Chapter 15 Atrial Septal Defect
Simple and Complex
Galen was aware of the foramen ovale and its normal postnatal closure.1 Botallo described a patent foramen ovale after birth without understanding its function in the fetus.1 Leonardo da Vinci wrote, “I have found from left auricle to right auricle the perforating channel.” Leonardo’s account of a true atrial septal defect is thought to be the first record of a congenital malformation of the human heart.2 In 1640, Pierre Gassendi based an entire treatise on observations of a patent foramen ovale in an adult cadaver.3
Karl von Rokitansky, in 1875, published superb observations on the pathologic anatomy of atrial septal defects and what he presumed to be their embryologic basis and distinguished septum primum from septum secundum defects. In 1921, Assmann’s4 description of the radiologic features of atrial septal defects paved the way for clinical recognition. In 1934, Roesler5 analyzed 62 necropsy cases of atrial septal defect, only one of which had been correctly diagnosed during life. In a landmark publication in 1941, Bedford, Papp, and Parkinson6 described the clinical features of atrial septal defects. Hudson’s7 1955 description of the normal and abnormal interatrial septum was refined in 1979 by Sweeney and Rosenquist.8 These early accounts have been extended by studies of the anatomy of the interatrial septum with tranesophageal echocardiography.9
The normal atrial septum viewed from its right side is a blade-shaped structure with a superoanterior margin that reflects the curvature of the ascending aorta, an inferior margin that borders the mitral annulus, and a posterior margin that is convex.8 The left side of the septum has a network of trabeculations that are remnants of the septum primum.
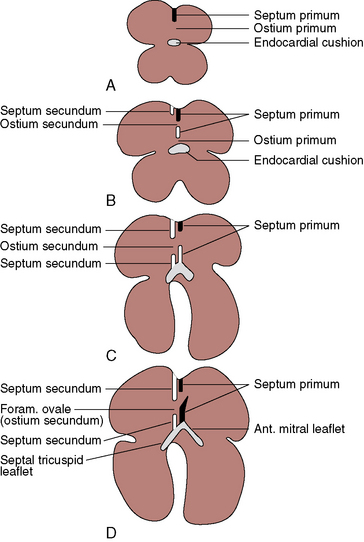
The fossa ovalis occupies about 28% of the septal area, irrespective of age, and is bordered by a limbus and guarded by a valve (see Figure 15-46C).8 After birth, the patent fetal foramen ovale closes via fusion of its valve with the limbus of the fossa ovalis as left atrial pressure exceeds right atrial pressure. The incidence of persistent patency of the foramen ovale declines from about one third during the first three decades of life to about one quarter during the fourth through eighth decades.10 The morphogenetic sequence of normal intrauterine formation and closure of the atrial septum is illustrated in Figure 15-1.7 Redundancy of the valve of the foramen is responsible for an atrial septal aneurysm (see Figure 15-47).11,12
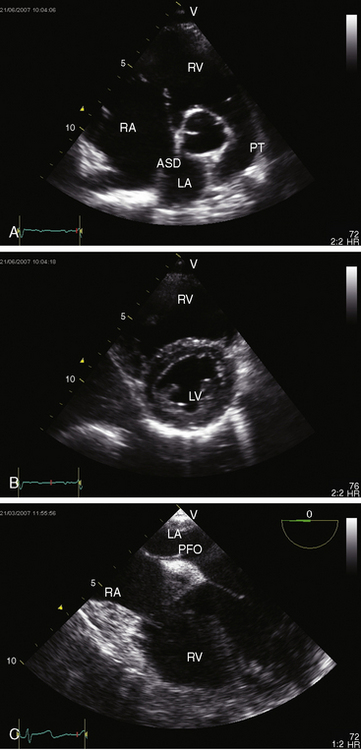
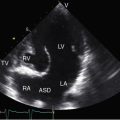
The most common type of atrial septal defect is in the ostium secundum or fossa ovalis location (Figures 15-2 and 15-3).13–15 These defects lie in a folded area rather than on a flat plane. Their anatomy is more complex on the right side than on the left side of the septum.16 Ostium secundum defects result from shortening of the valve of the foramen ovale, excessive resorption of the septum primum, or deficient growth of the septum secundum (see Figures 15-1, 15-2, and Figure 15-46C). Occasionally, the atrial septal perforations resembles Swiss cheese,17 or the interatrial communication is represented by multiple openings less than 5 mm in diameter.18
Next in frequency are ostium primum defects, also called atrioventricular septal defects because the atrioventricular septum is defective (absent; see Figures 15-1 and 15-2). Oddly and rarely, a communication exists in the atrioventricular septum when septal structures are intact.19
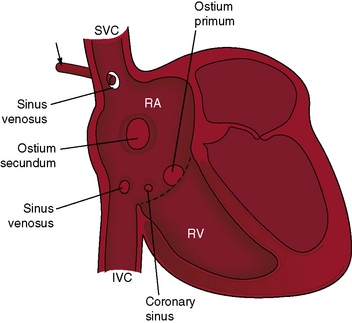
Sinus venosus atrial septal defects are uncommon, but not rare, and constitute 2% to 3% of interatrial communications. During normal embryogenesis, the inferior vena cava and the right superior vena cava are incorporated into the right horn of the sinus venosus. Faulty resorption results in a communication near the orifice of the superior or the inferior cava. The right valve of the sinus venosus is a broad membrane that almost partitions the developing right atrium. Both vena cavas are located on the left side of the membrane. The principle type of sinus venosus defect was described in 1868 as a “free communication between the auricles by deficiency of the upper part of the septum auricularum.”20
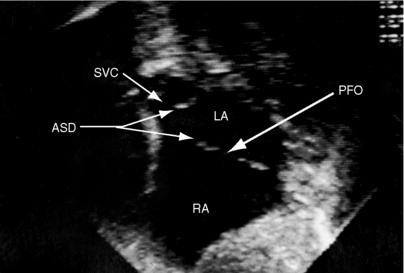
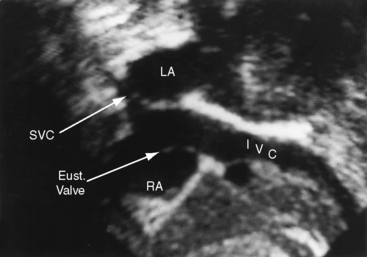
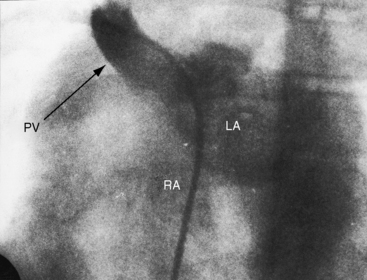
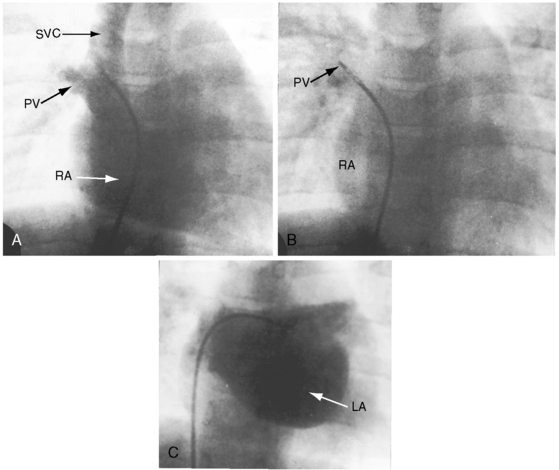
Superior vena caval sinus venosus defects are located immediately below the junction of the superior cava and the right atrium (see Figures 15-2 and 15-48) and vary from small to nonrestrictive. The orifice of the superior vena cava may override the defect, which is therefore biatrial.21 Inferior vena caval sinus venosus defects are located below the foramen ovale and merge with the floor of the inferior cava (see Figures 15-2 and 15-48).15,22 As the valve of the inferior vena cava resorbs, its rudiment becomes the fetal eustachian valve that directs inferior caval blood across the foramen ovale. Persistence of a large eustachian valve (Figure 15-4 and Videos 15-1A and 15-1B) channels inferior vena caval blood across an ostium secundum atrial septal defect (Figure 15-5 and Video 15-2) or across an inferior vena caval sinus venosus defect (see Figure 15-48).22,23 Atrial septal defects are usually located in only one of the foregoing locations, but separate ostium secundum, sinus venosus, and ostium primum defects occasionally coexist.24
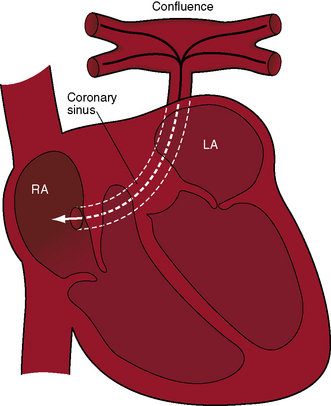
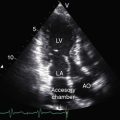
Coronary sinus atrial septal defects are uncommon but not rare. As the name implies, the defect is located at the site normally occupied by the right atrial ostium of the coronary sinus (see Figure 15-2)25 and is characterized by an opening in the wall of the distal end of the sinus or by unroofing caused by absence of the partition between the coronary sinus and left atrium. A left superior vena cava inserts into the upper left corner of the left atrium. A relatively rare combination consists of absence of the coronary sinus, a defect in the atrial septum in the location of the ostium of the coronary sinus, and a left superior vena cava connected to the left atrium.25 This combination is necessarily cyanotic because blood from the left superior vena caval enters the left atrium directly.
Spontaneous closure of an ostium secundum atrial septal defect refers to sealing of a true tissue defect, and not to cessation of a left-to-right shunt through a valve-incompetent foramen ovale.26,27 Ostium secundum atrial septal defects are seldom symptomatic in infants and young children (see Figure 15-46B and Videos15-3A through 15-3D); but when they are so manifest, approximately one third close spontaneously between 1 and 2 years of age.27 The mechanisms responsible for spontaneous closure remain to be established, but multiple small interatrial septal openings (diameters less than 5 mm) in newborns have a strong tendency to close during the first year of life.18
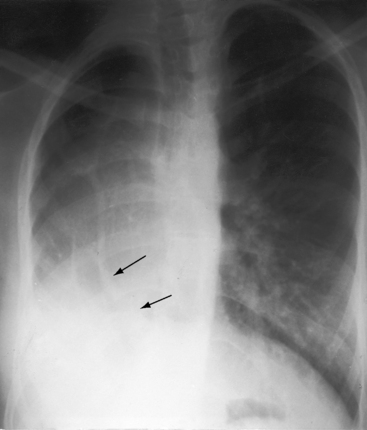
Anatomic connections and physiologic drainage of pulmonary veins are important distinctions in atrial septal defects. Connection refers to a pulmonary vein that is anatomically contiguous—connected—with a morphologic left atrium or a morphologic right atrium.28,29 Drainage refers to the physiologic pathway of blood from pulmonary veins into the left or right atrium. Pulmonary veins that connect normally can drain anomalously, but pulmonary veins that connect anomalously drain anomalously. Normal right pulmonary veins connect to the left atrium close to the rim of ostium secundum atrial septal defects,29,30 so a substantial portion of right pulmonary venous blood preferentially drains into the right atrium even though the veins connect anatomically to the left atrium (Figure 15-6). Partial anomalous pulmonary venous connection refers to one or more but not all pulmonary veins that connect anomalously to the right atrium.28,31 Total anomalous pulmonary venous connection exists when all four pulmonary veins connect anomalously to the right atrium, directly or indirectly. Ten percent to 15% of ostium secundum atrial septal defects are associated with partial anomalous pulmonary venous connections. Eighty percent to 90% of superior vena caval sinus venosus defects are associated with anomalous connection of the right superior pulmonary vein to the right atrium or superior vena cava (Figures 15-2 and 15-7).21 About 90% of partial anomalous pulmonary venous connections join the right upper or middle lobe pulmonary veins into the right atrium or superior vena cava.28,32 Partial anomalous connection of right pulmonary veins is usually associated with ostium secundum atrial septal defects,33 exceptionally is associated with an intact atrial septum, and may go unrecognized when associated with a restrictive sinus venosus defect (see Figure 15-7). Anomalous connection of left pulmonary veins is far less prevalent (incidence rate about 10%) than anomalous connection of right pulmonary veins and is represented by anomalous connection to the innominate vein or to a persistent left superior vena cava that attaches to the innominate vein. Bilateral partial anomalous pulmonary venous connections are rare.
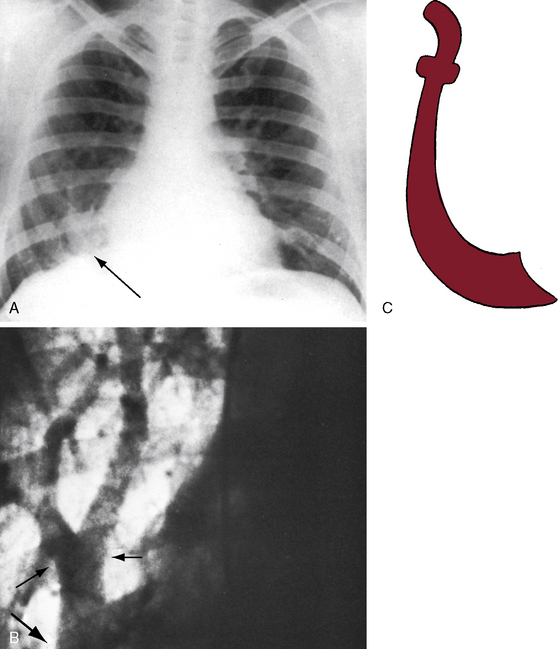
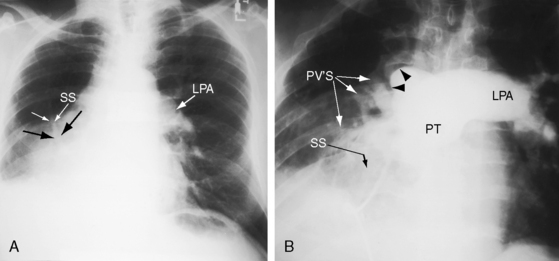
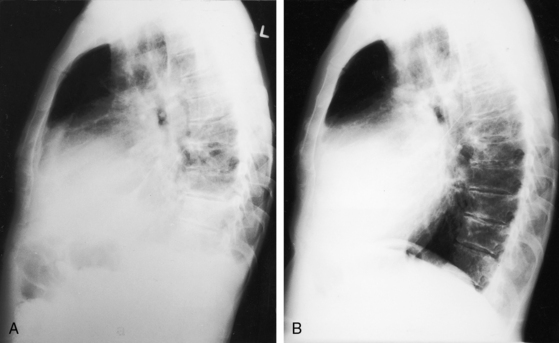
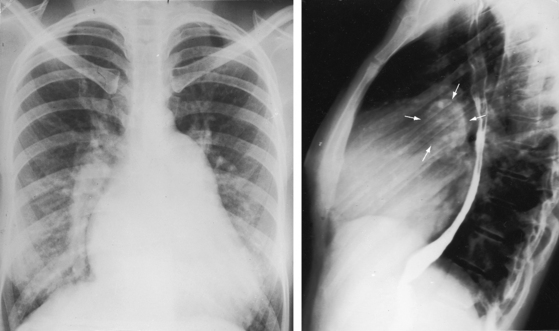
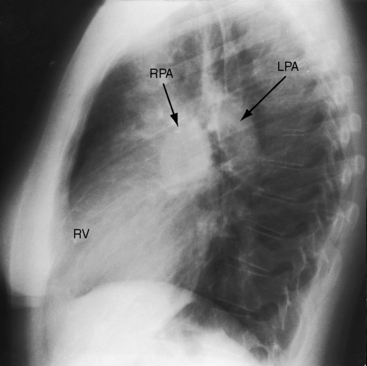
The scimitar syndrome, described in 1836 by Chassinat,34 is a rare anomaly characterized by connection of all of the right pulmonary veins into the inferior vena cava.35–38 The ipsilateral lung and pulmonary artery are usually hypoplastic (Figures 15-8, 15-9, and 15-10). The syndrome rarely involves the left lung.39 The term scimitar refers to a radiologic shadow that resembles the shape of a Turkish sword (see Figure 15-8C). The lower portion of the right lung is perfused by systemic arteries from the abdominal aorta.36,37
In ostium secundum atrial septal defects, anatomic studies of the mitral, tricuspid, and pulmonary valves have disclosed morphologic and architectural modifications.40 The mitral valve abnormalities consist of thickening and fibrosis of leaflets and chordae tendineae attributed to traumatic cusp movements that result from deformity of the left ventricular cavity. The lesions are thought to be the basis for age-related mitral regurgitation.41,42 Superior systolic displacement of the mitral leaflets (mitral valve prolapse) occurs because leaflets with normal area and chordal length are housed in a left ventricular cavity that is reduced in size and abnormal in shape from the leftward position of the ventricular septum (see Figure 15-46B).41
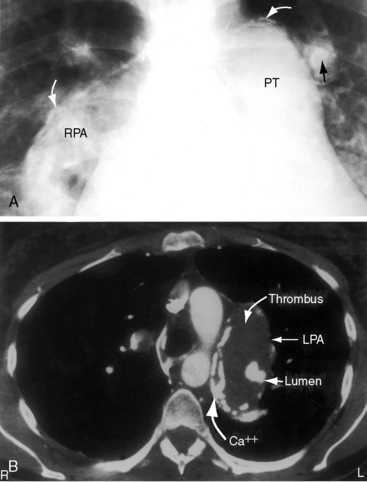
In patients with nonrestrictive atrial septal defects and pulmonary vascular disease, the hypertensive proximal pulmonary arteries dilate aneurysmally and contain mural calcification and intraluminal thrombi that can be massive and occlusive (Figure 15-11).43 Aneurysmal proximal pulmonary arteries may rupture.44 Abnormalities of medial smooth muscle, elastin, collagen, and ground substance reside in the walls of these pulmonary arteries and are held responsible for dilation that is out of proportion to hemodynamic or morphogenetic expectation.44
The physiologic consequences of atrial septal defects depend on the magnitude and chronicity of the left-to-right shunt and on the behavior of the pulmonary vascular bed. When the defect is restrictive, size per se determines the magnitude of the shunt.45 When the defect is nonrestrictive, there is no pressure difference between the right and left atrium, so shunt volume is determined by the relative compliance of the two ventricles.46 During diastole, all four cardiac chambers are in common communication, so blood can flow from the left atrium through the atrial septal defect into the right atrium and across the tricuspid valve into the right ventricle or can flow directly into the left ventricle across the mitral valve. Alternatively, blood from the right atrium can flow across the atrial septal defect into the left atrium across the mitral valve into the left ventricle or directly into the right ventricle across the tricuspid valve. In an ostium secundum atrial septal defect, the right ventricle is thinner and more compliant than the left ventricle, so blood flow is from left atrium through the atrial septal defect across the tricuspid valve into the relatively compliant right ventricle, which thus establishes a left-to-right shunt.46 The shunt reaches its peak in late systole and early diastole; it diminishes throughout diastole; and in late diastole, it is supplemented by atrial contraction (see Figure 15-5B).47,48 A small transient right-to-left shunt coincides with the onset of ventricular systole (see Figure 15-5B). Clinical and experimental studies of instantaneous flow across atrial septal defects have confirmed these flow patterns.47
The fetal circulation is not altered by an atrial septal defect because in utero interatrial flow is normally from right to left through a patent foramen ovale (see Figure 15-46C). At birth, there is little or no shunt in either direction across an atrial septal defect because the compliance of the right and left ventricles is virtually identical.20,49,50 The right ventricle gradually becomes thinner and more compliant than the left ventricle in response to the fall in neonatal pulmonary vascular resistance, so left atrial blood then flows across the atrial septal defect into the more compliant right ventricle.51 Pulmonary blood flow that is received by the right pulmonary veins is channeled into the right atrium because of proximity of the right pulmonary veins to the rim of the atrial septal defect (see previous; see Figure 15-6). Pulmonary blood flow received by the left pulmonary veins is channeled directly into the left atrium and is then shunted across the atrial septal defect. Accordingly, the right ventricle is volume overloaded and the left ventricle is volume underloaded.
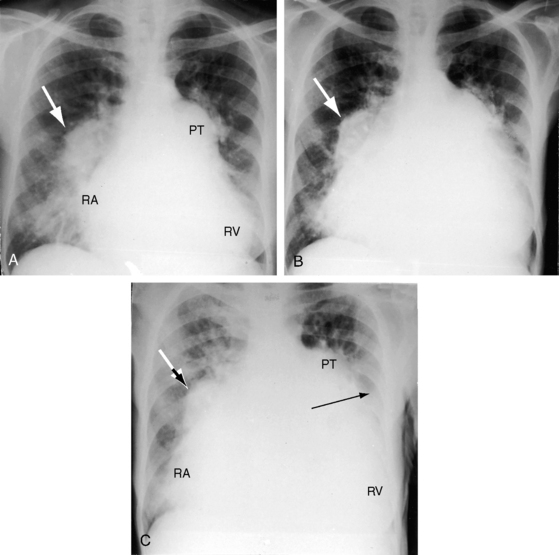
The mature right ventricle is a compliant chamber that readily adapts to volume overload and ejects its increased stroke volume into the low-resistance pulmonary vascular bed.51 Right ventricular function is usually maintained through the fourth decade.51 Ischemic heart disease and systemic hypertension conspire to reduce left ventricular compliance and thus to increase the left-to-right shunt. The additional volume overload of the right atrium provokes atrial fibrillation and atrial flutter, which further increase the left-to-right shunt and result in heart failure (Figure 15-12).
Left ventricular end-diastolic volume, stroke volume, ejection fraction, and cardiac output are decreased in infants and adults with an atrial septal defect,52–54 and ejection fraction tends to fall with exercise.52 Diminished left ventricular functional reserve is related to the mechanical effects of right ventricular volume overload, which displaces the ventricular septum into the left ventricular cavity, reducing its size and changing its shape from ovoid to crescentic (see previous; see Figure 15-46B).42,52,53 In addition, coronary reserve is compromised in the volume-overloaded right ventricle if the left main coronary artery is compressed by a dilated pulmonary trunk.55
An important and poorly understood deviation from the prevailing pattern of an asymptomatic onset of the left-to-right shunt across an atrial septal defect is the occasional infant with development of a large shunt and right ventricular failure.27,56 A left-to-right shunt that begins before the increase in right ventricular compliance has been attributed to more complete emptying of the right ventricle (reduced resistance to discharge). Right ventricular failure ensues because the neonatal right ventricle is volume overloaded before involution of its free wall thickness.57 Although right ventricular failure is occasionally intractable, there is a propensity for clinical improvement because of spontaneous closure of the atrial septal defect.18,27
The paucity of pulmonary vascular disease in patients with nonrestrictive atrial septal defects has been ascribed to the onset of the left-to-right shunt after pulmonary arterial pressure and pulmonary vascular resistance have normalized. A low-resistance low-pressure pulmonary vascular bed accommodates an appreciable increment in blood flow without a rise in pressure.58,59 An exception is the propensity for pulmonary hypertension in patients with atrial septal defects who are born at high altitude.60,61 Pulmonary vascular disease with a right-to-left shunt at sea level occurs in less than 10% of patients with an atrial septal defect and is believed to represent the coincidence in young females of primary pulmonary hypertension and an ostium secundum atrial septal defect (Figure 15-13; see Chapter 14).
Increased resistance to right ventricular discharge can also result from massive occlusive thrombus in dilated hypertensive proximal pulmonary arteries (see previous; see Figures 15-11B and 15-42). Older adults experience a moderate rise in pulmonary artery pressure with persistence of the left-to-right shunt. Thus, pulmonary hypertension with a nonrestrictive atrial septal defect at sea level is bimodal and is represented in young females with coexisting primary pulmonary hypertension or in older adults, male or female, who have moderate pulmonary hypertension with a persistent left-to-right shunt.
The physiologic consequences of an atrial septal defect with partial anomalous pulmonary venous connection are similar if not identical to those of an isolated atrial septal defect with an equivalent net shunt because the hemodynamic fault remains the left-to-right shunt at atrial level.21,62 However, flow through anomalous pulmonary veins into the right atrium is obligatory and is therefore established earlier than shunt flow across an atrial septal defect. When partial anomalous pulmonary venous connection occurs with an intact atrial septum, flow is increased in the segment of lung with the anomalous pulmonary veins because right atrial pressure is lower than left atrial pressure (intact atrial septum). The pressure gradient across the anomalously draining lung is therefore greater than across the normally draining lung.32,33
Anomalous systemic venous drainage from a normally aligned inferior vena cava results in cyanosis with increased pulmonary blood flow. In an ostium secundum atrial septal defect, small amounts of inferior vena caval blood transiently stream across the defect in early systole, a pattern appropriate for the direction of fetal blood from inferior vena cava across a foramen ovale.63 A large persistent eustachian valve sometimes extends from the orifice of the inferior vena cava to the margin of an ostium secundum atrial septal defect (see Figure 15-4), selectively channeling inferior caval blood into the left atrium and causing cyanosis.22,64 A large eustachian valve in the presence of an inferior vena caval sinus venosus atrial septal defect channels inferior caval blood directly into the left atrium.22
History
The female:male ratio is at least 2:1 in patients with an ostium secundum atrial septal defect,57 and the gender ratio in sinus venosus defects and in ostium primum defects is approximately equal.13 Ostium secundum atrial septal defects are sometimes familial, can recur in a several generations,65,66 and have been found in identical twins.67 Familial scimitar syndrome has been reported.68,69 Autosomal dominant inheritance is a feature of atrial septal defect with the Holt-Oram syndrome (see section Physical Appearance).70 Autosomal dominant inheritance tends to be the mode in inheritance in ostium secundum defects with prolonged atrioventricular conduction.71–73 In some members of a family, the atrial septal defect occurs with PR interval prolongation; other family members have PR prolongation with an intact atrial septum71; and still others experience sudden death. Mutations in the NKX2.5 gene have been associated with familial atrial septal defect and progressive prolongation of atrioventricular conduction.74 Concordant familial segregation of atrial septal defect has been reported with the Axenfeld-Reiger Anomaly (see section Physical Appearance).75
Atrial septal defects may go unrecognized for decades because symptoms are mild or absent and physical signs are subtle.13 The soft pulmonary midsystolic flow murmur in children and young adults is often dismissed as innocent (see Chapter 2). Conversely, an atrial septal defect may first come to light in a routine chest x-ray.76 An important exception is the symptomatic infant with an ostium secundum atrial septal defect (see Figure 15-46B) and congestive heart failure followed by spontaneous closure (see previous).26,27,57
About half of patients with a scimitar syndrome are either asymptomatic or only mildly symptomatic when the diagnosis is made, despite varying degrees of hypoplasia of the right lung (see Figures 15-8, 15-9, and 15-10).36,38 However, a small but important group of patients with scimitar syndrome consists of symptomatic infants with cyanosis and pulmonary hypertension.36 Older children and young adults come to light because an x-ray discloses the scimitar sign and the hypoplastic right lung or because of an atrial tachyarrhythmia, recurrent lower respiratory infections, or a murmur.36,38,77
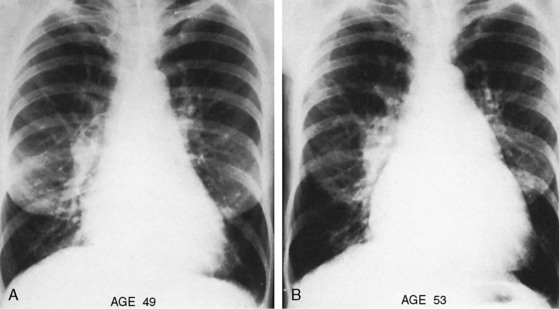
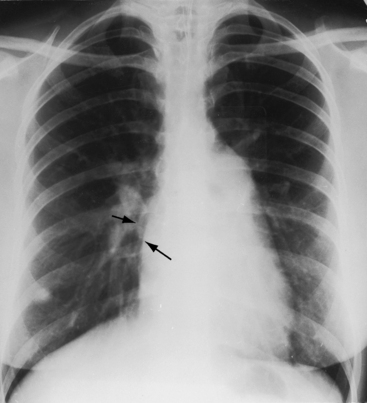
Paul Wood remarked, “In any series of geriatric necropsies atrial septal defect is always represented.”78 Ostium secundum atrial septal defects are among the most common congenital cardiac malformations in adults and account for 30% to 40% of patients over 40 years of age who have not undergone an operation.13,79–81 Patients often survive to advanced age, but life expectancy is not normal. Three quarters of patients are alive through the third decade, but three quarters are dead by age 50 years and 90% are dead by age 60 years.79 Sporadic survivals have been recorded beyond age 70 years, and rare examples are found of patients in their 80s or 90s (see Figure 15-43B).80–83 An exceptional case was a patient who died 3 months before his 95th birthday (see Figure 15-43A).80 Death is often unrelated to the malformation, but when a relationship exists, cardiac failure is the most common cause.
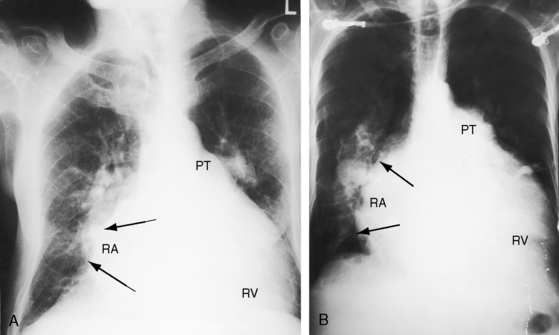
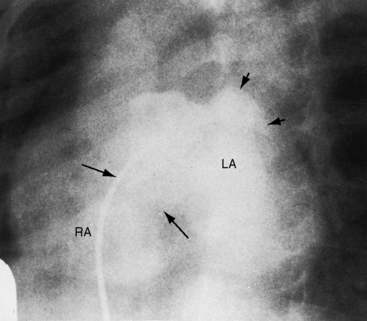
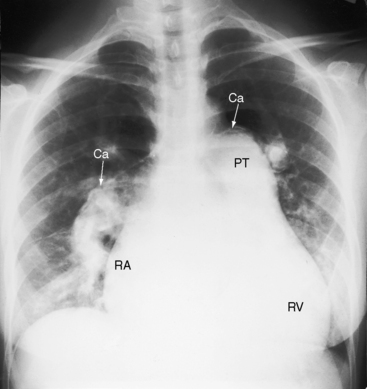
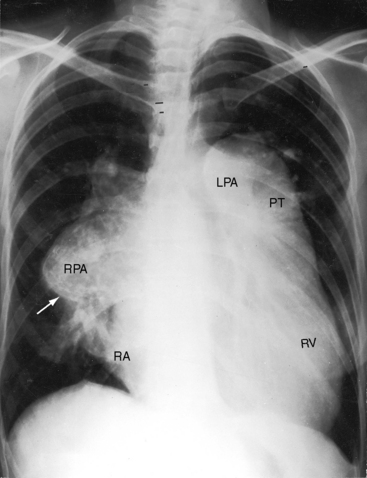
Figure 15-43 A, X-ray from the 95-year-old acyanotic man with a nonrestrictive ostium secundum atrial septal defect. The electrocardiogram (see Figure 15-32) shows atrial fibrillation. Pulmonary arterial and pulmonary venous vascularity are increased. The pulmonary trunk (PT) is dilated, but the ascending aorta is not border-forming. The right atrium (RA) and the right ventricle (RV) are strikingly enlarged. B, X-ray from an 87-year-old acyanotic woman with a nonrestrictive ostium secundum atrial septal defect, severe tricuspid regurgitation, and atrial fibrillation. Pulmonary vascularity is decreased. The pulmonary trunk (PT) is dilated, and the enlarged right branch contains eggshell calcification. The ascending aorta is not border-forming. The right atrium (RA) and right ventricle (RV) are strikingly enlarged. At necropsy, there was a large thrombus in the dilated right pulmonary artery and a smaller thrombus in the dilated left pulmonary artery.
The clinical course of ostium secundum atrial septal defects spans the reproductive years, and most patients are female. It is therefore reassuring that, despite the gestational increase in cardiac output and stroke volume, young gravida with an atrial septal defect generally endure pregnancy, even multiple pregnancies, without tangible ill effects.84 However, brisk hemorrhage during delivery provokes a rise in systemic vascular resistance and a fall in systemic venous return, a combination that augments the left-to-right shunt, sometimes appreciably.84 There is also a peripartum risk of paradoxical embolization from leg veins or pelvic veins because emboli carried by the inferior vena cava traverse the atrial septal defect and enter the systemic circulation.84
Dyspnea and fatigue are early symptoms of an ostium secundum atrial septal defect. The large left-to-right shunt is responsible for a decrease in pulmonary compliance and an increase in the work of breathing. Orthopnea may be experienced because the supine position increases the work of breathing in patients with reduced lung compliance. Platypnea-orthodeoxia is a rare syndrome characterized by orthostatic provocation of a right-to-left shunt across an atrial septal defect or a patent foramen ovale.85,86 Platypnea (dyspnea induced by the upright position and relieved by recumbency) and orthodeoxia (arterial desaturation in the upright position with improvement during recumbency) are features of this rare disorder. Clinical suspicion may originate from the patient who reports that dyspnea is provoked by standing upright.
Recurrent lower respiratory infections are common, especially in children. Although the pulmonary valve is theoretically susceptible to infective endocarditis because of the rapid rate of ejection, only a single case has been reported.87
The conditions of older patients deteriorate chiefly on three counts. First, a decrease in left ventricular distensibility associated with aging, ischemic heart disease, systemic hypertension, or acquired calcific aortic stenosis augments the left-to-right shunt.81,88 Second, an age-related increase in prevalence of paroxysmal atrial tachycardia, atrial fibrillation, and atrial flutter precipitates congestive heart failure (see Figure 15-45).89 Third, mild to moderate pulmonary hypertension in older adults occurs with a persistent left-to-right shunt, so the aging right ventricle is doubly beset by both pressure and volume overload. Pulmonary vascular disease with reversed shunt is believed to represent the coincidence of primary pulmonary hypertension with an ostium secundum atrial septal in young females who are predisposed to both lesions (see Figure 15-13; see previous).59 Importantly, the outlook is better when primary pulmonary hypertension occurs with an atrial septal defect or a patent foramen ovale that permits the right heart to decompress (see Chapter 14).
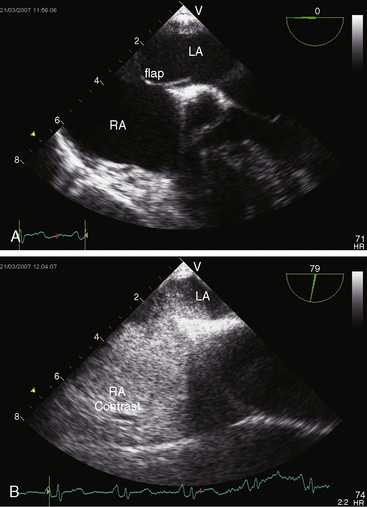
A patent foramen ovale is the most common remnant of the fetal circulation (see Figure 15-46C); it occurs in 10% to 15% of healthy adults and in 20% to 30% of normal hearts at postmortem.10,56,90 The patent foramen varies in anatomic and functional size and is implicated in paradoxical embolization, transient ischemic attacks, venoarterial gas embolism, decompression sickness, and platypnea-orthodeoxia (see previous).56,91
Atrial septal aneurysm is characterized by protrusion beyond the plane of the atrial septum and by rapid, dramatic phasic cardiorespiratory oscillations (see Figure 15-47 and Videos 15-4A through 15-4C).11,12,92 An atrial septal defect may coexist. Cerebral emboli originate from fibrin-platelet aggregates on the left atrial side of the aneurysm and are dislodged by the phasic excursions.92 Atrial septal aneurysms have been incriminated in atrial arrhythmias in children and adults and in the fetus.
Physical Appearance
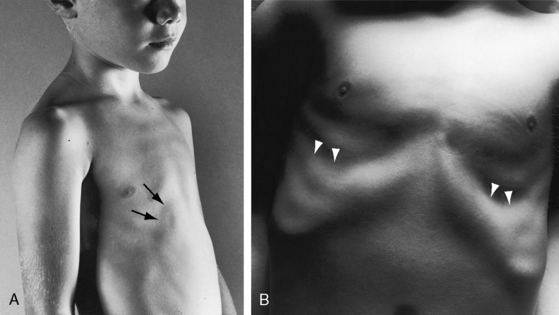
Children with an atrial septal defect may have a delicate gracile habitus, with weight more affected than height (Figure 15-14A), and may have a left precordial bulge with Harrison’s grooves (Figure 15-14B).93 Newborns subsequently found to have an atrial septal defect are on average smaller than their healthy siblings.94 Symptomatic infants may be cyanotic because of the effects of congestive heart failure. Cyanosis also occurs when a large eustachian valve (see previous) selectively channels inferior vena caval blood into the left atrium through an ostium secundum atrial septal defect (see Figure 15-4) or through an inferior vena caval sinus venosus defect (see Figure 15-48).22,63,64
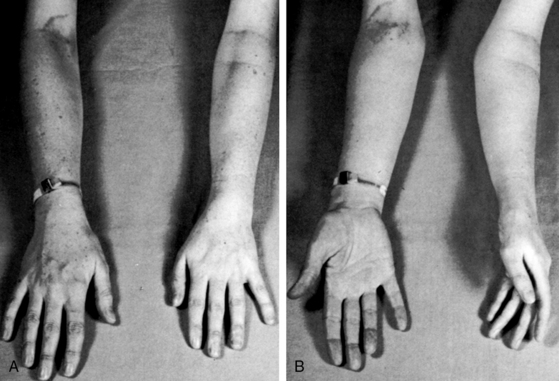
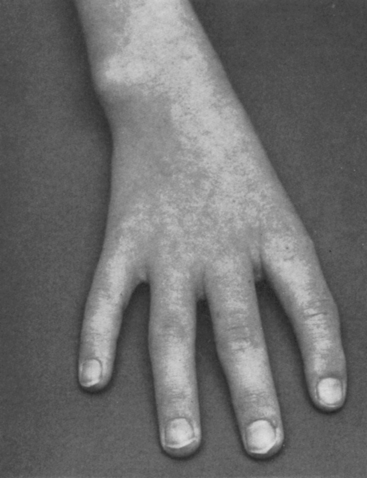
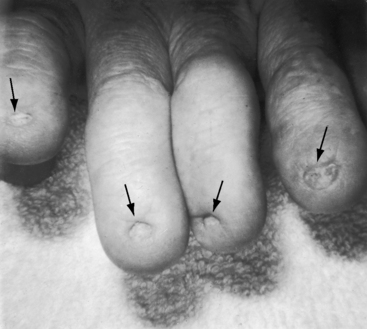
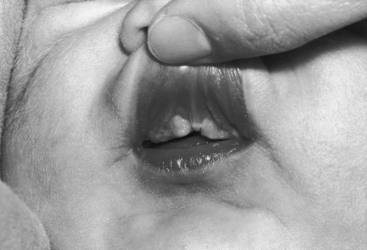
The distinctive physical appearance of the Holt-Oram syndrome heightens suspicion of a coexisting ostium secundum atrial septal defect (Figure 15-15),70,95,96 less commonly of an ostium primum defect. The thumb is hypoplastic with an accessory phalanx that results in triphalangism, a crooked appearance, and difficulty in apposition of thumb to fingertips. The abnormality becomes more obvious when the palms are supinated (see Figure 15-15B). The thumb may be rudimentary or absent, and the metacarpal bone may be small or absent with hypoplasia extending to the radius (Figure 15-16). The bony anomaly ranges from minor changes identified on x-ray to absence of the arm (abrachia) or absent arms with persistent underdeveloped hands (phocomelia).97 Other cardiac malformations occur without prevailing patterns.70 Mutations in a gene on chromosome 12q2 play an important role in skeletal and cardiac development and produce a wide range of partial phenotypes of the Holt-Oram syndrome.98
Patau’s syndrome (trisomy 13) is characterized by polydactyly, flexion deformities of the fingers, palmar crease, microcephaly, holoproscencephaly, cleft lip, cleft palate, and low-set malformed ears. Edward’s syndrome (trisomy 18) is characterized by clenched fists, rocker bottom feet, prominent occiput, low-set malformed ears, and micrognathia (see Figures 19-5 and 20-11). The most common congenital cardiac malformations in trisomy 13 and in trisomy 18 are atrial septal defect, ventricular septal defect, and patent ductus arteriosus.99 An atrial septal defect is associated with the Axenfeld-Rieger anomaly, a genetically heterogeneous autosomal dominant disorder characterized by ocular abnormalities with glaucoma and nonocular abnormalities that include maxillary hypoplasia, dental anomalies, umbilical hernia, and hypospadias.75
Jugular Venous Pulse
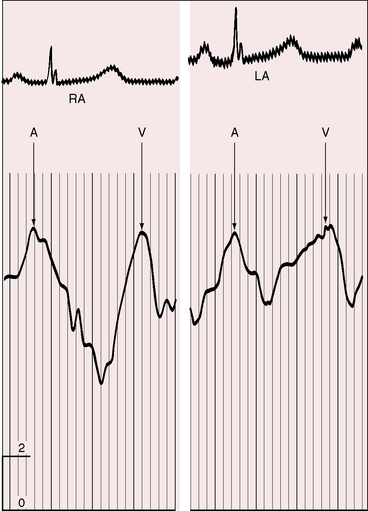
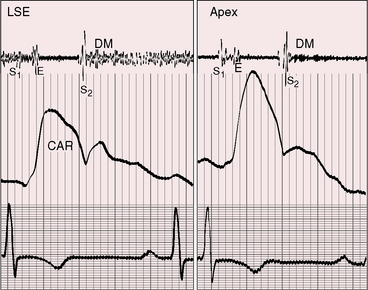
Most important is left atrialization of the jugular venous wave form.100 The crests of the A and V waves tend to be equal as they are in the left atrium (Figure 15-17) because the two atria are in common communication through a nonrestrictive atrial septal defect. The A wave amplitude varies with heart rate as in healthy subjects.101 When left ventricular compliance decreases, left atrial pressure rises, and with it, the right atrial pressure.100,101 Pulmonary vascular disease results in an increased force of right atrial contraction and a dominant, if not giant, A wave (Figure 15-18).
Precordial Movement and Palpation
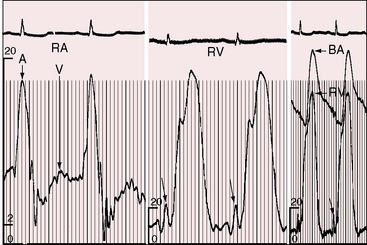
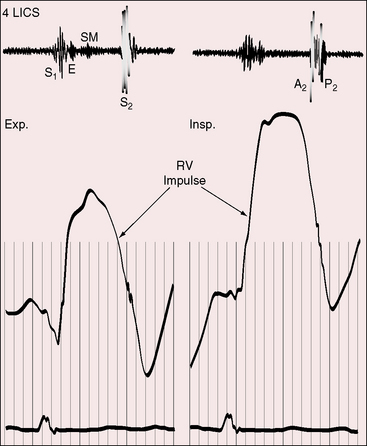
In 1934, Roesler5 called attention to the conspicuous thrust of the right ventricle in atrial septal defect. The impulse is hyperdynamic but not sustained because the volume-overloaded right ventricle contracts vigorously and empties rapidly into a low-resistance pulmonary vascular bed.102,103 The impulse is especially prominent at the left sternal border during held exhalation and in the subxyphoid area during held inspiration.103 Anterior movement at the left sternal border is accompanied by retraction at the apex because the enlarged right ventricle occupies the apex.6,103 A dilated pulsatile pulmonary trunk is palpable in the second left intercostal space, but a systolic thrill is seldom present despite hyperkinetic right ventricular ejection into a dilated pulmonary trunk.
Auscultation
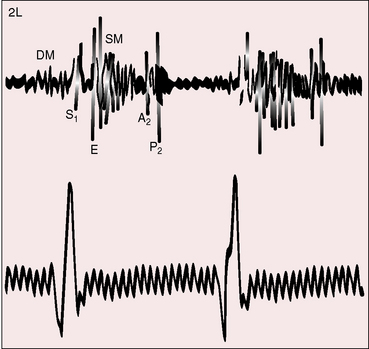
Auscultatory signs are the same in all varieties of isolated nonrestrictive atrial septal defects.104–106 The first heart sound is split at the lower left sternal edge and apex, and the tricuspid component is loud (Figure 15-19).103,105,107 Increased diastolic flow across the tricuspid valve depresses the bellies of the leaflets into the right ventricle, and vigorous right ventricular contraction causes abrupt cephalad excursion of the leaflets, generating a loud tricuspid component of the first heart sound.104,107,108 A pulmonary ejection sound is uncommon, despite dilation of the pulmonary trunk (Figure 15-20).106,109
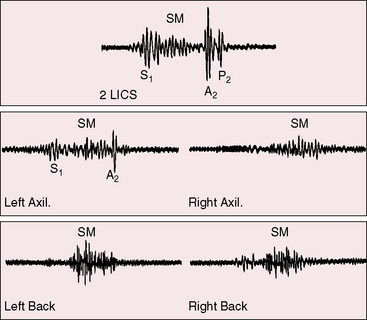
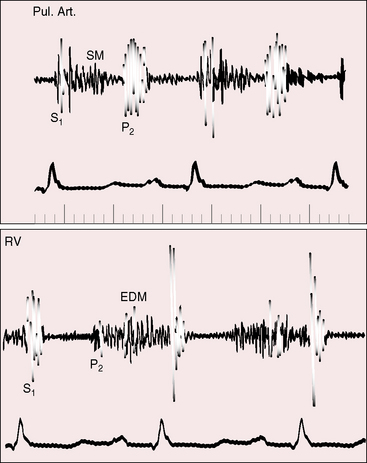
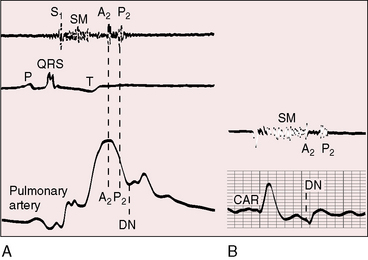
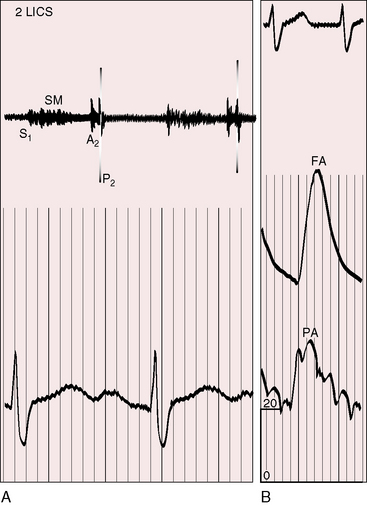
The pulmonary midsystolic flow murmur begins immediately after the first heart sound because right ventricular isovolumetric contraction is short. The murmur is grade 2/6 or 3/6, is maximal in the second left intercostal space over the pulmonary trunk, is impure and superficial because of proximity of the dilated pulmonary trunk to the chest wall, and is crescendo-decrescendo, peaking in early or mid systole and ending well before the second heart sound (Figures 15-21 and 15-22).6,93,104,105 Origin in the pulmonary trunk has been confirmed with intracardiac phonocardiography (see Figure 15-22)104 and with phonocardiograms recorded from the surface of the pulmonary trunk during surgery. The murmur radiates to the apex because the right ventricle occupies the apex.105 A louder murmur is reserved for coexisting pulmonary valve stenosis. Systolic murmurs widely distributed in the right chest, axillae, and back are generated by rapid flow through peripheral pulmonary arteries (see Figure 15-21).110,111
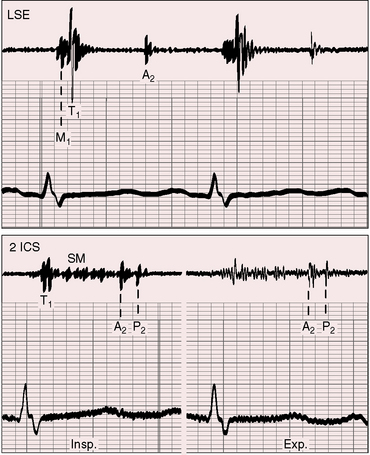
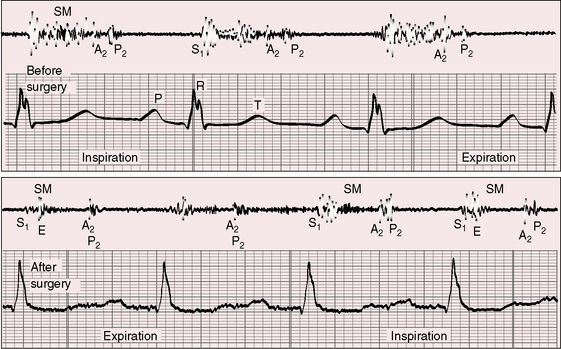
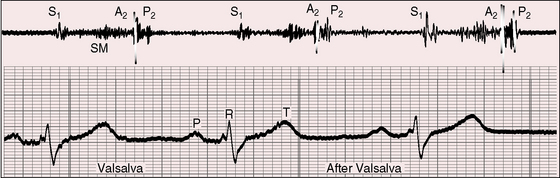
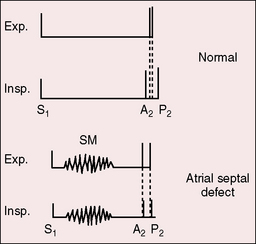
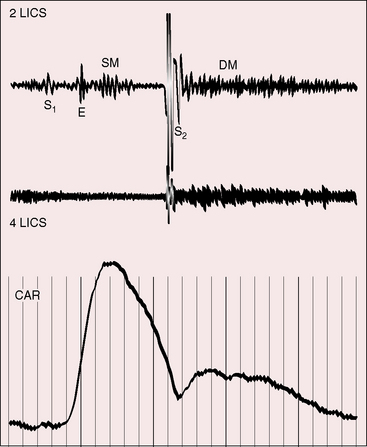
The pulmonary component of the second sound is prominent because of proximity of the dilated pulmonary trunk to the chest wall and because of brisk elastic recoil (Figure 15-23).105 Wide fixed splitting is an auscultatory hallmark of atrial septal defect.105,112,113 The aortic and pulmonary components are widely split during expiration, and the degree of splitting does not change during inspiration (Figures 15-20, 15-21, and 15-24) or during the Valsalva’s maneuver (Figure 15-25). Wide splitting is caused by a delay in the pulmonary component associated with an increase in pulmonary vascular capacitance and an increase in “hangout interval” between the descending limbs of the pulmonary arterial and right ventricular pressure pulses.114–117 With a rise in pulmonary arterial pressure, the hangout interval decreases. The split then becomes a function of the relative duration of right and left ventricular electromechanical systole, which is the same in both ventricles because a potential increase in duration of right ventricular systole from volume overload is countered by accelerated ejection.116 A healthy child examined in the supine position may exhibit relatively wide but not fixed splitting of the second heart sound, but in the sitting position, respiratory splitting is normal.103,118 The duration of diastole affects the degree of splitting by influencing the relative volumes of the right and left ventricles.119 As diastole shortens, the split narrows, and as diastole lengthens, the split widens,119 patterns that are evident in the beat-to-beat variations in cycle length with atrial fibrillation in which splitting tends to vary inversely with the duration of the preceding diastole.
Fixed splitting means that the width of the split remains constant throughout active respiration and during the Valsalva’s maneuver. Persistent splitting means that the split widens during inspiration and narrows during expiration. Atrial septal defect is characterized by splitting of the second heart sound that is wide and fixed (see Figure 15-20). During inspiration, the aortic and pulmonary components are equally delayed or do not move at all.120 In the normal heart, inspiratory splitting is chiefly the result of a delay in the pulmonary component of the second sound because the increase in pulmonary capacitance during inspiration is accompanied by an increase in the hangout interval (Figure 15-26). The high pulmonary capacitance in atrial septal defect precludes an additional increase during inspiration, so there is no inspiratory delay in the pulmonary component of the second sound. Normal phasic changes in systemic venous return during respiration are associated with reciprocal changes in volume of the left-to-right shunt, which minimize the respiratory variations in right and left ventricular filling.112,113,121 Inspiration is accompanied by an increase in systemic venous return, so right ventricular filling is maintained or increased while the left-to-right shunt decreases reciprocally; thus, left ventricular filling is maintained or is increased by the same amount. An inspiratory decrease in left-to-right shunt has been shown in experimental animals112 and in human subjects.122 Wide fixed splitting is unlikely in neonates because there is little or no shunt in either direction.123
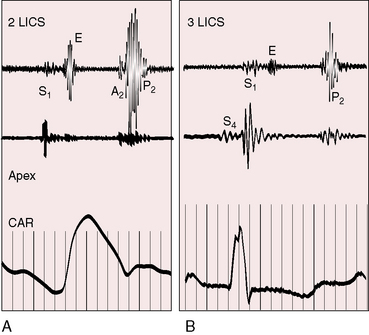
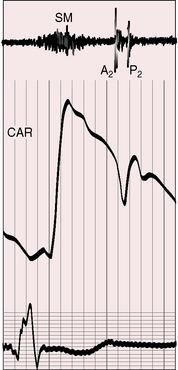
These patterns of splitting do not apply when partial anomalous pulmonary venous connection occurs with an intact atrial septum (Figure 15-27).113,124 Increased venous return during inspiration is not accompanied by a reciprocal fall in left-to-right shunt because the atrial septum is intact. Accordingly, the aortic component of the second heart sound moves toward the first heart sound and the pulmonary component moves away, so the split widens with inspiration and narrows with expiration (see Figure 15-27).
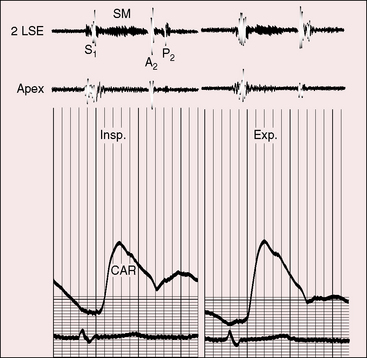
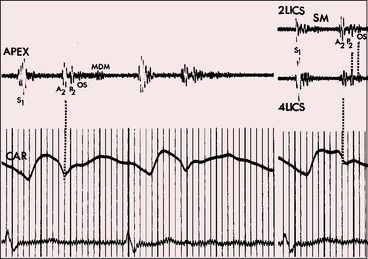
Figure 15-27 Phonocardiograms and carotid pulse (CAR) from a 16-year-old girl with anomalous pulmonary venous connection of the entire right lung to the inferior vena cava and an intact atrial septum (see Figure 15-9). The second heart sound is persistently split, but the split is not fixed. (A2 = aortic component; P2 = pulmonary component; SM = systolic murmur; 2 LSE = second intercostal space left sternal edge; Insp./Exp. = inspiration and expiration.)
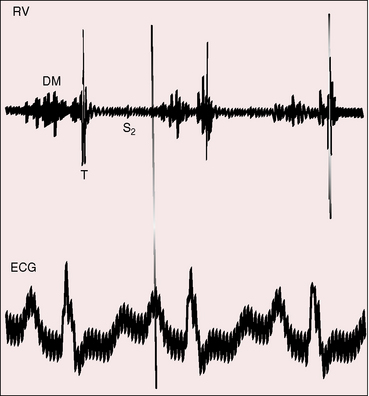
Rarely, an opening sound of the tricuspid valve follows the pulmonary component of the second heart sound.105,107 Echocardiographic timing confirms that the sound coincides with abrupt arrest of the opening movement of the tricuspid valve in early diastole.107 Mid-diastolic murmurs are the result of augmented tricuspid flow.104,105,125,126 Origin of the flow murmur at the tricuspid orifice has been shown experimentally in animals and with intracardiac phonocardiography in human subjects (Figure 15-28).104,127 Tricuspid flow murmurs are medium frequency, impure, soft, short, presystolic or mid-diastolic, and localized at the lower left sternal border and do not increase with inspiration despite their right-sided origin. Intracardiac phonocardiograms identify low-intensity inaudible diastolic murmurs within the atrial septal defect itself.125–127 The combination of superficial impure presystolic and mid-diastolic murmurs together with a superficial impure midsystolic murmur occasionally creates the impression of a pericardial rub. Occasionally, a rub in fact does occur, and attention has been called to roughened pericardium in patients with an atrial septal defect (Figure 15-29).128,129 A diastolic murmur of low-pressure pulmonary regurgitation is uncommon (see Figure 15-22) and is reserved for aneurysmal dilation of the pulmonary trunk.130 Continuous murmurs through restrictive atrial septal defects are rare.131 Atrial septal defects with pulmonary vascular disease and reversed shunts are accompanied by auscultatory signs of pulmonary hypertension (Figures 15-30 and 15-31; see Chapter 14).105,132,133
Electrocardiogram
Sinus node dysfunction has been identified as early as age 2 to 3 years,134–136 and accelerated atrial rhythms have been recorded on 24-hour ambulatory electrocardiograms in 35% of children with an atrial septal defect.134 The incidence of atrial fibrillation, atrial flutter, and supraventricular tachycardia increases in the fourth decade (Figures 15-32 and 15-33).89,136 Interestingly, sinus arrhythmia does not occur in adults with an atrial septal defect and is minimal or absent in children (see Figure 15-33). Sinus arrhythmia requires separation of the systemic and pulmonary venous returns. With an atrial septal defect, the two venous returns are by definition not separated.137
Atrioventricular conduction defects are intrinsic components of atrial septal defects and are usually age related.134,136,138 The PR interval tends to be prolonged.139 Atrioventricular node dysfunction begins in older children and is less frequent than sinus node dysfunction.135,136 Advanced first-degree atrioventricular nodal block occurs with familial or, less commonly, nonfamilial atrial septal defect and occasionally culminates in complete heart block.71–74 Some family members have an ostium secundum atrial septal defect and first-degree heart block, and others have PR prolongation with an intact atrial septum.71 In the Holt-Oram syndrome (see Figures 15-15 and 15-16), PR interval prolongation, sinus bradycardia, and ectopic atrial rhythms are relatively common.70
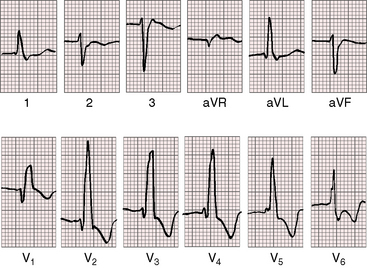
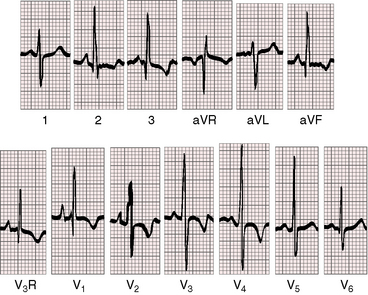
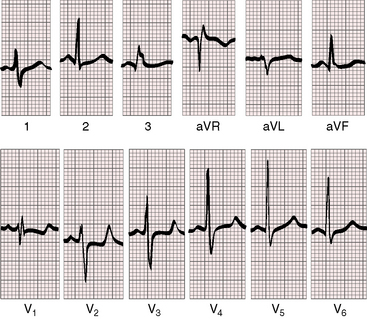
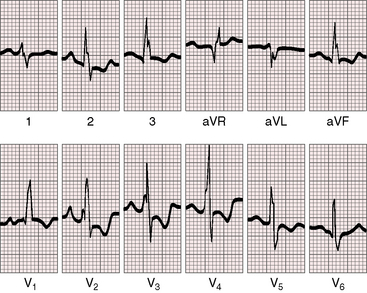
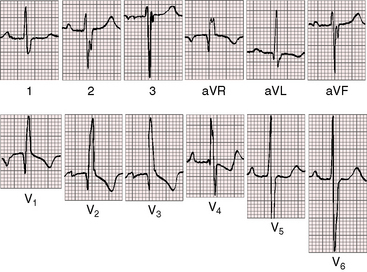
Abnormal right atrial P waves are peaked rather than tall (Figure 15-34), but P wave configurations are usually normal (Figure 15-35).140 Prolonged P wave duration occurs when the terminal force is written by an enlarged right atrium.141–143 The P wave axis with an ostium secundum atrial septal defect is inferior and to the left with upright P waves in leads 2, 3, and aVF (see Figures 15-34 and 35). With a superior vena vaval sinus venosus atrial septal defect, the atrial pacemaker is ectopic because the defect occupies the site of the sinoatrial node. The P wave axis is then leftward, and the P waves are inverted in leads 2, 3, and aVF and upright in lead aVL (Figure 15-36). Superior vana caval sinus venosus defects are occasionally accompanied by shifts from sinus rhythm with a normal P axis to an ectopic atrial rhythm with a leftward P axis.
The QRS duration is slightly prolonged in atrial septal defects because of slurring of the terminal force (see Figure 15-35). The duration increases with age and may culminate in a pattern that resembles complete right bundle branch block (see Figure 15-32). The QRS axis is vertical with clockwise depolarization that writes q waves in leads 2, 3, and aVF (see Figures 15-34 and 15-35). Right axis deviation is reserved for infants with symptomatic pulmonary hypertension or for young females with pulmonary vascular disease. Left axis deviation is exceptional and represents acquired left anterior fascicular block in older adults (see Figure 15-32).144
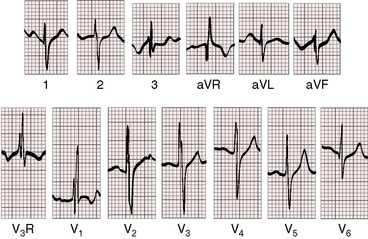
An electrocardiographic hallmark of atrial septal defect is an rSr prime or an rsR prime in right precordial leads (see Figures 15-34 and 15-35).6,145,146 The r prime in lead V1 and aVR is slurred in contrast to thin terminal r waves in 5% of normal electrocardiograms. Q waves are small or absent in left precordial leads because the shunt does not traverse the left ventricle (see Figures 15-34, 15-35, and 15-36). The outflow tract of the right ventricle is the last portion of the heart to depolarize. Enlargement and increased thickness caused by right ventricular volume overload are responsible for the rightward superior and anterior direction of the terminal force of the QRS and for increased QRS duration.147–149 The term incomplete right bundle branch block is a misnomer.149
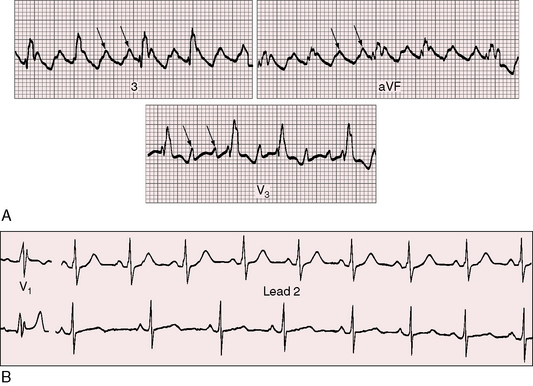
A notch near the apex of the R waves in inferior leads of ostium secundum and sinus venosus atrial septal defects has been called crochetage150 because the notch resembles the work of a crochet needle (Figure 15-37).151 Crochetage is independent of the terminal force direction of the QRS, but when the rSr prime pattern occurs with crochetage in each of the inferior limb leads, the specificity of the electrocardiographic diagnosis of atrial septal defect is remarkably high.150 Although crochetage has been correlated with shunt severity, the pattern has also been reported with a patent foramen ovale and has been suggested as an electrocardiographic marker of a patent foramen.152
X-Ray
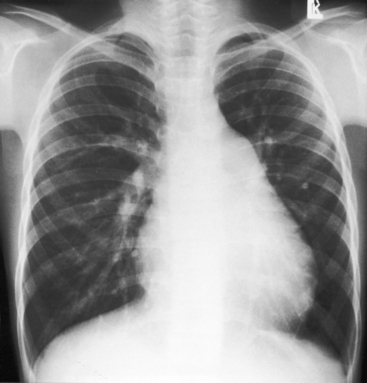
Increased pulmonary arterial vascularity extends to the periphery of the lung fields (Figures 15-38 and 15-39). The pulmonary trunk and its proximal branches are dilated (see Figure 15-39). The left branch is usually obscured by an enlarged pulmonary trunk (see Figure 15-39), but the lateral view discloses dilation of both branches (Figures 15-39 and 15-40). The ascending aorta is seldom border forming because the intracardiac shunt does not traverse the aortic root (see Figures 15-38 and 15-39).5,153 However, angiographic and echocardiographic assessments indicate that the intrinsic caliber of the ascending aorta is not significantly reduced. A sinus venosus atrial septal defect may be accompanied by localized ampullary dilation of the superior vena cava proximal to its attachment to the right atrium (Figure 15-41).15 Infants with large left-to-right shunts exhibit both pulmonary arterial and pulmonary venous vascularity with enlargement of all four cardiac chambers.123 In older adults with moderate pulmonary hypertension and persistent left-to-right shunt, the pulmonary trunk and proximal branches are occasionally aneurysmal (Figure 15-42). In young adults with pulmonary vascular disease and a balanced or reversed shunt, the pulmonary trunk and its branches are strikingly enlarged and calcified (see Figures 15-11 and 15-13).
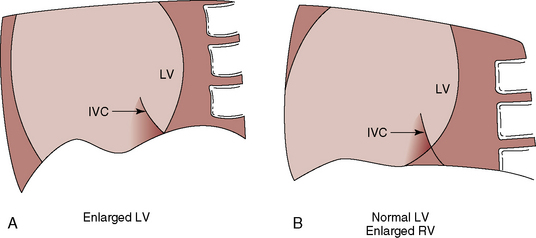
Right atrial enlargement is characteristic (Figures 15-39A and 15-43), but the left atrium seldom enlarges despite a left-to-right shunt (see Figure 15-39B) because a major portion of pulmonary venous return enters the right atrium directly owing to the proximity of the right pulmonary veins to the rim of the ostium secundum atrial septal defect (see previous).54,154 Left atrial enlargement is reserved for older adults with atrial fibrillation.89,155 Volume elastic properties of the right and left atrium also play a role in determining relative enlargement. For equal increments in volume, the right atrium is more distensible than the left.154
An enlarged right ventricle occupies the apex and forms an acute angle with the left hemidiaphragm (see Figure 15-38). Dilation of the outflow tract causes smooth continuity with the enlarged pulmonary trunk above (see Figure 15-38). In the lateral projection, the dilated right ventricle encroaches on the retrosternal space (see Figures 15-39B and 15-40), and displaces the left ventricle posteriorly. The size of the left ventricle is normal because its stroke volume is normal or reduced. The relationship between the inferior vena caval shadow and the left ventricular shadow in the lateral projection distinguishes posterior displacement of the left ventricle from intrinsic dilation (Figure 15-44).156 The lateral projection in adults often shows disproportionate anterior bowing of the upper third of the sternum (see Figure 15-39B). A progressive and often dramatic increase in heart size is initiated by atrial tachyarrhythmias, especially atrial fibrillation with congestive heart failure (Figures 15-12 and 15-45).155
In the scimitar syndrome, the confluence of right pulmonary veins forms a distinctive shadow parallel to or behind the right cardiac silhouette as the anomalous venous channels course downward to join the inferior vena cava (see Figures 15-8, 15-9, and 15-10).28,37,157 The heart is displaced into the right hemithorax because of hypoplasia of the right lung, but the anomalous venous channels usually remain visible (see Figures 15-9 and 15-10).
Echocardiogram
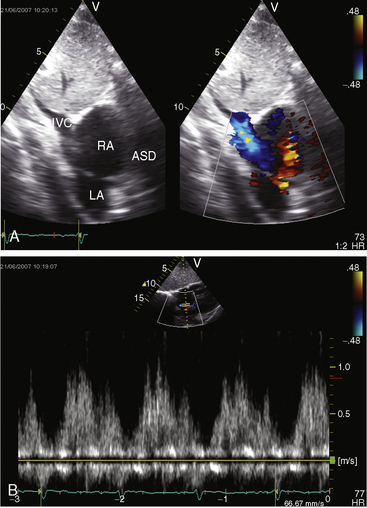
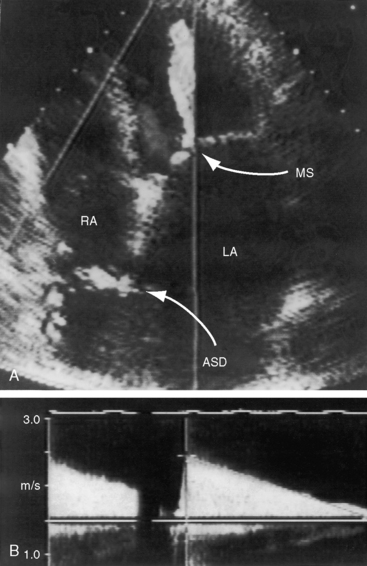
Echocardiography with color flow imaging and Doppler interrogation establishes the location and size of an atrial septal defect and its physiologic consequences.21 Transesophageal echocardiography has refined the anatomic assessment of the interatrial septum9,158 and identifies partial anomalous pulmonary venous connections.62,159,160 Pulsed Doppler imaging can identify anomalous pulmonary venous connections in the fetus.161 Real-time three-dimensional transesophageal echocardiography has optimized assessment of atrial septal defects.162 An ostium secundum atrial septal defect is represented by an echo-free space in the mid portion of the atrial septum (see Figure 15-5A). Color flow imaging confirms that the echo-free space is a true tissue defect, and pulsed Doppler imaging characterizes instantaneous flow patterns across the defect (see Figure 15-5B). A foramen ovale can be identified (Figure 15-46C and Video 15-2C), and color flow determines its patency.90,163 An in utero patent foramen can be distinguished from an ostium secundum atrial septal defect.27 Transthoracic and transesophageal echocardiography establish the diagnosis of atrial septal aneurysm (Figure 15-47A and Video 15-4A), and color flow imaging or echocontrast determines whether an atrial septal defect coexists (Figure 15-47B and Video 15-4B).12,164
Transesophageal echocardiography identifies a divided right atrium that consists of a shelf that separates an anterior supratricuspid component from a posterior systemic venous sinus, to which the cava are connected.165 A rare double atrial septum is imaged as a midline chamber between the left and right atrium.166
Real-time imaging discloses a dilated hyperkinetic right ventricle with paradoxical motion of the ventricular septum51,167 and vigorous pulsations of the pulmonary trunk and its branches. The size, shape, and functional reserve of the left ventricle can be determined (see Figure 15-46B).52,53
A superior vena caval sinus venosus defect lies near the junction of the superior cava and the right atrium (Figure 15-48A),21,158 and an inferior vena caval sinus venosus defect lies just beyond the rim of the inferior vena cava.163 An unroofed coronary sinus is identified by dilation of the sinus and by a defect between the sinus and left atrium.
Summary
Ostium secundum atrial septal defects in the young are among the most readily diagnosed congenital malformations of the heart, but these same defects sometimes defy clinical recognition because of diagnostic ambiguities. The diagnosis of mitral stenosis is entertained (Box 15-1) because of dyspnea, orthopnea, atrial fibrillation, an increased jugular venous V wave, a right ventricular impulse, a loud first heart sound, a delayed pulmonary component of the second heart sound mistaken for an opening snap, a tricuspid flow murmur mistaken for a mitral diastolic murmur, shunt vascularity mistaken for pulmonary venous congestion, and dilation of the pulmonary trunk, the right atrium, and occasionally, the left atrium. Mitral regurgitation may be misdiagnosed as acquired (Box 15-2) because the holosystolic murmur of tricuspid regurgitation is well heard at the apex, a delayed pulmonary component of the second sound followed by a tricuspid flow murmur is mistaken for an opening snap and the mid-diastolic murmur of mitral stenosis, and a tricuspid flow murmur is mistakenly attributed to flow across the mitral valve. Diagnostic ambiguity in older adults results from atrial arrhythmias, ischemic heart disease, systemic hypertension, and inverted left precordial T waves (Box 15-3).
Lutembacher’s syndrome
In 1811, Corvisart168 described the association of atrial septal defect with mitral stenosis; and in 1916, Lutembacher169 published the first comprehensive account of these two defects as a combination that has come to be called Lutembacher’s syndrome. Opinion differs regarding what lesions the syndrome should include. Lutembacher believed that mitral stenosis was congenital even though his patient was 61 years old. The current consensus is that Lutembacher’s syndrome consists of a congenital defect in the atrial septum together with acquired mitral stenosis.32,170,171 The syndrome has been broadened to include different anatomic types of congenital interatrial communications and different anatomic types of acquired mitral valve disease.
What then constitutes an acceptable type of left-to-right shunt in Lutembacher’s syndrome?
When the interatrial communication is an ostium secundum atrial septal defect, the answer is clear.32,172 However, a patent foramen ovale is not included in the definition, although Lutembacher stated that the high left atrial pressure of mitral stenosis might stretch the margins of a valve-incompetent foramen ovale and cause a left-to-right shunt.32,173 An age-related increase in mitral leaflet fibrosis and shortened fibrotic mitral chordae tendineae is found in ostium secundum atrial septal defects,41,174 the functional consequence of which is mitral regurgitation. But whether pure mitral regurgitation should be included in the definition of Lutembacher’s syndrome is debatable. Congenital obstruction to left atrial flow that accompanies cor triatriatum or a parachute mitral valve is occasionally associated with an atrial septal defect (see Chapter 9), but by convention, the Lutembacher eponym is not applied. Partial anomalous pulmonary venous connection with intact atrial septum is a left-to-right shunt at atrial level, and cases have been reported with acquired mitral stenosis.33,170 More recently, iatrogenic Lutembacher’s syndrome has emerged as a defect in the atrial septal acquired during percutaneous transseptal mitral valvotomy for rheumatic mitral stenosis (Figure 15-49).175 For the purposes of this chapter, Lutembacher’s syndrome is considered as the combination of a congenital atrial septal defect and acquired rheumatic mitral stenosis.
When these disorders coexist, each modifies the hemodynamic and clinical expressions of the other.176 Let us first examine the physiologic effects that mitral stenosis exerts on an atrial septal defect.176 The left-to-right shunt across a nonrestrictive atrial septal defect is determined principally by the relative resistances to flow from left atrium into left ventricle and from left atrium through the atrial septum into the right ventricle (see previous). Mitral stenosis increases the resistance to flow from the left atrium into the left ventricle and, in so doing, augments the left-to-right shunt in proportion to the severity of mitral stenosis.176,177 In the presence of a restrictive atrial septal defect, severe mitral stenosis generates a continuous left-to-right shunt because the pressure difference across the restrictive atrial septum exists throughout the cardiac cycle (see Figure 15-49).178,179
Now let us examine the effects that an atrial septal defect exerts on mitral stenosis.176 The idea that an interatrial communication might have a favorable hemodynamic effect was proposed in 1880 by Firkett180 and formalized in 1949 by Bland and Sweet181 who surgically treated mitral stenosis by anastomosing a pulmonary vein to the azygos vein, after which the left atrial pressure fell. The mitral valve is normally the only exit from the left atrium. An atrial septal defect constitutes an alternative exit that decompresses the left atrium and, in so doing, diminishes the transmitral gradient.171 In brief, mitral stenosis increases the shunt flow across an atrial septal defect, and an atrial septal defect decreases the gradient across a stenotic mitral valve. As the left-to-right shunt increases, left ventricular filling and stroke volume reciprocally fall. Conversely, an increase in pressure in the left atrium and pulmonary artery causes right ventricular hypertrophy that decreases right ventricular distensibility, reduces the left-to-right shunt, and improves left ventricular filling.171
History
Lutembacher’s syndrome has a predilection for females because ostium secundum atrial septal defect and rheumatic mitral stenosis are both more prevalent in females.32,176 The incidence rate of mitral stenosis in patients with atrial septal defect has been estimated at 4%.32,176 The incidence rate of atrial septal defect in patients with mitral stenosis has been estimated at 0.6% to 0.7%.32,176 Mitral stenosis is rheumatic even when Lutembacher’s syndrome is familial because it is the atrial septal defect that is genetically transmitted. The incidence rate of coexisting rheumatic mitral stenosis depends on the geographic prevalence of rheumatic fever.176 In underdeveloped countries, a history of rheumatic fever has been reported in 40% of patients with Lutembacher’s syndrome.176
The most important clinical consequence of a nonrestrictive atrial septal defect in Lutembacher’s syndrome is its ameliorating effect on the symptoms of mitral stenosis. Orthopnea, paroxysmal nocturnal dyspnea, pulmonary edema, and hemoptysis are infrequent and attenuated and replaced by fatigue from reduced left ventricular filling and low cardiac output.171 When mitral stenosis is severe and the atrial septal defect restrictive, the symptoms and clinical course resemble isolated mitral stenosis of equivalent severity.32,176
The ameliorating effects of an atrial septal defect were evident in Lutembacher’s original patient, a 61-year-old woman who had had seven pregnancies.169 Firkett’s patient was a 74-year-old woman who experienced 11 pregnancies.180 An 81-year-old woman had no cardiac symptoms until her 75th year,182 and survival to advanced age has been reaffirmed.172,183,184 However, these reports should not discount the unfavorable long-term effect exerted by an atrial septal defect on mitral stenosis that increases the left-to-right shunt and predisposes to atrial fibrillation.172,176 Susceptibility to infective endocarditis resides in the stenotic mitral valve in contrast to negligible susceptibility in an uncomplicated ostium secundum atrial septal defect.
Arterial Pulse
The arterial pulse is small because left ventricular stroke volume is small. Atrial fibrillation reduces the pulse still further (Figure 15-50).176
Auscultation
The auscultatory signs of mitral stenosis are attenuated on two counts (see Figure 15-50).32 First, flow across the stenotic mitral valve is reduced because left atrial blood has an alternative exit across the atrial septal defect, an explanation correctly proposed by Lutembacher to account for the atypical characteristics of the mitral diastolic murmur.169 Second, the auscultatory signs of uncomplicated mitral stenosis are heard best over the left ventricular impulse103; but in Lutembacher’s syndrome, the cardiac apex is occupied by the volume-overloaded right ventricle, so a topographic auscultatory advantage is lost.
When a nonrestrictive atrial septal defect occurs with mild mitral stenosis, the auscultatory signs resemble those of the atrial septal defect.32 The diagnostic yield is improved when the patient turns into a left lateral decubitus position and coughs briskly, while the bell of the stethoscope is applied to the approximate location of the apex.103 Mitral stenosis increases the left-to-right shunt and augments the midsystolic flow murmur across the pulmonary valve. Right ventricular dilation is accompanied by the holosystolic murmur of tricuspid regurgitation that transmits to the apex, inviting a mistaken diagnosis of mitral regurgitation.176 Inspiratory augmentation of the tricuspid murmur—Carvallo’s sign—should prevent error. When the atrial septal defect is restrictive, mitral stenosis results in a continuous gradient from left atrium to right atrium and a continuous murmur at the lower right sternal border because the murmur is generated within the right atrial cavity.178,179 The continuous murmur may increase with slow deep inspiration because of a delayed inspiratory increase in left atrial volume.179 During the straining phase of the Valsalva’s maneuver, the interatrial gradient is reduced or abolished, and the continuous murmur diminishes.179
Electrocardiogram
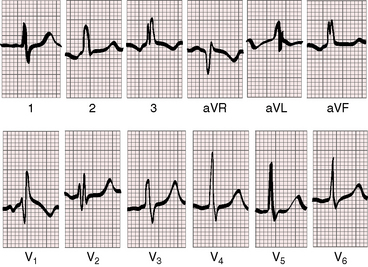
When the atrial septal defect is restrictive, the electrocardiogram resembles that of mitral stenosis. When the atrial septal defect is nonrestrictive, the electrocardiogram resembles that of an atrial septal defect. P waves show left atrial abnormalities with a broad bifid configuration in lead 2 and a deep prolonged P terminal force in lead V1 (Figure 15-51).32,185 However, atrial fibrillation is frequent. Right ventricular hypertrophy is more common than with isolated atrial septal defect.
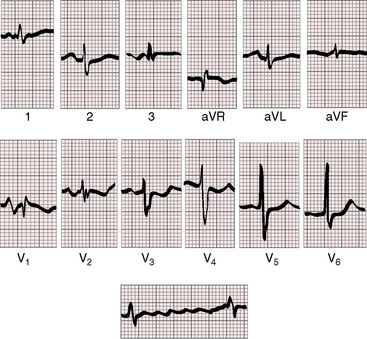
Figure 15-51 Electrocardiogram from the 42-year-old woman with Lutembacher’s syndrome. The phonocardiogram is shown in Figure 15-50. There are broad notched left atrial P waves in leads 2 and aVL, with a broad deep left atrial P terminal force in leads V1-2. The leftward P wave axis with inverted P waves in leads 3 and aVF was the result of an ectopic atrial pacemaker associated with a superior vena caval sinus venosus atrial septal defect. The QRS pattern resembles an ostium secundum defect with a prolonged terminal force directed to the right, superior, and anterior (rsr prime in lead V1, a qr in aVR, and a broad S wave in lead V6). The rhythm strip below shows atrial fibrillation.
X-Ray
A restrictive atrial septal defect results in an x-ray that resembles mitral stenosis with pulmonary venous congestion and left atrial enlargement. When the atrial septal defect is nonrestrictive, the x-ray shows increased pulmonary arterial blood flow but little or no pulmonary venous congestion because the left atrium is decompressed (Figure 15-52). Left atrial enlargement is less than expected for equivalent mitral stenosis but more than expected for an uncomplicated atrial septal defect (see Figure 15-52B).176,185 Left atrial size increases with the advent of atrial fibrillation.176 Dilation of the right atrium, right ventricle, and pulmonary trunk exceeds expectations for an uncomplicated atrial septal defect. Whether mitral valve calcification is infrequent or simply overlooked is unclear.
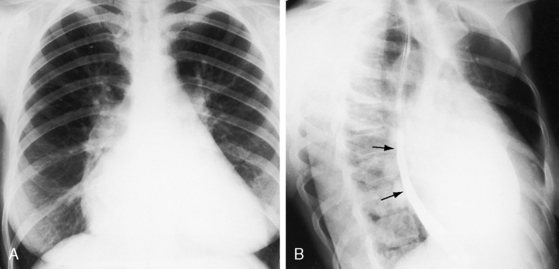
Figure 15-52 X-rays from the 42-year-old woman with Lutembacher’s syndrome (see Figures 15-50 and 15-51). A, The frontal projection shows no signs of pulmonary venous congestion. The pulmonary trunk is moderately prominent and merges with the shadow of the left atrial appendage, resulting in a straight left cardiac border. The ascending aorta is not border-forming. An enlarged right atrium occupies the lower right cardiac border, and an enlarged right ventricle occupies the apex. B, The right anterior oblique projection shows retrodisplacement of the barium esophagram by a moderately enlarged left atrium (paired arrows).
Echocardiogram
Echocardiography with color flow imaging and Doppler interrogation is used to establish the diagnosis of Lutembacher’s syndrome, identify the location and size of the atrial septal defect, and determine the degree of mitral stenosis (see Figure 15-49).178,186 A continuous murmur at the right lower sternal border coincides with Doppler flow patterns recorded with transesophageal echocardiography in the presence of a restrictive atrial septal defect.178
Summary
Lutembacher’s syndrome has a predilection for females because ostium secundum atrial septal defect and rheumatic mitral stenosis are both prevalent in females.* Despite the rheumatic etiology of mitral stenosis, a history of active rheumatic fever in childhood is obtained in less than half the cases.
Common atrium
Common atrium is a rare variety of interatrial communication characterized by absence or virtual absence of the atrial septum, vestigial remnants of which may remain as diaphanous strands of tissue.187 The right-sided portion of the common chamber has features of a morphologic right atrium (crista terminalis, pectinate muscles, right atrial appendage) and receives the superior and inferior vena cavae and coronary sinus.187 The left-sided portion of the common chamber has features of a morphologic left atrium (smooth nontrabeculated walls, a left atrial appendage) and receives the pulmonary veins.187 Common atrium therefore differs from atrial isomerism with a common atrial chamber that is either a bilateral morphologic right atrium or a bilateral morphologic left atrium (see Chapter 3). Absence of the atrial septum necessarily includes the ostium primum (atrioventricular septal) location, so there is a common atrioventricular valve (Figure 15-53) or a cleft anterior mitral leaflet (Figure 15-54).188,189 Partial anomalous pulmonary venous connections are frequent.187 Systemic venous anomalies consist of superior vena caval connection to the left-sided portion of the common atrium, persistent left superior vena cava, and a left hemiazygos vein.190
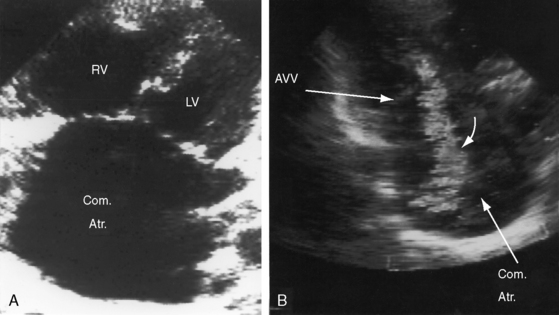
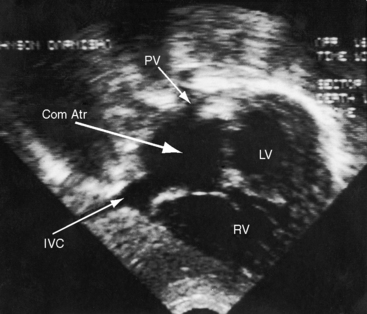
Figure 15-53 Echocardiogram with black and white print of a color flow image from the 57-year-old woman with common atrium (see Figures 15-54 and 15-59). A, Apical view showing the common atrium (Com. Atr.) and the common atrioventricular valve, whose left and right components lie at the same level. (LV = left ventricle; RV = right ventricle.) B, Black-and-white print of a color flow image showing regurgitation (curved arrow) across the left component of the common atrioventricular valve (AVV).
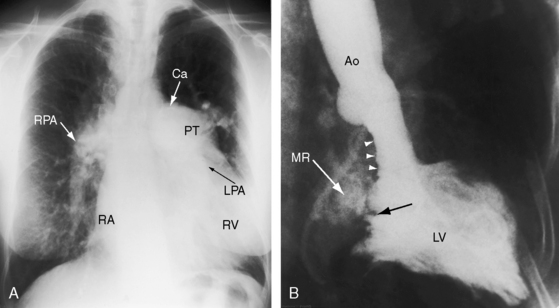
Figure 15-54 A, X-ray and left ventriculogram from the 57-year-old cyanotic woman with common atrium. The electrocardiogram is shown in Figure 15-59. The lung fields are oligemic. The ascending aorta is not border-forming, in contrast to the huge pulmonary trunk (PT) that contains a rim of calcium (Ca) in its upper margin. The right pulmonary artery (RPA) is dilated and tapers rapidly. A large left pulmonary artery (LPA) lies behind the pulmonary trunk. The right atrium (RA) is dilated, and an enlarged right ventricle (RV) occupies the apex. B, Left ventriculogram shows the typical gooseneck deformity (small white arrowheads) and the cleft anterior mitral leaflet (black arrow) of an atrioventricular septal defect. Mitral regurgitation (MR) was only moderate despite a malformed common atrioventricular valve. (Ao = aorta; LV = left vertricle.)
Physiologic consequences of common atrium resemble a nonrestrictive atrial septal defect except for obligatory venoarterial mixing.187 Common atrium is therefore a cyanotic malformation with increased pulmonary arterial blood flow.189 Despite absence of the atrial septum, venoarterial mixing is usually no more than moderate, with systemic arterial oxygen saturations that are often above 90%.187,191 The relative magnitude of left-to-right and right-to-left components of interatrial mixing are determined chiefly by the distensibility characteristics of the right and left ventricles, as in a nonrestrictive atrial septal defect (see previous).191 At one end of the spectrum are patients with low pulmonary vascular resistance, a distensible right ventricle, a large left-to-right shunt, and mild or absent cyanosis. At the other end of the spectrum are patients with high pulmonary vascular resistance, poorly distensible right ventricles, a significant right-to-left shunt, and conspicuous cyanosis.
History
Symptoms begin earlier and are more pronounced than with a nonrestrictive atrial septal defect.187 Most patients experience dyspnea, fatigue, respiratory infections, mild cyanosis, and physical underdevelopment in the first year of life. Occasional patients are relatively well into late childhood or early adolescence187,191 but are rarely into adulthood (see Figure 15-54).
Physical Appearance
Cyanosis occurs despite insufficient evidence of pulmonary hypertension to account for its presence and may be insignificant at rest but induced by exercise or crying. Small or intermittent right-to-left shunts express themselves as highly colored cheeks or digital erythema. The Down phenotype is not a feature of common atrium, despite the presence of elements of an atrioventricular septal defect. However, the Ellis-van Creveld syndrome, chondroectodermal dysplasia, is an important phenotype because 50% of patients with this syndrome have congenital heart disease and half of them have a common atrium.192–194 Ellis-van Creveld syndrome is autosomal recessive and is characterized by dwarfism with polydactyly of the hands that is invariable (Figures 15-55A), but polydactyly of the feet occurs in only 10% of cases (Figure 15-55B).194 Polycarpaly (a ninth or tenth carpal bone), clinodactyly (bent fingers), syndactyly (interdigital webbing), and hypoplasia of the nails194,195 are common features (Figures 15-55 and 15-56). Premature eruption of malformed maxillary incisors, gingival hypertrophy, and multiple frenula are distinctive (Figure 15-57).
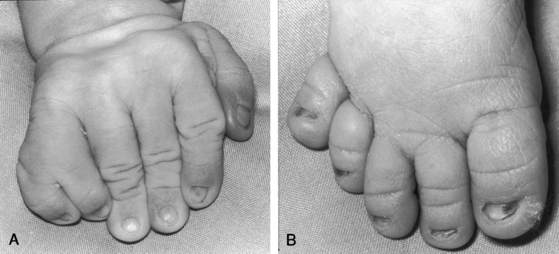
Figure 15-55 Photographs from a female neonate with Ellis-van Creveld syndrome and common atrium. A, The hand is characterized by a well-formed extra digit on its ulnar aspect (polydactyly), clinodactyly (bent fingers), and syndactyly (interdigital webbing). Hypoplasia of the nails is better seen in the foot (B) and in Figure 15-56.
Arterial Pulse, Jugular Venous Pulse, Precordial Movement and Palpation, and Auscultation
The arterial pulse, the jugular venous pulse, and precordial palpation are analagous to a nonrestrictive ostium secundum atrial septal defect (see previous). Auscultatory signs are also similar, with the important exception of an apical holosystolic murmur of mitral regurgitation caused by the malformed left atrioventricular valve (see Figure 15-54B). An increase in the jugular A wave and a loud pulmonary component of the second heart sound reflect the early development of pulmonary hypertension.
Electrocardiogram
Absence of the atrial septum assumes deficiency of the superior vena caval sinus venosus location, the ostium primum or atrioventricular septal location, and the ostium secundum location. The electrocardiogram reflects the deficiencies at all of these sites.188 Leftward deviation of the P wave axis with inverted P waves in the inferior leads is analogous to the ectopic atrial pacemaker in a superior vena caval sinus venous atrial septal defect with an absent sinus node.188,187,196 Left axis deviation of the QRS with counterclockwise depolarization (Figures 15-58 and 15-59) and splintering of S waves in the inferior leads (see Figure 15-59) is analogous to the electrocardiogram of an atrioventricular septal defect.21,187,188 The precordial leads are analogous to an ostium secundum atrial septal defect, but early development of pulmonary hypertension results in increased R wave amplitude in right precordial leads (see Figures 15-58 and 15-59). The electrocardiogram therefore reflects the combined absence of the superior, mid, and inferior portions of the atrial septum.
X-Ray
The chest x-ray is similar to that of a nonrestrictive ostium secundum atrial septal defect (Figures 15-54A and 15-60).187 Enlargement of the left atrial portion of the common atrium is seldom seen in the x-ray, even in the presence of significant mitral regurgitation.
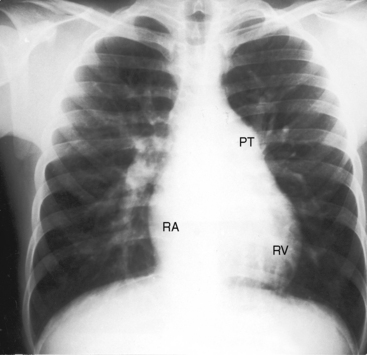
Figure 15-60 X-ray from a 5-year-old acyanotic boy with a common atrium. The electrocardiogram is shown in Figure 15-58. The x-ray resembles an ostium secundum atrial septal defect. Pulmonary arterial vascularity is increased, the pulmonary trunk (PT) and its right branch are dilated, the right atrium (RA) is convex, and a prominent right ventricle (RV) occupies the apex.
Echocardiogram
Echocardiography with color flow imaging and Doppler interrogation identifies a common atrial chamber (Figure 15-61), absence of the atrial septum, and a common atrioventricular valve or a cleft anterior mitral leaflet (see Figure 15-53). The right and left components of the common atrioventricular valve lie at the same level because the atrioventricular septum is absent (see Figure 15-53A). Color flow imaging establishes regurgitation across the left component of the common atrioventricular valve (see Figure 15-53B). The right ventricle is dilated (see Figure 15-53A), the ventricular septum moves paradoxically, and the pulmonary trunk is dilated and pulsatile.
Total anomalous pulmonary venous connection
In 1798, the Philosophical Transactions of the Royal Society of London published “A description of a very unusual formation of the human heart.”197 A 1942 review of 100 cases of anomalous pulmonary venous connections included 35 examples that were total, a term that applies when all four pulmonary veins connect anomalously to a systemic venous tributary of the right atrium or to the right atrium proper but have no connection to the left atrium.198 The malformation is isolated in approximately two thirds of patients so afflicted,199,200 occurs in approximately four to six per 100,000 live births,57 and accounts for about 2% of deaths from congenital heart disease in the first year of life. Total anomalous pulmonary venous connection with atrial isomerism is dealt with in Chapter 3.
Classifications take into account three features: (1) the pathway by which pulmonary venous blood reaches the right atrium; (2) the presence or absence of obstruction along the course of the pathway; and (3) the nature of the interatrial communication. The most widely used clinical classification recognizes supradiaphragmatic connections with or without obstruction and infradiaphragmatic or infracardiac connections that are always obstructed.31,199
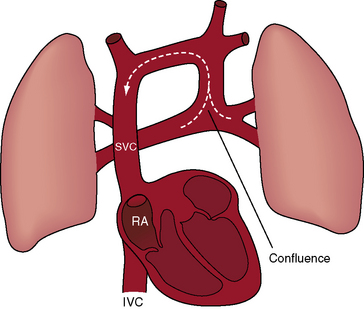
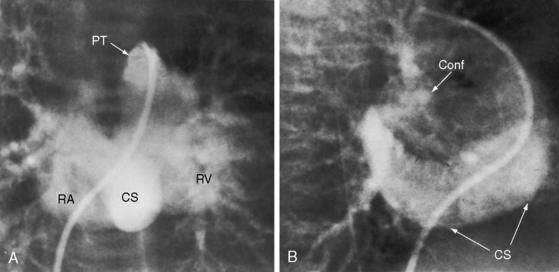
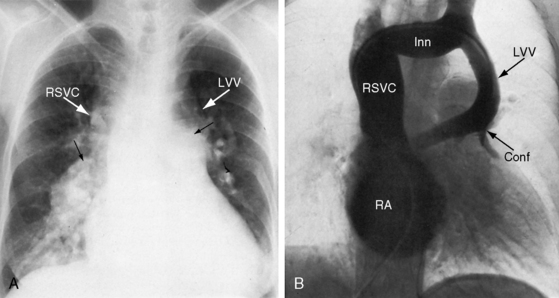
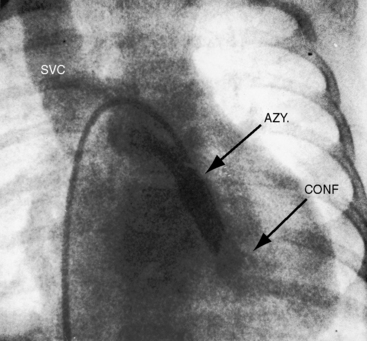
Supradiaphragmatic varieties constitute more than three quarters of cases.199,200 Except for mixed connections and connections of all four pulmonary veins directly to the right atrium, all varieties of total anomalous pulmonary venous connections incorporate a venous confluence that receives the four pulmonary veins (Figures 15-62 through 15-65). In the supradiaphragmatic varieties, the confluence joins the coronary sinus (see Figures 15-63 and 15-64) or a left vertical vein that ascends to join an innominate bridge to the right superior vena cava and right atrium (Figures 15-62, 15-65, and 15-66). Obstruction of the vertical vein is caused by a hemodynamic vise formed posteriorly by the left bronchus and anteriorly by the pulmonary trunk (Figures 15-65 and 15-67B).199 Less commonly, the vertical vein is intrinsically obstructed.201,202 When the coronary sinus receives the venous confluence (see Figures 15-63 and 15-64), obstruction results from stenosis of the right atrial ostium of the sinus.201–203 Rarely, the superior vena cava is obstructed at its junction with the right atrium. Uncommon connections include a venous confluence to the right superior vena cava via a right-sided anomalous venous channel (Figure 15-68) or via the azygos venous system (Figure 15-69).
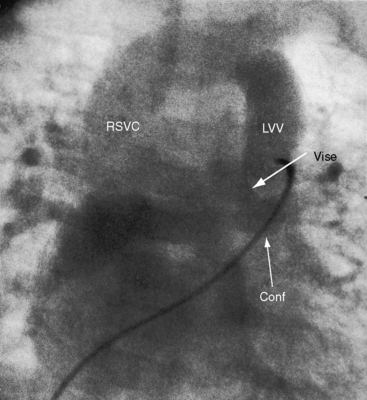
Figure 15-65 Levophase after injection of contrast material into the pulmonary trunk of a 3-month-old female with total anomalous pulmonary venous connection and obstruction of a left vertical vein (LVV) by a hemodynamic vise that consisted of the dilated anterior pulmonary trunk and the posterior left bronchus (compare with Figure 15-67B). (Conf = confluence; RSVC = right superior vena cava.)
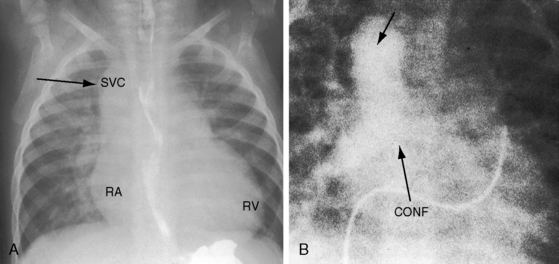
Figure 15-68 A, X-ray from a 10-month-old male with total anomalous pulmonary venous connection to a right vertical vein that joined a right superior vena cava (SVC). Arrow points to the shadow formed by the superimposed right vertical vein and superior vena cava. Pulmonary arterial vascularity is increased. The right atrium is prominent, and an enlarged right ventricle (RV) occupies the apex. B, Levophase after contrast injection into the pulmonary trunk. The right and left pulmonary veins form a confluence (CONF) that rises as a right-sided anomalous pulmonary venous channel (unmarked arrow) and joins the right superior vena cava. Compare with right SVC shadow in Figure 15-68A.
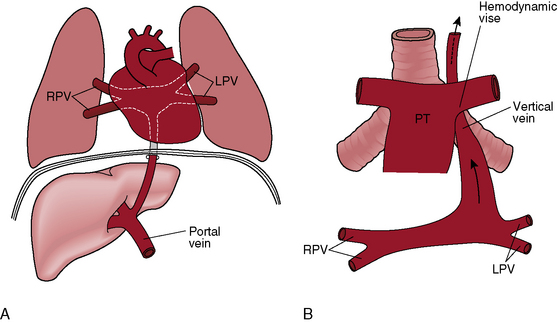
Subdiaphragmatic or infracardiac total anomalous pulmonary venous connection refers to a venous confluence from which a vascular channel originates and descends anterior to the esophagous, penetrates the esophageal hiatus, and terminates in the portal vein, or less commonly in the inferior vena cava or ductus venosus (Figures 15-67A and 15-70).31,199,200 Obstruction is almost invariable, and is certain when the connection is to the portal vein, because blood confronts resistance in the capillary bed of the liver. Alternatively, the descending venous channel is intrinsically obstructed or is compressed as it traverses the esophageal hiatus.199
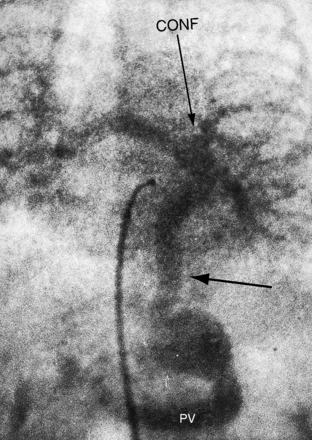
Figure 15-70 Levophase after injection of contrast material into the pulmonary trunk of a 4-day-old male with infradiaphragmatic total anomalous pulmonary venous connection. Right and left pulmonary veins form a confluence (CONF) that gives rise to a vascular channel (unmarked horizontal arrow) that enters the abdominal cavity through the diaphragmatic hiatus and terminates in the portal vein (PV). Compare with Figure 15-67A. Obstruction resided in the portal vein and hepatic bed rather than at the diaphragmatic hiatus, accounting for the subdiaphragmatic dilation.
An interatrial communication is the only exit from the right atrium and the only access to the left side of the heart. The communication varies from a restrictive patent foramen ovale to a nonrestrictive atrial septal defect.199,200,202
The embryologic fault responsible for total anomalous pulmonary venous connection is believed to be agenesis of the common pulmonary vein with persistence and enlargement of primitive communications between the pulmonary venous and the systemic venous beds.199,204 The common pulmonary vein is a temporary outgrowth of the developing left atrium and joins the pulmonary veins as they arise from lung buds. When the common pulmonary vein fails to develop, the pulmonary venous plexus of lung buds does not join the left atrium but instead communicates with systemic veins via anastomotic channels that represent persistence and enlargement of embryonic vascular channels.
Once the pulmonary and systemic venous returns converge in the right atrium, the response of the circulation depends on the size of the interatrial communication and the behavior of the pulmonary vascular bed.199,200 A restrictive patent foramen ovale represents an obstruction that results in an increase in right atrial and pulmonary venous pressure, pulmonary hypertension, and a decrease in flow to the left side of the heart. A nonrestrictive atrial septal defect provides free access to the left atrium, so the direction taken by right atrial blood becomes a function of the distensibility characteristics of the right and left ventricles. Low pulmonary vascular resistance is accompanied by a dilated compliant right ventricle that receives a large volume of blood from the right atrium and ejects the large volume into the pulmonary circulation. Systemic flow is generally maintained despite low left ventricular end-diastolic volume and ejection fraction and the small size of the left atrium and left ventricle. As pulmonary blood flow increases, pulmonary venous return to the right atrium increases, cyanosis is mild, and the systemic oxygen saturation is equal in the right atrium, right ventricle, pulmonary trunk, left atrium, and left ventricle. An increase in pulmonary vascular resistance induces right ventricular hypertrophy, so a smaller fraction of right atrial blood crosses the tricuspid valve into the hypertrophied low-compliant right ventricle. Pulmonary blood flow falls, and cyanosis increases. Elevated pulmonary vascular resistance occurs more frequently with total anomalous pulmonary venous connection than with an isolated nonrestrictive atrial septal defect and an equilavent shunt.199,200
Pulmonary venous obstruction, whether supradiaphragmatic or infradiaphragmatic, results in pulmonary hypertension, reduced pulmonary blood flow,199,200 and a decrease in volume of pulmonary venous blood that reaches the right atrium. Cyanosis increases as oxygen saturation of the right atrial mixture falls. Intrapulmonary and extrapulmonary veins exhibit intimal proliferation, abnormally thick walls, and a reduction in size.201 More malignant hemodynamic forces come into play when a left vertical venous channel is compressed between the pulmonary trunk and the left bronchus (see Figures 15-65 and 15-67B). A rise in pulmonary venous pressure provokes pulmonary hypertension and further dilation of the pulmonary trunk. The dilated hypertensive pulmonary trunk compresses the vertical venous channel still further, and the vicious cycle continues.
History
Gender ratio with supradiaphragmatic total anomalous pulmonary venous connections is about equal,200 and infrahepatic connection to the portal vein strongly favors males.200 These malformations have been reported in first cousins, siblings, and monozygotic and dizygotic twins and in a father and his two children.200,205,206 Total anomalous pulmonary venous connection with Holt-Oram syndrome has been described in a family with a history of atrial septal defect.143
The clinical course is abbreviated even in the absence of pulmonary venous obstruction. Seventy-five percent to 90% of symptomatic infants do not reach their first birthday, and most are dead within 3 to 6 months.199,200,207 Only 50% of patients who survive to age 3 months are alive at 1 year.207 The malformation may not be suspected when pulmonary blood flow is increased in the presence of mild cyanosis. Before long, the infant becomes dyspneic and overtly cyanotic, with difficulty feeding and poor growth and development. Those who survive their first year almost always have supradiaphragmatic connections, low pulmonary vascular resistance, and a nonrestrictive atrial septal defect. A 10-year-old boy had an unobstructed infradiaphragmatic connection to the inferior vena cava,208 and a 17-year-old had obstructed infradiaphragmatic connection to the hepatic vein. Exceptionally, patients reach their third, fourth, or fifth decade with relatively little disability and a clinical picture that resembles isolated ostium secundum atrial septal defect except for the mild cyanosis.209,210 A cyanotic woman with suprasystemic pulmonary vascular resistance was 54 years (see Figure 15-66), and a 62-year-old woman came to necropsy.211 The oldest reported patient underwent surgical repair at age 66 years.209
In total anomalous pulmonary venous connection with obstruction, lifespan is brief and the clinical course stormy. Tachypnea follows expansion of the lungs because neonatal pulmonary blood flow is obstructed (Figure 15-71).199,200 Cyanosis is conspicuous because only a small fraction of oxygenated pulmonary venous blood reaches the right atrium for mixing. When an infradiaphragmatic venous channel traverses the esophageal hiatus, feeding, crying, and straining induce additional compression that aggravates the dyspnea and increases the cyanosis. Death from pulmonary edema and right ventricular failure comes within days, weeks, or months after birth. Newborns with infradiaphragmatic connections and asplenia have major esophageal varices.212
Physical Appearance
In total anomalous pulmonary venous connection without obstruction, mild to moderate cyanosis occurs with increased pulmonary blood flow. Infants with congestive heart failure are catabolic.200 However, a relatively asymptomatic 46-year-old man had been cyanotic since childhood, and a 39-year-old man was never overtly cyanotic. The Holt-Oram syndrome, Klippel-Feil syndrome,200 and cat-eye syndrome sporadically coexist.213–215
Precordial Movement and Palpation
When the interatrial communication is nonrestrictive, precordial movement and palpation resemble nonrestrictive ostium secundum atrial septal defect, although these precordial signs begin earlier. A rise in pulmonary vascular resistance imposes afterload on the volume-loaded right ventricle, so its impulse becomes more striking (Figure 15-72).200 A hyperdynamic right ventricle is not a feature in neonates and infants with pulmonary vein obstruction because pulmonary blood flow is reduced.
Auscultation
When the interatrial communication is nonrestrictive, auscultatory signs resemble an ostium secundum atrial septal defect. The tricuspid component of the first heart sound is loud, there is wide fixed splitting of the second heart sound (Figures 15-73 and 15-74), a pulmonary midsystolic murmur is generated by rapid ejection of a large right ventricular stroke volume into a dilated pulmonary trunk (see Figures 15-73 and 15-74), and a tricuspid diastolic flow murmur is common (see Figure 15-73).200,216 The systolic murmur may have a wide thoracic distribution analogous to the peripheral pulmonary arterial murmurs with ostium secundum atrial septal defect and a hyperkinetic pulmonary circulation.111 Pulmonary hypertension is accompanied by a pulmonary ejection sound, attenuation of the pulmonary systolic flow murmur, loss of the tricuspid diastolic murmur, inspiratory splitting of the second heart sound (see Figure 15-72),200 and a high-frequency early diastolic Graham Steell murmur (Figure 15-75). An increased force of right atrial contraction generates a right ventricular fourth heart sound, and right ventricular failure is accompanied by a third heart sound and the holosystolic murmur of tricuspid regurgitation.200
A continuous murmur is a noteworthy although uncommon auscultatory sign that originates in continuous flow through the venous confluence, the left vertical vein, and left innominate vein (see Figure 15-62).217 The continuous murmur has the quality of a soft venous hum and is maximal at the left upper sternal border. Less commonly, a continuous murmur is heard along the right upper sternal border where it is believed to originate from flow through a right-sided anomalous pulmonary venous channel into a right superior vena cava (see Figure 15-68).