Chapter 39 Arthroscopic Debridement for Elbow Stiffness
Introduction
Limitations in elbow motion restricts the extent of hand reach and, depending on the degree of loss of motion, can cause major functional limitations. Most activities of daily living and work can be accomplished with 100° of flexion–extension motion and 100° of rotation.1 More severe restrictions of elbow motion are usually associated with significant loss of function.
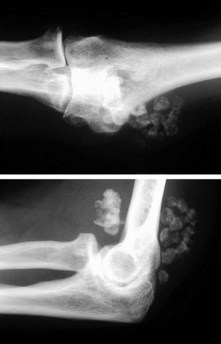
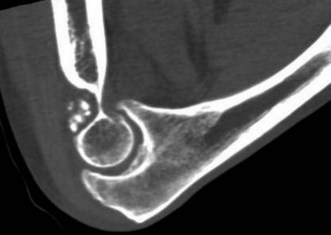
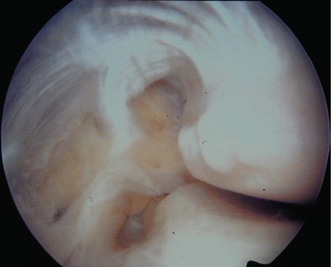
Loss of elbow motion can be caused by a number of osseous or soft-tissue abnormalities located within or around the joint. Intra-articular causes include joint incongruity after fractures, articular adhesions and posttraumatic or degenerative arthritis. Diseases affecting the synovium such as rheumatoid arthritis and synovial chondromatosis (Fig. 39.1) may also result in limitation of motion. Posttraumatic capsular thickening and soft tissue contractures or heterotopic bone formation are typical extra-articular causes of loss of motion. Trauma is by far the most common cause of restriction of elbow motion.
Pain may or may not be a prominent feature of elbow stiffness. This may be related to the fact that patients may be less likely to seek advice for their elbow problem if the elbow is not painful. In patients with slight or moderate stiffness, pain is usually the main problem. In very stiff or ankylotic elbows it is the lack motion that causes the functional limitations. Very stiff elbows may actually be pain-free, but often have secondary pain related to the shoulder or neck region. Non-operative management consists of gentle, active physical therapy and dynamic or static progressive splinting, which is particularly useful in posttraumatic cases.2,3 Surgical treatment may be indicated in patients with major functional limitations who have failed non-surgical management.
Advances in both open and in particular arthroscopic surgical approaches have led to a higher rate of clinically successful treatment of elbow stiffness and osteoarthritis of the elbow. In an recent state-of-the-art article, Bernhard Morrey stated that ‘without question, one of the greatest recent advances has been the application of techniques of elbow arthroscopy to the treatment of the stiff elbow’.4 The aim of this chapter is to discuss the role of arthroscopic release and debridement for elbow stiffness.
Background/aetiology
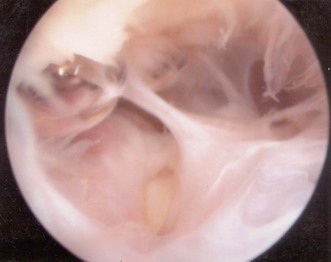
Intra-articular adhesions (Fig. 39.2), heterotopic ossification, scarring and contracture of the capsule all contribute to the stiffness. Scarring and shortening of the anterior capsule is usually the primary cause of the extension deficit and stiffness and shortening of the posterior oblique ligament medially and the posterior capsule result in flexion deficits.5 The degree of contracture is related to the severity of the initial trauma but other factors contribute including: non-anatomical reduction resulting in joint incongruency or inadequate fixation of the fractures not allowing early range of motion exercises. Prolonged immobilization can lead to capsular contracture and early active mobilization after injury is considered a significant factor in limiting residual stiffness.6–8
Heterotopic ossification may be caused by any type of trauma, including surgery, and is particularly common following elbow injuries when associated with head trauma. Heterotopic ossifications usually form in the flexor compartment of the elbow and can cause significant contractures. Resection should not be done until the ectopic bone exhibits a mature appearance on radiographs with trabeculations and well-defined cortical margins.7
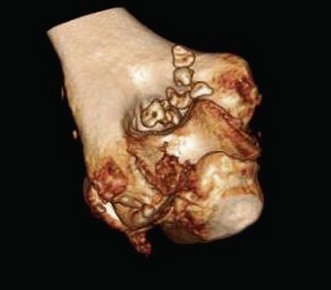
Primary elbow osteoarthritis is relatively rare in comparison with osteoarthritis of the shoulder and the joints of the lower extremities (Fig. 39.3). Approximately 2% of the population is affected. Men are much more frequently affected than women and usually the dominant arm is involved. Because of the low prevalence, studies on larger number of patients are not possible, and our knowledge on factors associated with initiation of and progression of the condition is limited, as is the interplay and relative importance of risk factors. A multifactorial aetiology of elbow osteoarthritis is presumed. A number of factors, predisposing to or influencing the development and course of elbow osteoarthritis have been suggested: genetic predisposition, malalignment, ageing, joint loading and repetitive micro-trauma.9
Several reports have described an association between heavy manual labour and the development of elbow osteoarthritis. Two early reports noted a high incidence of elbow osteoarthritis in coal miners using pneumatic tools. In a study that examined 774 coal miners who used pneumatic tools Rostock found that 32.8% had degenerative arthritis of the elbow.10 Lawrence found a prevalence of osteoarthritis of 31% in miners who used pneumatic drills compared with only 16% in miners not using pneumatic tools.11 In a more recent study Stanley found an association between strenuous manual work and the development of elbow osteoarthritis.12 In most of the published series dealing with the management of elbow osteoarthritis there is an overrepresentation of patients engaged in strenuous manual labour or sports activities that induce comparative high loads or forces across the elbow. Even though the elbow is not a weight-bearing joint, forces across the joint are significant during normal activities of living. Using various simulation and mathematical models it has been estimated that resultant forces across the humeroulnar joint may reach one-half times body weight during normal daily activities and up to two to three times body weight during occupational activities such as lifting and moving heavy objects.13 Throwing or heavy pounding produce even higher forces of up to an estimated six times body weight.
Degenerative arthritis begins primarily at the radiocapitellar joint and at all stages changes tends to be more advanced radially than in the humeroulnar articulation.14 The early changes found are posteromedial defects on the radial head with a corresponding defect on the posterior part of the crest separating the trochlea from the capitellar area.
Presentation, investigation and treatment options
Clinical presentation
Limitation of motion may also be related to a more severe trauma with joint incongruity, severe stiffness and pain causing significant impairment of function. A typical patient with elbow osteoarthritis is a manual worker in his mid-fifties, complaining of pain in the dominant arm on terminal elbow extension, flexion and forearm rotation. Usually the pain is elicited by more strenuous activities. Painful catching or locking sensations are common. The loss of motion is usually moderate with 20–30° of motion loss in both the flexion–extension and rotation arch.15 Between 25% and 50% of patients also have intermittent symptoms of ulnar neuropathy.16–18
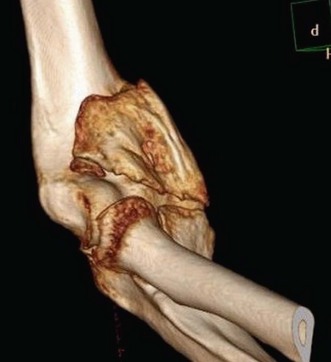
In primary osteoarthritis elbow motion may be compromised for several reasons. Flexion may be limited by osteophyte formation on the tip and on the medial facet of the coronoid (Fig. 39.4). In addition loose bodies or impacted loose bodies in the coronoid fossa may hinder flexion. Extension deficits or flexion contractures are usually caused by a combination of osteophytes along the olecranon process, loose bodies in the olecranon fossa and anterior capsular contracture (Figs 39.5, 39.6).
Assessment (investigations)
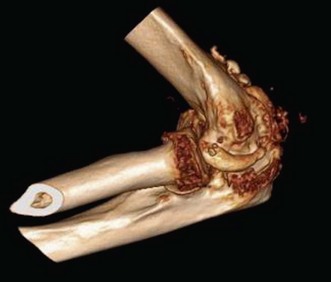
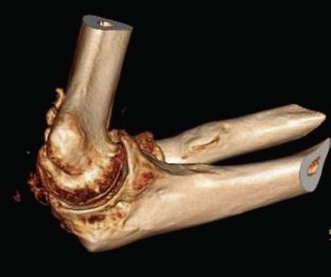
A goniometer is used to document active and passive motion in the extension–flexion plane (Fig. 39.7) as well as on rotation so that the response to treatment modalities can be documented. The presence of crepitus or pain in the motion arc is also noted. Standard anteroposterior and lateral radiographs are usually sufficient, but oblique and special projections may be helpful in evaluating possible intrinsic or extra-articular causes of restriction of motion: fracture deformity, joint incongruity, osteoarthritis, osteophytes, loose bodies, heterotopic ossifications and position of hardware. Computed tomography (CT) scans with three-dimensional reconstructions are very helpful in determining the extent and exact location of heterotopic ossification (Fig. 39.8), impinging osteophytes and the location of loose bodies in osteoarthritic elbows.19 CT scans are also routinely used if fracture deformity or joint incongruency is suspected.
Treatment options (indications)
Posttraumatic elbow stiffness
Trauma is by far the commonest cause of elbow contracture and prevention of stiffness is a primary goal in the rehabilitation after elbow trauma. Usually the main problem is an extension deficit as a result of a contracture of the anterior joint capsule. Less commonly, there is also heterotopic ossification present. Fracture deformity or joint incongruency can also cause flexion deficit. Finally, rotational deficit may be present after radial head fractures or in osteoarthritic elbows. In posttraumatic cases, physiotherapy, splinting, static or dynamic braces and other conservative treatment modalities should be tried to help improve range of motion. Some contractures, however, persist and do not improve over time in spite of therapy. A minimum of 6 months of non-operative treatment is recommended, but after 6 months there is usually only minimal additional gain in motion. There seems to be considerable individual clinical variation demonstrated by patients to similar degrees of trauma.20
Osteoarthritis
Osteoarthritis is the second most common cause of elbow stiffness. Patients usually present with both pain and restriction of motion and often also locking or catching. Pain is often at the extremes of motion but there may also be mid-range pain with activity. Non-operative treatment options are: rest, activity modification and antiinflammatory medication.15 Temporary relief may also be achieved using intra-articular injections of steroid and local anaesthetics; however, they rarely provide pain relief of longer duration. Long-term activity modification is the most important component of conservative management. In patients engaged in strenuous work activities this may necessitate a job change. Physiotherapy with strengthening or range of motion exercises is rarely indicated and may actually increase pain.
Indications
Indications for surgical intervention in elbow stiffness should be based on the individual patient’s needs and the competence of the surgeon to fulfil these needs without undue risks for the patient.1,21,22 If there is considerable loss of motion or even ankylosis the potential benefits from surgery are of course very significant. Functional demands in occupation, personal needs, sports and other activities, however, differ considerably. Restriction of motion, even if moderate, will not necessarily be tolerated by all. Surgical benefits and risks should always be carefully explained and discussed with each patient.
Open surgical releases using various techniques are well established and successful in the treatment of posttraumatic elbow stiffness. Depending on the type and localization of the pathology, limited anterior, lateral, medial or extensile posterior exposures may be used.6,23–26 (The tendency over the last two decades has been to use less invasive techniques in an attempt to minimize soft tissue trauma, reduce pain to allow early aggressive rehabilitation and minimize the risk of recurrence because of scarring. The complication rate with open techniques has also been a concern. Limiting the exposure and extent of surgery has not negatively influenced the functional outcomes.
More recently arthroscopic techniques have evolved, further reducing soft tissue trauma. With only small arthroscopic scars and reduced pain postoperatively, rehabilitation can be accelerated and hospital stays shortened. Indeed, it can now be undertaken as an outpatient procedure. Clinical outcomes after arthroscopic release have been encouraging.27–29 Arthroscopic elbow release is challenging because of the close proximity of neurovascular structures to portals and capsule, a limited working space within a stiff and non-compliant capsule and possibly joint incongruency. In addition it still is an uncommon procedure and only few surgeons perform a sufficient number of procedures to gain experience. Therefore, the indication for an arthroscopic procedure depends to a large extent on the surgeon’s ability and experience. Simple release of a moderate anterior capsular contracture in an otherwise intact joint with normal bony anatomy is not a technically demanding procedure, and an ideal case for an arthroscopic release. Very stiff joints with fracture sequelae or severely osteoarthritic joints with extensive osteophyte formation are more challenging and should be attempted only by the most experienced surgeons. Radial head resection is sometimes necessary as a part of the release procedure and again it may be performed arthroscopically. In patients with healed radial head fractures with deformity and radiocapitellar osteoarthritis with pain on forearm rotation and radiohumeral compression, resection is indicated. However, in osteoarthritis, there is still some controversy as to in which cases the procedure should be undertaken.30–32
Relative contraindications for an arthroscopic approach include extremely stiff or ankylotic joints, some cases of heterotopic ossifications, and joint incongruity where joint surface correctional surgery is anticipated (Fig. 39.9). Unless the ulnar nerve can be clearly identified by palpation after a previous ulnar nerve transposition, localization may require either establishing the anteromedial portal through a mini-incision or identifying the ulnar nerve through a mini open approach before creating the portal.33 Ultrasonography is usually very helpful in determining the exact location of nerves relative to ossification or the exact location of an anteposed ulnar nerve in different degrees of flexion. Heterotopic ossification is extracapsular and may be closely related to neurological structures.
Ulnar nerve intraneural pressure rises with increasing elbow flexion. Beyond 90° of flexion the intraneural pressure exceeds extraneural pressure and as a consequence it has been suggested that traction may be as important as compression in causing a cubital tunnel syndrome.34 It has been suggested that in patients with flexion of less than 90–100° as well as undertaking a release, ulnar nerve decompression with resection of the posterior band of the medial collateral ligament should be undertaken.1,33 Arthroscopic release is not an effective procedure for patients with restriction of forearm motion. Only rotation loss caused by adhesions, fracture deformity or osteophytes around the radial head may be dealt with this way. Open release is usually indicated if there is a need for articular reconstruction, as after malunited intraarticular fractures.
Surgical techniques and rehabilitation
Antibiotics
In a retrospective study of 473 elbow arthroscopies, Kelly et al31 found a 0.8% incidence of deep infection, 2% had superficial infection and prolonged drainage was observed in 5%. The infection risk seems to increase with the complexity of the procedure and the number of former surgeries. Most authors therefore recommend perioperative administration of antibiotics and some an even longer course.35
Portals
Surface landmarks and nerve courses are marked. Joint distension displaces the anterior neurovascular structures away from the portals and the 90° flexion position allows the best distension and optimal security with regard to neurovascular structures.36–38 Saline or local anaesthetic may be injected. In elbow stiffness the joint capacity and compliance is reduced.39 The lateral portals are closer to the radial nerve than the medial from the median nerve and the proximal portals are consistently farther away from the nerves than the distal.36,38,40,41 Some recommend the proximal anteromedial as the starting portal because of the greater distance to neurovascular structures.42,43 The second portal may be established using inside-out or outside-in technique. For most arthroscopic procedures and in particular for arthroscopic releases or debridement the proximal portals also give better access to the relevant parts of the joint.40 Two posterior portals are routinely used: a posterolateral and central. An additional lateral portal may also be necessary.
Surgical technique
Good results with significant improvements of range of motion and function have been reported using different arthroscopic techniques; arthroscopic debridement and fenestration32,44,45 capsulotomy which involves incising the capsule and releasing it off the anterior humerus.28,29,45,46 So far, any evidence of superiority of one technique over another is lacking. Aspects of surgical techniques and pathology-specific procedures are discussed in the personal technique section.
Rehabilitation
One of the advantages of arthroscopic release is that it allows an accelerated rehabilitation course because there is less soft tissue trauma, smaller scars and reduced pain postoperatively compared with an open approach. Postoperative splinting in extension is advocated by many to reduce swelling and help restore extension.3,22,47,48 Immediate range of motion exercises guided by a physiotherapist is generally recommended. Although CPM is used by many, its role is still unclear and not evidence based.21 CPM is often used in combination with regional blocks or catheters to relieve pain.
Administration of indometacin or other non-selective NSAIDs, as prophylaxis against heterotopic ossifications is recommended by many.24,47,49 However, the role of NSAIDs in prevention of heterotopic ossifications around the elbow is still unclear, and a comparative trial has yet to be undertaken.50
Personal technique
The patient is in the supine position lying with the side of the chest flush with the side of the operating table so that the arm is free. The hand and forearm are placed in a prefabricated forearm and wrist gauntlet connected to an overhead suspension device (Fig. 39.10). The upper arm is parallel to the floor at a right angle to the body and the elbow is flexed to 90°. It is a balanced suspension so that the elbow can be freely flexed, extended and rotated during surgery. The surgeon sits on the lateral side facing the elbow. The tourniquet is placed high on the upper arm. After exsanguination of the arm, the tourniquet is inflated to 250 mmHg.
Standard arthroscopic equipment is used: arthroscopes, instruments and portals. Standard portals for evaluating the anterior compartment are the anterolateral and anteromedial portals. Usually the anterolateral is the primary portal. This portal is located approximately 2 cm proximal and 1–2 cm anterior to the lateral epicondyle; depending on the size of the arm, the portal must be placed anteriorly enough for instruments to reach the bottom of the coronoid fossa. Because of the somewhat variable course of the radial cutaneous nerve branch, only the skin is incised and the joint is then entered careful by blunt dissection using a forceps (Fig. 39.11) followed by a blunt trochar.
Figure 39.11 Anatomical landmarks are marked. Skin incision only. Blunt dissection into the joint cavity.
Once the joint has been entered, the proximal anteromedial portal may be developed using either the inside-out or outside-in techniques. At this stage it is important to know that the ulnar nerve is not subluxed or has been transposed previously. There are also on the medial side cutaneous branches that may be injured. As a consequence only the skin should be incised and the portal established using blunt dissection. Care must also be taken that the portal is placed sufficiently anteriorly for instruments to be able to reach the bottom of the coronoid fossa. A 6 mm cannula is routinely used to ease switching of instruments and arthroscope. With the arthroscope in the anterolateral portal the anterior compartment and joint space is evaluated starting anteromedially and centrally (Fig. 39.12).
The radiocapitellar joint is best evaluated with the arthroscope in the medial portal. To minimize swelling, fluid pressure is kept as low as possible at the beginning of the procedure, although it may be necessary to increase it later. Using a shaver, the anterior compartment is gradually cleared of adhesions, fibrotic capsule and synovial irritation. The resection is started proximally and continued distally. Only non-aggressive shavers are used and the tip of the shaver should be fully visible at all times so that blind shaving is avoided. Suction is used intermittently and in a controlled fashion, both to avoid uncontrolled suction of tissue into the shaver and joint collapse because of loss of fluid pressure. To maximize control of how much tissue is being resected, the cutting edge of the shaver should be kept at a right angle to the capsule or tissue that is being removed. Pointing the cutting edge of the shaver directly towards the capsule should be avoided or done only in a very controlled fashion and under direct vision. Capsule and tissue resection is usually started medially proximally along the humeral shaft, which is the safest place in terms of distance to neurovascular structures. Usually after resection of adhesions and capsular tissue proximally, the fat pad will appear and it is then possible to lift the remaining capsule away from the anterior and anteromedial part of the humerus using a blunt trochar so that a reasonable working space is then created. If necessary the plane between the deep surface of the brachialis muscle and the anterior capsule can be developed using a low profile basket forceps. The capsule can then be resected under full vision. Repeatedly cutting the edge of the often very thick and stiff capsule with a forceps may facilitate resection with the shaver. Occasionally in extremely stiff elbows with almost no joint volume, it may be difficult to start resection from within the joint capsule, in which case resection must start from the outside after developing the plane between capsule and brachialis muscle.51 It is safest to start the resection proximally and the joint should be entered as soon as possible. Retractors can be used to maintain visualization without resorting to a high-pressure irrigation.
In osteoarthritic elbows there may be some synovitis but the capsule is usually not very thick and is easily resected (Fig. 39.13). Loose bodies in the fossa or attached to capsule are common and can either be removed using arthroscopic grabbing tools or, if large trimmed or divided, using an acromionizer pinning the loose body against the anterior humerus or fossa. There may also be impacted loose bodies at the bottom or the sides of the fossa necessitating a deepening of the fossa. Usually it is also necessary to trim osteophytes that have formed along the anteromedial and anterolateral facets of the coronoid process. The anteromedial osteophytes should be trimmed as far down on the medial side as possible. The tip of the coronoid is almost invariably prominent and will impinge on or sometimes block full flexion. Resection of the tip of the coronoid can be done in as much flexion as possible, thereby protecting the cartilage on the humeral side. Ossifications may also have formed just proximal to the joint surface of the capitellum, and should also be resected to ensure full flexion. In the posterior compartment there may also be loose bodies or impacted loose bodies filling up the olecranon fossa and hindering full extension. Re-creation of the depth and width of the fossa with trimming of osteophytes along the tip as well as the lateral and medial margins of the olecranon can be quite time consuming. Great care must be taken working in the medial recess as the ulnar nerve is very close; osteophytes can be trimmed with the acromionizer on reverse, soft tissue resection medially should be avoided or undertake with minimal or no suction, and the cutting edge of the shaver pointing away from the capsule and ulnar nerve. The posterolateral recess should be carefully evaluated; often there are loose bodies embedded in the synovial tissue (Fig. 39.2).
Figure 39.13 Anterior compartment. Radial head to the left, coronoid process in the centre. Synovitis.
Heterotopic ossification is most commonly located anteriorly and can usually be removed working from within the joint after careful dissection of the soft tissues covering the deep surface of the ossification (Figs 39.14, 39.15). Standard fine dissectors may be used. The heterotopic bone is usually fairly soft and can easily be removed using an acromionizer on reverse setting to minimize the risk of soft tissue damage. If the ossifications are attached to the humerus, the safest approach is often to resect the base, grab the bone fragment with a forceps and dissect of the soft tissues from the anterior surface, thereafter removing the fragment as a loose body. If it is suspected that the radial or median nerves are in contact with or run close to the ossifications, a preoperative ultrasonographic examination may help clarify the anatomical relationship.
Figure 39.14 Heterotopic ossifications. Preoperative flexion to 95°, extension deficit 70°, free rotation.
The posterior compartment is entered with the elbow in approximately 30° of flexion using two portals. A posterolateral portal is created 2 cm proximal to the tip of the olecranon at the lateral border of the thick part of the triceps aponeurosis and in line with the posterolateral recess. A posteromedial portal is placed 1 cm more proximally midway between the midline and the palpated ulnar nerve position. If the ulnar nerve is not freely moveable because of previous surgery or trauma, a more central position is preferred so as not to endanger the nerve. Placing the two portals too close together, however, will make triangulation difficult. If the olecranon fossa and posterior joint space is completely or nearly completely obliterated, a working space can be created by debriding the fossa blindly (Fig. 39.16) with a blunt forceps. Once a space is created the resection can be continued under direct arthroscopic vision. Dissection and resection medially under or along the course of the ulnar nerve must of course be done with great care, particularly in situations where the ulnar nerve does not have its normal mobility. Usually it is possible using fine dissectors to release the tissue of the posteromedial aspect of the humerus. Following posterior debridement the posterior capsule can gradually be lifted off the posterior part of the humerus. Finally both the medial and lateral recesses are inspected for synovitis and loose bodies (Fig. 39.17). If necessary an additional portal in the lateral soft spot is created.
Other pathology specific procedures include the following:
Outcome including literature review
The effectiveness of open surgical elbow release in restoring motion has been demonstrated by a number of authors.6,8,20,23–26,28,29,47,48,52–54 These surgical interventions have gradually developed into more limited exposures using sequential steps to address the specific causes of stiffness and limiting surgical exposure.1,22 The less invasive techniques minimize soft tissue trauma, reduce pain and allow early and more aggressive rehabilitation. The introduction of arthroscopic techniques has followed the same trend of attempting to reduce soft tissue trauma. More extensive approaches are now primarily reserved for ankylotic or nearly ankylotic joints, usually post-fracture cases with a need for articular reconstruction because of joint incongruency.
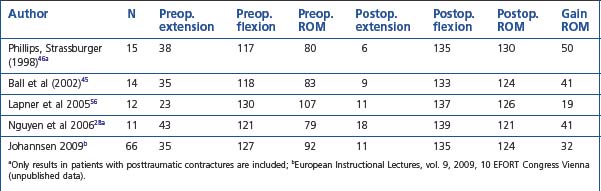
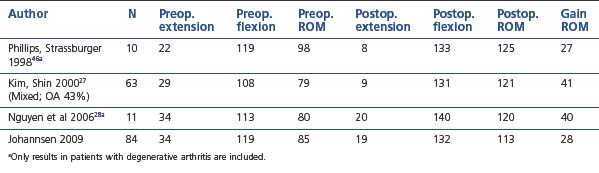
The number of reports on results after arthroscopic elbow release is still fairly limited compared with open techniques, but the results using arthroscopic techniques have been good and seem to match open surgery in all aspects.27–29,31,45,46,55,56 In most studies the results are reported for a mixture of pathologies resulting in elbow stiffness. Tables 39.1 and 39.2 give details from some of the series that have reported results for posttraumatic and osteoarthritic cases separately. Overall there seems to be only minor differences in results obtained, although the results in our series were slightly better for the posttraumatic cases. In most series the focus has been on improvements in arc of motion; however, the reported results in terms of pain relief, and functional improvements are also good.28,45,48 In an open series Ring et al found that final motion influenced physician-based rating scales but not patient-specific health status, which was dominated by pain and persistent ulnar neuropathy.48 In our series the results in terms of pain relief were slightly better for posttraumatic than for osteoarthritic cases. For patients with primarily soft tissue contractures the results were also good at 2–3-year follow-up, with close to 90% in the good or excellent category. Among patients with degenerative arthritis a slight tendency for the stiffness and pain to recur was observed and only 67% were satisfied at the 2–3-year follow-up.
Table 39.2 Elbow motion before and after arthroscopic release. Degenerative arthritis and mixed population24
Elbow arthroscopy has always been considered a demanding and potentially dangerous procedure because of the limited joint space and the close proximity of neurovascular structures. However, complications in the reported arthroscopic series are relatively few and usually minor. Complications after open contracture release include neuropathies, infection and recurrence of stiffness or heterotopic ossifications.6,7,21,23,25,47,49 Comparing complication rates after arthroscopic and open releases is difficult as there are no comparative studies, although complications after open procedures are not rare with reported rates up to 21%. A definite advantage of the arthroscopic techniques is that it can routinely be carried out as an outpatient procedure.
Complications of treatment
Over the past two decades there has been considerable evolution of arthroscopic elbow techniques. The number of procedures has increased, as has the complexity. In the beginning, elbow arthroscopy was primarily a diagnostic procedure with occasional removal of a loose body or partial synovectomy. Currently, much more extensive procedures are routinely performed: total synovectomy, arthroscopic arthrolysis, debridement with osteophytes resection and resection of heterotopic bone formation. Kelly et al,52 in a study on complications in elbow arthroscopies over an 18-year period, noted an increasing trend related to increasing complexity of procedures undertaken.
Very few studies have reported complication rates after arthroscopy in a systematic fashion.52 Most publications are case reports dealing with isolated and rare complications.58–61 Complications after elbow arthroscopy include prolonged drainage, superficial and deep infection, neurovascular injury, heterotopic ossification and compartment syndrome. In an analysis of 473 consecutive elbow arthroscopies the overall complication rate was about 10%.52 The majority of complications was minor and temporary: prolonged drainage (5%) and superficial infection (2%). There were four cases of deep infection (0.8%). In a consecutive series of 374 arthroscopic elbow procedures performed at our institution, we had four cases of deep infection and two patients with prolonged drainage (unpublished data). Prophylactic antibiotics were administered to all of our patients. In this series, six patients developed significant heterotopic ossification after arthroscopic release (1.6%). None of these patients had taken the prescribed indometacin for the recommended 3 weeks; most had taken indometacin for only a few days or not at all.
The most feared complication in elbow arthroscopy is neurovascular injury. In most textbooks on elbow arthroplasty the risk of neurovascular injury is repeatedly stressed. The posterior interosseous and ulnar nerves are closest to the portals.52,58,60,62 There have also been rare reports of anterior interosseous and median nerve injuries, and even a complete trans-section of both the median and radial nerves during an arthroscopic elbow release.59,61 Kelly et al found transient nerve palsies in 2% (four superficial radial, one medial superficial, five ulnar and one anterior interosseous). There were no permanent injuries. In our series there were three transient nerve palsies (one radial, two superficial radial) but no permanent. Many of the nerve injuries reported in the literature have been partial and transient. That reports of nerve injuries are so rare, in spite of the documented close proximity of routinely used portals to the nerves, may be because not all cases of nerve injury are reported. Another possible reason could be that surgeons performing elbow arthroscopies, in particular the more advanced high-risk procedures, are very experienced and take all necessary precautions to avoid injury.
1 Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg (Am). 1981;63:872-877.
2 Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg. 2000;82B:74-78.
3 Doornberg JN, Ring D, Jupiter JB. Static progressive splinting for posttraumatic elbow stiffness. J Orthop Trauma. 2006;20:400-404.
4 Morrey BF. The posttraumatic stiff elbow. Clin Orthop Rel Res. 2005;431:26-35.
5 Hida S. The collateral ligaments of the elbow joint; their functional anatomy with special reference to the pathology and treatment of post-traumatic stiff elbow. Nippon Seikeigeka Gakkai Zasshi. 1994;68(10):864-877.
6 Urbaniak JR, Hansen PE, Beissinger SF, et al. Correction of post-traumatic flexion contracture of the elbow by anterior capsulotomy. J Bone Joint Surg (Am). 1985;67:1160-1164.
7 Viola RW, Hastings HII. Treatment of ectopic ossification about the elbow. Clin Orthop Relat Res. 2000;370:65-86.
8 Itoh Y, Saegusa K, Ishiguro T, et al. Operation for stiff elbow. Int Orthop. 1989;13:263-268.
9 Felson DT. Risk factors for osteoarthritis; understanding joint vulnerability. Clin Orthop Rel Res. 2004;427(Suppl.):S16-S21.
10 Rostock P. Gelenkschaden durch arbeiten mit pressluftwerkzeugen und andere schwere korperliche arbeit. Medizinische Klinik. 1936:11341-11343.
11 Lawrence JS. Rheumatism in coal miners III. Occupational factors. Br J Ind Med. 1955;12:249-261.
12 Stanley D. Prevalence and etiology of of symptomatic elbow osteoarthritis. J Shoulder Elbow Surg. 1994;3:386-389.
13 Chadwick EK, Nicol EC. Elbow and wrist joint contact forces during occupational pick and place activity. J Biomech. 2000;33:591-600.
14 Goodfellow JW, Bullogh PG. The pattern of ageing of the articular cartilage of the elbow joint. J Bone Joint Surg (Br). 1967;49:175-181.
15 Gramstad GD, Galatz LM. Management of elbow osteoarthritis. Current Concepts review. J Bone Joint Surg (Am). 2006;88:421-430.
16 Wada T, Isogai S, Ischii S, et al. Debridement arthroplasty for primary osteoarthritis of the elbow. J Bone Joint Surg (Am). 2004;86:233-241.
17 Antuna SA, Morrey BF, Adams RA, et al. Ulnohumeral arthroplasty for primary degenerative arthritis of the elbow: long term outcome and complications. J Bone Joint Surg (Am). 2002;84:2168-2173.
18 Oka Y. Debridement arthroplasty for osteoarthritis of the elbow: 50 patients followed mean 5 years. Acta Orthop Scand. 2000;71:185-190.
19 Zubler V, Saupe N, Jost B, et al. Elbow stiffness: Effectiveness of conventional radiography and CT to explain osseous causes. AJR Am J Roentgenol. 2010;194:w515-w520.
20 Morrey BF. Posttraumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg (Am). 1990;72:601-618.
21 Ring D, Jupiter JB. Excision of heterotopic bone around the elbow. Tech Hand Up Extrem Surg. 2004;8:25-33.
22 Lindenhovius ALC, Jupiter J. Review article. The posttraumatic stiff elbow: a review of the literature. J Hand Surg (Am). 2007;32:1605-1619.
23 Husband JB, Hastings HII. The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg. 1990;72A:1353-1358.
24 Kraushaar BS, Nirschl RP, Cox W. A modified lateral approach for release of posttraumatic elbow flexion contracture. J Shoulder Elbow Surg. 1999;8:476-480.
25 Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrincic contracture of the elbow. J Bone Joint Surg (Am). 1998;80:1603-1615.
26 Wada T, Ishii S, Usui M, et al. The medial approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg (Br). 2000;82:68-73.
27 Kim SJ, Shin SJ. Arthroscopic treatment for limitation of motion of the elbow. Clin Orthop Rel Res. 2000;375:140-148.
28 Nguyen D, Proper SIW, MacDermid JC, et al. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;8:842-849.
29 Timmerman LA, Andrews JR. Arthroscopic treatment of posttraumatic elbow pain and stiffness. Am J Sports Med. 1994;22:230-235.
30 Menth-Chiari WA, Ruch DS, Poehling GG. Arthroscopic excision of the radial head: clinical outcome in 12 patients with post-traumatic arthritis after fracture of the radial head or rheumatoid arthritis. Arthroscopy. 2001;17:918-923.
31 Kelly EW, Bryce R, Coghlan J, et al. Arthroscopic debridement without radial head resection of the osteoarthritic elbow. Arthroscopy. 2007;23:151-156.
32 Savoie FH, Nunley PD, Field LD. Arthroscopic management of the arthritic elbow. Indications, technique, and results. J Shoulder Elbow Surg. 1999;8:214-219.
33 Sahajpal DT, Blonna D, O’Driscoll SW. Anteromedial elbow arthroscopy portals in patients with prior ulnar nerve transposition or subluxation. Arthroscopy. 2010;8:1045-1052.
34 Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. An experimental study in human cadaver. J Shoulder Elbow Surg. 1998;80:492-510.
35 Van Zeeland NL, Yamaguchi K. Arthroscopic capsular release of the elbow. J Shoulder Elbow Surg. 2010;19:13-19.
36 Thoreux P, Blondeau C, Durand S, et al. Anatomic basis of arthroscopic capsulotomy for elbow stiffness. Surg Radiol Anat. 2006;28:409-415.
37 Miller CD, Jobe CM, Wright MH. Neuroanatomy in elbow arthroscopy. J Shoulder Elbow Surg. 1995;4(3):168-174.
38 Unlu MC, Kesmezacar H, Akgun I, et al. Anatomic relationship between elbow arthroscopy portals and neurovascular structures in different elbow and forearm positions. J Shoulder Elbow Surg. 2006;15:457-462.
39 Gallay SH, Richards RR. O`Driscoll SW. Intraarticular capacity and compliance of stiff and normal elbows. Arthoscopy. 1993;9(1):9-13.
40 Stothers K, Day B, Regan WR. Arthroscopy of the elbow. Anatomy, portal sites, and a description of the proximal lateral portal. Arthroscopy. 1995;11:449-457.
41 Verhaar J, van Mameren H, Brandsma A. Risks of neurovascular injury in elbow arthroscopy: Starting anteromedially or anterolaterally. Arthroscopy. 1991;7:287-290.
42 Field LD, Altcheck DW, Warren RF, et al. Arthroscopic anatomy of the lateral elbow: a comparison of three portals. Arthroscopy. 1994;10:6027.
43 Drescher H, Schwering L, Jerosch J, et al. The risk of neurovascular damage in elbow joint arthroscopy. Which approach is better: anteromedial or anterolateral. Z Orthop Ihre Grenzgeb. 1994;132:120-125.
44 Redden JF, Stanley D. Arthroscopy. 1993;9:14–16.
45 Ball CM, Meunier M, Galatz LM, et al. Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 2002;11:624-629.
46 Phillips BB, Strasburger S. Arthroscopic treatment of arthrofibrosis of the elbow joint. Arthroscopy. 1998;14:38-44.
47 Cohen MS, Hastings HII. Post-traumatic contracture of the elbow. Operative release using a lateral collateral ligament sparing approach. J Bone Joint Surg (Br). 1998;80:805-812.
48 Ring D, Adey L, Zurakowski D, et al. Elbow capsulotomy for posttraumatic elbow stiffness. J Hand Surg (Am). 2006;31:1264-1271.
49 Tsionos I, Leclecq C, Rochet JM. Heterotopic ossifications of the elbow in patients with burns. Results after early excision. J Bone Joint Surg (Br). 2004;86:396-403.
50 Ellerin BE, Helfet D, Parikh S, et al. Current therapy in the management of heterotopic ossification of the elbow: a review with case studies. Am J Phys Med Rehab. 1999;78:259-271.
51 Kaminen S, Savoi FH3rd, ElAttrache N. Endoskopic extracapsular capsulectomy of the elbow: a neurovascular safe technique for high-grade contractures. Arthroscopy. 2007;23:789-792.
52 Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg (Am). 2001;83:25-34.
53 Mart RK, Kerkhoffs GM, Maas M, et al. Progressive surgical release of a posttraumatic stiff elbow. Technique and outcome after 2–18 years in 46 patients. Acta Orthop Scand. 2002;73:144-150.
54 Park MJ, Kim HG, Lee JY. Surgical treatment of post-traumatic stiffness of the elbow. J Bone Joint Surg (Br). 2004;86:1158-1162.
55 Jones GS, Savoie FH. Arthroscopic capsular release of flexion contractures (arthrofibosis) of the elbow. Arthroscopy. 1993;9:277-283.
56 Lapner PC, Leith JM, Reagan WD. Arthroscopicc debridement of the elbow for arthrofibrosis resulting from non-displaced fracture of the radial head. Arthroscopy. 2005;21:1492.
57 Soejbjerg JO. The stiff elbow. How I do it. Acta Orthop Scand. 1996;67:626-631.
58 Dumonski ML, Arciero RA, Mazzoca AD. Ulnar nerve palsy after elbow arthroscopy. Arthroscopy. 2006;22:577.E1-577.E3.
59 Haapaniemi T, Berggren M, Adolfsson L. Complete transsection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15:784-787.
60 Papilion JD, Neff RS, Shall LM. Compression neuropathy of the radial nerve as a complication of elbow arthroscopy. A case report and a review of the literature. Arthroscopy. 1988;4:284-286.
61 Ruch DS, Poehling GG. Anterior interosseus nerve injury following elbow arthroscopy. Arthroscopy. 1997;13:757-758.
62 Park JY, Cho CH, Choi JH, et al. Radial nerve palsy after arthroscopic anterior capsular release for degenerative elbow contracture. Arthroscopy. 2007;23:1360.e1-1360.e3.