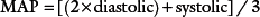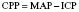Chapter 3 Assessment tools
3.1 Arterial Blood Gases (ABGs)
3.7 Breathing Pattern (See under 3.54 Respiratory Pattern – Page 148)
3.8 Breathlessness (Dyspnoea) Scales
3.10 (Invasive) Cardiac Monitoring
3.11 Central Venous Pressure (CVP)
3.12 Cerebral Perfusion Pressure (CPP)
3.14 Chest Imaging (Including Chest X-rays)
3.15 Chest X-ray (See under 3.14 Chest Imaging – Page 68)
3.23 Early Warning Scores (EWS)
3.24 Electrocardiogram (ECG) (See under 3.35 Heart Rhythms – Page 109)
3.25 Electrolytes (See under 3.6 Blood Results – page 49)
3.26 End-Tidal Carbon Dioxide (ETCO2) 92
3.27 Exercise (aerobic fitness) Testing
3.29 FEV1/FVC (See under 3.40 Lung Volumes and Lung Function Tests – Page 120)
3.30 Flow Volume Loops (See under 3.40 Lung Volumes and Lung Function Tests – Page 120)
3.31 Fluid Balance (Including Urine Output)
3.32 General Observation (See Chapter 1)
3.36 Inspiratory Muscle Testing (See under 3.40 Lung Volumes and Lung Function Tests – Page 120)
3.37 Intracranial Pressure (ICP)
3.38 ITU (critical care) Charts
3.40 Lung Volumes and Lung Function Tests (Pulmonary Function Tests)
3.41 Muscle Charting (Oxford Grading)
3.46 Peak Flow (See under 3.40 Lung Volumes and Lung Function Tests – Page 120)
3.50 Quality of Life Questionnaires
3.51 Rating of Perceived Exertion (RPE)
3.53 Renal Function (See under 3.6 Blood Results and 3.31 Fluid Balance – Pages 49 and 102)
3.61 TPR (Temperature, Pulse and Respiration) Chart
This chapter contains assessment procedures that may be used with respiratory patients. For each assessment performed, you need to select those that are most appropriate for each patient. If you are unsure which are appropriate, refer back to the assessment checklists in Chapter 2, which relate the tools to the assessment process.
Each technique has distinct sections:
• Definition: explains what the tool or measurement is about in simple terms.
• Purpose: suggests why the test/measurement is done and why this is important for physiotherapists.
• Procedure: gives step-by-step instructions on how the technique should be undertaken.
• Findings: looks at the findings and how this information may be of use to you, linking theoretical concepts to practical application.
• Documentation: demonstrates how this information should be recorded in your notes.
3.1 Arterial blood gases (ABGs)
Procedure
Physiotherapists need to be able to analyse the results (Fig. 3.1.1). The procedure itself involves taking an arterial blood sample and is normally carried out only by medical or nursing staff and some extended scope physiotherapists with specialized training.
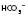
NB. Partial pressure refers to the pressure exerted by one specific gas (e.g. oxygen), which forms part of the total pressure exerted by the mixture of gases (e.g. oxygen, carbon dioxide and nitrogen) in the blood.
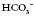 levels and make the blood more alkaline, thereby restoring the pH.
levels and make the blood more alkaline, thereby restoring the pH.
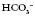 are outside the normal range and the pH is within the normal range.
are outside the normal range and the pH is within the normal range.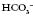 are both out of range but the pH is not quite within normal limits.
are both out of range but the pH is not quite within normal limits.6. Check for respiratory failure
Type One failure is when One gas (i.e. Oxygen) is affected – remember they both begin with O!
Type Two is when Two gases (i.e. oxygen and carbon dioxide) are affected.
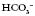 levels because it takes time (e.g. days) for adequate compensation to occur. Therefore, the pH is likely to be low (respiratory acidosis).
levels because it takes time (e.g. days) for adequate compensation to occur. Therefore, the pH is likely to be low (respiratory acidosis).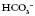 levels. Therefore, the pH may be normal, or only slightly lower than normal (compensated respiratory acidosis). Check any current ABG results against any previous or usual results as this will also help to confirm the diagnosis of acute or chronic failure.
levels. Therefore, the pH may be normal, or only slightly lower than normal (compensated respiratory acidosis). Check any current ABG results against any previous or usual results as this will also help to confirm the diagnosis of acute or chronic failure.3.2 Attachments
Definition
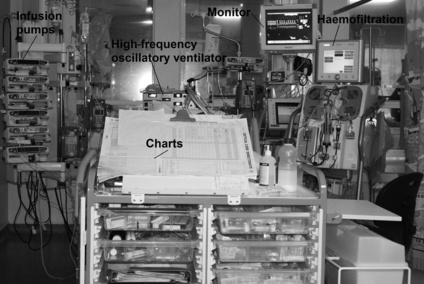
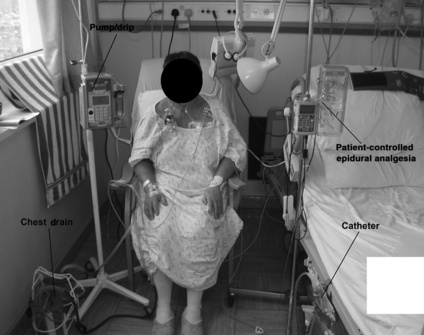
These may be any piece of equipment that is attached to the patient, e.g. intravenous (i.v.) lines, central venous line, ECG lines, surgical drains, ventilator tubing, saturations monitor and catheter (Figs 3.2.1 and 3.2.2).
Procedure
Be aware of all attachments and take care not to displace any equipment during your assessment.
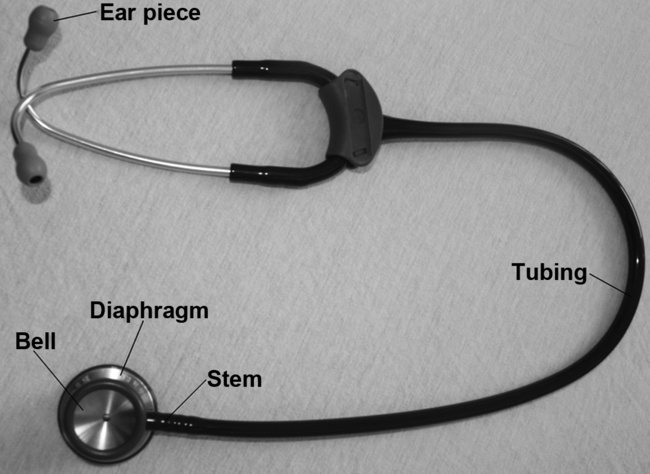
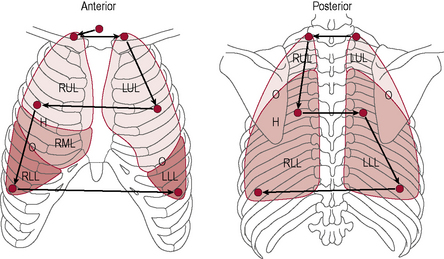
3.3 Auscultation
Procedure
| Anterior |
Findings
Breath sounds (Table 3.3.2) arise in the trachea and bronchi and are caused by the turbulence of the air as it flows in and out of these large airways. If you listen over the trachea (just below the cricoid cartilage or Adam’s apple) you will hear what they sound like at the source.You will notice that they are very loud here and also quite harsh sounding. Inspiration and expiration are heard clearly (both are loud) and there is a very slight pause between them. These sounds are known as bronchial breath sounds because they are generated in the bronchi (and trachea).
• Normal breath sounds. If you listen anywhere over the lung fields (including all of the zones shown in Fig. 3.3.2) the sounds are much quieter because you are over lung parenchyma and the air in the lungs has absorbed a lot of the sound. This attenuates the sound, making it smoother, gentler and more continuous, so it is harder to tell when inspiration changes to expiration. Since we expect to hear these sounds when auscultating over the lungs we call them ‘normal breath sounds’.
• Bronchial breathing (bronchial breath sounds heard over lung fields). If you are listening over the lungs and what you hear sounds loud, harsh and discontinuous (i.e. like bronchial breath sounds), it is not ‘normal’ and suggests that these sounds have not been dampened down by the air in the lungs – usually because in that area of lung the air has been replaced by something solid (consolidated). A solid area transmits sound very well because it vibrates more than air (ask the patient to say ‘99’ while auscultating and the sound will be very clear over consolidated lung tissue). These sounds may occur over an area of lobar pneumonia where the alveoli fill with clotted inflammatory exudate, or over collapsed lung tissue (occurring without a sputum plug), or at the lip of a pleural effusion.
• Breath sounds quieter than normal. If the breath sounds are very quiet it might be because the patient is very large and therefore the sounds have been dampened down by excess soft tissue. Make sure you press the stethoscope firmly to the chest wall and press through the adipose tissue to get the best sound transmission. It might, however, be because the breaths were quite small and not generating much turbulence. Perhaps the patient is not breathing very deeply? Does this fit with your observation and palpation? Is one side of the chest moving more than the other side?
• Absent breath sounds. A pleural effusion, sputum plugging or pneumothorax could prevent sounds from being heard by absorbing the sound completely before it can be transmitted to the chest wall.
• Breath sounds louder than normal. Loud breath sounds suggest more turbulence in the large airways. This is usually due to obstruction or narrowing of these airways, e.g. due to COPD. Narrower airways are harder to breathe through, so the patient may be working harder in order to breathe.
Table 3.3.3 Added sounds: crackles
| Crackles | Short, non-musical, popping sounds, fine or coarse | |
| Fine crackles | Atelectasis | |
| Intra-alveolar oedema | ||
| Secretions small airways | ||
| Coarse crackles | Obstruction more proximal and larger airways with sputum. May be inspiratory as well as expiratory | |
Table 3.3.4 Added sounds: wheezes
| Wheezes | Musical sounds due to vibration of wall of narrowed airway | |
| High pitched | Bronchospasm | |
| Low pitched | Sputum | |
| Localized | TumourForeign body | |
| Sounds like | Cause | |
|---|---|---|
| Pleural rub | ||
| Stridor |
! Alert medical staff as the patient’s airway is at great risk of compromise |
3.4 AVPU (see also 3.33 glasgow coma scale and 3.39 level of consciousness)
Purpose
This was adapted from paediatric practice to acute adult care as a method of quickly identifying change in neurological arousal. While effective, it does not replace formal neurological assessment such as the Glasgow Coma Scale (see 3.33 Glasgow Coma Scale and 3.57 Sedation/agitation score).
Procedure
1. Alert. The patient is alert and responsive and answers clearly and coherently. The patient could be sitting up reading the paper or talking to visitors.
2. Voice. The patient is responding only to voice commands and may be drowsy, possibly keeping eyes closed and not speaking coherently. There is a response to your voice. Does the patient wake up when you call their name? This response may be only a grunt.
3. Pain. The patient responds only to pain. To elicit any response from the patient requires a painful stimulus (for procedure and appropriate painful stimuli, see 3.33 Glasgow Coma Scale).
4. Unresponsive. The patient is unresponsive. This patient is unresponsive or unconscious. They may be in a ‘coma’ with no eye opening or movement.
3.5 Blood pressure (BP)
Definition
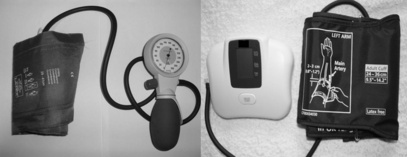
The force exerted by the blood against the walls of the arteries. Blood pressure (BP) can be measured in a large artery (e.g. brachial or femoral), and may be measured invasively through an arterial line or non-invasively through a cuff and sphygmomanometer (manually) or by using an automated BP machine (Figs 3.5.1 and 3.5.2).
Procedure
Non-invasive BP monitoring: cuff and sphygmomanometer (Fig. 3.5.3)
• Choose an appropriate sized cuff for the patient (Table 3.5.1). The inflatable part of the cuff should go round at least 75% of the arm circumference, but it should not be any longer than the arm circumference.
• Observe the patient’s arm carefully throughout the procedure. If there is any pain or numbness or if the arm starts to change colour, becoming blue or purple, the cuff should be immediately deflated and removed from the arm.
| Size of cuff | Upper arm circumference (measure at mid-point of humerus) |
|---|---|
| Child/small adult cuff | <23 cm |
| Standard/regular adult cuff | <33 cm |
| Large adult cuff | <50 cm |
| Adult thigh cuff | <53 cm |
Cuff and automated BP machine (Fig. 3.5.4)
• Make sure you are familiar with the machine and any manufacturer’s instructions before commencing.
• Carry out steps 1–5 above; then press the start button on the automated BP machine.
• It is very important to observe the patient’s arm carefully. If there is any pain or numbness or if the arm starts to change colour, becoming blue or purple, the cuff should be immediately deflated and removed from the arm.
• If the machine stays inflated too long, or continually reinflates over and over again, stop the procedure and check the patient’s circulation.
• Check the arm to ensure circulation (normal pink colour) is restored and no damage has been caused.
Findings
Average resting BP is 120/80 mmHg. The first figure (120 mmHg) represents the systolic pressure, achieved during ventricular contraction. The second figure (80 mmHg) is the diastolic pressure, achieved during ventricular diastole. Invasive measurements of BP will also calculate MAP (mean arterial pressure) (see 3.10 (Invasive) Cardiac monitoring).
• Inotropes (e.g. adrenaline, noradrenaline, enoximone, milrinone) help to increase BP. Patients who need this support are less stable, and need extra caution with physiotherapy techniques.
• Anti-hypertensives (e.g. beta-blockers, angiotensin-converting enzyme inhibitors, diuretics) help to reduce BP and may affect the patient’s response to exercise (see 3.22 Drugs).
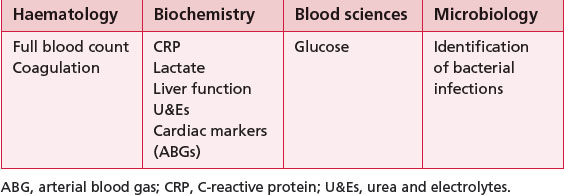
3.6 Blood results
Definition
There are a number of different types of blood tests; Table 3.6.1 summarizes those that are particularly relevant to physiotherapists. Other types of blood tests include those for immunology and virology.
Findings
Full blood count
A common test that assesses the state of health of the patient (Table 3.6.2). For definitions, see the further reading suggested at the end of this section.
| Test (normal range) | Too high | Too low |
|---|---|---|
| (WBC) (4–11 × 109/l) | May be increased with infections, inflammation, cancer, leukaemia | Decreased with some medications (e.g. chemotherapy), when immune system is compromised, some severe infections, bone marrow failure |
| WBC differentials, i.e. neutrophils, lymphocytes, monocytes, eosinophils and basophils | Measures the percentage of each type of WBC in blood. A low level is generally due to immunocompromise. Neutropenia is a low neutrophil count and happens after chemotherapy; neutropenic sepsis is when the patient becomes very unwell and has no reserves to fight off infection | |
DVT, deep vein thrombosis; F, female; Hb, haemoglobin; Hct, haematocrit; M, male; MCV, mean corpuscular volume; RBC, red blood cell; WBC, white blood cell.
Coagulation screen (Table 3.6.3)
| Test (normal range) | Too high | Too low |
|---|---|---|
| APTT (25–35 s) | Clotting deranged, bleeding time prolonged and interventions may have higher risk of bleeding; many treatments can be contraindicated. Check with senior! | Potential hypercoagulable state in which blood may clot too easily; this is unusual but could lead to, for example, DVT |
| PT (11–15 s) | ||
| INR (0.8–1.2) | ||
| Fibrinogen (2.0–4.0 g/l) | May rise sharply in any condition that causes inflammation or tissue damage. Rise will increase a person’s risk of developing a blood clot | Low levels impair the body’s ability to form a stable clot; may be an inherited disorder or can be the result of illness. Caution with treatment and discuss with senior! |
| Platelets | See Full blood count |
APTT, activated prothrombin time; DVT, deep vein thrombosis; INR, international normalized ratio; PT, prothrombin time.
C-reactive protein (CRP)
CRP (Table 3.6.4) is an inflammatory marker (often used alongside the white blood count). It serves as a general marker of infection and inflammation.
| Test | Too high | Very high |
|---|---|---|
CRP, C-reactive protein.
Lactate
Lactate (Table 3.6.4) is a by-product of anaerobic respiration and rises in patients with sepsis. It is also a marker of the severity of stress response. Hyperlactataemia is a mild-to-moderate persistent increase in blood lactate concentration (2–5 mmol l−1) without metabolic acidosis. Lactic acidosis is a persistently increased blood lactate level (usually >4–5 mmol l−1) in association with metabolic acidosis. It is not within the remit of this book to discuss lactic acidosis; see Further reading on this subject at the end of this section.
Urea and electrolytes (U&Es)
U&Es (Table 3.6.5) encompass renal function and electrolyte tests. Urea and creatinine are the main markers of renal function.
| Test | Measurement significance |
|---|---|
| Urea (2.1–8.0 mmol/l) | Urea is the final degradation product of proteins and amino acid metabolism and is the most important catabolic pathway for eliminating excess nitrogen in the body. Blood loss, fluid balance, renal failure and liver failure can also cause rises in urea |
| Creatinine (M = 80–115 μmol/l) (F = 70–100 μmol/l) |
A waste product produced by muscles; related to muscle mass (women and children tend to have lower levels). Creatinine blood levels can also increase temporarily as a result of muscle injury and are generally slightly lower during pregnancy Creatinine clearance measures how well creatinine is removed from your blood by your kidneys. It is completed using a blood sample and 24 hour urine collection |
F, female; M, male.
Sodium (Na+)
Na+ (Table 3.6.6) plays a vital role in maintaining the concentration and volume of the ECF. It is the main cation of the ECF and a major determinant of ECF osmolality. Na+ is important in maintaining irritability and conduction of nerve and muscle tissue and assists with the regulation of acid–base balance.
| Test | Too high | Too low |
|---|---|---|
| Sodium (135–145 mmol/l) |
ADH, antidiuretic hormone; GI, gastrointestinal.
Potassium (K+)
K+ (Table 3.6.6) is the major ion of the body. Nearly 98% of potassium is intracellular, with the concentration gradient maintained by the sodium- and potassium-activated adenosine triphosphatase pump. The ratio of intracellular to extracellular potassium is important in determining the cellular membrane potential.
Glucose
Blood sugar levels are important in maintaining acid–base balance in the body. It has previously been suggested that tight glycaemic control improved mortality, but this is currently under question. However, glucose levels (Table 3.6.7) are important because altered results may cause dramatic effects and yet can be easily treated.
| Test | Too high | Too low |
|---|---|---|
CNS, central nervous system.
3.7 Breathing pattern (See under 3.54 respiratory pattern – page 148)
3.8 Breathlessness (dyspnoea) scales
Purpose
Breathlessness scales have a number of uses:
• The Borg scale (CR10) can be used to rate exercise intensity during an exercise test and identify an appropriate level of intensity when prescribing exercise or activity. It may also be used to monitor progress during an exercise programme and as a guide when patients are learning to desensitize themselves to their breathlessness.
• The MRC scale can be used to classify patients according to the functional impact of their respiratory disease. This can be used to monitor progression or deterioration and in helping to select patients who are appropriate for pulmonary rehabilitation and medical interventions.
• Breathlessness scales can also be used as outcome measures for drug therapy and for physiotherapy interventions such as exercise programmes, breathing control or sputum clearance.
Procedure
Borg scale (Table 3.8.1)
| Number | Strength of perception of breathlessness |
|---|---|
| 0 0.3 0.5 0.7 1 2 2.5 3 4 5 6 7 8 9 10 * |
Nothing at all Extremely weak (just noticeable) Very weak Moderate Strong (heavy) Very strong Extremely strong (almost maximal) |
Note that some texts refer to the absolute maximum level of breathlessness as above 10; on some adapted scales, this may be marked with a symbol, e.g. ‘*’. There is also a Borg scale that uses 20 points (see 3.51 Rating of perceived exertion).
MRC scale (Table 3.8.2)
| MRC score | Descriptor |
|---|---|
| 1 | I only get breathless with strenuous exercise |
| 2 | I get short of breath when hurrying on the level or walking up a slight hill |
| 3 | I walk slower than other people of the same age on the level because of breathlessness, or I have to stop for breath when walking at my own pace on the level |
| 4 | I stop for breath after walking about 100 yards or after a few minutes on the level |
| 5 | I am too breathless to leave the house or garden by myself (i.e. I can only leave the house if I am with others and/or need a car to get out) |
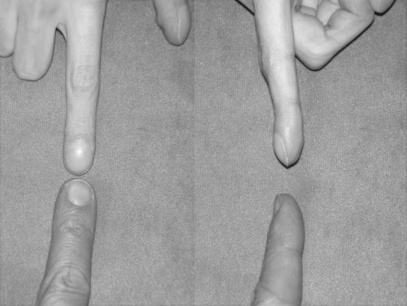
3.9 Capillary refill test
Procedure
• This should be performed at the level of the heart.
• Pressure is applied to the nail bed until it turns white, indicating that the blood has been forced from the tissue (blanching).
• Once the tissue has blanched, pressure is removed.
• The time taken for the tissue to return to its normal colour is then recorded.
• The tissue should normally take less than 2 seconds to return to its normal colour.
3.10 (Invasive) cardiac monitoring
Definition
Key terminology
• Stroke volume index : a calculation relating the stroke volume (the amount of blood pumped by the left ventricle in one contraction) to the body surface area (normal range = 33–47 ml/m2/beat).
• Cardiac index : a calculation that relates the cardiac output (stroke volume × heart rate) to body surface area (normal range = 2.5–4.0 l/min/m2).
• Global ejection fraction: the proportion of the volume of blood in the ventricles at the end of diastole that is ejected during systole; it is the stroke volume divided by the end-diastolic volume, often expressed as a percentage (normally range = 65 ± 8%).
• Mean arterial pressure: the average BP in an individual. It is not a true average as diastole counts twice as much as systole because two-thirds of the cardiac cycle is spent in diastole (normal range = 70–105 mmHg). It is calculated using the equation:
• Systemic vascular resistance: the resistance to flow that must be overcome to push blood through the circulatory system (normal range = 800–1200 dynes.sec/cm5).
Procedure
The insertion of all invasive cardiac monitoring is completed by specifically trained doctors.
Central venous pressure (CVP)
Reflects right ventricular filling and is usually monitored via a catheter which sits within the superior vena cava. The catheter is usually inserted via the subclavian vein within the thorax or the internal jugular vein. Less commonly, central venous catheters can be inserted via the femoral vein (see 3.11 Central venous pressure).
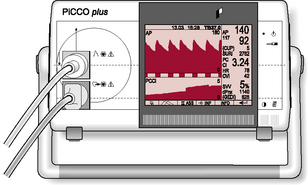
Continuous thermodilution (e.g. PiCCO®)
The PiCCO® system (Pulsion Medical Systems AG; Fig. 3.10.1) requires the insertion of a thermodilution catheter in the femoral or axillary artery and a central venous catheter (no catheter in right atrium required). By using a complex technique of transpulmonary thermodilution the monitor is able to provide specific parameters for arterial BP, heart rate, cardiac output, global end-diastolic blood pressure, intrathoracic blood volume index, cardiac function index, global ejection fraction, stroke volume, stroke volume variation and systemic vascular resistance.
Findings
For implications of arterial blood pressure, see 3.5 Blood pressure; for CVP, see 3.11 Central venous pressure.
3.11 Central venous pressure (CVP)
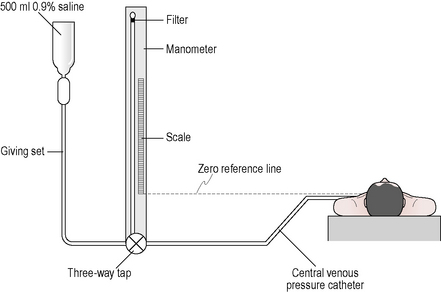
Procedure
The catheter can be inserted via the subclavian, the internal jugular or the femoral veins. The catheter is connected to a manometer that sits level with the right atrium of the heart (Fig. 3.11.1). A central line trace and value is seen on the patient’s monitor (this is often blue). Within critical care, continuous measurements are possible. Heart function is indirectly measured by CVP; therefore, in the critically unwell patient more specific invasive monitoring may be utilized (see 3.10 (Invasive) Cardiac monitoring).
Findings
• A high CVP may be associated with conditions that cause a rise in right atrial pressure such as heart failure, reduced right atrial compliance, tension pneumothorax, fluid overload or pulmonary hypertension.
• A low CVP may suggest hypovolaemia (reduced fluid) and gross vasodilatation of blood vessels (e.g. as in sepsis).
• Changes in CVP can provide information to the medical team on current fluid balance and also response to fluid loading.
3.12 Cerebral perfusion pressure (CPP)
Purpose
Monitoring CPP allows the team to identify whether the patient’s brain has enough blood flow to prevent further damage and provide enough oxygen to the brain tissue. Physiotherapists must be aware of the CPP value as our treatment techniques may have an impact on both BP and ICP (intracerebral pressure) and therefore alter CPP – see 3.37 ICP.
3.13 Chest drains
Definition
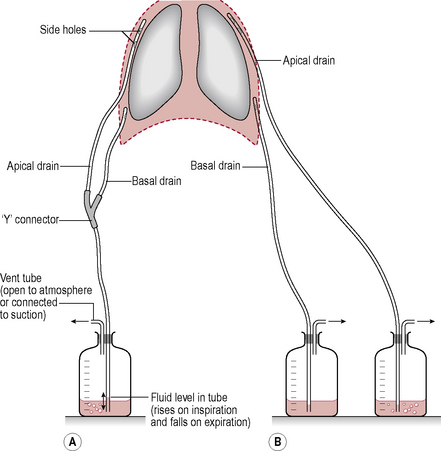
A chest (intercostal) drain is a tube inserted into the pleural space in order to drain air (pneumothorax) or fluid (pleural effusion, empyema, haemothorax or residual postoperative fluid) from the pleural space. The size of the tube can vary, from narrow bore (approximately 5 mm) to wide bore (approximately 1.5 cm). The tube leads away from the thorax to an underwater sealed unit which stops the drained fluid backtracking up the tube (Fig. 3.13.1). The drainage of fluid can be enhanced by the application of low-pressure suction. The tube will only be removed when it has stopped draining air or fluid.
Procedure
A simple look, listen and feel will tell you a lot:
• Look at where the drain enters the patient and the dressing around it. Do not take down the dressing, but look for oozing or leaks. Look at the tube and bottle forming the underwater seal and how the water level varies (Fig. 3.13.2).
• Listen to the patient – do they report any pain and how are they breathing?
Findings
The position of the chest drain will be assessed by the medical staff by chest radiograph.
Things to consider from a physiotherapy point of view:
• ‘Swinging’. The fluid in the tube should move during the respiratory cycle. If it is not swinging, check with the nursing staff. It could be that the tube is ready to come out, or that there is a problem with the tube. Check that the tube is not kinked or obviously blocked (by the patient sitting on it!). If not, discuss with the nursing or medical staff.
• ‘Bubbling’. Occurs if there is an air leak and air bubbles are seen in the drainage bottle. It is important to note whether bubbling happens in part of the respiratory cycle or just on coughing as this gives an indication of the severity of the leak. So what should you do if it is bubbling? Is this a new occurrence or not? If this has not happened previously, you will need to highlight this to the team.
• ‘Draining’. The volume of fluid drained needs to be measured. How is the volume measured? The volume is measured in millilitres (ml) by looking at the graduated lines on the side of the chest drain bottle. Remember that the bottle is filled with approximately 500 ml of water to create a negative suction pressure in the pleural space; therefore, 500 ml needs to be subtracted from the total amount of drainage in the bottle to determine how much fluid had drained from the pleural space – this is generally allowed for by our nursing colleagues when they chart drainage.
3.14 Chest imaging (including chest X-rays)
Purpose
Findings
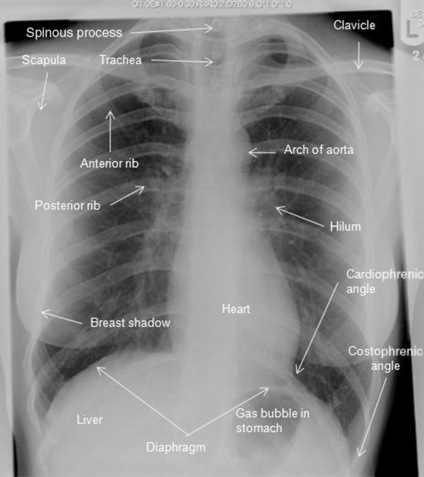
It is accepted practice that you view the image as if the patient were standing in front of you, so the right side of the image is the left side of the patient. To save confusion, the radiographers label the image with an ‘L’ or ‘R’ (see Fig. 3.14.1, which shows a PA film of a healthy 29 year old woman with some of the anatomical structures annotated). Note that the white areas represent densities (e.g. the heart) whereas the blacker areas represent less dense areas (air shows as black). Table 3.14.1 highlights some simple questions to consider when reviewing a CXR.
| Who? | Who is the film of? A simple question but it is all too easy to look at the film of the wrong patient! |
| What? | What part of the body was radiographed? With PACS systems it is easy to bring up an image from another part of the body |
| When? | When was the film taken? You can look at an old film and wonder why the patient looks so well! It is also good to compare previous images with the most recent film to look for changes |
| Where? | Where was the film taken? A film taken on the ward will not be of the same quality as one taken in the department. Departmental films are taken with the shoulders protracted, thus keeping the scapula away from the lung fields |
| Why? | Why was the film taken in the first place? There is always a good clinical reason |
| How? | How was the film taken? AP vs PA. With an AP film the heart shadow is larger |
AP, anteroposterior; PA, posteroanterior.
In Fig. 3.14.2, the same CXR of the 29 year old woman is shown. It has been marked for ease of interpretation using the system for interpretation of CXRs summarized in Table 3.14.2. Use the suggested points while looking at the radiograph in Fig. 3.14.2. This is just one system that can be used to go through CXRs – the key is to use the same system every time so that you do not miss anything out!
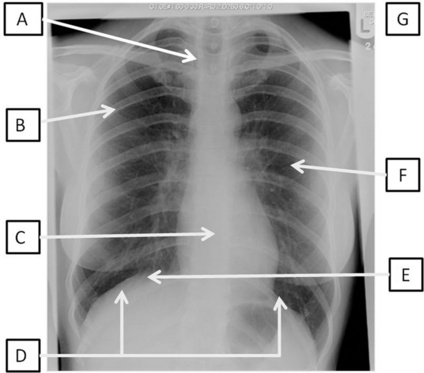
Fig 3.14.2 Posteroanterior film of a 29 year old woman with CXR analysis markers – see Table 3.14.2.
Table 3.14.2 A system for interpretation of CXR
| A | Alignment and A quick look! | Is there anything obviously wrong? Is it a straight film or rotated? If the patient was not straight at the time of the radiograph, the positions of the structures will appear to have moved. To assess this, look at the proximal ends of the clavicles – they should be an equal distance from the spinous processes. If they are not, the side with the bigger gap is the side it is rotated towards |
| B | Bones | Are they all there or are there bits that should not be there at all? Look for fractures, and not just of the ribs. In trauma patients these are frequently not picked up until much later on and it may be that you are the first person to notice it. There are no fractures in Fig. 3.14.2 |
| C | Cardiac | Look at the heart size and borders. The heart should be one-third of the diameter of the chest, with more on the left side than the right. The heart borders or lines around the heart should be smooth. There should also be a sharp angle seen between the heart borders and the diaphragm. These are known as cardiophrenic angles |
| D | Diaphragm | Can you see both of them clearly? The costophrenic angles between the ribs and the diaphragm should be clear and sharp. If not, there may be some fluid in the pleural space. Note that the right hemidiaphragm is always higher than the left hemidiaphragm owing to the position of the liver under the right lung |
| E | Expansion and Extrathoracic structures |
Expansion is a reflection on the volume of air in the lungs. The normal level of expansion is to the 10th rib at the mid-clavicular point posteriorly or the 6th rib anteriorly. Underexpansion may mean poor lung volumes; overexpansion may be related to other pathologies, such as emphysema. Extrathoracic structures should be looked at for obvious changes or warning signs such as subcutaneous emphysema (air in the soft-tissue structures) |
3.15 Chest X-ray (see under 3.14 chest imaging – page 68)
3.16 Chest wall shape
Purpose
• at an increased risk of respiratory complications due to a restrictive breathing pattern (e.g. after surgery)
• who might benefit from exercise or mobilization to improve chest wall mobility
• with severe airway obstruction where the effect on lung mechanics could limit their ability to breathe diaphragmatically or take part in exercise.
Findings
Variations in chest wall shape are shown in Fig. 3.16.1.
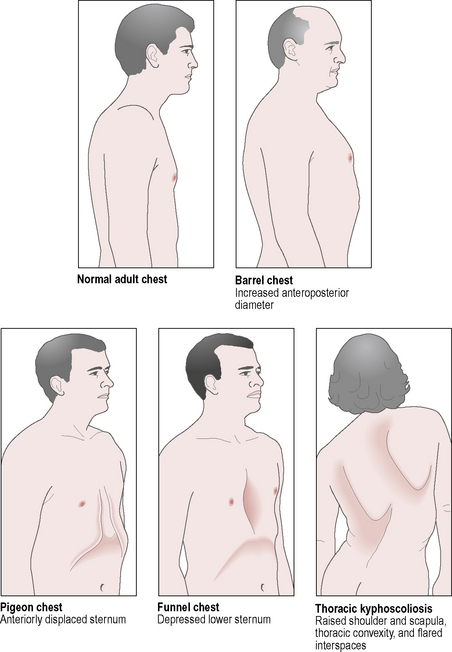
Fig 3.16.1 Chest wall shape.
Reproduced with kind permission from Lippincott, Williams and Wilkins 2004. Breath Sounds made Incredibly Easy. Springhouse Publishing Co.
Restrictive changes
• Pes excavatum (funnel chest): the sternum is indented.
• Pes carinatum (pigeon chest): the sternum projects forwards.
• Scoliosis: the thoracic spine is curved laterally and rotated.
• Kyphosis: the thoracic spine is curved anteroposteriorly.
• Stiff, immobile ribs due to osteoarthritis, osteoporosis or previous rib fractures.
3.17 (Digital) clubbing
Purpose
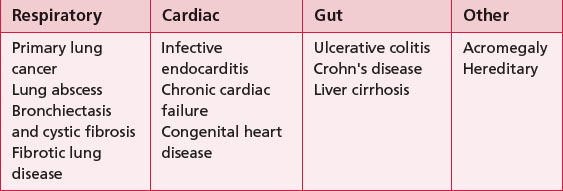
To identify patients with underlying respiratory or cardiac disease with significant hypoxia. The exact mechanism of developing clubbing is unclear; however, it is thought to be as a result of capillary proliferation. Digital clubbing usually occurs as a result of respiratory, cardiac or gut disease and occasionally can occur with no underlying pathology (Table 3.17.1).
3.18 Consent
3.19 Cough assessment
Purpose
• To determine whether cough is sufficient to maintain effective sputum clearance and identify factors adversely affecting cough effectiveness. Used to identify the most appropriate clearance intervention(s).
• To identify situations of excessive non-productive coughing that may respond to cough suppression (e.g. breathing control practice to reduce coughing).
• To identify problems associated with coughing that may be affecting the patient’s health or quality of life.
Findings
Potential factors affecting cough effectiveness:
• Can the patient take a deep breath in?
• Any inspiratory muscle weakness?
• Chest wall deformity or lack of mobility?
• Did you hear the glottis close, then open suddenly at the beginning of cough (the harsh sound that characterizes a cough) or did it sound more like a forced expiratory manoeuvre?
• Any pain that might be affecting cough? If so, consider pain control, or supported coughing if there is a wound or incision.
• Any wheezing? Patient may have airway obstruction affecting expiration – do they need bronchodilators?
• Does the patient have a condition which might cause weakness of the expiratory muscles? If so, could this respond to strengthening exercises or does the patient need some support such as manually assisted cough or the cough assist machine?
• Peak cough flow. The peak expiratory flow rate during cough gives an overall evaluation of cough efficiency; values below 160–270 l/min suggest poor airway clearance. A value of less than 160 l/min is not sufficient to clear secretions independently. It can be measured by coughing into a peak flow meter.
• Is the patient’s airway at risk? (Loss of bulbar function; see 3.60 Swallow assessment.)
• Is the patient unable to cough in response to direction? Are there any neurological reasons for inhibited cough reflex or lack of sensitivity in the airways? Has the patient lost other protective reflexes such as the gag reflex, swallow reflex or the expiratory reflex? These reflexes are controlled by the medulla oblongata and are referred to collectively as ‘bulbar function’.
3.20 Cyanosis
Purpose
Central cyanosis may indicate significant respiratory or cardiac failure, which is potentially life-threatening, requiring immediate respiratory or circulatory support (see Findings below).
Procedure
Cyanosis can be observed directly through observation of the lips, tongue and fingers in good light.
Findings
• Cyanosis of recent onset should be immediately reported to the medical team.
• Cyanosis shows best on those with good levels of Hb. It is less visible in patients with anaemia.
• Cyanosis is usually only visible when 5 g Hb per 100 ml blood is deoxygenated. For most people in an acute situation, this would suggest a severe lack of oxygen and the need for immediate intervention (e.g. airway management, CPR, ventilation).
• People with severe chronic respiratory disease are more likely to show signs of cyanosis (at higher SpO2) because they may have more Hb (because of polycythaemia). Additionally, their oxygen content may actually be acceptable despite low SpO2; this is the result of increased Hb, which enables more oxygen to be carried, so it is important to find out whether this is ‘usual’ for them before responding.
• Note that cyanosis is more difficult to see in Afro-Caribbean and Asian patients because of darker skin tone.
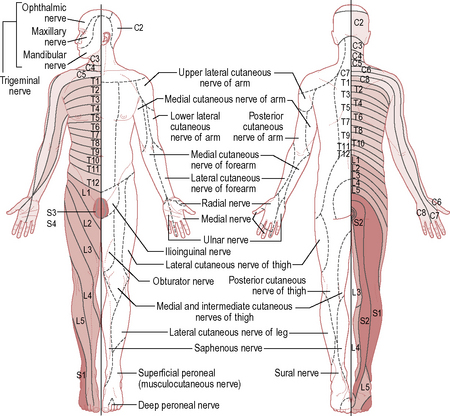
3.21 Dermatomes
Definition
A dermatome is an area of skin with sensory innervation by one main spinal nerve root (Fig. 3.21.1).
Purpose
Knowing which nerve supplies an area of skin can be used to ascertain the level of injury or deficit. It should be noted that there is some overlap between dermatomes. Dermatome assessment is often carried out alongside assessment of myotomes and muscle charting (see 3.42 Myotomes and 3.41 Muscle charting).
Dermatomes can also be used to assess the level of analgesia, e.g. with an epidural.
Procedure
• Make sure any clothing that may restrict the assessment is removed (remember to maintain dignity of the patient).
• Using your hand, or a cotton wool ball, touch an area of skin that is unaffected to make sure the patient can feel this sensation.
• Ask the patient to close his or her eyes.
• Working from the top of the body down, stroke over different dermatomes and ask the patient to report changes in sensation. Make sure to compare left and right as deficits may be unilateral!
3.22 Drugs
Findings
Tables 3.22.1–3.22.6 display some of the common classifications of medications patients may take. This is by no means comprehensive, and the reader is directed to other sources (such as a BNF (British National Formulary)) for more comprehensive information. Note that, if you come across a drug that is unfamiliar to you, you should ensure that you have an understanding of both what it is intended to treat and the implications for physiotherapy. The pharmacy team are an excellent resource if you cannot find what a drug is or why it is being used.
COPD, chronic obstructive pulmonary disease; GI, gastrointestinal.
Table 3.22.2 Common cardiac medications
| While these have been placed in broad categories, these agents can affect several aspects of cardiovascular physiology | ||
|---|---|---|
| Key drugs | Implication for physiotherapy | |
| Inotropes (increase myocardial contractility) | ||
| Anti-arrhythmia drugs (also known as anti-arrhythmics) | Be aware of an abnormal cardiac rhythm and seek help of senior staff if you are unsure whether the patient’s clinical picture has changed | |
| Beta (β)-blockers (reduce rate and strength of heart beat) | Patients may not be able to respond to increased workload; thus, you may not be able to use HR as a guide to exercise prescription. Consider using RPE as well | |
| Vasodilators (increase coronary blood flow and control angina) | GTN | |
| Calcium channel blockers (reduce myocardial contractility and BP) | Caution if mobilizing for the first time as BP may drop – be aware of resting BP; you may need to repeat BP measurement during your intervention | |
| Diuretics (increase urine production and can reduce BP) | Increase the need to go to the toilet and can induce muscle cramps | |
| Antihypertensive drugs | If a patient is on this medication, note that they have a history of increased blood pressure | |
BP, blood pressure; GTN, glyceryl trinitrate; HR, heart rate; RPE, rating of perceived exertion.
Table 3.22.3 Common antibiotics
| Key drugs | Implication for physiotherapy | |
|---|---|---|
| Penicillins |
Any patient who is on antibiotics will have an infection and is likely to feel unwell. Side-effects of many of these antibiotics include nausea, vomiting and diarrhoea. It is important to adhere to infection control principles Note: some antibiotics (e.g. in CF/bronchiectasis) may be nebulized and should be taken after airway clearance techniques |
|
| Cephalosporins | ||
| Tetracyclines | ||
| Aminoglycosides | Gentamicin |
Table 3.22.4 Analgesic medications
| The route of delivery is varied and can include inhaled, oral, intramuscular (i.m.) and intravenous (i.v.) routes and as a suppository. Care should be taken when mobilizing patients with epidurals in situ (in place) as they can become hypotensive (low BP) and may have altered motor control of their legs. | ||
|---|---|---|
| Key drugs | Implication for physiotherapy | |
| Paracetamol | Risk of liver toxicity, and is often contained in other preparations | |
| NSAIDs | Ibuprofen Voltarol |
Can cause irritation of the gut and cause GI bleeding. Some asthmatic patients are allergic to this group of drugs |
| Opiates | Morphine Diamorphine Codeine |
Can depress the respiratory drive if the dose is too high. Pinpoint pupils may indicate that the patient has had a large dose of opiates |
GI, gastrointestinal; NSAID, non-steroidal anti-inflammatory drug.
Table 3.22.5 Intensive therapy unit drugs
| Key drugs | Implication for physiotherapy | |
|---|---|---|
| Sedation agents | The patient may vary from being awake and settled to not being able to respond to you | |
| Paralysing agents | BE AWARE! The patient can make no muscular effort at all, so if removed from the ventilator the patient will stop breathing. As patients are paralysed, they will not be able to cough on suction |
Table 3.22.6 Other common drugs
| Key drugs | Implication for physiotherapy | |
|---|---|---|
| Anti coagulants (blood thinners) | There is an increased risk of bleeding, so care needs to be taken and some procedures considered with extreme caution, e.g. nasopharyngeal suction. Check the patient’s clotting screen! (see 3.6 Blood results) | |
| Insulins | If your patient is on insulin, it is good practice to ensure that blood sugars are stable prior to mobilizing. If the patient’s blood sugars are poorly controlled they may become unresponsive | |
| Immunosuppressant | Cyclosporin | As the immune system is suppressed, this leaves the patient more open to contracting infections |
| Steroids (oral/i.v.) | ||
| Anti-epileptics | Be aware that the patient may have a history of fitting |

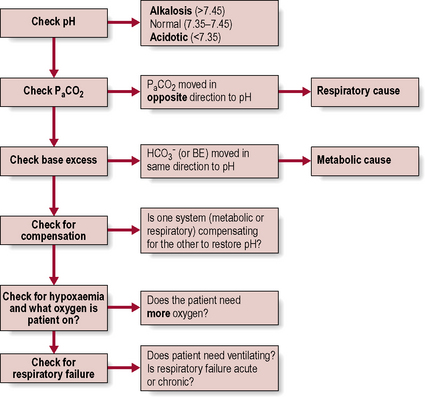
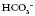 ) levels and base excess (BE). Blood gases can also be taken from a venous or capillary sample.
) levels and base excess (BE). Blood gases can also be taken from a venous or capillary sample.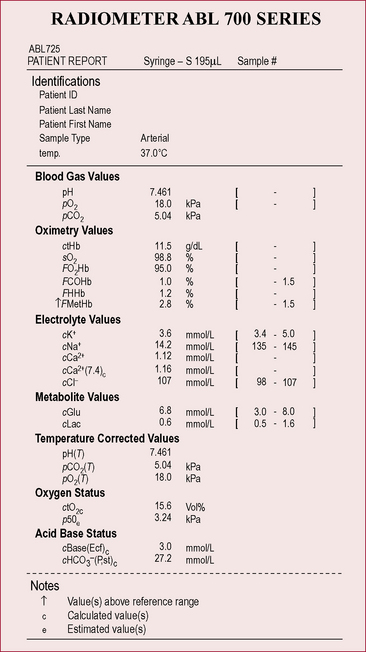
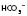 above or below the average value of 24 mmol l−1
above or below the average value of 24 mmol l−1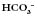 (or BE)
(or BE)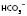 14 mmol l−1
14 mmol l−1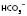 30 mmol l−1
30 mmol l−1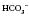 move in the same direction as if in an elevator (e.g. if the pH is high the
move in the same direction as if in an elevator (e.g. if the pH is high the 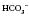 will also be high).
will also be high).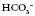 have moved in the opposite direction, this suggests that the metabolic system is compensating and the problem is respiratory.
have moved in the opposite direction, this suggests that the metabolic system is compensating and the problem is respiratory.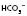 28
28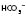 16
16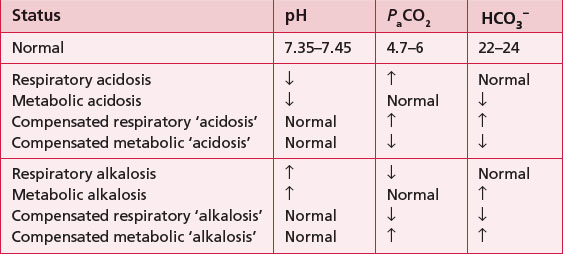
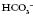 24
24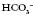 22
22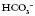 32
32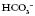 3.5
3.5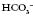 to increase and his pH is within the normal range, despite his high PaCO2 (remember he is an ex-miner; therefore, respiratory disease would not be uncommon).
to increase and his pH is within the normal range, despite his high PaCO2 (remember he is an ex-miner; therefore, respiratory disease would not be uncommon).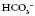 and therefore a fall in pH. There is some compensation because the PaCO2 has gone down, suggesting that he is breathing more in order to try and raise the pH. This is only partially successful because his pH is still below the normal range. Physiotherapy is not going to help his breathing at this point as he needs medical attention to stabilize his diabetes.
and therefore a fall in pH. There is some compensation because the PaCO2 has gone down, suggesting that he is breathing more in order to try and raise the pH. This is only partially successful because his pH is still below the normal range. Physiotherapy is not going to help his breathing at this point as he needs medical attention to stabilize his diabetes.