Atrial Tachycardia
Atrial tachycardias (ATs) are regular atrial rhythms at a constant rate ≥100 beats/min that originate outside the sinus node region and do not require participation of the atrioventricular node for maintenance of the arrhythmia.1 Atrial tachycardias constitute a major cause of supraventricular tachycardias. Although sustained AT occurs relatively infrequently, bursts of nonsustained AT are commonly observed on Holter or telemetry recordings. The mechanism of AT can be focal or macroreentrant. Over the last two decades, significant advances in our understanding of ATs have occurred. The use of advanced mapping tools and catheter ablation has enabled detailed assessment of the substrate and characteristic anatomic locations of ATs. This chapter reviews information about the classification, mechanisms, electrocardiographic localization, and electrophysiological characterization of ATs.
Classification and Mechanisms
An expert consensus group from the European Society of Cardiology and the North American Society of Pacing and Electrophysiology published a classification of atrial flutter and regular ATs based on mechanism and anatomy.1 Atrial tachycardias were classified as tachycardias with a regular atrial rate arising from the atrium and were further categorized as either focal or macroreentrant. Focal AT can be caused by automatic, triggered, or microreentrant mechanisms. Focal ATs are characterized by radial, circular, or centrifugal spread of activation from a single focus or point source and lack of electrical activation spanning the tachycardia cycle length. Macroreentrant ATs are due to reentry through relatively large, potentially well-characterized circuits. Macroreentrant ATs are characterized by a repetitive pattern of electrical activation encompassing the entire cardiac cycle. Patterns that have been described include a single loop (such as common isthmus-dependent flutter), two reentrant loops creating a figure of eight, and reentry through narrow channels adjacent to scars or natural anatomic barriers, such as the tricuspid or mitral annulus. Typical atrial flutter, lower loop reentry, double loop reentry, left atrial macro-reentrant AT, scar AT, reverse typical atrial flutter, and right atrial free-wall macro-reentry all are classified as macro-reentrant ATs. Atrial tachycardias occurring in the setting of congenital heart disease are described in Chapter 78, and those occurring after atrial fibrillation ablation in Chapter 123. Atrial tachycardias occurring after atrial fibrillation ablation may be extremely complex, involving multiple loops, and often are related to gaps in previously created ablation lines.
A new technique has been proposed to differentiate between focal and macroreentrant ATs based on the surface electrocardiogram (ECG).2 Using non-invasive methodologies that did not require mapping in the electrophysiology laboratory, the study investigators analyzed quantitative ECG metrics with computerized signal-averaging techniques. A short atrial activation time relative to the tachycardia cycle length was used to differentiate focal from macroreentrant ATs based on the principle that macroreentrant ATs have activation throughout the cycle length in contrast to focal ATs. The investigators were able to develop algorithms to quantify P or F wave duration with overlying T waves by transitions in slope relative to the expected T waves generated from scaling of the sinus rate T wave. Electrocardiographic P wave duration correlated with the duration of intraatrial activation. Focal ATs had shorter P wave durations and smaller ratios of P wave to cycle length. P waves less than 160 ms and P wave to cycle length ratios less than 45% differentiated focal AT from macroreentry with promising accuracy in patients who subsequently underwent invasive catheter mapping and ablation.2
Focal Atrial Tachycardias
Sustained, focal ATs in patients who are in an electrophysiology laboratory for mapping and catheter ablation almost always arise in the absence of significant, preexisting structural heart disease. In one large series, among 345 patients with focal AT who underwent radiofrequency ablation only 4% of patients (n = 14) had preexisting structural heart disease.3 Notably, however, incessant or frequent paroxysmal tachycardia can produce a tachycardia-mediated cardiomyopathy. In this series, approximately 10% of patients with focal AT developed a tachycardia-mediated cardiomyopathy.3 A slower incessant tachycardia was more frequently complicated by cardiomyopathy, and appendage sites were associated with a higher incidence of incessant tachycardia (84%) and LV dysfunction (42%). Virtually all patients had restoration of normal LV function after successful ablation at a mean of 3 months.
Atrial arrhythmias frequently complicate heart failure and atrial enlargement. In an experimental model, Stambler et al.4,5 demonstrated that heart failure induced by rapid ventricular pacing provides a substrate that predisposes to focal ATs. Sustained ATs could be induced in greater than 90% of animals after an average of 3 weeks of heart failure, but in less than 10% at baseline in the absence of heart failure. The mode of AT induction, responses to pacing maneuvers and Ca2+ antagonists, and the presence of delayed afterdepolarizations suggested triggered activity, resulting from intracellular Ca2+ overload, as the likely tachycardia mechanism. Strikingly similar to the anatomic distribution of focal ATs in humans, mapping and ablation studies in this heart failure model indicated that the ATs had a focal origin with sites of earliest activation located predominately along the crista terminalis (CT) and within or near the pulmonary veins (PVs). Rapid, left AT generating atrial fibrillation was localized and ablated inside the pulmonary vein in this model.
The autonomic nervous system likely has a critical role in initiating or triggering some ATs. Reports of AT triggered by changes in posture, belching, swallowing, as well as termination of tachycardia by the Valsalva maneuver, edrophonium, or β-adrenergic blockers, support a probable role of the autonomic nervous system in some patients. A close association between autonomic nervous system activity and paroxysmal atrial arrhythmias in animal models has been documented. In dogs with pacing-induced heart failure, spontaneous paroxysmal ATs are triggered by simultaneous sympathovagal discharges.6 Likewise in a canine model of intermittent rapid left atrial pacing, autonomic nerve discharge was shown to be an invariable trigger of paroxysmal atrial tachyarrhythmias.7 All spontaneous, AT and atrial fibrillation episodes were preceded (<5 seconds) by superior left ganglionated plexi nerve activity.
An interaction between respiration and supraventricular arrhythmias has been described in rare cases demonstrating bursts of atrial ectopic beats that emerge after the start of inspiration and cease during expiration. A recent report described in detail, respiratory cycle–dependent ATs.8 Among 71 nonreentrant focal ATs in 60 patients, 9 respiratory cycle–dependent ATs (13%) were seen in 7 patients (12%). The arrhythmias were incessant, had irregular tachycardia cycle lengths, and emerged in synchrony with respiratory cycles. The P-wave morphology was positive or biphasic in V1 and positive in I and II. Electroanatomical mapping demonstrated the earliest site located around the RSPV or inside the SVC, and radiofrequency ablation was effective in eliminating these ATs.
Chen et al.9 classified ATs in 36 patients into one of three categories based on the following groups of findings.
The effects of adenosine on AT have been studied extensively by Markowitz et al.,10 who examined the mechanism-specific effects of adenosine on 85 ATs in 82 patients. In response to adenosine, termination occurred in 67 cases (84%), two patients exhibited a nonspecific response, and six were insensitive (8%). Adenosine-sensitive ATs arose from a wide distribution of sites in both atria. These sites included the tricuspid annulus (n = 45), mitral annulus (n = 8), CT (n = 15), and other sites (posteroseptal right atrium, atrial appendage, and interatrial septum). Adenosine-insensitive ATs arose near the pulmonary veins and in the right atrium. In contrast to the study by Chen et al.9, Markowitz et al.10 provided evidence that adenosine-insensitive focal ATs are caused by microreentry.11 Electrograms at the site of origin of adenosine-insensitive AT typically were highly fractionated and had longer durations and lower amplitudes compared with ATs that were terminated or transiently suppressed by adenosine. Similar observations were made by De Groot and Schalij,12 who showed that the site of origin of focal ATs was characterized by double potentials and fractionated and low-voltage, long-duration electrograms—findings attributed to poor cell-to-cell coupling.12
Chiale et al.13,14 described the behavior and pharmacologic responses of a group of rate-related, repetitive uniform, focal ATs that were highly sensitive to intravenous lidocaine. These arrhythmias appear to be rare, show a variable response to intravenous adenosine and verapamil, and are neither consistently induced nor terminated by programmed premature atrial stimulation, suggesting a nonreentrant mechanism. These authors speculated that delayed afterdepolarizations could have a determining role in these ATs.
Evaluation of studies, such as those noted here, that have attempted to categorize the mechanism of focal AT based on their responses to pacing and pharmacologic maneuvers in the clinical electrophysiology laboratory is difficult, as most have included small, heterogeneous populations and patients with a variety of ATs. Thus, given the lack of definitive criteria to identify the mechanism of focal AT in the electrophysiology laboratory, these studies remain largely descriptive. A major limitation to an approach for identifying the electrophysiological mechanism of focal AT based on responses to drugs is that there is important overlap in the electrophysiological and pharmacologic characterization of mechanisms. For example, adenosine-sensitivity can be highly useful and specific for distinguishing focal from macroreentrant ATs, but it is less helpful in definitively identifying the electrophysiological mechanism of a focal AT. Whereas triggered ATs are usually terminated by adenosine and verapamil, the use of these pharmacologic agents to differentiate AT mechanisms has produced some inconsistent and variable results.15,16 For example, some studies suggest that verapamil and adenosine both terminate ATs caused by microreentry and triggered activity, whereas others suggest that adenosine-insensitive focal ATs are caused by microreentry.9,10 In addition, adenosine administration can result in tachycardia termination by nonspecific effects via induction of atrial extrasystoles. Thus, pharmacologic interventions might not be a reliable means to identify AT mechanism.
Impact of Mapping Technologies
Extensive activation sequence mapping is typically used in the electrophysiology laboratory to define the presumptive mechanism of AT and plan an ablation strategy. Technologies for mapping arrhythmias include basket mapping, electroanatomic mapping, and noncontact mapping systems. The mapping technologies have definite advantages over point-by-point catheter mapping, including the ability to map unstable, poorly reproducible, and nonsustained arrhythmias. With these technologies, short episodes of arrhythmias can be mapped with one or several beats, or the arrhythmia substrate can be mapped for areas of low voltage or double potentials. Many series describing the use of each of these new technologies to map and ablate ATs have been reported.17,18 These technologies typically are accompanied by decreased fluoroscopic exposure for the operator, more precise localization of tachycardia, and a better appreciation of atrial anatomy.
In one study, electroanatomic mapping (CARTO; Biosense, Webster, California) of the right atrium was performed during 13 tachycardia episodes in 10 patients who had previous cardiac surgery.19 Focal ATs were defined in three patients, and macroreentrant circuits were found in seven patients. The mapping system allowed rapid distinction between a focal AT and a macroreentrant mechanism. In the patients with focal mechanisms, a radial spread of activation was noted from the site of earliest activation in all directions on an isochronal map (Figure 73-1). The right atrial activation time was markedly shorter than the tachycardia cycle length, accounting for, an average of 14% of the tachycardia cycle length. In patients with a macroreentrant mechanism, isochronal maps showed a continuous progression of activation around the right or left atrium, with close proximity of earliest and latest local activation (Figure 73-2). The right atrial activation time was in a range similar to that of tachycardia cycle length, occupying 70% or more of the tachycardia cycle length.
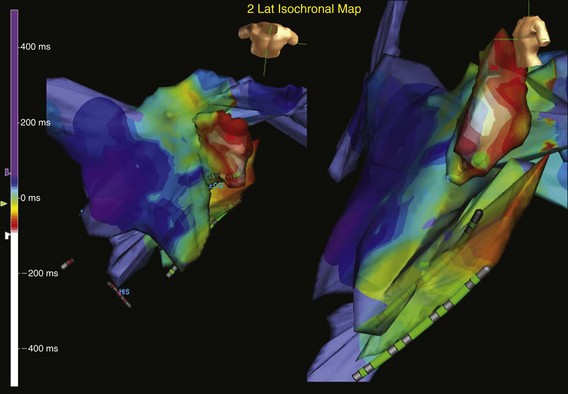
Figure 73-1 Computerized electroanatomic mapping from a patient with a focal atrial tachycardia arising from the epicardial surface of the left atrial appendage. Structural heart disease is absent, but the patient has an incessant tachycardia. The site of origin is focal, with rapid radial spread of atrial activation from the earliest site of activation.
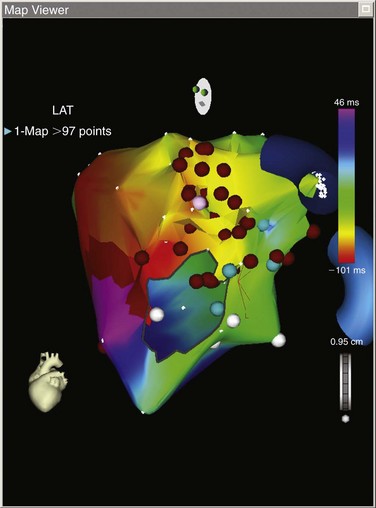
Figure 73-2 Left atrial electroanatomic map of a macroreentrant atrial tachycardia. This electroanatomic map shows a mitral isthmus reentrant tachycardia. This patient previously underwent minimally invasive surgical atrial fibrillation ablation More than 90 points were mapped, and the map shows an “earliest meets latest” or “head meets tail” on the lateral mitral valve isthmus. The postpacing interval minus the tachycardia cycle length (PPI-TCL) was less than 10 ms from the distal coronary sinus (see Figure 73-16).
Sanders et al.20 reported on the use of high-density mapping of focal AT. They used a high-density mapping catheter (PentaRay; Biosense) to characterize a group of patients in whom prior attempts at atria tachycardia ablation or atrial fibrillation ablation were unsuccessful.20 They noted that a localized focus was found in 70%, whereas 30% had evidence of localized reentry characterized by the ability to record electrograms during 95% of the tachycardia cycle length. They demonstrated that with high-density mapping, it was possible to define regions of preferential conduction and intraatrial conduction delay. Higa et al.21 associates made similar observations using the technique of noncontact mapping. These investigators demonstrated that focal AT originates from a small area of tissue but spreads through the atrium by way of regions of preferential (rather than centrifugal) conduction. These investigators made several other notable observations: most ATs originate near a border of a low-voltage zone or an area of anisotropic conduction, and the site of successful ablation is near the site of tachycardia origin or the proximal portion of the preferential conduction pathway, rather than at the “breakout” point.
Electrocardiographic Localization of Focal Atrial Tachycardia
These algorithms are useful despite considerable overlap in P wave morphology, because most ATs originate from relatively few sites. However, it is important to appreciate that the P wave configuration is in large part dependent on left atrial activation. Therefore, despite differences in site of origin, similar sequences of left atrial activation could yield nearly identical P waves. For example, P waves resulting from foci in the coronary sinus muscle can resemble flutter waves in patients with typical counterclockwise flutter. The overlap in P wave morphology reflects the limited spatial resolution of the P wave of at best 2 cm, based on discrimination between two different atrial pacing sites from mapping studies. In addition, the P wave often is obscured by the T wave or QRS complex during tachycardia. To better define P wave morphology and help to establish a fiducial point for mapping, spontaneous ventricular ectopy, ventricular pacing or adenosine infusion may be useful to uncover the true P wave morphology. Finally, the algorithms for differentiation of AT origin have been based largely on analysis of tracings from patients without structural heart disease or atrial dilatation. Notwithstanding these limitations, P wave morphology can provide useful clues to tachycardia location. Published algorithms help to determine the localization of AT site of origin22–26 (Figures 73-3, 73-4).
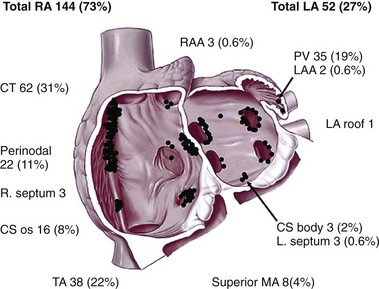
Figure 73-3 A schematic representation of the anatomic distribution of focal atrial tachycardias. The atrioventricular valvular annuli have been removed. CS, Coronary sinus; CT, crista terminalis; LA, left atrium; LAA, left atrial appendage; MA, mitral annulus; PV, pulmonary vein; RA, right atrium; RAA, right atrial appendage; TA, tricuspid annulus. (From Kistler PM, Roberts-Thompson KC, Haqqani HM, et al: P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol 48:1010-1017, 2006.)
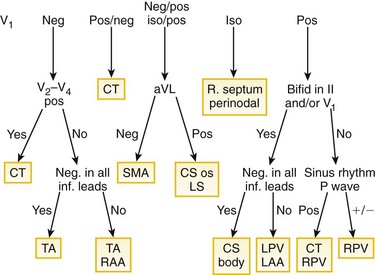
Figure 73-4 Algorithm for localizing the site of origin for focal atrial tachycardias. The algorithm, constructed on the basis of findings from 130 atrial tachycardias, correctly localized the focus in 93%. CS, Coronary sinus; CT, crista terminalis; inf., inferior; iso, isoelectric; LA, left atrium; LAA, left atrial appendage; MA, mitral annulus; PV, pulmonary vein; RA, right atrium; RAA, right atrial appendage; SMA, superior mitral annulus; TA, tricuspid annulus. (From Kistler PM, Roberts-Thompson KC, Haqqani HM, et al: P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol 48:1010-1017, 2006.)
Tang et al.22 constructed an algorithm to help localize the site of AT. They studied 12-lead ECGs from 31 patients with focal AT who underwent mapping and successful ablation, and they proposed an algorithm that distinguished right atrial from left atrial foci with a sensitivity of 88% to 93% and a specificity of 79% to 88%. The P wave configurations in leads aVL and V1 were most helpful in differentiating right atrial from left atrial foci. A positive or biphasic P wave in aVL predicted a right atrial focus, whereas a positive P wave in V1 predicted a left atrial focus. The algorithm incorrectly predicted the location of AT in patients with a right superior pulmonary vein focus, showing a positive P wave in aVL instead of the expected negative P wave, probably because of the close location of the right superior pulmonary vein to the high lateral right atrium. In these patients, close inspection of the P wave morphology during sinus rhythm and during AT showed the change in P wave morphology in V1 from biphasic to a positive P wave with a right superior pulmonary vein tachycardia.
This algorithm was further refined for right ATs by Tada et al.23 Based on the left anterior oblique view of the right atrium, tachycardias were classified as occurring at superolateral, inferolateral, and inferomedial locations. A negative P wave in lead aVR identified an AT lying on the CT with 100% sensitivity and 93% specificity. A positive P wave in the inferior leads differentiated superolateral ATs from inferolateral ATs with a sensitivity of 86% and a specificity of 100%. In any AT with inferomedial or inferolateral foci, the P wave in at least one of the inferior leads was negative. Negative P waves in leads V5 and V6 identified inferomedial ATs with a sensitivity of 92% and a specificity of 100%. In ATs coming from the triangle of Koch, the P wave duration in the inferior leads was shorter than during sinus rhythm (see Figure 73-4). Foci from the right atrial appendage have a characteristic P wave morphology, a negative P wave in lead V1 becoming progressively positive across the precordial leads, and low-amplitude positive P waves in the inferior leads in a majority of patients.
Another refinement of the algorithm for pulmonary vein foci was reported by Haissaguerre et al.24 Criteria were devised to distinguish right from left pulmonary vein sites: a positive and relatively flat P wave in lead aVL and a positive P wave in lead I of greater than 50 µV indicated a right pulmonary vein origin with a specificities of 100% and 97% and a sensitivities of 38% and 72%, respectively. The positive predictive values of these two criteria were 100% and 98%, respectively. A notched P wave in lead II was a predictor of left pulmonary vein origin, with a specificity of 95% and sensitivity of 39%. An amplitude ratio of lead III/II of 0.8 or greater and the duration of positivity in lead V1 (longer than 80 ms) also were helpful in differentiating left from right pulmonary vein origin. These two criteria had specificities of 75% and 73%, sensitivities of 96% and 85%, and positive predictive values for left pulmonary vein sites of 79% and 76%, respectively. Left pulmonary vein sites were characterized by low-amplitude and flat P waves in lead I, negative polarity in lead aVL, similar amplitudes in both limb leads III and II, and longer duration of positivity in lead V1. Superior pulmonary veins were distinguished from inferior pulmonary veins on the basis of P wave amplitude for superior pulmonary vein origin in lead II of 100 µV or greater. This value differentiated superior from inferior P waves, with a specificity of 74%, a sensitivity of 81%, and a positive predictive value of 86%. Focal ATs arising from the left atrial appendage can be distinguished from left or right pulmonary vein tachycardias by a deeply negative P wave in lead I. In addition, left atrial appendage (LAA) ATs also have a P wave that is upright or biphasic in lead V1 and highly positive in the inferior leads.
Hachiya et al.24 further refined and simplified the algorithm for left AT. Their algorithm involves two steps: a positive P wave in V1 localizes the site of origin to the pulmonary veins, and a biphasic or negative P wave in V1 localizes the septal or superior mitral annulus or left atrial appendage.25
Kistler et al.26, building on prior algorithms and using data from a series of 126 patients with 130 ATs, devised another algorithm that identifies tachycardia site of origin.26 This algorithm is presented as Figure 73-4; once again V1 was critical. They found that a negative or a positive-negative P wave in V1 demonstrated a specificity of 100% for a right atrial focus, and a positive or negative-positive P wave in V1 demonstrated a sensitivity of 100% for a left atrial focus. Potential limitations of this algorithm include possible inaccuracies in distinguishing between the left and right PVs and localizing tachycardias close to the interatrial septum and that it considers the CT as a single location.
A refinement of the P wave algorithm based on a computational simulation study aimed to improve this algorithm’s ability to distinguish between various focal origins along the CT.27 If the peak of the P wave in lead III is positive with the amplitude greater than that in V1, then the origin is in the superior CT. In addition, the larger the difference, the more superior the excitation. If the peak P wave in lead III is negative or has a smaller amplitude than the positive peak of V1, then the excitation origin is in the inferior CT.
A newer P wave morphology algorithm was designed to further improve the localization of focal AT.28 A combination of the inferior ECG leads along with the aVR lead and regrouping of atrial sites of origin into two areas that had similar ECG patterns (high atrial and right low septal origins) improved predictive accuracy compared with the algorithm by Kistler et al.26 Positive P waves in inferior leads and a negative P wave in the aVR lead indicated high atrial origins (high CT, superior PVs, and right atrial appendage [RAA]), and negative P waves in inferior leads and a positive P wave in the aVR lead suggested right low septal origins (coronary sinus [CS] ostium and inferior tricuspid annulus).
Noninvasive electrocardiographic imaging or electrocardiographic mapping (ECM) is a promising tool for diagnosis of tachycardia origin and could be useful in procedure planning before catheter ablation.29 As noted previously, the standard 12-lead surface ECG remains a useful tool for providing an initial approximation of the site of origin of AT, but it has numerous limitations. Body surface potential mapping incorporates a much larger number of electrodes, but does not provide anatomic information. The recent development of electrocardiographic imaging represents further advancement in high-resolution noninvasive mapping by combining body surface electrodes and heart-torso geometric information to produce detailed electroanatomic maps of the epicardial surface through the application of inverse solution mathematical algorithms. This methodology has permitted accurate localization of focal, microreentrant, and macroreentrant ATs.30–32
Roten et al.32 described a case report in which ECM was performed before an ablation procedure in a patient who developed AT 2 years after bilateral pulmonary transplantation. Tachycardia origin was located in a scar region medial to the anastomosis of the left inferior pulmonary donor vein. ECM matched with results of invasive electroanatomic (Carto) mapping and predicted both tachycardia mechanism and origin. In this case, tachycardia mechanism was microreentry from a scar region at the medial anastomosis of the left inferior pulmonary vein, as more than 75% of the tachycardia cycle length was recordable within a few millimeters.
Atrial Tachycardia Arising from the Crista Terminalis
Atrial tachycardias can be located all along the CT, which has been described as a “ring of fire” because it is a common location for ATs in patients without structural heart disease, accounting for two thirds of right ATs in one study (Figures 73-5, 73-6). One physiological factor contributing to the clustering of ATs in this area is that the CT demonstrates marked anisotropy because of poor transverse cell-to-cell coupling, which could contribute to slow conduction and microreentry. Another contributing factor is that the CT contains clusters of cells with automaticity. The ECG characteristics of these tachycardias were described earlier and depend on site of origin from the CT (e.g., superior versus inferior). Intracardiac echocardiography can be useful to demonstrate the close anatomic proximity of the tachycardia focus to the crista during mapping. Ablation of tachycardias arising from the superior CT carries a small risk of damage to the phrenic nerve resulting in diaphragmatic paralysis.
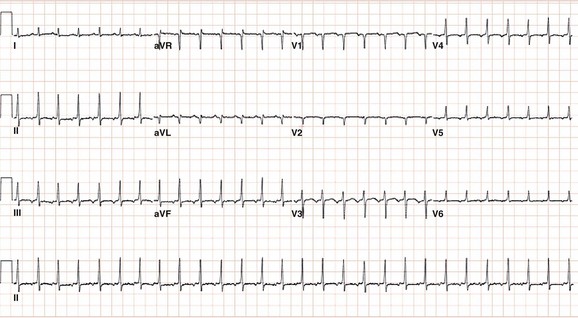
Figure 73-5 Electrocardiogram during atrial tachycardia arising from the crista terminalis in a 60-year-old female patient who developed a tachycardia-induced cardiomyopathy. Note that the P wave morphology is positive in lead aVL and biphasic (positive/negative) in lead V1 consistent with a right atrial focus.
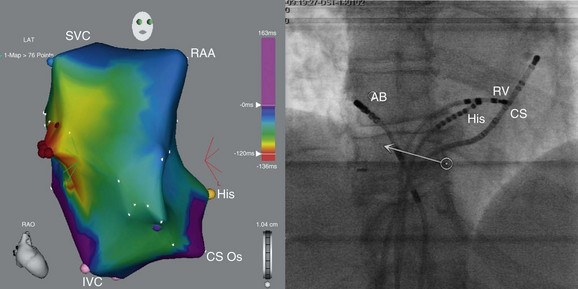
Figure 73-6 Electroanatomic map of the right atrium (right anterior oblique projection; left panel) and fluoroscopic image (left anterior oblique projection; right panel) obtained during mapping and ablation of a focal atrial tachycardia arising from the crista terminalis (electrocardiogram during this tachycardia is shown in figure 38-5). Red dots indicate the site of successful tachycardia ablation. The patient’s cardiomyopathy resolved after successful ablation of this tachycardia. AB, ablation catheter; His, His bundle catheter; SVC, superior vena cava; IVC, inferior vena cava; CS Os, coronary sinus ostium; RAA, right atrial appendage; RV, RV catheter).
Atrial Tachycardia Arising from the Tricuspid Atrioventricular Annulus
The tricuspid annulus probably represents the second most common location of right-sided ATs. In several series, tachycardias arising from the tricuspid annulus accounted for 13% to 22% of right ATs.33 Tricuspid annular AT has negative or notched P waves in V1 and invariably positive P waves in lead aVL. Reports describe foci from around the entire circumference of the tricuspid annulus. Inferior foci tend to have negative P waves in leads II, III, and aVF, whereas superior foci are usually isoelectric or positive in these leads. Foci from the superior tricuspid annulus and the RAA have similarity P wave morphologies, consistent with their close proximity. The presence of myocytes with AV nodal-type electrophysiologic characteristics around the entire tricuspid annulus has been described in animals.34 These cells are histologically similar to the atrial cells but resemble nodal cells in their cellular electrophysiology, response to adenosine, and lack of connexin43. These cells can serve as the substrate for AT originating from around the tricuspid annulus.
Atrial Tachycardia Arising from the Right Atrial Appendage and Superior Vena Cava
The RAA is an uncommon site of origin for AT (<5% of ectopic ATs in several series), although both appendages are a more common site for incessant ATs.35–37 The appendage is composed of ridges formed by pectinate muscles, which arise from the CT. The characteristic electrocardiographic pattern associated with RAA tachycardia shows negative P waves in lead V1 that become progressively positive across the rest of the precordial leads, along with upright P waves in the inferior leads, positive or isoelectric-positive in lead I, and an inverted P wave in lead aVR. As with other right ATs that arise from the superior crista, RAA can be confused with sinus tachycardia. At least one case series has suggested that RAA tachycardias arise more commonly in younger male patients and can manifest as an incessant tachycardia resulting in left ventricular dysfunction secondary to tachycardia-induced cardiomyopathy.35,38
Catheter ablation of focal RAA tachycardia is relatively straightforward and has high success rates.35,38 However, there are at least two case reports of RAA tachycardias that were more challenging to eliminate with catheter ablation. One case of RAA tachycardia that which originated in the inferior/lateral aspect of the appendage could not be ablated despite multiple attempts using manual catheter ablation, but it was successfully ablated using magnetic navigation.39 Another case of an AT that originated at the apex of the RAA was resistant to catheter ablation and required surgical right atrial appendectomy to eliminate the tachycardia.40
The SVC is an uncommon site of origin for focal ATs (<2%).41,42 Cardiac muscle extends for a distance into the SVC in human hearts, and the electrophysiological characteristics of the SVC and RA muscle are similar. AT originating in the SVC can arise from 1 to 3 cm above the SVC-RA junction and conduct to the right atrium in a 1 : 1 manner or with variable conduction delay or block. AT arising from the area of the SVC demonstrates a P wave morphology that is positive in leads I, II, III, and aVF, isoelectric or negative in lead aVL, biphasic (positive then negative) in lead V1, and positive or isoelectric in leads V2 to V6 (Figures 73-7, 73-8). Radiofrequency (RF) catheter ablation of SVC foci is usually successful in eliminating tachycardia. Rather than directly targeting the AT focus in the SVC, an alternative strategy is electrical disconnection of the SVC muscle sleeve at the SVC-RA junction in a circumferential or segmental fashion or isolation of the arrhythmogenic area from the rest of the SVC. Careful attention should be paid to avoid injury to the phrenic nerve during ablation in this region, and complete SVC isolation is best avoided because of the risk of SVC stenosis.43 The SVC also has been reported as having a role in arrhythmia initiation and maintenance in 5% to 10% of patients with paroxysmal AF.44 Fibrillatory conduction from a focus in the SVC with exit block to the right atrium masquerading as a focal right AT also has been reported.45
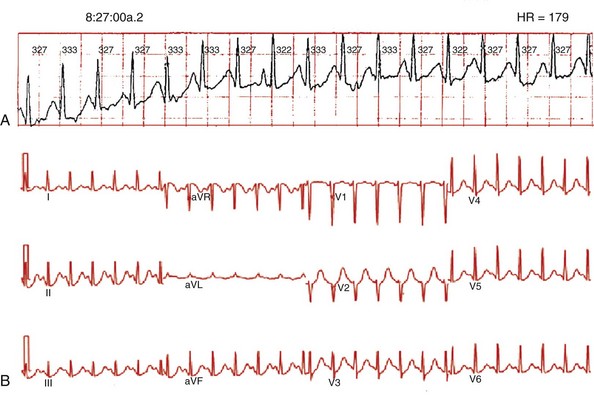
Figure 73-7 Spontaneous, ambulatory monitor recording (A, top panel) and electrocardiogram (ECG; B, bottom panel) during focal atrial tachycardia arising from the superior vena cava. The patient was a 25-year-old woman with recurrent episodes of highly symptomatic tachycardia at rates of 170 to 180 beats/min that were refractory to β-blockers, calcium channel blockers, and sotalol. During tachycardia, the P wave morphology is positive in leads I, II, III, and aVF, negative in lead aVR, isoelectric in lead aVL, biphasic (positive then negative) in lead V1, and positive or isoelectric in leads V2 to V6.
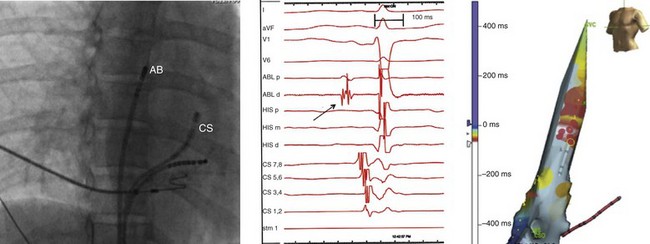
Figure 73-8 Fluoroscopic image (right anterior oblique projection; left panel), electrocardiographic and intracardiac electrograms (middle panel) and electroanatomic map (left anterior oblique projection; right panel) obtained during mapping and ablation of a focal atrial tachycardia arising from the superior vena cava (electrocardiogram shown in Figures 73-7). The electrogram recorded at the site of tachycardia origin (arrow, middle panel) preceded the onset of the P wave by 15 to 20 ms. Red dots on the electroanatomic map indicate a site of successful tachycardia ablation. The red dot with a yellow circle around it indicates the site of tachycardia termination during ablation along the medial aspect of the superior vena cava (SVC) 1 to 2 cm above the SVC–right atrial junction.
Atrial Tachycardia Arising from the Coronary Sinus
Focal ATs were reported to arise from the coronary sinus area in up to 10% of patients in one study.26 Most patients had tachycardia arising from outside the coronary sinus or just inside the os (Figures 73-9, 73-10).46 AT also arose from deep within the coronary sinus, presumably from the coronary sinus musculature, based on the observation that the tachycardia could not be ablated from the left atrial endocardium but could be ablated from within the coronary sinus.47 The arrhythmogenic substrate is believed to be the muscular sleeves on the epicardial atrial surface surrounding the coronary sinus and extending from the CS ostium to the lateral aspect of the mitral annulus. In human hearts, the CS musculature provides electrical connections to the LA most prominently at the proximal portion and activation from the RA can conduct to the LA via these electrical connections. A discrete potential is noted after the coronary sinus electrogram in sinus rhythm and preceding the surface P wave by 30 to 50 ms during tachycardia. Successful ablation sites typically have activation at least 20 ms before the onset of the P wave.47 Macroreentrant ATs can also involve the coronary sinus and require ablation on the left atrial endocardial surface as well as the coronary sinus.
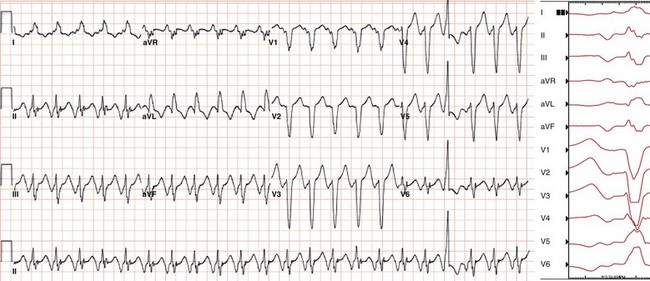
Figure 73-9 Electrocardiogram during focal atrial tachycardia arising from the coronary sinus ostium in a 60-year-old woman with an ischemic cardiomyopathy. The patient’s CRT-ICD recorded multiple episodes of supraventricular tachycardia that resulted in a loss of biventricular pacing. The atrial rate during tachycardia shown is 134 beats/min (450 ms). Note the P wave morphology during tachycardia resembles and may be confused with that of typical counterclockwise atrial flutter, as it is deeply negative in leads II, III, and aVF and markedly positive in lead aVR. Lead V1 is biphasic with an initial component that is isoelectric (when timed to onset of the P wave in inferior leads) followed by an upright component. Across the precordial leads, the initial component becomes more negative and the second component becomes isoelectric.
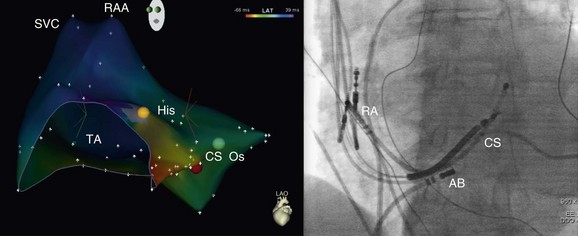
Figure 73-10 Electroanatomic map of the right atrium (left anterior oblique [LAO] projection; left panel) and fluoroscopic image (LAO projection; right panel) obtained during mapping and ablation of a focal atrial tachycardia arising from the coronary sinus os (electrocardiogram shown in Figure 38-9). The red dot indicates the site of successful tachycardia ablation at the coronary sinus os.
The electrocardiographic pattern of ATs arising from the CS musculature is somewhat typical, with deeply negative P waves in the inferior leads and positive P waves in aVR. As such, the morphology of the ECG during focal AT arising from CS can be similar to and can be confused with that of typical counterclockwise atrial flutter (AFL), which has an exit site at the CS ostium. Notably, tachycardia foci from the CS ostium have characteristic P wave morphologies in the precordial leads. Lead V1 is biphasic with an initial component that is either isoelectric or mildly inverted (when timed to onset of the P wave in inferior leads) followed by an upright component. Across the precordial leads, the initial component becomes more negative and the second component becomes isoelectric. Foci located within the body of the CS will have a P wave in V1 that is upright from the onset, without an isoelectric segment and P waves across the precordial leads that are frequently upright (Figure 73-11).
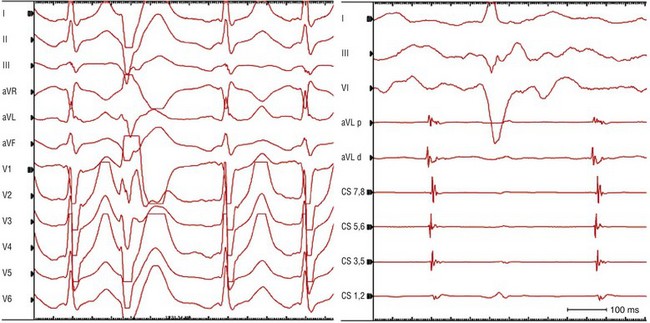
Figure 73-11 Electrocardiogram (ECG; left panel) and intracardiac electrograms (right panel) recorded during focal atrial tachycardia arising from the body of the coronary sinus. The patient was a 53-year-old man with recurrent episodes of adenosine-sensitive supraventricular tachycardia (SVT). During an electrophysiology study, typical slow–fast AV nodal reentrant tachycardia initially was induced. Following atrioventricular node slow pathway modification, another adenosine-sensitive SVT arose spontaneously during isoproterenol infusion. This latter SVT (cycle length, 430 ms) was demonstrated to be a focal atrial tachycardia. Note that a catheter-induced PVC (second beat on ECG at left) during tachycardia allows one to uncover the “true” P wave morphology, which is negative in the inferior leads and upright positive in V1. Earliest activation during tachycardia was recorded on the coronary sinus (CS) catheter at poles 5 and 6. An ablation catheter placed in the mid CS body recorded an electrogram that preceded CS activation and the onset of the P wave by 15 to 20 ms. Note that the electrogram recorded on the ablation catheter reveals two components: a near-field component (sharper, larger amplitude electrogram) from CS musculature that precedes a far-field component (lower amplitude and frequency) from left atrial myocardium consistent with an origin of atrial tachycardia in the CS musculature.
Atrial Tachycardia Arising from the Atrial Septum and Para-Hisian Regions
Focal ATs originating in the area of the atrial septum in the vicinity of the atrioventricular node include AT arising from the anterior, mid-, and posterior septal regions and from the apex of the triangle of Koch (e.g., the para-Hisian region).48–52 These tachycardias, like ones arising from near the tricuspid and mitral annuli, tend to be more sensitive to lower doses of adenosine than ATs arising from the CT. In addition, in one study, 45% of patients with a septal tachycardia required isoproterenol for tachycardia induction, compared with 32% of patients with AT originating in the right atrial free wall.
Atrial tachycardias can originate from near the apex of the triangle of Koch (i.e., anteroseptal tricuspid annulus, in close proximity to the His bundle recording) in 10% or more of patients with tachycardias arising from the right atrium. A location is considered para-Hisian when either a His deflection is observed at the site of earliest atrial activation during tachycardia or the successful ablation site along the tricuspid annulus is within 1 cm of a site recording the His bundle potential.52
It has been proposed that para-Hisian ATs have properties consistent with AT arising circumferentially along the tricuspid annulus, and as such should be considered a subset of this broader group of annular ATs.52 Atrial tissue surrounding the AV annuli is specialized and distinct from other atrial myocytes. Periannular cells are similar to atrial cells histologically, but can resemble AV nodal transitional cells electrophysiologically. There is controversy in the literature regarding the pharmacologic behavior and mechanism of these para-Hisian ATs. Some reports indicate that these tachycardias are adenosine and verapamil-sensitive, whereas others report that they are adenosine-insensitive.53 Some reports of ATs from the apex of the triangle of Koch suggest reentry as the underlying mechanism, whereas others argue that triggered activity is the most likely potential mechanism.52,54
ECG findings suggestive of a para-Hisian AT include narrowing of the P wave during AT compared with sinus rhythm, and biphasic P waves in V1, with the dominant positive or negative component having a polarity opposite to that observed in the inferior leads (Figures 73-12, 73-13). Some but not all reports suggest that a left-sided para-Hisian AT origin is associated with positive P waves in lead V1.50,51 The P waves are consistently positive or isoelectric in leads I and aVL. The P wave morphology in the inferior leads may show two distinct patterns: (1) negative or biphasic with terminal negativity or (2) positive or biphasic P waves and terminal positivity. The P wave morphology in the inferior leads during tachycardia is determined largely by the route of left atrial activation: via the posteroinferior input from the coronary sinus versus via the Bachmann bundle.
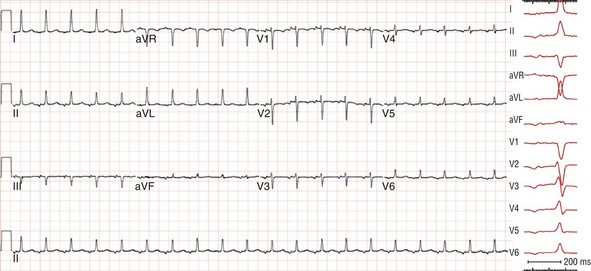
Figure 73-12 Electrocardiogram recorded during focal atrial tachycardia arising from the atrial septum in the para-Hisian region. The patient was a 75-year-old woman with an incessant long-RP supraventricular tachycardia at rates of 110 to 120 beats/min refractory to β-blockers that terminated with vagal maneuvers or adenosine. Note that the P wave during tachycardia is narrow (<80 ms), and its morphology is isoelectric in lead I and biphasic in lead V1 and the inferior leads.
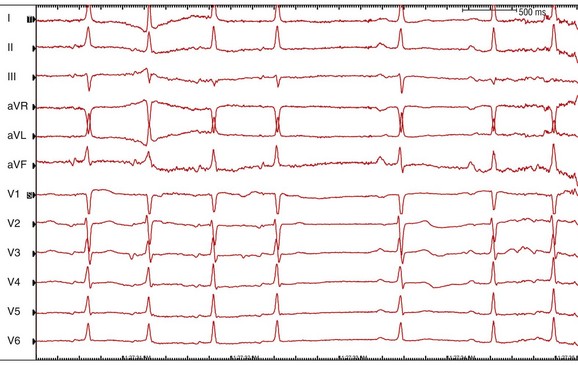
Figure 73-13 Electrocardiogram during termination of focal atrial tachycardia arising from the left atrial septum (see Figures 38-12, 38-14) in response to 6 mg of intravenous adenosine. Note the slowing of tachycardia rate without development of AV block followed by termination of tachycardia consistent with an atrial tachycardia. The P wave during tachycardia is much narrower than that during sinus rhythm consistent with an origin in the atrial septum.
Catheter ablation of this form of AT originating in the perinodal region is safe and effective. In most patients, these tachycardias can be ablated without damage to AV nodal conduction. In a recent detailed multicenter report of focal ATs arising exclusively from the para-Hisian region in 38 patients, catheter ablation was attempted in 30 patients and was successful in 26 (87%).52 Notably catheter ablation was not attempted in eight patients because of patient preference in avoiding the potential risk of complete AV block or left heart catheterization. RF energy was applied in 22 patients, and cryoenergy was applied in eight patients. Two patients had transient 2 : 1 AV block that resolved during the procedure. Two other patients had a prolonged PR interval after ablation.
In cases of para-Hisian AT, it is important to map both the right and left atrial septum (and the noncoronary cusp; Figure 73-14). Atrial activation mapping on the right side alone will not differentiate between a right and left atrial site of tachycardia origin, implying rapid direct transseptal conduction by way of the Bachmann bundle, which results in an early right-sided breakthrough. In one series of 16 patients with the earliest atrial activation in the triangle of Koch, a left atrial focus was found in nearly 40% of patients.
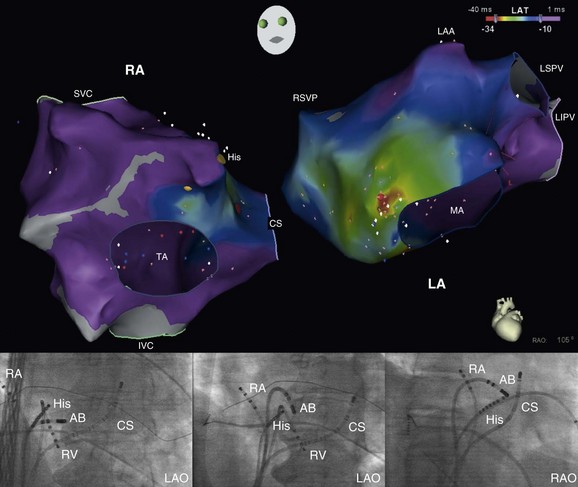
Figure 73-14 Biatrial electroanatomic map (right anterior oblique projection; upper panel) and fluoroscopic images (lower panels) obtained during mapping and ablation of a focal atrial tachycardia arising from the left side of the atrial septum in the para-Hisian region (electrocardiogram and electrograms are shown in Figures 73-12). The earliest activation in the right atrium during atrial tachycardia was recorded in the midseptum (red dot on the right atrial map on the left with the accompanying fluoroscopic image shown at the lower left in a left anterior oblique projection with the ablation catheter in the right atrium) less than 1 cm from the His bundle potential recording. Earliest activation and the site of successful tachycardia ablation was on the left side of the atrial septum (orange dot on the left atrial map on the right with accompanying fluoroscopic images shown in the left and right anterior oblique projections at the bottom with the ablation catheter in the left atrium). Note the close proximity of the ablation catheter in the left atrium to the His bundle catheter in the right atrium on the fluoroscopic images. The site of origin in the left atrium was less than 1.5 cm from the earliest activation site in the midseptum of the right atrium. Note that during tachycardia at a cycle length around 500 ms, there is rapid, biatrial activation in less than 50 ms, consistent with a focal atrial tachycardia arising from the atrial septum. RA, right atrium; LA, left atrium; LAA, left atrial appendage; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein.
There are a number of reports demonstrating that RF energy applications within the aortic sinuses of Valsalva can eliminate focal ATs originating from near the atrioventricular node or para-Hisian region.55–64 Of note, many of the patients in these reports had undergone multiple previous unsuccessful ablation procedures before successful ablation of ATs from the aortic sinuses. When earliest activation is recorded in the proximal electrode of the His-bundle catheter, but ablation in this region cannot eliminate tachycardia, then consideration should be given to attempting ablation from within the aortic sinus of Valsalva.
Each of the aortic sinuses of Valsalva, is in contact with the atrial myocardium, which enables mapping and ablation of some ATs from the aortic sinuses of Valsalva. In a recent series, among 113 patients with AT, 13 patients had midseptal to anteroseptal AT and 12 patients (9%) underwent successful ablation from the noncoronary cusp (n = 10), right coronary cusp (n = 1) or left coronary cusp (n = 1) without complication.64 During long-term follow up, AT recurred in only one patient.
A few ATs have been reported to have been successfully ablated within the right or left sinus of Valsalva.61–63 In one study, a negative/positive or isoelectric P wave in leads I and aVL was supportive of a left sinus of Valsalva AT versus an NSV AT in which positive P waves in leads I and aVL were observed.
Analysis of electrograms can be helpful for identifying the correct positioning of the mapping and ablation catheter at the aortic sinuses of Valsalva during the ablation procedure. NSV electrograms during sinus rhythm have a larger atrial amplitude than ventricular amplitude, which differs from left and right sinus of Valsalva electrograms.63
Left Atrial and Pulmonary Vein Tachycardias
Focal tachycardias originating in the LA and pulmonary veins not associated with prior catheter ablation for AF form an important subset of all ATs, reportedly constituting 10% to 40% of all focal ATs.26,65 These tachycardias can occur in a wide spectrum of clinical settings ranging from structurally normal hearts to advanced cardiac disease and in patients who have undergone a cardiac or lung transplant.32,65,66 Catheter ablation is a generally successful treatment for these focal ATs, including those refractory to pharmacologic therapy. Left atrial and pulmonary vein tachycardias occur commonly in patients following ablation for AF, but will not be discussed here.
The pulmonary veins and mitral valve annulus are the main source of focal left ATs. A majority of pulmonary vein tachycardias originate from the ostium, rather than from deep inside the veins with a strong propensity for the superior veins. In addition, it is rare for ATs to recur from different sites in the pulmonary veins or for atrial fibrillation to develop after a pulmonary vein focal ablation.67–69 Therefore, unlike patients with PV triggers and atrial fibrillation in which there are usually multiple triggers in multiple veins and the pathophysiology involves a diffuse, chronic process often with extensive atrial remodeling, patients whose sole clinical arrhythmia is AT appear to have an isolated or focal process. As such, patients with focal left-sided AT are curable in the long term with focal ablation.
Superior (Bachmann bundle), inferior (coronary sinus), and septal (fossa ovalis) interatrial electrical connections exist between the right and left atria. Additional posterior electrical connections between the right-sided pulmonary veins and right atrial posterior walls exist in human hearts. This can compound the difficulty differentiating between an AT originating from the pulmonary veins and one arising from the posterior right atrium. Focal AT from the PV usually has a positive P wave across the precordium, from V1 to V6. Careful analysis of the P wave configuration in V1 may be helpful; the posterior right atrial sites have a biphasic P wave in V1, with a positive-negative configuration, and the right superior pulmonary vein sites have a positive P wave in V1 (Figure 73-15). A second differentiating feature is that P waves from the right superior pulmonary vein show notching in lead II in 80%, but are smooth in those coming from the region of the SVC.
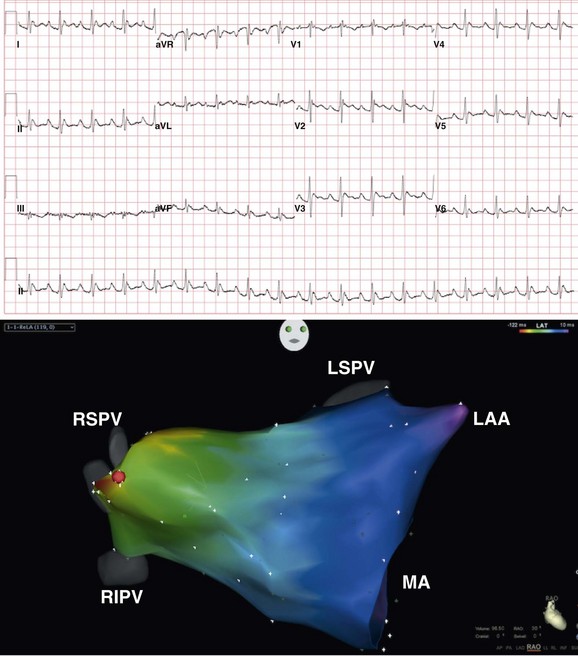
Figure 73-15 Electrocardiogram recorded during focal atrial tachycardia (top panel) arising from the ostium of the right superior pulmonary vein. Note that the P wave morphology during tachycardia is positive in lead V1, across the precordium and in leads, I, II, III, aVF, and aVL. Electroanatomic map of the left atrium (right anterior oblique projection; bottom panel) obtained during mapping and ablation of a focal atrial tachycardia arising from anterior surface of the left atrium at the ostium of the right superior pulmonary vein. A red dot indicates the site of successful tachycardia ablation. RIPV, right inferior pulmonary vein.
Analysis of electrograms recorded from the posterior right atrium, CS, and His bundle regions during AT often reveals two components consisting of near-field and far-field components, which can assist in determining whether the tachycardia originates in the right or left atria.70 In the right atrial electrograms, when the amplitude of the first component (near-field electrogram, a sharper electrogram) is higher than that of the second component, the AT originates in the right atrium. Likewise, analysis of CS electrograms consisting of a near-field component from CS musculature and a far-field component from left atrial myocardium can uncover LA activation preceding right atrial activation. Similarly, near-field and far-field components from RA and LA activation can be identified on the His bundle atrial electrograms.70 A near-field electrogram followed by a far-field signal on the His bundle electrogram during AT suggests a probable right atrial focal AT, whereas a far-field followed by a near-field electrogram sequence predicts the need for LA access.
Atrial Tachycardia Arising from the Mitral Atrioventricular Annulus
The mitral annulus is the second most common location of focal AT in the LA, after the pulmonary vein region. Interestingly, there appears to be a clustering of these foci around the superior aspect of the mitral annulus in immediate proximity to the region of the left fibrous trigone at the aortic-mitral continuity. A superior mitral annular location (as well as a location along the left side of the atrial septum or at the noncoronary cusp) should be considered for AT when the earliest right atrial activity is recorded in the para-Hisian region (approximately 0 to 20 ms before P wave onset) and the P wave demonstrates a characteristic morphology consisting of a biphasic (negative followed by positive) appearance in the precordial leads and a low amplitude appearance in the limb leads.71,72 Lead aVL is negative or isoelectric, and the inferior leads are positive during ATs arising from the superior mitral annulus. These ECG findings have a sensitivity of 88% and a specificity of 99%. The initial negative component of the P wave in lead V1 is unusual for left ATs, but it can be explained by the relatively anterior position of the mitral annulus in this region. Focal ablation of these tachycardias usually is successful. It is speculated that remnants of the developing specialized conduction system at the mitral annulus-aorta junction could be the underlying substrate of this arrhythmia.71 Myocardial cells at the mitral annulus-aorta junction exhibit AV node–like electrophysiologic properties, respond to adenosine, lack connexin, and give rise to catecholamine-induced triggered activity owing to delayed afterdepolarizations.73,74
Atrial Tachycardia Arising from the Left Atrial Appendage
Atrial tachycardia can arise from the left atrial appendage (~2% of sustained ATs). LAA tachycardias are often incessant and are associated with tachycardia-mediated cardiomyopathy in some cases. The P wave during LAA tachycardias is deeply negative in leads I and aVL, which has a sensitivity of 92% and a positive predictive accuracy of 92% with a specificity of 97%, highly positive in the inferior leads, and broad and positive in lead V1.75,76 The atrial activation sequence typically shows activation in the distal coronary sinus earlier than the P wave.
A recent report indicated that focal AT originating from the distal portion of the left atrial appendage could be ablated successfully and acutely with RF energy in 13 of 14 patients, with no long-term recurrences in the 13 successful cases.77 However, in some reported cases, elimination of AT arising from the body or apex of the LAA is refractory to endocardial catheter ablation.78 This result is similar to what was described earlier for tachycardias arising from the RAA and is likely related to the complex, blind cul-de-sac, heavily trabeculated anatomy of the left atrial appendage that makes it difficult to manipulate catheters safely or to apply sufficient contact force at the LAA apex. Rarely, a left atrial appendage tachycardia will need to be ablated epicardially, eliminated via surgical left atrial appendectomy, or abolished via thoracoscopic appendage exclusion.79–83
Inappropriate Sinus Tachycardia
The uncommon, poorly characterized entity of inappropriate sinus tachycardia is probably a heterogeneous disorder. The hallmark feature of this syndrome is a consistently elevated resting heart rate (>95 beats/min) and an exaggerated heart rate response to low levels of physical activity. The average sinus rate during 24-hour Holter monitoring usually is greater than 90 beats/min, and a persistent increase of at least 25 beats/min occurs when arising from supine to an upright position, in the absence of orthostatic arterial hypotension. Some affected patients have an elevated intrinsic heart rate, and others have increased responsiveness to infusions of isoproterenol. The ECG characteristics of this tachycardia include an identical P wave configuration in tachycardia and sinus rhythm. It is likely that some patients have primary autonomic abnormalities, including postural orthostatic tachycardia syndrome in some. Some patients may have primary abnormalities of the sinus node. A recent analysis of 18 patients with inappropriate sinus tachycardia receiving intravenous adenosine (0.1 to 0.15 mg/kg) showed that these patients demonstrated a blunted response, with less sinus cycle length prolongation than age-matched control subjects undergoing electrophysiology studies.84 The blunted response was also seen after pharmacologic autonomic blockade. These observations suggest that structural abnormalities of the sinus node are responsible for this syndrome. A recent report85 suggested that β-adrenergic receptor autoantibodies are involved in the mechanism of inappropriate sinus tachycardia. In the electrophysiology laboratory, a distinction should be made between inappropriate sinus tachycardia and a focal automatic AT with an origin close to the superior CT.85
Electrophysiological Differential Diagnosis of Focal Atrial Tachycardia
Adenosine administration is a useful diagnostic tool in patients with supraventricular tachycardia.86 In a report from Scheinman et al., 229 patients with supraventricular tachycardia during an electrophysiology study received intravenous boluses of 6 to 18 mg of adenosine. For patients with AT (n = 53), no consistent correlation was found between the response to adenosine and the location of the atrial focus. Termination or suppression of tachycardia with adenosine was observed in 56% of patients, but was not helpful for differentiating automatic tachycardias from triggered or reentrant tachycardias. Only patients with ATs demonstrated atrial cycle length oscillations (23%) before suppression with adenosine. AV block after adenosine was observed only in patients with AT, but it occurred uncommonly, being noted in only 27% of patients. Termination of AT renders the presence of a macroreentrant AT highly unlikely.
A variety of pacing maneuvers have been reported to be useful for the differentiation of AT from other forms of supraventricular tachycardia87,88 (Tables 73-1, 73-2). Ventricular pacing can be useful for differentiating AT from AV nodal reentry tachycardia and AV reentry tachycardia. Ventricular burst pacing for at least 3 to 6 beats at a cycle length shorter than the tachycardia cycle length results in tachycardia termination, entrainment of the tachycardia, or dissociation of the ventricle from the tachycardia. If the ventricle is dissociated from the tachycardia, a bypass tract is excluded. If burst pacing reproducibly terminates tachycardia without conduction to the atrium, AT is excluded as the mechanism.
Table 73-1
Prevalence and Diagnostic Value of Baseline Observations and Tachycardia Features
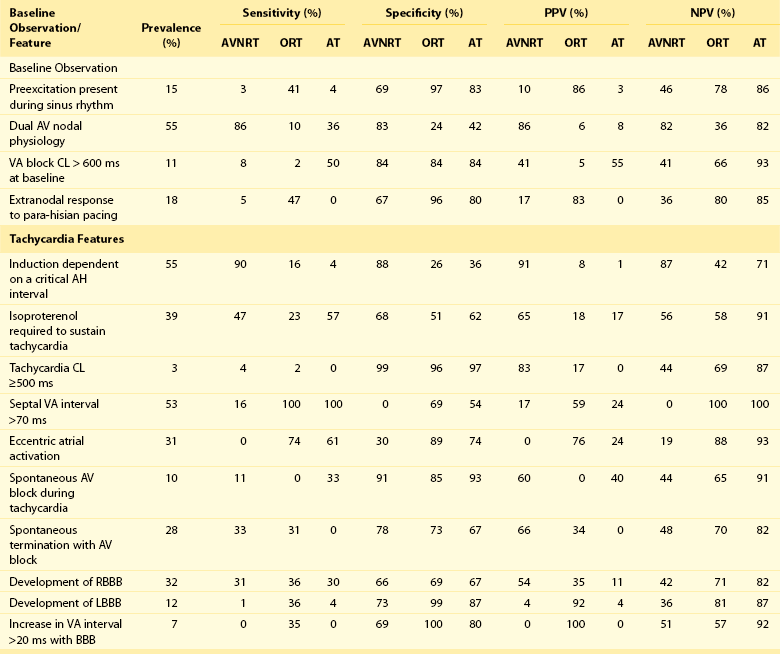
From Knight BP, Ebinger M, Oral H, et al: Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. J Am Coll Cardiol 36:574-582, 2000.
Table 73-2
Diagnostic Value of Pacing Maneuvers during Supraventricular Tachycardia
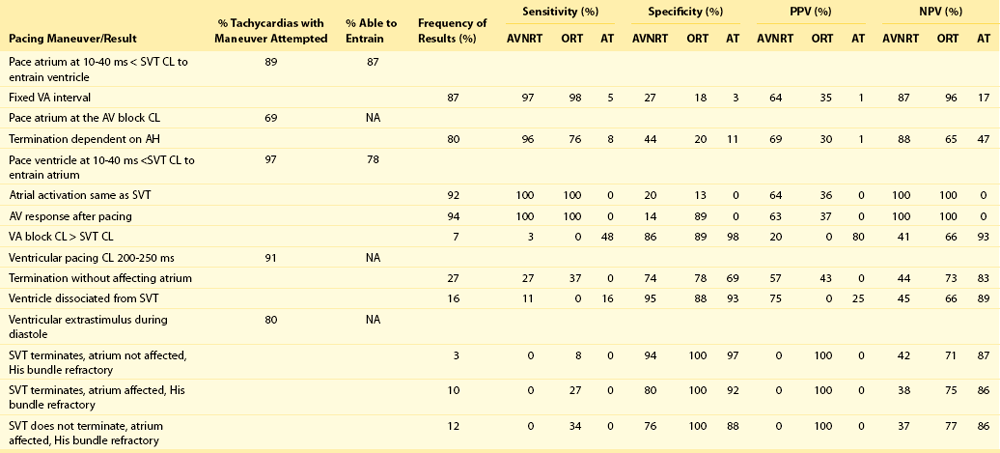
From Knight BP, Ebinger M, Oral H, et al: Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. J Am Coll Cardiol 36:574-582, 2000.
Tachycardia cycle length variability of 15 ms or more associated with a change in atrial cycle length that predicts a change in ventricular cycle length favors either AT or atypical AVNRT. A change in atrial cycle length that is preceded by the change in the preceding cycle length or H-H interval is suggestive of AVNRT or orthodromic reciprocating tachycardia.89
The postpacing interval response to atrial overdrive pacing during tachycardia also may be useful to localize the site of origin of focal AT. Mohamed et al.90 performed atrial overdrive pacing during AT at a rate slighter faster than the tachycardia rate. Measurement of the postpacing interval minus the tachycardia cycle length (PPI-TCL) from various sites in the right and left atria localized the tachycardia focus when the PPI-TCL was minimized (e.g., for the successful ablation site, 11 ± 8 ms). The investigators explained this observation by hypothesizing that the difference between the PPI and the TCL was proportional to the distance of the pacing site to the tachycardia focus and conduction time through the surrounding or perifocal tissue. In this study, overdrive suppression of the AT focus was not apparent. The PPI-TCL was never greater than 20 ms at a successful ablation site, suggesting minimal slowing by tissue near the atrial focus.
Macroreentrant Atrial Tachycardia
Classification
Macroreentrant ATs can be divided into those that are isthmus dependent (i.e., dependent on conduction through the tricuspid annulus–inferior vena cava isthmus) or not isthmus dependent.1 A majority of macroreentrant ATs in the adult population are isthmus dependent. A majority of non–isthmus-dependent macroreentrant ATs are related to an area of scar, often from a previous surgical incision. Some examples of macroreentrant ATs include typical atrial flutter, lower loop reentry, double-wave reentry, macroreentrant AT from the right atrial free wall, and macroreentrant ATs occurring in the right or left atria related to previous surgery, including atriotomy, surgical scarring, and the presence of septal prosthetic patches, suture lines, or other anatomic obstacles. An increasing frequency of macroreentrant left ATs is currently being seen after atrial fibrillation ablation. Some unusual causes of macroreentrant tachycardia include reentry at sites of donor recipient anastomosis from either orthotopic heart transplantation or lung transplantation.91 Recently there has been increased recognition of macroreentrant ATs arising from scarring in patients who have not undergone previous atrial surgery or catheter ablation. Such spontaneous scarring has been described in the posterior wall of the LA, as well as in the posterolateral and lateral right atrium, and is recognized as part of an atrial cardiomyopathy.92,93 The successful ablation electrogram in these cases is frequently characterized by a long fractionated signal, middiastolic signal, broad, fragmented signal that encompasses much of the tachycardia cycle length.
Diagnostic Maneuvers
Electrophysiological characterization, mapping, and ablation of macroreentrant AT has been performed using standard electrophysiological techniques to demonstrate entrainment by recording diastolic atrial potentials from an area of slow conduction. They extrapolated data from animal studies showing that anatomic barriers resulting from residual atrial scars provide the substrate for atrial reentry. Multiple endocardial recordings were made to define the protected zone of slow conduction, generally using standard techniques of entrainment. For example, potential target sites for ablation were defined by determining whether pacing from that site was associated with concealed entrainment. Atrial mapping and pacing were performed to look for the first PPI within 10 to 30 ms of the TCL (Figure 73-16). When a postsurgical structural abnormality was present or a natural anatomic barrier (e.g., venous orifice, valve annulus) was nearby, mapping was more detailed in that region. In contrast with focal AT ablation, in which a single site of origin can be ablated to terminate tachycardia, macroreentrant AT usually requires ablation performed over a larger region, often defined by anatomic barriers or by adjacent scars separated by an isthmus of tissue serving as a critical area of slow conduction. Often these sites are characterized by double potentials or areas with low-voltage, long-duration, and more fractionated electrograms. Cycle length variability (greater than 15%) is rarely seen with macroreentrant AT, but it is not uncommon with focal AT. Atrial macroreentrant tachycardias also occur less commonly in patients without previous cardiac surgery, catheter ablation, or obvious structural heart disease. In these patients, electrically silent areas act as barriers for tachycardia. Like focal ATs, macroreentrant ATs generally incorporate several “typical” areas as part of the reentrant circuit. For example, the most common site involved in the right atrium is the isthmus between the tricuspid valve and the inferior vena cava and in the LA the mitral isthmus, roof, or atrial septum.
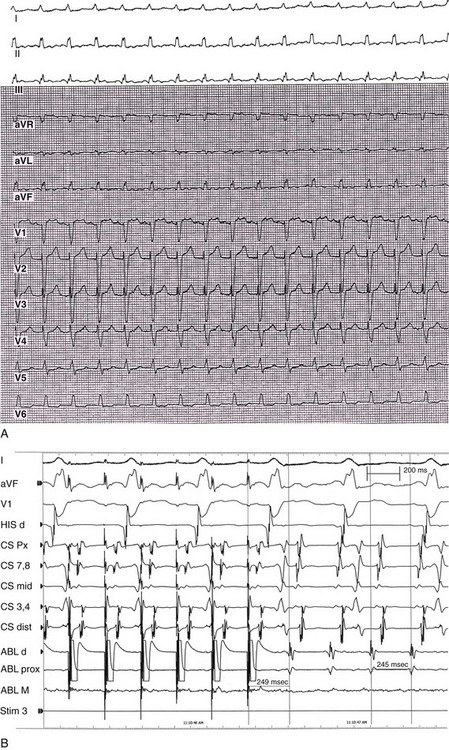
Figure 73-16 Entrainment of macrorentrant atrial tachycardia from the coronary sinus. A 12-lead electrocardiogram (A) and intracardiac tracings (B) are shown. Surface electrocardiographic tracings; intracardiac recordings are shown with pacing from an ablation catheter in the distal coronary sinus region. The postpacing interval minus the tachycardia cycle length is 4 ms. Pacing from other sites in the left atrium and in the right atrium was associated with much longer postpacing intervals.
The advent of computer mapping techniques has allowed a more precise and rapid identification of arrhythmia mechanisms. A second approach to studying the mechanism of macroreentrant ATs has focused on the concept of macroreentry around a scar or previous surgical incision. These new technologies have allowed anatomic reconstruction of the cardiac chambers with recording of local activation times and identification of atrial scar tissue, allowing for the construction of isochronal maps of a substantial portion of or the entirety of the tachycardia circuit in many patients. Nakagawa et al.94 made a fundamental contribution in this area with a study of 16 patients who had congenital heart disease surgery and underwent mapping and ablation of right atrial macroreentrant tachycardias. These workers mapped 15 macroreentrant ATs in 13 patients using the CARTO electroanatomic mapping system. First, they noted that all macroreentrant ATs propagated through narrow channels (<2.7 cm) defined by the mapping system as an area of decreased electrogram voltage between two close dense or thin scars. These workers coined the phrase “no channel–no macro-reentry.” Electrogram morphology, electrogram amplitude, and electrogram timing did not differentiate between sites within and those outside a narrow channel. Entrainment mapping failed to differentiate sites within the circuit but outside the channel (“outer loop” sites) from sites within the circuit and within the channel. Entrainment pacing was not done in many of the patients because it often resulted in the induction of a new tachycardia or in the termination of the original tachycardia. Additional limitations to entrainment mapping include difficulty measuring PPIs because of low amplitude or absence of atrial potentials in scarred atria or inability to pace the tissue. To overcome this limitation, measurements of the PPI-TCL can be made based on the N + 1 difference, or from recording sites remote from the pacing electrode.95 Entrainment mapping can also induce atrial fibrillation. Finally, the investigators were able to record double potentials during atrial pacing at slow rates, suggesting fixed sites of anatomic block. In general, it appears that the size and location of the scars or associated natural anatomic barriers (e.g., valve annulus, SVC, pulmonary veins, or inferior vena cava) determine the size and properties of the tachycardia circuit.
The sensitivity and specificity of classical criteria for concealed entrainment for identification of a critical isthmus are based on studies performed during ventricular tachycardia. The sensitivity and specificity of these criteria for defining entrainment in the atrium were examined by Morton et al.96 They used the model of typical atrial flutter and performed entrainment mapping from seven sites at four different cycle lengths. Concealed entrainment identified any isthmus site with 100% sensitivity with the flutter cycle length minus 10 ms but with a specificity of only 54%. Specificity increased to 98% with pacing at flutter cycle length minus 40 ms, but the sensitivity was decreased to 65%. At the flutter cycle length minus 30 ms, sensitivity was 85% and specificity was 90%. Concealed entrainment was seen from a nonisthmus site in 5 of 10 patients. Another caveat is that pacing at cycle lengths more than 10 to 20 ms faster than the tachycardia could results in PPIs that are higher than expected because of local pacing latency.97 These findings highlight the observation by Nakagawa et al.94 that targeting sites for ablation based solely on entrainment will result in a less than ideal success rate. Nevertheless, the techniques used by Nakagawa et al.94 have limitations as well, including the need for meticulous attention to labeling of points acquired by the electroanatomic mapping system, differentiation of electrograms with low voltage from poor contact, selection of the fiducial point on the electrogram for timing, and sensitivity of the map to incorrect labeling of electrograms. These findings underscore the difficulties of relying on any one criterion for defining sites of ablation of these complex macroreentrant tachycardias. In general, current approaches that combine the definition of anatomy with electrophysiologic mapping data are warranted for attempted ablation of these complex arrhythmias.
Electrocardiographic Characterization of Macroreentrant Atrial Tachycardia
The surface ECG is of limited value for precise anatomic localization of macroreentrant tachycardias. Its use certainly is much less straightforward and has many more limitations than electrocardiographic localization of focal ATs using P wave morphology. The ECG is most useful for establishing a diagnosis of typical counterclockwise AFL. Electrocardiographic localization of the macroreentrant tachycardia circuit is influenced by altered atrial anatomy, previous surgical incisions, and conduction of the atrial wavefront. For example, clockwise and counterclockwise atrial flutters are characterized by P waves of opposite polarity in the inferior leads. The tachycardia circuit encompassed by these two flutters is the same, but the P wave polarity is affected by the direction in which the atrial wavefront travels (e.g., craniocaudal or reverse direction) and the route of left atrial activation. Macroreentrant circuits near the subeustachian isthmus can result in P wave morphology similar or identical to that seen with typical atrial flutter.96
Atypical AFL from the right or left atria has no uniform electrocardiographic characteristics (other than being different from the stereotypic appearance of typical right AFL).98 An important point is that once the patient has had a previous ablation, the electrocardiographic appearance of the P wave morphology can be altered.99 Furthermore, a focal AT that arises near an area of previous ablation can appear electrocardiographically like a flutter because of the altered atrial activation sequence caused by the line of block.
Despite the known limitations, a number of studies have attempted to address whether there are general clues from the 12-lead ECG that might assist the clinician in the localization of an atrial macroreentrant circuits.100,101 V1 is the most useful lead for distinguishing left from right atrial origin, but much overlap exists. A broad-based upright V1 is predictive of a left-sided flutter. In addition, a negative P wave in lead aVL and low amplitude of the P waves in inferior leads are more consistent with left than right AFL. Conversely, when V1 is deeply inverted, it is suggestive of a right-sided flutter. In addition, right atrial macroreentrant AT tends to have a higher incidence of negative polarity in at least one precordial lead compared with LA macroreentry.
Left-sided atrial flutter originating from the posterior or lateral wall of the LA and involving the left pulmonary veins is characterized by coronary sinus activation that usually proceeds from the distal or mid coronary sinus to the proximal coronary sinus and demonstrates craniocaudal activation. Miyazaki et al. described a simplified approach to the rapid electrophysiological differentiation of left and right ATs.102 Entrainment was performed at three sites: the high right atrium, proximal coronary sinus, and distal coronary sinus. PPI-TCL less than versus greater than 50 ms at the high RA distinguished RA from LA reentrant circuits. PPI-TCL was less than 50 ms for right atrial circuits and greater than 50 ms from the high lateral right atrium for left atrial circuits. For left atrial circuits, the PPI-TCL difference at the proximal and distal coronary sinus greater than versus less than 50 ms differentiated perimitral reentry, from reentry involving the right pulmonary veins and septum. If the PPI-TCL, the difference was less than 50 ms from the proximal coronary sinus but greater than 50 ms at the distal coronary sinus, then the tachycardia was likely to involve the right pulmonary veins and septum (see Figure 73-16). The predictive accuracy for determining the successful ablation region was 93%.
Specific Types of Macroreentrant Atrial Tachycardia
Left Atrial Macroreentrant Flutters
A number of laboratories have characterized left atrial macroreentrant circuits, or “left atrial flutters.” In two early studies, macroreentrant left atrial flutters were mapped with the CARTO electroanatomic mapping system and were ablated successfully in more than 70% of patients.103,104 Most patients had structural heart disease, and 14% to 32% had mitral valve disease. Multiple left atrial scars and channels are typical. The most frequent locations are between an atriotomy scar and the mitral annulus. The investigators described macroreentrant ATs as having a continuous sequence of atrial activation, with adjacent earliest and latest activation and the range of activation times occupying 90% or more of the tachycardia cycle length. Entrainment mapping was done in some patients to locate a critical isthmus of slow conduction. A right atrial macroreentrant circuit was excluded by showing that the PPI in the right atrium was longer than the tachycardia cycle length by 40 ms or longer from three or more sites in the right atrium, including the cavotricuspid isthmus and right atrial free wall. Spontaneous variation of greater than 100 ms was seen in the right atrium, with concomitant variations in the LA of less than 20 ms, and right atrial activation accounted for less than 50% of the arrhythmia cycle length from more than eight points during sequential catheter mapping. The left atrial flutters typically were characterized by large reentrant circuits that rotated around the mitral annulus, electrically silent areas, and lines of double potentials near acquired or natural anatomic barriers, such as the pulmonary veins (Figure 73-17). Electrically silent areas were defined as those with no recordable electrogram or an electrogram amplitude of less than 0.05 mV. A dual-loop reentrant circuit was defined for 74% of tachycardias, and a single-loop circuit was defined in the remaining 26% of tachycardias in one series of patients. In some studies, the mapping can be highly complex and involve up to four loops rotating simultaneously. The isthmuses described for left ATs may be wider and have greater voltages indicating thicker myocardium.
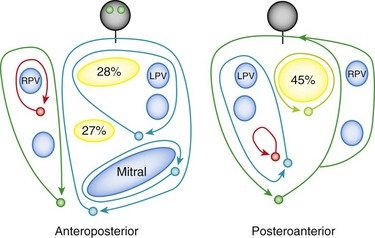
Figure 73-17 Schematic diagram of left atrial flutter circuits showing the location of silent areas and the percentage with circuits encountered in the left atrium. Nearly half of the silent areas were located in the posterior left atrium. LPV, Left pulmonary vein; RPV, right pulmonary vein. (From Jais P, Shah DC, Haissaguerre M, et al: Mapping and ablation of left atrial flutters. Circulation 101:2928-2934, 2000.)
Left AFL includes mitral annular AFL, pulmonary vein-related AFL, and left septal AFL. In some patients, the LA circuits are complex, with two or three loops rotating concomitantly. Electrically silent areas (“scars”) and anatomic obstacles (e.g., pulmonary veins, fossa ovalis, mitral annulus) have a critical role in the pathophysiology of these circuits. The mitral annular AFL macroreentrant circuit rotates around the mitral annulus, either counterclockwise or clockwise.105 The boundaries of the critical isthmus include the mitral annulus anteriorly and low-voltage zone or scars in the posterior or anterior LA walls. Radiofrequency ablation connecting the mitral annulus to an electrically silent area or an anatomic obstacle can cure this arrhythmia. The usual area of an ablation line at the isthmus between the left inferior pulmonary vein and the mitral annulus can eliminate this AFL. An alternative ablation line connecting the anterior–anterolateral mitral annulus with the left superior pulmonary vein for ablation of perimitral flutter has been suggested.106 Pulmonary vein–related AFL macroreentrant circuits can rotate around one or more pulmonary veins and a scar in the posterior wall or roof of the LA.107 Pulmonary vein reentrant circuits can be cured with ablation by creating a lesion from a pulmonary vein to the mitral annulus or to another pulmonary vein. The left septal AFL macroreentrant circuit rotates around the left septum primum, either counterclockwise or clockwise.108 The electrocardiographic characteristics are dominant positive P (“F”) waves in lead V1 and low-amplitude waves in the other leads. The critical isthmus is located between the septum primum and the pulmonary veins (posterior isthmus) or between the septum primum and the mitral annulus ring (anterior isthmus). Radiofrequency ablation of either the posterior or anterior isthmus can eliminate this left septal AFL. However, targeting the anterior isthmus might be more effective because the posterior isthmus may be thicker or more difficult to reach with a catheter.
Macroreentrant Atrial Tachycardia Following Cardiac Surgery
Markowitz et al.109 described a series of 10 patients with macroreentrant tachycardias after mitral valve surgery. Six tachycardias originated from the right atrium, four were from the LA, and one was biatrial. In 80% of patients, tachycardia circuits were defined by surgical atrial lesions or cannulation sites. Three macroreentrant tachycardias involved the left atrial septum and right pulmonary veins, with the tachycardia making a single reentrant loop in two patients and a figure-eight reentry in one patient involving a clockwise loop around an area of low voltage in the posterior wall and a counterclockwise loop around the mitral annulus with a central isthmus between two areas of low voltage. Of interest, the tachycardia had a focal origin in three patients.
Macroreentrant Atrial Tachycardia after Previous Atrial Fibrillation Ablation Procedures (Surgical or Catheter)
Atrial macroreentrant tachycardias after atrial fibrillation (catheter or surgical) ablation can be complex and is discussed in more detail in Chapters 78 and 123. These tachycardias typically are microreentrant or macroreentrant. The type of tachycardia observed after atrial fibrillation ablation depends significantly on the previous atrial fibrillation ablation strategy.110–112 Recurrent organized left ATs may be seen in 5% to 50% of patients and are more likely to be focal in patients with pulmonary vein isolation procedures and more likely to be macroreentrant in patients with linear lesions. Most patients with a focal origin for their tachycardia will have a site mapped to or near a reconnected pulmonary vein, whereas others have small-loop microreentry because of a focally ablatable small isthmus near a pulmonary vein. Typically, these patients will have fragmented, long-duration, and low-voltage electrograms, presumably owing to incomplete ablation of atrial tissue (e.g., gap-related) in their previous procedure (Figure 73-18). Others have been noted to have a macroreentrant tachycardia, which most commonly involves conduction across the mitral isthmus, the posterior wall, or gaps from previous ablation. The explanation for this abnormal atrial electrogram is believed to be altered intraatrial conduction and decreased left atrial voltage.113 Unusual forms of atrial macroreentry can occur after atrial fibrillation ablation, including circuits that incorporate the coronary sinus and its musculature, necessitating ablation inside the coronary sinus.114
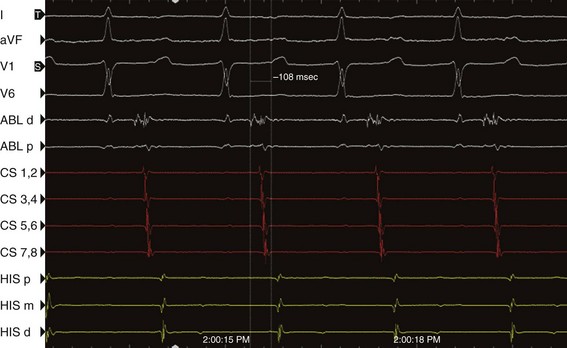
Figure 73-18 Surface (top) and intracardiac (bottom) recordings from a patient with a focal left atrial tachycardia at the base of the left atrial appendage who previously had undergone atrial fibrillation ablation. Note the complex fractionated atrial electrogram recorded at the site of successful ablation.
Macroreentrant Atrial Tachycardia without Structural Heart Disease or Previous Surgical or Catheter Intervention
In a recent study, electroanatomic mapping revealed that right-sided, electrically silent areas (electrogram amplitude < 0.03 mV) were involved in the tachycardia mechanisms in two thirds of patients with non–isthmus-dependent right AFL.115 In half of the cases, a single-loop reentry circuit rotated around the areas of electrical silence, which constituted a central obstacle. In the remaining half, a dual-loop reentry with a narrow isthmus within electrically silent areas was observed. The pathogenesis of these electrically silent scars is not known, but it could be related to volume and pressure overload, impaired vascular compliance and diastolic dysfunction, atrial ischemia, postinflammatory scarring, or atrial amyloidosis. Understanding of the anatomic basis of these circuits has been greatly aided by three-dimensional mapping systems. Searching for low voltage areas, double potentials, complex fractionated electrograms, and conventional entrainment could help to determine circuit boundaries and critical isthmuses.
References
1. Saoudi, N, Cosio, F, Waldo, A, et al. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: A statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol. 2001; 12:852–866.
2. Brown, JP, Krummen, DE, Feld, GK, et al. Using electrocardiographic activation time and diastolic intervals to separate focal from macro-reentrant atrial tachycardias. J Am Coll Cardiol. 2007; 49:1965–1973.
3. Medi, C, Kalman, JM, Haqqani, H, et al. Tachycardia-mediated cardiomyopathy secondary to focal atrial tachycardia: long-term outcome after catheter ablation. J Am Coll Cardiol. 2009; 53:1791–1797.
4. Stambler, BS, Fenelon, G, Shepard, RK, et al. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiology. 2003; 14:499–507.
5. Fenelon, G, Shepard, RK, Stambler, BS. Focal origin of atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiology. 2003; 14:1093–1102.
6. Ogawa, M, Zhou, S, Tan, AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007; 50:335–343.
7. Choi, EK, Shen, MJ, Han, S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010; 121:2615–2623.
8. Yamamoto, T, Hayashi, M, Miyauchi, Y, et al. Respiratory cycle–dependent atrial tachycardia: Prevalence, electrocardiographic and electrophysiologic characteristics, and outcome after catheter ablation. Heart Rhythm. 2011; 8:1615–1621.
9. Chen, S-A, Chiang, C-E, Yang, C-J, et al. Sustained atrial tachycardia in adult patients: Electrophysiological characteristics, pharmacological response, possible mechanisms and effects of radiofrequency ablation. Circulation. 1994; 90:1262–1278.
10. Markowitz, SM, Nemirovksy, D, Stein, KM, et al. Adenosine-insensitive focal atrial tachycardia. J Am Coll Cardiol. 2007; 49:1324–1333.
11. Markowitz, SM, Nemirovksy, D, Stein, KM, et al. Adenosine-insensitive focal atrial tachycardia. Evidence for de novo micro–re-entry in the human atrium. J Am Coll Cardiol. 2007; 49:1324–1333.
12. De Groot, NMS, Schalij, MJ. Fragmented, long-duration, low-amplitude electrograms characterize the origin of focal atrial tachycardia. J Cardiovasc Electrophysiol. 2006; 17:1–7.
13. Chiale, PA, Selva, HO, Militello, CA, et al. Lidocaine sensitive, rate-related, repetitive atrial tachycardia: a new arrhythmogenic syndrome. J Am Coll Cardiol. 2000; 36(5):1637–1645.
14. Chiale, PA, Faivelis, L, Garro, HA, et al. Distinct pharmacologic substrate in lidocaine-sensitive, repetitive atrial tachycardia. J Cardiovasc Pharmacol Ther. 2012; 17:146–152.
15. Kistler, PM, Kalman, JM. Atrial tachycardia: mechanisms, diagnosis and management. Curr Prob Cardiol. 2005; 30:529–573.
16. Iwai, S, Markowitz, SM, Stein, KM, et al. Response to adenosine differentiates focal from reentrant atrial tachycardia: validation using three-dimensional electroanatomic mapping. Circulation. 2002; 106:2793–2799.
17. Wetzel, U, Hindricks, G, Schirdewahn, P, et al. A stepwise mapping approach for localization and ablation of ectopic right, left, and septal atrial foci using electroanatomic mapping. Eur Heart J. 2002; 23:1387–1393.
18. Schmitt, H, Weber, S, Schwab, JO, et al. Diagnosis and ablation of focal right atrial tachycardia using a new high-resolution, non-contact mapping system. Am J Cardiol. 2001; 87:1017–1021.
19. Reithmann, C, Hoffmann, E, Dorwarth, U, et al. Electroanatomical mapping for visualization of atrial activation in patients with incisional atrial tachycardias. Eur Heart J. 2001; 22:237–246.
20. Sanders, P, Hocini, M, Jais, P, et al. Characterization of focal atrial tachycardia using high-density mapping. J Am Coll Cardiol. 2005; 46:2088–2099.
21. Higa, S, Tai, CT, Lin, YJ, et al. Focal atrial tachycardia: New insight from noncontact mapping and catheter ablation. Circulation. 2004; 109:84–91.
22. Tang, CW, Scheinman, MM, Van Hare, GF, et al. Use of P wave configuration during atrial tachycardia to predict site of origin. J Am Coll Cardiol. 1995; 26:1315–1324.
23. Tada, H, Nogami, A, Naito, S, et al. Simple electrocardiographic criteria for identifying the site of origin of focal right atrial tachycardia. Pacing Clin Electrophysiol. 1998; 21:2431–2439.
24. Yamane, T, Shah, DC, Peng, J-T, et al. Morphological characteristics of P waves during selective pulmonary vein pacing. J Am Coll Cardiol. 2001; 38:1505–1510.
25. Hachiya, H, Ernst, S, Ouyang, F, et al. Topographic distribution of focal left atrial tachycardias defined by electrocardiographic and electrophysiologic data. Circ J. 2005; 69:205–210.
26. Kistler, PM, Roberts-Thompson, KC, Haqqani, HM, et al. P-wave morphology in focal atrial tachycardia: Development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol. 2006; 48:1010–1017.
27. Colman, MA, Aslanidi, OV, Stott, J, et al. Correlation between P-wave morphology and origin of atrial focal tachycardia–insights from realistic models of the human atria and torso. IEEE Trans Biomed Eng. 2011; 58(10):2952–2955.
28. Qian, ZY, Hou, XF, Xu, DJ, et al. An algorithm to predict the site of origin of focal atrial tachycardia. Pacing Clin Electrophysiol. 2011; 34:414–421.
29. Ramanathan, C, Ghanem, RN, Jia, P, et al. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004; 10:422–428.
30. Wang, Y, Cuculich, PS, Woodard, PK, et al. Focal atrial tachycardia after pulmonary vein isolation: Noninvasive mapping with electrocardiographic imaging (ECGI). Heart Rhythm. 2007; 4:1081–1084.
31. Wang, Y, Schuessler, RB, Damiano, RJ, et al. Noninvasive electrocardiographic imaging (ECGI) of scar related atypical atrial flutter. Heart Rhythm. 2007; 4:1565–1567.
32. Roten, L, Pedersen, M, Pascale, P, et al. Noninvasive electrocardiographic mapping for prediction of tachycardia mechanism and origin of atrial tachycardia following bilateral pulmonary transplantation. J Cardiovasc Electrophysiol. 2012; 23:553–555.
33. Morton, JB, Sanders, P, Das, A, et al. Focal atrial tachycardia arising from the tricuspid annulus: electrophysiologic and electrocardiographic characteristics. J Cardiovasc Electrophysiol. 2001; 12:653–659.
34. McGuire, MA, De Bakker, JM, Vermeulen, JT, et al. Atrioventricular junctional tissue. Discrepancy between histological and electrophysiological characteristics. Circulation. 1996; 94:571–577.
35. Hillock, RJ, Stevenson, IH, Morton, JB, et al. Focal atrial tachycardias arising from the right atrial appendage: Electrocardiographic and electrophysiologic characteristics and radiofrequency ablation. J Cardiovasc Electrophysiol. 2007; 18:367–372.
36. Freixa, X, Berruezo, A, Mont, L, et al. Characterization of focal right atrial tachycardia. Eurospace. 2008; 10:105–109.
37. Zhang, T, Li, XB, Wang, YL, et al. Focal atrial tachycardia arising from the right atrial appendage: electrophysiologic and electrocardiographic characteristics and catheter ablation. Int J Clin Pract. 2009; 63(3):417–424.
38. Medi, C, Kalman, JM, Haqqani, H, et al. Tachycardia-mediated cardiomyopathy secondary to focal atrial tachycardia: long-term outcome after catheter ablation. J Am Coll Cardiol. 2009; 53:1791–1797.
39. Khan, MK, Elmouchi, D. Ablation of a resistant right atrial appendage tachycardia using a magnetic navigation system. PACE. 2013; 36:e15–e18.
40. Furushima, H, Chinushi, M, Hosaka, Y, et al. Focal atrial tachycardia refractory to radiofrequency catheter ablation originating from right atrial appendage. Europace. 2009; 11:521–522.
41. Dong, J, Schreieck, J, Ndrepepa, G, et al. Ectopic tachycardia originating from the superior vena cava. J Cardiovasc Electrophysiol. 2002; 13:620–624.
42. Zhao, Z, Li, X, Guo, J. Electrophysiologic characteristics of atrial tachycardia originating from the superior vena cava. J Interv Card Electrophysiol. 2009; 24:89–94.
43. Callans, DJ, Ren, JF, Schwartzman, D, et al. Narrowing of the superior vena cava-right atrium junction during radiofrequency catheter ablation for inappropriate sinus tachycardia: Analysis with intracardiac echocardiography. J Am Coll Cardiol. 1999; 33:1667–1670.
44. Tsai, CF, Tai, CT, Hsieh, MH, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000; 102:67.
45. Steven, D, Roberts-Thomson, KC, Seiler, J, et al. Fibrillation in the superior vena cava mimicking atrial tachycardia. Circ Arrhythm Electrophysiol. 2009; 2:e4–e7.
46. Chugh, A, Oral, H, Good, E, et al. Catheter ablation of atypical atrial flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol. 2005; 46:83–91.
47. Kistler, PM, Fynn, SP, Haqqani, H, et al. Focal atrial tachycardia from the ostium of the coronary sinus. Electrocardiographic and electrophysiological characterization and radiofrequency ablation. J Am Coll Cardiol. 2005; 45:1488–1493.
48. Lai, LP, Lin, JL, Chen, T-F, et al. Clinical, electrophysiological characteristics, and radiofrequency catheter ablation of atrial tachycardia near the apex of Koch’s triangle. Pacing Clin Electrophysiol. 1998; 21:367–374.
49. Chen, CC, Tai, CT, Chiang, CE, et al. Atrial tachycardias originating from the atrial septum: Electrophysiologic characteristics and radiofrequency ablation. J Cardiovasc Electrophysiol. 2000; 11:744–749.
50. Frey, B, Kreiner, G, Gwechenberger, M, et al. Ablation of atrial tachycardia originating from the vicinity of the atrioventricular node: Significance of mapping both sides of the interatrial septum. J Am Coll Cardiol. 2001; 38:394–400.
51. Marrouche, NF, Sippens Groenewegen, A, Yang, Y, et al. Clinical and electrophysiologic characteristics of left septal atrial tachycardia. J Am Coll Cardiol. 2002; 40:1133–1139.
52. Iwai, S, Badhwar, N, Markowitz, SM, et al. Electrophysiologic properties of para-hisian atrial tachycardia. Heart Rhythm. 2011; 8:1245–1253.
53. Zhou, Y, Guo, J, Xu, Y, et al. Electrophysiologic characteristics and radiofrequency ablation of focal atrial tachycardia arising from para-Hisian region. Int J Clin Pract. 2007; 61:385–391.
54. Yamabe, H, Tanaka, Y, Okumura, K, et al. Electrophysiologic characteristics of verapamil-sensitive atrial tachycardia originating from the atrioventricular annulus. Am J Cardiol. 2005; 95:1425–1430.
55. Tada, H, Naito, S, Miyazaki, A, et al. Successful catheter ablation of atrial tachycardia originating near the atrioventricular node from the noncoronary sinus of Valsalva. Pacing Clin Electrophysiol. 2004; 27:1440–1443.
56. Ouyang, F, Ma, J, Ho, SY, et al. Focal atrial tachycardia originating from the non-coronary aortic sinus. Electrophysiological characteristics and catheter ablation. J Am Coll Cardiol. 2006; 48:122–131.
57. Wang, Z, Liu, T, Shehata, M, et al. Electrophysiological characteristics of focal atrial tachycardia surrounding the aortic coronary cusps. Circ Arrhythm Electrophysiol. 2011; 4:902–908.
58. Das, S, Neuzil, P, Albert, CM, et al. Catheter ablation of peri-AV nodal atrial tachycardia from the noncoronary cusp of the aortic valve. J Cardiovasc Electrophysiol. 2008; 19:231–237.
59. Kriatselis, C, Roser, M, Min, T, et al. Ectopic atrial tachycardias with early activation at His site: Radiofrequency ablation through a retrograde approach. Europace. 2008; 10:698–704.
60. Rillig, A, Meyerfeldt, U, Birkemeyer, R, et al. Catheter ablation within the sinus of Valsalva: A safe and effective approach for treatment of atrial and ventricular tachycardias. Heart Rhythm. 2008; 5:1265–1272.
61. Gami, AS, Venkatachalam, KL, Friedman, PA, et al. Successful ablation of atrial tachycardia in the right coronary cusp of the aortic valve in a patient with atrial fibrillation: What is the substrate? J Cardiovasc Electrophysiol. 2008; 19:982–986.
62. Otomo, K, Nagata, Y, Uno, K, et al. “Left-variant” adenosine sensitive atrial reentrant tachycardia ablated from the left coronary aortic sinus. Pacing Clin Electrophysiol. 2008; 31:247–250.
63. Sasaki, T, Hachiya, H, Hirao, K, et al. Utility of distinctive local electrogram pattern and aortographic anatomical position in catheter manipulation at coronary cusps. J Cardiovasc Electrophysiol. 2011; 22:521–529.
64. Park, J, Wi, J, Joung, B, et al. Prevalence, risk, and benefits of radiofrequency catheter ablation at the aortic cusp for the treatment of mid to anteroseptal supra-ventricular tachyarrhythmias. Int J Cardiol. 2012 Mar 28.
65. Biviano, AB, Bain, W, Whang, W, et al. Focal left atrial tachycardias not associated with prior catheter ablation for atrial Fibrillation: Clinical and electrophysiological characteristics. PACE. 2012; 35:17–27.
66. Nazmul, M, Munger, TM, Powell, BD. Atrial tachycardia originating from a donor pulmonary vein in a lung transplant recipient. Circulation. 2011; 124(11):1288–1289.
67. Dong, J, Zrenner, B, Schreieck, J, et al. Catheter ablation of left atrial focal tachycardia guided by electroanatomic mapping and new insights into interatrial electrical conduction. Heart Rhythm. 2005; 2:578–591.
68. Kistler, PM, Sanders, P, Fynn, SP, et al. Electrophysiological and electrocardiographic characteristics of focal atrial tachycardia originating from the pulmonary veins. Acute and long-term outcomes of radiofrequency ablation. Circulation. 2003; 108:1968–1975.
69. Teh, AW, Kalman, JM, Medi, C, et al. Long-term outcome following successful catheter ablation of atrial tachycardia originating from the pulmonary veins: absence of late atrial fibrillation. J Cardiovasc Electrophysiol. 2010; 21:747–750.
70. Traykov, VB, Pap, R, Shalganov, TN, et al. Electrogram analysis at the His bundle region and the proximal coronary sinus as a tool to predict left atrial origin of focal atrial tachycardias. Europace. 2011; 13:1022–1027.
71. Gonzalez, MD, Contreras, LJ, Jongbloed, MRM, et al. Left atrial tachycardia originating from the mitral annulus-aorta junction. Circulation. 2004; 110:3187–3192.
72. Kistler, PM, Sanders, P, Hussin, A, et al. Focal atrial tachycardia arising from the mitral annulus. Electrocardiographic and electrophysiologic characterization. J Am Coll Cardiol. 2003; 41:2212–2219.
73. Wit, AL, Fenoglio, JJ, Jr., Wagner, BM, et al. Electrophysiological properties of cardiac muscle in the anterior mitral valve leaflet and the adjacent atrium in the dog. Possible implications for the genesis of atrial dysrhythmias. Circ Res. 1973; 32:731–745.
74. Wit, AL, Fenoglio, JJ, Jr., Hordof, AJ, et al. Ultrastructure and transmembrane potentials of cardiac muscle in the human anterior mitral valve leaflet. Circulation. 1979; 59:1284–1292.
75. Yamada, T, Murakami, Y, Yoshida, Y, et al. Electrophysiological and electrocardiographic characteristics and radiofrequency catheter ablation of focal atrial tachycardia originating from the left atrial appendage. Heart Rhythm. 2007; 4:1284–1291.
76. Wang, YL, Li, XB, Quan, X, et al. Focal atrial tachycardia originating from the left atrial appendage: electrocardiographic and electrophysiologic characterization and long-term outcomes of radiofrequency ablation. J Cardiovasc Electrophysiol. 2007; 18:459–464.
77. Yang, Q, Ma, J, Zhang, S, et al. Focal atrial tachycardia originating from the distal portion of the left atrial appendage: characteristics and long-term outcomes of radiofrequency ablation. Europace. 2012; 14:254–260.
78. Hillock, RJ, Singarayar, S, Kalman, JM, et al. Tale of two tails: the tip of the atrial appendages is an unusual site for focal atrial tachycardia. Heart Rhythm. 2006; 3:467–469.
79. Phillips, KP, Natale, A, Sterba, R, et al. Percutaneous pericardial instrumentation for catheter ablation of focal atrial tachycardias arising from left atrial appendage. J Cardiovasc Electrophysiol. 2008; 19:430–433.
80. Raczka, F, Granier, M, Mathevet, L, et al. Radiofrequency ablation of a left appendage focal tachycardia using intracardiac ultrasound image integration to guide catheter: minimizing the risk of left appendage perforation. Europace. 2009; 11:1253–1254.
81. Yamada, Y, Ajiro, Y, Shoda, M, et al. Video-assisted thoracoscopy to treat atrial tachycardia arising from left atrial appendage. J Cardiovasc Electrophysiol. 2006; 17:895–898.
82. Yamada, T, McElderry, T, Allison, S, et al. Focal atrial tachycardia originating from the epicardial left atrial appendage. Heart Rhythm. 2008; 5:766–767.
83. Pokushalov, E, Romanov, A, Artyomenko, S, et al. Left atrial appendectomy after failed catheter ablation of a focal atrial tachycardia originating in the left atrial appendage. Pediatr Cardiol. 2010; 31:908–911.
84. Still, A-M, Huikuri, HV, Juhani, KE, et al. Impaired negative chronotropic response to adenosine in patients with inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2002; 13:557–562.
85. Chiale, PA, Garro, HA, Schmidberg, J, et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006; 3:1182–1186.
86. Glatter, KA, Cheng, J, Dorostkar, P, et al. Electrophysiologic effects of adenosine in patients with supraventricular tachycardia. Circulation. 1999; 99:1034–1040.
87. Knight, BP, Ebinger, M, Oral, H, et al. Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2000; 35:574–582.
88. Knight, BP, Zivin, A, Souza, J, et al. A technique for the rapid diagnosis of atrial tachycardia in the electrophysiology laboratory. J Am Coll Cardiol. 1999; 33:775–781.
89. Crawford, TC, Mukerji, S, Good, E, et al. Utility of atrial and ventricular cycle length variability in determining the mechanism of paroxysmal supraventricular tachycardia. J Cardiovasc Electrophysiol. 2007; 18:698–703.
90. Mohamed, U, Skanes, AC, Gula, LJ, et al. A novel pacing maneuver to localize focal atrial tachycardia. J Cardiovasc Electrophysiol. 2007; 8:1–6.
91. Sacher, F, Vest, J, Raymond, JM, et al. Incessant donor-to-recipient atrial tachycardia after bilateral lung transplantation. Heart Rhythm. 2008; 5:149–151.
92. Stevenson, IH, Kistler, PM, Spence, SJ, et al. Scar-related right atrial macroreentrant tachycardia in patients without prior atrial surgery: Electroanatomic characterization and ablation outcomes. Heart Rhythm. 2005; 2:594–601.
93. Kottkamp, H. Fibrotic atrial cardiomyopathy: A specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol. 2012; 23:797–799.
94. Nakagawa, H, Shah, N, Matsudaira, K, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: Isolated channels between scars allow “focal” ablation. Circulation. 2001; 103:699–709.
95. Derejko, P, Szumowski, LJ, Sanders, P, et al. Clinical validation and comparison of alternative methods for evaluation of entrainment mapping. J Cardiovasc Electrophysiol. 2009; 20:741–748.
96. Morton, JB, Sanders, P, Deen, V, et al. Sensitivity and specificity of concealed entrainment for the identification of a critical isthmus in the atrium: Relationship to rate, anatomic location and antidromic penetration. J Am Coll Cardiol. 2002; 39:896–906.
97. Vollmann, D, Stevenson, WG, Luthje, L, et al. Misleading long post-pacing interval after entrainment of typical atrial flutter from the cavotricuspid isthmus. J Am Coll Cardiol. 2012; 59:819–824.
98. Della Bella, P, Fraticelli, A, Tonod, C, et al. Atypical atrial flutter: Clinical features, electrophysiological characteristics and response to radiofrequency catheter ablation. Europace. 2002; 4:241–253.
99. Gerstenfeld, EP, Dixit, S, Bala, R, et al. Surface electrocardiogram characteristics of atrial tachycardias occurring after pulmonary vein isolation. Heart Rhythm. 2007; 4:1136–1143.
100. Chang, SL, Tsao, HM, Lin, YJ, et al. Differentiating macroreentrant from focal atrial tachycardias occurred after circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011; 22:748–755.
101. Medi, C, Kalman, JM. Prediction of the atrial flutter circuit location from the surface electrocardiogram. Europace. 2008; 10:786–796.
102. Miyazaki, H, Stevenson, WG, Stephenson, K, et al. Entrainment mapping for rapid distinction of left and right atrial tachycardias. Heart Rhythm. 2006; 3:516–523.
103. Jais, P, Shah, DC, Haissaguerre, M, et al. Mapping and ablation of left atrial flutters. Circulation. 2000; 101:2928–2934.
104. Ouyang, F, Ernst, S, Vogtmann, T, et al. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: Critical isthmus block can prevent atrial tachycardia recurrence. Circulation. 2002; 105:1934–1942.
105. Jais, P, Hocini, M, Hsu, LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004; 110:2996–3002.
106. Tzeis, S, Luik, A, Jilek, C, et al. The modified anterior line: an alternative linear lesion in perimitral flutter. J Cardiovasc Electrophysiol. 2010; 21:665–670.
107. Jais, P, Shah, DC, Haissaguerre, M, et al. Mapping and ablation of left atrial flutter. Circulation. 2000; 101:2928–2934.
108. Marrouche, NF, Natale, A, Wazni, OM, et al. Left septal atrial flutter: electrophysiology, anatomy, and result of ablation. Circulation. 2004; 109:2440–2447.
109. Markowitz, SM, Brodman, RF, Stein, KM, et al. Lesional tachycardias related to mitral valve surgery. J Am Coll Cardiol. 2002; 39:1973–1983.
110. Gerstenfeld, EP, Marchlinski, FE. Mapping and ablation of left atrial tachycardias occurring after atrial fibrillation ablation. Heart Rhythm. 2007; 4:S65–S72.
111. Deisenhofer, I, Estner, H, Zrenner, B, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: Incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace. 2006; 8:573–582.
112. Wazni, O, Saliba, W, Fahmy, T, et al. Atrial arrhythmias after surgical maze. Findings during catheter ablation. J Am Coll Cardiol. 2006; 48:1405–1409.
113. Chugh, A, Latchamsetty, R, Oral, H, et al. Characteristics of cavotricuspid isthmus–dependent atrial flutter after left atrial ablation of atrial fibrillation. Circulation. 2006; 113:609–615.
114. Chugh, A, Oral, H, Good, E, et al. Catheter ablation of atypical atrial flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol. 2005; 45:83–91.
115. Fiala, M, Chovancík, J, Neuwirth, R, et al. Atrial macroreentry tachycardia in patients without obvious structural heart disease or previous cardiac surgical or catheter intervention: characterization of arrhythmogenic substrates, reentry circuits, and results of catheter ablation. J Cardiovasc Electrophysiol. 2007; 18:824–832.