Atrial Tachyarrhythmias in Adults With Congenital Heart Disease
Advances in pediatric cardiovascular care have resulted in a rapidly expanding population of adults with congenital heart disease (ACHD). However, improved surgical outcomes in the young have resulted in alternate sources of morbidity and mortality in the adult, central among which are cardiac arrhythmias. Atrial tachyarrhythmias (ATs) are the most commonly encountered.1 The etiology of these arrhythmias is multifactorial, and they can arise as a consequence of underlying hemodynamic derangements, chronic cyanosis, elevated pressures, or prior surgery in which patches and suture lines create the substrate for tachycardia, most frequently macroreentrant. The clinical burden of these arrhythmias is substantial, and the development of AT is associated with an increased risk of stroke, heart failure, and death.1,2 Ventricular dysfunction and a predisposition to bradyarrhythmias can constrain the choice of antiarrhythmic agents in ACHD and as such, catheter ablation—coupled with or instead of antiarrhythmic therapy—is being used increasingly. The advent of sophisticated mapping systems has improved procedural success rates of catheter ablation,3 although recurrences after successful ablation occur more frequently in this patient cohort, motivating active and ongoing research in this area. This chapter presents an overview of the epidemiology of congenital heart disease (CHD) and reviews the clinical effects, mechanisms, and management of AT in this population.
Epidemiology
Changing Epidemiology of Congenital Heart Disease
The development of advanced surgical techniques for patients with CHD and improved pediatric care have resulted in an increasing and aging population of ACHD, including those with complex lesions.1 More than 90% of children born with CHD are expected to survive to adulthood. In a large Canadian population-based database, the prevalence of CHD was 1.2% in children, 0.4% in adults, and 0.6% in the general population in the year 2000.4 For severe CHD lesions, the prevalence was 0.38 per 1000 adults, representing 9% of the total ACHD population. The prevalence of adults with severe CHD is rising faster than the prevalence of children with severe CHD and in the year 2000 there were nearly equal numbers of adults and children with severe CHD.4 Current estimates in the United States of people with CHD are in excess of 2 million with an approximate equal number of children and adults.5 It is expected that the number of adults with severe CHD will outnumber the number of children with severe CHD. These findings reflect the need for more specialized care facilities for ACHD patients to meet the needs of the additional morbidity burden including arrhythmias.
Risk of Atrial Tachyarrhythmias in Congenital Heart Disease
A large population-based study demonstrated that the prevalence of AT is approximately 15% in ACHD, which is nearly threefold higher than in the general population.1 In general, older patients with CHD are more likely to develop AT. For example, the 20-year risk of developing AT is 7% in a 20-year-old patient and 38% in a 55-year-old patient.1 Overall, approximately 50% of 20-year-old patients with CHD will develop AT during their lifetime.1 This risk is higher in those with severe CHD lesions and in those with right-sided lesions.1,6 The prevalence and mechanism of arrhythmias varies according to the underlying anatomic defect and method of surgical repair (Tables 78-1, 78-2). The prevalence of AT ranges from 4.8% in patients with patent ductus arteriosus to 33% in those with Ebstein anomaly.1 The following section discusses the congenital cardiac malformations at highest risk of developing AT, including Ebstein anomaly, complete transposition of the great arteries (TGA), univentricular heart (UVH), atrial septal defect (ASD), and tetralogy of Fallot (TOF).
Table 78-1
Prevalence of Atrial Tachyarrhythmias in Selected ACHD Cohorts
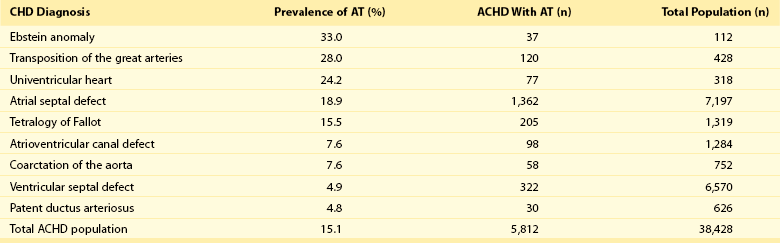
Modified from Bouchardy et al: Atrial arrhythmias in adults with congenital heart disease, Circulation 120:1679-1686, 2009.
Table 78-2
Relative Risk for Specific Tachyarrhythmias in Common Congenital Heart Defects
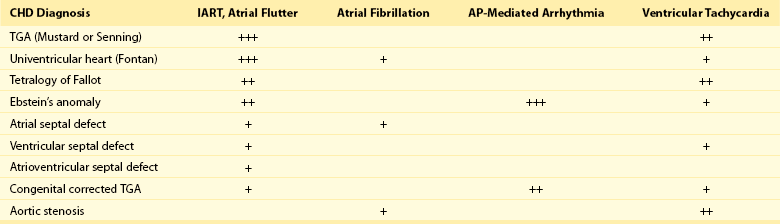
Modified from Walsh EP: Interventional electrophysiology in patients with congenital heart disease. Circulation 115:3224-3234, 2007.
Populations at Risk
Ebstein Anomaly
Ebstein anomaly is an uncommon congenital cardiac malformation accounting for less than 1% of CHD lesions. It is characterized by apical displacement and tethering of the septal and posterior leaflets of the tricuspid valve, creating a broad area of atrialized right ventricle. There is often coexistence of an ASD (84%), but other cardiac anomalies can also occur, including pulmonary stenosis (10%) and ventricular septal defect (4%).7 The pathophysiologic abnormalities that occur in Ebstein anomaly place these patients at increased risk of developing AT: a markedly enlarged right atrium (RA), left-to-right atrial shunting, and the not infrequent occurrence of accessory pathways.8 A recent study from the Mayo Clinic demonstrated that 36% of operated patients reported an episode of AT in adulthood.9 In patients experiencing AT, the most common mechanism of AT is atrial fibrillation or flutter (64%), followed by atrioventricular reentrant tachycardia (AVRT) (45%) and atrioventricular nodal reentrant tachycardia (AVNRT) (9%).8 Approximately 17% of patients with AT demonstrate multiple mechanisms of AT.8 AVRT can induce atrial fibrillation, and in conjunction with rapid antegrade conduction over an accessory pathway this can in turn provoke ventricular fibrillation.
Complete Transposition of the Great Arteries
Complete TGA is characterized by ventriculoarterial discordance and atrioventricular (AV) concordance. The prevalence of complete TGA is 5% to 7% of all congenital cardiac malformations. From the mid 1960s to the late 1980s, these patients underwent repair with atrial switch procedures using either artificial material (Mustard) or myocardial tissue (Senning) to redirect the systemic and pulmonary venous circulation, such that the morphologic right ventricle serves as the systemic ventricle and the morphologic left ventricle as the pulmonary venous ventricle. Although arterial switch surgery is the current procedure of choice, most adults currently have Mustard or Senning baffles. These surgeries involve extensive atrial reconstruction and predispose the patient to sinus node dysfunction and late AT.10 In a large cohort study of 478 early Mustard survivors (median age at operation, 1.3 years) the incidences of sinus node dysfunction and AT and were 60% and 24%, respectively, at 20 years after the index operation.10 Independent risk factors for the occurrence of late AT included perioperative bradycardia, permanent heart block, need for reoperation, and loss of sinus rhythm during follow-up. A later study in a subset of these patients demonstrated that by adulthood 48% of Mustard patients had experienced at least one episode of AT, with most patients having experienced intraatrial reentrant tachycardia (IART) or atrial flutter (AFL).11 Pulmonary hypertension, systemic ventricular dysfunction, and junctional rhythm before 18 years of age were independent predictors for AT.11 Documented AT has also been associated with sudden death in patients with atrial switch.12
The arterial switch operation is the most contemporary surgical approach for complete TGA. Besides restoring the morphologic left ventricle as the systemic ventricle, this procedure also reduces the incidence of sinus node dysfunction and late AT. A Japanese multicenter study of 624 1-year survivors (median age at operation, 2 months) demonstrated incidences of 1.0% and 4.3% for sinus node dysfunction and AT, respectively, during a mean follow-up duration of 10 years.13 This low incidence of AT appears to remain fairly constant during long-term follow-up, with a prevalence of approximately 4.5% reported at adulthood.14 These data support the notion that extensive atrial surgery is an important contributor to the development of AT in TGA patients.
Univentricular Heart with Fontan Surgery
The Fontan palliation was introduced in 1971 to treat tricuspid atresia. Four decades later, the Fontan circulation encompasses a spectrum of anatomic substrates, staging options, and operative techniques resulting in one functional ventricular chamber.15 Types of Fontan procedures include the atriopulmonary connection, a right atrioventricular connection, and total cavopulmonary connection (i.e., an intracardiac or extracardiac lateral tunnel connection, or an extracardiac conduit; Figure 78-1). ATs are highly prevalent, depending on type of surgery and age, and are associated with substantial morbidity.16,17 Earlier single-center cohort studies, in which a large proportion of patients had an atriopulmonary Fontan, reported a higher incidence of late AT ranging from 16% to 22%, with a mean follow-up duration of approximately 5 years after surgery.18,19 Risk factors for the development of late postoperative AT included RA enlargement, systemic AV regurgitation, sinus node dysfunction, older age at the time of cardiac surgery, elevated preoperative pulmonary artery pressure, early postoperative arrhythmias, lower functional status, and longer follow-up duration.17–19 Earlier canine models of atriopulmonary Fontan have shown that suture lines alone can contribute to the development of IART. As a consequence of these observations, the Fontan procedure was revised to minimize both RA dilatation and the extent of atrial surgery, resulting in the (1) lateral tunnel connection and (2) extracardiac total cavopulmonary conduit. In a recent multicenter cross-sectional pediatric cohort study of 521 early Fontan survivors (mean age, 12 years) the prevalences of IART, AVRT, and focal AT were 7.3%, 1.8%, and 0.8%, respectively. The risk of IART decreased after the Fontan operation until 4 to 6 years after completion, and it then increased with age thereafter.17 The prevalence of IART was related to the amount of RA dilatation and extent of atrial surgery: 19% in the atriopulmonary connection, 7% in the intracardiac lateral tunnel, 5% in the extracardiac lateral tunnel, and only 2% in the external conduit Fontan.17 Previously, a single-center study demonstrated a 15-year incidence of late AT of 13% for patients with intracardiac lateral tunnel repair versus 39% for atriopulmonary Fontan patients.15 Intracardiac lateral tunnel repair requires a lot of atrial surgery, whereas with the extracardiac Fontan, there is less extensive atrial surgery. The use of an extracardiac Fontan circulation has resulted in a low incidence of AT, 4.2% during a mean follow-up of 6 years.20 Although the duration of follow-up for these newer surgical variants is shorter than for the atriopulmonary Fontan, it is hoped that the avoidance of an extensive atriotomy and intraatrial suture lines will contribute to a reduction of AT in the long-term.
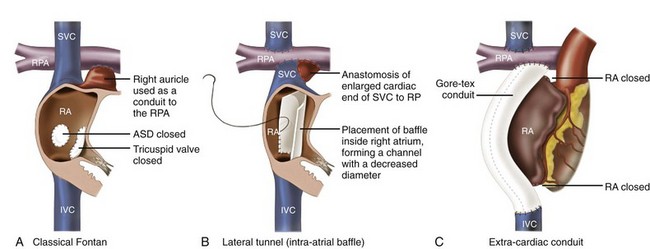
Figure 78-1 The different types of Fontan circulation. A, The atriopulmonary connection consists of closing the atrial septal defect (ASD) and connecting the right atrium (RA) directly to the right pulmonary artery (RPA). B, The intracardiac total cavopulmonary connection, or lateral tunnel procedure, connects the superior vena cava to the right pulmonary artery, so that the blood is directed from the inferior vena cava (IVC) to the pulmonary artery. C, The extracardiac cavopulmonary connection consists of a direct anastomosis of the superior vena cava (SVC) to the RPA and in the interposition of an extracardiac prosthesis between the IVC and the RPA. The advantage of the procedure is that it can be performed without myocardial ischemia, there are fewer suture lines in the RA, and there are no foreign material in the RA. (From d’Udekem et al: The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation 116:I157-I164, 2007.)
Atrial Septal Defect
Atrial septal defect is the most common congenital cardiac malformation in adults.4 Several anatomic types of ASD exist. Ostium secundum defects occur as a result of an increased resorption of the primum septum or an undergrowth of the flap of the foramen ovale, whereas ostium primum defects results from abnormal development of the endocardial cushions and are accompanied by anomalies of the mitral valve. The resultant atrial enlargement secondary to left-to-right atrial shunting—and mitral regurgitation in ostium primum defects—contributes to arrhythmia development. Sinus venosus defects result from the abnormal development of the sinus horn with a resulting defect in proximity to the sinus node. A shunt resulting from the ASD itself, anomalous pulmonary venous drainage, and prolonged AV node conduction predispose to subsequent arrhythmias. Secundum defects are the most common (60%), with primum defects accounting for 20% and superior sinus venosus defects 15%. In the absence of associated lesions, the development of late AT is low in those operated during childhood.21 Murphy et al.21 demonstrated that the incidence of late AT after closure increased from 17% in the 12- to 24-year-old age group to 55% in the over 41-year-old age group during late follow-up.21 The prevalence of AT is approximately 20% in patients undergoing surgical closure of ASD in adulthood, and the majority of patients with preoperative AT continues to experience AT during long-term follow-up after surgical closure.22,23 Furthermore, a randomized study showed that surgical closure at an older age (>40 years) does not appear to protect against new-onset AT compared with medical therapy.23 In the current era of predominantly percutaneous ASD closure, risk factors for the development of late AT after closure at adult age remain similar to those identified for the surgical cohort and include older age (>40 years) at time of closure and a prior history of AT.24 A prospective study of 200 patients with ASD demonstrated an incidence of AT of 16% during long-term follow-up after device closure.24 These data support the need to close defects at a young age to prevent the development of AT.
Tetralogy of Fallot
Tetralogy of Fallot is the most common cyanotic heart disease in adults, and it accounts for approximately 10% of CHD lesions. Surgical repair includes patch closure of the ventricular septal defect, relief of right ventricular outflow tract obstruction, and repair of associated anomalies. Ventriculotomies have largely been abandoned in favor of transatrial or transpulmonary approaches to accomplish the repair, because the latter appear to reduce the risk for subsequent ventricular tachyarrhythmias (VT). Although VT is recognized as a potentially life-threatening arrhythmia in this patient population, the development of AT is an important contributor to morbidity—including heart failure, reoperation, subsequent VT, and stroke—and mortality.25 In a large multicenter cohort study of 793 patients with TOF, sustained AT and VT occurred in 10% and 12%, respectively, at 35 years after repair.26 Older age at repair, previous palliative surgery, and significant tricuspid regurgitation at follow-up were independent predictors of AT.26 In a recent multicenter, cross-sectional study (n = 556; mean age, 37 ± 12 years), the prevalence of AT was 20.1%.27 Interestingly, this study identified risk factors associated with a specific AT type. Independent factors associated with IART/AFL were RA dilatation and hypertension, whereas atrial fibrillation was associated with older age, left ventricular dysfunction, and left atrial dilatation. The number of cardiac surgeries was a risk factor for both IART/AFL and atrial fibrillation. The prevalence of atrial fibrillation increased markedly after 45 years of age.27
Clinical Impact
Morbidity
Arrhythmias are an important cardiovascular reason for hospitalization of ACHD and are an increasingly frequent cause of morbidity and mortality.1,28 A population-based study in the Netherlands demonstrated that 31% of cardiovascular hospital admissions in the ACHD population were related to arrhythmias.28 The development of AT has been associated with a greater than 50% increase in the risk of stroke,1 whereas patients with intracardiac shunts and transvenous leads are deemed to be at even higher risk of stroke, estimated at a sixfold to sevenfold increase.29
New-onset AT can contribute to symptoms of palpitations, dizziness, chest pain, and syncope. In addition, it can herald worsening hemodynamic outcome, and the risk associated with AT can be amplified in the presence of the abnormal underlying circulation. For example, in patients with surgically corrected TOF, significant pulmonary regurgitation and left ventricular dysfunction are important risk factors for AT,25,27 whereas in other CHD defects elevated pulmonary artery pressures are an important risk factor for the development of AT.11,22 The development of AT confers a twofold to threefold increased risk of heart failure and has been associated with an increased risk of death from heart failure.1,2 Therefore, the onset of AT should prompt the clinician to look for hemodynamically reversible causes of AT.
Arrhythmias, especially AT, are common during pregnancy in women with CHD, with a prevalence of approximately 5% of completed pregnancies, and are associated with an increased risk of adverse fetal complications.30,31 Risk factors for developing arrhythmias during pregnancy are similar to those already described and include a prior history of arrhythmias and extensive atrial surgery (e.g., Fontan, Mustard, or Senning operation).30,31
Mortality
Bouchardy et al.1 demonstrated in a large ACHD population-based study (n = 38,428) that the presence of AT increases the risk of mortality by approximately 50%. A Dutch population-based study (n = 6933) also showed that AT was associated with all-cause mortality when corrected for age, gender, and severity of underlying CHD (adjusted hazard ratio of 1.8).2 Previous lesion-specific studies demonstrated an increase in mortality in patients with CHD and AT, particularly in Mustard or Senning patients,12 TOF patients,25 and Fontan patients.32 For the overall ACHD population, AT is associated with death owing to heart failure (hazard ratio of 5 : 1),2 whereas AT is also associated with sudden death in Mustard and Senning patients12 and thromboembolic death in Fontan patients.32
The ACHD population consists of different CHD subgroups with different anatomic diagnoses and background risks for death. Risk factors for mortality in the specific subgroup of ACHD with AT include poor functional class, UVH, pulmonary hypertension, and (residual) valvular lesions. ACHD with more than one risk factor have a 5-year mortality rate of approximately 30%.33
Mechanism of Atrial Tachyarrhythmias
Macroreentrant Atrial Tachycardia
The most commonly encountered AT mechanism in ACHD is that of macroreentrant AT, followed by atrial fibrillation.34 Focal AT, AVNRT, and AVRT occur less frequently.35 The term IART is used most commonly to describe the atrial reentrant tachycardias encountered in the patient with CHD. With the exception of Fontan patients, cavotricuspid isthmus (CTI)-dependent AFL remains the most common mechanism of macroreentrant AT in patients with CHD.36–39 Some authors prefer to use the term IART for both AFL and non-CTI-dependent IART.3,40 In patients with CHD, the propensity to IART is facilitated both by natural anatomic and surgically created barriers that result in delayed conduction. A variety of conduction barriers that confine IART circuits (e.g., the right AV valve, vena cavae, crista terminalis, surgical suture lines, patches, baffles, and conduits) have been described in these patients.38 In addition, chronic cyanosis, increased atrial wall stress, and increasing age contribute to the development of abnormal atrial myocardium and fibrosis (Figure 78-2). Patchy islands of scar surrounded by viable myocardium facilitating reentry have been described at the time of intracardiac mapping.41 The most common critical corridor of conduction delay in IART in ACHD is the area between the right lateral atriotomy scar and the inferior caval vein.36,38
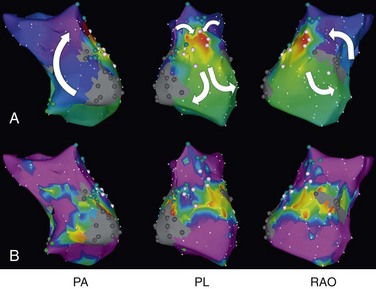
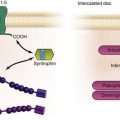
Figure 78-2 Electroanatomic mapping (CARTO XP) of intraatrial reentrant tachycardia (IART) in a Fontan patient with an atriopulmonary connection. A, Color-coded activation map showing a double-loop figure eight IART circuit rotating around two dense areas of scar (the right anterior oblique [RAO] view demonstrates a potential lateral right atriotomy scar). Tachycardia terminated by radiofrequency applications in the area between the two scars demonstrating fractionated atrial electrograms. No further atrial tachyarrhythmia could be induced. Local activation times are color-coded from red, orange, yellow, green, light blue, dark blue, and purple. The white arrows denote the direction of the IART circuit. B, Bipolar voltage map. The maximum peak-to-peak atrial electrogram amplitude is displayed with areas of very low (≤0.10 mV) or no voltage depicted in gray, and areas of normal endocardium (>0.50 mV) depicted in purple. All colors in between denote intermediate low-voltage zones (0.10-0.50 mV). Blue dots mark areas with double potentials, and pink dots mark areas with fractionated potentials. PA, Posterior anterior; PL, posterolateral view.
The arrhythmia mechanism can be reflected in the surface electrocardiogram, which can be helpful in making distinguishing between AFL and IART. Usually the P wave contour of these IARTs will differ from the familiar sawtooth appearance of typical AFL because of the course of the reentrant path. Furthermore, IART in ACHD usually has a longer cycle length than CTI-dependent AFL, although considerable overlap exists.39 Multiple IART circuits can be present in the same patient, most notably in Fontan patients who usually have the longest cycle lengths of macroreentrant AT because of their markedly enlarged RA (Figure 78-3).3,37,40
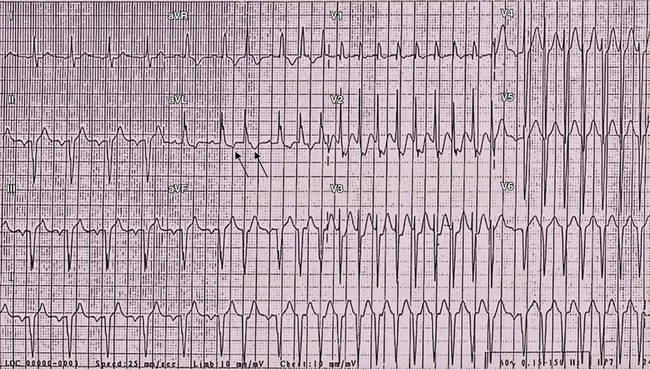
Figure 78-3 Electrocardiographic manifestations of intraatrial reentrant tachycardia (IART) in a patient with previous atriopulmonary Fontan connection. IART at relatively slow cycle length (340 ms) is present throughout the 12-lead electrocardiographic recording with 2 : 1 atrioventricular conduction evident in the first part of the tracing, converting to intermittent 1 : 1 conduction. Arrows denote the flutter waves.
It is well known that the incidence of AT in CHD increases with age,1 and the data suggest that in addition to the surgical and anatomic barriers,38 there is progressive atrial disease because of chronic atrial volume or pressure overload. Studies in Fontan patients have provided insight into the development of atrial myopathy and the relationship with IART. One study using three-dimensional (3D) electroanatomic mapping demonstrated progressive areas of low voltage with increasing age in adults with old-style Fontan modifications consistent with an evolving electroanatomic substrate in concert with the recognized progressive RA dilatation, stretch, and wall thickening.17,42
Focal Atrial Tachycardia
Although less common than IART/AFL, focal patterns of AT activation have been observed in ACHD.35,36 Focal AT can arise throughout both atria and are not limited to the site of suture lines.35 Low-amplitude fractionated potentials and continuous electrical activity have been demonstrated at the site of origin of focal AT, and it has been postulated that focal AT originates from areas with enhanced nonuniform anisotropic properties.35 The exact underlying mechanism of focal AT is not clear, and both a microreentrant mechanism and triggered activity could have roles.35
Atrial Fibrillation
Atrial fibrillation occurs in approximately 30% of ACHD with AT, most commonly in patients with residual left-sided lesions or unrepaired heart disease.33,34 Atrial fibrillation, in contrast to IART or AFL, is usually found in the older ACHD population.34 A lesion-specific study patients with TOF demonstrated that independent risk factors for the development of atrial fibrillation were older age, left ventricular dysfunction, left atrial dilatation, and number of cardiac surgeries.27 The exact mechanism of atrial fibrillation in ACHD is not well understood.
Accessory Pathways
The presence of an accessory pathway in CHD is most commonly associated with Ebstein anomaly of the tricuspid valve (34%).43 It has been postulated that failure of the AV annulus to fuse with the hypoplastic central cardiac fibrous skeleton allows persistence of fetal accessory pathways in these patients. As a result, the localization of the accessory pathway is usually on the right side near the dysplastic tricuspid valve, in contrast to the general population.8,43,44 Furthermore, multiple accessory pathways are common (18% to 52%) in patients with Ebstein anomaly and AVRT8,44,45 and the pathways are more likely to be broad bands rather than the discrete, microscopic pathways typically found in patients with structurally normal hearts.8 Mahaim fibers have been described in as many as 13% of patients with Ebstein anomaly and pre-excitation. In congenitally corrected TGA, it is thought that the increased incidence of Wolff-Parkinson-White syndrome is explained by the coexistence of Ebstein anomaly of the left-sided (systemic) tricuspid valve.46
Management
Drugs, Cardioversion, and Pacemaker Therapy
Direct current cardioversion for AT has a high conversion rate (94%).47 Although thromboembolism complicating cardioversion is rare,47 echocardiographic observation of atrial thrombi is not infrequent. Therefore, it is recommended that all patients with AT and high-risk cardiac anatomy, as well as those at lower risk who are not anticoagulated but have been in tachycardia of uncertain duration, undergo a transesophageal echocardiography examination before direct current cardioversion is attempted.47 Patients thought to be at high risk for intracardiac thrombi include those with complex CHD (e.g., TGA, TOF, Eisenmenger syndrome, Ebstein anomaly, intracardiac baffles, Fontan operation), and patients with mechanical valve prosthesis, in addition to those with conventional risk factors for stroke.47,48 The presence of a residual shunt in patients with intracardiac pacemakers also constitutes an increasingly recognized risk.29 Risk stratification for atrial fibrillation and thromboembolism tailored to patients with CHD in order to guide anticoagulation has not been established. Therefore, until such information is available, existing guidelines for anticoagulation before and after cardioversion should be used.48
In patients with CHD and AT, symptoms of palpitations for estimating duration of the arrhythmia are often unreliable because patients sometimes experience symptoms only when there is 1 : 1 conduction in IART. Furthermore, IART with 2 : 1 conduction can be mistaken as sinus rhythm when the arrhythmia cycle length is long and the atrial activity obscured by the QRS on alternate beats (see Figure 78-3).
In the acute setting, intravenous medications such as ibutilide, procainamide, and amiodarone have been reported to have success rates of 71%, 50%, and 15%, respectively, for cardioversion in young patients with CHD,49,50 whereas oral sotalol has also been shown to be effective with a 84% conversion rate in a similar aged cohort.51 When cardioversion is not appropriate, control of the ventricular response rate in AT can be attempted with β-blockers, calcium channel blockers, or digoxin. For patients with CHD and IART, 1 : 1 conduction is not infrequent and adequate rate control might be difficult to achieve. The experience with long-term antiarrhythmic drug therapy in the patient with CHD remains variable, and a number of factors such as ventricular dysfunction and predisposition to bradyarrhythmias must be considered when selecting an antiarrhythmic agent.47 Antiarrhythmic drugs are frequently poorly tolerated because of negative inotropic and chronotropic effects. The most commonly used antiarrhythmic drugs are digoxin, amiodarone, β-blockers, and sotalol.33 Amiodarone is notable for amiodarone-induced thyrotoxicosis, especially in those with a low body mass index (i.e., <21) and goiter.52 Furthermore, the potential proarrhythmic effects of antiarrhythmic agents must be kept in mind when being used for continued therapy, as should the risk of reduced atrial conduction and potential 1 : 1 AV conduction.
In ACHD requiring pacemaker therapy, the mode of pacing (physiological versus nonphysiological pacing) does not seem to influence the development of AT, in contrast to the general population.53 Interestingly, a few small studies have suggested that permanent atrial overdrive pacing suppresses the occurrence of IART/AFL.54 Pacemakers with algorithms for antitachycardia pacing have been demonstrated to have a 43% to 54% conversion rate for IART in CHD and may be a useful adjunctive therapy.55 However, atrial antitachycardia pacing can also be proarrhythmic accelerating the tachycardia, increasing the risk of conversion to atrial fibrillation and has been reported as precipitating ventricular arrhythmias.
Catheter Ablation in Congenital Heart Disease
The use of 3D electroanatomic mapping (e.g., CARTO; Biosense Webster, Diamond Bar, CA) and remote-controlled magnetic navigation (Niobe; Stereotaxis, St. Louis, MO) has greatly facilitated the identification of arrhythmia circuits in CHD, enabling catheter ablation to be applied successfully to many forms of tachycardia in this patient cohort. However, despite the introduction of advanced mapping techniques, catheter ablation of postoperative AT is time consuming and difficult, with a greater likelihood of recurrence during follow-up.3,40,56 Several challenges might be encountered when performing ablation procedures in patients with CHD, including the complexity of the underlying anatomy and prior surgeries, difficulty with vascular access, unstable reference catheter position, baffle or conduit obstruction, and the potential for multiple and nonsustained AT.46
General Mapping and Ablation Technique
The complexity of the macroreentrant circuits depends on the underlying CHD anatomy and type of repair. Patients with cardiac chambers in normal orientation sometimes have CTI-dependent AFL, and entrainment mapping can be used to determine whether the isthmus is involved in the circuit.40 After confirmation of involvement of the isthmus, linear ablation across this isthmus is warranted as a first step. Large-tip catheters or irrigated ablation catheters with high-output radiofrequency generators are often required for transmural lesions to interrupt the macroreentrant circuits in these patients because of the extent of atrial hypertrophy. Termination of the tachycardia in response to isthmus ablation confirms involvement of the isthmus, and demonstration of bidirectional block confirms a complete linear block across the isthmus.
If the tachycardia persists without a change in cycle length or activation or has changed, the possibility of IART macroreentry around a right lateral atriotomy scar or septal patch should be explored using activation and voltage mapping.41,57 Zones of slow conduction, possibly critical to the maintenance of the IART circuit, are usually identified by low-amplitude fractionated atrial electrograms (see Figure 78-2). These zones are further defined by their relation to known anatomic obstacles or acquired or surgical electrical conduction barriers. Care must be taken to identify the location of the right phrenic nerve, which is potentially susceptible to injury by ablation. Commonly, linear RF lesions are applied at the best and easiest accessible myocardial site in regard to catheter handling and at the narrowest possible isthmic zone, typically between the atriotomy scar and the tricuspid annulus or inferior vena cava. Although noninducibility during programmed atrial stimulation is the desired procedural end point, additional ATs are sometimes induced; however, whether to address these arrhythmias should be determined by the clinical relevance of these AT.
When the macroreentrant circuit cannot be identified because of the presence of multiple or unstable IART circuits, the RA needs to be explored extensively by voltage mapping to identify potential isthmuses that can be targeted for RFCA. Areas of scar can be identified by low-voltage areas with no or very low bipolar voltage (<0.1 mV).58 These areas and their relation to all naturally and surgical barriers of electrical conduction could identify potential RFCA targets.
If there is no wavefront circulating around a macroscopic anatomic structure or a functional line of conduction block, a focal AT should be suspected. Activation mapping can be supplemented by unipolar electrogram recordings from the distal ablation catheter demonstrating a QS pattern, and radiofrequency applications directed at the site of earliest activation can result in termination of the tachycardia.35,58
Challenges in Catheter Ablation in Patients With Congenital Heart Disease
Ablation of macroreentrant circuits becomes more complicated whenever RA anatomy is distorted by abnormalities of atrial situs, atresia of an AV valve, and intraatrial baffles. For example, in patients who have undergone the Mustard or Senning operation for complete TGA, the most common form of AT is a CTI-dependent AFL.36,37,59 However, depending on the surgical handling of the inferior suture line of the atrial baffle, most of the lateral critical isthmus may be located on the pulmonary venous side of the circulation, and successful ablation will need to be accomplished by ablation in both the systemic venous and pulmonary venous atria.40,59 The ablation catheter has to be delivered via a retrograde arterial approach, transbaffle puncture, or baffle leak to reach this site for ablation.40 Transbaffle puncture of synthetic material (e.g., Dacron, Gore-Tex) can sometimes be difficult, but provides greater catheter stability compared with a retrograde approach.
Fontan patients are at risk of multiple complex arrhythmia circuits.41,60 The location of successful ablation of macroreentrant tachycardias in Fontan patients is usually located on the lateral wall, and not the cavotricuspid isthmus as in other patients with CHD.36,40,60 The presence of multiple ATs with frequently changing activation sequences can hamper identification of the clinically relevant critical isthmus.41,60 In addition, the markedly enlarged RA limits the use of noncontact mapping because of the inaccuracy of electrogram reconstruction and poor definition of atrial scar.61 Although patients with a total cavopulmonary connection are at lower risk of developing AT, when AT ablation is required in these patients it is technically daunting because of the limited access to the RA, especially in those with an external conduit. Various novel approaches have been described, including a retrograde arterial approach, crossing a fenestration, puncturing the conduit itself, entering the roof of the atrium via the superior vena cava and left pulmonary artery,62 and direct transthoracic approach to the atrium.63 All these approaches are technically challenging and are associated with significant risk of complications.
Accessory pathway ablation in patients with CHD is common in patients with Ebstein anomaly.64 Successful accessory pathway ablation requires accurate localization of the AV groove and knowledge of AV node location. The appropriate level for ablation is the true AV groove, which is marked by the course of the right coronary artery. It can be difficult to establish the true AV groove with conventional fluoroscopy, and a right coronary angiogram may simplify this task.45,46 Radiofrequency ablation of septal accessory pathways in close proximity to the AV node poses a risk of heart block, and the recent availability of cryoablation may be protective of iatrogenic heart block in such cases.65 Catheter ablation of accessory pathways has a lower success rate in Ebstein anomaly (76% to 81%) than in patients with structurally normal hearts (>95%), and the risk of recurrence is higher.44,45 Therefore, some authors advocate the use of concomitant arrhythmia surgery at the time of corrective surgery in patients with Ebstein anomaly and documented AT.8
Outcome of Catheter Ablation in Patients With Congenital Heart Disease
Overall, the acute procedural success rate for AT ablation ranges from 63% to 100% (Table 78-3). Combining data for approximately 500 patients undergoing ablation in 11 centers results in a procedural success rate of 74%. Patient and technical factors influence the outcome of ablation. Predictors of procedural success include the use of irrigated ablation and 3D electro-anatomic mapping, absence of atrial fibrillation, and absence of complex atrial surgery (i.e., Mustard/Senning repair and Fontan palliation).3,40 Using 3D electro-anatomic mapping, recent ablation studies in TOF patients and Fontan patients have demonstrated a procedural success rates of >90% and 78%, respectively.60,66–67 The recent introduction of remote magnetic navigation in guiding RFCA in patients with CHD offers the potential to improve procedural success rates and may offer advantage in complex anatomic situations.68–69
Unfortunately, recurrence of AT remains disappointingly common, and has been estimated at approximately 50% at 4 years after successful RFCA.40 Recurrent ATs often follow circuits that are different from the initial AT, suggesting that these ATs are new and not caused by previous ablative lesions.39 It has been postulated that to reduce AT recurrences, both the cavotricuspid isthmus and lateral RA wall should be targeted even if the mapped circuit indicates only one region.67 Although AT recurrences are frequent, the ablative procedure can still provide effective palliation by reducing the frequency of AT episodes and eliminating the need for long-term drug therapy.3
Pulmonary Vein Isolation in Patients with Congenital Heart Disease
The experience with pulmonary vein isolation for treatment of drug-refractory atrial fibrillation in the ACHD population is limited. One single-center study of 36 patients demonstrated a freedom from atrial fibrillation of 42% at 1 year after one procedure.70 The most common CHD lesion was ASD (61%). Complication rates between patients with and without CHD were similar, except for a higher risk of vascular site complications in patients with CHD (8% versus 1%; P < 0.05).70
Surgical Intervention for Arrhythmias
Multiple modifications in technique and timing of corrective operations have significantly reduced the incidence of late postoperative AT. For example, the contemporary approach to patients with complete TGA is an arterial switch operation in the newborn period. This approach has effectively abolished the late complications of IART and sinus node dysfunction in this condition.13,14 Similarly, the use of the extracardiac conduit in the Fontan operation for UVH prevents the massive RA enlargement seen with the atriopulmonary connection and is associated with less atrial surgery. The adoption of these techniques has resulted in a decrease in the incidence of postoperative AT.20 In addition, recent decades have seen the advent of surgical techniques designed specifically to eliminate AT in patients with CHD, predominantly in ACHD whose defects were repaired with earlier techniques (e.g., atriopulmonary connection in Fontan patients).71 These operations usually have the combined goals of tachycardia elimination and improvement of hemodynamics.
Fontan Conversion and Atrial Maze
Recognition that atriopulmonary Fontan patients have recurrent, often refractory AT in addition to hemodynamic derangements led a group at the Children’s Memorial Hospital in Chicago to combine arrhythmia surgery with Fontan conversion to an extracardiac total cavopulmonary connection.71 Earlier surgical experience identified that hemodynamic conversion alone was insufficient to eliminate IART.72 In addition to RA reduction, arrhythmia surgery consists of a modified RA maze procedure, left atrial Cox maze III, and implantation of an epicardial dual chamber antitachycardia pacing system (Figure 78-4). A modified RA maze has been shown to be superior to anatomic isthmus block in preventing recurrence of AT. Analysis of 111 consecutive patients undergoing concomitant arrhythmia surgery demonstrated an AT recurrence rate in early survivors of 14% at 5 years after conversion.71 Other centers have achieved similar results with AT recurrence rates ranging from 14% to 28%.73,74 Although the perioperative mortality in the Chicago experience is less than 1%,71 other centers have demonstrated higher perioperative mortality rates ranging from 7% to 14%.73–75 This risk must be balanced against the potential electrophysiological benefit. Older age (>20 years) at Fontan conversion has been identified as a risk factor for perioperative mortality and could account for some of the variation in perioperative mortality between series.73 It is unresolved whether arrhythmia surgery should be considered primary therapy for AT in the absence of an underlying hemodynamic indication for surgery.
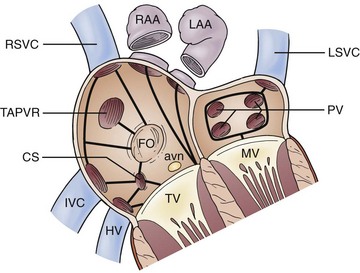
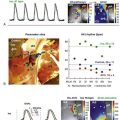
Figure 78-4 Surgical modifications of atrial maze procedure in complex anatomy. Solid black lines indicate sites of cryoablation. Avn, Atrioventricular node; CS, coronary sinus; FO, foramen ovale; HV, hepatic vein; IVC, inferior vena cava; LAA, left atrial appendage; LSVC, left superior vena cava; MV, mitral valve; PV, pulmonic vein; RAA, right atrial appendage; RSVC, right superior vena cava; TAPVR, total anomalous pulmonary venous return; TV, tricuspid valve. (Reprinted with permission from Mavroudis et al: Arrhythmia surgery in patients with and without congenital heart disease. Ann Thorac Surg 86:857-868, 2008.)
Pulmonary Valve Replacement in Tetralogy of Fallot
Significant pulmonary regurgitation has been shown to be a risk factor for the development of late AT in patients after TOF repair.25 Many patients with significant regurgitation undergo repeat pulmonary valve replacement. Although the hemodynamic benefit (positive RV remodeling) of this surgery has been well established,76 pulmonary valve replacement alone seems insufficient for reducing the propensity for AT.77 Therrien et al.77 demonstrated in a small substudy that concomitant surgical atrial cryoablation at the time of pulmonary valve implant is a helpful adjunct for preventing recurrent AT. Cryoablation was targeted at the cavotricuspid isthmus in those with a history of typical AFL and a modified RA maze procedure was performed in patients with IART or atrial fibrillation. In a larger cohort of these patient, a significant reduction in the incidence of recurrent AT was observed among those with a history of AT who underwent arrhythmia surgery compared with those who did not (25% versus 66% at 7.5 years).78
References
1. Bouchardy, J, Therrien, J, Pilote, L, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009; 120:1679–1686.
2. Verheugt, CL, Uiterwaal, CS, van der Velde, ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010; 31:1220–1229.
3. Triedman, JK, Alexander, ME, Love, BA, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002; 39:1827–1835.
4. Marelli, AJ, Mackie, AS, Ionescu-Ittu, R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007; 115:163–172.
5. Roger, VL, Go, AS, Lloyd-Jones, DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012; 125:e2–e220.
6. Bernier, M, Marelli, AJ, Pilote, L, et al. Atrial arrhythmias in adult patients with right- versus left-sided congenital heart disease anomalies. Am J Cardiol. 2010; 106:547–551.
7. Brown, ML, Dearani, JA, Danielson, GK, et al. The outcomes of operations for 539 patients with Ebstein anomaly. J Thorac Cardiovasc Surg. 2008; 135:1120–1136.
8. Khositseth, A, Danielson, GK, Dearani, JA, et al. Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. J Thorac Cardiovasc Surg. 2004; 128:826–833.
9. Brown, ML, Dearani, JA, Danielson, GK, et al. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. J Am Coll Cardiol. 2008; 52:460–466.
10. Gelatt, M, Hamilton, RM, McCrindle, BW, et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997; 29:194–201.
11. Puley, G, Siu, S, Connelly, M, et al. Arrhythmia and survival in patients >18 years of age after the mustard procedure for complete transposition of the great arteries. Am J Cardiol. 1999; 83:1080–1084.
12. Kammeraad, JA, van Deurzen, CH, Sreeram, N, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004; 44:1095–1102.
13. Hayashi, G, Kurosaki, K, Echigo, S, et al. Prevalence of arrhythmias and their risk factors mid- and long-term after the arterial switch operation. Pediatr Cardiol. 2006; 27:689–694.
14. Tobler, D, Williams, WG, Jegatheeswaran, A, et al. Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2010; 56:58–64.
15. d’Udekem, Y, Iyengar, AJ, Cochrane, AD, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007; 116:I157–I164.
16. Giannakoulas, G, Dimopoulos, K, Yuksel, S, et al. Atrial tachyarrhythmias late after Fontan operation are related to increase in mortality and hospitalization. Int J Cardiol. 2012; 157:221–226.
17. Stephenson, EA, Lu, M, Berul, CI, et al. Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010; 56:890–896.
18. Fishberger, SB, Wernovsky, G, Gentles, TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997; 113:80–86.
19. Gelatt, M, Hamilton, RM, McCrindle, BW, et al. Risk factors for atrial tachyarrhythmias after the Fontan operation. J Am Coll Cardiol. 1994; 24:1735–1741.
20. Giannico, S, Hammad, F, Amodeo, A, et al. Clinical outcome of 193 extracardiac Fontan patients: the first 15 years. J Am Coll Cardiol. 2006; 47:2065–2073.
21. Murphy, JG, Gersh, BJ, McGoon, MD, et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med. 1990; 323:1645–1650.
22. Gatzoulis, MA, Freeman, MA, Siu, SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999; 340:839–846.
23. Attie, F, Rosas, M, Granados, N, et al. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J Am Coll Cardiol. 2001; 38:2035–2042.
24. Silversides, CK, Haberer, K, Siu, SC, et al. Predictors of atrial arrhythmias after device closure of secundum type atrial septal defects in adults. Am J Cardiol. 2008; 101:683–687.
25. Harrison, DA, Siu, SC, Hussain, F, et al. Sustained atrial arrhythmias in adults late after repair of tetralogy of fallot. Am J Cardiol. 2001; 87:584–588.
26. Gatzoulis, MA, Balaji, S, Webber, SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000; 356:975–981.
27. Khairy, P, Aboulhosn, J, Gurvitz, MZ, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010; 122:868–875.
28. Verheugt, CL, Uiterwaal, CS, van der Velde, ET, et al. The emerging burden of hospital admissions of adults with congenital heart disease. Heart. 2010; 96:872–878.
29. Khairy, P, Landzberg, MJ, Gatzoulis, MA, et al. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation. 2006; 113:2391–2397.
30. Drenthen, W, Pieper, PG, Roos-Hesselink, JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007; 49:2303–2311.
31. Silversides, CK, Harris, L, Haberer, K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tachyarrhythmia and impact on fetal and neonatal outcomes. Am J Cardiol. 2006; 97:1206–1212.
32. Khairy, P, Fernandes, SM, Mayer, JE, Jr., et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008; 117:85–92.
33. Yap, SC, Harris, L, Chauhan, VS, et al. Identifying high risk in adults with congenital heart disease and atrial arrhythmias. Am J Cardiol. 2011; 108:723–728.
34. Kirsh, JA, Walsh, EP, Triedman, JK. Prevalence of and risk factors for atrial fibrillation and intra-atrial reentrant tachycardia among patients with congenital heart disease. Am J Cardiol. 2002; 90:338–340.
35. de Groot, NM, Zeppenfeld, K, Wijffels, MC, et al. Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery: role of circumscribed areas with heterogeneous conduction. Heart Rhythm. 2006; 3:526–535.
36. Lukac, P, Pedersen, AK, Mortensen, PT, et al. Ablation of atrial tachycardia after surgery for congenital and acquired heart disease using an electroanatomic mapping system: Which circuits to expect in which substrate? Heart Rhythm. 2005; 2:64–72.
37. Collins, KK, Love, BA, Walsh, EP, et al. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000; 86:969–974.
38. Love, BA, Collins, KK, Walsh, EP, et al. Electroanatomic characterization of conduction barriers in sinus/atrially paced rhythm and association with intra-atrial reentrant tachycardia circuits following congenital heart disease surgery. J Cardiovasc Electrophysiol. 2001; 12:17–25.
39. de Groot, NM, Atary, JZ, Blom, NA, et al. Long-term outcome after ablative therapy of postoperative atrial tachyarrhythmia in patients with congenital heart disease and characteristics of atrial tachyarrhythmia recurrences. Circ Arrhythm Electrophysiol. 2010; 3:148–154.
40. Yap, SC, Harris, L, Silversides, CK, et al. Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol. 2010; 56:1589–1596.
41. Nakagawa, H, Shah, N, Matsudaira, K, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation. 2001; 103:699–709.
42. Yap, SC, Harris, L, Downar, E, et al. Evolving electroanatomic substrate and intra-atrial reentrant tachycardia late after Fontan surgery. J Cardiovasc Electrophysiol. 2012; 23:339–345.
43. Chetaille, P, Walsh, EP, Triedman, JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart Rhythm. 2004; 1:168–173.
44. Reich, JD, Auld, D, Hulse, E, et al. The Pediatric Radiofrequency Ablation Registry’s experience with Ebstein’s anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol. 1998; 9:1370–1377.
45. Cappato, R, Schluter, M, Weiss, C, et al. Radiofrequency current catheter ablation of accessory atrioventricular pathways in Ebstein’s anomaly. Circulation. 1996; 94:376–383.
46. Walsh, EP. Interventional electrophysiology in patients with congenital heart disease. Circulation. 2007; 115:3224–3234.
47. Ammash, NM, Phillips, SD, Hodge, DO, et al. Outcome of direct current cardioversion for atrial arrhythmias in adults with congenital heart disease. Int J Cardiol. 2012; 154:270–274.
48. Fuster, V, Ryden, LE, Cannom, DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011; 123:e269–e367.
49. Chang, PM, Silka, MJ, Moromisato, DY, et al. Amiodarone versus procainamide for the acute treatment of recurrent supraventricular tachycardia in pediatric patients. Circ Arrhythm Electrophysiol. 2010; 3:134–140.
50. Hoyer, AW, Balaji, S. The safety and efficacy of ibutilide in children and in patients with congenital heart disease. Pacing Clin Electrophysiol. 2007; 30:1003–1008.
51. Rao, SO, Boramanand, NK, Burton, DA, et al. Atrial tachycardias in young adults and adolescents with congenital heart disease: conversion using single dose oral sotalol. Int J Cardiol. 2009; 136:253–257.
52. Stan, MN, Ammash, NM, Warnes, CA, et al. Body mass index and the development of amiodarone-induced thyrotoxicosis in adults with congenital heart disease-A cohort study. Int J Cardiol. 2012.
53. Walker, F, Siu, SC, Woods, S, et al. Long-term outcomes of cardiac pacing in adults with congenital heart disease. J Am Coll Cardiol. 2004; 43:1894–1901.
54. Snyder, CS, Dobson, G, Rollinson, N, et al. Suppression of intra-atrial reentrant tachycardia in patients with atrial overdrive pacing. Congenit Heart Dis. 2008; 3:200–204.
55. Stephenson, EA, Casavant, D, Tuzi, J, et al. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003; 92:871–876.
56. Paul, T, Windhagen-Mahnert, B, Kriebel, T, et al. Atrial reentrant tachycardia after surgery for congenital heart disease: endocardial mapping and radiofrequency catheter ablation using a novel, noncontact mapping system. Circulation. 2001; 103:2266–2271.
57. Kalman, JM, VanHare, GF, Olgin, JE, et al. Ablation of ‘incisional’ reentrant atrial tachycardia complicating surgery for congenital heart disease. Use of entrainment to define a critical isthmus of conduction. Circulation. 1996; 93:502–512.
58. de Groot, NM, Schalij, MJ, Zeppenfeld, K, et al. Voltage and activation mapping: how the recording technique affects the outcome of catheter ablation procedures in patients with congenital heart disease. Circulation. 2003; 108:2099–2106.
59. Zrenner, B, Dong, J, Schreieck, J, et al. Delineation of intra-atrial reentrant tachycardia circuits after mustard operation for transposition of the great arteries using biatrial electroanatomic mapping and entrainment mapping. J Cardiovasc Electrophysiol. 2003; 14:1302–1310.
60. de Groot, NM, Lukac, P, Blom, NA, et al. Long-term outcome of ablative therapy of postoperative supraventricular tachycardias in patients with univentricular heart: a European multicenter study. Circ Arrhythm Electrophysiol. 2009; 2:242–248.
61. Abrams, DJ, Earley, MJ, Sporton, SC, et al. Comparison of noncontact and electroanatomic mapping to identify scar and arrhythmia late after the Fontan procedure. Circulation. 2007; 115:1738–1746.
62. Mehta, C, Jones, T, De Giovanni, JV. Percutaneous transcatheter communication between the pulmonary artery and atrium following an extra-cardiac Fontan: an alternative approach to fenestration avoiding conduit perforation. Catheter Cardiovasc Interv. 2008; 71:936–939.
63. Nehgme, RA, Carboni, MP, Care, J, et al. Transthoracic percutaneous access for electroanatomic mapping and catheter ablation of atrial tachycardia in patients with a lateral tunnel Fontan. Heart Rhythm. 2006; 3:37–43.
64. Roten, L, Lukac, P, DEG, N, et al. Catheter ablation of arrhythmias in ebstein’s anomaly: a multicenter study. J Cardiovasc Electrophysiol. 2011; 22:1391–1396.
65. Bar-Cohen, Y, Cecchin, F, Alexander, ME, et al. Cryoablation for accessory pathways located near normal conduction tissues or within the coronary venous system in children and young adults. Heart Rhythm. 2006; 3:253–258.
66. de Groot, NM, Lukac, P, Schalij, MJ, et al. Long-term outcome of ablative therapy of post-operative atrial tachyarrhythmias in patients with tetralogy of Fallot: a European multi-centre study. Europace. 2012; 14:522–527.
67. Mah, DY, Alexander, ME, Cecchin, F, et al. The electroanatomic mechanisms of atrial tachycardia in patients with tetralogy of Fallot and double outlet right ventricle. J Cardiovasc Electrophysiol. 2011; 22:1013–1017.
68. Akca, F, Bauernfeind, T, Witsenburg, M, et al. Acute and long-term outcomes of catheter ablation using remote magnetic navigation in patients with congenital heart disease. Am J Cardiol. 2012; 110:409–414.
69. Ernst, S, Babu-Narayan, SV, Keegan, J, et al. Remote-controlled magnetic navigation and ablation with 3D image integration as an alternative approach in patients with intra-atrial baffle anatomy. Circ Arrhythm Electrophysiol. 2012; 5:131–139.
70. Philip, F, Muhammad, KI, Agarwal, S, et al. Pulmonary vein isolation for the treatment of drug-refractory atrial fibrillation in adults with congenital heart disease. Congenit Heart Dis. 2012.
71. Mavroudis, C, Deal, BJ, Backer, CL, et al. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg. 2007; 84:1457–1465.
72. Kreutzer, J, Keane, JF, Lock, JE, et al. Conversion of modified Fontan procedure to lateral atrial tunnel cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 1996; 111:1169–1176.
73. Takahashi, K, Fynn-Thompson, F, Cecchin, F, et al. Clinical outcomes of Fontan conversion surgery with and without associated arrhythmia intervention. Int J Cardiol. 2009; 137:260–266.
74. Aboulhosn, J, Williams, R, Shivkumar, K, et al. Arrhythmia recurrence in adult patients with single ventricle physiology following surgical Fontan conversion. Congenit Heart Dis. 2010; 5:430–434.
75. Mascio, CE, Pasquali, SK, Jacobs, JP, et al. Outcomes in adult congenital heart surgery: analysis of the Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg. 2011; 142:1090–1097.
76. Oosterhof, T, van Straten, A, Vliegen, HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007; 116:545–551.
77. Therrien, J, Siu, SC, Harris, L, et al. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001; 103:2489–2494.
78. Karamlou, T, Silber, I, Lao, R, et al. Outcomes after late reoperation in patients with repaired tetralogy of Fallot: the impact of arrhythmia and arrhythmia surgery. Ann Thorac Surg. 2006; 81:1786–1793.
79. Mavroudis, C, Deal, BJ, Backer, CL, et al. Arrhythmia surgery in patients with and without congenital heart disease. Ann Thorac Surg. 2008; 86:857–868.
80. Baker, BM, Lindsay, BD, Bromberg, BI, et al. Catheter ablation of clinical intraatrial reentrant tachycardias resulting from previous atrial surgery: localizing and transecting the critical isthmus. J Am Coll Cardiol. 1996; 28:411–417.
81. Akar, JG, Kok, LC, Haines, DE, et al. Coexistence of type I atrial flutter and intra-atrial re-entrant tachycardia in patients with surgically corrected congenital heart disease. J Am Coll Cardiol. 2001; 38:377–384.
82. Kannankeril, PJ, Anderson, ME, Rottman, JN, et al. Frequency of late recurrence of intra-atrial reentry tachycardia after radiofrequency catheter ablation in patients with congenital heart disease. Am J Cardiol. 2003; 92:879–881.
83. Liew, R, Catanchin, A, Behr, ER, et al. Use of non-contact mapping in the treatment of right atrial tachycardias in patients with and without congenital heart disease. Europace. 2008; 10:972–981.