25 Asthma
Asthma means ‘laboured breathing’ in Greek and was first described 3000 years ago. It is a broad term used to refer to a disorder of the respiratory system that leads to episodic difficulty in breathing. The national UK guidelines (BTS/SIGN, 2009) define asthma as ‘a chronic inflammatory disorder of the airways which occurs in susceptible individuals; inflammatory symptoms are usually associated with widespread but variable airflow obstruction and an increase in airway response to a variety of stimuli. Obstruction is often reversible either spontaneously or with treatment’.
Epidemiology
The exact prevalence of asthma remains uncertain because of the differing ways in which airway restriction is reported, diagnostic uncertainty (especially for children under 2 years) and the overlap with other conditions such as chronic obstructive pulmonary disease (COPD). Over 5 million people in the UK have asthma (Asthma UK, 2001) and around 300 million worldwide. Mortality from asthma is estimated at approximately 0.4 per 100,000 with around 1400 deaths per annum in the UK. Most deaths occur outside hospital; the most common reasons for death are thought to be inadequate assessment of the severity of airway obstruction by the patient and/or clinician and inadequate therapy with inhaled or oral steroids.
Aetiology
The two main causes of asthma symptoms are airway hyperresponsiveness and bronchoconstriction. Hyperresponsiveness is an increased tendency of the airway to react to stimuli or triggers to cause an asthma attack. Bronchoconstriction is a narrowing of the airways that causes airflow obstruction. Possible triggers are listed in Table 25.1. One of the most common trigger factors is the allergen found in the faeces of the house dust mite, which is almost universally present in bedding, carpets and soft furnishing. Pollen from grass (prevalent in June and July) can lead to seasonal asthma. The role of occupation in the development of asthma has become apparent with increased industrialisation. There are many causes of occupational asthma, and bronchial reactivity may persist for years after exposure to the trigger factor. Drug-induced asthma can be severe and the most common causes are β-blocker drugs and prostaglandin synthetase inhibitors. The administration of β-adrenoceptor blockers to a patient, even in the form of eye drops, can cause β2-receptor blockade and consequent bronchoconstriction. Selective β-adrenoceptor blockers are thought to pose slightly less risk, but as these lose their selectivity at higher doses, it is generally recommended that this group of drugs is avoided altogether in asthma patients. Aspirin and related non-steroidal anti-inflammatory drugs can cause severe bronchoconstriction in susceptible individuals. Aspirin inhibits the enzyme cyclo-oxygenase, which normally converts arachidonic acid to (bronchodilatory) prostaglandins. When this pathway is blocked, an alternative reaction predominates, leading to an increase in production of bronchoconstrictor (cys-) leukotrienes. Figures from differing studies vary, but between 2% and 20% of the adult asthma population are thought to be sensitive to aspirin.
| Trigger | Examples |
| Allergens | Pollens, moulds, house dust mite, animals (dander, saliva and urine) |
| Industrial chemicals | Manufacture of, for example, isocyanate-containing paints, epoxy resins, aluminium, hair sprays, penicillins and cimetidine |
| Drugs | Aspirin, ibuprofen and other prostaglandin synthetase inhibitors, β-adrenoceptor blockers |
| Foods | A rare cause but examples include nuts, fish, seafood, dairy products, food colouring, especially tartrazine, benzoic acid and sodium metabisulfite |
| Environmental pollutants | Traffic fumes. cigarette smoke, sulphur dioxide |
| Other industrial triggers | Wood or grain dust, colophony in solder, cotton, dust, grain weevils and mites |
| Miscellaneous | Cold air, exercise, hyperventilation, viral respiratory tract infections, emotion or stress, swimming pool chlorine |
Pathophysiology
Asthma can be classified according to the underlying pattern of airway inflammation with the presence or absence of eosinophils in the airways (eosinophilic vs. non-eosinophilic). Traditionally patients are described as having ‘extrinsic asthma’ when an allergen is thought to be the cause of their asthma. This is more common in children with a history of atopy, where triggers, such as dust mite, cause IgE production. Other environmental factors are also important, such as exposure to rhinovirus during the first 3 years of life (Holgate et al., 2010). ‘Intrinsic asthma’ develops in adulthood, with symptoms triggered by non-allergenic factors such as a viral infection, irritants which cause epithelial damage and mucosal inflammation, emotional upset which mediates excess parasympathetic input or exercise which causes water and heat loss from the airways, triggering mediator release from mast cells. In practice, patients often have features of both types of asthma and the classification is unhelpful and oversimplifies the pathogenesis of asthma.
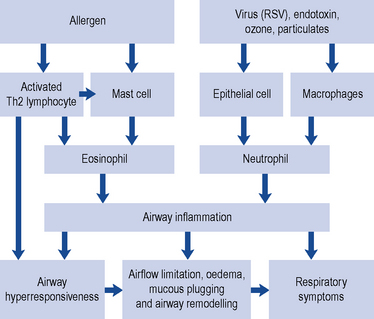
Mucus production is normally a defence mechanism, but in asthma patients, there is an increase in the size of bronchial glands and goblet cells that produce mucus. Mucus transport is dependent on its viscosity. If it is very thick, it plugs the airways, which also become blocked with epithelial and inflammatory cell debris. Mucociliary clearance is also decreased due to inflammation of epithelial cells. The environmental insults causing asthma are also thought to affect the structure and function of the airway epithelium. The exact role of these cytokines, cellular mediators and the interrelationships with each other and with the causative allergenic or non-allergenic mechanisms has, however, yet to be fully determined and may vary over time (Douwes et al., 2002; Holgate et al., 2010). Fig. 25.1 outlines the main cellular mechanisms involved.
Investigations
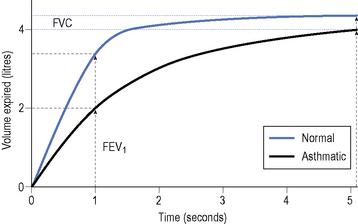
The most useful test for abnormalities in airway function is the forced expiratory volume (FEV). This is measured by means of lung function assessment apparatus such as a spirometer. The patient inhales as deeply as possible and then exhales forcefully and completely into a mouthpiece connected to a spirometer. The FEV1 is a measure of the FEV in the first second of exhalation. The forced vital capacity (FVC) can also be measured, which is an assessment of the maximum volume of air exhaled with maximum effort after maximum inspiration. The FEV1 is usually expressed as a percentage of the total volume of air exhaled, reported as the FEV1/FVC ratio. This ratio is a useful and highly reproducible measure of the capabilities of the lungs. Normal individuals can exhale at least 70% of their total capacity in 1 s. In obstructive lung disorders, such as asthma, the FEV1 is usually decreased, the FVC normal or slightly reduced and the FEV1/FVC ratio decreased, usually <0.7 (Fig. 25.2).
The diagnosis of asthma can be confirmed by measuring the response to a bronchodilator or by examining a patient’s day-to-day variation in PEF readings. A diurnal variability of 60 L/min (or more than 20%) is highly suggestive of asthma (GINA, 2009). However, individuals may not have airflow obstruction at the time of the test, so the absence of an improvement does not rule out asthma. In this situation, peak flow readings can be done at home with repeated pre- and post-bronchodilator readings taken at various times of the day.
Treatment
As asthma involves inflammation and bronchoconstriction, treatment should be directed towards reducing inflammation and increasing bronchodilation. Treatment aims should include a lack of day and nighttime symptoms, no asthma exacerbations, no need for rescue medication, normal PEFs and no unwanted side effects from medication (BTS/SIGN, 2009; GINA, 2009). Anti-inflammatory drugs should be given to all but those with the mildest of symptoms. Other measures, such as avoidance of recognised trigger factors, may also contribute to the control of this disease. The lowest effective dose of drugs should be given to minimise short-term and long-term side effects. It should, however, always be remembered that asthma is a potentially life-threatening illness, is often undertreated and not all patients will achieve optimal control. Common therapeutic and practice problems encountered in the management of asthma are outlined in Box 25.1.
Box 25.1 Management of common practice problems
Chronic asthma
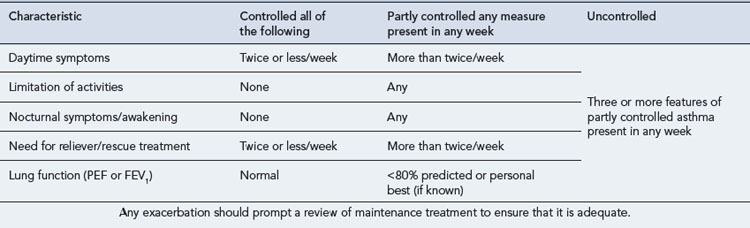
Treatment of chronic asthma should be managed in a stepwise progression. This section concentrates on management in adults, as outlined in Fig. 25.3, but corresponding management steps for children are available (BTS/SIGN, 2009). Therapy is moved up the steps according to the severity of the patient’s asthma symptoms and response to current treatment. When a patient has been stable for at least 3 months (GINA, 2009), therapy should be stepped back down; for example, by halving the inhaled corticosteroid (ICS) dose. International guidelines aim for management to achieve and maintain clinical control, which is defined in Table 25.2. A model for patient review and adjustment of therapy, based on assessment of asthma control, has been suggested (Crompton et al., 2006) and is shown in Fig. 25.4. To help in patient education, the terms used to describe the effects of asthma medication are similar across all manufacturers and sources of education. ‘Reliever’ is used for agents that give immediate relief of symptoms. Agents that act to reduce inflammation or give long-term bronchodilation are referred to as ‘controllers’ or ‘preventers’.
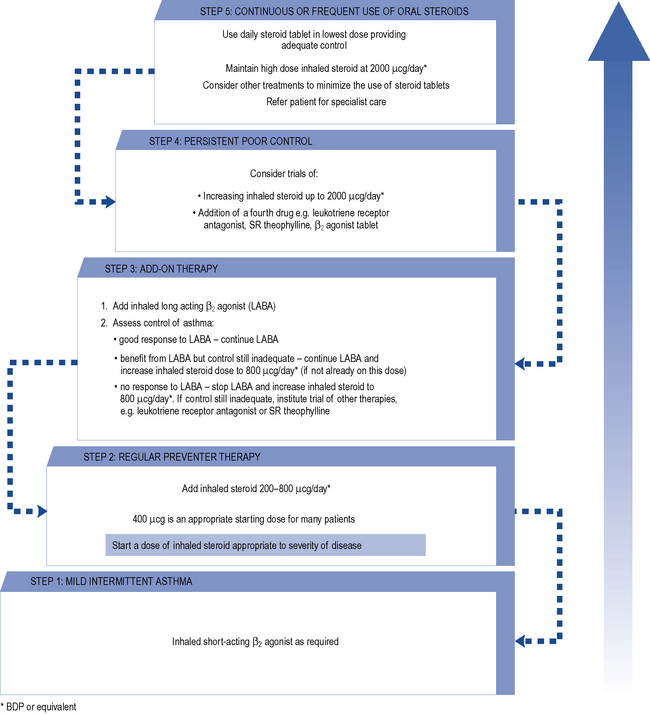
Fig. 25.3 Summary of stepwise management in adults
(reproduced by permission of the BMJ Publishing Group, from BTS/SIGN, 2009).
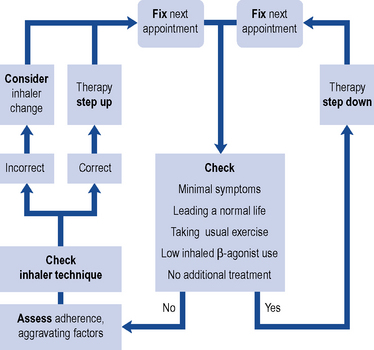
Fig. 25.4 Adjusting therapy to achieve asthma control
(from Crompton et al., 2006 reproduced by permission. Copyright Elsevier publishing).
Reliever medication
Additional bronchodilators
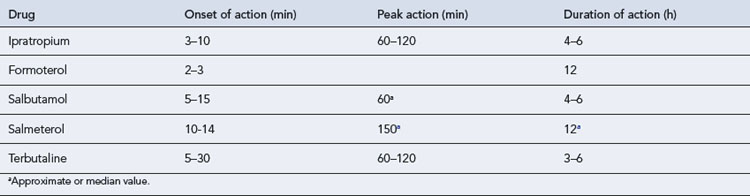
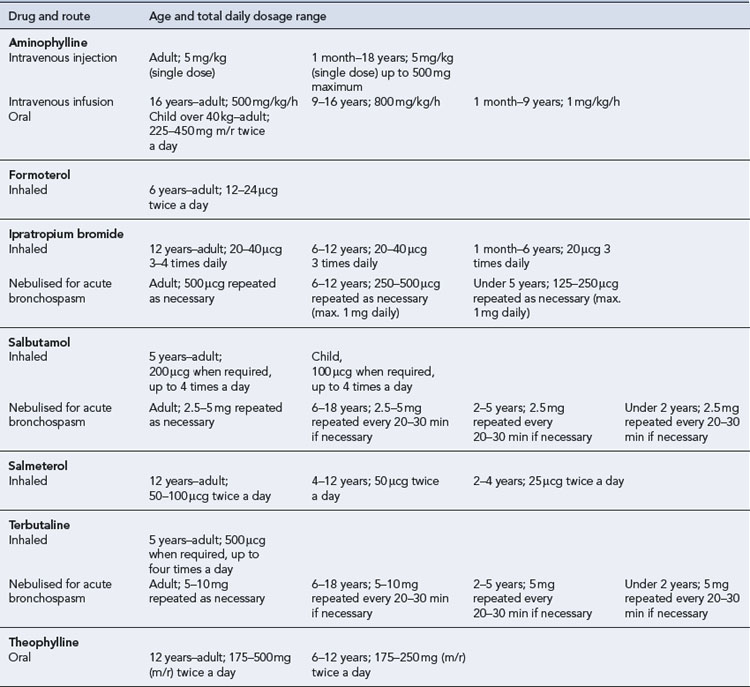
Additional bronchodilators may be required if the above therapy does not adequately control symptoms (Tables 25.3 and 25.4).
Long acting β-adrenoreceptor agonist bronchodilators
Meta-analysis of LABA trials has shown a potential increase in asthma deaths of 1 death in 1000 patient-years of use, but this increased risk is lessened when used alongside ICSs (Saltpeter et al., 2006). Taking this evidence into account, it is advised that LABAs should
A formoterol and budesonide combination inhaler can be given both as maintenance therapy and for symptomatic relief. Current trial evidence shows that this dosing method is an alternative at step 3 for adults who are poorly controlled on SABA and ICS, have experienced one or more severe exacerbations in the previous 12 months, or as an alternative to increasing the ICS dose to above 2 mg/day at step 4 (NPC, 2008).
Oral bronchodilators
Theophylline should be started at a dose of 400–500 mg/day in adults and, if required, increased after 7 days to 800–1000 mg/day. In children, higher doses may be required but this will be determined by the age of the child (see Chapter 10).
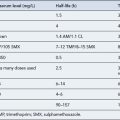
Theophylline has a narrow therapeutic index and its hepatic metabolism varies greatly between individuals. Theophylline clearance is affected by a variety of factors, including disease states and concurrent drug therapy. The dose used should, therefore, take into account these factors, which are listed in Table 25.5. Plasma levels may be taken after 3–4 days at the higher dose, and it has been normal practice to adjust the dose to keep the plasma level within a therapeutic window of 10–20 mg/L, although improvements in respiratory function are seen at levels as low as 5 mg/L in some patients. As the bronchodilating effects of theophylline are proportional to the log of the plasma concentrations, there is proportionally less bronchodilation as the plasma level increases. The mild side effects such as nausea and vomiting are seen at concentrations as low as 13 mg/L but are more common over 20 mg/L. Significant cardiac symptoms, tachycardia and persistent vomiting are usually seen at concentrations of 40 mg/L while severe CNS effects, such as seizures, have been seen at 30 mg/L but are more common above 50 mg/L.
| Decreased clearance | Increased clearance |
|---|---|
| Congestive cardiac failure | Cigarette smoking |
| Cor pulmonale | Children 1–12 years |
| Chronic obstructive pulmonary disease | High-protein, low-carbohydrate diet |
| Viral pneumonia | Barbecued meat |
| Acute pulmonary oedema | Carbamazepine |
| Cirrhosis | Phenobarbital |
| Premature and term babies | Phenytoin |
| Elderly | Sulfinpyrazone |
| Obesity | |
| High-carbohydrate, low-protein diet | |
| Cimetidine | |
| Erythromycin | |
| Oral contraceptives | |
| Ciprofloxacin | |
| Propranolol |
Preventer medication
Anti-inflammatory agents
Regular anti-inflammatory treatment should be used for patients who are not controlled on a SABA alone (BTS/SIGN, 2009). Corticosteroids are the most commonly used anti-inflammatory agents (Table 25.6), but others such as the cromones are available.
Table 25.6 Inhaled corticosteroids used for the prophylaxis of asthma
| Total daily dosage range (MDI) | ||
|---|---|---|
| Drug and age range | Standard dose | High dose |
| Beclometasone diproprionate or budesonidea | ||
| Adult | 100–400 μcg twice a day | 400–1000 μcg twice a day |
| 12–18 years | 100–400 μcg twice a day | 400–1000 μcg twice a day |
| Under 12 years | 100–200 μcg twice a day | 200–400 μcg twice a day |
| Ciclesonide | ||
| Adult | 80 μcg once daily | 160 μcg once daily |
| Fluticasone | ||
| Adult | 50–200 μcg twice a day | 400–1000 μcg twice a day |
| 12–18 years | 50–200 μcg twice a day | 200–500 μcg twice a day |
| 4–12 years | 50–100 μcg twice a day | 100–200 μcg twice a day |
| Mometasone | ||
| Adult | 200–400 μcg once daily | 400 μcg twice a day |
| 12-18 years | 200 μcg twice a day | Up to 400 μcg twice a day |
a There are bioavailability differences between CFC-free steroid inhalers. Always check dosing for specific brands.
Inhaled corticosteroids
The threshold frequency of β2-agonist use which prompts the start of ICSs has not been fully established but national guidance (BTS/SIGN, 2009) recommends considering ICS for patients with any of the following:
All ICSs have dose-related side effects. Adrenal suppression occurs at around doses of >1500 μcg/day of beclometasone in adults. In children, doses of 400 μcg/day of beclometasone or more are associated with growth failure and adrenal suppression; children treated at these doses should be under the care of a specialist paediatrician. Oropharyngeal side effects such as candidiasis are also more common at higher doses (Box 25.2). Measures to minimise this can be tried, such as using a large-volume spacer device and rinsing the mouth with water or brushing teeth after inhalation, but there is little evidence to confirm how effective these are.
Box 25.2 Adverse reactions associated with drugs used in the management of asthma
β2-Agonists
Oral corticosteroids
Ipratropium bromide
Anti-IgE monoclonal antibodies
The first of these, omalizumab, is used for the treatment of severe persistent IgE (30–1500 iu/mL)-mediated asthma as add-on therapy to existing optimised therapy in adults and individuals over 12 years of age who have severe unstable disease (NICE, 2007). Patient response should be measured and omalizumab discontinued after 16 weeks if no adequate response is seen.
Acute severe asthma
Immediate management
The immediate treatment of acute severe asthma should take place in the patient’s home if a moderate attack. Admission to hospital is considered if PEF drops below 50% predicted or normal, or the patient cannot complete sentences in one breath or is too breathless to talk, or if life-threatening features are present. A suggested treatment protocol for management in hospital is outlined in Fig. 25.5.
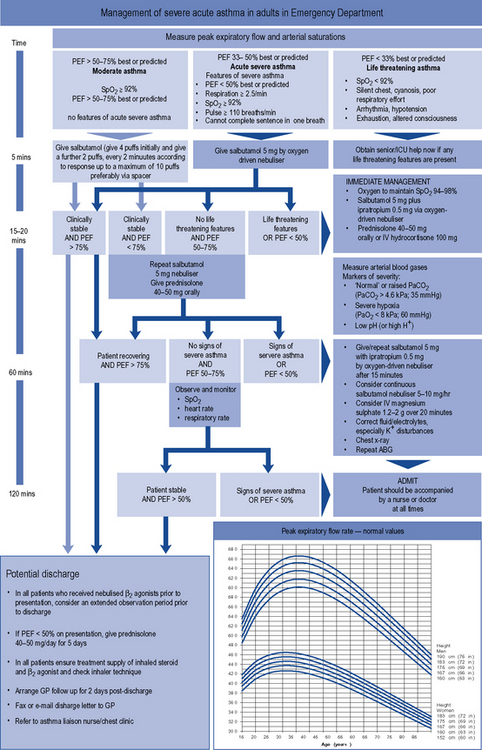
Fig. 25.5 Management of acute severe asthma in adults in hospital
reproduced by permission of the Scottish Intercollegiate Guidelines Network from BTS/SIGN, 2009.
Intravenous aminophylline can be given with a bolus dose of 250 mg over 30 min, followed by a continuous infusion of 500 μcg/kg/h. The bolus should be omitted if the patient is known to take oral theophylline or aminophylline. The choice between intravenous aminophylline and β2-agonist depends on concurrent therapy and side effect profiles. The dose of intravenous aminophylline used must also take into account recent theophylline therapy in addition to other factors (Table 25.7). Serious toxicity can occur with parenteral aminophylline and patients must be carefully monitored for nausea and vomiting, the most common early signs of toxicity. If the aminophylline infusion is continued for more than 24 h, the plasma theophylline concentration may be measured to guide any necessary alteration in infusion rate in order to maintain the level in the optimum range of 10–20 mg/L.
Table 25.7 Intravenous aminophylline dosing in acute severe asthma
| Aminophylline dose | Patient characteristics | |
|---|---|---|
| Loading dose | 5 mg/kg over 20–30 min | Adults and children |
| 3 mg/kg over 10–15 min | Previous theophylline therapy (although some authorities do not use a loading dose in these patients) | |
| Maintenance dose | 500 μcg/kg/h | Non-smoking adults |
| 700 μcg/kg/h | Children under 12 or smokers | |
| 200 μcg/kg/h | Cardiac failure, liver impairment, pneumonia |
Antibiotics are only indicated where there is evidence of a bacterial infection.
Ongoing management
As the patient responds to treatment, infusions can be stopped and other treatment changed or tailed off as described above. As improvement continues, an inhaled β2-agonist is substituted for the nebulised form and the oral corticosteroids stopped or reduced to a maintenance dose if clinically necessary. Throughout the treatment programme, potential drug interactions should be anticipated and managed appropriately (Table 25.8).
Table 25.8 Common clinically significant interactions with drugs used in the management of asthma
| Drug | Interacting drug | Probable mechanism and clinical result |
|---|---|---|
| β2-Agonists | Methyldopa | Acute hypotension possible with β2-agonist infusions |
| Corticosteroids | Anticoagulants | High-dose steroids enhance anticoagulant effect of coumarins |
| Antifungals | Metabolism of steroids possibly affected by antifungal agents | |
| Barbiturates | Accelerates steroid metabolism | |
| β2-Agonists | Increased risk of hypokalaemia with high doses | |
| Carbamazepine | Reduced steroid effect due to increased metabolism | |
| Ciclosporin | Increases plasma concentration of prednisolone | |
| Methotrexate | Increased risk of haematological toxicity | |
| Phenytoin | Reduced steroid effect due to increased metabolism | |
| Rifampicin | Reduced steroid effect due to increased metabolism | |
| Theophylline | Azithromycin | May increase theophylline plasma levels |
| β2-Agonists (high dose) | Increased risk of hypokalaemia | |
| Carbamazepine | Induction of theophylline metabolism resulting in decreased plasma levels | |
| Clarithromycin | Inhibition of theophylline metabolism resulting in increased plasma levels | |
| Cimetidine | Inhibition of theophylline metabolism resulting in increased plasma levels | |
| Ciprofloxacin | Increased plasma concentration. Possible risk of convulsions | |
| Diltiazem | Increased theophylline plasma levels | |
| Erythromycin (oral) | Inhibition of theophylline metabolism resulting in increased plasma levels | |
| Fluconazole | Possible increase in theophylline plasma level | |
| Dihydropyridine | ||
| calcium antagonists | May increase theophylline plasma levels | |
| Fluvoxamine | Increased theophylline plasma levels, halve theophylline dose | |
| Isoniazid | May increase theophylline plasma levels | |
| Ketoconazole | Possible increase in theophylline plasma level | |
| Lithium carbonate | Reducing plasma lithium concentrations as theophylline enhances lithium renal clearance | |
| Norfloxacin | Increased plasma concentration. Possible risk of convulsions | |
| Phenytoin | Plasma concentrations of theophylline and phenytoin both reduced | |
| Primidone | Induction of theophylline metabolism resulting in decreased plasma levels | |
| Rifampicin | Induction of theophylline metabolism resulting in decreased plasma levels | |
| Ritonavir | Metabolism of theophylline increased | |
| Smoking (tobacco) | Induction of theophylline metabolism resulting in decreased plasma levels | |
| St John’s wort | Reduced theophylline plasma levels | |
| Verapamil | Increased theophylline plasma levels |
Patient care
All members of the health care team should provide education and support for the asthmatic patient at regular intervals. The need for each patient to understand their asthma and its management must be balanced against the dangers of overwhelming the patient with information, particularly when the asthma has been newly diagnosed. To try to overcome this, a ‘ladder of asthma knowledge’ has been proposed. Patients are counseled in a gradual manner, each session adding to the previous one in content and reinforcing existing knowledge (Box 25.3).
Box 25.3 Ladder of asthma knowledge for patients
Inhalation device
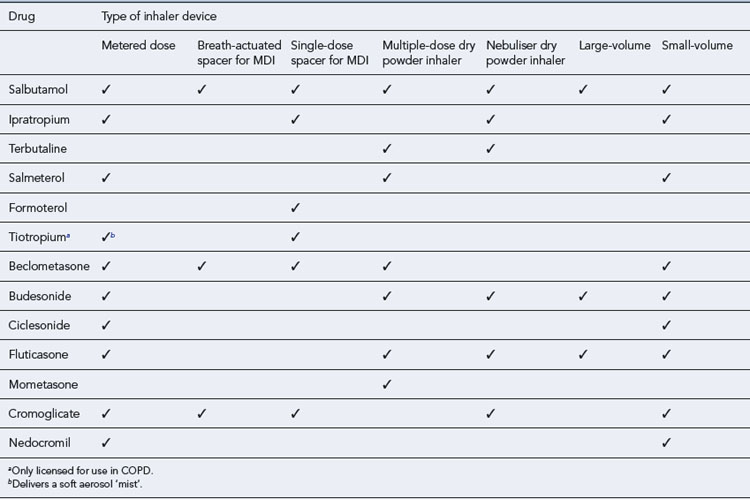
The choice of a suitable inhalation device is vital in asthma management. The incorrect use of inhalers will lead to suboptimal treatment. A review of inhaler technique studies has concluded that up to 50% of patients in Europe are unable to use their inhaler correctly (Crompton et al., 2006). There is no demonstrable difference in efficacy between the various devices available. Other factors, therefore, need to be considered when choosing the appropriate device, including the patient’s age, severity of disease, manual dexterity, co-ordination and personal preference. The range of different devices available for the drugs commonly used in asthma is shown in Table 25.9.
Metered dose aerosol inhalers
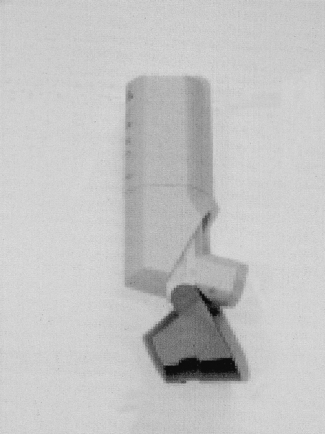
The pressurised MDI is the most widely prescribed inhalation device in the UK (Fig. 25.6). It usually contains a solution or suspension of active drug, with a typical particle size of 2–5 μm, in a liquefied propellant. Operation of the device releases a metered dose of the drug with a droplet size of 35–45 μm. The increased droplet size is due to the propellant, which evaporates when expelled from the inhaler. Inhalers have now been switched from chlorofluorocarbon (CFC) propellants to newer, non-CFC, hydrofluoroalkanes.
The correct technique for using MDIs is as follows:
Studies indicate that personal tuition improves inhaler technique, particularly if regularly repeated. Other methods of instruction include videos (see http://medguides.medicines.org.uk/demonstrations.aspx), package inserts and information leaflets or booklets provided by organisations such as Asthma UK and the pharmaceutical industry. Regular patient review, at least annually, is recommended. This can be used as an opportunity to check technique, along with assessment of the ability to generate the appropriate inspiratory flow for the device (Broeders et al., 2009).
Metered dose inhaler with a spacer extension
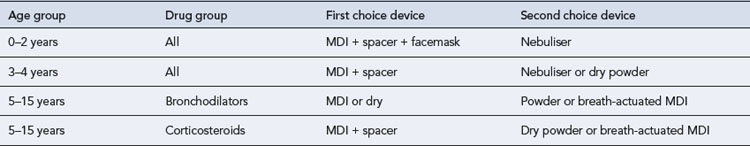
Extension devices allow greater evaporation of the propellant, so reducing particle size and velocity. This also reduces oropharyngeal deposition and potentially increases lung deposition. Oral candidiasis and dysphonia (impaired voice) from ICSs may also be reduced by using these devices. Spacers are useful for people who have poor co-ordination between inspiration and actuation and several types of spacer are available. In younger children, these offer advantages over MDIs alone with respect to adherence. Recommendations (see Table 25.10) have been published regarding device choice (NICE, 2000, 2002).
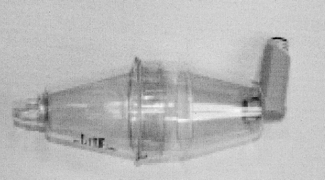
Large-volume (750 mL) spacers are available such as the Volumatic® (Fig. 25.7); these are manufacturer specific and have not been assessed or licensed for use with devices of other companies. These spacers have one-way valves that allow several inhalations of one dose from the spacer’s chamber. No co-ordination is required between actuation of the MDI and inhalation. A large-volume spacer can be used instead of a nebuliser to deliver high doses of a β2-agonist in acute severe asthma attacks. Disadvantages of these spacers include their large size, which renders them less portable, and their proven efficacy only with inhalers from the same manufacturer. Spacers should be washed regularly in warm, soapy water and left to drip dry without rinsing. Cloths should not be used for drying a spacer as this affects the antistatic coating of plastic spacers. All spacers should be replaced every 6–12 months. Facemasks are available for young children.
Small- and medium-volume spacer devices are available, either as an integral part of the design of some MDIs or as a separate device (Fig. 25.8). These spacers have also been used to compensate for poor inhaler technique in adults and reduce the oropharyngeal deposition of steroids. These are more convenient to carry around than the larger spacers. The published evidence of additional benefit from these devices in either increasing efficacy or decreasing adverse effects is more limited than with large volume spacers.
Breath-actuated metered dose inhalers
These MDIs are actuated automatically by inspiratory flow rates of about 22–36 L/min. A breath-actuated MDI is illustrated in Fig. 25.9. These eliminate the need for the correct co-ordination of inspiration and actuation but require priming before each actuation.
Dry powder inhalers
Several types of dry powder inhalers (DPIs) are available. These are propellant free and are designed to be easier to use than conventional MDIs. They are useful for those who have difficulty co-ordinating an MDI and can be used by children as young as 4 years old. Table 25.10 sets out the recommendations for device choice in children.
DPIs are available as either single-dose or multiple-dose devices (Fig. 25.10). Single-dose devices pierce or break a gelatin capsule to release the contents and must be regularly cleaned to avoid powder clogging the device. Multiple-dose devices are preferred by many patients since they avoid having to reload for each dose. Care must be taken to hold these devices in the correct orientation to avoid the powder falling out of the device before inhalation. Patients commenced on DPIs are sometimes concerned at the absence of any taste or spray plume which they have become accustomed to when using an MDI; reassurance that this is perfectly normal and that correct use of the DPI (including a check that the device is not empty) will ensure that the required dose is delivered should overcome this problem.
Nebulisers
Self-management programmes
Every individual with asthma should be considered for a self-management education programme. These programmes will contain structured education along with an individualised action plan. They aim to give the individual more confidence by involving them in the management of their own asthma. The individual should then be able to deal with any fluctuation in their condition and know when to seek medical advice. Personalised action plans have been shown to improve health outcomes in individuals with asthma (Gibson et al., 2002).
An action plan can also include details of when to increase the dose of an inhaled steroid, when to take a short course of oral corticosteroids and when to self-refer to a general medical practitioner or local hospital (Table 25.11).
Table 25.11 Example of a personalised action plan setting out the action required in response to a given peak flow reading and/or symptoms
| Peak flow | Example of symptoms | Action |
|---|---|---|
| >80% of personal best value | Intermittent or few symptoms | When required, β2-agonist for symptom relief, continue regular inhaler corticosteroid, consider reducing the dosage every 3 months if stable |
| 61–80% of best | Waking at night | Double dose of inhaled corticosteroid if taking <400 μcg day BDP |
| Symptoms of a cold | ||
| Start oral corticosteroid if taking >400 μcg/day BDP | ||
| 40–60% of best | Increasing breathlessness or using a β2-agonist every 2–3 h | Start oral corticosteroid course. Contact a doctor |
| <40% of best | Severe attack | Call emergency doctor or ambulance urgently |
| Poor response to β2-agonist |
BDP, equivalent dose of beclometasone dipropionate.
Questions
Answers
The level of asthma control for Mr GT can be determined by utilising tools such as the levels of asthma control in the GINA guidelines (GINA, 2009; Table 25.2) and the commonly used Royal College of Physicians, ‘3 questions’:
Management issues should also be discussed:
Answers
Practical information and treatment plans should be reinforced with written instruction; this can also be from patient support groups such as Asthma UK http://www.asthma.org.uk/. Every subsequent consultation with any health care professional should be an opportunity to review reinforce and extend both knowledge and skills.
Case 25.3
Salbutamol DPI 200 μcg when required (currently using three or four times every day)
Seretide-250® evohaler® 2 puffs twice daily
Answers
Asthma UK. Out in the open. A true picture of asthma in the United Kingdom today, National Asthma Campaign asthma audit 2001. Asthma J., 6. 2001: 1-14.
British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN). Update: British guideline on the management of asthma. Available at http://www.sign.ac.uk/guidelines/fulltext/101/index.html, 2009.
Broeders M., Sanchis J., Levy M., et al. The ADMIT series – Issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Primary Care Respir. J.. 2009;18:76-82. Available at http://www.thepcrj.org/journ/view_article.php?article_id=627
Crompton G.K., Barnes P.J., Broeders M., et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir. Med. 2006;100:1479-1494.
Douwes J., Gibson P., Pekkanen J., et al. Non-eosiniphilic asthma: importance and possible mechanisms. Thorax. 2002;57:643-648.
Getzsche P.C., Johansen H.K. House dust mite control measures from asthma. Cochrane Database of Systematic Reviews. 2008. Issue 2 Art No. CD001187. doi:10.1002/14651858. CD001187
Gibson P.G., Powell H., Wilson A., et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews. 2002. Issue 3. Art. No. CD001117. doi:10.1002/14651858. CD001117
GINA Global Initiative for Asthma. Global strategy for asthma management and prevention. GINA. 2009. Available at http://www.ginasthma.com/GuidelinesResources.asp?l1=2&l2=0
Holgate S., Arshad H.S., Roberts G.C., et al. A new look at the pathogenesis of asthma. Clin. Sci.. 2010;118:439-450.
Medicines and Healthcare products Regulatory Agency. Asthma: Long-Acting β2-Agonists. London: MHRA, 2008. Available at http://www.mhra.gov.uk/Safetyinformation/Generalsafetyinformationandadvice/Product-specificinformationandadvice/Asthma/index.htm
National Institute for Health and Clinical Excellence. Guidance on the Use of Inhaler Systems (Devices) in Children Under 5 Years with Chronic Asthma. London: NICE, 2000. Technology Appraisal 10. Available at http://guidance.nice.org.uk/TA10
National Institute for Health and Clinical Excellence. Inhaler Devices for Routine Treatment of Chronic Asthma in Older Children (Aged 5–15 Years). London: NICE, 2002. Technology Appraisal 38. Available at http://guidance.nice.org.uk/TA38
National Institute for Health and Clinical Excellence. Omalizumab for Severe Persistent Allergic Asthma. London: NICE, 2007. Technology Appraisal 133. Available at http://guidance.nice.org.uk/TA133
National Prescribing Centre (NPC). Current issues in the Drug Treatment of Asthma. MeReC Bull.. 2008;19:1-6. Available at http://www.npc.co.uk/ebt/merec/resp/asthma/merec_bulletin_vol19_no2.html
Saltpeter S.R., Buckley N.S., Ormiston T.M., et al. Meta-analysis: effect of long-acting β-agonists on severe asthma exacerbations and asthma-related deaths. Ann. Intern. Med.. 2006;144:904-912.
Asthma UK. Available at http://www.asthma.org.uk/index.html
Chanez P., Wenzel S.E., Anderson G.P., et al. Severe asthma in adults: what are the important questions? J. Allergy Clin. Immunol.. 2007;119:1337-1348.
Dolovici M.B., Ahrens R.C., Hess D.R., et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest. 2005;127:335-371. Available at http://chestjournal.chestpubs.org/content/127/1/335.long
Fitzgerald J.M., Gibson P.G. Asthma exacerbations – 4: prevention. Thorax. 2006;61:992-999.
Murphy A. Asthma in Focus. London: Pharmaceutical Press, 2007.
National Institute for Health and Clinical Excellence. Corticosteroids for the Treatment of Chronic Asthma in Adults and Children Aged 12 Years and Over. London: NICE, 2002. Technology Appraisal 138. Available at http://guidance.nice.org.uk/TA138
Pedersen S. From asthma severity to control: a shift in clinical practice. Primary Care Respir. J.. 2010;19:3-9. Available at http://www.thepcrj.org/journ/view_article.php?article_id=666
Pinnock H., Fletcher M., Holmes S., et al. Setting the standard for routine asthma consultations: a discussion of the aims, process and outcomes of reviewing people with asthma in primary care. Primary Care Respir. J.. 2010;19:75-83. Available at http://www.thepcrj.org/journ/view_article.php?article_id=684