90 Arterial and Venous Trauma and Great Vessel Injuries
• Thirty percent of patients with great vessel injury (GVI) die within 6 hours of hospital arrival.
• Thirty percent to 50% of patients with blunt aortic injury have no signs of trauma.
• A normal chest radiograph does not exclude GVI.
• Computed tomographic angiography is the diagnostic test of choice to rule out traumatic aortic injury in hemodynamically stable patients.
• For patients too large for a computed tomography scanner, transesophageal echocardiographic evaluation of the aorta should be considered.
• Medical management of GVI is typically used as a bridge to more definitive operative care.
• β-Adrenergic blockade is instituted before nitroprusside in the medical management of GVI to avoid possible reflex tachycardia.
Epidemiology
Few traumatic injuries are more devastating than great vessel injury (GVI). With an average circulating volume of 5 L and a flow rate of up to 4.8 L/min in the circulatory system, it is easy to see why GVI can result in catastrophic outcomes quickly. The true incidence of traumatic aortic injury may never be known; however, according to the National Trauma Data Bank, blunt thoracic aortic injury occurred in 0.3% of trauma patients admitted to the hospital during a 5-year period.1 When patients survive an initial injury to their great vessels, rapid diagnosis and treatment are imperative to prevent subsequent exsanguination within the next minutes to hours. This highlights the ever-emphasized “golden hour” of trauma resuscitation.
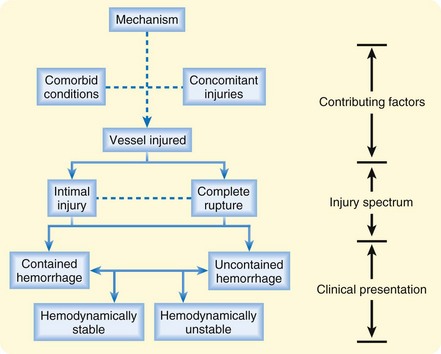
Several contributing factors are important when evaluating potential GVI (Fig. 90.1). Although the mechanism and specific vessel injured are the most important of these factors, significant attention must be paid to the role of concomitant injuries and comorbid conditions on patients’ morbidity and mortality. Unfortunately, on initial evaluation the emergency physician is often lucky to be privy to one, let alone all, of these factors.
The most important branch point for both the likelihood and the type of GVI is a penetrating versus blunt mechanism. Penetrating mechanisms are associated with greater than 90% of great vessel trauma, and any thoracic vascular structure is at risk.2 Patients who survive to arrive at the emergency department, particularly if they are not in hemorrhagic shock, have a survival rate that approaches 50%.3
In contrast, blunt traumatic injuries to the great vessels most often affect the aorta, although the innominate artery, pulmonary hilar vessels, and vena cava are also susceptible. Blunt aortic rupture carries an immediate mortality rate of greater than 80% and is responsible for 10% to 15% of motor vehicle accident fatalities.4 Because of the high association of blunt ascending aortic injury with fatal cardiac injury, the vast majority of those who survive to hospital evaluation have descending injuries. Of patients who survive until medical evaluation, 30% die within 6 hours and 40% within 24 hours.4 Because most of these injuries occur in young healthy males, the overall survival rate is much better than expected given the severity of injury.
Though incompletely understood, it is proposed that blunt aortic injury can result from any combination of shearing forces, rotational forces, increased intraluminal aortic pressure, or a pinching mechanism between the sternum and vertebral column. Given these forces, it is not a surprise that motor vehicle collisions cause the majority of blunt aortic injuries. This association increases with the speed of the accident.5,6 Shearing forces were originally thought to be the highest in frontal-impact accidents, where deceleration forces are the greatest. More recent studies, however, have shown that side-impact accidents are associated with a higher risk for blunt aortic injury. A review of 119 cases of known blunt aortic injury as a result of car accidents in the United Kingdom found that lateral impact direction to the same side was highly associated with aortic injury.7 A review of accident data from the United Kingdom and United States in 2004 mirrored these results and found that side impact involving the patient’s side of the vehicle carried a significantly higher risk for aortic injury than did frontal impact.8 Although motor vehicle accidents account for the majority of blunt GVI, falls from a height and crushing forces have also been known to cause the disease process.5
In part because of difficulty isolating the hilum, injuries to the pulmonary arteries, veins, and thoracic vena cava are associated with mortality rates greater than 60%, regardless of whether they are caused by blunt or penetrating force, although the latter is much more common.9
Concomitant injuries clearly play a role in the epidemiology, morbidity, and mortality of GVI. One study on blunt thoracic trauma showed that patient with traumatic aortic injury carried a mean injury severity score (ISS) of 40 whereas patients without vascular injury had a mean ISS of just 16.5 Another showed that closed head injury was diagnosed in more than half of patients with GVI, with one quarter having intracranial hemorrhage.3
PathopHysiology
Arterial System
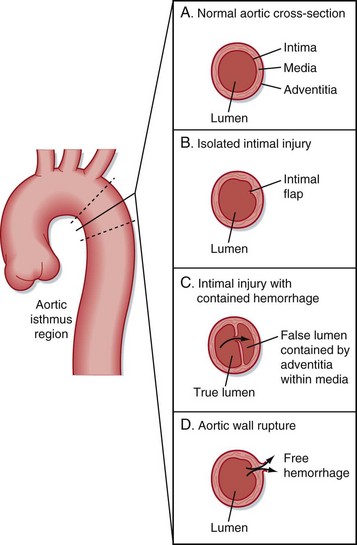
The microanatomy of the artery wall, with its intimal, medial, and adventitial layers, is integral in the spectrum of disease. Injuries range from isolated thrombogenic intimal flaps to full-thickness tears with free hemorrhage (Fig. 90.2).
Presenting Signs and Symptoms (Box 90.1)
Traditionally, aortic dissection as a result of nontraumatic causes is believed to be manifested as tearing pain radiating through the chest to the interscapular region of the back. This pain can be accompanied by various degrees of associated symptoms, including shortness of breath or vagal complaints. In patients with traumatic injury, this symptom pattern is seen less than 25% of the time; these patients most frequently either have vague chest-related complaints or no complaints at all because of distracting injuries. A remarkable 30% to 50% of patients with a blunt aortic injury may have no external signs of trauma.10
The signs and symptoms of GVI often result from its effect on blood flow, which can be secondary to direct vessel injury, traumatic thrombus formation, or vascular compression from surrounding hematoma. Of great concern are clinical signs of hemorrhagic shock such as hypotension, tachycardia, altered mental status, pallor, or diaphoresis. Frequently, hypertension occurs as a result of increased stimulation of sympathetic nerve fibers in close proximity to the aortic arch.11 Additionally, many of the other signs can be subtle. Dyspnea may result for any number of reasons, such as associated pulmonary injury, hemothorax, hypovolemia with poor tissue oxygenation, or tamponade. Neurologic symptoms can be found in patients with arterial injury involving the carotids or spinal arteries.
Unfortunately, none of the aforementioned signs and symptoms are sensitive or specific for making the diagnosis of GVI.10
Differential Diagnosis and Medical Decision Making
GVI is easily misdiagnosed in hemodynamically stable patients, particularly those without external signs of trauma, because of the nonspecific nature of the initial signs and symptoms of GVI. Penetrating injury in proximity to any of the great vessels mandates consideration of GVI. However, a diagnosis of blunt injury requires assessment of the severity of the causative mechanism (e.g., speed, forces), in combination with the patient’s complaints and findings on physical examination. This pretest probability will ultimately be used by the clinician to guide further diagnostic evaluation for GVI (Box 90.2).
Box 90.2 Pros and Cons of Imaging Modalities for Great Vessel Injury
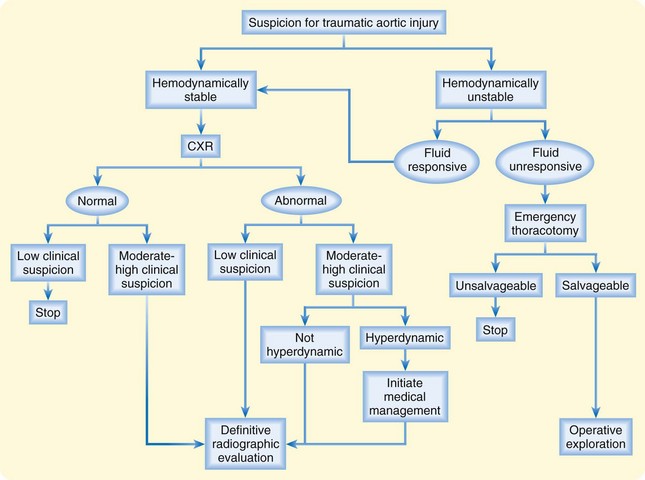
GVI is often associated with either significant multisystem trauma or distinct penetrating injury. In the first situation, the signs and symptoms of GVI are frequently obscured by other distracting injuries, altered mental status, or intubation, thereby necessitating a high level of suspicion and a low threshold for diagnostic testing. In the second situation, diagnostic testing is driven mainly by the pretest probability of GVI (i.e., the location and mechanism of the injury). Figure 90.3 depicts a diagnostic management algorithm for suspected traumatic aortic injury.
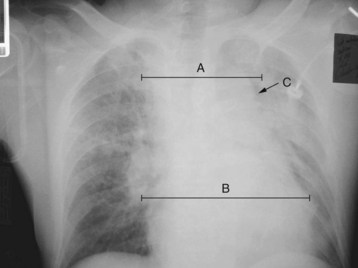
Chest radiography is not diagnostic of GVI; rather, it is used to identify any of the multiple findings suggestive of aortic injury. Box 90.3 reviews the classic findings on chest radiography associated with GVI; a widened superior mediastinum (50% to 92%), increased mediastinal width (67% to 85%), and indistinct aortic knob (21% to 24%) occur with the greatest frequencies.3,12 Figure 90.4 is a chest radiograph demonstrating these three findings.
Box 90.3
Radiographic Findings Associated with Great Vessel Injury
When GVI is a persistent concern following or despite the chest radiograph, computed tomographic angiography (CTA) of the chest should be carried out. This test provides significant information on patients with thoracic trauma, including identification of mediastinal hematoma and differentiation of the various causes. Recent advances in technology of helical computed tomography (CT) have improved its sensitivity and specificity for aortic injury, including isolated intimal tears, such that it approaches 100%.13,14 More recent studies focusing on the use of 64-slice multidetector CT have added to the evidence that sensitivity and specificity approaching 100% make this the preferred imaging modality.15 An additional benefit is its ability to identify concomitant or alternative injuries. Limitations of CTA of the chest for GVI include poor delineation of nonaortic vascular injuries and the need for relative hemodynamic stability in the patient.5,13
Because of the ease and accuracy of CTA, use of the traditional “gold standard” for traumatic aortic injury, aortography, has markedly diminished over the past decade. In fact, newer studies are questioning the role of aortography even in the setting of equivocal CT findings. Several studies have shown that catheter angiography following equivocal findings on CTA is unlikely to reveal GVI, which has led some authors to suggest that patients be monitored clinically with or without repeated cross-sectional imaging, thus obviating the need for aortography.16
Transesophageal echocardiography (TEE) is another modality that can be used to evaluate aortic injury. Availability reduces its usefulness in the emergency setting, but it should be considered for patients who are too unstable to leave the department or when body habitus prohibits the use of a CT scanner. Its use is contraindicated in patients with unstable cervical spine injuries or suspected esophageal trauma. TEE has similar sensitivity as CTA of the chest in the evaluation of traumatic aortic injury.14
Treatment
Definitive Treatment
Definitive treatment of GVI is usually surgical or endovascular repair. With mortality rates associated with these injuries increasing at an estimated rate of 1% per hour over the 48 hours after arrival at the hospital, expediting time to intervention is imperative.4 Surgical techniques usually include clamping and aortic reconstruction with or without vascular bypass. In part because of the need for vascular clamping, paraplegia can be a complication of repair in up to 4% to 20% of patients.3,17 Although cross-clamp times are not thought to directly correlate with the incidence of paraplegia, keeping times under 30 minutes is believed to be beneficial.3,17
More recently, endovascular stenting has become an alternative to open surgical repair.18,19 Potential benefits of endovascular repair include a less invasive approach and avoidance of aortic cross-clamping (which may lead to lower rates of paraplegia), cardiopulmonary bypass, and systemic heparinization. Endovascular stenting can be performed in less time and in patients too unstable for operative intervention. A metaanalysis of endovascular versus open repair published in 2008 showed lower mortality rates and lower risk for paraplegia with endovascular repair. It is thought that the mortality benefit relates to the less invasive approach and lack of systemic heparinization given that the majority of these patients have concomitant intraabdominal, intracerebral, or intrapulmonary injuries at high risk for bleeding complications.20
Before surgical or endovascular intervention, medical management is critical and should include pharmacologic reduction of wall tension and shearing forces to prevent propagation of intimal tears and minimize the risk for subsequent in-hospital catastrophic rupture of a contained hemorrhage. One study demonstrated that by starting pharmacologic management as soon as possible, even before a confirmative diagnostic test when suspicion is high enough, morbidity and mortality were reduced significantly.13
Beta-blockers are the first-line medication for reducing wall stress and controlling the heart rate. Esmolol is ideal because of its rapid onset of action and short half-life, which makes it easy to titrate in a continuous infusion. When further blood pressure control is desired, vasodilators such as nitroprusside can be added after beta-blockade has been established. Because of the potential for reflex tachycardia, which increases shearing forces, caution should be exercised when using nitroprusside alone.21,22 Table 90.1 lists drug dosages for esmolol and nitroprusside.
| DRUG | DOSE |
|---|---|
| Esmolol* | |
| Bolus | 0.5 mg/kg over 1-min period |
| Continuous infusion | 50-200 mcg/kg/min |
| Nitroprusside | 0.3-10 mcg/kg/min |
* A beta-blocker is first-line treatment; nitroprusside should be added if blood pressure control is not achieved. The goal is systolic blood pressure between 100 and 120 mm Hg.
A second but controversial intervention is permissive hypovolemia, in which blood pressure is controlled by limiting fluid administration. Lower systolic pressures of 60 to 90 mm Hg are thought to decrease the risk for clot rupture and minimize the shear force on traumatized vessels. Patients often have associated pulmonary contusions, which strengthens the rationale for limiting fluid administration before operative intervention.23,24 Animal studies using permissive hypovolemia have shown consistent benefit, whereas human trials have been few and the results conflicting.
Ideally, immediate surgical or endovascular repair of GVI has been recommended. However, recent studies have looked at delayed repair, particularly in patients with significant associated injuries or hemodynamic instability. Repair of great vessels has been delayed as long at 6 to 8 months.25 One prospective multicenter study in 2009 examined delayed repair of blunt traumatic aortic injury in stable patients and found significant survival benefits in all patient groups, particularly striking in those with associated injuries.26 Delayed repair is not routinely performed yet but may become more prevalent as further studies focus on this approach.
Fitzharris M, Franklyn M, Frampton R, et al. Thoracic aortic injury in motor vehicle crashes: the effect of impact direction, side of body struck, and seat belt use. J Trauma. 2004;57:582–590.
Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187–192.
Xenos E, Abedi N, Davenport D, et al. Meta-analysis of endovascular versus open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343–1351.
1 Garcia-Toca M, Naughton P, Matsumura J, et al. Endovascular repair of blunt traumatic thoracic aortic injuries. Arch Surg. 2010;145:679–683.
2 Campbell NC, Thomason SR, Muckart DJ, et al. Review of 1198 cases of penetrating cardiac trauma. Br J Surg. 1997;84:1737–1740.
3 Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: multicenter trial of the American Association of the Surgery of Trauma. J Trauma. 1997;42:374–383.
4 Parmley LF, Mattingly TW, Manion WC, et al. Nonpenetrating traumatic injury of the aorta. Circulation. 1958;17:1086–1101.
5 Dyer DS, Moore EE, Ilke DN, et al. Thoracic aortic injury: how predictive is mechanism and is chest computed tomography a reliable screening tool? J Trauma. 2000;48:673–683.
6 Ungar TC, Wolf SJ, Haukoos JS, et al. Derivation of a clinical decision rule to exclude thoracic aortic imaging in patients with blunt chest trauma following motor vehicle collisions. J Trauma. 2006;61:1150–1155.
7 Sastry P, Field M, Cuerden R, et al. Low-impact scenarios may account for two-thirds of blunt aortic rupture. Emerg Med J. 2010;27:341–344.
8 Fitzharris M, Franklyn M, Frampton R, et al. Thoracic aortic injury in motor vehicle crashes: the effect of impact direction, side of body struck, and seat belt use. J Trauma. 2004;57:582–590.
9 Mattox KL, Feliciano DV, Burch J, et al. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958-1988. Ann Surg. 1989;209:698–705. discussion 706–7
10 Sturm JT, Perry Jr, JF., Olson FR, et al. Significance of symptoms and signs in patients with traumatic aortic rupture. Ann Emerg Med. 1984;13:876–878.
11 Fox S, Pierce WS, Waldhausen JA. Acute hypertension: its significance in traumatic aortic rupture. J Thorac Cardiovasc Surg. 1979;77:622–625.
12 Mirvis SE, Bidwell JK, Buddemeyer EU, et al. Value of chest radiography in excluding traumatic aortic rupture. Radiology. 1987;163:487–493.
13 Fabian TC, Davis KA, Gavant ML, et al. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg. 1998;227:666–677.
14 Vignon P, Boncoeur MP, Francois B, et al. Comparison of multiplane transesophageal echocardiography and contrast-enhanced helical CT in the diagnosis of blunt traumatic cardiovascular injuries. Anesthesiology. 2001;94:615–622.
15 Steenburg S, Ravenel J. Acute traumatic thoracic injuries: experience with 64-MDCT. AJR Am J Roentgenol. 2008;191:1564–1569.
16 Sammer M, Wang E, Blackmore C, et al. Indeterminate CT angiography in blunt thoracic trauma: is CT angiography enough? AJR Am J Roentgenol. 2007;189:603–608.
17 Razzouk AJ, Gundry SR, Wang N, et al. Repair of traumatic aortic rupture: a 25-year experience. Arch Surg. 2000;135:913–918.
18 Rousseau H, Dambrin C, Marcheix B, et al. Acute traumatic aortic rupture: a comparison of surgical and stent-graft repair. J Thorac Cardiovasc Surg. 2005;129:1050–1055.
19 Agostinelli A, Saccani S, Borrello B, et al. Immediate endovascular treatment of blunt aortic injury: our therapeutic strategy. J Thorac Cardiovasc Surg. 2006;131:1053–1057.
20 Xenos E, Abedi N, Davenport D, et al. Meta-analysis of endovascular versus open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343–1351.
21 Walker WA, Pate JW. Medical management of acute traumatic rupture of the aorta. Ann Thorac Surg. 1990;50:965–967.
22 Maggisano R, Nathens A, Alexandrova NA, et al. Traumatic rupture of the thoracic aorta: should one always operate immediately? Ann Vasc Surg. 1995;9:44–52.
23 Bickell WH, Wall MJ, Pep P, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109.
24 Martin RR, Bickell WH, Pep PE, et al. Prospective evaluation of preoperative fluid resuscitation in hypotensive patients with penetrating truncal injury: a preliminary report. J Trauma. 1992;33:354–361.
25 Duhaylongsod FG, Glower DD, Wolfe WG. Acute traumatic aortic aneurysm: the Duke experience from 1970-1990. J Vasc Surg. 1992;15:331–342.
26 Demetriades D, Velmahos G, Scalea T, et al. Blunt traumatic thoracic aortic injuries: early or delayed repair—results of an American Association for the Surgery of Trauma prospective study. J Trauma. 2009;66:967–973.