Atrial Fibrillation
Paroxysmal, Persistent, and Permanent
Epidemiology and Societal Impact
The prevalence of AF is approximately 2% in North America and Europe, with more than 6 million Americans and more than 6 million Europeans affected.1–3 The prevalence is thought to be slightly lower in Asia and is estimated as 1% to 2%.4 The prevalence of AF increases with age to approximately 9% in octogenarians, and men are affected more frequently than women.5 The number of patients with AF in the United States is expected to exceed 12 million by 2050 (Figure 75-1).5 In the Framingham Heart Study, AF was associated with a 1.5-1.9 fold increase in mortality.6 Among Medicare beneficiaries with AF, the 1-year mortality rate was 25%.7 Annual health care expenditures resulting from AF were over $7 billion in 2001.8
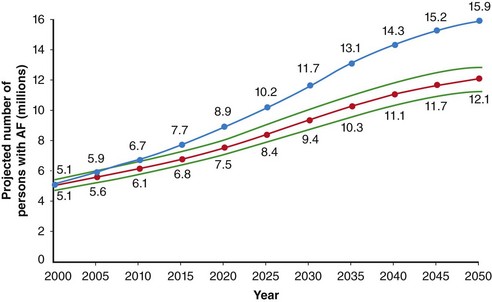
Figure 75-1 Secular trends in the incidence of atrial fibrillation (AF). Projected number of persons with AF in the United States between 2000 and 2050, assuming no further increase in age-adjusted AF incidence (solid curve) and assuming a continued increase in incidence rate as evident in 1980 to 2000 (dotted curve). (With permission from Miyasaka Y, Barnes ME, Gersh BJ, et al: Secular trends in incidence of atrial fibrillation in Olmsted county, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114:119–125, 2006.)
Classification
Clinical AF is defined as an episode that lasts longer than 30 seconds. Lone AF refers to AF in patients younger than 60 years without coexisting heart disease. Following initial presentation, AF can be categorized as9:
• Paroxysmal AF—episodes of AF terminating spontaneously within 7 days or cardioverted within 48 hours of onset
• Persistent AF—episodes of AF lasting greater than 7 days or cardioverted after 48 hours of onset
• Longstanding persistent AF—continuous AF for longer than 12 months
• Permanent AF—restoration and maintenance of sinus rhythm has either failed or a decision has been made to not attempt rhythm control
Pathophysiology
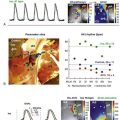
The pathophysiology of AF has been a subject of active investigation for more than a century. Reports as early as 190710 implicated multiple rapidly firing foci as a mechanism. This hypothesis was challenged in 1914 with the concept of reentry or “circus movement” enabling perpetuation of AF.11 A critical requirement for a reentrant mechanism is sufficient atrial dimension to accommodate the wavelength of the circuit, defined as the product of conduction velocity and effective refractory period (ERP). The multiple wavelets theory was explored in the 1960s and was proposed as the predominant mechanism for perpetuation of AF.12 More recent studies have reported on organized drivers of AF and fibrillatory conduction. The mechanisms of AF are also recognized as being multifactorial and sharing properties of reentry, automaticity, and triggered activity. These mechanisms might not be mutually exclusive (Figure 75-2).
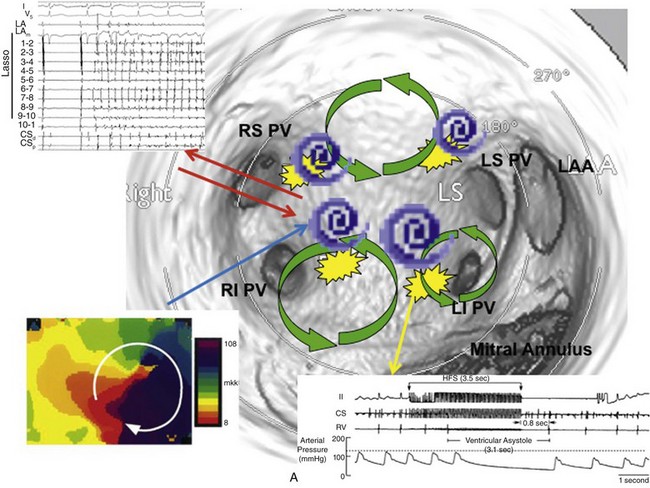
Figure 75-2 Multifactorial nature of the genesis of atrial fibrillation (AF). Shown is an endoscopic view of a three-dimensional computed tomographic image of the left atrium. Potential mechanisms of AF include PV tachycardias that initiate and perpetuate AF (left upper insert), rotors (magnified phase map in the left lower insert), multiple reentrant circuits (green circles), and autonomic modulation through ganglionated plexi (yellow patches and right lower inset). There is a dynamic interplay between the pulmonary veins (PVs) and other drivers of AF such that they continually activate and perpetuate each other. LS, left superior; RS, right superior; LI, left inferior; RI, right inferior; LAA, left atrial appendage. (Adapted with permission from Jalife J: Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol 14:776–780, 2003; and Po SS, Nakagawa H, Jackman WM: Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol 20:1186–1189, 2009.)
Multiple Wavelet Hypothesis
Reentry forms the basis of the multiple wavelet hypothesis.13 In this theory, reentrant waves perpetuate and drift in a seemingly random manner subject to heterogeneous excitability properties of adjacent tissue. These wavelets are continuously subject to extinction or generation of new circuits (daughter wavelets). It is possible that multiple wavelets and reentry with fibrillatory conduction have a role in perpetuation of AF, particularly in patients with atrial electroanatomic remodeling.14
Pulmonary and Thoracic Vein Arrhythmogenesis
The seminal observation that rapid depolarizations from the pulmonary veins (PVs) can initiate and perpetuate AF led to the development of novel mechanistic and therapeutic paradigms.15 The mechanism by which PVs become arrhythmogenic and initiate AF in some patients and remain dormant in others still is not clear. Genetic predisposition, stretch, neurohormonal milieu, and changes in autonomic tone have all been implicated.
During embryonic development, the common PV is incorporated into the left atrium. An immunohistochemical study demonstrated that the histologic characteristics of the PVs and the smooth-walled portion of the left atrium are identical (Figure 75-3),16 explaining why the antral regions of the PVs and the posterior left atrium also are arrhythmogenic. There appears to be a dynamic interplay between the PVs and the adjacent left atrium, as the site of rapid electrical activity alternates between the PV and the left atrium.
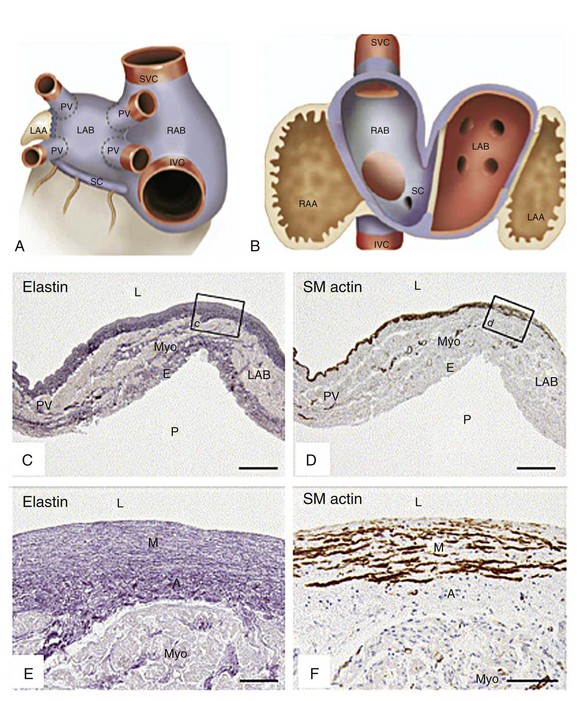
Figure 75-3 A, Schematic depiction of outer atrial chambers with pulmonary veins (PVs) and systemic veins. Left atrial body (LAB) and right atrial body (RAB) covered by myocardium with smooth-walled inner aspect (blue), which stretches over extracardiac segments of PV and over small peripheral part of systemic veins (blue area above and below dotted line). B, Schematic depiction of tissue types found inside left and right atria. Vessel wall tissue (red), myocardial tissue with smooth-walled inner aspect (blue), primary atrial segment tissue (brown), left-sided sinus venous tissue (*). C-F, Sections of segments of human neonatal heart. No histologic venoatrial demarcation was found at the transition of PV and LAB. LAA, Left atrial appendage; RAA, right atrial appendage; SVC, superior vena cava; IVC, inferior vena cava; SC, coronary sinus; Myo, myocardium; E, epicardium; L, lumen; P, pericardial cavity; SM, smooth muscle; M, medial layer; A, adventitial layer. (Adapted with permission from Douglas YL, Jongbloed MR, Gittenberger-de Groot AC, et al: Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol 97:662–670, 2006.)
Nonpulmonary thoracic veins (i.e., the superior and inferior venae cavae), the coronary sinus (CS), and the ligament of Marshall have all been reported to possess arrhythmogenic activity, but less frequently than the PVs. The CS seems to have a particularly complex role; it can harbor triggers of AF and participate in left atrial reentrant circuits.17 The complex, multilayered structure of the CS and adjacent left atrial myocardium with resultant anisotropy could facilitate reentry. A prior study suggested that the site of fastest electrical activity during AF alternates between the CS and left atrium.18 In that study, systematic disconnection of the CS from the left atrium rendered AF less likely to be inducible after PV isolation (PVI) in patients with paroxysmal AF. In a recent study, epicardial and endocardial ablation along and in the CS in patients who remained in AF after PVI prolonged AF cycle length and terminated AF in 35% of patients (46% paroxysmal AF, 30% persistent AF).19 These findings suggest a mechanistic role of the CS in initiation and perpetuation of AF.
High-Frequency Sources and Rotors
High frequency sources or rotors resulting from anisotropic reentry have been proposed as drivers of AF based on optical mapping studies and computer simulation and experimental models.20,21 A recent study using panoramic in vivo mapping of the atria also suggested the presence of rotors as drivers of AF in the human atrium.22 Wavebreak and fibrillatory conduction occur at the periphery of a rotor leading to complex fractionated atrial electrograms (CFAE).23 Anchor points of rotors may be more prevalent at the antral regions of the PVs. A shorter effective refractory period at sites of autonomic innervation within the PV antrum can also promote development of rotors. Propagation of atrial activity from high-frequency sources results in frequency gradients, such as from the PVs to the left atrium and to the right atrium.24 Elimination of PV tachycardias and ablation of CFAEs can result in a decrease in the frequency content. In persistent AF, reduction in dominant frequency after electrogram-guided ablation predicts maintenance of sinus rhythm.25
Autonomic Innervation
The heart has an intrinsic nervous system and an interactive atrial neural network. Both sympathetic and parasympathetic systems have significant influences, primarily by sympathetic mediated increases in intracellular Ca2+ and vagally mediated heterogeneous shortening of atrial ERP promoting reentry.26 Stimulation of ganglionated plexi (GP) promotes PV arrhythmogenicity and inducibility of AF, and GP have been considered as potential targets during ablation.27 However, the precise role of ablation of GP remains to be determined in patients with AF. It is important to note that strategies to ablate CFAEs and GPs are not mutually exclusive because targeted locations frequently overlap.28
Atrial Remodeling
AF leads to electroanatomic remodeling in the atria, which can include a decrease in atrial ERP; an increase in heterogeneity of refractoriness; shortening of action potential duration; changes in ion channel expression, cellular coupling, and conductivity; and development of fibrosis.29 Progressive electroanatomic remodeling of the atria facilitates perpetuation of AF.30 A variety of interventions to interfere with progression of remodeling both as a standalone therapy and also in conjunction with antiarrhythmic drug therapy or catheter ablation for rhythm control is the subject of ongoing research.
Treatment
Management of Thromboembolic Risk
Atrial fibrillation increases the risk of thromboembolic events by nearly fivefold.31 Left atrial mechanical dysfunction and stasis promote thrombus formation in the left atrial appendage. However, approximately 25% of all strokes in patients with AF are due to intrinsic cerebrovascular or atherosclerotic aortic disease.32 A hypercoagulable state with platelet and endothelial dysfunction can exist in patients with AF, with increases in thromboglobulin and platelet factor 4 levels and a trend toward higher fibrinogen levels.33 Further evidence of systemic involvement includes elevated CRP levels.34 It remains unclear whether the thromboembolic risk in patients with AF is primarily due to a hypercoagulable state, left atrial mechanical dysfunction, or both.
The risk of thromboembolism is considered similar among patients with paroxysmal, persistent, or permanent AF or atrial flutter. In patients younger than 60 years with lone AF, the benefit of even antiplatelet therapy using aspirin for primary prevention of stroke has not been established.35 In other patients, a commonly used algorithm proposes the classification of risk factors into low, moderate, or high-risk categories and selection of antithrombotic therapy on the composite score based on these risk factors (Table 75-1). The CHADS236,37 (Box 75-1) scoring system has been used to quantify thromboembolic risk in patients with AF by estimating an annual stroke rate based on the presence or absence of five clinical risk factors: heart failure, hypertension, age, diabetes, and previous stroke. The more recent CHA2DS2-VASc score38 (Box 75-1) also estimates annual stroke risk and incorporates incremental thromboembolic risk factors, including vascular disease, female sex, and two levels of risk based on age (Box 75-1). This scoring system has been incorporated into the European Society of Cardiology (ESC) guidelines for AF management.
Table 75-1
Guidelines for Antithrombotic Therapy in Patients With Atrial Fibrillation
| Risk Category | Recommended Therapy |
| No risk factors | Aspirin, 81-325 mg daily |
| One moderate-risk factor | Aspirin (81-325 mg daily) or warfarin (INR 2.0-3.0; target, 2.5) |
| Any high-risk factor or more than one moderate-risk factor | Warfarin (INR 2.0-3.0; target, 2.5) |
| Less Validated or Weaker Risk Factors | Moderate-Risk Factors | High-Risk Factors |
| Female gender | Age ≥ 75 yr | Previous stroke, TIA, embolism |
| Age 65-74 yr | Hypertension | Mitral stenosis |
| Coronary artery disease | Heart failure | Prosthetic heart valve* |
| Thyrotoxicosis | LV ejection fraction 35% or less | |
| Diabetes mellitus |
INR, international normalized ratio; TIA, transient ischemic attack; LV, left ventricular.
*If mechanical valve, target INR > 2.5.
With permission from Fuster V, Rydén LE, Cannom DS, et al: 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 57:e101–e198, 2011.
• HEMORR2HAGES—hepatic or renal disease, ethanol abuse, malignancy, older age (>75 years), reduced platelet count or function, rebleeding risk, hypertension (uncontrolled), anemia, genetic factors, excessive fall risk, and stroke39
• ATRIA—anticoagulation and risk factors in atrial fibrillation40
• HAS-BLED—hypertension (uncontrolled), abnormal renal or liver function, stroke, bleeding history or predisposition (anemia), labile international normalized ratio, elderly, drugs or alcohol concomitantly (Table 75-2)41
Table 75-2
Clinical Characteristics Composing the HAS-BLED Bleeding Risk Score
| Letter | Clinical Characteristic | Points Awarded |
| H | Hypertension | 1 |
| A | Abnormal renal and liver function (1 point each) | 1 or 2 |
| S | Stroke | 1 |
| B | Bleeding | 1 |
| L | Labile international normalized ratios | 1 |
| E | Elderly | 1 |
| D | Drugs or alcohol (1 point each) | 1 or 2 |
Based on data from Pisters R, Lane DA, Nieuwlaat R, et al: A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 138:1093–1100, 2010.
Among these, the HAS-BLED score is simple and readily applicable with identification of manageable risk factors, and it has a high predictive value.42 The HAS-BLED score can be used to identify patients with a high risk of bleeding, but it should not be considered prohibitive for antithrombotic therapy as patients with higher bleeding risk often are at higher thromboembolic risk and may still have incremental benefit with appropriate anticoagulation. Rather, a high HAS-BLED score (e.g., 3 or greater) should be used to individualize therapy for each patient, prompting more regular follow-up with higher vigilance for potential bleeding complications. Any reversible risk factor for bleeding should be addressed.
Although the bleeding risk is higher, warfarin is superior to aspirin in reducing thromboembolic events.43 Aspirin plus clopidogrel has bleeding rates similar to warfarin, but it is less effective in preventing thromboembolism.44 Current American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines for antithrombotic therapy are displayed in Table 75-1 and recommend consideration of warfarin in patients with one or more moderate thromboembolic risk factor. Recent ESC guidelines have incorporated the CHA2DS2-VASc algorithm and recommend oral anticoagulation unless contraindicated for a score of 2 or greater, and they suggest consideration for a score of 1 based on the bleeding risk and patient preference.45
Recent studies demonstrated the noninferiority or superiority of newer anticoagulants compared with warfarin. Dabigatran was reported to have a similar or lower stroke and major bleeding risk and a decrease in hemorrhagic strokes compared with warfarin, without a difference in overall mortality.46 Rivaroxaban is noninferior to warfarin in stroke prevention with similar overall bleeding risk, but is associated with fewer intracranial and fatal hemorrhages.47 Apixaban is superior to warfarin in stroke prevention with a lower risk of hemorrhagic complications.48 Other potential advantages of newer anticoagulants over warfarin include faster onset of action, no need for dietary restrictions, and potentially more time in the therapeutic range with proper compliance. It has been recognized that patients taking warfarin might not be in the optimal therapeutic anticoagulation range in up to 59% to 68% of the time.49 Limitations of the newer anticoagulant agents include lack of specific reversal agents, dose adjustment requirements in elderly patients and in patients with renal insufficiency, cost, and limited long-term safety data compared with warfarin. The latest ESC guidelines recommend the use of these newer anticoagulant agents as the preferred anticoagulant therapy for patients with AF (Figure 75-4).
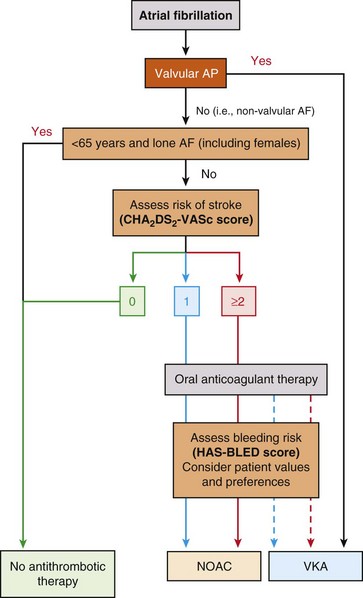
Figure 75-4 European Society of Cardiology guidelines on choice of anticoagulant therapy in patients with AF. Antiplatelet therapy with aspirin plus clopidogrel or, less effectively, aspirin only, should be considered in patients who refuse any oral anticoagulant (OAC), or cannot tolerate anticoagulants for reasons unrelated to bleeding. If there are contraindications to OAC or antiplatelet therapy, then left atrial appendage occlusion, closure, or excision should be considered. CHA2DS2-VASc: green = 0; blue = 1; red ≥ 2. Solid line indicates the best option; dashed line indicates an alternative option. AF, atrial fibrillation; NOAC, novel oral anticoagulants (direct thrombin or factor Xa inhibitors); VKA, vitamin K antagonist.
aIncludes rheumatic valvular disease and prosthetic valves. (Reproduced with permission from Camm AJ, Lip GY, De Caterina R, et al: 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace 14:1385–1413, 2012.)
Other options for stroke prevention, particularly in patients with contraindications to anticoagulation, are surgical or percutaneous left atrial appendage exclusion or occlusion. Reductions in the long term risk for stroke after surgical exclusion of the left atrial appendage, even among patients with high baseline risk factors and many who discontinued anticoagulation following surgery, have been reported.50 Surgical morbidity and mortality should be carefully considered. A high prevalence of residual communication between the left atrium and appendage after both surgical and percutaneous exclusion has been recognized; however, it is not clear whether this is associated with an increased risk of stroke.
Percutaneous techniques of left atrial appendage closure include endocardial deployment of an appendage occlusion device and epicardial ligation of the left atrial appendage with endocardial guidance (LARIAT; SentreHeart, Redwood City, CA). Clinical trials are underway in assessing the efficacy and safety of percutaneous left atrial appendage occlusion devices (Watchman, Atritech, Plymouth, MN; Amplatzer Cardiac Plug, St. Jude Medical, MN). Early studies suggested a significant effect of operator experience and a learning curve in minimizing the risk of complications. A single-center study evaluating the use of the LARIAT device demonstrated a high 1-year complete appendage closure rate (98%) with limited procedural complications.51 Limitations to the use of this device include the exclusion of patients with previous cardiac surgery or unfavorable appendage anatomy and the potential challenges and risk of obtaining percutaneous pericardial access. Large-scale randomized trials will be necessary to determine whether there will be a reduction in the risk of stroke.
Antiarrhythmic drug (AAD) use to maintain sinus rhythm has not been demonstrated to reduce the risk of stroke. However, early observational studies have suggested that stroke risk in the absence of anticoagulant therapy after successful catheter ablation of AF may be similar to the general population, at least in patients who have not had a prior stroke and are younger than 65 years.52,53 Another study showed that an older cohort (≥65 years) following successful ablation of AF also had a stroke rate of 0.7% per year even after discontinuing warfarin (Figure 75-5).54 However, these findings remain to be confirmed in longer-term and larger scale trials.
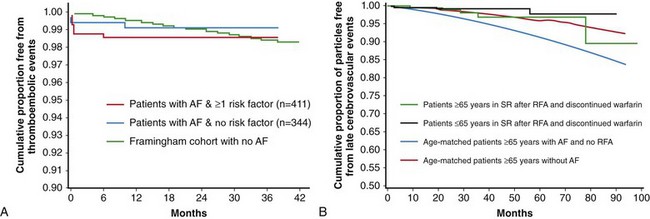
Figure 75-5 A, Cumulative proportion of patients free from thromboembolic events after atrial fibrillation (AF) ablation was similar to a hypothetical group of age-matched control subjects with no history of AF. B, Stroke risk remains low even in patients 65 years or older following successful ablation of AF. SR, sinus rhythm; RFA, radiofrequency ablation. (Adapted with permission from Oral H, Chugh A, Ozaydin M, et al: Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 114:759–765, 2006; Guiot A, Jongnarangsin K, Chugh A, et al: Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 23:36–43, 2012.)
Rate Versus Rhythm Control
In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study, 4060 patients 65 years or older with AF and other risk factors for stroke or death were randomized to a rate or rhythm control strategy.55 On an intention-to-treat analysis, there was a trend toward higher overall mortality in the rhythm control group (P = .08). Subsequent post hoc analyses suggested that potential adverse effects of AAD therapy might have negated any beneficial effects of sinus rhythm.56 An on-treatment analysis demonstrated that maintenance of sinus rhythm and warfarin use were associated with a decrease in mortality, whereas AAD therapy was associated with an increase in mortality.57 In the Rate Control versus Electrical Cardioversion (RACE) trial, there was no significant difference in the primary composite endpoint of cardiovascular mortality, heart failure, thromboembolic complications, bleeding, need for a pacemaker, and severe adverse AAD effects among the 522 patients who were randomized to rhythm or rate control strategy.58
Rate Control
A ventricular rate less than 100 beats/min (bpm) at rest, and 90 to 115 bpm during mild to moderate exertion is often targeted for rate control for AF.35 The RACE-2 trial randomized 614 patients to a strict (resting heart rate < 80 bpm, and moderate exercise heart rate < 110 bpm) versus lenient (resting heart rate < 110 bpm) rate control strategy. The lenient strategy was noninferior to a strict rate control strategy in the composite primary outcome (death from cardiovascular causes, hospitalization for heart failure, stroke, systemic embolism, bleeding, and life-threatening arrhythmias).59 A prospective randomized study of five different rate control regimens, demonstrated that combination of a β-blocker and digoxin was the most effective for ventricular rate control during daily activities.60
In patients who are not candidates for catheter ablation to eliminate AF and in whom ventricular rate control cannot be achieved pharmacologically, atrioventricular junction ablation plus ventricular pacing has been highly effective, with long-term improvement in quality of life and overall improvement in cardiac function. Some patients with left ventricular dysfunction and heart failure may benefit with initial biventricular pacing at the time of ablation, but this subset remains to be better defined. In patients who experience a decrease in cardiac function following right ventricular pacing, an upgrade to a biventricular pacing device can provide subsequent improvement in ejection fraction and heart failure symptoms.61
Rhythm Control
Maintenance of sinus rhythm has been associated with an improvement in quality of life, left ventricular ejection fraction, and left atrial size.62
Pharmacologic Therapy
Antiarrhythmic Drug Therapy
Once a decision for rhythm control is made, a trial of one or more AADs is usually warranted unless it is not preferable or contraindicated because of comorbid conditions or poor long-term risk/benefit ratio. The selection of AADs can be affected by the presence of structural heart disease, comorbidities, patient age, and the type of AF (Figure 75-6). In patients with preserved left ventricular function, no ischemia, and no significant left ventricular hypertrophy, class IC antiarrhythmic drugs are often the initial choice and can be administered daily to prevent AF or as needed in patients with infrequent, reasonably well-tolerated, and longer episodes of AF to shorten the duration of the arrhythmia (“pill in the pocket” approach). Class IC agents should be administered with a rate controlling agent to prevent paradoxic increases in ventricular rates during AF. Because of the risk of proarrhythmia, class IA agents are being used less often. However, disopyramide can be considered in patients with vagotonic AF because of its anticholinergic effects.
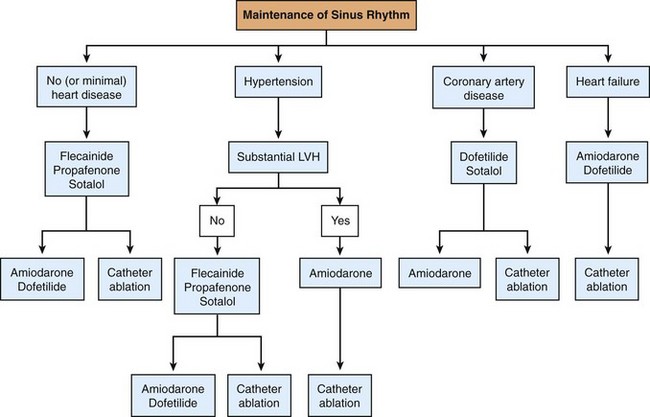
Figure 75-6 Choice of antiarrhythmic drug therapy for recurrent paroxysmal or persistent atrial fibrillation based on underlying cardiovascular comorbidities. (Reproduced with permission from Fuster V, Rydén LE, Cannom DS, et al: 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 57:e101–e198, 2011.)
In a randomized study, time to recurrence of AF was longer among patients who received amiodarone (487 days) compared with sotalol (74 days) or placebo (6 days).63 In a subgroup analysis of patients with ischemic heart disease, time to recurrence of AF was similar in the amiodarone and sotalol groups. Amiodarone is the AAD least likely to cause proarrhythmia in patients with structural heart disease and can be used in patients with renal insufficiency. However, its potential cumulative systemic side effects are a concern and should be monitored carefully.
Dofetilide, another class III agent, can also be used in patients with a reduced left ventricular ejection fraction. In the Danish Investigations of Arrhythmia and Mortality on Dofetilide—Congestive Heart Failure (DIAMOND-CHF) trial, 1518 patients with New York Heart Association class III or IV symptoms and severe LV dysfunction were randomized to dofetilide versus placebo.64 Dofetilide was associated with fewer hospitalizations for worsening heart failure and an improvement in sinus rhythm maintenance. Dofetilide has a narrow therapeutic window with complex pharmacokinetics and a 3.3% incidence of torsade de pointes.64 Dofetilide should be initiated as an inpatient; QT interval and renal function must be monitored regularly; and dose should be adjusted accordingly. Sotalol, another class III agent, also carries a risk of proarrhythmia, which can be more prevalent in women with a diminished repolarization reserve.65 Sotalol dosage should be adjusted according to the renal function.
Dronedarone has electrophysiological effects similar to amiodarone and may be considered in patients with paroxysmal AF. Lacking the iodine moiety, dronedarone was proposed to have fewer adverse effects than amiodarone; however, it appears to be less effective.66 It should not be used in patients with persistent AF and structural heart disease. It is contraindicated in patients with New York Heart Association class IV heart failure or recent decompensation. Rare cases of fulminant hepatic failure have also been reported.
Newer antiarrhythmic agents are atrioselective and primarily block early activating K+ channels (IKur). Intravenous vernakalant facilitates conversion from AF to sinus rhythm in recent onset (52%)67 and postoperative (47%) AF.68 Oral vernakalant can also help to maintain sinus rhythm following cardioversion.69 Overall safety and efficacy profiles remain to be determined, and vernakalant currently is being investigated in the United States.
Statins, Polyunsaturated Fatty Acids, Inhibitors of the Renin-Angiotensin-Aldosterone System
Statins can prevent AF by modulating inflammatory response and oxidative stress. Simvastatin attenuates atrial tachycardia pacing–induced electrophysiological remodeling through inhibition of the downregulation of L-type calcium channels, and it reduces atrial fibrosis and conduction abnormalities by attenuating fibroblast proliferation.70 In a recent metaanalysis including 7041 patients,71 statins only reduced the risk of AF in observational studies (23% reduction) and mostly in the postoperative setting. Statins decreased postoperative AF from 36.6% to 27.8% (odds ratio, 0.68; 95% confidence interval; 0.46 to 0.96).72
Omega-3 (n-3) polyunsaturated fatty acids (PUFAs) have antioxidant, antiinflammatory, and lipid-lowering effects. PUFAs also block Na and L-type calcium channels and modulate membrane fluidity.73 In an animal model of vagally induced AF, pretreatment with fish oil reduced expression of connexin40 and connexin43 by 50% and decreased inducibility of AF.74 In an observational, prospective study of 4815 adults 65 years or older, fish consumption was associated with a 30% reduction in AF incidence over 12 years.75 These results were not consistently confirmed in other observational or prospective randomized studies. In a randomized study, treatment with PUFAs reduced postoperative AF by 50% after coronary artery bypass surgery76; this benefit in postoperative AF was confirmed in a recent metaanalysis.77 Although secondary prevention AF trials do not consistently support the benefit of PUFAs, a limitation of many trials is the duration of therapy. Recently, 178 patients with persistent AF treated with fish oil 1 month before cardioversion and continued afterward had substantial 90-day (38.5% vs. 77.5%) and 1-year reductions in the recurrence of AF (67% vs. 90%).78 In another study, patients with higher levels of circulating n-3 PUFAs and docosahexaenoic acid levels had a lower incidence of AF.79
Activation of the renin-angiotensin-aldosterone system (RAAS) results in fibrosis and promotes electroanatomic remodeling.80 Renin-angiotensin gene polymorphisms are associated with an increased risk of AF.81 Local expression of the angiotensin-converting enzyme is increased in patients with persistent AF. Inhibition of RAAS can prevent AF by improving left ventricular remodeling and hemodynamics, decreasing left atrial stretch, attenuating angiotensin-induced fibrosis, and exerting direct effects on ion channels.82 In a metaanalysis of 11 studies involving 56,308 patients, treatment with an angiotensin-converting-enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) was associated with a 28% decrease in the relative risk of AF.83 The reduction in AF was similar with ACEI or ARBs, and it was most pronounced in patients with congestive heart failure. Except in patients with left ventricular hypertrophy, a similar reduction in AF risk was not observed in patients with hypertension. In another recent metaanalysis of 92,817 patients in 14 trials, ARBs more effectively reduced new onset AF (relative risk 78% compared with conventional therapy or placebo) than did ACEIs.84
Aldosterone also promotes inflammation and fibrosis. In an animal model of pacing-induced congestive heart failure, selective blockade of aldosterone decreased inducibility of atrial arrhythmias and prolonged atrial ERP.85 In the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF), eplerenone was associated with a reduction in new onset of AF or flutter (hazard ratio, 0.58).86 However, a recent metaanalysis showed no benefit of aldosterone antagonists in reducing new onset AF.84 Although there is preliminary evidence, the beneficial effects of statins, PUFA, and RAAS inhibitors on the pathogenesis of AF remain to be confirmed.
Catheter Ablation
Patient Selection
According to the current guidelines, after a trial of one or more AADs, catheter ablation is recommended for patients with symptomatic paroxysmal AF and is considered reasonable for patients with persistent AF. It can also be considered as first-line therapy for patients with symptomatic paroxysmal AF.9,45 Age, functional status, symptoms, long-term thromboembolic risk, structural remodeling associated with AF, long-term risk of anticoagulant therapy, presence of structural heart disease including tachycardia-mediated cardiomyopathy, left atrial size, duration of AF, and availability of effective and tolerable pharmacologic therapy should be weighed carefully against the long-term efficacy and risk of complications associated with catheter ablation.
Perioperative Anticoagulation
Uninterrupted warfarin is the preferred perioperative anticoagulation regimen in patients who undergo catheter ablation of AF. A recent metaanalysis of nine studies including 6400 patients demonstrated that uninterrupted anticoagulation with warfarin during ablation significantly decreased the thromboembolic risk without an increased risk of bleeding. There was no increase in the incidence or severity of pericardial tamponade.87,88 The optimal algorithm for the use of newer anticoagulants during the perioperative period remains to be defined because of different pharmacokinetics and limitation of specific antidotes available for the newer thrombin and Factor Xa inhibitors.
Techniques
The premise of anatomical ablation is that mechanisms of AF use similar locations, and empiric ablation at these sites should be effective in eliminating AF. PVs have a critical role in the initiation and perpetuation of AF15 and are targeted with a variety of techniques, including segmental ostial ablation, circumferential PV ablation, wide-area circumferential ablation, and antral PVI (APVI; Figure 75-7).89,90 Because of the arrhythmogenic potential of the PV antrum, APVI is often preferred and can exert its beneficial effects by: (1) elimination of arrhythmogenic sites within the PVs and their antra, (2) debulking of the left atrium by 25% to 30%, (3) elimination of anchor points of rotors, (4) ablation of GPs, and (5) possible ablation of the ligament of Marshall.
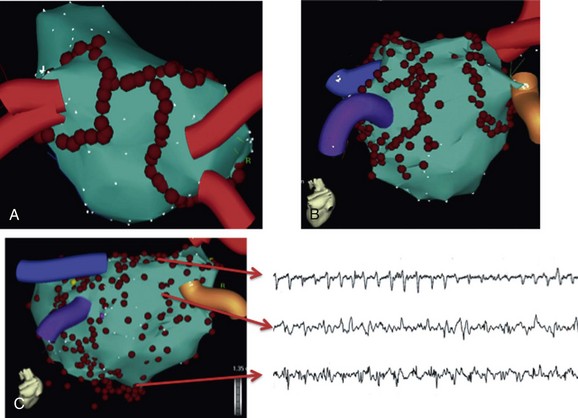
Figure 75-7 Electroanatomic maps of different strategies of atrial fibrillation ablation. A, Circumferential pulmonary vein isolation. B, Antral pulmonary vein ablation. C, Ablation of complex fractionated atrial electrograms (CFAE). Note the rapid, disorganized, and continuous electrograms represented at ablation points in the CFAE map. Adapted with permission from references 56 and 57.
APVI is highly effective for paroxysmal AF, although repeated procedures may be necessary in up to 20% to 50% of cases to eliminate recurrent PV conduction.91,92 Because of the complex mechanisms of persistent AF that extend beyond the PVs, the efficacy of APVI alone for persistent or long-standing persistent AF has been rather modest.93
The superior vena cava, ligament of Marshall, CS, and crista terminalis are often targeted when found to harbor arrhythmogenic foci. Some studies suggested that the CS might also have a role in the perpetuation of AF similar to a PV; these studies employed routine endocardial and/or epicardial isolation of the CS.19 However, the utility of routine CS isolation remains unaddressed in large prospective studies.
Sites of GP can be targeted based on typical anatomic patterns, such as near the PV ostia and septum in the left atrium,94 or identified by vagal responses to high frequency stimuli. Although GP ablation seems to be insufficient as standalone therapy for paroxysmal or persistent AF,95,96 few studies (evaluating both endocardial and surgical procedures) suggest an improvement in short- and long-term sinus rhythm maintenance when used in addition to PVI.97 The role of GP ablation remains to be better defined in larger-scale, prospective, randomized studies.
Electrophysiologically Guided Ablation
In a novel study, CFAEs were targeted to eliminate AF in paroxysmal and persistent AF with favorable clinical outcomes.98 CFAEs are usually defined by a short cycle length (≤120 ms), fractionation, or continuous electrical activity (see Figure 75-7). CFAEs can indicate sites of slow conduction, pivot points for reentrant circuits, wavefront collision, and conduction block; therefore, they also indicate sites of reentry.99 However, CFAEs are nonspecific and can reflect passive activation or summation of electrograms from overlapping layers of atrial myocardium and anisotropy. CFAEs can also be observed at the periphery of rotors where wave break and fibrillatory conduction occur.23 Ablation at these sites of CFAEs is sometimes effective by eliminating the nearby drivers.
Other approaches to identify AF drivers include near–real-time or real-time spectral mapping of AF to identify the sites with the highest dominant frequency. Offline spectral analysis of electrograms in patients with paroxysmal AF demonstrated that sites at which ablation terminated AF had a higher DF.100 The presence and elimination of DF gradients with ablation, whether within the left atrium or between the left and right atrium, has been associated with a more favorable clinical outcome.24,101 In one study, a decrease in DF by 11% following APVI and ablation of CFAEs was predictive of freedom from AF in patients with persistent AF.25 In another study, spectral components of AF other than the DF were suggested to indicate underlying macroreentrant atrial tachycardias that become manifest after elimination of drivers of AF with a higher DF.102 The feasibility and utility of real-time spectral mapping remains to be refined and subsequently validated in large-scale prospective trials.
In a recent study, localized rotors or rapidly firing sources were identified and ablated in paroxysmal and persistent AF using novel computational methods.22 The details remain undisclosed, but the methodology involves the application of a phase domain analysis (Hilbert transform) to derive a time-specific AF activation pattern. Pivot points for rotors or focal impulse sources were visualized and targeted (Figure 75-8).22 Compared with APVI (plus left atrial roof line for persistent AF), the focal impulse and rotor modulation (FIRM) method showed higher likelihood of acute termination or slowing of AF during ablation (86% vs. 20%; P < .001) and long-term freedom from AF (82.4% vs. 44.9%; P < .001) after a median of 273 days. Although the FIRM method incorporated APVI and was not associated with a decrease in total ablation time, median ablation time to termination of AF was 2.5 minutes. This promising approach will require detailed examination of the mapping algorithms and validation in multicenter randomized studies.
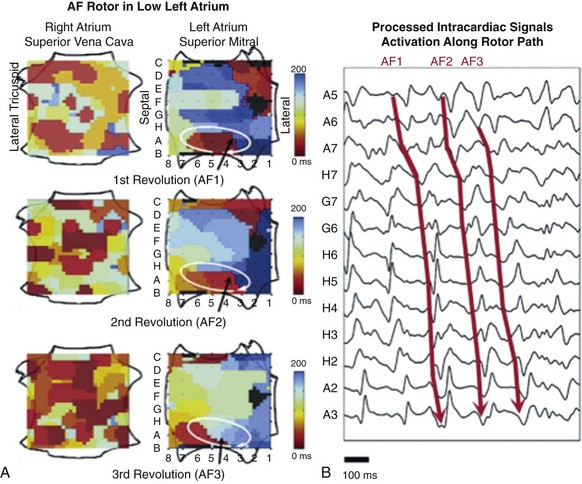
Figure 75-8 Mapping of an electrical rotor during atrial fibrillation (AF). A, Computational maps of the right and left atria depicting three cycles of a left atrial rotor during AF with clockwise revolution (activation time scale coded from red to blue). B, Processed and filtered intracardiac signals showing sequential activation over the rotor path for the three cycles. (Reproduced with permission from Narayan SM, Krummen DE, Shivkumar K, et al: Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation with or without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 60:628–636, 2012.)
During APVI, the optimal endpoint is complete PV isolation confirmed by elimination of PV potentials and demonstration of bidirectional block between the PVs and left atrium. Adenosine can also be used to unmask dormant conduction between the PVs and left atrium.103 However, it remains to be determined whether further ablation to eliminate adenosine-induced reconnection improves outcome. Isoproterenol can be used with or without rapid atrial pacing to identify other clinical supraventricular arrhythmias or non-PV triggers. Endpoints for individual applications of radiofrequency energy include elimination of target potentials, voltage abatement by greater than 80%, decreased impedance, slowing of the AF cycle length, and elimination of local activation gradients.104,105
Procedural endpoints are less well defined, particularly for nonparoxysmal AF. Several studies suggested that termination and noninducibility of AF during ablation predicts freedom from AF recurrence especially in patients with paroxysmal AF. Termination of AF indicates effective elimination of all triggers and drivers of AF active at a specific moment, whereas noninducibility suggests complete elimination of any residual triggers and drivers that could initiate and perpetuate AF. Alternative endpoints can include completion of selected anatomic lesion sets, elimination of CFAEs, decrease in AF cycle length, decrease in dominant frequency, or elimination of frequency gradients. Few studies have suggested that termination (and noninducibility whenever achievable) of AF is associated with a higher probability of freedom from AF in patients with nonparoxysmal AF. However, termination and noninducibility can be difficult to achieve in patients with nonparoxysmal AF; it can require substantial ablation with a long procedure duration, and it often prompts repeated ablation procedures primarily to eliminate atrial tachycardias.106
Outcomes and Cost Analysis
The efficacy of catheter ablation in maintaining sinus rhythm has been reported to vary between 60% and 85% in patients with paroxysmal AF and between 30% and 75% in patients with persistent AF.89,107–110 Efficacy is improved with concomitant antiarrhythmic drug therapy.107 However, assessment of clinical efficacy of catheter ablation can be complex and often highly dependent on the intensity of follow-up (extended ECG monitoring), patient characteristics, chronicity of AF, the extent of electroanatomic remodeling, and number of repeated ablation procedures.
Several studies have reported a superior efficacy of catheter ablation to AADs and cardioversion for maintenance of sinus rhythm with an improvement in quality of life and decrease in left atrial dimension in patients with paroxysmal or persistent AF.111,112 However, a recent study randomizing patients to catheter ablation versus AAD as an initial treatment strategy for paroxysmal AF showed no significant difference in cumulative AF burden over 2 years (Figure 75-9).113 In this study, a significant number of patients crossed over from the AAD to the ablation arm, but the results support an initial strategy of attempting antiarrhythmic medication. It remains to be determined whether earlier ablation confers longer-term benefit and decreases the likelihood of progression of AF. In a recent retrospective analysis, ablation of paroxysmal AF reduced or delayed progression to persistent AF over a mean follow-up of 27 months ± a standard deviation of 12 months (Figure 75-10).114
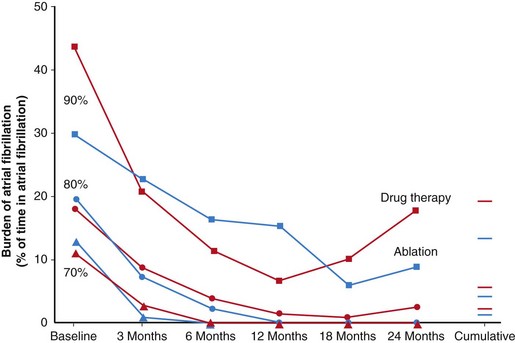
Figure 75-9 Percentiles (70%, 80%, and 90%) of the burden of atrial fibrillation in ablation and drug therapy groups at baseline and during follow-up. The same percentiles are also shown for the cumulative burden of atrial fibrillation. (Reproduced with permission from Cosedis Nielsen J, Johannessen A, Raatikainen P, et al: Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 367:1587–1595, 2012.)
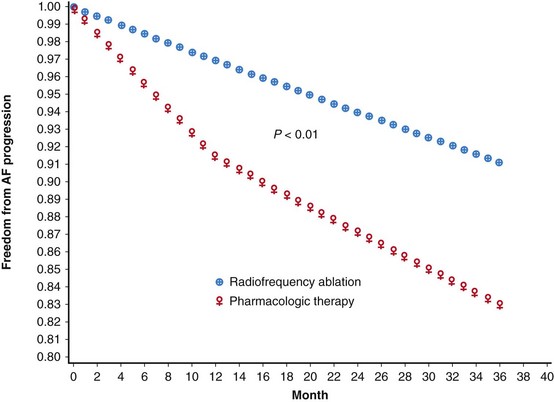
Figure 75-10 Rate of atrial fibrillation (AF) progression. The probability of AF progression was 0.6% per year after radiofrequency ablation in this study (blue line) versus 8.6% in the first year with slow but steady progression to 24.7% in 5 years in patients treated pharmacologically (red line). (Reproduced with permission from Jongnarangsin K, Suwanagool A, Chugh A, et al: Effect of catheter ablation on progression of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 23:9–14, 2012.)
A recent analysis of health care utilization using data from MarketScan Databases (Thomson Reuters; New York, NY) showed that health care expenditures fell by $2261 at 6 months after AF ablation compared with 6 months before ablation. Decreases in outpatient and emergency visits and hospitalization days were realized up to 30 months following ablation.115,116 In another study, projected cost effectiveness of ablation of AF versus use of amiodarone or a rate control strategy were compared. Based on an 80% efficacy rate for sinus rhythm maintenance, relative reduction in stroke risk would need to be greater than or equal to 42% or 11% to realize cost effectiveness ratios of less than $50,000 and $100,000, respectively, per quality adjusted life year.117 Another study demonstrated overall cost-neutral results at 2 years after ablation.118
Complications
Overall complication rates for catheter ablation of AF have been reported at 3.5% to 4.5%,119,120 with most being related to vascular access. Cardiac tamponade occurs in 1.3%, and thromboembolic events including stroke and TIA occur in less than 1%. Mortality remains rare at less than 0.2%. Atrial-esophageal fistula is a rare but potentially fatal complication; strategies to mitigate this risk include intraoperative barium swallow or radiopaque esophageal temperature probes, and avoidance of high power lesions on the posterior wall at areas adjacent to the esophagus. Uninterrupted warfarin throughout the perioperative period has shown a reduction in periprocedural stroke without an increase in bleeding risk.121 Phrenic nerve palsy is particularly a concern with cryoballoon ablation, with reports of up to 5% persisting after ablation, although the majority are reversible and can take up to 1 year to resolve (<0.4% persisted beyond 1 year).122 PV stenosis has significantly decreased with the shift from ostial PV isolation to an antral approach. A recent multicenter survey reported an incidence of 0.29% requiring intervention.107 Development or worsening of pulmonary hypertension and left atrial diastolic dysfunction following ablation (described as the “stiff left atrium syndrome” in postsurgical patients) was recently reported in 1.4% of patients in a prospective study of 1380 patients who underwent AF ablation.123 Patients exhibit dyspnea and in general respond well to diuresis.
Surgical Therapy
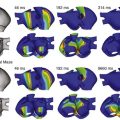
The classical surgical MAZE procedure with the cut and sew technique is highly effective in restoring sinus rhythm. Because of its technical complexity, the procedure has undergone several modifications (Figure 75-11).124–128 Currently, standalone surgical procedures for cure of AF often involve a thoracoscopic approach and use similar energy sources for percutaneous catheter ablation. A metaanalysis of 48 studies involving 3832 patients demonstrated sinus rhythm maintenance in 83% of the patients who underwent the classic cut and sew MAZE procedure and in 78% of patients who had ablation using alternative energy sources (P = .03).129 Stroke rates, partially attributed to left atrial appendage exclusion or amputation, are low, with one study citing a 0.2% annual risk following the Cox-MAZE procedure.130 Surgical ablation remains anatomically based with pulmonary vein isolation, linear lesions sets, ablation of GPs, and possible appendage exclusion.
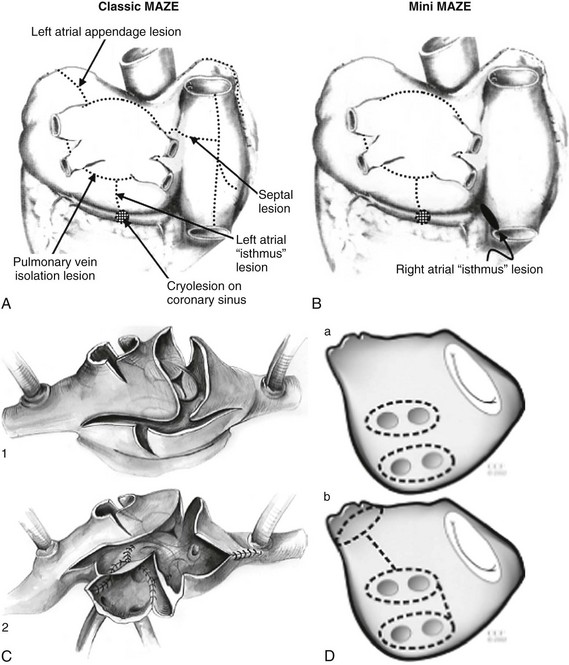
Figure 75-11 Examples of surgical classical and miniature MAZE procedures. Classical MAZE procedure is shown in A and C. Miniature MAZE procedure with slight modifications in the technique is shown in B and D. (Adapted with permission from Cox JL: Cardiac surgery for arrhythmias. J Cardiovasc Electrophysiol 15:250–262, 2004; Cox JL: Atrial fibrillation II: rationale for surgical treatment. J Thorac Cardiovasc Surg 126:1693–1699, 2003; Ballaux PK, Geuzebroek GS, van Hemel NM, et al: Freedom from atrial arrhythmias after classic maze III surgery: a 10-year experience. J Thorac Cardiovasc Surg 132:1433–1440, 2006; and Gillinov AM, McCarthy PM: Advances in the surgical treatment of atrial fibrillation. Cardiol Clin 22:147–157, 2004.)
References
1. Rich, MW. Epidemiology of atrial fibrillation. J Interv Card Electrophysiol. 2009; 25:3–8.
2. Roger, VL, Go, AS, Lloyd-Jones, DM, et al. Heart disease and stroke statistics–2012 update: A report from the American heart association. Circulation. 2012; 125:e2–e220.
3. Miyasaka, Y, Barnes, ME, Gersh, BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114:119–125.
4. Chiang, CE, Zhang, S, Tse, HF, et al. Atrial fibrillation management in asia: From the Asian expert forum on atrial fibrillation. Int J Cardiol. 2012.
5. Lip, GY, Brechin, CM, Lane, DA. The global burden of atrial fibrillation and stroke: A systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012.
6. Benjamin, EJ, Wolf, PA, D’Agostino, RB, et al. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998; 98:946–952.
7. Piccini, JP, Hammill, BG, Sinner, MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012; 5:85–93.
8. Coyne, KS, Paramore, C, Grandy, S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the united states. Value Health. 2006; 9:348–356.
9. Calkins, H, Kuck, KH, Cappato, R, et al. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012; 33:171–257.
10. Winterberg, H. Studien über herzflimmern: I. Über die wirkung des n. Vagus und accelerans auf das flimmern des herzens. 1907; 223–256.
11. Mines, GR. On dynamic equilibrium in the heart. J Physiol. 1913; 46:349–383.
12. Moe, GK. A conceptual model of atrial fibrillation. J Electrocardiol. 1968; 1:145–146.
14. Sahadevan, J, Ryu, K, Peltz, L, et al. Epicardial mapping of chronic atrial fibrillation in patients: Preliminary observations. Circulation. 2004; 110:3293–3299.
15. Haïssaguerre, M, Jaïs, P, Shah, DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339:659–666.
16. Douglas, YL, Jongbloed, MR, Gittenberger-de Groot, AC, et al. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol. 2006; 97:662–670.
17. Chugh, A, Oral, H, Good, E, et al. Catheter ablation of atypical atrial flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol. 2005; 46:83–91.
18. Oral, H, Ozaydin, M, Chugh, A, et al. Role of the coronary sinus in maintenance of atrial fibrillation. J Cardiovasc Electrophysiol. 2003; 14:1329–1336.
19. Haissaguerre, M, Hocini, M, Takahashi, Y, et al. Impact of catheter ablation of the coronary sinus on paroxysmal or persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2007; 18:378–386.
20. Jalife, J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol. 2003; 14:776–780.
21. Filgueiras-Rama, D, Martins, RP, Ennis, SR, et al. High-resolution endocardial and epicardial optical mapping in a sheep model of stretch-induced atrial fibrillation. J Vis Exp. 2011.
22. Narayan, SM, Krummen, DE, Shivkumar, K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation with or without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012; 60:628–636.
23. Kalifa, J, Tanaka, K, Zaitsev, AV, et al. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006; 113:626–633.
24. Atienza, F, Almendral, J, Jalife, J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009; 6:33–40.
25. Yoshida, K, Chugh, A, Good, E, et al. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2010; 7:295–302.
26. Bettoni, M, Zimmermann, M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation.. 2002; 105:2753–2759.
27. Po, SS, Scherlag, BJ, Yamanashi, WS, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006; 3:201–208.
28. Katritsis, D, Giazitzoglou, E, Sougiannis, D, et al. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009; 11:308–315.
29. Morillo, CA, Klein, GJ, Jones, DL, et al. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995; 91:1588–1595.
30. Wijffels, MC, Kirchhof, CJ, Dorland, R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995; 92:1954–1968.
31. Wolf, PA, Abbott, RD, Kannel, WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991; 22:983–988.
32. Miller, VT, Rothrock, JF, Pearce, LA, et al. Ischemic stroke in patients with atrial fibrillation: Effect of aspirin according to stroke mechanism. Stroke prevention in atrial fibrillation investigators. Neurology. 1993; 43:32–36.
33. Sohara, H, Amitani, S, Kurose, M, et al. Atrial fibrillation activates platelets and coagulation in a time-dependent manner: A study in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 1997; 29:106–112.
34. Aviles, RJ, Martin, DO, Apperson-Hansen, C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006–3010.
35. Fuster, V, Rydén, LE, Cannom, DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011; 57:e101–e198.
36. Gage, BF, Waterman, AD, Shannon, W, et al. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. J Am Med Assoc. 2001; 285:2864–2870.
37. van Walraven, C, Hart, RG, Wells, GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003; 163:936–943.
38. Lip, GY, Nieuwlaat, R, Pisters, R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137:263–272.
39. Gage, BF, Yan, Y, Milligan, PE, et al. Clinical classification schemes for predicting hemorrhage: Results from the national registry of atrial fibrillation (nraf). Am Heart J. 2006; 151:713–719.
40. Fang, MC, Go, AS, Chang, Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The atria (anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol. 2011; 58:395–401.
41. Pisters, R, Lane, DA, Nieuwlaat, R, et al. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest. 2010; 138:1093–1100.
42. Camm, AJ, Lip, GY, De Caterina, R, et al. Members ATF, (CPG) ECfPG, Reviewers D. 2012 focused update of the esc guidelines for the management of atrial fibrillation: An update of the 2010 esc guidelines for the management of atrial fibrillation developed with the special contribution of the european heart rhythm association. Europace. 2012; 14:1385–1413.
43. Atrial Fibrillation Investigators: Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994; 154:1449–1457.
44. Connolly, S, Pogue, J, Hart, R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (active w): A randomised controlled trial. Lancet. 2006; 367:1903–1912.
45. Camm, AJ, Lip, GY, De Caterina, R, et al. 2012 focused update of the esc guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation–developed with the special contribution of the european heart rhythm association. Europace. 2012; 14:1385–1413.
46. Connolly, SJ, Ezekowitz, MD, Yusuf, S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151.
47. Patel, MR, Mahaffey, KW, Garg, J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–891.
48. Granger, CB, Alexander, JH, McMurray, JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981–992.
49. Amouyel, P, Mismetti, P, Langkilde, LK, et al. Inr variability in atrial fibrillation: A risk model for cerebrovascular events. Eur J Intern Med. 2009; 20:63–69.
50. Pet, M, Robertson, JO, Bailey, M, et al. The impact of chads(2) score on late stroke after the cox maze procedure. J Thorac Cardiovasc Surg. 2012.
51. Bartus, K, Han, FT, Bednarek, J, et al. Percutaneous left atrial appendage suture ligation using the lariat device in patients with atrial fibrillation: Initial clinical experience. J Am Coll Cardiol. 2012.
52. Hunter, RJ, McCready, J, Diab, I, et al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart. 2012; 98:48–53.
53. Oral, H, Chugh, A, Ozaydin, M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006; 114:759–765.
54. Guiot, A, Jongnarangsin, K, Chugh, A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol. 2012; 23:36–43.
55. Wyse, DG, Waldo, AL, DiMarco, JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002; 347:1825–1833.
56. Steinberg, JS, Sadaniantz, A, Kron, J, et al. Analysis of cause-specific mortality in the atrial fibrillation follow-up investigation of rhythm management (affirm) study. Circulation. 2004; 109:1973–1980.
57. Corley, SD, Epstein, AE, DiMarco, JP, et al. Investigators A. Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow-up investigation of rhythm management (affirm) study. Circulation. 2004; 109:1509–1513.
58. Van Gelder, IC, Hagens, VE, Bosker, HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002; 347:1834–1840.
59. Van Gelder, IC, Groenveld, HF, Crijns, HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010; 362:1363–1373.
60. Farshi, R, Kistner, D, Sarma, JS, et al. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: A crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999; 33:304–310.
61. Leon, AR, Greenberg, JM, Kanuru, N, et al. Cardiac resynchronization in patients with congestive heart failure and chronic atrial fibrillation: Effect of upgrading to biventricular pacing after chronic right ventricular pacing. J Am Coll Cardiol. 2002; 39:1258–1263.
62. Oral, H, Pappone, C, Chugh, A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006; 354:934–941.
63. Singh, BN, Singh, SN, Reda, DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005; 352:1861–1872.
64. Torp-Pedersen, C, Møller, M, Bloch-Thomsen, PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish investigations of arrhythmia and mortality on dofetilide study group. N Engl J Med. 1999; 341:857–865.
65. Lehmann, MH, Hardy, S, Archibald, D, et al. Jtc prolongation with d,l-sotalol in women versus men. Am J Cardiol. 1999; 83:354–359.
66. Le Heuzey, JY, De Ferrari, GM, Radzik, D, et al. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: The dionysos study. J Cardiovasc Electrophysiol. 2010; 21:597–605.
67. Roy, D, Pratt, CM, Torp-Pedersen, C, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: A phase 3, randomized, placebo-controlled trial. Circulation. 2008; 117:1518–1525.
68. Kowey, PR, Dorian, P, Mitchell, LB, et al. Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: A randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009; 2:652–659.
69. Torp-Pedersen, C, Raev, DH, Dickinson, G, et al. A randomized, placebo-controlled study of vernakalant (oral) for the prevention of atrial fibrillation recurrence after cardioversion. Circ Arrhythm Electrophysiol. 2011; 4:637–643.
70. Shiroshita-Takeshita, A, Schram, G, Lavoie, J, et al. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004; 110:2313–2319.
71. Liu, T, Li, L, Korantzopoulos, P, et al. Statin use and development of atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials and observational studies. Int J Cardiol. 2008; 126:160–170.
72. Lertsburapa, K, White, CM, Kluger, J, et al. Preoperative statins for the prevention of atrial fibrillation after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2008; 135:405–411.
73. Reiffel, JA, McDonald, A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006; 98:50i–60i.
74. Sarrazin, JF, Comeau, G, Daleau, P, et al. Reduced incidence of vagally induced atrial fibrillation and expression levels of connexins by n-3 polyunsaturated fatty acids in dogs. J Am Coll Cardiol. 2007; 50:1505–1512.
75. Mozaffarian, D, Psaty, BM, Rimm, EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004; 110:368–373.
76. Calò, L, Bianconi, L, Colivicchi, F, et al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A randomized, controlled trial. J Am Coll Cardiol. 2005; 45:1723–1728.
77. He, Z, Yang, L, Tian, J, et al. Efficacy and safety of omega-3 fatty acids for the prevention of atrial fibrillation: A meta-analysis. Can J Cardiol. 2012.
78. Kumar, S, Sutherland, F, Morton, JB, et al. Long-term omega-3 polyunsaturated fatty acid supplementation reduces the recurrence of persistent atrial fibrillation after electrical cardioversion. Heart Rhythm. 2012; 9:483–491.
79. Wu, JH, Lemaitre, RN, King, IB, et al. Association of plasma phospholipid long-chain ω-3 fatty acids with incident atrial fibrillation in older adults: The cardiovascular health study. Circulation. 2012; 125:1084–1093.
80. Ehrlich, JR, Hohnloser, SH, Nattel, S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: Clinical and experimental evidence. Eur Heart J. 2006; 27:512–518.
81. Tsai, CT, Lai, LP, Lin, JL, et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004; 109:1640–1646.
82. Ehrlich, JR, Hohnloser, SH, Nattel, S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: Clinical and experimental evidence. Eur Heart J. 2006; 27:512–518.
83. Healey, JS, Baranchuk, A, Crystal, E, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: A meta-analysis. J Am Coll Cardiol. 2005; 45:1832–1839.
84. Khatib, R, Joseph, P, Briel, M, et al. Blockade of the renin-angiotensin-aldosterone system (raas) for primary prevention of non-valvular atrial fibrillation: A systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2012.
85. Shroff, SC, Ryu, K, Martovitz, NL, et al. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol. 2006; 17:534–541.
86. Swedberg, K, Zannad, F, McMurray, JJ, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: Results from the emphasis-hf (eplerenone in mild patients hospitalization and survival study in heart failure) study. J Am Coll Cardiol. 2012; 59:1598–1603.
87. Santangeli, P, Di Biase, L, Horton, R, et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: Evidence from a meta-analysis. Circ Arrhythm Electrophysiol. 2012; 5:302–311.
88. Latchamsetty, R, Gautam, S, Bhakta, D, et al. Management and outcomes of cardiac tamponade during atrial fibrillation ablation in the presence of therapeutic anticoagulation with warfarin. Heart Rhythm. 2011; 8:805–808.
89. Oral, H, Pappone, C, Chugh, A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006; 354:934–941.
90. Oral, H, Chugh, A, Yoshida, K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol.. 2009; 53:782–789.
91. Oral, H, Scharf, C, Chugh, A, et al. Catheter ablation for paroxysmal atrial fibrillation: Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003; 108:2355–2360.
92. Bhargava, M, Di Biase, L, Mohanty, P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: Results from a multicenter study. Heart Rhythm. 2009; 6:1403–1412.
93. Cappato, R, Calkins, H, Chen, SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:32–38.
94. Po, SS, Nakagawa, H, Jackman, WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009; 20:1186–1189.
95. Mikhaylov, E, Kanidieva, A, Sviridova, N, et al. Outcome of anatomic ganglionated plexi ablation to treat paroxysmal atrial fibrillation: A 3-year follow-up study. Europace. 2011; 13:362–370.
96. Pokushalov, E, Romanov, A, Artyomenko, S, et al. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace. 2010; 12:342–346.
97. Zhou, Q, Hou, Y, Yang, S. A meta-analysis of the comparative efficacy of ablation for atrial fibrillation with and without ablation of the ganglionated plexi. Pacing Clin Electrophysiol. 2011; 34:1687–1694.
98. Nademanee, K, McKenzie, J, Kosar, E, et al. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004; 43:2044–2053.
99. Jadidi, AS, Duncan, E, Miyazaki, S, et al. Functional nature of electrogram fractionation demonstrated by left atrial high-density mapping. Circ Arrhythm Electrophysiol. 2012; 5:32–42.
100. Sanders, P, Berenfeld, O, Hocini, M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005; 112:789–797.
101. Lin, YJ, Tsao, HM, Chang, SL, et al. Role of high dominant frequency sites in nonparoxysmal atrial fibrillation patients: Insights from high-density frequency and fractionation mapping. Heart Rhythm. 2010; 7:1255–1262.
102. Yokokawa, M, Chugh, A, Ulfarsson, M, et al. Effect of linear ablation on spectral components of atrial fibrillation. Heart Rhythm. 2010; 7:1732–1737.
103. Datino, T, Macle, L, Chartier, D, et al. Differential effectiveness of pharmacological strategies to reveal dormant pulmonary vein conduction: A clinical-experimental correlation. Heart Rhythm. 2011; 8:1426–1433.
104. Oral, H, Scharf, C, Chugh, A, et al. Catheter ablation for paroxysmal atrial fibrillation: Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003; 108:2355–2360.
105. Haissaguerre, M, Sanders, P, Hocini, M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: Critical structures for termination. J Cardiovasc Electrophysiol. 2005; 16:1125–1137.
106. Elayi, CS, Di Biase, L, Barrett, C, et al. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm. 2010; 7:1216–1223.
107. Cappato, R, Calkins, H, Chen, SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:32–38.
108. Oral, H, Chugh, A, Good, E, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006; 113:1824–1831.
109. Chao, TF, Tsao, HM, Lin, YJ, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: Results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012; 5:514–520.
110. Brooks, AG, Stiles, MK, Laborderie, J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: A systematic review. Heart Rhythm. 2010; 7:835–846.
111. Oral, H, Pappone, C, Chugh, A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006; 354:934–941.
112. Wazni, OM, Marrouche, NF, Martin, DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA. 2005; 293:2634–2640.
113. Cosedis Nielsen, J, Johannessen, A, Raatikainen, P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012; 367:1587–1595.
114. Jongnarangsin, K, Suwanagool, A, Chugh, A, et al. Effect of catheter ablation on progression of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2012; 23:9–14.
115. Ladapo, JA, David, G, Gunnarsson, CL, et al. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. J Cardiovasc Electrophysiol. 2012; 23:1–8.
116. Chao, TF, Tsao, HM, Lin, YJ, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: Results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012; 5:514–520.
117. Chan, PS, Vijan, S, Morady, F, Oral, H. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2006; 47:2513–2520.
118. Khaykin, Y, Wang, X, Natale, A, et al. Cost comparison of ablation versus antiarrhythmic drugs as first-line therapy for atrial fibrillation: An economic evaluation of the raaft pilot study. J Cardiovasc Electrophysiol. 2009; 20:7–12.
119. Cappato, R, Calkins, H, Chen, SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:32–38.
120. Baman, TS, Jongnarangsin, K, Chugh, A, et al. Prevalence and predictors of complications of radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2011; 22:626–631.
121. Di Biase, L, Burkhardt, JD, Mohanty, P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: The impact of periprocedural therapeutic international normalized ratio. Circulation. 2010; 121:2550–2556.
122. Andrade, JG, Khairy, P, Guerra, PG, et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: A systematic review of published studies. Heart Rhythm. 2011; 8:1444–1451.
123. Gibson, DN, Di Biase, L, Mohanty, P, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: Clinical characterization, prevalence, and predictors. Heart Rhythm. 2011; 8:1364–1371.
124. Weimar, T, Schena, S, Bailey, MS, et al. The cox-maze procedure for lone atrial fibrillation: A single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012; 5:8–14.
125. Cox, JL. Cardiac surgery for arrhythmias. J Cardiovasc Electrophysiol. 2004; 15:250–262.
126. Cox, JL. Atrial fibrillation II: rationale for surgical treatment. J Thorac Cardiovasc Surg. 2003; 126:1693–1699.
127. Ballaux, PK, Geuzebroek, GS, van Hemel, NM, et al. Freedom from atrial arrhythmias after classic maze III surgery: a 10-year experience. J Thorac Cardiovasc Surg. 2006; 132:1433–1440.
128. Gillinov, AM, McCarthy, PM. Advances in the surgical treatment of atrial fibrillation. Cardiol Clin. 2004; 22:147–157.
129. Khargi, K, Hutten, BA, Lemke, B, et al. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005; 27:258–265.
130. Pet, M, Robertson, JO, Bailey, M, et al. The impact of chads(2) score on late stroke after the cox maze procedure. J Thorac Cardiovasc Surg. 2012.