118–121
The pathogenesis of hyperthyroidism caused by struma ovarii is not clear. It has been suggested that thyroid-stimulating immunoglobulins stimulate ovarian strumal tissue or that struma ovarii may become autonomous.122 Treatment of struma ovarii, either with euthyroidism or thyrotoxicosis, should be effected by removal of the ovarian tumor. In the case of coexistent thyrotoxicosis, preparation for surgery should be done by administration of antithyroid drugs, sometimes in combination with β-adrenergic blocking agents. Because of the coexisting teratoma, it is sometimes difficult to determine whether the thyroid tissue in the tumor is benign or malignant. It is not advised that patients with thyrotoxic struma ovarii be treated with radioiodine because of the possibility that the tumor is malignant, which cannot be determined on clinical grounds, and because of the unknown radiation effects on the other ovarian elements.
References
1. Docter, R, Krenning, EP, de Jong, M, et al. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf). 1993;39:499.
2. Hamburger, JI. Evolution of toxicity in solitary non-functioning thyroid nodules. J Clin Endocrinol Metab. 1980;50:1089.
3. Bransom, CJ, Talbot, CH, Henry, J, et al. Solitary toxic adenoma of the thyroid gland. Br J Surg. 1997;66:590.
4. Horst, W, Rosler, H, Schneider, C, et al. 306 Cases of toxic adenoma: clinical aspects, findings in radioiodine diagnostics, radiochromatography and histology: Results of 131-I and surgical treatment. J Nucl Med. 1967;8:515.
5. Emrich, D, Erlenmaier, U, Pohl, M, et al. Determination of the autonomously functioning volume of the thyroid. Eur J Nucl Med. 1993;20:410.
6. Studer, H, Peter, HJ, Gerber, H. Natural heterogeneity of thyroid cells: the basis for understanding thyroid function and nodular goiter growth. Endocr Rev. 1989;10:125.
7. O’Sullivan, C, Barton, CM, Staddon, SI, et al. Activation point mutations of the GSP oncogene in human thyroid adenomas. Mol Carcinog. 1992;4:345.
8. Holtzapfel, H-P, Bergner, B, Wonerof, P, et al. Expression of Gas proteins and TSH receptor signaling in hyperfunctioning thyroid nodules with TSH receptor mutations. Eur J Endocrinol. 2002;147:109.
9. Trulzsch, B, Krohn, K, Wonerow, P, et al. Detection of thyroid-stimulating hormone receptor and Gsalpha mutations in 75 toxic thyroid nodules by denaturing gradient gel electrophoresis. J Mol Med. 2001;78:684.
10. Krohn, K, Fuhrer, D, Holzapfel, HP, et al. Clonal origin of toxic thyroid nodules with constitutively activating thyrotropin receptor mutations. J Clin Endocrinol Metab. 1998;83:130–134.
11. Derwahl, M. TSH receptor and Gs-alpha gene mutations in the pathogenesis of toxic thyroid adenoma: a note of caution [editorial]. J Clin Endocrinol Metab. 1996;81:1783.
12. Derwahl, M, Studer, H. Nodular goiter and goiter nodules: where iodine deficiency falls short of explaining the facts. Exp Clin Endocrinol Diabetes. 2001;109:250.
13. Van Sande, J, Massart, C, Costagliola, S, et al. Specific activation of the thyrotropin receptor by trypsin. Mol Cell Endocrinol. 1996;119:161.
14. Deleu, S, Allory, Y, Radulescu, A, et al. Characterization of autonomous thyroid adenoma: metabolism, gene expression, and pathology. Thyroid. 2000;10:131.
15. Fuhrer, D, Lewis, MD, Alkhafaji, F, et al. Biological activity of activating thyroid-stimulating hormone receptor mutants depends on the cellular context. Endocrinology. 2003;144:4018.
16. Sandler, MP, Fellmeth, B, Salhany, KE, et al. Thyroid carcinoma masquerading as a solitary benign hyperfunctioning nodule. Clin Nucl Med. 1988;30:410.
17. Hamburger, JI. Solitary autonomously functioning thyroid lesions: diagnosis, clinical features and pathogenetic considerations. Am J Med. 1975;58:740.
18. Eyre-Brook, IA, Talbot, CH. The treatment of autonomous functioning thyroid nodules. Br J Surg. 1982;69:577.
19. Ratcliffe, GE, Cooke, S, Fogelman, I, et al. Radioiodine treatment of solitary functioning thyroid nodules. Br J Radiol. 1986;59:385.
20. Ross, DS, Ridgway, EC, Daniels, GH. Successful treatment of solitary toxic nodules with relatively low-dose 131I with low prevalence of hypothyroidism. Ann Intern Med. 1984;101:488.
21. Goldstein, R, Hart, IR. Follow-up of solitary autonomous thyroid nodules treated with 131I. N Engl J Med. 1983;309:1473.
22. Mariotti, S, Martino, E, Francesconi, M, et al. Serum thyroid auto-antibodies as a risk factor for development of hypothyroidism after radioactive iodine therapy for single thyroid “hot” nodule. Acta Endocrinol (Copenh). 1986;113:500.
23. Lippi, F, Ferrari, C, Manetti, L, et al. Treatment of solitary autonomous thyroid nodules by percutaneous ethanol injection: results of an Italian multicenter study. The Multicenter Study Group. J Clin Endocrinol Metab. 1996;81:3261.
24. Monzani, F, Caraccio, N, Goletti, O, et al. Treatment of hyperfunctioning thyroid nodules with percutaneous ethanol injection: eight years’ experience. Exp Clin Endocrinol Diabetes. 1998;106(Suppl 4):S54.
25. Døssing, H, Bennedbaek, FN, Bonnema, SJ, et al. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol. 2007;157:95.
26. Tokuda, Y, Kasagi, K, Lida, Y, et al. Sonography of subacute thyroiditis: changes in the findings during the cause of the disease. J Clin Ultrasound. 1990;18:21.
27. Vitug, AC, Goldman, JM. Silent (painless) thyroiditis: evidence of a geographic variation in frequency. Arch Intern Med. 1985;145:437.
28. Schneeberg, NG. Silent thyroiditis. Arch Intern Med. 1983;143:2214.
29. Lazarus, JH, Ammari, F, Oretti, R, et al. Clinical aspects of recurrent postpartum thyroiditis. Br J Gen Pract. 1997;47:305.
30. Farid, NR, Hawe, BS, Walfish, PG. Increased frequency of HLA-D3 and 5 in the syndromes of painless thyroiditis with transient thyrotoxicosis: evidence for an auto immune etiology. Clin Endocrinol. 1983;19:669.
31. Chow, CC, Lee, S, Shek, CC, et al. Lithium-associated transient thyrotoxicosis in four Chinese women with autoimmune thyroiditis. Aust N Z J Psychiatry. 1993;27:246.
32. Sauter, MP, Atkins, MB, Meir, JW, et al. Transient thyrotoxicosis and persistent hypothyroidism during acute autoimmune-thyroiditis after interleukin-2 and interferon-alpha therapy for metastatic carcinoma: a case report. Am J Med. 1992;92:441.
33. Mittra, ES, McDougall, IR. Recurrent silent thyroiditis: a report of four patients and review of the literature. Thyroid. 2007;17:671.
34. Sakata, S, Nagai, K, Shibata, T, et al. A case of rheumatoid arthritis associated with silent thyroiditis. J Endocrinol Invest. 1992;15:377.
35. Yamamoto, M, Fuwa, Y, Chimori, K, et al. A case of progressive systemic sclerosis (PSS) with silent thyroiditis and anti-bovine thyrotropin antibodies. Endocrinol Jpn. 1991;38:265.
36. Itaka, M, Ishii, J, Ishikaea, N, et al. A case with Graves’ disease with false hyperthyrotropinemia who developed silent thyroiditis. Endocrinol Jpn. 1991;38:667.
37. Parker Klien, I, Fishman, LM, Levey, GS. Silent thyrotoxic thyroiditis in association with chronic adrenocortical insufficiency. Arch Intern Med. 1983;143:2214.
38. Magaro, M, Zoli, A, Altomonte, L, et al. The association with silent thyroiditis and active systemic lupus erythematosus. Clin Exp Rheumatol. 1992;10:67.
39. Mizukami, Y, Michigishi, T, Hashimoto, T, et al. Silent thyroiditis: a histologic and immunohistochemical study. Hum Pathol. 1988;19:423.
40. Wolff, PD. Transient painless thyroiditis with hyperthyroidism: a variant of lymphocytic thyroiditis? Endocr Rev. 1980;1:411.
41. Gegick, CG, Harring, WB. Painless subacute thyroiditis: a report of two cases. N C Med J. 1977;38:387.
42. Nicolai, TF, Coombs, GJ, McKenzie, AK, et al. Treatment of lymphocytic thyroiditis with spontaneously resolving hyperthyroidism (silent thyroiditis). Arch Intern Med. 1982;142:2281.
43. Nicolai, TF, Coombs, GJ, McKenzie, AK. Lymphocytic thyroiditis with spontaneously resolving hyperthyroidism and subacute thyroiditis: long-term follow-up. Arch Intern Med. 1981;141:1455.
44. Bogazzi, F, Bartalena, LA, Vitti, P, et al. Color flow Doppler sonography in thyrotoxicosis factitia. J Endocrinol Invest. 1996;19:603.
45. Kinney, JS, Hurwitz, ES, Fishbein, DB, et al. Community outbreak of thyrotoxicosis: epidemiology, immunogenetic characteristics and long-term outcome. Am J Med. 1988;84:10.
46. Hedberg, CW, Fishbein, DB, Janssen, RS, et al. An outbreak of thyrotoxicosis caused by the consumption of bovine thyroid gland in ground beef. N Engl J Med. 1987;316:993.
47. Nisula, BC, Ketelslegers, J-M. Thyroid-stimulating activity and chorionic gonadotropin. J Clin Invest. 1974;54:494.
48. Kenimer, JG, Hershman, JM, Higgins, HP. The thyrotropin in hydatidiform moles is human chorionic gonadotropin. J Clin Endocrinol Metab. 1975;40:482.
49. Nisula, BC, Taliadouros, GS. Thyroid function in gestational trophoblastic neoplasia: evidence that the thyrotropic activity of chorion gonadotropin mediates the thyrotoxicosis of choriocarcinoma. Am J Obstet Gynecol. 1980;77:138.
50. Carayon, P, Lefort, G, Nisula, BC. Interaction of human chorionic gonadotropin and human luteinizing hormone with human thyroid membranes. Endocrinology. 1980;106:1907.
51. Williams, JF, Davies, TF, Catt, KJ, et al. Receptor-binding activity of highly purified bovine luteinizing hormone and thyrotropin, and their subunits. Endocrinology. 1980;106:1353.
52. Yoshimura, M, Hershman, JM, Pang, XP, et al. Activation of the thyrotropin (TSH) receptor by human chorionic gonadotropin and luteinizing hormone in Chinese hamster cells expressing human TSH receptors. J Clin Endocrinol Metab. 1993;77:1009.
53. Yoshimura, M, Hershman, JM. Thyrotropic action of human chorionic gonadotropin. Thyroid. 1995;5:425.
54. Carayon, P, Amir, S, Nisula, B, et al. Effect of carboxypeptidase digestion of the human choriogonadotropin molecule on its thyrotropic activity. Endocrinology. 1981;108:1891.
55. Cole, LA, Kardana, A. Discordant results in human chorionic gonadotropin assays. Clin Chem. 1992;38:263.
56. Ouchimura, H, Nagataki, S, Ito, K, et al. Inhibition of the thyroid adenylate cyclase response to thyroid-stimulating immunoglobulin G and asialo-chorionic gonadotropin. J Clin Endocrinol Metab. 1982;55:347.
57. Hoerman, R, Broecker, M, Grossmann, M, et al. Interaction of human chorionic gonadotropin (hCG) and asialo-hCG with recombinant human thyrotropin receptor. J Clin Endocrinol Metab. 1994;78:933.
58. Hoerman, R, Amir, SM, Ingbar, SH. Evidence that partially desialylated variants of human chorionic gonadotropin (hCG) are the factors in crude hCG that inhibit the response to thyrotropin in human thyroid membranes. Endocrinology. 1988;123:1535.
59. Davies, TF, Platzer, M. hCG-induced TSH receptor activation and growth acceleration in FRTL-thyroid cells. Endocrinology. 1986;118:2149.
60. Hershman, JM, Lee, H-Y, Sugawara, M, et al. Human chorionic gonadotropin stimulates iodide uptake, adenylate cyclase, and deoxyribonucleic acid synthesis in cultured rat thyroid cells. J Clin Endocrinol Metab. 1988;67:74.
61. Glinoer, D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404.
62. Glinoer, D. Thyroid hyperfunction during pregnancy. Thyroid. 1998;8:859.
63. Goodwin, TM, Hershman, JM, Cole, L. Increased concentration of the free beta-subunit of human chorionic gonadotropin in hyperemesis gravidarum. Acta Obstet Gynecol Scand. 1994;73:770.
64. Rodien, P, Bremont, C, Rafin Sanson, M-L, et al. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med. 1998;339:1823.
65. Hershman, JM, Higgins, P. Hydatidiform mole: a cause of clinical hyperthyroidism. N Engl J Med. 1971;284:573.
66. Dowling, JT, Ingbar, SH, Frenkel, N. Iodine metabolism in hydatidiform mole and choriocarcinoma. J Clin Endocrinol Metab. 1960;20:1.
67. Kock, H, Vessel, HV, Stolte, L, et al. Thyroid function in molar pregnancy. J Clin Endocrinol Metab. 1966;26:1128.
68. Higgins, HP, Hershman, JM, Kenimer, JG, et al. The thyrotoxicosis of hydatidiform mole. Ann Intern Med. 1975;83:307.
69. Yoshimura, M, Pekary, AE, Pang, XP, et al. Thyrotropic activity of basic isoelectric forms of human chorionic gonadotropin extracted from hydatidiform mole tissues. J Clin Endocrinol Metab. 1994;78:862.
70. Anderson, NR, Lockich, JJ, McDermott, WV, Jr., et al. Gestational choriocarcinoma and thyrotoxicosis. Cancer. 1979;44:304.
71. Orgiazzi, JJ, Rousset, B, Consentino, C, et al. Plasma thyrotropic activity in a man with choriocarcinoma. J Clin Endocrinol Metab. 1974;39:653.
72. Fradkin, JE, Wolff, J. Iodine-induced thyrotoxicosis. Medicine (Baltimore). 1983;62:1.
73. Connolly, RJ, Vidor, GI, Stewart, JC. Increase in thyrotoxicosis in endemic goitre area after iodination of bread. Lancet. 1970;1:500.
74. Stewart, JC, Vidor, GI. Thyrotoxicosis induced by iodine contamination of food: a common unrecognized condition? Br Med J. 1976;1:372.
75. Stanbury, JB, Ermans, AE, Pourdiux, P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8:83.
76. Burman, KD, Wartofsky, L. Iodine effects on the thyroid gland: biochemical and clinical aspects. Rev Endocr Metab Disord. 2000;1:19–25.
77. Leger, AF, Massin, JP, Laurent, MF, et al. Iodine-induced thyrotoxicosis: analysis of eighty-five consecutive cases. Eur J Clin Invest. 1984;14:449.
78. Martin, FIR, Tress, BW, Colman, P, et al. Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly. Am J Med. 1933;95:78.
79. Bournaud, C, Orgiazzi, JJ. Iodine excess and thyroid autoimmunity. J Endocrinol Invest. 2003;26(2 Suppl):49–56.
80. Many, M-C, Mestdagh, C, van den Hove, M-F, et al. In vitro study of acute toxic effects of high iodide doses in human thyroid follicles. Endocrinology. 1992;131:621.
81. Hennemann, G, Docter, R, Friesema, ECH, et al. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451–476.
82. Bianco, AC, Salvatore, D, Gereben, B, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89.
83. Martino, E, Aghini-Lombardi, F, Bartalena, L, et al. Enhanced susceptibility to amiodarone-induced hypothyroidism in patients with thyroid autoimmune disease. Arch Intern Med. 1994;154:12.
84. Cappiello, E, Boldorini, R, Tosoni, A, et al. Ultrastructural evidence of thyroid damage in amiodarone-induced thyrotoxicosis. J Endocrinol Invest. 1995;18:862.
85. Bogazzi, F, Bartalena, L, Brogioni, S, et al. Color flow Doppler sonography differentiates type 1 and type 2 amiodarone-induced thyrotoxicosis. Thyroid. 1997;7:541.
86. Bartalena, L, Brogioni, S, Grasso, L, et al. Treatment of amiodarone-induced thyrotoxicosis, a difficult challenge: Results of a prospective study. J Endocrinol Metab. 1996;81:2930.
87. Reichert, LJ, de Rooy, HA. Treatment of amiodarone induced hyperthyroidism with potassium perchlorate and methimazole during amiodarone treatment. Br Med J. 1989;298:1547.
88. Roti, E, Minelli, R, Gardini, E, et al. Iodine-induced subclinical hypothyroidism in euthyroid subjects with a previous episode of amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 1992;75:1273.
89. Basaria, S, Cooper, DS. Amiodarone and the thyroid. Am J Med.. 2005;118:706–714.
90. Martino, E, Aghini-Lombardi, F, Mariotti, S, et al. Amiodarone: a common source of iodine-induced thyrotoxicosis. Horm Res. 1987;26:158.
91. Emerson, CH, Utiger, RD. Hyperthyroidism and excessive thyrotropin secretion. N Engl J Med. 1972;287:328.
92. Gershengorn, MC, Weintraub, BD. Thyrotropin-induced hyperthyroidism caused by selective pituitary resistance to thyroid hormone. J Clin Invest. 1975;56:633.
93. Rösler, A, Litvin, Y, Hage, C, et al. Familial hyperthyroidism due to inappropriate thyrotropin secretion successfully treated with triiodothyronine. J Clin Endocrinol Metab. 1982;54:76.
94. Catargi, B, Monsaingeon, M, Bex-Bachellerie, V, et al. A novel thyroid hormone receptor-beta mutation, not anticipated to occur in resistance to thyroid hormone, causes variable phenotypes. Horm Res. 2002;57:137.
95. Mindermann, T, Wilson, CB. Thyrotropin-producing pituitary adenomas. J Neurosurg. 1993;79:521.
96. Beck-Peccoz, P, Brucker-Davis, F, Persani, L, et al. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610.
97. McDermott, MT, Ridgway, EC. Central hyperthyroidism. Endocrinol Metab Clin North Am. 1998;27:187.
98. Sergi, E, Medri, G, Papandreou, MJ, et al. Polymorphism of thyrotropin and alpha subunit in human pituitary adenomas. J Endocrinol Invest. 1993;16:45–55.
99. Shimon, I, Melmed, S. Management of pituitary tumors. Ann Intern Med. 1998;129:472.
100. Kienitz, T, Quinkler, M, Strasburger, CJ, et al. Long-term management in five cases of TSH-secreting pituitary adenomas: a single center study and review of the literature. Eur J Endocrinol. 2007;157:39–46.
101. Salvatori, M, Saletnich, I, Rufini, V, et al. Severe thyrotoxicosis due to functioning pulmonary metastasis of well-differentiated cancer. J Nucl Med. 1998;39:1202.
102. Paul, SJ, Sisson, JC. Thyrotoxicosis caused by thyroid cancer. Endocrinol Metab Clin North Am. 1990;19:593.
103. Nakashima, T, Enue, K, Shiro-osu, A, et al. Predominant T3 synthesis in the metastatic thyroid carcinoma in a patient with T3-toxicosis. Metabolism. 1981;30:327.
104. Caillou, B, Troalen, F, Baudin, E, et al. NA+/I− symporter distribution in human thyroid tissues: an immunohistochemical study. J Clin Endocrinol Metab. 1998;83:4102.
105. Takano, T, Miyauchi, A, Ito, Y, et al. Thyroxine to triiodothyronine hyperconversion thyrotoxicosis in patients with large metastases of follicular thyroid carcinoma. Thyroid. 2006;16:615–618.
106. Cerletty, JM, Listwan, WJ. Hyperthyroidism due to functioning metastatic thyroid carcinoma: precipitation of thyroid storm with therapeutic radioactive iodine. JAMA. 1979;242:269.
107. Kasagi, K, Takeichi, R, Miyamoto, S, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clin Endocrinol. 1994;40:429.
108. Steffensen, FH, Aunsholt, NA. Hyperthyroidism associated with metastatic thyroid carcinoma. Clin Endocrinol. 1994;41:685.
109. Mazzaferri, EL. Thyroid cancer and Graves’ disease [editorial]. J Clin Endocrinol Metab. 1990;70:826.
110. Phitayakorn, R, McHenry, CR. Incidental thyroid carcinoma in patients with Graves’ disease. Am J Surg. 2008;195:292.
111. Belfiore, A, Charofalo, MR, Giuffrida, D. Increased aggressiveness of thyroid cancer in patients with Graves’ disease. J Clin Endocrinol Metab. 1990;70:830.
112. Hales, IB, McElduff, A, Crummer, P, et al. Does Graves’ disease or thyrotoxicosis affect the prognosis of thyroid cancer. J Clin Endocrinol Metab. 1992;75:886.
113. Ayhan, A, Yanik, F, Tuncer, R, et al. Struma ovarii. Int J Gynaecol Obstet. 1993;42:143.
114. Roth, LM, Karseladze, AI. Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol. 2008;27:213.
115. Tamsen, A, Mazur, MT. Ovarian strumal carcinoid in association with multiple endocrine neoplasia, type IIA. Arch Pathol Lab Med. 1992;116:200.
116. Sakura, H, Fujii, T, Okamoto, K. A study of human calcitonin in an ovarian carcinoid and ovarian cancers. Exp Clin Endocrinol. 1991;97:91.
117. Ozerwenka, KF, Schon, HJ, Bock, P. Immunochemical and ultrastructural studies of an ovarian strumal carcinoid. Wien Klin Wochenschr. 1990;102:687.
118. Kempers, RD, Dockerty, MB, Hoffman, DL, et al. Struma _ovarii-ascitic, hyperthyroid and asymptomatic syndromes. Ann Intern Med. 1970;72:883.
119. Devaney, K, Snyder, R, Norris, HJ, et al. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynaecol Pathol. 1993;12:333.
120. Pardo-Mindan, FJ, Vazquez, JJ. Malignant struma ovarii: light and electron microscopic study. Cancer. 1983;51:337.
121. Ross, DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am. 1998;27:169.
122. Bayot, MR, Chopra, IJ. Coexistence of struma ovarii and Graves’ disease. Thyroid. 1995;5:469.