CHAPTER 41 Atrial Septal Defect
Atrial septal defect (ASD) is one of the most commonly recognized congenital cardiac anomalies in adults. Knowledge of the embryologic development of the atrial septum provides the basis for understanding the pathophysiology, anatomy, and clinical manifestations of ASD. ASD is rarely diagnosed in infancy, and it even less commonly results in significant symptoms in infants. Patients, especially patients with small or isolated defects, are usually asymptomatic through the first 3 decades of life, although more than 70% become impaired by the fifth decade. Early surgical closure of most types of ASD is recommended.1
DEFINITION
An ASD is characterized by a defect in the interatrial septum that allows pulmonary venous return to pass from the left to the right atrium (i.e., left-to-right shunt), resulting in right atrial and right ventricular chamber dilation. There are four types of ASD. The first type is an ostium secundum defect. This type of defect is located in the area of the fossa ovalis and is the most common type of ASD, accounting for 75% of all cases. Although it usually consists of a single defect, fenestrated defects have also been reported. Secundum ASD can be associated with partial anomalous pulmonary venous return (<10%) and mitral valve prolapse. A second type of ASD is an ostium primum defect, which accounts for 15% of ASDs; this defect is located in the lower part of the interatrial septum and commonly involves other “endocardial cushion” defects. It can be associated with a “cleft” anterior mitral valve leaflet, which results in mitral regurgitation, or defects in the atrioventricular or membranous interventricular septum. The third type of ASD is a sinus venosus defect, which accounts for 10% of ASDs and involves the junction of the superior vena cava with the left atrium. Anomalous right upper lobe pulmonary venous return is a common manifestation with this type of ASD. The last type of ASD is a coronary sinus ASD, which is the rarest type and results from direct communication of the coronary sinus and the left atrium. Persistent left superior vena cava is commonly associated with coronary sinus ASD.1–4
PREVALENCE AND EPIDEMIOLOGY
ASD accounts for 10% of all congenital heart disease, and for 22% to 40% of congenital heart disease in adults. ASD is twice as common in girls as in boys. Most ASDs occur sporadically as a result of spontaneous genetic mutations; however, hereditary forms have been reported. Associated extracardiac congenital defects are present in 25% of infants, and about 30% have an ASD in association with a hereditary syndrome, such as Down syndrome, Holt-Oram syndrome, and Noonan syndrome.2
ETIOLOGY AND PATHOPHYSIOLOGY
Ostium secundum ASD results from incomplete adhesion between the original ridge of tissue of the valve of the foramen ovale and the septum secundum. The patent foramen ovale usually results from abnormal resorption of the septum primum during the formation of the foramen secundum. Resorption in abnormal locations causes a fenestrated or netlike septum primum. An abnormally large foramen ovale can occur as a result of defective development of the septum secundum. The normal septum primum does not close this type of abnormal foramen ovale at birth. A combination of excessive resorption of the septum primum and a large foramen ovale produces a large ostium secundum ASD.1,2
Ostium primum ASD is caused by incomplete fusion of septum primum with the endocardial cushion. The defect lies immediately adjacent to the atrioventricular valves, either of which may be deformed and incompetent. In most cases, only the anterior leaflet of the mitral valve is displaced, and it is commonly cleft. The tricuspid valve is usually not involved. The defect is often large, and a “complete” atrioventricular canal defect may occur with an ASD associated with a ventricular septal defect.1,2
Sinus venosus ASD occurs when abnormal fusion between the embryologic sinus venosus and the atrium occurs. These defects generally lie high in the atrial septum near the entry of the superior vena cava, and are generally associated with anomalous right upper pulmonary venous return. An uncommon inferior type is associated with partial anomalous return of the right lower pulmonary vein. Anomalous drainage can be into the right atrium, the superior vena cava, or the inferior vena cava.1,2
Coronary sinus ASD is characterized by an unroofed coronary sinus and persistent left superior vena cava that drains into the left atrium. A dilated coronary sinus often suggests this defect. The diagnosis can be made explicitly by MRI, CT, echocardiography, or angiography by injecting contrast agent into the left upper extremity. Coronary sinus opacification, which precedes right atrial opacification, confirms the diagnosis.1,2
A small ASD results in trivial shunting and has no hemodynamic consequences. Larger defects are associated with substantial shunting, which may lead to volume overload of the right atrium, right ventricle, and pulmonary arteries. The magnitude of left-to-right shunting depends on the size of the ASD, the relative compliance of the two ventricles, and the pulmonary and systemic vascular resistance. If left untreated, the left-to-right shunting may result in pulmonary hypertension, right ventricular failure, decreased right ventricular compliance, and potentially right-to-left shunting (Eisenmenger syndrome). Eisenmenger syndrome secondary to ASD is rare in adults, however, occurring in about 5% of patients.1,2
MANIFESTATIONS OF DISEASE
Clinical Presentation
ASD generally goes unnoticed because patients are usually asymptomatic in the first and second decades of life. The most common manifestations of this condition are development of fatigue, dyspnea on exertion, and exercise intolerance generally during the third and fourth decades. The most common presenting symptom is dyspnea and easy fatigability. Other symptoms include palpitations, syncope, and heart failure. The development of palpitations related to supraventricular arrhythmia is the most common symptom in adults. Occasionally, patients may present with paradoxical embolization or recurrent respiratory infection. If left untreated, patients with hemodynamically significant ASD develop symptoms of right-sided heart failure, including jugular venous distention and lower extremity edema. Over time, pulmonary pressure increases because of increased pulmonary resistance, and the shunt may reverse with predominant right-to-left shunting (Eisenmenger syndrome).3,4
The cardiac examination is generally characterized by signs of right heart overload. Cardiac auscultation reveals a normal S1, fixed splitting of S2, and a systolic outflow murmur as a result of the increased flow into the main pulmonary artery. Shunting across the ASD does not produce a murmur. In patients with ostium primum ASD and a cleft anterior mitral valve leaflet, a mitral regurgitation murmur can be appreciated at the apex. The development of pulmonary hypertension results in narrowing of the splitting of S2 and accentuation of the pulmonary closure component. The pulmonic systolic murmur decreases in intensity, and a diastolic pulmonic regurgitation murmur may appear. The development of Eisenmenger syndrome results in cyanosis and clubbing.3,4
Imaging Indications and Algorithm
If echocardiography does not provide adequate assessment of the ASD, CT may be performed; however, MRI is better at assessing ASD because it allows for quantitative assessment of shunts and determination of shunt fractions. Less commonly used modalities for the assessment of ASDs are nuclear medicine and angiography, which provide less anatomic information than the above-mentioned modalities, but yield quantitative assessment of shunt fractions and hemodynamic pressure measurements in the case of invasive angiography. The algorithm for the work-up generally can be accomplished with echocardiography, but rarely, other imaging modalities, most commonly MRI or angiography, can be applied if discrepancies exist.5
Imaging Technique and Findings
Radiography
A plain chest radiograph (Fig. 41-1) is characterized by increased caliber of the main pulmonary artery segment and the hilar and parenchymal pulmonary arteries (i.e., shunt vascularity). Until pulmonary resistance increases, resulting in pulmonary hypertension, the parenchymal vessels extend farther toward the pleura than expected, and appear to taper normally. The pulmonary vessels are sharp. The left-to-right shunt volume loads the right heart, resulting in right atrial and ventricular dilation and clockwise (leftward) cardiac rotation. Increased pulmonary venous return is decompressed across the defect in the interatrial septum; the left atrium and ventricle are normal in size (Fig. 41-2). The typical chest film of a patient with ASD is shunt vascularity with right heart enlargement and a normal left heart.4
When pulmonary resistance increases, causing pulmonary hypertension, the appearance of the chest film changes (Fig. 41-3). The central (extrahilar) pulmonary arteries remain enlarged, but the parenchymal pulmonary artery segments become vasoconstricted, producing the typical appearance of acute change of pulmonary artery caliber seen in pulmonary hypertension. Careful evaluation of the peripheral pulmonary artery segments reveals extension toward the pleura beyond that found in a normal examination, but in smaller caliber vessels. As long as the shunt is left-to-right, right heart enlargement and a normal left heart are seen. In the rare circumstance of shunt reversal, progressive right heart decompression and left heart enlargement may be seen.4,5
Ultrasonography
Echocardiography (Figs. 41-4 through 41-7) is generally the first-line assessment tool for the evaluation of ASD with TTE being the first method used. Echocardiography findings in the setting of ASD reveal evidence of right ventricular volume overload. TTE is the imaging study of choice for ostium primum or secundum ASD. Identification of a sinus venosus ASD usually requires TEE (see Fig. 41-6). Evaluation of the location, size, and direction of shunt can be performed by use of color flow Doppler and echocardiographic contrast agents. Important information such as estimated pulmonary artery pressure and the pressure of additional cardiac chambers can also be obtained. Accurate assessment of pulmonary-to-systemic flow (Qp/Qs) ratio can be obtained and corresponds to the shunt fraction. A Qp/Qs ratio of generally 1.5 to 2 indicates a significant left-to-right shunt. A patent foramen ovale generally does not cause a significant shunt, and Qp/Qs is generally around 1 to 1.05.6
TEE, although an invasive imaging modality, is an essential tool in the selection of patients who are candidates for percutaneous closure of secundum ASD. TEE allows for the sizing of the defect and the adequacy of the rim of tissue around the defect for proper attachment of the closure device. During the percutaneous closure, intracardiac echocardiography, which is now applied to many other percutaneous techniques, may be used for the guidance of the repair procedure.6–8
Computed Tomography
Findings on CT scans (Fig. 41-8), particularly multidetector CT scan, can be very specific; however, the CT scanner is less portable than the echocardiogram machine, and the radiation exposure related to the CT scan makes the assessment of ASD with CT less efficacious. Findings on CT include an incomplete interatrial septum, right heart enlargement, and pulmonary artery dilation. Size of the cardiac chambers can also be measured accurately. The three-dimensional capabilities of CT allow for the detection of anomalous pulmonary venous return associated with sinus venosus ASD.9
Magnetic Resonance Imaging
MRI (Figs. 41-9 through 41-12), especially cine MRI, has a sensitivity and specificity of greater than 90% in delineating septal defects. The high contrast that exists between the blood pool and cardiovascular structures because of the lack of signal from flowing blood using the spin-echo MRI technique or from the bright signal from blood using the gradient-echo (cine) MRI technique allows for excellent imaging capabilities. The three-dimensional capability of MRI allows for imaging to be performed in any plane, including planes parallel and perpendicular to the major axes of the ventricles.10,11
Ventricular volumes, mass, and function can be obtained by using cine MRI scans. Shunt volumes, valvular function, and pressure gradients, and blood flow and velocity across valves and conduits can be estimated using velocity-encoded phase contrast cine MRI. Gated cine MRI allows assessment of left and right ventricular function. MR angiography permits high-resolution three-dimensional evaluation of vessels and can noninvasively establish the presence of anomalous pulmonary veins.12,13
Nuclear Medicine and Positron Emission Tomography
Nuclear imaging is generally less useful in the assessment of ASD, but may be employed in some patients for assessing cardiac function. Equilibrium radionuclide angiography uses ECG gating to define the temporal relationship between the nuclear data and the components of the cardiac cycle. Several hundred heartbeats are sampled, quantified, and displayed in an endless-loop cine format for qualitative visual analysis and quantitative interpretation and analysis. The blood pool is labeled using a Tc 99m tracer. After a single labeling procedure, serial studies can be obtained for periods of 4 to 6 hours. Conventional scintillation cameras are used for these studies. The left anterior oblique view is used for qualitative analysis of global left ventricular ejection fractions and stroke volumes using a radioactivity count-based approach throughout the cardiac cycle.14–16
Right ventricular function is best evaluated using first-pass radionuclide angiography techniques. This technique involves sampling during the initial transit of the bolus through the central circulation. Analyzing right and left ventricular function independently during this brief transit is possible. Regional function also can be assessed from generated outlines of ventricular silhouettes. Tc 99m radiopharmaceuticals are also used for first-pass studies. The finding of abnormal right ventricular ejection fraction in the absence of intrinsic right ventricular disease is excellent evidence of acquired pulmonary hypertension in the presence of an ASD.14–16 Plain single photon emission computed tomography (SPECT) is not generally used in patients with ASD, but when it is performed, increased counts are seen in the right ventricle with dilation of the right ventricular cavity (Fig. 41-13).
Angiography
Invasive evaluation becomes necessary only when the results of noninvasive studies are inconclusive. When the aforementioned noninvasive techniques show the presence of an uncomplicated ASD in a child, routine cardiac catheterization is generally unnecessary. Invasive catheterization allows estimation of the magnitude of the shunt (Qp/Qs ratio) and measurement of the pulmonary artery pressure. Coronary angiography is recommended in patients with suspected coronary artery disease and in patients older than 40 years, especially if surgical repair of the ASD is planned. The diagnosis of ASD may also be confirmed by directly passing the catheter through the defect, and making serial oxygen saturation measurements to estimate the magnitude of the shunt. If high oxygen saturation is present in the superior vena cava, or if the catheter enters the pulmonary vein directly from the right atrium, an ASD of the sinus venosus type is likely present. In this case, partial anomalous pulmonary venous return is usually present and it affects the right upper pulmonary vein. Anomalous pulmonary venous return can also be seen with an ASD of the ostium secundum type.17
SYNOPSIS OF TREATMENT OPTIONS
Medical
ASD is generally considered a surgical disorder, and no specific medical therapy is available. Patients with ASD-associated symptoms of heart failure may require heart failure therapy, however, including but not limited to diuretics, digitalis, and angiotensin-converting enzyme inhibitors. Patients with arrhythmias may require antiarrhythmic therapy.18,19
Surgical and Interventional
The decision to repair any kind of ASD is based on clinical and echocardiographic information, including the size and location of the ASD, the magnitude and hemodynamic impact of the left-to-right shunt, and the presence and degree of elevated pulmonary pressures. Closure is advised for an ASD with echocardiographic evidence of right ventricular overload or with a clinically significant shunt (Qp/Qs ratio >1.5). Lack of symptoms is not a contraindication for repair. In patients with interatrial septal aneurysm and secundum ASD, spontaneous closure may occur, and patients may be followed conservatively for a period before repair is advised.20,21
For children and adults, surgical mortality rates are approximately 1% for uncomplicated secundum ASD. Elective closure in patients with small defects is controversial because patients with small defects generally have a good prognosis, and the risk of cardiopulmonary bypass may not be warranted. The benefit of catheter closure of small secundum defects especially in these patients remains to be determined. Generally, long-term prevention of complications is best achieved when the ASD is closed before age 25 years, and when the systolic pressure in the main pulmonary artery is <40 mm Hg. Even in elderly patients with large shunts, surgical closure can be performed at low risk and with good results in reducing symptoms. Repair in patients older than 40 years does not eliminate the risk of atrial arrhythmias and cerebrovascular accidents.18–21
Closure of an ASD is not recommended in patients who have severe pulmonary hypertension or severe pulmonary vascular disease (Qp/Qs ratio ≥0.7) without a clinically significant shunt or in patients who have a reversed shunt with at-rest arterial oxygen saturations of less than 90% with little or no residual left-to-right shunt. In addition to the high surgical mortality and morbidity risk, closure of the defect in these patients worsens outcome.18–21
INFORMATION FOR THE REFERRING PHYSICIAN
KEY POINTS
 Knowledge of the embryologic development of the atrial septum provides the basis for understanding the pathophysiology, anatomy, and clinical manifestations of ASD.
Knowledge of the embryologic development of the atrial septum provides the basis for understanding the pathophysiology, anatomy, and clinical manifestations of ASD. Patients with clinical findings suggestive of right heart overload or failure should undergo imaging to assess the presence of atrial level shunts owing to an ASD.
Patients with clinical findings suggestive of right heart overload or failure should undergo imaging to assess the presence of atrial level shunts owing to an ASD. The initial imaging modality generally should be a chest radiograph. Radiography gives certain clues for the presence or absence of an ASD.
The initial imaging modality generally should be a chest radiograph. Radiography gives certain clues for the presence or absence of an ASD. The next and generally more definitive modality that gives the clinician a great deal of information is ultrasound imaging with TTE.
The next and generally more definitive modality that gives the clinician a great deal of information is ultrasound imaging with TTE. If necessary, a patient may be sent for TEE, which can give better anatomic visualization of the less common types of ASD and associated anomalies.
If necessary, a patient may be sent for TEE, which can give better anatomic visualization of the less common types of ASD and associated anomalies.Boxt LM. Magnetic resonance and computed tomographic evaluation of congenital heart disease. J Magn Reson Imaging. 2004;18:827-847.
Fayad LM, Boxt LM. Chest film diagnosis of congenital heart disease. Semin Roentgenol. 1999;34:228-248.
Lee T, Tsai IC, Fu YC, et al. MDCT evaluation after closure of atrial septal defect with an Amplatzer septal occluder. AJR Am J Roentgenol. 2007;188:W431-W439.
Sandhu SK. Transcatheter closure of the atrial septal defect in the elderly. J Invasive Cardiol. 2007;19:513-514.
Smallhorn JF. Cross-sectional echocardiographic assessment of atrioventricular septal defect: basic morphology and preoperative risk factors. Echocardiography. 2001;18:415-432.
Taylor AM, Stables RH, Poole-Wilson PA, et al. Definitive clinical assessment of atrial septal defect by magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:43-47.
Wang ZJ, Reddy GP, Gotway MB, et al. Cardiovascular shunts: MR imaging evaluation. RadioGraphics. 2003;23(Spec No):S181-S194.
Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645-1653.
1 Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645-1653.
2 Bedford D. The anatomical types of atrial septal defect: their incidence and clinical diagnosis. Am J Cardiol. 1960;6:568.
3 Hopkins WE. Atrial septal defect. Curr Treat Options Cardiovasc Med. 1999;1:301-310.
4 Krasuski RA. When and how to fix a “hole in the heart”: approach to ASD and PFO. Cleve Clin J Med. 2007;74:137-147.
5 Espino-Vela J, Alvarado-Toro A. Natural history of atrial septal defect. Cardiovasc Clin. 1971;2:103-125.
6 Kowalski M, Hoffman P, Siudalska H, et al. Clinical and echocardiographic assessment of a right-to-left shunt across an atrial septal defect secondary to tricuspid regurgitation. Acta Cardiol. 2001;56:233-237.
7 Nanda NC, Ansingkar K, Espinal M, et al. Transesophageal three-dimensional echo assessment of sinus venosus atrial septal defect. Echocardiography. 1999;16:835-837.
8 Salem MI, Makaryus AN, Kort S, et al. Intracardiac echocardiography using the AcuNav ultrasound catheter during percutaneous balloon mitral valvuloplasty. J Am Soc Echocardiogr. 2002;15:1533-1537.
9 Berbarie RF, Anwar A, Dockery WD, et al. Measurement of right ventricular volumes before and after atrial septal defect closure using multislice computed tomography. Am J Cardiol. 2007;99:1458-1461.
10 Holmvang G, Palacios IF, Vlahakes GJ, et al. Imaging and sizing of atrial septal defects by magnetic resonance. Circulation. 1995;92:3473-3480.
11 Taylor AM, Stables RH, Poole-Wilson PA, et al. Definitive clinical assessment of atrial septal defect by magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:43-47.
12 Wang ZJ, Reddy GP, Gotway MB, et al. Cardiovascular shunts: MR imaging evaluation. RadioGraphics. 2003;23(Spec No):S181-S194.
13 Piaw CS, Kiam OT, Rapaee A, et al. Use of non-invasive phase contrast magnetic resonance imaging for estimation of atrial septal defect size and morphology: a comparison with transesophageal echo. Cardiovasc Intervent Radiol. 2006;29:230-234.
14 Jimenez-Angeles L, Medina-Banuelos V, Valdes-Cristerna R, et al. Analysis of ventricular contraction by factorial phase imaging with equilibrium radionuclide angiography. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1081-1084.
15 Chin BB, Metzler SD, Lemaire A, et al. Left ventricular functional assessment in mice: feasibility of high spatial and temporal resolution ECG-gated blood pool SPECT. Radiology. 2007;245:440-448.
16 Sharir T. Gated myocardial perfusion imaging for the assessment of left ventricular function and volume: from SPECT to PET. J Nucl Cardiol. 2007;14:631-633.
17 Yalonetsky S, Schwartz Y, Lorber A. Coronary angiography in patients undergoing transcatheter closure of interatrial shunt. J Invasive Cardiol. 2007;19:16-18.
18 Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45:505-507.
19 Konstantinides S, Geibel A, Olschewski M, et al. A comparison of surgical and medical therapy for atrial septal defect in adults. N Engl J Med. 1995;333:469-473.
20 Formigari R, Di Donato RM, Mazzera E, et al. Minimally invasive or interventional repair of atrial septal defects in children: experience in 171 cases and comparison with conventional strategies. J Am Coll Cardiol. 2001;37:1707-1712.
21 Jones DA, Radford DJ, Pohlner PG. Outcome following surgical closure of secundum atrial septal defect. J Paediatr Child Health. 2001;37:274-277.

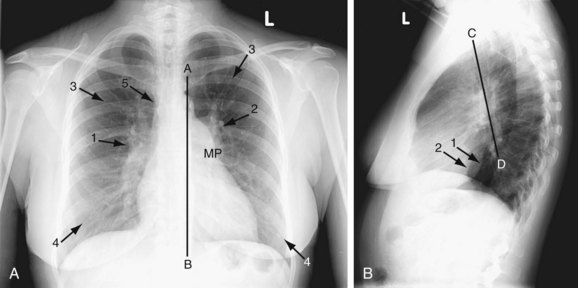
 FIGURE 41-1
FIGURE 41-1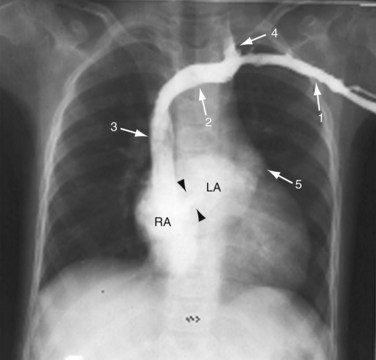
 FIGURE 41-2
FIGURE 41-2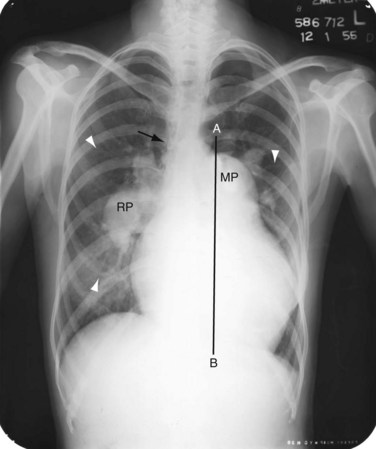
 FIGURE 41-3
FIGURE 41-3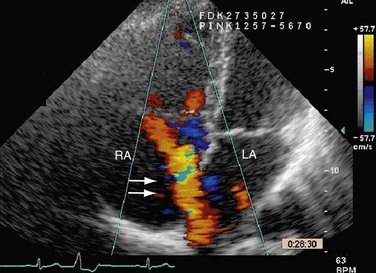
 FIGURE 41-4
FIGURE 41-4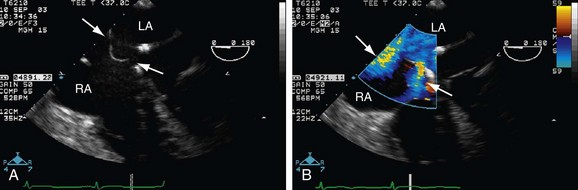
 FIGURE 41-5
FIGURE 41-5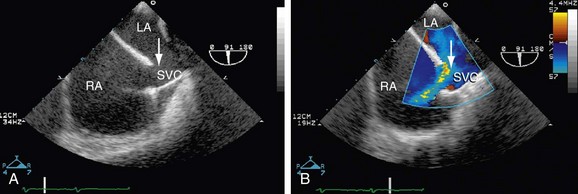
 FIGURE 41-6
FIGURE 41-6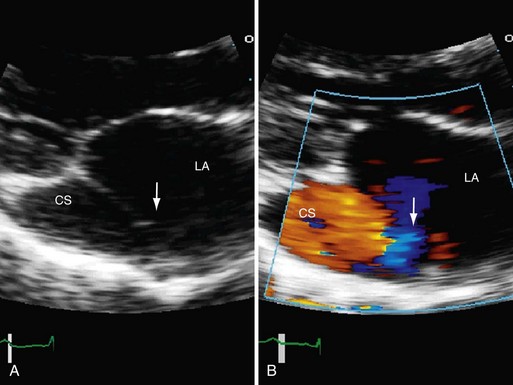
 FIGURE 41-7
FIGURE 41-7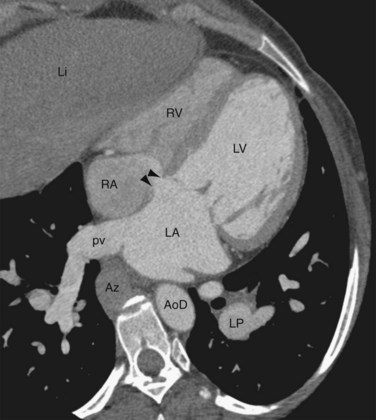
 FIGURE 41-8
FIGURE 41-8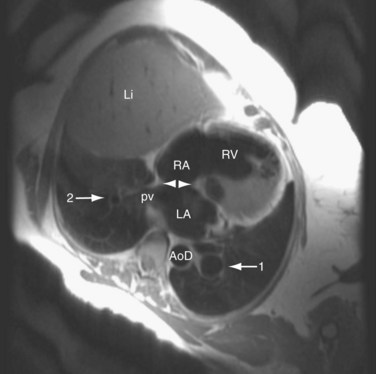
 FIGURE 41-9
FIGURE 41-9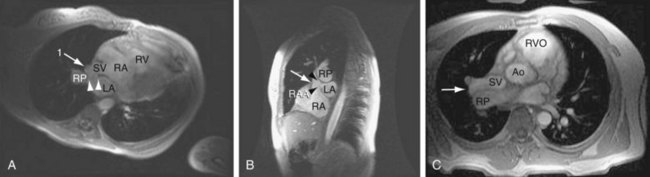
 FIGURE 41-10
FIGURE 41-10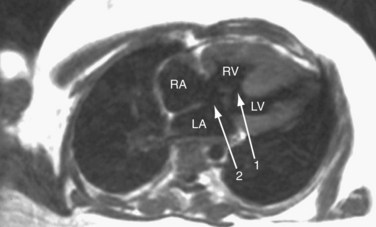
 FIGURE 41-11
FIGURE 41-11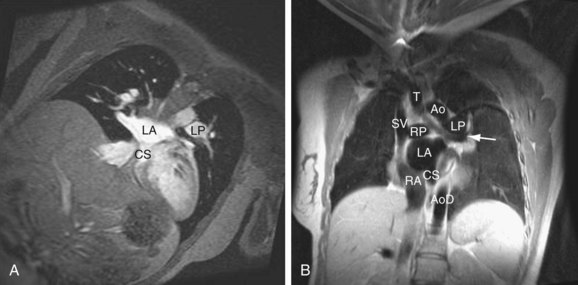
 FIGURE 41-12
FIGURE 41-12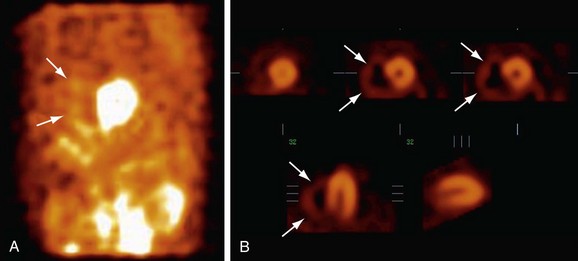
 FIGURE 41-13
FIGURE 41-13



