Chapter 1 Assessment of Cardiac Risk
The impetus for the development of a risk-adjusted scoring system was the need to compare adult cardiac surgery results in different institutions and to benchmark the observed complication rates.1 The first risk-scoring scheme for cardiac surgery was introduced by Paiement and colleagues at the Montreal Heart Institute in 1983.2 Since then, multiple preoperative cardiac surgery risk indices have been developed. The patient characteristics that affected the probability of specific adverse outcomes were identified and weighted, and the resultant risk indices have been used to adjust for case-mix differences among surgeons and centers where performance profiles have been compiled. In addition to comparisons among centers, the preoperative cardiac risk indices have been used to counsel patients and their families in resource planning, in high-risk group identification for special care or research, to determine cost-effectiveness, to determine effectiveness of interventions to improve provider practice, and to assess costs related to severity of disease.3
CARDIAC RISK ASSESSMENT AND CARDIAC RISK STRATIFICATION MODELS
Consistency among Risk Indices
Many different variables have been found to be associated with the increased risk during cardiac surgery, but only a few variables have consistently been found to be major risk factors across multiple and very diverse study settings. Age, female gender, left ventricular function, body habitus, reoperation, type of surgery, and urgency of surgery were some variables consistently present in most of the models (Box 1-1).
Predictors of Postoperative Morbidity and Mortality
A risk-scoring scheme for cardiac surgery (coronary artery bypass graft [CABG] and valve) was introduced by Paiement and colleagues at the Montreal Heart Institute in 1983.2 Eight risk factors were identified: (1) poor LV function, (2) congestive heart failure, (3) unstable angina or recent (within 6 weeks) myocardial infarction, (4) age older than 65 years, (5) severe obesity (body mass index > 30 kg/m2), (6) reoperation, (7) emergency surgery, and (8) other significant or uncontrolled systemic disturbances. Three classifications were identified: patients with none of these factors (normal), those presenting with one risk factor (increased risk), and those with more than one factor (high risk). In a study of 500 consecutive cardiac surgical patients, it was found that operative mortality increased with increasing risk (confirming their scoring system).
One of the most commonly used scoring systems for CABG was developed by Parsonnet and colleagues4 (Table 1-1). Fourteen risk factors were identified for in-hospital or 30-day mortality after univariate regression analysis of 3500 consecutive operations. An additive model was constructed and prospectively evaluated in 1332 cardiac procedures. Five categories of risk were identified with increasing mortality rates, complication rates, and length of stay. The Parsonnet Index frequently is used as a benchmark for comparison between institutions.
Table 1-1 Components of the Additive Model
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From Parsonnet V, Dean D, Bernstein A: A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation 79:I3, 1989.
Higgins and associates5 developed a Clinical Severity Score for CABG at the Cleveland Clinic. Independent predictors of in-hospital and 30-day mortality wereemergency procedure, preoperative serum creatinine level of greater than 168 μmol/L, severe left ventricular dysfunction, preoperative hematocrit of less than 34%, increasing age, chronic pulmonary disease, prior vascular surgery, reoperation, and mitral valve insufficiency. Predictors of morbidity (acute myocardial infarction and use of intra-aortic balloon pump [IABP], mechanical ventilation for 3 or more days, neurologic deficit, oliguric or anuric renal failure, or serious infection) included diabetes mellitus, body weight of 65 kg or less, aortic stenosis, and cerebrovascular disease. Each independent predictor was assigned a weight or score, with increasing mortality and morbidity associated with an increasing total score.
The New York State model of Hannan and coworkers6 collected data from 1989 through 1992, with 57,187 patients in a study with 14 variables. It was validated in 30 institutions. The mortality definition was “in hospital.” Observed mortality was 3.7%, and the expected mortality rate was 2.8%. These researchers included only isolated CABG operations.
The Society of Thoracic Surgeons national database represents the most robust source of data for calculating risk-adjusted scoring systems.7 Established in 1989, the database had grown to include 638 participating hospitals by 2004. This provider-supported database allows participants to benchmark their risk-adjusted results against regional and national standards. New patient data are brought into the Society of Thoracic Surgeons database on an annual and, now, semiannual basis. Since 1990, when more complete data collection was achieved, risk stratification models were developed for both CABG and valve replacement surgery.
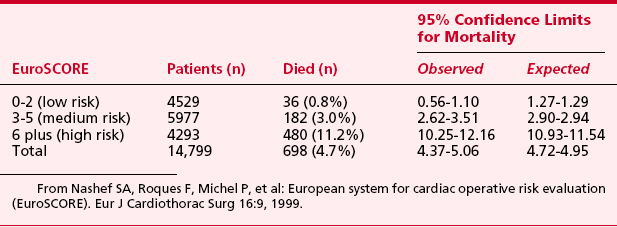
European System for Cardiac Operative Risk Evaluation (EuroSCORE) for cardiac operative risk evaluation was constructed from an analysis of 19,030 patients undergoing a diverse group of cardiac surgical procedures from 128 centers across Europe8 (Tables 1-2 and 1-3). The following risk factors were associated with increased mortality: age, female gender, serum creatinine, extracardiac arteriopathy, chronic airway disease, severe neurologic dysfunction, previous cardiac surgery, recent myocardial infarction, left ventricular ejection fraction, chronic congestive heart failure, pulmonary hypertension, active endocarditis, unstable angina, procedure urgency, critical preoperative condition, ventricular septal rupture, noncoronary surgery, and thoracic aortic surgery.
Table 1-2 EuroSCORE: Risk Factors, Definitions, and Weights (Score)
| Patient-Related Factors | Definition | Score |
|---|---|---|
| Age | Per 5 years or part thereof over 60 years | 1 |
| Sex | Female | 1 |
| Chronic pulmonary disease | Long-term use of bronchodilators or corticosteroids for lung disease | 1 |
| Extracardiac arteriopathy | Any one or more of the following: claudication, carotid occlusion or > 50% stenosis, previous or planned intervention on the abdominal aorta, limb arteries, or carotid arteries | 2 |
| Neurologic dysfunction | Disease severely affecting ambulation or day-to-day functioning | 2 |
| Previous cardiac surgery | Requiring opening of the pericardium | 3 |
| Serum creatinine level | >200 μmol/L preoperatively | 2 |
| Active endocarditis | Patient still under antibiotic treatment for endocarditis at the time of surgery | 3 |
| Critical preoperative state | Any one or more of the following: ventricular tachycardia or fibrillation or aborted sudden death, preoperative cardiac massage, preoperative ventilation before arrival in the anesthetic room, preoperative inotropic support, intra-aortic balloon counterpulsation or preoperative acute renal failure (anuria or oliguria < 10 mL/hr) | 3 |
| Cardiac-Related Factors | ||
| Unstable angina | Rest angina requiring intravenous administration of nitrates until arrival in the anesthetic room | 2 |
| Left ventricular dysfunction | Moderate or LVEF 30%–50% | 1 |
| Poor or LVEF > 30% | 3 | |
| Recent myocardial infarct | (<90% days) | 2 |
| Pulmonary hypertension | Systolic pulmonary artery pressure > 60 mmHg | 2 |
| Surgery-Related Factors | ||
| Emergency | Carried out on referral before the beginning of the next working day | 2 |
| Other than isolated CABG | Major cardiac procedure other than or in addition to CABG | 2 |
| Surgery on thoracic aorta | For disorder of ascending aorta, arch, or descending aorta | 3 |
| Postinfarct septal rupture | 4 |
CABG = coronary artery bypass graft surgery; LVEF = left ventricular ejection fraction.
From Nashef SA, Roques F, Michel P, et al: European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 16:9, 1999.
During the first years of this decade, this additive EuroSCORE has been widely used and validated across different centers in Europe and across the world, making it a primary tool for risk stratification in cardiac surgery.9 Although its accuracy has been well established for CABG and isolated valve procedures, its predictive ability in combined CABG and valve procedures has been less well studied.
Dupuis and colleagues10 attempted to simplify the approach to risk of cardiac surgical procedures in a manner similar to the original American Society of Anesthesiologists (ASA) physical status classification. They developed a score that uses a simple continuous categorization, using five classes plus an emergency status (Table 1-4). The Cardiac Anesthesia Evaluation Score (CARE) model collected data from 1996 to 1999 and included 3548 patients to predict both in-hospital mortality and a diverse group of major morbidities. It combined clinical judgment and the recognition of three risk factors previously identified by multifactorial risk indices: comorbid conditions categorized as controlled or uncontrolled, the surgical complexity, and the urgency of the procedure. The CARE score demonstrated similar or superior predictive characteristics compared with the more complex indices.
Table 1-4 Cardiac Anesthesia Risk Evaluation Score
| Score | Description |
|---|---|
| 1 | Patient with stable cardiac disease and no other medical problem. A noncomplex surgery is undertaken. |
| 2 | Patient with stable cardiac disease and one or more controlled medical problems.* A noncomplex surgery is undertaken. |
| 3 | Patient with any uncontrolled medical problem† or patient in whom a complex surgery is undertaken.‡ |
| 4 | Patient with any uncontrolled medical problem and in whom a complex surgery is undertaken. |
| 5 | Patient with chronic or advanced cardiac disease for whom cardiac surgery is undertaken as a last hope to save or improve life. |
| E | Emergency: surgery as soon as diagnosis is made and operating room is available. |
* Examples: controlled hypertension, diabetes mellitus, peripheral vascular disease, chronic obstructive pulmonary disease, controlled systemic diseases, others as judged by clinicians.
† Examples: unstable angina treated with intravenous heparin or nitroglycerin, preoperative intra-aortic balloon pump, heart failure with pulmonary or peripheral edema, uncontrolled hypertension, renal insufficiency (creatinine level > 140 μmol/L, debilitating systemic diseases, others as judged by clinicians.)
‡ Examples: reoperation, combined valve and coronary artery surgery, multiple valve surgery, left ventricular aneurysmectomy, repair of ventricular septal detect after myocardial infarction, coronary artery bypass of diffuse or heavily calcified vessels, others as judged by clinicians.
From Dupuis JY, Wang F, Nathan H, et al: The cardiac anesthesia risk evaluation score: A clinically useful predictor of mortality and morbidity after cardiac surgery. Anesthesiology 94:194, 2001.
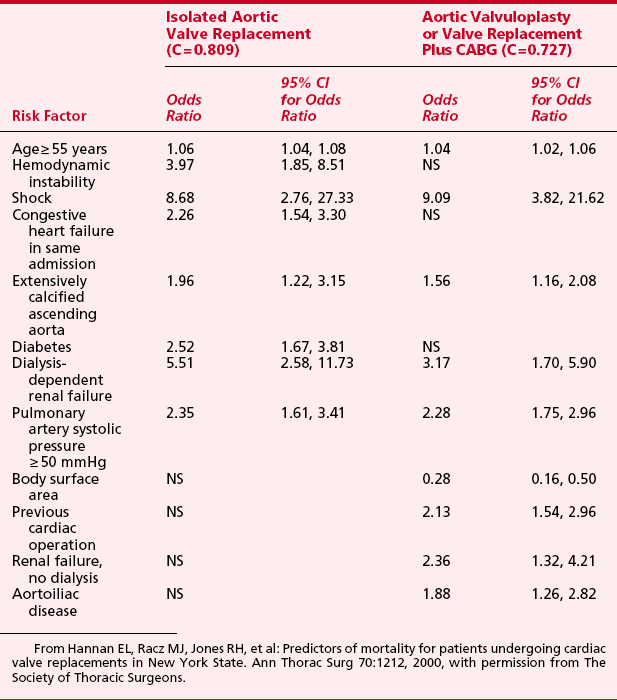
Hannan and colleagues11 evaluated predictors of mortality after valve surgery. A total of 18 independent risk factors were identified in the six models of differing combinations of valve and CABG. Shock and dialysis-dependent renal failure were among the most significant risk factors in all models. The risk factors and odds ratios are shown for aortic valve surgery in Table 1-5. Eleven risk factors were found to be independently associated with higher readmission rates: older age, female sex, African American race, greater body surface area, previous acute myocardial infarction within 1 week, and six comorbidities.
CARDIOVASCULAR TESTING
Patients who present for cardiac surgery have extensive cardiovascular imaging before surgery to guide the procedure. Coronary angiography provides a static view of the coronary circulation, whereas exercise and pharmacologic testing provide a more dynamic view. Because both tests may be available, it is useful to review some basics of cardiovascular imaging (Box 1-2).
In patients with a normal baseline ECG without a prior history of coronary artery disease, the exercise ECG response is abnormal in up to 25% and increasesup to 50% in those with a prior history of myocardial infarction or an abnormal resting ECG. The mean sensitivity and specificity are 68% and 77%, respectively, for detection of single-vessel disease; 81% and 66% for detection of multivessel disease; and 86% and 53% for detection of three-vessel or left main coronary artery disease.12
Nonexercise (Pharmacologic) Stress Testing
Pharmacologic stress testing has been advocated for patients in whom exercise tolerance is limited, both by comorbid diseases and by symptomatic peripheral vascular disease. Often, these patients may not stress themselves sufficiently during daily life to provoke symptoms of myocardial ischemia or congestive heart failure. Pharmacologic stress testing techniques either increase myocardial oxygen demand (dobutamine) or produce coronary vasodilatation leading to coronary flow redistribution (dipyridamole/adenosine).13 Echocardiographic or nuclear scintigraphic imaging (SPECT) is used in conjunction with the pharmacologic therapy to perform myocardial perfusion imaging for risk stratification and myocardial viability assessment (Box 1-3).
Dipyridamole-Thallium Scintigraphy
Dipyridamole works by blocking adenosine reuptake and increasing adenosine concentration in the coronary vessels. Adenosine is a direct coronary vasodilator. After infusion of the vasodilator, flow is preferentially distributed to areas distal to normal coronary arteries, with minimal flow to areas distal to a coronary stenosis.14 A radioisotope, such as thallium or technetium-99m sestamibi, is then injected. Normal myocardium will show up on initial imaging, whereas areas of either myocardial necrosis or ischemia distal to a significant coronary stenosis will demonstrate a defect. After a delay of several hours, or after infusion of a second dose of technetium-99m sestamibi, the myocardium is again imaged. Those initial defects that remain as defects are consistent with old scar, whereas those defects that demonstrate normal activity on subsequent imaging are consistent with areas at risk for myocardial ischemia.
SOURCES OF PERIOPERATIVE MYOCARDIAL INJURY IN CARDIAC SURGERY
Myocardial necrosis is the result of progressive pathologic ischemic changes that start to occur in the myocardium within minutes after the interruption of its blood flow, as seen in cardiac surgery (Box 1-4). The duration of the interruption of bloodflow, either partial or complete, determines the extent of myocardial necrosis. This is consistent with the finding that both the duration of the period of aortic cross-clamping and the duration of cardiopulmonary bypass consistently have been shown to be the main determinants of postoperative outcomes in virtually all studies.
Reperfusion of an Ischemic Myocardium
Surgical interventions requiring interruption of blood flow to the heart must, out of necessity, be followed by restoration of perfusion. Numerous experimental studies have provided compelling evidence that reperfusion, although essential for tissue and/or organ survival, is not without risk, owing to the extension of cell damage as a result of reperfusion itself. Myocardial ischemia of limited duration (<20 minutes), followed by reperfusion, are accompanied by functional recovery without evidence of structural injury or biochemical evidence of tissue injury.15
Adverse Systemic Effects of Cardiopulmonary Bypass
In addition to the effects of disruption and restoration of myocardial blood flow, cardiac morbidity may result from many of the components used to perform cardiovascular operations, which lead to systemic insults that result from cardiopulmonary bypass circuit-induced contact activation. Inflammation in cardiac surgical patients is produced by complex humoral and cellular interactions, including activation, generation, or expression of thrombin, complement, cytokines, neutrophils, adhesion molecules, mast cells, and multiple inflammatory mediators.16 Because of the redundancy of the inflammatory cascades, profound amplification occurs to produce multiorgan system dysfunction that can manifest as coagulopathy, respiratory failure, myocardial dysfunction, renal insufficiency, and neurocognitive defects.
ASSESSMENT OF PERIOPERATIVE MYOCARDIAL INJURY IN CARDIAC SURGERY
Traditionally, acute myocardial infarction was determined electrocardiographically. Cardiac biomarkers are elevated postoperatively and can be used for postoperative risk stratification, in addition to being used to diagnose acute morbidity (Box 1-5).
Assessment of Cardiac Function
Regional wall motion abnormalities follow the onset of ischemia in 10 to 15 seconds. Echocardiography can therefore be a very sensitive and rapid monitor for cardiac ischemia/injury. If the abnormality is irreversible, this indicates irreversible myocardial necrosis. The importance of transesophageal echocardiographic assessment of cardiac function is further enhanced by its value as a predictor of long-term survival. In patients undergoing CABG, a postoperative decrease in left ventricular ejection fraction compared with preoperative baseline predicts decreased long-term survival.17
Electrocardiography
The presence of new persistent Q waves of at least 0.03-second duration, broadening of preexisting Q waves, or new QS deflections on the postoperative ECG have been considered evidence of perioperative acute myocardial infarction. However, new Q waves may also be due to unmasking of an old myocardial infarction. Crescenzi and colleagues18 demonstrated that the association of a new Q wave and high levels of biomarkers was strongly associated with postoperative cardiac events; whereas the isolated appearance of a new Q wave had no impact on the postoperative cardiac outcome.
Serum Biochemical Markers to Detect Myocardial Injury
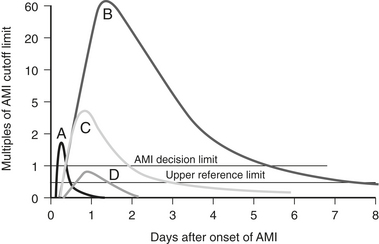
Serum biomarkers have become the primary means of assessing the presence and extent of acute myocardial infarction after cardiac surgery. Serum biomarkers that indicate myocardial damage include the following (with postinsult peak time givenin parentheses): myoglobin (4 hours), total creatine kinase (16 hours), CK-MB isoenzyme (24 hours), troponins I and T (24 hours), and lactate dehydrogenase (LDH) (76 hours). The CK-MB isoenzyme has been most widely used, but studies have suggested that troponin I is the most sensitive and specific in depicting myocardial ischemia and infarction19 (Fig. 1-1). Recently, a universal definition of myocardial infarction has been published, and following CABG it includes an elevation of biomarkers to 5 times baseline levels plus either new Q waves or a new LBBB, or evidence of new loss of viable myocardium by imaging techniques.20
SUMMARY
1. Kouchoukos N.T., Ebert P.A., Grover F.L., et al. Report of the Ad Hoc Committee on Risk Factors for Coronary Artery Bypass Surgery. Ann Thorac Surg. 1988;45:348.
2. Paiement B., Pelletier C., Dyrda I., et al. A simple classification of the risk in cardiac surgery. Can Anaesth Soc J. 1983;30:61.
3. Smith P.K., Smith L.R., Muhlbaier L.H. Risk stratification for adverse economic outcomes in cardiac surgery. Ann Thorac Surg. 1997;64:S61. 1997; discussion S80
4. Parsonnet V., Dean D., Bernstein A. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79:I-3.
5. Higgins T., Estafanous F., Loop F., et al. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. JAMA. 1992;267:2344.
6. Hannan E.L., Kilburn H.Jr., O’Donnell J.F., et al. Adult open heart surgery in New York State: An analysis of risk factors and hospital mortality rates. JAMA. 1990;264:2768.
7. Shroyer A.L., Grover F.L., Edwards F.H. 1995 Coronary artery bypass risk model: The Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg. 1998;65:879.
8. Nashef S.A., Roques F., Michel P., et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9.
9. Toumpoulis I.K., Anagnostopoulos C.E., Swistel D.G., et al. Does EuroSCORE predict length of stay and specific postoperative complications after cardiac surgery? Eur J Cardiothorac Surg. 2005;27:128.
10. Dupuis J.Y., Wang F., Nathan H., et al. The cardiac anesthesia risk evaluation score: A clinically useful predictor of mortality and morbidity after cardiac surgery. Anesthesiology. 2001;94:194.
11. Hannan E.L., Racz M.J., Jones R.H., et al. Predictors of mortality for patients undergoing cardiac valve replacements in New York State. Ann Thorac Surg. 2000;70:1212.
12. Horacek B.M., Wagner G.S. Electrocardiographic ST-segment changes during acute myocardial ischemia. Cardiol Electrophysiol Rev. 2002;6:196.
13. Grossman G.B., Alazraki N. Myocardial perfusion imaging in coronary artery disease. Cardiology. 2004;10:1.
14. Klocke F.J., Baird M.G., Bateman T.M., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionucleotide imaging: Executive summary. Circulation. 2003;108:1404.
15. Bolli R. Mechanism of myocardial “stunning.”. Circulation. 1990;82:723.
16. Levy J.H., Tanaka K.A. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715.
17. Jacobson A., Lapsley D., Tow D.E., et al. Prognostic significance of change in resting left ventricular ejection fraction early after successful coronary artery bypass surgery: A long-term follow-up study. J Am Coll Cardiol. 1995:184A.
18. Crescenzi G., Bove T., Pappalardo F., et al. Clinical significance of a new Q wave after cardiac surgery. Eur J Cardiothorac Surg. 2004;25:1001.
19. Greenson N., Macoviak J., Krishnaswamy P., et al. Usefulness of cardiac troponin I in patients undergoing open heart surgery. Am Heart J. 2001;141:447.
20. Thygesen K., Alpert J.S., White H.D., et al. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173.