Chapter 92
Autogenous Grafts
Scott A. Berceli
Despite ongoing attempts to develop prosthetic or bioengineered materials for bypass grafting in the lower extremities, autologous vein graft remains the conduit of choice for infrainguinal revascularization. Though it was sporadically used for repair of popliteal aneurysms in the early 20th century, the first report of the routine use of autogenous vein for the treatment of occlusive arterial disease was provided in 1944 by Dos Santos, who used segments of vein as patch material after superficial femoral artery endarterectomy.1 This was followed several years later by the report of Kunlin, who described the use of reversed saphenous vein grafts for the treatment of focal lesions within the superficial femoral artery.2 Based on these initial reports, expanded use of the saphenous vein for both endarterectomy and grafting was attempted, but creation of the anastomoses with the standard surgical techniques of the day proved difficult. With long-term patency negatively influenced by a high rate of anastomotic stricture and an inability to replicate the promising results of Kunlin, the majority of practitioners in the 1940s and 1950s used arterial homografts or the newly developed prosthetic grafts for infrainguinal arterial reconstruction. Reinvigorating the use of autogenous vein, however, was a small group of surgeons who traveled to Europe to observe Kunlin’s vein grafting procedures.3 Characterized by a spatulated end-to-side anastomosis using a small-caliber running silk suture, these meticulous techniques allowed Linton, Darling, and others to duplicate the reported success with autogenous vein grafting.4
In the 1960s, wider interest in the use of autogenous vein was fueled by the observation that acceptable long-term patency of prosthetic bypass grafts could be achieved only with distal anastomoses placed in the above-knee position.5 Treatment of critical limb ischemia in patients with compromised outflow via femorotibial bypass was a natural extension for autogenous vein grafting, with the first published report by Dale in 1963.6 Although initial published reports of the use of saphenous vein for tibial bypass grafts suggested it to be notably inferior to femoropopliteal grafts,7,8 further refinements in the techniques have proved the durability of tibial as well as inframalleolar bypass grafting with autologous vein.9 Along with these technical improvements in the 1980s came increasing experience with alternative autogenous vein sources (i.e., arm, small saphenous, and femoral veins),10 thereby expanding the potential for autogenous tissue reconstruction to 95% of patients undergoing infrainguinal bypass.11
Histology
Analogous to other vascular structures, veins are composed of three layers—the tunicae intima, media, and adventitia (Fig. 92-1). The intima consists of a continuous monolayer of cuboidal endothelial cells supported by a subendothelial layer of loose connective tissue. In contrast to the arterial endothelium, where cells are tightly packed and highly aligned in the direction of flow, venous endothelial cells are cuboidal with poorly developed interendothelial tight junctions.12,13 The resulting enhanced permeability to blood solutes, in conjunction with the underlying physiologic differences between arterial and venous endothelium, is thought to be among the underlying mechanisms for the accelerated intimal hyperplasia characteristic of vein grafts.14 The intima is separated from the media by a poorly developed internal elastic lamina with large fenestrae that allow diffusion of substances into the wall. The media is composed of vascular smooth muscle cells and extracellular matrix, with type I collagen being the dominant component of this layer. Unlike arteries, which function in a pulsatile pressure environment and correspondingly have high elastin content, veins are exposed to small excursions in pressure and demonstrate only sparse elastin fragments. Transposition of a vein into the arterial circulation induces a marked increase in intraluminal wall tension that greatly exceeds the dynamic range of the elastic elements and therefore leads to a rigid graft with substantial compliance mismatch in comparison to the adjacent artery.15
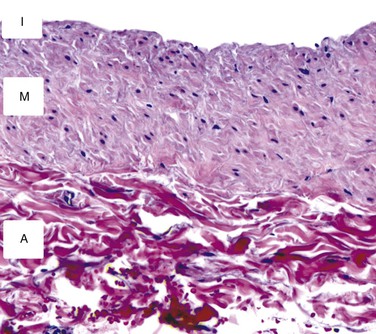
Figure 92-1 Cross-sectional histology of the normal saphenous vein. The intima (I) is composed of a single layer of endothelial cells and a thin basement membrane. The media (M) consists of smooth muscle cells surrounded by a tightly packed extracellular matrix. The adventitia (A) is a loose network of collagen-dominated extracellular matrix with sporadic inflammatory cells and fibroblasts.
External to a poorly developed external elastic lamina is the adventitia, a loose connection of extracellular matrix with a sparse vasa vasorum. Though traditionally thought to have limited influence on biologic function of the vein, evolving evidence suggests that remodeling of an implanted vein graft is very much dependent on adaptation within the adventitia. Recruitment of myofibroblasts, production of cytokines, and the influx of inflammatory cells into the adventitia are thought to have an important influence on the biology of the early vein graft.
Superficial extremity veins are prone to the development of a range of pathologies such as varicose degeneration, thrombosis with luminal recanalization, and phlebitis with intramural calcification. Limitations in available autogenous conduit frequently require these “compromised” vein segments to be included as conduit in a revascularization procedure. Histologic evaluation of harvested vein segments, coupled with long-term clinical follow-up, has demonstrated that grafts with low endothelial cell coverage, stenosis of the lumen, and thick walls are at an increased risk for early graft failure.16 Grafts with subendothelial spindle-shaped cells, which demonstrate a proliferative smooth muscle phenotype on electron microscopic examination, and calcification within the wall at the time of graft implantation are notably prone to the development of aggressive hyperplastic lesions.17 Unfortunately, preoperative ultrasound is unable to reliably identify many of these abnormalities.18
Anatomy
Lower Extremity Veins
The veins of the lower extremities are classified into three general groups: the deep, superficial, and perforating systems. The deep veins are encompassed by thick fascia and are contained within the muscular compartment of the leg. Superficial veins are bounded deeply by the muscular fascia and superficially by the dermis. Short veins piercing through the fascia and running between the superficial and deep systems are the perforating veins. Increasing interest in accurate description of the venous structures in the leg and confusion between the clinical and anatomic terminology used in naming them have prompted a review and standardization of the nomenclature. The following descriptions are based on this consensus terminology,19,20 with reference to the older nomenclature as appropriate.
The great saphenous vein (formerly called the greater or long saphenous vein) begins just anterior to the medial malleolus, crosses the tibia, traverses medial to the knee, and ascends in the medial-posterior aspect of the thigh to the groin. Duplicated systems within the great saphenous vein are relatively common and occur at a 25% and 8% incidence in the calf and thigh, respectively.21 The accessory great saphenous veins run parallel to the main trunk within the thigh and leg, in an anterior or posterior location. Although these veins are at times mistaken for the great saphenous vein during surgical exposure, they are usually of inadequate diameter for use in bypass grafting. The saphenous veins, but not their tributaries, are covered by a fibrous sheath termed the saphenous fascia. Less well developed than the deep fascia, this fascia, in combination with the deeper muscular fascia, forms a subcompartment that extends along the length of the lower extremity. Recognition of this fascia can be useful in localizing the great saphenous vein during surgical dissection. In the proximal portion of the thigh, the great saphenous vein receives one or two large tributaries from the anterior or posterior accessory great saphenous vein (or both) and enters the fossa ovalis approximately 4 cm inferior and lateral to the pubic tubercle, where it joins with the superficial circumflex iliac, superficial epigastric, and external pudendal veins to create the confluence of superficial inguinal veins (formerly called the saphenofemoral junction) on the anterior surface of the common femoral vein.
The small saphenous vein (formerly called the lesser or short saphenous vein) ascends lateral to the Achilles tendon in the calf. It runs in the subcutaneous adipose tissue in the lower two thirds of the calf, pierces the muscular fascia, and courses between the medial and lateral heads of the gastrocnemius. The small saphenous vein usually joins the popliteal vein in the popliteal fossa about 5 cm proximal to the knee crease. Rarely, the small saphenous vein ends high, either emptying into the femoral vein or running superficially to the posteromedial aspect of the thigh to join the great saphenous vein.
Particularly useful in arterial reconstructions within infected fields, the femoral vein (formerly called the superficial femoral vein) has increasingly become a potential source of autogenous conduit. The femoral vein originates from the popliteal vein at the upper margin of the popliteal fossa and courses in the femoral canal. The femoral vein is lateral to the femoral artery in the distal part of the thigh but crosses under and runs medial to the femoral artery in the proximal portion of the leg before joining the deep femoral (profunda femoris) veins approximately 9 cm below the inguinal ligament. During harvest of the femoral-popliteal vein segment for use as an autogenous graft, preservation of the junction of the profunda femoris vein with the common femoral vein is critical for maintaining adequate venous outflow from the limb.22,23
Upper Extremity Veins
Veins of the upper extremity are also divided into superficial and deep groups, although direction of flow from superficial to deep is not as distinct as in the lower extremity. The cephalic vein, which originates on the posterolateral aspect of the wrist, gradually swings across the lateral portion of the forearm, ascends lateral to the biceps muscle into the deltopectoral groove, and passes through the clavipectoral fascia to join the axillary vein. The basilic vein swings across the posteromedial side of the forearm, passes lateral to the antecubital fossa, and ascends medially in the arm, where it joins the brachial veins to become the axillary vein. The median cubital vein starts at the apex of the antecubital fossa as a branch of the cephalic vein and ascends medially to enter the basilic vein.
Approximately 10 cm proximal to the antecubital fossa, the basilic vein passes through the deep fascia to reside along the medial side of the brachial artery. Harvest of the basilic vein proximal to this location requires longitudinal division of this fascia and ligation of multiple communicating branches coursing between the basilic and brachial veins. During this portion of the dissection, the medial cutaneous nerve of the forearm is often first identified as it travels anterior to the basilic vein. Injury to this nerve can lead to paresthesias along the medial portion of the forearm.
Vein Graft Preparation
Preoperative Vein Mapping
As surgeons have increased the complexity of lower extremity reconstruction and become more aggressive with repeat revascularization after graft failure, assessment of available autogenous conduit has evolved into an essential component of preoperative planning. Initial attempts to evaluate available conduit centered around the use of routine preoperative saphenous venography, with information obtained from these studies influencing the operative approach in up to 30% of patients.24 Although useful in defining the anatomy and dimensions of the greater saphenous system, broad application of this technique was limited by its requirement for percutaneous access, frequent underestimation of conduit diameter, and the small but worrisome risks of nephrotoxicity or chemically induced thrombophlebitis.
With refinements in ultrasound instrumentation and the increasing availability of skilled technologists, investigations into the merits of B-mode imaging for preoperative conduit evaluation were initiated.25–28 By providing the ability to determine vein size and quality in a physiologic environment and facilitate operative exposure, duplex vein mapping generated significant enthusiasm. Early reports were mixed, however, with concern that duplicate saphenous systems were frequently not identified and diameters measured on B-mode imaging were not representative of in vivo graft geometries.25 Although the former issue has essentially been solved by increased experience of vascular technologists and an understanding of anatomic variants, difficulties with direct correlation of preoperative mapping and graft size remain. Among the important issues in minimizing this variability is the development of a standard preoperative evaluation protocol.
Warming the examination room, placing warmed blankets on the extremities, and using prewarmed ultrasonic gel minimize peripheral venous vasoconstriction. Imaging with a high-frequency probe (≥8 MHz) provides maximum resolution with 0.3-mm point-to-point discrimination. Veins are assessed in cross-section to evaluate compressibility, wall thickening, and the presence of intraluminal echoes, and sites of thrombus, intraluminal webs, and sclerotic walls are noted. Vein wall abnormalities have been described in 12% of potential conduits, with preoperative duplex evaluation correctly identifying 62% of these diseased segments.29 To assess the great saphenous vein, patients are placed in a modified reversed Trendelenburg position and images collected for measurement of lumen diameter at six locations along the length of the limb. Evaluation of the small saphenous vein is performed with the patient in a prone position and the diameter measured at three locations within the calf. If either of the saphenous veins is of good quality but inadequate size, the patient is placed in a standing position and diameter measurements are repeated. Alternatively, a tourniquet may be placed on the proximal part of the thigh to facilitate this reassessment. For segments identified to be of adequate size and quality for bypass grafting, the overlying skin is marked with indelible ink. Routine evaluation of the femoral vein may also be warranted depending on local clinical practice. In addition to lumen diameter, examination should include a thorough assessment for deep venous thrombosis and valvular incompetence. Use of standardized reference zones facilitates the reporting of relevant information, with zones 2, 3, and 4 corresponding to the proximal, middle, and distal portions of the thigh and zones 5, 6, and 7 corresponding to the proximal, middle, and distal portions of the calf, respectively.30 Evaluation of the upper extremity cephalic and basilic veins should follow a similar protocol. An upper arm tourniquet may be used selectively or routinely to facilitate these measurements. Reference zones have also been established for the upper extremity, where zones 3, 4, and 5 correspond to the proximal, middle, and distal portions of the arm and zones 6, 7, and 8 correspond to the proximal, middle, and distal portions of the forearm, respectively.30 Measurements of upper extremity vein diameter seem even more variable than those of the lower extremity. Although the underlying etiology is open to speculation, this variability should be recognized, and repeat testing of borderline upper extremity veins may prove valuable in preoperative planning.
Despite multiple attempts to define acceptable conduit size, no clear consensus has emerged. Smaller-diameter veins have repeatedly been shown to negatively influence long-term patency,31,32 and a minimum suitable diameter ranging between 2.0 and 3.0 mm has been proposed.33 Among the confounding issues is the variable effect of the mapping technique on approaching an accurate in vivo graft diameter. These variations are nonlinear and in part dependent on the initial size of the conduit; whereas a 2.5-mm vein increases 27% in diameter when placed in the arterial circulation, a 6-mm vein increases only 8%.27 In the absence of a universally accepted vein mapping protocol, absolute delineation of a minimum acceptable vein size is problematic, and development of size criteria within an institutional practice is most appropriate.
Vein Handling Considerations
Research over the last decade has uncovered the importance of the endothelium in vascular injury and repair. Previously thought to be a passive monolayer with predominantly barrier functions, endothelial cells have been identified as central mediators in the maintenance of vascular homeostasis. Advanced by the work of Ross and his development of the response-to-injury hypothesis,34–36 loss of endothelial integrity induces platelet deposition and elaboration of growth factors. Coupled with recruitment of inflammatory mediators, these growth factors stimulate smooth muscle proliferation and matrix deposition, which lead to the formation of an occlusive arterial lesion. Analogous mechanisms are thought to be fundamental in the pathologic remodeling of vein grafts, with early endothelial injury among the initiating events in the development of intimal hyperplasia.37 Accordingly, maintenance of an intact endothelial monolayer during vein graft harvest is thought to provide improved long-term patency of autogenous vein grafts. Work by LoGerfo38 and others39 in a canine model supports this contention by demonstrating that smooth muscle cells are maintained in a contractile (nonproliferative) phenotype and that leukocyte infiltration is reduced when endothelial injury is avoided. Analogous work by Conte’s group confirmed that surgical manipulation of human vein segments results in a significant increase in monocyte adhesion.40 However, direct evidence linking improved endothelial integrity to a reduction in intimal hyperplasia, enhanced outward remodeling, or improved patency is lacking. Nonetheless, significant indirect evidence supports the concept that endothelial preservation minimizes the early biologic perturbations within vein grafts, and therefore harvest techniques aimed at reducing endothelial dysfunction seem reasonable.
Dissection
Precise, atraumatic dissection and minimal direct manipulation of the vein via a “no-touch” technique have been demonstrated to reduce endothelial damage during harvest (Fig. 92-2).41–44 Though somewhat a misnomer, the “no-touch” technique involves limited handling of the vein without direct application of forceps or vascular clamps. Major branches are ligated away from the wall to avoid narrowing of the lumen and promote outward remodeling after implantation. Opinions regarding the importance of maintaining continuity of the vein during dissection versus early ligation for intermittent infusion of isotonic fluids are mixed. Despite not being studied in a directed manner, continued continuity of vein has been shown to reduce the formation of small thrombi on the wall, and cannulation and division of the distal vein promote the direct application of antithrombotic and smooth muscle relaxants directly into the lumen. Although limited vein graft handling is universally considered an important component of promoting both short- and long-term graft patency, this concept is challenged by the nearly identical clinical outcomes for reversed, nonreversed, and in situ vein grafting techniques.45,46 If limiting vein manipulation is a dominant factor, one might intuitively expect improved outcomes with in situ grafting, where direct vein manipulation can be essentially eliminated, and compromised outcomes with nonreversed/excised grafts, which require complete mobilization with valvulotomy. The absence of such differences suggests that careful dissection of the vein is prudent, but absent extensive injury, it is of only modest importance to final outcomes.
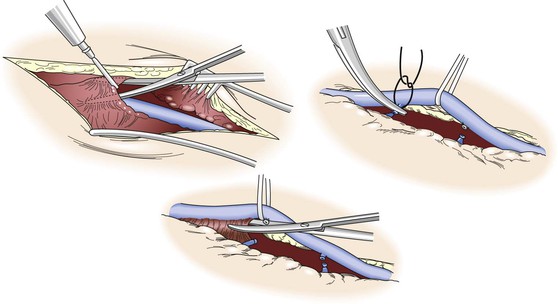
Figure 92-2 “No-touch” technique used in the harvest of autogenous vein. To minimize spasm, papaverine (120 mg/L) is injected into the periadventitial plane before surgical exposure. Side branches are identified and ligated several millimeters away from the wall to prevent luminal narrowing after implantation and outward remodeling of the vein graft. Using a Silastic vessel loop to control the vein without direct manipulation with surgical instruments, the periadventitial tissues are divided and the vein is excised.
Though not examined in the setting of peripheral vein grafts, saphenous vein encased by a thick pedicle of surrounding adipose tissue has been used for coronary bypass grafting with promising results. Morphologic and physiologic studies have confirmed reduced endothelial injury and increased expression of endothelial nitric oxide synthase when compared with the standard technique for vein harvest.47,48 Prospective randomized clinical testing has demonstrated improved short- and long-term patency with use of the pedicle harvest technique, with 90% versus 76% patency rates at 8.5 years for pedicle versus standard grafts.49,50 Although this technique may not be applicable to the long-segment grafts required for lower extremity revascularization, it is interesting to speculate on the underlying mechanisms for these improved outcomes, which could include decreased endothelial injury, maintenance of an intact vasa vasorum, or the potential delivery of biologic mediators from the surrounding periadventitial tissue.
Distention Pressure
Cannulation of the vein is required before implantation to evaluate the potential diameter of the graft, assess for leaks, and identify adventitial bands compromising the lumen. Use of a standard small-volume syringe can produce intraluminal pressure in excess of 700 mm Hg.51 A number of investigations have examined the effect of distention pressure on endothelial integrity and almost uniformly have observed that pressure in excess of 100 mm Hg causes patchy endothelial denudation and that pressure in excess of 500 mm Hg leads to disruption of the underlying media.52–54 The intrinsic biomechanical properties of the graft are negatively impacted, with a resultant decrease in wall compliance secondary to disruption of the elastic elements within the wall.55 Biologic function is also influenced, with distention injury initiating an increase in c-fos expression, an upstream regulator of platelet-derived growth factor production.56 Among the few studies to contradict this observation is the work of LoGerfo et al,54 who observed no significant injury up to a distention pressure of 500 mm Hg and speculated that their aggressive use of papaverine prevented vasospasm and minimized the effect of overdistention. Nevertheless, a variety of pressure-sensing devices have been developed to allow the surgeon to monitor or control distention pressure. Ranging from syringes with intrinsic pressure transducers to reservoir inflation bulbs that generate a fixed pressure, these devices have undergone only sporadic testing and have never been accepted into widespread clinical use.
Irrigating Solution
An array of irrigating solutions ranging from crystalloids to colloids to autogenous blood has been used during vein harvest. Among the first and still the mostly widely used solutions are the isotonic crystalloids normal saline (pH 5.5, 308 mOsm), lactated Ringer’s (pH 6.5, 273 mOsm), and Plasma-Lyte (pH 7.4, 292 mOsm). Although the extent of injury and the magnitude of biochemical perturbations with these solutions are variable and depend on confounding variables such as temperature and the use of pharmacologic adjuncts, the bulk of the literature would suggest that simple crystalloid solutions are most damaging to the endothelium.39,42,51,57 Modified crystalloid solutions such as tissue culture and whole-organ preservation media have also been investigated and have demonstrated modest reductions in endothelial denudation and no improvement in endothelial function when compared with an isotonic saline perfusate.54,58,59 Without ready access to these solutions in the operating room and because of only modest benefit, these modified crystalloids have not been widely accepted for clinical use. Despite some concern for fibrin deposition, predominantly at higher temperatures, heparinized autologous blood probably provides the best option for use during vein harvest.39,42,51 Published reports, however, are not unanimous, with several suggesting equivalent results when using standard crystalloid solutions with pharmacologic adjuncts.58,60
Temperature
Focusing on maintaining endothelial integrity, LoGerfo et al put forward the concept that warmer perfusate temperatures during active dissection would prevent vasospasm, whereas storage of the graft and minimization of metabolic insult would be best accomplished at a much cooler temperature.54 Supporting this hypothesis, they demonstrated that infusion of 37° C perfusate during active vein harvest and storage of the distended vein at 4° C minimized the extent of endothelial damage. Notably, the influence of this dual-temperature approach was most prominent in saline-treated grafts. Subsequent investigations using a single-temperature approach also favored perfusion with a 4° C irrigation solution,39,54,57 with blood preferred over saline because of the mural edema associated with cold saline infusion.42
Although initial studies focused on endothelial morphology as the primary endpoint, further work in the field diversified to examine the effect of solution temperature on biologic activity of the vein graft wall. Nitric oxide–mediated vasodilatation is greatly impaired after storage at 4° C, with room-temperature blood and Plasma-Lyte demonstrating little deterioration when compared with control veins tested immediately after harvest.61 Analogous studies examining endothelial prostacyclin production and functional thrombomodulin activity support the use of room-temperature perfusates as the best option for preserving metabolic activity.62,63
With conflicting experimental evidence, delineation of the most favorable storage temperature is difficult, and reasonable arguments for either cold- or room-temperature treatments can be crafted. Although no definitive recommendation can be proposed, the increased effort required to supply and maintain cold perfusate in the operating suite has prompted most clinicians to favor the use of room-temperature solutions.
Pharmacologic Adjuncts
Unfractionated heparin has universally been included in most vein harvest solutions. Aimed at reducing fibrin deposition and the formation of microthrombi, doses ranging from 4 to 10 U/mL are typically used.39,57,64
Prevention of vasospasm is widely identified as being critical in maintaining an intact endothelium, and the use of pharmacologic agents to facilitate this goal may be the most important component of the vein harvest regimen. The most widely studied vasodilator for this purpose is papaverine. Although its exact mechanism of action is unclear, papaverine appears to induce smooth muscle cell relaxation through inhibition of phosphodiesterase and an increase in intracellular cyclic adenosine monophosphate levels. Percutaneous injection of papaverine (120 mg/L) along the outside of the vein before skin incision has been described as an important step to minimize spasm,64,65 although this can prove challenging in all but the thinnest patients. Application of papaverine along periadventitial tissues and within the lumen perfusate is more easily accomplished and continued throughout vein harvest. Though not rigorously studied as an independent variable, most regimens containing papaverine have shown reduced endothelial injury in comparison to controls.39,54,57,60
Other vasodilators have been examined, and a combination of glyceryl trinitrate (8.3 mg/L) and verapamil (16.7 mg/L) appears to be particularly beneficial.66 In direct comparison to papaverine, glyceryl trinitrate/verapamil demonstrated notable improvement in endothelial coverage.
Even though extensive research has been conducted in this area, no consensus has been reached regarding the optimal techniques for vein graft harvest, with each variable demonstrating unique advantages and shortcomings. Box 92-1 provides a practical summary protocol for the preparation of autogenous vein conduit. The relative importance of these variables is best illustrated by the work of Adcock et al, who examined the combined benefits of papaverine, autologous blood, and limited pressurization in maintaining endothelial integrity.39 Even though this combined regimen achieved a significant reduction in endothelial loss at the time of implantation, 71% of the endothelial cells exhibited morphologic abnormalities by the second postoperative day (versus 92% for the “standard” regimen). Although the majority of published regimens have sought to optimize variables to maintain an intact endothelial monolayer at the time of implantation, few reports have challenged their techniques to extended exposure in an in vivo environment. Widespread endothelial injury and metabolic perturbation of the harvested vein are probably unavoidable, with only secondary improvements gained by the various methodologies just described.
Minimally Invasive Vein Harvest
Among the significant complications associated with a single continuous incision for lower extremity vein harvest are wound infection and dehiscence, observed in up to 40% of patients.67 Minimally invasive approaches to saphenous vein harvest have been developed to reduce incisional length and diminish the attendant morbidity. Early investigations of this technique were initiated without specialized instrumentation but used a series of small, sequential incisions overlying the vein. The use of such “skip incisions” significantly improved primary wound healing, with wound complications developing in 28% of patients with a continuous incision as opposed to 9.6% of patients in the “skip incision” group.68 Over the past 2 decades the concept has evolved with the marriage of endoscopic visualization and specialized instrumentation to facilitate minimally invasive vein harvest. Several different devices have been developed for this purpose, all using three small incisions to access the vein and ligate side branches. Used predominantly for harvesting of vein for coronary bypass surgery, prospective randomized human trials have demonstrated significant reductions in wound morbidity when compared with single-incision saphenectomy.69–71 Increased manipulation of the vein is inherent with this approach and raises concern about increased endothelial damage and accelerated graft failure. Blinded morphologic examination of harvested human vein specimens72,73 and clinical outcome studies71 have demonstrated no significant differences in vein injury or graft patency. Universally noted throughout these reports is the steep learning curve that accompanies endoscopic saphenous vein harvest, with many centers identifying a core group of practitioners who focus on this aspect of the procedure. Although performance of these studies at large-volume medical centers can provide insight into the optimum outcomes with this approach, extrapolation to lower volume centers may not be as promising.
Probably related to the long segments of vein required for lower extremity bypass and the prolonged learning curve inherent in this approach, endoscopic vein harvest for peripheral revascularization has not been uniformly embraced. A small number of centers have championed its use, with mixed results. With no randomized trials comparing single-incision with endoscopic saphenous vein harvest for peripheral bypass, experiences have predominantly been reported through case series with retrospective controls.74–78 These reports generally support the observations in the cardiac literature and suggest that endoscopic harvest offers reduced wound complications with no notable deterioration in short- and long-term graft patency. Not all reports have been favorable, however, with one investigative team detailing inferior patency rates with little improvement in wound complications after endoscopic vein harvest.78 Among the important conclusions in this study is the significant learning curve inherent in this technique, with an estimated 40- to 50-patient experience required to become fully competent in harvesting the more difficult below-knee saphenous vein.79,80 It is noteworthy that the center reporting inferior results performed an average of only one endoscopic harvest per month, probably an insufficient volume to master this technique. Concerns about increased cost of the procedure secondary to additional equipment expenses are relatively unfounded, with these costs more than offset by decreased length of stay during the primary admission and a reduction in the number of readmissions for wound complications.76
In an effort to reduce instrument costs while retaining the benefits of a minimally invasive approach, a limited-incision saphenectomy was proposed in which 2-cm-long incisions flanked by 6-cm skin bridges were used to extract the vein with standard surgical instrumentation. When compared with historical controls, this limited-incision approach offered no improvement in wound complications and a notable reduction in long-term patency.78 The authors speculated that the significant manipulation of the vein required for exposure beneath the skin bridges is the probable cause of the inferior performance of these grafts.
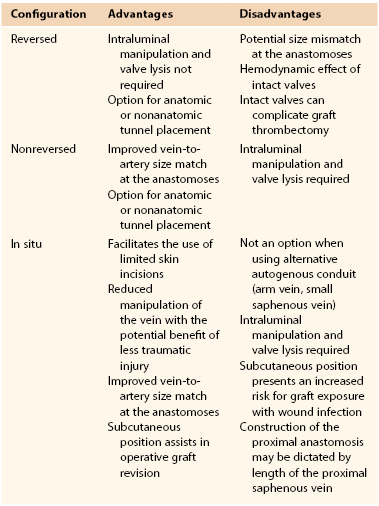
Autogenous Vein Graft Configurations
In the preoperative planning stage before open lower extremity revascularization, the surgeon is faced with a number of variables concerning the suitability of various inflow and outflow arteries and the length and quality of the available conduit. Invariably, because of some discordance among the available options, assessment of the potential risks and benefits for each choice is required. Preoperative arteriography and duplex vein mapping provide the baseline data through which primary and secondary operative plans are developed. Direct operative assessment of the intended proximal and distal anastomotic sites and the quality of the autogenous conduit may initiate reevaluation and modification of the intended plan during the course of the operation. A summary of the various factors that influence this decision is provided in Table 92-1.
The conduit of choice is the ipsilateral great saphenous vein, with the contralateral saphenous vein being an appropriate secondary option. To preserve the great saphenous vein in the contralateral limb for future revascularization procedures, some surgeons have advocated the use of alternative conduit as a secondary option.81 Studies examining this issue are in general agreement that in the absence of advanced ischemia (disabling claudication, rest pain, or tissue loss), use of the contralateral saphenous vein is recommended over alternative conduit because of its length, superior performance, and minimal risk to the donor limb.82–84 Independent risk factors predictive of the need for future vascular intervention in the contralateral limb include age younger than 70 years, diabetes, coronary artery disease, and an ankle-brachial index (ABI) of less than 0.7. In patients demonstrating three out of these four risk factors, the potential for requiring contralateral revascularization within the next 5 years ranges from 25% to 43%, and use of alternative conduit over the contralateral saphenous vein may be a reasonable consideration in this select population.85
The approach of using the “best” available autogenous conduit has been advocated86; this practice is used routinely by most vascular surgeons. Although vein segments with chronic phlebitis, calcification, or organized intraluminal thrombus are prone to accelerated failure and should be excluded from use,16,17 the other characteristics that define the “best” vein have been an active area of investigation. Even though single-segment saphenous vein has universally been identified as the most durable conduit, an array of single-institution, retrospective studies have attempted to define the effect of such characteristics as vein diameter, graft type, and orientation on long-term patency.11,31,87–91 Though useful in framing this question, the most comprehensive examination of this issue can be obtained from the prospective, multi-institutional PREVENT III (Project of Ex-vivo Vein Graft Engineering via Transfection) database.32,92 Examining conduit diameter and using veins greater than 3.5 mm as the reference standard, this trial found that grafts ranging from 3.0 to 3.5 mm had a 1.5-fold risk for primary failure. Grafts smaller than 3.0 mm in diameter had a 2.4-fold higher risk for primary failure and a 37% loss of secondary patency at 1 year. In terms of graft type, composite veins had a 1.5-fold increase and arm veins a 1.6-fold increase in primary failure when compared with single-segment great saphenous vein. Graft orientation did not influence patency, with reversed and nonreversed configurations demonstrating equivalent patency rates. As these data demonstrate, deviation from using an adequately sized single-segment saphenous vein carries increased risk for failure, and understanding the magnitude of these risks is critical in operative planning and maximizing the potential for a durable outcome.
Reversed Vein Grafts
Excision of the vein with orientation in a reversed configuration while maintaining antegrade flow through intact valves offers the most straightforward method for vein graft implantation and is suitable for most clinical situations. Difficulty may arise when significant tapering of the saphenous vein in the distal part of the limb creates a size mismatch between the artery and vein graft at both the proximal and distal anastomoses. The proximal vein graft appears to be a common site for luminal narrowing and vein graft failure, and use of small-diameter conduit at this location may accentuate this problem.93 The size mismatch can be most problematic, however, when performing a tibial or pedal artery anastomosis. A fourfold to fivefold difference in diameters may be encountered, and tailoring the proximal great saphenous vein into a suitable anastomotic configuration can be challenging in these situations.
Examination of normal venous physiology has demonstrated that complete effacement of the valves does not occur during periods of prograde flow. In the low-flow, nonpulsatile hemodynamic environment that characterizes the superficial venous system, the mild to moderate stenosis created by these partially open valves does not translate into a significant loss of pressure or impediment to flow. After implantation into the arterial system, the hemodynamic significance of the valves becomes more pronounced. Though not significantly narrowing the lumen during peak systole, a transition to turbulence with localized regions of stasis is noted within the valves.94 Lysis of these valves leads to a 15% decrease in hydrodynamic resistance and a 15% to 30% increase in graft flow rates.95–97 Intact valves have not been proved to adversely influence the long-term patency of reversed vein grafts, but based on these observations, routine valve lysis within these antegrade flow grafts has been proposed.
Less well studied is the biologic effect of the hemodynamic microenvironment within the valve regions, which serve as a nidus for the development of a hyperplastic lesion and focal graft narrowing. Strandness et al prospectively evaluated the long-term remodeling of these valves in reversed saphenous vein grafts.98,99 Ten percent of the valves were associated with greater than 50% stenosis, but only 2.5% progressed to a critical stenosis requiring operative intervention; these valves accounted for 17% of the total graft revisions within the 18-month follow-up period. Notably, valve morphology was dynamic, with 60% of the hemodynamically significant valve lesions showing regression to less than 20% stenosis in a mean period of 3 months.
Nonreversed Vein Grafts
Challenges in constructing a reversed-configuration vein graft include significant proximal-to-distal tapering of a vein and the resulting size mismatch at the proximal or distal anastomoses (or both). Although use of an in situ grafting technique can be one solution to this issue, the combination of complete excision of the vein, lysis of the valves, and orientation in a nonreversed configuration offers an alternative technique. Despite the fact that initial attempts with this approach suggested results inferior to the in situ method, these early efforts were probably compromised by the use of an eversion valvulectomy technique and extensive injury to the vein wall.100 With subsequent refinements in the approach to valve lysis, nonreversed vein grafts have proved as durable as other available techniques.101–104
Although nonreversed vein grafts inherently require increased manipulation with the potential for injury during valve lysis, this shortcoming is theoretically offset by the improved hemodynamics offered by this approach. After valve lysis, nonreversed grafts offer a 20% improvement in flow rates over nonlysed, reversed conduits.96 This hemodynamic effect is most pronounced in smaller diameter vein grafts ranging from 2.0 to 2.5 mm, where the leaflets from intact valves encompass 45% of the luminal cross-sectional area.95
In Situ Vein Grafts
The concept of using the great saphenous vein as a graft with mobilization of only the proximal and distal segments while maintaining the interval region within its subcutaneous bed was initially suggested by Rob’s group in 1959.105 Interested in the potential hemodynamic advantages offered by removal of the valves and retrograde perfusion of the conduit, Rob believed the procedure to be too time consuming for routine application. Hall, a visiting fellow from Norway, became intrigued by this approach, refined the techniques for routine clinical application, and published the first report of the in situ vein graft technique in 1962.106 His version of the procedure, requiring valve excision by opening the vein at multiple locations along the graft, was tedious, required significant technical expertise, and failed to gain widespread acceptance. The development of efficient instrumentation for valve lysis provided the opportunity to reduce vein manipulation and maintain an intact vasa vasorum and fueled renewed enthusiasm for the in situ approach. Initial reports of this approach, championed by Leather et al,107 suggested improved patency over excised vein grafts.108 These early analyses are compromised by the use of historical controls, however, and contemporary comparisons of in situ versus reversed or nonreversed grafts fail to demonstrate significant differences in long-term outcomes.46,103,109
Preparation of the saphenous vein for use in situ as an arterial bypass entails (1) mobilization of the proximal and distal segments for construction of the anastomoses, (2) removal of the valvular obstructions to arterial flow, and (3) interruption of the venous side branches to prevent the formation of arteriovenous fistulae. The objective is to accomplish these tasks in the most expeditious manner while minimizing traumatic injury to the vein.
Proximal and Distal Anastomoses
The common femoral artery serves as the most common source of inflow for infrainguinal revascularization, and the relative position of this artery and the confluence of the superficial inguinal veins dictates construction of the proximal anastomosis. Mobilization of the proximal great saphenous vein is accomplished by secure ligation of the superficial branches, placement of a side-biting vascular clamp across the common femoral vein, transection of the great saphenous vein flush with the wall, and repair of the femoral vein with running nonabsorbable suture. Lysis of the most proximal great saphenous valve can be difficult with standard valvulotomes, but localized eversion of this segment permits excision of the valve under direct vision. Intrinsic atherosclerotic disease in the common femoral artery may dictate more proximal placement of the anastomosis. Additional proximal vein length can be obtained by harvesting a segment of the superficial epigastric vein and extending the venotomy proximally along the posterior surface of this vein to provide an autologous patch for repair of proximal common femoral artery disease. Care should be taken to minimize longitudinal stretching of the graft, which can accelerate the development of intimal hyperplasia.110 Extensive common femoral or profunda femoris origin disease (or both) may necessitate endarterectomy with patch angioplasty or graft replacement of the common femoral artery. In the absence of significant proximal artery occlusive disease, defined as greater than a 50% reduction in lumen diameter, the superficial femoral, profunda femoris, or popliteal artery can be considered for placement of the proximal anastomosis.111
The posterior tibial, peroneal, and dorsalis pedis arteries provide the most straightforward options for placement of the distal anastomosis. Although the below-knee popliteal artery can also be used, fashioning the anastomosis can be problematic because of the nearly 90-degree angle that is formed between the graft and artery. With most hemodynamic analyses detailing an increase in flow separation and activation of proliferative metabolic pathways with right-angle anastomoses, grafts configured to this segment of the popliteal artery may benefit from excision and placement in an anatomic tunnel. Distal anastomoses to the above-knee popliteal artery do not offer the same constraints on their configuration; however, after mobilization of the proximal and distal graft, the segment that remains in situ can be quite short and of limited benefit.
Valve Lysis
Although limited transverse venotomies were used in initial development of the in situ bypass graft, this approach to render the valves incompetent has given way to less invasive techniques. Valvulotomes have undergone a variety of revisions over the last several decades, and the current generation of devices can be classified into three categories—the modified Mills valvulotome, the adjustable valvulotome, and the fixed valvulotome (Fig. 92-3).
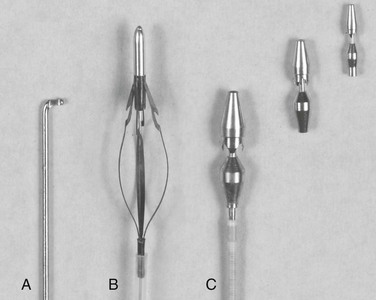
Figure 92-3 Valvulotomes. A, Modified Mills. B, LeMaitre adjustable. C, Uresil fixed with 2-, 3-, and 4-mm cutting heads.
The modified Mills valvulotome (see Fig. 92-3A) was initially described in 1976 for application to coronary artery bypass surgery.112 With limited modification, this device was rapidly adopted for use in peripheral vascular surgery. Introduced into a side branch or the distal end of the vein, the device is advanced through the valve leaflets (Fig. 92-4). The proximal vein graft is distended by arterial inflow or manual infusion to induce valve closure, and the valvulotome is slowly withdrawn until the reverse cutting blade engages the valve leaflet. The tip of the valvulotome is maneuvered toward the center of the lumen, and a short burst of inferior traction is applied to transect the valve.
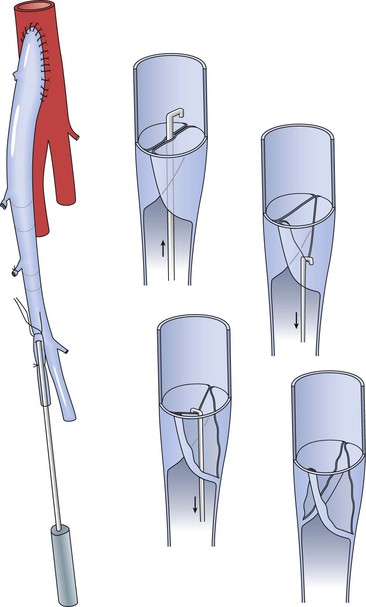
Figure 92-4 Valve lysis using the modified Mills valvulotome. The valvulotome is inserted into a long side branch and advanced into the proximal vein. Competent valves within the graft are maintained in closed position by arterial inflow pressure, and the valvulotome is withdrawn until resistance is encountered. After ensuring that the instrument has not engaged a side branch, a short burst of inferior traction is delivered and the valve leaflet transected. Short advancement and 180-degree rotation of the valvulotome permit division of the opposite valve in analogous fashion.
Popularized by LeMaitre, the expandable valvulotome offers four cutting blades encompassed within a self-centering series of wire hoops (see Fig. 92-3B).113 With the blades encased in a protective sheath, the device is advanced to the proximal end of the vein graft. The vein is distended, the cutting system deployed, and the device slowly withdrawn. Because of the mobility of the wire hoops, the position of the cutting blades is self-adjusting to accommodate vein diameters between 1.8 and 6 mm.
Fixed-diameter valvulotomes were developed from the original work of Hall, in which a blunt-tipped vein stripper was passed proximally to distally through the graft to avulse the valve leaflets and render them incompetent.114 The addition of cutting blades and detachable heads of varying size led to the current generation of devices (see Fig. 92-3C).115 A 2-, 3-, or 4-mm head is secured to a flexible shaft and reintroduced into the lumen of the vein. As with the other devices, the proximal vein is distended and the valves are lysed with a rapid burst of inferior traction.
Visualization studies after the application of valvulotomes report that complete disruption occurs in approximately 70% to 95% of the treated valve leaflets.116,117 Proximal segments of the vein, where the size mismatch is most pronounced, are most likely to demonstrate only partial valve disruption; thus careful attention to this region seems warranted.116 Limited data providing a direct comparison among the types are available, with one study suggesting incomplete valve lysis to be more common with expandable valvulotomes117 and another suggesting no differences among the groups.118 A prospective randomized trial comparing clinical outcomes for fixed and expandable devices failed to show any difference in short-term patency.118
Intraoperative angiography, duplex scanning, and angioscopy have been proposed as suitable adjuncts to evaluate the completeness of valve lysis during in situ bypass grafting. Angiography and duplex scanning are relatively insensitive modalities for identifying retained valves and detect only about 20% of such valves; angioscopy is much more sensitive.119 Used for either direct visualization during valve lysis or graft surveillance, angioscopy is successful, with a sensitivity of identifying retained valve cusps approaching 100%. The clinical implications of identifying these valve abnormalities are uncertain, and several studies suggest no long-term improvement in graft patency with the routine use of angioscopy.120,121 Potentially the most notable benefit from routine angioscopy may be the identification of significant preexisting venous disease. Veins with preexisting pathology have a high rate of early graft failure, and intraoperative recognition of these compromised vein segments may be the most important role for pre-bypass angioscopy.68,122 Angioscopy may also be especially important in the evaluation of arm vein conduits because of their documented increased frequency of intrinsic vein abnormalities (see Arm Vein and Composite Grafts section later in this chapter).
Side Branch Ligation
Without the need to mobilize the vein from its subcutaneous bed, multiple options are available for treatment of the venous side branches. The standard approach would be complete exposure of the great saphenous vein with a single continuous incision that extends the length of the leg. Although this open approach simplifies side branch ligation and valve lysis, it has been associated with a wound complication rate in excess of 40% in some reported series.67,123 These complications are of even more significance because of the superficial location of the in situ bypass. Even though extended rehospitalization and operative débridement may be required, these wounds can usually be managed without loss of graft patency.
Noninvasive identification of the major side branches offers the opportunity for a limited-incision or semiclosed in situ technique. Intraoperative venography and duplex scanning are two readily available options for this task, but these methods can be surprisingly inaccurate and fail to recognize almost 50% of the side branches.119 Using the angioscope to identify the orifice and transcutaneous illumination to guide the location of the skin incision has proved somewhat more successful, with detection of approximately two thirds of patent side branches.119,123 Commensurate with the smaller size of the incisions, these approaches offer notable improvement in wound complication rate.120,123 Graft patency rates are relatively unaffected by the operative approach, but an increased incidence of persistent arteriovenous fistulae has been documented with most semiclosed techniques. Studies examining persistent fistulae have generally recommended their ligation; however, such an aggressive approach has been called into question. Conservative management of these fistulae results in spontaneous closure in a third of cases, eventual revision for reduced distal graft velocity in a third, and a third remaining stable without intervention.124,125
Arm Vein and Composite Grafts
The ipsilateral great saphenous vein is clearly the most desired conduit, but reports suggest that it is absent in up to 45% of patients requiring infrainguinal revascularization.126 Use of single-segment or composite arm grafts provides an important alternative in such challenging patients. The most extensive experience with arm vein bypass is detailed through a series of reports from the Beth Israel Deaconess Hospital.81,90,127 Preoperative duplex evaluation of the upper extremity veins is critical in the operative planning for patients with inadequate or previously harvested ipsilateral saphenous vein. A summary of the advantages and disadvantages of available arm vein conduits, along with an estimate of their relative frequency of use, is provided in Figure 92-5.81
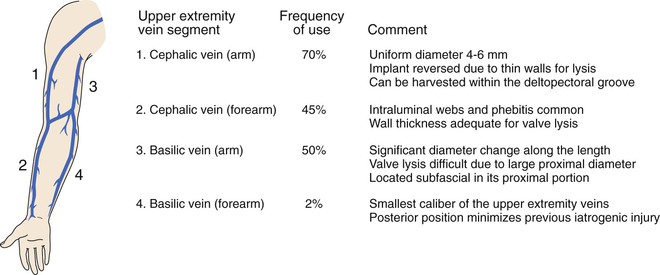
Figure 92-5 Superficial upper extremity veins available for use in bypass grafting, including individual considerations and relative frequency of their use.81
To take advantage of the larger veins in the upper part of the arm, the basilic-cephalic loop graft has been proposed.128,129 Using the median cubital vein as the connecting segment, the cephalic and basilic veins are harvested in a continuous loop from the antecubital fossa to their termination in the axillary vein. With the larger basilic vein serving as the proximal vein graft, a valvulotome is introduced into a midgraft side branch to facilitate lysis of valves in the basilic and median cubital segments. The cephalic vein segment, which tends to be thin walled and troublesome for valve lysis, is maintained in a reversed configuration and used for the distal anastomosis.
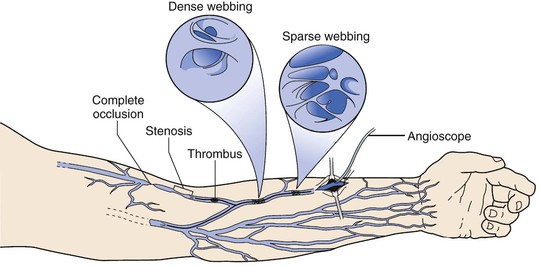
Similar to the approach when using lower extremity veins, single-segment arm vein is preferable. Through extensive use of the median antecubital vein as a bridge, success can be achieved in up to 80% of cases requiring the use of arm vein.81 Limiting this effort, however, is the inherent iatrogenic injury that occurs from repeated cannulation (Fig. 92-6). Veins that are most superficial and anatomically accessible, such as the forearm cephalic and median antecubital, are most frequently damaged, with 30% to 50% of these veins demonstrating a variety of pathologies.130 Angioscopic evaluation of the quality of the arm vein and identification of compromised segments are thus fundamental to achieving long-term patency in these grafts. Minor abnormalities such as sparse webbing or an adherent thrombus can be successfully treated by endoluminal repair to restore a widely patent lumen. Other pathologies such as dense webbing or focal stenosis may require local repair with vein patch angioplasty. Marked abnormalities such as sclerosis with long-segment stenosis or occlusion cannot be repaired locally and require resection of the abnormal segment. Using such a treatment strategy, 25% of arm veins required local repair and 16% required resection, with no difference in long-term patency when compared with good-quality arm vein that required no intervention.81 In the subset of grafts in which a segment of suboptimal-quality vein was used, failure within 6 months of implantation was common.
Small Saphenous Vein Grafts
Although lacking the length offered by the great saphenous vein, the small saphenous vein can be a useful source of autogenous conduit in the vein-limited patient. Arising from tributaries lateral to the calcaneus and terminating in the popliteal fossa, this conduit is predominantly used in combination with other vein segments in a composite configuration. Preoperative duplex evaluation is critical to evaluate adequacy, with a lumen diameter of 3.0 mm considered suitable for grafting. Small saphenous vein is usually the third choice of autogenous vein (behind great saphenous and arm vein) because of the inherent difficulties in surgical exposure and harvest. While initial reports described use of a medial leg incision with development of a subfascial plane along the enveloping fascia of the superficial posterior compartment,131 most surgeons currently use a posterior leg incision for surgical exposure of this conduit.132–135 Short, unilateral segments of vein can be harvested with the patient in the traditional supine position by elevating the leg, with the surgeon positioned inferior to the extremity. However, extensive unilateral or bilateral harvests are best accomplished using an initial prone position, in which the veins are excised, wounds closed, and the patient rotated into a supine position for completion of the procedure. In an attempt to use the posterior leg veins as a single conduit for femorotibial bypasses, the Giacomini vein can be harvested in continuity with the small saphenous vein.136 Although this extension of the small saphenous vein into the thigh is present in approximately two thirds of individuals, details regarding the size distribution for this conduit and intermediate to long-term patency have not been reported.
Patients with isolated tibial artery occlusive disease may be candidates for in situ bypass using the small saphenous vein. Employing a posterior approach for exposure of the proximal and distal anastomotic sites, popliteal-crural bypasses to each of the three tibial arteries can be performed. Cumulative 1- and 3-year primary patency for these grafts is 75% and 60%, respectively,134,135 approximating the same results observed with in situ grafting using the great saphenous vein. However, the durability of small saphenous vein used in composite grafting is notably inferior, with 1- and 3-year primary patency of 50% and 35%, respectively.135
Femoral-Popliteal Vein Grafts
Although primarily used for intra-abdominal vascular reconstructions in most current day treatment algorithms, the femoral-popliteal vein was initially investigated as the potential conduit of choice for lower extremity bypass procedures.137–140 With promising results demonstrated in a retrospective review of their clinical practice, Shulman et al initiated a prospective, randomized trial comparing autogenous femoral-popliteal vein to saphenous vein as the preferred conduit for infrainguinal bypass.140 Based on a total enrollment approaching 100 patients, no significant differences in 5-year primary patency (55%) and limb salvage rates (70%) were observed. Thirty-day mortality and early postoperative complications were also similar in both groups. Follow-up examination to evaluate the long-term sequelae of deep vein harvest demonstrated chronic calf swelling and an obstructive pattern on venous plethysmography but no significant increase in stasis dermatitis or venous ulceration.141 Although no objective differences were observed between the femoral-popliteal and saphenous vein groups, the authors concluded that the increased complexity associated with femoral vein harvest made saphenous vein the preferred conduit for lower extremity revascularization. Nonetheless, these data support the use of femoral-popliteal vein as a viable source of alternative conduit in select patients.
Superficial Femoral Artery Grafts
Patency of an occluded segment of excised superficial femoral artery can be restored via an eversion endarterectomy, providing an autogenous conduit up to 25 cm in length that is suitable for short interposition or composite grafting.142 Although conceptually, these conduits were thought to be useful in the salvage of patients with prosthetic graft infection, rupture of the anastomosis was observed in 75% of those graft placed in an infected field.143 When used in combination with autogenous vein, patency rates for these composite grafts have demonstrated marginal 1-year primary patency rates (60%).143,144 Frequently, the mechanism of failure for these conduits was acute thrombosis in the absence of a stenotic lesion, which is different from what is usually seen in autogenous vein grafts.144
Hypogastric Artery Grafts
Facilitated by the rich collateral network in the pelvis, the hypogastric artery offers an available source of autogenous conduit for short segment, intra-abdominal reconstructions. With a cascade of associated branches, this graft offers the ability to create several distal anastomoses from the primary graft, an advantageous configuration for use in complex aortorenal bypass procedures.145 Although used sporadically in the adult population,146,147 this segment of artery is the conduit of choice for renovascular reconstructions in the pediatric population.148,149 Autogenous vein grafts, because of the propensity for late aneurysmal dilatation in children, are rarely used for pediatric aortorenal bypass procedures. Although no detailed analysis of hypogastric artery patency has been performed, anecdotal experience across multiple centers support that a normal autograft placed without technical error should provide lifelong patency.145–149
Etiology of Vein Graft Failure
Technical Factors
Traditionally, graft occlusions within 30 days of implantation have been thought to be related to technical factors that could potentially have been corrected at the time of graft placement. Although flow-limiting lesions within the bypass graft are a clear component of these failures, estimated at about 20%,150 the events leading to graft thrombus are clearly more complex. Similar to the etiology of venous thrombosis, Virchow’s classic triad is probably at the intersection of early graft failure. Not uncommon is the scenario in which early graft failure prompts reexploration, which fails to identify any clear underlying cause.150 Endothelial injury related to manipulation of the graft, hypercoagulability in the early postoperative period, and reduced graft flow because of inflow, outflow, or graft stenosis are factors that contribute to these early failures.
With insufficient time for formation of significant intimal hyperplasia, graft failures within 30 days are thought to be technical if they are associated with one of four categories: inadequate inflow, inadequate outflow, extrinsic lesions, and intrinsic lesions.151 Inadequate inflow may be secondary to an unrecognized lesion in the proximal arteries or inflow graft. More common is the scenario in which the importance of the proximal lesion is underestimated, only to have its hemodynamic significance unmasked after placement of the distal graft. The resulting increase in flow leads to an accentuated drop in pressure across the lesion and converts a previously innocuous lesion into one that now has important hemodynamic implications. Cardiovascular compromise, such as systemic hypotension or a low cardiac output state (e.g., cardiogenic shock), can also be the cause of inflow-related graft failure.
Inadequate outflow as a result of extensive, preexisting tibial and inframalleolar occlusive disease is an established risk factor for early graft failure; patients demonstrating the most severe disease have a 10-fold increased risk for 30-day graft thrombosis.152,153 Vasospasm of the target artery is a less well-studied but possible contributor to early postoperative outflow compromise. Though difficult to quantify and monitor postoperatively, a subset of patients demonstrate severe luminal narrowing and delayed reperfusion despite minimal atherosclerotic disease in the distal artery (Fig. 92-7).
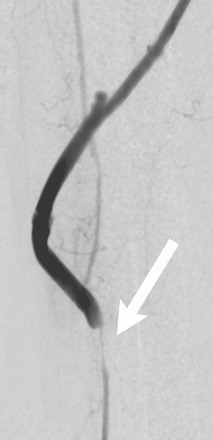
Figure 92-7 Intraoperative digital subtraction arteriogram of a vein bypass graft to the distal anterior tibial artery. Severe distal spasm of the outflow artery (arrow) has led to diminished diastolic flow in the graft.
Extrinsic causes of early graft failure include extrinsic compression because of tunneling errors or hematomas that may compromise graft flow. Other extrinsic causes of graft failure, such as hypercoagulability, are systemic. Alterations in the coagulation system are detectable in more than 25% of patients undergoing lower extremity revascularization.154 Although some studies have suggested that 10% of early graft failures are primarily related to an identified hypercoagulable state,150 the contribution of transient abnormalities in coagulation is unclear and perhaps underestimated.
Intrinsic vein graft defects include focal, flow-limiting lesions such as a retained valve leaflet, an intimal flap, or inappropriate placement of anastomotic sutures. Limitations in flow can result from the use of an undersized conduit, with vein grafts less than 3.0 mm in diameter being at significant risk for early failure.153 Independent of luminal narrowing, intrinsic vein graft defects, such as sclerosis and calcification, also contribute to graft thrombosis.17
Recent data from the multi-institutional PREVENT III trial have detailed a 5% incidence of thrombosis or reexploration within 30 days of graft implantation.92 Whereas endovascular management of these early graft occlusions is of limited value, open surgical revision provides a reasonable opportunity for durable graft salvage, with a 90% 1-year patency rate reported after successful exploration and revision.155
Intimal Hyperplasia and Pathologic Remodeling
Surgical manipulation of the vein combined with the acute exposure to elevated shear and tensile forces elicits a complex series of biologic events in the vein wall that begins immediately on implantation. Analogous to the events that occur in generalized wound healing, repair of the damaged vein graft is initiated by the local synthesis of a wide variety of cytokines and growth factors. Chemokines induce the recruitment of neutrophils and mononuclear cells, which further amplifies these pathways. Activated by these chemoattractants and mitogens, smooth muscle cells begin to dedifferentiate from a contractile to a synthetic phenotype and replicate within the media. By tracing the gradient of chemokines, liberated smooth muscle cells migrate from the media to the intima, where continued proliferation and abundant matrix deposition are stimulated. Progressive thickening of the intima leads to narrowing of the lumen, a reduction in graft flow, and intraluminal thrombosis (Fig. 92-8).
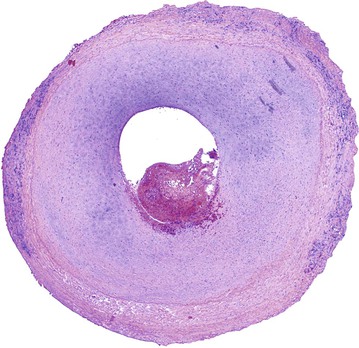
Figure 92-8 Cross-sectional histologic image of an 8-month-old vein graft demonstrating severe intimal hyperplasia. Adherent thrombus is present within the severely compromised lumen.
Despite the relative uniformity of the surgical trauma and hemodynamic insult, vein graft lesions demonstrate a strong tendency to be focal rather than diffuse.156 With a peak incidence at 4 to 12 months after implantation, 30% to 45% of autogenous grafts will fail or require revision secondary to significant narrowing of the lumen.92,157 Stenotic lesions are more likely to be associated with the proximal or distal anastomosis (53% incidence) but occur not infrequently in the midbody of the graft (30% incidence).93 It has been postulated that these midgraft lesions are associated with valve leaflets; detailed evaluation of this issue demonstrated substantial remodeling within valve sites, but progression to high-grade stenosis was rare and accounted for less than 20% of the intrinsic graft lesions requiring repair.99 Despite such general observations, the underlying biologic mechanisms for development of this segmental pattern of intimal hyperplasia remain poorly understood. Although the local flow environment plays a significant role, physical forces in isolation fail to explain these observations. More likely, critical lesion development occurs at the intersection of localized trauma, ongoing hemodynamic stress, and an altered ability for local repair after injury.
Although much of the emphasis in vein graft pathology has focused on intimal hyperplasia, graft morphology is actually dictated by the balance between outward expansion and wall thickening. As Glagov et al demonstrated, the normal vascular adaptive response is an increase in overall wall circumference to compensate for luminal narrowing in the presence of a developing occlusive lesion.158 In response to hemodynamic and biochemical stimuli, smooth muscle cells degrade and reorganize the rigid matrix structure of the wall to facilitate these changes. Examination of early vein graft morphology demonstrated a 20% increase in diameter within the first month after implantation; failure of this outward remodeling has been correlated with subsequent graft failure.159 Inflammatory pathways appear to further modulate this shear-mediated adaptive response, thus providing potential mechanistic insight into the heterogeneity that is observed clinically.160
Graft Atherosclerosis
Although the peak incidence of vein graft failure occurs within the first year, delayed progression of intrinsic lesions leads to a 4% annual loss of graft patency in the 2- to 10-year time frame.9,157 Because studies have focused predominantly on the mechanisms of early graft failure, insight into the pathology of delayed lesion development in peripheral vein grafts is limited. Much of our understanding of late graft failure is derived from coronary bypass grafts examined at the time of autopsy. Replacement of the fibrotic wall with lipid-laden macrophages and intramural calcification is common.161–163 Degeneration of the vein graft wall with intramural thrombus formation, characteristic of ruptured atherosclerotic plaque, is also frequently observed.164,165 Supporting these observations is the clinical impact of lipid-lowering therapy on coronary artery graft patency, where reductions in total serum cholesterol significantly enhance vein graft durability.166–168
With few relevant animal models, examination of these events in peripheral bypass grafts has been relegated to the analysis of sporadic samples obtained at the time of graft revision. The initial report published by Szilagyi et al in 1973 identified atherosclerotic changes in 8 of 21 grafts ranging in age from 8 to 96 months.156 These observations are supported by Walton et al, who described apolipoprotein B deposits, ulceration, calcification, and aneurysm formation in the majority of failed grafts that had been implanted for more than 2 years.169 More recent reports, however, present a more variable picture and predominantly describe a fibrocellular hyperplastic intima with rare atherosclerotic degeneration as the cause of late failure.170 Although investigators have speculated that modulation of early intimal hyperplasia will have an impact on cholesterol-driven atherogenesis and provide an avenue to improve graft patency, this view remains speculative with limited data to support it.171,172
Autogenous Graft Modifications to Improve Durability
For the last 2 decades, the prevailing concept in vein graft biology was that the initial stages of adaptation were critical in defining the long-term future of the conduit. Specifically, it has been hypothesized that inhibition of the early wave of smooth muscle cell proliferation would mitigate the development of stenotic lesions months to years following implantation.173 Emanating from this strategy, edifoligide emerged as the most promising approach to improve vein graft durability. The regulation of cell cycle kinetics and DNA replication are under the control of the E2F pathway, and the double-stranded oligodeoxynucleotide edifoligide was designed to bind and inactivate the E2F family of transcription factors. Applied to the harvested vein, this therapeutic agent was developed to inhibit the burst of smooth muscle cell proliferation that is initiated in the first several days following implantation.
Based on successful preclinical studies in a rabbit vein graft model, human phase I and II clinical studies examining edifoligide were initiated.174–176 Demonstrating safety and a suggestion of possible efficacy,177,178 two phase III clinical trials (PREVENT III and IV) were undertaken to investigate the use of edifoligide in infrainguinal and coronary artery revascularization. Following randomization of 4400 patients, treatment with edifoligide was demonstrated to have no influence on vein bypass graft durability.179,180 Although these were landmark trials in their attempts to directly modulate the biology of an implanted vein graft, the lack of a significant clinical effect has led to a reassessment of the fundamental paradigms in the field and the current wave of investigative research.
• Delivery vehicles—Founded in the concept that mitigation of the early vein graft response to injury prevents unchecked intimal hyperplasia, the PREVENT trials used a naked oligonucleotide approach to inhibit E2F binding to the promoter sites. The lack of clinical efficacy has brought into question such approaches using a single, ex vivo application of a therapeutic agent at the time of graft implantation. A more sustained but still transient delivery method, such as that offered by adenovirus, or the stable integration of a biologic modifier into the genome, using adeno-associated virus or lentivirus, may be more effective strategies for achieving a durable clinical outcome.181,182
• Biologic processes—Interrupting E2F binding, along with the transition from G1 to S phase within smooth muscle cells, was thought to be the critical event in improving vein graft survival. Although the conceptual model that smooth muscle cell proliferation leads to intimal hyperplasia and results in luminal narrowing and graft thrombosis is attractive in its simplicity, vein graft injury and repair occurs via a coordinated series of overlapping events.183 Endothelial damage and recovery, local thrombosis, smooth muscle cell apoptosis, and matrix deposition are all components of the adaptive process. Accentuating or inhibiting one or more of these parallel events may be required.
• Molecular targets—Genomic analyses following vein graft implantation have failed to identify a single target that dominates the adaptive process.184 Instead, the enhancement or repression of various biologic processes occurs via the broad modulation of the entire set of integrated pathways.185 Systems-based analyses offer the potential to identify the optimum time and duration for targeted interventions, such that the interconnections between biologic elements are augmented or disrupted. It is likely that a multitarget approach at specific times during the adaptation process is required for a robust and durable impact on vein graft morphology. An alternate approach is to develop a response system that adapts to the local biology of the environment. This concept is the foundation for use of autogenous cells, which, when placed in a perivascular position, modify the signaling milieu to positively impact the local biology of the vein graft wall. Such approaches have been examined in early phase human trials and are moving towards phase III testing.
Prevention of Vein Graft Failure
Intraoperative Evaluation
Despite improvements in surgical technique, 5% of autogenous vein grafts fail within 30 days of implantation.92 Retained valve leaflets, abnormal twists or kinks, intimal flaps, other unrecognized obstructive lesions, and technical defects at the time of graft placement are estimated to account for between 15% and 25% of early failures.186,187 The initial technical adequacy of an infrainguinal bypass graft can be judged intraoperatively by the external appearance of the bypass and anastomoses and by the restoration of distal pulses and perfusion. Continuous wave Doppler is a useful intraoperative adjunct that permits evaluation of anastomoses and areas of concern within the body of the graft for localized increases in the audible sound frequency. Though adequate for gross defects, a more detailed assessment to ensure technical success and to optimize short- and intermediate-term graft patency has been advocated. Recent investigations report a 15% intraoperative graft revision rate for abnormalities identified on secondary imaging.188 Although it is unclear to what extent these irregularities increase the risk for graft failure, the significant morbidity resulting from early reintervention for graft failure supports an aggressive approach to intraoperative repair.189
Angiography
Contrast-enhanced angiography remains the diagnostic standard for arterial anatomic imaging and is the most widely used method for intraoperative vein graft assessment. Facilitated by the increasing availability of high-quality intraoperative angiographic equipment, this technique provides the current standard for determination of technical adequacy of a bypass graft. Though particularly useful for identifying a kink or twist in the graft, a patent side branch or branch ligature stenosis, and distal arterial disease requiring sequential bypass,190 angiography has several notable shortcomings. Limited by a single projection plane and the dense opacification provided by iodinated contrast material, retained valve leaflets and intraluminal webs can be difficult to identify, with one study reporting failure rates approaching 80%.119 Particularly troublesome is angiographic evaluation of the distal anastomosis and delineation of a structural abnormality versus spasm in the outflow artery (see Fig. 92-7). Significant spasm occurs in approximately 50% of tibial and pedal artery bypasses, but it is of no clinical consequence in the large majority of cases.191,192 Routine intraluminal injection of papaverine hydrochloride (30 to 60 mg in 1 to 2 mL of 0.9% saline) into the vein graft before performing completion angiography has been advocated to alleviate vasospasm.193
With these technical limitations, the use of a standard evaluation algorithm (consisting of visual inspection, palpation, and interrogation with continuous wave Doppler) with selective arteriography has been proposed. Mills et al noted that these simpler techniques failed to identify 80% of the vein graft and outflow abnormalities that were detected by completion angiography and required revision.194 Focusing on 1-year patency as the primary endpoint, other investigators examining this issue arrived at the opposite conclusion and stated that the additional expense of routine arteriography outweighs its benefit.195 In the latter retrospective review, where no difference in graft patency was observed in association with routine or selective angiography, patient enrollment was modest and statistical analysis insufficiently powered to allow firm conclusions. Completion angiography provides useful anatomic information, especially with regard to the status of the outflow arteries; its major weakness is that it fails to provide any hemodynamic assessment.
Flow Rate Measurements
Non–imaging-based modalities such as measurement of blood flow rates have been advocated as useful adjuncts in the intraoperative evaluation of vein grafts. Based on the assumption that flow-limiting stenoses could be detected by reductions in flow, investigators identified 80 mL/min as the critical threshold below which there was a high probability of a technical problem within the reconstruction and for which further evaluation with angiography was suggested.196 Although of interest, this approach has largely been supplanted by duplex imaging. The use of intraoperative flow measurements has also been described as an independent predictor of graft patency. Despite being unreliable in identifying grafts at risk for early (<30 day) failure,197 extensive literature confirms the importance of graft flow rates in determining intermediate- and long-term patency.198–200 Not surprisingly, flow rates demonstrate high correlation with the angiographic run-off score152 and can serve as a surrogate for this well-established risk factor with limited direct application to intraoperative decision making. More quantitative assessments of graft hemodynamics, such as the resistive index and longitudinal impedance, have also been proposed.201,202 Similar to flow rate measurements, these parameters have prognostic significance but little utility in therapeutic decision making.
Duplex Scanning
After angiography, duplex scanning has developed into the next most commonly used intraoperative method for evaluation of vein grafts. Duplex uses B-mode imaging and velocity spectrum analysis to detect lesions and grade lesion severity, thus providing both anatomic and hemodynamic assessment. Initially described by Bandyk et al for the assessment of in situ bypass grafts,203 duplex scanning proved effective in the identification of competent valve leaflets, anastomotic narrowing, arteriovenous fistulae, hemodynamic stenosis caused by small-caliber conduit, and the intraoperative formation of fibrin-platelet aggregates. With evolving diagnostic criteria, duplex scanning was initially thought to be too sensitive and best suited for use as a screening tool in combination with selective arteriography to better define suspect areas. Subsequent refinements in both instrumentation and interpretation have yielded improved accuracy, with multiple studies supporting the replacement of intraoperative angiography with duplex scanning.204–206 Although the published literature has been unequivocal regarding the value of intraoperative duplex, application has been limited predominantly to centers of excellence where sufficient skilled vascular technologists are available to support these efforts.
A standardized procedure for intraoperative duplex graft scanning has been proposed by Johnson et al.188 Before scanning, papaverine (30 to 60 mg) is injected into the distal vein graft segment with a 27-gauge needle. Using a linear-array 7- to 15-MHz transducer (for superficial grafts) or 5-MHz transducer (for tunneled grafts), velocity spectra along the distal anastomosis and outflow artery are recorded while maintaining a 60-degree Doppler angle. The entire length of the graft is then scanned in a distal to proximal direction to evaluate color-flow images and velocity spectra for retained valves and residual arteriovenous fistulae (if applicable). Evaluation is completed with scanning of the proximal anastomosis and inflow artery. Diagnostic criteria for elevated velocities and an appropriate management algorithm are presented in Table 92-2. In one center using this protocol, a normal intraoperative duplex scan has been associated with a reduction in 90-day graft thrombosis to 0.4%.207
Table 92-2
Intraoperative Duplex Monitoring—Diagnostic Criteria and Management Options
PSV, Peak systolic velocity; PVR, peripheral vascular resistance—low (antegrade flow throughout the pulse cycle) or high (antegrade flow only during systole with minimal diastolic flow); Vr, velocity ratio (PSVat lesion/PSVproximal).
Adapted from Johnson BL, et al: Intraoperative duplex monitoring of infrainguinal vein bypass procedures. J Vasc Surg 31:678-690, 2000.
A significant advantage of this technique is its ability to detect low-flow bypass grafts, defined as an average peak systolic velocity (PSV) of less than 45 cm/sec (see Table 92-2). Subsequent analysis of these low-flow grafts has identified two clinical subgroups based on the presence or absence of diastolic flow. The majority of grafts with high peripheral vascular resistance and absent diastolic flow failed within 6 months of implantation,208 and surgical revision consisting of either a sequential graft to an alternative target or placement of an arteriovenous fistula has been advocated to salvage these threatened grafts. Low-flow grafts with low peripheral vascular resistance (characterized by antegrade flow throughout the pulse cycle) were at intermediate risk of failure (30% failure rate at 6 months) and may benefit from postoperative anticoagulation.188
Angioscopy
Angioscopy provides an alternative image-based modality for intraoperative assessment of vein grafts. Not only does it assist in preparation of the conduit by allowing accurate and complete valvulotomy, detecting unsuspected intraluminal pathology, and assisting in the selection of optimal-quality vein segments for composite grafting, it also provides a tool for assessment of technical errors at completion of the procedure. However, successful application of angioscopy requires an investment in new instrumentation and a commitment to overcome its inherent learning curve. Several groups have examined the potential benefits of completion angioscopy, with a generalized consensus that angioscopy is significantly more sensitive in identifying intraluminal abnormalities than either angiography209,210 or duplex scanning.119 The most informative study to date, conducted by Miller et al, was a 250-patient prospective randomized evaluation of angioscopy versus angiography.211 Intraluminal abnormalities were identified in a fifth of the enrolled grafts, with 75% of them determined to be of sufficient severity to prompt surgical correction. Intraoperative revisions were fourfold more likely to be performed after angioscopic evaluation and led to a 50% reduction in 30-day graft failure (3% after angioscopy versus 6% after angiography). Although this difference did not reach statistical significance, it suggested that some improvement in early graft patency may be obtained with the use of completion angioscopy and aggressive intraoperative revision of any abnormalities identified. Not examined in this trial were the potential intermediate- and long-term benefits in graft patency afforded by this approach, which may have been even more pronounced than the effects on early patency.
Postoperative Surveillance
Physical Examination and Physiologic Assessment
As initially established by the seminal work of Szilagyi156 and re-enforced by multiple other studies, it is now known that autogenous grafts develop segmental foci of luminal narrowing that lead to reductions in flow and precipitate thrombosis. Attempts to salvage thrombosed grafts outside of the early postoperative period have largely been unsuccessful, with 30% 1-year patency rates after thrombectomy.212 These observations contributed to the concept of routine graft surveillance, or periodic evaluation to identify grafts with evolving lesions before the development of thrombosis. Resulting from a combination of intimal hyperplasia and pathologic (inward) remodeling, these lesions have a peak incidence within the first 12 months, with 30% becoming hemodynamically significant within this 1-year time frame.93,213 These observations form the basis for graft surveillance protocols in which frequent evaluations are recommended during the first 12 to 18 months after implantation.
Clinical protocols for graft surveillance focus on physical examination with physiologic assessment of distal perfusion. Patient histories are elicited to detect new onset of claudication or ischemic pain at rest, and physical examination is performed to identify notable changes in the lower extremity pulse. The ABI is used as the quantitative measure of perfusion, with a reduction of greater than 0.15 being considered significant. Changes in any of these parameters should prompt further evaluation to identify the lesion or lesions that have precipitated the hemodynamic deterioration. To improve the sensitivity of physiologic testing, pulse volume recording with a transfer function index protocol has been proposed.214 Pulse volume recordings are collected from upper extremity (inflow) and ankle (outflow) cuffs and subjected to a Fourier transform algorithm to produce discrete spectra for review. Direct comparison of the frequency response curves provides a numeric value that can be used to identify at-risk grafts. Proof-of-concept testing of this device has suggested accuracy equivalent to that of duplex scanning, but this approach has failed to gain acceptance outside the research arena.
Duplex Scanning
With the recognition that clinical surveillance parameters were insensitive until the lumen was severely compromised,194,215 duplex scanning was identified as a method for earlier detection of these developing lesions. After an initial focus on reductions in PSV and the absence of diastolic flow,216,217 changes in velocity along the length of the graft were recognized as a more useful approach for lesion localization.218,219 Though not generated through a formal series of receiver operating characteristic curves, a duplex classification has evolved to correlate PSV and the velocity ratio (Vr) with the approximate degrees of vein graft stenosis (Table 92-3).220 Complicating management of vein graft stenosis is the dynamic remodeling that occurs in 12 to 18 months after implantation, during which the appearanceand subsequent regression of focal regions of luminal narrowing are frequently observed.99,221 Retrospective reviews correlating duplex-derived hemodynamic data with lesion morphology suggested a PSV of 300 cm/sec (or a Vr of 4.0) to be the critical threshold above which lesion regression was relatively unlikely (Fig. 92-9).222,223 Intermediate stenoses (200 cm/sec < PSV < 300 cm/sec; 2 < Vr < 4) are notably dynamic, with 20% to 30% of these lesions demonstrating significant regression within 2 years. Complicating these retrospective analyses, however, was the preexisting bias that high-grade stenoses mandate intervention, so the natural history of these high-grade lesions has been incompletely defined. Although the number of grafts with high-grade stenoses that did not undergo elective revision is relatively small, the risk of occlusion of these unrepaired grafts appears to be in excess of 75%.217,223 Predominantly based on these observations, an algorithm for the management of postoperative vein graft stenosis has been proposed,220 with the key elements of this approach outlined in Table 92-3. Of particular concern are grafts with a PSV of greater than 300 cm/sec and a change in the ABI of greater than 0.15 or significant dampening of the distal waveforms. These findings are generally thought to be preocclusive lesions, and initiation of systemic anticoagulation with prompt repair is indicated.
Table 92-3
Postoperative Duplex Monitoring—Diagnostic Criteria and Management Options
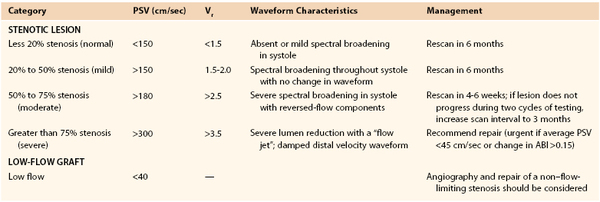
ABI, Ankle-brachial index; PSV, peak systolic velocity; Vr, velocity ratio (PSVat lesion/PSVproximal).
Adapted from Bandyk DF: Infrainguinal vein bypass graft surveillance: how to do it, when to intervene, and is it cost-effective? J Am Coll Surg 194(1 Suppl):S40-S50, 2002.
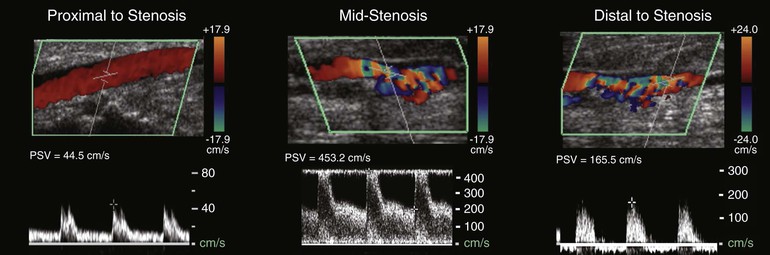
Figure 92-9 Postoperative duplex scan of a vein graft demonstrating markedly elevated peak systolic velocity and spectral broadening within an area of stenosis. Distal to the lesion, disordered flow is evident on color-flow imaging, and a significant reduction in diastolic velocity is observed.
In parallel with these observational studies, multiple single-institution cohort studies have clarified the role of duplex surveillance in improving patient outcomes. Results from these studies are in general agreement and demonstrate an approximate 15% improvement in graft patency rates with routine duplex evaluation versus clinical follow-up alone.224–226 Recommendations for the frequency of vein graft surveillance have been proposed and focus on the intermediate period (1 to 18 months), when lesion remodeling is most active. After an intraoperative or early postoperative scan to identify technical problems, outpatient duplex evaluations at 1, 3, 6, 12, and 18 months and annually thereafter are recommended. The development of moderate stenosis or the need for vein graft revision would prompt closer follow-up, with interval scans at approximately 6 weeks until lesion stabilization or regression is confirmed.
The economic benefits of a graft surveillance program have been investigated by a number of investigators, who have focused on major amputation as the dominant outcome variable.227,228 Duplex scan surveillance was the least expensive ($2823) and resulted in the fewest major amputations (17 per 1000 patients examined) when compared with clinical follow-up alone ($5072 and 77 amputations per 1000 patients).227 With the recognition that 90% of lesions that will require revision between 1 and 18 months will demonstrate some element of flow abnormality within 4 weeks,229 investigators have questioned the need for continued aggressive surveillance after normal 1- and 3-month graft scans.230 Longer-term follow-up has clarified this issue; one study reported that 30% of the flow abnormalities that ultimately required revision were not observed until after 6 months.231 Annual examination to evaluate for graft atherosclerosis or aneurysmal degeneration and progression of native inflow-outflow arterial disease therefore seems warranted.
Although the large array of cohort studies presents compelling data to support a routine duplex surveillance program, consensus on this issue is not uniform. Three randomized prospective trials were performed to evaluate vein graft surveillance and reached variable conclusions regarding its benefits. The first and smallest of these studies, published by Lundell et al in 1995, randomized 156 patients after vein bypass grafts to either intense duplex surveillance or intermittent clinical follow-up.232 Although major amputation was not explored as an endpoint, implementation of an intensive duplex protocol provided a 25% absolute improvement in both primary-assisted and secondary graft patency rates at 3 years. Among the confounding factors in this trial, however, was their use of frequent (every 3 months) follow-up in the duplex group but only annual follow-up in the clinical group. Consequently, a definitive conclusion on the value of duplex scanning versus more frequent clinical follow-up could not be obtained. The second trial randomized 342 patients between duplex and clinical surveillance and demonstrated no difference in graft patency or limb salvage at 1 year.233 Though methodologically sound, the duration of follow-up was probably insufficient to realize the full benefit of serial duplex evaluation. The largest trial, in which 594 patients were randomized to a duplex or clinical protocol, demonstrated no difference in major amputation or graft patency at 18 months.234 Despite the use of a prospective randomized methodology, significant concern has been raised in regard to the validity of these findings.235,236 Use of nonstandard duplex criteria resulting in an aggressive treatment approach to intermediate stenoses, delay in randomization until 4 to 6 weeks postoperatively (reducing the early benefit of duplex examination), and the short follow-up time were among the major issues that were raised.
Despite these potential methodologic flaws in the randomized trials, the available literature presents two diametrically opposing views on the relative value of a duplex surveillance program. Multiple cohort studies have exhaustively detailed the natural history of vein graft lesions and the perceived benefits of early detection and repair of high-grade lesions; yet all lack an appropriate control group. In contrast, the randomized trials suggest little benefit of duplex surveillance, but such generalization may be limited by notable methodologic flaws. Faced with this dilemma, the 2007 Inter-Society Consensus for the Management of Peripheral Arterial Disease came forth with a grade C recommendation supporting clinical surveillance only, with a focus on history taking for new symptoms, complete vascular examination of the extremity, and resting and postexercise ABI at 6-month intervals for a minimum of 2 years.237 The consensus statement notes that the previous recommendation for routine duplex scanning after autogenous lower extremity bypass has not yet proved cost effective. Nevertheless, most surgeons continue a program of vein graft surveillance and remain skeptical of these negative findings.
Selected Key References
Adcock OT Jr, Adcock GL, Wheeler JR, Gregory RT, Snyder SO Jr, Gayle RG. Optimal techniques for harvesting and preparation of reversed autogenous vein grafts for use as arterial substitutes: a review. Surgery. 1984;96:886–894.
Bandyk DF. Infrainguinal vein bypass graft surveillance: how to do it, when to intervene, and is it cost-effective? J Am Coll Surg. 2002;194(Suppl 1):S40–S50.
Caps MT, Cantwell-Gab K, Bergelin RO, Strandness DE Jr. Vein graft lesions: time of onset and rate of progression. J Vasc Surg. 1995;22:466–474.
Longitudinal evaluation of early and intermediate vein graft lesion development and regression..
Davies AH, Hawdon AJ, Sydes MR, Thompson SG. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the vein graft surveillance randomised trial (VGST). Circulation. 2005;112:1985–1991.
Hölzenbein TJ, Pomposelli FB Jr, Miller A, Contreras MA, Gibbons GW, Campbell DR, Freeman DV, LoGerfo FW. Results of a policy with arm veins used as the first alternative to an unavailable ipsilateral greater saphenous vein for infrainguinal bypass. J Vasc Surg. 1996;23:130–140.
Marcaccio EJ, Miller A, Tannenbaum GA, Lavin PT, Gibbons GW, Pomposelli FB Jr, Freeman DV, Campbell DR, LoGerfo FW. Angioscopically directed interventions improve arm vein bypass grafts. J Vasc Surg. 1993;17:994–1002.
Mills JL, Fujitani RM, Taylor SM. The characteristics and anatomic distribution of lesions that cause reversed vein graft failure: a five-year prospective study. J Vasc Surg. 1993;17:195–204.
Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, Moneta GL, Conte MS. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46:1180–1190.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Dos Santos JC. Sur la desobstruction des thromboses arterielle anciennes. Memorandes Acadamie Chirurgie. 1944;73:409–411.
2. Kunlin JL. Le traitement de l’arterite obliterante par le greffe veineuse. Arch Mal Coeur Vaiss. 1949;42:371–374.
3. Mannick JA. Presidential address: Remembrance of things past—New England surgeons and infrainguinal reconstruction. J Vasc Surg. 1996;24(1):1–5.
4. Linton RR, et al. Autogenous saphenous vein bypass grafts in femoropopliteal obliterative arterial disease. Surgery. 1962;51:62–73.
5. Couch NP, et al. Factors influencing limb survival after femoropopliteal reconstruction. Arch Surg. 1967;95(2):163–169.
6. Dale WA. Grafting small arteries. Experience with 19 shunts below the knee. Arch Surg. 1963;86:22–33.
7. Mannick JA, et al. Salvage of extremities by vein graft in far-advanced peripheral vascular disease. Surgery. 1964;55:154–164.
8. Mannick JA, et al. Success of bypass vein grafts in patients with isolated popliteal artery segments. Surgery. 1967;61(1):17–25.
9. Pomposelli FB, et al. a decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37(2):307–315.
10. Campbell DR, et al. The use of arm veins in femoral-popliteal bypass grafts. Ann Surg. 1979;190(6):740–742.
11. Donaldson MC, et al. Further experience with an all-autogenous tissue policy for infrainguinal reconstruction. J Vasc Surg. 1993;18(1):41–48.
12. Berceli SA, et al. Mechanisms of vein graft atherosclerosis: LDL metabolism and endothelial actin reorganization. J Vasc Surg. 1991;13(2):336–347.
13. Kevil CG, et al. Expression of zonula occludens and adherens junctional proteins in human venous and arterial endothelial cells: role of occludin in endothelial solute barriers. Microcirculation. 1998;5(2-3):197–210.
14. Davies MG, et al. The integrity of experimental vein graft endothelium—implications on the etiology of early graft failure. Eur J Vasc Surg. 1993;7(2):156–165.
15. Berceli SA, et al. Biomechanics of the venous wall under simulated arterial conditions. J Biomech. 1990;23(10):985–989.
16. Kouzi-Koliakos K, et al. Prebypass histological and ultrastructural evaluation of the long saphenous vein as a predictor of early graft failure. Cardiovasc Pathol. 2006;15(6):336–346.
17. Marin ML, et al. Saphenous vein biopsy: a predictor of vein graft failure. J Vasc Surg. 1993;18(3):407–414.
18. Giannoukas AD, et al. Pre-bypass quality assessment of the long saphenous vein wall with ultrasound and histology. Eur J Vasc Endovasc Surg. 1997;14(1):37–40.
19. Caggiati A, et al. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg. 2002;36(2):416–422.
20. Mozes G, et al. New discoveries in anatomy and new terminology of leg veins: clinical implications. Vasc Endovascular Surg. 2004;38(4):367–374.
21. Thomson H. The surgical anatomy of the superficial and perforating veins of the lower limb. Ann R Coll Surg Engl. 1979;61:198–205.
22. Clagett GP, et al. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg. 1997;25(2):255–266.
23. Smith ST, et al. Femoral vein harvest for vascular reconstructions: pitfalls and tips for success. Semin Vasc Surg. 2008;21(1):35–40.
24. Veith FJ, et al. Preoperative saphenous venography in arterial reconstructive surgery of the lower extremity. Surgery. 1979;85(3):253–256.
25. Leopold PW, et al. Initial experience comparing B-mode imaging and venography of the saphenous vein before in situ bypass. Am J Surg. 1986;152(2):206–210.
26. Salles-Cunha SX, et al. Preoperative noninvasive assessment of arm veins to be used as bypass grafts in the lower extremities. J Vasc Surg. 1986;3(5):813–816.
27. Seeger JM, et al. Preoperative saphenous and cephalic vein mapping as an adjunct to reconstructive arterial surgery. Ann Surg. 1987;205(6):733–739.
28. Buchbinder D, et al. B-mode ultrasonic imaging in the preoperative evaluation of saphenous vein. Am Surg. 1987;53(7):368–372.
29. Panetta TF, et al. Unsuspected preexisting saphenous vein disease: an unrecognized cause of vein bypass failure. J Vasc Surg. 1992;15(1):102–110.
30. Meissner MH, et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007;46(Suppl S):4S–24S.
31. Towne JB, et al. The effect of vein diameter on patency of in situ grafts. J Cardiovasc Surg (Torino). 1991;32(2):192–196.
32. Schanzer A, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46(6):1180–1190.
33. Buxton B, et al. The significance of vein wall thickness and diameter in relation to the patency of femoropopliteal saphenous vein bypass grafts. Surgery. 1980;87(4):425–431.
34. Ross R, et al. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976;295(8):420–425.
35. Ross R, et al. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976;295(7):369–377.
36. Ross R. The pathogenesis of atherosclerosis—an update. N Engl J Med. 1986;314(8):488–500.
37. Conte MS, et al. Genetic interventions for vein bypass graft disease: a review. J Vasc Surg. 2002;36(5):1040–1052.
38. Quist WC, et al. Prevention of smooth muscle cell phenotypic modulation in vein grafts: a histomorphometric study. J Vasc Surg. 1992;16(2):225–231.
39. Adcock GD, et al. Arterialization of reversed autogenous vein grafts: quantitative light and electron microscopy of canine jugular vein grafts harvested and implanted by standard or improved techniques. J Vasc Surg. 1987;6(3):283–295.
40. Eslami MH, et al. Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury? J Vasc Surg. 2001;34(5):923–929.
41. Abbott WM, et al. Structural changes during preparation of autogenous venous grafts. Surgery. 1974;76(6):1031–1040.
42. Gundry SR, et al. Optimal preparation techniques for human saphenous vein grafts. Surgery. 1980;88(6):785–794.
43. Gundry SR, et al. Intraoperative trauma to human saphenous veins: scanning electron microscopic comparison of preparation techniques. Ann Thorac Surg. 1980;30(1):40–47.
44. Gottlob R. The preservation of the venous endothelium by “dissection without touchin” and by an atraumatic technique of vascular anastomosis. The importance for arterial and venous surgery. Minerva Chir. 1977;32(11):693–700.
45. Taylor LM Jr, et al. Reversed vein bypass to infrapopliteal arteries. Modern results are superior to or equivalent to in-situ bypass for patency and for vein utilization. Ann Surg. 1987;205(1):90–97.
46. Mamode N, et al. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2000;(2).
47. Dashwood MR, et al. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg. 2005;80(3):939–944.
48. Souza DS, et al. “No-touch” technique using saphenous vein harvested with its surrounding tissue for coronary artery bypass grafting maintains an intact endothelium. Scand Cardiovasc J. 1999;33(6):323–329.
49. Souza DSR, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg. 2002;73(4):1189–1195.
50. Souza DSR, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. The J Thorac Cardiovasc Surg. 2006;132(2):373.
51. Ramos JR, et al. Histologic fate and endothelial changes of distended and nondistended vein grafts. Ann Surg. 1976;183(3):205–228.
53. Bush HL Jr, et al. Functional injury of vein graft endothelium. Role of hypothermia and distention. Arch Surg. 1984;119(7):770–774.
54. LoGerfo FW, et al. An improved technique for preservation of endothelial morphology in vein grafts. Surgery. 1981;90(6):1015–1024.
55. Zhao J, et al. Manual pressure distension of the human saphenous vein changes its biomechanical properties—implication for coronary artery bypass grafting. Journal of Biomechanics. 2007;40(10):2268–2276.
56. Galea J, et al. Alterations in c-fos expression, cell proliferation and apoptosis in pressure distended human saphenous vein. Cardiovasc Res. 1999;44(2):436–448.
57. Sottiurai VS, et al. Ultrastructure of human and transplanted canine veins: effects of different preparation media. Surgery. 1983;93(1 Pt 1):28–38.
58. Cavallari N, et al. Functional and morphological evaluation of canine veins following preservation in different storage media. J Surg Res. 1997;68(2):106–115.
59. Cavallari N, et al. Short-term preservation of autogenous vein grafts: effectiveness of University of Wisconsin solution. Surgery. 1997;121(1):64–71.
60. LoGerfo FW, et al. Integrity of vein grafts as a function of initial intimal and medial preservation. Circulation. 1983;68(3 Pt 2):II117–II124.
61. Lawrie GM, et al. Endothelium-dependent relaxation in human saphenous vein grafts. Effects of preparation and clinicopathologic correlations. J Thorac Cardiovasc Surg. 1990;100(4):612–620.
62. Cook JM, et al. Thrombomodulin activity on human saphenous vein grafts prepared for coronary artery bypass. J Vasc Surg. 1991;14(2):147–151.
63. Bush J, et al. Functional injury of vein graft endothelium. Role of hypothermia and distention. Arch Surg. 1984;119(7):770–774.
64. LoGerfo FW, et al. A clinical technique for prevention of spasm and preservation of endothelium in saphenous vein grafts. Arch Surg. 1984;119(10):1212–1214.
65. Adcock OT Jr, et al. Optimal techniques for harvesting and preparation of reversed autogenous vein grafts for use as arterial substitutes: a review. Surgery. 1984;96(5):886–894.
66. Roubos N, et al. Improved preservation of saphenous vein grafts by the use of glyceryl trinitrateû verapamil solution during harvesting. Circulation. 1995;92(9):31–36.
67. Reifsnyder T, et al. Wound complications of the in situ saphenous vein bypass technique. J Vasc Surg. 1992;15(5):843–848.
68. Wengrovitz M, et al. Wound complications of autogenous subcutaneous infrainguinal arterial bypass surgery: predisposing factors and management. J Vasc Surg. 1990;11(1):156–161.
69. Kiaii B, et al. A prospective randomized trial of endoscopic versus conventional harvesting of the saphenous vein in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2002;123(2):204–212.
70. Wang S, et al. Saphenous Vein Harvest With SaphLITE System Versus Conventional Technique: A Prospective, Randomized Study. Ann Thorac Surg. 2005;79(6):2018–2023.
71. Yun KL, et al. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: Six-month patency rates. J Thorac Cardiovasc Surg. 2005;129(3):496–503.
72. Fabricius AM, et al. Minimally invasive saphenous vein harvesting techniques: morphology and postoperative outcome. Ann Thorac Surg. 2000;70(2):473–478.
73. Meyer DM, et al. Histologic evidence of the safety of endoscopic saphenous vein graft preparation. Ann Thorac Surg. 2000;70(2):487–491.
74. Erdoes LS, et al. Encouraging results with endoscopic vein harvest for infrainguinal bypass. J Vasc Surg. 2005;42(3):442–448.
75. Gazoni LM, et al. Endoscopic versus open saphenous vein harvest for femoral to below the knee arterial bypass using saphenous vein graft. J Vasc Surg. 2006;44(2):282–287.
76. Illig KA, et al. Financial impact of endoscopic vein harvest for infrainguinal bypass. J Vasc Surg. 2003;37(2):323–330.
77. Jordan WD Jr, et al. The durability of endoscopic saphenous vein grafts: a 5-year observational study. J Vasc Surg. 2001;34(3):434–439.
78. Pullatt R, et al. Compromised bypass graft outcomes after minimal-incision vein harvest. J Vasc Surg. 2006;44(2):289–294.
79. Erdoes LS. Endoscopic vein harvest in peripheral vascular surgery. J Vasc Surg. 2006;44(2):417–418.
80. Jimenez JC, et al. Technical modifications in endoscopic vein harvest techniques facilitate their use in lower extremity limb salvage procedures. J Vasc Surg. 2007;45(3):549–553.
81. Holzenbein TJ, et al. Results of a policy with arm veins used as the first alternative to an unavailable ipsilateral greater saphenous vein for infrainguinal bypass. J Vasc Surg. 1996;23(1):130–140.
82. Chew DK, et al. Bypass in the absence of ipsilateral greater saphenous vein: safety and superiority of the contralateral greater saphenous vein. J Vasc Surg. 2002;35(6):1085–1092.
83. Poletti LF, et al. Should vein be saved for future operations? A 15-year review of infrainguinal bypasses and the subsequent need for autogenous vein. Ann Vasc Surg. 1998;12(2):143–147.
84. Robin C, et al. Distal bypass for limb salvage: should the contralateral great saphenous vein be harvested? Ann Vasc Surg. 2006;20(6):761–766.
85. Tarry WC, et al. Fate of the contralateral leg after infrainguinal bypass. J Vasc Surg. 1998;27(6):1039–1048.
86. Masser PA, et al. Technique of reversed vein bypass for lower extremity ischemia. Ann Vasc Surg. 1996;10(2):190–200.
87. Armstrong PA, et al. Optimizing infrainguinal arm vein bypass patency with duplex ultrasound surveillance and endovascular therapy. J Vasc Surg. 2004;40(4):724–731.
88. Belkin M, et al. Preferred strategies for secondary infrainguinal bypass: lessons learned from 300 consecutive reoperations. J Vasc Surg. 1995;21(2):282–293.
89. Faries PL, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32(6):1080–1090.
90. Faries PL, et al. The use of arm vein in lower-extremity revascularization: results of 520 procedures performed in eight years. J Vasc Surg. 2000;31(1 Pt 1):50–59.
91. Kent KC, et al. Short-term and midterm results of an all-autogenous tissue policy for infrainguinal reconstruction. J Vasc Surg. 1989;9(1):107–114.
92. Conte MS, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751.
93. Mills JL, et al. The characteristics and anatomic distribution of lesions that cause reversed vein graft failure: a five-year prospective study. J Vasc Surg. 1993;17(1):195–204.
94. McCaughan JJ Jr, et al. In vitro observations of greater saphenous vein valves during pulsatile and nonpulsatile flow and following lysis. J Vasc Surg. 1984;1(2):356–361.
95. Chin AK, et al. The effect of valvulotomy on the flow rate through the saphenous vein graft: clinical implications. J Vasc Surg. 1988;8(3):316–320.
96. Ku DN, et al. The contribution of valves to saphenous vein graft resistance. J Vasc Surg. 1987;6(3):274–279.
97. Walsh DB, et al. Valvular obstruction of blood flow through saphenous veins. J Surg Res. 1987;42(1):39–42.
98. Tullis MJ, et al. Detection of “functional” valves in reversed saphenous vein bypass grafts: identification with duplex ultrasonography. J Vasc Surg. 1997;25(3):522–527.
99. Vesti BR, et al. Follow-up of valves in saphenous vein bypass grafts with duplex ultrasonography. J Vasc Surg. 2001;33(2):369–374.
100. Vanttinen E, et al. Femoropopliteal and femorotibial arterial reconstructive surgery. Special reference to the autogenous venous bypass procedure using unreversed vein after eversion valvectomy. Acta Chir Scand. 1975;141(5):341–352.
101. Banz M, et al. Infrainguinal arterial reconstruction with non-reversed autologous vein after angioscopy guided valvulotomy ex situ. Eur J Vasc Endovasc Surg. 1995;10(2):211–214.
103. Belkin M, et al. Infrainguinal arterial reconstruction with nonreversed greater saphenous vein. J Vasc Surg. 1996;24(6):957–962.
104. Stierli P, et al. Angioscopy guided in situ bypass versus angioscopy guided non reversed bypass for infrainguinal arterial reconstructions. A comparison of outcome. J Cardiovasc Surg (Torino). 1995;36(3):211–217.
105. May AG, et al. Arterialized in situ saphenous vein. Arch Surg. 1965;91(5):743–750.
106. Hall KV. The great saphenous vein used in situ as an arterial shunt after extirpation of the vein valves. A preliminary report. Surgery. 1962;51:492–495.
107. Leather RP, et al. A reappraisal of the in situ saphenous vein arterial bypass: its use in limb salvage. Surgery. 1979;86(3):453–461.
108. Leather RP, et al. Further experience with the saphenous vein used in situ for arterial bypass. Am J Surg. 1981;142(4):506–510.
109. Wengerter KR, et al. Prospective randomized multicenter comparison of in situ and reversed vein infrapopliteal bypasses. J Vasc Surg. 1991;13(2):189–197.
110. Jackson ZS, et al. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res. 2002;90(8):918–925.
111. Veith FJ, et al. Short vein grafts in limb-saving arterial reconstructions. J Vasc Interv Radiol. 1990;1(1):57–61.
112. Mills NL, et al. Valvulotomy of valves in the saphenous vein graft before coronary artery bypass. J Thorac Cardiovasc Surg. 1976;71(6):878–879.
113. LeMaitre GD, et al. In situ grafting made easy. Modification of a technique. Arch Surg. 1988;123(1):101–103.
114. Skagseth E, et al. In situ vein bypass. Experiences with new vein valve strippers. Scand J Thorac Cardiovasc Surg. 1973;7(1):53–58.
115. Leather RP, et al. Instrumental evolution of the valve incision method of in situ saphenous vein bypass. J Vasc Surg. 1984;1(1):113–123.
116. Alback A, et al. Valvulotomy of non-reversed saphenous vein bypass grafts: a randomised, blinded, angioscopy-controlled study. Eur J Vasc Endovasc Surg. 1999;18(2):144–148.
117. Parent FN III, et al. Angioscopic evaluation of valvular disruption during in situ saphenous vein bypass. Ann Vasc Surg. 1994;8(1):24–30.
118. Malmstedt J, et al. A randomized prospective study of valvulotome efficacy in in situ reconstructions. Eur J Vasc Endovasc Surg. 2005;30(1):52–56.
119. Gilbertson JJ, et al. A blinded comparison of angiography, angioscopy, and duplex scanning in the intraoperative evaluation of in situ saphenous vein bypass grafts. J Vasc Surg. 1992;15(1):121–127.
120. Clair DG, et al. Randomized prospective study of angioscopically assisted in situ saphenous vein grafting. J Vasc Surg. 1994;19(6):992–999.
121. Maini BS, et al. A modified, angioscopically assisted technique for in situ saphenous vein bypass: impact on patency, complications, and length of stay. J Vasc Surg. 1993;17(6):1041–1047.
122. Sales CM, et al. Prospective study of the value of prebypass saphenous vein angioscopy. Am J Surg. 1995;170(2):106–108.
123. Wilson YG, et al. Angioscopically-assisted in situ saphenous vein bypass for infrainguinal revascularisation. Eur J Vasc Endovasc Surg. 1996;12(2):223–229.
124. Lundell A, et al. Do residual arteriovenous fistulae after in situ saphenous vein bypass grafting influence patency? J Vasc Surg. 1999;30(1):99–110.
125. van Dijk LC, et al. Residual arteriovenous fistulae after “closed” in situ bypass grafting: an overrated problem. Eur J Vasc Endovasc Surg. 1997;13(5):439–442.
126. Taylor LM Jr, et al. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11(2):193–205.
127. Faries PL, et al. Arm vein conduit is superior to composite prosthetic-autogenous grafts in lower extremity revascularization. J Vasc Surg. 2000;31(6):1119–1127.
128. Holzenbein TJ, et al. The upper arm basilic-cephalic loop for distal bypass grafting: technical considerations and follow-up. J Vasc Surg. 1995;21(4):586–594.
129. LoGerfo FW, et al. A new arm vein graft for distal bypass. J Vasc Surg. 1987;5(6):889–891.
130. Marcaccio EJ, et al. Angioscopically directed interventions improve arm vein bypass grafts. J Vasc Surg. 1993;17(6):994–1002.
131. Shandall AA, et al. Use of the short saphenous vein in situ for popliteal-to-distal artery bypass. Am J Surg. 1987;154(2):240–244.
132. Chang BB, et al. The lesser saphenous vein: an underappreciated source of autogenous vein. J Vasc Surg. 1992;15(1):152–156.
133. Goyal A, et al. Popliteal-crural bypass through the posterior approach with lesser saphenous vein for limb salvage. J Vasc Surg. 2002;36(4):708–712.
134. Ouriel K. The posterior approach to popliteal-crural bypass. J Vasc Surg. 1994;19(1):74–80.
135. Weaver FA, et al. The lesser saphenous vein: autogenous tissue for lower extremity revascularization. J Vasc Surg. 1987;5(5):687–692.
136. Delis KT, et al. The Giacomini vein as an autologous conduit in infrainguinal arterial reconstruction. J Vasc Surg. 2004;40(3):578–581.
137. Schulman ML, et al. Deep veins of the leg as femoropopliteal bypass grafts. Arch Surg. 1981;116(9):1141–1145.
138. Schulman ML, et al. A saphenous alternative: preferential use of superficial femoral and popliteal veins as femoropopliteal bypass grafts. Am J Surg. 1986;152(2):231–237.
139. Schulman ML, et al. A saphenous alternative: preferential use of superficial femoral and popliteal veins as femoropopliteal bypass grafts. Am J Surg. 1986;152(2):231–237.
140. Schulman ML, et al. Superficial femoral-popliteal veins and reversed saphenous veins as primary femoropopliteal bypass grafts: a randomized comparative study. J Vasc Surg. 1987;6(1):1–10.
141. Schanzer H, et al. Use of lower extremity deep veins as arterial substitutes: functional status of the donor leg. J Vasc Surg. 1991;14(5):624–627.
142. Mukherjee D, et al. The superficial femoral artery as a conduit: an alternative to prosthetic material. J Vasc Surg. 1985;2(5):739–740.
143. Presti C, et al. Superficial femoral eversion endarterectomy combined with a vein segment as a composite artery-vein bypass graft for infrainguinal arterial reconstruction. J Vasc Surg. 1999;29(3):413–421.
144. Taylor SM, et al. Superficial femoral artery eversion endarterectomy: a useful adjunct for infrainguinal bypass in the presence of limited autogenous vein. J Vasc Surg. 1997;26(3):439–445.
145. Stoney RJ, et al. EX vivo renal artery reconstruction. Arch Surg. 1978;113(11):1272–1278.
146. Hansen KJ, et al. Contemporary surgical management of renovascular disease. J Vasc Surg. 1992;16(3):319–330.
147. Reilly LM, et al. The role of arterial reconstruction in spontaneous renal artery dissection. J Vasc Surg. 1991;14(4):468–477.
148. Piercy KT, et al. Renovascular disease in children and adolescents. J Vasc Surg. 2005;41(6):973–982.
149. Stanley JC, et al. Pediatric renovascular hypertension: a thirty-year experience of operative treatment. J Vasc Surg. 1995;21(2):212–226.
150. Seeger JM, et al. Potential predictors of outcome in patients with tissue loss who undergo infrainguinal vein bypass grafting. J Vasc Surg. 1999;30(3):427–435.
151. Conte MS, et al. Design and rationale of the PREVENT III clinical trial: edifoligide for the prevention of infrainguinal vein graft failure. Vasc Endovascular Surg. 2005;39(1):15–23.
152. Alback A, et al. Prediction of the immediate outcome of femoropopliteal saphenous vein bypass by angiographic runoff score. Eur J Vasc Endovasc Surg. 1998;15(3):220–224.
153. Blankensteijn JD, et al. Intraoperative determinants of infrainguinal bypass graft patency: a prospective study. Eur J Vasc Endovasc Surg. 1995;9(4):375–382.
154. Taylor LM Jr, et al. Antiphospholipid antibodies in vascular surgery patients. A cross-sectional study. Ann Surg. 1994;220(4):544–550.
155. Berceli SA, et al. Surgical and endovascular revision of infrainguinal vein bypass grafts: analysis of midterm outcomes from the PREVENT III trial. J Vasc Surg. 2007;46(6):1173–1179.
157. Roddy SP, et al. Gender-related differences in outcome: an analysis of 5880 infrainguinal arterial reconstructions. J Vasc Surg. 2003;37(2):399–402.
158. Glagov S, et al. Mechanical determinants of plaque modeling, remodeling and disruption. Atherosclerosis. 1997;131(Suppl):S13–S14.
159. Owens CD, et al. Early biomechanical changes in lower extremity vein grafts–distinct temporal phases of remodeling and wall stiffness. J Vasc Surg. 2006;44(4):740–746.
160. Owens CD, et al. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008;47(6):1235–1242.
161. Bulkley BH, et al. Accelerated “atherosclerosis.” A morphologic study of 97 saphenous vein coronary artery bypass grafts. Circulation. 1977;55(1):163–169.
162. Garratt KN, et al. Differential histopathology of primary atherosclerotic and restenotic lesions in coronary-arteries and saphenous-vein bypass grafts-analysis of tissue obtained from 73 patients by directional atherectomy. J Am Coll Cardiol. 1991;17(2):442–448.
163. Kalan JM, et al. Morphologic findings in saphenous veins used as coronary arterial bypass conduits for longer than 1 year: Necropsy analysis of 53 patients, 123 saphenous veins, and 1865 five-millimeter segments of veins. Am Heart J. 1990;119(5):1164–1184.
164. Walts AE, et al. Ruptured atheromatous plaques in saphenous vein coronary artery bypass grafts: a mechanism of acute, thrombotic, late graft occlusion. Circulation. 1982;65(1):197–201.
165. Pregowski J, et al. Incidence and clinical correlates of ruptured plaques in saphenous vein grafts: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45(12):1974–1979.
166. Knatterud GL, et al. Long-Term Effects on Clinical Outcomes of Aggressive Lowering of Low-Density Lipoprotein Cholesterol Levels and Low-Dose Anticoagulation in the Post Coronary Artery Bypass Graft Trial. Circulation. 2000;102(2):157–165.
167. Motwani JG, et al. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916–931.
168. Shah SJ, et al. Intensive lipid-lowering with atorvastatin for secondary prevention in patients after coronary artery bypass surgery. J Am Coll Cardiol. 2008;51(20):1938–1943.
169. Walton KW, et al. Atherosclerosis in vascular grafts for peripheral vascular disease. Part 1. Autogenous vein grafts. Atherosclerosis. 1985;54(1):49–64.
170. Nielsen TG, et al. Histopathological features of in situ vein bypass stenoses. Eur J Vasc Endovasc Surg. 1997;14(6):492–498.
171. Mann MJ, et al. Genetic-engineering of vein grafts resistant to atherosclerosis. Proc Natl Acad Sci USA. 1995;92(10):4502–4506.
172. Mann MJ. Novel strategies for the prevention of bypass graft failure. Biodrugs. 2004;18(1):1–8.
173. Schwartz SM. The intima: a new soil. Circ Res. 1999;85(10):877–879.
174. Ehsan A, et al. Endothelial healing in vein grafts: proliferative burst unimpaired by genetic therapy of neointimal disease. Circulation. 2002;105(14):1686–1692.
175. Ehsan A, et al. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Surg. 2001;121(4):714–722.
176. Mann MJ, et al. Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest. 2000;106(9):1071–1075.
177. Mann MJ, et al. Transcription factor decoys for the prevention of vein bypass graft failure. Am J Cardiovasc Drugs. 2003;3(2):79–85.
178. Mann MJ, et al. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354(9189):1493–1498.
179. PREVENT IV, I: Efficacy and safety of edifoligide, an e2f transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: prevent IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454.
180. Conte MS, et al. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751.
181. Conte MS. Molecular engineering of vein bypass grafts. J Vasc Surg. 2007;45(6 Suppl):A74–A81.
182. Southerland KW, et al. Gene therapy for the prevention of vein graft disease. Transl Res. 2013;161(4):321–338.
183. Conte MS, et al. Genetic interventions for vein bypass graft disease: a review. J Vasc Surg. 2002;36(5):1040–1052.
184. Berceli SA, et al. Hemodynamically driven vein graft remodeling: a systems biology approach. Vascular. 2009;17(Suppl 1):S2–S9.
185. Wang Y, et al. How to cluster gene expression dynamics in response to environmental signals. Brief Bioinform. 2012;13(2):162–174.
186. Stept LL, et al. Technical defects as a cause of early graft failure after femorodistal bypass. Arch Surg. 1987;122(5):599–604.
187. Walsh DB, et al. Intragraft drug infusion as an adjunct to balloon catheter thrombectomy for salvage of thrombosed infragenicular vein grafts: a preliminary report. J Vasc Surg. 1990;11(6):753–759.
188. Johnson BL, et al. Intraoperative duplex monitoring of infrainguinal vein bypass procedures. J Vasc Surg. 2000;31(4):678–690.
189. Robinson KD, et al. Long-term outcome after early infrainguinal graft failure. J Vasc Surg. 1997;26(3):425–438.
190. Liebman PR, et al. Intraoperative arteriography in femoropopliteal and femorotibial bypass grafts. Arch Surg. 1981;116(8):1019–1021.
191. Sato O, et al. Completion arteriography paramalleolar bypasses: effect of configurational changes, with special reference to spasm, on long-term outcome. Int Angiol. 1996;15(3):219–224.
192. Sugawara Y, et al. Significance of arterial defects on completion arteriograms following infrainguinal bypasses. Vasc Surg. 1998;32(1):33–41.
193. Samson RH, et al. Arterial spasm complicating distal vascular bypass procedures. Arch Surg. 1982;117(7):973–975.
194. Mills JL, et al. Contribution of routine intraoperative completion arteriography to early infrainguinal bypass patency. Am J Surg. 1992;164(5):506–511.
195. Dalman RL, et al. Is completion arteriography mandatory after reversed-vein bypass grafting? J Vasc Surg. 1996;23(4):637–644.
196. Schwierz T, et al. Interpretation of the results of doppler ultrasound flow volume measurements of infrainguinal vein bypasses. Eur J Vasc Endovasc Surg. 2005;29(5):452–456.
197. Wolfle KD, et al. The importance of graft blood flow and peripheral outflow resistance for early patency in infrainguinal arterial reconstructions. Vasa J Vasc Dis. 1999;28(1):34–41.
198. Alback A, et al. Preoperative angiographic score and intraoperative flow as predictors of the mid-term patency of infrapopliteal bypass grafts. Eur J Vasc Endovasc Surg. 2000;20(5):447–453.
199. Stirnemann P, et al. Intraoperative flow measurement of distal runoff: a valid predictor of outcome of infrainguinal bypass surgery. Eur J Surg Acta Chir. 1994;160(8):431–436.
200. Ihlberg LHM, et al. Intraoperative flow predicts the development of stenosis in infrainguinal vein grafts. J Vasc Surg. 2001;34(2):269–276.
201. Brennan JA, et al. Perioperative monitoring of blood flow in femoroinfragenicular vein grafts with Doppler ultrasonography: a preliminary report. J Vasc Surg. 1991;13(4):468–474.
202. Schwartz LB, et al. Validation of a new and specific intraoperative measurement of vein graft resistance. J Vasc Surg. 1997;25(6):1033–1043.
203. Bandyk DF, et al. Intraoperative assessment of in situ saphenous vein arterial grafts using pulsed Doppler spectral analysis. Arch Surg. 1986;121(3):292–299.
204. Bandyk DF, et al. Intraoperative duplex scanning of arterial reconstructions: fate of repaired and unrepaired defects. J Vasc Surg. 1994;20(3):426–433.
205. Papanicolaou G, et al. Intraoperative color duplex scanning for infrainguinal vein grafts. Ann Vasc Surg. 1996;10(4):347–355.
206. Sawaqed RS, et al. Prospective comparison of intraoperative angiography with duplex scanning in evaluating lower-extremity bypass grafts in a community hospital. Am Surg. 2001;67(6):601–604.
208. Rzucidlo EM, et al. Prediction of early graft failure with intraoperative completion duplex ultrasound scan. J Vasc Surg. 2002;36(5):975–981.
209. Baxter BT, et al. A comparative study of intraoperative angioscopy and completion arteriography following femorodistal bypass. Arch Surg. 1990;125(8):997–1002.
210. Woelfle KD, et al. Intraoperative imaging techniques in infrainguinal arterial bypass grafting: completion angiography versus vascular endoscopy. Eur J Vasc Surg. 1994;8(5):556–561.
211. Miller A, et al. Comparison of angioscopy and angiography for monitoring infrainguinal bypass vein grafts: results of a prospective randomized trial. J Vasc Surg. 1993;17(2):382–398.
212. Eagleton MJ, et al. Outcome of surgical and endoluminal intervention for infrainguinal bypass anastomotic strictures. Vasc Endovascular Surg. 2006;40(1):11–22.
213. Caps MT, et al. Vein graft lesions: time of onset and rate of progression. J Vasc Surg. 1995;22(4):466–474.
214. Ihlberg LHM, et al. Transfer function index of pulse volume recordings: a new method for vein graft surveillance. J Vasc Surg. 2001;33(3):546–553.
215. Bandyk DF, et al. Hemodynamics of vein graft stenosis. J Vasc Surg. 1988;8(6):688–695.
216. Bandyk DF, et al. A low flow velocity predicts failure of femoropopliteal and femorotibial bypass grafts. Surgery. 1985;98(4):799–809.
217. Mills JL, et al. The importance of routine surveillance of distal bypass grafts with duplex scanning: a study of 379 reversed vein grafts. J Vasc Surg. 1990;12(4):379–386.
218. Grigg MJ, et al. Detection and grading of femorodistal vein graft stenoses: duplex velocity measurements compared with angiography. J Vasc Surg. 1988;8(6):661–666.
219. Sladen JG, et al. Color flow duplex screening of infrainguinal grafts combining low- and high-velocity criteria. Am J Surg. 1989;158(2):107–112.
220. Bandyk DF. Infrainguinal vein bypass graft surveillance: how to do it, when to intervene, and is it cost-effective? J Am Coll Surg. 2002;194(1 Suppl):S40–S52.
221. Gahtan V, et al. Duplex criteria for predicting progression of vein graft lesions: which stenoses can be followed? J Vasc Technol. 1995;19(4):211–215.
222. Mills JL, et al. The natural history of intermediate and critical vein graft stenosis: recommendations for continued surveillance or repair. J Vasc Surg. 2001;33(2):273–280.
223. Westerband A, et al. Prospective validation of threshold criteria for intervention in infrainguinal vein grafts undergoing duplex surveillance. Ann Vasc Surg. 1997;11(1):44–48.
224. Idu MM, et al. Impact of a color-flow duplex surveillance program on infrainguinal vein graft patency: a five-year experience. J Vasc Surg. 1993;17(1):42–53.
225. Mattos MA, et al. Does correction of stenoses identified with color duplex scanning improve infrainguinal graft patency? J Vasc Surg. 1993;17(1):54–66.
226. Moody P, et al. Vein graft-surveillance improves patency in femoro-popliteal bypass. Eur J Vasc Surg. 1990;4(2):117–121.
227. Visser K, et al. Duplex scan surveillance during the first year after infrainguinal autologous vein bypass grafting surgery: costs and clinical outcomes compared with other surveillance programs. J Vasc Surg. 2001;33(1):123–130.
228. Wixon CL, et al. An economic appraisal of lower extremity bypass graft maintenance. J Vasc Surg. 2000;32(1):1–12.
229. Mills JL, et al. The origin of infrainguinal vein graft stenosis: a prospective study based on duplex surveillance. J Vasc Surg. 1995;21(1):16–22.
230. Erickson CA, et al. Ongoing vascular laboratory surveillance is essential to maximize long-term in situ saphenous vein bypass patency. J Vasc Surg. 1996;23(1):18–26.
231. Passman MA, et al. Do normal early color-flow duplex surveillance examination results of infrainguinal vein grafts preclude the need for late graft revision? J Vasc Surg. 1995;22(4):476–481.
232. Lundell A, et al. Femoropopliteal-crural graft patency is improved by an intensive surveillance program: a prospective randomized study. J Vasc Surg. 1995;21(1):26–34.
233. Ihlberg L, et al. Does a completely accomplished duplex-based surveillance prevent vein-graft failure? Eur J Vasc Endovasc Surg. 1999;18(5):395–400.
234. Davies AH, et al. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the vein graft surveillance randomised trial (VGST). Circulation. 2005;112(13):1985–1991.
235. Lindsay TF, et al. Vascular viewpoint. Vasc Med. 2006;11(2):137–138.
236. Mills JL Sr. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the Vein Graft Surveillance Randomised Trial (VGST). Perspect Vasc Surg Endovasc Ther. 2006;18(2):194–196.
237. Norgren L, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67.