Assessment of Implantable Devices
Patients with implanted pacemakers or automatic implantable cardioverter-defibrillators (AICDs) are commonly seen in the emergency department (ED). Fortunately, the increased reliability of these devices has prevented a marked increase in patients with true emergencies related to device malfunction, but such patients clearly have serious underlying medical problems that must be considered. Pacemaker complications are not uncommon, with rates ranging from 2.7% to 5%.1 Many pacemakers fail within the first year.2 AICD complication rates, including inadvertent shocks, occur in up to 34% of patients with the device.3 The basic evaluation and treatment of patients with cardiac complaints are not substantially different in patients with pacemakers and AICDs than in those without. However, a general knowledge of the range of problems, complications, and techniques for evaluating or inactivating pacemakers or AICDs is important for emergency clinicians. These devices are complicated, so appropriate consultation may be necessary, depending on the clinical situation.
Pacemaker Characteristics
In essence, a pacemaker consists of an electrical pulse–generating device and a lead system that senses intrinsic cardiac signals and then delivers a pulse. The pulse generator is hermetically sealed with a lithium-based battery device that weighs about 30 g and has an anticipated lifetime of 7 to 12 years. A semiconductor chip serves as the device’s central processing unit. The generator is connected to sensing and pacing electrodes that are inserted into various locations in the heart, depending on the configuration of the pacemaker. Newer models are programmable for rate, output, sensitivity, refractory period, and modes of response.4 They can be reprogrammed radiotelemetrically after implantation.
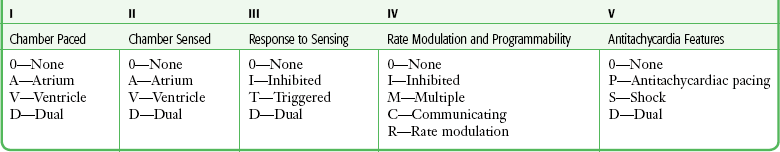
Pacemakers are classified according to a standard five-letter code developed by the North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group (Table 13-1). Known as the NBG code, it consists of five positions or digits. The first letter designates the chamber that receives the pacing current; the second, the sensing chamber; and the third, the pacemaker’s response to sensing. The fourth letter refers to the pacemaker’s rate modulation and programmability, and the fifth describes the pacemaker’s ability to provide an antitachycardia function. Whereas standard pacemakers generally do not have an antitachycardia function, AICDs do have this capability and overdrive pacing is the device’s first response to tachycardia. In normal practice, only the first three letters are used to describe the pacemaker (e.g., VVI or DDD).5
Pacemaker wires are embedded in plastic catheters. The terminal electrodes, which may be unipolar or bipolar, travel from the generator unit to the heart via the venous system. In a unipolar system, the lead electrode functions as the negatively charged cathode, and the pulse generator case acts as the positively charged anode into which electrons flow to complete the circuit. The pulse generator casing must remain in contact with tissue and be uninsulated for pacing to occur. In the case of bipolar systems, both electrodes are located within the heart. The cathode is at the tip of the lead, and the anode is a ring electrode roughly 2 cm proximal to the tip. Bipolar leads are thicker, draw more current than unipolar leads, and are commonly preferred because of several advantages, including a decreased likelihood of pacer inhibition as a result of extraneous signals and decreased susceptibility to interference by electromagnetic fields.6
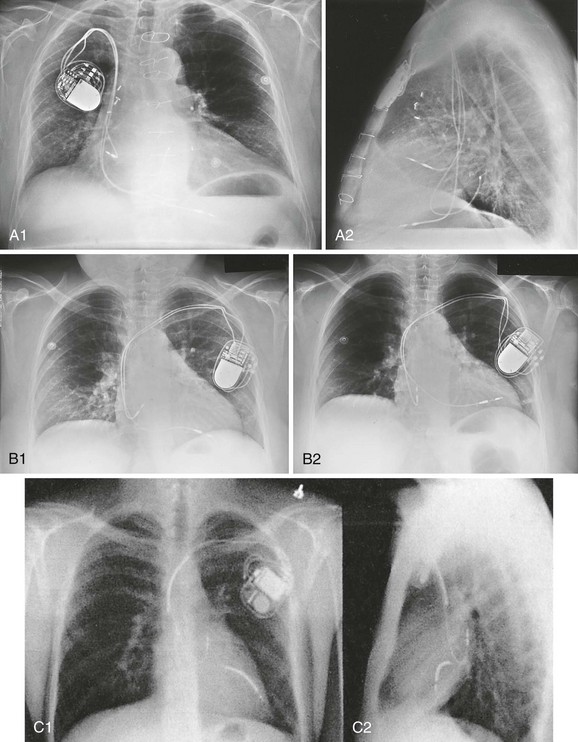
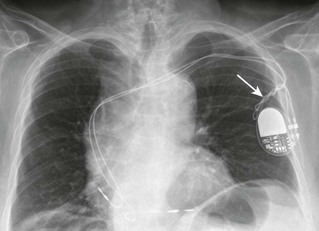
The typical entry point for inserting the leads is the central venous system, which is generally accessed via the subclavian or cephalic vein. The terminal electrodes are placed either in the right ventricle or in both the right ventricle and the atrium under fluoroscopic guidance. Proper lead placement and sensing and pacing thresholds are assessed with electrocardiograms (ECGs).7 The typical radiographic appearance of an implanted pacemaker is shown in Figure 13-1.
The pacemaker is typically programmed to pace at a rate of 60 to 80 beats/min. A significantly different rate usually indicates malfunction. When the battery is low, the rate generally begins to drop and gets slower as the battery fades. Sensing of intracardiac electrical activity is a combination of recognizing the characteristic waveforms of P waves or QRS complexes while discriminating them from T waves or external interfering signals, such as muscle activity or movement. The pacing electrical stimulus is a triphasic wave consisting of an intrinsic deflection, far-field potential, and an injury current, which typically delivers a current of 0.1 to 20.0 mA for 2 msec at 15 V.8
Several innovations in rate regulation have been incorporated into some pacemakers. When present, the hysteresis feature causes pacing to be triggered at a rate greater than the intrinsic heart rate. When the hysteresis feature is used in a single-chamber ventricular pacemaker, it is designed to maintain atrioventricular (AV) synchrony at rates that are lower than what would be normal for a ventricular-paced rhythm alone. To illustrate, were the hysteresis feature of the pacemaker set at 50 beats/min, an intrinsic rate lower than 50 beats/min would trigger ventricular pacing. Unlike a standard ventricular pacemaker, the hysteresis feature might be set to offer a ventricular pacing rate at 70 beats/min or greater once the pacer is triggered.9
Rate modulation by sensor-mediated methods is an additional feature triggered and mediated by a sensed response to various physiologic stimuli.9 The primary application for this rate modulation feature is in patients with pacemakers who continue to engage in vigorous physical activity. When present, the rate regulation feature is engaged and modulated through motion sensors installed within a pulse generator device, with a corresponding increase or decrease in the pacing rate depending on the degree of motion sensed by the pacemaker device. Other physiologic sensors that may be installed as part of the pacemaker system include those designed to sense minute ventilation, the QT interval, temperature, venous oxygen saturation, and right ventricular contractions. The latter sensors generally require that additional leads be placed.
Characteristics of AICDs
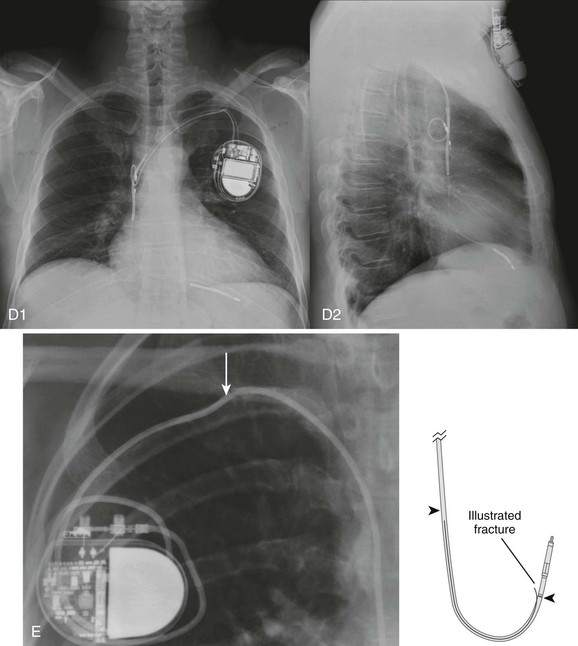
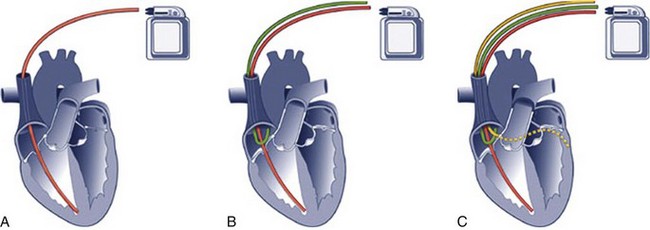
The basic components of an AICD, including sensing electrodes, defibrillation electrodes, and a pulse generator (Fig. 13-2), can be seen on a chest radiograph. Transvenous electrodes have obviated the previous need for surgical placement. They are inserted into the pectoralis muscle. Many transvenous systems consist of a single lead containing a distal sensing electrode and one or more defibrillation electrodes in the right atrium and ventricle.10 Leads are inserted through the subclavian, axillary, or cephalic vein into the right ventricular apex. The left side is preferred because of a smoother venous route to the heart and a more favorable shocking vector.11 In an effort to improve the efficiency of defibrillation, an additional defibrillation coil may be used.11 Various placements of AICDs are demonstrated in Figure 13-3.
The pulse generator is a sealed titanium casing that encloses a lithium–silver–vanadium oxide battery. It has voltage converters and resistors, capacitors to store charge, microprocessors and integrated circuits to control analysis of the rhythm and delivery of therapy, memory chips to store electrographic data, and a telemetry module.12 Whereas a pacemaker can draw the voltage required for function from its component battery, the energy needed for defibrillation requires a battery that is prohibitively large.6 To circumvent this problem, an AICD contains a capacitor that maximizes the voltage required by transferring energy from the battery before discharge. To achieve the energy required, AICDs use capacitors that are charged over a period of 3 to 10 seconds by the battery and then release this energy rapidly for defibrillation.10 The maximal output is 30 J in most units and 45 J in higher-energy units.6 This energy is high enough that a discharge is very obvious and often distressing to the patient.
Most AICDs use a system in which the pulse generator is part of the shocking circuit, often described as a “can” technology, and most of them have a dual-coil lead with a proximal coil in the superior vena cava and a distal coil in the right ventricle.13 Current flows in a three-dimensional configuration from the distal coil to both the proximal coil and the generator.14 This dispersion of the electrical field increases the likelihood of depolarizing the entire myocardium at once, thereby leading to successful defibrillation.14
AICDs may have the same programming capabilities as pacemakers and can be single chambered, dual chambered, or used with cardiac resynchronization therapy (CRT) .15 Single-chamber devices have only a right ventricular lead. They often have difficulty identifying atrial arrhythmias, which can result in inappropriate defibrillation of atrial tachycardias. Dual-chamber AICDs have right atrial and right ventricular leads and improved ability to discriminate rhythms. In most studies, dual devices have been found to offer improved discrimination between ventricular and supraventricular arrhythmias, thus decreasing inappropriate shocks as a result of rapid supraventricular rhythms or physiologic sinus tachycardia.16 Approximately 50% of AICDs implanted in the United States are dual-chamber devices.17 CRT devices add an additional left ventricular lead that is placed in the coronary sinus or epicardium. In patients requiring both AICD and pacemaker functions, both these devices are placed together. The advent of technology has allowed placement of a single device that can perform both pacemaker and defibrillator functions.
AICDs use a combination of antitachycardia pacing, low-energy cardioversion, defibrillation, and bradycardiac pacing in a combination also known as tiered therapy. They are programmed with specific algorithms that identify and treat specific rhythms. Ventricular arrhythmias may initially be converted (or undergo attempts at conversion) with antitachycardiac pacing as opposed to immediate defibrillation. This overdrive pacing may terminate the rhythm without the need for electrical defibrillation in up to 90% of events. It is most successful for terminating monomorphic ventricular tachycardia with a rate of less than 200 beats/min.1 Overdrive pacing is better tolerated by patients than cardioversion and reduces the risk for inducing atrial fibrillation.18 These events may be silent, not felt by the patient, and discovered only by interrogating the device.
If unsuccessful, the next intervention may be low-energy cardioversion (<5 J). The device may be programmed to very low levels of electricity that, again, are better tolerated by the patient. This works best for ventricular rates higher than 150 and lower than 240 beats/min.14 This may be followed by a high-energy defibrillation. Traditionally, the energy level of the first shock is set at least 10 J above the threshold of the last defibrillation measured.12 If the first shock fails, a backup shock may be required, but this may induce or aggravate ventricular arrhythmias (see the later section “Pacemaker-Mediated Tachycardia”). Unlike the proarrhythmic effects of medication, these arrhythmias are almost never fatal, although they may be associated with increased morbidity.11 Currently used biphasic waveforms have improved defibrillation thresholds.12
This tiered approach obviates the need for unnecessary energy requirements. The devices also have antibradycardiac pacing that allows these patients to have one device instead of separate units. Additional complications associated with AICDs that have antibradycardiac pacing algorithms include a tendency toward oversensing, increased current drain, potential detection problems, and an increased incidence of hardware and software design problems.1 At the time of insertion the amount of energy required for various AICD functions, such as the defibrillation threshold, is determined for any given patient, and output and sensing functions can be adjusted by reprogramming as needed.
Indications for Placement of Implantable Pacemakers and Aicds
The most common indication for placement of a cardiac pacemaker is for the treatment of symptomatic bradyarrhythmias.19 Roughly 50% of pacemakers are placed in such patients for the treatment of sinus node dysfunction (sick sinus syndrome). Other diagnoses include symptomatic sinus bradycardia, atrial fibrillation with a slow ventricular response, high-grade AV block (including Mobitz type II and third-degree AV block), tachycardia-bradycardia syndrome, chronotropic incompetence, and selected prolonged QT syndromes. Though not classified as absolute indications, pacemakers are sometimes placed for the treatment of severe refractory neurocardiogenic syncope, paroxysmal atrial fibrillation, and hypertrophic or dilated cardiomyopathy.
In recent years, CRT has emerged as a primary approach for patients with severe diastolic dysfunction and a low left ventricular ejection fraction (LVEF).19 Commonly, such patients manifest low-grade AV blocks and left bundle branch block.20 The resultant delay in left ventricular conduction often results in corresponding biomechanical delays in ventricular contraction, which in turn causes a further decrement in cardiac output and worsening congestive heart failure. Such prolongation may occur in as many as 33% of patients with advanced heart failure.20 This electromechanical “dyssynchrony” has been associated with increased risk for sudden cardiac death.21
CRT comprises atrial-synchronized, biventricular pacemaking, which overcomes the atrial and ventricular blocks while optimizing both preload and LVEF.22 Clinical trials and systematic reviews have confirmed the efficacy of CRT, with decrements in mortality of 22% to 30%, as well as improved LVEF and quality of life.23,24 It is therefore likely that emergency physicians will see the CRT configuration with increasing frequency in patients with implanted pacemakers and AICDs.
The 2008 American Heart Association guidelines for implantation of a cardiac pacemaker are summarized in Box 13-1.19 AICD technology is used principally for both primary and secondary prevention in patients at risk for sudden death. Primary prevention is an attempt to avoid a potentially malignant ventricular arrhythmia in patients identified as being at high risk.25 Secondary prevention is for patients who have already had a ventricular arrhythmia and are at risk for further events. In addition, AICDs are implanted for a number of other congenital or familial cardiac conditions. Box 13-2 is a summary of class I indications for the placement of AICDs.26
Pacemaker and AICD Response to Magnet Placement
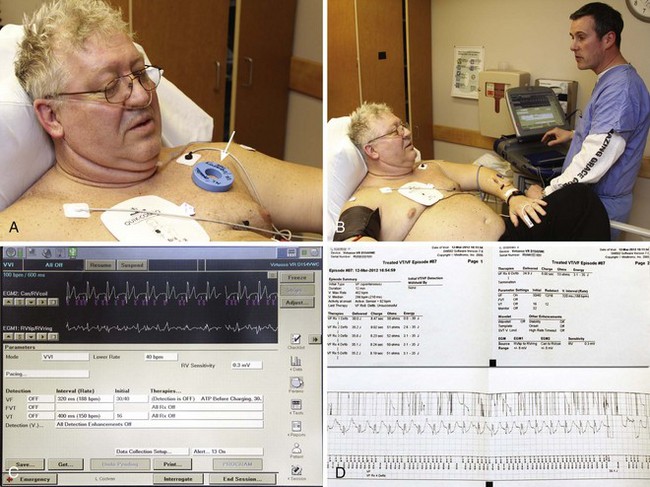
Placing a magnet over any of the currently available AICD models will temporarily disable tachyarrhythmia intervention (Fig. 13-4). An ECG should be obtained before and after magnet placement for comparison (Fig. 13-5). Most commercially available pacer magnets are 7 cm in size and can be used with most implantable devices. Each of the present models may have a slightly different response to the magnet. The magnetic field closes a reed switch in the generator circuit that will disable recognition of tachyarrhythmias and subsequent firing of the device. There may be a variety of tones (continuous, intermittent, or silence) during activation or inactivation with the magnet, which are dependent on the manufacturer. Some devices may be programmed to not respond at all.27 After the desired effect is obtained, the magnet should be secured to maintain inactivation.
Of further note, in some obese patients or those with heavily developed chest wall musculature, the magnetic field emitted by a single magnet device may not be strong enough to elicit the desired effect on the implanted device. In such cases the clinician may find greater efficacy by using two magnets, one on top of the other.28
Clinical Evaluation of Patients with Implanted Pacemakers and Aicds
Radiography
The imaging modality of choice, at least initially, in a patient with complaints related to an implanted pacemaker or AICD is the plain chest radiograph. If the patient’s stability is in question, obtain a portable anteroposterior film. In addition to the standard cardiac, pulmonary, vascular, and skeletal evaluation, this study will probably confirm the location of the pulse generator case, as well as the current location of any leads. Survey the radiograph for evidence of lead fracture or displacement (see Fig. 13-1E). Compare with previous chest films. If the patient does not have a device identification card in immediate possession, take an overpenetrated radiograph to look for a radiopaque marker identifying the model type.
Electrocardiography
Perform an ECG to seek evidence of pacing, ectopy, and ST-segment or T-wave abnormalities. A comparison ECG may be helpful. If an AICD shock has been delivered, the shock itself can cause transient electrocardiographic changes, and waiting for several minutes to repeat the ECG may identify whether the changes are due to the discharge or an ongoing disease process.29
Cardiopulmonary Resuscitation, ACLS Interventions, and External Cardiac Defibrillation in Patients with Implanted Pacemakers or AICDs
External cardiac defibrillation may be performed safely in patients with pacemakers and AICDs with the standard expected efficacy; however, it is recommended that external paddles or defibrillator pads be placed at a location approximately 10 cm distant from the pulse generator if possible.30 A transcutaneous cardiac pacemaker may also be used in similar fashion, again with a recommendation that the pacing pads be placed in anatomically appropriate locations but preferably at a distance of 10 cm from the pulse generator.
Placing the external defibrillation or transcutaneous pacemaker pads in an anteroposterior configuration is advised because this configuration may circumvent energy shunting and shielding.11 Every attempt should be made to avoid application of the defibrillators directly over the device. Use of the lowest possible energy setting for cardioversion or defibrillation is recommended. If available, biphasic cardioverter-defibrillators are further suggested.30 In the event of successful resuscitation and return of spontaneous circulation, the pacemaker or AICD should be interrogated expeditiously by a cardiologist or electrophysiologist to ensure that no damage was sustained as a result of the resuscitation effort.
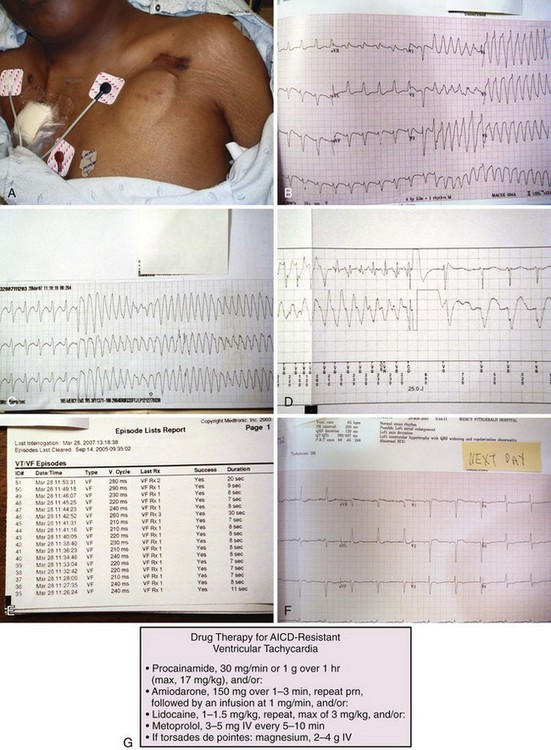
Regarding pharmacologic adjuncts, amiodarone has been reported to be more effective for the treatment of potentially lethal arrhythmias in the setting of implanted devices.31 Antiarrhythmic medications may be required for a resistant malignant rhythm when the AICD is functioning properly but the arrhythmia persists (Fig. 13-6G).
Complications and Malfunction of Implanted Pacemakers
Complications associated with pacemakers are listed in Box 13-3. In addition, patients with previously implanted and otherwise stable pacemakers may experience complications related to direct or indirect trauma affecting the pulse generator or leads. Major complications of pacemaker placement or those caused by subsequent injuries that the emergency clinician might encounter include local or systemic infections resulting from pacemaker placement, thrombophlebitis involving the transvenous route of the pacemaker leads, a venous thromboembolic event, pneumothorax or hemothorax, pericarditis, air embolism, localized hematoma interfering with pacemaker operation or sensing, lead dislodgment,32 cardiac perforation, hemopericardium with possible progression to cardiac tamponade, and development of the phenomenon known as pacemaker syndrome. This condition is often seen in patients with single-chamber ventricular pacemakers who have an underlying component of congestive heart failure. It is believed to be a consequence of the loss of AV synchrony resulting from the ventricular pacing and may be manifested as vertigo, syncope, hypotension, and signs specific to the exacerbation of congestive heart failure.
Pacemaker Output Failure
Pacemaker generator output failure is present when no pacing “spike” is noted on the ECG despite an indication for pacing. This condition may result from battery failure, fracture or loss of insulation in the pacer leads, oversensing of extraneous signals resulting in pacer inhibition, disconnection of the leads from the pacer generator, or in the case of a dual-chamber pacer, erroneous sensing of the pacemaker’s atrial signal by a ventricular sensor.33 The latter phenomenon is commonly referred to as crosstalk.34
Failure to Capture
In the case of failure to capture, pacemaker spikes are present on the ECG. However, some or all of the spikes are not followed by atrial or ventricular complexes, as appropriate for the pacemaker model in question. Failure to capture may result from deterioration of lead insulation; fracture or dislodgment of the leads; electrolyte disturbances, including hyperkalemia or hypocalcemia; a new condition requiring an elevated pacing threshold; acid-base disturbance; direct damage to the myocardium, which is in contact with the pacer lead’s tip (such as myocardial infarction or direct trauma); or dysfunction of the microcircuitry of the pulse generator. In addition, flecainide, a class IC antiarrhythmic medication, has been identified as an acute cause of the rise in ventricular capture thresholds in patients with implanted pacemakers.32 Likewise, all class I antiarrhythmic agents (sodium channel antagonists) may affect pacer capture thresholds and should therefore be identified as potential etiologic agents in patients suffering failure to capture.
Failure to Sense
Failure of an implanted cardiac pacemaker to sense the patient’s intrinsic cardiac rhythm may be subdivided into conditions related to oversensing or undersensing. Oversensing is present when the pacemaker erroneously identifies extrinsic electrical signals, such as those from skeletal muscle potentials or electromagnetic interference (EMI), and is inhibited from delivering an appropriate pacemaker pulse. For additional information on extrinsic EMI, refer to “Electromagnetic Interference and Implantable Devices” later in this chapter.
Pacemaker-Mediated Tachycardia
A third instance of pacemaker-mediated tachycardia occurs in patients with AICD units that have backup antibradycardia pacing capability. It appears that if this pacemaker feature is switched on in such patients and an ectopic ventricular stimulus is delivered after a sudden pause in the intrinsic ventricular depolarization cycle, a ventricular tachyarrhythmia may be triggered.2,35
Diagnosis of Acute Myocardial Infarction in the Presence of a Paced Cardiac Rhythm
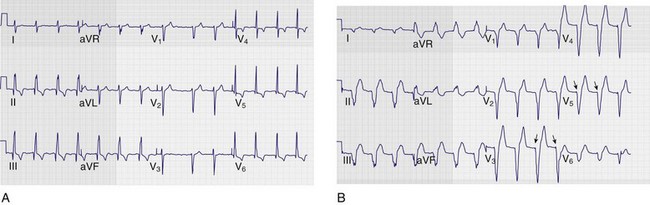
Patients undergoing active ventricular pacing from an implanted pacemaker device will normally have ECGs that resemble a left bundle branch block pattern. As a result, electrocardiographic diagnosis of acute ischemic changes is equally challenging in both populations. Sgarbossa and coworkers36 published a series of criteria in 1996 that offer some utility in the interpretation of ECGs in patients with active ventricular pacing in whom acute coronary syndromes are suspected. These criteria are depicted in Table 13-2.7
Automatic Implantable Cardioverter-Defibrillators—Unique Malfunctions
Issues with sensing problems, lead migration, and battery failure are similar to pacemaker complications, and most occur within 3 months after implantation.37 A potential malfunction unique to AICDs is inappropriate or lack of defibrillation of the device. An analysis of 23,000 Medicare patients with AICDs revealed a complication rate of 11%, with lead dislodgement being the most common problem followed by hematoma or hemorrhage, infection, and pneumothorax.38
The AICD may not terminate ventricular arrhythmias, which may or may not be the result of malfunction of the device. AICD malfunction may be a result of battery depletion, component failure, failure-to-sense, or lead malfunction. Failure to cardiovert or defibrillate occurring in the setting of a functioning AICD system may be caused by inappropriate cutoff rates, failure to satisfy multiple detection criteria, completed and exhaustion of therapies, and cross-inhibition by a separate pacemaker.11 The advent of AV or dual-chamber AICD devices has improved the sensitivity of detecting arrhythmias and thus preventing the delivery of inappropriate shocks.
Inappropriate AICD-delivered shocks occur in 20% to 25% of patients38 and are the most common adverse events observed in AICD patients.32 The main causes are atrial arrhythmias, sinus tachycardia, nonsustained ventricular tachycardia, lead fracture or EMI, or electrical storm.38 By definition, this phenomenon occurs when three or more shocks are delivered in a 24-hour period, occurs in 10% to 20% of AICD patients,39 and constitutes a medical emergency.40 AICD patients who experience this phenomenon may have end-stage cardiac failure and increased long-term mortality.41 Specific causes of electrical storm are unclear, but it is associated with ventricular tachycardia in the setting of LVEF lower than 30% and occurs more frequently in patients with demonstrated coronary artery disease who have not as yet undergone revascularization procedures. Suggested initial treatment includes the administration of amiodarone and β2-adrenergic antagonists to pharmacologically suppress the arrhythmias and urgent cardiology consultation for possible pacemaker interrogation, overdrive pacing, or even catheter ablation.41–43
The AICD may discharge inappropriately in response to rapid supraventricular rhythms such as atrial fibrillation, supraventricular tachycardia, or even sinus tachycardia. Multiple shocks may be a manifestation of inefficient termination of tachycardia, such as inappropriately low-energy delivery at the first shock, increased defibrillation thresholds, and migration or dislodgment of the defibrillation lead system or failure of the defibrillator system. Shocks that occur every few minutes may suggest that recurring ventricular tachyarrhythmias are being terminated appropriately (see Fig. 13-6).
AICD discharges in the setting of chest pain may be a result of myocardial ischemia–induced tachyarrhythmias.28 As noted earlier, any electrocardiographic abnormalities noted immediately after shocks should be interpreted with caution because ST elevation or depression can occur immediately after a shock.29 If the patient receives shocks in association with chest pain, ischemia is suggested, but other causes, including hypokalemia, hypomagnesemia, drug-induced proarrhythmia, or drugs that can prolong the QT interval (such as phenothiazines), should also be considered as underlying causes.11
In some settings, the AICD may fail to sense sustained ventricular tachycardia or fibrillation. Such failure may be caused by an intrinsic arrhythmia rate below the programmed detection rate, usually as a result of concurrent pharmacologic therapy. If the patient is hemodynamically stable, it may be advantageous for the cardiologist to interrogate the pacer before initiating further antiarrhythmic therapy. If unsuccessful or if the patient is experiencing a nonperfusing ventricular arrhythmia, other pharmacologic interventions include procainamide26,44 or amiodarone. Box 13-4 depicts the outcome of AICD placement in a general population.
Use of a Magnet for AICD
Technique: The method for inactivating an AICD device is outlined in Box 13-5. The orientation of the device in the abdominal pocket should be determined, with the lead connections normally being cephalad. A ring magnet is then placed over the corner adjacent to the lead connections (usually the upper right-hand corner of the device). A series of beeping tones will sound that correspond to the sensed QRS complexes. In the absence of organized QRS activity, random beeps will sound.45 When the magnet is left in place for 30 seconds, a continuous beep is heard. This indicates that the AICD is inactivated. The magnet should then be removed, and the AICD will remain inactivated. The AICD may be reactivated by applying the magnet for 30 seconds and removing it when the steady beep changes to intermittent beeping. Note that unlike a pacemaker, in which a magnet will turn a demand pacemaker to a fixed-rate pacemaker, a magnet will not affect the pacing function of an AICD.
“Twiddler’s Syndrome”
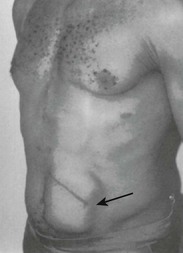
In some cases, patients with implantable pacemakers choose to “twiddle” with the device: they manipulate the pulse generator case within its physiologic pocket under the skin in the chest. Note that a generator may also be placed in the abdominal wall (Fig. 13-7). This practice of “twiddling” may result in coiling, dislodgment, or disconnection of the pacemaker leads (Fig 13-8). It may even lead to actual displacement of the pulse generator case. At a minimum it may result in physical discomfort and may, in fact, precipitate cardiac arrhythmias or other complications local to the site of pacemaker placement. After initial stabilization of the complaint, these patients may require readjustment or replacement of their pacemaker devices. As part of their care, pacemaker patients should be educated to avoid manipulating their pacemakers.46
Mental Health Issues Related to Implanted Pacemakers and AICDs
Patients with these devices may manifest a number of anxiety-related complications, including adjustment disorder, panic attacks, depression, imaginary shock, and defibrillator dependence, abuse, or withdrawal.47,48 These patients may benefit from psychiatric referral either as an outpatient or as part of the admission evaluation if applicable. The conditions may be severe enough that the device is removed by patient request.
Implantable Pacemaker and AICD Recalls
Since 1990 there have been approximately 29 Food and Drug Administration safety alerts and recalls affecting nearly 337,000 AICDs.49 These advisories were issued as a result of unanticipated failure of devices identified after release of the product and widespread clinical use.49 The decision to remove the devices is complex, and there has been difficulty reaching consensus on the optimal management of patients with these recalled devices. The decision to replace them should be multifactorial and take into consideration the estimated malfunction rate of the device, anticipated consequences of failure of the device, the individual center’s procedural risk for complications resulting from generator replacement, and patient preferences and desired level of risk tolerance.50
Electromagnetic Interference and Implantable Devices
The most common response of implanted devices to EMI is inappropriate inhibition or triggering of pacemaker stimuli, reversion to asynchronous pacing, and spurious detection of tachyarrhythmias by the AICD. Reprogramming of operating parameters and permanent damage to the circuitry of the device or the electrode-tissue interface can also occur but are much less frequent.30 Additional adverse effects that may occur include inhibition of bradycardia pacing, inadvertent delivery of a shock, or antitachycardia pacing.
The use of hermetic shielding in metal cases, filtering, interference rejection circuits, and bipolar sensing have helped mitigate most of this interference.50 Nonetheless, the clinician should be familiar with common sources of EMI that may affect pacemakers and AICDs.
Several caveats will help avoid the deleterious effects of EMI on implantable devices. Cell phones should not be kept in a pocket over the device. When in use, they should be held at least 6 inches away from the device. In the case of hands-free headphones, when used these devices should be placed in the ear opposite the implanted pacemaker or AICD. In addition, patients should be advised to not linger in theft detection areas or airport metal detectors. Box 13-6 identifies different devices and the corresponding potential EMI that can occur with pacemakers and AICDs.51,52
Out-of-Hospital Discharge of AICD
Patients are told to adhere to standard advice defining an appropriate response to out-of-hospital discharges of the AICD (Box 13-7). Many, however, come to the ED for evaluation after every shock. There is no standard ED intervention mandated by historical information, and clinical decisions are made on an individual basis corresponding to the current scenario. Options include prolonged ED observation, consultation, cardiac monitoring, laboratory testing (such as electrolytes and cardiac enzymes), or interrogation.
Disposition Criteria
With regard to AICD malfunctions and disposition, patients who have had a single shock and no other specific complaints or comorbidity can be discharged with follow-up in 24 to 48 hours. Patients with symptoms concerning for ischemia, potentially lethal arrhythmias, or symptomatic illness should be admitted and specialty consultation obtained expeditiously. Patients who have had multiple shocks will need admission for observation and interrogation of their AICD device. Interrogation reveals significant information about the device, such as why it fired, the rhythm history, and an accurate assessment of the underlying problem (Fig. 13-9).
References
1. Trohman, RG, Kim Michael, H, Pinski, SL. Cardiac pacing: the state of the art. Lancet. 2004;364:1701.
2. Vukmir, RB. Emergency cardiac pacing. Am J Emerg Med. 1993;11:166.
3. Munter, DW, DeLacey, WA. Automatic implantable cardioverter-defibrillators. Emerg Med Clin North Am. 1994;12:579.
4. Ludmer, PL, Goldschlager, N. Cardiac pacing in the 1980s. N Engl J Med. 1984;311:1671.
5. Coppola, M, Yealy, DM. Transvenous pacemakers. Emerg Med Clin North Am. 1994;12:633.
6. Stone, KR, McPherson, CA. Assessment and management of patients with pacemakers and implantable cardioverter defibrillators. Crit Care Med. 2004;32:155.
7. Munter, DW. Assessment of implanted pacemaker/AICD devices. In: Roberts JR, Hedges JR, Chanmugan AS, et al, eds. Clinical Procedures in Emergency Medicine. 4th ed. Philadelphia: Saunders; 2004:258.
8. Furman, S, Hurzeler, P, DeCaprio, V. Appraisal and reappraisal of cardiac therapy. Am Heart J. 1977;93:794.
9. Bunch, TJ, Hayes, DL, Friedman, PA. Clinically-relevant basics of pacing and defibrillation. In Hayes DL, Friedman PA, eds.: Cardiac Pacing, Defibrillation and Resynchronization: A Clinical Approach, 2nd ed, West Sussex, UK: Wiley-Blackwell, 2008.
10. Clemo, HF, Ellenbogen, KA, Belz, MK, et al. Safety of pacemaker implantation in patients with transvenous (nonthoracotomy) implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 1994;17:2285.
11. Pinski, SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med. 2000;28(10 Suppl):N174.
12. DiMarco, JP. Implantable cardioverter-defibrillators. N Engl J Med. 2003;349:1836.
13. Wilbur, SL, Marchlinski, FE. Implantable cardioverter-defibrillator follow-up: what everyone needs to know. Cardiol Rev. 1999;7:176.
14. Moses, HW, Mullin, JC. Implantable cardioverter defibrillator and antitachycardia pacing. In: Moses HW, Mullins JC, eds. A Practical Guide to Cardiac Pacing. Philadelphia: Lippincott; 2007:112.
15. Kadish, A, Mehra, M. Heart failure devices: implantable cardioverter-defibrillators and biventricular pacing therapy. Circulation. 2005;111:3327.
16. Goldberger, Z, Lampert, A. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA. 2006;295:809.
17. Goldberger, A, Elbel, B, McPherson, CA, et al. Cost advantage of dual chamber versus single chamber cardioverter-defibrillator implantation. J Am Coll Cardiol. 2005;46:850.
18. Estes, NAM, Haugh, CJ, Wang, PJ, et al. Antitachycardia pacing and low energy cardioversion: a clinical perspective. Am Heart J. 1994;127:1038.
19. Epstein, AE, DiMarco, JP, Eleenbogen, KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5(6):e1–e62.
20. Doval, HC, Nul, DR, Grancelli, HO, et al. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344:493–498.
21. Naccarelli, GV, Luck, JC, Wolbrette, DL, et al. Pacing therapy for congestive heart failure: is it ready for prime time? Curr Opin Cardiol. 1999;14:1–3.
22. Bristow, MR, Saxon, LA, Boehmer, J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150.
23. Bradley, DJ, Bradley, EA, Baughman, KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730–740.
24. McAlister, FA, Ezekowitz, J, Hooten, N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction. JAMA. 2007;297:2502–2514.
25. Kusumoto, F, Goldschlager, N. Implantable cardiac arrhythmia devices—part II: implantable cardioverter defibrillators and implantable loop recorders. Clin Cardiol. 2006;29:189.
26. Zipes, DP, Camm, AJ, Borggrefe, M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for practice guidelines. J Am Coll Cardiol. 2006;48:1064.
27. McPherson, CA, Manthous, C. Permanent pacemakers and implantable defibrillators: considerations for intensivists. Am J Respir Crit Care Med. 2004;170:933.
28. Pinski, SL, Trohman, RG. Implantable cardioverter-defibrillators: implications for the nonelectrophysiologist. Ann Intern Med. 1995;122:770.
29. Eysmann, SB, Marchlinski, FE, Buxton, AE, et al. Electrocardiographic changes after cardioversion of ventricular arrhythmias. Circulation. 1986;73:73.
30. Pinski, SL, Trohman, RG. Interference in implanted cardiac devices, part II. Pacing Clin Electrophysiol. 2002;25:1496.
31. Pinter, A, Dorian, P. Approach to antiarrhythmic therapy in patients with ICDs and frequent activations. Curr Cardiol Rep. 2005;7:376.
32. Rosenqvis, M, Beyer, T, Block, M, et al. Adverse events with transvenous implantable cardioverter-defibrillators: a prospective multicenter study. Circulation. 1998;98:663.
33. Hayes, DL, Vlietstra, RE. Pacemaker malfunction. Ann Intern Med. 1993;119:828.
34. Pinski, SL, Trohman, RG. Interference in implanted cardiac devices, part I. Pacing Clin Electrophysiol. 2002;25:1367.
35. Himmrich, E, Przibille, O, Zellerhoff, A, et al. Proarrhythmic effect of pacemaker stimulation in patients with implanted cardioverter-defibrillators. Circulation. 2003;108:192.
36. Sgarbossa, E, Piniski, SL, Gates, KB, et al. Early electrocardiographic diagnosis of acute myocardial infarction in the presence of ventricular paced rhythm. Am J Cardiol. 1996;77:423–424.
37. Becker, R, Ruf-Rickter, J, Senges-Becker, JC, et al. Patient alert in implantable cardioverter defibrillators: toy or tool? J Am Coll Cardiol. 2004;44:95.
38. Reynolds, MR, Cohen, DJ, Kugelmass, AD, et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2493.
39. Bailey, SM, Wilkoff, BL. Complications of pacemakers and defibrillators in the elderly. Am J Geriatr Cardiol. 2006;15:102.
40. Emkanjoo, Z, Alihasani, N, Alizadeh, A, et al. Electrical storm in patients with implantable cardioverter-defibrillators: can it be forecast? Tex Heart Inst J. 2009;36:563–567.
41. Srivasta, UN, Ebrahimi, R, El-Bialy, A, et al. Electrical storm: case series and review of management. J Cardiovasc Pharmacol Ther. 2003;8:237.
42. Sesslberg, HW, Mosss, AJ, McNitt, S, et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm. 2007;4:1395–1402.
43. Carbucicchio, C, Santamaria, M, Trevisi, N, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short and long term outcomes in a prospective single-center study. Circulation. 2008;117:462.
44. Gorgels, APM, van den Dool, A, Hofs, A, et al. Comparison of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Am J Cardiol. 1996;78:43.
45. Chapman, PD, Veseth-Rogers, JL, Duquette, SE. The implantable defibrillator and the emergency physician. Ann Emerg Med. 1989;18:579.
46. Nicholson, WJ, Tuohy, KA, Tilkemeier, P. Twiddler’s syndrome. N Engl J Med. 2003;348:1726–1727.
47. Vlay, SC, Olson, LC, Fricchione, GL, et al. Anxiety and anger in patients with ventricular tachyarrhythmias. Responses after automatic internal cardioverter defibrillator implantation. Pacing Clin Electrophysiol. 1993;16:186.
48. Fricchione, GL, Olson, LC, Vlay, SC. Psychiatric syndromes in patients with the automatic implantable cardioverter defibrillator: anxiety, psychological dependence, abuse and withdrawal. Am Heart J. 1989;117:1411.
49. Maisel, WH. Safety issues involving medical devices: implications of recent implantable cardioverter-defibrillator malfunctions. JAMA. 2005;294:955.
50. Amin, MS, Matchr, DB, Wood, MA, et al. Management of recalled pacemaker and implantable cardioverter-defibrillators: a decision analysis model. JAMA. 2006;296:412.
51. Pinski, SL, Trohman, RG. Interference with cardiac pacing. Cardiology Clin North Am. 2000;18:219.
52. Spencer, WH, Block, PC. What interferes with pacemakers and AICD’s? Cardiosource. Available at www.cardiosource.com.