Artificial Perfusion during Cardiac Arrest
Cardiopulmonary resuscitation (CPR) can be lifesaving for a patient in cardiac arrest, particularly in conjunction with other therapies such as defibrillation or delivery of medications. In several large clinical studies, data have shown that prompt delivery of CPR serves as an important predictor of successful outcome and increases the chance of survival by up to twofold. Each minute without treatment, on the other hand, is associated with a 10% to 15% decrease in the probability of survival.1,2
The quality of CPR is an important technical issue and has a direct effect on patient outcome. For example, shallow chest compressions have an adverse impact on the success of defibrillation.3 Because of these and related data, emphasis has recently been placed on improving the quality of CPR, and such priority has been codified in consensus CPR guidelines promulgated by the American Heart Association. These guidelines are formulated through a formalized data evaluation process and are updated every 5 years.4
Worrisome data have shown that the quality of CPR during actual resuscitation is endemically poor.5,6 Specifically, chest compressions are often administered too slowly with inadequate depth. In addition, pauses in chest compressions are too long, and hyperventilation of arrest patients is common. These deficiencies may be due to a variety of factors, including infrequent training, lack of awareness of the quality of CPR during resuscitation, and inadequate team leadership during resuscitation efforts.7
Conventional CPR
Although CPR is widely taught to health care personnel and reassessed periodically, the importance of high-quality CPR cannot be stressed enough. High-quality CPR immediately before defibrillation increases the chance of successful restoration of circulation.3,8 Although another recent multicenter investigation of out-of-hospital arrest did not support this claim,9 it is generally believed that for unwitnessed arrest or arrest events with a long downtime, early CPR and defibrillation have a significant impact on patient survival and recovery.10,11 Quality chest compression also increases the efficacy of drugs administered during resuscitation, whereas inadequate circulation leads to minimal effects from peripherally delivered drugs.12 Hyperventilation is also widely prevalent and dramatically compromises hemodynamics. In animal studies, hyperventilation leads to reduced survival from arrest. In this section we review the key procedural aspects of manual CPR.
Compressions
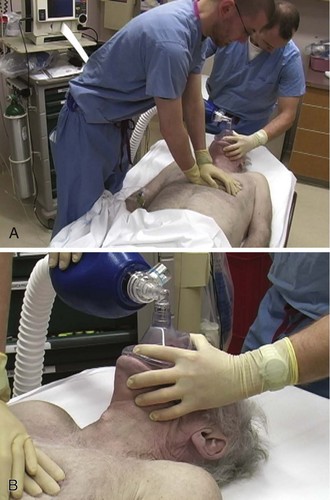
The 2010 resuscitation guidelines emphasize the importance of quality chest compression4 by recommending that clinicians focus on maintaining proper chest compression depth and rate. Compress the sternum to a depth of at least 2 inches with a rate of at least 100 compressions/min. Box 17-1 provides a summary of procedural recommendations for CPR. If possible, place a backboard under the victim to ensure appropriate thoracic compression. In addition, adjust the height of the bed or have the rescuer stand on top of a stepstool so that the entire weight of the rescuer above the waist is directed onto the patient’s sternum (Fig. 17-1A). This enhances the depth of compressions and helps prevent leaning on the patient’s chest between compressions, which is another key deficiency that has been widely observed. Extend the arms fully and place them perpendicular to the patient’s chest while making sure to pull away from the chest sufficiently between compressions to allow full chest recoil. Rotate rescuers aggressively (approximately every 2 to 3 minutes) to avoid deteriorating quality of compressions because of exhaustion. Properly delivered compressions are highly fatiguing, and rescuer bravado often interferes with the realization of declining CPR quality over time.
Minimize pauses in chest compressions because even short pauses have profound effects on coronary perfusion pressure and outcomes.13 As stated earlier, long pauses in chest compressions before delivery of a shock are associated with failure of defibrillation.3 Do not stop CPR to deliver medications because the drugs can be administered at the same time as the compressions. Keep pauses in chest compressions to a minimum (e.g., for procedures such as intubation or pulse checks).
Ventilations
Deliver ventilations at a rate of 8 to 10 breaths/min (see Fig. 17-1B). Hyperventilation (e.g., ventilation rates greater than 30/min) is common during resuscitation. To prevent unwittingly hyperventilating the patient, ask the rescuer who is providing ventilations to remove his or her hand completely off the bag-valve-mask apparatus between ventilations. The team leader should be vigilant in the observation of delivery of ventilations and should be ready to verbally prompt rescuers to ventilate the patient at the appropriate rate if hyperventilation is performed.
Pulse Checks
Monitoring end-tidal CO2 pressure (Petco2) also affords an opportunity to detect a pulse during CPR. During ongoing resuscitation of a pulseless patient, capnography will generally remain low (often less than 20 mm Hg), which is indicative of low blood flow. If the patient achieves return of spontaneous circulation (ROSC), a sharp increase in the Petco2 value (usually greater than 25 to 30 mm Hg) is consistent with return of adequate perfusion.14
Leadership and Teamwork
Cardiac arrest resuscitations are often crowded, chaotic events filled with stress and anxiety. To maximize calm and efficiency and to ensure quality of care, establish a team protocol. Designate someone to be the leader of the resuscitation, and make sure that all participants are clearly aware of this designation. The designated team leader should be responsible for monitoring the rhythm, for giving orders to initiate and terminate chest compressions, and for delivery of drugs and other therapies. The team leader should be situated either at the head of the bed or at a place where you can direct the resuscitation. As the team leader, it is important that you do not actually perform compressions, ventilations, or other specific procedures unless absolutely necessary because you may quickly lose control of the resuscitation. Since most rescuers are unable to detect when their own quality of compressions is diminishing, observe CPR closely and order rescuer rotations throughout the duration of the resuscitation.15
New Directions: CC-CPR
Chest compression–only cardiopulmonary resuscitation (CC-CPR) has been shown in a number of investigations to be as effective as standard CPR in resuscitation efforts initiated by members of the lay public.16,17 Give compressions at a rate of at least 100/min.
Because of its simplicity, CC-CPR minimizes pauses in chest compressions while maintaining proper rate and depth. Lay rescuers in the community may be less experienced with standard CPR and uncomfortable with the performance of mouth-to-mouth resuscitation. The simplicity of CC-CPR makes it relatively easy for first responders to initiate resuscitation efforts and for emergency medical dispatchers to guide lay rescuers remotely. The American Heart Association’s 2010 guidelines have shifted emphasis from “ABC” (“airway, breathing, compressions”) to “CAB” (“compressions, airway, breathing”) for lay rescuers. Their endorsement of “hands-only CPR” (a synonym for CC-CPR) educational programs reflects additional evidence that a focus on chest compressions during CPR may lead to an increase in bystander CPR, as well as improvements in patient outcomes.18 Recent investigations have shown that CC-CPR is associated with improved survival of patients with out-of-hospital cardiac arrest when performed by lay bystanders. A period of CC-CPR before intubation and rhythm evaluation also improves outcomes when used by emergency medical service (EMS) personnel.19,20 The EMS community is likely to see more widespread adoption and use of CC-CPR by lay public educational programs in the upcoming years.
Adjuncts to Improve the Quality of CPR
ACD-CPR
Active compression-decompression cardiopulmonary resuscitation (ACD-CPR) is a variant of CPR in which the passive relaxation phase of CPR is converted into an active phase by means of a handheld or mechanical suction device, which can theoretically improve both myocardial and cerebral circulation when compared with traditional CPR.21,22 However, data on these devices are mixed; there have been studies on out-of-hospital cardiac arrest using this technique that did not find any improvements in either initial outcome or survival to discharge, and as with many devices, there are instances when its application is impractical.23,24
ITD
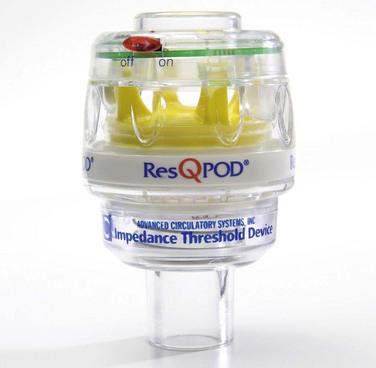
The impedance threshold device (ITD) optimizes chest compression hemodynamics via manipulation of intrathoracic pressure. From a practical standpoint, the ITD is a relatively simple device that is placed between the endotracheal tube and the bag-valve apparatus, much like a colorimetric Petco2 detector, which is familiar to most ED clinicians (Fig. 17-2). The ITD contains a valve that prevents air from flowing through the device that is less than 10 cm H2O in pressure. During resuscitation, the ITD prevents air from entering the thorax during recoil of the chest wall after each compression by generating a small but hemodynamically significant negative pressure within the chest. In laboratory studies this negative pressure enhances venous return to the heart and results in increased cardiac output with each subsequent chest compression.
Current data are conflicting on whether ITDs improve clinical outcomes when used as an adjunct to resuscitation efforts. Numerous studies and clinical trials using one particular model of ITD (Res-Q-Pod, Advanced Circulatory Systems, Inc., Eden Prairie, MN) have demonstrated improved hemodynamics during CPR and have suggested that use of an ITD during resuscitation efforts may lead to improved survival and patient outcomes.25–27 However, the findings from recent randomized controlled trials of ITD use in patients suffering out-of-hospital cardiac arrest have offered opposing data, thus suggesting that there is not a significant improvement in patient outcomes when these devices have been used.28
Monitoring and Feedback Devices
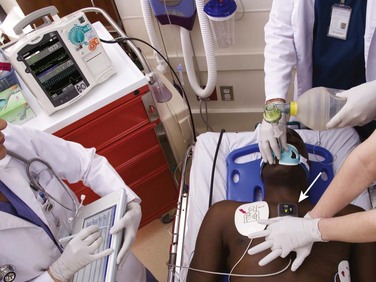
One method of monitoring chest compressions involves placing a relatively small external device on the patient’s sternum and performing chest compressions on top of the device (Fig. 17-3). The device measures the quality of compressions via a force detector or accelerometer (or both) that determines the rate and depth of chest compressions. Different versions of these CPR quality–monitoring and feedback devices are on the market. Some are incorporated into defibrillators (MRx-QCPR, Philips Healthcare, Andover, MA; R series with Real CPR Help, Zoll Medical Corp, Chelmsford, MA), whereas others are stand-alone devices applied to the chest. In recent trials, use of such a defibrillator with CPR monitoring and feedback improved CPR performance and, in one out-of-hospital trial, improved the rate of initial resuscitation.29 Further research will be required to assess the magnitude of improvement in survival that these devices can offer and what training mechanisms can maximize team responses to feedback messages.
Mechanical CPR Devices
Recent studies to determine the efficacy of the Autopulse have had mixed results. Although initial smaller investigations appeared promising, a large multicenter randomized trial was stopped early because patients in the manual CPR arm had survival equivalent to those receiving care via the Autopulse, with a trend toward worse outcomes in the Autopulse group.30
A separate nonrandomized trial showed a marked improvement in survival when using the device.31 A recent randomized trial in Europe has demonstrated the utility and feasibility of automated compression devices (in this case the Autopulse) in the resuscitation of out-of-hospital cardiac arrest patients.32 The survival benefit of such devices may very much depend on the specifics of how they are applied and used; an upcoming large clinical trial (the Circulation Improving Resuscitation Care [CIRC] Trial) seeks to examine the effectiveness of the Autopulse device, improve EMS education and proper use of automated compression devices, and minimize confounders that may have affected previous investigations.33
Another mechanical CPR device has been developed in Europe (LUCAS, Jolife Corp., Lund, Sweden) and is currently being evaluated in clinical trials outside the United States (Fig. 17-4). This device, in contrast to the band mechanism of the Autopulse, uses a piston/suction cup to compress the anterior aspect of the chest, much like during manual CPR, with the suction cup providing some degree of active compression-decompression, as described earlier in this chapter. A pilot study found no difference in survival to discharge between patients who received manual chest compressions and those who were resuscitated using the LUCAS device.34 A larger clinical trial involving the LUCAS device is currently under way and will provide additional information on the clinical impact of this particular mechanical CPR device.35
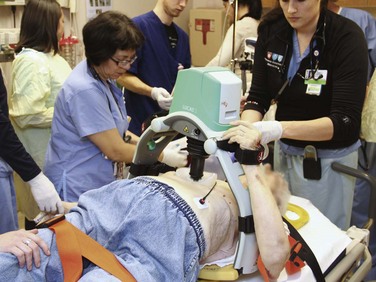
Figure 17-4 The LUCAS-2 mechanical cardiopulmonary resuscitation device (Jolife Corp., Lund, Sweden).
To highlight an intriguing opportunity available with mechanical CPR devices, there has been much discussion about the potential utility of these tools in clinical situations in which coronary angiography might be performed concurrently with ongoing resuscitation efforts. If clinical evidence suggests a major coronary event as the cause of the arrest, mechanical devices could be used to perform high-quality, continuous chest compressions as percutaneous coronary intervention is being performed. Case studies have demonstrated the feasibility of using the LUCAS device during intra-arrest coronary angiography with good patient outcomes,36,37 but additional data will be necessary to draw more clear conclusions about clinical practices and patient outcomes in these situations.
Emergency Cardiac Bypass
Extracorporeal cardiopulmonary resuscitation (E-CPR) is an emergency technique that has been investigated as a “last resort” for cardiac arrest patients who have failed to achieve ROSC despite ongoing resuscitation efforts. Several clinical studies have demonstrated successful outcomes for patients in whom E-CPR was used (and thus indicate that E-CPR could be a feasible addition to resuscitation efforts).35–37 One prospective trial in Japan, which identified patients who failed to respond to other traditional resuscitation efforts, demonstrated a favorable neurologic outcome in patients who were able to undergo both emergency cardiac bypass and therapeutic hypothermia treatment; rapid initiation of E-CPR and attainment of the target temperature were associated with positive neurologic outcomes in this cohort.38 The specialized training necessary to perform the procedure, as well as significant logistic issues surrounding rapid establishment of extracorporeal membrane oxygenation in the emergency department (ED) setting, raises concern about the widespread applicability of this intervention. Other investigations have highlighted the potential complications related to E-CPR in these critically ill patients.39,40 Additional research is needed on this topic, and more information will be necessary to clearly identify patients who are likely to benefit from E-CPR, examine the cost of such an intervention, and determine the impact of E-CPR on the survival of cardiac arrest victims.41
Monitoring during CPR
No blood testing is considered routine or standard during the initial stages of cardiopulmonary arrest, although early serum potassium and blood glucose monitoring is prudent if resuscitation is successful.14
Capnography
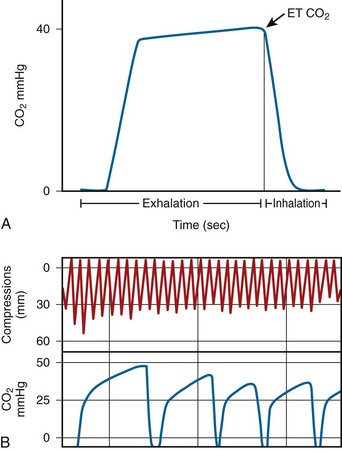
Capnography measures respiratory CO2, which is delivered to the lungs and expelled during exhalation (Fig. 17-5). The highest CO2 levels occur at the end of each exhalation, called Petco2. During cardiac arrest, Petco2 falls abruptly at the onset of cardiac arrest, increases during the delivery of effective CPR, and returns to physiologic levels after ROSC. Petco2 correlates with cardiac output under low-flow states such as CPR.42 Because of this relationship with cardiac output, Petco2 has been regarded as a probable indicator of the quality of CPR. During effective CPR in animal trials, Petco2 positively correlates with cardiac output, coronary perfusion pressure, efficacy of cardiac compression, ROSC, and even survival. Research is currently being done to further understand the use of Petco2 during CPR. At the other end of the spectrum, Petco2 could be useful in determining when to terminate resuscitation efforts.43
Although capnography is a common method of confirming correct endotracheal tube placement, it has also been regarded as a potential method of measuring hemodynamics and perfusion during cardiac arrest, as well as for determining the outcome of resuscitation efforts (specifically, detection of ROSC). The 2010 resuscitation guidelines recommend continuous waveform capnography for all intubated patients during resuscitation efforts.14
Ultrasound Monitoring
With advances in ultrasound equipment, properly trained users can portably and accurately monitor cardiac function in real time. Preliminary studies have demonstrated that trained physicians can assess cardiac function and obtain adequate images rapidly by using a subcostal approach to standard echocardiography in the cardiac arrest setting.44 If you are adequately trained in this technology, use it during resuscitation efforts to clinically diagnose conditions such as pulseless electrical activity (PEA) and to make a global assessment of cardiac motion during CPR and pulse restoration. Use ultrasound during arrest to rapidly diagnose and treat conditions such as cardiac tamponade. Get the ED ultrasound machine ready to use when preparing for an incoming cardiac arrest. Remember, however, that ultrasound is only a secondary diagnostic adjunct and should not interfere with the performance of high-quality CPR. Minimize interruptions to perform ultrasound and use it only during resuscitation for specific purposes (e.g., diagnosis of PEA versus hypotensive sinus rhythm). In most cases of arrest, ultrasound is probably of little value. Finally, there is ongoing research on the use of transcranial Doppler ultrasound to determine the prognosis after cardiac arrest. One preliminary study concluded that patients with severely disabling or fatal outcomes could be identified within the first 24 hours with this method.45
References
1. Larsen, MP, Eisenberg, MS, Cummins, RO, et al. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Ann Emerg Med. 1993;22:1652.
2. Valenzuela, T, Roe, D, Cretin, S, et al. Estimating effectiveness of cardiac arrest interventions: a logistic regression survival model. Circulation. 1997;96:3308.
3. Edelson, DP, Abella, BS, Kramer-Johansen, J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71:137.
4. Hazinski, MF, Nolan, JP, Billi, JE, et al. Part 1: executive summary: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 suppl 2):S250–S275.
5. Wik, L, Kramer-Johansen, J, Myklebust, H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299.
6. Abella, BS, Alvarado, JP, Myklebust, H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305.
7. Abella, BS, Kim, S, Edelson, DP, et al. Difficulty of cardiac arrest rhythm identification does not correlate with length of chest compression pause before defibrillation. Crit Care Med. 2006;34:S427.
8. Wik, L, Hansen, TB, Fylling, F, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389.
9. Stiell, IG, Nichol, G, Leroux, BG, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. for the ROC Investigators. N Engl J Med. 2011;365:787–797.
10. Stiell, IG, Wells, GA, Field, B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. for the Ontario Prehospital Advanced Life Support Study Group. N Engl J Med. 2004;351:647–656.
11. Weisfeldt, ML, Sitlani, CM, Ornato, JP, et al. Survival after application of automatic external defibrillators before arrival of the emergency medical system: evaluation in the Resuscitation Outcomes Consortium population of 21 million. for the ROC Investigators. J Am Coll Cardiol. 2010;55:1713–1720.
12. Pytte, M, Kramer-Johansen, J, Eilevstjonn, J, et al. Haemodynamic effects of adrenaline (epinephrine) depend on chest compression quality during cardiopulmonary resuscitation in pigs. Resuscitation. 2006;71:369.
13. Kellum, MJ, Kennedy, KW, Ewy, GA. Cardiocerebral resuscitation improves survival of patients with out-of-hospital cardiac arrest. Am J Med. 2006;119:335.
14. Morrison, LJ, Deakin, CD, Morley, PT, et al. Part 8: advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. for the Advanced Life Support Chapter Collaborators. Circulation. 2010;122(16 suppl 2):S345–S421.
15. Hightower, D, Thomas, SH, Stone, CK, et al. Decay in quality of closed-chest compressions over time. Ann Emerg Med. 1995;26:300.
16. Heidenreich, JW, Sanders, AB, Higdon, TA, et al. Uninterrupted chest compression CPR is easier to perform and remember than standard CPR. Resuscitation. 2004;63:123.
17. Hallstrom, A, Cobb, L, Johnson, E, et al. Cardiopulmonary resuscitation by chest compression alone or with mouth-to-mouth ventilation. N Engl J Med. 2000;342:1546.
18. Shuster, M, Lim, SH, Deakin, CD, et al. Part 7: CPR techniques and devices: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. for the CPR Techniques and Devices Collaborators. Circulation. 2010;122(16 suppl 2):S338–S344.
19. Bobrow, BJ, Spaite, DW, Berg, RA, et al. Chest compression–only CPR by lay rescuers and survival from out-of-hospital cardiac arrest. JAMA. 2010;304:1447–1454.
20. Bobrow, BJ, Clark, LL, Ewy, GA, et al. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. JAMA. 2008;299:1158–1165.
21. Cohen, TJ, Goldner, BG, Maccaro, PC, et al. A comparison of active compression-decompression cardiopulmonary resuscitation with standard cardiopulmonary resuscitation for cardiac arrests occurring in the hospital. N Engl J Med. 1993;329:1918.
22. Lurie, KG, Shultz, JJ, Callaham, ML, et al. Evaluation of active compression-decompression CPR in victims of out-of-hospital cardiac arrest. JAMA. 1994;271:1405.
23. Schwab, TM, Callaham, ML, Madsen, CD, et al. A randomized clinical trial of active compression-decompression CPR vs standard CPR in out-of-hospital cardiac arrest in two cities. JAMA. 1995;273:1261.
24. Skogvoll, E, Wik, L. Active compression-decompression cardiopulmonary resuscitation: a population-based, prospective randomized clinical trial in out-of-hospital cardiac arrest. Resuscitation. 1999;42:163.
25. Thayne, RC, Thomas, DC, Neville, JD, et al. Use of an impedance threshold device improves short-term outcomes following out-of-hospital cardiac arrest. Resuscitation. 2005;67:103.
26. Pirrallo, RG, Aufderheide, TP, Provo, TA, et al. Effect of an inspiratory impedance threshold device on hemodynamics during conventional manual cardiopulmonary resuscitation. Resuscitation. 2005;66:13.
27. Plaisance, P, Soleil, C, Lurie, KG, et al. Use of an inspiratory impedance threshold device on a facemask and endotracheal tube to reduce intrathoracic pressures during the decompression phase of active compression-decompression cardiopulmonary resuscitation. Crit Care Med. 2005;33:990.
28. Aufderheide, TP, Nichol, G, Rea, TD, et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. for the Resuscitation Outcomes Consortium (ROC) Investigators. N Engl J Med. 2011;365:798–806.
29. Kramer-Johansen, J, Myklebust, H, Wik, L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71:283.
30. Hallstrom, A, Rea, TD, Sayre, MR, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA. 2006;295:2620.
31. Ong, ME, Ornato, JP, Edwards, DP, et al. Use of an automated, load-distributing band chest compression device for out-of-hospital cardiac arrest resuscitation. JAMA. 2006;295:2629.
32. Krep, H, Mamier, M, Breil, M, et al. Out-of-hospital cardiopulmonary resuscitation with the AutoPulse system: a prospective observational study with a new load-distributing band chest compression device. Resuscitation. 2007;73:86–95.
33. Lerner, EB, Persse, D, Souders, CM, et al. Design of the Circulation Improving Resuscitation Care (CIRC) Trial: a new state of the art design for out-of-hospital cardiac arrest research. Resuscitation. 2011;82:294–299.
34. Smekal, D, Johansson, J, Huzevka, T, et al. A pilot study of mechanical chest compressions with the LUCAS device in cardiopulmonary resuscitation. Resuscitation. 2011;82:702–706.
35. Perkins, GD, Woollard, M, Cooke, MW, et al. Prehospital randomised assessment of a mechanical compression device in cardiac arrest (PaRAMeDIC) trial protocol. for the PARAMEDIC trial collaborators. Scand J Trauma Resusc Emerg Med. 2010;18:58.
36. Larsen, AI, Hjørnevik, A, Bonarjee, V, et al. Coronary blood flow and perfusion pressure during coronary angiography in patients with ongoing mechanical chest compression: a report on 6 cases. Resuscitation. 2010;81:493–497.
37. Grogaard, HK, Wik, L, Eriksen, M, et al. Continuous mechanical chest compressions during cardiac arrest to facilitate restoration of coronary circulation with percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1093–1094.
38. Nagao, K, Kikushima, K, Watanabe, K, et al. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ J. 2010;74:77–85.
39. Liu, Y, Cheng, YT, Chang, JC, et al. Extracorporeal membrane oxygenation to support prolonged conventional cardiopulmonary resuscitation in adults with cardiac arrest from acute myocardial infarction at a very low-volume centre. Interact Cardiovasc Thorac Surg. 2011;12:389–393.
40. Thiagarajan, RR, Brogan, TV, Scheurer, MA, et al. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87:778–785.
41. Topjian, A, Nadkarni, V. E-CPR … is there E-nough E-vidence to reach a “tipping point” for rapid deployment? Crit Care Med. 2008;36:1607–1613.
42. Weil, MH, Bisera, J, Trevino, RP, et al. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13:907.
43. Hatlestad, D. Capnography as a predictor of the return of spontaneous circulation. Emerg Med Serv. 2004;33:75.
44. Niendorff, DF, Rassias, AJ, Palac, R, et al. Rapid cardiac ultrasound of inpatients suffering PEA arrest performed by nonexpert sonographers. Resuscitation. 2005;67:81.
45. Wessels T, Harrer JU, Jacke C, et al: The prognostic value of early transcranial Doppler ultrasound following cardiopulmonary resuscitation. Ultrasound Med Biol. 2006;32:1845.