CHAPTER 16 Attention-Deficit/Hyperactivity Disorder
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurobehavioral health condition among children. Although it has been the subject of great controversy, it is also the most studied neurobehavioral condition of childhood with the greatest empirical basis for evaluation and treatment. Affected children usually present with behavioral problems or academic difficulties. It is important to determine whether these concerns arise from true ADHD, from a condition that mimics ADHD, from ADHD complicated with by a comorbid diagnosis, or from normal activity for the child’s age. Understanding the current recommendations for the evaluation, diagnosis, treatment, and management is imperative to provide these children the best care possible. Guidelines have been published by experts in pediatrics1,2 and mental health3,3a for the diagnosis and treatment of ADHD. These recommendations, along with the criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV),4 provide for greater uniformity of the diagnosis, treatment, and management processes in the care of children with this complicated symptom complex.
HISTORY
In the media, ADHD is often represented as a newly discovered entity. In reality, the core symptoms of ADHD have been puzzling health care providers since the mid-1800s. The first literary description was provided in a children’s book written in 1848 by a German physician, Heinrich Hoffmann5:
The poem continues, giving a description of Fidgety Phil’s antics, which resemble hyperactive/impulsive symptoms. Hoffmann also described inattentive symptoms in another character, Johnny Head-In-Air. Johnny was watching the birds and the sun and never knew what hit him when he fell headlong into the river and had to be fished out.5
A perhaps more parent-friendly description appeared soon after in 1851 in a story by George Sand. In this story, a mother dies young, leaving three children in the care of their father and grandparents. The woman’s father encourages the father to remarry because the grandparents cannot keep up with the care of the children, particularly “Sylvain, who is not four years old, and who is never quiet day or night. He has a restless disposition like yours; that will make a good workman of him, but it makes a dreadful child, and my old wife cannot run fast enough to save him when he almost tumbles into the ditch, or when he throws himself in front of the tramping cattle.”6 This description highlights the familial nature of the disorder, its early onset, and the burden of care that it places upon families.
At first, in dealing with this disorder, the primary focus was on conduct. In 1902, Still described children with ADHD symptoms and believed these children had a “defect in moral control.”7 He stated the “problem resulted in a child’s inability to internalize rules and limits, and additionally manifested itself in patterns of restlessness, inattentive, and over-aroused behaviors.”7 This stress on control of behavior has returned, renamed as response inhibition or executive dysfunction.8 In 1937, a stimulant, racemic amphetamine (Benzedrine), was noted to improve the behaviors in children affected with these core symptoms.9 Methylphenidate, whose effects were similar to those of the amphetamines, was released for general use in 1957.10
As research has revealed more about this troubling symptom complex of inattention, hyperactivity, and impulsivity, there have been many causal theories and name changes. The cause of the disorder was first thought to be brain damage when some of the children recovering from encephalitis caused by the worldwide influenza epidemic in 1917 exhibited symptoms of restlessness, inattention, impulsivity, easy arousability, and hyperactivity11,12 When brain damage was found to be less evident in many children exhibiting symptoms, the name was changed to minimal brain damage and minimal cerebral dysfunction. It shifted from an etiological name to a behavioral descriptive name in the late 1960s. In the Diagnostic and Statistical Manual of Mental Disorders, 2nd edition (DSM-II), it was labeled Hyperkinetic Impulse Disorder,13 which reflected a focus on the hyperactive symptoms. In the third edition (DSM-III),14 the name underwent further change as the focus shifted from hyperactive symptoms to inattention, with the name Attention Deficit Disorder, on the basis of research by Douglas that demonstrated deficiencies in continuous performance and similar vigilance tasks.15 The name Attention-Deficit/Hyperactivity Disorder was introduced in the revision of the third edition (DSM-III-R).16 The latest terminology is defined in the fourth edition (DSM-IV),4 in which Attention-Deficit/Hyperactivity Disorder is divided into three subtypes: primarily inattentive type, primarily hyperactive-impulsive type, and combined type.4
The confusion over the causes and even the specific definition of this symptom complex is demonstrated by the frequent name changes. This is perhaps not surprising, inasmuch as the intimate interrelationship between attention and intention was pointed out as early as 1890 by William James: “The essential achievement of the will, in short, when it is most ‘voluntary,’ is to attend to a difficult object and hold it fast before the mind. The so-doing is the fiat; and it is a mere physiological incident that when the object is thus attended to, immediate motor consequences should ensue.”17
PREVALENCE
Researchers have identified individuals with ADHD symptoms in every nation and culture studied,18 but determining the true prevalence rate of ADHD has been a challenging task. Prevalence estimates for ADHD vary, depending on the diagnostic criteria used, the population studied, and the number of sources necessary to make the diagnosis.2 Several features of the disorder are major contributors to the challenge. First, there are no known specific biological markers (laboratory tests or image studies) that can discriminate children with ADHD from children with another neurobehavioral disorder or from normal controls. Second, the behaviors observed in ADHD differ in quantity, not quality, from those of typical children. This contrasts to disorders such as schizophrenia, in which the presence of auditory hallucinations is qualitatively distinct from normal experience, or conduct disorder, in which a child may willfully engage in criminal activity. Third, the frequency of these behaviors is observed and reported by the child’s caregivers; therefore, the diagnosis must rely on the judgment of persons who do not share any uniform training or view of child development and whose interrater reliability is unknown. Fourth, there is no consensus about what frequency of any given behavior is normal at any given age; for example, in assessing intelligence, there are clear normative guidelines for which tasks can be accomplished at which age. Fifth, the behaviors are context specific; in situations of stress, most people exhibit inattention, overactivity, and impulsive behaviors.18a Sixth, the ADHD core symptoms and signs are not specific to ADHD; for example, the continuous performance task that was used to establish the attentional component of ADHD was first developed to study subjects with schizophrenia.
The modifications in diagnostic criteria over time have further complicated the process of determining the true prevalence of ADHD. The most recent change, from only one subtype in DSM-III-R to three subtypes in DSM-IV, has increased the prevalence rates. In addition to the challenges to making accurate diagnoses, studies of prevalence rates are dependent on the sample studied. The rates are different in a sample referred to a mental health clinic from those in a primary care sample or from a community/school sample. In view of these challenges, it is not surprising that varying rates have been reported. The prevalence has ranged from 4% to 12% (median, 5.8%).2 Rates are higher in community samples (10.3%) than in school samples (6.9%), and higher among male subjects (9.2%) than among female subjects (3.0%).2 This effect also seems to extend even into population-based studies. One population-based survey in which identical interview strategies were used in four different communities revealed prevalence rates that varied from 1.6% to 9.4 % (pooled mean, 5.1%).19
As with other neurodevelopmental disorders, ADHD is more common in boys and men, and male : female ratios are 5 : 1 for predominantly hyperactive/impulsive type and 2 : 1 for predominantly inattentive type.20,21 Many experts believe this gender difference exists partially because boys commonly present with the externalizing hyperactive/impulsive symptoms such as aggression and overactivity, whereas girls often present with internalizing inattentive symptoms such as underachievement and daydreaming.20,21 This difference is thought to lead to an earlier referral for boys and a later referral and, possibly, underdiagnosis for girls.
ETIOLOGY
Approximately 20% to 25% of children who have ADHD also have a diagnosis that can be associated with an organic cause. Prenatal exposure to some substances may be dangerous to the developing fetal brain. For example, children born with fetal alcohol syndrome may exhibit the same hyperactivity, inattention, and impulsivity as do children with ADHD22 (see Chapter 11). Exposure to other toxins, including cocaine, nicotine, and lead, or the occurrence of trauma or infection that leads to central nervous system damage may produce the ADHD symptom complex.22 In the other 75% to 80% of affected children, ADHD is thought to have a polygenic basis. Genetic evidence of ADHD has been provided by studies involving adoption, twins, siblings, and parents. In twin studies, the heritability of ADHD has been estimated at 0.75 (75% of the variance in phenotype can be attributed to genetic factors). If a child with ADHD has an identical twin, the twin has a greater than 50% chance of developing ADHD.23 Family studies have also demonstrated that adoptive relatives of children with ADHD are less likely to have the disorder24,25 and that first-degree relatives have a greater risk than do controls.26–28
Neuroimaging studies with magnetic resonance imaging, positron emission tomography, and single photon emission computed tomography have demonstrated differences in brain structure and function between individuals with ADHD and controls in the basal ganglia, cerebellar vermis, and frontal lobes. These areas are thought to regulate attention: The basal ganglia help inhibit automatic responses, the vermis is thought to regulate motivation, and the prefrontal cortex helps filter out distractions.23,29,30 Investigations of the brain’s response to stimulants have implicated the dopaminergic system as a possible contributor to the disorder. Dopamine can inhibit or intensify the activity of other neurons. It is also possible that the norepinephrine receptors may be involved; however, this has yet to be confirmed. Specific gene associations have been identified in a small proportion of individuals with ADHD. These include the dopamine transporter gene (DAT1), the D4 receptor gene (DRD4), and the human thyroid receptor–β gene.31–34 Currently, imaging and genetic analysis are not helpful on a clinical basis because of the wide variation of size and function of the brain in individuals with ADHD and those without ADHD and the small numbers who have identified gene abnormalities.
PROGNOSIS
It was once thought that children outgrew ADHD. It is now known that 70% to 80% of children who have ADHD continue to have difficulty through adolescence and adulthood.35 The manifestation of symptoms usually changes through a child’s lifetime. In general, hyperactive core symptoms decrease over time, whereas inattentive symptoms persist.35 Some children learn to adapt and are able to build on their strengths and minimize their impairment. The majority continues to struggle, with their impairment manifesting in different ways. The true outcome depends on the severity of symptoms, presence or absence of coexisting conditions, social circumstances, intelligence, socioeconomic status, and treatment history.35 Adolescents with ADHD have higher rates of school failure, motor vehicle accidents, substance abuse, and encounters with law officials than do the general population.36 Adults with ADHD may achieve lower socioeconomic status and have more marital problems than do the general population.36
EVALUATION AND DIAGNOSIS
To establish the degree of symptoms and their functional significance, and to rule out alternative causes, information must be gathered from many sources. This includes obtaining a thorough history and physical examination, reviewing ADHD-specific behaviors in multiple settings, and determining the presence of any comorbid conditions. For children, the sources must include, at a minimum, their parents and teachers.1 Teachers observe children for up to 6 hours a day. They see them in comparison with a group of same-age peers and in situations that require the children to pay attention, control their activity level, and resist their impulses. When possible, it is also helpful to obtain information from other observers, such as coaches, scout leaders, and grandparents. Direct observation of a child’s behavior in the classroom can provide some of the most objective information, if it is available, but this is labor intensive and therefore has to be limited to small samples of time.1,4 Observations in the pediatric office are frequently not useful because they may not be well correlated with the child’s behavior in the home, classroom, and community.
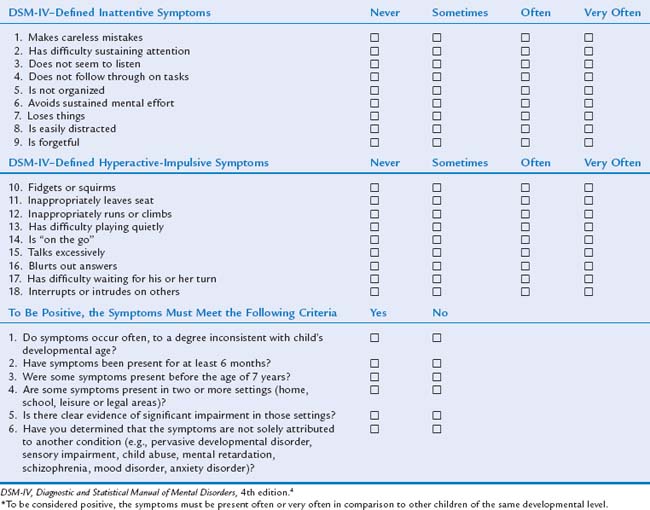
ADHD remains a clinical diagnosis based on specific criteria and clinical impression. It is important to use a structured, systematic approach in evaluating children with behavioral problems and not to rely on clinical judgment alone. Table 16-1 is a general overview of the recommended guidelines for diagnosing ADHD.1,3,3a,37 Depending on the situation, many health care providers obtain information from behavioral rating scales before proceeding to an office evaluation. Scales in which DSM-IV criteria are used are helpful1 (e.g., the Vanderbilt Assessment Scales,21 DuPaul and associates’ ADHD Rating Scale-IV38; the Revised Conners Rating Scales39). Broadband scales (e.g., Child Behavior Checklist40 and Behavior Assessment System for Children41) were not found to be as helpful in making an ADHD diagnosis but do help screen for co-occurring conditions.1 Other clinicians are more comfortable gathering information from an office visit to gain a clearer picture of the problems before proceeding to the next step. In evaluation of a child for ADHD, the differential diagnosis and common comorbid diagnoses are quite extensive (Tables 16-2 and 16-3). Keeping these in mind when the history and physical examination are updated is important because many conditions can mimic or coexist with ADHD. The correct diagnosis dictates the proper treatment and prognosis for patients. Young children most commonly have comorbid complications of developmental delays, communication disorders, developmental coordination disorder, reading and writing problems, tic disorder, oppositional defiant disorder, anxiety, or autistic behaviors, whereas older children and adults may have comorbid symptoms related to depression, anxiety, substance abuse disorder, or conduct disorder. One extensive review revealed the following percentages of comorbid diagnoses: 35%, oppositional defiant disorder; 26%, conduct disorder; 18%, depression; 26%, anxiety; and 12%, learning disorders.2
ADHD, attention-deficit/hyperactivity disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition.4
TABLE 16-2 Differential and/or Comorbid Diagnoses
TABLE 16-3 Comorbid Protocol: Does Child Have Symptoms of Comorbid Conditions?
| Learning disorder or language disorder | |
DSM-IV Criteria
The DSM-IV4,4a provides the diagnostic criteria currently used in the United States. It contains a description of 18 core symptoms focusing on the main problems of inattention, hyperactivity, and impulsivity (Table 16-4). The child must exhibit at least six of nine inattentive behaviors inappropriately often to meet the criteria for ADHD, inattentive subtype; at least six of nine hyperactive/impulsive behaviors inappropriately often to meet the criteria for ADHD, hyperactive/impulsive subtype; and six of nine behaviors in both dimensions to meet the criteria for ADHD, combined subtype. In addition to the presence of the core symptoms:
It is important to remember that attention is inherently an interaction between child and environment. A child’s behavior varies with setting, situation, and stimulus. It is typical for symptoms to be minimal when there is novelty, immediate reinforcement, or increased stimulus salience involved (such as a movie, video game, or doctor visit). Symptoms are often most intense when the situation is less interesting or unstructured and requires concentration, such as listening to instructions, doing homework, or sitting in religious services.36,42
Associated problems can increase the attentional demands of a situation. Cognitive or learning disabilities, family disruption, or dysfunctional classrooms can all increase inattentive behaviors. For these differences, it is important to obtain information from multiple sources. Parent and teacher behavioral rating scales specific for ADHD can effectively provide information required to make a specific diagnosis. Broadband scales are less useful for establishing specific diagnoses but can be useful in screening for comorbid conditions.2 This can also be achieved by verbal interview, if the clinician has the time, and is systematic in the interview process. The interview can sometimes reveal biases of some reporters: for example, teachers who believe that ADHD does not exist or parents who resist accepting diagnoses of learning disorders. Sometimes a child has few symptoms in a very structured special education setting but exhibits impairment in typical settings such as regular education, in the community, and at home.
Common Noncore Symptoms of Attention-Deficit/Hyperactivity Disorder
In addition to the DSM-IV core symptoms, there are a number of symptoms that are frequently seen in ADHD, but do not imply an additional comorbid diagnoses. These include social skills dysfunction, problems with self-esteem, motor coordination, and sleep. Social skills deficits have been documented in preschool, middle childhood, and adolescent children with ADHD.42a,42b In long-term follow-up studies of hyperactive children, investigators have reported reduced numbers of friends, low measures of self-esteem, and an increase in antisocial behavior.43 Results of one study suggested that some of these difficulties may arise from an inappropriately high level of self-esteem or “positive illusory self-concept” on the basis of sociometric analyses of child, peer, and teacher ratings of social competence.44
Sleep disturbances are common in children with ADHD45–47 but may not come to the attention of the clinician until after the presenting behavioral crisis has resolved. There may then be confusion as to whether the sleep disturbance is secondary to the ADHD or is a side effect of stimulant medication. In most placebo-controlled studies of stimulant medication, investigators have reported an increase in sleep problems, although several sleep laboratory studies have not demonstrated worsening of sleep disturbance with stimulant therapy.48,49 Reports of increased rates of inattention in children referred for sleep evaluations and improvement after tonsillectomy and adenoidectomy50–52 underscore the importance of a good sleep history in an initial ADHD evaluation. In one comparison of children with “significant” ADHD symptoms with those with “mild” symptoms, findings suggested that obstructive apnea was uncommon in “significant” ADHD but caused a syndrome of “mild” inattention and distractibility.53
TREATMENT
It is important to understand that ADHD is a chronic illness for which there is no cure. However, even though there is no curative treatment for the condition, ongoing management can minimize the extent of impairment. First, it is important to educate the parents and patients about the condition and its treatment. This education can help to demystify the condition and clarify many misconceptions raised in the popular press or prevalent in the community. Educated families are better able to work as partners with the clinician in establishing and maintaining an effective treatment program. The treatment plan should be carefully tailored for each individual patient (Table 16-5). When a family is invested in the treatment plan, there is an increased chance of adherence to the regimen.3 This investment can be maximized by educating the family about their options and by taking individual needs, family preferences, opinions, and lifestyle into account in designing the regimen. For the most favorable outcomes, it is necessary to develop a multidisciplinary approach involving the child, caregiver, educators, and clinician. Communication between home, school, and clinician is needed for monitoring outcomes and making quick changes when needed. Three treatment strategies have been studied and shown effective in treating ADHD: medication, behavioral modification, and a combination of both.54,55
|
1. With input from information gathered from parent, teacher, and child, main problem areas are identified at home and school
|
IDEA, Individuals with Disabilities Education Act of 2004; IEP, Individualized Education Plan.
Family Education
Educating the caregivers and child about ADHD is essential to good treatment outcomes. Education can help the family come to grips with the diagnosis (Table 16-6). Understanding that ADHD is a brain-based problem and not caused by poor parenting or that it is not intentional misbehavior by the child can relieve guilt and help alleviate stress and frustration that have been present for many years. Raising, teaching, or being a child who has difficulty sustaining attention, filtering out stimuli, learning from past mistakes, and regulating activity level can be very challenging. Being able to change the focus on helping the child improve function instead of always pointing out bad behaviors will improve satisfaction with the child’s response to treatment. ADHD runs in families, and many parents of affected children had difficult school experiences themselves. A physician or other medical personnel moderating interaction between home and school can help build trust and cooperation.
TABLE 16-6 Education for Child, Parents/Caregivers, and Teacher
CHADD, Children and Adults with Attention De. cit/Hyperactivity Disorder.
As a chronic condition, the symptoms and impairment change throughout the child’s life and developmental stages (Table 16-7). Providing updated information to the child and family as these developmental stages approach helps the family face new challenges and anticipate and prepare for the future. This information can be provided through a variety of resources, including trained staff, handouts, suggested reading lists, Internet Web sites, local and national support groups, and community resources. (See Appendix: Helpful Internet Resources)56
TABLE 16-7 Symptoms and Manifestation of Attention-Deficit/Hyperactivity Disorder through a Lifetime
| Symptoms | Life Stage | Possible Presentation |
|---|---|---|
| Hyperactivity | Preschool-aged child | Motoric hyperactivity |
| Impulsivity | Aggressiveness | |
| Inattention | Elementary school–aged child | Underachievement |
| Distractibility | Lack of motivation | |
| Frustration | Reputation as class clown | |
| Boredom | Difficulty following class rules | |
| Poor social skills | ||
| Poor organizational skills | Older school-aged child | Difficulty completing homework independently |
| Difficulty learning from mistakes | Teenager/college student | Increased social problems |
| Trouble with long-term projects | ||
| Car accidents | ||
| Adults | Trouble juggling demands of marriage/family and work | |
| Trouble interacting with colleagues | ||
| Difficulty keeping a job | ||
| Difficulty managing money |
Medication
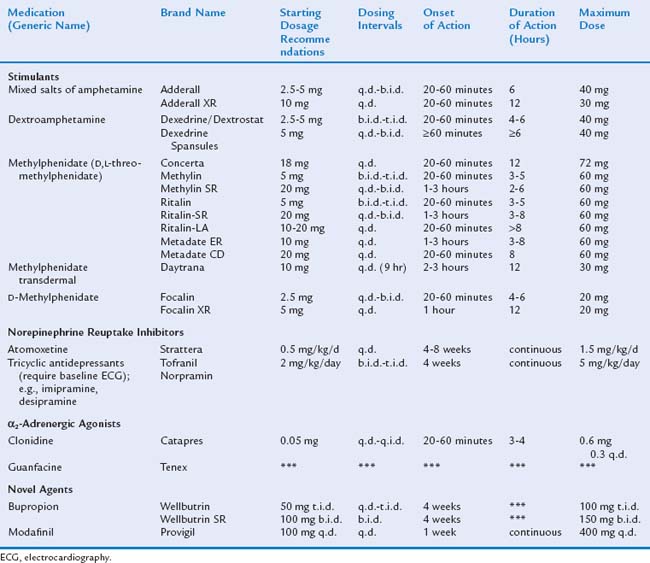
Medications used for ADHD include stimulants, norepinephrine reuptake inhibitors, antihypertensives, bupropion, and modafinil. Common medications are listed in Table 16-8.
STIMULANTS
The stimulants have been the most extensively studied and are considered the first choice of pharmacological management for ADHD because of both their efficacy and safety.2 The stimulant medications include dextroamphetamine, methylphenidate, and mixed salts of amphetamine. More than 300 studies with 6000 subjects have demonstrated the short-term efficacy of stimulants.57 Most researchers have studied the effects of stimulants on elementary school–aged children. The medications often offer immediate and dramatic improvement to a child’s symptom complex. Improvements are present only as long as medication is taken. Stimulants are effective in 70% to 80% of affected children.36 The stimulant medications reduce the core symptoms of inattention, hyperactivity, and impulsivity. They also improve academic productivity (e.g., the number of problems completed on a math sheet), although they do not improve cognitive abilities or performance on standardized academic testing. Furthermore, in some children, they reduce oppositional and aggressive behaviors. Although the evidence for the short-term efficacy of stimulant medications is quite strong, the evidence for long-term efficacy is not as clear.35 Evidence from the National Institute of Mental Health Multimodal Treatment Study of ADHD (MTA)55 supports efficacy for 72 months; this has been the longest careful follow-up of children on stimulant medications.
Unlike most pediatric medications, stimulant dosing is not based on milligrams per kilogram.3,37 Instead, current recommendations are to start at the lowest dosage possible and titrate up according to information gathered from parents and teachers about treatment effectiveness. For titrating, it is important to warn parents that there may be many changes in dosage and that the initial dosage has been selected to be low; otherwise, they may interpret the need for changes as evidence that the medication is ineffective or that excessive dosages are being reached. Depending on the child’s daily schedule, multiple doses each day may be required. In most studies of methylphenidate since 2000, three-times-a-day dosing has been used, and some adolescents with evening activities or homework, or children who have a return of severe disruptive behaviors in the evening, may require a fourth dose. Many families and practitioners have experienced a phenomenon referred to as “rebound” in which returning symptoms are worse than baseline for a short time as medication wears off. In behavioral studies in which behaviors over the day are observed and counted, no true rebound has been identified, but a return of symptoms has been documented. This has been treated with half a dose of stimulant, given just before the rebound is anticipated. Initial medication titration necessitates follow-up at least weekly by phone or office visit. The best dosage is that at which the child has maximum success reaching individualized target goals and has the fewest side effects. Once response is stable, monitoring can be stretched to monthly and eventually quarter-yearly office visits. Table 16-9 lists monitoring guidelines.
Although most of the studies of medication efficacy have been performed on children with ADHD and normal intelligence, stimulant medications also are effective for many children with mental retardation.58,59 Stimulant medication is reportedly used by 3.4% of children with moderate mental retardation60 and 15% of children with mild mental retardation.61 Although individuals with mild mental retardation respond in essentially the same way as individuals without intellectual impairments, those with severe intellectual impairment are much less likely to respond.62
Dextroamphetamine, methylphenidate, and mixed salts of amphetamine have similar effects, side effects, and safety. Although methylphenidate may increase the probability of a seizure in a person with a seizure disorder63,64 and dextroamphetamine does not, both medications have been used to treat children with ADHD and seizure disorders with no recurrence of seizures as long as the children’s seizure disorder is adequately treated.63,64 Even in children with other comorbid conditions such as anxiety or mood disorders, it is preferable to treat the ADHD first, because the mood disorder or depressive symptoms may diminish significantly if the stress caused by the ADHD is reduced.
There are several misconceptions about stimulant medications. The effects of the medications are not paradoxical: the same effects are seen in children without ADHD and in adults. Therefore, a response to medication cannot be used as a diagnostic test. Children with ADHD do not find stimulant medications pleasurable and do not commonly abuse them. In the more usual clinical situation, an adolescent for whom stimulants have had documented benefit refuses to take medication. There is some suggestion that children with ADHD who are appropriately managed have a lower risk of substance abuse disorders than do those who are not appropriately managed.65
Stimulant medications act as dopamine and norepinephrine reuptake inhibitors, increasing norepinephrine and dopamine activity primarily in the caudate nucleus and prefrontal cortex.66 Methylphenidate is a piperidine derivative that is a racemic compound. The levo isomer is rapidly metabolized and essentially inactive.39 Short-acting methylphenidate has a half-life of 2 to 3 hours and a duration of action of about 4 hours.67 d-Methylphenidate has become available under the brand name Focalin; the manufacturers suggest that it may have fewer side effects but there is little reason to believe that l-methylphenidate contributes to side effects or efficacy of methylphenidate.68a One randomized, double-blind, placebo-controlled, comparison study of d-methylphenidate and d,l-methylphenidate found similar efficacy at half of the milligram dose of d-methylphenidate and suggested a longer duration of action in twice daily dosing.68 Amphetamine is also active in the dextro isomer. d-Amphetamine has a similar half-life and duration of action to methylphenidate. However, l-amphetamine is converted to d-amphetamine in vivo, lengthening the duration of action. This conversion, combined with the slower dissociation and absorption of the saccharate and aspartate salts, accounts for the slightly increased duration of action of mixed amphetamine salt.69
Extended-Release Preparation
A number of longer acting stimulant medications have become available. There has been an interest in longer acting medications because, although short-acting stimulants have been shown to be safe and effective, their administration is more challenging.70 Appreciation that ADHD affects important nonacademic functions has resulted in a desire for “coverage” of longer periods of time each day. When short-acting stimulants are used, symptoms return at the end of each 3- to 6-hour period, often at the most unstructured times of the day (while getting up and ready for school, at lunchtime, and on the bus ride home).71 The need to take medication at school presents difficulties with remembering to take the dose, stigmatization of students who take medication, refusal to take medication at school, school policies that discourage the taking of medication at school, opportunities for diversion of controlled substances, and personnel costs for schools. After school, the problems of taking a third dose at the end of the school day or at daycare also cause difficulties for many families. The unevenness of the effect over the 3- to 4-hour course, repeated two to three times each day, is problematic for children with ADHD and their caretakers. The cost of medication goes up with the number of pills used, which often results in costs for generic preparations that would rival those of a once-a-day branded preparation. A medication that can be taken once daily offers many potential benefits. Two reports of stimulant medication use patterns, one in a Medicaid population and the other in a managed care population, demonstrated increased continuity of treatment with use of extended-release preparations in clinical practice.72,73
The evidence of the efficacy of long-acting medications is derived almost entirely from studies financed by their manufacturers. The older preparations, developed before the advent of advertising and the U.S. Food and Drug Administration’s (FDA’s) “pediatric rule,”73a have almost no published support. Since 1998, as the market for ADHD medication has become more competitive, a number of studies assessing the newer preparations have been published. There are no large federally funded studies, such as the MTA,55 of long-acting medications.
Criteria for the adoption of longer-acting medications should include
Older Medications
Three medications with longer duration of action than immediate-release methylphenidate have been available for a number of years. These are Ritalin SR (methylphenidate in a wax matrix) and Dexedrine Spansules (dextroamphetamine in small beads that release an initial immediate dose and release the remainder of the dose slowly over about 8 hours). Pemoline (Cylert), a third longer acting medication, has been removed from the market because of a potentially severe side effect of liver toxicity. The published literature on these medications is limited. Ritalin SR has not been as effective as expected. The onset of action is slow, often necessitating the coadministration of immediate-release methylphenidate in the morning, and afternoon efficacy requires higher drug levels not achieved by this slow-release preparation. In the one small double-blind comparison of methylphenidate, 10 mg twice a day, and methylphenidate SR 20 mg once a day, twice a day methylphenidate was comparable with the slow-release preparation on measures of cognition and social performance and superior on measures of disruption.74 In other small studies there were no differences.75,76 Diagnostic criteria and outcome measures employed in these studies were much different from those of recent studies of stimulants in ADHD. In one small (N = 22) crossover trial in a summer treatment program, Dexedrine Spansules and pemoline were more frequently recommended than methylphenidate SR or twice-a-day methylphenidate, on the basis of behavioral observations.
Newer Sustained-Release Stimulants
The first of the reformulated stimulant medications was OROS methylphenidate (Concerta). OROS methylphenidate is an osmotically active capsule with an overcoat of methylphenidate. The overcoat is delivered rapidly, and the osmotic capsule delivers medication at a rate that produces a gradually increase in blood levels over the day. The capsules are designed so that 18 mg of OROS methylphenidate should mimic the effect of 5 mg of immediate-release methylphenidate given three times a day. In an analogue classroom, double-blind, crossover situation, it has been shown to have effects comparable to three-times-a-day methylphenidate for over 12 hours.77 In another large (N = 282), multisite, short-term (4 weeks), parallel group design, OROS methylphenidate was again shown to be comparable to three-times-a-day methylphenidate.78 The reported effect sizes for both OROS methylphenidate and three-times-a-day methylphenidate were about the same. In another report, the open-label long-term follow-up of children previously studied in short-term double-blind studies (N = 407) revealed that 71% of patients remained on the medication for at least 1 year with maintenance of behavior ratings and a modest average increase in average dosage.79 In a study of simulated driving in adolescents, positive effects on driving performance persisted longer into the evening with OROS methylphenidate than with three-times-a-day methylphenidate.80
There are two preparations now available in which delayed-release beads simulate twice-a-day administration of methylphenidate. Methylphenidate extended-release capsules (Metadate CD) are divided into 30% immediate-release and 70% delayed-release doses, and methylphenidate extended release capsules (Ritalin LA) are divided into 50% immediate-release and 50% delayed-release doses. Methylphenidate extended-release capsules (Metadate CD), 20, 40, or 60 mg, was compared with OROS methylphenidate, 18, 36, or 54 mg (in a trial funded by the manufacturer of Metadate CD); methylphenidate extended-release capsules had a quicker onset of morning action, was comparable in strength in the afternoon, and wore off sooner in the evening.81 In a postmarketing, uncontrolled study of 308 children, 65% had significant improvement on the Clinical Global Impressions scale, and 87% of parents reported satisfaction with the treatment. In a double-blind, placebo-controlled study, methylphenidate extended-release capsules (Ritalin LA) was shown safe and effective, as expected.82 In a double-blind crossover comparison with OROS methylphenidate, methylphenidate extended-release capsules (Ritalin LA) was shown, as expected, to have a quicker onset.83
The longer acting preparation of mixed amphetamine salts (Adderall XR) has also been shown to last more than 12 hours and to be safe and effective in a large parallel-group, double-blind, multisite study.84 The children in this study were followed in an open-label extension for 2 years, and the medication was well tolerated.85 A later analysis of cardiovascular effects revealed minimal effects (increased systolic blood pressure of 3.5 mm Hg, increased pulse of 3.4 beats per minute, and no change in corrected Q-T interval).86 However, there have been reports of sudden death in adolescents taking mixed amphetamine salts. These events have been rare, and the baseline rate of sudden death in an equivalent number of untreated adolescents is unknown. The FDA has revised its recommendations to include an encouragement of physicians to identify existing cardiac conditions in patients before initiating treatment and to monitor cardiac conditions closely, but a more stringent warning (Black Box) was not believed to be warranted.87
A transdermal d-methylphenidate patch (Daytrana) has been approved by the FDA. Only preliminary data have been published. Results of one dose-ranging study of 36 children,88 a study of the patch in combination with behavior modification in 27 children,89 and a 1-week placebo-controlled crossover study of 80 children90 all suggest efficacy and tolerability similar to those with oral treatments.
The d-threo enantiomer of methylphenidate has also been isolated in a new medication, dexmethylphenidate (Focalin). It has properties similar to those of the racemic compound but is twice as potent. It is rapidly absorbed, reaching a maximum level in the fasting state after 1 to 1.5 hours. The levels obtained were similar to those of the racemic compound. The mean elimination half-life is 2.7 hours. The metabolism, like that for the racemic compound, is principally a deesterification to ritalinic acid. In children with ADHD, it produced significant improvement in comparison with a placebo.91 It does not appear to provide benefits or risks different from those of racemic methylphenidate.68a It is also available in an extended-release formulation that utilizes microbead technology (Focalin XR).
Cost
Newer, patented preparations of generically available medications cost more per pill than the generic preparations that are often preferred by third-party payer pharmacy committees. However, if fewer pills are taken and other costs are taken into account, long-acting medications may be less expensive than short-acting generic medications. In an econometric study funded by the makers of Metadate CD, the investigators attempted to factor in cost of medication, cost of school personnel to store and distribute medication, and the cost of physician evaluations for combined methylphenidate immediate-release/extended-release, methylphenidate immediate-release three times daily, extended-release methylphenidate (Metadate CD) once daily, OROS methylphenidate once daily, methylphenidate (Ritalin) three times daily, and mixed amphetamine salts (Adderall) twice daily. (Mixed amphetamine salts [Adderall XR] and extended-release methylphenidate [Ritalin LA] were not yet available at the time.)91a Costs ranged from $639 to $1124 per year for medication alone. However, when costs for in-school administration by school secretarial staff (estimated at $531 per year) were added, the total costs of once-a-day medication were less than those of generic methylphenidate. The savings are higher if more highly trained school personnel (nurses rather than secretaries) dispense medication at school. The analysis did not account for the costs of disruptive behavior if medication wears off at midday.
Side Effects
The most common side effects of all the stimulant medications are anorexia, headache, and sleep disturbance. The anorexia frequently diminishes after several months. In most placebo-controlled studies, the mean weight of the treated group has begun at higher than the 50th percentile and has decreased but not gone below the 50th percentile. For most patients, monitoring of weight is all that is necessary. If a patient has problematic weight loss, use of calorie-enriched food may be helpful. It is important to determine the patient’s current and previous history of sleep and headaches. Sleep problems are frequently present in patients with ADHD before they begin treatment, and in prospective studies, stimulants do not appear to worsen sleep patterns in most children with ADHD.48,49,53 However, in placebo-controlled trials, there is an increased rate of reports of sleep disturbance in the treated group. According to clinical anecdotes, in some children, increased activity as the medication is wearing off interferes with bedtime routine. In these cases, a later dose of medication may actually improve sleep. Headaches usually improve with a decrease in dose, but a change in medications may be required. The effects on growth have been ambiguous; some studies have demonstrated no effect,92–94 and some have demonstrated some effect.95,96
Less common side effects include dysphoria (in extreme cases, psychotic symptoms), “overfocusing” (usually manifested by the patient’s becoming listless or what parents refer to as “appearing like a zombie”), and tics. These side effects can often be resolved by lowering the dosage. Dysphoria is a feeling of being unwell, similar to depression. This may necessitate a change in medications. It is important not to confuse this side effect with worsening of the primary symptoms, because an increase in dosage can worsen these symptoms. Stimulant use has been associated with an increase in tics in some children, but because transient tics are common in children, and because the usual course of tic disorders is for the tics to wax and wane, it can be difficult to determine the relationship between the stimulant medications and the tics. In the OROS methylphenidate clinical trials, there was no significant difference between the rates of tics in the children taking OROS methylphenidate, those taking methylphenidate three times a day, and the controls taking placebo.97 Of the children who have tics and require treatment with stimulant medication, about one fourth to one third have an increase in the tics and about a fourth have a decrease.98 In these cases, it is important to weigh the degree of impairment caused by the ADHD symptoms against the impairment attributable to the tics when therapeutic decisions are made.
In summary, although side effects are common, most are mild, and they can be managed by careful monitoring and by slight alterations in dosage and times given. It is rarely necessary to discontinue medication because of side effects. Table 16-10 lists simple strategies for minimizing side effect complications.
TABLE 16-10 Possible Regimen Modifications to Minimize Side Effects
| Side Effect | Regimen Modifications |
|---|---|
| Decreased appetite |
Note: If stimulant is not working or side effects are intolerable, another stimulant or preparation should be tried. If other stimulants do not work or create intolerable side effects, the clinician should consider second-line drugs or referral to a mental health or developmental-behavioral specialist.
Several studies have demonstrated benefits when behavioral therapy was given in addition to stimulant medication. This combined treatment plan has produced greater satisfaction in treatment, according to child, parent, and teacher report.99,100 In the MTA,55 combined treatment and medication-only treatment did not differ significantly in decreasing core ADHD symptoms. However, the combined treatment did produce more improvement in oppositional and internalizing symptoms, as well as in teacher-rated social skills, parent-child relations, and reading achievement. This improvement was accomplished with a lower daily dosage of medication than when medication was used alone.
If, after appropriate trials of two or three stimulants or stimulant preparations, an optimal dosage is not obtained, the diagnosis and management plan should be reexamined (see Table 16-9).37 If the questions in Table 16-9 have been adequately addressed, it is appropriate to prescribe second-line medications, including norepinephrine reuptake inhibitors and α2-adrenergic agents. These medications may be appropriate in some cases but should be used carefully. They can have more serious side effects and tend to necessitate more monitoring.
NOREPINEPHRINE REUPTAKE INHIBITORS
Tricyclic Antidepressants
The tricyclic antidepressants imipramine, and desipramine have been used in children with ADHD. Their mechanism of action is believed to be the inhibition of the reuptake of norepinephrine, but they also inhibit the reuptake of serotonin and have anticholinergic and quinidine-like effects. Their efficacy in the treatment of ADHD has been supported by approximately 20 randomized control trial studies. They have a much longer half-life than do stimulants; thus, they can be taken once daily, and they have no rebound effect. They also pose minimal risk for abuse. However, side effects are much more serious and include cardiovascular, neurological, and anticholinergic difficulties. Baseline electrocardiography is required before the medication is started, because of the quinidine-like cardiac effects. Acceptable parameters include a heart rate of less than 130, a P-R interval of less than 200 milliseconds, a QRS interval not increased more than 30% from baseline, and a corrected Q-T interval of less than 480 milliseconds.3 Electrocardiography and measurement should be repeated at each major dosage change.3 Once the maintenance dosage is determined, a serum level should be obtained, because levels higher than 150 ng/mL have been associated with electrocardiographic changes.3 High dosages have also resulted in several sudden deaths from cardiac arrhythmias. Because of the greater side effects, particularly cardiac side effects, and the narrow margin of safety, tricyclic antidepressants are currently used only infrequently to treat ADHD.
Atomoxetine
Atomoxetine is the first new nonstimulant agent developed specifically for the treatment of ADHD in children. There are a number of studies in children and adults of various designs (parallel-group, crossover, placebo-controlled, methylphenidate controlled, double-blind, open-label, daily dosing, twice-daily dosing, faster and slower dose escalation).101–108 Reported effect sizes are moderate (about 0.7 in children and 0.4 in adults, in comparison with the usual 1.0 in stimulant trials). Advantages of atomoxetine are its low abuse/diversion potential, its activity early in the morning before stimulants become effective (time-course, placebo-controlled trials of stimulants often show symptoms are worse with stimulants than with placebo for the first interval after a dose). In contrast to stimulants, dosing is on a milligram-per-kilogram basis. The starting dosage is about 0.5 mg/kg each morning, increasing every 4 to 7 days to a maximum dosage of 1.4 mg/kg. If side effects are excessive, the dosage can be divided to twice a day. Disadvantages are the probably mildly lower effect size and the slow onset of effect (weeks). The slow onset and round-the-clock effects of atomoxetine necessitate closer, more quantitative monitoring of symptom changes than is usual with stimulants to determine their effects, because these gradual, consistent changes are less evident to caretakers than are the rapid, daily changes observed with stimulants. This is especially true with children who have previous experience with stimulant medications whose parents may view some of the quick changes seen with stimulants as evidence that “the medication is working” and conclude that there is no medication effect with atomoxetine, even if there is a decrease in core ADHD symptoms that is demonstrable in behavior ratings. Side effects include appetite suppression, sedation if the dosage is escalated too rapidly, and irritability. A rare (probably less than 1 per 1,000,000 prescriptions) complication of reversible liver failure has been reported. Such complications are much less common and less severe than what was found with pemoline. In both reported cases, enzyme levels returned to normal after the medication was stopped, and the lack of any elevation in liver function test results before development of this syndrome suggest that routine monitoring of liver function is not useful. There have also been rare reports of suicidal ideation, although no cases of suicide have been reported.
α2-ADRENERGIC AGONISTS
The α2-adrenergic medications used to treat patients with ADHD are clonidine and guanfacine. They were developed as antihypertensive agents. However, they affect the central nervous system more broadly. In a meta-analysis of 11 studies of clonidine treatment of ADHD, the effect size of clonidine treatment was estimated at 0.58 (stimulants usually produce an effect size of about 1.0; atomoxetine, 0.7).109 The side effects of the α2-adrenergic medications include sedation, fatigue, anorexia, dry mouth, and hypotension. There have been several cases of sudden death in patients treated with a combination of clonidine and methylphenidate, but it could not be confirmed that these deaths were caused by the medications.109,110 Because of the potential side effects and the limited evidence for efficacy, the α2-adrenergic medications should be prescribed only if stimulant medications and noradrenergic reuptake inhibitors have failed after an adequate trial and behavioral alternatives are not effective, available, or acceptable to the family.3 Blood pressure and pulse measurements, supine and standing, should be obtained weekly during the titration phase.3
Clonidine had also been used as a treatment for delayed onset of sleep in children with ADHD. One chart review of a pediatric psychopharmacology clinic showed reported that of children taking clonidine for ADHD-associated sleep disturbances, 85% experienced much or very much improvement,111 but no properly controlled studies have been published.
BUPROPION
Bupropion is an antidepressant medication whose mechanism of action is mostly unclear. It is a weak dopamine agonist, and it decreases whole body norepinephrine levels, but neither of these effects explains its clinical results. Its reputation for efficacy in treating patients with ADHD is based on one multisite study in which it was significantly better than the placebo but not as potent as stimulant medications.112 The side effects of bupropion include agitation, reduction in the seizure threshold, anorexia, insomnia, and nausea/vomiting.113 Because bupropion has more sedative effects and there is sparse evidence of its efficacy, it should be prescribed only if stimulant medications, atomoxetine, and behavioral interventions have failed after adequate trials. It may take as long as 4 weeks to demonstrate effectiveness.3
MODAFINIL
Modafinil, which is marketed for excessive daytime sleepiness in adults, has been studied as a treatment for ADHD. Modafinil has a different chemical structure than stimulants and is believed to activate cortex directly without causing widespread central nervous system stimulation. In the largest study published to date, investigators reported an effect size between 0.6 and 0.7 for core ADHD symptoms reported by parents and teachers. These investigators used specially prepared film-coated tablets, not currently available, at dosages from 85 to 425 mg, titrated by clinical effect.114 Modafinil has not been approved by the FDA for use in children and is a Schedule IV drug. The manufacturer has stopped development because some patients developed Stevens-Johnson syndrome in clinical trials.
Psychosocial Interventions
Psychosocial interventions include all of the interventions in which counseling or behavior management is used. The intervention most frequently employed and with the strongest scientific evidence for its efficacy is behavior modification training performed by the significant caretakers in the child’s environment. Techniques shown to be effective involve contingency reinforcement, including token economies, timeouts, and response cost (earning or losing privileges).54 Social skills therapy is an attempt to address the deficit that many children with ADHD have in social situations; however, because of the difficulty that the children have in generalizing what they learn, there is limited evidence for its efficacy unless the training takes place in actual situations with other children. Family therapy may be helpful, particularly for issues such as sibling relationships, but the evidence for its efficacy is weak. Play and cognitive therapy have not been found to be efficacious treatments for children with ADHD.54
Parent training occurs in different forms, depending on the severity of the behavioral problems. With children whose behavioral problems are mild and with parents who are adept at behavior management, simple advice from their primary care clinician, combined with reading material, may suffice, although this limited intervention has not been studied to determine its efficacy. Most parents are likely to require more intensive instruction that is available in many communities and consists of training groups of parents in behavior modification techniques.115 When parents find it difficult to understand or implement the techniques and/or their children demonstrate more severe behavior problems, individualized training tailored to their needs, such as parent-child interaction therapy, is required.116 The most severe situations, short of removing a child from the home, may require implementing the parent training directly in the home or using a day treatment situation that can train the parent and at the same time shape the child’s behavior.
Parent training usually consists of three elements: (1) providing clear commands and rules to the children and then keeping them aware of those rules, (2) providing positive attention and reinforcing the children for positive behaviors, and (3) providing punishment and the removal of the positive attention for rule violations and inappropriate behaviors.117 It is essential for caregivers (e.g., parents, teachers, childcare workers) to provide positive attention and reinforcement to children. Many times, because of the child’s difficult behaviors, caregivers of children with ADHD get into a cycle in which most of their interactions are negative and involve punishment for unwanted behaviors. Unless they are able to develop a systematic method for providing quality time in the form of positive attention and for reinforcing appropriate behaviors, the punishments are ineffective, and the desired goals will not be achieved. Positive attention requires providing undivided attention to the child for activities that are mutually enjoyed by both parties. The caregivers also need to learn to recognize and reward appropriate behaviors.
One systematic method for providing reinforcement is a token system. A token system consists of identifying the appropriate behaviors that parents want their child to increase. The three or four most salient behaviors are targeted, and the child can earn points for performing the appropriate behavior. For example, if the parents want their child to say, “Please” when the child requests something, the child earns points every time he or she uses “please” appropriately. For young children between 3 and 6 to 7 years of age, tangible tokens may work better than the point system. The parents need to set up a system such as a chart to keep track of the points, and the child needs to know how many points he or she needs to achieve a reward. The target behaviors and the number of points necessary to earn rewards can be revised as the child progresses or if the system does not seem to be working. The rewards can be special privileges, such as increased television time or increased time with a parent, or they can be tangible, such as baseball cards. Rewards are most effective when the child participates in selection of the reward. Immediate praise for earning points can help enhance the effects.
School Interventions
Children with ADHD can receive services from their public schools on the basis of Section 504 of the Rehabilitation Act for milder cases and the Individuals with Disabilities Education Act (IDEA) for more severe cases.118 The Rehabilitation Act (Section 504) requires schools to provide accommodations so that the child can function in his or her class. All children with the diagnosis of ADHD are eligible. However, the act does not provide any added compensation to the school. Therefore, the adaptations provided are of a limited nature, and the procedures are not well defined or scrutinized. Adaptations include preferential class seating, assignment and homework reduction, and consultation with the teacher in helping her or him set up a behavioral program.
The IDEA is a much more comprehensive program, but it is available only to children with ADHD in which the ADHD interferes with their ability to learn or to those with cognitive comorbid conditions such as learning disabilities. The school system is required to provide comprehensive testing, including intellectual, achievement, speech and language, and motor evaluation if appropriate. Testing provided by an external source such as a private psychologist can be used in place of school testing if school personnel believe it is accurate; however, most frequently, outside testing has to be obtained at the parents’ expense. On the basis of the test results, the school system is required to develop an Individualized Education Plan (IEP) with clearly measurable goals. Services must be provided so that the child is placed as close to the mainstream as possible (least restrictive environment). As a result, most children with ADHD spend a small portion of the day in a resource room with a teacher trained in special education or with help from an aid in the classroom. Psychological, speech/language and occupational therapy services are also provided as necessary. In cases in which behavior problems are refractory, a functional behavior analysis can be requested. This entire process, from obtaining the testing through providing the services, must be accomplished with informed parental consent. The IDEA is able to put in place such specific guidelines and services because the schools are provided with increased funding that is based on the number of students in special education that they serve and the severity of the students’ needs that they address. However, the additional funds rarely cover the full cost of the services. More detailed information about Section 504 and IDEA can be found on several Web sites (see Appendix: Table of Helpful Internet Resources).
Daily report cards are an adaptation of behavioral therapy to the school setting and are an excellent way of monitoring a child’s functioning over time. An example of a report card and an explanation of how to establish one can be downloaded from the Comprehensive Treatment for Attention Deficit Disorder Web site.119 By selecting two to three specific goals to work on at home and at school and by establishing an appropriate reward system, parents and teachers can provide immediate feedback to the child concerning his or her behavior. This feedback can be very motivating for the child and the caregivers as they are able to see target goals met. Once established, they take little time from the teacher and caregiver but provide ongoing monitoring of progress, important daily communication between the teacher and parent, and discovery of problem behaviors early. This is also a good method for monitoring therapy and medication management. In general, a 20% improvement over baseline is targeted for each goal, and the child should have a success rate of 66%.100 If the success rate target is lower, it does not provide enough encouragement, and if it is close to 100%, the tasks are too easily accomplished. As the child’s behavior improves, the requirements for success should be modified to maintain the same level of success. Positive report cards should be rewarded with reinforcements that are of value to the child, such as increased privileges or tangible prizes.100
Behavioral interventions do have some limitations. Behavioral interventions alone are frequently insufficient to bring a child with ADHD to a normal range of functioning and are not effective for all children.55 However, some families are uncomfortable beginning treatment with stimulants and wish to start with behavioral treatment. Beginning with behavioral therapy can also provide baseline measures that allow more precise evaluations of medication effects. In children younger than 6 years, for whom stimulant therapy is not approved by the FDA, an initial period of behavior therapy has been advocated. In addition, parental satisfaction is usually high when behavioral therapy is used. The effects of combining both stimulant medications and behavioral intervention can also lower the dose of medication required, and a less intense behavioral intervention may be needed to reach optimal treatment outcomes.89,120
Alternative Treatments
DIETS
The three diets recommended to treat children with ADHD have been the Feingold diet; the oligoantigenic, or elimination, diet; and a restricted sugar diet. The Feingold diet was proposed by an allergist, Dr. Ben Feingold, who suggested that some children with ADHD have an allergic-type reaction to certain dietary elements.121 The elements included additives, preservatives, food dyes, and salicylate compounds. His clinical impression was that a number of children with hyperactivity had this problem. However, subsequent blinded studies revealed that very few children (approximately 1% of the children studied) responded adversely when challenged with dyes or additives.122 In addition, a strict adherence to this diet can result in inadequate vitamin C intake. Current recommendations have dropped the natural salicylate restrictions, so that the low vitamin C intake should no longer be a problem.
Similar to the Feingold diet, the oligoantigenic, or elimination, diet is based on the hypothesis that some children with ADHD are responding adversely to specific foods and dietary ingredients. This diet also restricts additives, dyes, and preservatives, but it also initially limits the patient’s diet to two meats, two vegetables, two fruits, and two carbohydrates. If a positive response is seen after several weeks, other foods are gradually reintroduced, one at a time, in order to determine which foods adversely affect the patient’s behavior. The hypothesis is that affected individuals have sensitivity to certain foods that adversely affect their behavior. About five studies have been performed to examine this intervention with blinded and controlled conditions.123 Although some effects were demonstrated, methodological weaknesses, such as problems with blinding, preclude making a definitive conclusion about its efficacy.
Sugar was first believed to adversely affect behavior according to several studies in which an association was found between worse behavior and increased sugar intake. Authors discussing sugar have usually referred to refined and added sugars as the offending agents. These sugars are usually sucrose or fructose. However, 23 rigorous studies have demonstrated no association between sugar and behavior.124 The main complication of trying to modify sugar intake is the difficulty in having the children comply, because pursuing compliance usually increases the parent-child conflicts. A further drawback is that it further stigmatizes the child with behavior and social skills problems as “different.”
DIETARY SUPPLEMENTS
The dietary supplements recommended for treating children with ADHD include essential fatty acids, megavitamins, zinc, antioxidants, and herbs. The two primary essential fatty acids under consideration are linoleic and linolenic acids. There is no clear evidence that these supplements benefit any children, and it is not known whether there is any physical risk.125 Megavitamins consist of large quantities (at least 10 times the recommended daily allowance) of most vitamins. There is no clear evidence of their efficacy, and there is the physical side effect of elevated liver function test findings.126,127 Zinc has been recommended for the treatment of some patients with ADHD because some children were found to have zinc deficiency on the basis of hair analysis. This treatment with zinc has not been studied rigorously; therefore, there is no information about its benefits or risks. Antioxidants include melatonin, ginkgo biloba, and pycnogenol. There have been no scientific studies of their effects on patients with ADHD; thus, their potential benefits and side effects remain unknown.125 Herbal compounds have been recommended for treating patients with ADHD mainly because of their sedative properties. The herbal compounds recommended are chamomile, kava hops, lemon balm, valerian root, and passionflower. There have not been any rigorous studies of their efficacy in patients with ADHD, and their potential side effects remain unknown.
ALTERNATIVE MEDICATIONS
The hypothesis behind antifungal therapy is that children treated on multiple occasions with broad-spectrum antibiotics, such as for otitis media, have alterations in their intestinal flora that make them susceptible to the growth of Candida and the absorption of candidal toxins. These toxins then produce behavioral disturbances. The treatment consists of using antifungal agents such as nystatin or ketoconazole and eliminating sugar and foods made with molds and yeast from the diet.125 No studies have been completed to assess efficacy, and the risks are those of the side effects of the medication and the stigma of requiring a diet different from everybody else’s, as noted previously. Nootropic medications are cerebral metabolic enhancers that stave off aging. Those recommended for individuals with ADHD are piracetam and dimethylaminoethanol. There have been no rigorous studies of their efficacy, and there are no reported significant side effects, although the side effects have also not been studied systematically in children.125
BIOFEEDBACK
Electroencephalographic biofeedback is based on the premise that the electroencephalographic pattern reflects the behavior of individuals; thus, if their electroencephalographic pattern could be changed with suppression of θ activity and enhancement of β wave production, their behavior would change. Furthermore, individuals can be trained to control these activities. Although there have been a number of positive nonrandomized studies and a few with wait-list comparison groups, there have been no randomized controlled trials.128
EXERCISE
Sensory integration, developed by Dr. Jean Ayres, is based on the theory that improvement in the ability to integrate the senses improves the ability to behave and pay attention. It consists of exercises to improve the integration of the senses. Although there have been a number of positive nonrandomized or methodologically flawed studies, there have been no randomized control trials demonstrating its efficacy.128 The potential harm is the expense and time required to perform the intervention and parent-child conflicts in children who are unwilling to cooperate.
1 Diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. American Academy of Pediatrics. Pediatrics. 2000;105:1158-1170.
2 Brown RT, Freeman WS, Perrin JM, et al. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics. 2001;107(3):E43.
3 Pliszka SR, Greenhill LL, Crismon ML, et al. The Texas Children’s Medication Algorithm Project: Report of the Texas Consensus Conference Panel on Medication Treatment of Childhood Attention-Deficit/Hyperactivity Disorder. Part II: Tactics. Attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:920-927.
3a American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medication in the treatment of children, adolescents and adults. J Am Acad Child Adolesc Psychiatry:. 2002;41(2 Supplement):26S-49S.
4 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association, 1994.
4a American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association, 2000.
5 Hoffman H. Der Struwwelpeter. Leipzig: Imsel Verlag, 1848;11-15.
6 Sand G. The devil’s pool. In: Eliot CW, editor. The Harvard Classics Shelf of Fiction: French Fiction. New York: PF Collier; 1917:289.
7 Still GF. The Coulstonian lectures on some abnormal physical conditions in children. Lancet. 1902;1:1008-1012.
8 Barkley R. Response inhibition in attention-deficit hyperactivity disorder. Ment Retard Dev Disabil Res Rev. 1999;5:177-184.
9 Bradley C. The behavior of children receiving Benzedrine. Am J Psychiatry. 1937;94:577-585.
10 Laufer M, Denhoff E. Hyperkinetic behavior syndrome in children. J Pediatr. 1957;50:463-474.
11 Hohman LB. Post-encephalitic behavior disorder in children. Johns Hopkins Hosp Bull. 1922;33:372-375.
12 Ebaugh FG. Neuropsychiatric sequelae of acute epidemic encephalitis in children. Am J Dis Child. 1923;25:89-97.
13 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 2nd ed. Washington, DC: American Psychiatric Association, 1967.
14 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Washington, DC: American Psychiatric Association, 1980.
15 Douglas V. Stop, look and listen: The problem of sustained attention and impulse control in hyperactive and normal children. Can J Behav Sci. 1972;4:259-282.
16 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Washington, DC: American Psychiatric Association, 1987. revised
17 James W. The Principles of Psychology. Cambridge, MA: Harvard University Press, 1983;1166. (Originally published1890.)
18 Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child Adolesc Psychiatr Clin North Am. 2000;9:541-555.
18a de Fockert JW, Rees G, Frith CD, LaVie N. The role of working memory in visual selective attention. Science. 2001;291:1803-1806.
19 Jensen PS, Kettle L, Roper MT, et al. Are stimulants overprescribed? Treatment of ADHD in four U.S. communities. J Am Acad Child Adolesc Psychiatry. 1999;38:797-804.
20 Baumgaertel A, Wolraich ML, Dietrich M. Comparison of diagnostic criteria for attention deficit disorders in a German elementary school sample. J Am Acad Child Adolesc Psychiatry. 1995;34:629-638.
21 Wolraich ML, et al. Comparison of diagnostic criteria for attention deficit hyperactivity disorder in a county-wide sample. J Am Acad Child Adolesc Psychiatry. 1996;35:319-323.
22 Swanson JM, Castellanos FX. Biological bases of ADHD: Neuroanatomy, genetics and pathophysiology. Paper presented at: NIH Consensus Development Conference on Diagnosis and Treatment of Attention Deficit Hyperactivity Disorder, 1998; Bethesda, MD.
23 Barkley RA. Attention-deficit hyperactivity disorder. Sci Am. 1998;279:66-71.
24 Alberts-Corush J, Firestone P, Goodman JT. Attention and impulsivity characteristics of the biological and adoptive parents of hyperactive and normal control children. Am J Orthopsychiatry. 1986;56:413-423.
25 Morrison JR, Stewart MA. The psychiatric status of the legal families of adopted hyperactive children. Arch Gen Psychiatry. 1973;28:888-891.
26 Biederman J, Faraone SV, Keenan K, et al. Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1990;29:526-533.
27 Morrison JR, Stewart MA. A family study of the hyperactive child syndrome. Biol Psychiatry. 1971;3:189-195.
28 Cantwell DP. Psychiatric illness in the families of hyperactive children. Arch Gen Psychiatry. 1972;27:414-417.
29 Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity. N Engl J Med. 1999;340:40-46.
30 Shaywitz BA, Fletcher JM, Pugh KR, et al. Progress in imaging attention deficit hyperactivity disorder. Ment Retard Dev Disabil Res Rev. 1999;5:185-190.
31 Hauser P, Zametkin AJ, Martinez P, et al. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993;328:997-1001.
32 Cook EHJr, Stein MA, Krasowski MD, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993-998.
33 Gill M, Daly G, Heron S, et al. Confirmation of an association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry. 1997;2:311-313.
34 Swanson JM, Sunohara GA, Kennedy JL, et al. Association of the dopamine receptor D4(DRD4) gene with a refined phenotype of attention deficit-hyperactivity disorder (ADHD): A family-based approach. Mol Psychiatry. 1998;3:38-41.
35 Ingram S, Hechtman L, Morgenstern G. Outcome issues in ADHD: Adolescent and adult long-term outcome. Ment Retard Dev Disabil Res Rev. 1999;5:243-250.
36 Miller A, Lee S, Raina P, et al. A review of therapies for attention-deficit/hyperactivity disorder. Vancouver: Research Institute for Children’s and Women’s Health and University of British Columbia, 1998.
37 American Academy of Pediatrics, Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement. Clinical practice guideline: Treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1104. 4
38 DuPaul GJ, Power TJ, Anastopoulos AD, et al. Teacher ratings of attention deficit hyperactivity disorder symptoms: Factor structure and normative data. Psychol Assess. 1997;9:436-444.
39 Conners CK, Pliszka SR, Wolraich ML. Paying Attention to ADHD: Accurate Diagnosis, Effective Treatment. Philadelphia: Medical Education Systems, 1999.
40 Achenbach TM, Edelbrock L. Manual for the Child Behavior Checklist 4–18 and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry, 1991.
41 Reynolds C, Kamphaus RW. The Clinician’s Guide to the Behavior Assessment System for Children (BASC). New York: Guilford, 2002.
42 Wolraich M. Attention deficit hyperactivity disorder: The most studied yet most controversial diagnosis. Ment Retard Dev Disabil Res Rev. 1999;5:163-168.
42a Du Paul GJ, McGoey KE, Eckert TL, Van Brakle J. Preschool children with attention-deficit/hyperactivity disorder: Impairments in behavioral, social and school functioning. J Am Acad Child Adolesc Psychiatry. 2001;49:508-515.
42b Bagwell CL, Molina BSG, et al. Attention-deficit hyperactivity disorder and problems with peer relations: Predictions from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40:1285-1292.
43 Hechtman L, Weiss G, Perlman T. Self-esteem and social skills. Can J Psychiatry. 1980;25:478-483.
44 Hoza B, Pelham WE, et al. Do boys with attention-deficit/hyperactivity disorder have positive illusory self-concepts? J Abnorm Psychol. 2002;111:268-278.
45 Crabtree V, Ivenenki A, Gozal D. Clinical and parental assessment of sleep in children with attention-deficit/hyperactivity disorder referred to a pediatric sleep medicine center. Clin Pediatr. 2003;42:807-813.
46 Marcotte AC, Thacher PV, Butters M, et al. Parental report of sleep problems in children with attentional and learning disorders. J Dev Behav Pediatr. 1998;19:178-186.
47 Lebourgeois M, Avis K, Mixon M, et al. Snoring, sleep quality, and sleepiness across attention-deficit/hyperactivity disorder subtypes. Sleep. 2004;27:520-525.
48 O’Brien L, Ivanenko A, Crabtree VM, et al. The effect of stimulants on sleep characteristics in children with attention deficit/hyperactivity disorder. Sleep Med. 2003;4:309-316.
49 Tirosh E, Sadeh A, Munvez R, et al. Effects of methylphenidate on sleep in children with attention-deficit hyperactivity disorder. Am J Dis Child. 1993;147:1313-1315.
50 Avior G, Fishman G, Leor A, et al. The effect of tonsillectomy and adenoidectomy on inattention and impulsivity as measured by the Test of Variables of Attention (TOVA) in children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2004;131:367-371.
51 Mazza S, Pepin JL, Naegele B, et al. Most obstructive sleep apnoea patients exhibit vigilance and attention deficits on an extended battery of tests. Eur Respir J. 2005;25:75-80.
52 Schechter M. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome: Technical report: Diagnosis and management of childhood obstructive apnea syndrome. Pediatrics. 2002;109(4):e69.
53 O’Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111:554-563.
54 Pelham WEJ, Wheeler T, Chronis A. Empirically supported psychosocial treatments for attention deficit hyperactivity disorder. J Clin Child Psychol. 1998;27:190-205.
55 Jensen PS, Hinshaw SP, Swanson JM, et al. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): Implications and applications for primary care providers. J Dev Behav Pediatr. 2001;22:60-73.
56 Children and Adults With Attention-Deficit/Hyper-activity Disorders. Ment Retard Dev Disabil Res Rev. 2001;5:215. http://www.chadd.org/
57 Wigal T, Swanson JM, Regino R, et al: Stimulant medications for the treatment of ADHD: Efficacy and 1224, 1999.
58 Handen BL, Breaux AM, Janosky J, et al. Effects and noneffects of methylphenidate in children with mental retardation and ADHD. J Am Acad Child Adolesc Psychiatry. 1992;31:455-461.
59 Handen BL, Breaux AM, Gosling A, et al. Efficacy of methylphenidate among mentally retarded children with attention deficit hyperactivity disorder. Pediatrics. 1990;86:922-930.
60 Gadow KD. Prevalence and efficacy of stimulant drug use with mentally retarded children and youth. Psychopharmacol Bull. 1985;21:291-303.
61 Cullinan D, Gadow KD, Epstein MH. Psychotropic drug treatment among learning-disabled, educable mentally retarded, and seriously emotionally disturbed students. J Abnorm Child Psychol. 1987;15:469-477.
62 Aman MG, Marks RE, Turbott SH, et al. Clinical effects of methylphenidate and thioridazine in intellectually subaverage children. J Am Acad Child Adolesc Psychiatry. 1991;30:246-256.
63 Feldman H, Crumrine P, Handen BL, et al. Methylphenidate in children with seizures and attention-deficit disorder. Am J Dis Child. 1989;143:1081-1086.
64 Gross-Tsur V, Manor O, van der Meere J, et al. Epilepsy and attention deficit hyperactivity disorder: Is methylphenidate safe and effective? J Pediatr. 1997;130:670-674.
65 Biederman J, Wilens T, Mick E, et al. Psychoactive substance use in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;52:1652-1658.
66 Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder. Behav Brain Res. 1998;94:127-152.
67 [Ritalin (methylphenidate) product information.] East Hanover, NJ: Novartis Pharmaceuticals, 1998. (Available at: http://www.pharma.us.novartis.com/product/pi/pdf/ritalin_sr.pdf; accessed 1/8/06.)
68 Wigal S, Swanson JM, Feifel D, et al. A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and D, L-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1406-1414.
68a Heal DJ, Pierce C. M. Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs. 2006;20:713-738.
69 Swanson JM, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull. 1998;34:55-60.
70 Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26S-49S.
71 Pelham WE, Gnagy EM, Burrows-Maclean L, et al. Once-a-day Concerta methylphenidate versus three-times daily methylphenidate in laboratory and natural settings: Safety and efficacy. Pediatrics. 2001;107(6):E105.
72 Marcus S, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159:572-578.
73 Lage M, Hwang P. Effect of methylphenidate formulation for attention deficit hyperactivity disorder on patterns and outcomes of treatment. J Child Adolesc Psychopharmacol. 2004;14:575-581.
73a Regulations Requiring Manufacturers to Assess the Safety and Effectiveness of New Drugs and Biological Products in Pediatric Patients, 21 C.F.R. §§ 201, 312, 314, 601. Fed Register. 1998;63:66632.
74 Pelham WE, Hoza J. Behavioral assessment of psychostimulant effects on ADD children in a summer day treatment program. Adv Behav Assess Child Fam. 1987;3:3-34.
75 Whitehouse D, Shah U, Palmer FB. Comparison of sustained-release and standard methylphenidate in the treatment of minimal brain dysfunction. J Clin Psychiatry. 1980;41:282-285.
76 Fitzpatrick P, Klorman R, Brumaghim JT, et al. Effects of sustained-release and standard preparations of methylphenidate on attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:226-234.
77 Pelham W, Gnagy EM, Burrows-Maclean L, et al. Once-a-day Concerta methylphenidate versus three-times-dai ly methylphenidate in laboratory and natural settings: Safety and efficacy. Pediatrics. 2001;107(6):E105.
78 Wolraich ML, Greenhill LL, Pelham W, et al. Randomized controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:883-892.
79 Wilens T, Pelham W, Stein M, et al. ADHD treatment with once-daily OROS methylphenidate: Interim 12-month results from a long-term open-label study. J Am Acad Child Adolesc Psychiatry. 2003;42:424-433.
80 Cox DJ, Humphrey JW, Merkel RL, et al. Controlled-release methylphenidate improves attention during on-road driving by adolescents with attention-deficit/hyperactivity disorder. J Am Board Fam Pract. 2004;17:235-239.
81 Swanson JM, Wigal SB, Wigal T, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study). Pediatrics. 2004;113(3 Part 1):e206-e216.
82 Biederman J, Quinn D, Weiss M, et al. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs. 2003;5:833-841.
83 Lopez F, Silva R, Pestreich L, et al. Comparative efficacy of two once daily methylphenidate formulations (Ritalin LA and Concerta) and placebo in children with attention deficit hyperactivity disorder across the school day. Paediatr Drugs. 2003;5:545-555.
84 Biederman J, Lopez FA, Boellner SW, et al. A randomized, double-blind, placebo-controlled parallel-group study of SLI 381(Adderall XR) in children with attention-deficit/hyperactivity disorder. Pediatrics. 2002;110:258-266.
85 McGough JJ, Biederman J, Wigal SB, et al. Long-term tolerability and effectiveness of once-daily mixed amphetamine salts (Adderall XR) in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44:530-538.
86 Findling R, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr. 2005;147:348-354.
87 Bridges A: Advisers Reject Strong ADHD Warnings. Associated Press, March 23, 2006.
88 Pelham WEJr, Manos MJ, Ezzell CE, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44:522-529.
89 Pelham WE, Burrows-Maclean L, Gnagy EM, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Psychopharmacol. 2005;13:111-126.
90 McGough JJ, Wigal SB, Abikoff H, et al. A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord. 2006;9:476-485.
91 Keating GM, Figgitt DP. Dexmethylphenidate. Drugs. 2002;62:1899-1908.
91a Marchetti A, Magar R, Lan H, Murphy EL, Jensen PS, Connors CK, Findling R, Wineburg E, Carotenuto I, Einarson TR, Iskedjian M. Pharmacotherapies for attention-deficit/hyperactivity disorder: expected-cost analysis. Clinical Therapeutics. 2001;23:1904-1921.
92 Kramer JR, Loney J, Ponto LB, et al. Predictors of adult height and weight in boys treated with methylphenidate for childhood behavior problems. J Am Acad Child Adolesc Psychiatry. 2000;39:517-524.
93 Sund AM, Zeiner P. Does extended medication with amphetamine or methylphenidate reduce growth in hyperactive children? Nord J Psychiatry. 2002;56:53-57.
94 Spencer T, Biederman J, Wilens T, et al. Pharmaco-therapy of attention-deficit hyperactivity disorder across the life span. J Am Acad Child Adolesc Psychiatry. 1996;35:409-432.
95 Lisska MC, Rivkees SA. Daily methylphenidate use slows the growth of children: A community based study. J Pediatr Endocrinol Metab. 2003;16:711-718.
96 Poulton A, Cowell CT. Slowing of growth in height and weight on stimulants: A characteristic pattern. J Paediatr Child Health. 2003;39:180-185.
97 Palumbo D, Spencer T, Lynch J, et al. Emergence of tics in children with ADHD: Impact of once-daily OROS methylphenidate therapy. J Child Adolesc Psychopharmacol. 2004;14:185-194.
98 Gadow K, Sverd J. Stimulants for ADHD in child patients with Tourette’s syndrome: The issue of relative risk. J Dev Behav Pediatr. 1990;11:269-271.
99 Jensen P: Behavioral and medication treatments for ADHD: Comparisons and combinations. Presented at the NIH Consensus Conference on Diagnosis and Treatment of Attention Deficit Hyperactivity Disorder, Washington, DC, November 16–18, 1998.
100 Pelham WE, Gnagy EM. Psychosocial and combined treatments for ADHD. Ment Retard Dev Disabil Res Rev. 1999;5:225-236.
101 Biederman J, Heiligenstein JH, Faries DE, et al. Efficacy of atomoxetine versus placebo in school-age girls with attention-deficit/hyperactivity disorder. Pediatrics. 2002;110(6):e75.
102 Kratochvil C, Bohac D, Harrington M, et al. An open-label trial of tomoxetine in pediatric attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:167-170.
103 Kratochvil C, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry. 2002;41:776-784.
104 Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled, dose-response study. Pediatrics. 2001;108(5):E83.
105 Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: A randomized, placebo-controlled study. Am J Psychiatry. 2002;159:1896-1901.
106 Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: Two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112-120.
107 Spencer T, Biederman J, Heiligenstein J, et al. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:251-265.
108 Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63:1140-1147.
109 Connor D, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:1551-1559.
110 Wilens TE, Spencer TJ. A clinically sound medication option. J Am Acad Child Adolesc Psychiatry. 1999;38:5.
111 Prince J, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: A systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35:599-605.
112 Conners CK, Casat CD, Gualtieri CT, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996;35:1314-1321.
113 Physicians’ Desk Reference, 54th ed. Montvale, NJ: Medical Economics, 2000, pp 1301–1308.
114 Biederman J, Swanson JM, Wigal SB, et al. Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: Results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics. 2005;116(6):e777-e784.
115 Cunningham C, Bremner R, Boyle M. Large group community-based parenting programs for families of preschoolers at risk for disruptive behaviour disorders: Utilization, cost, effectiveness, and outcome. J Child Psychol Psychiatry. 1995;36:1141-1159.
116 Herschell AD, Calzada E, Eyberg SM, et al. Parent-child interaction therapy: New directions in research. Cogn Behav Pract. 2002;9:9-16.
117 Hannah JN. Parenting a child with attention-deficit/hyperactivity disorder. Austin, TX: Pro-Ed, 1999.
118 Davila RR, Williams ML, MacDonald JT. Memorandum on clarification of policy to address the needs of children with attention deficit disorders within general and/or special education. In: Parker HC, editor. The ADD Hyperactivity Handbook for Schools. Plantation, FL: Impact Publications; 1991:261-268.
119 Pelham WE: How to Establish a Daily Report Card. Center for Treatment of Attention Deficit Disorder, 2005. (Available at: http://ccf.buffalo.edu/defoul.php; accessed 5/27/07.)
120 Pelham W, Gnagy EM, Greiner AR, et al. Behavioral versus behavioral and pharmacological treatment in ADHD children attending a summer treatment program. J Abnorm Child Psychol. 2000;28:507-525.
121 Feingold B. Why Your Child Is Hyperactive. New York: Random House, 1975.
122 Wender EH. The food additive-free diet in the treatment of behavior disorders: A review. J Dev Behav Pediatr. 1986;7:35-42.
123 Wolraich M: Attention Deficit Hyperactivity Disorder: Current Diagnosis and Treatment. Presented at the annual meeting of the American Academy of Pediatrics, Chicago, October 28, 2000. (Available at: http://medscape.com/viewarticle/420198; accessed 5/27/07.)
124 Wolraich ML, Wilson DB, White JW. The effect of sugar on behavior or cognition in children: A meta-analysis. JAMA. 1995;274:1617-1621.
125 Baumgaertel A. Alternative and controversial treatments for attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 1999;46:977-992.
126 Arnold LE. Megavitamins for MBD: A placebo-controlled study. JAMA. 1978;20:24.
127 Haslam R, Dalby J, Rademaker A. Effects of megavi-tamin therapy on children with attention deficit disorders. Pediatrics. 1984;74:103-111.
128 Goldstein S, Goldstein M. Managing Attention Deficit Hyperactivity Disorder in Children: A Guide for Practitioners, 2nd ed. New York: Wiley, 1998.