Atherosclerosis46
6.2 Thrombosis48
6.3 Embolism49
Atherosclerosis, a common degenerative disease of arteries characterised by thickening of the intima as a result of deposition of lipids, is a common cause of illness in industrialised countries and is the main pathological process that leads to cardiovascular disease. Infarction can result from atherosclerosis, and also from the intravascular events of thrombosis and embolism.
6.1. Atherosclerosis
Atherosclerosis causes narrowing and/or weakening of arteries, and is the pathological process underlying many common diseases such as myocardial infarction (heart attacks), strokes and aneurysms. It is an acquired, degenerative condition which affects large- and medium-sized arteries, e.g. aorta, carotid and coronary arteries, where it begins in the innermost intimal layer. It is characterised by lipid deposition with consequent inflammation and fibrosis. Atheroma (from the Greek word for porridge that describes the necrotic material in the core of the lesions) is a term which is often used synonymously with atherosclerosis.
Epidemiology and risk factors
Atherosclerosis is common in industrialised countries but less common elsewhere. If individuals from countries with a low incidence migrate to one with a high incidence, their risk of atherosclerosis increases, presumably as a result of adopting a ‘Western’ lifestyle.
The risk factors for atherosclerosis can be divided into those that are non-modifiable (e.g. age, sex) and those that are modifiable as a result of lifestyle changes or drug therapy. Important risk factors are:
• Age. Death rates from the complications of atherosclerosis increase with age.
• Male sex. Premenopausal women have a low rate of death from complications of atherosclerosis; the protective factor is thought to be high oestrogen levels. After the menopause, the incidence of complications increases and by old age it equals that of males.
• Family history. A family history of atherosclerotic-related disease confers an increased risk. This familial predisposition is probably polygenic in most cases, but in some patients there is a specific inherited abnormality of lipid metabolism causing hyperlipidaemia.
• Hyperlipidaemia. The cholesterol in low-density lipoprotein (LDL) is particularly important as a risk factor. The ratio of LDL to high-density lipoprotein (HDL) is also important, since a low LDL/HDL ratio appears to have a protective effect. The mechanism is thought to be related to their functions: LDL delivers lipids to tissues, whereas HDL transports it to the liver for metabolism; therefore, HDL could remove lipids from developing plaques. The LDL/HDL ratio is lowered by exercise, consumption of polyunsaturated fatty acids and moderate amounts of alcohol, whereas it is increased by smoking, obesity and a diet rich in saturated fats. Circulating cholesterol can also be reduced by drugs (the statins).
• Hypertension. Reduction of blood pressure in hypertensive patients reduces the risk of strokes and ischaemic heart disease.
• Cigarette smoking. Tobacco can be atherogenic through damage to endothelial cells by toxins and through the increase in LDL/HDL ratio.
• Diabetes mellitus. This important risk factor could act through hypercholesterolaemia or a direct toxic effect on endothelial cells.
• Physical inactivity. Lack of exercise promotes obesity and increases LDL/HDL ratios.
Pathogenesis
The earliest visible lesion of atherosclerosis is the fatty streak which results from accumulation of lipid-laden macrophages within the intima and which can be seen in the arteries of children. Fatty streaks are flat, pale yellow spots.
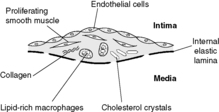
Atheromatous plaques occur later in life and contain macrophages intermingled with proliferating smooth muscle cells, capped by fibrous tissue. Lipid, particularly cholesterol, may be present within both macrophages and smooth muscle cells. Angiogenesis (proliferation of small blood vessels) is stimulated at the periphery of the lesions. A mature fibrolipid plaque is composed of:
• a core of necrotic lipid-rich material
• a chronic inflammatory infiltrate containing lymphocytes, macrophages, smooth muscle cells and myofibroblasts surrounding the core
• a fibrous cap
• small blood vessels entering the periphery of the plaque.
These plaques protrude into the vessel lumen and can cause significant narrowing. The underlying arterial wall is weakened by the chronic inflammation.
The response to injury hypothesis
Several different theories have been proposed to explain the initiation and evolution of atherosclerosis. The current favoured idea is the ‘response to injury hypothesis’, which brings some of these theories together. Damage to the arterial endothelium leads to increased permeability of the vessel wall and attachment of platelets and monocytes. The increased permeability allows lipid to enter the vessel wall. Meanwhile, the attached platelets and monocytes produce growth factors which stimulate smooth muscle cells of the arterial media to migrate into the intima and proliferate. One of these growth factors is platelet-derived growth factor (PDGF). This is a locally acting polypeptide which binds to cell surface receptors and triggers a chain of events which may enable genes to switch on and produce proteins which promote cell proliferation. Proliferating smooth muscle cells acquire some fibroblast-like characteristics and produce collagens and proteoglycans, which form the fibrotic cap and matrix of the atherosclerotic plaque. The monocytes transform into macrophages within the intima. These macrophages ingest the lipid that has entered the intima through the leaky endothelium (Figure 15). Macrophages with lipid in their cytoplasm have a foamy appearance and are called foam cells. The macrophages oxidise the lipids they have ingested, and these oxidised lipids have a number of effects that seem to be important in the development of the plaque. In particular, oxidised lipids:
• are chemotactic for circulating monocytes
• inhibit motility of macrophages already in the plaque
• stimulate the macrophages to release growth factors and cytokines which attract inflammatory cells into and around the plaque
• upregulate endothelial cell adhesion molecules, thus promoting adherence of inflammatory cells (see Ch. 9)
• are cytotoxic and cause further endothelial damage.
Lipids and atherosclerosis
Low-density lipoproteins (LDL) are rich in cholesterol and raised blood levels of LDL are an important factor in plaque genesis. Both genetic and environmental dietary factors can determine the LDL levels, but the precise mechanism of this relationship with plaque development is not known. Genetic abnormalities can lead to increased blood levels in very young people, who may suffer heart attacks and strokes in their late teens or early twenties. There is a strong epidemiological correlation between cardiovascular disease and high LDL blood levels. In contrast, there is a reduced risk of atherosclerosis with high levels of high-density lipoproteins (HDL).
Fish oils
Populations which have a high dietary intake of fish oil containing omega-3 fatty acids (a special type of polyunsaturated fatty acid) seem to be protected from developing complicated atherosclerosis. Fish oils may have a lipid-lowering effect in the blood, resulting in reduced levels of LDL and raised levels of HDL. Fish oils may also reduce levels of thromboxane A2, which is metabolised from arachidonic acid in platelets and increases their capacity to aggregate. Reducing thromboxane A2 levels may reduce the risk of thrombosis.
Complications of atherosclerotic plaques
Atherosclerotic plaques are prone to a number of possible complications, namely:
• ulceration and thrombosis
• haemorrhage
• aneurysm
• calcification.
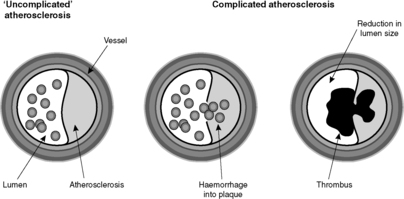
The reason atherosclerosis is a serious condition is that some of the above listed complications can produce potentially fatal diseases. Since the plaque is intimal and protrudes into the lumen of the vessel, haemorrhage or thrombosis can cause narrowing (stenosis) or complete blockage (occlusion) of the artery (Figure 16). If the artery is an end-artery (see Box 5), the tissue supplied by the artery cannot get blood from elsewhere, and it will die, causing infarction. Alternatively, weakening of the wall by the inflammation associated with the atherosclerotic plaque can lead to aneurysm formation.
Box 5
Some tissues receive blood from several different arteries via a collateral circulation. Obstruction of one of these arteries, whether by embolism, thrombosis, atherosclerosis or other cause, is unlikely to cause critical ischaemia because the blood supply can be maintained from the other arteries. However, in some tissues only one artery supplies any particular area, and obstruction of such a vessel is likely to cause an infarct; these arteries are called end-arteries. Organs with end-arteries include the heart and brain.
Haemorrhage
Blood can be forced into the plaque from the lumen of the vessel or bleeding can occur from the vessels vascularising the base and edges of the plaque. The sudden increase in size of the plaque can cause critical ischaemia in the territory supplied by the artery.
Aneurysm formation
The presence of an intimal plaque leads to atrophy and weakening of the underlying media. The weakened vessel wall may dilate and eventually a large sac is formed. This is called an aneurysm (the definition of aneurysm is a permanent abnormal dilatation of an artery). Aneurysms can rupture, with consequent life-threatening haemorrhage. In addition, they commonly contain thrombus, fragments of which can become dislodged and embolise (see Section 6.3 Embolism).
Calcification
Old atherosclerotic plaques often undergo dystrophic calcification. Hence atherosclerotic arteries can sometimes be seen on radiographs. This complication, unlike the three mentioned previously, does not have lethal effects.
6.2. Thrombosis
Thrombosis is the formation of a blood clot within vascular spaces during life, and the resulting clot is called a thrombus. This is in contrast to the term clotting, which can also be applied after death or to blood in a test tube. There are three main predisposing factors for thrombus formation, known as Virchow’s triad:
• changes in blood flow (turbulence or stasis)
• increased coagulability of blood.
These predisposing factors are associated with particular conditions or lifestyles (see Table 6).
| Examples | |
|---|---|
| Endothelial damage | Trauma, atherosclerosis, smoking, bacterial toxins |
| Stasis/turbulence | Postoperative immobility (inactive legs) and blood pooling; postmyocardial infarction (sluggish blood flow around body); turbulence around atherosclerotic plaques or within aneurysms |
| Increased coagulability | Pregnancy; oral contraceptives; leukaemia; cancer |
How does a thrombus form?
Any of the components of Virchow’s triad, alone or in combination, may predispose to the formation of a thrombus. Thrombi build up from the initial platelet plug. The clotting cascade is triggered by endothelial damage and factors released by the aggregation of platelets. As the blood flows past, red cells become trapped in the fibrin mesh which is thus formed. The thrombus grows in layers in the direction of the blood flow, a process known as propagation. To the naked eye, a thrombus is a dark-red mass of blood in which delicate white lines of fibrin called lines of Zahn can be seen. When a pathologist finds clotted blood at autopsy, it is necessary to determine whether the clot occurred during life (i.e. is a thrombus) or after death. If lines of Zahn are present, they show the clot formed in flowing blood and are a useful indicator that the clot is, in fact, a thrombus; other distinguishing features are the granularity of the thrombus and the fact that it may be attached to the vessel wall.
Thrombi can occur in arteries, veins or within the heart chambers (mural thrombus). The outcome of thrombus formation can be:
• propagation leading to complete occlusion of vessel lumen
• removal by fibrinolysis with restoration of normal blood flow
• organisation, i.e. ingrowth of granulation tissue converting the thrombus into a fibrous scar; sometimes this process can recanalise an occlusive thrombus, i.e. capillary channels through the thrombus are produced, allowing some blood to flow through it
• embolism, i.e. carriage of thrombus through the circulation to lodge in a distant vessel.
6.3. Embolism
An embolus is a mass of material which is carried in the bloodstream and becomes lodged within a blood vessel, thus blocking it. The material may be a solid, liquid or gas. The effects of an embolus follow from the obstruction of blood flow to the tissues and depend on the precise site at which this occurs and whether or not there is an alternative tissue blood supply (see Box 5). Emboli travel through the circulatory system and obstruct the vessel lumen when the diameter prevents further passage. Table 7 gives the sources and types and of embolism; by far the most common in general clinical practice is thrombo-embolism.
| Type | Source |
|---|---|
| Thrombus (thrombo-embolus) | Commonly arise in deep veins of leg/pelvis (deep venous thrombosis, DVT), or in the heart (mural thrombus, e.g. over a myocardial infarct or within a fibrillating atrium) |
| Atherosclerotic plaque debris (cholesterol or atheromatous embolus) | Exposure of the necrotic core in an ulcerated plaque can release debris that embolises to distant sites downstream |
| Vegetations | Cardiac vegetations can embolise to lungs (right heart valves) or systemic circulation (left heart valves). In infective endocarditis, the bacteria-laden emboli can cause infection where they lodge, e.g. brain abscess |
| Amniotic fluid | Uterus at delivery: embolism to lungs via torn uterine veins |
| Nitrogen | Inadequate decompression in deep-sea divers: nitrogen bubbles can cause problems in joints, lungs, brain and spinal cord, bones, skin and elsewhere |
| Air | Typically introduced into the large veins of the neck or chest through trauma or surgery |
| Fat | Occurs after serious trauma, especially bone fractures and widespread burns. Although fragments of fatty marrow can embolise from fractured bones, most cases of fat embolism are due to coalescence of serum lipid into globules. Organs affected by emboli include lungs, central nervous system and skin |
| Foreign body | Occasionally, foreign bodies introduced into vessels can embolise, e.g. catheter tips |
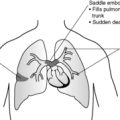
Pulmonary embolism
Pulmonary emboli arise in the systemic veins or the right side of the heart and lodge in the pulmonary circulation. The vast majority arise from a thrombus in the deep leg or pelvic veins, i.e. a deep venous thrombosis (DVT). Small thrombo-emboli may go unnoticed and dissolve by fibrinolysis, whereas a large embolus which lodges in a main pulmonary artery may cause sudden death. Other emboli may cause severe respiratory and cardiac symptoms, relating to either infarction of the lung or right-sided heart strain.
Risk factors for DVT can be understood in terms of Virchow’s triad.
• Change in blood flow, i.e. poor flow through the veins, as seen in:
• immobile or bedridden patients
• heart failure.
• Damage to endothelium, i.e. inflammation of the vein wall due to:
• trauma or surgery
• vascular stasis.
• Hypercoagulability of the blood, due to:
• release of pro-coagulant factors following trauma or surgery
• inherited hypercoagulability (e.g. deficiency in antithrombin III, protein C or protein S)
• increased oestrogen (pregnancy, contraceptive pill)
• polycythaemia
• cancer.
DVT and consequent pulmonary thrombo-embolism are feared complications of major surgery, since postoperatively patients exhibit many of these predisposing factors. All postoperative patients should receive prophylaxis to reduce the risk of DVT.
Systemic embolism
Paradoxical embolism
On rare occasions, an embolus can pass between the left and right sides of the circulation through an atrial or ventricular septal defect or through a patent ductus arteriosus. In these circumstances, a systemic embolism can result from material originating in the systemic veins. This is called paradoxical embolism.
6.4. Infarction
Infarction is defined as death of tissue caused by ischaemia, i.e. lack of oxygen. It may result from a partial or complete reduction of the blood supply (arterial infarction) or diminished drainage of blood from the tissue (venous infarction). The shape and size of the infarct depends on the territory normally supplied or drained by the occluded blood vessel. The appearance of an infarct varies, depending on how long the process has been going on, and on the nature of the tissue affected. The majority of infarcts are arterial in nature and are caused by thrombosis or embolism.
Obstruction of venous outflow causes intensely congested (haemorrhagic) infarcts. It occurs as a result of:
• twisting of an organ or other structure on its pedicle (e.g. torsion of testis, volvulus of sigmoid colon)
• constriction of an organ or structure by the neck of a hernia (e.g. entrapment of a loop of bowel in an inguinal or femoral hernia sac)
• compression of a vein by a tumour mass.
The appearances of an infarct change with time. The macroscopic (visible to the naked eye) and microscopic features of the resulting necrosis take time to develop. The morphological changes in a myocardial infarct are given in Table 8.
| Time after infarct | Naked eye | Light microscope |
|---|---|---|
| 8 hours | No visible changes | Cytoplasm looks more pink (eosinophilic) than normal |
| 12 hours | No visible changes, or possible slight pallor or redness | Neutrophils start to appear at junction with surrounding viable tissue |
| 24 hours | Pallor or redness | Features of coagulative necrosis, i.e. loss of nuclei and cross-striations |
| 48 hours | Pallor or redness with a yellow rim, representing neutrophilic infiltrate | Macrophages start to ingest necrotic myocytes; increasing numbers of neutrophils |
| 3–10days | Brown, soft; yellow or red rim representing granulation tissue | Granulation tissue forms around the infarct |
| 2–6weeks | Brown, soft, with fibrosis developing at periphery | Granulation tissue grows into the infarct, organising it into a fibrous scar |
| After 6–8weeks | Mature fibrous scar | Mature fibrous scar |
Self-assessment: questions
One best answer questions
2. A pathologist performing an autopsy finds clotted blood in the pulmonary artery. Which one of the following features of the clot is likely to indicate that it formed during life rather than after death?
a. ‘chicken fat’ appearance
b. lines of Zahn
c. presence of fibrin in the clot
d. presence of platelets in the clot
e. ‘redcurrant jelly’ appearance
3. Which of the following is most likely to form a significant component of an atheromatous plaque?
a. adipocytes
b. chondrocytes
c. germ cells
d. mesothelial cells
e. smooth muscle cells
4. Which one of the following lesions is the precursor of atherosclerosis?
a. aneurysm
b. fatty streak
c. squamous metaplasia
d. thrombo-embolus
e. vegetation
5. A 70-year-old man collapses suddenly while walking his dog. In hospital, a diagnosis of stroke is made. Radiological investigations reveal a brain infarct. He also has an abdominal aortic aneurysm. Which of the following pathological processes is most likely to be the cause of the stroke?
a. calcification of the wall of the basilar artery
b. deep venous thrombosis
c. nitrogen bubbles in cerebral vessels
d. thrombo-embolism from the abdominal aortic aneurysm
e. ulcerated atherosclerotic plaque of the left carotid artery
True-false questions
1. The following statements are true:
a. mural thrombus in the left ventricle may embolise to the brain
b. dietary cholesterol levels are the most important determinant of plasma cholesterol levels
c. vascular endothelium produces prothrombin
d. atherosclerosis only occurs in large arteries with turbulent blood flow
e. thrombus commonly develops on atherosclerotic plaques
2. The following are correctly paired:
a. raised plasma high-density lipoprotein (HDL) – atherosclerosis
b. atherosclerosis – aneurysm formation
c. abdominal aortic aneurysm – leg ischaemia
d. myocardial infarction – ventricular mural thrombus
e. foamy macrophages – oxidised LDL
Case history questions
Case history 1
A 35-year-old woman gave birth to healthy twin babies. She went home after 5days but was readmitted to hospital 2days later complaining of feeling breathless with pleuritic chest pain (a sharp stabbing pain in the side of her chest which was worse on breathing in). Her left leg was slightly swollen and she was tender over the calf. Investigations showed that she had a small infarct in the left lower lung lobe. She was treated with the antifibrinolytic agent streptokinase and the anticoagulant heparin and made a good recovery. On discharge she was prescribed a course of warfarin.
1. What has caused the infarct in her lung (i.e. what is the diagnosis)?
2. State three ways in which pregnancy could have contributed to the development of this condition.
3. Describe the rationale for treatment with streptokinase, heparin and warfarin.
Case history 2
A 68-year-old man presented to his doctor with a painful, black big toe. This was gangrenous on examination and he was admitted to hospital urgently for treatment and surgical amputation. He made a good recovery and was well for a year. He then started to develop pain in the lower back which came and went but was not severe enough for him to go to his doctor. On one occasion, however, he experienced excruciating pain in the lower right back and rapidly became shocked and collapsed with a pulse rate of 120 per minute and a systolic blood pressure of 60mmHg. He was rushed to hospital but died in the ambulance.
1. How might you connect the episode of gangrene in the toe with the autopsy findings in this man?
2. List four modifiable risk factors for atherosclerosis.
3. State three complications of aortic aneurysm.
Essay question
1. What is an infarct? Discuss the different pathological processes within vessels that can cause an infarct.
Self-assessment: answers
One best answer
2. b. Lines of Zahn are produced in flowing blood. In contrast, post-mortem clots often resemble either redcurrant jelly or chicken fat, and pathologists sometimes use these terms descriptively. Both post-mortem and ante-mortem clots would be expected to contain platelets and fibrin.
3. e. Smooth muscle cells proliferate in the lesion. They take part in production of matrix materials like collagen and mucopolysaccharides. They may also acquire phagocytic properties and, along with macrophages, ingest lipid.
4. b. The fatty streak is the earliest visible lesion in the evolution of atherosclerosis.
5. e. Ulceration of a plaque in a carotid artery could cause thrombosis with consequent occlusion of the artery or thrombo-embolism to a cerebral vessel. Alternatively, the exposed necrotic core might produce cholesterol emboli. Either way, there would be occlusion of an end-artery supplying brain tissue. Calcification of arterial walls can complicate atherosclerosis or occur as an ageing phenomenon, but does not cause clinical problems. Deep venous thrombosis would be expected to cause pulmonary embolism (except for the very rare phenomenon of paradoxical embolism). Nitrogen bubbles occur in decompression sickness. Embolism of thrombus in aortic aneurysms is not uncommon, but the emboli would pass downstream, e.g. into the mesenteric or iliac arteries, but not the cerebral circulation.
True-false answers
1.
a. True. Mural thrombus in the left ventricle may develop after myocardial infarction. If pieces break off and embolise then they can end up anywhere in the systemic circulation. The brain is a common site for this and an embolus travelling through the carotid arteries and then into a cerebral artery will cause ischaemic infarction of the brain (stroke). Cardiac mural thrombus can also embolise and cause infarcts in, for example, the spleen, gut or kidney.
b. False. Although there is a relationship between high blood cholesterol levels and a high risk of coronary heart disease in Western countries and this is associated with diets which are high in saturated fat, dietary cholesterol consumption has only a modest influence on plasma cholesterol levels.
c. False. Prothrombin is produced by the liver and is the inactive precursor of thrombin, the protein which causes fibrin to be formed and hence coagulation to occur. Vascular endothelium does produce plasminogen, which tends to counteract any tendency to coagulate.
d. False. Atherosclerosis can occur in arteries of large or medium calibre. The presence of turbulent blood flow, smoking and high circulating LDL levels will all predispose to atherosclerosis.
e. True. Superimposed thrombus is an important complication of atherosclerosis. It develops as a result of activation of the clotting cascade when plaque contents or blood vessel collagen are exposed to the blood. Platelet aggregation also occurs and triggers clotting. The resulting thrombus may propagate and cause occlusion of the vessel, for example in a coronary artery causing coronary thrombosis and myocardial infarction. Alternatively, the thrombus may break off and embolise to a distant vessel and cause occlusion there.
2.
a. False. In fact, the opposite is true. HDL is involved in the transport of cholesterol from peripheral tissues to the liver. There is strong epidemiological evidence that high levels of HDL-cholesterol are associated with a reduced risk of heart disease and coronary artery atherosclerosis. High levels of LDLs are associated with heart disease.
b. True. The atherosclerotic plaque is a proliferative and destructive lesion. The arterial wall becomes progressively less elastic and weaker as atherosclerosis progresses. Aneurysms commonly form as a result of this weakening. The most common site is the distal abdominal aorta.
c. True. As the aneurysm enlarges because of the weakening of the aortic wall, there will be stasis of blood flow and thrombus forming in layers, gradually filling the sac. This thrombus may cause occlusion of blood flow to the legs via the iliac arteries or bits may break off and embolise to leg arteries, causing ischaemia of the foot.
e. True. Macrophages in atherosclerotic plaques ingest lipid derived from circulating LDL and become foam cells. The lipid is oxidised principally by reactive oxygen species produced by the macrophages.
Case history answers
Case history 1
1. The diagnosis is pulmonary thrombo-embolism. The leg swelling and tenderness are features of DVT, the source of the embolism in this case. A thrombus has detached itself and embolised to a branch of the pulmonary artery. The segment of infarcted lung has become necrotic and haemorrhagic. The pleural surface of the lung will be involved and give rise to the sharp pain that the patient experiences. Sometimes patients with pulmonary embolus and infarction develop haemoptysis (coughing up of blood) from the bleeding into the dead lung segment. Because the lung has a dual blood supply from the pulmonary and bronchial arterial trees, infarction of the lung does not always occur with pulmonary embolism, and is more likely if the circulation is otherwise compromised, e.g. heart failure.
2. Three ways in which pregnancy could have contributed to the development of this condition are:
• Pregnancy, especially with twins, may have led to stasis of blood in the pelvic veins.
• Pregnancy is associated with an increase in the tendency of the blood to clot.
• Immobility is another possible cause, since the patient spent 5days in hospital just before the event.
3. Streptokinase is a fibrinolytic drug which dissolves the thrombo-embolus and restores blood flow to the infarcted area of tissue. Heparin and warfarin are anticoagulant drugs that prevent new thrombus forming. Heparin is quick acting and inhibits factors IX and X. It is given subcutaneously or intravenously. Warfarin is taken orally and takes about 24 hours to work. It inhibits the synthesis of vitamin-K-dependent clotting factors (II, VII, IX and X).
Case history 2
1. Occlusion of a digital artery would have caused ischaemic infarction with subsequent infection by anaerobic organisms, leading to gangrene. In this case, it is most likely that atheromatous or thrombotic emboli from the abdominal aortic aneurysm were responsible.
2. Four modifiable risk factors for atherosclerosis are: smoking, lack of exercise, hyperlipidaemia, diabetes mellitus.
3. Atherosclerotic or thrombotic embolism to the lower limbs or abdominal organs. Rupture with massive haemorrhage into the retroperitoneum and peritoneal cavity. Atrophy of the lumbar vertebrae (this is thought to cause the backache experienced by many of these patients and is due to pressure from the expanding aneurysm).
Essay answer
Comment: To answer the first part of the question, define infarct as an area of tissue death due to a reduction in blood supply or venous drainage causing hypoxic cell death. In general, the changes in vessels relate to occlusion of an end-artery or to obstruction of venous drainage. You could discuss each of these under its own heading. Arterial obstruction can be caused by a number of different processes. One way of organising the answer is to divide the causes into changes within the vessel lumen, changes in the wall of the vessel, and changes outside the wall, again using headings: within the lumen you should mention emboli; important lesions of the arterial wall include atherosclerosis, arteritis and spasm; and lesions outside the wall include tumours and cysts. You could give examples of each. Obstruction of venous drainage is typically caused by torsion or by strangulation in a hernia. Make sure you are selective in what you write so you have time to discuss all these factors. It would be easy to get carried away writing about, for example, atherosclerosis, leaving no time to mention anything else. Note that the question does not ask for a discussion of infarcts themselves or the cellular changes that occur in hypoxic tissue, so inclusion of this material would not attract any marks.