Chapter 139 Atopic Dermatitis (Atopic Eczema)
Pathology
Acute AD skin lesions are characterized by spongiosis, or marked intercellular edema, of the epidermis. In AD, dendritic antigen-presenting cells (APCs) in the epidermis, such as Langerhans cells (LCs), exhibit surface-bound immunoglobulin (Ig) E molecules. These APCs play an important role in cutaneous allergen presentation to T helper type 2 (Th2) cells (Chapter 134). There is a marked perivenular T-cell infiltrate with occasional monocyte-macrophages in acute AD lesions. Mast cells are found in normal numbers but in different stages of degranulation. Chronic, lichenified AD is characterized by a hyperplastic epidermis with hyperkeratosis, and minimal spongiosis. There are predominantly IgE-bearing LCs in the epidermis, and macrophages in the dermal mononuclear cell infiltrate. Mast cell and eosinophil numbers are increased. Eosinophils contribute to allergic inflammation by secreting cytokines and mediators that augment inflammatory responses and induce tissue injury in AD through the production of reactive oxygen intermediates and release of toxic granule proteins.
Clinical Manifestations
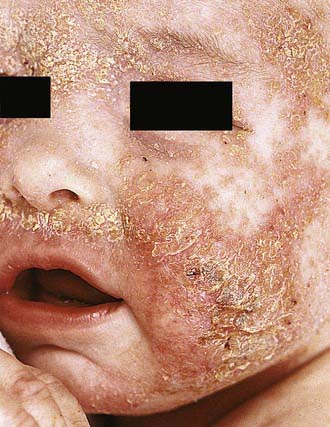
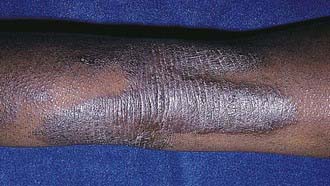
Acute AD skin lesions are intensely pruritic with erythematous papules (Figs. 139-1 and 139-2). Subacute dermatitis manifests as erythematous, excoriated, scaling papules. In contrast, chronic AD is characterized by lichenification (Fig. 139-3), or thickening of the skin with accentuated surface markings, and fibrotic papules (prurigo nodularis). In chronic AD, all three types of skin reactions may coexist in the same individual. Most patients with AD have dry, lackluster skin irrespective of their stage of illness. Skin reaction pattern and distribution vary with the patient’s age and disease activity. AD is generally more acute in infancy and involves the face, scalp, and extensor surfaces of the extremities. The diaper area is usually spared. Older children and children with chronic AD have lichenification and localization of the rash to the flexural folds of the extremities. AD often goes into remission as the patient grows older, leaving an adolescent or adult with skin prone to itching and inflammation when exposed to exogenous irritants.
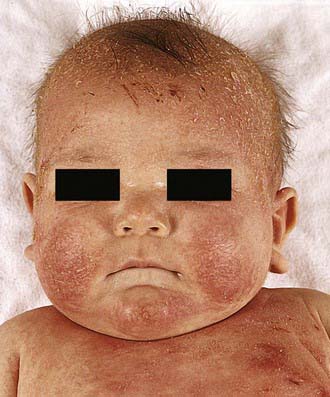
Figure 139-1 Atopic dermatitis, typical cheek involvement.
(From Eichenfield LF, Friedan IJ, Esterly NB: Textbook of neonatal dermatology, Philadelphia, 2001, WB Saunders, p 242.)
Diagnosis and Differential Diagnosis
AD is diagnosed on the basis of 3 major features: pruritus, an eczematous dermatitis that fits into a typical presentation, and a chronic or chronically relapsing course (Table 139-1). Associated features, such as a family history of asthma, hay fever, elevated IgE, and immediate skin test reactivity, are variably present.
Table 139-1 CLINICAL FEATURES OF ATOPIC DERMATITIS
MAJOR FEATURES
ASSOCIATED FEATURES
Many inflammatory skin diseases, immunodeficiencies, skin malignancies, genetic disorders, infectious diseases, and infestations share symptoms with AD and should be considered and excluded before a diagnosis of AD is established (Table 139-2). Severe combined immunodeficiency syndrome (Chapter 120.1) should be considered for infants presenting in the 1st year of life with diarrhea, failure to thrive, generalized scaling rash, and recurrent cutaneous and/or systemic infection. Histiocytosis (Chapter 501) should be excluded in any infant with AD and failure to thrive. Wiskott-Aldrich syndrome (Chapter 120.2), an X-linked recessive disorder associated with thrombocytopenia, immune defects, and recurrent severe bacterial infections, is characterized by a rash almost indistinguishable from that in AD. Hyper-IgE syndrome (Chapter 120.2) is characterized by markedly elevated serum IgE values, recurrent deep-seated bacterial infections, chronic dermatitis, and refractory dermatophytosis.
Table 139-2 DIFFERENTIAL DIAGNOSIS OF ATOPIC DERMATITIS
CONGENITAL DISORDERS
CHRONIC DERMATOSES
INFECTIONS AND INFESTATIONS
MALIGNANCIES
AUTOIMMUNE DISORDERS
IMMUNODEFICIENCIES
METABOLIC DISORDERS
From Leung DYM, Sampson HA, Geha RS, et al: Pediatric allergy principles and practice, St Louis, 2003, Mosby, p 562.
Adolescents who present with an eczematous dermatitis but no history of childhood eczema, respiratory allergy, or atopic family history may have allergic contact dermatitis (Chapter 647). A contact allergen may be the problem in any patient whose AD does not respond to appropriate therapy. Sensitizing chemicals, such as parabens and lanolin, can be irritants for patients with AD and are commonly found as vehicles in therapeutic topical agents. Topical glucocorticoid contact allergy has been reported in patients with chronic dermatitis who are undergoing topical corticosteroid therapy. Eczematous dermatitis has also been reported with HIV infection as well as with a variety of infestations such as scabies. Other conditions that can be confused with AD include psoriasis, ichthyoses, and seborrheic dermatitis.
Treatment
The treatment of AD requires a systematic, multifaceted approach that incorporates skin hydration, topical anti-inflammatory therapy, identification and elimination of flare factors, and, if necessary, systemic therapy. Assessment of the severity also helps direct therapy (Table 139-3).
Table 139-3 CATEGORIZATION OF PHYSICAL SEVERITY OF ATOPIC ECZEMA
From Lewis-Jones S, Mugglestone MA; Guideline Development Group: Management of atopic eczema in children aged up to 12 years: summary of NICE guidance, BMJ 335:1263–1264, 2007.
Topical Corticosteroids
Topical corticosteroids are the cornerstone of anti-inflammatory treatment for acute exacerbations of AD. Patients should be carefully instructed on their use of topical glucocorticoids in order to avoid potential adverse effects. There are 7 classes of topical glucocorticoids, ranked according to their potency as determined by vasoconstrictor assays (Table 139-4). Because of their potential adverse effects, the ultra-high-potency glucocorticoids should not be used on the face or intertriginous areas and should be used only for very short periods on the trunk and extremities. Mid-potency glucocorticoids can be used for longer periods to treat chronic AD involving the trunk and extremities. Long-term control can be maintained with twice-weekly applications of topical fluticasone or mometasone to areas that have healed but are prone to relapse, once control of AD is achieved after a daily regimen of topical corticosteroids. Compared with creams, ointments have a greater potential to occlude the epidermis, resulting in enhanced systemic absorption. Adverse effects of topical glucocorticoids can be divided into local adverse effects and systemic adverse effects, the latter of which result from suppression of the hypothalamic-pituitary-adrenal axis. Local adverse effects include the development of striae and skin atrophy. Systemic adverse effects are related to the potency of the topical corticosteroid, site of application, occlusiveness of the preparation, percentage of the body surface area covered, and length of use. The potential for adrenal suppression from potent topical corticosteroids is greatest in infants and young children with severe AD requiring intensive therapy.
Table 139-4 SELECTED TOPICAL CORTICOSTEROID PREPARATIONS*
GROUP 1
GROUP 2
GROUP 3
GROUP 4
GROUP 5
GROUP 6
GROUP 7
Hydrocortisone (Hytone) 2.5% & 1% ointment/cream
* Representative corticosteroids are listed by group from 1 (superpotent) through 7 (least potent).
Adapted from Stoughton RB: Vasoconstrictor assay-specific applications. In Malbach HI, Surber C, editors: Topical corticosteroids, Basel, Switzerland, 1992, Karger, pp 42–53.
Avoiding Triggers
Foods
Food allergy is co-morbid in approximately 40% of infants and young children with moderate to severe AD (Chapter 145). Undiagnosed food allergies in patients with AD may induce eczematous dermatitis in some patients and urticarial reactions, wheezing, or nasal congestion in others. Increased severity of AD symptoms and younger age correlate directly with the presence of food allergy. Removal of food allergens from the diet leads to significant clinical improvement but requires a great deal of education, because most common allergens (egg, milk, peanut, wheat, soy) contaminate many foods and are difficult to avoid.
Alm B, Aberg N, Erdes L, et al. Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child. 2009;94:11-15.
Baumer JH. Atopic eczema in children, NICE. Arch Dis Child Educ Pract Ed. 2008;93:93-97.
Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
Boguniewicz M, Nicol N, Kelsay K, et al. A multidisciplinary approach to evaluation and treatment of atopic dermatitis. Semin Cutan Med Surg. 2008;27:115-127.
Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al: Probiotics for treating eczema (review), Cochrane Database System Rev (4): CD006135, 2008.
De Benedetto A, Agnihothri R, McGirt LY, et al. Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol. 2009;129:14-30.
Esparza-Gordillo J, Weidinger S, Fölster-Holst R, et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596-601.
Jung T, Stingl G. Atopic dermatitis: therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol. 2008;122:1074-1081.
Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116-121.
Krakowski AC, Eichenfield LF, Dohil MA. Management of atopic dermatitis in the pediatric population. Pediatrics. 2008;122:812-824.
Lewis-Jones S, Mugglestone MA. Guideline Development Group: Management of atopic eczema in children aged up to 12 years: summary of NICE guidance. BMJ. 2007;335:1263-1264.
O’Regan GM, Sandilands A, McLean WH, et al. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689-693.
Ownby DR, Johnson CC. Does exposure to cats or dogs in early life alter a child’s risk of atopic dermatitis? J Pediatr. 2010;158(2):184-186.
Paller AS, Eichenfield LF, Kirsner RS, et al. Three times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for use. Pediatrics. 2008;122:e1210-e1218.
Rosenlund H, Bergström A, Aim JA, et al. Allergic disease and atopic sensitization in children in relation to measles vaccination and measles infection. Pediatrics. 2009;123:771-778.
Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
Treadwell PA. Eczema and infection. Pediatr Infect Dis J. 2008;27:551-552.