Arthritis and Differential Inflammatory Joint Disorders
Rheumatologic conditions in children are myriad in presentation, with overlapping imaging features that may sometimes superficially mimic infection. Rheumatologic conditions in children do not follow the typical course and presentation compared with their counterparts in adults. As a consequence, the International League of Associations for Rheumatology (ILAR) formulated a revised nomenclature to describe the various pediatric rheumatologic conditions (Box 137-1).1 In this chapter, we discuss imaging of pediatric arthritis from the point of view of noninfectious synovial proliferation using the ILAR classification, including juvenile idiopathic arthritis (JIA) and its differentials: hemophilic arthropathy, lipoma arborescens, synovial chondromatosis, pigmented villonodular synovitis, and reactive synovitis (Box 137-2). Subtypes of JIA, enthesitis-related arthritis and psoriatic arthritis, will be discussed in their own subsections because of their unique presentation and imaging findings.
Juvenile Idiopathic Arthritis
JIA, which occurs worldwide, is the most frequent cause of chronic musculoskeletal pain in youths and the most common chronic musculoskeletal disease of childhood.2–4 It is a nonmigratory, chronic, monoarticular or polyarticular arthropathy of childhood.2
The diagnostic criteria for JIA include disease onset before the age of 16 years, the presence of arthritis in one or more joints for at least 6 weeks, onset type defined by type of disease in the first 6 months of diagnosis (Box 137-1), and exclusion of other forms of juvenile arthritis.1 JIA may be associated with systemic manifestations that include fever, erythematous rashes, nodules, leukocytosis, and, less commonly, iridocyclitis, pleuritis, pericarditis, anemia, fatigue, and growth failure.5 At the time of presentation, other causes of inflammation should be excluded. JIA differs from the adult type of rheumatoid arthritis because of the age of presentation, its preference for large joints, its tendency to generate joint contractures and muscle wasting, and its association with extraarticular clinical manifestations.6
A new internationally accepted classification system was established in 19951,7 and revised in 20011 (Box 137-1). The previously used terms “juvenile chronic arthritis” and “juvenile rheumatoid arthritis” were incorporated under the term JIA.
The clinical and laboratory tests that are currently available for assessment of JIA are poor for characterization of early inflammatory, hypoxic, and vascular changes, which are the primary physiologic events involved in the disease.8 Hence because the clinical and laboratory diagnosis of early joint changes in JIA is suboptimal,9,10 imaging becomes an ideal noninvasive method for early diagnosis and outcome measure during follow-up of joint changes in persons with this disease.
Epidemiology
The incidence and prevalence of JIA, respectively, is between 5-18 and 30-150 per 100,000 children younger than 16 years in Europe and North America.11 Twice as many girls as boys have JIA.12 Although few data are available on geographic or racial groups of persons with JIA, studies suggest that in the United States, proportionately fewer African American than white children have JIA.13 The onset of JIA before the age of 6 months is distinctly unusual; nevertheless, the age at onset is often quite young, with the highest frequency occurring between 1 and 3 years.14
Radiographic changes are seen most frequently in patients with JIA who have a polyarticular course.15,16 Large joints are most commonly affected in persons with this disease. The knee is the most frequently affected joint, followed by the ankle. Occasionally, changes may develop in the cervical spine or temporomandibular joint.17 It has been suggested that patients with JIA who have polyarthritis and wrist disease are at high risk of experiencing radiographic progression.18 The wrist is the most vulnerable site for early radiographic changes in patients with JIA.16,19
Pathophysiology
Although the etiology of JIA is unknown, some investigators believe that it is multifactorial given the heterogeneity of presentations and course of the disease.20 JIA is characterized by an acute synovitis that leads to synovial proliferation and formation of a highly cellular pannus.21 The pannus erodes the adjacent articular cartilage and subchondral bone, leading to centripetal articular destruction; that is, the articular damage starts at the periphery of the joint and progresses toward its center. Inflammatory changes also can involve tendon sheaths and bursa and can give rise to periostitis. With prolonged inflammation, more extensive joint changes including cartilage destruction, bone erosions, and joint malalignment often are present.
Despite the fact that JIA is usually transient and self-limited, without active synovitis in adulthood, up to 10% of children become severely disabled in adulthood. Despite therapy, 28% to 54% of children have progressive disease and experience cartilage or bone erosions, with a median onset of radiographic findings between 2.2 to 5.4 years after the initial disease presentation.22 The disease process leads to joint instability, subluxation, and ankylosis.23,24 Disturbance of joint growth can be consequent to the disease itself and/or to the treatment.17
Imaging
Imaging often plays a key role in establishing the presence, severity, and extent of joint disease, and it can also help monitor for disease complications, exclude other diagnoses, and assess treatment response. Imaging can provide early diagnosis and visualization of inflammatory abnormalities, including synovitis and osteochondral damage.25–27
Radiographs are the standard imaging tools for the diagnosis of JIA; however, they have low sensitivity (50%) and moderate specificity (85%) for detection of cartilage destruction.8
Both magnetic resonance imaging (MRI) and ultrasound can detect synovial hypertrophy, cartilage erosion and joint effusion in peripheral joints, and clinically meaningful response to treatment in children with JIA. Ultrasound is less sensitive than MRI for assessment of both soft tissue findings (sensitivity, 62%) and superficial cartilage loss (sensitivity, 60%).8 Overall, MRI is the imaging modality of choice for evaluation of joints in children with JIA. However, ultrasound can be an excellent initial imaging tool for evaluation of young children who otherwise would require sedation for MRI.8
Radiography
Conventional radiography is not effective in the evaluation of soft tissue abnormalities, which are precursors of cartilage degeneration in persons with JIA.26 Moreover, available radiographic scoring systems for assessment of JIA have poor internal consistency and poor criterion and construct validity because they do not take into consideration patients’ sex and age.28 Despite the aforementioned limitations and the strong evidence for low sensitivity (50%) and moderate specificity (85%) in the detection of cartilage destruction,8,28 in many centers this technique remains the standard practice for imaging evaluation of disease progression in persons with JIA, with an expanding role for ultrasound and MRI.29
A variety of radiographic features can be encountered with joint disease. Specific joint findings will depend on the underlying abnormality, the chronicity of the disease, and the response to therapy. A systematic approach to the imaging interpretation of any joint is highly recommended. One popular approach is the “ABCDS” of joint disease, featuring assessment of joint Alignment, Bone density and other bone changes, Cartilage loss, Distribution of joint disease (whether monoarticular, oligoarticular, or polyarticular) and Soft tissue abnormalities (Box 137-3).
The earliest abnormalities include soft tissue swelling, osteopenia, and effusion. Periosteal reaction occasionally may be seen. Typically, the osteopenia is initially periarticular (Fig. 137-1), becoming more diffuse with time. Osteopenia may be subtle and better recognized by comparison with the contralateral extremity (if it is unaffected). With long-standing disease, uniform bone loss may occur with a thin cortex. Uncommonly, a linear subphyseal demineralization can be observed, but this finding is nonspecific and can also be seen in persons with other conditions such as leukemia.30
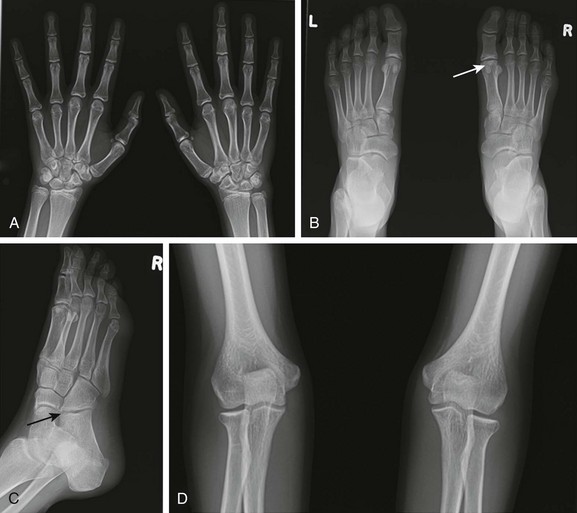
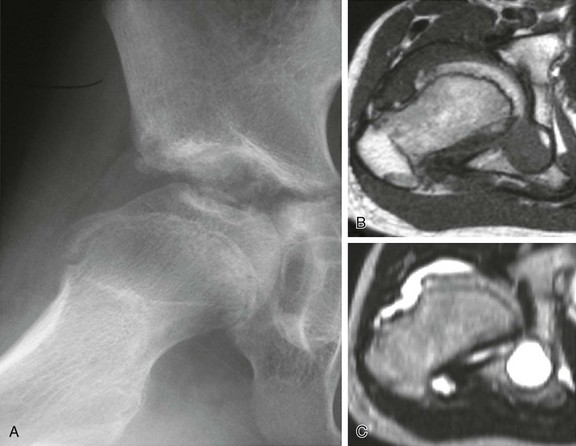
Figure 137-1 A 15-year-old girl with polyarticular juvenile idiopathic arthritis.
A frontal radiograph of the hands and wrists (A) shows periarticular osteopenia, erosive changes in the scaphoid, capitates, hamate, and triquetrum bilaterally, and joint space narrowing at the radiocarpal and carpal-metacarpal joints of the second and third digits. Radiographs of the feet (B and C) show flattening and subchondral sclerosis of the metatarsal head of the first right toe, suggesting avascular necrosis (arrow, B), and a right calcaneocuboid joint space narrowing (arrow, C) that was believed to be related to underlying inflammatory changes. A frontal radiograph of the elbows (D) demonstrates overgrowth of epiphyses (medial humeral epicondyles and radial heads) bilaterally.
Joint effusions are encountered commonly and can be seen in inflammatory or noninflammatory joint disease. A sign of knee effusions is fullness in the suprapatellar region, which is best seen on the lateral view. In the elbow, knee, and ankle, adjacent fat lines and fat pads are displaced. Periosteal reaction, when present, is commonly seen in the phalanges, metacarpals, and metatarsals but also can occur in the long bones. Joint space narrowing may be caused by cartilage loss (Fig. 137-1). In persons with JIA, the joint space narrowing is usually uniform. In some patients with rheumatoid factor positive polyarthritis or systemic arthritis, early erosive disease can occur (Fig. 137-2).
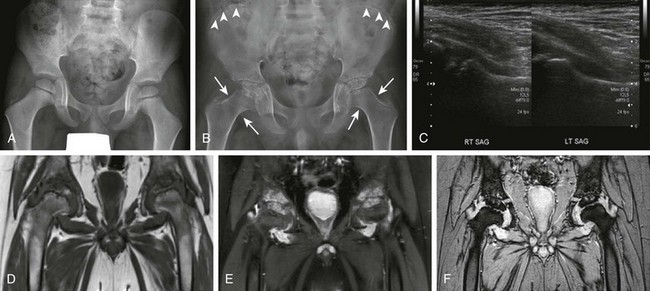
Figure 137-2 A patient with systemic juvenile idiopathic arthritis after having multiple hip infections.
At the age of 4 years, only slight irregularity of the contour of the proximal femoral epiphyses is noted on a frontal radiograph (A) representing a variation of normal, with preserved joint spaces. At the age of 11 years, extensive erosive changes are seen in the femoral heads and acetabula with further joint space loss at the hips bilaterally, as seen on radiographs (B). Interval increased sclerosis is noted along the acetabular roof bilaterally. Nonspecific periosteal reaction is seen along the medial aspect of the femoral necks bilaterally (arrows). Sclerotic lines are shown along the iliac wings (arrowheads) compatible with previous bisphosphonate therapy. Gray-scale ultrasound images (C) obtained at the age of 11 years, 1 month before the corresponding magnetic resonance (MR) imaging scan, demonstrate moderate left hip joint effusion and mild right joint effusion. On an unenhanced multiplanar gradient recalled acquisition MR image (D), a contrast-enhanced coronal T1-weighted spectral presaturation inversion recovery MR image (E), and a multiplanar gradient recalled acquisition MR image (F) at the age of 11 years, markedly thickened, lobulated, heterogeneously enhancing synovium is seen in both hip joints. Enhancing signal abnormality, subchondral cysts, and surface irregularity are seen along the superior compartment of the hip joints. Marked reduction in the hip joint spaces is seen bilaterally, with flattening of femoral heads. The findings are likely to represent severe progression of inflammatory arthritis with diffuse pannus formation. Bilateral secondary avascular necrosis of the femoral heads is noted.
Bone erosions are typically located at joint margins in the bare areas but also may occur at tendinous insertions. Bone erosions also can be seen in persons with septic arthritis or hemophilic arthritis related to the inflammatory reaction caused by intraosseous hemorrhage. Large erosions can be seen in the camptodactyly arthropathy coxa vara pericarditis syndrome31 (e-Fig. 137-3). Deformity of the fingers, whether with boutonniere (proximal interphalangeal [PIP] flexion with distal interphalangeal [DID] extension) or swan neck (PIP extension with DIP flexion) deformity, can be seen in a variety of disorders, including JIA (Fig. 137-4), camptodactyly arthropathy coxa vara pericarditis syndrome, or systemic lupus erythematosus. Enlarged or irregular epiphyseal ossification centers can be seen in persons with hemophilia, JIA, and tuberculous arthritis. Atlantoaxial subluxation or cervical vertebrae pseudosubluxation and ankylosis (e-Fig. 137-5) may be noted in persons with JIA, the arthropathy of Down syndrome, dysostosis multiplex, and systemic lupus erythematosus.
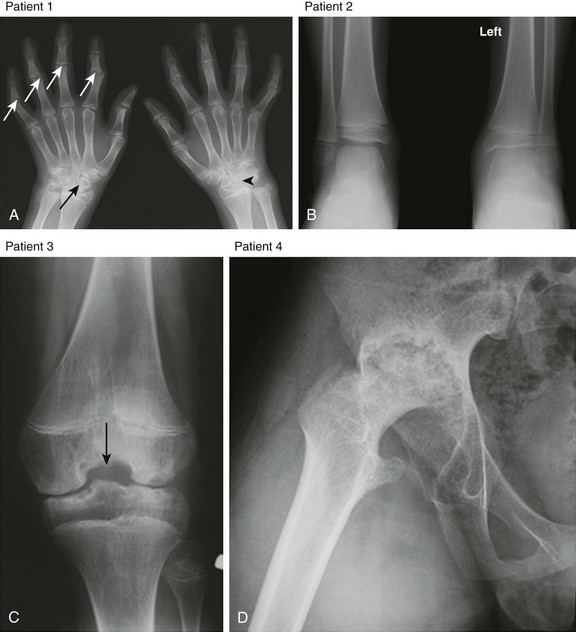
Figure 137-4 Radiographic findings of advanced juvenile idiopathic arthritis in the peripheral skeleton in different patients.
A, Patient 1: Deformity of the digits as a result of erosive changes is seen at the phalangeal heads and bases (white arrows) and metacarpal and carpal bones (black arrow), along with joint space narrowing, carpal ankylosis (arrowhead), and array subluxation. B, Patient 2: A radiograph shows joint space narrowing, advanced maturation, and epiphyseal overgrowth (shown in the distal left tibia and fibula in comparison with normal-appearing right counterparts). C, Patient 3: Widening of intercondylar notch of the knee. D, Patient 4: Protrusio acetabuli.
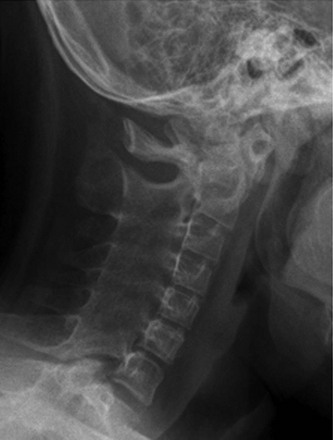
e-Figure 137-5 A child with polyarticular juvenile idiopathic arthritis.
A lateral cervical radiograph demonstrates diffuse ankylosis of the posterior elements of C3-T1.
Changes in bone growth and maturation with changes in the normal size of ossification centers and alteration of normal bone modeling can be seen in persons with JIA and in persons with infections and hemophilia. Enlargement of ossification centers and epiphyses (Fig. 137-4), contour irregularity, trabecular changes, and squaring (typically of the patella) can be seen. Tibiotalar slant (ankle valgus) also can be noted in persons with JIA.32
Late sequelae of JIA include epiphyseal deformity, abnormal angular carpal bones, widening of the intercondylar notch of knees (Fig. 137-4), and premature fusion of the growth plates. Growth disturbances are more frequent if disease onset is early. Joint space narrowing and osseous erosions are usually late manifestations. At the hip, protrusio acetabuli (Fig. 137-4), premature degenerative changes, coxa magna, and coxa valga can be seen. Joint space loss can progress to ankylosis, particularly in the apophyseal joints of the cervical spine (Fig. 137-5) and wrist. Rarely, ankylosis also can be seen in larger joints, including the hips. Subluxation of the joints, especially at the wrist, may be evident. Growth disturbance of the temporomandibular joint may lead to micrognathia and temporomandibular disk abnormality.32
Magnetic Resonance Imaging
MRI is an optimal tool for evaluation of both soft tissues and osteochondral abnormalities with superb tissue contrast.33,34 Contrast-enhanced MRI is extremely sensitive for detecting active disease and for early detection of cartilage loss, bone erosions, and synovial hypertrophy in children and adolescents.34 MRI can define vascular anatomy, often without the need for intravenous contrast.35 Higher cost, limited availability, and more frequent need for sedation in younger patients have limited its more widespread use. MRI provides multiplanar evaluation with a combination of available imaging sequences, including T1 and fast spin echo T2-weighted sequences, gradient echo sequences, and postcontrast studies all tailored to the specific clinical problem.36–38
Three-dimensional (3D) isovolumetric sequences also can be obtained, making it possible to reformat images in any desired plane. Cartilaginous structures including the growth plate are well visualized with gradient-recalled echo techniques39 or fat-suppressed fast proton density sequences. Gadolinium-enhanced MRI can help differentiate physeal from unossified epiphyseal cartilage and can visualize normal vessels present within the chondroepiphysis.40 MRI also is helpful in detecting synovial abnormalities within the joint41 and can be used to assess for changes in the synovium with therapy.42 Additionally, MRI can be used to demonstrate muscle pathology, typically demonstrating nonuniform increased signal intensity on T2-weighted images and normal signal on T1-weighted sequences. These findings are not specific but may help in the selection of a biopsy site.
Without use of contrast material, proliferating synovium on MRI appears as soft tissue thickening with an intermediate T1- and T2-weighted signal (Fig. 137-6). It may have slightly higher signal intensity than adjacent fluid on unenhanced T1-weighted images. Pannus appears as a thickened intermediate to dark signal intensity on T2-weighted images and is best seen when outlined by bright signal joint fluid. Its variable signal intensity reflects the relative amount of fibrous tissue and hemosiderin. Intravenous administration of gadolinium-based contrast agents improves visualization of thickened synovium, especially with use of fat-suppression techniques. Proliferating synovium appears as enhancing linear, villous, or nodular tissue. Images should be obtained immediately after contrast injection because diffusion of contrast material from the synovium into joint fluid occurs over time. Hypervascular inflamed pannus enhances significantly (see Fig. 137-2, E), whereas fibrous inactive pannus shows much less enhancement. Subchondral cysts and bone erosions (see Fig. 137-2) appear as low signal areas on T1-weighted sequences,36 with overlying articular and epiphyseal cartilage loss better delineated on fluid-sensitive sequences. Meniscal hypoplasia can be seen in some cases of JIA (e-Fig. 137-7).34
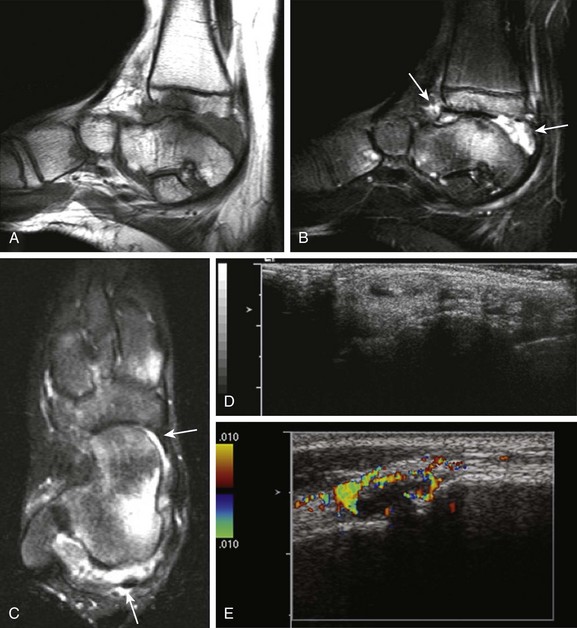
Figure 137-6 A 14-year-old girl with juvenile idiopathic arthritis and symptomatic tenosynovitis.
Sagittal T1-weighted (A) and fat-saturated T2-weighted (B) magnetic resonance (MR) images of the right ankle show synovial overgrowth within the tibiotalar (arrows) and intertarsal joints. Extensive erosive changes and bone marrow edema are noted at the tibiotalar joint (A and B). A small amount of fluid is seen within the synovial sheaths of the tendons of the medial aspect of the right foot, including the tibialis posterior (long arrow) and flexor hallucis longus (short arrow) on axial T2-weighted MR images, suggesting tenosynovitis (C). Longitudinal gray-scale (D) and color Doppler (E) ultrasound scans demonstrate a small amount of fluid noted within the synovial sheaths of the tendons of the medial aspect of the right foot (D) with associated local hyperemia (E).
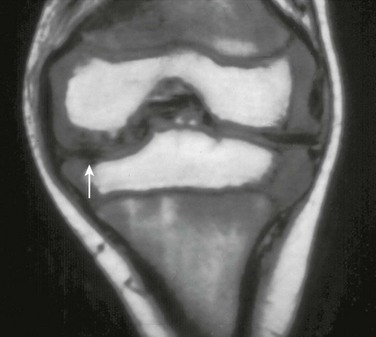
e-Figure 137-7 A 5-year-old patient with juvenile idiopathic arthritis.
A coronal T1-weighted magnetic resonance image shows meniscal hypoplasia (arrow) in the medial meniscus of the left knee.
Quantitative techniques have been developed for synovial volume.43,44 MRI is more sensitive than clinical evaluation in detecting some specific joint involvement, including the temporomandibular joint, which often demonstrates inflammatory change in the absence of clinical symptoms.45
With prolonged synovial inflammation, well-defined intraarticular nodules termed “rice bodies” (Fig. 137-8) may be present. Rice bodies likely arise from detached fragments of hypertrophied synovial villi. On MRI, rice bodies have dark signal on T2-weighted images because of their fibrous tissue composition and are associated with joint effusion, synovial hypertrophy, and synovial enhancement after gadolinium administration.46 Bone marrow edema is represented by areas of low T1-weighted and high T2-weighted signal intensity (e-Fig. 137-9) and should be differentiated from normal marrow speckling seen on fluid-sensitive sequences, most frequently in the ankle and foot.
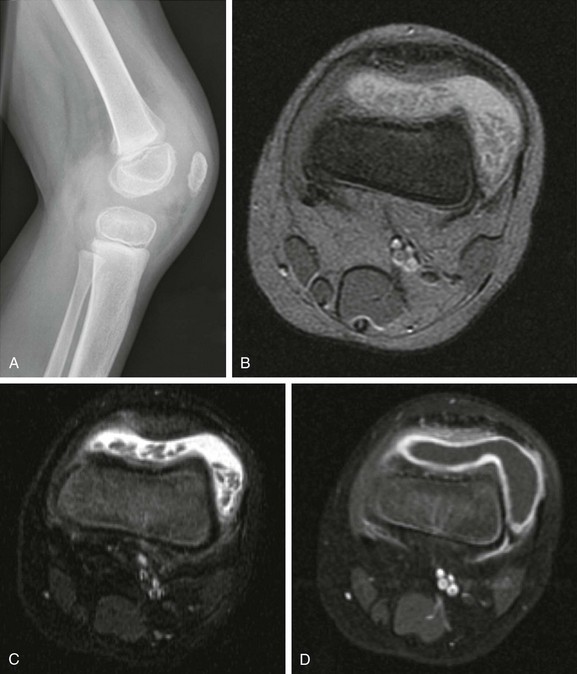
Figure 137-8 Rice bodies.
A 3-year-old boy had persistent knee pain 1 month after sustaining a trauma. The lateral radiograph view (A) shows moderately large joint effusion distending the suprapatellar bursa. Intraarticular elongated bodies are seen on axial multiplanar gradient-recalled acquisition (B) and T2-weighted fat-saturated (C) and contrast-enhanced T1-weighted fat-saturated (D) magnetic resonance images; these bodies, which extend into the recesses of the joint space and layer in dependent portions, are so-called “rice bodies.” Marked synovial enhancement also is noted. These findings suggest an underlying inflammatory arthritic process without associated joint destruction.
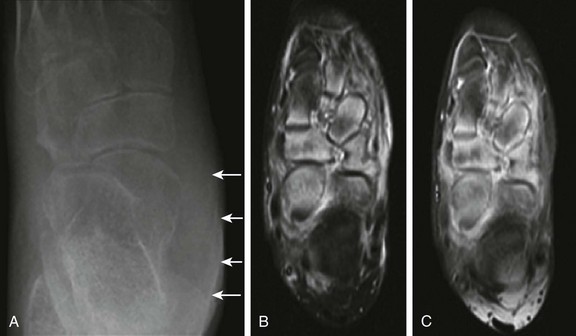
e-Figure 137-9 An 11-year-old girl with juvenile idiopathic arthritis presenting with a swollen left ankle and mid foot.
The radiograph (A) and magnetic resonance imaging (MRI) examination (B and C) were obtained 1 week apart. Unenhanced axial inversion-recovery (B) and postcontrast axial T1-weighted spin echo images (C) of the left foot obtained with fat saturation are shown. Soft tissue swelling is present along the left ankle (A) overlying the medial malleolus (arrows). Also present are marked osteopenia of the ankle, hindfoot, and midfoot and talonavicular, calcaneocuboid, and navicular-cuneiform joint space narrowing (A). Assessment of joint space narrowing is best performed with radiography. Although no erosions are identified on MRI, bone marrow edema is noted predominantly within the anterior aspect of the talus, navicular, and cuneiforms. Increased signal intensity on inversion recovery images (B) and contrast enhancement (C) is also noted in the soft tissues surrounding the involved bones, but the metatarsophalangeal and interphalangeal joints are unremarkable (not shown).
Recently, studies in adults47–49 have shown an association between presence of subchondral bone marrow edema in persons with inflammatory arthritis and radiographic erosive progression.
Although MRI has being extensively investigated for use in persons with JIA,8,50 standardized measures for data acquisition and interpretation are not currently available51; hence this technique is underutilized both in clinical practice and in research. In addition, the imaging evaluation of growing joints is challenging because thinning of articular cartilage can be either a physiologic or a pathologic process. As a result, early subchondral abnormalities can be masked in joints of young children on MRI because of the greater thickness of epiphyseal cartilage in these joints, which makes evaluation less accurate.8 Very few MRI scales have been designed to specifically assess morphologic changes with JIA.52,53
Novel MRI Techniques: A number of novel MRI techniques are under evaluation for improved assessment of synovial, cartilaginous, or osseous abnormalities (Table 137-1). These MRI techniques include diffusion-weighted imaging (DWI) and perfusion imaging, delayed gadolinium-enhanced cartilage imaging, and T2 quantification. DWI evaluates the translational movement (Brownian motion) of water molecules that occurs in all tissues, including synovium and cartilage. Alteration of normal diffusion can occur in diseases including infection, inflammation, and infarction.54 Diffusion tensor imaging (DTI) is a variant of DWI and has been used to study the structure of ordered biological tissue.55 DTI-derived metrics correlate with inflammatory cytokines and adhesion molecules and holds potential to delineate synovial inflammation; however, it is not superior to conventional MRI in the detection and assessment of therapeutic response.56 Despite the contrast-free characteristics of DWI and the possibility of using 3D steady-state sequences to enable imaging of short-T2 species with high signal-to-noise ratio, the use of DTI to assess cartilage in knee joints is limited because of the short T2 relaxation time of cartilage (30 to 70 msec).57
Table 137-1
Matching of Magnetic Resonance Imaging Sequences to Tissue of Interest
| Tissue | Measurement | MRI Sequence/Technique |
| Synovium | Fluid | T2-weighted fast spin echo MRI |
| Rate of transfer of contrast between plasma and extravascular extracellular space | Dynamic contrast-enhanced MRI | |
| Restricted water motion (inflammation proxy) | Diffusion tensor imaging | |
| Cartilage | Cartilage hydration and collagen orientation | T2-mapping MRI |
| Glycosaminoglycan content | Delayed gadolinium-enhanced MRI | |
| Proteoglycan depletion | 23Na MRI | |
| Proteoglycan content | T1 rho MRI | |
| Bone | Erosions | T1-weighted MRI |
| Bone marrow edema | T2-weighted fast spin echo or short tau inversion recovery MRI |
Modified from Borrero CG, Mountz JM, Mountz JD. Emerging MRI methods in rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:85-95.
Contrast-enhanced perfusion MRI assesses blood flow using intravenously administered paramagnetic contrast agents and may be helpful in characterizing ischemic or hyperemic areas. Potential uses of this technique include recognition of epiphyseal ischemia and quantification and monitoring of synovial inflammation.58 The rate of synovial enhancement depends in part on tissue vascularization and capillary permeability, both of which are highly correlated with synovial inflammation.59,60 Rapid enhancement suggests active synovial inflammation, whereas gradual delayed enhancement suggests subacute/chronic synovial inflammation.
The use of pharmacokinetic modeling for quantitative mapping of the perisynovial tissue61,62 can add information to subjective synovial assessment. In this physiologically based model, signal enhancement time courses are described by a plasma compartment and the extracellular extravascular space. Pharmacokinetic parameters that are representative of tissue signal enhancement have been shown to decrease in children with arthritis under treatment over time and provide specific information on synovial inflammation.62
Because cartilage is one of the earliest sites of damage in persons with JIA, it is an important area to be evaluated with MRI. Cartilage has bright signal on both fast spin echo and fat-suppressed proton density sequences; hyaline cartilage has the highest intensity40 and can be differentiated from epiphyseal and physeal cartilage. Articular cartilage should be assessed for areas of altered signal, thinning, erosions, or deep cartilage loss that may extend to the subchondral bone. The development of a variety of fast imaging methods with increased signal-to-noise ratio provide greater cartilage-synovial fluid contrast and have improved the MRI evaluation of cartilage morphology. Fat-suppressed 3D spoiled gradient-recalled echo imaging provides excellent contrast because cartilage has a bright signal compared with adjacent structures, but it is limited in differentiating epiphyseal, articular, and physeal cartilage. Other valuable sequences in cartilage assessment include driven equilibrium Fourier transform, dual-echo steady-state imaging, Dixon water-fat separation technique, and steady-state free precession.35
Delayed gadolinium-enhanced MR cartilage imaging is a sensitive technique for assessing cartilage proteoglycan content using the negative charge of the intravenously administered paramagnetic MR contrast agent.63 The contrast agent distributes into cartilage inversely to the fixed charge density of negatively charged glycosaminoglycan (GAG). T1 relaxation time in the presence of gadolinium agent is approximately linearly related to GAG content. Delayed gadolinium-enhanced MR cartilage imaging may be used to assess early cartilage injury with depletion of GAG before anatomic changes are visible by conventional cartilage sequences.
Cartilage assessment can also be provided by mapping T2 relaxation time measurements. These measurements may help characterize the structural integrity of the cartilaginous tissue and quantitatively assess the degree of cartilaginous hydration and collagen orientation.64 Typically an overall decrease in T2 relaxation from the cartilage surface to the deeper layers occurs.65,66 In persons with JIA, increased cartilage T2 relaxation time is thought to be an early marker of disease progression in JIA, because it can identify microstructural changes before damage becomes visible.67 In a longitudinal study evaluating patients with JIA from 3-month to 2-year follow-up, the clinical assessments improved, whereas T2 maps showed increased T2 values.68 This increase likely represents progressive microstructural changes, even though clinical symptoms improved with treatment.
Another alternative MRI method is Na23 MRI,69,70 which identifies areas of proteoglycan depletion through bonding of the positively charged sodium with negatively charged GAG molecules.71 The major limitation of this technique is the low overall sodium content in cartilage, which limits the signal-to-noise ratio. MRI protocols using higher strength field MRI scanners (7 tesla) have been devised to try to overcome this technical challenge.72–74 With the improved signal-to-noise ratio of higher strength field MRI scanners and dedicated transmit-receive radiofrequency coils, signal-to-noise ratio can be increased while specific absorption rate is reduced, which should facilitate imaging of small joints.75,76
Ultrasonography
Recent advances in ultrasonography, including better transducers and more pediatric musculoskeletal experience, have stimulated increased use of ultrasound in the assessment of pediatric joint disease. Ultrasound is ideal for assessing the pediatric musculoskeletal system largely because of its ability to visualize intraarticular structures such as cartilage and thickened synovium without the need for radiation. Ultrasound is very sensitive in detecting joint effusion, particularly in the hip (see Fig. 137-2, C) and shoulder, where radiographs are insensitive.77 Intraarticular masses also may be detected with ultrasound, although their appearance is often nonspecific. Tendons and ligaments also can be assessed with higher frequency transducers.78 Fluid within the synovial sheath appears as an anechoic halo surrounding the tendon (Fig. 137-10), whereas synovial thickening appears as a hypoechoic thickening around the tendon. Vascular anatomy can be assessed by combining sonography with Doppler. Synovial hyperemia leads to increased Doppler signal.79 Power Doppler has been shown to detect residual disease activity more sensitively than clinical examination and/or MRI both in active disease and when JIA is in remission80,81 and could be used to predict short-term relapse in patients with JIA who appear to be in remission clinically.82
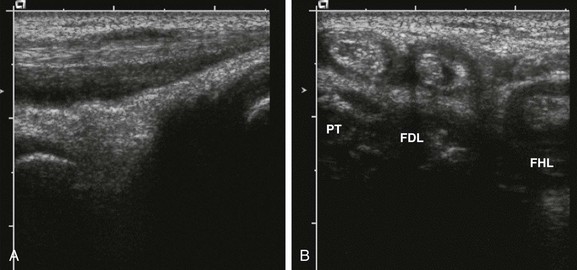
Figure 137-10 A 10-year-old girl with polyarticular juvenile idiopathic arthritis.
The longitudinal gray-scale ultrasound scan along the medial aspect of the patient’s left ankle shows an increased amount of fluid within the posterior tibialis tendon (A). Corresponding transverse (B) sonograms demonstrate tenosynovitis involving the posterior tibialis (PT), flexor digitorum longus (FDL), and flexor hallucis longus (FHL) tendons of this ankle.
Sonography can also be used to assess other periarticular soft tissue abnormalities, including popliteal cysts (e-Fig. 137-11) or other soft tissue masses, and to guide aspiration or injection of joints.83
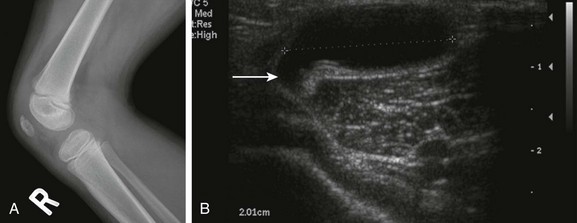
e-Figure 137-11 A Baker’s cyst.
A 4-year-old girl has an asymptomatic lump in the popliteal fossa of the right knee. The lateral radiograph (A) is unremarkable, but the transverse sonogram (B) through the popliteal fossa demonstrates a large cyst with a tail (arrow) extending toward the knee joint.
Disadvantages of ultrasound include lack of standardization of ultrasound techniques for assessment of growing joints and normative literature data, lack of capability to visualize the central aspect of some joints, and difficulty in assessing some joints, such as the temporomandibular joints (Fig. 137-12).45,84 Data on the diagnostic accuracy of ultrasound in children with JIA are limited. Assessment of joint effusion, synovial hypertrophy, and cartilage erosions by ultrasound can provide information about the severity of the disease.
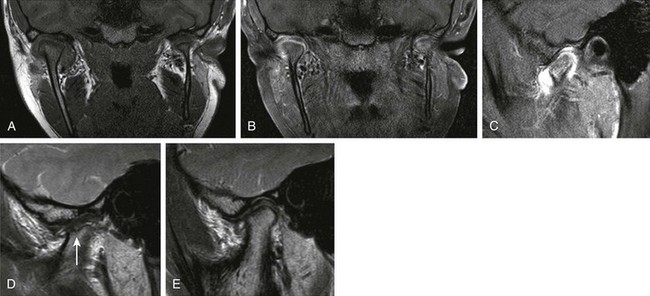
Figure 137-12 A 12-year-old girl with longstanding juvenile idiopathic arthritis and symptomatic and asymmetrically involved temporomandibular (TMJ) joints clinically.
A coronal T1 image (A), a contrast-enhanced coronal T1 spectral presaturation inversion recovery (SPIR) image (B), a contrast-enhanced T1 SPIR image (C, right TMJ), a sagittal proton density image (D, left TMJ), and a sagittal PD image (E, right TMJ) show moderate right acute TMJ synovitis and mild left acute synovitis. Established chronic arthropathic changes are seen in the left TMJ joint with definite erosive changes. Incipient evidence of chronic arthropathy is noted in the right TMJ. Note a hypoplastic left mandibular ramus in A and B.
Erosions and focal or diffuse thinning of the articular cartilage also can be detected, but only peripherally in the joint. Color Doppler ultrasound enables the detection of perisynovial hyperemia. Studies in children85,86 have demonstrated the ability of color and power Doppler sonography, with or without intravenous injection of contrast agents, to estimate synovial activity in JIA. Resistive indices and fraction of color pixels may be used as quantitative measurements of the blood flow.87,88 Contrast-enhanced sonography holds potential for detection of active synovial inflammatory disease in persons with subclinical JIA and may help guide early treatment.85
Very limited information is available about the diagnostic performance of ultrasound compared with MRI or clinical examination (in knees, sensitivity for joint effusion is 62%,89 ranging between 60% and 90% for clinically active joints and approximately 70% for clinically inactive joints90–92; for superficial cartilage destruction, overall sensitivity is 60%).89 Ultrasound-determined synovial thickness of the knee seems to correlate with clinical and laboratory (sedimentation rate and C-reactive protein levels) disease activity scores and with biomarkers of disease activity.92 In ankles, however, very poor agreement was observed comparing clinical and ultrasound scores.93
Treatment and Follow-Up
A recent systematic review on the best evidence for treatment of JIA94 showed that nonsteroidal antiinflammatory drugs are effective only for a minority of patients, mainly those with oligoarthritis. Intraarticular corticosteroid injections are very effective for persons with oligoarthritis. Methotrexate is effective for the treatment of persons with extended oligoarthritis and polyarthritis and less effective for persons with systemic arthritis. Sulfasalazine and leflunomide may be alternatives to methotrexate. Antitumor necrosis factor medications are highly effective for polyarticular JIA that is not responsive to methotrexate but are less effective in persons with systemic arthritis. Therefore despite many advances in the treatment of persons with JIA, evidence is still lacking for treatment of several disease subtypes.
With regard to the use of intraarticular corticosteroids, studies have shown that as many as 70% of patients with oligoarthritis do not have reactivation of disease in the injected joint for at least 1 year, and 40% do not have reactivation for more than 2 years.95–97 Radiographic and MRI studies have shown a marked decrease in synovial volume after injection without deleterious effects on the cartilage.98
Imaging in the Assessment of Response to Therapy
Radiography: Radiography is able to demonstrate epiphyseal overgrowth and osteopenia after the injection of intraarticular triamcinolone hexacetonide.99 Carpal length, defined as the radiometacarpal length plotted against the length of the second metacarpal bone on a chart with normal growth carpal scores, as described by Poznanski et al.,100 is another parameter that has been used in follow-up with an interval increase in carpal length (a positive change) indicating improvement.
Sonography: Eich et al.99 used ultrasound to determine the presence of effusion, pannus, popliteal cysts, and lymphadenopathy in 10 children with JIA affecting 15 joints (11 knee and 4 hip joints) before and after intraarticular therapy and concluded that ultrasound was as sensitive as MRI in demonstrating joint effusion and/or pannus but that differentiation between the two was difficult, particularly in the hip joint. Sureda et al.90 reported that 2 out of 16 patients (12.5%) had marked clinical improvement with a corresponding decrease in cartilage thickness (insufficient evidence).
Magnetic Resonance Imaging: Although CT is able to demonstrate joint space narrowing, erosions, and condylar flattening,101 MRI is currently the modality of choice to document changes before and after therapy. MRI can be used to monitor cartilage and bone erosions, effusion, pannus, and synovial volumes.39 In studies of children with arthritis who received intraarticular steroid injections, MRI has shown that intraarticular steroid therapy has a long-lasting beneficial effect, with suppression of synovial inflammation and reversion of pannus formation.98,99,103
Quantitative dynamic contrast-enhanced MRI based on pharmacokinetic modeling can be used to evaluate disease activity in the knee. A study of pharmacokinetic parameters and synovial volumes showed significant decreases at 12 months after intraarticular steroid therapy; however, improvement in synovial volume appeared to lag behind dynamic parameters, reflecting delay or subclinical synovitis.62 Of all the imaging modalities, MRI has been shown to be the most sensitive modality in the assessment of temporomandibular joint arthritis in children and has been used as a reference standard measure for comparison of clinical examination and ultrasound in clinical studies.45 Ultrasound has been shown to be less useful than clinical examination to exclude active temporomandibular joint arthritis in patients with JIA.45
Complications and Adverse Effects of Joint Injections
It is difficult to determine whether other postinjection changes in JIA patients reflect the underlying severity of the disease or the effects of the local treatment.104 The most common radiographic finding after injection is intraarticular calcification (e-Fig. 137-13). This calcification can resolve or persist for some time. It typically does not impair joint function. Damage to cartilage during the procedure is a potential complication.98 Additional complications of intraarticular steroid injections include skin depigmentation, overlying skin atrophy, and iatrogenic infection.
Enthesitis-Related Arthritis
Etiology, Pathophysiology, and Clinical Presentation
Enthesitis-related arthritis (ERA) affects 1% to 7% of patients with JIA and includes patients with arthritis and enthesitis, or with arthritis or enthesitis, as well as two of five defined criteria, including a history of sacroiliac joint tenderness and/or lumbosacral pain, the presence of the HLA-B27 antigen, the onset of arthritis in a male patient older than 6 years, uveitis, or a history of ankylosing spondylitis, inflammatory bowel disease, or uveitis in a first-degree relative.1 The term “ERA” was introduced in lieu of the term “pediatric spondyloarthropathies” to emphasize that (1) axial skeletal involvement in children is rare and large joint appendicular involvement is more common at presentation, and (2) the disease commonly involves tendon/ligamentous insertions and origins in children.
Imaging
Radiographic findings of ERA and other spondyloarthropathies are similar to those encountered in other forms of JIA with the exception of sacroiliitis and enthesitis, which are more specific for spondyloarthropathy (Box 137-4).105,106 In the appendicular skeleton, radiography typically shows asymmetrical involvement of the large joints of the lower limb, that is, the hip, ankle, knee, and tarsal joints. The interphalangeal joint of the hallux is also frequently involved. Radiographs may be normal initially or can demonstrate soft tissue swelling, effusion, ossification and epiphyseal overgrowth, erosions (Fig. 137-14), osteopenia, joint space narrowing, or, rarely, fusion. Bone erosions may be associated with irregular bone apposition at joint margins, referred to as “whiskering.” With hip involvement, these proliferative changes are noted at the junction of the femoral head and neck. Dactylitis may be seen with soft tissue swelling and periosteal reaction along the shaft of metacarpals, metatarsals, or phalanges.
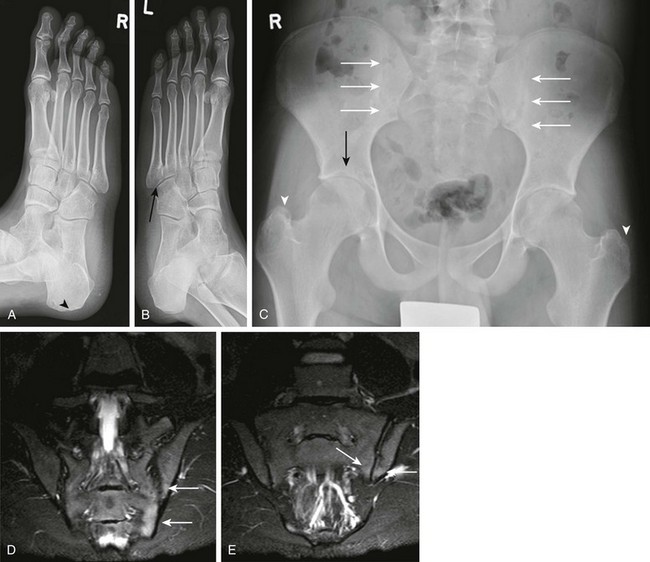
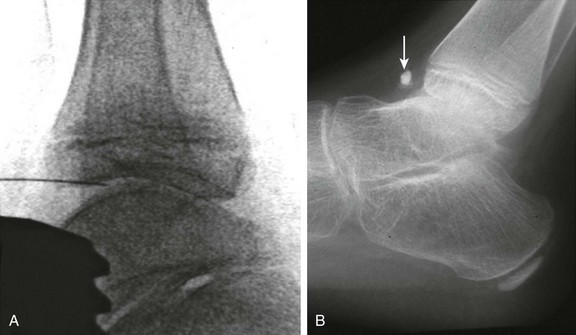
Figure 137-14 Oblique radiographs of the feet in a 16-year-old boy newly diagnosed with enthesitis-related B27 negative arthritis.
A, An erosion is seen at the Achilles tendon insertion of the right calcaneal tuberosity (arrowhead). B, Mild narrowing is noted at the left fifth tarsometatarsal joint (arrow). C, On the frontal radiograph of the pelvis, slight irregularity and sclerosis is seen along both sacroiliac joints (white arrows) suggestive of bilateral sacroiliitis. Uniform narrowing is noted in both hip joints in association with erosions and a subchondral cyst (black arrow) along the superior acetabulum and erosive changes at both greater trochanters (arrowheads), representing signs of enthesitis. D and E, Coronal T2-weighted magnetic resonance images with fat saturation obtained 2 months after the aforementioned radiographs show heterogeneous high bone marrow signal involving the iliac and sacral aspect of the left sacroiliac joint (arrows) confirming the presence of sacroiliitis.
In ERA, changes in the spine and sacroiliac joints generally are not seen until the latter part of the second decade or even adulthood. It is unusual for ERA to present initially with spinal involvement in children. Spinal involvement includes localized osteitis, erosions, and sclerosis, particularly at vertebral margins. Syndesmophytes and atlantoaxial subluxation are rarely seen in children. Radiographs may demonstrate unilateral or bilateral sacroiliitis (Fig. 137-14) with indistinct articular margins (also known as pseudowidening), erosions, and sclerosis, particularly on the iliac side of the joint. Radiography shows asymmetrical sacroiliac joint space widening initially, but eventually the classic bilateral, symmetrical joint involvement can be seen, with joint space narrowing and ankylosis. Radiographic evaluation of the sacroiliac joints is often especially difficult in teenagers. Diffuse osteopenia of the pelvic bones is also seen as a late change.
Sonography
On sonography, enthesitis may show loss of the normal fibrillar echotexture of the tendon and irregular fusiform thickening.107 Doppler sonography can be used to assess low-velocity flow in small synovial vessels.108 Using Doppler sonography, Tse et al.80 demonstrated the ability of color Doppler sonography to show decreased hyperemia at the cortical bone insertion of enthesis and along the adjacent synovium in children undergoing treatment, suggesting that this technique may add valuable information to gray-scale sonography.
Magnetic Resonance Imaging
Bone marrow edema, tenosynovitis, granulation tissue, or cortical erosion at the site of enthesitis in the appendicular skeleton may be seen with MRI.38 MRI can demonstrate early inflammatory changes in the sacroiliac joints and spine and is especially sensitive for evaluation of subchondral bone marrow edema not shown on other types of imaging.109
The administration of contrast material improves the detection of early sacroiliitis and enthesitis-related arthritis (Fig. 137-15). On MRI, a periarticular low signal may be seen on T1-weighted images, with a high signal on T2-weighted images from inflammatory changes in bone marrow, whereas a low signal on both sequences will be seen with bone sclerosis. MRI also may demonstrate erosions in articular cartilage.38 Evaluation of more widespread anatomic assessment, including whole body MRI, is currently under way and appears useful at least in demonstrating the presence of multiple sites of enthesitis-related disease.
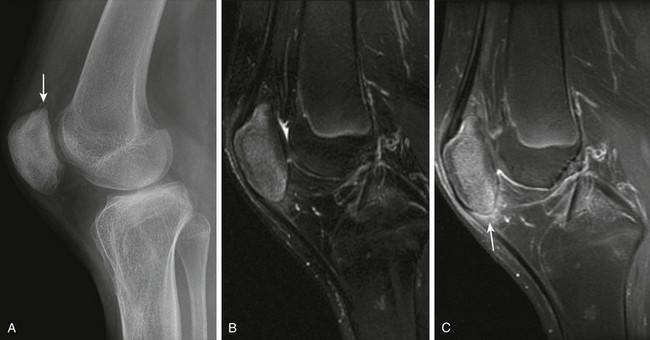
Figure 137-15 A 13-year-old boy with enthesitis-related arthritis.
A, A lateral radiograph shows spurring at the upper aspect of the patella (arrow). Corresponding sagittal T2 fast spin echo fat-saturated (B) and contrast-enhanced sagittal T1-weighted fat-saturated (C) magnetic resonance images reveal increased signal intensity in the patellar and postcontrast enhancement at the origin of the patellar tendon and adjacent Hoffa’s fat pad (arrow).
Treatment and Follow-Up
Evidence for the optimal treatment of enthesitis-related arthritis is lacking.94 Conventional treatments frequently offer limited efficacy. Treatment with nonsteroidal antiinflammatory drugs and corticosteroids may provide symptomatic improvement but do not alter disease progression. It was found that sulfasalazine is not significantly better than placebo.110 Methotrexate has an uncertain effect and has not been demonstrated to modify disease course.111 Treatment directed against tumor necrosis factor-α seems to significantly improve the arthritis and enthesitis.111,112 Infliximab treatment yielded responses in young adults presenting with peripheral enthesitis inflammatory heel pain.113
Psoriatic Arthritis
Etiology, Pathophysiology, and Clinical Presentation
Psoriatic arthritis involves 2% to 15% of patients with JIA and may involve either larger joints such as the knees and ankles or interphalangeal joints of hands and feet, resulting in the characteristic “sausage digits.”20 Unlike in adults, in children, arthritis actually may precede skin manifestations.
Imaging
Radiographs obtained in the initial phase of the disease may be normal or show juxtaarticular osteoporosis. Characteristic radiographic features of psoriatic arthritis include asymmetric involvement, sausage digits (Fig. 137-16), joint erosions, joint space narrowing, bony proliferation including periarticular and shaft periostitis, enthesitis, osteolysis including “pencil-in-cup” deformity (Fig. 137-17), acro-osteolysis, spur formation, and ankylosis.35 The bone erosions tend to be larger and more asymmetrical than those seen in persons with JIA, but the radiographic features may be indistinguishable from other forms of JIA. The characteristic adult changes seen at the distal interphalangeal joints are uncommon in children.
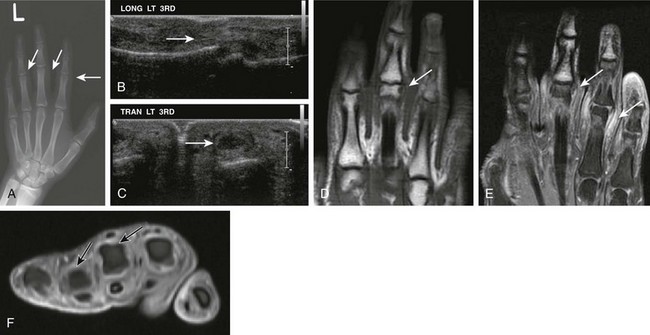
Figure 137-16 A 16-year-old boy with psoriatic arthritis.
A, A frontal radiograph of the patient’s left hand shows soft tissue swelling at the proximal interphalangeal joint level at the right second, third, and fourth digits. Corresponding longitudinal (B) and transverse (C) ultrasound images demonstrate fusiform swelling surrounding the proximal interphalangeal joints of the right hand. Localized echogenic thickened tissue compatible with synovial hypertrophy is seen (arrows) within the left third interphalangeal joint. Corresponding unenhanced coronal non–fat-saturated (D) and contrast-enhanced fat-saturated coronal (E) and axial T1-weighted images (F) of the left hand show prominent synovial hypertrophy and soft tissue enhancement (arrows) of the left third and fourth proximal interphalangeal joints.
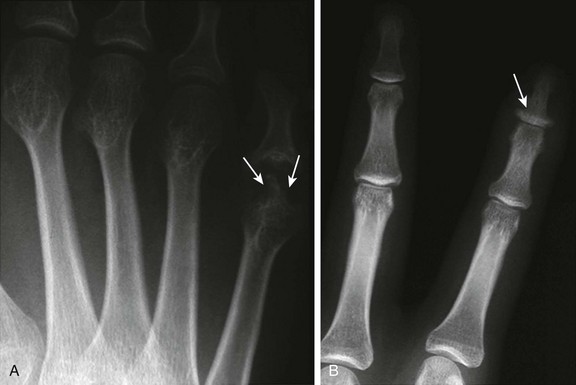
Figure 137-17 A patient with advanced psoriatic arthritis.
Radiographs of the digits reveal extensive erosive changes at the distal end of the fifth metacarpal, resulting in a “pencil-in-cup” appearance at the proximal interphalangeal joint (A, arrows), and bony proliferation at the base of the distal phalanx (B, arrow).
Sacroiliitis and vertebral involvement typically manifest later during the progression of the disease. The sacroiliitis of juvenile psoriasis is usually asymmetric and resembles that of reactive arthritis. Syndesmophytes, paraspinal calcification, and atlantoaxial subluxation are rare in children.35
Magnetic Resonance Imaging
In persons with psoriatic arthritis, MRI demonstrates erosive changes, joint space narrowing, ligament disruption, and tenosynovitis. Sausage digits are seen in patients with psoriatic arthritis and reactive arthritis. These swollen fingers or toes result from tenosynovitis, soft tissue edema, and synovial proliferation.114 MRI may also be used to evaluate the responsiveness of therapy as noted by a significant reduction in gadolinium uptake after treatment with infliximab.115 In the axial skeleton, contrast-enhanced MRI can demonstrate synovitis (e-Fig. 137-18) and may be a useful tool for follow-up of response to therapy.
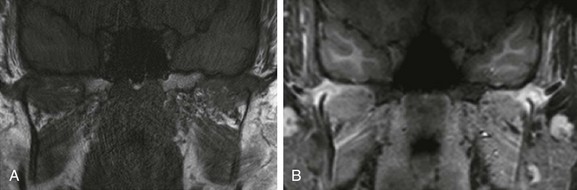
e-Figure 137-18 A 15-year-old boy with newly diagnosed psoriatic arthritis but long-standing symptoms who presented with microretrognathia on physical examination.
Precontrast and postcontrast coronal T1-weighted magnetic resonance images demonstrate severely dysplastic mandibular condyles with tapering of the mandibular condyles bilaterally (A). Intense pericondylar synovial enhancement is noted bilaterally after administration of intravenous gadolinium (B).
Computed Tomography
CT may be useful in assessing spine disease, but it has little role in the assessment of peripheral joints. Previous studies have shown that CT is as accurate as MRI for assessment of erosions in the sacroiliac joints but is not as affective for identifying synovial inflammation.114a CT can be used to guide sacroiliac joint injection.115
Hemophilic Arthritis
Etiology, Pathophysiology, and Clinical Presentation
Hemophilia, an X-linked recessive disorder characterized by abnormality of the coagulation mechanism, affects 20 in every 100,000 males in North America.116 It may be a result of a deficiency in factor VIII as in classic hemophilia (hemophilia A), or it may result from a deficiency of factor IX in persons with Christmas disease (hemophilia B).116
Hemarthrosis occurs in 75% to 90% of patients with hemophilia117,118 and starts in the first and second decades of life. The most commonly affected joints include the knee, elbow, and ankle. Recurrent joint bleeding leads to secondary inflammation and hypertrophy of the synovium in response to blood products, and the cartilage degeneration and development of erosions and subchondral cysts119 are similar to a primary inflammatory arthritis. Hemophilic pseudotumors, which are often intramuscular in location, may develop in these patients.
Imaging
The radiographic changes may be identical to those in children with JIA, but clinical findings and typical joint involvement help distinguish these entities.120 Radiodense joint effusions and subchondral cystic changes are more common in persons with hemophilic arthropathy. In the knee, the classic radiographic findings include squaring of the femoral condyles (Fig. 137-19), multiple erosions, a widened intercondylar notch, and squaring of the patella.25
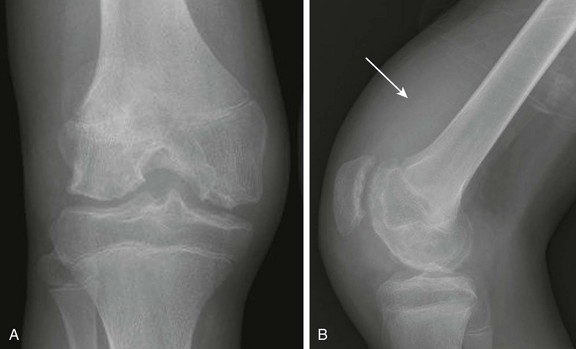
Figure 137-19 An adolescent boy with hemophilic arthritis.
Frontal (A) and lateral (B) radiographs demonstrate widening of the intercondylar notch, epiphyseal cortical angular deformities related to innumerable erosions, and a large radiodense suprapatellar soft tissue density related to hemarthrosis (arrow).
Magnetic Resonance Imaging
MRI can detect acute and subclinical prior hemarthrosis.117 In a cohort study evaluating ankles, elbows, and knees of 24 boys with severe hemophilia examined by MRI at a median age of 8.8 years (range, 6.2 to 11.5 years), hemosiderin deposition was detected by MRI in 26% of joints (ankles, 63%; elbows, 16%; and knees, 12%) with no history of clinically evident bleeding.120
Acute hemarthrosis and chronic joint effusion may be indistinguishable with a low signal on T1-weighted images and a high signal on T2-weighted images.121 Subacute hemarthrosis usually has a high signal on both T1- and T2-weighted images, which is related to the presence of extracellular methemoglobin.121 Hemosiderin deposition can occur in persons with JIA but is more frequently seen in persons with other disorders, including hemophilic arthropathy, pigmented villonodular synovitis, synovial venous malformation, and posttraumatic synovitis.122–124
On MRI, gradient echo sequences are most sensitive in detecting synovial hemosiderin deposition, with signal loss occurring because of increased magnetic susceptibility (Fig. 137-20). Synovitis that contains hemosiderin appears very low in signal on all MRI sequences, and this finding is accentuated on gradient echo sequences.125,126 The synovial thickening often has areas of low signal on T1- and T2-weighted imaging related to fibrosis or hemosiderin deposition.121 Although gadolinium can better delineate the extent of synovial thickening, which shows less enhancement compared with rheumatoid arthritis, previous studies127 have shown that dynamic MRI is not useful for evaluating hemophilic arthropathy, and gadolinium contrast agents are not routinely indicated.
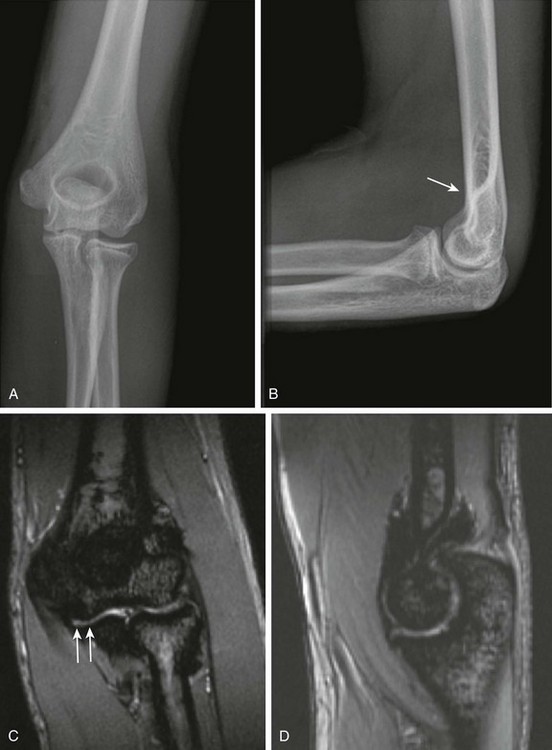
Figure 137-20 Hemophilia.
Frontal (A) and lateral (B) radiographs of the left elbow of a 16-year-old hemophilic boy. A subtle anterior fat pad is visible as a sign of joint effusion/synovial thickening (arrow). Note is made of slight overgrowth of the radial head. Coronal (C) and sagittal (D) multiplanar gradient-recalled acquisition magnetic resonance images of this elbow show severe synovial hypertrophy and hemosiderin deposition, as well as surface erosions and cartilage loss (arrows).
Sonography
A major advantage of ultrasound over MRI in the assessment of hemophilic joints is the lack of interference of susceptibility artifacts, which are seen commonly on gradient-echo MRI sequences128 and may obscure the synovium in the joint. In a patient with intraarticular bleeding with significant hemosiderin deposition, gradient-echo MRI sequences cannot tell whether the patient has mild, moderate, or large synovial hypertrophy. Ultrasound, on the other hand, is able to quantify the amount of synovial hypertrophy regardless of the amount of the hemosiderin deposition (e-Fig. 137-21). This advantage of ultrasound is particularly important for evaluating patients who may be candidates for radiosynovectomy.
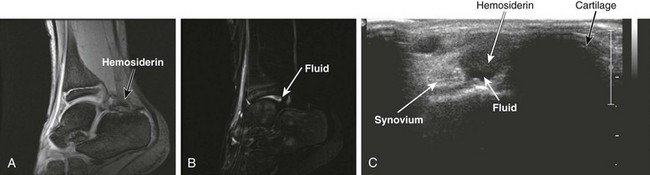
e-Figure 137-21 Hemophilia.
Sagittal gradient echo (A) and T2-weighted (B) images of a hemophilic ankle show a mild amount of hemosiderin deposition and joint effusion. Only minimal “blooming” (susceptibility artifact) is noted within the posterior synovial recess of the ankle; however, the synovium is not promptly identified on the gradient-echo images. A corresponding ultrasound image (C) allows easy discrimination between synovium (echogenic, “white”), hemosiderin (hypoechoic, “gray”), and fluid (anechoic, “black”).
Recently, a few systematic protocols for ultrasound imaging of hemophilic joints have been developed,129–131 which enable systematization of imaging protocols and future comparative clinical trials. Although a prior study found a very close relationship between ultrasound and MRI in the assessment of the synovium and a good correlation between the degree of cartilage damage in ultrasound and the progression of bone changes in radiographs,132 further investigation is required to determine the value of ultrasound for assessment of cartilage changes. Ultrasound has limitations in the assessment of deeper cartilage surface abnormalities that are expected to occur in early stages of hemophilic arthropathy, given the centrifugal progression of cartilage degeneration.133,134
Treatment and Follow-Up
Treatments for hemarthrosis include injection of the deficient coagulation factor, cryotherapy, arthrocentesis of intraarticular blood (guided by ultrasound), and arterial embolization (in cases in which an arteriogram has identified a bleeding vessel).135 Radioactive injections into joints (radionuclide synovectomy) initially were used in cases of JIA but have been shown to be effective in reducing bleeding and effusion in selected cases of hemophilic arthropathy. Radionuclide synovectomy has a good record for low complication rates; however, concern remains that the radiation exposure in young children could lead to radiation necrosis or tumor induction.136
Prophylaxis is the first therapeutic option in noninhibitor hemophilic patients.137 This therapy reduces the joint symptoms and avoids further degeneration of the joints and should be started prior to the development of cartilage lesions. MRI is used to detect early hemophilic disease and to help direct the appropriate therapy. The role of ultrasound for detecting early cartilage lesions is still under investigation.138
Transient Synovitis
Etiology, Pathophysiology, and Clinical Presentation
Transient synovitis is the most common cause of childhood hip pain and can be mimicked by a number of more serious hip disorders, including Legg-Calvé-Perthes disease, slipped capital femoral epiphysis, JIA, septic arthritis, and malignancy.139 Transient synovitis is an acute, self-limiting disorder of unknown origin.139
Imaging
Imaging is usually performed with conventional radiography or ultrasound. It has been proposed that radiography should not be used in the primary evaluation of most children with hip pain because the results are generally normal or show only subtle findings of joint effusion. Exceptions should be made in infants younger than 1 year of age and in children older than 8 years because of the lower incidence of transient synovitis in these age groups, with the higher risk of child abuse and septic arthritis in infancy, and the occurrence of slipped capital femoral epiphysis in the older age group.139 Although sonography is a sensitive and noninvasive method of detecting hip joint effusion in persons with transient synovitis (Fig. 137-22),140 it is unable to distinguish between different types of synovitis. Thus the combination of the patient’s age, history of fever, and laboratory studies is useful to distinguish transient synovitis and septic arthritis.141
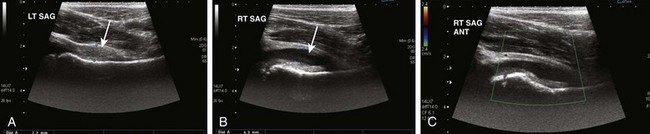
Figure 137-22 A 7-year-old girl with transient synovitis.
Longitudinal gray-scale ultrasound images of the right hip show marked synovial hypertrophy (A, arrow) and joint effusion (B, arrow). No significant synovial hyperemia is noted on color Doppler (C). ANT, Anterior; LT, left; RT, right; SAG, sagittal.
Nevertheless, the major contribution of ultrasound is to confirm the presence of joint effusion.141 An ultrasound joint space thickness difference >2.0 mm between asymptomatic and symptomatic hips has been considered valid for the presence of effusion.142 Other modalities such as bone scans, CT, and MRI are not used initially in the evaluation of irritable hips because of their higher cost and limited benefit.25
Treatment and Follow-Up
Aspiration is required if infection is a consideration and is readily accomplished with ultrasound guidance.143 Treatment for hip pain related to transient synovitis can include traction of the hip in 45° of flexion, which may minimize intracapsular pressure. Treatment with ibuprofen may shorten the duration of symptoms.144
Malignancies
Etiology, Pathophysiology, and Clinical Presentation
Any of the childhood malignancies, especially the leukemias and neuroblastoma, can mimic rheumatic disease. These malignancies may (Fig. 137-23) or may not involve the joint itself. Leukemic arthritis typically presents with transient arthralgias and joint pain, often involving the knees, shoulders, and ankles.30 Joint effusion is postulated to occur in relation to either synovial infiltration with leukemic cells and/or an autoimmune response related to the leukemia. However, differentiating leukemic arthritis from infection is not possible, and aspiration may be necessary.
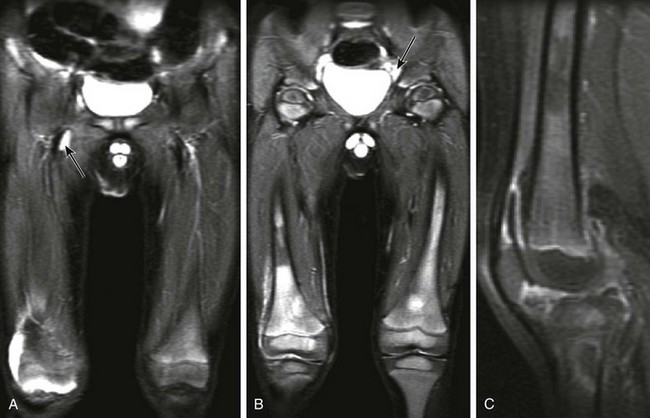
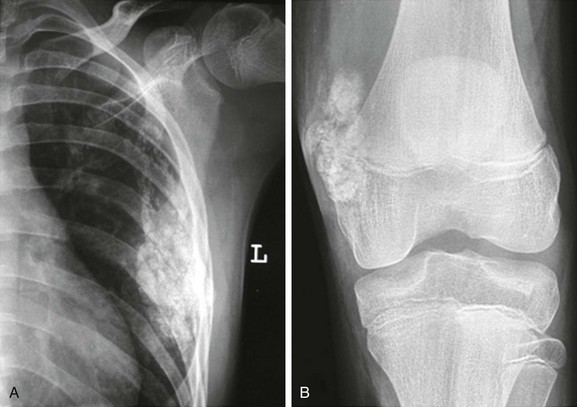
Figure 137-23 A patient with leukemia.
Coronal inversion-recovery (A and B) and contrast-enhanced sagittal T1-weighted fat-saturated (C) magnetic resonance images of the lower extremities show a large right joint effusion (A) with synovial enhancement representing synovitis (C). Diffuse heterogeneous signal abnormality is seen within the bone marrow of the femora and right proximal tibia, along with generalized adenopathy (A). Note is made of patchy metaphyseal enhancement in the proximal right tibia likely reflecting early bone marrow ischemia. This patient was diagnosed with acute lymphocytic leukemia.
Imaging
Although radiologic findings often are normal, they can include joint effusion, osteopenia, periostitis, lytic or sclerotic bone lesions, and metaphyseal radiolucent bands.145,146 Diffuse abnormal signal intensity of bone marrow, which is low on T1-weighted sequences and high on T2-weighted sequences, can be seen on MRI (Fig. 137-23).
Pigmented Villonodular Synovitis
Etiology, Pathophysiology, and Clinical Presentation
Pigmented villonodular synovitis (PVNS) is a benign proliferative disorder of uncertain etiology that affects synovial lined joints, bursae, and tendon sheaths (where it is sometimes termed “cell tumor of tendon sheath”). PVNS mostly occurs in the third and fourth decades of life147 but can affect persons of any age.148
Gross pathologic features include a thickened synovium with a combination of villous and nodular proliferation, depending on the site of involvement. PVNS can either be focal or diffuse (i.e., involving nearly the entire synovial lining). On microscopy, PVNS is characterized by the presence of hemosiderin-laden, multinucleated giant cells. In addition, lipid-laden macrophages, fibroblasts, and other large, mononuclear cells are present. Hemosiderin also is found within the surrounding tissues. The ubiquitous presence of hemosiderin lends the tissue a characteristic pigmented appearance. The lesions tend to be hypervascular and demonstrate synovial hyperplasia.148 PVNS typically invades local tissues and has the potential for extensive local destruction, even though it rarely metastasizes.149
Imaging
Radiographs show soft tissue swelling without calcification; in advanced stages, findings are of degenerative arthritis, including bony erosions and subchondral cysts. The invasion of the subchondral bone, with resultant cyst formation, is a characteristic finding.150 When PVNS involves relatively constricted joint spaces such as the hip, erosions related to intraarticular mass effect may be seen earlier compared with more capacious joints such as the knee.
On MRI, diffuse or nodular synovial thickening is noted, with low to intermediate T1 signal. Thick frondlike synovial excrescences often are identified extending into a joint effusion. Hemosiderin deposition results in linear low T2 signal, particularly at the periphery of the thickened synovium, which is more prominent on gradient-echo sequences; anecdotally, this finding is less likely to be seen in younger children. On postcontrast imaging, homogeneous enhancement is seen (Fig. 137-24).20,151 Focal PVNS usually is a well-defined solitary masslike lesion that superficially may not appear to arise from synovium (Fig. 137-25), whereas masses related to diffuse PVNS originate from the synovium (see Fig. 137-24).
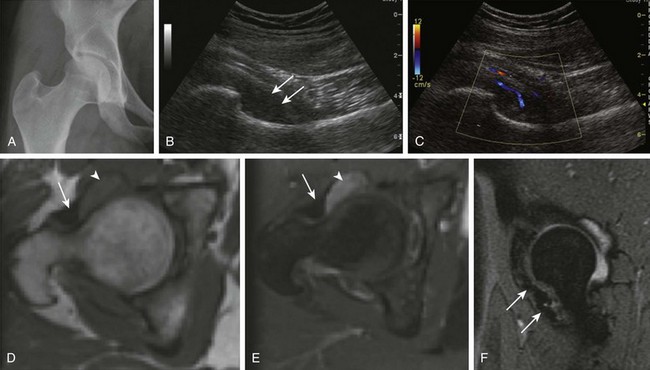
Figure 137-24 Pigmented villonodular synovitis (PVNS).
A 17-year-old girl with a history of right hip pain for 3 years has a biopsy-proven diagnosis of PVNS. A frontal radiograph of the pelvis (A) shows a slight increase in the right femoroacetabular joint space medially. A subsequent longitudinal ultrasound of the right hip (B and C) demonstrates an area of synovial thickening within the anteroinferior aspect of the right hip with fine internal solid echogenicity (B, arrows) and internal vascularity on color Doppler (C). Mild right hip effusion was confirmed on the corresponding magnetic resonance imaging (MRI) examination. Unenhanced (D) and contrast-enhanced (E) axial T1-weighted MR images of the right hip reveal moderate thickening and enhancement of the synovium (arrowheads). A sagittal multiplanar gradient-recalled acquisition (F) MR image shows low signal intensity within the anteroinferior aspect of the right hip with mild blooming artifact consistent with extracellular hemosiderin (arrows). No invasion of the adjacent bone is noted.
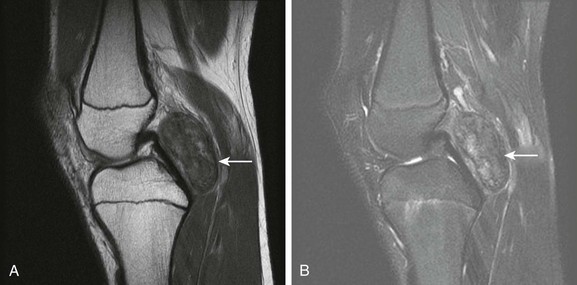
Figure 137-25 A 15-year-old girl with focal pigmented villonodular synovitis (PVNS).
Sagittal proton density (A) and T2-weighted fat saturated sagittal (B) images of the knee demonstrate a well-circumscribed mass with heterogeneous signal on fluid sensitive sequences in the posterior knee joint space (arrow), with a biopsy confirming PVNS.
Synovial Chondromatosis/Osteochondromatosis
Etiology, Pathophysiology, and Clinical Presentation
Synovial chondromatosis/osteochondromatosis is a benign condition of unknown etiology characterized by synovial membrane proliferation and metaplasia. It is rare in children,152,153 and mostly occurs in the third to fifth decades of life.154 The synovium undergoes neoplastic nodular proliferation, and fragments may break off into the joint. There, nourished by synovial fluid, the fragments may grow, calcify, or ossify. The fragments may be found free within the joint cavity, or they may be embedded within the proliferating synovium, which may extend into the surrounding soft tissues.154 The natural history of synovial osteochondromatosis is gradual progression of disease, joint deterioration, and secondary osteoarthritis.
Imaging
Imaging findings depend on the degree of mineralization of the chondro-osseous bodies. Ossification (synovial osteochondromatosis) is seen in 70% of patients.20 Unlike with adults, children rarely present with radiographically detectable ossified loose bodies. Therefore the initial diagnosis often is suggested from MRI, where these particles may mimic pannus, rice bodies, and PVNS. On MRI, a noncalcified lesion is seen as an isointense T1-weighted and high T2-weighted signal mass. In a calcified lesion, focal areas of bone marrow signal are noted on all sequences (Fig. 137-26) but appear most conspicuous on gradient-echo sequences.20
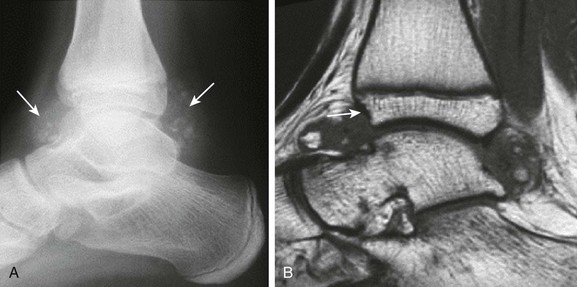
Figure 137-26 Synovial osteochondromatosis.
An adolescent had a history of pain in the right ankle for years. A lateral radiograph (A) shows soft tissue swelling with multifocal calcific concretions within the anterior and posterior synovial recesses of the ankle (arrows). The corresponding sagittal T1-weighted image (B) of this ankle demonstrates large nodular synovial proliferation within the anterior and posterior synovial recesses with bony fragments seen within the synovial tissue. Associated incipient erosive changes at the anterior distal tibial epiphysis are best demonstrated by magnetic resonance imaging (arrow).
Lipoma Arborescens
Etiology, Pathophysiology, and Clinical Presentation
Lipoma arborescens is a rare intraarticular lesion of unknown etiology characterized by replacement of synovial cells by fat cells, producing villous transformation of the synovium. It is not a true neoplasm and most commonly affects the suprapatellar bursa of the knee in patients between the fifth and seventh decades of life.155,156 Patients present with painless joint swelling and effusions.155
Lipoma arborescens associated with degenerative joint disease and chronic inflammatory arthritis (secondary lipoma arborescens) is more common in the adult population, whereas primary lipoma arborescens (without a primary etiology) is seen predominantly in a younger population.157
Imaging
The characteristic MRI appearance is frondlike synovial proliferation with fat signal intensity on all pulse sequences (Fig. 137-27), but it is best seen on T1-weighted and fat-suppressed T2-weighted sequences.155,156
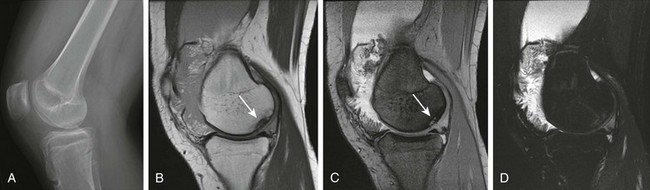
Figure 137-27 A 15-year-old girl with a diagnosis of psoriatic arthritis, lipoma arborescens, and osteochondritis dissecans.
A lateral view of the radiograph of the right knee (A) shows a large joint effusion and an osteochondral defect (in the medial right femoral condyle; arrow). Corresponding sagittal proton-density (B), multiplanar gradient-recalled acquisition (C), and T2-weighted fat-saturated fast spin echo (D) magnetic resonance images of the patient’s right knee demonstrate, in addition to the known osteochondral defect, a large effusion with fat signal frondlike synovial proliferation in keeping with lipoma arborescens.
Vascular Malformations
Etiology, Pathophysiology, and Clinical Presentation
Vascular malformations are benign lesions that usually are diagnosed in childhood or young adulthood. The Mulliken and Glowacki classification for these lesions separates these lesions as high flow (arteriovenous malformations and fistulas) and low flow (venous, lymphatic, capillary) lesions and reserves the term hemangioma for vascular tumors that present during infancy.160 The most frequent vascular malformation that may present with arthropathic changes is a venous malformation with synovial involvement,161 although in the literature, these lesions often are erroneously referred to as “synovial hemangiomas.”162 Clinically, they mimic chronic arthritis because of recurrent bleeding of the involved synovium, and not uncommonly, diagnosis is delayed. They may have associated cutaneous lesions, recurrent hemarthrosis, and arthropathy (Fig. 137-28). The knee is the most commonly affected joint, but other joints such as the elbow, wrist, and ankle also can be affected, along with tendon sheaths. These lesions often have both a synovial component and a large extrasynovial component.161
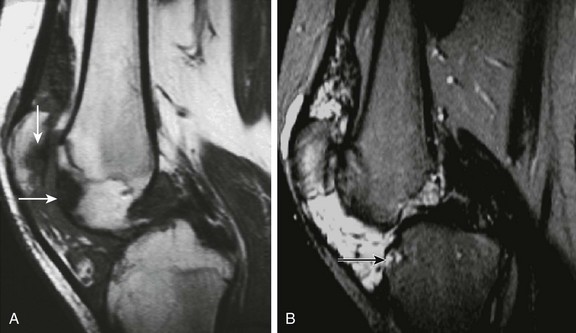
Figure 137-28 Venous malformation.
An adolescent presented with chronic pain in the left knee. Sagittal T1-weighted (A) and T2-weighted fat-saturated (B) magnetic resonance images show an extensive soft tissue mass with predominant low signal intensity on a T1-weighted image (A) and high signal intensity on a T2-weighted image (B) involving the suprapatellar bursa and Hoffa’s fat pad of this knee. Focal areas of low signal intensity on both T1- and T2-weighted images suggest the presence of hemosiderin deposition from previous hemarthrosis. Erosive and subchondral abnormalities are noted along the articular surface of the tibial plateau, patella, and anterior aspect of the femoral condyle (arrows) as a result of inflammatory arthropathy generated by the presence of intraarticular blood products.
Imaging
Radiography can demonstrate phleboliths in the setting of synovial venous malformations, a soft tissue mass, joint effusion, osteoporosis, advanced epiphyseal maturation, leg length discrepancy, arthropathy simulating hemophilia, and periosteal reaction.161
The MRI appearance varies from slightly lobulated, nonencapsulated masses to infiltrating serpentine vascular masses to a combination of these morphologies. They are usually isointense to hypointense to muscle on T1-weighted images and hyperintense on T2-weighted images, with variable contrast enhancement.161,162 Venous components tend to enhance and may demonstrate hematocrit levels within their tubular channels related to sluggish flow and microthrombi. Phleboliths are characteristic of venous malformations and typically present with low signal intensity on all sequences. Intraarticular fluid-fluid levels, synovial blooming artifact on gradient-echo images because of the presence of hemosiderin, or bone erosions due to a reactive arthritis as a result of the presence of intraarticular blood may be present (e-Fig. 137-29).
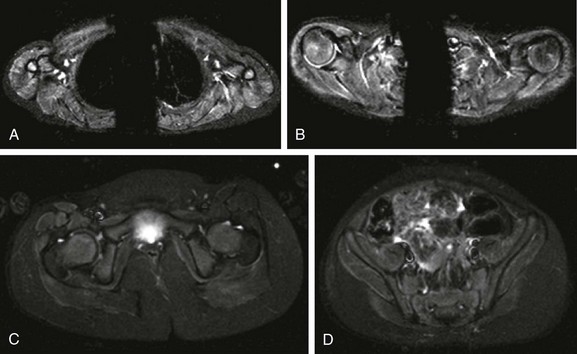
e-Figure 137-29 A 3-year-old girl with juvenile dermatomyositis.
Axial inversion-recovery images of the shoulder girdle (A and B) demonstrate diffuse symmetric increased signal intensity involving the anterior belly of the right deltoid muscle and posterior belly of the left deltoid muscle, supraspinatus, infraspinatus, and triceps muscles bilaterally. In the pelvic girdle (C and D), faint asymmetric increased signal intensity is noted within the obturator externus, pectineus, rectus femoris, sartorius, vastus lateralis, and gluteus muscles, predominately on the right side.
Juvenile Dermatomyositis
Etiology, Pathophysiology, and Clinical Presentation
Juvenile dermatomyositis (JDM) is an autoimmune inflammatory myopathy characterized by diffuse nonsuppurative inflammation of muscle fibers and skin.163 The inflammatory infiltrates are predominantly perivascular, in the interfascicular septa, or around the fascicles.164 The incidence of JDM in the United States is 3.2 per million children per year.165 The ratio of girls to boys is 2.3 to 1.166 JDM most commonly presents between the ages of 5 and 14 years.167 Clinical findings include severe proximal muscle weakness, fatigue, heliotrope rash (a pink or purple rash on the face and knuckles), and underlying vasculitic pathology.167,168
Imaging
In the acute stage of JDM, radiologic changes are minimal. However, incipient soft tissue swelling and subcutaneous edema represented by blurring of fat planes can be noted in the proximal appendicular skeleton in some cases. The muscles of the scapular and pelvic girdles are most frequently affected, and their involvement is typically symmetrical (Figs. 137-29 and 137-30).169,170 In chronic disease, radiographs demonstrate soft tissue loss and muscle atrophy. Marked osteoporosis of the long bones and vertebral bodies also may be present. The most characteristic finding of chronic disease, however, is the deposition of calcium in the soft tissues (Fig. 137-31), which is identified in 25% to 50% of cases.171 The calcium deposits may present as subcutaneous plaques, nodules, periarticular calcific foci, and large clumps or sheets of calcium within muscle or subcutaneous tissue.172 The arthritis associated with JDM is usually transient and nondeforming in nature.172
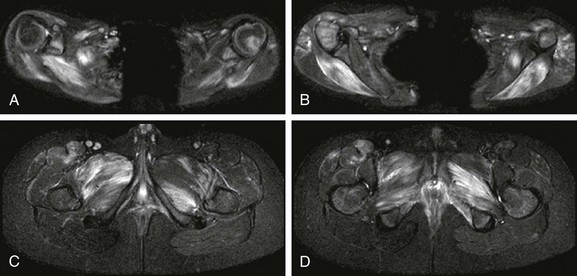
Figure 137-30 An 11-year-old boy with juvenile dermatomyositis.
Axial inversion-recovery images of the upper extremities (A and B) show patchy asymmetric involvement of bilateral deltoid muscles, pectoralis minor muscle, triceps, supraspinatus, infraspinatus, teres minor, teres major, paraspinal and subscapularis muscles. Within the lower extremities (C and D), patchy asymmetric signal abnormality was noted in the adductor compartment, internal obturator, tensor fascia lata, quadriceps, hamstring, iliopsoas, gluteus maximum, and gluteus medius and minimum muscles.
Ultrasonography
Ultrasound scanning of the involved musculature shows diffusely increased echogenicity of the soft tissues and acoustic shadowing within the musculature in regions of calcium deposits. Histopathologically, muscle lipomatosis significantly correlates to muscle echogenicity.164 Other sonographic findings include atrophy and decreased muscular bulk, soft tissue fasciculation, tenosynovitis, and soft tissue nodularities.173
Magnetic Resonance Imaging
Ultrasound and MRI have a similar capacity to demonstrate the features of inflammatory muscle disease, but MRI is more sensitive for the detection of edema, representing inflammation, in the acute phase of the disease. Both MRI and ultrasound allow guided biopsy and aspiration of muscle abnormalities.174–177 MRI reveals increased water content (edema) as increased signal intensity on T2-weighted and short tau inversion recovery MR images (Figs. 137-29 and 137-30) within infarcted muscles as a result of vasculitis.178 In fact, T2 relaxation time can be used as a quantitative measure of muscle inflammation and correlates well with other measures of disease activity.179 Because physical exercise can induce changes that mimic inflammation of muscle, children with JDM should be at rest for at least 30 minutes before MR imaging to assess disease activity.180
In chronic disease, focal areas of low signal intensity usually are seen on all MRI sequences, which represent calcific and fibrotic foci. Focal areas of increased signal intensity on T1-weighted images representing partial fatty replacement of muscles and tenosynovitis-related changes also may be identified.178
Computed Tomography
Although CT does not detect inflammatory changes in muscle tissue, it is the modality of choice for identifying soft tissue calcifications in soft tissues associated with JDM.181 It also allows quantification of muscle atrophy and fatty replacement in deep muscles.
Scintigraphy
Whole-body thallium-201 chloride and technetium-99m-methylene diphosphonate (99mtechnetium-MDP) muscle scintigraphy a potentially useful tool to investigate occult muscle groups affected by dermatomyositis; however, further investigation in the pediatric population is required.182,183 Furthermore, bone scans with 99mtechnetium-MDP can function as auxiliary tools to evaluate calcinosis in patients with JDM.181–183
Treatment and Follow-Up
Since the 1970s, standard treatment for JDM has been high-dose daily oral corticosteroids, which is continued until clinical and laboratory improvement are evident, and then slowly reduced over at least a 2-year period.184 Methotrexate is an important ancillary treatment. Early studies suggested that methotrexate improves strength and reduces other signs of disease activity in steroid-resistant patients185 with acceptable adverse effects.186,187 Other treatments, including cyclosporine, hydroxychloroquine, tacrolimus, azathioprine, mycophenolate mofetil, and cyclophosphamide for severe disease, have shown some benefit in cases of refractory disease.188
Biological agents, which are used widely in persons with other rheumatic diseases, are being developed for the treatment of childhood myositis. The few studies189,190 of rituximab in persons with JDM suggest a favorable response; however, biologic agents currently are reserved for patients with recalcitrant disease.191
Physical disability from muscle weakness or contractures represents a significant problem. Physiotherapy plays an important role in the rehabilitation of patients with JDM.192 Studies using MRI T2-weighted relaxation time suggest that moderate exercise does not increase muscle inflammation.193 In another study,194 aerobic exercise limitation in JDM was shown to correlate best with measures of disease damage (e.g., global damage assessment, T1-weighted MRI, and disease duration).
Chronic Recurrent Multifocal Osteomyelitis
Etiology, Pathophysiology, and Clinical Presentation
Chronic recurrent multifocal osteomyelitis (CRMO) is a skeletal disorder of unknown origin mainly occurring in children and adolescents.195 It is characterized by multifocal nonpyogenic inflammatory bone lesions, a course of exacerbations and remissions, and an association with other inflammatory disorders.196 The association of CRMO with dermatologic disorders (such as psoriasis) and inflammatory bowel disease and its response to steroids has led to the suggestion of an autoimmune cause.197–199 More recently, a genetic origin for CRMO has been suggested as a result of observation of disease in siblings and monozygotic twins.200–202
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is the adult equivalent of CRMO.203 Whereas CRMO typically manifests in the first decade of life, the mean age of onset for SAPHO syndrome is 28 years.204 CRMO is a diagnosis of exclusion, distinct from bacterial osteomyelitis, based on the following criteria:
1. Bone lesions with a radiographic picture suggesting subacute or chronic osteomyelitis
2. An unusual location of lesions when compared with infectious osteomyelitis and frequent multifocality
3. No abscess formation, fistula, or sequestra
4. Lack of a causative organism
5. Nonspecific histopathologic and laboratory findings compatible with subacute or chronic osteomyelitis
6. A characteristic prolonged, fluctuating course with recurrent episodes of pain
Imaging
The imaging evaluation of CRMO should start with radiographic evaluation of the symptomatic sites. If the radiographs have negative findings in the presence of significant clinical symptoms, further evaluation with MRI should be considered to evaluate for bone marrow edema. Whole-body evaluation traditionally has been performed with 99mtechnetium bone scintigraphy,205 although whole-body MRI is being used increasingly for evaluation of multifocal bone lesions in persons with CRMO.206 The diagnosis of CRMO typically is confirmed by means of a bone biopsy; with MRI guidance, a biopsy of CRMO bone lesions that are visible on MRI but are occult on radiography and CT can be performed.207
The three most common sites of disease at initial presentation are the lower extremities (39.7%) (Fig. 137-32 and e-Fig. 137-33), spine (25.9%), and pelvis (20.7%),203 followed by the clavicle, the spine, the mandible (see Fig. 137-34), and pelvic bones.208,209 In tubular bones, metaphyseal lesions are the most common, accounting for 49% of all long bone lesions.203 When the shoulder girdle is involved, the term sternocostoclavicular hyperostosis is used.
Figure 137-32 An adolescent girl with bilateral distal tibial juxtaphyseal metaphyseal widening seen on T1-weighted coronal sequences.
After infection was excluded, the diagnosis of chronic recurrent multifocal osteomyelitis was established.
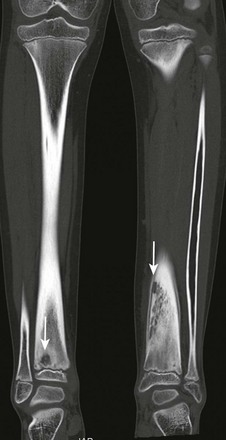
e-Figure 137-33 An 8-year-old girl with bilateral distal tibial lytic lesions.
One lesion is located in the right distal tibial juxtaphyseal metaphysis, and a secondary lytic lesion is located in the left tibia metadiaphysis (arrows). The left tibial metadiaphyseal lesion was biopsied, and the diagnosis of chronic recurrent multifocal osteomyelitis was established.
Figure 137-34 Chronic recurrent multifocal osteomyelitis.
At the age of 6 years, this girl started to present with pain in the left mandible. At that time the bone scan (A) showed a very hyperemic focus in the left mandibular ramus. Eight months later, diffuse increased activity was still noted in the left mandible on a bone scan (B); however, it was less intense than in the previous study. Subsequently, the patient started treatment with pamidronate. Three years later, coronal (C) and axial (D) computed tomography (CT) scans of the mandibles showed a lytic left mandibular lesion (9 × 6 mm) posterior to an unerupted molar (arrows). Magnetic resonance imaging (MRI) (postcontrast axial T1-weighted turbo spin echo spectral presaturation inversion recovery [SPIR], E and F; coronal T1-weighted three-dimensional fast field echo postcontrast, G) performed 2 months after the CT scan confirmed the cysticlike lesion noted within the inferior aspect of the left mandibular ramus, which likely represented previous sequela from the local inflammatory process. The MRI also showed the interval development of an inflammatory process involving the bone and adjacent soft tissues in the mid and superior aspects of the left mandibular ramus (arrows) with associated mild reactive synovitis (arrowheads). Three years after the performance of the CT and MRI scans, pain started to develop in the right knee. The frontal radiograph (H) and MRI of the right knee (coronal T1-weighted, I; coronal short tau inversion recovery, J; and postcontrast axial T1-weighted SPIR, K) showed patchy bone marrow signal changes involving the right distal femoral and proximal tibial metaphysis and epiphysis with mild associated soft tissue changes adjacent to the right proximal tibia (arrow).
The differential diagnosis of CRMO includes subacute and chronic infectious osteomyelitis, histiocytosis, hypophosphatasia, and malignancies such as leukemia, lymphoma, and Ewing sarcoma.210 The imaging features may mimic pyogenic, chronic osteomyelitis, and ultimately, it may be necessary to perform a biopsy in clinically equivocal cases. Multifocal metaphyseal-based lesions favor a diagnosis of CRMO after pyogenic infection has been excluded. Langerhans cell histiocytosis is a less likely consideration when multifocal lesions involve the metaphysis because Langerhans cell histiocytosis tends to affect the axial skeleton or diaphysis.
CRMO of long bones can result in orthopedic complications such as bony overgrowth, angular deformities, and limb-length discrepancy.211 Because of its tendency to occur near the physes, CRMO can cause premature physeal closure, resulting in growth arrest. The hyperemia caused by chronic inflammation may result in diffuse demineralization, predisposing to fractures.212
Treatment and Follow-Up
Suggestion of the diagnosis by a radiologist could help avoid unnecessary diagnostic procedures and antibiotic therapy and initiate an appropriate therapy.203 Many different treatments have been used in persons with CRMO, including nonsteroidal antiinflammatory agents, corticosteroids,213 azithromycin, tumor necrosis factor-blocker (infliximab),214,215 and interferon.216 Bisphosphonates have been used in persons with CRMO for pain relief, control of disease progression,217 and when simple therapies fail to control symptoms or disease progression.217
Chan, WP, Liu, GC. MR imaging of primary skeletal muscle diseases in children. AJR Am J Roentgenol. 2002;179:989–997.
Doria, A, Lundin, B. Imaging modalities for assessment of hemophilic arthropathy. In: Lee C, Berntorp E, Hoots K, eds. Textbook of hemophilia. West Sussex, UK: Wiley-Blackwell; 2009:191–199.
Khanna, G, Sato, TS, Ferguson, P. Imaging of chronic recurrent multifocal osteomyelitis. Radiographics. 2009;29:1159–1177.
Miller, E, Doria, AS. Imaging for early assessment of peripheral joints in juvenile idiopathic arthritis. In: Santiago Medina L, Applegate K, Blackmore C, eds. Evidence-based imaging in pediatrics: optimizing imaging in pediatric patient care. New York, NY: Springer; 2010:219–273.
References
1. Petty, RE, Southwood, TR, Manners, P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392.
2. Cassidy, JT. Juvenile rheumatoid arthritis. In: Kelly W, Harris E, Ruddy S, et al, eds. Textbook of rheumatology. Philadelphia: WB Saunders; 2003:1189–1208.
3. Schanberg, LE, Sandstrom, MJ. Causes of pain in children with arthritis. Rheum Dis Clin North Am. 1999;25:31–53. [vi].
4. Schanberg, LE, Anthony, KK, Gil, KM, et al. Daily pain and symptoms in children with polyarticular arthritis. Arthritis Rheum. 2003;48:1390–1397.
5. Hollister, J. Juvenile rheumatoid arthritis. In: Hay W, Levin M, Sondheimer J, et al, eds. Current diagnosis & treatment in pediatrics. New York: Lange Medical Books/McGraw-Hill Medical Publishing Division, 2007.
6. Wright, D. Juvenile idiopathic arthritis. In: Morrissy R, Weinstein S, eds. Lovell & Winter’s pediatric orthopedics. Philadelphia: Lippincott Williams & Wilkins; 2006:406–410.
7. Fink, CW. Proposal for the development of classification criteria for idiopathic arthritides of childhood. J Rheumatol. 1995;22:1566–1569.
8. Miller, E, Doria, AS. Imaging for early assessment of peripheral joints in juvenile idiopathic arthritis. In: Santiago Medina L, Applegate K, Blackmore C, eds. Evidence-based imaging in pediatrics: optimizing imaging in pediatric patient care. New York: Springer; 2010:219–273.
9. Guzman, J, Burgos-Vargas, R, Duarte-Salazar, C, et al. Reliability of the articular examination in children with juvenile rheumatoid arthritis: interobserver agreement and sources of disagreement. J Rheumatol. 1995;22:2331–2336.
10. Forslind, K, Larsson, EM, Johansson, A, et al. Detection of joint pathology by magnetic resonance imaging in patients with early rheumatoid arthritis. Br J Rheumatol. 1997;36:683–688.
11. Gare, BA. Epidemiology. Baillieres Clin Rheumatol. 1998;12:191–208.
12. Aaron, S, Fraser, PA, Jackson, JM, et al. Sex ratio and sibship size in juvenile rheumatoid arthritis kindreds. Arthritis Rheum. 1985;28:753–758.
13. Hanson, V, Kornreich, H, Bernstein, B. Three subtypes of juvenile rheumatoid arthritis (correlations of age at onset, sex, and serologic factors). Arthritis Rheum. 1977;20(suppl):184.
14. Cassidy, J, Petty, R. Juvenile rheumatoid arthritis. In: Textbook of pediatric rheumatology. Philadelphia: W.B. Saunders; 1995:133–223.
15. Ravelli, A. Toward an understanding of the long-term outcome of juvenile idiopathic arthritis. Clin Exp Rheumatol. 2004;22:271–275.
16. Oen, K. Long-term outcomes and predictors of outcomes for patients with juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2002;16:347–360.
17. Davidson, J. Juvenile idiopathic arthritis: a clinical overview. Eur J Radiol. 2000;33:128–134.
18. Cassone, R, Falcone, A, Rossi, F, et al. Unilateral destructive wrist synovitis in juvenile idiopathic arthritis. Clin Exp Rheumatol. 2004;22:637–642.
19. Lang, BA, Schneider, R, Reilly, BJ, et al. Radiologic features of systemic onset juvenile rheumatoid arthritis. J Rheumatol. 1995;22:168–173.
20. Kim, HK, Zbojniewicz, AM, Merrow, AC, et al. MR findings of synovial disease in children and young adults: part 1. Pediatr Radiol. 2011;41:495–511.
21. Yulish, BS, Lieberman, JM, Newman, AJ, et al. Juvenile rheumatoid arthritis: assessment with MR imaging. Radiology. 1987;165:149–152.
22. Wallace, CA, Levinson, JE. Juvenile rheumatoid arthritis: outcome and treatment for the 1990s. Rheum Dis Clin North Am. 1991;17:891–905.
23. Minden, K, Niewerth, M, Listing, J, et al. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2392–2401.
24. Zak, M, Pedersen, FK. Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology (Oxford). 2000;39:198–204.
25. Babyn, P, Doria, AS. Radiologic investigation of rheumatic diseases. Pediatr Clin North Am. 2005;52:373–411. [vi].
26. Azouz, EM. Juvenile idiopathic arthritis: how can the radiologist help the clinician? Pediatr Radiol. 2008;38(suppl 3):S403–S408.
27. Southwood, T. Juvenile idiopathic arthritis: clinically relevant imaging in diagnosis and monitoring. Pediatr Radiol. 2008;38(suppl 3):S395–S402.
28. Doria, AS, Babyn, PS, Feldman, B. A critical appraisal of radiographic scoring systems for assessment of juvenile idiopathic arthritis. Pediatr Radiol. 2006;36:759–772.
29. Lamer, S, Sebag, GH. MRI and ultrasound in children with juvenile chronic arthritis. Eur J Radiol. 2000;33:85–93.
30. Spilberg, I, Meyer, GJ. The arthritis of leukemia. Arthritis Rheum. 1972;15:630–635.
31. Laxer, RM, Cameron, BJ, Chaisson, D, et al. The camptodactyly-arthropathy-pericarditis syndrome: case report and literature review. Arthritis Rheum. 1986;29:439–444.
32. Babyn, P, Doria, AS. Radiologic investigation of rheumatic diseases. Rheum Dis Clin North Am. 2007;33:403–440.
33. Backhaus, M, Kamradt, T, Sandrock, D, et al. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999;42:1232–1245.
34. Gylys-Morin, VM, Graham, TB, Blebea, JS, et al. Knee in early juvenile rheumatoid arthritis: MR imaging findings. Radiology. 2001;220:696–706.
35. Babyn, P, Doria, A. Radiologic investigation of rheumatic diseases. In: Cassidy J, Petty R, Lindsley C, eds. Textbook of pediatric rheumatology. Philadelphia: Saunders, 2010.
36. Gylys-Morin, VM. MR imaging of pediatric musculoskeletal inflammatory and infectious disorders. Magn Reson Imaging Clin N Am. 1998;6:537–559.
37. Lamer, S, Sebag, GH. MRI and ultrasound in children with juvenile chronic arthritis. Eur J Radiol. 2000;33:85–93.
38. Daldrup-Link, HE, Steinbach, L. MR imaging of pediatric arthritis. Magn Reson Imaging Clin N Am. 2009;17:451–467. [vi].
39. Cakmakci, H, Kovanlikaya, A, Unsal, E. Short-term follow-up of the juvenile rheumatoid knee with fat-saturated 3D MRI. Pediatr Radiol. 2001;31:189–195.
40. Laor, T, Jaramillo, D. MR imaging insights into skeletal maturation: what is normal? Radiology. 2009;250:28–38.
41. McQueen, FM. The MRI view of synovitis and tenosynovitis in inflammatory arthritis: implications for diagnosis and management. Ann N Y Acad Sci. 2009;1154:21–34.
42. Ostergaard, M, Stoltenberg, M, Henriksen, O, et al. Quantitative assessment of synovial inflammation by dynamic gadolinium-enhanced magnetic resonance imaging. A study of the effect of intra-articular methylprednisolone on the rate of early synovial enhancement. Br J Rheumatol. 1996;35:50–59.
43. Graham, TB, Laor, T, Dardzinski, BJ. Quantitative magnetic resonance imaging of the hands and wrists of children with juvenile rheumatoid arthritis. J Rheumatol. 2005;32:1811–1820.
44. Ostergaard, M, Hansen, M, Stoltenberg, M, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(5):918–929.
45. Muller, L, Kellenberger, CJ, Cannizzaro, E, et al. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: a pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology (Oxford). 2009;48:680–685.
46. Chung, C, Coley, BD, Martin, LC. Rice bodies in juvenile rheumatoid arthritis. AJR Am J Roentgenol. 1998;170:698–700.
47. Haavardsholm, EA, Bøyesen, P, Østergaard, M, et al. MRI-detected bone marrow edema is a predictor of subsequent radiographic progression in early rheumatoid arthritis. Ann Rheum Dis. 2007;66(suppl II):94.
48. McQueen, FM. A vital clue to deciphering bone pathology: MRI bone oedema in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2007;66:1549–1552.
49. Palosaari, K, Vuotila, J, Takalo, R, et al. Bone oedema predicts erosive progression on wrist MRI in early RA—a 2-yr observational MRI and NC scintigraphy study. Rheumatology (Oxford). 2006;45(12):1542–1548.
50. Miller, E, Uleryk, E, Doria, AS. Evidence-based outcomes of studies addressing diagnostic accuracy of MRI of juvenile idiopathic arthritis. AJR Am J Roentgenol. 2009;192:1209–1218.
51. Johnson, K. Imaging of juvenile idiopathic arthritis. Pediatr Radiol. 2006;36:743–758.
52. Malattia, C, Damasio, MB, Pistorio, A, et al. Development and preliminary validation of a paediatric-targeted MRI scoring system for the assessment of disease activity and damage in juvenile idiopathic arthritis. Ann Rheum Dis. 2011;70:440–446.
53. Hemke, R, Maas, M, van Veenendaal, M, et al. MRI findings in the knee of juvenile idiopathic arthritis: experience with a newly developed juvenile arthritis MRI scoring system (JAMRIS). Ann Rheum Dis. 2010;69:632.
54. Friedrich, KM, Mamisch, TC, Plank, C, et al. Diffusion-weighted imaging for the follow-up of patients after matrix-associated autologous chondrocyte transplantation. Eur J Radiol. 2010;73:622–628.
55. Basser, PJ, Pajevic, S, Pierpaoli, C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632.
56. Agarwal, V, Kumar, M, Singh, JK, et al. Diffusion tensor anisotropy magnetic resonance imaging: a new tool to assess synovial inflammation. Rheumatology (Oxford). 2009;48:378–382.
57. Miller, KL, Hargreaves, BA, Gold, GE, et al. Steady-state diffusion-weighted imaging of in vivo knee cartilage. Magn Reson Med. 2004;51:394–398.
58. Malattia, C, Damasio, MB, Basso, C, et al. Dynamic contrast-enhanced magnetic resonance imaging in the assessment of disease activity in patients with juvenile idiopathic arthritis. Rheumatology (Oxford). 2010;49:178–185.
59. Konig, H, Sieper, J, Wolf, KJ. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990;176:473–477.
60. Yamato, M, Tamai, K, Yamaguchi, T, et al. MRI of the knee in rheumatoid arthritis: Gd-DTPA perfusion dynamics. J Comput Assist Tomogr. 1993;17:781–785.
61. Workie, DW, Dardzinski, BJ. Quantifying dynamic contrast-enhanced MRI of the knee in children with juvenile rheumatoid arthritis using an arterial input function (AIF) extracted from popliteal artery enhancement, and the effect of the choice of the AIF on the kinetic parameters. Magn Reson Med. 2005;54:560–568.
62. Workie, DW, Graham, TB, Laor, T, et al. Quantitative MR characterization of disease activity in the knee in children with juvenile idiopathic arthritis: a longitudinal pilot study. Pediatr Radiol. 2007;37:535–543.
63. Bashir, A, Gray, ML, Boutin, RD, et al. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205:551–558.
64. Dardzinski, BJ, Laor, T, Schmithorst, VJ, et al. Mapping T2 relaxation time in the pediatric knee: feasibility with a clinical 1.5-T MR imaging system. Radiology. 2002;225:233–239.
65. Dardzinski, BJ, Mosher, TJ, Li, S, et al. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546–550.
66. Mosher, TJ, Dardzinski, BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368.
67. Kight, AC, Dardzinski, BJ, Laor, T, et al. Magnetic resonance imaging evaluation of the effects of juvenile rheumatoid arthritis on distal femoral weight-bearing cartilage. Arthritis Rheum. 2004;50:901–905.
68. Kim, HK, Laor, T, Graham, TB, et al. T2 relaxation time changes in distal femoral articular cartilage in children with juvenile idiopathic arthritis: a 3-year longitudinal study. AJR Am J Roentgenol. 2010;195:1021–1025.
69. Perman, WH, Turski, PA, Houston, LW, et al. Methodology of in vivo human sodium MR imaging at 1.5 T. Radiology. 1986;160:811–820.
70. Shapiro, EM, Borthakur, A, Gougoutas, A, et al. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47:284–291.
71. Reddy, R, Insko, EK, Noyszewski, EA, et al. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39:697–701.
72. Madelin, G, Jerschow, A, Regatte, RR. Sodium relaxation times in the knee joint in vivo at 7T. NMR Biomed. 2012;25(4):530–537.
73. Schmitt, B, Zbyn, S, Stelzeneder, D, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260:257–264.
74. Wang, L, Wu, Y, Chang, G, et al. Rapid isotropic 3D-sodium MRI of the knee joint in vivo at 7T. J Magn Reson Imaging. 2009;30:606–614.
75. Chavhan, GB, Babyn, PS. Pediatric musculoskeletal imaging at 3 Tesla. Semin Musculoskelet Radiol. 2009;13:181–195.
76. Friedrich, KM, Chang, G, Vieira, RL, et al. In vivo 7.0-tesla magnetic resonance imaging of the wrist and hand: technical aspects and applications. Semin Musculoskelet Radiol. 2009;13:74–84.
77. Backhaus, M. Ultrasound and structural changes in inflammatory arthritis: synovitis and tenosynovitis. Ann N Y Acad Sci. 2009;1154:139–151.
78. Ruhoy, MK, Tucker, L, McCauley, RG. Hypertrophic bursopathy of the subacromial-subdeltoid bursa in juvenile rheumatoid arthritis: sonographic appearance. Pediatr Radiol. 1996;26:353–355.
79. Terslev, L, Torp-Pedersen, S, Qvistgaard, E, et al. Estimation of inflammation by Doppler ultrasound: quantitative changes after intra-articular treatment in rheumatoid arthritis. Ann Rheum Dis. 2003;62:1049–1053.
80. Tse, SM, Laxer, RM, Babyn, PS, et al. Radiologic Improvement of juvenile idiopathic arthritis-enthesitis-related arthritis following anti-tumor necrosis factor-alpha blockade with etanercept. J Rheumatol. 2006;33:1186–1188.
81. Rebollo-Polo, M, Koujok, K, Weisser, C, et al. Ultrasound findings on patients with juvenile idiopathic arthritis in clinical remission. Arthritis Care Res (Hoboken). 2011;63:1013–1019.
82. Scire, CA, Montecucco, C, Codullo, V, et al. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology (Oxford). 2009;48:1092–1097.
83. Laurell, L, Court-Payen, M, Nielsen, S, et al. Ultrasonography and color Doppler in juvenile idiopathic arthritis: diagnosis and follow-up of ultrasound-guided steroid injection in the ankle region. A descriptive interventional study. Pediatr Rheumatol Online J. 2011;9:4.
84. Weiss, PF, Arabshahi, B, Johnson, A, et al. High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis, as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum. 2008;58:1189–1196.
85. Doria, AS, Kiss, MH, Lotito, AP, et al. Juvenile rheumatoid arthritis of the knee: evaluation with contrast-enhanced color Doppler ultrasound. Pediatr Radiol. 2001;31:524–531.
86. Shahin, AA, el Mofty, SA, el Sheikh, EA, et al. Power Doppler sonography in the evaluation and follow-up of knee involvement in patients with juvenile idiopathic arthritis. Z Rheumatol. 2001;60:148–155.
87. Klauser, A, Frauscher, F, Schirmer, M, et al. The value of contrast-enhanced color Doppler ultrasound in the detection of vascularization of finger joints in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:647–653.
88. Terslev, L, Torp-Pedersen, S, Qvistgaard, E, et al. Spectral Doppler and resistive index. A promising tool in ultrasonographic evaluation of inflammation in rheumatoid arthritis. Acta Radiol. 2003;44:645–652.
89. El Miedany, YM, Housny, IH, Mansour, HM, et al. Ultrasound versus MRI in the evaluation of juvenile idiopathic arthritis of the knee. Joint Bone Spine. 2001;68:222–230.
90. Sureda, D, Quiroga, S, Arnal, C, et al. Juvenile rheumatoid arthritis of the knee: evaluation with US. Radiology. 1994;190:403–406.
91. Frosch, M, Foell, D, Ganser, G, et al. Arthrosonography of hip and knee joints in the follow up of juvenile rheumatoid arthritis. Ann Rheum Dis. 2003;62:242–244.
92. Magni-Manzoni, S, Epis, O, Ravelli, A, et al. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:1497–1504.
93. Pascoli, L, Wright, S, McAllister, C, et al. Prospective evaluation of clinical and ultrasound findings in ankle disease in juvenile idiopathic arthritis: importance of ankle ultrasound. J Rheumatol. 2010;37:2409–2414.
94. Hashkes, PJ, Laxer, RM. Medical treatment of juvenile idiopathic arthritis. JAMA. 2005;294:1671–1684.
95. Balogh, Z, Ruzsonyi, E. Triamcinolone hexacetonide versus betamethasone. A double-blind comparative study of the long-term effects of intra-articular steroids in patients with juvenile chronic arthritis. Scand J Rheumatol Suppl. 1987;67:80–82.
96. Zulian, F, Martini, G, Gobber, D, et al. Comparison of intra-articular triamcinolone hexacetonide and triamcinolone acetonide in oligoarticular juvenile idiopathic arthritis. Rheumatology(Oxford). 2003;42:1254–1259.
97. Zulian, F, Martini, G, Gobber, D, et al. Triamcinolone acetonide and hexacetonide intra-articular treatment of symmetrical joints in juvenile idiopathic arthritis: a double-blind trial. Rheumatology (Oxford). 2004;43:1288–1291.
98. Huppertz, HI, Tschammler, A, Horwitz, AE, et al. Intraarticular corticosteroids for chronic arthritis in children: efficacy and effects on cartilage and growth. J Pediatr. 1995;127:317–321.
99. Eich, GF, Halle, F, Hodler, J, et al. Juvenile chronic arthritis: imaging of the knees and hips before and after intraarticular steroid injection. Pediatr Radiol. 1994;24:558–563.
100. Poznanski, AK, Hernandez, RJ, Guire, KE, et al. Carpal length in children—a useful measurement in the diagnosis of rheumatoid arthritis and some congenital malformation syndromes. Radiology. 1978;129:661–668.
101. Ringold, S, Torgerson, TR, Egbert, MA, et al. Intraarticular corticosteroid injections of the temporomandibular joint in juvenile idiopathic arthritis. J Rheumatol. 2008;35:1157–1164.
102. Reference deleted in proofs.
103. Neidel, J, Boehnke, M, Kuster, RM. The efficacy and safety of intraarticular corticosteroid therapy for coxitis in juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1620–1628.
104. Sparling, M, Malleson, P, Wood, B, et al. Radiographic follow-up of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33:821–826.
105. Jacobs, JC, Berdon, WE, Johnston, AD. HLA-B27-associated spondylarthritis and enthesopathy in childhood: clinical, pathologic, and radiographic observations in 58 patients. J Pediatr. 1982;100:521–528.
106. Prieur, AM. Spondyloarthropathies in childhood. Baillieres Clin Rheumatol. 1998;12:287–307.
107. Balint, PV, Kane, D, Wilson, H, et al. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–910.
108. Kamel, M, Eid, H, Mansour, R. Ultrasound detection of heel enthesitis: a comparison with magnetic resonance imaging. J Rheumatol. 2003;30:774–778.
109. Bollow, M, Braun, J, Biedermann, T, et al. Use of contrast-enhanced MR imaging to detect sacroiliitis in children. Skeletal Radiol. 1998;27:606–616.
110. Burgos-Vargas, R, Vazquez-Mellado, J, Pacheco-Tena, C, et al. A 26 week randomised, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Ann Rheum Dis. 2002;61:941–942.
111. Tse, SM, Burgos-Vargas, R, Laxer, RM. Anti-tumor necrosis factor alpha blockade in the treatment of juvenile spondylarthropathy. Arthritis Rheum. 2005;52:2103–2108.
112. Henrickson, M, Reiff, A. Prolonged efficacy of etanercept in refractory enthesitis-related arthritis. J Rheumatol. 2004;31:2055–2061.
113. D’Agostino, MA, Said-Nahal, R, Hacquard-Bouder, C, et al. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–533.
114. Lee, EY, Sundel, RP, Kim, S, et al. MRI findings of juvenile psoriatic arthritis. Skeletal Radiol. 2008;37:987–996.
114a. Puhakka, KB, Jurik, AG, Egund, N, et al. Imaging of sacroiliitis in early seronegative spondylarthropathy. Assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003;44:218–229.
115. Ory, PA, Gladman, DD, Mease, PJ. Psoriatic arthritis and imaging. Ann Rheum Dis. 2005;64(Suppl 2):ii55–57.
116. Venkateswaran, L, Wilimas, JA, Jones, DJ, et al. Mild hemophilia in children: prevalence, complications, and treatment. J Pediatr Hematol Oncol. 1998;20:32–35.
117. Rand, T, Trattnig, S, Male, C, et al. Magnetic resonance imaging in hemophilic children: value of gradient echo and contrast-enhanced imaging. Magn Reson Imaging. 1999;17:199–205.
118. Resnick, D. Diagnosis of bone and joint disorders, 4th ed. Philadelphia: Saunders; 2002.
119. Kerr, R. Imaging of musculoskeletal complications of hemophilia. Semin Musculoskelet Radiol. 2003;7:127–136.
120. Kraft, J, Blanchette, V, Babyn, P, et al. Magnetic resonance imaging and joint outcomes in boys with severe hemophilia A treated with tailored primary prophylaxis in Canada. J Thromb Haemost. 2012;10:2494–2502.
121. Baunin, C, Railhac, JJ, Younes, I, et al. MR imaging in hemophilic arthropathy. Eur J Pediatr Surg. 1991;1:358–363.
122. Cotten, A, Flipo, RM, Herbaux, B, et al. Synovial haemangioma of the knee: a frequently misdiagnosed lesion. Skeletal Radiol. 1995;24:257–261.
123. Hughes, TH, Sartoris, DJ, Schweitzer, ME, et al. Pigmented villonodular synovitis: MRI characteristics. Skeletal Radiol. 1995;24:7–12.
124. Bravo, SM, Winalski, CS, Weissman, BN. Pigmented villonodular synovitis. Radiol Clin North Am. 1996;34:311–326. [x-xi].
125. Kilcoyne, RF, Nuss, R. Radiological assessment of haemophilic arthropathy with emphasis on MRI findings. Haemophilia. 2003;9(suppl 1):57–63.
126. Kilcoyne, RF, Lundin, B, Pettersson, H. Evolution of the imaging tests in hemophilia with emphasis on radiography and magnetic resonance imaging. Acta Radiol. 2006;47:287–296.
127. Lundin, B, Berntorp, E, Pettersson, H, et al. Gadolinium contrast agent is of limited value for magnetic resonance imaging assessment of synovial hypertrophy in hemophiliacs. Acta Radiol. 2007;48:520–530.
128. Doria, A, Lundin, B. Imaging modalities for assessment of hemophilic arthropathy. In: Lee C, Berntorp E, Hoots K, eds. Textbook of hemophilia. West Sussex, UK: Wiley-Blackwell; 2009:191–199.
129. Merchan, EC, De Orbe, A, Gago, J. Ultrasound in the diagnosis of the early stages of hemophilic arthropathy of the knee. Acta Orthop Belg. 1992;58:122–125.
130. Zukotynski, K, Jarrin, J, Babyn, PS, et al. Sonography for assessment of haemophilic arthropathy in children: a systematic protocol. Haemophilia. 2007;13:293–304.
131. Muca-Perja, M, Riva, S, Grochowska, B, et al. Ultrasonography of haemophilic arthropathy. Haemophilia. 2012;18(3):364–368.
132. Klukowska, A, Czyrny, Z, Laguna, P, et al. Correlation between clinical, radiological and ultrasonographical image of knee joints in children with haemophilia. Haemophilia. 2001;7:286–292.
133. Roosendaal, G, van Rinsum, AC, Vianen, ME, et al. Haemophilic arthropathy resembles degenerative rather than inflammatory joint disease. Histopathology. 1999;34:144–153.
134. Speer, DP. Early pathogenesis of hemophilic arthropathy. Evolution of the subchondral cyst. Clin Orthop Relat Res. 1984;185:250–265.
135. Rodriguez-Merchan, EC, Jimenez-Yuste, V, Aznar, JA, et al. Joint protection in haemophilia. Haemophilia. 2011;17(suppl 2):1–23.
136. Kilcoyne, RF. Imaging in musculoskeletal complications of hemophilia, WebMD. (website) http://emedicine.medscape.com/article/401842-overview. [Accessed January 8, 2013].
137. Manco-Johnson, MJ, Abshire, TC, Shapiro, AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544.
138. Doria, AS, Keshava, S, Mohanta, A, et al. Reliability of blinded and unblinded acquisition and interpretation of ultrasound for assessment of hemophilic knees and ankles. Buenos Aires, Argentina: Presented at the XXIX International Congress of the World Federation of Hemophilia; 2010.
139. Murray, JRD, Holmes, EJ, Misra, RR. Irritable hip/transient synovitis. In: Murray JRD, Holmes EJ, Misra RR, eds. A-Z of musculoskeletal and trauma radiology. Cambridge, UK: Cambridge University Press; 2008:74–75.
140. Martinoli, C, Garello, I, Marchetti, A, et al. Hip ultrasound. Eur J Radiol. 2012;81(12):3824–3831.
141. Merino, R, de Inocencio, J, Garcia-Consuegra, J. Differentiation between transient synovitis and septic arthritis of the hip with clinical and ultrasound criteria. An Pediatr (Barc). 2010;73:189–193.
142. Yabunaka, K, Ohue, M, Morimoto, N, et al. Sonographic measurement of transient synovitis in children: diagnostic value of joint effusion. Radiol Phys Technol. 2012;5(1):15–19.
143. Marchal, GJ, Van Holsbeeck, MT, Raes, M, et al. Transient synovitis of the hip in children: role of US. Radiology. 1987;162:825–828.
144. Kermond, S, Fink, M, Graham, K, et al. A randomized clinical trial: should the child with transient synovitis of the hip be treated with nonsteroidal anti-inflammatory drugs? Ann Emerg Med. 2002;40(3):294–299.
145. Evans, TI, Nercessian, BM, Sanders, KM. Leukemic arthritis. Semin Arthritis Rheum. 1994;24:48–56.
146. Gallagher, D, Heinrich, SD, Craver, R, et al. Skeletal manifestations of acute leukemia in childhood. Orthopedics. 1991;14:485–492.
147. Neubauer, P, Weber, AK, Miller, NH, et al. Pigmented villonodular synovitis in children: a report of six cases and review of the literature. Iowa Orthop J. 2007;27:90–94.
148. Goldman, AB, DiCarlo, EF. Pigmented villonodular synovitis. Diagnosis and differential diagnosis. Radiol Clin North Am. 1988;26:1327–1347.
149. Layfield, LJ, Meloni-Ehrig, A, Liu, K, et al. Malignant giant cell tumor of synovium (malignant pigmented villonodular synovitis). Arch Pathol Lab Med. 2000;124:1636–1641.
150. Dorwart, RH, Genant, HK, Johnston, WH, et al. Pigmented villonodular synovitis of the shoulder: radiologic-pathologic assessment. AJR Am J Roentgenol. 1984;143:886–888.
151. Llauger, J, Palmer, J, Roson, N, et al. Pigmented villonodular synovitis and giant cell tumors of the tendon sheath: radiologic and pathologic features. AJR Am J Roentgenol. 1999;172:1087–1091.
152. Kistler, W. Synovial chondromatosis of the knee joint: a rarity during childhood. Eur J Pediatr Surg. 1991;1:237–239.
153. Carey, RP. Synovial chondromatosis of the knee in childhood. A report of two cases. J Bone Joint Surg Br. 1983;65:444–447.
154. Murphey, MD, Vidal, JA, Fanburg-Smith, JC, et al. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27:1465–1488.
155. Donnelly, LF, Bisset, GS, III., Passo, MH. MRI findings of lipoma arborescens of the knee in a child: case report. Pediatr Radiol. 1994;24:258–259.
156. Martin, S, Hernandez, L, Romero, J, et al. Diagnostic imaging of lipoma arborescens. Skeletal Radiol. 1998;27:325–329.
157. Vilanova, JC, Barcelo, J, Villalon, M, et al. MR imaging of lipoma arborescens and the associated lesions. Skeletal Radiol. 2003;32:504–509.
158. Sola, JB, Wright, RW. Arthroscopic treatment for lipoma arborescens of the knee: a case report. J Bone Joint Surg Am. 1998;80:99–103.
159. Erselcan, T, Bulut, O, Bulut, S, et al. Lipoma arborescens; successfully treated by yttrium-90 radiosynovectomy. Ann Nucl Med. 2003;17:593–596.
160. Mulliken, JB, Glowacki, J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422.
161. Jans, L, Ditchfield, M, Jaremko, JL, et al. MRI demonstrates the extension of juxta-articular venous malformation of the knee and correlates with joint changes. Euro Radiol. 2010;20:1792–1798.
162. Sasho, T, Nakagawa, K, Matsuki, K, et al. Two cases of synovial haemangioma of the knee joint: Gd-enhanced image features on MRI and arthroscopic excision. Knee. 2011;18:509–511.
163. Pachman, LM. Juvenile dermatomyositis. Pathophysiology and disease expression. Pediatr Clin North Am. 1995;42:1071–1098.
164. Reimers, CD, Fleckenstein, JL, Witt, TN, et al. Muscular ultrasound in idiopathic inflammatory myopathies of adults. J Neurol Sci. 1993;116:82–92.
165. Mendez, EP, Lipton, R, Ramsey-Goldman, R, et al. US incidence of juvenile dermatomyositis, 1995-1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300–305.
166. Pachman, LM, Lipton, R, Ramsey-Goldman, R, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53:166–172.
167. Park, JH, Niermann, KJ, Ryder, NM, et al. Muscle abnormalities in juvenile dermatomyositis patients: P-31 magnetic resonance spectroscopy studies. Arthritis Rheum. 2000;43:2359–2367.
168. Chan, WP, Liu, GC. MR imaging of primary skeletal muscle diseases in children. AJR Am J Roentgenol. 2002;179:989–997.
169. Ozonoff, MB, Flynn, FJ, Jr. Roentgenologic features of dermatomyositis of childhood. Am J Roentgenol Radium Ther Nucl Med. 1973;118:206–212.
170. Steiner, RM, Glassman, L, Schwartz, MW, et al. The radiological findings in dermatomyositis of childhood. Radiology. 1974;111:385–393.
171. Sullivan, DB, Cassidy, JT, Petty, RE. Dermatomyositis in the pediatric patient. Arthritis Rheum. 1977;20:327–331.
172. Cassidy, JT, Petty, RC. Juvenile dermatomyositis. In: Textbook of paediatric rheumatology. Philadelphia: Saunders; 1995:323–364.
173. Rose, AL. Childhood polymyositis. A follow-up study with special reference to treatment with corticosteroids. Am J Dis Child. 1974;127:518–522.
174. Bureau, NJ, Cardinal, E, Chhem, RK. Ultrasound of soft tissue masses. Semin Musculoskelet Radiol. 1998;2:283–298.
175. Reimers, CD, Finkenstaedt, M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol. 1997;9:475–485.
176. Fornage, BD. The case for ultrasound of muscles and tendons. Semin Musculoskelet Radiol. 2000;4:375–391.
177. Yosipovitch, G, Beniaminov, O, Rousso, I, et al. STIR magnetic resonance imaging: a noninvasive method for detection and follow-up of dermatomyositis. Arch Dermatol. 1999;135:721–723.
178. Hernandez, RJ, Keim, DR, Sullivan, DB, et al. Magnetic resonance imaging appearance of the muscles in childhood dermatomyositis. J Pediatr. 1990;117:546–550.
179. Maillard, SM, Jones, R, Owens, C, et al. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology (Oxford). 2004;43:603–608.
180. Summers, RM, Brune, AM, Choyke, PL, et al. Juvenile idiopathic inflammatory myopathy: exercise-induced changes in muscle at short inversion time inversion-recovery MR imaging. Radiology. 1998;209:191–196.
181. Agarwal, V, Sachdev, A, Dabra, AK. Case 104: calcinosis in juvenile dermatomyositis. Radiology. 2007;242:307–311.
182. Wu, Y, Seto, H, Shimizu, M, et al. Extensive soft-tissue involvement of dermatomyositis detected by whole-body scintigraphy with 99mTc-MDP and 201TL-chloride. Ann Nucl Med. 1996;10:127–130.
183. Bar-Sever, Z, Mukamel, M, Harel, L, et al. Scintigraphic evaluation of calcinosis in juvenile dermatomyositis with Tc-99m MDP. Clin Nucl Med. 2000;25:1013–1016.
184. Bowyer, SL, Blane, CE, Sullivan, DB, et al. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr. 1983;103:882–888.
185. Fischer, TJ, Rachelefsky, GS, Klein, RB, et al. Childhood dermatomyositis and polymyositis. Treatment with methotrexate and prednisone. Am J Dis Child. 1979;133:386–389.
186. Miller, LC, Sisson, BA, Tucker, LB, et al. Methotrexate treatment of recalcitrant childhood dermatomyositis. Arthritis Rheum. 1992;35:1143–1149.
187. Al Mayouf, S, Al Mazyed, A, Bahabri, S. Efficacy of early treatment of severe juvenile dermatomyositis with intravenous methylprednisolone and methotrexate. Clin Rheumatol. 2000;19:138–141.
188. Feldman, BM, Rider, LG, Reed, AM, et al. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–2212.
189. Miles, L, Bove, KE, Lovell, D, et al. Predictability of the clinical course of juvenile dermatomyositis based on initial muscle biopsy: a retrospective study of 72 patients. Arthritis Rheum. 2007;57:1183–1191.
190. Tezak, Z, Hoffman, EP, Lutz, JL, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;168:4154–4163.
191. Bader-Meunier, B, Decaluwe, H, Barnerias, C, et al. Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol. 2011;38:1436–1440.
192. Stringer, E, Feldman, BM. Advances in the treatment of juvenile dermatomyositis. Curr Opin Rheumatol. 2006;18:503–506.
193. Maillard, SM, Jones, R, Owens, CM, et al. Quantitative assessments of the effects of a single exercise session on muscles in juvenile dermatomyositis. Arthritis Rheum. 2005;53:558–564.
194. Hicks, JE, Drinkard, B, Summers, RM, et al. Decreased aerobic capacity in children with juvenile dermatomyositis. Arthritis Rheum. 2002;47:118–123.
195. Jurik, AG. Chronic recurrent multifocal osteomyelitis. Semin Musculoskelet Radiol. 2004;8:243–253.
196. Ferguson, PJ, El Shanti, HI. Autoinflammatory bone disorders. Curr Opin Rheumatol. 2007;19:492–498.
197. Laxer, RM, Shore, AD, Manson, D, et al. Chronic recurrent multifocal osteomyelitis and psoriasis—a report of a new association and review of related disorders. Semin Arthritis Rheum. 1988;17:260–270.
198. Dagan, O, Barak, Y, Metzker, A. Pyoderma gangrenosum and sterile multifocal osteomyelitis preceding the appearance of Takayasu arteritis. Pediatr Dermatol. 1995;12:39–42.
199. Bousvaros, A, Marcon, M, Treem, W, et al. Chronic recurrent multifocal osteomyelitis associated with chronic inflammatory bowel disease in children. Dig Dis Sci. 1999;44:2500–2507.
200. El Shanti, HI, Ferguson, PJ. Chronic recurrent multifocal osteomyelitis: a concise review and genetic update. Clin Orthop Relat Res. 2007;462:11–19.
201. Grosse, J, Chitu, V, Marquardt, A, et al. Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease. Blood. 2006;107:3350–3358.
202. Jansson, A, Renner, ED, Ramser, J, et al. Classification of non-bacterial osteitis: retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford). 2007;46:154–160.
203. Khanna, G, Sato, TS, Ferguson, P. Imaging of chronic recurrent multifocal osteomyelitis. Radiographics. 2009;29:1159–1177.
204. Hayem, G, Bouchaud-Chabot, A, Benali, K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum. 1999;29:159–171.
205. Mandell, GA, Contreras, SJ, Conard, K, et al. Bone scintigraphy in the detection of chronic recurrent multifocal osteomyelitis. J Nucl Med. 1998;39:1778–1783.
206. Fritz, J, Tzaribatchev, N, Claussen, CD, et al. Chronic recurrent multifocal osteomyelitis: comparison of whole-body MR imaging with radiography and correlation with clinical and laboratory data. Radiology. 2009;252:842–851.
207. Fritz, J, Tzaribachev, N, Thomas, C, et al. Magnetic resonance imaging-guided osseous biopsy in children with chronic recurrent multifocal osteomyelitis. Cardiovasc Intervent Radiol. 2012;35:146–153.
208. Huber, AM, Lam, PY, Duffy, CM, et al. Chronic recurrent multifocal osteomyelitis: clinical outcomes after more than five years of follow-up. J Pediatr. 2002;141:198–203.
209. Job-Deslandre, C, Krebs, S, Kahan, A. Chronic recurrent multifocal osteomyelitis: five-year outcomes in 14 pediatric cases. Joint Bone Spine. 2001;68:245–251.
210. Girschick, HJ, Mornet, E, Beer, M, et al. Chronic multifocal non-bacterial osteomyelitis in hypophosphatasia mimicking malignancy. BMC Pediatr. 2007;7:3.
211. Duffy, CM, Lam, PY, Ditchfield, M, et al. Chronic recurrent multifocal osteomyelitis: review of orthopaedic complications at maturity. J Pediatr Orthop. 2002;22:501–505.
212. Prose, NS, Fahrner, LJ, Miller, CR, et al. Pustular psoriasis with chronic recurrent multifocal osteomyelitis and spontaneous fractures. J Am Acad Dermatol. 1994;31:376–379.
213. Holden, W, David, J. Chronic recurrent multifocal osteomyelitis: two cases of sacral disease responsive to corticosteroids. Clin Infect Dis. 2005;40:616–619.
214. Carpenter, E, Jackson, MA, Friesen, CA, et al. Crohn’s-associated chronic recurrent multifocal osteomyelitis responsive to infliximab. J Pediatr. 2004;144:541–544.
215. Deutschmann, A, Mache, CJ, Bodo, K, et al. Successful treatment of chronic recurrent multifocal osteomyelitis with tumor necrosis factor-alpha blockage. Pediatrics. 2005;116:1231–1233.
216. Gallagher, KT, Roberts, RL, MacFarlane, JA, et al. Treatment of chronic recurrent multifocal osteomyelitis with interferon gamma. J Pediatr. 1997;131:470–472.
217. Simm, PJ, Allen, RC, Zacharin, MR. Bisphosphonate treatment in chronic recurrent multifocal osteomyelitis. J Pediatr. 2008;152:571–575.