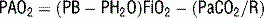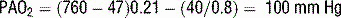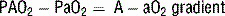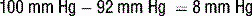Chapter 4 Assessments of Oxygenation
3 What alternatives exist to measure respiratory gases and gas exchange?
 Pulse oximetry: Pulse oximetry is a noninvasive, continuous method of measuring the saturation of hemoglobin by using the differential absorption of different wavelengths of light depending on the loading conditions of oxygen that can significantly reduce the number of ABGs measured in critically ill patients. Further, recent studies have validated the use of respiratory variation in pulse oximetry to detect fluid responsiveness in critically ill patients.
Pulse oximetry: Pulse oximetry is a noninvasive, continuous method of measuring the saturation of hemoglobin by using the differential absorption of different wavelengths of light depending on the loading conditions of oxygen that can significantly reduce the number of ABGs measured in critically ill patients. Further, recent studies have validated the use of respiratory variation in pulse oximetry to detect fluid responsiveness in critically ill patients.
 End-tidal carbon dioxide: In patients without significant pulmonary pathologic conditions, an end-tidal carbon dioxide monitor can approximate the plasma levels of carbon dioxide, indicating ongoing metabolic production and adequacy of ventilation.
End-tidal carbon dioxide: In patients without significant pulmonary pathologic conditions, an end-tidal carbon dioxide monitor can approximate the plasma levels of carbon dioxide, indicating ongoing metabolic production and adequacy of ventilation.
 Bicarbonate: Levels of serum bicarbonate can provide guidance about serum levels of carbon dioxide and pH, especially in the chronic setting. Elevations of serum bicarbonate can indicate chronic retention of carbon dioxide.
Bicarbonate: Levels of serum bicarbonate can provide guidance about serum levels of carbon dioxide and pH, especially in the chronic setting. Elevations of serum bicarbonate can indicate chronic retention of carbon dioxide.
 Exhaled carbon dioxide: The content of exhaled carbon dioxide over a given period of time can be measured by several commercially available devices. This can provide information regarding total body carbon dioxide production, as well as the adequacy of carbon dioxide elimination. Specifically, with use of the Bohr equation, the dead-space fraction can be calculated to assess the adequacy of ventilation and underlying degrees of pulmonary pathologic conditions.
Exhaled carbon dioxide: The content of exhaled carbon dioxide over a given period of time can be measured by several commercially available devices. This can provide information regarding total body carbon dioxide production, as well as the adequacy of carbon dioxide elimination. Specifically, with use of the Bohr equation, the dead-space fraction can be calculated to assess the adequacy of ventilation and underlying degrees of pulmonary pathologic conditions.
 Calculated minute ventilation: The overall minute ventilation can be calculated by multiplying the tidal volume by respiratory rate. Again, the minute ventilation can provide information about metabolic demands and production.
Calculated minute ventilation: The overall minute ventilation can be calculated by multiplying the tidal volume by respiratory rate. Again, the minute ventilation can provide information about metabolic demands and production.
6 How is the A − aO2 gradient useful? What can cause significant elevations of the A − aO2 gradient?
Clinically significant hypoxemia associated with a widened A − aO2 gradient is commonly due to:
 V/Q mismatch: The normal heterogeneity of V/Q matching in the lung can be significantly altered by many disease states, resulting in a widened A − aO2 gradient and clinical hypoxemia. Commonly, this can be seen with atelectasis, pneumonia, aspiration, pulmonary edema, and/or acute lung injury or adult respiratory distress syndrome (ARDS).
V/Q mismatch: The normal heterogeneity of V/Q matching in the lung can be significantly altered by many disease states, resulting in a widened A − aO2 gradient and clinical hypoxemia. Commonly, this can be seen with atelectasis, pneumonia, aspiration, pulmonary edema, and/or acute lung injury or adult respiratory distress syndrome (ARDS).
 Right-to-left shunt: A right-to-left shunt exists when blood passes from the right to the left side of the heart without being oxygenated. Numerous anatomic shunts exist physiologically that contribute to the normal A − aO2 gradient, such as the bronchial and thebesian circulation. Pathologic shunts can develop when nonventilated alveoli are perfused (atelectasis, pneumonia, edema, ARDS, pulmonary fibrosis).
Right-to-left shunt: A right-to-left shunt exists when blood passes from the right to the left side of the heart without being oxygenated. Numerous anatomic shunts exist physiologically that contribute to the normal A − aO2 gradient, such as the bronchial and thebesian circulation. Pathologic shunts can develop when nonventilated alveoli are perfused (atelectasis, pneumonia, edema, ARDS, pulmonary fibrosis).
 Diffusion impairment: Diffusion impairment occurs when a limitation exists to the movement of oxygen from the alveoli into the pulmonary vasculature. This can be due to overall destruction of lung parenchyma, such as severe emphysema, or pathologic changes in the air-blood interface in the lung, such as fibrosis.
Diffusion impairment: Diffusion impairment occurs when a limitation exists to the movement of oxygen from the alveoli into the pulmonary vasculature. This can be due to overall destruction of lung parenchyma, such as severe emphysema, or pathologic changes in the air-blood interface in the lung, such as fibrosis.
7 What clinical conditions can present with a normal A − aO2 gradient?
 Low FiO2: This can be seen in a variety of clinical situations, such as hypoxic mixtures of inhaled gases. These are also readily correctable by administration of higher concentrations of oxygen; failure to respond to such therapy should prompt investigation of other causes of hypoxemia.
Low FiO2: This can be seen in a variety of clinical situations, such as hypoxic mixtures of inhaled gases. These are also readily correctable by administration of higher concentrations of oxygen; failure to respond to such therapy should prompt investigation of other causes of hypoxemia.
 Low PB: Atmospheric pressure at sea level is approximately 760 mm Hg. As altitude increases, PB decreases; with significant elevations of altitude, low alveolar oxygen levels can develop. The mean measured PAO2 measured at 8400 m (descent from summit of Mount Everest) above sea level was 30 mm Hg with an A − aO2 gradient of 5.41 mm Hg.
Low PB: Atmospheric pressure at sea level is approximately 760 mm Hg. As altitude increases, PB decreases; with significant elevations of altitude, low alveolar oxygen levels can develop. The mean measured PAO2 measured at 8400 m (descent from summit of Mount Everest) above sea level was 30 mm Hg with an A − aO2 gradient of 5.41 mm Hg.
 Significant hypercarbia (elevated PaCO2): Elevations of arterial carbon dioxide levels can result in decreases in alveolar oxygen levels. Pure hypercarbia is often the result of hypoventilation syndromes. These can be related to multiple factors:
Significant hypercarbia (elevated PaCO2): Elevations of arterial carbon dioxide levels can result in decreases in alveolar oxygen levels. Pure hypercarbia is often the result of hypoventilation syndromes. These can be related to multiple factors:
 Central nervous system (CNS) depression, such as drug overdose, structural CNS lesions, or ischemic CNS lesions that affect the respiratory center
Central nervous system (CNS) depression, such as drug overdose, structural CNS lesions, or ischemic CNS lesions that affect the respiratory center Impaired neural conduction, such as amyotrophic lateral sclerosis, Guillain-Barré syndrome, high cervical spine injury, or phrenic nerve paralysis
Impaired neural conduction, such as amyotrophic lateral sclerosis, Guillain-Barré syndrome, high cervical spine injury, or phrenic nerve paralysis10 Given that oximetry is so readily available, painless, and accurate, why is ABG analysis necessary?
 Hypercarbia: An increased PCO2 from hypoventilation (e.g., in a patient receiving narcotics) can often be missed by a reassuring oxygen saturation. Although the pulse oximeter provides information regarding systemic oxygenation, it cannot provide data regarding systemic conditions of carbon dioxide. An ABG or venous blood gas measurement is necessary for further evaluation, because oxygenation can be maintained despite rising PCO2 and impending respiratory failure.
Hypercarbia: An increased PCO2 from hypoventilation (e.g., in a patient receiving narcotics) can often be missed by a reassuring oxygen saturation. Although the pulse oximeter provides information regarding systemic oxygenation, it cannot provide data regarding systemic conditions of carbon dioxide. An ABG or venous blood gas measurement is necessary for further evaluation, because oxygenation can be maintained despite rising PCO2 and impending respiratory failure.
 Carbon monoxide (CO): CO diffuses rapidly across the pulmonary capillary membrane and binds to hemoglobin with approximately 240 times the affinity of oxygen. Standard pulse oximetry cannot screen for CO exposure, because it cannot differentiate carboxyhemoglobin from oxyhemoglobin, inasmuch as they absorb the same emitted light wavelength. Arterial blood gas measurements tend to be normal because PO2 reflects oxygen dissolved in blood, and this process is not affected by CO. Acute CO poisoning must be clinically suspected on the basis of a suggestive history and associated physical examination findings (e.g., singed nasal hair, soot); specialized eight-wavelength pulse oximeters and ABG analysis with CO-oximetry are required to detect systemic CO.
Carbon monoxide (CO): CO diffuses rapidly across the pulmonary capillary membrane and binds to hemoglobin with approximately 240 times the affinity of oxygen. Standard pulse oximetry cannot screen for CO exposure, because it cannot differentiate carboxyhemoglobin from oxyhemoglobin, inasmuch as they absorb the same emitted light wavelength. Arterial blood gas measurements tend to be normal because PO2 reflects oxygen dissolved in blood, and this process is not affected by CO. Acute CO poisoning must be clinically suspected on the basis of a suggestive history and associated physical examination findings (e.g., singed nasal hair, soot); specialized eight-wavelength pulse oximeters and ABG analysis with CO-oximetry are required to detect systemic CO.
 Abnormal hemoglobin or hemoglobin variants: Methemoglobin is an altered state of hemoglobin in which the ferrous (Fe2 +) irons of heme are oxidized to the ferric (Fe3 +) state, which are unable to bind oxygen. In addition, the oxygen affinity of any remaining ferrous hemes is increased, resulting in a leftward shift of the oxygen dissociation curve. Large amounts of methemoglobin production can be induced by various drugs, including antibiotics and local anesthetics, such as benzocaine (commonly used for oropharyngeal topicalization). Methemoglobinemia may be clinically suspected by the presence of clinical cyanosis in the presence of a normal arterial PO2 as obtained by ABGs. Classically, oxygen saturation as measured by pulse oximetry drops to 85%, as methemoglobin absorbs both wavelengths of light (660 and 940 nm) emitted by pulse oximetry resulting in an average value regardless of the true percentage of oxyhemoglobin.
Abnormal hemoglobin or hemoglobin variants: Methemoglobin is an altered state of hemoglobin in which the ferrous (Fe2 +) irons of heme are oxidized to the ferric (Fe3 +) state, which are unable to bind oxygen. In addition, the oxygen affinity of any remaining ferrous hemes is increased, resulting in a leftward shift of the oxygen dissociation curve. Large amounts of methemoglobin production can be induced by various drugs, including antibiotics and local anesthetics, such as benzocaine (commonly used for oropharyngeal topicalization). Methemoglobinemia may be clinically suspected by the presence of clinical cyanosis in the presence of a normal arterial PO2 as obtained by ABGs. Classically, oxygen saturation as measured by pulse oximetry drops to 85%, as methemoglobin absorbs both wavelengths of light (660 and 940 nm) emitted by pulse oximetry resulting in an average value regardless of the true percentage of oxyhemoglobin.
1 Barton C.W. Correlation of end-tidal CO2 measurements to arterial PaCO2 in non-intubated patients. Ann Emerg Med. 1994;23:562–563.
2 Durbin C.G., Rostow S.K. More reliable oximetry reduces the frequency of arterial blood gas analyses and hastens weaning after cardiac surgery: a prospective, randomized trial of the clinical impact of a new technology. Crit Care Med. 2002;30:1735–1740.
3 Feissel M., Teboul J.L., Merlani P., et al. Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med. 2007;33:993–999.
4 Fu E.S., Downs J.B., Schweiger J.W., et al. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126:1552–1558.
5 Hampson N.B. Pulse oximetry in severe carbon monoxide poisoning. Chest. 1998;114:1036–1041.
6 Hess D., Schlottag A., Levin B., et al. An evaluation of the usefulness of end-tidal PCO2 to aid weaning from mechanical ventilation following cardiac surgery. Respir Care. 1991;36:837–843.
7 Michael P.W., Grocott M.B., Martin D.S., et al. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–149.
8 Mithoefer J.C., Bossman O.G., Thibeault D.W., et al. The clinical estimation of alveolar ventilation. Am Rev Respir Dis. 1968;98:868–871.
9 Nuckton T.J., Alonso J.A., Kallet R.H., et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286.
10 Perkins G.D., McAuley D.F., Giles S., et al. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care. 2003;7:R67.
11 Rodríguez-Roisin R., Roca J. Mechanisms of hypoxemia. Intensive Care Med. 2005;31:1017–1019.
12 Severinghaus J.W., Astraup P., Murray J.F. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med. 1998;157:S114–S122.
13 Siddiki H., Kojicic M., Li G., et al. Bedside quantification of dead-space fraction using routine clinical data in patients with acute lung injury: secondary analysis of two prospective trials. Crit Care. 2010;14:R141.
14 Story D.A. Alveolar oxygen partial pressure, alveolar carbon dioxide partial pressure, and the alveolar gas equation. Anesthesiology. 1996;84:101.
15 Tobin M.J. Respiratory monitoring in the intensive care unit. Am Rev Respir Dis. 1988;138:1625–1642.