Chapter 14 Atrial Tachyarrhythmias in Congenital Heart Disease
Pathophysiology
Patients with congenital heart disease have a high prevalence of atrial tachycardias (ATs), particularly after they have undergone reparative or palliative surgical procedures. For macroreentrant ATs in adults with repaired congenital heart disease, three right atrial (RA) circuits are generally identified: lateral wall circuits with reentry around or related to the lateral atriotomy scar, septal circuits with reentry around an atrial septal patch, and typical atrial flutter (AFL) circuits using the cavotricuspid isthmus (CTI). Typical clockwise or counterclockwise isthmus-dependent AFL is the most common macroreentrant tachycardia in this patient population. Left atrial (LA) macroreentrant circuits are infrequent in this group of patients. Atrial macroreentry in the right free wall is the most common form of non–isthmus-dependent RA macroreentry (atypical flutter). Very complex or multiple reentry circuits can be seen after placement of an intraatrial baffle (Mustard or Senning correction for transposition of the great vessels) in an extremely dilated RA, after a Fontan procedure, and in patients with a univentricular heart.1,2
Anatomical factors promoting macroreentry in patients with congenital heart disease include abnormalities of the underlying cardiac anatomy, surgically created anastomoses, and atriotomy scars, resulting in anatomical barriers to impulse propagation. Additionally, extensive cardiac surgery and hemodynamic overload result in myocardial hypertrophy and diffuse areas of atrial scarring with surviving myocardial fibers embedded within scar areas, which provide the substrate for potential reentry circuits.3,4
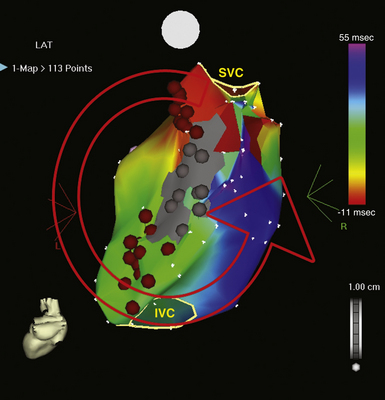
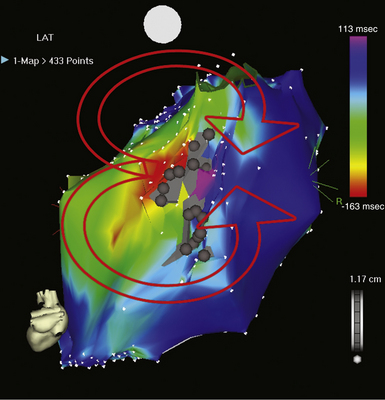
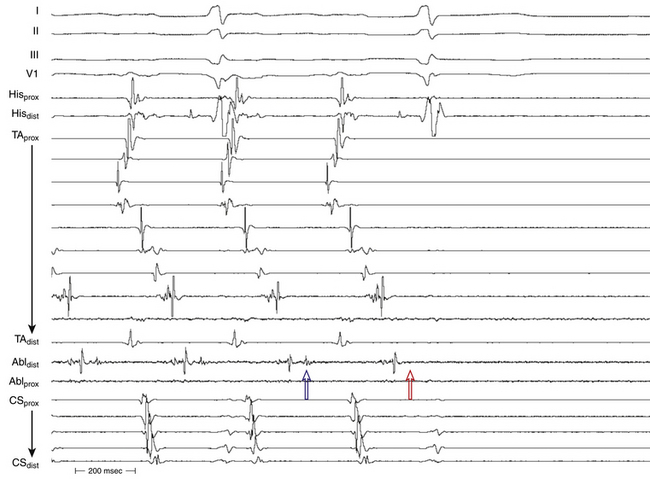
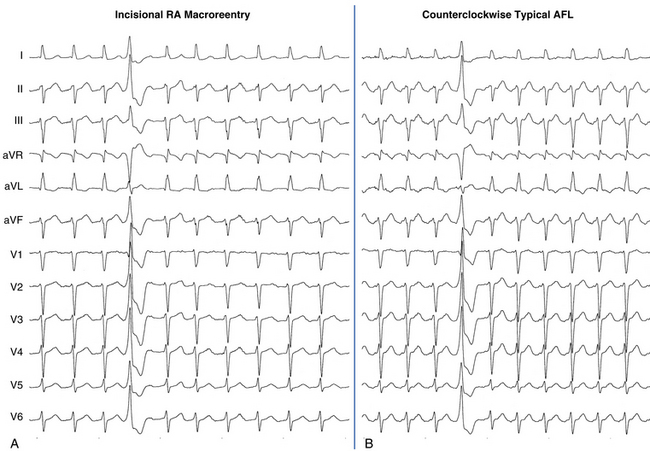
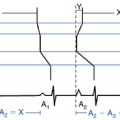
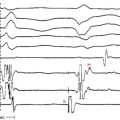
The best characterization of macroreentrant AT caused by atriotomy is activation around an incision scar in the lateral RA wall, with a main superoinferior axis (Fig. 14-1). This is a common problem in patients who have undergone surgery for congenital or valvular heart disease. The length, location, and orientation of the atriotomy incisions, as well as potential electrical conduction gaps across the atriotomy, are important determinants of arrhythmogenicity.5 Typically, the reentry circuit is located in the lateral RA wall. Not only does the central obstacle include the scar, but also functional block can magnify this obstacle to include the superior vena cava (SVC). The anterior RA wall is commonly activated superoinferiorly (descending activation pattern), as in counterclockwise typical AFL. However, the septal wall frequently lacks a clear-cut inferosuperior (ascending) activation pattern. Participation of the anterior RA wall in the circuit can be confirmed with entrainment mapping. A line of double potentials can be recorded in the lateral RA, extending superoinferiorly. Double potential separation can be more marked and demonstrate a voltage lower than in typical AFL. Narrow passages (isthmuses) in the circuit can be found between the SVC and the superior end of the atriotomy scar, between the inferior vena cava (IVC) and the inferior end of the atriotomy, between the atriotomy scar and the tricuspid annulus, between the atriotomy and the crista terminalis, or even within the scar itself (Fig. 14-2). These isthmuses can be areas of slow conduction. Stable pacing of the critical isthmus can be difficult or impossible in RA atriotomy tachycardia because of tachycardia interruption. Isthmus participation in the circuit is often proven by tachycardia interruption with catheter pressure (Fig. 14-3), as well as by tachycardia interruption and noninducibility after radiofrequency (RF) application in the area. A single, wide, fractionated electrogram can be recorded from the lower pivot point of the circuit (Fig. 14-4) in the low lateral RA, close to the IVC, and perhaps also from other isthmuses of the circuit. The line of double potentials or fractionated, low-voltage electrograms can also often be recorded in normal sinus rhythm, to allow tentative localization of the scar and the associated anatomical isthmuses.2
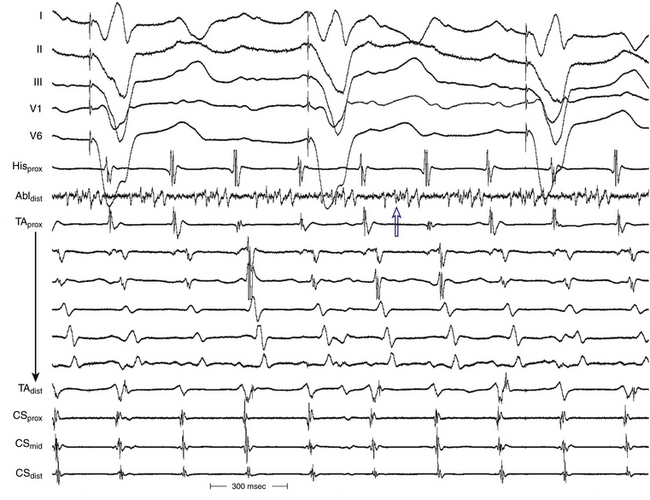
Typical AFL is also often associated with RA atriotomy. In fact, the single most common form of AT among patients with congenital heart disease appears to be isthmus-dependent AFL, particularly in patients with simpler anatomical lesions (e.g., tetralogy of Fallot, atrial and ventricular septal defects) (Fig. 14-5). Moreover, the CTI was found to be part of the reentrant circuit in approximately 70% of patients with postoperative intraatrial reentrant tachycardia. In one report, ablation of this isthmus alone resulted in elimination of the tachycardia in 27% of these patients. Factors such as an atriotomy or atrial fibrosis and hypertrophy serve as promoters for early development of the typical form of AFL.
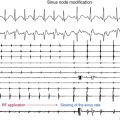
Reentry circuits can also occur in the sinus node region, possibly as a result of injury related to the superior atrial cannulation site for the bypass pump. These circuits can be quite small, often manifesting as focal tachycardia in the sinus node region, and they frequently can be ablated in a single location without establishing a particular line of block.4
Focal mechanisms underlying postoperative AT have been rarely reported in this patient population. Nonautomatic focal ATs are predominantly found in adults, with most foci in the RA. The reasons behind this laterality are unknown. The mechanism underlying focal AT is unknown. Both triggered and microreentrant mechanisms have been suggested.6 Viable myocardial fibers embedded within areas of scar tissue, which play a pivotal role in the initiation and perpetuation of macroreentrant tachycardias, can also be the site of origin of a focal AT and thus play an important role in the pathogenesis of these ATs.3
Arrhythmias are also frequently observed in the early postoperative period after corrective surgery in children, occurring in 14% to 48% in the first few days after surgery. The most common arrhythmia in this period is junctional tachycardia, occurring in 5% to 10% of the operated children and usually self-limiting. Other supraventricular arrhythmias are also seen in 4%. The occurrence of early postoperative arrhythmias seems to be related to procedural factors of cardiac surgery, which are, in turn, related to the complexity of the congenital malformation. Early postoperative arrhythmias influence the long-term outcome of patients with congenital heart disease and have been found to be a predictor of late complications, such as ventricular dysfunction, late arrhythmias, and late mortality. Whether preventing these arrhythmias will influence the long-term survival of patients with congenital heart disease is unknown.7
Clinical Considerations
Epidemiology
Interestingly, compared with patients with structurally normal hearts, the incidence of atrial fibrillation (AF) in patients with congenital heart disease is relatively low. AF is less common than one would anticipate, especially considering the often extremely dilated RA in this patient population.8 AF is typically associated predominantly with markers of left-sided heart disease (i.e., lower left ventricular ejection fraction and LA dilation) and is most commonly seen in patients with congenital aortic stenosis, mitral valve disease, palliated single ventricles, or end-stage heart disease.1,9
Atrial Septal Defect
Macroreentrant ATs are the most frequent arrhythmias encountered in patients with secundum and sinus venosus atrial septal defects. In the absence of surgical repair, the prevalence of supraventricular arrhythmias increases with age. These arrhythmias have been reported in 20% of these patients at the age of 40 years, and typical AFL is the most common circuit. In the presence of atriotomy incisions, sutures, or patches, non–isthmus-dependent macroreentrant circuits can occur or coexist with typical AFL. Common substrates include macroreentry along the lateral RA wall and double-loop or figure-of-8 circuits. The septal patch itself is rarely a critical conduction obstacle. Surgical closure of an atrial septal defect during childhood provides a substantially lower incidence of arrhythmias. In contrast, surgical closure at adult age is far less effective; approximately 60% of these patients continue to have atrial arrhythmias during follow-up after surgery. The impact of transcatheter atrial septal defect closure on atrial arrhythmias is less clear. In one series, all patients with persistent arrhythmias remained in AF or AFL after closure.7,8,10
Univentricular Hearts with Fontan Palliation
Among patients with congenital heart disease, the incidence of macroreentrant AT is highest (16% to 56%) among older patients who have undergone older-style Fontan (atriopulmonary anastomosis) operations, in which extensive suture lines and long-term hemodynamic stress result in marked atrial hypertrophy and fibrosis.4 The incidence of atrial tachyarrhythmias appears lower in patients with total cavopulmonary connections in comparison with classical atriopulmonary connections. Overall, the most common arrhythmia is atrial macroreentry. Tachycardia circuits may be complex or multiple. Single circuits that are quite amenable to catheter ablation can occasionally be encountered.11
Tetralogy of Fallot
ATs occur commonly (12% to 34%) during extended follow-up after tetralogy of Fallot repair. The observed prevalence of atrial arrhythmias (20.1%) is modestly higher than that of ventricular arrhythmias (14.6%).12 The most common atrial circuit is typical clockwise or counterclockwise AFL. Other circuits often involve the lateral RA wall and may be multiple, often with a double-loop type of reentry. Nonautomatic focal ATs most commonly arise adjacent to suture points, with radial spread of activation.
The prevalence of AF increases with advancing age. In the first few decades of life, AF is far less common than macroreentrant AT, but it becomes more common (more than 30%) than macroreentrant AT after 55 years of age.12
d-Transposition of the Great Arteries
The Mustard or Senning atrial switch procedures were performed from the early 1960s until approximately 1985 as the major long-term surgical palliation procedures for young children having d-transposition of the great arteries. Hence, there is a population of patients in their late 20s to early 50s who have undergone these operations and who are at great risk of having supraventricular arrhythmias (15% to 48%), with similar rates in patients with Mustard and Senning baffles.4,11 Most ATs in this patient group are typical AFL, but non–isthmus-dependent macroreentrant ATs with critical zones of slow conduction between a suture line and the SVC orifice, mitral valve annulus, and pulmonary vein orifice have all been described. Focal ATs adjacent to suture lines are also not uncommon. Currently, arterial switch surgery has supplanted atrial redirection as the procedure of choice for d-transposition of the great arteries, and it has been associated with a lower risk of arrhythmias.8,10
Reports suggest that atrial tachyarrhythmias are important contributors to sudden death. Contributing factors may include longer cycle lengths (CLs) than in typical AFL (favoring 1:1 conduction), impaired atrioventricular (AV) transport with failure to augment right ventricular filling rates during tachycardia, systemic ventricular dysfunction, and subendocardial ischemia resulting from right coronary circulation irrigation of a systemic ventricle.8
Clinical Presentation
Macroreentrant ATs are typically chronic or long-lasting. As with AF and typical AFL, patients can present with symptoms related to rapid ventricular response, tachycardia-induced cardiomyopathy, or deterioration of preexisting cardiac disease.
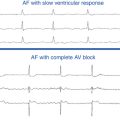
Generally, in the adult population with congenital heart disease, macroreentrant ATs tend to be slower than typical AFL, with atrial rates in the range of 150 to 250 per minute. In the setting of a healthy AV node (AVN), such rates frequently conduct in a rapid 1:1 AV pattern and can potentially result in hypotension, syncope, or possibly circulatory collapse in patients with limited myocardial reserve. This phenomenon can potentially be compounded by ineffective atrial transport and ventricular dysfunction. Even if the ventricular response rate is well controlled, sustained macroreentrant AT can cause debilitating symptoms in some patients because of the loss of AV synchrony and can contribute to thromboembolic complications when the duration is protracted.9
Late-onset supraventricular arrhythmias can potentially have a major impact notably not only on morbidity but also on mortality in patients with congenital heart disease. Not only can arrhythmias be the cause of rapid hemodynamic deterioration, but also they have been linked to an increased risk of sudden death. A multicenter study of defibrillator recipients suggested that supraventricular arrhythmias can trigger ventricular arrhythmias (tachycardia-induced tachycardia). It is likely that the rapid ventricular response is not well tolerated by the impaired ventricle, thus resulting in ventricular tachycardia or fibrillation.7,11
Initial Evaluation
In patients with congenital heart disease, arrhythmia onset can herald a changing hemodynamic profile and can be the first sign of deterioration. Therefore, if arrhythmias occur, a thorough evaluation of the hemodynamic status is warranted.7,13
Principles of Management
Although the use of antiarrhythmic agents from every class has been reported, none of these drugs have demonstrated clear long-term efficacy. Furthermore, most antiarrhythmic agents carry the risk of proarrhythmia, and many agents aggravate sinus node dysfunction and compromise ventricular function, thus diminishing their utility in these patients, particularly in the absence of pacemaker therapy.9
Because the general experience with long-term pharmacological therapy has been discouraging, catheter ablation has been adopted as an early intervention for recurrent macroreentrant AT. Catheter ablation carries short-term success rates of nearly 90%, although later tachycardia recurrence is still disappointingly common. The recurrence risk appears to be particularly high in the population of patients who have undergone Fontan procedures. These patients tend to have multiple AT circuits and the thickest and largest atrial dimensions. Nonetheless, ablation results are far superior to the extent of control obtained with medications alone.9 It is important to recognize that macroreentrant ATs in patients with congenital heart disease can have complex reentrant circuits, the ablation of which can be far more difficult than ablation of typical AFL. This complexity requires that the electrophysiologist be well acquainted with the details of congenital heart lesions and the types of surgical repairs. Therefore, referral to an experienced center should be considered.
Pacemaker implantation can be useful for those patients who have concomitant sinus node dysfunction as a prominent component of their clinical picture. In these patients, prevention of severe sinus bradycardia not only allows for the use of drugs necessary for rate and rhythm control but also can potentially improve the hemodynamic status and often result in marked reduction in AT frequency. Pacemakers with advanced programming features that incorporate AT detection and automatic burst pacing can also be beneficial in select cases, but they carry the risk of accelerating the atrial rate and must thus be used cautiously in patients with rapid AV conduction.9
Surgical ablation (RA maze procedure) can be considered in patients with AT refractory to medical therapy and catheter ablation or in those requiring reoperation for hemodynamic reasons. This procedure is used most commonly for patients with failing Fontan procedures and the most refractory variety of macroreentrant AT and is usually combined with a revision of the Fontan connection or conversion from an older atriopulmonary anastomosis to a cavopulmonary connection. This typically includes debulking the RA, removing thrombus, excising RA scar tissue, implanting an epicardial pacemaker, performing a modified RA maze procedure, and, in patients with prior documented AF, performing a left-sided maze procedure as well.9 Case series with short-term follow-up report promising results, with arrhythmia recurrence rates of 13% to 30%.11
It is also important to understand that although effective tachycardia therapy appears to reduce symptoms and the need for cardioversion and improves quality of life in patients with congenital heart disease, it is unclear at present that it improves survival free of major events. If the target arrhythmia is a marker, rather than a cause of poor outcome, therapy should be directed toward the suppression of symptoms while exposing the patient to minimal risk of procedure-related adverse events. Therefore, depending on the expected complexity of the arrhythmia substrate and the underlying structural heart disease, ablation may be reserved for symptomatic patients who are not responding to, or intolerant of, antiarrhythmic medications. Therapeutic decisions are made case by case.1
Electrocardiographic Features
The surface ECG morphology of RA free wall macroreentry in a patient with a previous atriotomy is highly variable. The presence of complex anatomy secondary to congenital abnormalities, prior atrial surgery, or large low-voltage zones can modify P wave propagation in nonuniform manner, with resulting altered atrial activation vectors or low-amplitude P waves.14
The morphology of the atrial complex on the surface ECG can range from that similar to typical AFL to that characteristic of focal AT (see Fig. 14-5). Often, inverted flutter waves can be observed in lead V1. Depending on the predominant direction of septal activation, RA free wall macroreentrant AT can mimic either clockwise or counterclockwise typical AFL.14
Mapping
Detailed knowledge of the of the patient’s congenital and operative anatomy is important in the interpretation of the results of mapping, in ascertaining how to access the RA, in determining the feasibility of the transseptal puncture, and in deciding whether fluoroscopy is helpful in localizing the catheters or whether intracardiac or transesophageal echocardiography is needed. Careful preprocedural planning includes a detailed review of prior operative notes, hemodynamic catheterization reports, angiography, and other imaging studies (echocardiography, cardiac computed tomography [CT], or MRI).13 Table 14-1 includes those conditions that are expected to pose vascular or cardiac chamber access challenges and methods that have been reported to allow catheter access. There is often limited opportunity to place the standard number of diagnostic electrode catheters; alternative approaches to pacing and recording may include the esophagus and hepatic veins and a retrograde approach to the atria from the ventricles.10
TABLE 14-1 Conditions Associated with Challenges for Vascular and Cardiac Chamber Access
| OCCLUSION OR ACCESS CHALLENGE | ASSOCIATED CONDITIONS | ALTERNATE STRATEGIES |
|---|---|---|
| Iliofemoral venous occlusion |
BAS = balloon atrial septostomy; D-TGA = d-transposition of the great arteries; TCPC = total cavopulmonary connection.
Adapted with permission from Kanter RJ: Pearls for ablation in congenital heart disease, J Cardiovasc Electrophysiol 21:223-230, 2010.
Exclusion of Cavotricuspid Isthmus Dependence
Exclusion of the CTI as part of the AT circuit is an important initial step because typical clockwise or counterclockwise AFL is the most common macroreentrant AT in patients with congenital heart disease (particularly in patients with simpler anatomical lesions such as the tetralogy of Fallot and atrial and ventricular septal defects), even if the P wave morphology is not characteristic for these arrhythmias. Isthmus-dependent AFL can be rapidly diagnosed or excluded by mapping of the tricuspid annulus and by entrainment maneuvers at the CTI. Exclusion of the CTI as part of the reentrant circuit can be established by any of the following: (1) demonstration of bidirectional activation of the CTI during AT, with resulting collision or fusion within the isthmus by activation from opposing directions (the low lateral RA and coronary sinus [CS]); (2) recording of double potentials separated by an isoelectric and constant interval throughout the full extent of the CTI during tachycardia; or (3) entrainment mapping from the CTI demonstrating manifest atrial fusion with a long post-pacing interval.4
Identification of Barriers and Potential Lines of Block
Mapping of macroreentrant ATs in patients with congenital heart disease follows the same principles discussed for mapping of other types of macroreentrant ATs (see Chap. 13 for detailed discussion). Electromagnetic three-dimensional (3-D) mapping (CARTO mapping system [Biosense Webster, Inc., Diamond Bar, Calif.] or EnSite NavX system [St. Jude Medical, St. Paul, Minn.]) is typically used to help facilitate identification of the macroreentrant circuit and the sequence of atrial activation during tachycardia and rapid visualization of the activation wavefront in the context of the relevant anatomy. Integration of preacquired cardiac CT or MRI images on the 3-D shell of the cardiac chamber created with the electroanatomical system can further facilitate the procedure. Angiography of the desired chamber may be considered.4,13 Noncontact mapping may also be used to define the atrial substrate and reentrant circuit, and it can be of significant value when the flutter is short-lived or cannot be reproducibly initiated.
Initially, potential tachycardia circuit barriers are identified (during sinus rhythm or tachycardia) and marked on the electroanatomical map to help understand propagation of the reentrant activation wavefront in relation to these barriers, identify potential slow-conducting pathways critical to the reentrant circuit, identify sites to target by entrainment mapping, and plan subsequent ablation strategy to abolish the tachycardia. The tricuspid annulus often provides one important barrier. Other naturally fixed barriers (i.e., independent of the precise form of activation and present also in sinus rhythm) include the IVC, SVC, and CS ostium. Acquired barriers include surgical incisions or patches, lines of block, and electrical scars. Silent areas are defined as having an atrial potential amplitude of less than 0.05 mV and the absence of atrial capture at 20 mA. Such areas and surgically related scars, such as atriotomy scars in the lateral RA or atrial septal defect closure patches, are tagged as “scar” (see Figs. 14-1 and 14-2).3,4,13
Identification of the Complete Reentrant Circuit
It is very important to remain vigilant during the mapping process and identify any change in CL or activation sequence, or both, that can result from catheter manipulation, pacing maneuvers, or ablation. Such changes can indicate transformation of a multiple loop tachycardia by interruption of one loop, change in bystander activation, or transition to another tachycardia, which requires reassessment. The transition may be obvious but also is often quite subtle and sometimes imperceptible if only the CS activation is analyzed. Simultaneous recording RA activation (using a Halo catheter around the tricuspid annulus) and CS activation (using a decapolar catheter) can help rapid identification of tachycardia transformation.15
Sometimes, it can be impossible to map the entire circuit, especially in patients with repaired congenital heart disease, because of the complex suture lines, baffles, or both. In this setting, a combination of activation, entrainment, and voltage mapping data is used to identify the potential isthmus or zone of slow conduction critical to the reentrant circuit, which is then targeted by catheter ablation.4
Identification of the Critical Isthmus
Once one or more potential isthmuses are identified during activation mapping and propagation mapping in relation to atrial scars, barriers, and lines of block, entrainment is performed to determine their role for supporting the reentrant circuit. Entrainment with concealed fusion should be sought; this indicates that the pacing site is in a protected isthmus located within or attached to the reentrant circuit. It is important to understand, however, that in patients with complex anatomy and surgical repairs, critical corridors of slow conduction can exist anywhere in the RA mass, and multiple circuits are the rule. As a consequence, entrainment with concealed fusion is highly nonspecific for most circuits in these patients. Therefore, whether the protected isthmus is crucial to the reentrant circuit or just a bystander site needs to be verified by comparing the post-pacing interval with the tachycardia CL and the stimulus-exit interval with the electrogram-exit interval. Features of entrainment when pacing from different sites are listed in Table 13-2.10,16,17
Ablation
Target of Ablation
Although success in most of these studies has been achieved by targeting the “slow zone” of atrial conduction presumed necessary for maintenance of the reentry, some investigators have emphasized the need for considering both the anatomy and electrophysiology simultaneously to identify a susceptible bridge of tissue, which connects two areas of electrical block. Such an approach requires careful review of the details of the atrial surgery, in addition to detailed mapping of the atria in its “usual” rhythm and during reentry.4
Because typical isthmus-dependent AFL is the most common mechanism underlying macroreentrant AT in the patient population with congenital heart disease, determination of the role of the CTI in supporting reentry is evaluated first, and this isthmus is targeted by ablation if it is proved to be critical to the AT circuit (see Chap. 12 for detailed discussion). Ablation of the CTI may also be reasonable in every patient with congenital heart disease, even patients presenting with non–isthmus-dependent RA macroreentry, because the CTI can support reentry in most patients with prior right atriotomy.
Successful CTI ablation can result in termination of the tachycardia and restoration of sinus rhythm, thus confirming participation of the CTI in the circuit. Alternatively, the tachycardia may transform to a different arrhythmia, as indicated by a change in activation sequence or CL, or both. Additionally, after extensive ablation and even appearance of dissociated electrograms in the CTI, AT may persist with little discernible change in CL or activation sequence. This usually indicates multiple circuits, often with a double-loop type of reentry that uses the lateral wall as well as the CTI, and is now dependent on a different isthmus (e.g., the lateral RA around or within the atriotomy scar).18
If participation of the CTI in the reentrant circuit is excluded, or if the tachycardia persists or transforms into a different tachycardia after ablation of the CTI, the participation of the lateral RA wall in the AT circuit should be assessed. Non–isthmus-dependent RA macroreentry is frequently localized to the free wall of the RA, anchored by the atriotomy scar, in which setting the ablation strategy includes any of the following: (1) to target the slow conduction area (critical isthmus of the reentrant circuit) as identified by detailed activation and entrainment mapping; (2) to extend the atriotomy (double potential or scar) to the IVC (see Fig. 14-1); or (3) to extend the scar area to the SVC. The last approach can result in injury of the sinus node or phrenic nerve. Therefore, when possible, extending the atriotomy to the IVC is preferable. As noted, additional ablation of the CTI may be considered in these patients because these circuits are often combined with a circuit around the tricuspid annulus in a figure-of-8 reentry, such that ablation of both the CTI and one of the lateral wall options must be performed to eliminate and prevent reentrant AT.4,18
Another focus for reentry circuits is the sinus node region, possibly because of injury related to the superior atrial cannulation site for the bypass pump. These circuits may be quite small, often manifesting as focal tachycardia in the sinus node region, and they can often be ablated in a single location without establishing a particular line of block.4
In patients with incomplete maps, a combination of activation, entrainment, and voltage mapping data is used to identify the potential isthmus or zone of slow conduction critical to the reentrant circuit, which is then targeted by catheter ablation. When limitations to this approach still exist (from poor inducibility or frequent change in morphology of the tachycardia), a stepwise ablation strategy may be employed. Because the reentrant circuit in most patients is anchored to the CTI or the atriotomy scar, or both, ablation of the CTI is initially performed. Then, if the tachycardia persists, linear ablation is carried out by targeting the isthmuses related to the lateral atriotomy scar (i.e., connecting the atriotomy to either the IVC or SVC). Additional ablation lines connecting other areas of conduction block (scar, surgical incision, septal patch, baffle, or natural barriers) may be considered based on substrate mapping findings.4
Ablation Technique
Once the ablation target is identified, ablation involves placing a series of RF lesions to sever the critical isthmus to connect two anatomical or surgical barriers. Because some congenital malformations and surgical repairs are associated with myocardial hypertrophy and extensive fibrotic regions, thus making the achievement of a transmural lesion difficult, ablation is performed with an irrigated (25 to 50 W) or 8-mm (target temperature, 60° to 70°C; 50 to 60 W) electrode-tip RF catheter.18
During delivery of RF energy, the tachycardia can terminate, or its CL can increase transiently or permanently. These findings indicate that the lesions have affected the circuit and should lead to continuation of RF delivery or extension of the lesion to ensure the achievement of complete conduction block across the isthmus.Alternatively, the tachycardia may transform to a different loop or to a different tachycardia (rather than terminate), as indicated by a change in activation sequence or CL, or both, in which setting reassessment of the new tachycardia mechanism and location is necessary before continued RF ablation. However, if ablation across an isthmus that was initially found to be critical to the tachycardia circuit (by entrainment mapping techniques) appears to be not affecting the tachycardia, it is important to verify whether the isthmus is still critical to the reentrant circuit by repeating entrainment mapping. Sometimes, complete isthmus block is already achieved (as suggested by the presence of double potentials along the ablation line), and the isthmus is no longer participating in the tachycardia circuit (as suggested by entrainment mapping findings), as a result of a change in the tachycardia circuit that occurred during ablation.15,19,20
Ablation near the region of the superior crista terminalis and SVC can result in right phrenic nerve injury and diaphragmatic paralysis. Pacing with a high output (10 mA at 2 milliseconds) from the ablation catheter at the target site can help identify the location of the right phrenic nerve. Additionally, suspicion of phrenic nerve injury should be considered in the case of hiccup, cough, or decrease in diaphragmatic excursion during energy delivery. Early recognition of phrenic nerve injury during RF delivery allows the immediate interruption of the application prior to the onset of permanent injury and is associated with the rapid recovery of phrenic nerve function.4
Endpoints of Ablation
Tachycardia Termination During Radiofrequency Energy Application
Sudden termination of AT during RF application suggests that the lesion has affected a critical isthmus, and that site should be targeted for additional lesions. However, AT termination during RF application as the sole criterion of a successful ablation is hazardous because such an AT can also terminate spontaneously, secondary to premature atrial complexes induced by RF energy delivery, and by partial, rather than complete, isthmus block. Therefore, termination of AT during RF application is insufficient by itself as an endpoint.4,18
Noninducibility of Tachycardia
To use this criterion as a reliable endpoint, careful assessment of inducibility should be performed prior to ablation, and the feasibility and best method of reproducible induction of the AT should be documented at baseline before ablation. In the setting of easy inducibility prior to ablation, one can consider the lack of inducibility as an indicator of successful ablation. Noninducibility of the arrhythmia is inapplicable if the original arrhythmia either is noninducible at baseline or was inadvertently terminated mechanically. Noninducibility may also reflect conduction delay in the critical isthmus, and not stable block, or it may be secondary to changes in autonomic tone.4,18
Documentation of a Line of Block
Complete stable conduction block within the reentry path is the most useful and objective endpoint for ablation of macroreentrant AT. Complete conduction block following linear ablation across the isthmus can be confirmed by atrial pacing on one side of the ablation line and mapping along the line demonstrating absence of electrograms or the presence of widely separated parallel double potentials recorded all along the line with marked delay and reversal in the direction of activation on the opposite side of the linear lesion. For a vertically oriented RA free wall atriotomy, assessment of conduction block can be facilitated by the use of a multielectrode Halo-type catheter, which can document activation sequences anterior and posterior to the scar, so that when pacing close to the contiguously ablated isthmus, the absence of a wavefront penetrating the scar and isthmus is used to indicate conduction block. Additionally, assessment of electrical block across the ablation line can be facilitated by the use of the noncontact mapping system (EnSite, St. Jude Medical, St. Paul, Minn.), which allows for simultaneous mapping of the entire chamber of interest (versus sequential point-by-point mapping) during pacing on either side of the ablation line.4,18
Outcome
Atrial enlargement and myocardial hypertrophy and extensive scarring in patients with congenital heart disease can interfere with RF energy delivery and limit RF lesion depth. Additionally, the complexity of anatomy, vascular access issues, and the multiplicity of circuits in some patients can render the mapping and ablation procedure very challenging. Nonetheless, the combined use of 3-D mapping and tip cooling techniques has improved acute success rates to nearly 90%. However, high recurrence rates and the onset of new arrhythmias remain problematic (up to 30% to 45% within the first year), which may be related, at least in part, to ongoing changes in the arrhythmia substrate.8
Importantly, in some congenital heart malformations (e.g., AV canal defects), the AVN is displaced just anterior to the mouth of the CS. Consequently, ablating in the right inferior paraseptal region can cause AV block. Therefore, for patients with CTI-dependent macroreentry, lateral ablation lines are generally preferred, to prevent AV block.10
1. Triedman J.K. Atypical atrial tachycardias in patients with congenital heart disease. Heart Rhythm. 2008;5:315-317.
2. Garan H. Atypical atrial flutter. Heart Rhythm. 2008;5:618-621.
3. de Groot N.M., Zeppenfeld K., Wijffels M.C., et al. Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery: role of circumscribed areas with heterogeneous conduction. Heart Rhythm. 2006;3:526-535.
4. Saul J.P. Role of catheter ablation in postoperative arrhythmias. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S7-S12.
5. Anselme F. Macroreentrant atrial tachycardia: pathophysiological concepts. Heart Rhythm. 2008;5(Suppl):S18-S21.
6. Seslar S.P., Alexander M.E., Berul C.I., et al. Ablation of nonautomatic focal atrial tachycardia in children and adults with congenital heart disease. J Cardiovasc Electrophysiol. 2006;17:359-365.
7. Roos-Hesselink J.W., Karamermer Y. Significance of postoperative arrhythmias in congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S2-S6.
8. Khairy P., Balaji S. Cardiac arrhythmias in congenital heart diseases. Indian Pacing Electrophysiol J. 2009;9:299-317.
9. Warnes C.A., Williams R.G., Bashore T.M., et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118:e714-e833.
10. Kanter R.J. Pearls for ablation in congenital heart disease. J Cardiovasc Electrophysiol. 2010;21:223-230.
11. Khairy P., Van Hare G.F. Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm. 2009;6:283-289.
12. Khairy P., Aboulhosn J., Gurvitz M.Z., et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;31(122):868-875.
13. Khairy P. EP challenges in adult congenital heart disease. Heart Rhythm. 2008;5:1464-1472.
14. Medi C., Kalman J.M. Prediction of the atrial flutter circuit location from the surface electrocardiogram. Europace. 2008;10:786-796.
15. Morady F., Oral H., Chugh A. Diagnosis and ablation of atypical atrial tachycardia and flutter complicating atrial fibrillation ablation. Heart Rhythm. 2009;6(Suppl):S29-S32.
16. Miyazaki H., Stevenson W.G., Stephenson K., et al. Entrainment mapping for rapid distinction of left and right atrial tachycardias. Heart Rhythm. 2006;3:516-523.
17. Deo R., Berger R. The clinical utility of entrainment pacing. J Cardiovasc Electrophysiol. 2009;20:466-470.
18. Khairy P., Stevenson W.G. Catheter ablation in tetralogy of Fallot. Heart Rhythm. 2009;6:1069-1074.
19. Weerasooriya R., Jais P., Wright M., et al. Catheter ablation of atrial tachycardia following atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2009;20:833-838.
20. Jais P., Matsuo S., Knecht S., et al. A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: importance of localized reentry. J Cardiovasc Electrophysiol. 2009;20:480-491.